Published online Feb 7, 2020. doi: 10.3748/wjg.v26.i5.524
Peer-review started: November 19, 2019
First decision: December 12, 2019
Revised: January 6, 2020
Accepted: January 15, 2020
Article in press: January 15, 2020
Published online: February 7, 2020
Processing time: 79 Days and 22.3 Hours
Accelerated therapeutic treatment should be considered in patients with progressive Crohn’s disease (CD) to prevent complications as well as surgery. Therefore, screening for risk factors and predicting the need for early surgery are of great importance in clinical practice.
To establish a model to predict CD-related early surgery.
This was a retrospective study collecting data from CD patients diagnosed at our inflammatory bowel disease center from January 1, 2012 to December 31, 2016. All data were randomly stratified into a training set and a testing set at a ratio of 8:2. Multivariable logistic regression analysis was conducted with receiver operating characteristic curves constructed and areas under the curve calculated. This model was further validated with calibration and discrimination estimated. A nomogram was finally developed.
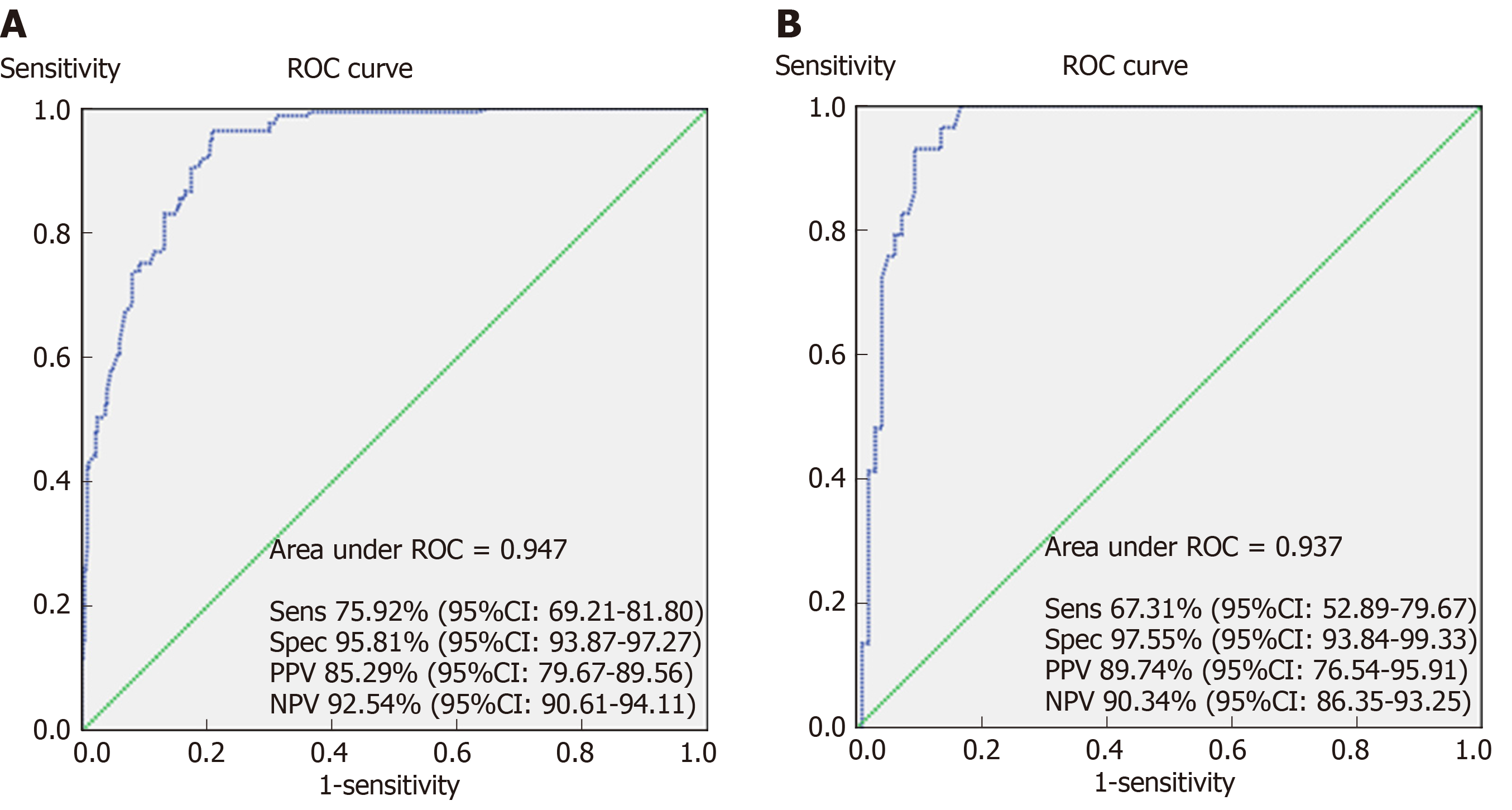
A total of 1002 eligible patients were enrolled with a mean follow-up period of 53.54 ± 13.10 mo. In total, 24.25% of patients received intestinal surgery within 1 year after diagnosis due to complications or disease relapse. Disease behavior (B2: OR [odds ratio] = 6.693, P < 0.001; B3: OR = 14.405, P < 0.001), smoking (OR = 4.135, P < 0.001), body mass index (OR = 0.873, P < 0.001) and C-reactive protein (OR = 1.022, P = 0.001) at diagnosis, previous perianal (OR = 9.483, P < 0.001) or intestinal surgery (OR = 8.887, P < 0.001), maximum bowel wall thickness (OR = 1.965, P < 0.001), use of biologics (OR = 0.264, P < 0.001), and exclusive enteral nutrition (OR = 0.089, P < 0.001) were identified as independent significant factors associated with early intestinal surgery. A prognostic model was established and further validated. The receiver operating characteristic curves and calculated areas under the curves (94.7%) confirmed an ideal predictive ability of this model with a sensitivity of 75.92% and specificity of 95.81%. A nomogram was developed to simplify the use of the predictive model in clinical practice.
This prognostic model can effectively predict 1-year risk of CD-related intestinal surgery, which will assist in screening progressive CD patients and tailoring therapeutic management.
Core tip: Predicting the likelihood of Crohn’s disease-related early surgery is of great importance in treatment strategy monitoring. Disease behavior, smoking, body mass index and C-reactive protein level at diagnosis, previous perianal or intestinal surgery, maximum bowel wall thickness, use of biologics, and exclusive enteral nutrition were identified as independent significant factors associated with early intestinal surgery. A validated prognostic model and a nomogram were established to aid clinical practice.
- Citation: Yao JY, Jiang Y, Ke J, Lu Y, Hu J, Zhi M. Development of a prognostic model for one-year surgery risk in Crohn’s disease patients: A retrospective study. World J Gastroenterol 2020; 26(5): 524-534
- URL: https://www.wjgnet.com/1007-9327/full/v26/i5/524.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i5.524
There has been a sharp rise in the prevalence of Crohn’s disease (CD) in Asian countries over the last decade[1]. CD patients exhibit heterogenous disease progression. Some patients have quiescent CD[2,3], which remains stable for long periods of time, while others have progressive CD[4-6], and tend to progress to complex complications such as intestinal stenosis or perforation requiring surgery within a year after diagnosis. It is of extreme importance to be able to accurately characterize the differences in disease progression among patients and institute targeted accelerated therapy as necessary.
According to published research, disabling CD is associated with factors such as age less than 40 years old[7], perianal disease[8,9], initial steroid requirement[10,11], and involvement of the upper gastrointestinal tract[10]. Population-based cohort studies have reported that 37%-61% of CD patients require surgery within 10 years after diagnosis, 43%-57% of which were identified as having disabling disease[12-14]. Progressive CD patients have a higher rate of surgery and shorter interval between diagnosis and surgery[15,16]. However, with the emergence of “treat-to-target” theory and widespread application of biologics, factors predicting early CD-related intestinal surgery and progressive CD have varied. Until recently, prognostic models for progressive CD have been lacking. Moreover, region-specific data is necessary, given that disease phenotypes differ among regions and races. Therefore, we conducted a retrospective cohort study aiming to establish a prognostic model to predict the likelihood of intestinal surgery at 1 year after diagnosis, with an aim to guide targeted accelerated therapy for patients at high risk of progressive CD.
This was a retrospective cohort study collecting data from 1203 CD patients from the inflammatory bowel disease (IBD) center of the Sixth Affiliated Hospital of Sun Yat-Sen University from January 1, 2012 to December 31, 2016. All patients were followed until September 30, 2019 with an average follow-up period of 53 mo and a minimum follow-up period of 32 mo. In total, 201 patients were excluded from the analysis due to incomplete data, loss to follow-up, or death. This study was approved by the ethics committee of Sun Yat-Sen University (2019ZSLYEC-058) and was registered in the Chinese Clinical Trial Registry (ChiCTR1900025751).
Diagnosis of CD was made based on a combination of radiographic, endoscopic, and histologic criteria[17]. Disease phenotype was classified according to the Montreal classification[18]. Serum biomarkers such as C-reactive protein, erythrocyte sedimentation rate, and albumin were collected at the time of diagnosis. Intestinal stenosis was defined by luminal narrowing as well as prestenotic dilation evident on radiologic examinations, or intestinal narrowing which was unable to be passed by endoscope. Penetrating disease was defined as the presence of an abscess or fistula. Perianal disease included perianal fistulas and abscesses. Early abdominal surgery was defined as bowel resection or stoma creation within 1 year after diagnosis due to complications or disease relapse. Therapeutic treatment was categorized as follows: 5-aminosalicylates (oral and/or topical use), corticosteroids (systematic and/or topical use), immunosuppressants (azathioprine, 6-mercaptopurine, methotrexate, cyclophosphamide, or thalidomide), biologics (infliximab or adalimumab), and surgery (abdominal surgery or perianal surgery). Smoking was defined as daily consumption of more than 10 cigarettes for more than 1 year. Alcohol intake was dichotomized around a cut-off of 80 grams of ethanol a day for at least 1 year. Bowel sonography was performed by two experienced gastroenterologists who were blinded to the other procedures. The entire intestinal tract was scanned and bowel wall thickness (BWT) measured in both the longitudinal and transverse slices. A BWT ≥ 4 mm was considered abnormal.
Continuous variables are presented as the mean ± SD or median ± interquartile range, while categorical variables are presented as percentages or proportions. Continuous variables were analyzed using Student’s t-tests or paired t-tests as appropriate, while categorical variables were analyzed using the chi-square or Fisher’s exact test. We randomly stratified all the data into a training set and a testing set at a ratio of 8:2 using a random seed of 666. Multivariable logistic regression analysis was conducted to establish a forward stepwise model with receiver operating characteristic curves constructed and areas under the curve (AUC) calculated. The results were then confirmed using a backward elimination procedure. The model was eventually validated with calibration using the Hosmer-Lemeshow goodness-of-fit test, and discrimination was assessed using AUC. A nomogram was established using R software (R Foundation for Statistical Computing, Vienna, Austria). A two-tail P value < 0.05 was considered statistically significant. Analyses were performed using IBM SPSS (version 22.0, IBM Corp., Armonk, NY, United States). The statistical methods used in this study were reviewed by Jinxin Zhang from the Department of Medical Statistics, Sun Yat-Sen University.
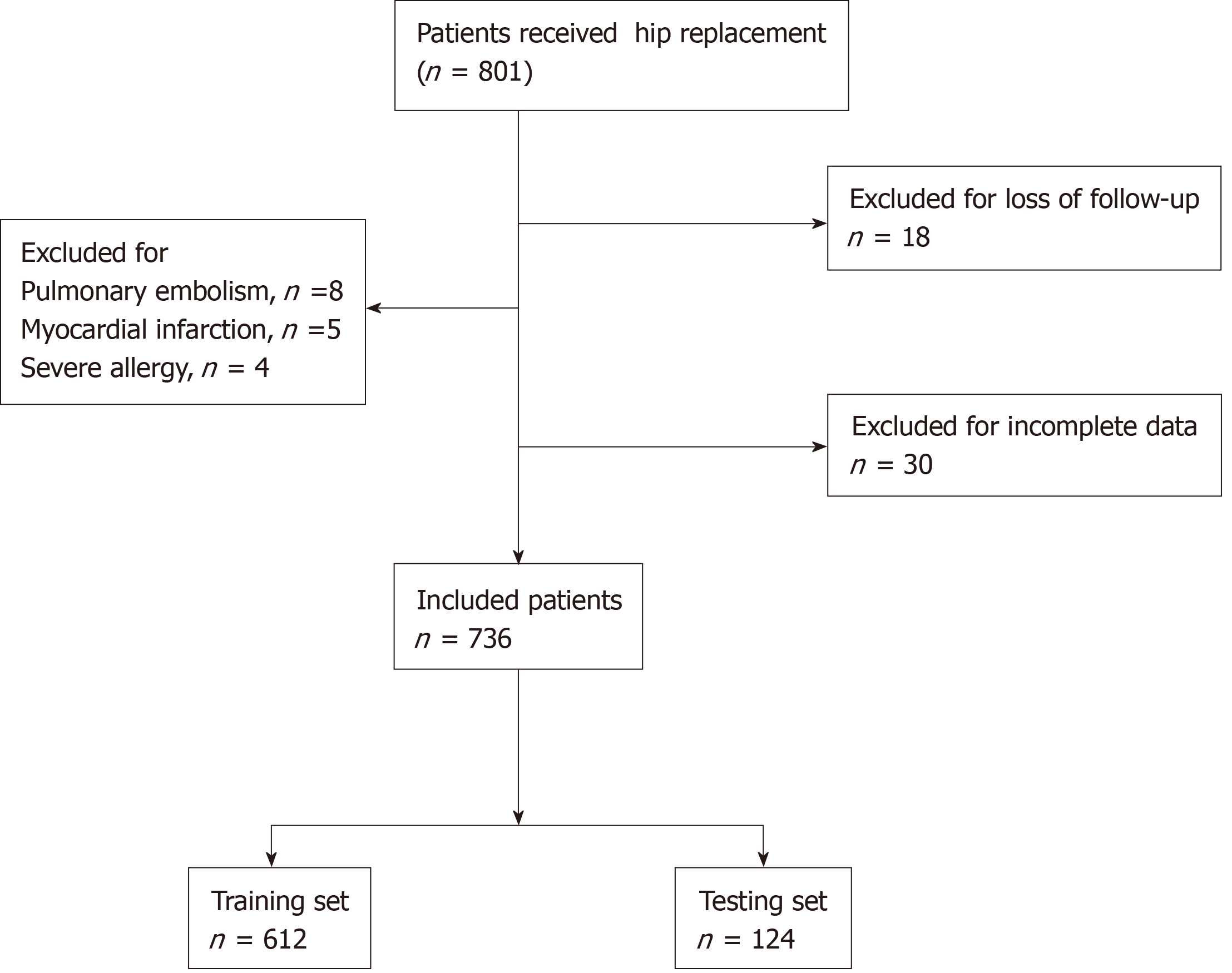
A total of 1203 patients with a confirmed diagnosis of CD were enrolled in our study. Of these, 201 (16.7%) patients were excluded for the sake of incomplete data (n = 42, 20.9%), loss to follow-up (n = 156, 77.6%), or death (n = 3, 1.5%). Causes of death included severe infection associated with bone marrow suppression (n = 2, 66.7%) and cardiac arrest (n = 1, 33.3%). Of the enrolled patients, 73.65% were male (n = 738) (Figure 1), and the mean age at diagnosis was 28.41 ± 11.05 years. The mean follow-up period was 53.54 ± 13.10 mo with a maximum follow-up time of 81 mo.
According to the Montreal classification, the majority of patients were classified as A2 (A1, n = 119, 11.88%; A2, n = 744, 74.25%; A3, n = 139, 13.87%), L3 (L1, n = 145, 14.47%; L2, n = 104, 10.38%; L3, n = 678, 67.66%; L4, n = 75, 7.49%), and B1 (B1, n = 614, 61.28%; B2, n = 185, 18.46%; B3, n = 203, 20.26%). In this cohort, 40.82% (n = 409) of patients had previous CD-related intestinal surgery, while 29.84% (n = 299) had previous perianal surgery. The main therapies included corticosteroids (n = 529, 52.79%), immunosuppressants (n = 746, 74.45%), biologics (n = 462, 46.11%), and exclusive enteral nutrition (n = 230, 22.95%), whereas relatively few patients were receiving 5-aminosalicylates (n = 202, 20.16%). Throughout the study period, 12.87% patients developed complications, including stenosis (n = 129, 12.87%), perforation (n = 139, 13.87%), and gastrointestinal bleeding (n = 19, 1.90%). Nearly a quarter (n = 243, 24.25%) of patients received intestinal surgery within 1 year. Detailed information regarding patient characteristics is listed in Table 1.
| Characteristic | n (%)/mean ± SD |
| Male/female | 738/264 (73.65/26.35) |
| Age at diagnosis (yr) | 28.41 ± 11.05 |
| Drinking/not drinking | 58/944 (5.79/94.21) |
| Smoking/not smoking | 150/852 (14.97/85.03) |
| Body mass index at diagnosis (kg/m2) | 18.58 ± 2.90 |
| Year of follow-up (mo) | 53.54 ± 13.10 |
| Montreal classification | |
| Age at diagnosis (%) | |
| A1 | 119 (11.88) |
| A2 | 744 (74.25) |
| A3 | 139 (13.87) |
| Location at diagnosis | |
| L1 | 145 (14.47) |
| L2 | 104 (10.38) |
| L3 | 678 (67.66) |
| L4 | 75 (7.49) |
| Behavior at diagnosis | |
| B1 | 614 (61.28) |
| B2 | 185 (18.46) |
| B3 | 203 (20.26) |
| CRP at diagnosis | 19.84 ± 24.25 |
| ESR at diagnosis | 34.58 ± 28.48 |
| Alb at diagnosis | 39.14 ± 25.65 |
| Previous perianal surgery | 299 (29.84) |
| Previous intestinal surgery | 409 (40.82) |
| Complication | 287 (28.64) |
| Stenosis | 129 (12.87) |
| Perforation | 139 (13.87) |
| GI bleeding | 19 (1.90) |
| Treatment | |
| 5-aminosalicylic acid | 202 (20.16) |
| Immunosuppressants | 746 (74.45) |
| Corticosteroids | 529 (52.79) |
| Biologics | 462 (46.11) |
| EEN | 230 (22.95) |
| Surgery within 1 yr after diagnosis | 243(24.25) |
| Surgery within the follow-up period | 473 (47.21) |
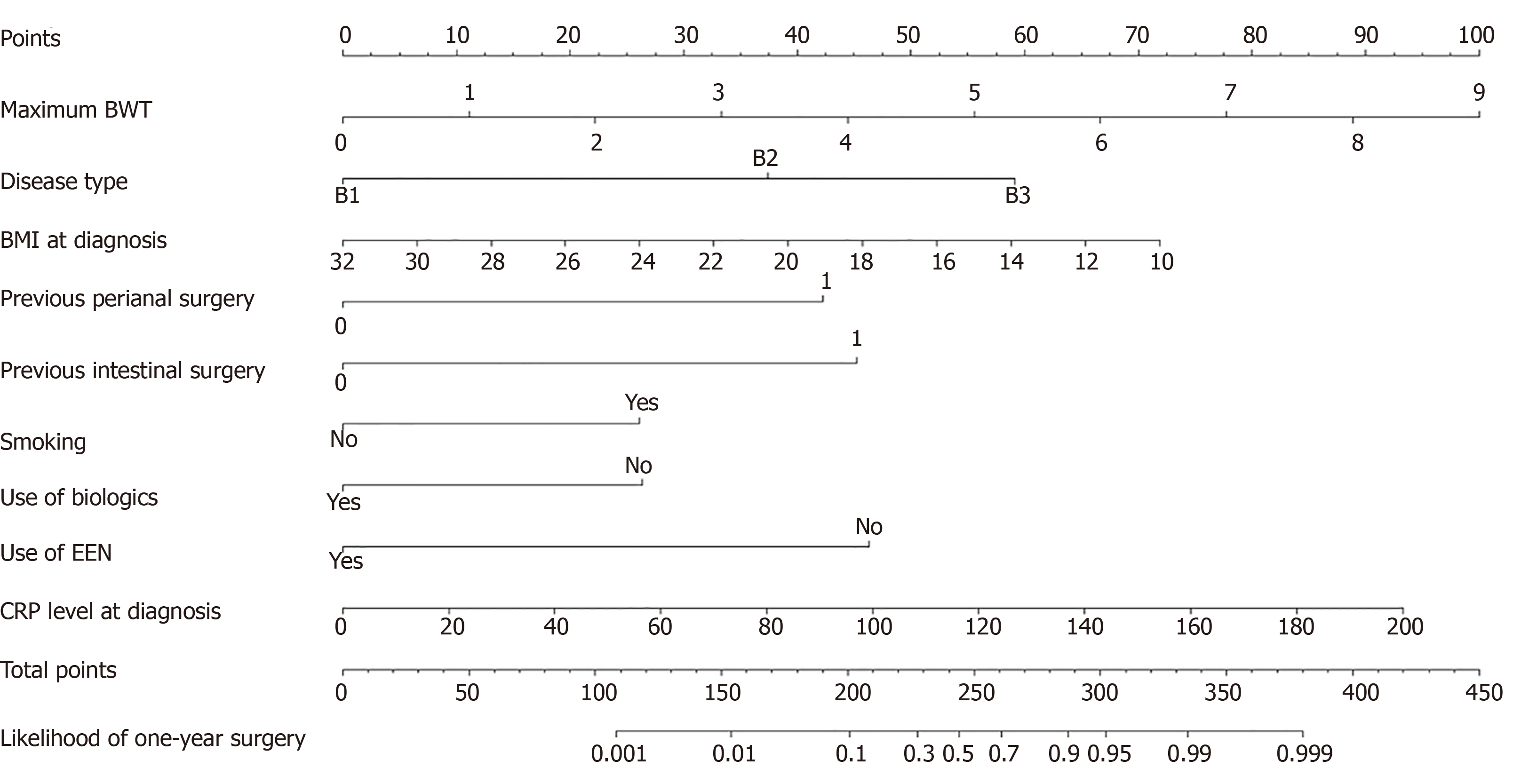
According to univariate analysis, disease behavior, smoking, body mass index (BMI) and C-reactive protein (CRP) at diagnosis, previous perianal and intestinal surgery, use of biologics, exclusive enteral nutrition, and maximum BWT were significantly associated with early intestinal surgery. The abovementioned factors were evaluated in a multivariable logistic regression analysis, and a prognostic model was established, as shown in Figure 2 [X1 = Maximum BWT (mm); X2 = Smoking (0: No, 1: Yes); X3 = BMI at diagnosis (m/kg2); X4 = Previous perianal surgery (0: No, 1: Yes); X5 = Previous intestinal surgery (0: No, 1: Yes); X6 = Disease type (stricturing or penetrating disease); X7 = Use of biologics; X8 = Use of exclusive enteral nutrition; X9 = CRP at diagnosis (Table 2)].
| Factor | Univariate analysis | Multivariable logistic regression | ||||
| OR | 95%CI | P | OR | 95%CI | P | |
| Gender (male vs female) | 0.793 | 0.565-1.113 | 0.180 | |||
| Age (yr) | 1.012 | 0.999-1.025 | 0.070 | |||
| Location (L1 vs L2/L3/L4) | ||||||
| L2 | 0.729 | 0.401-1.324 | 0.300 | |||
| L3 | 0.853 | 0.568-1.282 | 0.445 | |||
| L4 | 0.988 | 0.526-1.855 | 0.971 | |||
| Behavior (B1 vs B2/B3) | ||||||
| B2 | 5.771 | 3.837-8.680 | < 0.001 | 6.693 | 3.437-13.032 | < 0.001 |
| B3 | 14.998 | 10.113-22.242 | < 0.001 | 14.405 | 7.208-28.790 | < 0.001 |
| Maximum BWT | 1.391 | 1.278-1.514 | < 0.001 | 1.965 | 1.660-2.327 | < 0.001 |
| Drinking | 1.702 | 0.970-2.985 | 0.064 | |||
| Smoking | 4.359 | 3.034-6.262 | < 0.001 | 4.135 | 2.149-7.953 | < 0.001 |
| BMI | 0.901 | 0.854-0.950 | < 0.001 | 0.873 | 0.786-0.968 | 0.01 |
| Previous perianal surgery | 6.776 | 4.943-9.288 | < 0.001 | 9.483 | 5.317-16.912 | < 0.001 |
| Previous intestinal surgery | 5.199 | 3.794-7.125 | < 0.001 | 8.887 | 5.045-15.656 | < 0.001 |
| 5-aminosalicylic acid use | 0.709 | 0.485-1.038 | 0.077 | |||
| Immunosuppressants use | 0.974 | 0.700-1.355 | 0.877 | |||
| Corticosteroid use | 0.931 | 0.697-1.243 | 0.627 | |||
| Biologics use | 0.283 | 0.204-0.392 | < 0.001 | 0.264 | 0.146-0.476 | < 0.001 |
| Exclusive enteral nutrition use | 0.359 | 0.235-0.550 | < 0.001 | 0.089 | 0.038-0.205 | < 0.001 |
| CRP at diagnosis (pathological vs normal) | 1.020 | 1.014-1.026 | < 0.001 | 1.022 | 1.009-1.036 | 0.001 |
| ESR at diagnosis (pathological vs normal) | 1.005 | 1.000-1.010 | 0.058 | |||
| Alb at diagnosis (pathological vs normal) | 0.981 | 0.962-1.001 | 0.058 | |||
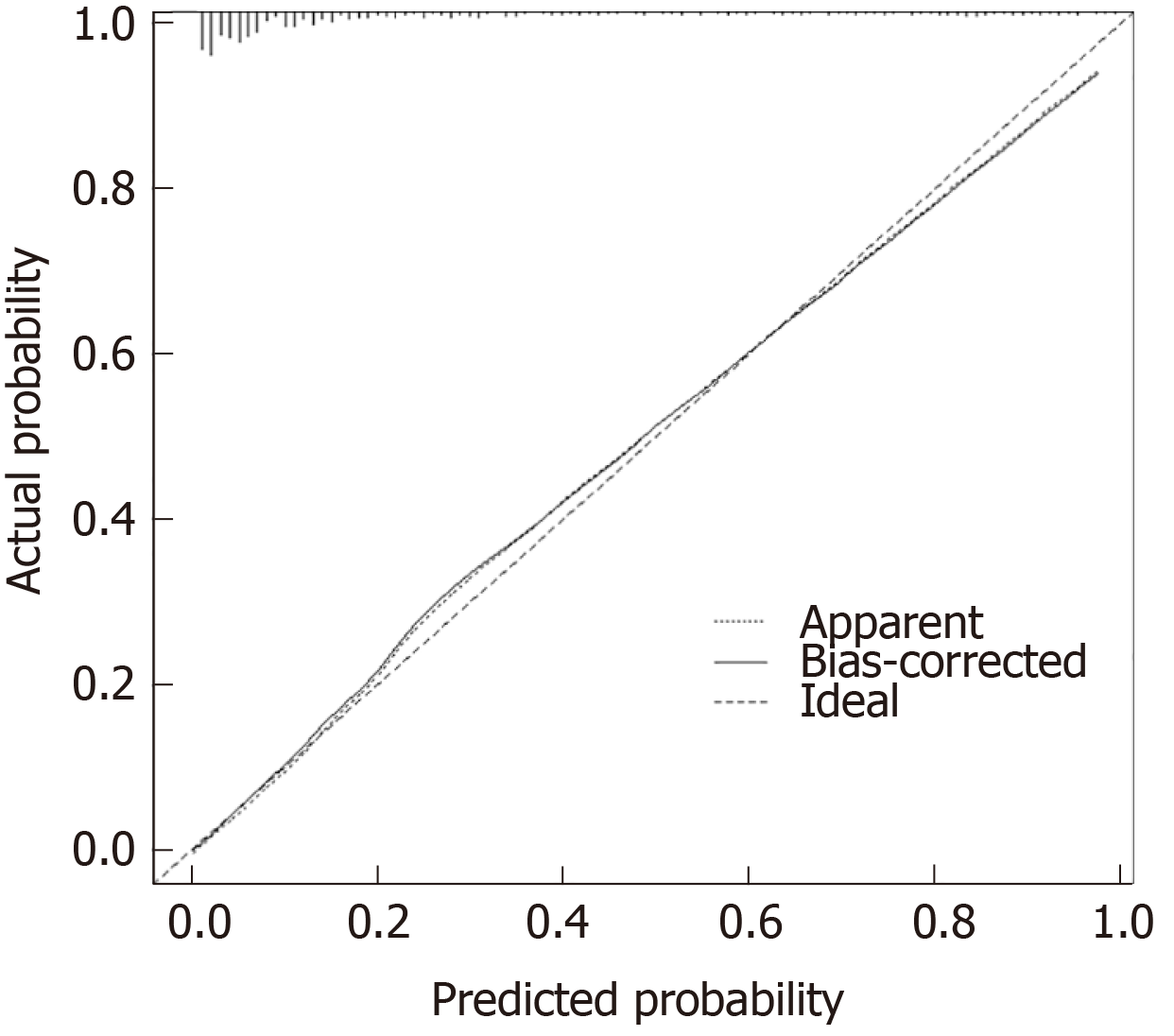
The receiver operating characteristic curve and calculated AUC (94.7%) confirmed an ideal predictive ability of this model with a sensitivity of 75.92%, specificity of 95.81%, positive predictive value of 85.29%, negative predictive value of 92.54%, positive likelihood ratio of 18.10, and negative likelihood ratio of 0.25 (Figure 3, panel A). The prognostic model was subsequently applied to the validation cohort. Discrimination of the model was acceptable, which confirms a good predictive power and discriminatory ability (Figure 3B). With respect to calibration, this model showed a good fit using the Hosmer-Lemeshow goodness-of-fit test, with a high level of agreement between calculated risk and the observed outcomes (Figure 4).
A nomogram was developed to simplify the use of the predictive model in clinical practice (Figure 5). Probability of early intestinal surgery was easily obtained by calculating the total number of points and matching vertically downward to the risk of surgery at 1 year.
In this retrospective study enrolling 1002 CD patients with a mean follow-up period of 53 mo, we identified factors associated with intestinal surgery at 1 year, as follows: Disease behavior, smoking, BMI at diagnosis, previous perianal surgery, previous intestinal surgery, use of biologics, exclusive enteral nutrition, maximum BWT, and CRP level at diagnosis. We combined all associated factors in a prognostic model and established a nomogram to facilitate convenient use in clinical practice so that clinicians can identify patients at risk of early surgery, and implement more aggressive medical management as required. Further, for patients with a high risk of surgery at 1 year, it may be beneficial to consider proactive early surgery, such as to minimize both the duration and expenditure associated with drug therapy.
According to recent cohort studies from Denmark and Canada, 13%, 21%, and 26% of the patients underwent surgical resections after 1, 5, and 10 years, respectively[19]. The rate of intestinal surgery at our IBD center was high (24.25%) for the following reasons: Our IBD center was the largest in China for CD patients and a referral center for the entire country; most referred patients had complications and did not respond to medical therapy; our center was particularly famous for gastrointestinal surgery, and thus, a certain part of patients sought surgical treatment here.
Previous research conducted by Zallot et al[20] suggested that ileal disease and a stricturing phenotype significantly increase the risk of resection. Further, changes in disease location as well as progression in behavior were closely linked to a cumulative higher risk of early surgery, as shown in a population-based inception cohort from Denmark[21]. According to this prognostic model, disease behavior, including stricturing and penetrating phenotypes, was considered to be a risk factor for early intestinal surgery. However, there was no solid evidence to confirm the link between disease location and surgery risk. In our data, the majority of disease locations at diagnosis were L3 (67.66%), followed by L1 (14.47%) and L2 (10.38%), while L4 accounted for less than 1/10 (7.49%) of all cases. Indeed, disease location distribution differed from the previous report on patients in Denmark[21], which might explain why disease location was not a risk factor in our study.
Smoking is known to increase the risk of surgery in CD patients, with a recent study concluding that it approximately doubled the risk of surgery[22]. In our study, we confirmed that smoking exacerbated disease progression and eventually enhanced risk of surgery, as expected based on results from previous studies[23,24]. Further, CRP level at diagnosis, indicative of inflammatory processes, was also associated with early surgery, and BMI as a proxy for nutritional status was negatively correlated with the risk of intestinal surgery. Bhattacharya et al[25] showed that elevated CRP was a predictor of disease relapse as well as exacerbations, based on a clinically quiescent CD cohort, which is comparable to our results. In previous studies, perianal and intestinal surgeries have been associated with a disabling course[7], recurrence[26], and subsequent surgery[27]; such factors were found to be independent predictors of early intestinal surgery in this study.
Transabdominal bowel ultrasound can effectively estimate IBD-associated morphological changes in the bowel wall[28]. Increased BWT has been observed in active CD patients with intestinal inflammatory infiltration, and patients with intestinal stricture or even obstruction[29]. According to our prognostic model, maximum BWT was a significant indicator for early surgery in CD patients, which is consistent with previous research. Since bowel ultrasonography has the advantages of being noninvasive, easily available, patient-friendly, and repeatable, it is widely used in diagnosis, complication screening, and directing treatment.
According to the “treat-to-target” approach, mucosal healing[30], histologic remission[31], and even “deep remission” (clinical, endoscopic, and biomarker normalization)[32] are the therapeutic goals for gastroenterologists. Therefore, tailored treatments should be considered. Previous research has shown that exclusive enteral nutrition has a similar treatment effect as steroids, especially for remission in pediatric CD[33]. Recently, two published studies[34,35] from our IBD center have confirmed the therapeutic effect of exclusive enteral nutrition (both through nasogastric tube and orally) in inducing early clinical remission and mucosal healing in adult CD patients with complications. This study showed that 230 (22.95%) patients had received exclusive enteral nutrition treatment during the follow-up period, 76.10% (n = 175) of which had complications including intestinal fistula, abdominal abscess, and stricture. Use of exclusive enteral nutrition reduced the risk of surgery, and was found to be an independent protective factor in the prognostic model. To our knowledge, this is the first prognostic model using exclusive enteral nutrition.
In the last decade, CD-related surgery rates have declined. At the same time, use of biologics has become widespread, with biologics becoming a leading therapeutic strategy in CD. As reported by Arieira et al[22], biologics prevent phenotype changes and reduce surgery risk in CD patients. In our study, nearly half (45.1%) of the patients had received biologic therapy during the follow-up period, the percentage of which was larger than previous studies. This was because most patients hospitalized in our IBD center had moderate-to-severe active CD with a disabling course, complex complications, or steroid resistance. With treatment goals progressing to deep remission and biologics becoming significantly cheaper to access via national medical insurance coverage, it is expected that the use of biologics will increase. In our cohort, we confirmed that use of biologics was a protective factor for early surgery, in keeping with previous studies[22,36,37].
The strength of our study was the relatively large number of CD patients with a confirmed diagnosis and complete follow-up data. Our clinical center is the biggest IBD center in China, with a strong team composed of gastroenterologists, pathologists, radiologists, and gastrointestinal surgeons. Given the size of our cohort and center, it is possible that the cohort presented here somewhat approximates the national cohort. Further, development of the nomogram, a simplified visual tool predicting likelihood of surgery at 1 year, will allow for easy incorporation of our results into clinical practice.
A number of limitations are present in this study. This was a retrospective single-center study, lacking data from different regions of China. Clinical activity scores, endoscopy estimation, and genetic markers were not included in our prognostic model. Future studies with a broader population base and more associated factors are needed to confirm our findings.
To summarize, we have identified that smoking, CRP at diagnosis, previous perianal and intestinal surgery, disease behavior, and maximum BWT are independent risk factors for intestinal surgery at 1 year, while BMI, use of biologics, and exclusive enteral nutrition are protective factors. A prognostic model and nomogram have been established to facilitate clinical application, serving as the basis for tailored therapy.
Patients with progressive Crohn’s disease (CD) should be given accelerated therapy.
Predicting CD-related early surgery risk is challenging and important in treatment strategy monitoring.
This study aimed to establish a model to predict CD-related early surgery.
This was a retrospective study collecting data from CD patients from January 1, 2012 to December 31, 2016. A prognostic model was established and further validated. A nomogram was developed to facilitate clinical practice.
A total of 1002 eligible patients were enrolled, and 24.25% received intestinal surgery within 1 year after diagnosis. Disease behavior, smoking, body mass index and C-reactive protein level at diagnosis, previous perianal or intestinal surgery, maximum bowel wall thickness, use of biologics, and exclusive enteral nutrition were identified as independent significant factors associated with early intestinal surgery.
This prognostic model can effectively predict CD-related early surgery, serving as the basis for tailored therapy.
Future studies with a broader population base and more associated factors including endoscopy estimation and genetic markers are needed to perfect this model.
The authors thank Professor Xiao-Ming He from the Department of Nutrition Sciences, University of Alabama at Birmingham (Birmingham, USA) for her assistance in improving the language.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dutta A, Mijandrusic-Sincic B, Rostami-Nejad M S-Editor: Zhang L L-Editor: Wang TQ E-Editor: Ma YJ
| 1. | Vegh Z, Kurti Z, Lakatos PL. Epidemiology of inflammatory bowel diseases from west to east. J Dig Dis. 2017;18:92-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 2. | Horta D, Lira A, Sanchez-Lloansi M, Villoria A, Teggiachi M, García-Rojo D, García-Molina S, Figuerola A, Esteve M, Calvet X. A Prospective Pilot Randomized Study: Electroacupuncture vs. Sham Procedure for the Treatment of Fatigue in Patients with Quiescent Inflammatory Bowel Disease. Inflamm Bowel Dis. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Amitai MM, Zarchin M, Lahat A, Yablecovitch D, Neuman S, Levhar N, Klang E, Avidan B, Ben-Horin S, Eliakim R, Kopylov U; Israeli IBD research Nucleus (IIRN). Structural bowel damage in quiescent Crohn's disease. Dig Liver Dis. 2017;49:490-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Rispo A, Imperatore N, Testa A, Bucci L, Luglio G, De Palma GD, Rea M, Nardone OM, Caporaso N, Castiglione F. Combined Endoscopic/Sonographic-based Risk Matrix Model for Predicting One-year Risk of Surgery: A Prospective Observational Study of a Tertiary Centre Severe/Refractory Crohn's Disease Cohort. J Crohns Colitis. 2018;12:784-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 5. | Forcione DG, Rosen MJ, Kisiel JB, Sands BE. Anti-Saccharomyces cerevisiae antibody (ASCA) positivity is associated with increased risk for early surgery in Crohn's disease. Gut. 2004;53:1117-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 106] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Nahon S, Lahmek P, Paupard T, Lesgourgues B, Chaussade S, Peyrin-Biroulet L, Abitbol V. Diagnostic Delay Is Associated with a Greater Risk of Early Surgery in a French Cohort of Crohn's Disease Patients. Dig Dis Sci. 2016;61:3278-3284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Beaugerie L, Seksik P, Nion-Larmurier I, Gendre JP, Cosnes J. Predictors of Crohn's disease. Gastroenterology. 2006;130:650-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 630] [Cited by in RCA: 639] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 8. | Solberg IC, Vatn MH, Høie O, Stray N, Sauar J, Jahnsen J, Moum B, Lygren I; IBSEN Study Group. Clinical course in Crohn's disease: results of a Norwegian population-based ten-year follow-up study. Clin Gastroenterol Hepatol. 2007;5:1430-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 526] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 9. | Lakatos PL, Czegledi Z, Szamosi T, Banai J, David G, Zsigmond F, Pandur T, Erdelyi Z, Gemela O, Papp J, Lakatos L. Perianal disease, small bowel disease, smoking, prior steroid or early azathioprine/biological therapy are predictors of disease behavior change in patients with Crohn's disease. World J Gastroenterol. 2009;15:3504-3510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 92] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Romberg-Camps MJ, Dagnelie PC, Kester AD, Hesselink-van de Kruijs MA, Cilissen M, Engels LG, Van Deursen C, Hameeteman WH, Wolters FL, Russel MG, Stockbrügger RW. Influence of phenotype at diagnosis and of other potential prognostic factors on the course of inflammatory bowel disease. Am J Gastroenterol. 2009;104:371-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 234] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 11. | Loly C, Belaiche J, Louis E. Predictors of severe Crohn's disease. Scand J Gastroenterol. 2008;43:948-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 220] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 12. | Peyrin-Biroulet L, Harmsen WS, Tremaine WJ, Zinsmeister AR, Sandborn WJ, Loftus EV. Surgery in a population-based cohort of Crohn's disease from Olmsted County, Minnesota (1970-2004). Am J Gastroenterol. 2012;107:1693-1701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 237] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 13. | Munkholm P, Langholz E, Davidsen M, Binder V. Intestinal cancer risk and mortality in patients with Crohn's disease. Gastroenterology. 1993;105:1716-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 236] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 14. | American Gastroenterological Association. American Gastroenterological Institute Guideline on the Management of Crohn's Disease After Surgical Resection: Clinical Decision Support Tool. Gastroenterology. 2017;152:276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Cushing KC, Mclean R, McDonald KG, Gustafsson JK, Knoop KA, Kulkarni DH, Sartor RB, Newberry RD. Predicting Risk of Postoperative Disease Recurrence in Crohn's Disease: Patients with Indolent Crohn's Disease Have Distinct Whole Transcriptome Profiles at the Time of First Surgery. Inflamm Bowel Dis. 2019;25:180-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Park Y, Cheon JH, Park YL, Ye BD, Kim YS, Han DS, Kim JS, Hong SN, Kim YH, Jeon SR, Kim WH; IBD Study Group of the Korean Association for the Study of Intestinal Diseases (KASID). Development of a Novel Predictive Model for the Clinical Course of Crohn's Disease: Results from the CONNECT Study. Inflamm Bowel Dis. 2017;23:1071-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Gomollón F, Dignass A, Annese V, Tilg H, Van Assche G, Lindsay JO, Peyrin-Biroulet L, Cullen GJ, Daperno M, Kucharzik T, Rieder F, Almer S, Armuzzi A, Harbord M, Langhorst J, Sans M, Chowers Y, Fiorino G, Juillerat P, Mantzaris GJ, Rizzello F, Vavricka S, Gionchetti P; ECCO. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn's Disease 2016: Part 1: Diagnosis and Medical Management. J Crohns Colitis. 2017;11:3-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1585] [Cited by in RCA: 1448] [Article Influence: 181.0] [Reference Citation Analysis (0)] |
| 18. | Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1970] [Cited by in RCA: 2350] [Article Influence: 123.7] [Reference Citation Analysis (2)] |
| 19. | Burr NE, Lord R, Hull MA, Subramanian V. Decreasing Risk of First and Subsequent Surgeries in Patients with Crohn's Disease in England From 1994 through 2013. Clin Gastroenterol Hepatol. 2019;17:2042-2049.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | Zallot C, Peyrin-Biroulet L. Clinical risk factors for complicated disease: how reliable are they? Dig Dis. 2012;30 Suppl 3:67-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Lo B, Vester-Andersen MK, Vind I, Prosberg M, Dubinsky M, Siegel CA, Bendtsen F, Burisch J. Changes in Disease Behaviour and Location in Patients with Crohn's Disease After Seven Years of Follow-Up: A Danish Population-based Inception Cohort. J Crohns Colitis. 2018;12:265-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 22. | Arieira C, Cúrdia Gonçaves T, Dias de Castro F, João Moreira M, Cotter J. Clinical course in Crohn's disease: factors associated with behaviour change and surgery. Scand J Gastroenterol. 2018;53:1222-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | To N, Gracie DJ, Ford AC. Systematic review with meta-analysis: the adverse effects of tobacco smoking on the natural history of Crohn's disease. Aliment Pharmacol Ther. 2016;43:549-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 129] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 24. | Golovics PA, Lakatos L, Mandel MD, Lovasz BD, Vegh Z, Kurti Z, Szita I, Kiss LS, Pandur T, Lakatos PL. Prevalence and predictors of hospitalization in Crohn's disease in a prospective population-based inception cohort from 2000-2012. World J Gastroenterol. 2015;21:7272-7280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Bhattacharya A, Rao BB, Koutroubakis IE, Click B, Vargas EJ, Regueiro M, Schwartz M, Swoger JM, Babichenko D, Hartmann D, Rivers CR, Barrie A, Hashash JG, Dunn MA, Binion DG. Silent Crohn's Disease Predicts Increased Bowel Damage During Multiyear Follow-up: The Consequences of Under-reporting Active Inflammation. Inflamm Bowel Dis. 2016;22:2665-2671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Wolters FL, Russel MG, Sijbrandij J, Ambergen T, Odes S, Riis L, Langholz E, Politi P, Qasim A, Koutroubakis I, Tsianos E, Vermeire S, Freitas J, van Zeijl G, Hoie O, Bernklev T, Beltrami M, Rodriguez D, Stockbrügger RW, Moum B. Phenotype at diagnosis predicts recurrence rates in Crohn's disease. Gut. 2006;55:1124-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 177] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 27. | Solberg IC, Cvancarova M, Vatn MH, Moum B; IBSEN Study Group. Risk matrix for prediction of advanced disease in a population-based study of patients with Crohn's Disease (the IBSEN Study). Inflamm Bowel Dis. 2014;20:60-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 28. | Lied GA, Milde AM, Nylund K, Mujic M, Grimstad T, Hausken T, Gilja OH. Increased wall thickness using ultrasonography is associated with inflammation in an animal model of experimental colitis. Clin Exp Gastroenterol. 2012;5:195-201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Taylor SA, Mallett S, Bhatnagar G, Baldwin-Cleland R, Bloom S, Gupta A, Hamlin PJ, Hart AL, Higginson A, Jacobs I, McCartney S, Miles A, Murray CD, Plumb AA, Pollok RC, Punwani S, Quinn L, Rodriguez-Justo M, Shabir Z, Slater A, Tolan D, Travis S, Windsor A, Wylie P, Zealley I, Halligan S; METRIC study investigators. Diagnostic accuracy of magnetic resonance enterography and small bowel ultrasound for the extent and activity of newly diagnosed and relapsed Crohn's disease (METRIC): a multicentre trial. Lancet Gastroenterol Hepatol. 2018;3:548-558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 175] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 30. | Ben-Horin S, Lahat A, Amitai MM, Klang E, Yablecovitch D, Neuman S, Levhar N, Selinger L, Rozendorn N, Turner D, Chowers Y, Odes S, Schwartz D, Yanai H, Dotan I, Braun T, Haberman Y, Kopylov U, Eliakim R; Israeli IBD Research Nucleus (IIRN). Assessment of small bowel mucosal healing by video capsule endoscopy for the prediction of short-term and long-term risk of Crohn's disease flare: a prospective cohort study. Lancet Gastroenterol Hepatol. 2019;4:519-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 31. | Pai RK, Geboes K. Disease activity and mucosal healing in inflammatory bowel disease: a new role for histopathology? Virchows Arch. 2018;472:99-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Pineton de Chambrun G, Blanc P, Peyrin-Biroulet L. Current evidence supporting mucosal healing and deep remission as important treatment goals for inflammatory bowel disease. Expert Rev Gastroenterol Hepatol. 2016;10:915-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 33. | Gorard DA, Hunt JB, Payne-James JJ, Palmer KR, Rees RG, Clark ML, Farthing MJ, Misiewicz JJ, Silk DB. Initial response and subsequent course of Crohn's disease treated with elemental diet or prednisolone. Gut. 1993;34:1198-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 117] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 34. | Yang Q, Gao X, Chen H, Li M, Wu X, Zhi M, Lan P, Hu P. Efficacy of exclusive enteral nutrition in complicated Crohn's disease. Scand J Gastroenterol. 2017;52:995-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 35. | Chen JM, He LW, Yan T, Guo XF, Hu PJ, Peng JS, Cheng WJ, Li LL, He Q. Oral exclusive enteral nutrition induces mucosal and transmural healing in patients with Crohn's disease. Gastroenterol Rep (Oxf). 2019;7:176-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 36. | Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, Rachmilewitz D, Wolf DC, Olson A, Bao W, Rutgeerts P; ACCENT I Study Group. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002;359:1541-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2987] [Cited by in RCA: 3055] [Article Influence: 132.8] [Reference Citation Analysis (0)] |
| 37. | Magro F, Rodrigues-Pinto E, Coelho R, Andrade P, Santos-Antunes J, Lopes S, Camila-Dias C, Macedo G. Is it possible to change phenotype progression in Crohn's disease in the era of immunomodulators? Predictive factors of phenotype progression. Am J Gastroenterol. 2014;109:1026-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |