Published online Mar 21, 2021. doi: 10.3748/wjg.v27.i11.1090
Peer-review started: November 1, 2020
First decision: November 30, 2020
Revised: January 11, 2021
Accepted: March 7, 2021
Article in press: March 7, 2021
Published online: March 21, 2021
Processing time: 128 Days and 21.4 Hours
Colonoscopy remains the gold standard for detection of colonic disease. An optimal evaluation depends on adequate bowel cleansing. Patients with inflammatory bowel disease (IBD), require frequent endoscopic assessment for both activity and dysplasia assessment. Two commonly used bowel preparations in Australia are Prep Kit-C (Pc) and Moviprep (Mp). Little is known about tolerability, efficacy and safety of split protocols of Mp and Pc in both IBD and non-IBD patients.
To primary aim was to compare the tolerability, efficacy and safety of split protocols of Mp and Pc in patients having a colonoscopy. The secondary aim was to compare the efficacy, tolerability and safety of either preparation in patients with or without IBD.
Patients were randomized to Pc or Mp bowel preparation. Patients completed a questionnaire to assess tolerability. Efficacy was assessed using the Ottawa Bowel Preparation Score. Serum electrolytes and renal function were collected one week prior to colonoscopy and on the day of colonoscopy.
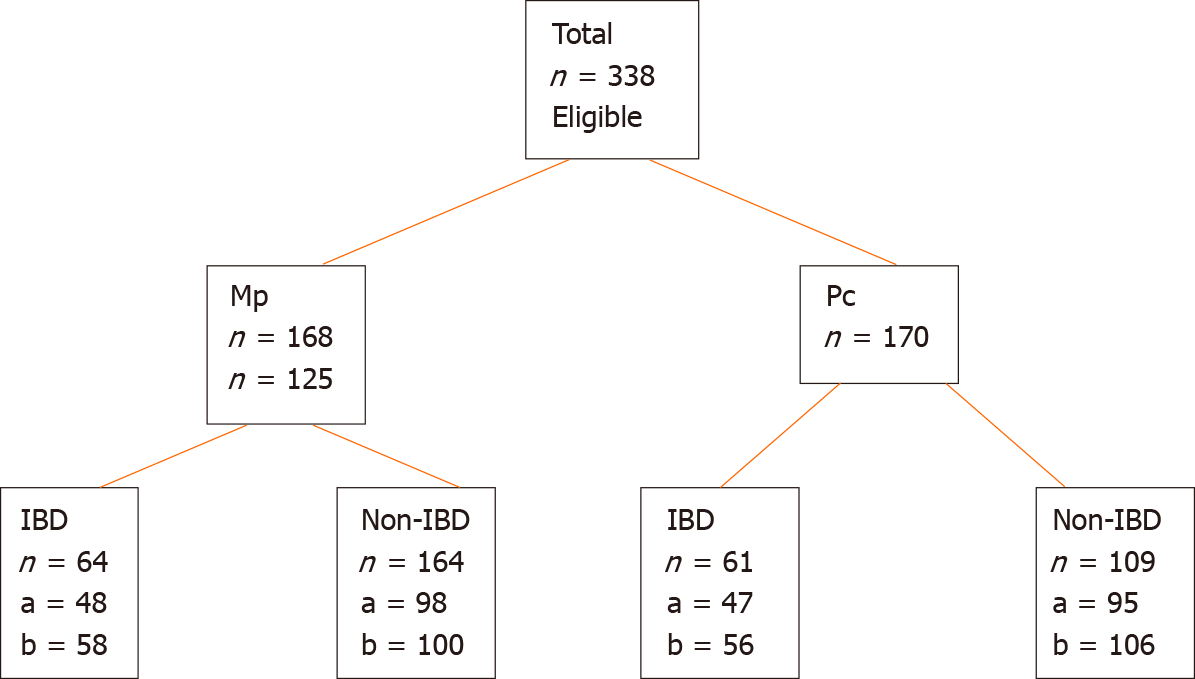
Of 338 patients met the inclusion criteria. Of 168 patients randomized to Mp and 170 to Pc. The efficacy of bowel preparation (mean Ottawa Bowel Preparation Score) was similar between Mp (5.4 ± 2.4) and Pc (5.1 ± 2.1) (P = 0.3). Mean tolerability scores were similar in Mp (11.84 ± 5.4) and Pc (10.99 ± 5.2; P = 0.17). 125 patients had IBD (73 had Crohn’s Disease and 52 had Ulcerative colitis). Sixty-four IBD patients were allocated to Mp and 61 to Pc. In non-IBD patients, 104 were allocated to Mp and 109 to Pc. The mean tolerability score in the IBD group was lower than the non-IBD group (mean tolerability scores: IBD: 10.3 ± 5.1 and non-IBD: 12.0 ± 5.3; P = 0.01). IBD patients described more abdominal pain with Mp when compared with Pc; (Mp: 5.7 ± 4.4 vs Pc: 3.6 ± 2.6, P = 0.046). Serum magnesium level increased with Pc compared with Mp in all patients (mean increase in mmol/L: Mp: 0.03 ± 0.117 and Pc: 0.11 ± 0.106; P < 0.0001).
In this study, the efficacy, tolerability and safety of Mp and Pc were similar in all patients. However, patients with IBD reported lower tolerability with both preparations. Specifically, IBD patients had more abdominal pain with Mp. These results should be considered when recommending bowel preparation especially to IBD patients.
Core Tip: When comparing Moviprep (Mp) and Prep-Kit C (Pc) in patients with and without inflammatory bowel disease (IBD): (1) Efficacy, tolerability and safety of Mp and Pc are similar; (2) Participants with IBD reported lower tolerability with both preparations; (3) IBD participants described more abdominal pain with Mp. Consideration of these results are important when discussing bowel preparation with IBD patients.
- Citation: Mohsen W, Williams AJ, Wark G, Sechi A, Koo JH, Xuan W, Bassan M, Ng W, Connor S. Prospective single-blinded single-center randomized controlled trial of Prep Kit-C and Moviprep: Does underlying inflammatory bowel disease impact tolerability and efficacy? World J Gastroenterol 2021; 27(11): 1090-1100
- URL: https://www.wjgnet.com/1007-9327/full/v27/i11/1090.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i11.1090
Colonoscopy remains the gold standard for detection of colonic disease. An optimal evaluation depends on adequate bowel cleansing. Suboptimal preparation occurs in up to 25% of colonoscopies and results in aborted or incomplete examinations in up to 7% of procedures[1,2]. Suboptimal preparation is associated with longer procedural time, increased need for repeat procedures, lower overall polyp detection rates, including detection of flat (non-polypoid) lesions, small polyps (< 10 mm) and large polyps (> 10 mm)[1,3]. The American Society for Gastrointestinal Endoscopy recommends the rate of inadequate bowel preparation should not exceed 15%[4].
Efficacious bowel preparation is not solely dependent on the type of preparation used. Preparation is enhanced when instructions regarding bowel preparation are explained thoroughly, interpreters are used (when required), a split regime is used and when the type of preparation is individualized to the patient’s age and comorbidities[5,6].
Adequate bowel preparation is particularly important in patients with inflammatory bowel disease (IBD). These patients have an increased risk of developing colonic dysplasia and neoplasia. The increasingly adopted technique of chromoendoscopy is also highly dependent on excellent bowel cleansing[7]. With the increasing annual incidence (24 per 100000) and prevalence (345 per 100000) of IBD in Australia[8], efficacious colonoscopy is crucial. Low tolerability of bowel preparation is reported in IBD patients, although this has not been prospectively validated[9]. The exact mechanism driving such low tolerability is unclear. It may relate to abdominal pain, nausea and vomiting[1,9]. Additional factors that have been reported include previous surgery, intestinal stenosis, altered motility, anxiety, or heightened visceral sensitivity and pre-procedure dietary recommendations[1,9].
In the general population, poor bowel preparation is more commonly seen in males, smokers, the elderly, patients with a history of stroke, dementia, diabetes, previous colonic resection and in patients who take opioids, psychotropic drugs and calcium channel blockers[4,10-12]. Tolerability is one of the most significant factors contributing to efficacy of preparation. Efficacy and tolerability are related, and synergistically both contribute to “effectiveness” of a preparation[13]. If the preparation is not well tolerated, even if otherwise efficacious, it will not be consumed, leading to reduced effectiveness.
In Australia, several bowel cleansing agents are available. Bowel preparations are usually based on solutions of polyethylene glycol (PEG)[14,15]. Prep Kit-C (Pc) is a combination of Picoprep (Sodium picosulphate⁄magnesium citrate) and glycoprep (PEG). Picoprep is a small volume, hyperosmotic solution, primarily exerting its action through osmotically drawing fluid into the intestinal lumen. Moviprep (Mp) is a combination of low volume PEG solution with ascorbic acid. The ascorbic acid has osmotic laxative effects and a pleasant taste[14,16]. Both Pc and Mp are approved for use under the Australian therapeutic goods administration.
At present, there are no prospective studies which examine tolerability, efficacy and safety of Pc when compared with Mp in both the general and IBD populations. This study’s primary aim was to compare tolerability, efficacy and safety of split protocols of Mp with Pc in participants having a colonoscopy. The secondary aim was to compare the efficacy and tolerability of either preparation in participants with or without IBD.
A prospective, randomized, single blinded trial was conducted at a single tertiary referral center. Recruitment of patients occurred from March 2013 to December 2016. Approval was obtained from the Institutional Human Ethics and Research Office (reference HREC/12/LPOOL/108).
All patients aged between 18-75 years requiring an outpatient colonoscopy were invited to participate in this study. Patients identified as having IBD required histological evidence of Crohn’s disease or ulcerative colitis from a previous colonoscopy.
The following were exclusion criteria: non–English speaking, renal insufficiency (defined as an estimated Glomerular Filtration Ratio of less than 50 mL/min), cardiac failure (New York Heart Association Class greater than two), advanced liver disease (Child-Pugh B or C), poorly controlled diabetes mellitus (uninterrupted Hba1c > 8.0% for greater than one year and/or end organ complications from diabetes mellitus), bowel obstruction, total or limited colonic resection, megacolon, dysphagia and pregnancy or planning to become pregnant during the trial period. Patients with IBD who had a preceding colectomy or ileocolonic resection (that involved or extended beyond the hepatic flexure) were also excluded from this study.
All participants were randomly allocated to a bowel preparation regime (Mp or Pc) at time of study recruitment in a 1:1 ratio. The allocation sequence was provided by the coordinating investigator. The investigator drew the patient allocated preparation out of an envelope which had equal numbers of both preparations. Patients were provided with their assigned bowel cleansing preparation at the time of randomization. The cohort was then stratified according to presence of IBD. Patients were unable to be blinded to their allocated preparation due to associated packaging and the differences in administration. Written information about the bowel preparation including appropriate diet and timing of consumption was provided and explained in detail at a clinic review prior to colonoscopy. These instructions are provided in Supplementary material 1 . All assessing endoscopists were blinded to the assigned bowel preparation.
The primary endpoint was the tolerability and efficacy of each bowel preparation in the entire cohort. The secondary endpoints were comparison of the tolerability and efficacy of the allocated bowel preparation in patients with and without IBD, as well as overall safety of bowel preparation.
Tolerability was assessed using a Tolerability Questionnaire modified from Lawrance et al[17] (Supplementary material 2). Patients received the questionnaire at their pre-assessment visit and completed it after finishing their bowel preparation on the day of their colonoscopy. The questionnaire included a five-point Likert scale to assess tolerability (ranging from 0 to 5) and palatability (ranging from 0 to 5) of the preparation. A lower score indicated poorer tolerance. Common side effects (abdominal discomfort, abdominal pain, nausea, vomiting, abdominal distension, dizziness and shortness of breath) were also measured on a five-point Likert scale (ranging from 0 to 5). A higher score indicated worse reported side effects.
Patients were provided with written instructions and the bowel preparation explained in detail by the recruiting investigator at the time of study recruitment (full preparation instructions are available in Supplementary material 2). Apart from the preparation agent, preparation was standardized between the two groups including split dosing and 24 h of clear fluids. Colonoscopies were performed by experienced consultant colonoscopists (n = 4) or advanced gastroenterology trainees under the direct supervision of one of the colonoscopists. All procedures were performed using intravenous sedation administered by an anesthetist.
Efficacy of colon cleansing was assessed using the validated Ottawa Bowel Preparation Score (OBPS)[18]. All colonoscopists attended calibrating sessions prior to study commencement. Two colonoscopists were blinded to the allocated bowel preparation, independently assessed the efficacy of bowel cleansing regime during insertion of the colonoscope, prior to washing. The OBPS grades the quality of bowel preparation (0 to 4, with 0 being no fluid and 4 pertaining to fluid/fecal material unable to be cleared) in three colonic segments (right, left, recto-sigmoid) in addition to an overall fluid score. The total score out of 14 was provided for each patient and an average score calculated from both scores. A score of zero represents excellent preparation and 14 represents solid stool in each segment and excessive fluid. Inadequate bowel preparation is defined as an OBPS score equal to or greater than 8[19,20].
Safety of each bowel preparation included determination of electrolyte alteration. Blood was collected from each patient within one week before bowel preparation and on the day of colonoscopy prior to the procedure for serum electrolytes. Changes in serum sodium, chloride, potassium, bicarbonate, urea, creatinine, magnesium, calcium and phosphate were measured.
For the primary analysis in the entire cohort, an estimated sample size of 127 patients in each group was calculated to detect a 15% difference in the tolerability of bowel preparation between Mp and Pc, with 95% confidence and 90% power. Preliminary data using the same Tolerability Questionnaire which reported the mean tolerability of Moviprep of 13.3 (standard deviation 4.9) in patients undergoing colonoscopy was used to guide the sample size calculation[21]. The difference in tolerability of 15% between bowel preparation regimes was selected as this was also used in another study assessing tolerability of different bowel preparations[17]. Assuming a completion rate of 80%, a target of at least 159 participants for recruitment in each group was sought, giving a sample size of at least 318. The student t-test was used to compare the differences in mean scores of tolerability and efficacy. Associations between categorical variables and outcomes were assessed using Chi-square test. The IBM Statistical Package for the Social Sciences for Windows version 25.0 (IBM corporation, Armonk, NY, United States) was used to analyze the data.
From March 2013 to December 2016, 338 patients were enrolled in the study. 168 patients were randomized to Mp and 170 to Pc (Figure 1). One hundred and twenty-five patients had a pre-existing diagnosis of IBD (58% patients with Crohn’s disease and 42% with ulcerative colitis). In the IBD group, 64 patients had Mp and 61 had Pc. In the non-IBD group, 104 patients had Mp and 109 had Pc (Figure 1). Within both the IBD and non-IBD groups, there was no difference in age or gender distribution across the allocated bowel preparation groups (Table 1). Forty percent (n = 86) of the non-IBD cohort were male, compared with 52% (n = 65) in the IBD cohort. The mean ages of the IBD and non-IBD groups were 40.3 ± 14.7 and 50.3 ± 13.4 years respectively (P = 0.65).
| IBD cohort (n = 125) | Non-IBD cohort (n = 213) | P value | |||||
| mean age +/- SD (yr) | 40.3 ± 14.7 | 50.3 ± 13.4 | 0.65 | ||||
| Prep Kit -C | Moviprep | P value | Prep Kit -C | Moviprep | P value | ||
| mean ± SD, age (yr) | 39.7 ± 14.27 | 40.9 ± 15.1 | 0.65 | 52.98 ± 12.97 | 53.65 ± 13.98 | 0.72 | |
| Male (n) | 35 | 30 | 0.13 | 39 | 47 | 0.93 | |
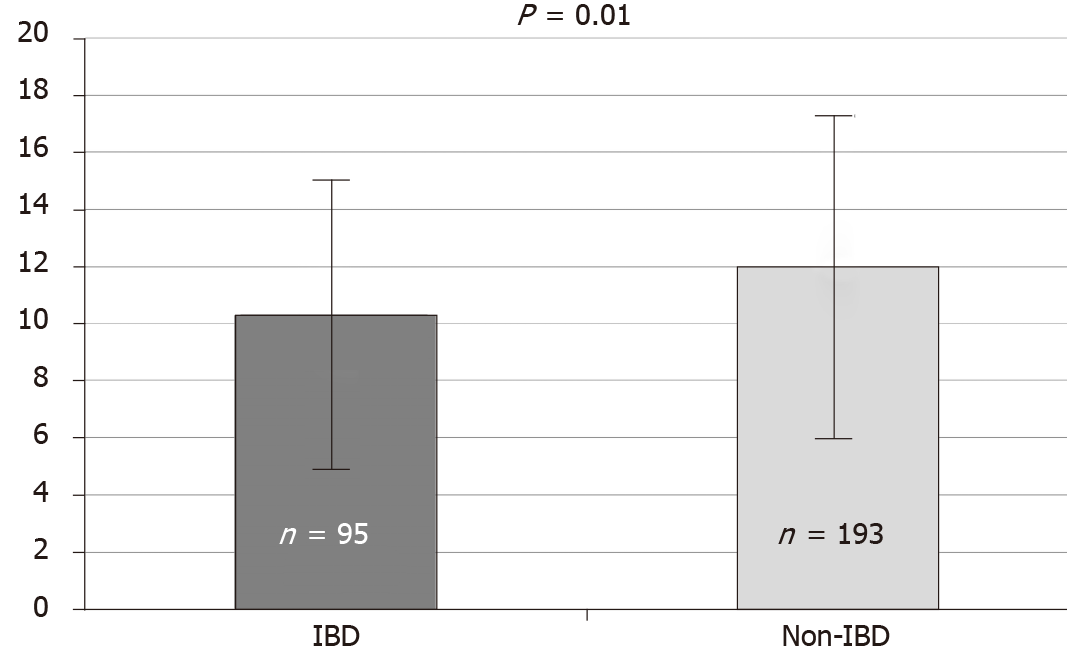
Of the 338 patients, 288 (85%) completed the questionnaire assessing tolerability (Figure 1), this proportion was similar in both the Mp and Pc groups. There were no significant differences in the mean scores for tolerability between Mp (11.84 ± 5.4) and Pc groups (10.99 ± 5.2; P = 0.17). Thirty and 20 patients from the IBD and non-IBD groups respectively did not complete the tolerability questionnaire. The tolerability score in the IBD (n = 95) group was significantly lower than the non-IBD group (n = 193) (10.3 ± 5.1 vs 12.0 ± 5.3, P = 0.01) (Figure 2), indicating poorer tolerability in this group of patients.
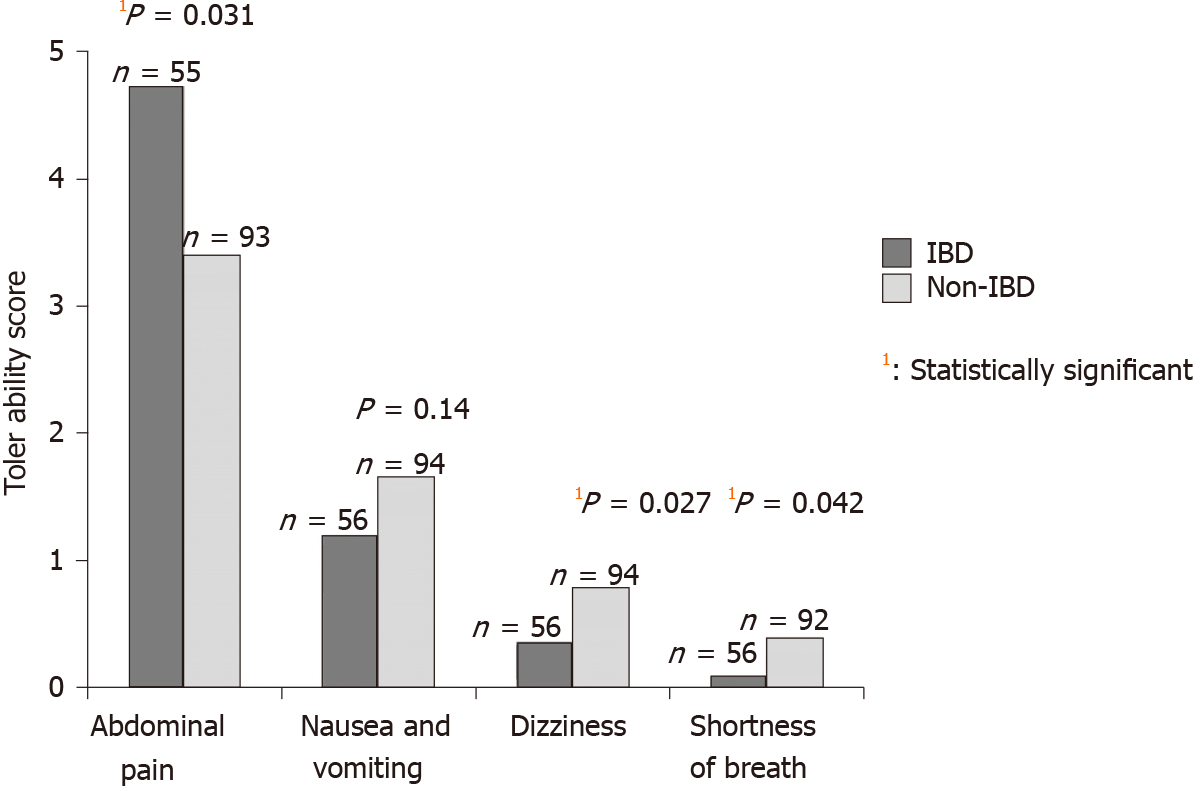
The IBD group reported higher score (indicating worse) for abdominal pain (mean 4.78 vs 3.39; P = 0.031) and lower mean score for dizziness (0.37 vs 0.78; P = 0.03), and shortness of breath (mean 0.09 vs 0.39; P = 0.042) compared with the non-IBD group. The mean scores for nausea/vomiting were similar in both groups (mean 1.15 vs 1.65; P = 0.14) (Figure 3). Within the IBD group, patients who had Mp reported more abdominal pain when compared with Pc (mean 5.7 vs 3.62; P = 0.046). There were no other significant differences in the mean scores for other symptoms within the non-IBD or IBD group.
When comparing the overall tolerability of Pc (n = 145) with Mp (n = 143) in both the IBD and non-IBD groups, there was no statistically significant difference in mean tolerability scores between the two bowel preparations, although the study may not have been powered to detect a significant difference (Table 2).
| Prep Kit-C (n = 145) | Moviprep (n = 143) | P value | |
| IBD, n = 95 | 9.67 ± 4.87; n = 47 | 10.89 ± 5.21; n = 48 | 0.25 |
| Non-IBD, n = 193 | 11.61 ± 5.32; n = 98 | 12.32 ± 5.35; n = 95 | 0.36 |
Data on efficacy of the bowel preparation was available in 320 patients (95%). There was no difference in the efficacy within the entire group when comparing Mp to Pc [mean OBPS: Mp (n = 158; 5.4 ± 2.4) and Pc (n = 162; 5.1 ± 2.1; P = 0.73)], nor within both the IBD [mean OBPS: Mp (n = 58; 4.8 ± 2.9) and Pc (n = 56; 5.2 ± 3.3; P = 0.53)] and non-IBD [mean OBPS: Mp (n = 100; 5.5 ± 2.4) and Pc (n = 106; 5.4 ± 2.1; P = 0.84)] groups.
Efficacy of bowel preparation when comparing the IBD (n = 114) to the non-IBD (n = 206) group was not significantly different (P = 0.26). Inadequate bowel preparation (defined as an OBPS of greater than or equal to 8)[17,19] was present in 8.9% (n = 29) of all patients: 10.5% (n = 12) of the IBD group and 8% (n = 17) of the non-IBD group.
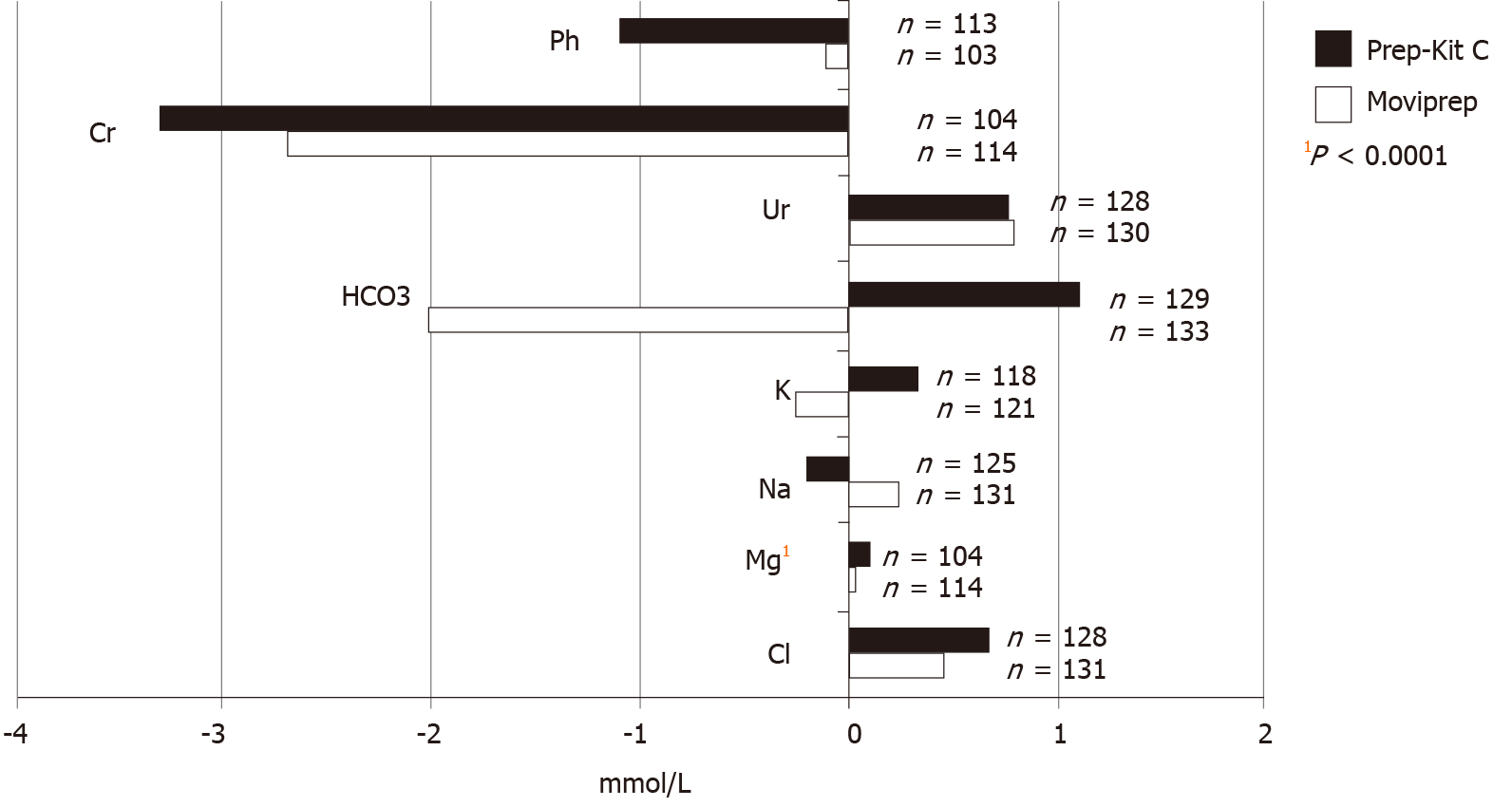
Electrolyte data was available for 256 patients (78%). There was a statistically significant increase in magnesium in patients who received Pc compared with Mp (mean increase in mmol/L: Mp 0.03 ± 0.117 and Pc 0.11 ± 0.106; P < 0.0001) (Figure 4). There were no additional differences detected in the remaining electrolytes. There were no reported clinical concerns attributed to electrolyte abnormalities during the peri-procedural period, such as arrhythmias, exacerbation of congestive cardiac failure or acute pulmonary edema.
This study has demonstrated no significant differences in the tolerability and efficacy of bowel preparation when comparing Mp with Pc. However, subgroup analysis revealed IBD patients were less tolerant of bowel preparation when compared with patients without IBD. IBD patients reported more abdomen pain with both preparations when compared with the non-IBD group. Within the IBD group, Mp produced more abdomen pain compared with Pc. Safety was comparable for IBD and non-IBD patients, although Pc resulted in a higher magnesium level than Mp.
The influence of effective bowel preparation on the quality of colonoscopy is substantial, as recently highlighted by the inclusion of bowel preparation adequacy and safety in the Australian Colonoscopy Care Standards formulated by the Commission on Safety and Quality in Health Care[22]. Systematic reviews have not demonstrated superiority of any specific bowel preparation regimes when assessing efficacy in both the non-IBD population as well as in those with IBD[14,23,24]. At our center, as well as many in Australia, Mp and Pc are commonly recommended bowel preparations. Prior to this study, there have been no prospective studies which compare the efficacy of Pc with Mp in non-IBD or IBD populations. Consistent with systematic reviews for other bowel preparations, our study demonstrated no significant difference in bowel preparation efficacy between Mp and Pc in both IBD and non-IBD populations. Our findings supported both Pc and Mp as suitable choices when considering efficacy of bowel preparation regimes in patients with and without IBD[1,19,20]. Nine percent of our overall study population had inadequate bowel preparation, which falls within the American Society for Gastrointestinal Endoscopy guidelines for adequate bowel preparation in at least 85% of patients[4].
Our study was unique in that both our IBD and non-IBD patients prospectively completed tolerability questionnaires at the time of bowel preparation ingestion. It was observed that IBD patients were less tolerant of bowel preparation when compared with patients without IBD, though the type of bowel preparation did not affect the total tolerability score when comparing IBD with the non-IBD groups. IBD patients also reported more abdominal pain when compared to non-IBD patients.
Poorer tolerability of bowel preparation within IBD cohorts is consistent with previously published literature. Denters et al[25] reported significantly more psychological and physical burden from bowel preparation in patients with IBD when compared with other patient groups. In another study, IBD patients most commonly cited difficulty with bowel preparation as the most important reason for failed compliance with scheduled colonoscopies for colorectal cancer surveillance[9]. Tolerability of bowel preparation in IBD patients may not be entirely related to luminal pathology. In another study, tolerance of bowel preparation was similar when comparing IBD and non-IBD cohorts, however co-morbid anxiety played a role in symptom development during bowel preparation in IBD patients[26].
Our study provides further impetus to reinforce the importance of educating IBD patients about bowel preparation, including the possibility for reduced tolerance and more abdomen pain. IBD patient awareness about potentially poor tolerance prior to ingestion may positively impact on the bowel preparation quality and compliance with surveillance protocols. Dietary liberalization, specifically using the white or low residue diet has been shown to be better tolerated and as efficacious as a clear fluid diet[27]. Tolerability of the white diet in comparison with the clear fluid diet, prior to colonoscopy, within the IBD population is a future research area.
Our study supports the safety of both Mp and Pc. There were no reported adverse clinical outcomes. A statistically significant increase in serum magnesium level with the use of Pc when compared with Mp was identified but it was of a small magnitude and unlikely to be clinically significant. Whilst there have been no prospective studies comparing electrolyte changes or adverse outcomes in patients taking Mp compared with Pc, our study is in line with other studies which have shown that Pc can cause electrolyte derangement[24]. Thus, Pc should be avoided in the elderly and patients with renal impairment[24].
Our study has several limitations. In relation to assessment of bowel preparation tolerability, our study utilized a modified, un-validated questionnaire developed by our study team based on an existing questionnaire[17]. Whilst we acknowledge this limitation, the same questionnaire was used in all study arms (Mp and Pc; IBD and non-IBD), and the questionnaire completion rate was equivalent amongst all study arms. Furthermore, tolerability of bowel preparation may have been influenced by the volume of fluid (e.g., water) replacement consumed by each participant in addition to the actual bowel preparation. This was not standardized between groups (Supplementary material 1). The tolerability questionnaire was completed just prior to the colonoscopy. As a result, delayed tolerability side effects from the allocated preparation may have been missed. Lastly, we did not collect data about variables which may influence bowel preparation efficacy. These variables include smoking history, medication history, history of Diabetes Mellitus or disease activity in IBD.
Our prospective, randomized controlled study has compared the tolerability, efficacy and safety of Mp and Pc in non-IBD and IBD patients. We demonstrated that both Mp and Pc had similar efficacy of bowel preparation in either the non-IBD or IBD cohorts. However, IBD patients were less tolerant of bowel preparation and reported more abdomen pain compared with patients without IBD. Furthermore, IBD patients reported more abdominal pain with Mp compared with Pc. Future research opportunities in this field include assessing factors contributing to poor bowel preparation tolerability in IBD patients is required.
Colonoscopy remains the gold standard for detection of colonic disease. An optimal evaluation depends on adequate bowel cleansing. Patients with inflammatory bowel disease (IBD), require frequent endoscopic assessment for both activity and dysplasia assessment. Two commonly used bowel preparations in Australia are Prep Kit-C (Pc) and Moviprep (Mp). Little is known about tolerability, efficacy and safety of split protocols of Mp and Pc in both IBD and non-IBD patients.
To determine which bowel preparation is tolerable and effective in both IBD and non-IBD patients. Efficacy and tolerability are related, and both contribute to effectiveness. By maximizing effectiveness we minimise the chances of inadequate bowel cleansing and incomplete colonoscopy. This ensures that hospital and patient resources are not wasted.
This study’s primary aim was to compare tolerability, efficacy and safety of split protocols of Mp with Pc in participants having a colonoscopy. The secondary aim was to compare the efficacy and tolerability of either preparation in participants with or without IBD.
Patients were randomized to Pc or Mp bowel preparation. Patients completed a questionnaire to assess tolerability. Efficacy was assessed using the Ottawa Bowel Preparation Score. Serum electrolytes and renal function were collected one week prior to colonoscopy and on the day of colonoscopy.
Of 338 patients met the inclusion criteria. Of 168 patients randomized to Mp and 170 to Pc. The efficacy of bowel preparation (mean Ottawa Bowel Preparation Score) was similar between Mp and Pc. Mean tolerability scores were similar in Mp and Pc. The mean tolerability score in the IBD group was lower than the non-IBD group. IBD patients described more abdominal pain with Mp when compared with Pc. Serum magnesium level increased with Pc compared with Mp in all patients.
In this study, the efficacy, tolerability and safety of Mp and Pc were similar in all patients. However, patients with IBD reported lower tolerability with both preparations. Specifically, IBD patients had more abdominal pain with Mp.
These results should be considered when recommending bowel preparation especially to IBD patients. More prospective studies are required in this field.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sha WH S-Editor: Zhang L L-Editor: A P-Editor: Ma YJ
| 1. | Nett A, Velayos F, McQuaid K. Quality bowel preparation for surveillance colonoscopy in patients with inflammatory bowel disease is a must. Gastrointest Endosc Clin N Am. 2014;24:379-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Harewood GC, Sharma VK, de Garmo P. Impact of colonoscopy preparation quality on detection of suspected colonic neoplasia. Gastrointest Endosc. 2003;58:76-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 560] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 3. | Heron V, Parmar R, Ménard C, Martel M, Barkun AN. Validating bowel preparation scales. Endosc Int Open. 2017;5:E1179-E1188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Rex DK, Schoenfeld PS, Cohen J, Pike IM, Adler DG, Fennerty MB, Lieb JG 2nd, Park WG, Rizk MK, Sawhney MS, Shaheen NJ, Wani S, Weinberg DS. Quality indicators for colonoscopy. Am J Gastroenterol. 2015;110:72-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 356] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 5. | Sharara AI, Bou Daher H. Bowel Cleansing Strategies After Suboptimal Bowel Preparation. Clin Gastroenterol Hepatol. 2019;17:1239-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Australian Commission on Safety and Quality in Health Care. Colonoscopy Clinical Care Standard. [cited 23 February 2021]. In: Clinical Care Standards, 2020 [Internet]. Available from: https://www.safetyandquality.gov.au/standards/clinical-care-standards/colonoscopy-clinical-care-standard. |
| 7. | Kiesslich R, Neurath MF. Surveillance colonoscopy in ulcerative colitis: magnifying chromoendoscopy in the spotlight. Gut. 2004;53:165-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Studd C, Cameron G, Beswick L, Knight R, Hair C, McNeil J, Desmond P, Wilson J, Connell W, Bell S. Never underestimate inflammatory bowel disease: High prevalence rates and confirmation of high incidence rates in Australia. J Gastroenterol Hepatol. 2016;31:81-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Friedman S, Cheifetz AS, Farraye FA, Banks PA, Makrauer FL, Burakoff R, Farmer B, Torgersen LN, Wahl KE. Factors that affect adherence to surveillance colonoscopy in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:534-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Yee R, Manoharan S, Hall C, Hayashi A. Optimizing bowel preparation for colonoscopy: what are the predictors of an inadequate preparation? Am J Surg. 2015;209:787-92; discussion 792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Chung YW, Han DS, Park KH, Kim KO, Park CH, Hahn T, Yoo KS, Park SH, Kim JH, Park CK. Patient factors predictive of inadequate bowel preparation using polyethylene glycol: a prospective study in Korea. J Clin Gastroenterol. 2009;43:448-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 151] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 12. | Lim SW, Seo YW, Sinn DH, Kim JY, Chang DK, Kim JJ, Rhee JC, Shim SG, Kim YH. Impact of previous gastric or colonic resection on polyethylene glycol bowel preparation for colonoscopy. Surg Endosc. 2012;26:1554-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Rex DK. Bowel preparation for colonoscopy: entering an era of increased expectations for efficacy. Clin Gastroenterol Hepatol. 2014;12:458-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Barkun A, Chiba N, Enns R, Marcon M, Natsheh S, Pham C, Sadowski D, Vanner S. Commonly used preparations for colonoscopy: efficacy, tolerability, and safety--a Canadian Association of Gastroenterology position paper. Can J Gastroenterol. 2006;20:699-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 91] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 15. | Munsterman ID, Cleeren E, van der Ploeg T, Brohet R, van der Hulst R. 'Pico-Bello-Klean study': effectiveness and patient tolerability of bowel preparation agents sodium picosulphate-magnesium citrate and polyethylene glycol before colonoscopy. A single-blinded randomized trial. Eur J Gastroenterol Hepatol. 2015;27:29-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Bitoun A, Ponchon T, Barthet M, Coffin B, Dugué C, Halphen M; Norcol Group. Results of a prospective randomised multicentre controlled trial comparing a new 2-L ascorbic acid plus polyethylene glycol and electrolyte solution vs. sodium phosphate solution in patients undergoing elective colonoscopy. Aliment Pharmacol Ther. 2006;24:1631-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Lawrance IC, Willert RP, Murray K. A validated bowel-preparation tolerability questionnaire and assessment of three commonly used bowel-cleansing agents. Dig Dis Sci. 2013;58:926-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Rostom A, Jolicoeur E. Validation of a new scale for the assessment of bowel preparation quality. Gastrointest Endosc. 2004;59:482-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 343] [Article Influence: 16.3] [Reference Citation Analysis (2)] |
| 19. | Chan M, Birnstein E, Patel N, Chan L, Kline M. Ottawa Score of 8 or Greater Is an Optimal Cut-off Point for Inadequate Bowel Preparation: 1156. Am J Gastroenterol. 2011;106:S431-S432. [DOI] [Full Text] |
| 20. | Parmar R, Martel M, Rostom A, Barkun AN. Validated Scales for Colon Cleansing: A Systematic Review. Am J Gastroenterol. 2016;111:197-204; quiz 205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 130] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 21. | Lee SH, Lee DJ, Kang JK. A randomized controlled trial of comparison on time and rate of cecal and terminal ileal intubation according to adult-colonoscope length: intermediate versus long. J Gastroenterol Hepatol. 2014;29:44-46. |
| 22. | Australian Commission on Safety and Quality in Health Care. Colonoscopy Clinical Care Standard 2017. [cited 23 February 2021]. Available from: https://www.safetyandquality.gov.au/standards/clinical-care-standards/colonoscopy-clinical-care-standard. |
| 23. | Restellini S, Kherad O, Bessissow T, Ménard C, Martel M, Taheri Tanjani M, Lakatos PL, Barkun AN. Systematic review and meta-analysis of colon cleansing preparations in patients with inflammatory bowel disease. World J Gastroenterol. 2017;23:5994-6002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 24. | Belsey J, Epstein O, Heresbach D. Systematic review: oral bowel preparation for colonoscopy. Aliment Pharmacol Ther. 2007;25:373-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 151] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 25. | Denters MJ, Schreuder M, Depla AC, Mallant-Hent RC, van Kouwen MC, Deutekom M, Bossuyt PM, Fockens P, Dekker E. Patients' perception of colonoscopy: patients with inflammatory bowel disease and irritable bowel syndrome experience the largest burden. Eur J Gastroenterol Hepatol. 2013;25:964-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 26. | Bessissow T, Van Keerberghen CA, Van Oudenhove L, Ferrante M, Vermeire S, Rutgeerts P, Van Assche G. Anxiety is associated with impaired tolerance of colonoscopy preparation in inflammatory bowel disease and controls. J Crohns Colitis. 2013;7:e580-e587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Butt J, Bunn C, Paul E, Gibson P, Brown G. The White Diet is preferred, better tolerated, and non-inferior to a clear-fluid diet for bowel preparation: A randomized controlled trial. J Gastroenterol Hepatol. 2016;31:355-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |