Published online Apr 21, 2021. doi: 10.3748/wjg.v27.i15.1630
Peer-review started: December 21, 2020
First decision: January 10, 2021
Revised: January 24, 2021
Accepted: March 22, 2021
Article in press: March 22, 2021
Published online: April 21, 2021
Processing time: 113 Days and 21.3 Hours
Recurrent acute pancreatitis (RAP) may be a presenting feature of and an indication for resection of pancreatic cysts, including intra-ductal papillary mucinous neoplasm (IPMN). Few data are available regarding the prevalence of malignancy and post-operative RAP in this population.
To study the role of resection to help prevent RAP and analyze if presentation as RAP would be a predictor for malignancy.
This retrospective study assessed 172 patients who underwent surgical resection of pancreatic cystic neoplasms at a university hospital between 2002 and 2016. The prevalence of preoperative high-risk cyst features, and of neoplasia was compared between patients with and without RAP. To identify the cause of pancreatitis, all the patients had a detailed history of alcohol, smoking, medications obtained, and had cross-sectional imaging (contrast-enhanced computed tomography/magnetic resonance imaging) and endoscopic ultrasound to look for gallstone etiology and other structural causes for pancreatitis. The incidence of RAP post-resection was the primary outcome.
IPMN accounted for 101 cases (58.7%) {[branch duct (BD) 59 (34.3%), main duct (MD) 42] (24.4%)}. Twenty-nine (16.9%) presented with RAP (mean 2.2 episodes): 15 had BD-IPMN, 8 MD-IPMN, 5 mucinous cystic neoplasm and 1 serous cystic neoplasm. Malignancy was similar among those with vs without RAP for all patients [6/29 (20.7%) vs 24/143 (16.8%)] and IPMN patients [6/23 (26.1%) vs 23/78 (29.5%)], although tended to be higher with RAP in BD-IPMN, [5/15 (33.3%) vs 3/44 (6.8%), P = 0.04]. At mean follow-up of 7.2 years, 1 (3.4%) RAP patient had post-resection RAP. The mean episodes of acute pancreatitis before vs after surgery were 3.4 vs 0.02 (P < 0.0001).
Malignancy was not increased in patients with pancreatic cystic neoplasms who have RAP compared to those without RAP. In addition, specific cyst charac-teristics were not clearly associated with RAP. The incidence of RAP was markedly decreased in almost all patients following cyst resection.
Core Tip: Our findings from this original study support international consensus guidelines recommendations for surgical resection of pancreatic cystic neoplasms presenting with acute pancreatitis but more for the effective treatment of recurrent acute pancreatitis (RAP) rather than for the identification of malignancy. The objective of this research was to study the role of resection to help prevent RAP and analyze if presentation as RAP would be a predictor for malignancy.
- Citation: Muniraj T, Aslanian HR, Laine L, Jamidar PA, Farrell JF, Mitchell KA, Salem RR. Resection of pancreatic cystic neoplasms in recurrent acute pancreatitis prevents recurrent pancreatitis but does not identify more malignancies. World J Gastroenterol 2021; 27(15): 1630-1642
- URL: https://www.wjgnet.com/1007-9327/full/v27/i15/1630.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i15.1630
Acute pancreatitis is among the most frequent gastrointestinal causes of hospital admission in the United States with nearly 280000 hospitalizations annually[1]. While pancreatic cysts in the setting of pancreatitis are frequently assumed to be pseudocysts, neoplastic pancreatic cysts are recognized as a cause of acute pancreatitis. Intra-ductal papillary mucinous neoplasms (IPMN) are the most common cystic pancreatic neoplasms. They arise within the main pancreatic duct or side-branches and are characterized by production of thick mucinous fluid. Additional pancreas cystic neoplasms include mucinous cystic neoplasm and serous cystic neoplasm. Pancreatic cysts may cause acute pancreatitis by compression of the pancreatic duct or by obstruction of the pancreatic duct with mucus. The reported rate of acute pancreatitis among patients with IPMN varies between 7% and 67%[2-5]. A careful clinical history could help to differentiate such neoplastic cysts from a mature, encapsulated fluid collection without necrosis (pseudocyst) or with necrosis (walled-off necrosis, WON) that may occur as a cystic lesion after 4 wk from the onset of acute pancreatitis[6]. The presence of a pancreatic cyst in imaging at diagnosis of index pancreatitis is probably a neoplastic cyst, while the detection of a cyst in the follow-up of pancreatitis with a normal first imaging exam likely a post inflammatory pseudocyst or WON.
The diagnosis of acute pancreatitis is based on the presence of two of the following three features: (1) Abdominal pain consistent with acute pancreatitis (acute onset of a persistent, severe, epigastric pain often radiating to the back); (2) Serum lipase activity (or amylase activity) at least three times greater than the upper limit of normal; And (3) Characteristic findings of acute pancreatitis on contrast-enhanced computed tomography (CECT) or magnetic resonance imaging (MRI) or transabdominal ultrasonography[6]. Recurrent acute pancreatitis (RAP), defined as two or more definite episodes of acute pancreatitis, occurs in approximately 17% to 29% of patients who present with a first episode of acute pancreatitis[5,7-9]. Approximately 80% of RAP is related to alcohol and gallstones. Other etiologies include medications, pancreas divisum, sphincter of Oddi dysfunction, pancreatic cancer, hypertriglyceridemia, hypercalcemia, infections, and genetic predisposition[10,11].
Few data are available regarding the prevalence of malignancy and on the course of RAP associated with pancreatic cystic neoplasms, such as IPMN, and the potential benefit of surgical resection in patients with acute pancreatitis due to pancreatic cysts. We, therefore, performed a retrospective study to assess the characteristics of patients with pancreatic cystic neoplasms who present with unexplained RAP and the effect of surgical resection on the natural history.
We conducted a retrospective cohort study at the Yale-New Haven Hospital to identify patients who underwent surgical resection of pancreatic cystic neoplasms between January 1, 2002 and December 1, 2016. Those who had RAP, defined as 2 or more episodes of acute pancreatitis without an identifiable cause prior to resection[12], and imaging [computed tomography, magnetic resonance imaging, and/or endoscopic ultrasound (EUS)] documenting the cyst pre-operatively were defined as the RAP cohort. To identify the cause of pancreatitis, all the patients had detailed history of alcohol, smoking, medications obtained and had cross-sectional imaging (CECT/MRI) and EUS to look for gallstone etiology and other structural causes for pancreatitis. The diagnosis was double-checked. Patients with resection who did not have prior RAP served as the control cohort. Patient follow-up extended until September 2018. The study was approved by the institutional review board at Yale University.
Preoperative classification was based on preoperative imaging and results of fine-needle aspiration, if performed. Cysts were classified as IPMNs and non-IPMNs. The IPMNs were sub-classified as main-duct (MD) and branch-duct (BD). Mixed-type IPMNs were included in the MD group. Preoperative diagnosis of IPMNs were based on clinical findings from the images obtained. Histopathology from post-surgical resection specimens was used as the gold standard to ascertain the final diagnosis. The pathological classification and pre-surgical classification were made on the basis of World Health Organization criteria and current literature[13-16]. Performing metastatic urothelial cancer (MUC) staining or gene sequencing
Malignancy was defined as the presence of invasive carcinoma or high-grade dysplasia on final surgical pathology. Patients with no follow-up data post-operatively, known pseudocysts and predominantly solid pancreatic masses were excluded. Patient demographic, clinical, radiographic, and pathologic data were extracted from the medical record. In patients with multiple cysts, the features of the largest cyst or the cyst with the most concerning high-risk features (e.g., mural nodules) were the characteristics recorded for the patient. Patient data post-resection were also extracted, with the primary goal of identifying episodes of acute pancreatitis. On surgically resected specimens, MUC staining, histologic examination and gene sequencing were used for pathologic subclassification[17,18].
The primary analysis involved a comparison of the number of episodes of acute pancreatitis pre- and post-resection of pancreas cyst in patients who had RAP. We also compared baseline patient and cyst characteristics between patients with RAP and those without RAP. Comparisons between the RAP cohort and control (non-RAP) cohort and between the preoperative and post-operative time period were performed with the Mantel-Haenszel risk difference calculation for continuous variables and Mantel-Haenszel risk ratio for proportions. Statistical analysis was performed by using the statistical algorithms in Cochrane Review Manager Software 5.3.
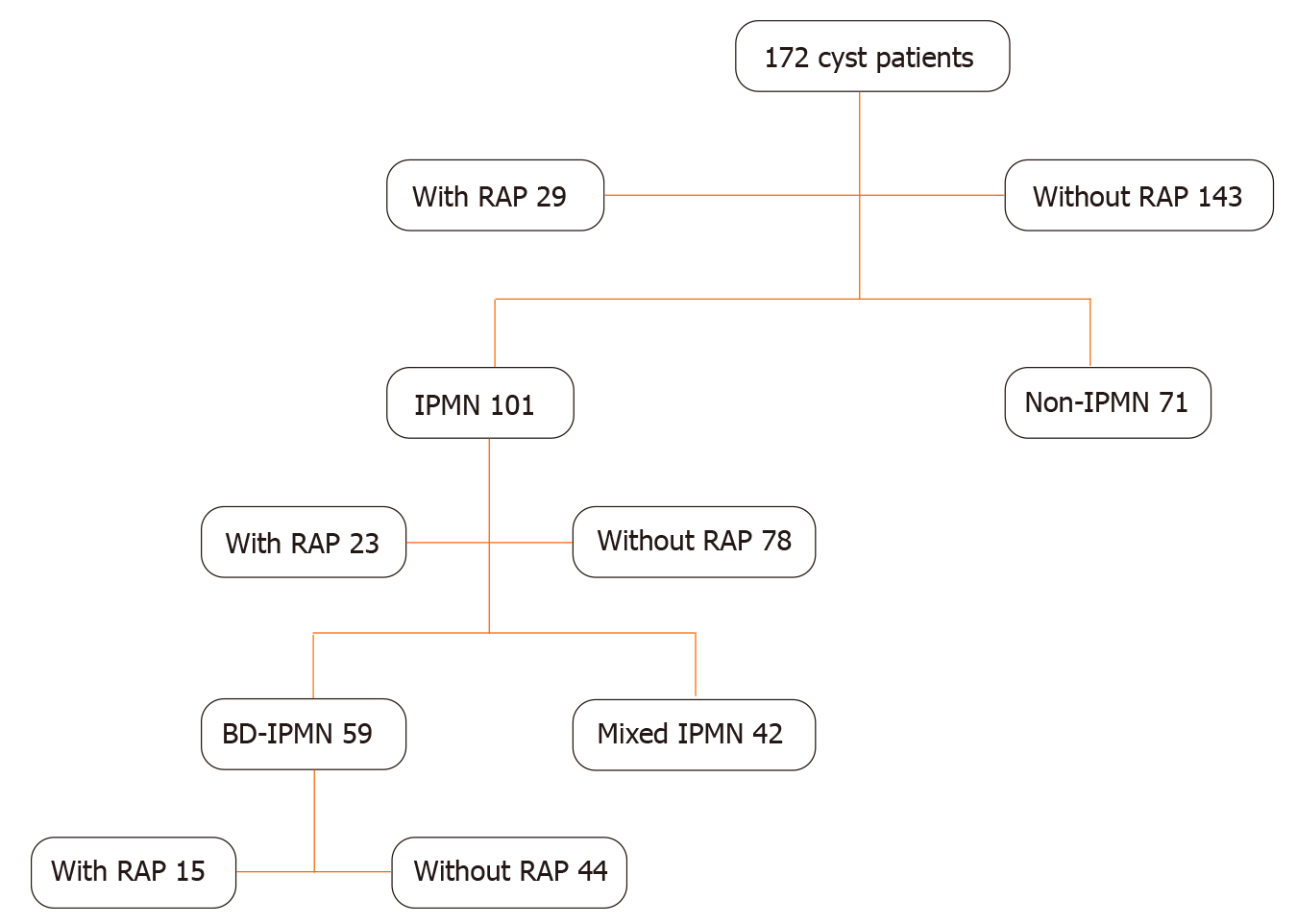
A total of 172 patients with pancreatic cysts underwent surgery between 2002 and 2016. The indication for surgery was RAP along with cyst characteristics in 11 patients and among all other patients, the indication for surgery was based on the cyst morphology. IPMNs accounted for 101 (58.7%) of these 172 cases {BD-IPMN [59 (34.3%)] and MD-IPMN [42 (24.4%)]} (Table 1 and Figure 1). The next most common types of lesions were mucinous cystic neoplasms [32/172 (18.6%)] and serous cystic neoplasms [16/172 (9.3%)] (Table 1). Since preoperative diagnosis on cysts were made using radiologic findings, the final diagnosis might have varied based on surgical findings. Twenty-nine (16.9%) patients had RAP without other identifiable cause prior to resection, while the remaining 143 (83.1%) patients had no episodes of acute pancreatitis prior to resection. No patient had evidence of chronic pancreatitis. Among the 29 patients with RAP with cystic neoplasms, the surgical procedure performed was pancreaticoduodenectomy (n = 18) or distal pancreatectomy (n = 11) with surgical mortality 0%, morbidity 3/29 (10%) (one patient had mesenteric bleeding requiring immediate exploratory laparotomy, one had afferent limb syndrome 2 wk later required exploratory laparotomy for adhesion lysis, and other with a delayed abscess in left upper quadrant managed with external drainage.
| IPMN cysts (total) | 101 |
| BD-IPMN | 59 |
| MD-IPMN | 42 |
| Non-IPMN cysts (total) | 71 |
| Mucinous cystic neoplasm | 32 |
| Serous cystic neoplasm | 16 |
| Pancreatic neuroendocrine tumor | 11 |
| Solid pseudopapillary epithelial neoplasm | 6 |
| Squamoid | 3 |
| Benign | 2 |
| Cystic degeneration | 1 |
The mean number of pancreatitis episodes per patient prior to resection in the RAP cohort was 2.2 (range 2-32 episodes). The median elapsed time between the first episode of acupuncture (AP) and surgery was 17 mo (range 1.5 mo to 7 years). The mean follow-up after surgery was 7.2 years (range 1.9-13.8 years).
Only 1 (3%) of 29 RAP patients (with BD-IPMN) had acute pancreatitis documented post-resection. The mean number of recurrent episodes of acute pancreatitis per patient-year before vs after surgery was 3.40 vs 0.02 (absolute difference = 3.38, 95%CI 1.76 to 5.00; P < 0.0001)). All the episodes were mild acute pancreatitis and none of the patients in this cohort had severe acute pancreatitis[3]. A marked decrease was seen in all types of cysts (Table 2). The one patient with recurrent pancreatitis after resection had BD-IPMN; he had 8 episodes before resection and 32 after resection and progressed to chronic pancreatitis with ductal calcification at 6 years after resection. No patient without prior RAP developed acute pancreatitis after resection.
| Episodes per patient-year (mean + SD) | Mean difference [95%CI] | P value | ||
| Pre-resection | Post-resection | |||
| All cysts (n = 29) | 3.40 (4.46) | 0.02 (0.08) | 3.38 [1.76, 5.00] | < 0.0001 |
| IPMN (n = 23) | 2.32 (1.69) | 0.03 (0.09) | 2.29 [1.60, 2.98] | < 0.0001 |
| BD-IPMN (n = 15) | 2.06 (1.85) | 0.04 (0.11) | 2.02 [1.08, 2.96] | < 0.0001 |
| MD-IPMN (n = 8) | 2.81 (1.32) | 0 (0)1 | 2.81 [1.83, 3.79] | < 0.0001 |
| Non-IPMN (n = 6) | 7.52 (8.53) | 0 (0)1 | 7.52 [0.69, 14.35] | 0.03 |
Selected characteristics of the RAP and control cohorts are shown in Table 3. Median age and proportion who were male were comparable. No clear differences were identified between the cohorts in characteristics of the cystic lesions, including in size, or proportion with malignancy except for location in the tail of pancreas where most cyst patients were without RAP. IPMNs tended to be more common among patients with RAP than in those without RAP (79.3% vs 54.5%; risk difference = 0.25; 95%CI: 0.08, 0.41, P = 0.004) with most of the difference seen in the BD-IPMN patients.
| With RAP (n = 29) | Without RAP (n = 143) | Risk difference [95%CI] | P value | |
| n (%) | n (%) | |||
| Median age | 61 (IQR 18) | 65 (IQR 15) | NA | 0.65 |
| Sex (M) | 13 (44.8) | 58 (40.6) | NA | 0.67 |
| Location of cyst | ||||
| Head | 16 (55.2) | 65 (45.5) | 0.10 [-0.10, 0.30] | 0.34 |
| Neck | 2 (6.9) | 7 (4.9) | 0.02 [-0.08, 0.12] | 0.69 |
| Body | 8 (27.6) | 32 (22.4) | 0.05 [-0.12, 0.23] | 0.56 |
| Tail | 3 (10.3) | 37 (25.9) | -0.16 [-0.29, -0.02] | 0.02 |
| Diffuse | 0 (0) | 2 (1.4) | -0.01 [-0.06, 0.04] | 0.59 |
| Main pancreas duct > 10 | 4 (13.8) | 10 (7.0) | 0.07 [-0.06, 0.20] | 0.31 |
| Cyst size > 3 | 6 (20.7) | 36 (25.2) | -0.04 [-0.21, 0.12] | 0.59 |
| Solid component | 7 (24.1) | 46 (32.2) | -0.08 [-0.25, 0.09] | 0.36 |
| Malignancy | 6 (20.7) | 24 (16.8) | 0.04 [-0.12, 0.20] | 0.63 |
| Pathological subtype | ||||
| Intestinal | 10 (34.5) | 11 (7.7) | 0.27 [0.09, 0.45] | 0.003 |
| Gastric | 3 (10.3) | 43 (30.1) | -0.20 [-0.33, -0.06] | 0.004 |
| Pancreatobiliary | 7 (24.1) | 15 (10.5) | 0.14 [-0.03, 0.30] | 0.10 |
| Oncocytic | 0 (0) | 3 (2.1) | -0.02 [-0.07, 0.03] | 0.43 |
| Unknown | 9 (31.0) | 71 (49.7) | -0.19 [-0.37, 0.001] | 0.05 |
| Cyst type | ||||
| IPMN total | 23 (79.3) | 78 (54.5) | 0.25 [0.08, 0.41] | 0.004 |
| BD-IPMN | 15 (51.7) | 44 (30.8) | 0.21 [0.01, 0.41] | 0.04 |
| MD-IPMN | 8 (27.6) | 34 (23.8) | 0.04 [-0.14, 0.22] | 0.67 |
| Non-IPMN | 6 (20.7) | 65 (45.5) | -0.25 [-0.42, -0.08] | 0.004 |
Table 4 shows the results for the RAP and control cohorts in all patients with IPMN. Patients with RAP were more likely to have intestinal and less likely to the gastric IPMN cysts (intestinal: 34.8% vs 12.8%; risk difference: 0.22 (95%CI: 0.01, 0.43); P = 0.04; gastric: 13% vs 53.9%; risk difference: -0.41 (95%CI: -0.58, -0.23); P < 0.0001). The pathological subtypes of 21.8% of RAP patients and 10.3% of non-RAP patients was unknown.
| With RAP (n = 23) | Without RAP (n = 78) | Risk difference [95%CI] | P value | |
| n (%) | n (%) | |||
| Median age | 64 (IQR 17) | 67 (IQR 12) | NA | 0.64 |
| Sex (M) | 13 (56.5) | 38 (48.7) | NA | 0.51 |
| Location of cyst | ||||
| Head | 16 (69.6) | 46 (59) | 0.11 [-0.11, 0.32] | 0.34 |
| Body/Tail | 7 (30.4) | 32 (41) | -0.11 [-0.32, 0.11] | 0.34 |
| Main pancreas duct > 10 | 4 (17.4) | 10 (12.8) | 0.05 [-0.13, 0.22] | 0.60 |
| Cyst size > 3 cm | 6 (26) | 36 (46.2) | -0.20 [-0.41, 0.01] | 0.06 |
| Solid component | 5 (21.7) | 20 (25.6) | -0.04 [-0.23, 0.16] | 0.69 |
| Malignancy | 6 (26.1) | 23 (29.5) | -0.03 [-0.24, 0.17] | 0.75 |
| Pathological subtype | ||||
| Intestinal | 8 (34.8) | 10 (12.8) | 0.22 [0.01, 0.43] | 0.04 |
| Gastric | 3 (13) | 42 (53.9) | -0.41 [-0.58, -0.23] | < 0.0001 |
| Pancreatobiliary | 7 (30.4) | 15 (19.2) | 0.11 [-0.10, 0.32] | 0.29 |
| Oncocytic | 0 (0%) | 3 (3.8) | -0.04 [-0.11, 0.03] | 0.30 |
| Unknown | 5 (21.8) | 8 (10.3) | 0.11 [-0.07, 0.30] | 0.22 |
Table 5 is restricted to patients with BD-IPMN. Again, no clear differences were seen between cohorts in patient or cyst characteristics with the possible exception of a tendency to a higher prevalence of malignancy in the RAP group vs non-RAP group with BD-IPMNs {33.3% vs 6.8%, [risk difference = 0.27 (95%CI: 0.02, 0.52); P = 0.04]} although the numbers in each cohort were small. A similar difference in pathological subtypes were noted. Patients with RAP were more likely to have BD-IPMN of an intestinal subtype and less likely to have a gastric subtype vs the control cohort [intestinal: 46.7% vs 9.1%; risk difference = 0.38 (95%CI: 0.11, 0.64); P = 0.006; gastric: 0% vs 68.2%; risk difference = -0.68 (95%CI: -0.84, -0.52); P < 0.001]. However, it should be noted that the pathological subtype of 26.7% of BD-IPMNs was not recorded. Based on the significant results from Univariate analysis, multivariable analysis logistic regression performed, however, the results did not vary in any significance (Tables 6-8).
| With RAP (n = 15) | Without RAP (n = 44) | Risk difference [95%CI] | P value | |
| n (%) | n (%) | |||
| Median age | 60 (IQR 23) | 65 (IQR 12.5) | NA | 0.50 |
| Sex (M) | 8 (53.3) | 24 (54.6) | NA | 0.94 |
| Location of cyst | ||||
| Head | 11 (73) | 28 (64) | 0.10 [-0.17, 0.36] | 0.47 |
| Body/Tail | 4 (9.1) | 16 (36) | -0.10 [-0.36, 0.17] | 0.47 |
| Main pancreas duct > 10 | 2 (13.3) | 2 (4.6) | 0.09 [-0.10, 0.27] | 0.35 |
| Cyst size > 3 cm | 6 (40) | 21 (47.7) | -0.08 [-0.37, 0.21] | 0.60 |
| Solid component | 2 (13.3) | 4 (9.1) | 0.04 [-0.15, 0.23] | 0.66 |
| Malignancy | 5 (33.3) | 3 (6.8) | 0.27 [0.02, 0.52] | 0.04 |
| Pathological subtype | ||||
| Intestinal | 7 (46.7) | 4 (9.1) | 0.38 [0.11, 0.64] | 0.006 |
| Gastric | 0 | 30 (68.2) | -0.68 [-0.84, -0.52] | < 0.001 |
| Pancreatobiliary | 4 (26.7) | 7 (16) | 0.11 [-0.14, 0.36] | 0.4 |
| Oncocytic | 0 | 0 | NA | NA |
| Unknown | 4 (26.7) | 3 (6.8) | 0.20 [-0.04, 0.43] | 0.10 |
| With RAP (n = 29) | Without RAP (n = 143) | P value | |
| Odds ratio | 95%CI | ||
| Location of cyst | |||
| Head | - | ||
| Neck | 1.240013 | [0.19933, 7.713993] | 0.818 |
| Body | 1.736854 | [0.570778, 5.285173] | 0.331 |
| Tail | 0.686678 | [0.155679, 3.028833] | 0.62 |
| Diffuse | - | ||
| Pathological subtype | |||
| Intestinal | |||
| Gastric | 0.059886 | [0.013402, 0.267608] | < 0.001 |
| Pancreatobiliary | 0.454606 | [0.122387, 1.688636] | 0.239 |
| Oncocytic | - | ||
| Unknown | 0.355592 | [0.088518, 1.428472] | 0.145 |
| Cyst type | |||
| IPMN total | 3.462462 | [0.782586, 15.31927] | 0.102 |
| BD-IPMN | 1.660612 | [0.558816, 4.934779] | 0.361 |
| MD-IPMN | - | ||
| With RAP (n = 29) | Without RAP (n = 143) | P value | |
| Odds ratio | 95%CI | ||
| Location of cyst | |||
| Head | - | - | |
| Neck | 1.265446 | [0.169046, 9.472912] | 0.819 |
| Body | 0.506621 | [0.111835, 2.295024] | 0.378 |
| Tail | 0.572817 | [0.090659, 3.619267] | 0.554 |
| Diffuse | - | - | |
| Pathological subtype | |||
| Intestinal | - | - | |
| Gastric | 0.096091 | [0.020977, 0.440165] | 0.003 |
| Pancreatobiliary | 0.773074 | [0.199147, 3.001027] | 0.71 |
| Oncocytic | - | ||
| Unknown | 1.034683 | [0.214488, 4.991279] | 0.966 |
| Cyst type | |||
| IPMN total | |||
| BD-IPMN | 1.571892 | [0.530571, 4.65695] | 0.414 |
| MD-IPMN | - | ||
| With RAP (n = 29) | Without RAP (n = 143) | P value | |
| Odds ratio | 95%CI | ||
| Location of cyst | |||
| Head | - | - | |
| Neck | - | - | |
| Body | 0.61173 | [0.059835, 6.254136] | 0.679 |
| Tail | 0.537057 | [0.050552, 5.705601] | 0.606 |
| Diffuse | - | - | |
| Pathological subtype | |||
| Intestinal | - | - | |
| Gastric | - | - | |
| Pancreatobiliary | 0.461648 | [0.063565, 3.352753] | 0.445 |
| Oncocytic | - | - | |
| Unknown | 1.458186 | [0.15039, 14.13861] | 0.745 |
Surgical resection has been recommended for patients with pancreatic cystic neoplasms who present with acute pancreatitis[16], regardless of cyst type. Reasons for resection include identification and removal of underlying malignancy, prevention of the development of malignancy, and prevention of additional attacks of pancreatitis[16]. The characteristics of cystic neoplasms associated with acute pancreatitis and the impact of surgical resection on the prevention of malignancy and future episodes of RAP, however, have not been well defined. We found that cyst characteristics, such as location, size, the proportion with solid component, and prevalence of malignancy were not clearly different between patients with vs without RAP. IPMN was the most common cystic neoplasm among patients with a history of RAP undergoing surgical resection. Although a prior report found MD/combined type IPMN to be more frequently associated with RAP (%)[3] and another study found no difference[5], we found that among patients with IPMN, RAP was more often associated with BD-IPMN (65%) than MD-IPMN (35%). Our results are different from Jang et al[3] where MD/combined IPMN resulting more RAP was thought to be due to mechanical obstruction of the main pancreatic duct by thick mucin, resulting in ductal hypertension, thereby premature activation and release of pancreatic enzymes[3]. However, BD-IPMN leading to AP/RAP is assumed primarily due to the obstruction of the main pancreatic duct by the migration of mucin from the BD or may not be related to mucin but just to the viscosity of cyst fluid[3]. Patients with RAP also were more likely to have intestinal histological subtype vs the control cohort, although histologic subtype was unknown in a third of patients.
Malignancy was present in approximately one-fifth of patients undergoing surgical resection of pancreatic cysts, with similar proportions in those with and without RAP. Malignancy tended to be more common in BD-IPMN patients with RAP (33.3% vs 6.8% without RAP), although given relatively small numbers and multiple comparisons, this finding must be viewed as hypothesis-generating. Prior studies vary in the reported association of malignancy within pancreatic cysts in patients presenting with acute pancreatitis[19]. Some report no increased risk[20-22]. However, a recent study identified IPMN presenting with acute pancreatitis to be associated with an increased risk of malignancy as compared to IPMN without pancreatitis on multivariable analysis[23]. Morales-Oyarvide et al[23] also identified a significant association of AP with IPMN of intestinal subtype (OR = 4.7, 2.5-8.8)[23]. The intestinal phenotype of IPMN has a propensity for mucin hypersecretion, which may promote AP due to ductal obstruction[24-27]. In addition, the intestinal phenotype has been associated with an increased risk of malignancy and with colloid carcinoma[25].
Among the 15 BD-IPMN patients with RAP who had surgical resection in our study, pre-operatively only 1 (6.7%) demonstrated all 3 high-risk features for malignancy (dilated main pancreatic duct > 10 mm, size > 3 cm, solid component)[28] and 3 (20%) had 2 high-risk features. One-third, however, had malignancy identified on surgical pathology. Based on the presence of high-risk stigmata/worrisome features alone, per international revised consensus guidelines of 2017[16,29] or European guidelines[30], resection would not have been indicated in 14 of the 15 BD-IPMN patients if they had not presented with acute pancreatitis. And based on American Gastroenterological Association guidelines, if these patients did not have RAP, only 3 of 15 would have had EUS based on the requirement for at least 2 high-risk features[28]. American College of Gastroenterology guidelines recommends EUS or referral to a multidisciplinary team for pancreatic cystic lesions in the presence of pancreatitis regardless of high-risk features[31]. While observational studies have suggested an increased risk of pancreatic cancer in all patients presenting with acute pancreatitis[19,32], further studies are needed to evaluate whether acute pancreatitis may be an independent predictor of malignancy within pancreatic cysts.
The primary goal of our study was to determine the post-resection course of patients with RAP associated with pancreatic cystic neoplasms. We found that episodes of RAP are rare following surgical resection of pancreatic cystic neoplasms, suggesting that the pancreatic cystic neoplasms were indeed the cause of RAP in almost all cases. Only 1 (3%) of 29 patients presenting with RAP had RAP after resection, and the rate of acute pancreatitis per patient-year decreased from 3.4 to 0.02 (P < 0.0001), with marked reductions in all types of pancreatic cystic lesions. It is also important to note that as the pancreatic exocrine function decreases after pancreas resection, the mere resection itself may reduce the frequency of RAP[33]. Pancreatic surgery for an indication of RAP alone is a very invasive procedure and therefore, risk/benefits should be considered while making the decision. In such cases, since the pancreatic ductal occlusion at the site of the papilla has been postulated to be the main reason for RAP in IPMN, performing an endoscopic pancreatic sphincterotomy with or without stenting may be effective in preventing pancreatitis recurrence[34].
We recognize several limitations to this study, including the retrospective review of data. In addition, this cohort is limited to patients who have undergone surgical resection of pancreas cystic neoplasms, which makes it a selected population not representative of the overall group of patients with pancreatic cystic neoplasms. For example, most patients with BD-IPMN or serous cystic neoplasms do not undergo resection. Patient cases were reviewed to exclude other identifiable causes of acute pancreatitis; however, given the study’s retrospective nature, the evaluation was not standardized. All of the patients in our surgical cohort had RAP, with 2 or more episodes. This is reflective of clinical practice for BD-IPMN, as resection in the absence of multiple high-risk features is typically not pursued following a single episode of idiopathic pancreatitis. Although, this study clearly shows the risk reduction of RAP after the pancreatic resection, further investigation with large population prospective study is needed to prove causal relationship between the cause of RAP and pancreatic resection.
In conclusion, we found that malignancy is not increased in patients with pancreatic cystic neoplasms who have RAP compared to those without RAP. In addition, specific cyst characteristics were not clearly associated with RAP, although intestinal histological subtype appeared to be more common in patients with RAP. Most importantly, episodes of RAP were markedly reduced after cyst resection, with only one of 29 patients having recurrent episodes. Our findings support international consensus guidelines recommendations for surgical resection of pancreatic cystic neoplasms presenting with acute pancreatitis but more for the effective treatment of RAP rather than for the identification of malignancy[16].
Pancreatic cystic neoplasms may present recurrent acute pancreatitis (RAP). Little is known on the role of resection for preventing RAP and if any correlation of higher prevalence of malignancy is seen among these patients.
Predicting malignancy among the pancreatic cystic neoplasms and management of RAP is challenging.
The objective of this research was to study the role of resection to help prevent RAP and analyze if presentation as RAP would be a predictor for malignancy.
We adopted a retrospective cohort study model, enrolling all the patients with pancreatic cystic neoplasms who underwent surgical resection and compare between those who presented with RAP and without RAP. Incidence of RAP after resection and prevalence of malignancy among those who presented with RAP were the primary outcomes.
Malignancy was similar among those with vs without RAP for all patients 20.7% vs 16.8%. The mean episodes of acute pancreatitis before vs after surgery were 3.4 vs 0.02 (P < 0.0001). These findings clearly contribute much to this area of science. Although, this study clearly shows the risk reduction of RAP after the pancreatic resection, further investigation with large population prospective study is needed to prove a causal relationship between the cause of RAP and pancreatic resection.
When patients with RAP are noted to have pancreatic cysts, it should be emphasized to differentiate if those cysts are the cause or the effect of RAP, i.e., if cystic neoplasms are causing the RAP or if cysts are pseudocysts as a result of RAP. Larger data is needed to see if there exists a causal relationship. Similar to the presentation of jaundice as a predictor for malignancy among pancreas cystic neoplasms, RAP may have a role as a predictor of high-risk cysts.
Future research to be focused on a larger prospective cohort to answer the above two questions.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: American Gastroenterological Association; and American Society for Gastrointestinal Endoscopy.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Faias S, Gupta V, Inchingolo R, Sato H S-Editor: Fan JR L-Editor: A P-Editor: Ma YJ
| 1. | Peery AF, Crockett SD, Murphy CC, Lund JL, Dellon ES, Williams JL, Jensen ET, Shaheen NJ, Barritt AS, Lieber SR, Kochar B, Barnes EL, Fan YC, Pate V, Galanko J, Baron TH, Sandler RS. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2018. Gastroenterology 2019; 156: 254-272. e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 776] [Cited by in RCA: 1082] [Article Influence: 180.3] [Reference Citation Analysis (1)] |
| 2. | Venkatesh PG, Navaneethan U, Vege SS. Intraductal papillary mucinous neoplasm and acute pancreatitis. J Clin Gastroenterol. 2011;45:755-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Jang JW, Kim MH, Jeong SU, Kim J, Park DH, Lee SS, Seo DW, Lee SK, Kim JH. Clinical characteristics of intraductal papillary mucinous neoplasm manifesting as acute pancreatitis or acute recurrent pancreatitis. J Gastroenterol Hepatol. 2013;28:731-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Tibayan F, Vierra M, Mindelzun B, Tsang D, McClenathan J, Young H, Trueblood HW. Clinical presentation of mucin-secreting tumors of the pancreas. Am J Surg. 2000;179:349-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Crippa S, Fernández-Del Castillo C, Salvia R, Finkelstein D, Bassi C, Domínguez I, Muzikansky A, Thayer SP, Falconi M, Mino-Kenudson M, Capelli P, Lauwers GY, Partelli S, Pederzoli P, Warshaw AL. Mucin-producing neoplasms of the pancreas: an analysis of distinguishing clinical and epidemiologic characteristics. Clin Gastroenterol Hepatol. 2010;8:213-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 233] [Article Influence: 15.5] [Reference Citation Analysis (2)] |
| 6. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4342] [Article Influence: 361.8] [Reference Citation Analysis (45)] |
| 7. | Ahmed Ali U, Issa Y, Hagenaars JC, Bakker OJ, van Goor H, Nieuwenhuijs VB, Bollen TL, van Ramshorst B, Witteman BJ, Brink MA, Schaapherder AF, Dejong CH, Spanier BW, Heisterkamp J, van der Harst E, van Eijck CH, Besselink MG, Gooszen HG, van Santvoort HC, Boermeester MA. Risk of Recurrent Pancreatitis and Progression to Chronic Pancreatitis After a First Episode of Acute Pancreatitis. Clin Gastroenterol Hepatol. 2016;14:738-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 213] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 8. | Yadav D, O'Connell M, Papachristou GI. Natural history following the first attack of acute pancreatitis. Am J Gastroenterol. 2012;107:1096-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 245] [Article Influence: 18.8] [Reference Citation Analysis (2)] |
| 9. | Sankaran SJ, Xiao AY, Wu LM, Windsor JA, Forsmark CE, Petrov MS. Frequency of progression from acute to chronic pancreatitis and risk factors: a meta-analysis. Gastroenterology 2015; 149: 1490-1500. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 273] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 10. | Coyle WJ, Pineau BC, Tarnasky PR, Knapple WL, Aabakken L, Hoffman BJ, Cunningham JT, Hawes RH, Cotton PB. Evaluation of unexplained acute and acute recurrent pancreatitis using endoscopic retrograde cholangiopancreatography, sphincter of Oddi manometry and endoscopic ultrasound. Endoscopy. 2002;34:617-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 96] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 11. | Sajith KG, Chacko A, Dutta AK. Recurrent acute pancreatitis: clinical profile and an approach to diagnosis. Dig Dis Sci. 2010;55:3610-3616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Testoni PA. Acute recurrent pancreatitis: Etiopathogenesis, diagnosis and treatment. World J Gastroenterol. 2014;20:16891-16901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 65] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 13. | Hruban RPM, Klimstra DS. Tumors of the Pancreas. Washington, DC: American Registry of Pathology, 2007. |
| 14. | Adsay NV, Fukushima N. Bosman FT, Carneiro F, Hruban RH, Theise ND. Intraductal neoplasms of the pancreas, WHO Classification of Tumors. 4th ed. Lyon, France: WHO Press, 2010. |
| 15. | Ma GK, Goldberg DS, Thiruvengadam N, Chandrasekhara V, Kochman ML, Ginsberg GG, Vollmer CM, Ahmad NA. Comparing American Gastroenterological Association Pancreatic Cyst Management Guidelines with Fukuoka Consensus Guidelines as Predictors of Advanced Neoplasia in Patients with Suspected Pancreatic Cystic Neoplasms. J Am Coll Surg 2016; 223: 729-737. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB, Schmidt CM, Shimizu M, Wolfgang CL, Yamaguchi K, Yamao K; International Association of Pancreatology. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1714] [Cited by in RCA: 1614] [Article Influence: 124.2] [Reference Citation Analysis (0)] |
| 17. | Patra KC, Bardeesy N, Mizukami Y. Diversity of Precursor Lesions For Pancreatic Cancer: The Genetics and Biology of Intraductal Papillary Mucinous Neoplasm. Clin Transl Gastroenterol. 2017;8:e86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 18. | Furukawa T, Hatori T, Fujita I, Yamamoto M, Kobayashi M, Ohike N, Morohoshi T, Egawa S, Unno M, Takao S, Osako M, Yonezawa S, Mino-Kenudson M, Lauwers GY, Yamaguchi H, Ban S, Shimizu M. Prognostic relevance of morphological types of intraductal papillary mucinous neoplasms of the pancreas. Gut. 2011;60:509-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 214] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 19. | Goh BK, Tan YM, Cheow PC, Chung YF, Chow PK, Wong WK, Ooi LL. Cystic lesions of the pancreas: an appraisal of an aggressive resectional policy adopted at a single institution during 15 years. Am J Surg. 2006;192:148-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 104] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 20. | Kitagawa Y, Unger TA, Taylor S, Kozarek RA, Traverso LW. Mucus is a predictor of better prognosis and survival in patients with intraductal papillary mucinous tumor of the pancreas. J Gastrointest Surg. 2003;7:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 109] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Sugiyama M, Izumisato Y, Abe N, Masaki T, Mori T, Atomi Y. Predictive factors for malignancy in intraductal papillary-mucinous tumours of the pancreas. Br J Surg. 2003;90:1244-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 308] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 22. | Pelletier AL, Hammel P, Rebours V, Couvelard A, Vullierme MP, Maire F, Hentic O, Aubert A, Sauvanet A, Lévy P, Ruszniewski P. Acute pancreatitis in patients operated on for intraductal papillary mucinous neoplasms of the pancreas: frequency, severity, and clinicopathologic correlations. Pancreas. 2010;39:658-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Morales-Oyarvide V, Mino-Kenudson M, Ferrone CR, Gonzalez-Gonzalez LA, Warshaw AL, Lillemoe KD, Fernández-del Castillo C. Acute pancreatitis in intraductal papillary mucinous neoplasms: A common predictor of malignant intestinal subtype. Surgery. 2015;158:1219-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Aso T, Ohtsuka T, Ideno N, Kono H, Nagayoshi Y, Mori Y, Ohuchida K, Ueda J, Takahata S, Morimatsu K, Aishima S, Igarashi H, Ito T, Ishigami K, Mizumoto K, Tanaka M. Diagnostic significance of a dilated orifice of the duodenal papilla in intraductal papillary mucinous neoplasm of the pancreas. Gastrointest Endosc. 2012;76:313-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Adsay NV, Merati K, Basturk O, Iacobuzio-Donahue C, Levi E, Cheng JD, Sarkar FH, Hruban RH, Klimstra DS. Pathologically and biologically distinct types of epithelium in intraductal papillary mucinous neoplasms: delineation of an "intestinal" pathway of carcinogenesis in the pancreas. Am J Surg Pathol. 2004;28:839-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 337] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 26. | Adsay NV, Merati K, Andea A, Sarkar F, Hruban RH, Wilentz RE, Goggins M, Iocobuzio-Donahue C, Longnecker DS, Klimstra DS. The dichotomy in the preinvasive neoplasia to invasive carcinoma sequence in the pancreas: differential expression of MUC1 and MUC2 supports the existence of two separate pathways of carcinogenesis. Mod Pathol. 2002;15:1087-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 202] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 27. | Hata T, Sakata N, Okada T, Aoki T, Motoi F, Katayose Y, Egawa S, Unno M. Dilated papilla with mucin extrusion is a potential predictor of acute pancreatitis associated with intraductal papillary mucinous neoplasms of pancreas. Pancreatology. 2013;13:615-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Vege SS, Ziring B, Jain R, Moayyedi P; Clinical Guidelines Committee; American Gastroenterology Association. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148:819-22; quize12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 629] [Cited by in RCA: 759] [Article Influence: 75.9] [Reference Citation Analysis (1)] |
| 29. | Tanaka M, Fernández-Del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T, Salvia R, Shimizu Y, Tada M, Wolfgang CL. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017;17:738-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 868] [Cited by in RCA: 1157] [Article Influence: 144.6] [Reference Citation Analysis (1)] |
| 30. | European Study Group on Cystic Tumours of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut. 2018;67:789-804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1006] [Cited by in RCA: 896] [Article Influence: 128.0] [Reference Citation Analysis (1)] |
| 31. | Elta GH, Enestvedt BK, Sauer BG, Lennon AM. ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am J Gastroenterol. 2018;113:464-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 423] [Article Influence: 60.4] [Reference Citation Analysis (1)] |
| 32. | Kirkegård J, Cronin-Fenton D, Heide-Jørgensen U, Mortensen FV. Acute Pancreatitis and Pancreatic Cancer Risk: A Nationwide Matched-Cohort Study in Denmark. Gastroenterology. 2018;154:1729-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 145] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 33. | Moore JV, Tom S, Scoggins CR, Philips P, Egger ME, Martin RCG 2nd. Exocrine Pancreatic Insufficiency After Pancreatectomy for Malignancy: Systematic Review and Optimal Management Recommendations. J Gastrointest Surg. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 34. | Bernardoni L, Crinò SF, De Conti G, Conti Bellocchi MC, De Pretis N, Amodio A, Frulloni L, Gabbrielli A. Preliminary experience with pancreatic sphincterotomy as treatment for intraductal papillary mucinous neoplasm-associated recurrent pancreatitis. Endosc Int Open. 2017;5:E1144-E1150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |