Published online Mar 14, 2021. doi: 10.3748/wjg.v27.i10.959
Peer-review started: December 3, 2020
First decision: December 11, 2020
Revised: December 30, 2020
Accepted: February 24, 2021
Article in press: February 24, 2021
Published online: March 14, 2021
Processing time: 97 Days and 16 Hours
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of mortality in patients with nonalcoholic fatty liver disease (NAFLD). Weight loss is a key factor for successful NAFLD and CVD therapy. Ursodeoxycholic acid (UDCA), which is one of the first-line therapeutic agents for treatment of NAFLD, is reported to have a beneficial effect on dyslipidemia and ASCVD risk because of antioxidant properties.
To evaluate the effects of 6 mo of UDCA treatment on hepatic function tests, lipid profile, hepatic steatosis and fibrosis, atherogenesis, and ASCVD risk in men and women with NAFLD, as well as to assess the impact of > 5% weight reduction on these parameters.
An open-label, multicenter, international noncomparative trial was carried out at primary health care settings and included 174 patients with ultrasound-diagnosed NAFLD who received 15 mg/kg/d UDCA for 6 mo and were prescribed lifestyle modification with diet and exercise. The efficacy criteria were liver enzymes, lipid profile, fatty liver index (FLI), noninvasive liver fibrosis tests (nonalcoholic fatty liver disease fibrosis score and liver fibrosis index), carotid intima-media thickness (CIMT), and ASCVD risk score. To test statistical hypotheses, the Wilcoxon test, paired t-test, Fisher’s exact test, and Pearson's chi-squared test were used.
The alanine aminotransferase (ALT) level changed by -14.1 U/L (-31.0; -5.3) from baseline to 3 mo and by -6.5 U/L (-14.0; 0.1) from 3 to 6 mo. The magnitude of ALT, aspartate transaminase, and glutamyltransferase decrease was greater during the first 3 mo of treatment compared to the subsequent 3 mo (P < 0.001, P < 0.01, P < 0.001, respectively). At 6 mo, in the total sample, we observed a statistically significant decrease in body weight and levels of FLI: 84.9 ± 10.4 vs 72.3 ± 17.6, P < 0.001, total cholesterol: 6.03 ± 1.36 vs 5.76 ± 1.21, Р < 0.001, low-density lipoprotein: 3.86 ± 1.01 vs 3.66 ± 0.91, Р < 0.001, and triglyceride: 3.18 (2.00; 4.29) vs 2.04 (1.40; 3.16), Р < 0.001. No effect on nonalcoholic fatty liver disease fibrosis score or liver fibrosis index was found. The CIMT decreased significantly in the total sample (0.985 ± 0.243 vs 0.968 ± 0.237, P = 0.013), whereas the high-density lipoprotein (Р = 0.036) and 10-year ASCVD risk (Р = 0.003) improved significantly only in women. Fifty-four patients (31%) achieved > 5% weight loss. At the end of the study, the FLI decreased significantly in patients with (88.3 ± 10.2 vs 71.4 ± 19.6, P < 0.001) and without > 5% weight loss (83.5 ± 10.3 vs 72.8 ± 16.7, P < 0.001). The changes in ALT, aspartate transaminase, glutamyltransferase, total cholesterol, and low-density lipoprotein levels were similar between the subgroups.
UDCA normalizes liver enzymes greatly within the first 3 mo of treatment, improves lipid profile and hepatic steatosis independent of weight loss, and has a positive effect on CIMT in the total sample and 10-year ASCVD risk in women after 6 mo of treatment.
Core Tip: An open-label, multicenter, international noncomparative trial demonstrated the effect of ursodeoxycholic acid (UDCA) on hepatic steatosis and fibrosis, atherogenesis, and atherosclerotic cardiovascular disease risk in 174 ultrasound-diagnosed nonalcoholic fatty liver disease patients who received 15 mg/kg/d UDCA for 6 mo and were prescribed lifestyle modifications with diet and exercise. UDCA had a bidirectional positive effect on the liver and cardiovascular system in patients with and without > 5% weight loss: It improved liver enzymes, lipid profiles, and hepatic steatosis in addition to improving carotid intima-media thickness and atherosclerotic cardiovascular disease risk.
- Citation: Nadinskaia M, Maevskaya M, Ivashkin V, Kodzoeva Kh, Pirogova I, Chesnokov E, Nersesov A, Kaibullayeva J, Konysbekova A, Raissova A, Khamrabaeva F, Zueva E. Ursodeoxycholic acid as a means of preventing atherosclerosis, steatosis and liver fibrosis in patients with nonalcoholic fatty liver disease. World J Gastroenterol 2021; 27(10): 959-975
- URL: https://www.wjgnet.com/1007-9327/full/v27/i10/959.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i10.959
Nonalcoholic fatty liver disease (NAFLD) is currently the leading cause of liver disease and liver transplantation in developed countries; the number of people with this pathology is steadily growing[1,2]. NAFLD-related morbidity in the world is approximately 25%, being the highest in Middle Eastern and South American countries, while in Europe and Asia, the morbidity is approximately the same[3].
According to the DIREG 1, DIREG_L_01903, and DIREG 2 studies, up to 27% of the population suffers from NAFLD in Russia[4]. In Kazakhstan, the morbidity is almost 30%[5], and in Uzbekistan, it reaches 37%[6]. Among the NAFLD risk factors, obesity is considered to be one of the key factors: a body mass index (BMI) increase of 1 kg/m2 is associated with a 13%-38% increased risk of NAFLD development[7]. Such a high morbidity puts this disease among the most important diseases in primary health care.
NAFLD is often associated with cardiovascular diseases (CVDs), which have become the main cause of decreased life expectancy in patients[8,9]. According to one prospective study with over almost three decades of follow-up, 30% of deaths in patients with NAFLD were due to CVD, and 19% were due to liver disease[10].
The gold standard methods for the diagnosis of NAFLD are liver biopsy, proton magnetic resonance spectroscopy, and quantitative fat/water selective magnetic resonance imaging.
An ultrasound examination (US), a less expensive and more accessible method, is preferred for hepatic steatosis detection in primary health care settings. NAFLD is diagnosed after steatosis secondary causes are excluded. At all stages of the disease, the following validated scales are used: the fatty liver index (FLI), nonalcoholic fatty liver disease fibrosis score (NFS), and liver fibrosis index (FIB-4)[11].
Validated scales are also used to assess the CVD risk in patients with NAFLD. To accomplish this task, the Atherosclerotic Cardiovascular Disease (ASCVD) 2013 Risk Calculator and the Framingham Risk Score (2008) are used, the results of which, according to a recent study, correlate with the severity of steatosis according to US data and fibrosis severity, as assessed by the NFS and FIB-4[12].
In a number of studies, it has been noted that hepatic steatosis is an early predictor of coronary atherosclerosis and is associated with an increase in carotid intima-media thickness (CIMT)[13,14] as well as with uncalcified atherosclerotic plaque formation[15].
There is currently no approved standard therapy for NAFLD. The main factor in successful treatment is weight loss, which is a key link in both NAFLD itself and CVD pathogenesis[16]. Among the NAFLD drug treatments, those that affect various disease pathogenesis links may be considered, including increased sensitivity to insulin (pioglitazone, rosiglitazone and liraglutide), lipid-lowering agents (statins), and antioxidant and cytoprotective drugs such as ursodeoxycholic acid (UDCA), vitamin E, obeticholic acid, and omega-3 fatty acid-polyunsaturated fatty acids[17-24].
Several antioxidant mechanisms may explain the beneficial effect of UDCA on dyslipidemia and CVD risk. UDCA treatment seems to protect against iron-dependent and hydroxyl radical-dependent oxidative damage, inhibit lipid peroxidation products, and prevent reactive oxygen species-induced oxidative stress by activation of the PI3K/Akt/Nrf2 pathway in hepatocytes[25-33]. Recent studies demonstrated that the oxidative stress products increase significantly along with the thickening of artery intima, and elevated peroxidative glutathione redox status is associated with atherosclerosis progressing[34,35].
Study Purpose: To evaluate the effects of 6 mo of UDCA treatment on liver function tests (LFT), lipid profile, hepatic steatosis and fibrosis, atherogenesis, and CVD risk in men and women with NAFLD, as well as to assess the impact of > 5% weight reduction on these parameters.
An open international noncomparative study with the code USPEH –Ursodeoxycholic acid as a means of preventing atherosclerosis, steatosis and liver fibrosis in patients with nonalcoholic fatty liver disease – was carried out at primary health care settings.
The study protocol passed the review procedure and was approved at a meeting of an independent interdisciplinary committee on the ethical review of clinical trials in each center of the Russian Federation, Kazakhstan and Uzbekistan. The study took place from November 1, 2017, until August 31, 2018.
Age over 18; proven NAFLD case based on US abdominal data; FLI index value > 60; the physician's decision to prescribe UDCA regardless of the patient's inclusion in the study; and availability of the patient's written informed consent to participate in the program and use of personal data in accordance with the legislation of the participating countries.
Pregnancy; hepatic decompensation (serum albumin ≤ 35 g/L, international normalized ratio ≥ 1.2, platelets < 150 × 109/L); UDCA allergy in past medical history; presence of atherosclerosis complications; use of statins; use of other drugs that potentially affect the studied parameters; use of medications in past medical history associated with secondary hepatic steatosis development (amiodarone, methotrexate, tamoxifen, glucocorticoids, valproic acid, and antiretroviral drugs); unhealthy alcohol use (40 g ethanol per day for men and 20 g ethanol per day for women); scores on the AUDIT (Alcohol Use Disorders Identification Test) questionnaire > 8 for both men and women; type 1 diabetes mellitus; parenteral nutrition; fasting; and presence of a secondary etiology of liver disease (viral, metabolic, autoimmune, cholestatic, or drug etiology).
A patient's decision to discontinue participation in the study at any stage; acute hepatocellular or cholestatic injury that occurred during the study: Increased alanine aminotransferase (ALT), aspartate transaminase (AST), glutamyltransferase (GGT), or alkaline phosphatase levels by two or more times; and the need to take other drugs that potentially affect the studied parameters, arising during the study.
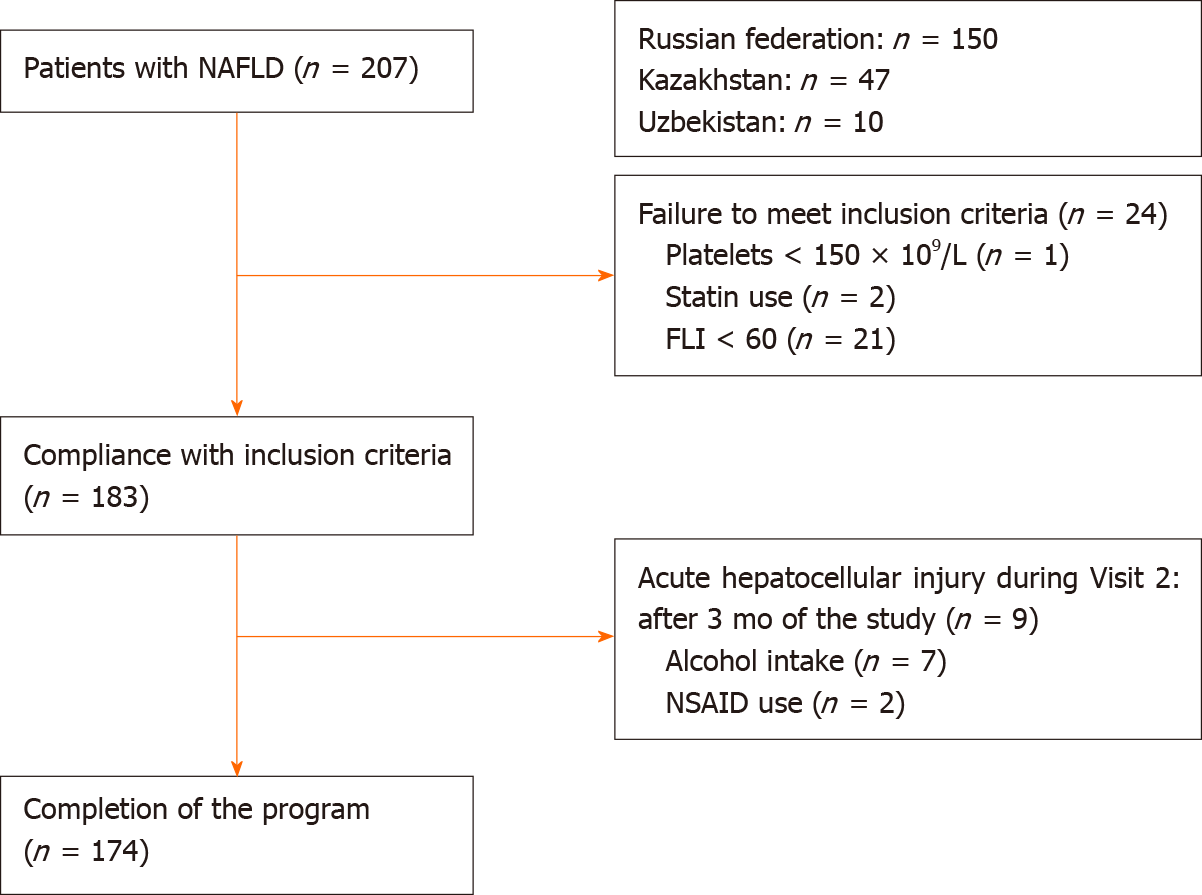
To select study participants, a total of 207 Caucasian patients from regional registries of patients with NAFLD were screened as follows: 150 people from 3 centers in the Russian Federation (Moscow, Tyumen, Chelyabinsk), 47 people from 3 centers in Kazakhstan (Almaty, Nur-Sultan, Shymkent), and 10 people from 1 center in Uzbekistan (Tashkent).
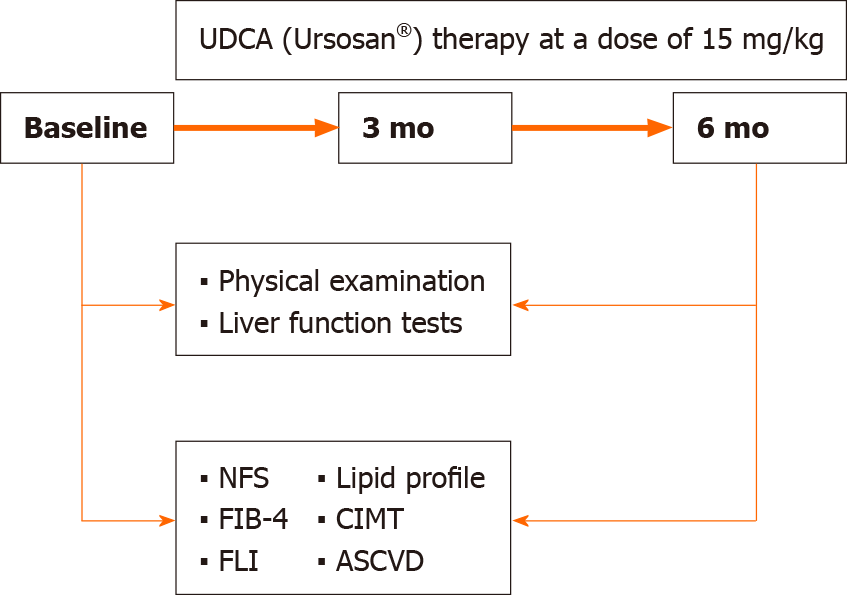
The inclusion criteria were met by 183 patients who had been receiving daily UDCA (Ursosan®) therapy at a dose of 15 mg/kg body weight for 6 mo. In addition, total sample were given standard recommendations to modify their lifestyle and diet: strength or aerobic exercise for at least 150 min per week, Mediterranean diet, and consumption of no more than 1500 kcal/d[36].
Each patient was assessed according to the following parameters: weight, height, BMI (weight, kg/height, m2), waist circumference (WC), smoking, alcohol consumption, metabolic syndrome criteria according to National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III)[37], complete blood count and LFT, and lipid profile.
The criteria for the treatment effectiveness evaluation were as follows: LFT [ALT, AST, and GGT upper limit of normal (ULN) = 40 U/L]; lipid profile [total cholesterol (TC), high-density lipoprotein (HDL), low-density lipoprotein (LDL), and triglyceride (TG)]; noninvasive hepatic steatosis assessment by the FLI; noninvasive fibrosis assessment by the NFS and FIB-4; 10-year and lifetime ASCVD risk; and CIMT.
FLI was calculated using the formula FLI = ey /(1 + ey) × 100, where y = 0.953 × ln (TG, mg/dL) + 0.139 × BMI, kg/m2 + 0.718 × ln (GGT, U/L) + 0.053 × WC, cm - 15.745).
FLI index values that exceeded a value of 60 corresponded to a high probability of hepatic steatosis, indicators from 30 to 59 constituted the "gray zone", and a result less than 30 excluded the likelihood of hepatic steatosis[38].
The noninvasive liver fibrosis assessment was carried out using the NFS score, which was calculated by the formula NFS = -1.675 + [0.037 × age (years)] + [0.094 × BMI (kg/m2)] + [1.13 × Impaired fasting glucose/diabetes (yes = 1, no = 0)] + (0.99 × AST/ALT ratio) – [0.013 × platelet count (× 109/L)] – [0.66 × albumin (g/dL)] URL: https://www.mdcalc.com/nafld-non-alcoholic-fatty-liver-disease-fibrosis-score. Fibrosis index values of -1.455 and below have made it possible to exclude the presence of advanced liver fibrosis, and a value of more than 0.675 testified in favor of the F3-F4 fibrosis stages[39].
Another method for liver fibrosis assessment was the calculation of the fibrosis index FIB-4 according to the formula FIB-4 = age (years) × AST (U/L)/platelets (109/L) × √ ALT (U/L). A FIB-4 value less than 1.45 excluded the presence of severe liver fibrosis, and a value greater than 3.25 indicated the presence of advanced fibrosis.
The risk of developing complications from the cardiovascular system was assessed using the ASCVD 2013 calculator. In accordance with the age limits, the CVD risk over 10 years in patients over 40 years of age and the risk over a lifetime in those under 59 years of age were calculated. A program presented in the public domain was used for calculation[40].
The CIMT was assessed with a standard method in accordance with the European Society of Cardiology recommendations. CIMT was measured from longitudinal images of both the right and left common carotid arteries during B-mode ultrasonography as the distance from the echogenic lumen-intima interface to the echogenic media-adventitia interface; the highest value for each patient was used for analysis[41]. Values of the 95th percentile for the corresponding age and sex were used as the ULN[42].
All data were entered into the patient’s Case Report Form. After 3 mo of the study, 9 patients were excluded due to acute hepatocellular or cholestatic injury associated with nonsteroidal anti-inflammatory drug intake and alcohol consumption during the study. The study was completed by 174 patients who were included in the final analysis (Figure 1).
To assess treatment effectiveness, the comparisons were made for the total sample and for men and women separately. The body weight, BMI, WC, and LFT were compared between baseline and 3 mo as well as between 3 and 6 mo of the treatment. The lipid profile, FLI, NFS, FIB-4, 10-year and lifetime ASCVD risk, and CIMT were compared between baseline and the end of the follow-up (Figure 2).
Considering that the patients were given dietary and lifestyle recommendations, it was assumed that some patients would lose weight by the end of the study, which in itself could affect the parameters studied. Based on this assumption, a subgroup analysis with and without weight loss > 5% from baseline was planned.
For the statistical processing of the study results, all data from the case report forms were entered by two researchers (M.N., Kh.K.) independently into a database created on the basis of Excel spreadsheets (Microsoft, United States). In case of data discrepancies, the indicators were manually checked from the case report forms. The missing data for the studied parameters were 2.1% ± 0.8%. Data are presented as absolute and relative indicators, mean ± SD for normally distributed values and for the rest of the values, as median (Me) and the 25th and 75th percentiles-interquartile range (IQR). To test statistical hypotheses, the Wilcoxon test, paired t-test, Fisher’s exact test, and Pearson's chi-squared test were used. The critical value of the statistical significance level, when testing the null hypotheses, was equal to 0.05. Statistical analysis was performed using SPSS Statistics v.23.0 (IBM Corporation, United States).
The study involved 121 (69.5%) men and 53 (30.5%) women aged 24 to 68 years. The patients’ baseline data are presented in Table 1. Among the concomitant diseases, type 2 diabetes mellitus (T2DM) was diagnosed in 54 (31.0%) patients, and arterial hypertension (AH) was diagnosed in 41 (23.6%) patients. Tobacco smoking was noted in every third patient. Obesity (Classes I-III) was observed in 121 (69.5%) patients, and the remaining patients were overweight. Seventy-nine (45.4%) patients met the NCEP ATP III criteria for metabolic syndrome. The mean age of women was significantly higher than that of men (P < 0.001); the metabolic syndrome was more common in women compared with men (P < 0.002). Men and women did not differ in frequency of T2DM, AH, tobacco smoking, and obesity (classes I-III) (Table 1).
| Parameter | Total sample (n = 174) | Men (n = 121) | Women (n = 53) | P value |
| Age, years, Ме (IQR) | 45.2 ± 10.1 | 42.9 ± 8.7 | 50.7 ± 10.5 | < 0.001 |
| T2DM | 54 (31.0) | 35 (28.9) | 19 (35.8) | 0.466 |
| AH | 41 (23.6) | 27 (22.3) | 14 (26.4) | 0.695 |
| Smoking | 58 (33.3) | 45 (37.2) | 13 (24.5) | 0.146 |
| Obesity, classes I-III | 121 (69.5) | 79 (65.3) | 42 (79.2) | 0.097 |
| Metabolic syndrome, NCEP ATP III | 79 (45.4) | 45 (37.2) | 34 (64.2) | 0.002 |
During the study, smoking status, need for AH treatment, and the number of T2DM cases did not change.
As compared with their initial levels, body weight, BMI, and WC decreased significantly (P < 0.001) in men and women after 3 mo of treatment and continued to reduce between 3 and 6 mo (P < 0.001) (Table 2). No significant difference was reported in body weight change between the 0- to 3-mo and 3- to 6-mo intervals, whereas the WC was greatly reduced between baseline and 3 mo of treatment (P < 0.001).
| Parameter | Baseline | 3 mo | 6 mo |
| Weight (total sample), kg | 94.8 (86.7; 102.1) | 93.0 (86.0; 100.0)c | 92.9 (85.0; 98.3)c |
| Men | 95.0 (92.0; 104.2) | 94.0 (90; 101.1)c | 93.7 (88.7; 100.0)c |
| Women | 86.7 (82.0; 96.0) | 85.0 (80.0; 94.8)c | 85.0 (79.0; 95.0)b |
| ∆ total sample, kg | -1.0 (-2.4; 0.0) | -1.0 (-2.4; 0.0) | |
| Weight loss > 5% (total sample) | 11 (6.3) | 54 (31)c | |
| Men | 8 (6.6) | 39 (32.2)c | |
| Women | 3 (5.6) | 15 (28.3)b | |
| BMI (total sample), kg/m2 | 31.2 (29.4; 34.0) | 30.9 (29.3; 33.3)c | 30.6 (29.0; 33.1)c |
| Men | 30.7 (29.0; 33.3) | 30.4 (29.0; 32.6)c | 30.1 (28.4; 32)c |
| Women | 32.0 (30.7; 36.2) | 31.5 (30.1; 34.6)c | 31.6 (29.7; 34.4)b |
| ∆ total sample, kg/m2 | -0.4 (-0.9; 0.0) | -0.3 (-0.8; 0.0) | |
| Waist circumference (total sample), cm | 102.0 (97.0; 111.8) | 100.0 (96.0; 108.0)c | 100.0 (94.0; 106.0)c |
| Men | 103.0 (98.0; 112.0) | 102.0 (97.0; 110.0)c | 100 (97.0; 106.0)c |
| Women | 99.0 (93.0; 106.0) | 98.0 (92.0; 105.0)c | 97.0 (90.0; 102.0)c |
| ∆ total sample, сm | -2.0 (-3.0; 0.0) | 0.0 (-2.0; 0.0)c | |
| ALT (total sample), U/L | 53.0 (34.0; 78.0) | 35.0 (26.0; 45.0)c | 29.0 (24.0; 38.0)c |
| Men | 55.8 (37.0; 78.0) | 38.0 (29.0; 46.0)c | 32.0 (25.9; 41.0)c |
| Women | 44.0 (30.8; 69.0) | 28.5 (21.6; 38.0)c | 26.0 (16.5; 33.2)c |
| ∆ total sample, U/L | -14.1 (-31.0; -5.3) | -6.5 (-14.0; 0.1)c | |
| ALT (total sample) 10-19.9/20-39.9/≥ 40 U/L | 5 (2.8)/53 (30.5)/116 (66.7) | 15 (8.6)/95 (54.6)/64(36.8)b | 26 (14.9)/108 (62.1)/40 (23.0)c |
| Men | 1 (0.8)/35 (28.9)/85 (70.2) | 5 (4.1)/64 (52.9)/52 (43)c | 10 (8.3)/79 (65.3)/32 (26.4)a |
| Women | 4 (7.5)/18 (34)/31 (58.5) | 10 (18.9)/31 (58.5)/12 (22.6)c | 16 (30.2)/29 (54.7)/8 (15.1) |
| AST (total sample), U/L | 31.0 (24.0; 42.0) | 26.5 (21.0; 34.0)c | 25.0 (21.0; 29.0)c |
| Men | 29.1 (23.5; 42.0) | 26.0 (21.0; 33.0)c | 25.0 (21.0; 28.0)c |
| Women | 32.0 (24.0; 42.0) | 28.0 (22.0; 35.0)c | 26.0 (23.0; 32.0) |
| ∆ total sample, U/L | -4.0 (-12.0; 1.0) | -2.0 (-7.0; 2.2)b | |
| GGT (total sample), U/L | 36.0 (26.0; 48.0) | 28.0 (21.0; 38.0)c | 26.0 (19.0; 35.0)c |
| Men | 36.0 (28.0; 46.0) | 31.0 (24.0; 41.0)c | 26.0 (21.0; 35.0)c |
| Women | 35.0 (23.0; 53.0) | 23.0 (18.0; 30.0)c | 21.0 (12.0; 34.0) |
| ∆ total sample, U/L | -5.0 (-15.0; 2.0) | -3.0 (-8.0; 2.0)c |
After 3 mo of treatment, 11 (6.3%) patients lost > 5% weight, whereas at the end of treatment, 54 (31%) patients had > 5% weight reduction, a 3-mo difference being statistically significant (P < 0.001) (Table 2).
The levels of ALT, AST, and GGT decreased significantly from baseline to 3 mo and from 3 to 6 mo. The ALT level initially exceeded 39.9 U/L in 116 (66.7%) of patients but decreased to 64 (36.8%) by the 3rd mo and to 40 (23%) by the end of the study. Low-normal ALT values (0-19.9) were noted in 5 (2.8%) at baseline, in 15 (8.6%) at 3 mo, and in 26 (14.9%) after treatment. The ALT level changed by -14.1 U/L (IQR = -31.0 U/L to -5.3 U/L) from baseline to 3 mo and by -6.5 U/L (IQR = -14.0 U/L to 0.1 U/L) from 3 to 6 mo. Similar changes were seen with AST and GGT levels. From baseline to 3 mo, the levels of AST and GGT decreased by -4.0 U/L (IQR = -12.0 U/L to 1.0 U/L) and -5.0 U/L (IQR = -15.0 U/L to 2.0 U/L), respectively. From 3 to 6 mo, AST and GGT levels dropped by -2.0 U/L (IQR = -7.0 U/L to 2.2 U/L) and -3.0 U/L (IQR = -8.0 U/L to 2.0 U/L), respectively.
The magnitude of ALT, AST, and GGT decrease was greater during the first 3 mo of treatment compared to the subsequent 3 mo (P < 0.001, P < 0.01, P < 0.001, respectively). The baseline ALT, AST, and GGT levels did not differ between the sexes, in contrast to the rates of change of these parameters over the trial. Men had a significant decrease in ALT, AST, and GGT levels both from baseline to 3 mo and from 3 to 6 mo, whereas women had the only significant decrease in ALT over the both periods. The AST and GGT levels decreased to normal levels during the first 3 mo of treatment in most women (Table 2).
During the study, there was a statistically significant change in lipid profile: a decrease in the ТС, TG, and LDL levels in the total sample and by sex. The HDL level did not change significantly in men, but significantly increased in women (Table 3).
| Parameter | Baseline | 6 mo | P value |
| ТС (total sample), mmol/L | 6.03 ± 1.36 | 5.76 ± 1.21 | < 0.001 |
| Men | 5.99 ± 1.39 | 5.77 ± 1.24 | 0.003 |
| Women | 6.11 ± 1.32 | 5.73 ± 1.16 | 0.019 |
| LDL (total sample), mmol/L | 3.86 ± 1.01 | 3.66 ± 0.91 | < 0.001 |
| Men | 3.81 ± 1.04 | 3.67 ± 0.90 | 0.033 |
| Women | 4.0 ± 0.94 | 3.65 ± 0.93 | 0.006 |
| HDL (total sample), mmol/L | 1.24 ± 0.32 | 1.24 ± 0.27 | 0.910 |
| Men | 1.24 ± 0.33 | 1.21 ± 0.22 | 0.160 |
| Women | 1.23 ± 0.29 | 1.31 ± 0.34 | 0.036 |
| TG (total sample), mmol/L | 3.18 (2.00; 4.29) | 2.04 (1.40; 3.16) | < 0.001 |
| Men | 3.13 (2.10; 4.29) | 2.04 (1.4; 2.79) | < 0.001 |
| Women | 3.45 (1.80; 4.36) | 2.26 (1.35; 3.62) | < 0.001 |
| FLI (total sample) | 84.9 ± 10.4 | 72.3 ± 17.6 | < 0.001 |
| Men | 86.3 ± 9.0 | 73.6 ± 17.2 | < 0.001 |
| Women | 81.9 ± 12.7 | 69.4 ± 18.4 | < 0.001 |
| FLI (total sample) ≥ 60/30-59/< 30 | 174 (100)/-/- | 133 (76.4)/40 (23.0)/1 (0.6) | < 0.001 |
| Men | 121 (100)/-/- | 96 (79)/24 (20)/1 (1) | < 0.001 |
| Women | 53 (100)/-/- | 37 (70)/16 (30)/- | < 0.001 |
| NFS (total sample) < -1.455 (F0-F2), n (%) | 141 (81.0) | 148 (85.0) | 0.353 |
| Men | 102 (84.3) | 112 (92.6) | 0.071 |
| Women | 39 (76.6) | 34 (64.2) | 0.402 |
| FIB 4 (total sample) < 1.45 (F0-F1), n (%) | 157 (90.2) | 163 (93.7) | 0.256 |
| Men | 111 (91.7) | 114 (94.2) | 0.615 |
| Women | 46 (86.8) | 49 (92.5) | 0.402 |
| CIMT (total sample), mm | 0.985 ± 0.243 | 0.968 ± 0.237 | 0.013 |
| Men | 0.993 ± 0.224 | 0.977 ± 0.217 | 0.073 |
| Women | 0.967 ± 0.284 | 0.947 ± 0.276 | 0.052 |
| CIMT (total sample) exceeding ULN for the corresponding age and sex | 143 (84) | 139 (82) | 0.549 |
| Men | 103 (85.1) | 101 (83.5) | 0.860 |
| Women | 40 (75.5) | 38 (72) | 0.826 |
| ASCVD (total sample, n = 112) 10-year risk | 5.1 (2.9; 9.1) | 4.8 (2.6; 8.0) | 0.053 |
| Men (n = 71) | 6.0 (3.6; 11.1) | 5.8 (3.4; 8.8) | 0.720 |
| Women (n = 41) | 3.5 (2.3; 7.8) | 3.2 (2.0; 6.0) | 0.003 |
| ASCVD (total sample, n = 152) lifetime risk | 50 (39; 60) | 50 (39; 64) | 0.370 |
| Men (n = 112) | 50 (46; 69) | 50 (46; 69) | 0.870 |
| Women (n = 40) | 39 (39; 50) | 39 (39; 50) | 0.160 |
By the end of the study, the FLI had decreased to a value of < 30 in one men, and in another 40 patients [24 (20%) men 16 (30%) women], it had gone into the “gray zone.” No significant changes in fibrosis were noted according to the NFS and FIB-4.
The CIMT decreased significantly by the end of 6 mo in the total sample. When men and women were analyzed separately, the rate of CIMT change remained close to significant with Р values of 0.073 and 0.052, respectively. At the same time, no statistically significant changes in the proportion of persons with reference values exceeding the ULN for the corresponding sex and age were established (Table 3).
A significant decrease in the 10-year ASCVD risk score was observed only in women (Р = 0.003). The same patient's 10-year risk with optimal risk factors was 1.5 (0.9; 2.9) in both sexes.
The lifetime ASCVD risk did not change during the study and remained significantly increased relative to lifetime risk with an optimal risk factor of 5.0 (5.0; 8.0).
Weight loss of more than 5% of the initial weight was observed in 54 patients (31%); in 54 patients (31%), the weight decreased to 5% of the initial weight; in 35 patients (20%), it did not change; and in 31 patients (18%), it increased by an average of 3%. A comparison was made between subgroups with a weight loss of more than 5% and all remaining patients.
The delta of the studied parameters ALT, AST, GGT, TC, and LDL did not differ between the subgroups (Table 4). In the subgroup with more than 5% weight loss, the FLI baseline was higher, and during the study, there was a more pronounced decrease than in the other group. By the end of the study, the subgroups with and without weight loss of more than 5% did not differ in FLI. Additionally, in the subgroup with a weight loss of more than 5%, there was a tendency toward a more pronounced decrease in TG (P was close to statistically significant).
| Parameter | Weight loss > 5% (n = 54) | Weight loss ≤ 5% (n = 120) | P value |
| ∆ALT, U/L | -21.1 (-45.0; -6.0) | -25.0 (-42.5; -6.0) | 0.683 |
| ∆ALT, % | -46 (-63; -18) | -43 (-54; -23) | 0.281 |
| ∆AST, U/L | -6.0 (-15.0; 1.6) | -5.0 (-18.0; 1.0) | 0.673 |
| ∆AST, % | 22 (-41; 0) | -17 (-42; 0) | 0.446 |
| ∆GGT, U/L | -5.0 (-17.0; 0) | -8.0 (-22.5; 0) | 0.492 |
| ∆GGT, % | -19 (-41; 0) | -32 (-50; 0) | 0.354 |
| ∆TC, mmol/L | -0.10 (-0.66; 0.30) | -0.10 (-0.57; 0.2) | 0.666 |
| ∆TG, mmol/L | -1.04 (-1.80; -0.23) | -0.70 (-1.33; -0.11) | 0.079 |
| ∆LDL, mmol/L | -0.20 (-0.7; 0.2) | -0.14 (-0.58; 0.3) | 0.401 |
| FLI | |||
| Initially | 88.3 ± 10.2 | 83.5 ± 10.3 | 0.002 |
| After 6 mo | 71.4 ± 19.6c | 72.8 ± 16.7c | 0.916 |
| ∆FLI | -14.6 (-21.8; -6.3) | -8.45 (-17.9; -2) | 0.003 |
The NAFLD prevalence rate in the population, as well as associated complications and risk factors, is growing steadily. Thus, a 10-fold increase in hepatocellular carcinoma related to NAFLD has been reported over the past decade. Moreover, it has been shown that patients with more advanced NAFLD are 4 times more likely to have a fatal CVD event and twice as likely to suffer a nonfatal CVD event[43]. These data demonstrate the urgency of NAFLD-related problems and the need to find a modern drug to treat it effectively.
Male sex is one of the nonmodifiable risk factors for NAFLD and ASCVD. In our study, the number of men with NAFLD was 2 times higher than the number of women, which is consistent with the NAFLD prevalence data in other populations[44-46]. The patients’ average age in our study was 45 years, which generally corresponds to the NAFLD epidemiological study data. Thus, according to individual studies, in Europe, the average age of patients with NAFLD is 56 years in Italy and 58 years in Spain[47,48]. In Asia, this parameter is lower; in the Chinese population, the average age is 45 years, and in the Japanese population, it is 51 years[49,50]. In a NAFLD study conducted in Kazakhstan, the patients’ average age was 55 years[5].
It is well known that NAFLD and CVD share common modifiable risk factors[43]. In our study, one-third of patients had T2DM, most (69.5%) of the observed participants were obese (Classes I-III obesity), and the rest were overweight. A total of 45.4% of patients met the criteria for metabolic syndrome based on the NCEP ATP III, which is consistent with a large-scale epidemiological study data conducted by Younossi et al[3], in which the number of patients with metabolic syndrome was 42.5%.
Factors released from fatty liver that could link NAFLD to CVD are proinflammatory and proatherogenic cytokines (IL-6, tumor necrosis factor-alpha), procoagulant factors (fibrinogen, factors VII and VIII, plasminogen activator type 1 inhibitor), and pro-oxidant factors (reactive oxygen species); other pathogenic mechanisms include progression of insulin resistance and dyslipidemia[10,13].
In this study, patients were given lifestyle and dietary modifications, and UDCA was prescribed. For 6 mo of the study, there was a statistically significant decrease in weight, BMI, and WC, which is associated with the adherence of a certain proportion of the subjects to the recommendations on lifestyle modification and diet, which were discussed with patients prior to the UDCA administration and three months later.
In a randomized study by Wong et al[51], the participation of patients with NAFLD in lifestyle and diet modification programs contributed to a decrease in body weight, liver TG content and disease remission achievement. At the same time, in a study by Dudekula et al[52], it has been shown that in a primary care setting, prescribing only lifestyle recommendations for significant (> 5%) weight loss in NAFLD patients is insufficient. In this study, the degree of weight loss was correlated with the frequency of outpatient visits.
During the study, a statistically significant decrease in ALT level was achieved; the number of patients whose ALT level exceeded 40 U/L decreased almost 3-fold. The Me ALT reduction was 45.3%. A decrease in ALT levels in NAFLD patients during treatment with UDCA has been demonstrated in previous studies. For example, in a randomized controlled trial by Ratziu et al[19], high-dose UDCA therapy for one year was associated with a decrease in ALT level by 28.3% and ALT level normalization in a quarter of the patients, regardless of weight loss. An even more pronounced decrease in ALT level during a 6-mo course of UDCA at a dose of 15 mg/kg/d, as in our study, was noted in a prospective study by Ozel Coskun et al[20]: The ALT level decreased after therapy by 54% in patients with histologically confirmed NAFLD.
In addition, during the study, there was a significant decrease in AST and GGT levels by 6 and 10 U/L, respectively, which was repeatedly noted in other similar studies[21-24]. The percentage of AST reduction was 19.4% and 27.8% for GGT, which is consistent with previously mentioned publications: a decrease in AST of 18.9% and GGT of 62% in a study by Ratziu et al[19], and a decrease in AST of 43% and GGT of 41% in a study by Ozel Coskun et al[20]. A statistically significant decrease in AST levels of 8.5 U/L and GGT of 16.5 U/L was also demonstrated after a relatively short (3-wk) course of UDCA 20 mg/kg/d[53].
In comparison with other studies, we also analyzed the rates of LFT change during the first and subsequent 3-mo intervals. The magnitude of ALT, AST, and GGT decrease was significantly higher during the first 3 mo of treatment, whereas the rates of weight and BMI change were the same in both periods.
Improvement in LFT is probably associated with the antioxidant effect of UDCA, the ability to suppress the production of tumor necrosis factor-alpha, which is associated with an improvement in LFT and histological findings[54,55].
Positive changes have also been observed in relation to lipid profile. The Me ТС during follow-up decreased significantly by 0.27 mmol/L, which is consistent with the results of a recently published meta-analysis, which, based on an analysis of 20 studies, demonstrated a significant decrease in TC with UDCA therapy by 0.36 mmol/L[56]. The Me of other lipid parameters (LDL and TG) also decreased during the study. The HDL level increased significantly only in women.
Administration of UDCA improves the lipid spectrum by decreasing cholesterol absorption and synthesis and the activity of the farnesoid X-receptor and increasing the synthesis of bile acids[56].
The effect of UDCA therapy on the FLI has not been previously shown. In our study, the FLI, a noninvasive assessment of steatosis, decreased significantly. This is due to a decrease in the parameters included in the FLI algorithm, including BMI, WC, GGT, and TG. At the end of the program, 1/4 of the patients moved to the "gray zone". Similarly, Laurin et al[22] previously reported a histologic improvement in steatosis after UDCA treatment.
The literature contains a limited number of clinical studies evaluating the effects of drug therapy on fibrosis in patients with NAFLD. In our work, liver fibrosis was assessed using the NFS and FIB-4 scales, which have similar diagnostic value according to the Sun meta-analysis[57]. We observed no significant effect on fibrosis, which is consistent with a 52-wk study by Parikh et al[58] using the NFS scale to compare the fibrosis severity before and after UDCA therapy. Notably, in another study, there was a decrease in fibrosis severity when assessed using the FibroTest, but the study duration and the UDCA dose were higher than those in our trial[19].
NAFLD is currently known to be associated with increased CIMT, an early predictor of atherosclerosis and CVD. In our study, patients achieved a statistically significant decrease in CIMT with UDCA treatment. Similar data have been demonstrated in the prospective Coscun study (6 mo of UDCA at a dose of 15 mg/kg/d). The mechanism for reducing CIMT appears to be associated with the antioxidant, anti-inflammatory, and hypolipidemic effects of UDCA, as well as its effect on insulin resistance[20]. In turn, CIMT reduction plays an important role in CVD prevention. Notably, CIMT, exceeding the ULN for the corresponding sex and age, remained unchanged during the study, which can probably be explained by the short duration. The duration of cardiac studies, which showed a more pronounced decrease in CIMT, was 24 and 36 mo[59].
In our study, the impact of UDCA on CVD risk was assessed using the ASCVD risk calculator for the first time. We found a statistically significant decrease in the 10-year ASCVD risk only in women. Of all the parameters assessed in ASCVD, this one was due to a decrease in the TC level in both sexes and the HDL level only in women.
At the same time, in the present study, the predicted 10-year risk of CVD at the end of 6 mo was 3 times higher than the same patient's 10-year risk with optimal risk factors. There was no reduction in the predicted lifetime CVD risk. Indeed, it was 10 times higher than the same individual's lifetime risk with optimal risk factors.
Achieving more significant reductions in CIMT and ASCVD risk appears to take longer and requires control of other modifiable risk factors such as sedentary lifestyle, T2DM, AH, and smoking. Cardiological and endocrinological studies have shown that regular physical activity for 1 year or more, smoking cessation, blood pressure control, and glycemic control using new-generation hypoglycemic agents such as sodium–glucose cotransporter 2 inhibitor (dapagliflozin) or inhibitor of dipeptidyl peptidase-4 (sitagliptin) contribute to CIMT and ASCVD risk decrease[60-65].
Weight loss is believed to be a key factor in successful NAFLD treatment and in reducing the risk of CVD. A recent meta-analysis has shown clinical and laboratory improvements in NAFLD patients with 5%-10% weight loss[66]. To assess the effect of weight loss on the studied efficacy parameters in our patients, we divided them into two subgroups: weight loss of more than 5% and weight loss of 5% or less. The cutoff point of 5% was chosen due to the short 6-mo study period.
When comparing groups, there was no statistically significant difference in absolute and relative reductions in ALT, AST, GGT, TC, and LDL levels. At the same time, in the group with a weight loss of more than 5%, we observed a close to significant decrease in TG level and a significant decrease in FLI steatosis. Since the FLI formula includes the GGT, TG, BMI, and WC values, it is likely that the steatosis improvement in the group of patients with a decrease in body weight of more than 5% is associated with a greater change in the values of the last three parameters. However, even in patients who did not achieve more than 5% weight loss, a statistically significant decrease in the FLI index was observed.
A recent meta-analysis[66], which assessed the relationship between weight loss and LFT, found that weight reduction resulted in a decrease in ALT by –9.81 U/L; 95%CI: -13.12; -6.50. In our study, patients with a weight loss of more than 5% had a decrease in ALT levels by an average of 21.1 U/L; this reduction is greater than that in the meta-analysis, demonstrating the UDCA effectiveness. Moreover, in the current study, both the subgroups with and without a loss of > 5% body weight achieved significant improvement in FLI, while the above mentioned meta-analysis did not show any differences in FLI under UDCA therapy.
In addition, in contrast to the meta-analysis data, our study achieved a statistically significant decrease in FLI both in the subgroup with a weight loss of more than 5% and in the subgroup without weight loss; in the meta-analysis, the weight loss effect on FLI was not established. The study was conducted in a primary health care setting, which expands the possibilities of its transfer to regular practice. Some limitations of our study should also be noted, namely, the open design, the absence of a control group, and the relatively short follow-up period.
The study suggests that 6-mo UDCA therapy at a dose of 15 mg/kg/d may improve ALT, AST, GGT, TC, and TG levels and FLI in the primary care setting independent of weight loss. A positive effect of UDCA on ALT, AST, and GGT levels is greater during the first 3 mo of treatment. Our study also demonstrates a beneficial effect of UDCA on the progression of CIMT in both sexes and reduction in 10-year CVD risk in women. Further long-term studies with control groups are needed to confirm these findings.
Nonalcoholic fatty liver disease (NAFLD) is currently the leading cause of liver disease and liver transplantation in developed countries. Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of mortality in patients with NAFLD. Weight loss is a key factor for successful NAFLD and cardiovascular disease therapy. Ursodeoxycholic acid (UDCA), which is one of the first-line therapeutic agents for treatment of NAFLD, is reported to have a beneficial effect on dyslipidemia and ASCVD risk because of antioxidant properties.
The main motivation was our wish to improve the treatment outcomes of patients with NAFLD. Each author of our international study had a personal positive experience of treating NAFLD with UDCA. We sincerely wanted to combine our efforts to gain a new knowledge.
To evaluate the effects of 6-mo administration of UDCA on liver function tests (LFT), lipid profile, hepatic steatosis and fibrosis, carotid intima-media thickness (CIMT), and ASCVD risk in total sample with NAFLD, separately in men and women, in patients with and without > 5% weight loss.
An open international noncomparative study was carried out at primary health care settings. The study was completed by 174 patients who were included in the final analysis and received UDCA (Ursosan®) therapy at a dose of 15 mg/kg body weight daily for 6 mo. In addition, total sample was given recommendations to modify lifestyle behaviors and diet. The body mass index, waist circumference, and LFT were compared between baseline and 3 mo as well as between 3 and 6 mo of the treatment. The lipid profile, fatty liver index (FLI), nonalcoholic fatty liver disease fibrosis score, liver fibrosis index, 10-year and lifetime ASCVD risk, and CIMT were compared between baseline and the end of the follow-up.
The magnitude of LFT decrease was greater during the first 3 mo of treatment compared to the subsequent 3 mo. At 6 mo, in the total sample, we observed a statistically significant decrease in body mass index, waist circumference, and levels of FLI (P < 0.001), total cholesterol (TC) (Р < 0.001), low-density lipoprotein (LDL) (Р < 0.001), triglyceride (TG) (Р < 0.001), and the CIMT (P = 0.013), whereas the high-density lipoprotein (HDL) (Р = 0.036) and 10-year ASCVD risk improved significantly only in women (Р = 0.003). No effect on nonalcoholic fatty liver disease fibrosis score, and liver fibrosis index was found. At the end of the study, the FLI decreased significantly in patients with and without > 5% weight loss; the changes in LFT, TC, TG, and LDL levels were similar between the subgroups.
The study suggests that 6-mo UDCA therapy at a dose of 15 mg/kg/d may improve LFT, TC, LDL, and TG levels, FLI, and CIMT in total sample with NAFLD. These changes are observed independent of sex and weight loss. UDCA has also a beneficial effect on HDL and 10-year ASCVD risk in women.
Further long-term studies with control groups are needed to confirm these findings. For further research, we suggest to compare lipid profile and hepatic steatosis between baseline to 3 mo and 3 to 6 mo; evaluate the effects of long-term UDCA therapy (during 1-year follow-up or more) on HDL and 10-year and lifetime risk ASCVD in men and women.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Russia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen GX S-Editor: Zhang L L-Editor: A P-Editor: Liu JH
| 1. | Kwong A, Kim WR, Lake JR, Smith JM, Schladt DP, Skeans MA, Noreen SM, Foutz J, Miller E, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2018 Annual Data Report: Liver. Am J Transplant. 2020;20 Suppl s1:193-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 313] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 2. | Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313:2263-2273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1508] [Cited by in RCA: 1757] [Article Influence: 175.7] [Reference Citation Analysis (0)] |
| 3. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7538] [Article Influence: 837.6] [Reference Citation Analysis (0)] |
| 4. | Ivashkin VT, Drapkina OM, Mayev IV, Trukhmanov AS, Blinov DV, Palgova LK, Tsukanov VV, Ushakova TI. Prevalence of nonalcoholic fatty liver disease in out-patients of the Russian Federation: DIREG 2 study results. (In Russian). Ross z Gastroenterol Gepatol Koloproktol. 2015;6:31-41. |
| 5. | Nersesov AV, Raissova AM, Kaibullayeva JA, Jumabayeva AE, Novitskaya MS. Epidemiological Investigation of Fatty Liver Disease and Abnormal Liver Function in the Republic of Kazakhstan. Open J Epidemiol. 2019;09:309-320. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Daminov BT, Usmanova US, Alavi BA, Sobirova GN. Risk factors progression of a non-alcoholic fat liver disease and state of gut microflora in patients with overbody and obesity. Int J Adv Sci Technol. 2020;29:3241-3246. |
| 7. | European Association for the Study of the Liver (EASL). The HEPAHEALTH project report risk factors and the burden of liver disease in Europe and selected Central Asian countries. 2018 [accessed 2020 Oct 25]. Available from: https://easl.eu/wp-content/uploads/2018/09/EASLHEPAHEALTH-Report.pdf. |
| 8. | Mahfood Haddad T, Hamdeh S, Kanmanthareddy A, Alla VM. Nonalcoholic fatty liver disease and the risk of clinical cardiovascular events: A systematic review and meta-analysis. Diabetes Metab Syndr. 2017;11 Suppl 1:S209-S216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 133] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 9. | Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. J Hepatol. 2016;65:589-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1049] [Cited by in RCA: 1005] [Article Influence: 111.7] [Reference Citation Analysis (0)] |
| 10. | Söderberg C, Stål P, Askling J, Glaumann H, Lindberg G, Marmur J, Hultcrantz R. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 2010;51:595-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 562] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 11. | Sberna AL, Bouillet B, Rouland A, Brindisi MC, Nguyen A, Mouillot T, Duvillard L, Denimal D, Loffroy R, Vergès B, Hillon P, Petit JM. European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD) and European Association for the Study of Obesity (EASO) clinical practice recommendations for the management of non-alcoholic fatty liver disease: evaluation of their application in people with Type 2 diabetes. Diabet Med. 2018;35:368-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 12. | Pisetta C, Chillè C, Pelizzari G, Pigozzi MG, Salvetti M, Paini A, Muiesan ML, De Ciuceis C, Ricci C, Rizzoni D. Evaluation of Cardiovascular Risk in Patient with Primary Non-alcoholic Fatty Liver Disease. High Blood Press Cardiovasc Prev. 2020;27:321-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Bonora E, Targher G. Increased risk of cardiovascular disease and chronic kidney disease in NAFLD. Nat Rev Gastroenterol Hepatol. 2012;9:372-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 14. | Pais R, Giral P, Khan JF, Rosenbaum D, Housset C, Poynard T, Ratziu V; LIDO Study Group. Fatty liver is an independent predictor of early carotid atherosclerosis. J Hepatol. 2016;65:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 15. | Lee SB, Park GM, Lee JY, Lee BU, Park JH, Kim BG, Jung SW, Jeong ID, Bang SJ, Shin JW, Park NH, Yang DH, Kang JW, Lim TH, Kim HK, Choe J, Lee HC. Association between non-alcoholic fatty liver disease and subclinical coronary atherosclerosis: An observational cohort study. J Hepatol. 2018;68:1018-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 112] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 16. | Musso G, Gambino R, Cassader M, Pagano G. A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology. 2010;52:79-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 437] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 17. | Paumgartner G, Beuers U. Mechanisms of action and therapeutic efficacy of ursodeoxycholic acid in cholestatic liver disease. Clin Liver Dis. 2004;8:67-81, vi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 126] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Harnois DM, Angulo P, Jorgensen RA, Larusso NF, Lindor KD. High-dose ursodeoxycholic acid as a therapy for patients with primary sclerosing cholangitis. Am J Gastroenterol. 2001;96:1558-1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Ratziu V, de Ledinghen V, Oberti F, Mathurin P, Wartelle-Bladou C, Renou C, Sogni P, Maynard M, Larrey D, Serfaty L, Bonnefont-Rousselot D, Bastard JP, Rivière M, Spénard J; FRESGUN. A randomized controlled trial of high-dose ursodesoxycholic acid for nonalcoholic steatohepatitis. J Hepatol. 2011;54:1011-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 236] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 20. | Ozel Coskun BD, Yucesoy M, Gursoy S, Baskol M, Yurci A, Yagbasan A, Doğan S, Baskol G. Effects of ursodeoxycholic acid therapy on carotid intima media thickness, apolipoprotein A1, apolipoprotein B, and apolipoprotein B/A1 ratio in nonalcoholic steatohepatitis. Eur J Gastroenterol Hepatol. 2015;27:142-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Kiyici M, Gulten M, Gurel S, Nak SG, Dolar E, Savci G, Adim SB, Yerci O, Memik F. Ursodeoxycholic acid and atorvastatin in the treatment of nonalcoholic steatohepatitis. Can J Gastroenterol. 2003;17:713-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 134] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 22. | Laurin J, Lindor KD, Crippin JS, Gossard A, Gores GJ, Ludwig J, Rakela J, McGill DB. Ursodeoxycholic acid or clofibrate in the treatment of non-alcohol-induced steatohepatitis: a pilot study. Hepatology. 1996;23:1464-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 361] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 23. | Dufour JF, Oneta CM, Gonvers JJ, Bihl F, Cerny A, Cereda JM, Zala JF, Helbling B, Steuerwald M, Zimmermann A; Swiss Association for the Study of the Liver. Randomized placebo-controlled trial of ursodeoxycholic acid with vitamin e in nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2006;4:1537-1543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 256] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 24. | Leuschner UF, Lindenthal B, Herrmann G, Arnold JC, Rössle M, Cordes HJ, Zeuzem S, Hein J, Berg T; NASH Study Group. High-dose ursodeoxycholic acid therapy for nonalcoholic steatohepatitis: a double-blind, randomized, placebo-controlled trial. Hepatology. 2010;52:472-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 230] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 25. | Lapenna D, Ciofani G, Festi D, Neri M, Pierdomenico SD, Giamberardino MA, Cuccurullo F. Antioxidant properties of ursodeoxycholic acid. Biochem Pharmacol. 2002;64:1661-1667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 77] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Qiao L, Yacoub A, Studer E, Gupta S, Pei XY, Grant S, Hylemon PB, Dent P. Inhibition of the MAPK and PI3K pathways enhances UDCA-induced apoptosis in primary rodent hepatocytes. Hepatology. 2002;35:779-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 113] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 27. | Lukivskaya O, Zavodnik L, Knas M, Buko V. Antioxidant mechanism of hepatoprotection by ursodeoxycholic acid in experimental alcoholic steatohepatitis. Adv Med Sci. 2006;51:54-59. [PubMed] |
| 28. | Bernstein C, Payne CM, Bernstein H, Garewal H. Activation of the metallothionein IIA promoter and other key stress response elements by ursodeoxycholate in HepG2 cells: relevance to the cytoprotective function of ursodeoxycholate. Pharmacology. 2002;65:2-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Ljubuncic P, Fuhrman B, Oiknine J, Aviram M, Bomzon A. Effect of deoxycholic acid and ursodeoxycholic acid on lipid peroxidation in cultured macrophages. Gut. 1996;39:475-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 92] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 30. | Schwarzenberg SJ, Bundy M. Ursodeoxycholic acid modifies gut-derived endotoxemia in neonatal rats. Pediatr Res. 1994;35:214-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Lim SC, Duong HQ, Parajuli KR, Han SI. Pro-apoptotic role of the MEK/ERK pathway in ursodeoxycholic acid-induced apoptosis in SNU601 gastric cancer cells. Oncol Rep. 2012;28:1429-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Arisawa S, Ishida K, Kameyama N, Ueyama J, Hattori A, Tatsumi Y, Hayashi H, Yano M, Hayashi K, Katano Y, Goto H, Takagi K, Wakusawa S. Ursodeoxycholic acid induces glutathione synthesis through activation of PI3K/Akt pathway in HepG2 cells. Biochem Pharmacol. 2009;77:858-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 33. | Li C, Zhang S, Li L, Hu Q, Ji S. Ursodeoxycholic Acid Protects Against Arsenic Induced Hepatotoxicity by the Nrf2 Signaling Pathway. Front Pharmacol. 2020;11:594496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 34. | Huang YS, Wang LX, Sun L, Wu Y, Lu JM, Zhao SC, Dai FM, Xu BS, Wang SR. Elevated peroxidative glutathione redox status in atherosclerotic patients with increased thickness of carotid intima media. Chin Med J (Engl). 2009;122:2827-2832. [PubMed] |
| 35. | Leach NV, Dronca E, Vesa SC, Sampelean DP, Craciun EC, Lupsor M, Crisan D, Tarau R, Rusu R, Para I, Grigorescu M. Serum homocysteine levels, oxidative stress and cardiovascular risk in non-alcoholic steatohepatitis. Eur J Intern Med. 2014;25:762-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 36. | Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF, Loria CM, Millen BE, Nonas CA, Pi-Sunyer FX, Stevens J, Stevens VJ, Wadden TA, Wolfe BM, Yanovski SZ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63:2985-3023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1247] [Cited by in RCA: 1509] [Article Influence: 125.8] [Reference Citation Analysis (0)] |
| 37. | Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC Jr, Spertus JA, Fernando Costa. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement: Executive Summary. Crit Pathw Cardiol. 2005;4:198-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 374] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 38. | Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, Tiribelli C. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1238] [Cited by in RCA: 2035] [Article Influence: 107.1] [Reference Citation Analysis (0)] |
| 39. | European Association for Study of Liver. EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63:237-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1177] [Cited by in RCA: 1332] [Article Influence: 133.2] [Reference Citation Analysis (0)] |
| 40. | Lloyd-Jones DM, Huffman MD, Karmali KN, Sanghavi DM, Wright JS, Pelser C, Gulati M, Masoudi FA, Goff DC Jr. Estimating Longitudinal Risks and Benefits From Cardiovascular Preventive Therapies Among Medicare Patients: The Million Hearts Longitudinal ASCVD Risk Assessment Tool: A Special Report From the American Heart Association and American College of Cardiology. J Am Coll Cardiol. 2017;69:1617-1636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (1)] |
| 41. | Simova I. Intima-media thickness: appropriate evaluation and proper measurement, described. An article from the e-journal of the ESC Council for Cardiology Practice. European Society of Cardiology 2015. Available from: https://www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volume-13/Intima-media-thickness-Appropriate-evaluation-and-proper-measurement-described. |
| 42. | Engelen L, Ferreira I, Stehouwer CD, Boutouyrie P, Laurent S; Reference Values for Arterial Measurements Collaboration. Reference intervals for common carotid intima-media thickness measured with echotracking: relation with risk factors. Eur Heart J. 2013;34:2368-2380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 204] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 43. | Marjot T, Moolla A, Cobbold JF, Hodson L, Tomlinson JW. Nonalcoholic Fatty Liver Disease in Adults: Current Concepts in Etiology, Outcomes, and Management. Endocr Rev. 2020;41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 148] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 44. | Ballestri S, Nascimbeni F, Baldelli E, Marrazzo A, Romagnoli D, Lonardo A. NAFLD as a Sexual Dimorphic Disease: Role of Gender and Reproductive Status in the Development and Progression of Nonalcoholic Fatty Liver Disease and Inherent Cardiovascular Risk. Adv Ther. 2017;34:1291-1326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 411] [Cited by in RCA: 397] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 45. | Wong VW, Wong GL, Yeung DK, Lau TK, Chan CK, Chim AM, Abrigo JM, Chan RS, Woo J, Tse YK, Chu WC, Chan HL. Incidence of non-alcoholic fatty liver disease in Hong Kong: a population study with paired proton-magnetic resonance spectroscopy. J Hepatol. 2015;62:182-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 136] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 46. | Yun KE, Nam GE, Lim J, Park HS, Chang Y, Jung HS, Kim CW, Ko BJ, Chung EC, Shin H, Ryu S. Waist Gain Is Associated with a Higher Incidence of Nonalcoholic Fatty Liver Disease in Korean Adults: A Cohort Study. PLoS One. 2016;11:e0158710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 47. | Soresi M, Noto D, Cefalù AB, Martini S, Vigna GB, Fonda M, Manzato E, Cattin L, Fellin R, Averna MR, Notarbartolo A; Metabolic Syndrome Study Group. Nonalcoholic fatty liver and metabolic syndrome in Italy: results from a multicentric study of the Italian Arteriosclerosis society. Acta Diabetol. 2013;50:241-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 48. | Caballería L, Arteaga I, Pera G, Rodríguez L, Alumà A, Auladell MA, Torán P. [Risk factors associated with non-alcoholic fatty liver disease: a case-control study]. Med Clin (Barc). 2013;141:233-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 49. | Yan J, Xie W, Ou WN, Zhao H, Wang SY, Wang JH, Wang Q, Yang YY, Feng X, Cheng J. Epidemiological survey and risk factor analysis of fatty liver disease of adult residents, Beijing, China. J Gastroenterol Hepatol. 2013;28:1654-1659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 50. | Eguchi Y, Hyogo H, Ono M, Mizuta T, Ono N, Fujimoto K, Chayama K, Saibara T; JSG-NAFLD. Prevalence and associated metabolic factors of nonalcoholic fatty liver disease in the general population from 2009 to 2010 in Japan: a multicenter large retrospective study. J Gastroenterol. 2012;47:586-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 401] [Article Influence: 30.8] [Reference Citation Analysis (2)] |
| 51. | Wong VW, Chan RS, Wong GL, Cheung BH, Chu WC, Yeung DK, Chim AM, Lai JW, Li LS, Sea MM, Chan FK, Sung JJ, Woo J, Chan HL. Community-based lifestyle modification programme for non-alcoholic fatty liver disease: a randomized controlled trial. J Hepatol. 2013;59:536-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 253] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 52. | Dudekula A, Rachakonda V, Shaik B, Behari J. Weight loss in nonalcoholic Fatty liver disease patients in an ambulatory care setting is largely unsuccessful but correlates with frequency of clinic visits. PLoS One. 2014;9:e111808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 53. | Mueller M, Thorell A, Claudel T, Jha P, Koefeler H, Lackner C, Hoesel B, Fauler G, Stojakovic T, Einarsson C, Marschall HU, Trauner M. Ursodeoxycholic acid exerts farnesoid X receptor-antagonistic effects on bile acid and lipid metabolism in morbid obesity. J Hepatol. 2015;62:1398-1404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 234] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 54. | Neuman M, Angulo P, Malkiewicz I, Jorgensen R, Shear N, Dickson ER, Haber J, Katz G, Lindor K. Tumor necrosis factor-alpha and transforming growth factor-beta reflect severity of liver damage in primary biliary cirrhosis. J Gastroenterol Hepatol. 2002;17:196-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 111] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 55. | Lindor KD, Kowdley KV, Heathcote EJ, Harrison ME, Jorgensen R, Angulo P, Lymp JF, Burgart L, Colin P. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology. 2004;39:770-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 516] [Cited by in RCA: 492] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 56. | Simental-Mendía LE, Simental-Mendía M, Sánchez-García A, Banach M, Serban MC, Cicero AFG, Sahebkar A. Impact of ursodeoxycholic acid on circulating lipid concentrations: a systematic review and meta-analysis of randomized placebo-controlled trials. Lipids Health Dis. 2019;18:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 57. | Sun W, Cui H, Li N, Wei Y, Lai S, Yang Y, Yin X, Chen DF. Comparison of FIB-4 index, NAFLD fibrosis score and BARD score for prediction of advanced fibrosis in adult patients with non-alcoholic fatty liver disease: A meta-analysis study. Hepatol Res. 2016;46:862-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 165] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 58. | Parikh P, Ingle M, Patel J, Bhate P, Pandey V, Sawant P. An open-label randomized control study to compare the efficacy of vitamin e versus ursodeoxycholic acid in nondiabetic and noncirrhotic Indian NAFLD patients. Saudi J Gastroenterol. 2016;22:192-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 59. | Kim JS, Park S, Yan P, Jeffers BW, Cerezo C. Effect of inter-individual blood pressure variability on the progression of atherosclerosis in carotid and coronary arteries: a post hoc analysis of the NORMALISE and PREVENT studies. Eur Heart J Cardiovasc Pharmacother. 2017;3:82-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 60. | Byrkjeland R, Stensæth KH, Anderssen S, Njerve IU, Arnesen H, Seljeflot I, Solheim S. Effects of exercise training on carotid intima-media thickness in patients with type 2 diabetes and coronary artery disease. Influence of carotid plaques. Cardiovasc Diabetol. 2016;15:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 61. | Suhadi R, Virginia DM, Setiawan CH. The Effect of Health Education by Pharmacists on 10-Year Atherosclerotic Cardiovascular Disease Risk: A Cluster-Randomized Control Study in a Low Socioeconomic Status Javanese Population. J Prim Care Community Health. 2018;9:2150132718773674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 62. | Kweon SS, Lee YH, Shin MH, Choi JS, Rhee JA, Choi SW, Ryu SY, Kim BH, Nam HS, Jeong SK, Park KS. Effects of cumulative smoking exposure and duration of smoking cessation on carotid artery structure. Circ J. 2012;76:2041-2047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 63. | Puato M, Boschetti G, Rattazzi M, Zanon M, Pesavento R, Faggin E, Fania C, Benetti E, Palatini P, Pauletto P. Intima-media thickness remodelling in hypertensive subjects with long-term well-controlled blood pressure levels. Blood Press. 2017;26:48-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 64. | Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Bansilal S, Bhatt DL, Leiter LA, McGuire DK, Wilding JP, Gause-Nilsson IA, Langkilde AM, Johansson PA, Sabatine MS. The design and rationale for the Dapagliflozin Effect on Cardiovascular Events (DECLARE)-TIMI 58 Trial. Am Heart J. 2018;200:83-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 113] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 65. | Mita T, Katakami N, Shiraiwa T, Yoshii H, Onuma T, Kuribayashi N, Osonoi T, Kaneto H, Kosugi K, Umayahara Y, Yamamoto T, Matsumoto K, Yokoyama H, Tsugawa M, Gosho M, Shimomura I, Watada H; Collaborators on the Sitagliptin Preventive Study of Intima-Media Thickness Evaluation (SPIKE) Trial. Erratum. Sitagliptin Attenuates the Progression of Carotid Intima-Media Thickening in Insulin-Treated Patients With Type 2 Diabetes: The Sitagliptin Preventive Study of Intima-Media Thickness Evaluation (SPIKE). A Randomized Controlled Trial. Diabetes Care 2016; 39: 455-464. Diabetes Care. 2017;40:808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 66. | Koutoukidis DA, Astbury NM, Tudor KE, Morris E, Henry JA, Noreik M, Jebb SA, Aveyard P. Association of Weight Loss Interventions With Changes in Biomarkers of Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-analysis. JAMA Intern Med. 2019;179:1262-1271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 174] [Article Influence: 29.0] [Reference Citation Analysis (0)] |