Published online Mar 28, 2021. doi: 10.3748/wjg.v27.i12.1226
Peer-review started: December 17, 2020
First decision: January 23, 2021
Revised: January 28, 2021
Accepted: February 25, 2021
Article in press: February 25, 2021
Published online: March 28, 2021
Processing time: 97 Days and 16.7 Hours
Coronavirus disease 2019 (COVID-19) pandemic is still evolving globally, and Brazil is currently one of the most affected countries. It is still debated whether patients with inflammatory bowel disease (IBD) are at a higher risk for developing COVID-19 or its complications.
To assess geographical distribution of IBD patients at the highest risk and correlate these data with COVID-19 mortality rates in Brazil.
The Brazilian IBD Study Group (Grupo de Estudos da Doença Inflamatória Intestinal do Brasil) developed a web-based survey adapted from the British Society of Gastroenterology guidelines. The included categories were demographic data and inquiries related to risk factors for complications from COVID-19. Patients were categorized as highest, moderate or lowest individual risk. The Spearman correlation test was used to identify any association between highest risk and mortality rates for each state of the country.
A total of 3568 patients (65.3% females) were included. Most participants were from the southeastern and southern regions of Brazil, and 84.1% were using immunomodulators and/or biologics. Most patients (55.1%) were at moderate risk, 23.4% were at highest risk and 21.5% were at lowest risk of COVID-19 complications. No association between the proportion of IBD patients at highest risk for COVID-19 complications and higher mortality rates was identified in different Brazilian states (r = 0.146, P = 0.467).
This study indicates a distinct geographical distribution of IBD patients at highest risk for COVID-19 complications in different states of the country, which may reflect contrasting socioeconomic, educational and healthcare aspects. No association between high risk of IBD and COVID-related mortality rates was identified.
Core Tip: The coronavirus disease 2019 pandemic is still evolving globally, and Brazil is currently one of the most affected countries. The Brazilian Inflammatory Bowel Diseases Study Group developed a web-based survey of 3568 patients that was adapted from the guidelines of the British Society of Gastroenterology. This study indicates a distinct geographical distribution of patients with inflammatory bowel disease at higher risk for coronavirus disease 2019 complications in different states of the country, which may reflect contrasting socioeconomic, educational and health aspects. No association between high risk and coronavirus disease 2019-related mortality rates has been identified.
- Citation: Queiroz NSF, Teixeira FV, Motta MP, Chebli LA, Hino AAF, Martins CA, Quaresma AB, Silva AAPD, Damião AOMC, Saad-Hossne R, Kotze PG. Risk stratification and geographical mapping of Brazilian inflammatory bowel disease patients during the COVID-19 outbreak: Results from a nationwide survey. World J Gastroenterol 2021; 27(12): 1226-1239
- URL: https://www.wjgnet.com/1007-9327/full/v27/i12/1226.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i12.1226
The World Health Organization declared coronavirus disease 2019 (COVID-19) a pandemic on March 11, 2020. Although many countries are already registering a reduction in the incidence of infections and starting vaccination programs, Brazil is currently one of the leaders in the world on a daily basis for both numbers of new cases and deaths. As of November 28, 2020, we have confirmed 6238250 infected patients and 171998 COVID-related deaths[1].
Available data suggests that patients with inflammatory bowel disease (IBD) are not at a higher risk for severe acute respiratory syndrome coronavirus-2 infection or the development of COVID-19 complications[2]. Moreover, the evolution of COVID-19 does not seem to be worse in patients with IBD, irrespective of their treatment. A recent analysis of 525 IBD patients from the Surveillance Epidemiology of Coronavirus Under Research Exclusion for Inflammatory Bowel Disease revealed that increasing age, comorbidities and corticosteroids are associated with worse outcomes of COVID-19 and that treatment with tumor necrosis factor inhibitors (TNFi) was not associated with severe COVID-19. The number of reported patients exposed to other medical options was insufficient to drive conclusions regarding risk for severe outcomes in this population[3]. Recently, another report from the Surveillance Epidemiology of Coronavirus Under Research Exclusion for Inflammatory Bowel Disease registry aimed to evaluate the association of IBD medications and their combinations on the risk of adverse COVID-19 outcomes. Based on data from over 1400 IBD patients, it was demonstrated that the combination of thiopurines with TNFi and thiopurine monotherapy are associated with a significantly increased risk of severe COVID-19 as compared with TNFi monotherapy. Anti-interleukins and anti-integrins were not associated with a significantly different risk than TNFi monotherapy[4]. Regardless of the risk of IBD medications, it is noteworthy that maintaining patients in remission with steroid-sparing treatments may be crucial during the pandemic period.
As the effect of immunosuppressive agents in IBD patients remains unclear during the pandemic, the British Society of Gastroenterology has issued guidance on risk stratification for IBD patients depending on medications in use, age, comorbidities and other risk factors[5]. Patients at highest risk for COVID-related complications are those who have a comorbidity and/or are over 70-years-old and are on any immunosuppressant therapy for IBD. Those of any age that are receiving ≥ 20 mg of prednisolone, undergoing new induction therapy with biologics and immunomodul-ators (combination therapy) within 6 wk with moderate to severe active disease with short gut syndrome or patients requiring parenteral nutrition are also considered at highest risk for severe COVID-19 outcomes. Patients receiving biological therapy, thiopurines, calcineurin inhibitors, Janus kinase inhibitors or combination therapy are stratified as moderate risk and the remaining IBD patients as lowest risk. Depending on the risk stratification, recommendations can be suggested. Patients at highest risk are advised to self-isolate, while patients at moderate risk or lowest risk should follow strict social distancing or social distancing as for the general population, respectively. Specific guidance on instructions for self-isolation (shielding education) and social distancing measures to reduce spread of the disease within the population and to protect vulnerable groups has been recently issued[6].
In order to identify Brazilian IBD patients who could be at high risk for COVID-19 complications, a taskforce group from the Brazilian IBD Study Group (Grupo de Estudos da Doença Inflamatória Intestinal do Brasil, GEDIIB) developed an anonymous web-based survey that allows self-identification risk assessment adapted from the British Society of Gastroenterology guidelines. Through a decision tree, patients were self-identified into different groups according to their risk of developing COVID-19 complications and received updated information according to their respective group (Table 1).
| Highest risk |
| Stay home at all times and do not leave, even to buy food, medicine or to do exercises |
| Maintain attendance to infusions (only time to leave home) and the use of prescribed IBD medications |
| Stay at least 2 m (3 steps) from other people, including family members at home, whenever possible |
| Delivered products must be left outside the house by the courier |
| Anyone entering home must wash their hands thoroughly with soap for at least 20 s |
| Do not receive any visitors, unless help is needed |
| Moderate risk |
| Avoid contact with people with symptoms of COVID-19 |
| Avoid using public transportation, crowds, public spaces and meetings with friends or family |
| Home office whenever possible |
| Use smartphones or virtual technology to contact physicians or other essential services |
| Lowest risk |
| All risk groups must follow the recommendations of the World Health Organization |
| Wash hands thoroughly with soap for at least 20 s, frequently |
| Use 70% alcohol gel if soap or water is not available |
| Avoid touching the face |
| Clean objects and surfaces that are frequently touched (such as door handles and phones) |
| Stay home to help prevent the spread of the virus |
| Leave home for very limited purposes: buying food and medication, exercise once a day (running, walking or cycling) alone or with a family member, help a vulnerable person or donate blood |
| Travel for professional purposes only if strictly necessary |
| When leaving home, minimize the time spent away and keep 2 m away from others |
Currently, Brazil still has one of the fastest growing severe acute respiratory syndrome coronavirus-2 outbreaks in the world with one of the highest mortality rates, just behind the United States. Given that a higher absolute number of deaths in the context of an epidemic may reflect a strained healthcare system and economy, mapping IBD patients at highest risk for COVID-19 complications could help public authorities to delineate protection strategies to this possibly vulnerable population. Our study aimed to assess geographical distribution of IBD patients at highest risk and correlate these data with COVID-19 mortality rates in different states of Brazil.
The GEDIIB COVID taskforce members in collaboration with experts in the field developed a questionnaire and established a decision tree to evaluate IBD patients pertaining to their risk of serious complications from COVID-19. The national survey was available on the website (diicovid.com.br). Data was collected from April 14, 2020 to June 2, 2020. An informative article concerning this survey was posted at the official GEDIIB website, official mailing lists for patients and in national IBD patient association communication medias. All identified erroneous reports were removed for higher accuracy of the data.
The questionnaire consisted of fourteen questions (Supplementary Table 1). The included categories were demographic data (state and city of residence in Brazil, age, sex, smoking status) and questions related to the risk factors for complications from COVID-19 in IBD populations according to current previously published guidance[5,6]. The questions included IBD medications in use, self-reported comorbidities (hypertension, diabetes, cardiovascular disease and chronic pulmonary disease) and abdominal surgery for IBD performed in the past 30 d. Through a decision tree, patients were categorized as highest, moderate or lowest individual risk for the potential to develop serious complications from COVID-19 and received updated recommendations according to their risk level. Respondents were distributed according to their respective domiciliary states.
States of the national federation with a proportion of respondents at the highest risk for COVID-19 infection that were higher than the median rate of the overall country were considered for the analysis. Additionally, states with higher COVID-related cumulative mortality rates than the median of the country (as of June 2, 2020, the last available date of the survey) were also grouped. A possible correlation of these findings would suggest if there could be any coincidental relation between highest risk for COVID-19 complications and mortality in the same states.
Demographic and clinical characteristics of the study population were summarized by descriptive statistics. Categorical variables were expressed as absolute counts and percentages and continuous variables as means and standard deviations. Data was presented initially for the total study population. Thereafter, it was organized according to the five Brazilian regions and respective states to evaluate their geographical distribution. The frequencies of patients using each therapeutic IBD regimen in combination with oral steroids were analyzed as subcategories.
Categorical variables were compared using a Chi-squared test, and continuous data was analyzed using Student’s t-test. A two-tailed P value of 0.05 was used for statistical significance. The Spearman correlation test was performed to study a possible correlation between the proportion of highest risk patients and COVID-19 cumulative mortality in states with higher rates compared with the median national cutoffs for each variable. Data was exported and analyzed in SPSS Statistics 23 (IBM Corporation, Armonk, NY, United States).
Data regarding COVID-19 cumulative death rates from March 3 (first death registered in Brazil) to June 2 were obtained from the Brazilian Ministry of Health COVID-19 website, (https://covid.saude.gov.br/). We computed the COVID-19 mortality per 100000 people using the estimated populational data of 2019 available at the Statistical and Geographical Brazilian Institute for each of the Brazilian states and the federal district (https://datasus.saude.gov.br/populacao-residente/). In order to represent the mortality of COVID-19, we used classification into deciles. ArcMap 10.3® was used to generate the map representation.
The study was approved by the GEDIIB ethical review board under the protocol No. 002/2020 on October 28, 2020. Informed consent was waived because the survey recruitment was self-selective. In addition, data were de-identified. Individual participant data were not published, which maintained confidentiality in all steps of study analysis. This study was conducted in compliance with regulations stated in the 1975 Declaration of Helsinki.
A total of 3568 IBD patients participated in the national web-based survey and had data included. Six patients were excluded from the analysis due to inconsistent reported data. Overall demographic and baseline characteristics of respondents are illustrated in Table 2. Most respondents (55.6%) were 20-39-years-old, and 65.3% were females. Current smoking status was reported by 5.1% of the participants. The states with the highest response rates to the survey were São Paulo (29.6%), Rio de Janeiro (9.4%) Santa Catarina (7.7%), Paraná (7.7%), Bahia (6.0%) and the Federal District (5.3%). Details of the distribution of respondents per state are described in Supplementary Table 2.
| Characteristics | n = 3568 (%) |
| Age (yr) | 38.1 ± 12.3 |
| 0-9 | 10 (0.3) |
| 10-19 | 120 (3.4) |
| 20-29 | 769 (21.6) |
| 30-39 | 1214 (34) |
| 40-49 | 848 (23.8) |
| 50-59 | 379 (10.6) |
| 60-69 | 177 (5.0) |
| ≥ 70 | 51 (1.4) |
| Sex | |
| Male | 1238 (34.7) |
| Smoking | 183 (5.1) |
| Clinical risk factors | |
| Hypertension | 402 (11.3) |
| Diabetes | 119 (3.3) |
| Cardiovascular disease | 107 (3.0) |
| Chronic pulmonary disease | 164 (4.6) |
| Recent abdominal surgery for IBD (< 30 d) | 136 (3.8) |
| Overall IBD medications | |
| No medication | 339 (9.5) |
| Oral steroids | 473 (13.3) |
| 5-ASA | 1221 (34.2) |
| AZA/6-MP/MTX | 1169 (32.8) |
| Biologics | 1832 (51.3) |
| Therapeutic regimen | |
| Oral steroids monotherapy1 | 83 (2.3) |
| 5-ASA monotherapy1 | 758 (21.2) |
| 5-ASA + oral steroids2 | 115 (15.2) |
| AZA/6-MP/MTX monotherapy1 | 556 (15.6) |
| AZA/6-MP/MTX + oral steroids2 | 90 (16.2) |
| Biologic monotherapy1 | 1219 (34.2) |
| Biologic + oral steroids2 | 100 (8.2) |
| Combo therapy3 | 613 (17.2) |
| Combo therapy3 + oral steroids1 | 85 (13.9) |
| COVID-related complications risk | |
| Highest | 768 (23.4) |
| Moderate | 1965 (55.1) |
| Lowest | 836 (21.5) |
The proportion of patients presenting with at least one self-reported comorbidity was 21.6%, and the most prevalent was hypertension (11.3%) followed by chronic pulmonary disease (4.6%) and recent (< 30 d) IBD-related abdominal surgery (3.8%). Most patients (84.1%) were on immune-mediated therapy (biologics 51.3% and/or immunomodulators 32.8%), 34.2% of respondents were using aminosalicylates and 13.3% had been recently treated with corticosteroids. Demographic, clinical and treatment characteristics by states is presented in Tables 3 and 4. The uptake of biological therapy was slightly higher among patients from the southeastern (52.9%) and southern (52.2%) regions compared with those from the northern region (33.3%). Demographic, clinical and treatment characteristics by regions are described in detail in Supplementary Table 3.
| Characteristics | São Paulo | Rio de Janeiro | Minas Gerais | Santa Catarina | Paraná | Bahia | Distrito Federal | Rio Grande do Sul | Espírito Santo | Ceará | Pernambuco | Maranhão | Goiás |
| n = 1057 (%) | n = 336 (%) | n = 329 (%) | n = 278 (%) | n = 277 (%) | n = 217 (%) | n = 190 (%) | n = 183 (%) | n = 144 (%) | n = 107 (%) | n = 74 (%) | n = 57 (%) | n = 49 (%) | |
| Clinical risk factors | |||||||||||||
| Age ≥ 70 yr | 7 (0.7) | 2 (0.6) | 2 (0.6) | 7 (2.5) | 9 (3.2) | 1 (0.5) | 2 (1.1) | - | 8 (5.6) | 5 (4.7) | 2 (2.7) | - | - |
| Hypertension | 133 (12.6) | 43 (12.8) | 34 (10.3) | 27 (9.7) | 31 (11.2) | 29 (13.4) | 19 (10.0) | 11 (6.0) | 13 (9.0) | 14 (13.1) | 6 (8.1) | 2 (3.5) | 4 (8.2) |
| Diabetes | 33 (3.1) | 14 (4.2) | 7 (2.1) | 8 (2.9) | 13 (4.7) | 9 (4.1) | 9 (4.7) | - | 8 (5.6) | 6 (5.6) | 2 (2.7) | - | 3 (6.1) |
| Cardiovascular diseases | 23 (2.2) | 10 (3.0) | 4 (1.2) | 10 (3.6) | 12 (4.3) | 9 (4.1) | 9 (4.7) | 3 (1.6) | 8 (5.6) | 6 (5.6) | 2 (2.7) | 3 (5.3) | 1 (2.0) |
| Liver diseases | 52 (4.9) | 15 (4.5) | 12 (3.6) | 17 (6.1) | 11 (4.0) | 8 (3.7) | 10 (5.3) | 11 (6.0) | 5 (3.5) | 1 (0.9) | 3 (4.1) | 2 (3.5) | 2 (4.1) |
| Abdominal surgery for IBD (< 30 d) | 38 (3.6) | 12 (3.6) | 7 (2.1) | 11 (4.0) | 16 (5.8) | 5 (2.3) | 8 (4.2) | 1 (0.5) | 16 (11.1) | 5 (4.7) | 4 (5.4) | 1 (1.8) | 2 (4.1) |
| Overall IBD medications | |||||||||||||
| No medication | 112 (10.6) | 40 (11.9) | 26 (7.9) | 22 (7.9) | 28 (10.1) | 16 (7.4) | 16 (8.4) | 15 (8.2) | 15 (10.4) | 6 (5.6) | 5 (6.8) | 7 (12.3) | 1 (2.0) |
| Oral steroids | 137 (13.0) | 50 (14.9) | 52 (15.8) | 32 (11.5) | 30 (10.8) | 34 (15.7) | 27 (14.2) | 23 (12.6) | 10 (6.9) | 16 (15.0) | 11 (14.9) | 6 (10.5) | 7 (14.3) |
| 5-ASA | 357 (33.8) | 90 (26.8) | 124 (37.7) | 110 (39.6) | 90 (32.5) | 88 (40.6) | 68 (35.8) | 73 (39.9) | 20 (13.9) | 21 (19.6) | 37 (50.0) | 18 (31.6) | 19 (38.8) |
| AZA/6-MP/MTX | 308 (29.1) | 101 (30.1) | 130 (39.5) | 93 (33.5) | 95 (34.3) | 71 (32.7) | 51 (26.8) | 68 (37.2) | 62 (43.1) | 52 (48.6) | 27 (36.5) | 17 (29.8) | 21 (42.9) |
| Biologics | 575 (54.4) | 164 (48.8) | 156 (47.4) | 145 (52.2) | 153 (55.2) | 95 (43.8) | 93 (48.9) | 87 (47.5) | 92 (63.9) | 64 (59.8) | 28 (37.8) | 29 (50.9) | 29 (59.2) |
| Therapeutic regimen | |||||||||||||
| Oral steroid monotherapy1 | 17 (1.6) | 14 (4.2) | 8 (2.4) | 5 (1.8) | 2 (0.7) | 8 (3.7) | 12 (6.3) | 4 (2.2) | 1 (0.7) | 1 (0.9) | 3 (4.1) | - | - |
| 5-ASA monotherapy1 | 212 (20.1) | 57 (17.0) | 67 (20.4) | 67 (24.1) | 49 (17.7) | 68 (31.3) | 45 (23.7) | 46 (25.1) | 11 (7.6) | 12 (11.2) | 23 (31.1) | 12 (21.1) | 14 (28.6) |
| 5-ASA + oral steroids2 | 39 (18.4) | 4 (7.0) | 9 (13.4) | 12 (17.9) | 5 (10.2) | 11 (16.2) | 5 (11.1) | 6 (13.0) | 1 (9.1) | 1 (8.3) | 1 (4.3) | 2 (16.7) | 6 (42.9) |
| AZA/6-MP/MTX monotherapy1 | 141 (13.3) | 61 (18.2) | 72 (21.9) | 39 (14.0) | 45 (16.2) | 30 (13.8) | 24 (12.6) | 31 (16.9) | 25 (17.4) | 24 (22.4) | 15 (20.3) | 9 (15.8) | 5 (10.2) |
| AZA/6-MP/MTX + oral steroids2 | 28 (19.9) | 12 (19.7) | 11 (15.3) | 2 (5.1) | 10 (22.2) | 6 (20.0) | 3 (12.5) | 2 (6.5) | 2 (8.0) | 6 (25.0) | 2 (13.3) | 2 (22.2) | - |
| Biologic monotherapy1 | 408 (38.6) | 124 (36.9) | 98 (29.8) | 91 (32.7) | 103 (37.2) | 54 (24.9) | 66 (34.7) | 50 (27.3) | 55 (38.2) | 36 (33.6) | 16 (21.6) | 21 (36.8) | 13 (26.5) |
| Biologic + oral steroids2 | 21 (5.1) | 17 (13.7) | 18 (18.4) | 6 (6.6) | 9 (8.7) | 2 (3.7) | 5 (7.6) | 5 (10.0) | 4 (7.3) | 2 (5.6) | 3 (18.8) | 1 (4.8) | 1 (7.7) |
| Combo therapy3 | 167 (15.8) | 40 (11.9) | 58 (17.6) | 54 (19.4) | 50 (18.1) | 41 (18.9) | 27 (14.2) | 37 (20.2) | 37 (25.7) | 28 (26.2) | 12 (16.2) | 8 (14.0) | 16 (32.7) |
| Combo therapy3 + oral steroids2 | 32 (19.2) | 3 (7.5) | 6 (10.3) | 7 (13.0) | 4 (8.0) | 7 (17.1) | 2 (7.4) | 6 (16.2) | 2 (5.4) | 6 (21.4) | 2 (16.7) | 1 (12.5) | - |
| Risk classification | |||||||||||||
| Low | 221 (21.0) | 71 (21.1) | 67 (20.4) | 65 (23.4) | 53 (19.1) | 51 (23.5) | 50 (26.3) | 46 (25.1) | 15 (10.4) | 16 (15.0) | 20 (27.0) | 11 (19.3) | 11 (22.4) |
| Medium | 579 (54.8) | 184 (54.8) | 193 (58.7) | 139 (50.0) | 156 (56.3) | 118 (54.4) | 91 (47.9) | 108 (59.0) | 86 (59.7) | 62 (57.9) | 40 (54.1) | 40 (70.2) | 24 (49.0) |
| High | 256 (24.8) | 81 (24.1) | 69 (21.0) | 74 (26.6) | 68 (24.5) | 48 (22.1) | 49 (25.8) | 29 (15.8) | 43 (29.9) | 29 (27.1) | 14 (18.9) | 6 (10.5) | 14 (28.6) |
| Characteristics | Piauí | Amazonas | Pará | Alagoas | Rio Grande do Norte | Mato Grosso | Paraíba | Acre | Sergipe | Mato Grosso do Sul | Tocantins | Rondônia | Roraima | Amapá |
| n = 41 (%) | n = 38 (%) | n = 32 (%) | n = 31 (%) | n = 25 (%) | n = 22 (%) | n = 18 (%) | n = 16 (%) | n = 13 (%) | n = 12 (%) | n = 7 (%) | n = 6 (%) | n = 6 (%) | n = 3 (%) | |
| Clinical risk factors | ||||||||||||||
| Age ≥ 70 yr | - | - | - | - | - | - | 3 (16.7) | 2 (12.5) | - | 1 (8.3) | - | - | - | - |
| Hypertension | 5 (12.2) | 4 (10.5) | 1 (3.1) | 4 (12.9) | 1 (4.0) | 2 (9.1) | 5 (27.8) | 3 (18.8) | 1 (7.7) | 3 (25.0) | - | 3 (50.0) | 3 (50.0) | 1 (33.3) |
| Diabetes | - | 2 (5.3) | - | 1 (3.2) | - | - | 1 (5.6) | 2 (12.5) | - | 1 (8.3) | - | - | - | - |
| Cardiovascular diseases | 1 (2.4) | - | - | - | 1 (4.0) | 1 (4.5) | - | 1 (6.3) | - | 2 (16.7) | - | - | 1 (16.7) | - |
| Liver diseases | 2 (4.9) | 2 (5.3) | 5 (15.6) | 1 (3.2) | 1 (4.0) | 2 (9.1) | 2 (11.1) | - | - | - | - | - | - | - |
| Abdominal surgery for IBD (< 30 d) | 2 (4.9) | 4 (10.5) | 2 (6.3) | 1 (3.2) | - | - | - | 1 (6.3) | - | - | - | - | - | - |
| Overall IBD medications | ||||||||||||||
| No medication | 5 (12.2) | 5 (13.2) | 5 (15.6) | 1 (3.2) | - | 2 (9.1) | - | 2 (12.5) | 1 (7.7) | 3 (25.0) | 2 (28.6) | 1 (16.7) | 3 (50.0) | - |
| Oral steroids | 8 (19.5) | 7 (18.4) | 6 (18.8) | - | 3 (12.0) | 5 (22.7) | 3 (16.7) | 3 (18.8) | 1 (7.7) | 1 (8.3) | - | 1 (16.7) | - | - |
| 5-ASA | 11 (26.8) | 15 (39.5) | 21 (65.6) | 8 (25.8) | 6 (24.0) | 12 (54.5) | 10 (55.6) | 4 (25.0) | 7 (53.8) | 3 (25.0) | 2 (28.6) | 4 (66.7) | 2 (33.3) | 1 (33.3) |
| AZA/6-MP/MTX | 9 (22.0) | 11 (28.9) | 7 (21.9) | 13 (41.9) | 5 (20.0) | 5 (22.7) | 7 (38.9) | 4 (25.0) | 4 (30.8) | 2 (16.7) | 3 (42.9) | 1 (16.7) | - | 2 (66.7) |
| Biologics | 20 (48.8) | 19 (50.0) | 6 (18.8) | 19 (61.3) | 21 (84.0) | 5 (22.7) | 8 (44.4) | 6 (37.5) | 7 (53.8) | 6 (50.0) | 2 (28.6) | 1 (16.7) | 1 (16.7) | 1 (33.3) |
| Therapeutic regimen | ||||||||||||||
| Oral steroid monotherapy1 | 5 (12.2) | - | 1 (3.1) | - | - | - | - | 2 (12.5) | - | - | - | - | - | - |
| 5-ASA monotherapy1 | 9 (22.0) | 9 (23.7) | 15 (46.9) | 4 (12.9) | 3 (12.0) | 10 (45.5) | 8 (44.4) | 4 (25.0) | 3 (23.1) | 2 (16.7) | 1 (14.3) | 4 (66.7) | 2 (33.3) | 1 (33.3) |
| 5-ASA + oral steroids2 | - | 3 (33.3) | 4 (26.7) | - | - | 4 (40.0) | 1 (12.5) | - | - | - | - | 1 (25.0) | - | - |
| AZA/6-MP/MTX monotherapy1 | 2 (4.9) | 5 (13.2) | 5 (15.6) | 7 (22.6) | 1 (4.0) | 5 (22.7) | 2 (11.1) | 2 (12.5) | 2 (15.4) | 1 (8.3) | 2 (28.6) | - | - | 1 (33.3) |
| AZA/6-MP/MTX + oral steroids2 | - | 1 (20.0) | - | - | - | 1 (20.0) | - | 1 (50.0) | - | 1 (100) | - | - | - | - |
| Biologic monotherapy1 | 13 (31.7) | 13 (34.2) | 4 (12.5) | 13 (41.9) | 17 (68.0) | 5 (22.7) | 3 (16.7) | 4 (25.0) | 5 (38.5) | 5 (41.7) | 1 (14.3) | - | 1 (16.7) | - |
| Biologic + oral steroids2 | 1 (7.7) | 3 (23.1) | - | - | 1 (5.9) | - | - | - | 1 (20.0) | - | - | - | - | - |
| Combo therapy3 | 7 (17.1) | 6 (15.8) | 2 (6.3) | 6 (19.4) | 4 (16.0) | - | 5 (27.8) | 2 (12.5) | 2 (15.4) | 1 (8.3) | 1 (14.3) | 1 (16.7) | - | 1 (33.3) |
| Combo therapy3 + oral steroids2 | 2 (28.6) | - | 1 (50.0) | - | 2 (50.0) | - | 2 (40.0) | - | - | - | - | - | - | - |
| Risk classification | ||||||||||||||
| Low | 10 (24.4) | 7 (18.4) | 13 (40.6) | 4 (12.9) | 3 (12.0) | 9 (40.9) | 6 (33.3) | 4 (25.0) | 4 (30.8) | 3 (25.0) | 2 (28.6) | 3 (50.0) | 2 (33.3) | - |
| Medium | 24 (58.5) | 22 (57.9) | 14 (43.8) | 22 (71.0) | 16 (64.0) | 11 (50.0) | 4 (22.2) | 6 (37.5) | 7 (53.8) | 7 (58.3) | 4 (57.1) | 2 (33.3) | 3 (50.0) | 3 (100) |
| High | 7 (17.1) | 9 (23.7) | 5 (15.6) | 5 (16.1) | 6 (24.0) | 2 (9.1) | 8 (44.4) | 6 (37.5) | 2 (15.4) | 2 (16.7) | 1 (14.3) | 1 (16.7) | 1 (16.7) | - |
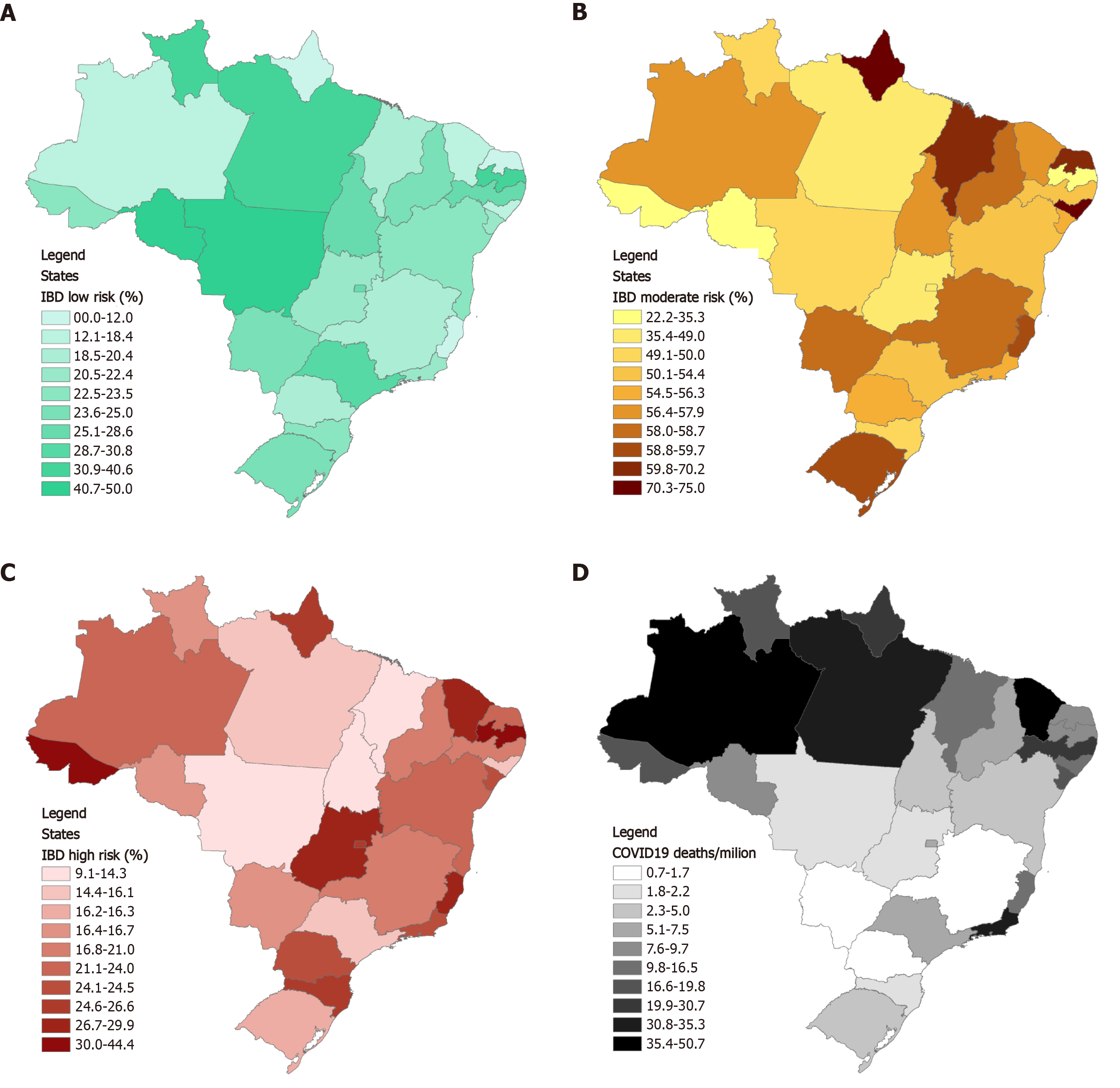
Overall, the majority (55.1%) of patients were at moderate risk, 23.4% were at highest risk and 21.5% were at lowest risk of developing COVID-19 complications. The proportion of IBD patients at highest, moderate and lowest risk for each state is represented in Figure 1A-C and for each county/city in Supplementary Figure 1. Thirteen states had higher proportional rates of patients at highest risk than the national median cutoff of 22.1% (Amapá, Rio Grande do Norte, Rio de Janeiro, São Paulo, Paraná, Amapá, Federal District, Santa Catarina, Ceará, Goiás, Espírito Santo, Acre and Paraíba). Paraíba was the state with the greatest proportion of IBD patients at highest risk (44.4%), followed by Acre (37.5%), Espírito Santo (29.9%) and Goiás (28.6%).
Geographical distribution of cumulative deaths from COVID-19 in Brazil as of June 2, 2020 by state (per 100000 people) is represented in Figure 1D. The national mortality rate median cutoff in June 2 was 9.7/100000. The 13 states with mortality rates above the national median were Rio Grande do Norte, Maranhão, Alagoas, Espírito Santo, São Paulo, Acre, Roraima, Amapá, Pernambuco, Rio de Janeiro, Pará, Ceará and Amazonas. The states with higher mortality rates were Amazonas (50.7), Ceará (37.5), Pará (35.3), Rio de Janeiro (32.9) and Amapá (28).
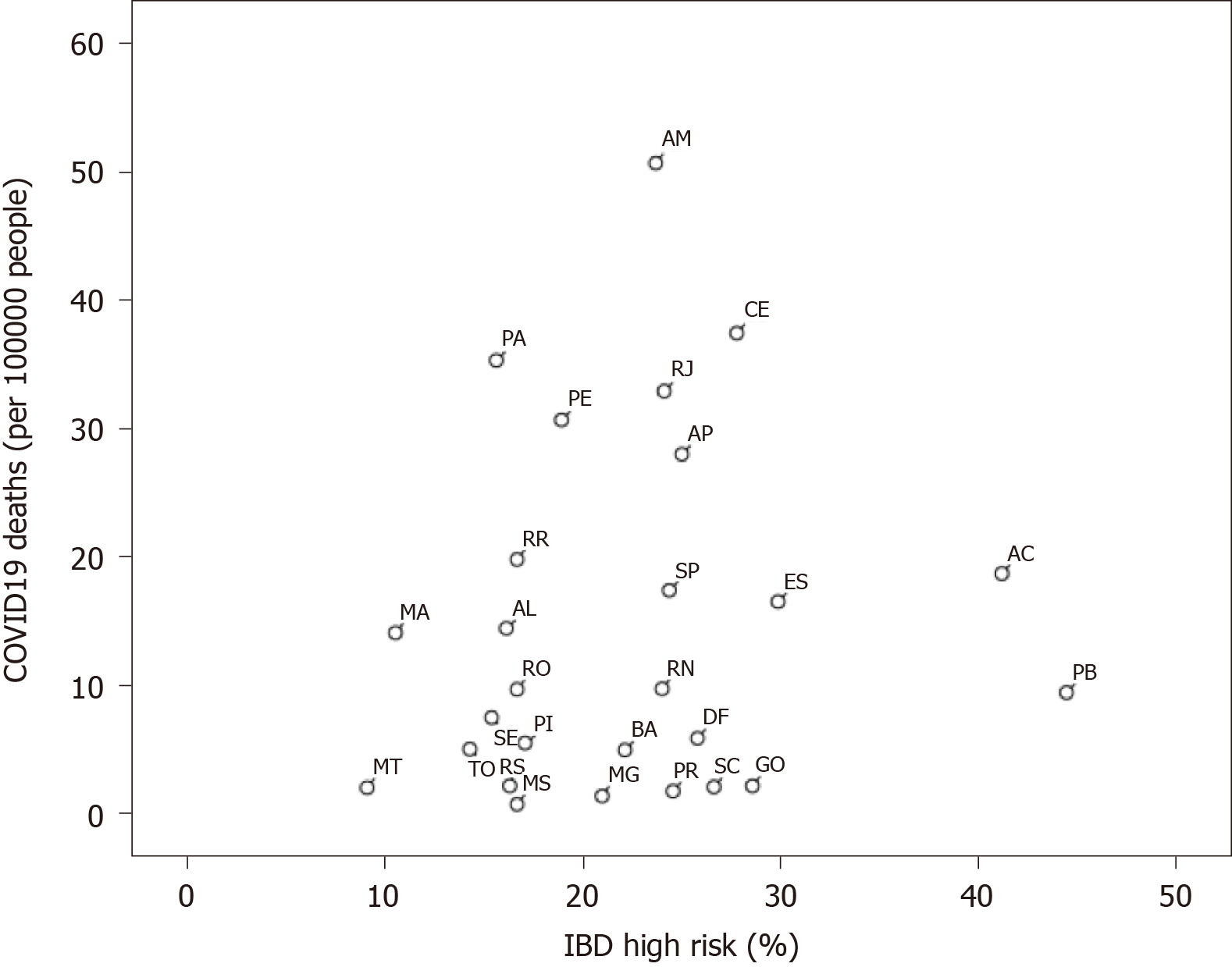
When crossing overall proportion of highest risk patients with cumulative mortality rates, the Spearman rank correlation coefficient was not statistically significant (r = 0.146, P = 0.467). These data are illustrated in Figure 2.
This web-based survey analyzed important patient and treatment characteristics that could influence the IBD-related risk of having COVID-19 complications at a national level. Brazil is a continental country with different socioeconomic realities between its five different geographic regions (i.e. Northern, Northeastern, Southern, Southeastern and Midwestern). Most patients who participated in the survey were from the southeastern (n = 1886) and southern (n = 738) regions, which are more developed areas of the country. This may reflect patients who more often follow official mailing lists from the study group (as the call for participation in the survey) and might be treated in IBD tertiary referral centers. This could also mirror a higher prevalence of IBD in these regions of Brazil as compared to others as stated in a systematic review and some population-based studies[7-10]. Indeed, the findings of our study may not reflect a full national reality, as patients from the northern and northeastern regions may have a different IBD treatment profile. In the same line, it is noteworthy that the northern region had the highest proportion of patients with no current IBD medication (17.6%), and the southern and southeastern regions the highest proportion of patients under biological therapy (52.2% and 52.9%, respectively).
Our study suggests a different geographical distribution of IBD patients at highest risk for COVID-19 complications in different states of the country, which may reflect different socioeconomic, educational and healthcare issues that could potentially have affected our findings. Despite the fact that the states of Paraíba, Acre, Espírito Santo and Goiás had the greatest proportion of patients at highest risk, this was not reflected in higher COVID-related mortality according to official data from the Ministry of Health. Higher mortality for COVID-19 as of June 2, 2020 (last available date capturing responses in the survey) was observed in the states of Amazonas, Ceará and Rio de Janeiro.
Nevertheless, a clear correlation between the risk of COVID-19 complications in IBD patients and mortality was not demonstrated according to the Spearman test. This important finding is in tune with other previously published data from IBD cohorts, which did not identify worse COVID-19 disease courses in IBD patients[3,11]. This analysis underscores the important finding that the majority of respondents (55.1%) were classified at moderate risk, which means that they were currently having adequate IBD treatment with no increased rates of steroid therapy. Despite being treated with immunosuppressant agents, this population may not be as vulnerable as expected[12]. Although this still needs to be proven, speculation is undertaken if the reduction of the COVID-related “cytokine storm” can be achieved with adequate medical therapy for IBD[13]. More studies in this field are warranted. It is important to state that no longitudinal follow-up of these patients was evaluated in this study. The exact number of infected patients with IBD within our sample was out of the focus of the survey.
When data is analyzed by regions, the proportion of IBD patients at highest risk was 20%-25%, and the majority of patients comprised those using immunomodulators or biologics with no active disease, did not undergo recent IBD-related surgery and were not under steroid therapy. This may reinforce the fact that the survey reached more patients who were likely under regular follow-up with their specialists, a common practice in more developed areas. Another important point is that no significant difference between the regions in the low, moderate or high risk of COVID-related complications was demonstrated (P = 0.118; Supplementary Table 2). This may also be demonstrated by the fact that even in the same region, a particular state could have a different frequency of high risk in comparison to its neighbors. As an example, the proportion of patients at highest risk was 28.6% in the state of Goiás and only 14.3% in Tocantins and 16.7% in Mato Grosso do Sul, all states from the midwestern region. This can also be justified by different types of IBD care and patient profiles in terms of comorbidities between the states, which demonstrates the complexity of analyzing data in a heterogeneous country such as Brazil.
Regarding COVID-related mortality rates in Brazil, it seems clear that the higher rates found in the states of Amazonas, Ceará, Pará, Rio de Janeiro and Amapá reflect difficulties in healthcare in these specific areas. Most of these states belong to less developed regions of the country (northern and northeastern). These are states with chronic difficulties in the public health system over the last decades, with limited resources, reduced numbers of hospitals and consequently disproportional intensive care unit beds per 100000 inhabitants[14]. Recent data revealed that Brazil has 15.6 intensive care unit beds per 100000 inhabitants. Considering only intensive care unit beds from the public health system, the average drops to 7.1 per 100000 inhabitants, and there are significant differences between the regions of the country. Among the population exclusively dependent on the public health system, 30.5% of the Northeast, 22.6% of the North and 21% of the Midwest regions live in places without intensive care unit beds[15].
Socioeconomical limitations may also be illustrated by the assessment of the Human Development Index (HDI) in these states because it is based on three aspects: Health, as measured by life expectancy at birth; Knowledge, as measured by the adult literacy rate; and A decent standard of living, as measured by gross domestic product per capita. Concerning the five geographic regions of Brazil, the first five states with the highest HDI are from the South, Southeast and Midwest regions and the last five states with the lowest HDI are from the North and Northeast regions[16]. Despite the northern and northeastern regions having a lower prevalence of IBD patients, the significant percentage of patients at highest risk for COVID-19 complications might reflect a similar healthcare system limitation, possibly with few available IBD specialists in the respective regions.
It is not being an easy task for Brazilian health authorities to deal with the COVID-19 pandemic. The country is facing important economic and political challenges that likely contribute to the significant increase of the number of cases and deaths throughout the country. The Brazilian government has been a recurrent target for scientific and regular media worldwide[17]. We truly hope this manuscript can raise awareness and call the attention from national health authorities with respect to vulnerability of specific populations during this critical period our country is facing.
The present study is associated with some limitations, which must be considered for adequate interpretation of the results. First, as previously mentioned, the higher proportion of respondents coming from the southeastern and southern regions may not reflect the reality in other states, mostly from the northern and northeastern regions, which have more rural areas and lower HDI. Another important point is that the survey was simple, objective and analyzed a limited number of variables. As an example, even the simple diagnostic difference between ulcerative colitis and Crohn’s disease was not evaluated. Disease activity at the time of the survey was not captured, which could have influenced the results. Data is also derived from self-reported personal and treatment-related characteristics, which may be biased by individual intellectual issues. Important additional limitations of our study include the absence of follow-up of the patients who replied to the survey. By not having this information, we could not describe in detail if patients who had COVID-19 infection continued their medications, possible differences between ulcerative colitis and Crohn’s disease or common manifestations of severe acute respiratory syndrome coronavirus-2 among included patients due to methodological issues. Despite these limitations, the study’s strength is based in the large number of patients who responded to the survey from all states of Brazil, and this represents one of the largest databases regarding COVID-19 risk for complications in IBD patients globally.
In summary, no correlation between the proportion of IBD patients at highest risk for COVID-19 complications and higher mortality rates was identified among Brazilian states. This can be related to the fact that the majority of the IBD patients are at moderate risk, which could possibly reflect adequate treatment and controlled disease. More epidemiological data comparing IBD and COVID-19 outcomes are suggested in large countries such as Brazil to properly position which IBD patients are more vulnerable in this pandemic period.
The coronavirus disease 2019 (COVID-19) pandemic is a public health emergency of international concern, and Brazil is currently one of the most affected countries.
It is uncertain whether patients with inflammatory bowel disease (IBD) are at a greater risk for developing COVID-19 or its complications. There are scarce data in large countries correlating IBD patients, the risk of COVID-19 complications and mortality.
This study aimed to evaluate geographical distribution of IBD patients at highest risk and correlate these data with COVID-19 mortality rates in different states of Brazil.
It was a web-based survey adapted from the British Society of Gastroenterology guidelines. We included demographic data and risk factors for complications from COVID-19. Patients were categorized as highest, moderate or lowest individual risk.
The proportion of IBD patients at highest risk for COVID-19 complications depends on individual aspects and can vary in specific regions. No correlation between patients with IBD at highest risk and COVID-related mortality rates was demonstrated in different regions of the country.
This is one of the largest studies analyzing the risk of patients with IBD during COVID-19 pandemic globally.
These data can be important to large countries such as Brazil, United States, Russia and India, which are currently facing significant problems in terms of controlling the pandemic. Even European countries, facing a second wave of COVID-19 infection, can base future research or decisions using these data as an example.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cassell III AK S-Editor: Gao CC L-Editor: Filipodia P-Editor: Li JH
| 1. | Painel Coronavirus. Ministério da Saúde. 16 July 2020. [cited 28 November 2020]. In: Coronavirus Brasil [Internet]. Available from: https://covid.saude.gov.br/. |
| 2. | Allocca M, Fiorino G, Zallot C, Furfaro F, Gilardi D, Radice S, Danese S, Peyrin-Biroulet L. Incidence and Patterns of COVID-19 Among Inflammatory Bowel Disease Patients From the Nancy and Milan Cohorts. Clin Gastroenterol Hepatol. 2020;18:2134-2135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 102] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 3. | Brenner EJ, Ungaro RC, Gearry RB, Kaplan GG, Kissous-Hunt M, Lewis JD, Ng SC, Rahier JF, Reinisch W, Ruemmele FM, Steinwurz F, Underwood FE, Zhang X, Colombel JF, Kappelman MD. Corticosteroids, But Not TNF Antagonists, Are Associated With Adverse COVID-19 Outcomes in Patients With Inflammatory Bowel Diseases: Results From an International Registry. Gastroenterology 2020; 159: 481-491. e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 491] [Cited by in RCA: 580] [Article Influence: 116.0] [Reference Citation Analysis (0)] |
| 4. | Ungaro RC, Brenner EJ, Gearry RB, Kaplan GG, Kissous-Hunt M, Lewis JD, Ng SC, Rahier JF, Reinisch W, Steinwurz F, Underwood FE, Zhang X, Colombel JF, Kappelman MD. Effect of IBD medications on COVID-19 outcomes: results from an international registry. Gut. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 228] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 5. | Kennedy NA, Jones GR, Lamb CA, Appleby R, Arnott I, Beattie RM, Bloom S, Brooks AJ, Cooney R, Dart RJ, Edwards C, Fraser A, Gaya DR, Ghosh S, Greveson K, Hansen R, Hart A, Hawthorne AB, Hayee B, Limdi JK, Murray CD, Parkes GC, Parkes M, Patel K, Pollok RC, Powell N, Probert CS, Raine T, Sebastian S, Selinger C, Smith PJ, Stansfield C, Younge L, Lindsay JO, Irving PM, Lees CW. British Society of Gastroenterology guidance for management of inflammatory bowel disease during the COVID-19 pandemic. Gut. 2020;69:984-990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 211] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 6. | Queiroz NSF, Barros LL, Azevedo MFC, Oba J, Sobrado CW, Carlos AS, Milani LR, Sipahi AM, Damião AOMC. Management of inflammatory bowel disease patients in the COVID-19 pandemic era: a Brazilian tertiary referral center guidance. Clinics (Sao Paulo). 2020;75:e1909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Kotze PG, Underwood FE, Damião AOMC, Ferraz JGP, Saad-Hossne R, Toro M, Iade B, Bosques-Padilla F, Teixeira FV, Juliao-Banos F, Simian D, Ghosh S, Panaccione R, Ng SC, Kaplan GG. Progression of Inflammatory Bowel Diseases Throughout Latin America and the Caribbean: A Systematic Review. Clin Gastroenterol Hepatol. 2020;18:304-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 125] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 8. | Gasparini RG, Sassaki LY, Saad-Hossne R. Inflammatory bowel disease epidemiology in São Paulo State, Brazil. Clin Exp Gastroenterol. 2018;11:423-429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Lima Martins A, Volpato RA, Zago-Gomes MDP. The prevalence and phenotype in Brazilian patients with inflammatory bowel disease. BMC Gastroenterol. 2018;18:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 10. | Parente JM, Coy CS, Campelo V, Parente MP, Costa LA, da Silva RM, Stephan C, Zeitune JM. Inflammatory bowel disease in an underdeveloped region of Northeastern Brazil. World J Gastroenterol. 2015;21:1197-1206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 60] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 11. | Norsa L, Indriolo A, Sansotta N, Cosimo P, Greco S, D'Antiga L. Uneventful Course in Patients With Inflammatory Bowel Disease During the Severe Acute Respiratory Syndrome Coronavirus 2 Outbreak in Northern Italy. Gastroenterology. 2020;159:371-372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 120] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 12. | Rubin DT, Feuerstein JD, Wang AY, Cohen RD. AGA Clinical Practice Update on Management of Inflammatory Bowel Disease During the COVID-19 Pandemic: Expert Commentary. Gastroenterology. 2020;159:350-357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 188] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 13. | Feldmann M, Maini RN, Woody JN, Holgate ST, Winter G, Rowland M, Richards D, Hussell T. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. Lancet. 2020;395:1407-1409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 405] [Cited by in RCA: 439] [Article Influence: 87.8] [Reference Citation Analysis (0)] |
| 14. | Moreira RDS. COVID-19: intensive care units, mechanical ventilators, and latent mortality profiles associated with case-fatality in Brazil. Cad Saude Publica. 2020;36:e00080020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 15. | Rache B, Rocha R, Nunes L, Spinola P, Malik AM MA. Necessidades de infraestrutura do SUS em preparo à COVID-19: leitos de UTI, respiradores e ocupação hospitalar. São Paulo Inst Estud para Políticas Saúde. 2020;. |
| 16. | Instituto Brasileiro de Geografia e Estatística. Índice de Desenvolvimento Humano. 16 July 2020. [cited 28 November 2020]. In: São Paulo. Governo. [Internet]. Available from https://cidades.ibge.gov.br/brasil/sp/pesquisa/37/0?tipo=ranking. |
| 17. | The Lancet. COVID-19 in Brazil: "So what? Lancet. 2020;395:1461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 303] [Cited by in RCA: 272] [Article Influence: 54.4] [Reference Citation Analysis (0)] |