Published online Apr 7, 2021. doi: 10.3748/wjg.v27.i13.1330
Peer-review started: January 9, 2021
First decision: February 11, 2021
Revised: February 19, 2021
Accepted: March 7, 2021
Article in press: March 7, 2021
Published online: April 7, 2021
Processing time: 79 Days and 18 Hours
The factors affecting the short-term and long-term prognosis of hepatocellular carcinoma (HCC) patients with portal vein tumor thrombosis (PVTT) receiving transarterial chemoembolization (TACE) are still unclear.
To clarify the predictors correlated with the short-term and long-term survival of HCC patients with PVTT who underwent TACE.
The medical records of 181 HCC patients with PVTT who underwent TACE at the Second Affiliated Hospital of Chongqing Medical University from January 2015 to July 2019 were retrospectively analyzed. We explored the short-term and long-term prognostic factors by comparing the preoperative indicators of patients who died and survived within 3 mo and 12 mo after TACE. Multivariate analyses were conducted using logistic regression. The area under the receiver operating characteristic curve (area under curve) was used to evaluate the predictive ability of the factors related to the short-term and long-term prognosis.
The median survival time was 4.8 mo (range: 2.5-8.85 mo). The 3 mo, 6 mo, and 12 mo survival rates were 68.5%, 38.7%, and 15.5%, respectively. In multivariable analysis, total bilirubin, sex, and aspartate aminotransferase (AST) were closely linked to short-term survival. When AST ≥ 87 U/L and total bilirubin ≥ 16.15 µmol/L, the 3-mo survival rate after TACE was reduced significantly (P < 0.05). AST had the best predictive ability, followed by total bilirubin, while sex had the worst predictive ability for short-term survival area under curve: 0.763 (AST) vs 0.707 (total bilirubin) vs 0.554 (sex)]. The long-term survival outcome was significantly better in patients with a single lesion than in those with ≥ three lesions (P = 0.009). Patients with massive block HCC had a worse long-term survival than patients with nodular and diffuse HCC (P = 0.001).
AST, total bilirubin, and sex are independent factors associated with short-term survival. The number of tumors and the gross pathological type of tumor are related to the long-term outcome.
Core Tip: It is unclear which factors affect the short-term and long-term prognosis of hepatocellular carcinoma patients with portal vein tumor thrombosis receiving transarterial chemoembolization. In our research, we clarified the predictors correlated with the short-term and long-term survival of hepatocellular carcinoma patients with portal vein tumor thrombosis who underwent transarterial chemoembolization by analyzing preoperative indicators. Results showed that aspartate aminotransferase, sex, and total bilirubin were independent factors associated with short-term survival. The number of lesions and the gross pathological type of tumor were related to the long-term outcome.
- Citation: Chen KL, Gao J. Factors influencing the short-term and long-term survival of hepatocellular carcinoma patients with portal vein tumor thrombosis who underwent chemoembolization. World J Gastroenterol 2021; 27(13): 1330-1340
- URL: https://www.wjgnet.com/1007-9327/full/v27/i13/1330.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i13.1330
Primary liver cancer is currently one of the most common malignant tumors, and it is among the top five causes of death and morbidity from malignant tumors around the world[1]. Hepatocellular carcinoma (HCC) is the most common pathological type, accounting for approximately 90% of all primary liver cancers. Because of its biological and anatomical characteristics, HCC easily invades the portal vein during the progression of liver cancer and forms portal vein tumor thrombosis (PVTT). Some reports have proposed that the incidence of HCC with PVTT is approximately 44%-62.2%[2]. HCC accompanied by PVTT is closely associated with a poor prognosis and is likely to lead to intrahepatic metastasis, liver function damage, portal hypertension, upper gastrointestinal bleeding, and other complications. The median overall survival time of HCC patients with PVTT who receive no treatment is only 2.7 mo[3].
There is still controversy about the treatment of HCC with PVTT in Eastern and Western countries. Previous studies have suggested that HCC with PVTT should be classified as stage C of Barcelona Clinic Liver Cancer and is no longer suitable for surgical treatment[4]. Some researchers have mentioned that compared with conservative treatment, TACE is a safe and effective therapy for selected HCC patients with PVTT[5-7]. However, some patients die in the short term after TACE, and patients who have expected postoperative survival times of less than 3 mo may not be suitable for TACE.
At present, the factors affecting the short-term (3 mo) and long-term (12 mo) prognosis of HCC patients with PVTT treated with TACE are still unclear. We aimed to identify the preoperative factors related to the short-term and long-term prognosis by comparing the preoperative clinical data of patients who died in the short-term (< 3 mo) with those who survived into the long-term (> 12 mo) after TACE. The area under the curve (AUC) was utilized to assess the predictability of the factors related to short-term and long-term survival to provide some help for doctors when screening HCC patients with PVTT to identify those who can benefit from TACE.
We enrolled a total of 181 HCC patients with PVTT who received TACE in the Second Affiliated Hospital of Chongqing Medical University from January 2015 to July 2019. The inclusion criteria were as follows: (1) Histopathology confirmed as HCC or clinically diagnosed as HCC; (2) Abdominal color Doppler ultrasound, digital subtraction angiography, contrast-enhanced computerized tomography, or magnetic resonance imaging showed signs of PVTT; (3) No treatment, such as surgery, radiotherapy, chemotherapy, liver transplantation, targeted, or biological therapy, was administered before and after TACE; (4) Age ≥ 18 years; and (5) Complete clinical data were available. Patients with other malignancies or severe heart and lung diseases were eliminated. The follow-up endpoint was July 2020 or the date of death, whichever came first. The follow-up was conducted by telephoning or outpatient visits. During our follow-up, 14 patients received other treatments, such as targeted therapy and High Intensity Focused Ultrasound, and one patient was diagnosed with and treated for a hematologic malignancy. Twenty-six patients were lost to follow-up. The above patients were not included in the study. The study complied with the ethical guidelines of the Declaration of Helsinki in 1964 and passed the review of the Review Committee of the Second Affiliated Hospital of Chongqing Medical University.
We collected the basic data of the patients, including sex, age, cause of hepatitis, ascites, hepatoportal arteriovenous fistula, liver cirrhosis, cavernous transformation of the portal vein, Child-Pugh grade, the model for end-stage liver disease score, the Eastern Cooperative Oncology Group score, and albumin-bilirubin grade (ALBI grade). The biochemical parameters included the following indicators: Prealbumin, serum albumin (ALB), total bilirubin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alpha-fetoprotein, activated partial thromboplastin time, prothrombin time, international normalized ratio, hemoglobin, platelet count, white blood cell count, serum cholinesterase, and gamma-glutamyl transpeptidase. The characteristics of the tumors included the number and size of tumors, the type of PVTT, gross pathological type, invasion of the left and right liver lobes and inferior vena cava tumor thrombus. Cheng’s classification of PVTT was applied[8]. The formula for calculating the ALBI score was as follows: ALBI = log10 bilirubin × 0.66 + albumin × (-0.085)[9]. Gross pathological types of liver cancer included the massive block type, the nodular type, and the diffuse type. The clinical diagnostic criteria for patients with HCC are in line with the European Society for Medical Oncology Clinical Practice Guidelines[10].
The puncture of the artery used Seldinger technology. The right femoral artery was often selected. The catheter was delivered to the celiac trunk and the common hepatic artery, and arteriography was performed to identify the tumor's nutrient artery. The catheter was sent into the tumor-feeding artery and injected with chemotherapy drugs. Chemoembolization drugs commonly included lipiodol, polyvinyl alcohol particles, cisplatin, bleomycin, gelatin sponge particles, etc.
The mean ± SD or median were used to express the continuous variables. Categorical variables are presented as n (%). Two groups of data were compared using independent-samples t tests, Mann-Whitney U tests, χ2 tests or Fisher's exact tests. The statistically significant indicators in the univariate analysis (P < 0.05) were selected in multivariate analysis in the study. The independent predictors for survival were determined by logistic regression analysis. The predictive ability of the independent predictors for short-term survival was assessed by the AUC. The cutoff value was calculated by the receiver operating characteristic (ROC) curves. The Kaplan-Meier method was used to plot the survival curves. SPSS 25.0 software (Armonk, NY, United States) was used to perform all statistical analyses. When P < 0.05, the statistical results were considered significant.
A total of 181 HCC patients with PVTT who underwent TACE were enrolled in our study. Their baseline data are shown in Table 1. The average age of the patients was 52.16 ± 9.73 years. The 3 mo, 6 mo, and 12 mo survival rates were 68.5%, 38.7%, and 15.5%, respectively. In our study, 159 patients (87.8%) were men and 22 patients (12.2%) were women, hepatitis B patients accounted for 90.1%, and 133 (73.5%) patients had a background of cirrhosis. According to the type of PVTT, 29 (16%) patients, 77 (42.5%) patients, 68 (37.6%) patients, and 7 (3.9%) patients were classified as having type I, II, III and IV PVTT, respectively. For the Child-Pugh grade, 123 (68%) had grade A, 55 (30.4%) had grade B, and 3 (1.6%) had grade C. Patients with greater than or equal to 3 lesions accounted for 48.1%. The median survival time was 4.8 mo.
| Parameters | Patients, n = 181 |
| Age in yr | 52.16 ± 9.73 |
| Sex, men/women | 159/22 |
| Cause of liver disease, hepatitis B/C/B and C/others | 163/3/1/14 |
| Tumor size, < 5 cm/≥ 5 cm, < 10 cm/≥ 10 cm | 39/86/56 |
| Number of tumors, 1/2/≥ 3 | 82/12/87 |
| Liver cirrhosis, no/yes | 48/133 |
| Ascites, no/small/moderate-massive | 91/70/20 |
| CTPV, no/yes | 158/23 |
| Invade left and right liver lobes, no/yes | 119/62 |
| Type of gross pathology, massive/nodular/diffuse | 112/59/10 |
| PVTT type, I/II/III/IV | 29/77/68/7 |
| Inferior vena cava tumor thrombus, no/yes | 171/10 |
| Arteriovenous fistula, no/yes | 125/56 |
| Total bilirubin, µmol/L | 17.1 (12.3-24.7) |
| Prealbumin, mg/L | 110 (79-147) |
| Albumin, g/L | 37 ± 4.8 |
| Hemoglobin, g/L | 127 (115-142) |
| WBC, 109/L | 5.1 (3.925-6.505) |
| PLT, 109/L | 116 (81- 179.5) |
| INR | 1.12 (1.055-1.205) |
| PT, S | 14.3 (13.7-15.25) |
| APTT, S | 39.5 (37-42.8) |
| ALT, U/L | 46 (31-71.5) |
| AST, U/L | 65 (46-109.5) |
| GGT, U/L | 211 (124.5-352.5) |
| Cholinesterase, kU/L | 4.5 (3.065-5.78) |
| Creatinine, µmol/L | 68 (57.7-78.1) |
| AFP, µg/L | 1210 (82.905-1210) |
| Child-Pugh grade, A/B/C | 123/55/3 |
| ECOG score, 0/1/2/3 | 8/142/29/2 |
| ALBI grade, 1/2/3 | 56/120/5 |
| MELD score | 7.67 (6.08-9.54) |
| Overall survival time, mo | 4.8 (2.5-8.85) |
Of the 181 patients, 56 patients died within 3 mo after interventional therapy, and 125 patients survived. The clinical data of patients who died and survived within 3 mo after treatment were compared. The results of the univariate analysis are shown in Table 2. Multivariate analysis showed that total bilirubin [odds ratio (OR): 1.027, 95% confidence interval (CI): 1-1.054 P = 0.046), sex (OR: 2.832, 95%CI: 1.025-7.828, P = 0.045] and AST (OR: 1.014, 95%CI: 1.006-1.021, P < 0.01) were significant independent predictors of short-term survival (Table 3).
| Parameters | Survivors, n = 125 | Non-survivors, n = 56 | P value |
| Age in yr | 52.56 ± 9.966 | 51.27 ± 9.212 | 0.18 |
| Sex, men/women | 114/11 | 45/11 | 0.04 |
| Cause of liver disease, hepatitis B/C/B and C/others | 113/1/0/11 | 50/2/1/3 | 0.157 |
| Tumor size, < 5 cm/≥ 5 cm, < 10 cm/≥ 10 cm | 29/57/39 | 10/29/17 | 0.659 |
| Number of tumors, 1/2/≥ 3 | 62/10/53 | 20/2/34 | 0.06 |
| Liver cirrhosis, no/yes | 36/89 | 12/44 | 0.299 |
| Ascites, no/small/moderate-massive | 68/47/10 | 23/23/10 | 0.087 |
| CTPV, no/yes | 110/15 | 48/8 | 0.67 |
| Invade left and right liver lobes, no/yes | 85/40 | 34/22 | 0.34 |
| Type of gross pathology, massive / nodular and diffuse | 78/47 | 34/22 | 0.829 |
| PVTT type, I/II/III/IV | 24/52/44/5 | 5/25/24/2 | 0.354 |
| Inferior vena cava tumor thrombus, no/yes | 119/6 | 52/4 | 0.775 |
| Arteriovenous fistula, no/yes | 89/36 | 36/20 | 0.352 |
| Total bilirubin, µmol/L | 15 (11.15-21.75) | 22.25 (17.175-32) | < 0.01 |
| Prealbumin, mg/L | 119 (87-159.5) | 91.5 (67.25-120.5) | < 0.01 |
| Albumin, g/L | 36.407 ± 4.749 | 37.205 ± 4.853 | 0.758 |
| Hemoglobin, g/L | 127 (115-142) | 127 (113.25-143) | 0.89 |
| WBC, 109/L | 5.06 (4.06-6.27) | 5.09 (3.61-6.72) | 0.87 |
| PLT, 109/L | 119.00 (86.00-180.50) | 111.00 (69.25-178.25) | 0.15 |
| INR | 1.11 (1.06-1.19) | 1.12 (1.05-1.26) | 0.33 |
| PT, S | 14.20 (13.70-15.20) | 14.35 (13.70-15.78) | 0.38 |
| APTT, S | 39.60 (37.25-42.80) | 39.35 (36.32-42.85) | 0.47 |
| ALT, U/L | 41.00 (29.50-62.00) | 58.00 (41.25-85.75) | < 0.01 |
| AST, U/L | 56.00 (41.00-81.00) | 109.00 (72.00-161.75) | < 0.01 |
| GGT, U/L | 188.00 (109.00-300.50) | 256.00 (186.00-415.25) | < 0.01 |
| Cholinesterase, kU/L | 4.88 (3.44-5.84) | 3.89 (2.69-5.4675) | 0.02 |
| Creatinine, µmol/L | 68.60 (58.20-77.25) | 66.95 (54.98-79.60) | 0.63 |
| AFP, µg/L | 1210 (47.86-1210) | 1210 (270-1210) | 0.34 |
| Child-Pugh grade, A/B/C | 94/30/1 | 29/25/2 | < 0.01 |
| ECOG score, 0/1/2/3 | 8/102/14/1 | 0/40/15/1 | 0.016 |
| ALBI grade, 1/2/3 | 43/79/9 | 13/41/2 | 0.31 |
| MELD score | 7.53 (5.88-9.02) | 9.10 (7.08-11.19) | < 0.01 |
| Variables | Short-term survival | Long-term survival | ||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Total bilirubin, µmol/L | 1.027 (1-1.054) | 0.046 | ||
| AST, U/L | 1.014 (1.006-1.021) | P < 0.01 | ||
| Sex | ||||
| Men | 1 | |||
| Women | 2.832 (1.025-7.828) | 0.045 | ||
| Type of gross pathology | ||||
| Massive | 1 | |||
| Nodular and diffuse | 0.197 (0.075-0.521) | 0.001 | ||
| Number of tumors | ||||
| 1 | 1 | |||
| 2 | 1.365 (0.283-6.581) | 0.698 | ||
| 3 | 5.809 (1.563-21.594) | 0.009 | ||
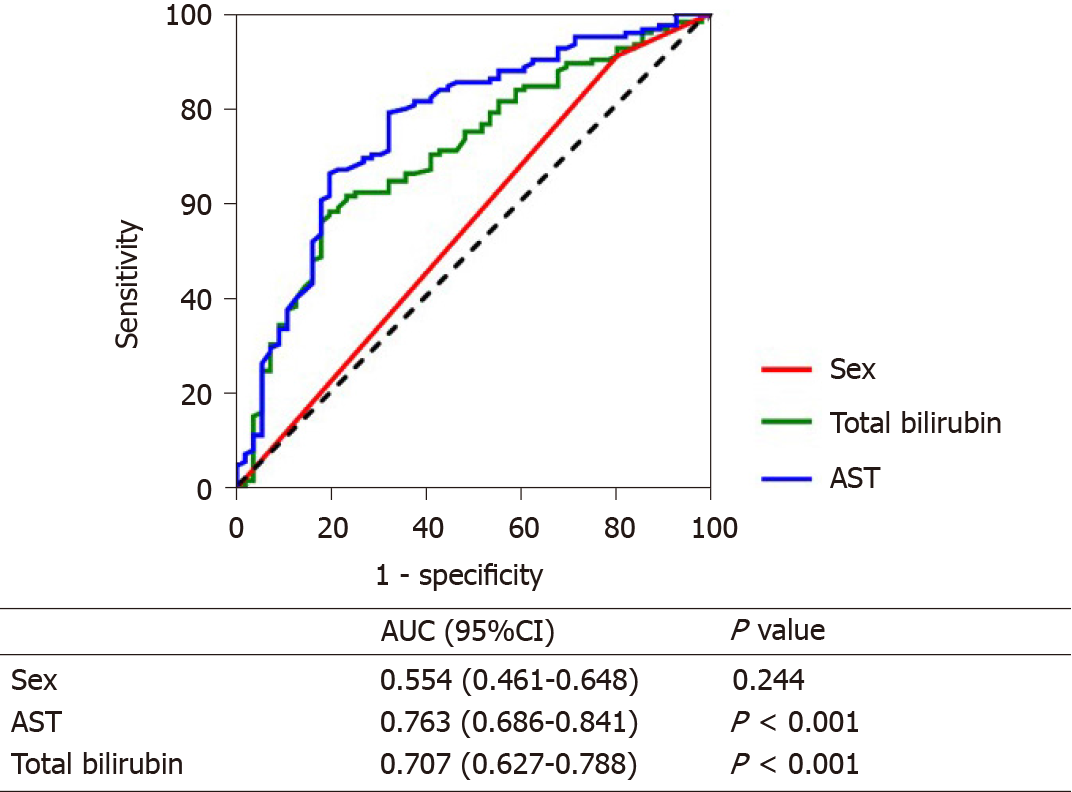
The AUC was used to evaluate the predictive ability of these indicators for short-term survival. AST (AUC: 0.763, 95%CI: 0.686-0.841) had the best predictive ability, followed by total bilirubin (AUC: 0.707, 95%CI: 0.627-0.788), while sex (AUC: 0.554, 95%CI: 0.461-0.648) had the worst predictive ability (Figure 1).
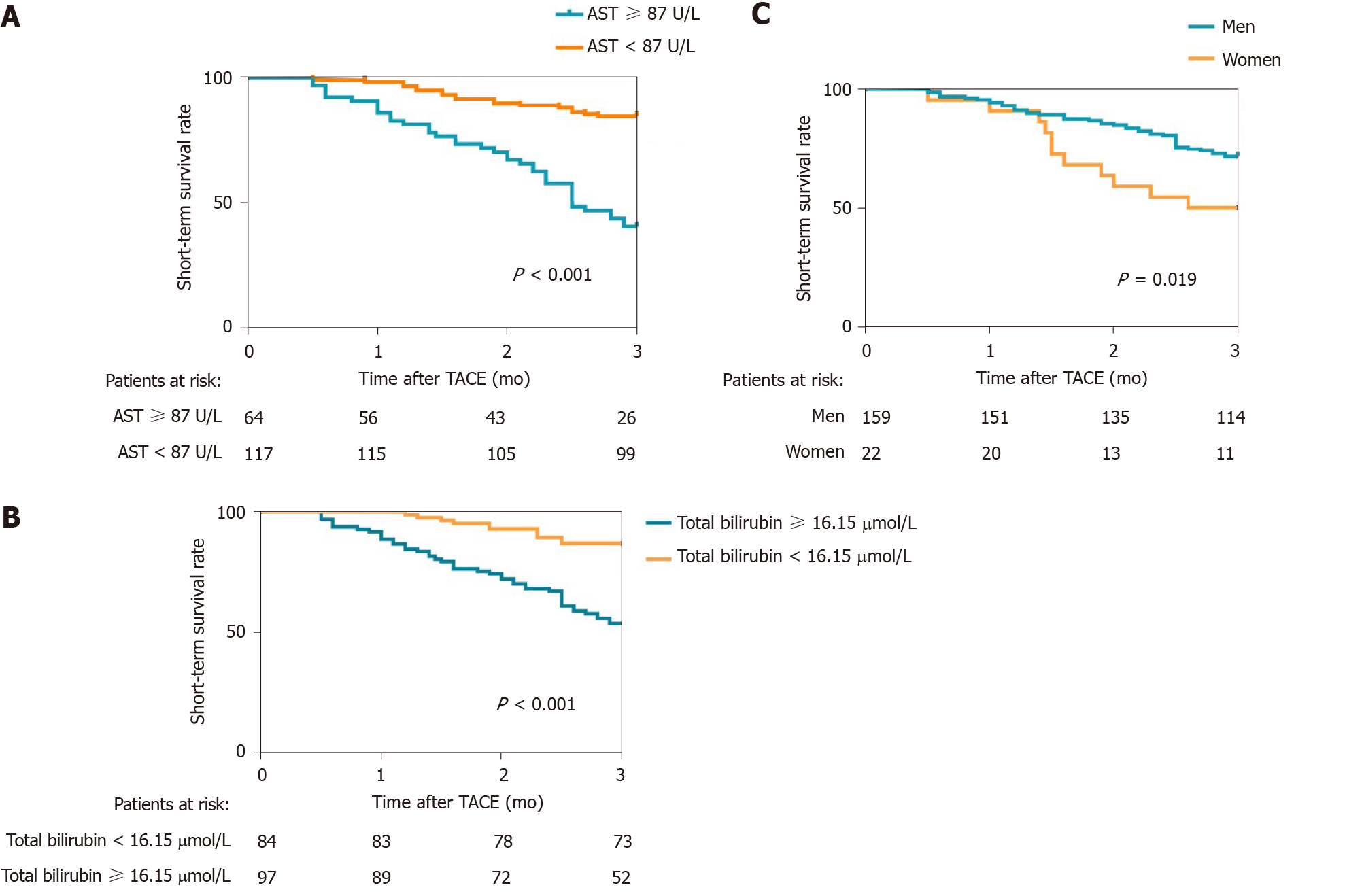
The cutoff values of the above indicators were calculated by using ROC curves, and the 3-mo cumulative survival rates of patients with AST < 87 U/L and AST ≥ 87 U/L were 84.6% and 40.6% (Figure 2A), respectively. The difference was significant (P < 0.001). The 3-mo cumulative survival rates of the patients with total bilirubin < 16.15 µmol/L and bilirubin ≥ 16.15 µmol/L were 86.9% and 53.6% (Figure 2B), respectively, and the difference was statistically significant (P < 0.001). The 3-mo cumulative survival rates of men and women were 71.1% and 50% (Figure 2C), respectively. The discrepancy was statistically significant (P = 0.019).
Of the 181 patients, 150 died within 12 mo after interventional therapy, and 31 survived. Univariate analysis showed that the number of tumors, invasion of the left and right liver lobes, pathological type, platelet count, and gamma-glutamyl transpeptidase were correlated with the long-term survival of patients (Table 4), and logistic regression analysis showed that the number of lesions and pathological type were independent predictors of long-term survival (Table 3). The long-term survival outcome was significantly better in patients with a single lesion than in those with ≥ three lesions (OR: 5.809, 95%CI: 1.563-21.594, P = 0.009). Patients with massive block HCC had a worse long-term survival than patients with nodular and diffuse HCC (OR: 0.197, 95%CI: 0.075-0.521, P = 0.001).
| Parameters | Survivors, n = 31 | Non-survivors, n = 150 | P value |
| Age in yr | 54.770 ± 10.724 | 51.800 ± 9.400 | 0.225 |
| Sex, men/women | 28/3 | 131/19 | 0.871 |
| Cause of liver disease, hepatitis B/C/B and C/others | 30/0/0/1 | 133/3/1/13 | 0.751 |
| Tumor size, < 5 cm/≥ 5 cm, < 10 cm/≥ 10 cm | 11/14/6 | 28/72/50 | 0.081 |
| Number of tumors, 1/2/≥ 3 | 21/4/6 | 61/8/81 | 0.002 |
| Liver cirrhosis, no/yes | 4/27 | 44/106 | 0.059 |
| Ascites, no/small/moderate-massive | 16/11/4 | 75/59/16 | 0.892 |
| CTPV, no/yes | 26/5 | 132/18 | 0.74 |
| Invade left and right liver lobes, no/yes | 26/5 | 93/57 | 0.019 |
| Type of gross pathology, massive/nodular and diffuse | 13/18 | 99/51 | 0.012 |
| PVTT type, I/II, III/IV | 7/10/11/3 | 22/67/57/4 | 0.155 |
| Inferior vena cava tumor thrombus, no/yes | 30/1 | 141/9 | 0.854 |
| Arteriovenous fistula, no/yes | 21/10 | 104/46 | 0.861 |
| Total bilirubin, µmol/L | 19.550 (11.200-33.400) | 16.600 (12.900-24.000) | 0.436 |
| Prealbumin, mg/L | 124.500 (86.750-160.500) | 105.000 (78.000-142.000) | 0.163 |
| Albumin, g/L | 37.047 ± 5.635 | 36.813 ± 4.622 | 0.669 |
| Hemoglobin, g/L | 132.500 (117.500-146.500) | 125.000 (115.000-142.000) | 0.316 |
| WBC, 109/L | 4.565 (3.398-5.850) | 5.100 (4.050-6.610) | 0.284 |
| PLT, 109/L | 89.000 (63.250-126.000) | 119.000 (87.000-182.000) | 0.046 |
| INR | 1.120 (1.070-1.253) | 1.110 (1.050-1.200) | 0.281 |
| PT, S | 14.400 (13.850-15.875) | 14.300 (13.700-15.200) | 0.266 |
| APTT, S | 39.200 (36.450-42.075) | 39.700 (37.100-42.900) | 0.541 |
| ALT, U/L | 46.500 (38.500-72.000) | 45.000 (30.000-72.000) | 0.770 |
| AST, U/L | 56.500 (41.750-83.750) | 71.000 (46.000-115.000) | 0.108 |
| GGT, U/L | 177.500 (72.250-265.250) | 216.000 (132.000-356.000) | 0.036 |
| Cholinesterase, kU/L | 4.25 (2.775-5.905) | 4.51 (3.08-5.79) | 0.945 |
| Creatinine, µmol/L | 71.300 (61.475-79.500) | 67.600 (57.400-78.100) | 0.220 |
| AFP, µg/L | 568 (15.13~1210) | 1210 (97.335-1210) | 0.188 |
| Child-Pugh grade, A/B/C | 19/11/1 | 104/44/2 | 0.373 |
| ECOG score, 0/1/2/3 | 4/24/3/0 | 4/118/26/2 | 0.086 |
| ALBI grade, 1/2/3 | 13/16/2 | 43/104/3 | 0.09 |
| MELD score | 7.130 (5.548-9.315) | 7.860 (6.070-9.790) | 0.348 |
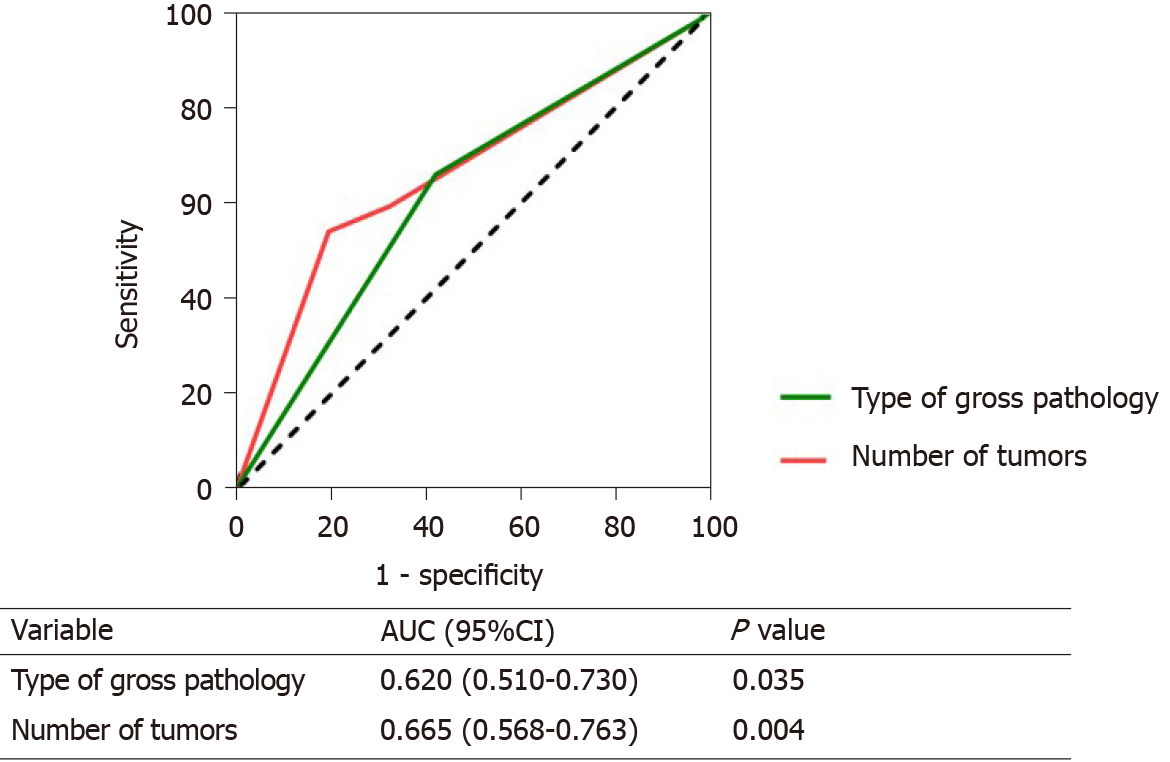
We used ROC curves to evaluate the predictability of the number of tumors and pathology for long-term survival. Number of tumors (AUC: 0.665, 95%CI: 0.568-0.763) had the best predictive ability, followed by gross pathology (AUC: 0.620, 95%CI: 0.510-0.730) (Figure 3).
In this paper, we found that sex, AST, and total bilirubin were independent influential factors for short-term survival. The incidence and mortality of HCC in men were higher than in women[11]. This was different from the conclusion in our article. The main reason was the imbalance of gender ratio in the article. The AUC of sex was not high (AUC: 0.554, 95%CI: 0.461-0.648, P > 0.05), which showed that although sex was an independent factor influencing short-term prognosis, its predictability was not well.
Total bilirubin was a biochemical indicator of liver metabolic function. Carr et al[12] believed that high levels of serum bilirubin can increase the risk of death in HCC patients with PVTT. In our study, it was also found that elevated bilirubin levels were associated with poor short-term survival in HCC patients with PVTT who underwent TACE. According to the AUC of bilirubin, we thought that this indicator had good predictive power. When total bilirubin was greater than 16.15 µmol/L, the patients’ mortality within 3 mo after TACE was significantly increased. This suggested that for patients with preoperative total bilirubin greater than 16.15 µmol/L, the treatment of lowering the bilirubin level may be beneficial in reducing the short-term postoperative mortality.
AST level is elevated in patients with liver disease, reflecting the degree of liver damage[13]. In this study, AST was found to be closely related to short-term survival after TACE, while ALT was not found to be associated with short-term survival after interventional treatment.
Xie et al[14] suggested that the total cause mortality rate, liver disease mortality and liver cancer mortality rate of those with elevated AST were higher than those of the correspondingly elevated ALT patients. This may mean that patients with preoperative high levels of AST do not obtain good short-term survival from TACE. When AST ≥ 87 U/L, the 3-mo mortality rate after TACE increased significantly, which indicated that correcting high levels of AST before TACE may be an effective measure to reduce short-term mortality.
In long-term survival analysis, we found that patients with only one lesion had better long-term survival than those with ≥ three lesions. Liu et al[15] obtained the same conclusion. Second, patients with massive block liver cancer had a worse long-term outcome than patients with nodular and diffuse liver cancer. This study used the gross pathological type of liver cancer because some patients were clinically diagnosed with liver cancer, and it was not possible to obtain liver tissue biopsy results. The gross pathological type could be judged by imaging, and its clinical applicability was better. However, in this research, the sample size of patients with the diffuse type of liver cancer was small, and this conclusion may need to be further validated in a larger sample size.
In addition, most patients had a background of viral hepatitis in the study, which was different from some areas. This may limit the application of our findings in regions where the cause of liver cancer was non-viral hepatitis. Therefore, research in other countries or regions may be needed to make up for the shortcoming of our study. Because patients with viral hepatitis accounted for the majority, model for end-stage liver disease scores in our study were higher than those of patients whose cause of liver cancer was alcohol or cholestasis.
The following limitations existed in this study. First, this study was a single-center, retrospective study, and it was difficult to avoid selection bias. Second, perhaps because of the small sample size, AUC value was not high, but the AUC value was still statistically significant. The larger and multicenter studies are needed to validate further the results in the future.
In summary, sex, AST, and total bilirubin were associated with the short-term survival outcomes in HCC patients with PVTT who underwent TACE. According to the AUC, AST was the best predictor of short-term survival, followed by total bilirubin. Multiple tumor lesions and massive block types of liver cancer were closely related to long-term adverse survival outcomes in HCC patients with PVTT who underwent TACE. In the future, multi-center, prospective and large sample studies are needed to verify these results.
Hepatocellular carcinoma (HCC) patients with portal vein tumor thrombosis (PVTT) have poor prognosis. Transarterial chemoembolization (TACE) is an effective treatment for HCC patients with PVTT. The factors affecting the short-term and long-term prognosis of HCC patients with PVTT receiving TACE are still unclear.
The main aim of this study was to clarify the predictors correlated with the short-term and long-term survival of HCC patients with PVTT who underwent TACE.
We can provide some guidance to clinicians for selecting suitable patients for TACE by analyzing preoperative indicators.
A total of 181 HCC patients with PVTT who underwent TACE were enrolled in this retrospective study. We explored the short-term and long-term prognostic factors by comparing the preoperative indicators of patients who died and survived within 3 mo and 12 mo after TACE. Multivariate analyses were conducted using logistic regression. The area under the receiver operating characteristic curve was used to evaluate the predictive ability of the factors related to the short-term and long-term prognosis.
Total bilirubin, sex, and aspartate aminotransferase (AST) were closely linked to short-term survival. When AST ≥ 87 U/L and total bilirubin ≥ 16.15 µmol/L, the 3-mo survival rate after TACE was reduced significantly. In long-term survival analysis, we found that patients with only one lesion had better long-term survival than those with ≥ three lesions. Patients with massive block liver cancer had a worse long-term outcome than patients with nodular and diffuse liver cancer.
Sex, AST, and total bilirubin were associated with short-term survival outcomes in HCC patients with PVTT who underwent TACE. According to the area under the curve, AST was the best predictor of short-term survival, followed by total bilirubin. Multiple tumor lesions and massive block types of liver cancer were closely related to long-term adverse survival outcomes in HCC patients with PVTT who underwent TACE.
Larger and multicenter studies are needed to validate further the results in the future.
We would like to thank Miss Huang F for her help in the preparation of this manuscript.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Pang H, Sempokuya T, Tarazov PG S-Editor: Zhang L L-Editor: Filipodia P-Editor: Liu JH
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55806] [Article Influence: 7972.3] [Reference Citation Analysis (132)] |
| 2. | Zhang ZM, Lai EC, Zhang C, Yu HW, Liu Z, Wan BJ, Liu LM, Tian ZH, Deng H, Sun QH, Chen XP. The strategies for treating primary hepatocellular carcinoma with portal vein tumor thrombus. Int J Surg. 2015;20:8-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 132] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 3. | Wang JC, Xia AL, Xu Y, Lu XJ. Comprehensive treatments for hepatocellular carcinoma with portal vein tumor thrombosis. J Cell Physiol. 2019;234:1062-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Lu J, Zhang XP, Zhong BY, Lau WY, Madoff DC, Davidson JC, Qi X, Cheng SQ, Teng GJ. Management of patients with hepatocellular carcinoma and portal vein tumour thrombosis: comparing east and west. Lancet Gastroenterol Hepatol. 2019;4:721-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 129] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 5. | Luo J, Guo RP, Lai EC, Zhang YJ, Lau WY, Chen MS, Shi M. Transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: a prospective comparative study. Ann Surg Oncol. 2011;18:413-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 273] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 6. | Xiang X, Lau WY, Wu ZY, Zhao C, Ma YL, Xiang BD, Zhu JY, Zhong JH, Li LQ. Transarterial chemoembolization vs best supportive care for patients with hepatocellular carcinoma with portal vein tumor thrombus: a multicenter study. Eur J Surg Oncol. 2019;45:1460-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 7. | Silva JP, Berger NG, Tsai S, Christians KK, Clarke CN, Mogal H, White S, Rilling W, Gamblin TC. Transarterial chemoembolization in hepatocellular carcinoma with portal vein tumor thrombosis: a systematic review and meta-analysis. HPB (Oxford). 2017;19:659-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 8. | Shuqun C, Mengchao W, Han C, Feng S, Jiahe Y, Guanghui D, Wenming C, Peijun W, Yuxiang Z. Tumor thrombus types influence the prognosis of hepatocellular carcinoma with the tumor thrombi in the portal vein. Hepatogastroenterology. 2007;54:499-502. [PubMed] |
| 9. | Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, O'Beirne J, Fox R, Skowronska A, Palmer D, Yeo W, Mo F, Lai P, Iñarrairaegui M, Chan SL, Sangro B, Miksad R, Tada T, Kumada T, Toyoda H. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1962] [Cited by in RCA: 2009] [Article Influence: 200.9] [Reference Citation Analysis (0)] |
| 10. | Chen LT, Martinelli E, Cheng AL, Pentheroudakis G, Qin S, Bhattacharyya GS, Ikeda M, Lim HY, Ho GF, Choo SP, Ren Z, Malhotra H, Ueno M, Ryoo BY, Kiang TC, Tai D, Vogel A, Cervantes A, Lu SN, Yen CJ, Huang YH, Chen SC, Hsu C, Shen YC, Tabernero J, Yen Y, Hsu CH, Yoshino T, Douillard JY. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with intermediate and advanced/relapsed hepatocellular carcinoma: a TOS-ESMO initiative endorsed by CSCO, ISMPO, JSMO, KSMO, MOS and SSO. Ann Oncol. 2020;31:334-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 164] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 11. | Wallace MC, Preen D, Jeffrey GP, Adams LA. The evolving epidemiology of hepatocellular carcinoma: a global perspective. Expert Rev Gastroenterol Hepatol. 2015;9:765-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 281] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 12. | Carr BI, Guerra V, Giannini EG, Farinati F, Ciccarese F, Ludovico Rapaccini G, Di Marco M, Benvegnù L, Zoli M, Borzio F, Caturelli E, Chiaramonte M, Trevisani F; Italian Liver Cancer (ITA. LI.CA) Group. Association of abnormal plasma bilirubin with aggressive hepatocellular carcinoma phenotype. Semin Oncol. 2014;41:252-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Iluz-Freundlich D, Zhang M, Uhanova J, Minuk GY. The relative expression of hepatocellular and cholestatic liver enzymes in adult patients with liver disease. Ann Hepatol. 2020;19:204-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 14. | Xie K, Chen CH, Tsai SP, Lu PJ, Wu H, Zeng Y, Ye Y, Tu H, Wen C, Huang M, Zhang Y, Lee JH, Tsai MK, Wen CP, Wu X. Loss of Life Expectancy by 10 Years or More From Elevated Aspartate Aminotransferase: Finding Aspartate Aminotransferase a Better Mortality Predictor for All-Cause and Liver-Related than Alanine Aminotransferase. Am J Gastroenterol. 2019;114:1478-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Liu L, Zhang C, Zhao Y, Qi X, Chen H, Bai W, He C, Guo W, Yin Z, Fan D, Han G. Transarterial chemoembolization for the treatment of advanced hepatocellular carcinoma with portal vein tumor thrombosis: prognostic factors in a single-center study of 188 patients. Biomed Res Int. 2014;2014:194278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |