Published online Dec 28, 2020. doi: 10.3748/wjg.v26.i48.7664
Peer-review started: July 22, 2020
First decision: September 30, 2020
Revised: October 9, 2020
Accepted: November 29, 2020
Article in press: November 29, 2020
Published online: December 28, 2020
Processing time: 156 Days and 6 Hours
Primary liver cancer includes three subtypes: Hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma (CCA), and combined hepatocellular carcinoma. Patients with primary liver cancer experienced poor prognosis and high mortality, so early detection of liver cancer and improved management of metastases are both key strategies to reduce the death toll from liver cancer. Prostate-specific membrane antigen (PSMA) expression in the tumor-associated neovasculature of nonprostate malignancies including liver cancer has been reported recently, but conclusive evidence of PSMA expression based on the pathological type of liver cancer remains limited.
To study the expression of PSMA in HCC, CCA, and liver cirrhosis.
A total of 446 formalin-fixed paraffin-embedded (FFPE) liver tumor and liver cirrhosis tissue samples were obtained retrospectively from the Pathology Department of Tongji Hospital. Immunohistochemistry was used to detect PSMA expression in these 446 FFPE liver biopsy specimens (213 HCC, 203 CCA, and 30 liver cirrhosis). The tumor compartment and the associated neovascular endothelium were separately analyzed. PSMA expression was examined by two certified pathologists, and the final results were presented in a 4-point scoring system (0-3 points). Correlation between PSMA expression and clinicopathological information was also assessed.
PSMA was expressed primarily in the neovascular endothelium associated with tumors. The positive rate of PSMA staining in HCC was significantly higher than that in CCA (86.8% vs 79.3%; P = 0.001) but was only 6.6% in liver cirrhosis (P = 0.000). HCC cases had more 3-score PSMA staining than CCA had (89/213, 41.8% vs 35/203, 17.2%; P = 0.001). PSMA expression correlated positively with the stage and grade of HCC and CCA. In both liver cancer subtypes, there were more PSMA+ cases in stages III–V diseases than in stages I and II. High staining intensity of PSMA was more frequently observed in liver cancers at high grade and advanced stage. There was no significant association of PSMA expression with sex, age, region, α-fetoprotein, hepatitis B surface antigen, or tumor size in both tumor subtypes.
Neovascular PSMA may be a promising marker to differentiate HCC from liver cirrhosis and a prognostic marker for anti-tumor angiogenesis therapy for HCC.
Core Tip: Immunohistochemistry was used to detect prostate-specific membrane antigen (PSMA) expression in hepatocellular carcinoma (HCC), cholangiocellular carcinoma (CCA), and liver cirrhosis. PSMA is specifically expressed in tumor-associated vasculature in HCC and CCA. The positive rate of PSMA staining in HCC was significantly higher than that in CCA (86.8% vs 79.3%), meanwhile, it was only 6.6% in liver cirrhosis, thus the potential of using PSMA-targeted imaging to distinguish HCC from liver cirrhosis may be true. PSMA expression correlated positively with stage and grade both in HCC and CCA; high staining intensity of PSMA was more frequently observed in liver cancers at high grade and advanced stage.
- Citation: Chen LX, Zou SJ, Li D, Zhou JY, Cheng ZT, Zhao J, Zhu YL, Kuang D, Zhu XH. Prostate-specific membrane antigen expression in hepatocellular carcinoma, cholangiocarcinoma, and liver cirrhosis. World J Gastroenterol 2020; 26(48): 7664-7678
- URL: https://www.wjgnet.com/1007-9327/full/v26/i48/7664.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i48.7664
Primary liver cancer can be categorized according to its pathological characteristics into hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma (CCA), and combined hepatocellular carcinoma (CHC)[1]. HCC accounts for 85%–90% cases of primary liver cancer, which is highly prevalent in China due to the epidemic of chronic hepatitis B. Most patients with primary liver cancer are diagnosed at advanced stages when treatment options are limited and subsequently experience poor prognosis and high mortality[1]. Therefore, early detection of liver cancer as well as improved management of metastases are both critical approaches to reducing the death toll from liver cancer.
Prostate-specific membrane antigen (PSMA), also known as folate hydrolase I or glutamate carboxypeptidase II, is a new biomarker that was initially defined by 7E11 immunoglobulin G monoclonal antibody[2]. PSMA is a 100 kDa transmembrane glycoprotein that can transduce extracellular signals into cytoplasm[3-6]. Originally found to be highly expressed in prostate cancer and high-grade intraepithelial neoplasia of prostate, PSMA has been extensively studied in recent decades for prostate cancer imaging and theranostic applications[7]. For example, a large number of clinical trials have underpinned the advantage of PSMA–targeted radionuclide therapy for metastatic prostate cancer[8].
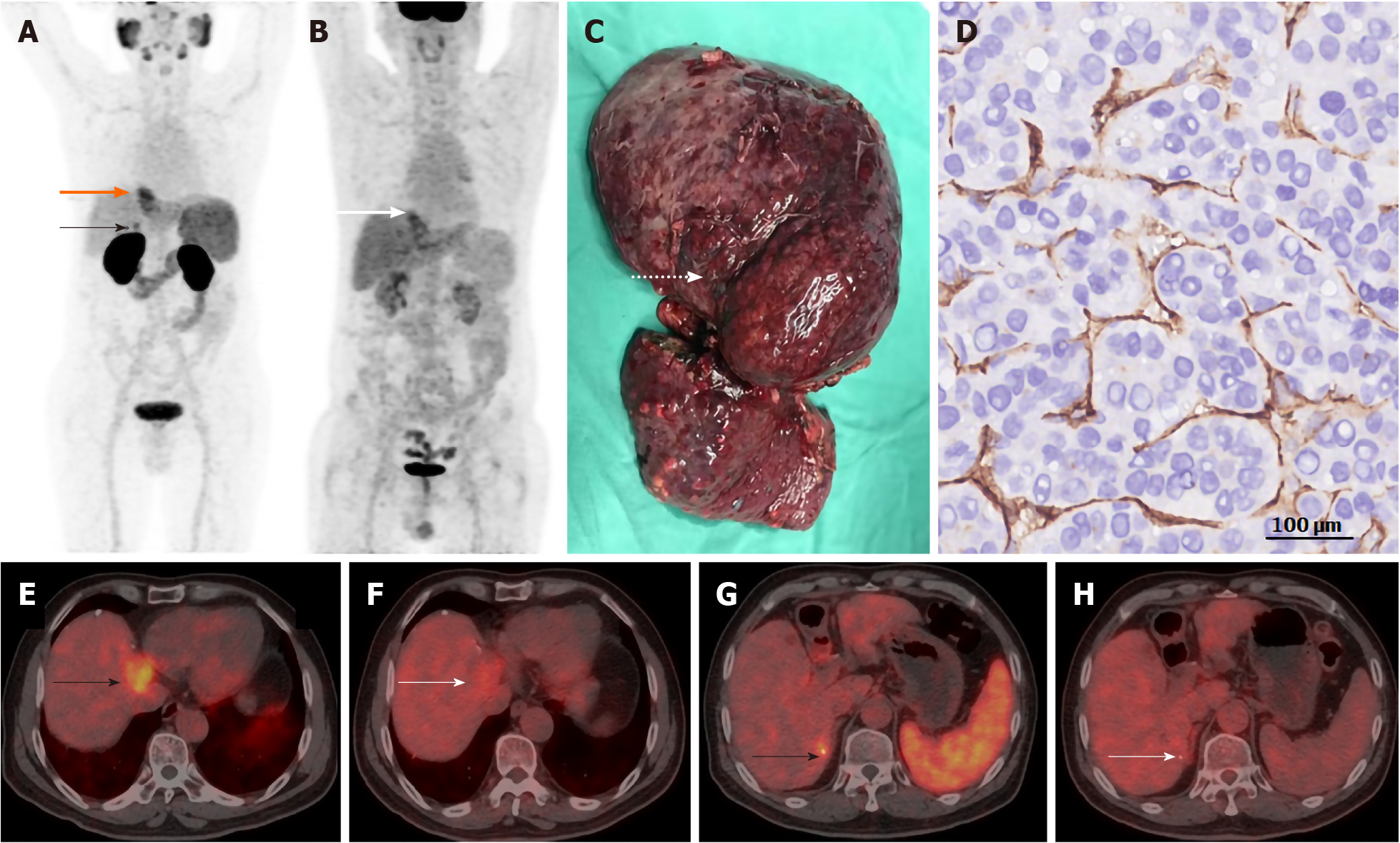
Despite its nomenclature, PSMA expression is also observed in the neovasculature of a wide range of nonprostate cancers, including glioblastoma multiforme; esophageal, gastric, breast, ovarian, colorectal, lung, adrenal, hepatocellular, pancreatic, renal cell, bladder, and testicular germ cell carcinoma; malignant melanoma; mesothelioma tumor and malignant neoplasms of the thyroid[9-26]. Several case reports have shown that HCC, CCA, and CHC have high uptake of radiotracer in PSMA-targeted positron emission tomography (PET) imaging[20-23]. A recent prospective pilot study in seven HCC patients demonstrated that the HCC lesions are hypervascular with 68Ga-PSMA-positive microvessels, suggesting that 68Ga-PSMA PET is more suitable for imaging HCC patients than the conventional 18F-fluorodeoxyglucose (FDG)-PET[24]. We recently compared PSMA-PET with FDG-PET in HCC imaging and found that PSMA-PET exhibited higher standardized uptake value in the tumor region and higher tumor-to-background ratios (Figure 1). In addition to the findings from noninvasive imaging, a pathological evaluation of 103 HCC specimens confirmed that PSMA was expressed on 74% of tumor-associated blood vessels. PSMA expression has oncogenic consequences, including an association with tumor stage, differentiation, lymph node metastasis, and Ki67 index[25]. High vascular expression of PSMA is correlated with poor prognosis, indicating that it is an independent prognostic factor for liver cancer and subsequently a target for antiangiogenic therapy[25].
However, HCC is often accompanied with cirrhosis, which may acquire a nodular architecture with altered vascularity that resembles the regenerated nodules of early-stage HCC. As a result, the correlation between PSMA expression and the pathological classification of liver cancers remain elusive. In this retrospective study, we examined PSMA expression in 446 liver specimens (213 HCC, 203 CCA, and 30 cirrhosis) by immunohistochemistry (IHC), investigated the relationship between PSMA expression and clinicopathological findings, and discussed the potential of using PSMA-targeted imaging to distinguish HCC from liver cirrhosis.
This study was approved by the Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology (No. 2019-S951). Formalin-fixed paraffin-embedded liver tumor and liver cirrhosis tissue samples from hospitalized patients were obtained retrospectively from the Pathology Department of Tongji Hospital from January 2013 to December 2017. All samples were deidentified before analysis. A total of 446 liver specimens, including 213 HCC, 203 CCA, and 30 cirrhosis specimens, were studied. HCC and CCA were classified according to the World Health Organization and Edmondson pathological classification criteria as grade I (low), grade II (intermediate), and grade III (high)[1,26,27]. Patient characteristics and pathological features are summarized in Table 1.
| Clinicopathological parameters | No. of cases (%) | ||
| HCC | CCA | ||
| Total | 213 | 203 | |
| Gender | Male | 185 (86.9) | 112 (55.2) |
| Female | 28 (13.1) | 91 (44.8) | |
| Age of diagnosis | < 50 | 106 (49.8) | 49 (24.1) |
| ≥ 50 | 107 (50.2) | 154 (75.9) | |
| Mean (range) | 50 (19-85) | 57 (41-78) | |
| Region | Country | 82 (38.5) | 112 (55.2) |
| Urban | 131 (61.5) | 91 (44.8) | |
| AFP | < 400 | 134 (62.9) | - |
| ≥ 400 | 79 (37.1) | - | |
| HBsAg | + | 166 (77.9) | 140 (69.0) |
| - | 47 (22.1) | 63 (31.0) | |
| Tumor size | < 5 cm | 92 (43.2) | 98 (48.3) |
| ≥ 5 cm | 121 (56.8) | 105 (51.7) | |
| Stage | pT1 | 9 (4.2) | 14 (6.9) |
| pT2 | 73 (34.3) | 105 (51.7) | |
| pT3 | 24 (11.3) | 21 (10.3) | |
| pT4 | 107 (50.2) | 63 (31.0) | |
| Nodal status | N0 | 190 (89.2) | 182 (89.7) |
| N1 | 23 (10.8) | 21 (10.3) | |
| Metastasis | M0 | 188 (88.2) | 182 (89.7) |
| M1 | 25 (11.8) | 21 (10.3) | |
| UICC stage at diagnosis | I | 16 (7.5) | 14 (6.9) |
| II | 106 (49.8) | 98 (48.3) | |
| III | 28 (13.6) | 21 (10.3) | |
| IV | 63 (29.6) | 70 (34.5) | |
| Tumor grading | I | 79 (37.1) | 75 (36.9) |
| II | 76 (35.7) | 72 (35.7) | |
| III | 58 (27.2) | 56 (27.6) | |
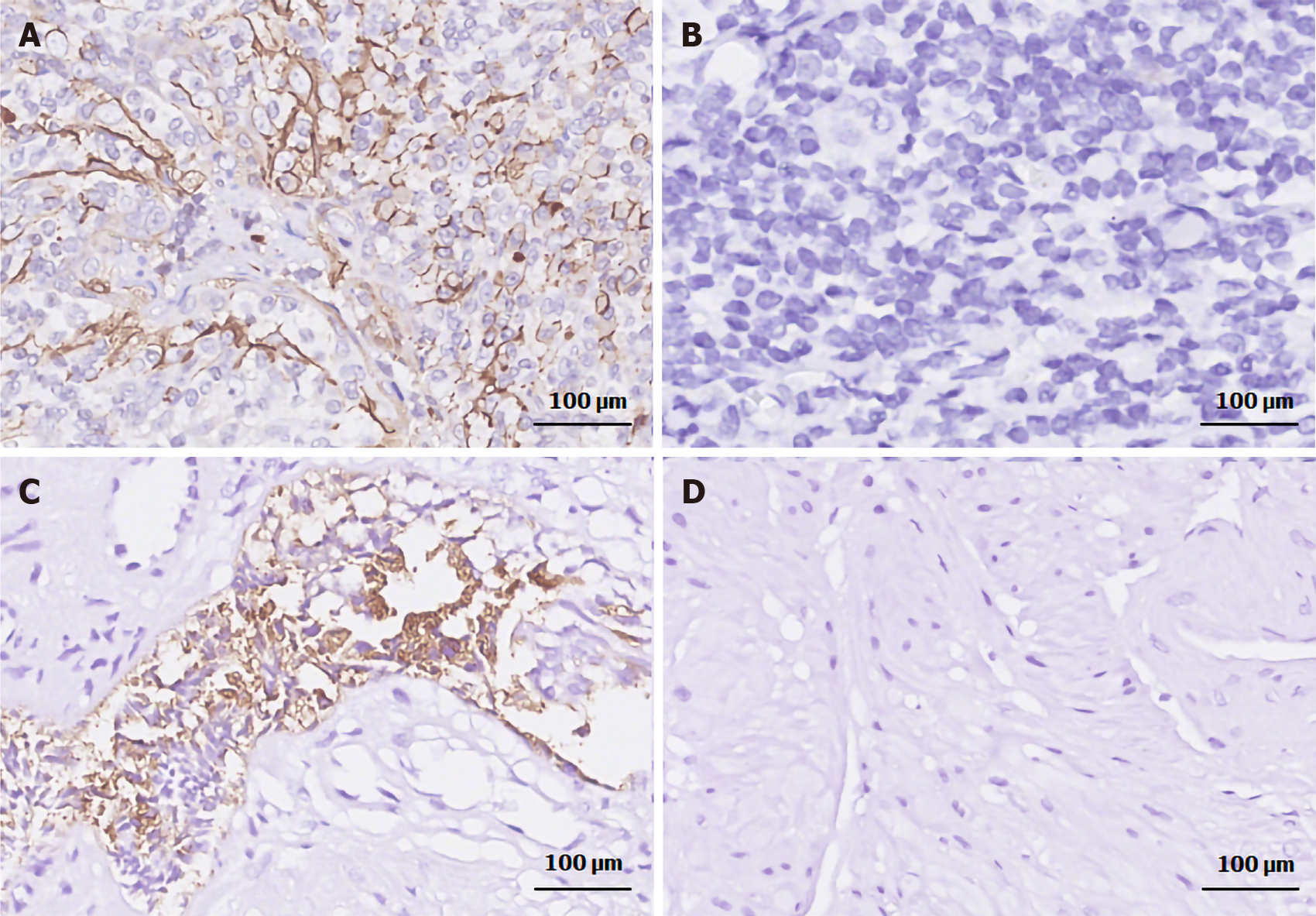
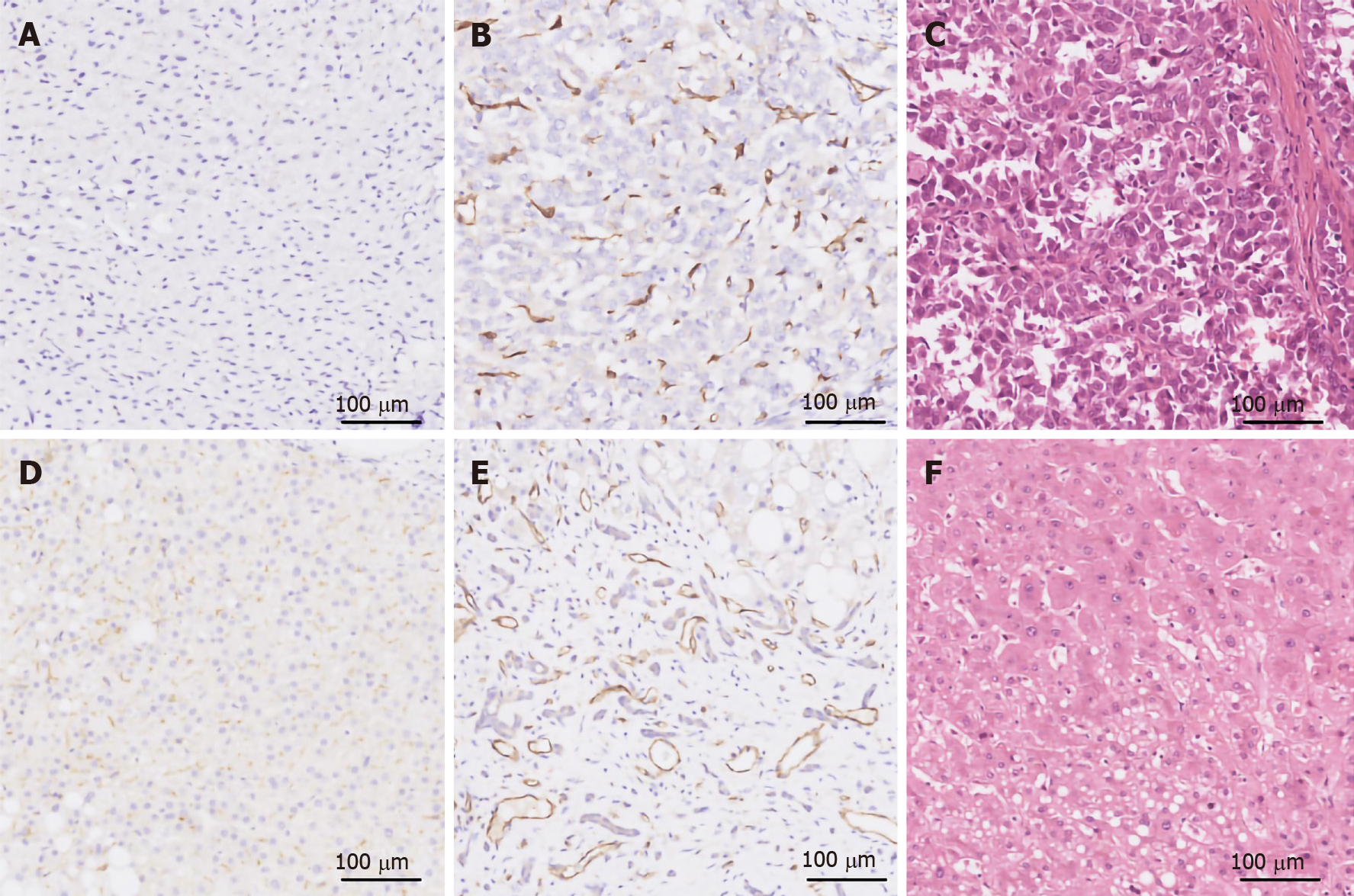
IHC was performed as previously described[11]. PSMA was stained with an anti-PSMA rabbit monoclonal antibody (ab133579; Abcam, Cambridge, MA, United States; 1:250 dilution) on a Leica Bond-Max autostainer and visualized with the Bond Polymer Refine Detection System (Leica Biosystems Newcastle, Newcastle upon Tyne, United Kingdom). Vascular structures were confirmed by staining with an anti-CD31 rabbit polyclonal (ab28264; Abcam; 1:100 dilution). Primary antibody-null staining was used as a negative control. Prostatic adenocarcinoma specimens with confirmed PSMA expression and tonsil specimens were used as the positive controls for PSMA and CD31 staining, respectively (Figure 2). All specimens were routinely stained with hematoxylin and eosin to verify tumor morphology prior to IHC.
The tumor compartment and the associated neovascular endothelium (ANVE) were separately analyzed on a minimum of three randomly chosen sections and observed at three different magnifications (40 ×, 100 ×, and 400 ×) per section. Protein expression was examined by two certified pathologists who were blinded to all the clinical data. Each pathologist assigned a score of 0 (no staining on any tumor cells or neovascular endothelium); 1 (low staining intensity in < 10% of tumor cells or ANVE); 2 (low staining intensity in 10%–50% of tumor cells or ANVE, or high staining intensity in ≤ 25% of tumor cells or ANVE); and 3 (low staining intensity in > 50% of tumor cells or ANVE, or high staining intensity in > 25% of tumor cells or ANVE) (Table 2)[11]. The two scores for each section were then averaged to give the final score. A consensus review was performed in case where there was substantial disagreement between the two pathologists.
| Score | Stain intensity | Percent of vessels staining |
| 0 | None | 0 |
| 1 | Low | ≤ 10% |
| 2 (type 1) | Low | 10%-50% |
| 2 (type 2) | High | ≤ 25% |
| 3 (type 1) | Low | ≥ 50% |
| 3 (type 2) | High | > 25% |
Data were analyzed using SPSS version 25.0 (SPSS, Armonk, NY, United States). P < 0.05 was considered statistically significant. Quantitative data were expressed as mean ± standard deviation. The χ2 test was used to compare categorical variables. Spearman's correlation coefficient (nonparametric) was used to determine the correlation between IHC scores and clinical variables.
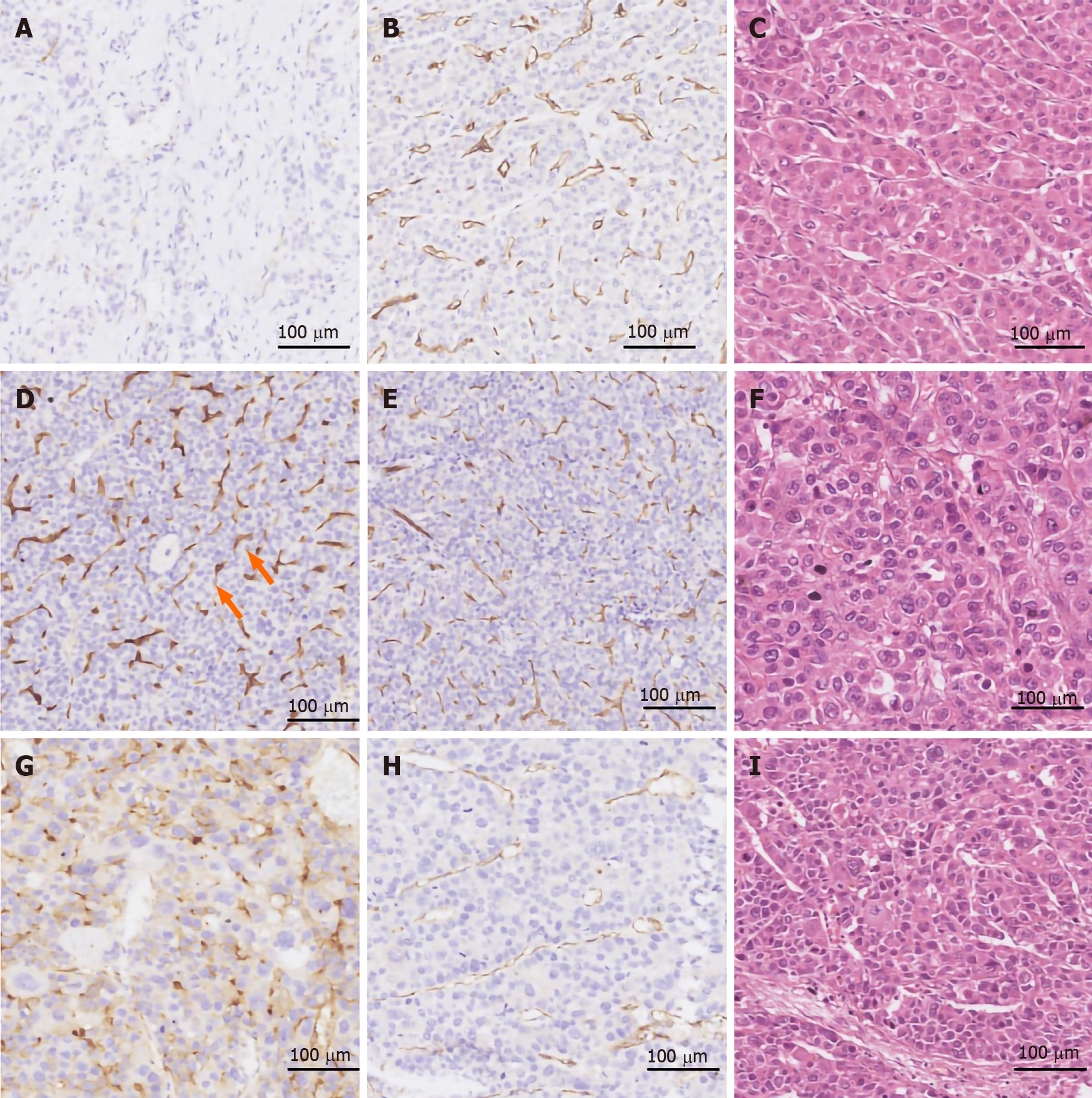
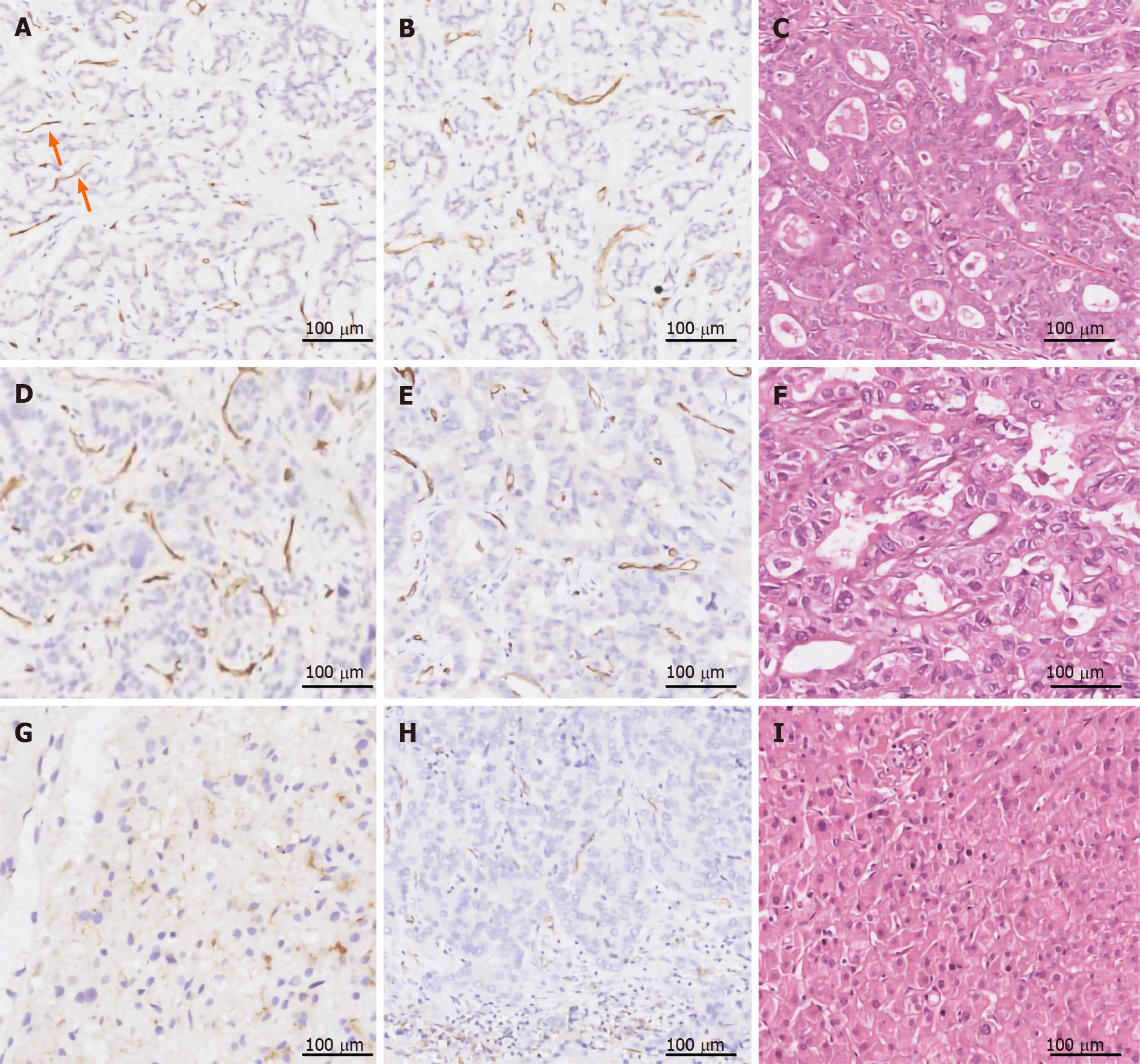
PSMA was expressed in the tumor-associated neovascular endothelium that was also positively stained with the pan-endothelial marker CD31 (Figures 3 and 4). In contrast, blood vessels in the peritumoral normal tissues were exclusively CD31+, indicating that PSMA is a specific marker for the tumor-associated neovasculature. The percentage of PSMA+ cases in HCC (185/213, 86.8%) and CCA (161/203, 79.3%) was 13- and 12-fold higher, respectively, than that in liver cirrhosis (2/30, 6.6%) (P < 0.0001, Table 3), while the percentage of PSMA+ cases in HCC was significantly higher than that in CCA (86.8% vs 79.3%, P = 0.001). There were more sections with a score of 3 for PSMA expression in HCC (89/213, 41.8%) than in CCA (35/203, 17.2%, P = 0.001). PSMA expression correlated positively with the stage and grade of HCC and CCA. In both liver cancer subtypes, stages III–V disease had more PSMA+ cases than stage I and II had, while high staining intensity of PSMA was more frequently observed in liver cancers of high grade and advanced stage. There was no significant association of PSMA expression with sex, age, region, AFP, hepatitis B surface antigen (HBsAg), or tumor size.
| Number | PSMA expression score, n | Positive staining, n (%) | |||||
| 0 | 1 | 2 | 3 | ||||
| HCC | Cells | 213 | 187 | 8 | 16 | 2 | 26 (12.2) |
| NECs | 213 | 29 | 31 | 64 | 89 | 184 (86.4) | |
| Total | 213 | 28 | 32 | 64 | 89 | 185 (86.8) | |
| CCA | Cells | 203 | 196 | 0 | 7 | 0 | 7 (3.4) |
| NECs | 203 | 42 | 42 | 84 | 35 | 161 (79.3) | |
| Total | 203 | 42 | 42 | 84 | 35 | 161 (79.3) | |
| Cirrhosis | Cells | 30 | 28 | 2 | 0 | 0 | 2 (6.6) |
Neovascular expression of PSMA was observed in 184/213 (86.4%) HCC cases, while no PSMA staining was found in normal vascular endothelial cells or peritumoral normal tissues. Among the 184 cases with PSMA+ neovasculature, 31 (14.6%) had an expression score of 1, 64 (30.0%) a score of 2, and 89 (41.8%) a score of 3. In comparison, only 26/213 HCC cases had PSMA+ tumor cells, with most of the staining in the cytoplasm and cell membrane. The PSMA staining score was 1 in eight (3.7%) cases, 2 in 16 (7.5%) cases, and 3 in two (0.9%) cases (Table 3 and Figure 3). Among these 26 cases, one case showed PSMA staining exclusively in tumor cells, while the remaining 25 cases had PSMA staining in both tumor cells and neovasculature. Furthermore, in 3/213 (1.4%) cases, positive PSMA staining of tumor cells was not accompanied by nearby CD31 expression, which may be attributed to tumor necrosis. In 2/213 cases, the vessel-like structures within the tumor compartment were exclusively stained with PSMA rather than CD31 (score of 3, Figure 3D and E).
PSMA expression correlated positively with stage (Spearman r = 0.226, P = 0.001) and grade (Spearman r = 0.224, P = 0.004) of HCC. Eighty-seven of 91 (95.5%) stage III and IV HCC cases were PSMA+, which was significantly higher than stage I and II HCC (97/122, 79.5%, P = 0.001). There was a higher positive rate for PSMA expression in the neovasculature of grade III (high) HCCs (57/58, 98.2%) than in those of grade II (intermediate, 65/76, 86.5%) or grade I (low, 62/79, 78.4%, P = 0.004) HCC cases. There was no significant association of PSMA expression with sex, age, region, alpha fetoprotein (AFP), HBsAg, or tumor size (Table 4).
| Clinicopathological parameters | No. of cases | Tumor PSMA-positive, n | P value | |||
| 0 | 1 | 2 | 3 | |||
| Gender | ||||||
| Male | 185 | 25 | 27 | 52 | 81 | 0.912 |
| Female | 28 | 4 | 4 | 12 | 8 | |
| Age of diagnosis | ||||||
| < 50 | 106 | 12 | 14 | 29 | 51 | 0.331 |
| ≥ 50 | 107 | 17 | 17 | 35 | 38 | |
| Mean (range) | 50 (19-85) | |||||
| Region | ||||||
| Urban | 131 | 15 | 16 | 44 | 56 | 0.080 |
| Country | 82 | 14 | 15 | 20 | 33 | |
| AFP | ||||||
| < 400 | 134 | 21 | 18 | 38 | 57 | 0.254 |
| ≥ 400 | 79 | 8 | 13 | 26 | 32 | |
| HBsAg | ||||||
| + | 166 | 24 | 23 | 49 | 70 | 0.990 |
| - | 47 | 5 | 8 | 15 | 19 | |
| Tumor size | ||||||
| < 5 cm | 92 | 14 | 10 | 23 | 45 | 0.552 |
| ≥ 5 cm | 121 | 15 | 21 | 41 | 44 | |
| Stage | ||||||
| pT1 | 9 | 1 | 2 | 4 | 2 | 0.812 |
| pT2 | 73 | 19 | 15 | 18 | 54 | |
| pT3 | 24 | 2 | 4 | 6 | 12 | |
| pT4 | 107 | 7 | 10 | 36 | 21 | |
| Nodal status | ||||||
| N0 | 190 | 27 | 24 | 58 | 82 | 0.466 |
| N1 | 23 | 2 | 7 | 6 | 7 | |
| Metastasis | ||||||
| M0 | 188 | 23 | 31 | 57 | 77 | 0.136 |
| M1 | 25 | 6 | 0 | 7 | 12 | |
| UICC stage at diagnosis | ||||||
| I-II | 122 | 25 | 17 | 37 | 43 | 0.001a, r = 0.226 |
| III-IV | 91 | 4 | 14 | 27 | 46 | |
| Tumor grading | ||||||
| I | 79 | 17 | 8 | 17 | 37 | 0.004a, r = 0.224 |
| II | 76 | 11 | 7 | 25 | 33 | |
| III | 58 | 1 | 16 | 22 | 19 | |
| All case | 213 | 29 | 31 | 64 | 89 | |
Variable levels of PSMA expression were found in tumor neovasculature but in neither normal liver tissue nor peritumoral tissue (Table 3 and Figure 4). One hundred and sixty-one (79.3%) of 203 primary CCA cases were PSMA+ in the tumor neovasculature, among which, 42 cases (20.7%) had an expression score of 1, 84 (41.4%) a score of 2, and 35 (17.2%) a score of 3 (Table 3 and Figure 4). Seven (3.4%) cases had PSMA staining in both tumor cells (cytoplasm and cell membrane) and tumor-associated neovasculature endothelium, with an expression score of 2. Like HCC, one CCA case exhibited vessel-like structures within the tumor compartment that was weakly stained with PSMA (score = 1) but negative with CD31 staining (Figure 4A and B).
PSMA expression correlated positively with the stage (Spearman r = 0.211, P = 0.002) and grade (Spearman r = 0.253, P = 0.001) of CCA. Positive staining of PSMA was more frequent in stage III and IV CCAs (81/91, 89.0%) than in stage I and II CCA (80/112, 71.4%, P = 0.002). There was a higher rate of positive staining for PMSA in the tumor neovasculature of grade III (high) CCA cases (53/56, 94.6) compared to that of grade II (intermediated, 57/72, 79.0%) or grade I (low, 51/75, 68.0, P = 0.001). There was no significant correlation between PSMA expression and other clinicopathological features of CCA patients (Table 5).
| Clinicopathological parameters | No. of cases | Tumor PSMA-positive, n | P value | |||
| 0 | 1 | 2 | 3 | |||
| Gender | ||||||
| Male | 112 | 24 | 13 | 46 | 29 | 0.773 |
| Female | 91 | 18 | 29 | 38 | 6 | |
| Age of diagnosis | ||||||
| < 50 | 49 | 8 | 22 | 6 | 13 | 0.387 |
| ≥ 50 | 154 | 34 | 20 | 78 | 22 | |
| Mean (range) | 57 (41-78) | |||||
| Region | ||||||
| Urban | 91 | 16 | 23 | 47 | 5 | 0.325 |
| Country | 112 | 26 | 19 | 37 | 30 | |
| HBsAg | ||||||
| + | 140 | 29 | 26 | 69 | 16 | 0.990 |
| - | 63 | 13 | 16 | 15 | 19 | |
| Tumor size | ||||||
| < 5 cm | 98 | 23 | 27 | 30 | 18 | 0.178 |
| ≥ 5 cm | 105 | 19 | 15 | 54 | 17 | |
| Stage | ||||||
| pT1 | 14 | 5 | 3 | 4 | 2 | 0.293 |
| pT2 | 105 | 23 | 21 | 49 | 12 | |
| pT3 | 21 | 5 | 5 | 7 | 4 | |
| pT4 | 63 | 9 | 13 | 24 | 17 | |
| Nodal status | ||||||
| N0 | 182 | 36 | 39 | 74 | 33 | 0.346 |
| N1 | 21 | 6 | 3 | 10 | 2 | |
| Metastasis | ||||||
| M0 | 182 | 37 | 40 | 76 | 29 | 0.709 |
| M1 | 21 | 5 | 2 | 8 | 6 | |
| UICC stage at diagnosis | ||||||
| I-II | 112 | 32 | 26 | 38 | 16 | 0.002a, r = 0.211 |
| III-IV | 91 | 10 | 16 | 46 | 19 | |
| Tumor grading | ||||||
| I | 75 | 24 | 18 | 28 | 5 | 0.001a, r = 0.253 |
| II | 72 | 15 | 18 | 35 | 6 | |
| III | 56 | 3 | 6 | 21 | 5 | |
| All case | 203 | 42 | 42 | 84 | 35 | |
CD31+ blood vessels were observed in all 30 liver cirrhosis specimens (Figure 5). However, only two of 30 specimens showed weak PSMA staining in the cytoplasm and cell membrane of liver cells (score = 1). The remaining 28 specimens were PSMA- in either hepatocytes or vascular endothelium.
HCC is the fourth most common malignancy and the third leading cause of tumor-related death in China, accounting for 85%-90% of all primary liver cancer cases[1]. Early radical intervention or effective management at late stage are both important strategies to reduce the death toll from HCC.
PSMA is a type II transmembrane glycoprotein that has attracted extensive attention due to its specific and high expression in prostate cancer cells. PSMA was first identified by Holmes et al[7] from a crude membrane extract of an androgen-dependent prostate cancer cell line LNCaP[7]. Other than tumor tissue, PSMA is also highly expressed in pancreatic islets and skeletal muscle, moderately expressed in brain and ganglia of gastrointestinal tract, and weakly expressed in prostate, endometrial glands, kidney tubules, and urinary bladder. No PSMA expression was observed in the liver, spleen, or other tissues[12]. In addition to prostate cancer cells, PSMA has previously been detected in the tumor-associated neovasculature of solid tumors including HCC[9-26]. Notably, PSMA is absent in blood vessels of normal tissue due to the lack of PSMA transcription enhancement regions[28,29].
HCC is a highly vascularized tumor that is characterized by early angiogenesis. The hepatic artery is the main route to supply oxygen and nutrients to HCC, therefore making antiangiogenic therapy promising for HCC. In contrast, PSMA facilitates the invasion of endothelial cells during angiogenic sprouting and thereby supports tumor growth through provision of oxygen and nutrients[29,30]. As a result, targeted therapy against PSMA-expressing neovasculature represents a feasible option in treating rapidly growing solid tumors. Recently, several PSMA-targeted PET imaging studies reported high uptake of radiotracers in the tumor region of HCC, CCA, and CHC[20-22]. Kuyumcu et al[31] studied 68Ga-PSMA PET imaging in 19 patients with liver cancer and found tumor uptake of radiotracers in 16 patients[31]. A multi-center phase II trial found that a PSMA-targeted therapy using an antiangiogenic drug mipsagargin led to long-term stable disease in patients with advanced liver cancer[32]. Magnetic resonance imaging after the mipsagargin treatment revealed a decrease in blood flow in liver lesions, confirming that PSMA plays an important role in liver cancer progression[32]. Jiao et al[25] found that PSMA was specifically expressed in the vasculature in 76 of 103 (74%) HCC specimens[25]. However, PSMA expression in liver cancer subtypes other than HCC remains to be elucidated.
Here, for the first time, we demonstrated that PSMA was expressed in the tumor-associated neovasculature of most HCC (86.8%) and CCA (79.3%) cases in a large sample set. PSMA expression was restricted to the neovasculature of HCC and CCA, while normal liver and peritumoral tissues were largely PSMA-. A few vessel-like structures in the tumor compartment was PSMA+ but CD31-, suggesting that PSMA is a useful biomarker for early-stage tumor-associated angiogenesis. This temporal mismatch between PSMA and CD31 underscores the role of PSMA in the invasion of endothelial cells. It is worth mentioning that HCC (86.8%) exhibited a higher positive rate of PSMA staining than CCA (79.3%) did and that the HCC cases had more 3-score PSMA staining than CCA had (89/213, 41.7% vs 35/203, 17.2%). Therefore, PSMA could provide better diagnostic power in HCC than in CCA and functions as a valuable therapeutic target in HCC.
In some HCC and CCA cases, PSMA staining was observed in the cytoplasm and cell membrane of tumor cells, albeit with lower staining intensity than in tumor-associated neovasculature. Similarly, Nomura et al[10] found that < 2% of tumor cells were stained with PSMA in grade II and III glioma[10]. In contrast, Kesler et al[24] recently reported that three out of five HCC specimens had intense PSMA staining in intratumoral microvessels[24]. However, they did not observe any PSMA staining in the epithelial tumor cells. Such discrepancies in terms of PSMA expression can be attributed to the difference in sample size and biopsy locations.
Cirrhosis caused by viral hepatitis, especially type B and C, is the leading risk factor for HCC. The regenerated nodules of early-stage HCC are often indistinguishable from the accompanying cirrhosis, which makes ablative therapy more challenging. In our study, only two (6.7%) cases of liver cirrhosis showed weak PSMA staining in tumor cell cytoplasm and cell membrane, with an expression score of 1. In contrast, the positive staining of PSMA was more frequent and with higher intensity in HCC and CCA. Therefore, our study proves that PSMA could be a useful biomarker to distinguish HCC from liver cirrhosis. Accordingly, PSMA-targeted PET imaging can potentially pinpoint the regenerated nodules of HCC.
In this study, PSMA expression correlated positively with the stage and grade of HCC and CCA, and stage III and IV disease tended to have higher positive rate of PSMA than stage I and II diseases. High PSMA expression was more likely to be found in the neovasculature of HCC and CCA with high grade or stage III or IV. There was no significant association of PSMA expression with sex, age, region, AFP, HBsAg, or tumor size in HCC and CCA. Jiao et al[25] reported that vascular PSMA expression correlated with tumor stage, tumor differentiation, lymph node metastasis, and Ki67 index[25]. They did not find any significant association between the vascular PSMA expression and age or sex, which was in accordance with our results.
PSMA was expressed primarily in the tumor-associated neovascular endothelium of liver cancer. We discovered a potential role of PSMA-targeted imaging in the detection and staging of liver cancer patients, especially those with HCC. The PSMA-targeted imaging may also be useful to distinguish liver cancer from cirrhosis. As a result, PSMA-targeted approaches represent a feasible alternative to current antiangiogenic cancer therapy.
Prostate-specific membrane antigen (PSMA) is a transmembrane glycoprotein expressed in the neovasculature of various nonprostate malignancies.
PSMA expression in the tumor-associated neovasculature of nonprostate malignancies including liver cancer has been reported, but conclusive evidence of PSMA expression based on the pathological type of liver cancers remains limited.
This retrospective study was performed to study the expression of PSMA in hepatocellular carcinoma (HCC), cholangiocarcinoma (CCA), and liver cirrhosis.
Immunohistochemistry was used to detect PSMA expression in 446 formalin-fixed paraffin-embedded liver biopsy specimens (213 HCC, 203 CCA, and 30 liver cirrhosis).
PSMA was expressed primarily in the neovascular endothelium associated with tumors. The positive rate of PSMA staining in HCC was significantly higher than that in CCA.
Neovascular PSMA may be used as a promising marker to differentiate HCC from liver cirrhosis and a prognostic marker for antitumor angiogenesis for HCC.
Vascular PSMA may be used as a prognostic marker for anti-tumor angiogenesis therapy for HCC.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: Nuclear Medicine Branch of the Chinese Medical Doctor Association.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bordonaro M, Ciccone MM S-Editor: Fan JR L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Department of Medical Administration, National Health and Health Commission of the People's Republic of China. [Guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition)]. Zhonghua Gan Zang Bing Za Zhi. 2020;28:112-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 65] [Reference Citation Analysis (0)] |
| 2. | Ross JS, Sheehan CE, Fisher HA, Kaufman RP, Kaur P, Gray K, Webb I, Gray GS, Mosher R, Kallakury BV. Correlation of primary tumor prostate-specific membrane antigen expression with disease recurrence in prostate cancer. Clin Cancer Res. 2003;9:6357-6362. [PubMed] |
| 3. | Troyer JK, Beckett ML, Wright GL. Detection and characterization of the prostate-specific membrane antigen (PSMA) in tissue extracts and body fluids. Int J Cancer. 1995;62:552-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 256] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 4. | O'Keefe DS, Su SL, Bacich DJ, Horiguchi Y, Luo Y, Powell CT, Zandvliet D, Russell PJ, Molloy PL, Nowak NJ, Shows TB, Mullins C, Vonder Haar RA, Fair WR, Heston WD. Mapping, genomic organization and promoter analysis of the human prostate-specific membrane antigen gene. Biochim Biophys Acta. 1998;1443:113-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 137] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 5. | Conway RE, Petrovic N, Li Z, Heston W, Wu D, Shapiro LH. Prostate-specific membrane antigen regulates angiogenesis by modulating integrin signal transduction. Mol Cell Biol. 2006;26:5310-5324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 167] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 6. | Rajasekaran AK, Anilkumar G, Christiansen JJ. Is prostate-specific membrane antigen a multifunctional protein? Am J Physiol Cell Physiol. 2005;288:C975-C981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 159] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 7. | Holmes EH. PSMA specific antibodies and their diagnostic and therapeutic use. Expert Opin Investig Drugs. 2001;10:511-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Ahmadzadehfar H, Rahbar K, Kürpig S, Bögemann M, Claesener M, Eppard E, Gärtner F, Rogenhofer S, Schäfers M, Essler M. Early side effects and first results of radioligand therapy with (177)Lu-DKFZ-617 PSMA of castrate-resistant metastatic prostate cancer: a two-centre study. EJNMMI Res. 2015;5:114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 214] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 9. | Chang SS, Reuter VE, Heston WD, Bander NH, Grauer LS, Gaudin PB. Five different anti-prostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer Res. 1999;59:3192-3198. [PubMed] |
| 10. | Nomura N, Pastorino S, Jiang P, Lambert G, Crawford JR, Gymnopoulos M, Piccioni D, Juarez T, Pingle SC, Makale M, Kesari S. Prostate specific membrane antigen (PSMA) expression in primary gliomas and breast cancer brain metastases. Cancer Cell Int. 2014;14:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 155] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 11. | Haffner MC, Kronberger IE, Ross JS, Sheehan CE, Zitt M, Mühlmann G, Ofner D, Zelger B, Ensinger C, Yang XJ, Geley S, Margreiter R, Bander NH. Prostate-specific membrane antigen expression in the neovasculature of gastric and colorectal cancers. Hum Pathol. 2009;40:1754-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 187] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 12. | Mhawech-Fauceglia P, Zhang S, Terracciano L, Sauter G, Chadhuri A, Herrmann FR, Penetrante R. Prostate-specific membrane antigen (PSMA) protein expression in normal and neoplastic tissues and its sensitivity and specificity in prostate adenocarcinoma: an immunohistochemical study using mutiple tumour tissue microarray technique. Histopathology. 2007;50:472-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 225] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 13. | Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3:81-85. [PubMed] |
| 14. | Kinoshita Y, Kuratsukuri K, Landas S, Imaida K, Rovito PM, Wang CY, Haas GP. Expression of prostate-specific membrane antigen in normal and malignant human tissues. World J Surg. 2006;30:628-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 265] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 15. | Verburg FA, Krohn T, Heinzel A, Mottaghy FM, Behrendt FF. First evidence of PSMA expression in differentiated thyroid cancer using [68Ga] PSMA-HBED-CC PET/CT. Eur J Nucl Med Mol Imaging. 2015;42:1622-1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 107] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 16. | Nimmagadda S, Pullambhatla M, Chen Y, Parsana P, Lisok A, Chatterjee S, Mease R, Rowe SP, Lupold S, Pienta KJ, Pomper MG. Low-Level Endogenous PSMA Expression in Nonprostatic Tumor Xenografts Is Sufficient for In Vivo Tumor Targeting and Imaging. J Nucl Med. 2018;59:486-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Haffner MC, Laimer J, Chaux A, Schäfer G, Obrist P, Brunner A, Kronberger IE, Laimer K, Gurel B, Koller JB, Seifarth C, Zelger B, Klocker H, Rasse M, Doppler W, Bander NH. High expression of prostate-specific membrane antigen in the tumor-associated neo-vasculature is associated with worse prognosis in squamous cell carcinoma of the oral cavity. Mod Pathol. 2012;25:1079-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | Derlin T, Kreipe HH, Schumacher U, Soudah B. PSMA Expression in Tumor Neovasculature Endothelial Cells of Follicular Thyroid Adenoma as Identified by Molecular Imaging Using 68Ga-PSMA Ligand PET/CT. Clin Nucl Med. 2017;42:e173-e174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Unterrainer M, Niyazi M, Ruf V, Bartenstein P, Albert NL. The endothelial prostate-specific membrane antigen is highly expressed in gliosarcoma and visualized by [68Ga]-PSMA-11 PET: a theranostic outlook for brain tumor patients? Neuro Oncol. 2017;19:1698-1699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Sasikumar A, Joy A, Nanabala R, Pillai MR, Thomas B, Vikraman KR. (68)Ga-PSMA PET/CT imaging in primary hepatocellular carcinoma. Eur J Nucl Med Mol Imaging. 2016;43:795-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 21. | Marafi F, Usmani S, Esmail A. 68Ga-Prostate-Specific Membrane Antigen PET/CT in Cholangiocarcinoma: A Potential Biomarker for Targeted Radioligand Therapy? Clin Nucl Med. 2019;44:e439-e441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Alipour R, Gupta S, Trethewey S. 68Ga-PSMA Uptake in Combined Hepatocellular Cholangiocarcinoma With Skeletal Metastases. Clin Nucl Med. 2017;42:e452-e453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Huang HL, Zhen Loh TJ, Hoe Chow PK. A Case of Well-differentiated Hepatocellular Carcinoma Identified on Gallium-68 Prostate-specific Membrane Antigen Positron Emission Tomography/Computed Tomography. World J Nucl Med. 2018;17:102-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 24. | Kesler M, Levine C, Hershkovitz D, Mishani E, Menachem Y, Lerman H, Zohar Y, Shibolet O, Even-Sapir E. 68Ga-PSMA is a novel PET-CT tracer for imaging of hepatocellular carcinoma: A prospective pilot study. J Nucl Med. 2019;60:185-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 25. | Jiao D, Li Y, Yang F, Han D, Wu J, Shi S, Tian F, Guo Z, Xi W, Li G, Zhao A, Yang AG, Qin W, Wang H, Wen W. Expression of Prostate-Specific Membrane Antigen in Tumor-Associated Vasculature Predicts Poor Prognosis in Hepatocellular Carcinoma. Clin Transl Gastroenterol. 2019;10:1-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 26. | Pirisi M, Leutner M, Pinato DJ, Avellini C, Carsana L, Toniutto P, Fabris C, Boldorini R. Reliability and reproducibility of the edmondson grading of hepatocellular carcinoma using paired core biopsy and surgical resection specimens. Arch Pathol Lab Med. 2010;134:1818-1822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 27. | Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383:2168-2179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1072] [Cited by in RCA: 1374] [Article Influence: 124.9] [Reference Citation Analysis (1)] |
| 28. | Murphy GP, Kenny GM, Ragde H, Wolfert RL, Boynton AL, Holmes EH, Misrock SL, Bartsch G, Klocker H, Pointner J, Reissigl A, McLeod DG, Douglas T, Morgan T, Gilbaugh J. Measurement of serum prostate-specific membrane antigen, a new prognostic marker for prostate cancer. Urology. 1998;51:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Noss KR, Wolfe SA, Grimes SR. Upregulation of prostate specific membrane antigen/folate hydrolase transcription by an enhancer. Gene. 2002;285:247-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 30. | Ghosh A, Heston WD. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J Cell Biochem. 2004;91:528-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 644] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 31. | Kuyumcu S, Has-Simsek D, Iliaz R, Sanli Y, Buyukkaya F, Akyuz F, Turkmen C. Evidence of Prostate-Specific Membrane Antigen Expression in Hepatocellular Carcinoma Using 68Ga-PSMA PET/CT. Clin Nucl Med. 2019;44:702-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 32. | Mahalingam D, Peguero J, Cen P, Arora SP, Sarantopoulos J, Rowe J, Allgood V, Tubb B, Campos L. A Phase II, Multicenter, Single-Arm Study of Mipsagargin (G-202) as a Second-Line Therapy Following Sorafenib for Adult Patients with Progressive Advanced Hepatocellular Carcinoma. Cancers (Basel). 2019;11:833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |