Published online Apr 14, 2021. doi: 10.3748/wjg.v27.i14.1362
Peer-review started: December 24, 2020
First decision: February 11, 2021
Revised: February 14, 2021
Accepted: March 17, 2021
Article in press: March 17, 2021
Published online: April 14, 2021
Processing time: 106 Days and 16.7 Hours
Colorectal cancer (CRC) is among the most prevalent cancers worldwide, and its prevention and reduction of incidence is imperative. The presence of diabetes has been associated with a 30% increased risk of CRC, likely through the mechanism of hyperinsulinemia, which promotes tumorigenesis via the insulin receptor in the epithelium or by insulin-like growth factor pathways, inflammation, or adipokines, inducing cancer cell proliferation and cancer spread. Metformin, the first-line agent in treating type 2 diabetes, has a chemopreventive role in CRC development. Additionally, preclinical studies suggest synergistic effects of metformin with oxaliplatin in inhibiting in vitro models of colon cancer. Although preclinical studies on the post diagnostic use of metformin were promising and suggested its synergistic effects with chemotherapy, the data on the possible effects of metformin after surgery and other CRC treatment in the clinical setting are less conclusive, and randomized controlled trials are still lacking.
Core Tip: Metformin is one of the oldest oral antidiabetic agents used to treat type 2 diabetes mellitus. While there is substantial evidence that metformin may have a chemopreventive role in colorectal cancer (CRC) development, the data on the possible effects of metformin after surgery and other CRC treatment is much less conclusive.
- Citation: Berkovic MC, Mikulic D, Bilic-Curcic I, Mrzljak A. How far along are we in revealing the connection between metformin and colorectal cancer? World J Gastroenterol 2021; 27(14): 1362-1368
- URL: https://www.wjgnet.com/1007-9327/full/v27/i14/1362.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i14.1362
Colorectal cancer (CRC) is one of the most prevalent cancers worldwide, irrespective of gender. Factors contributing to the risk of development of CRC include lifestyle, genetics, and chronic diseases such as diabetes mellitus and obesity[1-3]. Although patient survival has increased over the years due to more effective treatments, there is still a problem with adverse effects and therapy costs. The prevention and reduction of CRC incidence is imperative. Several agents with chemopreventive effects have emerged. Cyclooxygenase-2 inhibitors offered the most promising results in CRC prevention, but their use was associated with elevated cardiovascular risk. Considering that CRC patients often have diabetes mellitus and obesity, which also confers cardiovascular risk, novel targets for CRC chemoprevention are needed. Accumulating data indicates that metformin, the first-line treatment for type 2 diabetes mellitus, may be a candidate chemoprevention agent for cancer, including CRC.
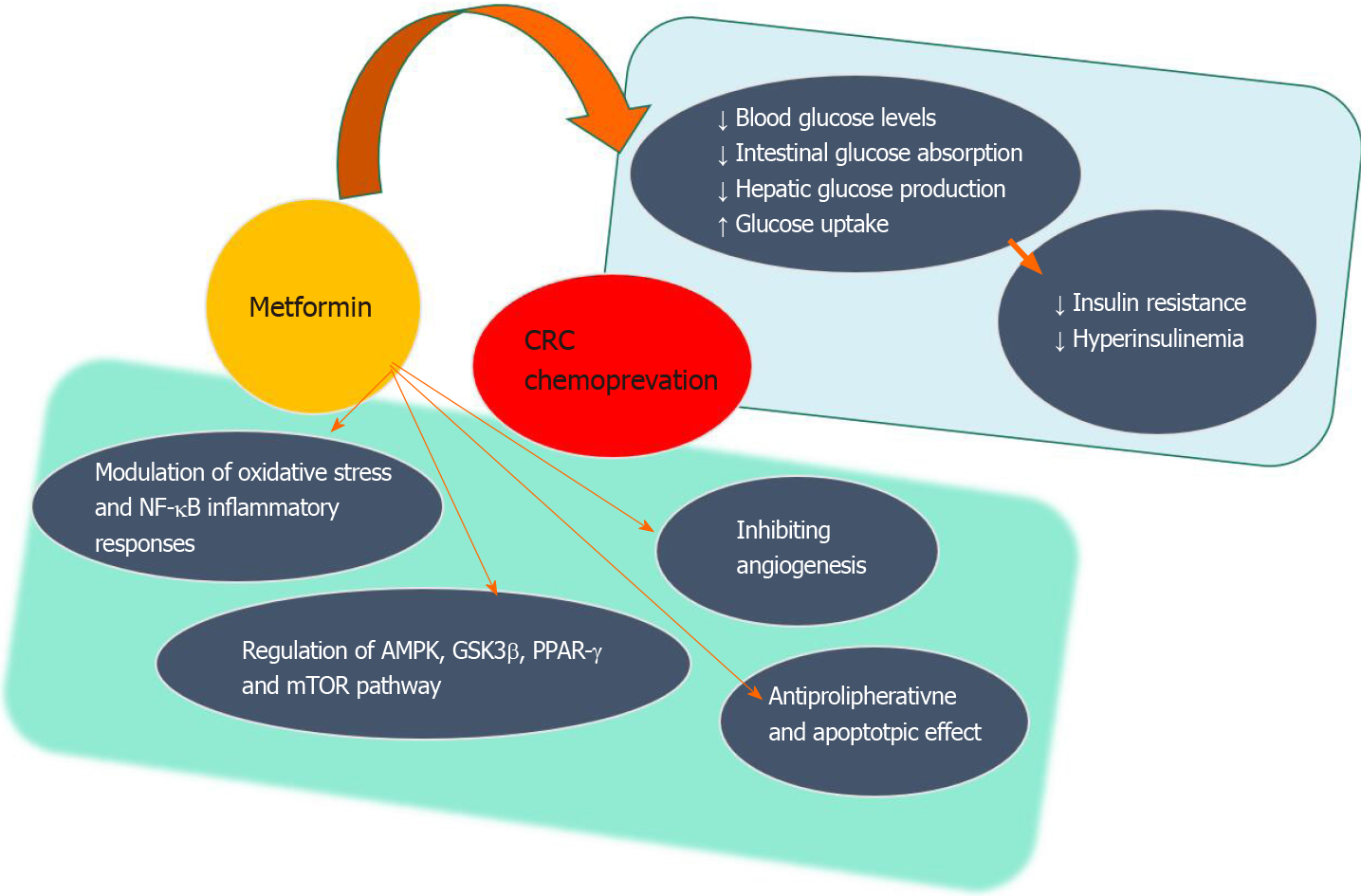
Metformin is one of the oldest oral antidiabetic agents used to treat type 2 diabetes mellitus alone or in combination with other oral or injectable agents. It lowers blood glucose levels by reducing hepatic glucose production, stimulating glucose uptake by peripheral tissues (muscle and fat), and lowering intestinal glucose absorption[4]. The presence of diabetes has been associated with a 30% increased risk of CRC. This potentially occurs through the mechanism of hyperinsulinemia accompanying insulin resistance, which promotes tumorigenesis via action on the insulin receptor in the epithelium or by influencing insulin-like growth factor pathways, inflammation, or adipokines inducing cancer cell proliferation and metastasis[5-7]. Therefore, metformin might provide preventive effects by reducing insulin resistance and lowering hyperinsulinemia[8] (Figure 1). Additionally, the use of metformin in various cancer models provided anticarcinogenic action by inhibiting angiogenesis or through antimetabolic and radio-chemosensitizer effects[9]. This is mediated by the synergistic regulation of metformin on adenosine monophosphate-activated protein kinase (AMPK), glycogen synthase kinase-3β, and proliferator-activated receptor-γ as observed in the case of pancreatic cancer[10].
The mechanistic target of rapamycin (mTOR) pathway, with complex multiple feedback loops, plays a central role in coordinating cell growth and proliferation in CRC carcinogenesis. Different mTOR inhibitors have been extensively studied for preventing and/or treating CRC, but no single agent has shown a significant therapeutic efficacy towards CRC potentially because many of the genetic pathways involved in CRC development lie upstream of mTOR and elicit the oncogenic effect through the mTOR signaling pathway[11]. According to the contemporary understanding, the mTOR pathway is continuously activated by various hormones, inflammation, and energy-related factors such as glucose, insulin, and insulin-like growth factor 1. The hypothesis to combine inhibition of these pathways seems promising in providing more effective tumor suppression[12]. Metformin inhibits mitochondrial mammalian respiratory chain complex I followed by activation of liver kinase B1 and downstream target AMPK that results in an inhibition of mTOR activity[13,14]. Metformin also exerts its metabolic effects by improving insulin resistance, hyperinsulinemia, and glycemia. Metformin has apoptotic effects on cancer stem cells and potentially has a synergistic effect with other chemotherapeutics[15,16].
Animal and in vitro studies indicate that the role of metformin as an antiproliferative agent on CRC cells is through the activation of AMPK[17,18]. One of the first reports on anticancer effect of metformin in a CRC cell model showed the concentration and time-dependent effects on AMPK activation and the reduction of cancer cell proliferation[19]. Other research provided further evidence of the apoptotic effects of metformin by modulating oxidative stress and nuclear factor-κB inflammatory responses[20-22]. In the in vitro models, metformin was used either alone or in combination with other agents, primarily fluorouracil (commonly referred to as 5-FU)[20,23-25], which is similar to its use in clinics.
Previous studies have indicated that metformin may decrease the risk of development of colorectal carcinoma[26,27]. Observational data indicates lower tumor incidence in diabetic patients taking metformin, and results from interventional studies show a reduction in the incidence of colorectal adenomas in patients without diabetes taking low-dose metformin[28-30]. Specifically, in a phase 3 randomized trial, a 1-year treatment with low-dose metformin reduced the number and prevalence of premalignant colorectal lesions, such as polyps and adenomas, in patients without diabetes post polypectomy[30]. A recently published retrospective cohort study including 47351 people found an inverse association between long-term (> 5 years) exposure to metformin in diabetic patients and the risk of CRC[31], suggesting its chemopreventive potential. This finding was substantiated with the meta-analysis results of 58 studies showing that metformin usage significantly reduced the incidence of colorectal adenoma and CRC, improving overall and CRC-specific survival rates[32].
On the other hand, data on the role metformin plays in CRC carcinogenesis in nondiabetic patients are not extensive. According to the randomized placebo-controlled trial results, metformin has the chemopreventive potential in nondiabetic patients with a high risk for CRC development[33]. Although metformin therapy was usually well tolerated by patients with different cancers and side effects did not differ from those seen in diabetic patients (dose-related gastrointestinal adverse effects, rarely lactic acidosis, and vitamin B12 deficiency), more data are needed on the optimal dose, schedule, route of administration, treatment duration, and combinations with other chemotherapeutic agents for CRC patients (especially nondiabetic patients) to be able to minimize the potential side effects[34-36].
While there is substantial evidence that metformin may have a chemopreventive role in CRC development, the data on the possible effects of metformin after surgery and other CRC treatments are much less conclusive.
Lee et al[37] first observed a protective effect of metformin after CRC diagnosis in a single institution cohort of 595 patients. Metformin use showed a lower risk of overall mortality [hazard ratio (HR) = 0.66; 95% confidence interval (CI): 0.476-0.923] and CRC-specific mortality (HR = 0.66; 95%CI: 0.45-0.975) in patients with diabetes. However, this analysis suffered from immortal time bias, which may have influenced the results[38]. Fransgaard et al[39] examined the association between metformin and overall survival after resection for CRC in patients from the national Danish CRC database. They found better overall survival of patients treated with metformin compared to patients treated with insulin. However, cancer-specific mortality was not differentiated from diabetes-specific mortality, and it is not clear what their respective roles were in all-cause mortality. Therefore, it is impossible to ascertain whether better overall survival in the metformin-treated group can be attributed to metformin or the antitumor effects of other factors. Interestingly, in a recent paper, the same group found no association between metformin use and recurrence-free survival or disease-free survival in patients with surgically treated CRC[40]. The findings of a large population-based analysis from the United Kingdom also do not support a protective association between post-diagnostic metformin use and survival in a cohort of CRC patients with type 2 diabetes mellitus[41].
Several meta-analyses have been published on the topic, and most of them have reported a reduction in CRC-specific mortality with metformin use compared with nonuse in CRC patients[42-45]. However, the studies included in these meta-analyses are very heterogeneous regarding their design and inclusion criteria (e.g., analyzing patients with different CRC stages). Also, information is often not available on the types of surgeries and administered chemotherapy in the included studies.
Evidence exists that metformin in combination with adjuvant therapy may be associated with a better prognosis in CRC patients treated with metformin post-surgery. In preclinical studies, metformin has shown synergistic effects with oxaliplatin in inhibiting in vitro models of colon cancer[46]. Based on such evidence, it was hypothesized that metformin combined with adjuvant chemotherapy might be associated with a better prognosis in patients with resected CRC. This was not confirmed in the setting of a randomized study in patients with resected high-risk stage II or stage III CRC who received FOLFOX-4/XELOX adjuvant therapy[47]. Another study exploring the impact of metformin on overall survival and recurrence-free survival in a homogenous group of stage III CRC patients receiving FOLFOX adjuvant therapy failed to find a significant association[48].
These findings stand in contrast to the findings of studies that found an association between metformin use and improved outcomes. However, these were the studies that considered patients with stages I-IV treated with different chemotherapy regimens, including both patients with low stages without adjuvant chemotherapy and patients with advanced stages who received multiple chemotherapeutics[37,49,50]. Therefore, the effect of metformin on overall survival and recurrence-free survival in patients with CRC receiving chemotherapy after resection remains uncertain. Extensive prospective studies that include homogenous patient populations, ideally within randomized trials, with longer follow-up would provide more evidence on the possible association of metformin use and improved survival of patients after CRC treatment.
Mounting evidence suggests a chemopreventive role of metformin on CRC development, but the data on its role in diagnosed CRC in adjunct to surgery and chemotherapy are still inconclusive. Additional evidence from prospective, randomized controlled trials are required.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Croatia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chan DKH, Matowicka-Karna J S-Editor: Fan JR L-Editor: A P-Editor: Liu JH
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13300] [Cited by in RCA: 15470] [Article Influence: 2578.3] [Reference Citation Analysis (2)] |
| 2. | Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 2073] [Article Influence: 188.5] [Reference Citation Analysis (0)] |
| 3. | Vargas AJ, Thompson PA. Diet and nutrient factors in colorectal cancer risk. Nutr Clin Pract. 2012;27:613-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 150] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 4. | Buse JB, Wexler DJ, Tsapas A, Rossing P, Mingrone G, Mathieu C, D'Alessio DA, Davies MJ. 2019 Update to: Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2020;43:487-493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 856] [Cited by in RCA: 817] [Article Influence: 163.4] [Reference Citation Analysis (0)] |
| 5. | Handelsman Y, Leroith D, Bloomgarden ZT, Dagogo-Jack S, Einhorn D, Garber AJ, Grunberger G, Harrell RM, Gagel RF, Lebovitz HE, McGill JB, Hennekens CH. Diabetes and cancer--an AACE/ACE consensus statement. Endocr Pract. 2013;19:675-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 6. | Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst. 2005;97:1679-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 719] [Cited by in RCA: 753] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 7. | Godsland IF. Insulin resistance and hyperinsulinaemia in the development and progression of cancer. Clin Sci (Lond). 2009;118:315-332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 163] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 8. | Salani B, Del Rio A, Marini C, Sambuceti G, Cordera R, Maggi D. Metformin, cancer and glucose metabolism. Endocr Relat Cancer. 2014;21:R461-R471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 9. | Leone A, Di Gennaro E, Bruzzese F, Avallone A, Budillon A. New perspective for an old antidiabetic drug: metformin as anticancer agent. Cancer Treat Res. 2014;159:355-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 10. | Elmaci İ, Altinoz MA. A Metabolic Inhibitory Cocktail for Grave Cancers: Metformin, Pioglitazone and Lithium Combination in Treatment of Pancreatic Cancer and Glioblastoma Multiforme. Biochem Genet. 2016;54:573-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Slattery ML, Herrick JS, Lundgreen A, Fitzpatrick FA, Curtin K, Wolff RK. Genetic variation in a metabolic signaling pathway and colon and rectal cancer risk: mTOR, PTEN, STK11, RPKAA1, PRKAG2, TSC1, TSC2, PI3K and Akt1. Carcinogenesis. 2010;31:1604-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | Slattery ML, Fitzpatrick FA. Convergence of hormones, inflammation, and energy-related factors: a novel pathway of cancer etiology. Cancer Prev Res (Phila). 2009;2:922-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Zi F, Zi H, Li Y, He J, Shi Q, Cai Z. Metformin and cancer: An existing drug for cancer prevention and therapy. Oncol Lett. 2018;15:683-690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 14. | Vial G, Detaille D, Guigas B. Role of Mitochondria in the Mechanism(s) of Action of Metformin. Front Endocrinol (Lausanne). 2019;10:294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 213] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 15. | Miranda VC, Braghiroli MI, Faria LD, Bariani G, Alex A, Bezerra Neto JE, Capareli FC, Sabbaga J, Lobo Dos Santos JF, Hoff PM, Riechelmann RP. Phase 2 Trial of Metformin Combined With 5-Fluorouracil in Patients With Refractory Metastatic Colorectal Cancer. Clin Colorectal Cancer 2016; 15: 321-328. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 16. | Yang M, Liu P, Huang P. Cancer stem cells, metabolism, and therapeutic significance. Tumour Biol. 2016;37:5735-5742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 17. | Aljada A, Mousa SA. Metformin and neoplasia: implications and indications. Pharmacol Ther. 2012;133:108-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 18. | Liu X, Chhipa RR, Pooya S, Wortman M, Yachyshin S, Chow LM, Kumar A, Zhou X, Sun Y, Quinn B, McPherson C, Warnick RE, Kendler A, Giri S, Poels J, Norga K, Viollet B, Grabowski GA, Dasgupta B. Discrete mechanisms of mTOR and cell cycle regulation by AMPK agonists independent of AMPK. Proc Natl Acad Sci USA. 2014;111:E435-E444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 181] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 19. | Zakikhani M, Dowling RJ, Sonenberg N, Pollak MN. The effects of adiponectin and metformin on prostate and colon neoplasia involve activation of AMP-activated protein kinase. Cancer Prev Res (Phila). 2008;1:369-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 233] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 20. | Saber MM, Galal MA, Ain-Shoka AA, Shouman SA. Combination of metformin and 5-aminosalicylic acid cooperates to decrease proliferation and induce apoptosis in colorectal cancer cell lines. BMC Cancer. 2016;16:126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Cho SY, Lee HJ, Jung DB, Kim H, Sohn EJ, Kim B, Jung JH, Kwon BM, Kim SH. Activation of AMP-Activated Protein Kinase α and Extracelluar Signal-Regulated Kinase Mediates CB-PIC-Induced Apoptosis in Hypoxic SW620 Colorectal Cancer Cells. Evid Based Complement Alternat Med. 2013;2013:974313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Nguyen TT, Ung TT, Li S, Lian S, Xia Y, Park SY, Do Jung Y. Metformin inhibits lithocholic acid-induced interleukin 8 upregulation in colorectal cancer cells by suppressing ROS production and NF-kB activity. Sci Rep. 2019;9:2003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 23. | Abu El Maaty MA, Strassburger W, Qaiser T, Dabiri Y, Wölfl S. Differences in p53 status significantly influence the cellular response and cell survival to 1,25-dihydroxyvitamin D3-metformin cotreatment in colorectal cancer cells. Mol Carcinog. 2017;56:2486-2498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Feng YH, Wu CL, Shiau AL, Lee JC, Chang JG, Lu PJ, Tung CL, Feng LY, Huang WT, Tsao CJ. MicroRNA-21-mediated regulation of Sprouty2 protein expression enhances the cytotoxic effect of 5-fluorouracil and metformin in colon cancer cells. Int J Mol Med. 2012;29:920-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Huang WS, Lin CT, Chen CN, Chang SF, Chang HI, Lee KC. Metformin increases the cytotoxicity of oxaliplatin in human DLD-1 colorectal cancer cells through down-regulating HMGB1 expression. J Cell Biochem. 2018;119:6943-6952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Singh S, Singh H, Singh PP, Murad MH, Limburg PJ. Antidiabetic medications and the risk of colorectal cancer in patients with diabetes mellitus: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2013;22:2258-2268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 27. | Sehdev A, Shih YC, Vekhter B, Bissonnette MB, Olopade OI, Polite BN. Metformin for primary colorectal cancer prevention in patients with diabetes: a case-control study in a US population. Cancer. 2015;121:1071-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 28. | Liu F, Yan L, Wang Z, Lu Y, Chu Y, Li X, Liu Y, Rui D, Nie S, Xiang H. Metformin therapy and risk of colorectal adenomas and colorectal cancer in type 2 diabetes mellitus patients: A systematic review and meta-analysis. Oncotarget. 2017;8:16017-16026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 29. | Hosono K, Endo H, Takahashi H, Sugiyama M, Sakai E, Uchiyama T, Suzuki K, Iida H, Sakamoto Y, Yoneda K, Koide T, Tokoro C, Abe Y, Inamori M, Nakagama H, Nakajima A. Metformin suppresses colorectal aberrant crypt foci in a short-term clinical trial. Cancer Prev Res (Phila). 2010;3:1077-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 244] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 30. | Higurashi T, Hosono K, Takahashi H, Komiya Y, Umezawa S, Sakai E, Uchiyama T, Taniguchi L, Hata Y, Uchiyama S, Hattori A, Nagase H, Kessoku T, Arimoto J, Matsuhashi N, Inayama Y, Yamanaka S, Taguri M, Nakajima A. Metformin for chemoprevention of metachronous colorectal adenoma or polyps in post-polypectomy patients without diabetes: a multicentre double-blind, placebo-controlled, randomised phase 3 trial. Lancet Oncol. 2016;17:475-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 239] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 31. | Bradley MC, Ferrara A, Achacoso N, Ehrlich SF, Quesenberry CP Jr, Habel LA. A Cohort Study of Metformin and Colorectal Cancer Risk among Patients with Diabetes Mellitus. Cancer Epidemiol Biomarkers Prev. 2018;27:525-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 32. | Ng CW, Jiang AA, Toh EMS, Ng CH, Ong ZH, Peng S, Tham HY, Sundar R, Chong CS, Khoo CM. Metformin and colorectal cancer: a systematic review, meta-analysis and meta-regression. Int J Colorectal Dis. 2020;35:1501-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 33. | Higurashi T, Takahashi H, Endo H, Hosono K, Yamada E, Ohkubo H, Sakai E, Uchiyama T, Hata Y, Fujisawa N, Uchiyama S, Ezuka A, Nagase H, Kessoku T, Matsuhashi N, Yamanaka S, Inayama Y, Morita S, Nakajima A. Metformin efficacy and safety for colorectal polyps: a double-blind randomized controlled trial. BMC Cancer. 2012;12:118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 34. | Molenaar RJ, van de Venne T, Weterman MJ, Mathot RA, Klümpen HJ, Richel DJ, Wilmink JW. A phase Ib study of everolimus combined with metformin for patients with advanced cancer. Invest New Drugs. 2018;36:53-61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Mastroianni A, Ciniselli CM, Panella R, Macciotta A, Cavalleri A, Venturelli E, Taverna F, Mazzocchi A, Bruno E, Muti P, Berrino F, Verderio P, Morelli D, Pasanisi P. Monitoring Vitamin B12 in Women Treated with Metformin for Primary Prevention of Breast Cancer and Age-Related Chronic Diseases. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Aldea M, Craciun L, Tomuleasa C, Berindan-Neagoe I, Kacso G, Florian IS, Crivii C. Repositioning metformin in cancer: genetics, drug targets, and new ways of delivery. Tumour Biol. 2014;35:5101-5110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 37. | Lee JH, Kim TI, Jeon SM, Hong SP, Cheon JH, Kim WH. The effects of metformin on the survival of colorectal cancer patients with diabetes mellitus. Int J Cancer. 2012;131:752-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 162] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 38. | Suissa S, Azoulay L. Metformin and the risk of cancer: time-related biases in observational studies. Diabetes Care. 2012;35:2665-2673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 377] [Cited by in RCA: 419] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 39. | Fransgaard T, Thygesen LC, Gögenur I. Metformin Increases Overall Survival in Patients with Diabetes Undergoing Surgery for Colorectal Cancer. Ann Surg Oncol. 2016;23:1569-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 40. | Fransgaard T, Thygesen LC, Gögenur I. Association between metformin use after surgery for colorectal cancer and oncological outcomes: A nationwide register-based study. Int J Cancer. 2018;143:63-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 41. | Mc Menamin ÚC, Murray LJ, Hughes CM, Cardwell CR. Metformin use and survival after colorectal cancer: A population-based cohort study. Int J Cancer. 2016;138:369-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 42. | Singh PP, Singh S, Gonsalves WI, Grothey A. Association of metformin with reduced mortality in patients with colorectal cancer: A systematic review and meta-analysis of observational studies. J Clin Oncol. 2014;32:522a. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 43. | Coyle C, Cafferty FH, Vale C, Langley RE. Metformin as an adjuvant treatment for cancer: a systematic review and meta-analysis. Ann Oncol. 2016;27:2184-2195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 347] [Cited by in RCA: 332] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 44. | Du L, Wang M, Kang Y, Li B, Guo M, Cheng Z, Bi C. Prognostic role of metformin intake in diabetic patients with colorectal cancer: An updated qualitative evidence of cohort studies. Oncotarget. 2017;8:26448-26459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 45. | Meng F, Song L, Wang W. Metformin Improves Overall Survival of Colorectal Cancer Patients with Diabetes: A Meta-Analysis. J Diabetes Res. 2017;2017:5063239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 46. | Richard SM, Martinez Marignac VL. Sensitization to oxaliplatin in HCT116 and HT29 cell lines by metformin and ribavirin and differences in response to mitochondrial glutaminase inhibition. J Cancer Res Ther. 2015;11:336-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 47. | Vernieri C, Galli F, Ferrari L, Marchetti P, Lonardi S, Maiello E, Iaffaioli RV, Zampino MG, Zaniboni A, De Placido S, Banzi M, Damiani A, Ferrari D, Rosati G, Labianca RF, Bidoli P, Frassineti GL, Nicolini M, Pavesi L, Tronconi MC, Buonadonna A, Ferrario S, Re GL, Adamo V, Tamburini E, Clerico M, Giordani P, Leonardi F, Barni S, Ciarlo A, Cavanna L, Gori S, Cinieri S, Faedi M, Aglietta M, Antista M, Dotti KF, Di Bartolomeo M; TOSCA (Three or Six Colon Adjuvant) Investigators. Impact of Metformin Use and Diabetic Status During Adjuvant Fluoropyrimidine-Oxaliplatin Chemotherapy on the Outcome of Patients with Resected Colon Cancer: A TOSCA Study Subanalysis. Oncologist. 2019;24:385-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 48. | Singh PP, Shi Q, Foster NR, Grothey A, Nair SG, Chan E, Shields AF, Goldberg RM, Gill S, Kahlenberg MS, Sinicrope FA, Sargent DJ, Alberts SR. Relationship Between Metformin Use and Recurrence and Survival in Patients With Resected Stage III Colon Cancer Receiving Adjuvant Chemotherapy: Results From North Central Cancer Treatment Group N0147 (Alliance). Oncologist. 2016;21:1509-1521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 49. | Lee GE, Aung T, Kim TH, Tan WS, Tai WMD, Tan H, Tan IB. Examining the effects of metformin on survival outcome in stage II/III colorectal cancer patients with diabetes mellitus. J Clin Oncol. 2012;30 (Suppl 15):3589a. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 50. | Spillane S, Bennett K, Sharp L, Barron TI. A cohort study of metformin exposure and survival in patients with stage I-III colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2013;22:1364-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |