Published online Mar 14, 2021. doi: 10.3748/wjg.v27.i10.976
Peer-review started: November 23, 2020
First decision: January 7, 2021
Revised: January 12, 2021
Accepted: February 26, 2021
Article in press: February 26, 2021
Published online: March 14, 2021
Processing time: 107 Days and 17.5 Hours
Somatostatin analogues are an established first-line therapy for well differentiated small bowel neuroendocrine tumours (Wd-SBNETs), while and peptide receptor radionuclide therapy (PRRT) is frequently used as a second-line therapy. Adequate treatment selection of third-line treatment remains challenging due to the limited prospective data currently available on the best therapeutic sequence.
To understand current practice and rationale for decision-making by physicians in the 3rd-line setting by building an online survey.
Weighted average (WA) of likelihood of usage between responders (1 very unlikely; 4 very likely) was used to reflect the relevance of factors explored.
Replies from representatives of 28 centers were received (5/8/2020-21/9/2020); medical oncologist (53.6%), gastroenterologist (17.9%); United Kingdom (21.4%), Spain (17.9%), Italy (14.3%). Majority from European Neuroendocrine Tumor Society (ENETS) Centres of Excellence (57.1%), who followed ENETS guidelines (82.1%). Generally speaking, 3rd-line treatment for Wd-SBNETs was: everolimus (EVE) (66.7%), PRRT (18.5%), liver embolization (LE) (7.4%) and interferon-alpha (IFN) (3.7%); chemotherapy (0%); decision was based on clinical trial data (59.3%), or personal experience (22.2%). EVE was most likely used if Ki-67 < 10% (WA 3.27/4) or age < 70 years (WA 3.23/4), in the 3rd-line setting (WA 3.23/4); regardless of presence/absence of carcinoid syndrome (CS), rate of progression or extent of disease. Chemotherapy was mainly utilised only if rapid progression (within 6 mo) (WA 3.35/4), Ki-67 10%-20% (WA 2.77/4), negative somatostatin receptor imaging (WA 2.65/4) or high tumour burden (WA 2.77/4); temozolomide or streptozocin was used with capecitabine or 5-fluorouracil (5-FU) (57.7%), FOLFOX (5-FU combined with oxaliplatin) (23.1%). LE was selected if presence of CS (WA 3.24/4) or Ki-67 < 10% (WA 2.8/4), after progression to other treatments (WA 2.8/4). IFN was rarely used (WA 1.3/4).
Everolimus was the most frequently used therapeutic option in the third-line setting. The most important factors for decision-making included Ki-67, rate of progression, functionality and tumour burden; since this decision is based on multiple factors, it highlights the need for a multidisciplinary assessment.
Core Tip: Our survey delineates a possible treatment algorithm in patients with advanced small bowel neuroendocrine tumour (SBNET). While somatostatin analogues (SSAs), peptide receptor radionuclide therapy (PRRT) and everolimus are usually considered preferred first, second and third-line options respectively, chemotherapy is generally used when all other available treatments have failed. Locoregional therapies appear particularly useful when facing patients with functioning tumours, but their use is mainly limited to after at least two prior lines of treatment. We were also able to identify relevant unanswered questions in the field of advanced SBNET treatment, mainly in regards to the role of maintenance SSA after PRRT for non-functioning tumours. Multiple factors were identified as relevant at time of decision making; among them, Ki-67, rate of progression, tumour functionality and tumour burden may have a key role in helping physicians tailoring the treatment. Based on this, we would encourage for treatment decisions to be made within a multidisciplinary setting.
- Citation: Lamarca A, Cives M, de Mestier L, Crona J, Spada F, Öberg K, Pavel M, Alonso-Gordoa T. Advanced small-bowel well-differentiated neuroendocrine tumours: An international survey of practice on 3rd-line treatment. World J Gastroenterol 2021; 27(10): 976-989
- URL: https://www.wjgnet.com/1007-9327/full/v27/i10/976.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i10.976
Neuroendocrine neoplasms are rare and heterogeneous[1,2]. One of the main primary sites of development of well-differentiated neuroendocrine tumours (NETs) is the small bowel (SBNETs)[1]. These are characterised by the production of certain bioactive compounds (such as serotonin) which may derive on specific symptoms, such as carcinoid syndrome (CS)[3].
Nearly half of SBNETs are diagnosed at a non-resectable, advanced stage, being only suitable for palliative therapeutic approaches[4]. For treatment decision making, assessment of hormonal syndrome, disease stage, burden of disease, somatostatin receptor (SSTR) expression and tumour grade are crucial[5,6], with available guidelines informing on treatment options[7-11].
In recent years, management of SBNETs has changed significantly, with development of new anti-tumour treatment options. There is now high-level evidence phase III clinical trial data supporting the use of somatostatin analogues (SSAs)[12,13], which have shown both anti-tumour and anti-secretory effects. Above-label dosages of SSAs may also provide antitumour activity after progression on standard doses of SSAs, particularly in patients with slow-growing tumours[14]. After progression to SSA, the results of the NETTER-1 trial support the use of peptide-receptor radionuclide therapy (PRRT) with 177Lu-DOTATATE in SBNETs with high SSTRs expression (expected to account for above 80% of cases)[15]. In addition, the RADIANT-4 trial supported the use of everolimus over placebo for non-functioning SBNETs after progression to one prior line of treatment[16].
SBNETs have a better prognosis than other gastrointestinal malignancies, with estimated median survival from time of identification of metastatic disease of above 5 years[1,2]. In view of this, many patients are fit enough for multiple lines of treatment. In view of increasing treatment options[4], and despite available guidelines[7,8], selection of the best treatment strategy remains challenging. The use of SSAs in the first-line setting for patients diagnosed with SBNETs is supported by randomised clinical trial results and reflected in multiple guidelines. As mentioned above, options for second-line treatment could include PRRT or everolimus, with most recent guidelines supporting the use of PRRT over everolimus in the second-line setting especially if tumour expresses SSTR[8].
Thus, it is expected that majority of patients will receive first-line SSA and second-line PRRT as the predominant treatment strategy. However, the right choice for third-line treatment remains unclear and practice regarding the use of everolimus, chemotherapy and other therapy options is likely to vary significantly between different institutions. Such therapeutic options may include everolimus[16] (approved for patients with non-functioning SBNETs), liver embolization[17], chemotherapy (mainly alkylating-based or oxaliplatin-based and generally combined with fluoropyrimidine)[18,19] and interferon-alpha[20,21].
This survey of practice aims to provide an understanding of current practice and main factors used for treatment selection in advanced SBNETs, with special focus on third-line treatment strategies.
An online survey was built and shared with European Neuroendocrine Tumor Society (ENETS) Centres of Excellence (CoE)[22] and other health care professionals with expertise in NETs (via NETConnect group[23]). The survey contained questions regarding current practice, with special focus on factors currently influencing treatment decision making in SBNETs and especially on third-line treatment options. Even though baseline characteristics of responders (i.e., country, specialty, whether they practiced in an ENETS CoE or not) were collected, participation was anonymous and aimed to reflect their personal views.
The primary aim of this survey was to provide information on the patterns of use of third-line treatments in SBNET patients. The secondary aim was to define the factors associated with treatment selection in the third-line setting. Replies were collected between August 5, 2020 until September 21, 2020 and all data was analysed together. Duplicate replies from an individual centre were excluded, if all provided replies concurred. In order to assess for likelihood of prescribing a specific treatment in a specific scenario, respondents were asked to determine how likely they were to do so and replies collected in numbers ranging between 1 (very unlikely) to 4 (very likely). Joined analysis was performed by calculating weighted average (WA) of likelihood of usage between responders for each scenario explored, to reflect the relevance of each factor in the decision making; reported WA ranged between 1 and 4 (1 very unlikely; 4 very likely).
Answers from 28 health care providers were collected. Characteristics of responders are summarized in Table 1. Most responders were medical oncologist (53.6%) or gastroenterologist (17.9%), from United Kingdom (21.4%), Spain (17.9%) or Italy (14.3%). Majority were completed by healthcare professionals working in ENETS CoEs (57.1%) who reported using ENETs guidelines for informing their practice (82.1%).
| Number of responses | Percentage (%) | ||
| Specialty | Medical oncology | 15 | 53.6% |
| Clinical oncology | 3 | 10.7% | |
| Gastroenterology | 5 | 17.7% | |
| Endocrinology | 3 | 10.7% | |
| Surgery | 2 | 7.1% | |
| Country of practice | Belgium | 2 | 7.1% |
| Germany | 1 | 3.6% | |
| France | 2 | 7.1% | |
| Italy | 4 | 14.3% | |
| Netherlands | 2 | 7.1% | |
| Spain | 5 | 17.9% | |
| Sweden | 2 | 7.1% | |
| Switzerland | 2 | 7.1% | |
| United Kingdom | 6 | 21.4% | |
| Other | 2 | 7.1% | |
| Practice at ENETS Centre of Excellence | Yes | 16 | 57.1% |
| No | 12 | 42.9% | |
| Use of guidelines to inform management of SBNETs | Yes | 28 | 100.0% |
| No | 0 | 0.0% | |
| Guidelines used | ENETS guidelines | 23 | 82.1% |
| ESMO guidelines | 0 | 0.0% | |
| NCCN guidelines | 0 | 0.0% | |
| NANETS guidelines | 0 | 0.0% | |
| Other | 5 | 17.7% |
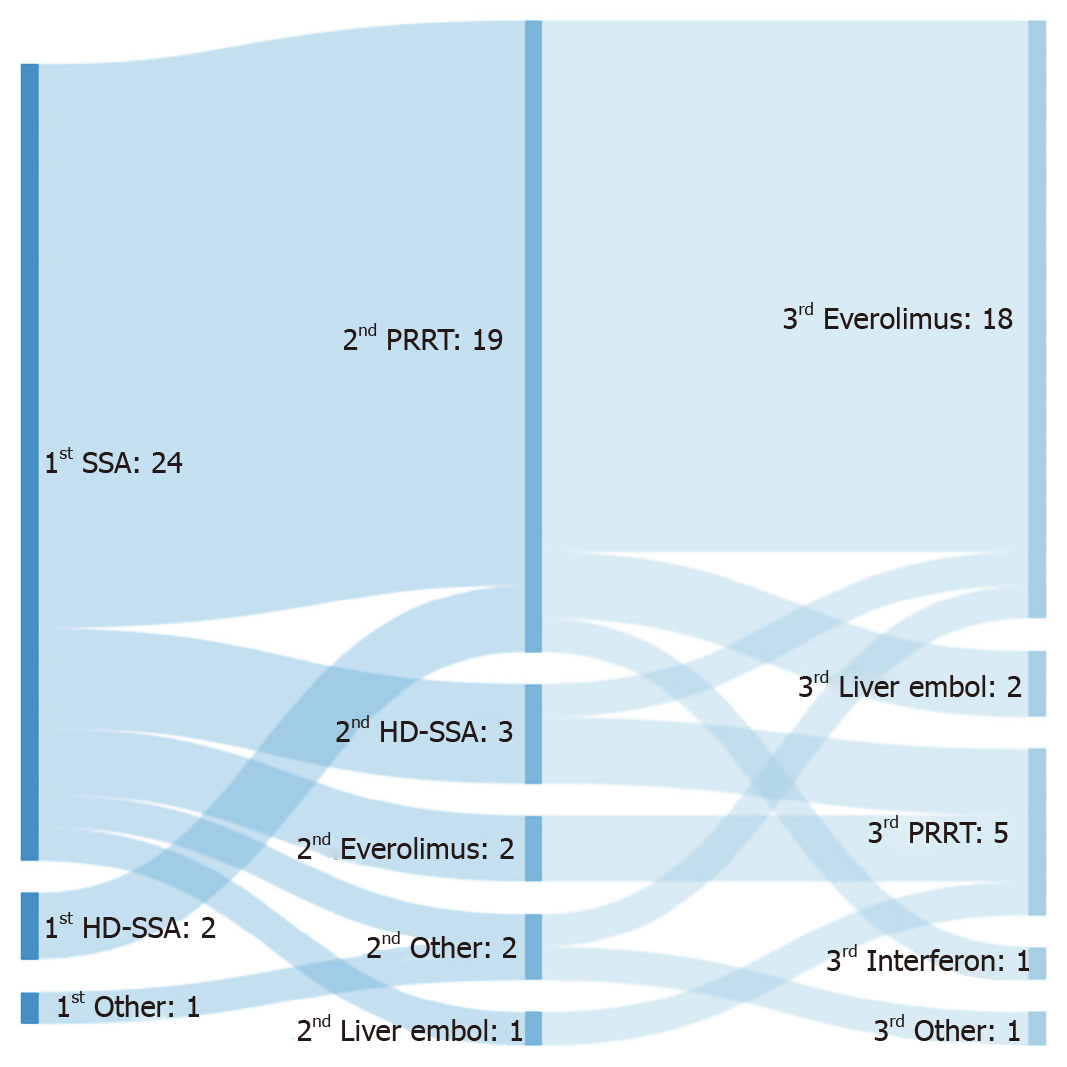
The most frequently used first- and second-line therapies for advanced SBNET were SSAs (88.9%) and PRRT (70.4%), respectively (Figure 1). Rationale for such choice was based on clinical trial evidence in 96.3% and 74.1% of responses, respectively. A proportion of responders selected alternative second-line options after SSA, such as high-dose SSA (3/24; 12.5%) or everolimus (2/24; 8.3%) (Figure 1).
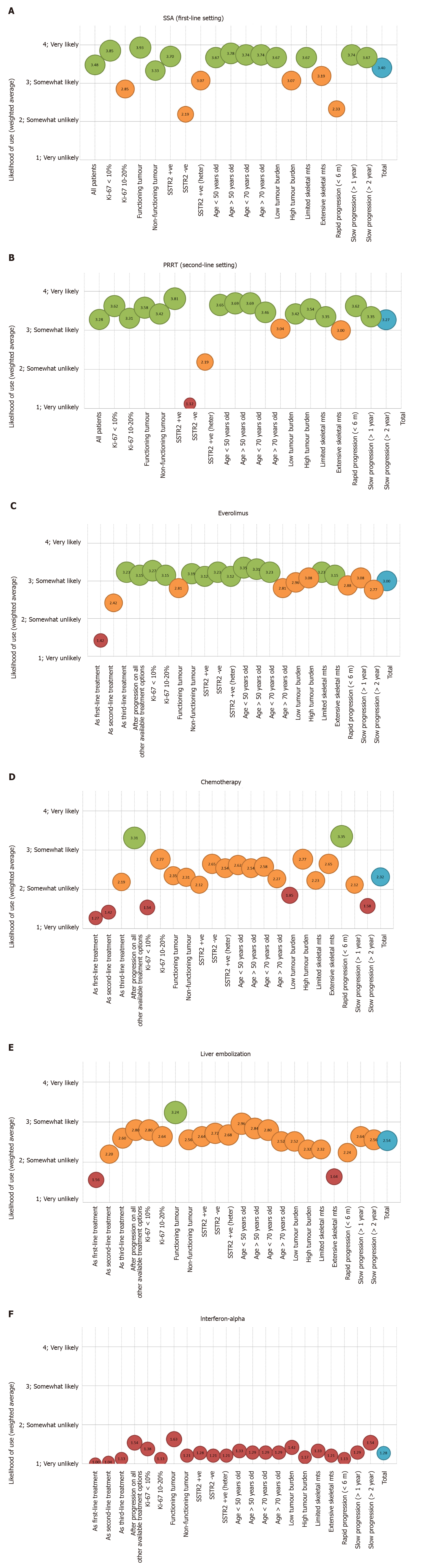
The likelihood of using SSAs for all SBNET patients (regardless of baseline characteristics) in the first-line setting had a weighted average of 3.48 (out of a maximum on 4) (Figure 2A; full details in Supplementary Figure 1A). The patients who were most likely to receive SSA in the first-line setting were those with functioning tumours (CS) (weighted average 3.93/4) and/or Ki-67 < 10% (weighted average 3.85/4), among others. Patients with rapid progression (<6 mo) (weighted average 2.33/4), negative SSTR imaging (weighted average 2.19/4) and/or Ki-67 10%-20% (weighted average 2.85/4) were the least likely to receive this treatment.
In terms of the use of PRRT in the second-line setting, the patients who were most likely to receive PRRT (Figure 2B; full details in Supplementary Figure 1B) were those with positive SSTR imaging (weighted average 3.81/4) and slow progression (> 1 year) (weighted average 3.62/4), among others. Patients with negative (weighted average 1.12/4) or heterogenous (weighted average 2.19/4) SSTR imaging were the least likely to be offered this treatment, followed by patients with low tumour burden (weighted average 3.04/4) and rapid progression (< 6 mo) (weighted average 3.00/4).
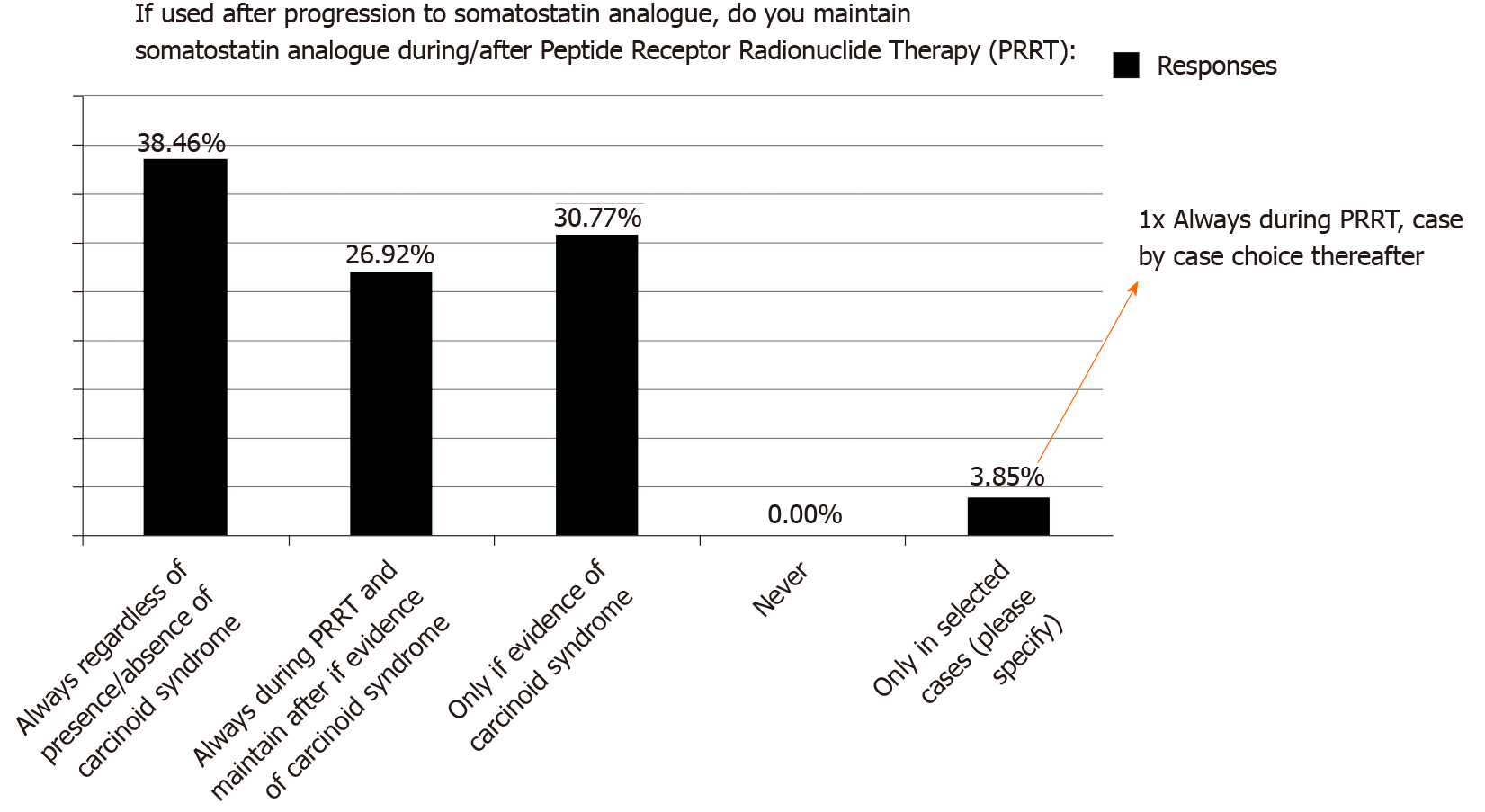
Regarding the use of PRRT, most considered this only after clinical or radiological progression on SSA (76.9%); however, there was no consensus regarding the use of concomitant SSA during/after PRRT (Figure 3): 38.5% always used SSA during/after PRRT (regardless presence/absence of CS), 26.9% used SSA during PRRT and maintain after PRRT only if evidence of CS and 30.8% used SSA during/after PRRT only in the presence of CS. One respondent (3.9%) specified that SSA was given during PRRT and individualised decision thereafter (details of factors involved not provided).
Third-line treatment choice was as follows: everolimus (66.7%), PRRT (18.5%), liver embolization (7.4%) and interferon-alpha (3.7%) (Figure 1); none of the responders selected chemotherapy as their preferred third-line therapy (0%). These replies were mainly based on clinical trial evidence (59.3%) and personal experience (22.2%). All five responders who selected PRRT as a third-line treatment did so following second-line treatment with high-dose SSA (2 responders), everolimus (2 responders) or liver embolization (1 responder); none of the responders considered rechallenge with PRRT as a predominant strategy.
Half of the responders (51.9%) would consider a trial at any point during patient’s pathway; 11.1%, 11.1%, 14.8% and 11.1% would do so in the first-, second-, third- and beyond third-line setting, respectively.
Everolimus was mainly used in the third-line setting (weighted average 3.23/4) or after progression to all other available treatment options (weighted average 3.15/4). The patients who were most likely to receive everolimus (Figure 2C; full details in Supplementary Figure 1C) were those with Ki-67 < 10% (weighted average 3.27/4) and/or age < 70 years (weighted average 3.23/4), within others. Choice of everolimus did not seem to vary depending on presence (weighted average 2.81/4) or absence (weighted average 3.19/4) of CS, rate of progression, extent of disease or Ki-67 (weighted average 3.27/4 if Ki-67 < 10% vs weighted average 3.15/4 if Ki67 10%-20%); full details Figure 2C; full details in Supplementary Figure 1C.
None of the responders selected chemotherapy as the preferred main third-line treatment option. Despite these, factors associated with its use were explored. Similar to the scenario described with everolimus, chemotherapy was mainly used predominantly after progression on all other available treatment options (weighted average 3.31/4) (Figure 2D; full details in Supplementary Figure 1D). In addition, chemotherapy was mainly chosen for patients with rapid progression to prior treatment (within 6 mo) (weighted average 3.35/4), Ki-67 10%-20% (weighted average 2.77/4), negative SSTR (weighted average 2.65/4) and high tumour burden (weighted average 2.77/4). Combination of temozolomide or streptozocine with capecitabine or 5-fluorouracil (5-FU) was the most popular choice of chemotherapy (57.7%), followed by FOLFOX (5-FU combined with oxaliplatin) (23.1%).
The use of liver-directed therapies, such as transarterial liver embolization, was also limited to second-line (weighted average 2.20/4) and third-line scenarios (weighted average 2.6/4); with a predominance of use after progression to all other available treatment options (weighted average 2.80/4) (Figure 2E; full details in Supplementary Figure 1E). The patients who were most likely to receive liver embolization were those with presence of CS (weighted average 3.24/4) and Ki-67 < 10% (weighted average 2.8/4), within others. Patients with extensive skeletal metastases were the least likely to be offered liver embolization (weighted average 1.64/4).
Use of interferon-alpha was rarely reported, with overall weighted average 1.3/4 (Figure 2F; full details in Supplementary Figure 1F). According to responses collected, no specific factors were associated with its use.
This survey aimed to address one of the main caveats in the management of SBNET: treatment decision making in terms of therapy sequencing, with special interest in the third-line setting. Our results identified everolimus as the preferred option of third-line therapy, with multiple factors identified as relevant at time of decision making, such as Ki-67, rate of progression, tumour functionality (CS) and tumour burden. The data underline that treatment decisions should be made within a multidisciplinary setting, where all available therapeutic options can be considered.
Choice of SSAs and PRRT as predominant first- and second-line treatments was expected. This is in keeping with current available guidelines[7,8] and can be accepted as current standard of care as supported by phase III clinical trial evidence[12,13,15]. The only exception would be those patients with SSTR2 negative disease who would not be eligible for PRRT and had been excluded from the NETTER-1 clinical trial[15]. Results of our survey do reflect this as well (Figure 2B; full details in Supplementary Figure 1B).
As shown in Figure 1, a small proportion of responders chose treatment with high-dose SSAs following progression to standard-dose SSAs. While the specific factors involved in this choice were not explored in the present survey, it is worth highlighting that this therapeutic option is suggested by guidelines mainly for patients with slowly-progressive SBNET and refractory functioning syndrome[7,8]. Results of the recently reported CLARINET-FORTE trial would support its anti-proliferative use following progression to SSA in selected scenarios, especially in case of Ki-67 ≤ 10%[14]. This phase II study included a cohort of 51 patients diagnosed with SBNET, who following progression to standard 4-weekly 120 mg lanreotide, received an increased dose of this same drug (120 mg 2-weekly); median duration of prior lanreotide exposure was 1.3 years (95%CI: 1.0-1.9) and 82.4% of patients had low-burden liver disease (affecting < 25% of liver parenchyma). Among the 51 patients with advanced SBNET included, median progression-free survival (PFS) was 8.3 (95%CI: 5.6-11.1) mo, with median PFS of 8.6 mo (95%CI: 5.6-13.8) and 5.5 mo (95%CI: 2.6-nr) for Ki-67 ≤ 10% and > 10% tumours, respectively. In view of this, authors concluded that high-dose SSAs could be recommended in selected patients, “before switching to an alternative, more toxic treatment”.
Our survey highlights three main unanswered questions around the use of PRRT in SBNETs.
First, there was discrepancy in terms of the current use of concomitant SSAs during and after PRRT (especially in non-functioning tumours). The NETTER-1 clinical trial recruited patients with SBNETs and as part of the study protocol, all patients (regardless of presence or absence of CS) received concomitant 4-weekly long-acting octreotide injection[15]. Thus, strictly speaking, NETTER-1 trial showed that PRRT was superior to high dose SSA when PRRT was given concomitantly with SSA (during and after PRRT). There is some evidence suggesting that combination of “hot” (PRRT) and “cold” (SSA) somatostatin analogue therapy could impact biodistribution of PRRT and may enable higher doses of PRRT to be delivered to tumour sites[24]. However, the benefit of maintenance SSA following PRRT completion is of much controversy. Some retrospective studies have suggested a benefit in terms of prolonged PFS[25], but selection bias (due to retrospective design) may confound findings. Thus, in the absence of CS, there is still disagreement regarding the benefit derived from maintenance SSAs after completion of PRRT and further studies are required to inform practice.
Second, the potential role of rechallenge with PRRT remains unclear. None of the responders chose rechallenge with PRRT in this survey as part of the main treatment pathway. However, it is likely that this is still a valid option of treatment for selected patients. There are data supporting the safety and efficacy of rechallenge with PRRT, mainly for patients with prolonged PFS to prior PRRT. In a series of 35 patients (23 SBNET), with median PFS of 33 mo (95%CI: 30-36) to prior PRRT, rechallenge with PRRT was shown to be safe (both in terms of kidney toxicity and myelosuppression)[26]. Main benefit was in the form of disease stabilisation (identified in 81.3% of patients), with partial response identified in 1 patient (3.1%). Median PFS reported with rechallenge PRRT was 6 mo (95%CI: 0-16). Other series also identified a possible association between initial response (in terms of prolonged PFS) to PRRTon first use and PFS benefit on PRRT rechallenge[27]. In view of these findings, and despite absence of prospective trial data, rechallenge PRRT may be a treatment option in selected patients (mainly patients with prolonged PFS to prior PRRT), despite low partial response rate and limited median PFS.
Third, the potential role of PRRT earlier in patients’ pathway remains undefined. There is currently no clinical trial data supporting this and available guidelines do consider PRRT after SSA for SBNETs[7,8]. This was reflected in our survey results, in which none of the responders selected PRRT as a first-line treatment, highlighting that further clinical trials would be required prior to any changes in current clinical practice. In this scenario, ongoing studies such as COMPETE (NCT03049189) and NETTER-2 (NCT03972488) are likely to provide useful insight to inform future practice.
There was a clear trend in favour of everolimus as the preferred choice of treatment in the third-line setting and this is likely based on vast clinical experience with everolimus. It is worth noting that the presence or absence of CS did not play a significant role on the likelihood of everolimus being considered. The fact that the weighted average was higher for non-functioning tumours (3.19/4) was not really surprising, in view of the benefit from everolimus over placebo in the setting of non-functioning SBNETs from the RADIANT-4 trial results[16]. However, the weighted average for functioning tumours SBNETs was high (2.81/4). This was despite the fact that everolimus is approved only in the setting of non-functioning SBNETs[28], which is reflected in current guidelines[7,8] and derived from the negative results of the RADIANT-2 study[29].
Based on our findings, interferon-alpha seems to have a minor role in the management of SBNETs and to be rarely used in clinical practice. Liver embolization was a preferred treatment option for patients with functioning tumours, in keeping with data from recent systematic review and meta-analysis[17], which reported a pooled symptomatic response of 55.2%. Chemotherapy was mainly used as a rescue treatment when progression to other available treatments was shown, and was mainly suggested for patients with rapid progression. These findings were expected and in keeping with current guideline recommendations[7,8]. As shown in our survey, most suitable choice of chemotherapy remains unclear, and despite a preference for temozolomide and capecitabine-based combinations, many responders reported to use other schedules. Collaborative work and trials in this setting are urgently needed.
Our results identified that many factors are involved in treatment choices, not only in second-line but also in the third-line setting, when choosing between a variety of treatment options such as everolimus, chemotherapy, liver embolization and interferon-alpha. The main factors identified as relevant for decision making were Ki-67, rate of progression, tumour functionality and tumour burden. It is therefore clear that “one size does not fit all” when selecting treatment options, especially in the third-line setting. Based on this, discussion within multidisciplinary meetings when disease progression to prior therapies is documented is recommended, to secure that the best treatment choice is made.
The main strength of this work is the fact that responders were clinicians with wide experience in the management of NETs, thus including the insights of healthcare professionals with adequate expertise to answer the proposed questions and dilemmas. Unfortunately, the number of replies was limited, which is the main limitation of our work. In addition, variability in drug access between different countries was likely to impact on some of the decision making steps, although it was not interrogated in the present work. Since treatment choices are based on approval and accessibility to drugs as well as reimbursement it remains unclear how much our findings are applicable to other countries including outside Europe.
In conclusion, our survey delineates a possible treatment algorithm in patients with advanced SBNET. While SSAs, PRRT and everolimus are usually considered preferred first, second and third-line options respectively, chemotherapy is generally used when all other available treatments have failed. Locoregional therapies appear particularly useful when facing patients with functioning tumours, but their use is mainly limited to after at least two prior lines of treatment. We were also able to identify relevant unanswered questions in the field of advanced SBNET treatment, mainly in regards to the role of maintenance SSA after PRRT for non-functioning tumours. Multiple factors were identified as relevant at time of decision making; among them, Ki-67, rate of progression, tumour functionality and tumour burden may have a key role in helping physicians tailoring the treatment. Based on this, we would encourage for treatment decisions to be made within a multidisciplinary setting.
Adequate sequencing of treatment for small bowel neuroendocrine tumour (SBNET) is unclear and likely to rely on multiple factors.
We aimed to understand current practice for advanced SBNET.
This international survey aimed to collect data on current algorithms and main factors involved in decision making process.
An international survey was completed by many health care professionals with an expertise in neuroendocrine tumours.
Our survey delineates a possible treatment algorithm in patients with advanced SBNET. While somatostatin analogues (SSAs), peptide receptor radionuclide therapy (PRRT) and everolimus are usually considered preferred first, second and third-line options respectively, chemotherapy is generally used when all other available treatments have been used. Locoregional therapies appear particularly useful when facing patients with functioning tumours, but their use commonly follows the first two lines of treatment.
We were also able to identify relevant unanswered questions in the field of advanced SBNET treatment, mainly in regards to the role of maintenance SSA after PRRT for non-functioning tumours. Multiple factors were identified as relevant at time of decision making; among them, Ki-67, rate of progression, tumour functionality and tumour burden may have a key role in helping physicians tailoring the treatment.
Based on this, we would encourage for treatment decisions to be made within a multidisciplinary setting.
Dr Angela Lamarca received funding from The Christie Charity. Dr. Joakim Crona received funding from Cancerfonden. The design of the survey and its distribution was supported by COR2ED and the NETConnect group (funded by Ipsen).
Dr. Louis de Mestier received advisory honoraria from Ipsen, Novartis and Pfizer, and speaker honoraria from Ipsen, Novartis, Pfizer and Keocyt. Marianne Pavel received honoraria for presentations and/or advisory role from Novartis, IPSEN, AAA, Pfizer, Riemser, Lexicon, Prime Oncology, Boehringer Ingelheim. Teresa Alonso-Gordoa declares consultant or advisory honoraria from IPSEN, Adacap, Roche, Pfizer, Sanofi, Bayer, Janssen, Astellas, BMS, Merck and Eisai and speaker honoraria from IPSEN, Adacap, Pfizer, Janssen, Astellas. All coauthors are member of the NETConnect Initiative supported by Ipsen.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kim HS S-Editor: Zhang H L-Editor: A P-Editor: Ma YJ
| 1. | Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, Shih T, Yao JC. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017;3:1335-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1510] [Cited by in RCA: 2461] [Article Influence: 307.6] [Reference Citation Analysis (4)] |
| 2. | Auernhammer CJ, Spitzweg C, Angele MK, Boeck S, Grossman A, Nölting S, Ilhan H, Knösel T, Mayerle J, Reincke M, Bartenstein P. Advanced neuroendocrine tumours of the small intestine and pancreas: clinical developments, controversies, and future strategies. Lancet Diabetes Endocrinol. 2018;6:404-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 3. | Lyseng-Williamson KA. Telotristat Ethyl: A Review in Carcinoid Syndrome Diarrhoea. Drugs. 2018;78:941-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Scott AT, Howe JR. Management of Small Bowel Neuroendocrine Tumors. J Oncol Pract. 2018;14: 471-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 5. | Lloyd RV, Osamura RY, Klöppel G, Rosai J. WHO classification of tumours: pathology and genetics of tumours of endocrine organs. 4th ed. Lyon: IARC, 2017. |
| 6. | Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. 4th ed. Europe: World Health Organization, 2010. |
| 7. | Pavel M, O'Toole D, Costa F, Capdevila J, Gross D, Kianmanesh R, Krenning E, Knigge U, Salazar R, Pape UF, Öberg K; Vienna Consensus Conference participants. ENETS Consensus Guidelines Update for the Management of Distant Metastatic Disease of Intestinal, Pancreatic, Bronchial Neuroendocrine Neoplasms (NEN) and NEN of Unknown Primary Site. Neuroendocrinology. 2016;103:172-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 655] [Cited by in RCA: 738] [Article Influence: 82.0] [Reference Citation Analysis (0)] |
| 8. | Pavel M, Öberg K, Falconi M, Krenning EP, Sundin A, Perren A, Berruti A; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:844-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 691] [Article Influence: 138.2] [Reference Citation Analysis (0)] |
| 9. | de Mestier L, Lepage C, Baudin E, Coriat R, Courbon F, Couvelard A, Do Cao C, Frampas E, Gaujoux S, Gincul R, Goudet P, Lombard-Bohas C, Poncet G, Smith D, Ruszniewski P, Lecomte T, Bouché O, Walter T, Cadiot G; Thésaurus National de Cancérologie Digestive (TNCD). Digestive Neuroendocrine Neoplasms (NEN): French Intergroup clinical practice guidelines for diagnosis, treatment and follow-up (SNFGE, GTE, RENATEN, TENPATH, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, SFR). Dig Liver Dis. 2020;52:473-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 10. | Kvols LK, Brendtro KL; North American Neuroendocrine Tumor Society (NANETS). The North American Neuroendocrine Tumor Society (NANETS) guidelines: mission, goals, and process. Pancreas. 2010;39:705-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | National Comprehensive Cancer Network (NCCN) Guidelines. [cited October 26, 2020]. Available from: https://www. nccn. org/professionals/physician_gls/pdf/hepatobiliary.pdf. |
| 12. | Caplin ME, Pavel M, Ćwikła JB, Phan AT, Raderer M, Sedláčková E, Cadiot G, Wolin EM, Capdevila J, Wall L, Rindi G, Langley A, Martinez S, Blumberg J, Ruszniewski P; CLARINET Investigators. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371:224-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1142] [Cited by in RCA: 1286] [Article Influence: 116.9] [Reference Citation Analysis (0)] |
| 13. | Rinke A, Müller HH, Schade-Brittinger C, Klose KJ, Barth P, Wied M, Mayer C, Aminossadati B, Pape UF, Bläker M, Harder J, Arnold C, Gress T, Arnold R; PROMID Study Group. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27:4656-4663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1609] [Cited by in RCA: 1737] [Article Influence: 108.6] [Reference Citation Analysis (0)] |
| 14. | Pavel ME, Ćwikła J, Lombard-Bohas C, Borbath I, Shah T, Pape U, Truong Thanh X, Houchard A, Ruszniewski P. Efficacy and safety of lanreotide autogel (LAN) 120 mg every 14 days in progressive pancreatic or midgut neuroendocrine tumours (NETs): CLARINET FORTE study results. Ann Oncol. 2020;31:S711-S724. [DOI] [Full Text] |
| 15. | Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, Mittra E, Kunz PL, Kulke MH, Jacene H, Bushnell D, O'Dorisio TM, Baum RP, Kulkarni HR, Caplin M, Lebtahi R, Hobday T, Delpassand E, Van Cutsem E, Benson A, Srirajaskanthan R, Pavel M, Mora J, Berlin J, Grande E, Reed N, Seregni E, Öberg K, Lopera Sierra M, Santoro P, Thevenet T, Erion JL, Ruszniewski P, Kwekkeboom D, Krenning E; NETTER-1 Trial Investigators. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med. 2017;376:125-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1702] [Cited by in RCA: 2226] [Article Influence: 278.3] [Reference Citation Analysis (0)] |
| 16. | Yao JC, Fazio N, Singh S, Buzzoni R, Carnaghi C, Wolin E, Tomasek J, Raderer M, Lahner H, Voi M, Pacaud LB, Rouyrre N, Sachs C, Valle JW, Fave GD, Van Cutsem E, Tesselaar M, Shimada Y, Oh DY, Strosberg J, Kulke MH, Pavel ME; RAD001 in Advanced Neuroendocrine Tumours; Fourth Trial (RADIANT-4) Study Group. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet. 2016;387:968-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 749] [Cited by in RCA: 896] [Article Influence: 99.6] [Reference Citation Analysis (0)] |
| 17. | Kanabar R, Barriuso J, McNamara MG, Mansoor W, Hubner RA, Valle JW, Lamarca A. Liver embolisation for patients with neuroendocrine neoplasms: systematic review. Neuroendocrinology. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Oberg K. Chemotherapy and biotherapy in the treatment of neuroendocrine tumours. Ann Oncol. 2001;12 Suppl 2:S111-S114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 78] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Lamarca A, Elliott E, Barriuso J, Backen A, McNamara MG, Hubner R, Valle JW. Chemotherapy for advanced non-pancreatic well-differentiated neuroendocrine tumours of the gastrointestinal tract, a systematic review and meta-analysis: A lost cause? Cancer Treat Rev. 2016;44:26-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 20. | Yao JC, Guthrie KA, Moran C, Strosberg JR, Kulke MH, Chan JA, LoConte N, McWilliams RR, Wolin EM, Mattar B, McDonough S, Chen H, Blanke CD, Hochster HS. Phase III Prospective Randomized Comparison Trial of Depot Octreotide Plus Interferon Alfa-2b Versus Depot Octreotide Plus Bevacizumab in Patients With Advanced Carcinoid Tumors: SWOG S0518. J Clin Oncol. 2017;35:1695-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 117] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 21. | Oberg K. Interferon in the management of neuroendocrine GEP-tumors: a review. Digestion. 2000;62 Suppl 1:92-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 114] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 22. | ENETS. ENETS Centre of Excellence (CoE) Centres. [cited October 26, 2020]. Available from: https://www.enets.org/coe.html. |
| 23. | NETConnnect. NET CONNECT. [cited October 26, 2020]. Available from: https://net-connect.info/#:~:text=NET%20CONNECT%20is%20a%20group%20of%20both%20established,experts%20in%20the%20field%20of%20neuroendocrine%20tumors%20%28NET%29. |
| 24. | Cherk MH, Kong G, Hicks RJ, Hofman MS. Changes in biodistribution on 68Ga-DOTA-Octreotate PET/CT after long acting somatostatin analogue therapy in neuroendocrine tumour patients may result in pseudoprogression. Cancer Imaging. 2018;18:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 25. | Yordanova A, Wicharz MM, Mayer K, Brossart P, Gonzalez-Carmona MA, Strassburg CP, Fimmers R, Essler M, Ahmadzadehfar H. The Role of Adding Somatostatin Analogues to Peptide Receptor Radionuclide Therapy as a Combination and Maintenance Therapy. Clin Cancer Res. 2018;24:4672-4679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 26. | Rudisile S, Gosewisch A, Wenter V, Unterrainer M, Böning G, Gildehaus FJ, Fendler WP, Auernhammer CJ, Spitzweg C, Bartenstein P, Todica A, Ilhan H. Salvage PRRT with 177Lu-DOTA-octreotate in extensively pretreated patients with metastatic neuroendocrine tumor (NET): dosimetry, toxicity, efficacy, and survival. BMC Cancer. 2019;19:788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 27. | van der Zwan WA, Brabander T, Kam BLR, Teunissen JJM, Feelders RA, Hofland J, Krenning EP, de Herder WW. Salvage peptide receptor radionuclide therapy with [177Lu-DOTA,Tyr3]octreotate in patients with bronchial and gastroenteropancreatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2019;46:704-717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 28. | EMC. Afinitor 10mg tablets (SPC). [cited October 28, 2020]. Available from: https://www.medicines.org.uk/emc/product/6658/smpc#gref. |
| 29. | Pavel ME, Baudin E, Öberg KE, Hainsworth JD, Voi M, Rouyrre N, Peeters M, Gross DJ, Yao JC. Efficacy of everolimus plus octreotide LAR in patients with advanced neuroendocrine tumor and carcinoid syndrome: final overall survival from the randomized, placebo-controlled phase 3 RADIANT-2 study. Ann Oncol. 2019;30:2010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |