Published online Feb 7, 2020. doi: 10.3748/wjg.v26.i5.535
Peer-review started: November 7, 2019
First decision: December 7, 2019
Revised: January 6, 2020
Accepted: January 11, 2020
Article in press: January 11, 2020
Published online: February 7, 2020
Processing time: 91 Days and 21.8 Hours
There are few effective tools to predict survival in patients with invasive intraductal papillary mucinous neoplasms of the pancreas.
To develop comprehensive nomograms to individually estimate the survival outcome of patients with invasive intraductal papillary mucinous neoplasms of the pancreas.
Data of 1219 patients with invasive intraductal papillary mucinous neoplasms after resection were extracted from the Surveillance, Epidemiology, and End Results database, and randomly divided into the training (n = 853) and the validation (n = 366) cohorts. Based on the Cox regression model, nomograms were constructed to predict overall survival and cancer-specific survival for an individual patient. The performance of the nomograms was measured according to discrimination, calibration, and clinical utility. Moreover, we compared the predictive accuracy of the nomograms with that of the traditional staging system.
In the training cohort, age, marital status, histological type, T stage, N stage, M stage, and chemotherapy were selected to construct nomograms. Compared with the American Joint Committee on Cancer 7th staging system, the nomograms were generally more discriminative. The nomograms passed the calibration steps by showing high consistency between actual probability and nomogram prediction. Categorial net classification improvements and integrated discrimination improvements suggested that the predictive accuracy of the nomograms exceeded that of the American Joint Committee on Cancer staging system. With respect to decision curve analyses, the nomograms exhibited more preferable net benefit gains than the staging system across a wide range of threshold probabilities.
The nomograms show improved predictive accuracy, discrimination capability, and clinical utility, which can be used as reliable tools for risk classification and treatment recommendations.
Core tip: Due to its rarity, it is difficult to develop a prognostic nomogram for intraductal papillary mucinous neoplasms (IPMNs) of the pancreas in a single institution; however, the Surveillance, Epidemiology, and End Results database has provided useful data on prognosis. To date, no study has focused on a predictive model for the prognosis of IPMNs. In this study, we developed nomograms to predict the probability of overall survival and cancer-specific survival at different time points in patients with invasive IPMNs of the pancreas based on the Surveillance, Epidemiology, and End Results dataset. Compared with the American Joint Committee on Cancer 7th staging system, the formulated nomograms in this study showed perfect performance in terms of discrimination, calibration, reclassification, and clinical usefulness.
- Citation: Wu JY, Wang YF, Ma H, Li SS, Miao HL. Nomograms predicting long-term survival in patients with invasive intraductal papillary mucinous neoplasms of the pancreas: A population-based study. World J Gastroenterol 2020; 26(5): 535-549
- URL: https://www.wjgnet.com/1007-9327/full/v26/i5/535.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i5.535
Intraductal papillary mucinous neoplasms (IPMNs) of the pancreas are characterized by intraductal proliferation of neoplastic mucinous cells that arise in the epithelium of the main pancreatic duct (MPD) or its major branches. Patients with IPMNs are generally asymptomatic, and the condition is only discovered accidentally. However, some cases may present symptoms, including abdominal pain, jaundice, or acute pancreatitis. Given the widespread use of high-resolution cross-sectional imaging and the aging population, IPMNs are more commonly diagnosed and account for approximately half of incidentally detected pancreatic cysts[1]. Although the actual prevalence of IPMNs is unknown, it reportedly varies from 0.31 to 4.35 per 100000 persons, they are slightly more predominant in males and occur in the 6th to 7th decade of life[2]. IPMNs exhibit a significant progressive course from low-grade dysplasia (benign) to high grade dysplasia (carcinoma in situ) to invasive carcinoma, accounting for approximately 20% of pancreatic cancers[3]. Patients with IPMNs have better prognosis than patients with pancreatic cancer, but the prognosis is poor when IPMNs transform into invasive carcinoma. A comprehensive study demonstrated that the prognosis of malignant IPMNs was as poor as that of sporadic pancreatic carcinoma[4].
When an IPMN is determined, a clinical decision has to be made for surgical resection of invasive cysts or for surveillance of noninvasive lesions. Based on the site of origin, IPMNs can be sub-categorized into the following three groups: Main duct type (MD-IPMN), which is characterized by diffuse or segmental dilation of the MPD; branch duct type (BD-IPMN), which is characterized by cyst-forming dilation of lateral branches in communication with a normal MPD; and mixed type (MT-IPMN), which includes characteristics of the other types[5]. Differentiation is important for the management of IPMNs, as involvement of the main duct is significantly associated with malignant risk. The malignant risk of MD-IPMN (62.2%) resembles that of MT-IPMN (57.6%), but is much higher than that of BD-IPMN (24.4%)[6]. However, a BD-IPMN may develop distinct pancreatic cancer synchronously and metachronously; thus, its surveillance is controversial[7]. IPMN management, especially the indication for resection, continues to be a problem. Surgery is the only therapeutic option for patients with high malignant risk; thus, some experts recommend surgical resection for most patients with MD-IPMNs and MT-IPMNs, whereas the conservative approach is recommended for selected patients with BD-IPMNs[8].
Associated invasive carcinoma may be detected in 40%-60% of resected IPMN lesions[9]. Invasive IPMNs confer a distinct unfavorable prognosis with a 5-year overall survival (OS) of 24%-40%, whereas that of noninvasive IPMNs is higher than 90%[10,11]. Thus, clinicians need an effective prognostic tool to predict the survival probability of individual patients and to plan further clinical management. The nomogram is a simple and convenient mathematical tool with graphical representation, and it is widely applied in clinical practice to predict the probability of a specific event. Compared with the traditional TNM staging system, nomograms can more accurately estimate survival probability for individual patients by incorporating important prognostic factors[12]. To our knowledge, a prognostic nomogram for patients with invasive IMPNs has not yet been reported. Considering the rarity and the indolent process of IPMNs, survival analysis designed with prospective cohort studies are unlikely, and small retrospective series in a single-institution have limited evidence quality. Therefore, the prognostic research on IPMNs is best performed in large population-based registries with long-term follow-up periods to achieve the best conclusion. The Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute provides cancer statistics for determination of the clinical relationship and survival outcomes in tumor-related research[13]. In particular, it provides a broad path for research on rare tumors. In this study, we aimed to develop and validate nomograms to quantify the probability of long-term OS and cancer-specific survival (CSS) of patients with invasive IPMNs who underwent surgical resection based on the SEER database.
A retrospective cohort study was conducted by extracting the data collected between 1996 and 2016 from the SEER database. This study was reviewed and approved by the Ethics Committee of the Affiliated Hospital of Guangdong Medical University. Informed consent was waived, because the SEER research data are anonymous and publicly available. Moreover, we received permission from SEER to access the original data (accession number: 11250-Nov 2018). We utilized the incidence - SEER 18 Regs Custom Data (with additional treatment fields), Nov 2018 Sub (1975-2016 varying) as the data source. Only patients who had a histologic diagnosis after 1996 were included, as IPMN was first defined by the World Health Organization in that year[14]. Patients with IPMNs were first identified through the “SEER Site Recode” using the term “pancreas”, and then identified along with the label “malignant” using the variable “Histologic Type ICD-O-3” (International Classification of Disease for Oncology, 3rd edition) with the following codes: 8050, 8260, 8450, 8453, 8471, 8480, 8481, and 8503[15]. Only patients older than 18 years who underwent surgical resection of invasive IPMNs were included. Patients were excluded if they were diagnosed with in situ carcinoma or when their diagnosis was based only on an autopsy report or death certificate. Patients with concurrent malignant tumors of the pancreas were also excluded to avoid the inclusion of IPMNs that were incidentally discovered during pancreatic resection of other aggressive tumors, such as pancreatic adenocarcinoma.
Demographic, clinicopathologic, and therapeutic data were extracted for each patient, including age at diagnosis, sex, race, marital status, histological grade, American Joint Committee on Cancer (AJCC) TNM stage, tumor size, tumor location, surgical approach, radiation sequence with surgery, chemotherapy, and survival duration (in months). Death due to all factors or cancer, namely, OS and CSS, were the primary endpoints of this study. Patients with poorly differentiated and undifferentiated grades were placed in a subgroup for survival analysis due to the low number of undifferentiated tumors. We adopted the AJCC cancer stages system based on the 7th edition for further analysis to ensure uniform staging classification across all years of research. For patients diagnosed before 2003, there was no direct AJCC TNM staging data from SEER. Instead, we analyzed the extended information fields to assign a suitable AJCC stage definition. Besides AJCC staging (7th and 6th editions), the T stage was also derived from the variables of “CS tumor size (2004+)”, “CS extension (2004+)”, “EOD 10 - size (1988-2003)” and “EOD 10 - extent (1988-2003)”. N stage was derived from AJCC staging and “Regional nodes positive (1988+)”. M stage was derived from AJCC staging and “SEER historic stage A”[16].
All eligible patients were divided into the training and the validation cohorts through the simple randomization grouping method (roughly 7:3). The data from the training cohort were used to perform survival analysis and formulate nomograms, whereas the data from the validation cohort were used to validate the prediction models. Univariate and multivariate Cox proportional hazards regression analyses were used to screen the prognostic factors. Nomograms for 3-, 5-, and 10-year OS and 3-, 5-, and 10-year CSS were constructed based on the results of the multivariate Cox regression analyses.
The performance of the nomogram was first quantified in the training cohort and then the validation cohort in terms of discrimination, calibration, and clinical utility.
The discriminative performances of the nomogram were quantitatively evaluated by the concordance index (C-index) and the area under curve (AUC) of the time-dependent receiver operating characteristics curve[17]. C-index and AUC ranged from 0.5 to 1, and large values indicated increased prediction accuracy. The net reclassification improvement (NRI) and integrated discrimination improvement (IDI) were calculated to determine the overall improvement in the predictive accuracy using the novel nomogram in place of the AJCC 7th staging system. NRI refers to the difference in the proportion of patients with events correctly assigned a higher probability and that of patients without events correctly assigned a low probability by an updated model compared with the initial model[18]. IDI represents the improvement in average sensitivity (i.e., the true positive rate) without reducing the average specificity (i.e., the true negative rate) of a new model compared with a baseline model[19]. A marginal estimate versus model was applied to plot calibration to investigate the consistency between predicted probabilities and the actual outcomes from the graphical representations[20]. Finally, we conducted decision curve analysis (DCA), a novel algorithm, to evaluate the clinical usefulness of the predicted model and the traditional TNM staging system. In the present study, DCA was used to assess whether the nomogram increased the net benefits compared with the AJCC staging system throughout the range of threshold probabilities[21,22]. All these analyses were conducted using R version 3.5.1 (http://www.r-project.org/). A two-sided P value less than 0.05 was considered statistically significant.
After strict selection, 1219 patients with invasive IPMNs and undergoing surgical resection were included in this study. Of these patients, 853 and 366 comprised the training and the validation cohorts, respectively. In the entire cohort, 840 (68.9%) patients suffered from all-cause death at the end of the follow-up with a median follow-up period of 30 mo, whereas 557 (45.7%) patients died of cancer with a median survival time of 51 mo. The 3-, 5-, and 10-year OS rates of the entire cohort were 45.1%, 34.7%, and 21.4%, respectively. The 3-, 5-, and 10-year CSS rates were 55.5%, 47.3%, and 39.8%, respectively. Patient age ranged from 26 years to 94 years with a median of 68 years. Most of the patients were male (55.0%), married (64.9%), white (82.9%), tumor located in the pancreatic head (64.4%), received pan-creatoduodenectomy (68.5%), and did not undergo radiotherapy (70.3%). Regarding the histologic grade, 253 (20.8%) patients had well differentiated tumors, 477 (39.1%) patients had moderately differentiated tumors, 235 (19.3%) patients had poorly differentiated or undifferentiated tumors, and 254 (20.8%) patients had unknown differentiation status. The proportions of Stages I, II, III, and IV were 29.7% (362/1219), 60.0% (731/1219), 4.3% (53/1219), and 6.0% (73/1219), respectively. The characteristics of the patients in the training cohort and validation cohort are shown in Table 1.
| Characteristics | Training cohort (n = 853) | Validation cohort (n = 366) | ||
| Number of patients | Percent (%) | Number of patients | Percent (%) | |
| Age (yr) | ||||
| ≤ 60 | 218 | 25.6 | 70 | 19.1 |
| 60-69 | 276 | 32.4 | 127 | 34.7 |
| 70-79 | 281 | 32.8 | 131 | 35.8 |
| ≥ 80 | 78 | 9.2 | 38 | 10.4 |
| Sex | ||||
| Male | 470 | 55.1 | 201 | 54.9 |
| Female | 383 | 44.9 | 165 | 45.1 |
| Race | ||||
| White | 694 | 81.4 | 316 | 86.3 |
| Others1 | 159 | 18.6 | 50 | 13.7 |
| Marital status | ||||
| Married | 558 | 65.4 | 233 | 63.7 |
| Unmarried2 | 295 | 34.6 | 133 | 36.3 |
| Grade | ||||
| Well differentiated | 187 | 21.9 | 66 | 18.0 |
| Moderately differentiated | 320 | 37.5 | 157 | 42.9 |
| Poorly differentiated/Undifferentiated | 162 | 19.0 | 73 | 19.9 |
| Unknown | 184 | 21.6 | 70 | 19.1 |
| Tumor location | ||||
| Head | 181 | 21.2 | 232 | 63.4 |
| Body/tail | 553 | 64.8 | 75 | 20.5 |
| Other3 | 119 | 14.0 | 59 | 16.1 |
| T stage | ||||
| T1 | 124 | 14.5 | 57 | 15.6 |
| T2 | 206 | 24.2 | 71 | 19.4 |
| T3 | 468 | 54.9 | 207 | 56.5 |
| T4 | 55 | 6.4 | 31 | 8.5 |
| N stage | ||||
| N0 | 497 | 58.3 | 209 | 57.1 |
| N1 | 356 | 41.7 | 157 | 42.9 |
| M stage | ||||
| M0 | 800 | 93.8 | 346 | 94.5 |
| M1 | 53 | 6.2 | 20 | 5.5 |
| Clinical stage | ||||
| I | 267 | 31.3 | 95 | 26.0 |
| II | 500 | 58.6 | 231 | 63.0 |
| III | 33 | 3.9 | 20 | 5.5 |
| IV | 53 | 6.2 | 20 | 5.5 |
| Tumor size | ||||
| < 3 cm | 302 | 35.4 | 146 | 39.9 |
| 3-6 cm | 417 | 48.9 | 168 | 45.9 |
| > 6 cm | 134 | 15.7 | 52 | 14.2 |
| Surgical type | ||||
| Partial/localized pancreatectomy | 152 | 17.8 | 65 | 17.8 |
| Total pancreatectomy | 122 | 14.3 | 45 | 12.3 |
| Extensive pancreatoduodenectomy | 579 | 67.9 | 256 | 69.9 |
| Radiation | ||||
| None | 609 | 71.4 | 248 | 67.8 |
| Adjuvant radiotherapy solely | 222 | 26.0 | 107 | 29.2 |
| Others4 | 22 | 2.6 | 11 | 3.0 |
| Chemotherapy | ||||
| No/unknown | 435 | 51.0 | 180 | 49.2 |
| Yes | 418 | 49.0 | 186 | 50.8 |
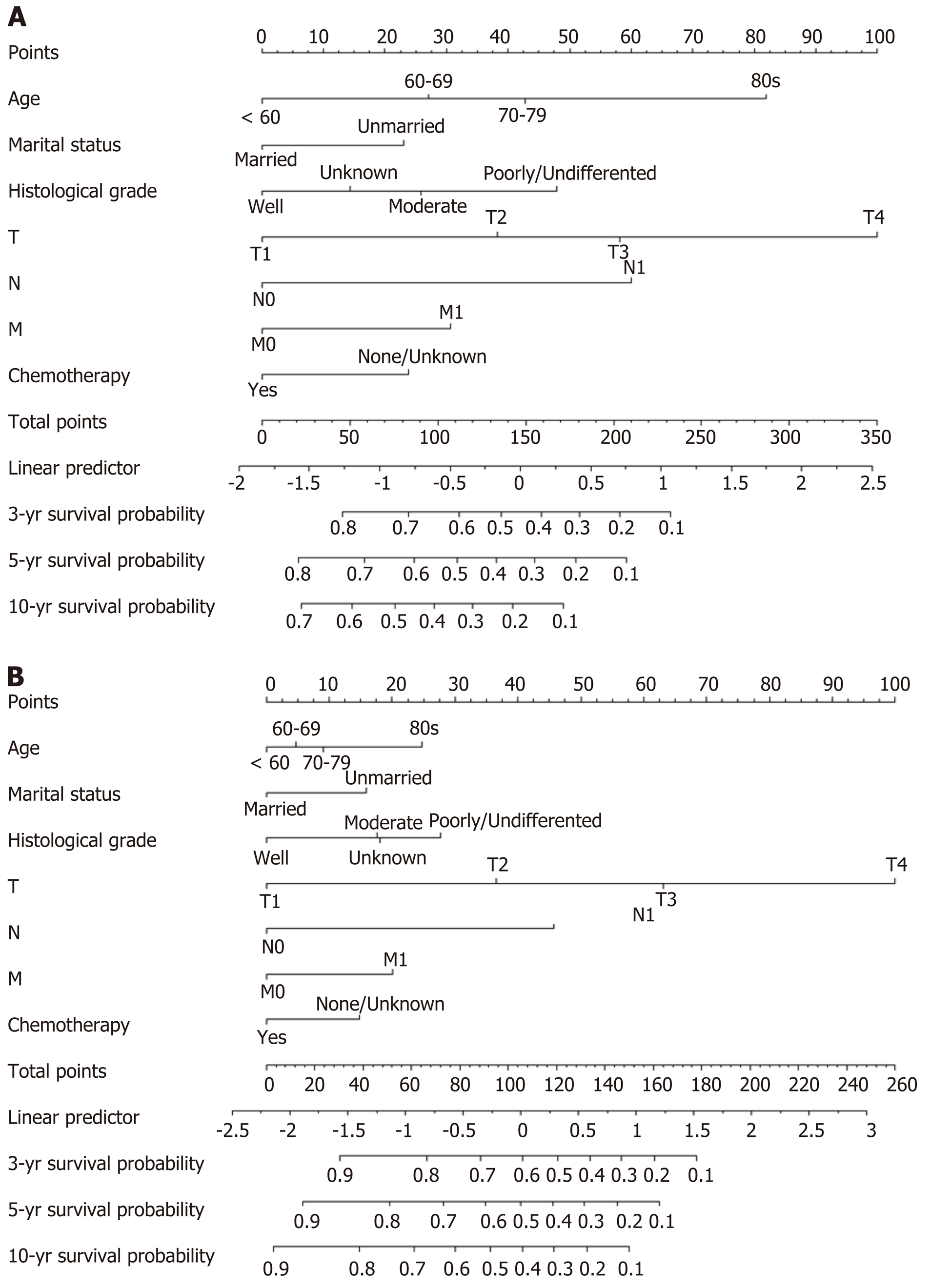
In the training cohort, all variables except sex, tumor location, and surgical type were considered significant risk factors of OS according to the univariate analysis (Table 2). Following multivariate analysis, age, marital status, histological grade, T stage, N stage, M stage, and chemotherapy were the selected independent prognostic factors (Table 2). These elements were then used to establish a nomogram to predict the 3-, 5-, and 10-year OS probability for patients with invasive IPMNs (Figure 1A).
| Variables | OS | CSS | ||||||
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | |
| Age (yr) | ||||||||
| ≤ 60 | Reference | Reference | Reference | Reference | ||||
| 60-69 | 1.33 (1.06-1.67) | 0.014 | 1.41 (1.12-1.79) | 0.003 | 1.10 (0.83-1.46) | 0.525 | 1.10 (0.82-1.47) | 0.512 |
| 70-79 | 1.74 (1.39-2.16) | < 0.001 | 1.68 (1.33-2.12) | < 0.001 | 1.22 (0.91-1.62) | 0.181 | 1.16 (0.86-1.56) | 0.344 |
| ≥ 80 | 2.47 (1.81-3.35) | < 0.001 | 2.82 (2.04-3.91) | < 0.001 | 1.52 (1.20-2.21) | 0.007 | 1.81 (1.14-2.87) | 0.012 |
| Sex | ||||||||
| Male | Reference | Reference | Reference | Reference | ||||
| Female | 1.12 (0.95-1.32) | 0.168 | 1.10 (0.92-1.31) | 0. 300 | 1.23 (0.98-1.52) | 0.068 | 1.27 (0.99-1.60) | 0. 051 |
| Race | ||||||||
| White | Reference | Reference | Reference | Reference | ||||
| Others1 | 0.77 (0.61-0.96) | 0.018 | 0.84 (0.67-1.06) | 0.142 | 0.73 (0.54-0.98) | 0.040 | 0.80 (0.59-1.08) | 0.143 |
| Marital status | ||||||||
| Married | Reference | Reference | Reference | Reference | ||||
| Unmarried2 | 1.31 (1.10-1.55) | 0.002 | 1.31 (1.10-1.57) | 0.003 | 1.23 (1.05-1.54) | 0.004 | 1.34 (1.05-1.71) | 0.017 |
| Grade | ||||||||
| Well differentiated | Reference | Reference | Reference | Reference | ||||
| Moderately differentiated | 1.58 (1.25-1.99) | < 0.001 | 1.36 (1.08-1.72) | 0.001 | 1.77 (1.29-2.43) | < 0.001 | 1.39 (1.01-1.92) | 0.047 |
| Poorly differentiated/undifferentiated | 2.46 (1.90-3.18) | < 0.001 | 1.73 (1.32-2.28) | < 0.001 | 2.71 (1.90-3.86) | < 0.001 | 1.70 (1.16-2.48) | 0.006 |
| Unknown | 1.08 (0.82-1.40) | 0.598 | 1.17 (0.89-1.55) | 0.253 | 1.08 (0.74-1.57) | 0.707 | 1.51 (1.02-2.23) | 0.040 |
| Tumor location | ||||||||
| Head | Reference | Reference | Reference | Reference | ||||
| Body/tail | 1.06 (0.86-1.29) | 0.605 | 1.10 (0.83-1.45) | 0.524 | 0.97 (0.74-1.29) | 0.853 | 0.93 (0.62-1.37) | 0.700 |
| Others3 | 0.81 (0.63-1.03) | 0.088 | 0.91 (0.69-1.20) | 0.503 | 0.80 (0.58-1.11) | 0.184 | 0.86 (0.60-1.24) | 0.424 |
| T stage | ||||||||
| T1 | Reference | Reference | Reference | Reference | ||||
| T2 | 1.70 (1.23-2.34) | 0.001 | 1.49 (1.03-2.14) | 0.033 | 2.35 (1.36-4.05) | 0.002 | 1.97 (1.09-3.56) | 0.026 |
| T3 | 2.84 (2.12-3.80) | < 0.001 | 1.94 (1.36-2.76) | < 0.001 | 5.38 (3.28-8.82) | < 0.001 | 2.63 (1.36-5.09) | 0.004 |
| T4 | 4.94 (3.36-7.28) | < 0.001 | 3.19 (2.01-5.07) | < 0.001 | 8.03 (5.70-12.61) | < 0.001 | 5.56 (2.17-14.22) | < 0.001 |
| N stage | ||||||||
| N0 | Reference | Reference | Reference | Reference | ||||
| N1 | 2.51 (2.13-2.96) | < 0.001 | 2.06 (1.69-2.50) | < 0.001 | 3.33 (2.66-4.18) | < 0.001 | 2.29 (1.72-3.04) | < 0.001 |
| M stage | ||||||||
| M0 | Reference | Reference | Reference | Reference | ||||
| M1 | 2.35 (1.75-3.15) | < 0.001 | 1.47 (1.03-2.08) | 0.032 | 2.77 (1.93-3.98) | < 0.001 | 2.65 (1.29-5.44) | 0.008 |
| Clinical stage | ||||||||
| I | Reference | Reference | Reference | Reference | ||||
| II | 2.37 (1.94-2.89) | < 0.001 | 1.13 (0.90-1.41) | 0.377 | 2.72 (1.79-3.96) | < 0.001 | 1.66 (0.96-2.88) | 0.071 |
| III | 3.55 (2.38-5.29) | < 0.001 | 1.59 (0.74-3.42) | 0.235 | 3.79 (2.53-7.49) | < 0.001 | 1.78 (0.66-4.81) | 0.253 |
| IV | 4.28 (3.08-5.96) | < 0.001 | 1.72 (0.85-3.81) | 0.176 | 4.99 (3.58-9.68) | < 0.001 | 1.89 (0.82-4.63) | 0.155 |
| Tumor size | ||||||||
| < 3 cm | Reference | Reference | Reference | Reference | ||||
| 3-6 cm | 1.66 (1.38-2.00) | < 0.001 | 1.13 (0.91-1.40) | 0.261 | 1.80 (1.40-2.31) | < 0.001 | 1.07 (0.81-1.41) | 0.645 |
| > 6 cm | 1.25 (1.04-1.74) | 0.023 | 1.03 (0.77-1.37) | 0.848 | 1.43 (1.01-2.00) | 0.041 | 1.06 (0.74-1.54) | 0.741 |
| Surgical type | ||||||||
| Partial/localized pancreatectomy | Reference | Reference | Reference | Reference | ||||
| Total pancreatectomy | 0.90 (0.67-1.21) | 0.496 | 0.97 (0.69-1.35) | 0.846 | 0.78 (0.53-1.16) | 0.224 | 0.75 (0.48-1.17) | 0.201 |
| Extensive pancreatoduodenectomy | 0.96 (0.77-1.19) | 0.716 | 0.96 (0.71-1.29) | 0.767 | 0.97 (0.73-1.29) | 0.815 | 0.95 (0.64-1.42) | 0.809 |
| Radiotherapy | ||||||||
| None | Reference | Reference | Reference | Reference | ||||
| Adjuvant radiotherapy solely | 1.25 (1.04-1.50) | 0.015 | 1.13 (0.90-1.42) | 0.303 | 1.45 (1.14-1.83) | 0.002 | 1.14 (0.86-1.52) | 0.430 |
| Others4 | 0.75 (0.44-1.28) | 0.290 | 0.78 (0.44-1.36) | 0.376 | 1.01 (0.52-1.98) | 0.966 | 0.76 (0.37-1.53) | 0.440 |
| Chemotherapy | ||||||||
| No/unknown | Reference | Reference | Reference | Reference | ||||
| Yes | 0.64 (0.55-0.76) | < 0.001 | 0.72 (0.58-0.89) | 0.002 | 1.27 (1.02-1.58) | 0.036 | 0.70 (0.53-0.92) | 0.012 |
It was found that age, race, marital status, histological grade, T stage, N stage, M stage, clinical stage, tumor size, radiotherapy, and chemotherapy were statistically significantly associated with CSS based on the outcomes of univariate analysis (Table 2). In multivariate analysis, the following seven variables were selected as independent prognostic factors of CSS: Age, marital status, histological grade, T stage, N stage, M stage, and chemotherapy (Table 2). Therefore, a nomogram predicting the 3-, 5-, and 10-year CSS probability was created by considering these variables (Figure 1B).
For OS prediction, the nomogram provided a higher C-index (0.756, 95%CI: 0.734-0.778) than the AJCC 7th staging system (0.645, 95%CI: 0.623-0.667; P < 0.001) in the training cohort. Similarly, the C-index of the validation cohort (0.748, 95%CI: 0.715-0.781) was also higher than the traditional TNM staging system (0.654; 95%CI: 0.623-0.685; P < 0.001). For CSS prediction, a higher C-index was observed in the training cohort (0.769, 95%CI: 0.742-0.796) than the AJCC 7th staging system (0.671, 95%CI: 0.646-0.696; P < 0.001). Moreover, the validation cohort exhibited a higher C-index (0.752, 95%CI: 0.713-0.791) compared with the classic tumor staging system (0.667, 95%CI: 0.632-0.702; P < 0.001). These results indicated that these nomograms were more robust than the existing AJCC staging system.
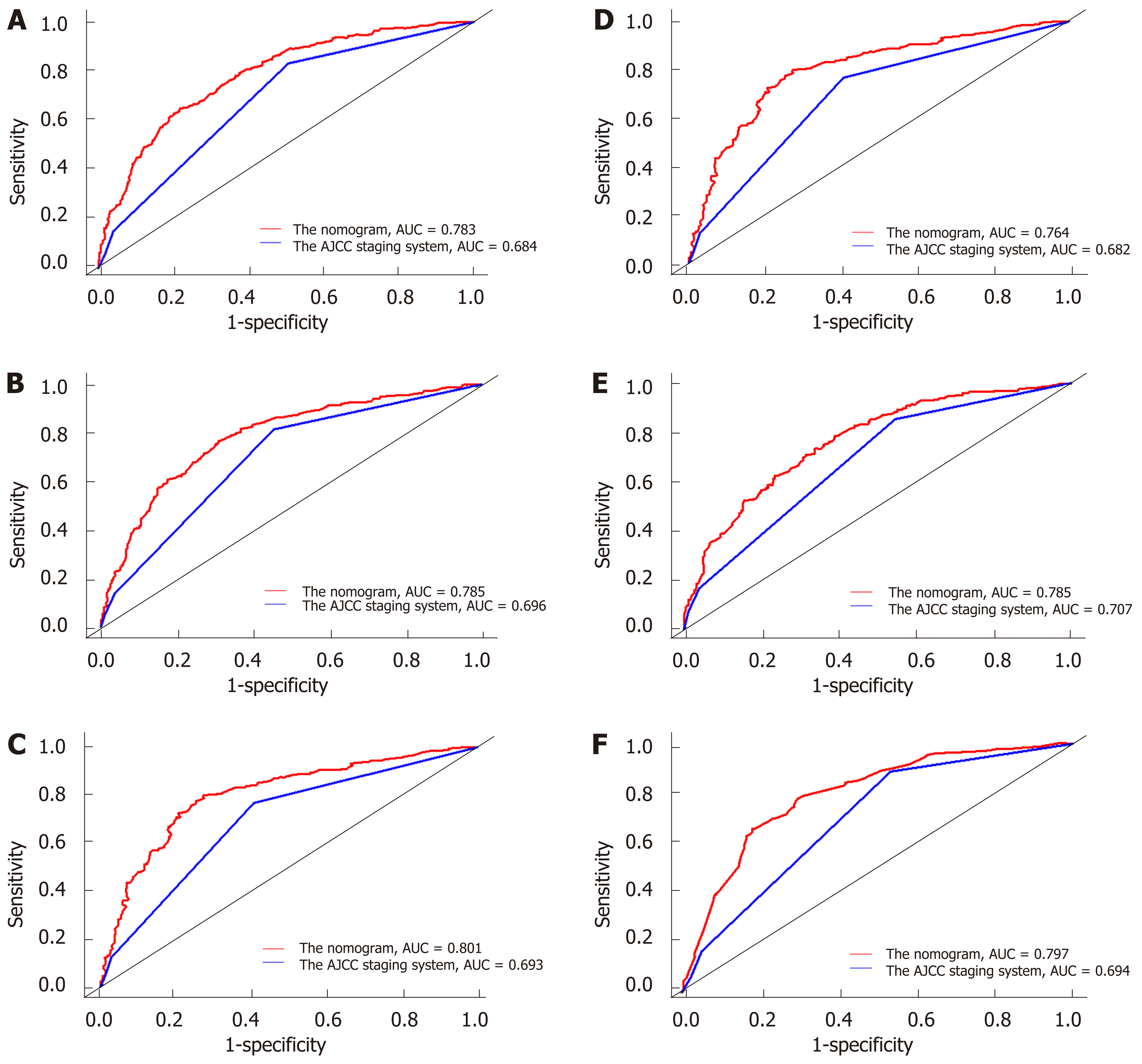
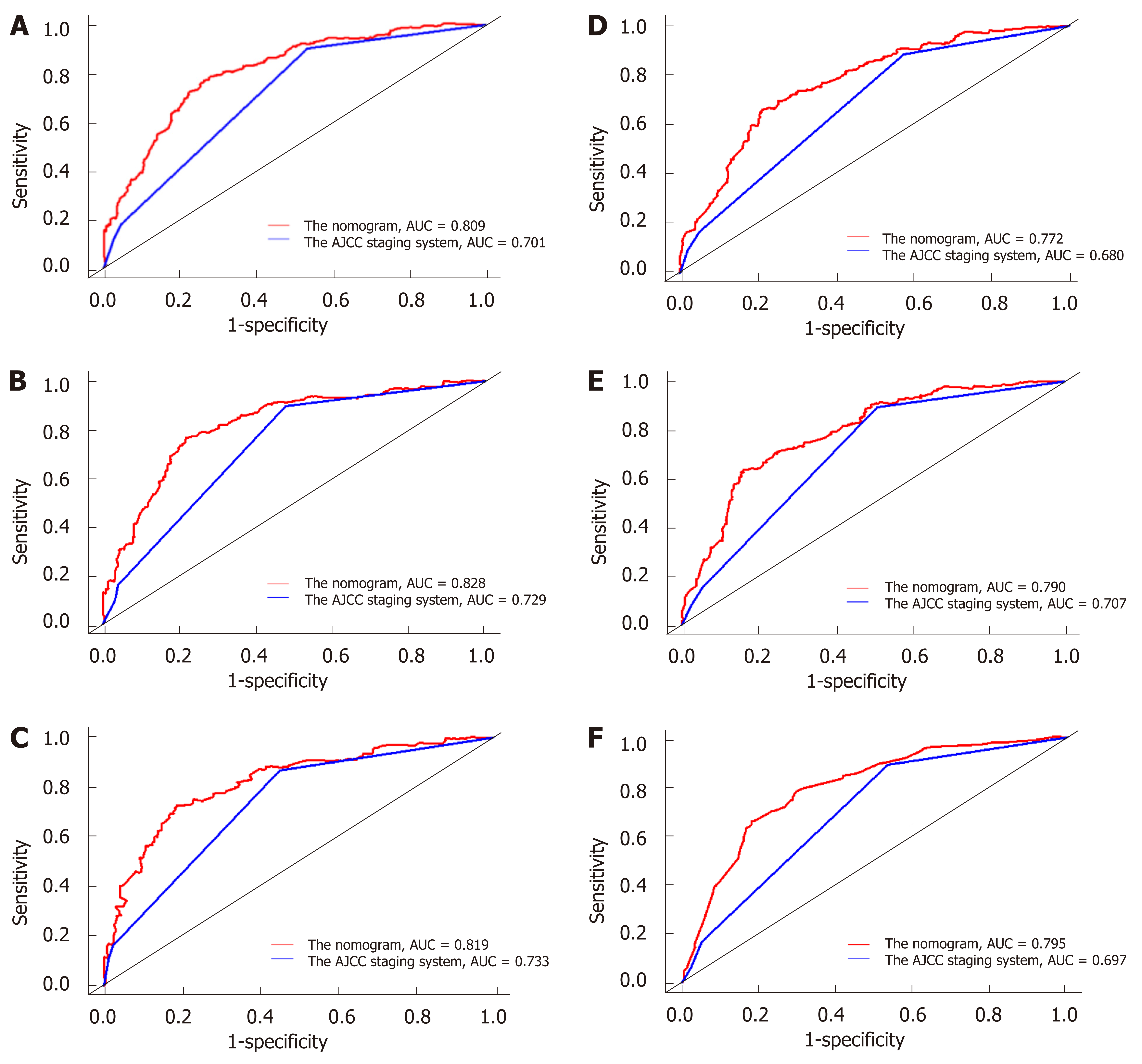
The discriminatory abilities of the nomogram and the AJCC 7th staging system were compared by calculating the AUC values. For the training cohort (Figure 2A-2C), the AUC values of the nomogram for the 3-, 5-, and 10-year OS were 0.783, 0.785, and 0.801, whereas these values were 0.684, 0.696, and 0.693, respectively, for the AJCC 7th staging system. Regarding the validation cohort (Figure 2D-2F), the AUC values of the nomogram for the 3-, 5-, and 10-year OS were 0.764, 0.785, and 0.797, whereas those of the AJCC 7th staging system were 0.682, 0.707, and 0.694, respectively. The same trend was observed for the 3-, 5-, and 10-year CSS in both the training cohort (Figure 3A-3C) and the validation cohort (Figure 3D-3F). These results demonstrated that the nomograms had higher discriminative capacity for predicting OS and CSS compared with the AJCC 7th staging system.
The results of NRI and IDI calculations are shown in Table 3. The comprehensive nomogram significantly improved the risk reclassification for 3-, 5-, and 10-year OS and CSS predictions compared with the AJCC 7th staging system in the training and validation cohorts.
| Category | NRI (95%CI) | P value | IDI (95%CI) | P value |
| OS | ||||
| Training set | ||||
| 3-yr OS | 0.564 (0.413-0.731) | < 0.001 | 0.106 | < 0.001 |
| 5-yr OS | 0.467 (0.292-0.653) | < 0.001 | 0.104 | < 0.001 |
| 10-yr OS | 0.458 (0.323-0.715) | < 0.001 | 0.091 | < 0.001 |
| Validation set | ||||
| 3-yr OS | 0.526 (0.344-0.714) | < 0.001 | 0.095 | < 0.001 |
| 5-yr OS | 0.455 (0.225-0.697) | < 0.001 | 0.092 | < 0.001 |
| 10-yr OS | 0.529 (0.234-0.764) | < 0.001 | 0.076 | < 0.001 |
| CSS | ||||
| Training set | ||||
| 3-yr OS | 0.570 (0.293-0.763) | < 0.001 | 0.076 | < 0.001 |
| 5-yr OS | 0.376 (0.225-0.673) | < 0.001 | 0.072 | < 0.001 |
| 10-yr OS | 0.421 (0.174-0.662) | < 0.001 | 0.068 | < 0.001 |
| Validation set | ||||
| 3-yr OS | 0.461 (0.246-0.752) | < 0.001 | 0.065 | < 0.001 |
| 5-yr OS | 0.368 (0.197-0.680) | < 0.001 | 0.060 | < 0.001 |
| 10-yr OS | 0.436 (0.188-0.711) | < 0.001 | 0.059 | < 0.001 |
The calibration plots exhibited a good agreement between predicted and observed survival for the 3-, 5-, and 10-year OS in the training (Supplement Figure 1A-1C) and validation cohorts (Supplement Figure 1D-1F). Furthermore, the nomograms showed excellent consistency with the observed probabilities for CSS at different time points in the training (Supplement Figures 2A-2C) and validation cohorts (Supplement Figures 2D-2F). These findings suggested the appreciable reliability of these nomograms.
As shown in Figure 4, DCA showed that the nomograms had good clinical validity in predicting the 3-, 5-, and 10-year OS and CSS of patients with invasive IPMNs due to the wide field of threshold probability. Furthermore, preferable net benefit was also obtained with the formulated nomogram in comparison with the AJCC 7th staging system at different time points, thereby indicating the favorable clinical utility of these nomograms.
Surgical resection is the mainstay of treatment for invasive IPMNs. Several authoritative groups have weighed the evidence and issued guidelines to address the appropriate management of IPMNs, and surgical indications for invasive IPMNs are now widely accepted[23-25]. The survival of patients with invasive IPMNs after surgery is assumed to be higher compared with patients with non-IPMN-related pancreatic carcinoma. However, invasive IPMN is rare, and most published results are based on a small number of patients. Thus, the prognosis of patients with invasive IPMNs who underwent resection is not well understood. In this study, the 5-year survival of patients with surgical invasive IPMNs of 34.0% was slightly higher than those of the previous large-population studies, which ranged from 19.4% to 24.1%[16,26]. This difference may be due to improvements in the therapeutic technology for invasive IPMNs and the exclusion of patients with other types of pancreatic tumors. This negative prognosis of invasive IPMN is probably attributed to the considerable risk of recurrence after resection[27]. Postoperative follow-up for invasive IPMN is recommended due to its similarity to pancreatic ductal adenocarcinoma. However, the correct approach for follow-up has not been fully resolved, including which patients experience a poor outcome, when this happens, and how it manifests.
The proposed nomograms in our study comprised seven independent prognostic factors, namely, age, marital status, histological grade, T stage, N stage, M stage, and chemotherapy. Here, we demonstrated several novel findings. First, older patients had higher mortality than younger patients, which was partly because older patients have more comorbidities and higher perioperative risks[28]. Second, married patients had better prognosis than unmarried patients. An explanation for this might be that married patients have less distress, depression, and anxiety than their unmarried counterparts, because a spouse can share the emotional burden and provide appropriate social support[29]. Third, the magnitude of poor prognosis was consistent with the change in histological grade, T stage, N stage, and M stage, which are the main components of the TNM staging system. According to the nomograms, patients with the different abovementioned features were assigned different points and had different survival outcomes, even if the TNM stage was the same. These results could partly explain the advantage of the nomograms for predicting prognosis compared with the TNM staging system[30]. Fourth, chemotherapy was associated with improved OS and CSS in patients with invasive IPMN. However, Marchegiani[31] found that chemotherapy could only prolong the survival period for patients with nodal disease and tubular differentiation of invasive pancreatic cysts. Further prospective trials should be conducted to improve the level of evidence regarding the application of chemotherapy for invasive IPMN due to the lack of evidence-based recommendations. Finally, given that T stage had the strongest prognostic weight for OS and CSS, it was converted into 100 points. The remaining variables were assigned a smaller number of points proportional to their effect size, which presented the relative importance of the remaining factors compared with the most significant factor.
A positive surgical margin is associated with tumor recurrence[32]. In theory, patients with invasive IPMNs who underwent total pancreatectomy and extended pancreatoduodenectomy, in which the pancreatic lesions are completely removed, and local recurrence is eliminated, should achieve a better prognosis than patients who received local or partial pancreatectomy. However, our study demonstrated an equivalent survival outcome among patients who received different surgical approaches. The reason for this may be the slow malignant progression of this tumor. It was reported that a benign IPMN takes 5-7 years to become malignant, whereas pancreatic neoplasms take 10-12 years to gain metastatic ability[33,34]. In our study, the mean age at diagnosis of invasive IPMN was 66.31 years. Thus, the risk of a recurrent lesion might be limited if we consider the average life span of the United States population, which was 79.2 years in 2018. Another reason might be that IPMN favors recurrence at distant sites, rather than pancreatic remnants. Nearly 1/3 of IPMN patients harbor other type of tumors, and 13%-61% of deaths among IPMN cases were caused by extra-pancreatic cancers[35,36]. Therefore, even if pancreatic cysts are completely removed, the risk of relapse cannot be eliminated[37].
By applying the independent prognostic factors from multivariate analysis, we constructed the first prognostic nomogram to predict 3-, 5-, and 10-year OS and CSS for patients with invasive IPMNs who underwent surgery. The nomograms work by ranking the effect estimates, and are influenced by the presence of the included variables[38]. In these nomogram models, each included factor was ascribed a weighted point, and the total score calculated from the various factors correspond to the predicted survival probability of a patient. Patients with a higher score had worse prognosis. Using these predictive tools, we can easily and precisely calculate the survival probability of individual patients at certain time points. Follow-up even after resection with negative margins is required for invasive IPMN due to the high risk of recurrence and the low survival rate. The current guidelines recommend surveillance after resection for all invasive IPMNs regardless of the postoperative prognosis of individual patients. These nomograms may help with such recommendations. For example, patients with favorable prognosis should be monitored with less unnecessary exposure to imaging and radiation, thereby resulting in low healthcare costs. Moreover, these predictive tools may also contribute to the planning of postoperative treatment. Thus, patients with an isolated tumor recurrence after resection may be managed with surgical reintervention if the prognostic prediction is favorable. Otherwise, secondary surgery may not be appropriate.
At present, the AJCC staging system is commonly used in clinical practice to predict the prognosis of malignant patients. The TNM staging system depends purely on the anatomical extent of the cancer and ignores the effect of other possible prognostic variables. Significant heterogeneity existed for patients at the same stage, and consequently, the predictive efficiency based on these issues may not be entirely precise. Compared with the AJCC 7th staging system, the current nomograms showed excellent predictive accuracy and discriminative ability. Moreover, the NRI and IDI quantitatively proved that these nomograms have a significantly increased likelihood of unfavorable prognostic identification, and are more effective than the traditional TNM classification. Perfect predictive accuracy is not equal to usefulness in practice. When the threshold probabilities of the net benefit are unrealistic, a model with good performance might also have limited applicability[39]. Therefore, we applied the DCA curves to investigate the clinical validity of our nomograms, and the results further proved their superiority to the traditional AJCC TNM classification.
Although the SEER database provides a large population sample, there were several limitations. First, retrospective analyses of secondary data were inevitably influenced by selection bias. Second, because determining the subtype preoperatively was not possible, data regarding classification of MD-IPMN, BD-IPMN, and MT-IPMN were unavailable in the SEER dataset but potentially influenced the prognosis. Moreover, we could not conduct this study by following the newest classification and concept of the classification of IPMN. Third, the detailed information on radiotherapy, chemotherapy, the side effects of the operation, and the long-term complications, which may also affect patients’ prognosis, were not available in the SEER database. Fourth, recurrence is the primary cause of death of invasive IPMN, but we could not evaluate it as one of the endpoints due to the lack of relevant data in the SEER database. Fifth, the diagnostic criteria and therapeutic approach for invasive IPMN have been significantly changed during the recruitment time of this study (1996-2016). However, we could not explore the impact of these changes on prognosis due to insufficient information. Finally, due to the retrospective nature of our study, the nomograms were developed and validated in the same database. Thus, it is more reliable to validate them prospectively or at least in another dataset.
In conclusion, we constructed nomograms to estimate the probability of OS and CSS in patients with invasive IPMNs who underwent surgical resection using a large, population-based dataset with long-term follow-up. These nomograms outperformed the 7th edition of the AJCC staging system in predictive accuracy, discrimination capability, and clinical applicability. These predictive tools effectively help clinicians identify patients who are at high risk of postoperative death at different timepoints, and in making more precise clinical decisions on secondary surgery, adjuvant therapy, and follow-up strategies.
Patients with invasive intraductal papillary mucinous neoplasms (IPMNs) of the pancreas after resection have a distinct unfavorable prognosis. Clinicians need an effective prognostic tool to predict the survival probability of individual patients and to plan further clinical management. To date, no previous study has focused on a predictive model for the prognosis of IPMNs.
Considering the rarity and the indolent course of IPMNs, it is difficult to develop a prognostic nomogram for IPMNs in a single institution. Thus, a prognostic nomogram should be performed based on a population-based cohort with long-term follow-up to achieve the best conclusion. The Surveillance, Epidemiology, and End Results database has provided useful data on prognosis in patients with IPMNs.
We aimed to develop and validate comprehensive nomograms to estimate the probability of long-term overall survival and cancer-specific survival in individual patients with invasive IPMNs of the pancreas who underwent surgical resection.
The information on patients with invasive IPMNs after resection was extracted from the Surveillance, Epidemiology, and End Results database, and then randomly divided into the training and the validation cohorts (roughly 7:3). Based on the Cox regression model, nomograms were constructed to predict the probability of overall survival and cancer-specific survival at different time points for an individual patient. The performance of the nomogram was measured with respect to discrimination, calibration, and clinical utility. Moreover, we compared the predictive accuracy of the nomograms with that of the traditional staging system.
In the training cohort, age, marital status, histological type, T stage, N stage, M stage, and chemotherapy were selected to construct nomograms. Compared to the American Joint Committee on Cancer 7th staging system, the formulated nomograms in this study showed perfect performance with respect to discrimination, calibration, reclassification, and clinical usefulness.
The nomograms showed improved predictive accuracy, discrimination capability, and clinical utility.
These new predictive models need to be validated by a prospective study or at least in another dataset.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bramhall SR, Suzuki S, Wada R S-Editor: Dou Y L-Editor: Webster JR E-Editor: Zhang YL
| 1. | Stark A, Donahue TR, Reber HA, Hines OJ. Pancreatic Cyst Disease: A Review. JAMA. 2016;315:1882-1893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 177] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 2. | Klibansky DA, Reid-Lombardo KM, Gordon SR, Gardner TB. The clinical relevance of the increasing incidence of intraductal papillary mucinous neoplasm. Clin Gastroenterol Hepatol. 2012;10:555-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 3. | Attiyeh MA, Fernández-Del Castillo C, Al Efishat M, Eaton AA, Gönen M, Batts R, Pergolini I, Rezaee N, Lillemoe KD, Ferrone CR, Mino-Kenudson M, Weiss MJ, Cameron JL, Hruban RH, D'Angelica MI, DeMatteo RP, Kingham TP, Jarnagin WR, Wolfgang CL, Allen PJ. Development and Validation of a Multi-institutional Preoperative Nomogram for Predicting Grade of Dysplasia in Intraductal Papillary Mucinous Neoplasms (IPMNs) of the Pancreas: A Report from The Pancreatic Surgery Consortium. Ann Surg. 2018;267:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 113] [Article Influence: 14.1] [Reference Citation Analysis (1)] |
| 4. | Wasif N, Bentrem DJ, Farrell JJ, Ko CY, Hines OJ, Reber HA, Tomlinson JS. Invasive intraductal papillary mucinous neoplasm versus sporadic pancreatic adenocarcinoma: a stage-matched comparison of outcomes. Cancer. 2010;116:3369-3377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 5. | Tanaka M, Fernández-Del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T, Salvia R, Shimizu Y, Tada M, Wolfgang CL. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017;17:738-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 868] [Cited by in RCA: 1157] [Article Influence: 144.6] [Reference Citation Analysis (1)] |
| 6. | Pusateri AJ, Krishna SG. Pancreatic Cystic Lesions: Pathogenesis and Malignant Potential. Diseases. 2018;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Nakamura M, Miyasaka Y, Sadakari Y, Date K, Ohtsuka T. Comparison of guidelines for intraductal papillary mucinous neoplasm: What is the next step beyond the current guidelines? Ann Gastroenterol Surg. 2017;1:90-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Basar O, Brugge WR. My Treatment Approach: Pancreatic Cysts. Mayo Clin Proc. 2017;92:1519-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Yopp AC, Allen PJ. Prognosis of invasive intraductal papillary mucinous neoplasms of the pancreas. World J Gastrointest Surg. 2010;2:359-362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Rong Y, Wang D, Xu C, Ji Y, Jin D, Wu W, Xu X, Kuang T, Lou W. Prognostic value of histological subtype in intraductal papillary mucinous neoplasm of the pancreas: A retrospective analysis of outcome from one single center. Medicine (Baltimore). 2017;96:e6599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Murakami Y, Uemura K, Sudo T, Hayashidani Y, Hashimoto Y, Nakashima A, Sueda T. Invasive intraductal papillary-mucinous neoplasm of the pancreas: comparison with pancreatic ductal adenocarcinoma. J Surg Oncol. 2009;100:13-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Song W, Lv CG, Miao DL, Zhu ZG, Wu Q, Wang YG, Chen L. Development and validation of a nomogram for predicting survival in patients with gastrointestinal stromal tumours. Eur J Surg Oncol. 2018;44:1657-1665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 13. | Boero IJ, Paravati AJ, Hou J, Gillespie EF, Schoenbrunner A, Unkart J, Wallace AM, Einck JP, Mell LK, Murphy JD. The Impact of Surgeons on the Likelihood of Mastectomy in Breast Cancer. Ann Surg. 2019;269:951-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Kloppel G, Solcia E, Longnecker DS, Capella C, Sobin LH. Histological typing of tumours of the exocrine pancreas. 2nd edition. Berlin: Springer, 1996: 7-9. |
| 15. | Riall TS, Stager VM, Nealon WH, Townsend CM, Kuo YF, Goodwin JS, Freeman JL. Incidence of additional primary cancers in patients with invasive intraductal papillary mucinous neoplasms and sporadic pancreatic adenocarcinomas. J Am Coll Surg. 2007;204:803-13; discussion 813-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Gaitanidis A, Alevizakos M, Tsaroucha A, Tsalikidis C, Simopoulos C, Pitiakoudis M. Conditional survival analysis for patients with intraductal papillary mucinous neoplasms (IPMNs) undergoing curative resection. Eur J Surg Oncol. 2018;44:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Yang J, Tian G, Pan Z, Zhao F, Feng X, Liu Q, Lyu J. Nomograms for predicting the survival rate for cervical cancer patients who undergo radiation therapy: a SEER analysis. Future Oncol. 2019;15:3033-3045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Thomas LE, O'Brien EC, Piccini JP, D'Agostino RB, Pencina MJ. Application of net reclassification index to non-nested and point-based risk prediction models: a review. Eur Heart J. 2019;40:1880-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 19. | Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157-72; discussion 207-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4325] [Cited by in RCA: 5066] [Article Influence: 298.0] [Reference Citation Analysis (0)] |
| 20. | Alba AC, Agoritsas T, Walsh M, Hanna S, Iorio A, Devereaux PJ, McGinn T, Guyatt G. Discrimination and Calibration of Clinical Prediction Models: Users' Guides to the Medical Literature. JAMA. 2017;318:1377-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 632] [Cited by in RCA: 1051] [Article Influence: 131.4] [Reference Citation Analysis (1)] |
| 21. | Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26:565-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3515] [Cited by in RCA: 3481] [Article Influence: 183.2] [Reference Citation Analysis (1)] |
| 22. | Vickers AJ, Cronin AM, Elkin EB, Gonen M. Extensions to decision curve analysis, a novel method for evaluating diagnostic tests, prediction models and molecular markers. BMC Med Inform Decis Mak. 2008;8:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 596] [Cited by in RCA: 993] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 23. | Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB, Schmidt CM, Shimizu M, Wolfgang CL, Yamaguchi K, Yamao K; International Association of Pancreatology. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1714] [Cited by in RCA: 1614] [Article Influence: 124.2] [Reference Citation Analysis (0)] |
| 24. | Vege SS, Ziring B, Jain R, Moayyedi P; Clinical Guidelines Committee; American Gastroenterology Association. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148:819-22; quize12-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 629] [Cited by in RCA: 759] [Article Influence: 75.9] [Reference Citation Analysis (1)] |
| 25. | Del Chiaro M, Verbeke C, Salvia R, Klöppel G, Werner J, McKay C, Friess H, Manfredi R, Van Cutsem E, Löhr M, Segersvärd R; European Study Group on Cystic Tumours of the Pancreas. European experts consensus statement on cystic tumours of the pancreas. Dig Liver Dis. 2013;45:703-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 334] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 26. | Worni M, Akushevich I, Gloor B, Scarborough J, Chino JP, Jacobs DO, Hahn SM, Clary BM, Pietrobon R, Shah A. Adjuvant radiotherapy in the treatment of invasive intraductal papillary mucinous neoplasm of the pancreas: an analysis of the surveillance, epidemiology, and end results registry. Ann Surg Oncol. 2012;19:1316-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Yogi T, Hijioka S, Imaoka H, Mizuno N, Hara K, Tajika M, Tanaka T, Ishihara M, Shimizu Y, Hosoda W, Yatabe Y, Niwa Y, Yoshimura K, Bhatia V, Fujita J, Yamao K. Risk factors for postoperative recurrence of intraductal papillary mucinous neoplasms of the pancreas based on a long-term follow-up study: proposals for follow-up strategies. J Hepatobiliary Pancreat Sci. 2015;22:757-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | Shen W, Sakamoto N, Yang L. Prognostic models and nomograms for predicting survival of patients with maxillary sinus carcinomas. Int Forum Allergy Rhinol. 2017;7:741-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Wang XD, Qian JJ, Bai DS, Li ZN, Jiang GQ, Yao J. Marital status independently predicts pancreatic cancer survival in patients treated with surgical resection: an analysis of the SEER database. Oncotarget. 2016;7:24880-24887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 30. | Wu Q, Wang WJ, Huang YQ, Fang SY, Guan YJ. Nomograms for estimating survival in patients with liver-only colorectal metastases: A retrospective study. Int J Surg. 2018;60:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Marchegiani G, Andrianello S, Dal Borgo C, Secchettin E, Melisi D, Malleo G, Bassi C, Salvia R. Adjuvant chemotherapy is associated with improved postoperative survival in specific subtypes of invasive intraductal papillary mucinous neoplasms (IPMN) of the pancreas: it is time for randomized controlled data. HPB (Oxford). 2019;21:596-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 32. | Marchegiani G, Mino-Kenudson M, Ferrone CR, Morales-Oyarvide V, Warshaw AL, Lillemoe KD, Castillo CF. Patterns of Recurrence After Resection of IPMN: Who, When, and How? Ann Surg. 2015;262:1108-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 33. | Sereni E, Luchini C, Salvia R, Pea A. Molecular and clinical patterns of local progression in the pancreatic remnant following resection of pancreatic intraductal papillary mucinous neoplasm (IPMN). Chin Clin Oncol. 2019;8:21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 34. | Canto MI, Almario JA, Schulick RD, Yeo CJ, Klein A, Blackford A, Shin EJ, Sanyal A, Yenokyan G, Lennon AM, Kamel IR, Fishman EK, Wolfgang C, Weiss M, Hruban RH, Goggins M. Risk of Neoplastic Progression in Individuals at High Risk for Pancreatic Cancer Undergoing Long-term Surveillance. Gastroenterology. 2018;155:740-751.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 305] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 35. | Panic N, Macchini F, Solito S, Boccia S, Leoncini E, Larghi A, Berretti D, Pevere S, Vadala S, Marino M, Zilli M, Bulajic M. Prevalence of Extrapancreatic Malignancies Among Patients With Intraductal Papillary Mucinous Neoplasms of the Pancreas. Pancreas. 2018;47:721-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Kawakubo K, Tada M, Isayama H, Sasahira N, Nakai Y, Takahara N, Miyabayashi K, Yamamoto K, Mizuno S, Mohri D, Kogure H, Sasaki T, Yamamoto N, Tateishi R, Hirano K, Ijichi H, Tateishi K, Koike K. Risk for mortality from causes other than pancreatic cancer in patients with intraductal papillary mucinous neoplasm of the pancreas. Pancreas. 2013;42:687-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 37. | Wang X, Hong X, Pang H, Dai H, You L, Wu W, Zhao Y. Selecting optimal surgical procedures for intraductal papillary mucinous neoplasm (IPMN): An analysis based on the Surveillance, Epidemiology, and End Result registry database. Eur J Surg Oncol. 2016;42:1526-1532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 38. | Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26:1364-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1306] [Cited by in RCA: 2307] [Article Influence: 135.7] [Reference Citation Analysis (0)] |
| 39. | Rouprêt M, Hupertan V, Seisen T, Colin P, Xylinas E, Yates DR, Fajkovic H, Lotan Y, Raman JD, Zigeuner R, Remzi M, Bolenz C, Novara G, Kassouf W, Ouzzane A, Rozet F, Cussenot O, Martinez-Salamanca JI, Fritsche HM, Walton TJ, Wood CG, Bensalah K, Karakiewicz PI, Montorsi F, Margulis V, Shariat SF; French National Database on Upper Tract Tumors; Upper Tract Urothelial Carcinoma Collaboration. Prediction of cancer specific survival after radical nephroureterectomy for upper tract urothelial carcinoma: development of an optimized postoperative nomogram using decision curve analysis. J Urol. 2013;189:1662-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |