Published online Dec 28, 2020. doi: 10.3748/wjg.v26.i48.7652
Peer-review started: September 5, 2020
First decision: September 30, 2020
Revised: October 15, 2020
Accepted: November 21, 2020
Article in press: November 21, 2020
Published online: December 28, 2020
Processing time: 110 Days and 12.8 Hours
Screening provides earlier colorectal cancer (CRC) detection and improves outcomes. It remains poorly understood if these benefits are realized with screening guidelines in remote northern populations of Canada where CRC rates are nearly twice the national average and access to colonoscopy is limited.
To evaluate the participation and impact of CRC screening guidelines in a remote northern population.
This retrospective cohort study included residents of the Northwest Territories, a northern region of Canada, age 50-74 who underwent CRC screening by a fecal immunohistochemical test (FIT) between January 1, 2014 to March 30, 2019. To assess impact, individuals with a screen-detected CRC were compared to clinically-detected CRC cases for stage and location of CRC between 2014-2016. To assess participation, we conducted subgroup analyses of FIT positive individuals exploring the relationships between signs and symptoms of CRC at the time of screening, wait-times for colonoscopy, and screening outcomes. Two sample Welch t-test was used for normally distributed continuous variables, Mann-Whitney-Wilcoxon Tests for data without normal distribution, and Chi-square goodness of fit test for categorical variables. A P value of < 0.05 was considered to be statistically significant.
6817 fecal tests were completed, meaning an annual average screening rate of 25.04%, 843 (12.37%) were positive, 629 individuals underwent a follow-up colonoscopy, of which, 24.48% had advanced neoplasia (AN), 5.41% had CRC. There were no significant differences in stage, pathology, or location between screen-detected cancers and clinically-detected cancers. In assessing participation and screening outcomes, we observed 49.51% of individuals referred for colonoscopy after FIT were ineligible for CRC screening, most often due to signs and symptoms of CRC. Individuals were more likely to have AN if they had signs and symptoms of cancer at the time of screening, waited over 180 d for colonoscopy, or were indigenous [respectively, estimated RR 1.18 95%CI of RR (0.89-1.59)]; RR 1.523 (CI: 1.035, 2.240); RR 1.722 (CI: 1.165, 2.547)].
Screening did not facilitate early cancer detection but facilitated higher than anticipated AN detection. Signs and symptoms of CRC at screening, and long colonoscopy wait-times appear contributory.
Core Tip: This 5-year retrospective cohort study evaluates the participation and impact of colorectal cancer (CRC) screening guidelines in a northern region of Canada. We evaluated CRC screening results of 6817 participants January, 2014 to March, 2019. We compared the stage and location of screen-detected CRC to clinically-detected CRC cases in 2014-2016. We observed no difference in screen-detected CRC vs clinically detected cases. During the 5-year observation period, we observed a higher incidence of advance neoplasia than anticipated, especially among patients presenting with signs and symptoms of cancer at the time of screening, who experienced long colonoscopy wait-times, and/or who identified as indigenous.
- Citation: Smith HA, Scarffe AD, Brunet N, Champion C, Kandola K, Tessier A, Boushey R, Kuziemsky C. Impact of colorectal cancer screening participation in remote northern Canada: A retrospective cohort study. World J Gastroenterol 2020; 26(48): 7652-7663
- URL: https://www.wjgnet.com/1007-9327/full/v26/i48/7652.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i48.7652
The benefits of colorectal cancer (CRC) screening have been well established in multiple prospective studies, including earlier detection, removal of pre-cancerous lesions, and reduction in CRC-associated mortality [RR 0.84, 95%CI: (0.78, 0.90)][1,2]. While guidelines for CRC screening have been widely adopted, the extent to which the desired benefits of screening have been realized among remote northern populations remains poorly understood[3]. Remote northern populations experience multiple geographic and systemic barriers to health care which may impact CRC screening guideline implementation and adherence[4,5]. This is particularly important for indigenous populations who represent a high proportion of northern residents and are known to experience important sociocultural barriers to healthcare[6]. Significant disparities in CRC outcomes have been observed among remote and indigenous residents[6-9]. Therefore, it is imperative to evaluate if the benefits of CRC screening are realized in these regions.
The Northwest Territories (NWT) is a northern region of Canada with 44900 residents living in remote and isolated communities dispersed across 1.1 million km2, of which, 50.7% of residents identify as indigenous. The population of the NWT has been shown to have a higher age-standardized incidence rate of CRC and a higher incidence of CRC-associated mortality than other areas of Canada; however, the reasons for these trends have not been explored[10]. Nonetheless, screening guidelines have been established since 2009 and recommend that average risk individuals age of 50-74 years undergo fecal immunochemical testing (FIT) every 1-2 years[11-13]. If the FIT is positive, the patient should receive a colonoscopy within 60 d. Higher risk individuals are advised to undergo regular screening colonoscopy (i.e., those with a family history of CRC, relevant genetic syndrome, or inflammatory bowel disease). Semi-structured interviews with clinicians in the NWT indicate that implementation of these guidelines has been challenging particularly with regards to recruiting of participants, determining their eligibility for FIT, and arranging timely colonoscopy access for residents[4]. This study aims to understand the impact of screening guidelines in this remote population with a high incidence of CRC by assessing the participation and outcomes of CRC screening.
This study was approved by the Aurora College Research Ethics Committee, protocol No. 20190404.
A population-based, retrospective cohort study was conducted of individuals who underwent CRC screening by FIT in the NWT between January 1, 2014 to March 30, 2019. Individuals were identified in the prospectively collected Public Health Registries, the most reliable form of capturing FIT participation in the NWT. We included all individuals who, at the time of testing, were between the ages of 50-74 and had a valid NWT health card. Of those included, we collected their demographic details including community of residence and indigenous status (based on health card data).
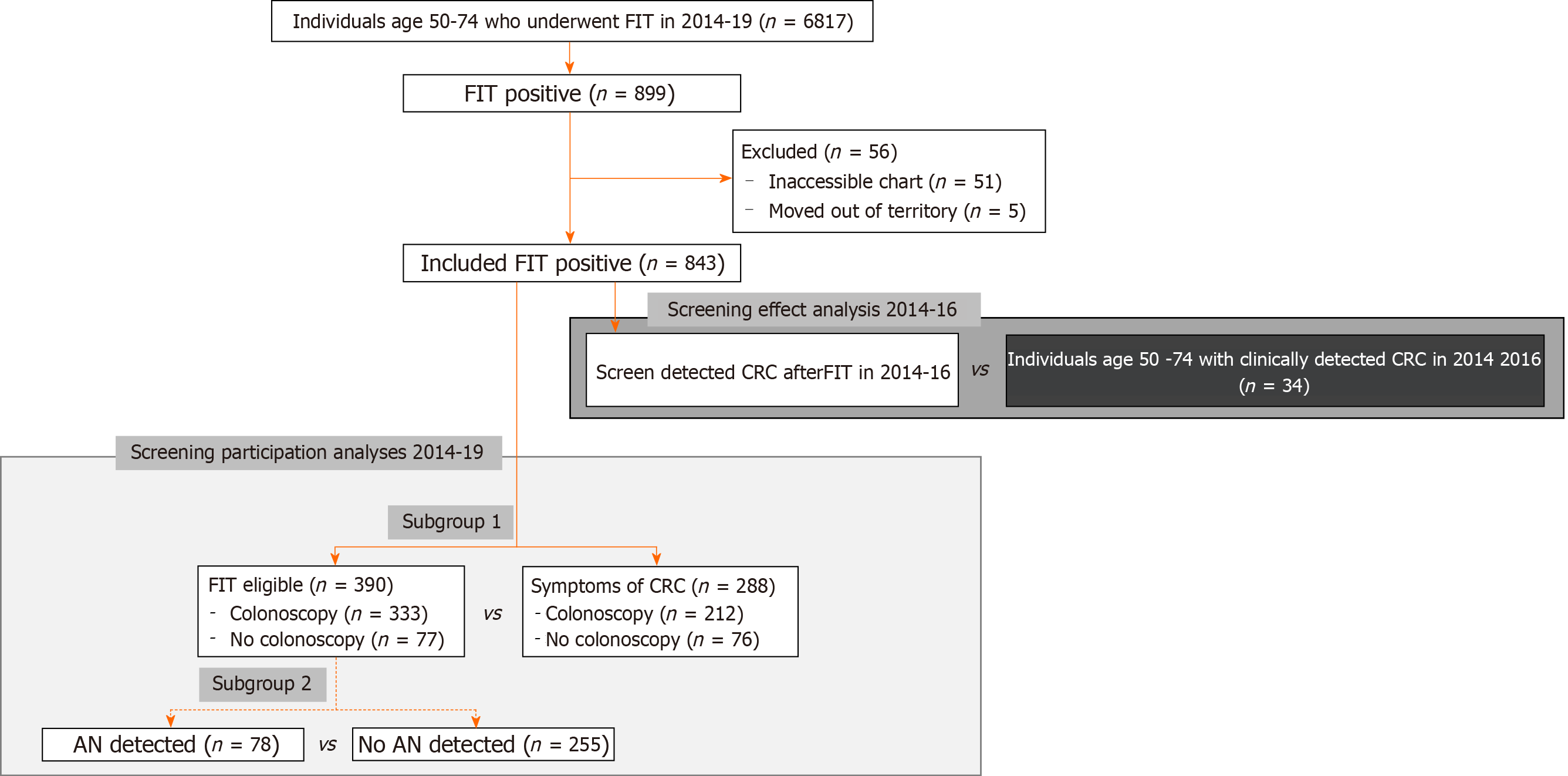
Individuals with a positive FIT result were included in further analysis of FIT follow-up, excluding individuals without accessible health records, or who moved out of territory during the observation period. FIT-eligibility and colonoscopy results was derived from the patient’s chart using manual chart review and patient identifiers (name and health card number). FIT-eligibility was defined as per the NWT screening guidelines: Individuals age 50-74 who are average risk and asymptomatic. This definition excluded individuals with signs and symptoms concerning for CRC (rectal bleeding, melena, anemia, abdominal pain, change in bowel habits, and/or unexplained weight loss), candidates for high risk screening, and/or indications for surveillance colonoscopy[14].
For colonoscopy results, participants were classified by the highest-risk pathology identified (Table 1). Clinically-detected cases of CRC were identified using the most recent data available in the NWT Cancer Registry (only available prior to 2017). Individuals who were between the ages of 50-75 years at the time of diagnosis and were diagnosed between January 1, 2014 to December 31, 2016 were included. We collected data regarding the patient demographics, cancer pathology, stage, and location.
| Classification | Description |
| LRA | One to three tubular adenomas or sessile serrated adenomas that are each < 10 mm diameter |
| HRA | Three or more tubular adenomas or an AA described as having at least one of the following features: Size > 10 mm diameter, villous or tubulovillous histology, and/or high-grade dysplasia |
| AN | Either an AA or cancer |
| CRC | Cancer identified including early stage (I and II), and late stage (III and IV) |
The screening participation rate was calculated using the Canadian Partnership Against Cancer definitions and the estimated cohort of screen eligible individuals age 50-75 from the NWT Bureau of Statistics[15,16]. To assess CRC screening impact, we compared screen-detected cases of CRC to clinically-detected cases. To assess CRC participation, we conducted a subgroup analysis of FIT positive individuals comparing individual with signs and symptoms of CRC to FIT eligible individuals. Statistical analyses were conducted using Microsoft Excel (16.16.19) and RStudio (1.1463). The following tests were used to complete the statistical analyses found in this paper: Two sample Welch t-tests for normally distributed continuous variables, Mann-Whitney-Wilcoxon Tests for data without a normal distribution, Chi-Square Goodness of Fit Tests for categorical variables, and Fisher’s Exact Tests for scenarios where categorical variables did not meet the requisite criteria for Chi-Square testing (i.e., observed frequencies less than 5). Relative risks were calculated using the “epitools” package in R-Studio, which uses the Wald unconditional maximum likelihood estimation and has the option of a small sample adjustment (where appropriate); age, gender and other potentially confounding factors and/or comorbidities were not considered in the calculation of relative risk. A P value of < 0.05 was considered to be statistically significant; all confidence intervals are reported at a 95 percent confidence interval.
Between 2014-2019, 6817 FITs were completed by individuals between the age of 50-74 years, translating to an estimated biannual screening rate of 25.04% on average, 843 (12.37%) FITs were positive after 56 were excluded due to incomplete records or moving out of territory (Figure 1). We compared included and excluded individuals and observed a higher number of excluded individuals identified as Inuit (18 vs expected 5.17), and/or were from the Beaufort Delta region (22 vs expected 7.41). Fewer excluded patients were from Yellowknife (11 vs expected 27.26) (Supplementary Table 1). There was no significant difference in mean age or sex of individuals of included and excluded individuals.
When comparing cases of screen-detected cancer to clinically-detected cancer in 2014-2016 (Table 2), we observed no differences in age, sex, or indigenous status of individuals. In comparing the histology, location, and stage, we did not observe any statistically significant differences between the screen-detected and the clinically-detected cancers (Table 2).
| Baseline characteristics | Screen detected (n = 19) | Clinically detected (n = 34) | P value |
| Age, mean years (SD) | 63.2 (7.47) | 59.1 (7.27) | 0.061 |
| Sex, female (%; Std. Res) | 6 (31.58; -0.56) | 15 (68.42; 0.45) | 0.37 |
| Indigenous (%; Std. Res) | 11 (33.33; -0.24) | 22 (66.66; 0.18) | 0.62 |
| Region of residence (%; Std. Res) | 0.892 | ||
| Beaufort Delta | < 5 (21.05; -0.31) | 9 (26.47; 0.23) | |
| Dehcho | N/A | N/A | |
| Fort Smith | < 5 (5.26; -0.59) | < 5 (11.76; 0.44) | |
| Hay River | < 5 (15.79; 0.31) | < 5 (11.76; -0.23) | |
| Sahtu | < 5 (0; -0.85) | < 5 (5.88; 0.63) | |
| Tilcho | < 5 (10.53; 0.16) | < 5 (8.82; -0.12) | |
| Yellowknife | 9 (47.37; 0.54) | 12 (35.29; -0.40) | |
| Histology type, n (%; Std. Res) | 0.132 | ||
| Adenocarcinoma | 18 (94.74; 0.37) | 28 (82.35; -0.28) | |
| Carcinoid | < 5 (5.26; 1.07) | < 5 (0; -0.80) | |
| Mucinous | < 5 (0; -0.85) | < 5 (5.88; 0.63) | |
| Other | < 5 (0; -1.20) | < 5 (11.76; 0.90) | |
| Location, n (%; Std. Res) | 0.252 | ||
| Proximal | < 5 (10.53; -1.23) | 11 (32.35; 0.92) | |
| Distal | 17 (89.47; 0.70) | 23 (67.65; -0.53) | |
| Stage, n (%; Std. Res) | 0.18 | ||
| Early | 12 (63.16; 0.75) | 15 (44.12; -0.56) | |
| Late | 7 (36.83; -0.76) | 19 (55.88; 0.57) |
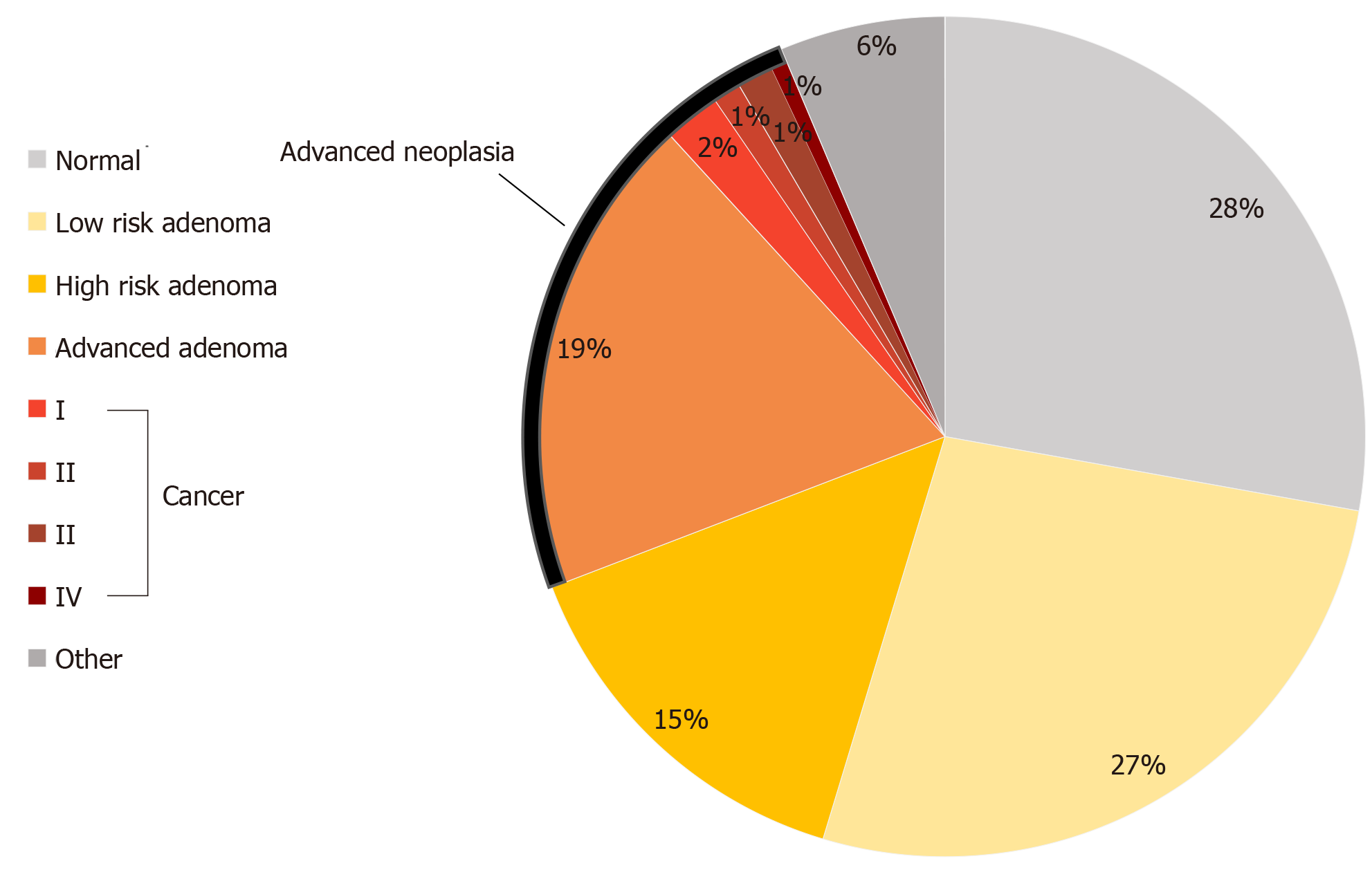
Of the 843 FIT positive individuals who were FIT positive between 2014-2019, 629 (74.61%) underwent a colonoscopy after waiting a median of 133.00 d (IQR 166.5; SD 236.40; mean 207.20). On colonoscopy exam, 380 (60.41%) were found to have adenomas, of which, 120 were AA(s) (Figure 2). Thirty-four individuals were found to have a cancer. This translated to a positive predictive value (PPV) for FIT of 24.48% for AN.
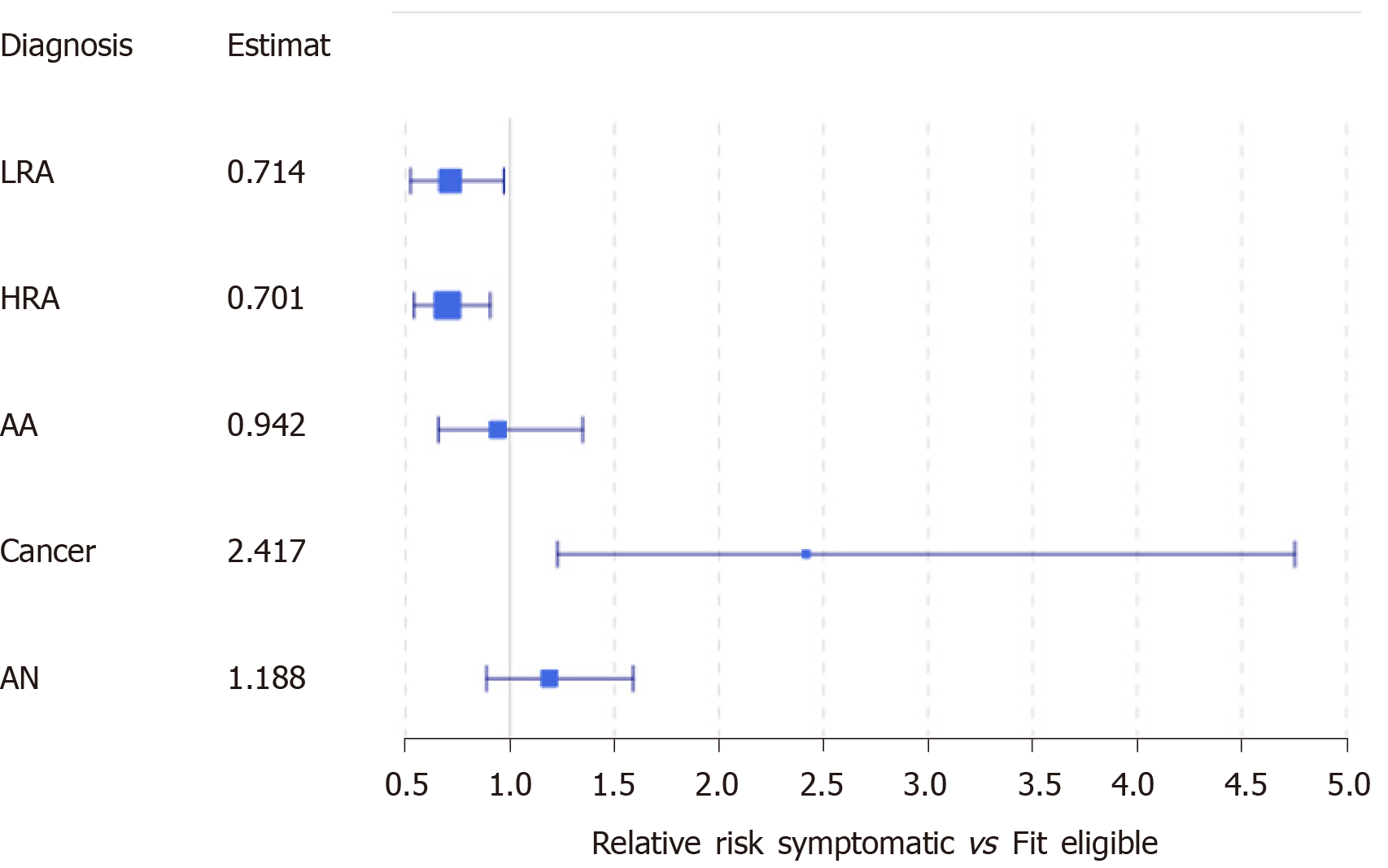
At the time of referral for colonoscopy, 802 individuals were referred for a colonoscopy, of which, 398 (49.62%) met at least one exclusion criteria for FIT screening (Supplementary Figure 1). Among these individuals, we identified 288 (35.91%) with signs or symptoms concerning for CRC. In our first subgroup analysis, we compared symptomatic individuals to FIT eligible individuals, and observed symptomatic individuals were, on average, older than FIT eligible individuals at the time of FIT [61.18 vs 60.15 years, P = 0.047; 95%CI of difference (0.01, 2.06)] (Table 3). Indigenous patients were 49.04% more likely to have symptoms at the time of referral than non-indigenous patients [95%CI of RR: (1.248, 1.780)]. When comparing by the region of residence, we observed that the region of residence was not independent of FIT eligibility (P < 0.01): More patients than expected were referred from Fort Smith with symptoms, and fewer patients than expected from Yellowknife, but neither observation was statistically significant (Table 3 and Supplementary Figure 2). Similar proportions of individuals underwent a colonoscopy however, patients who were symptomatic waited, on average, significantly longer than patients who were asymptomatic [199.5 vs 149.0 d, P < 0.05; 95%CI of difference: (5.07, 41.92)]. Symptomatic individuals were at least 22.8% more likely to have cancer identified on colonoscopy than screen eligible individuals [95%CI of RR: (1.228, 4.754)] (Figure 3).
| Baseline characteristics | FIT eligible (n = 390) | Symptomatic (n = 288) | P value |
| Age, mean years (SD) | 60.15 (6.55) | 61.18 (6.82) | 0.04741 |
| Sex, female, n (%; Std. Res) | 157 (40.26; -0.52) | 127 (44.10; 0.58) | 0.316 |
| Indigenous, n (%; Std. Res) | 152 (38.97; -2.15) | 162 (56.25; 2.49) | 0.000008 |
| Region of residence, n (%; Std. Res) | 0.0092 | ||
| Beaufort Delta | 42 (10.77; -0.26) | 34 (11.81; 0.30) | |
| Dehcho | 17 (4.36; -0.58) | 17 (5.90; 0.67) | |
| Fort Smith | 26 (6.67; -1.62) | 36 (12.50; 1.88) | |
| Hay River | 39 (10.00; -0.80) | 38 (13.19; 0.93) | |
| Sahtu | 21 (5.38; 0.06) | 15 (5.21; -0.08) | |
| Tilcho | 18 (4.62; -0.94) | 21 (7.29; 1.09) | |
| Yellowknife | 227 (58.20; 1.64) | 127 (44.10; -1.91) | |
| Size of health centre, n (%; Std. Res) | 0.0002 | ||
| Yellowknife | 219 (56.15; 1.94) | 115(39.93; -2.26) | |
| Regional | |||
| Fort Smith | 26 (6.67; -1.62) | 36 (12.50; 1.88) | |
| Hay River | 36 (9.23; -0.84) | 36 (12.50; 0.98) | |
| Inuvik | 21(5.38; 0.60) | 11(3.82; -0.70) | |
| Small community | 88 (22.56; -1.42) | 90 (31.25; 1.66) | |
| Colonoscopy, n (%) | 333 (85.38) | 212 (74.65) | 0.29 |
| Completed, n (%; Std. Res) | 318 (95.50; 0.15) | 198 (93.40; -0.19) | |
| Incomplete, n (%; Std. Res) | 15 (4.50; -0.65) | 14 (6.60; 0.81) | |
| FIT to colonoscopy, d | |||
| Mean | 159.9 | 183.4 | 0.011 |
| Median | 149.0 | 199.5 | 0.02 |
| < 60 d, n (%; Std. Res) | 79 (23.87; 0.88) | 38 (18.10; -1.10) | |
| 60-180 d, n (%; Std. Res) | 111 (33.53; 0.69) | 59 (28.10; -0.86) | 0.04 |
| > 180 d, n (%; Std. Res) | 141 (42.60; -1.16) | 113 (53.80; 1.45) | 0.011 |
| Findings, n (%; Std. Res) | |||
| Low risk adenoma | 99 (29.73; 1.17) | 45 (21.23; -1.47) | 0.03 |
| High risk adenoma | 130 (39.03; 1.41) | 58 (27.36; -1.77) | 0.005 |
| Advanced adenoma | 65 (19.52; 0.18) | 39 (18.40; -0.23) | 0.75 |
| Cancer | 13 (3.90; -1.60) | 20 (9.43; 2.00) | 0.008 |
| Advanced neoplasia | 78 (23.42; -0.62) | 59 (27.83; 0.78) | 0.25 |
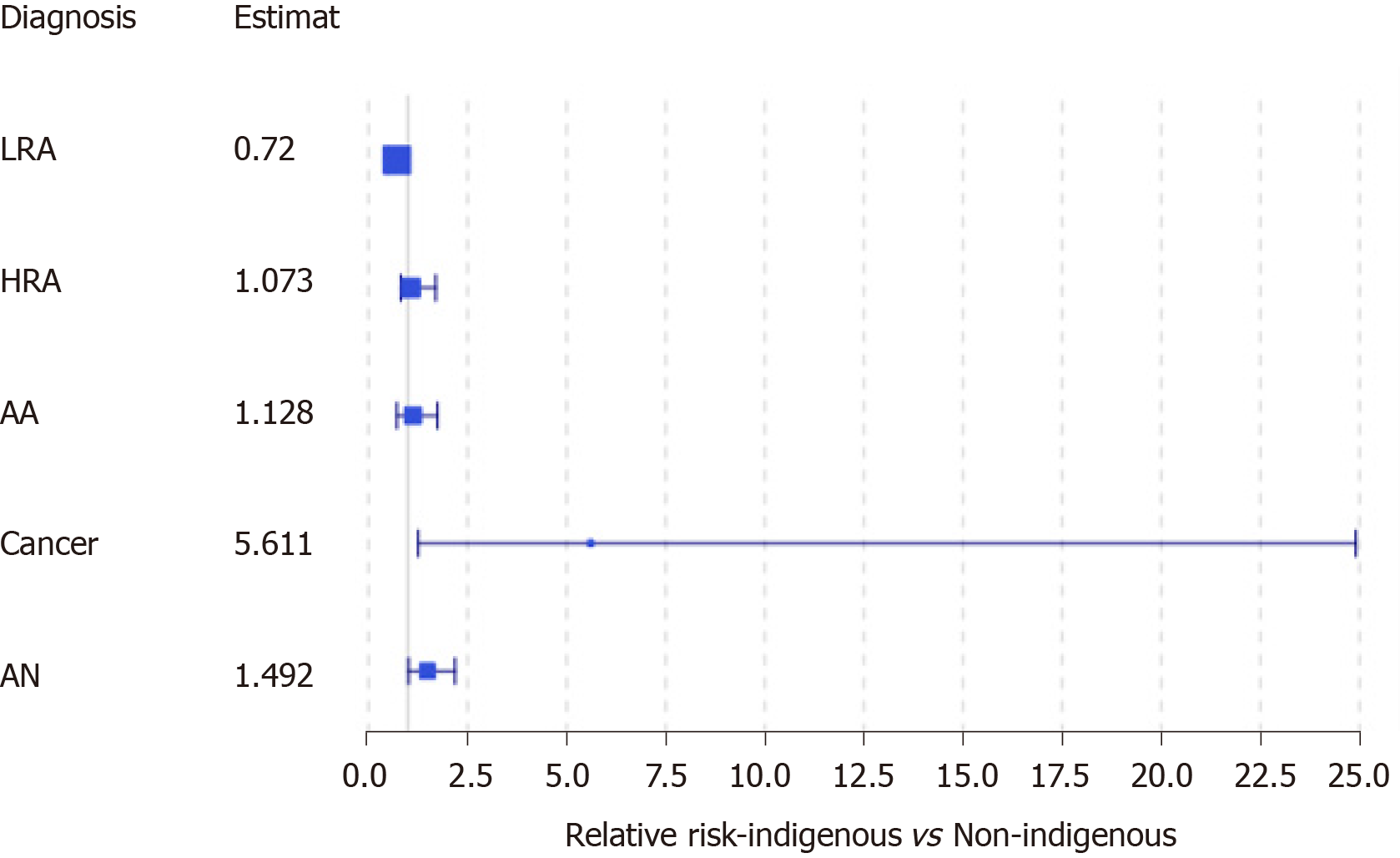
When looking at the outcomes of FIT eligible individuals alone who underwent a colonoscopy, 229 of 333 had adenomas, of which, 130 were higher risk adenomas (HRAs). We identified 13 FIT eligible individuals who were diagnosed with CRC, the majority of which were early stage (I or II, 63.1%). The PPV for FIT among asymptomatic eligible individuals was 42.9% for HRA and advanced neoplasia (AN) combined, 23.4% for AN and 3.9% for cancer. We conducted a second subgroup analysis to look at only FIT eligible individuals comparing those with AN to those without. We observed no significant difference in sex (P = 0.30), or age (59.69 years with AN vs 59.96 years without; P = 0.746). However, we did observe that indigenous patients experienced an estimated 49.2% higher relative risk of AN compared to non-indigenous patients [95%CI of RR (adjusted for small sample): (1.0145, 2.194)] (Figure 4). We also observed that those with AN, on average, experienced a longer wait-time for colonoscopy than those without AN [194.54 vs 148.09 d; 95%CI of difference: (20.04, 72.85), P = 0.0007]. We found that that the relative risk of having an AN for FIT eligible patients who wait more than 180 d is estimated to be 68.21% more than those who wait less than 180 d [95%CI (adjusted for small sample size): (1.138, 2.487)]. The availability of colonoscopy services within the patients’ community of residence was not associated with a diagnosis of AN.
Disparities in CRC incidence and outcomes exist between populations and could be reduced through CRC screening[17]. In this retrospective cohort study of CRC screening in a remote northern population, known to experience significant disparities in CRC, FIT-based CRC screening did not facilitate earlier CRC detection. This may be due to the limited participation of only 25% of eligible individuals, and frequent participation of ineligible individuals (49.6% of FIT positive individuals who underwent colonoscopy)[18]. Nonetheless, CRC screening appears to have facilitated effective adenoma detection, a majority of which would be amenable to removal at index colonoscopy and therefore, may reduce the risk of CRC in the long-term[19]. The positivity rate and PPV of FIT were higher than observed in a recent prospective trial by Liles et al[20] which employed the same brand and FIT threshold for 2761 individuals undergoing screening and observed a FIT positivity rate of 8.1%, and PPV for HRA or cancer of 21.9%-24.8% (in contrast to this study we observed a positivity rate of 12.3% and PPV of 43.8%). In reviewing CRC screening participation and outcomes, we observed three factors which appear to contribute to the relatively high rate of AN in this population which warrant further discussion: (1) Individuals with signs and symptoms of CRC frequently participated in screening, (2) Patients experienced long wait-times for colonoscopy, and (3) Indigenous individuals experienced a higher burden of CRC than non-indigenous.
Over 1 in 3 FIT positive individuals had signs or symptoms of CRC at the time of screening, despite the recommendations for their exclusion from screening. Several studies have similarly evaluated the symptoms of FIT positive individuals and observed even higher rates among participants of 47%-78%[21-23]. This may have important implications for CRC screening positivity and definition of “asymptomatic” screen-detected cancer[24]. We observed higher rates of CRC among individuals with reported signs or symptoms than FIT eligible individuals. However, the predictive value of red flag symptoms for colorectal pathology has been found to be variable[21-23]. De Klerk et al[23] (2018) reviewed 527 FIT positive patients and the 41% of individuals who had symptoms had a higher odds of CRC but the results were not statistically significant (OR 1.64 CI 0.86-3.13). In their analysis by individual symptoms, only a change in bowel habits or blood in the stool were associated with CRC detection at colonoscopy (OR 2.86, CI 1.23-6.62 and OR 8.65, CI 2.35-32.0). Previous systematic reviews summarizing the predictive value of red flag signs and symptoms, independent of FIT, have found rectal bleeding has diagnostic value, but other signs and symptoms only provide modest diagnostic value[25,26]. At present, guidelines clearly recommend against FIT screening of symptomatic individuals. The observed frequent use of FIT by symptomatic patients, may be partially attributed to the long wait-times for colonoscopy in the NWT. Previous interviews with clinicians in the NWT suggest that clinicians use FIT as a mechanism to accelerate a patient’s access to colonoscopy[4]. This strategy does not appear to be effective, patients in this study with symptoms waited significantly longer for colonoscopy than FIT eligible individuals (P = 0.036)[13]. Further research is needed to discern the reasons individuals with red flag symptoms to undergo screening and the impact of their participation on the diagnostic yield of FIT in order to guide screening and endoscopy protocols.
Patients in this study experienced long wait-times for colonoscopy following a positive FIT. Longer wait-times were associated with more advanced pathology at colonoscopy. National screening quality guidelines in Canada recommend a follow-up colonoscopy be completed within 60 d of a positive FIT and define a follow-up colonoscopy as one completed within 180 d of FIT[16]. In this study, only 23.87% of FIT eligible individuals met the benchmark of 60 d. Individuals who waited more than 180 d were 68.21% more likely to have AN than those waited ≤ 180 d. Other studies have similarly observed wait-times for colonoscopy after fecal screening test to be associated with more advanced pathology at colonoscopy[27,28]. Corley et al[27] found wait-times of 10-12 mo associated with a higher odds of CRC [OR 1.49 (95%CI 1.05-2.08)]. Flugelman et al[28] identified a significant relationship between increasing wait-time interval and stage of disease at presentation, as well as an association between wait-times more than 12 mo and a higher risk of CRC mortality [adjusted hazard ratio 1.53 (1.13-2.12)]. Colonoscopy wait-times could be contributing the overall CRC disparities experienced by this population and these findings reinforce the expert recommendations for prompt colonoscopy follow-up after FIT to enhance the quality of screening, and potentially, detect AN earlier. The underlying cause for long colonoscopy wait-times cannot be fully elucidated by this study but is likely multifactorial as demonstrated in our recent analysis of colonoscopy cancellations in this region[29]. Increasing colonoscopy access in this remote region is complex but could provide substantial benefit to patients.
Finally, we observed significantly higher rates of AN and cancer among indigenous individuals compared to non-indigenous. Indigenous Canadians have been found to experience important barriers to accessing cancer care and disparities in cancer outcomes in Canada[30-32]. This study provides an important contribution by reporting CRC rates among indigenous residents in northern Canada. A study of Alaskan indigenous populations observed that they experienced a higher CRC incidence than other ethnic groups in the United States. Boardman et al[33] assessed this further by comparing the tumour genetics among Alaskans but found no significant differences, and concluded that the cause of the higher incidence of CRC is likely multifactorial and attributable to recent diet changes, namely higher intake in fat, refined carbohydrates. Cancer is increasingly a public health concern among northern indigenous populations and our study is the first demonstrate this in CRC screening results[34]. The higher incidence of malignancy we observed advocates for further research and a potentially a re-evaluation of the current screening protocol for this cohort. Individuals with a first degree relative with a history of CRC have a 1.9-4.4 relative risk of CRC compared to average risk individual and are recommended to undergo colonoscopy screening every 5-10 years in Canada[35]. As such, indigenous individuals may benefit from enhanced screening to optimize CRC detection and control. This would require further analysis of the risks and benefits in discussion with indigenous healthcare leaders in the NWT.
The generalizability of our results is limited to the retrospective data collected in the health records and small sample size. Cancer registry data for clinically detected CRC was only available prior to 2018 which limited the timeframe of comparing screen-detected and clinically detected CRC. FIT eligibility was derived from clinicians notes and therefore dependent on the consistency of provider documentation. We excluded 51 individuals due to inaccessible records, a higher than anticipated proportion of these individuals were from Beaufort Delta region and/or were Inuit. This is not surprising given that accessing paper records in this region was challenging. The included Inuit and Beaufort Delta residents did not experience any significant difference in colonoscopy outcomes than the remainder of the cohort. Finally, the population of the NWT is small, and factors not captured in this study such as patient comorbidities, and substance use may confound patients’ cancer risk.
In this study of a northern remote population, FIT-based CRC screening did not appear to prevent CRC or provide earlier detection but did result in more frequent positive pathology results than anticipated for average risk screening. Individuals were more likely to have CRC at the time of screening if they experienced long wait-times for colonoscopy, had clinical signs and symptoms of CRC, and/or were indigenous. Increasing access to colonoscopy, and effective triaging of FIT eligible individuals could enhance CRC screening effectiveness. Further research is needed to understand how to increase colonoscopy access in this remote region, and to discern if colonoscopy screening should be adopted among indigenous populations given their relative risk of CRC.
Screening provides earlier colorectal cancer (CRC) detection and improves outcomes. However, it remains poorly understood if these benefits are realized with screening guidelines in remote northern populations where access to colonoscopy is limited. This study provides a critical contribution to this knowledge gap by providing an overview of the participation in, and impact of, CRC screening guidelines in a remote northern region of Canada that experiences higher rates of CRC: The Northwest Territories (NWT).
Previous studies suggest that remote and indigenous populations may experience significant geographic and systemic barriers to accessing CRC screening as well as a higher rate of CRC than other populations. To optimize CRC screening, a better understanding of current participation and screening outcomes in northern populations is critical.
This study aimed to evaluate the participation and outcomes of CRC screening in a remote northern population. In particular, we sought to understand the effectiveness of screening in the NWT and identify factors which may contribute to the likelihood of advanced neoplasia being detected among participants. Realizing these objectives will help inform future CRC screening in the NWT and our understanding of CRC screening access and effectiveness for northern populations.
A population-based, retrospective cohort study was conducted of individuals who underwent CRC screening in the NWT in the last 5 years. This is the first study to review the results of CRC screening in a remote northern population.
Screen-detected cancer cases were not detected earlier than clinically-detected cases which suggests screening was not particularly effective and warrants further research. Potentially contributing to this trend were the findings that individuals experienced a higher incidence of CRC if they had signs and symptoms of CRC at screening, experienced long colonoscopy wait-times, or were indigenous. Further research is needed to further characterize the risk of CRC among indigenous individuals and inform strategies to improve colonoscopy access in the NWT.
These findings suggest that critical gaps in colonoscopy access, triaging of eligible individuals, and knowledge of CRC risk among indigenous individuals may be impairing the CRC screening effectiveness for this remote northern population. This highlights the importance of pragmatic evaluation of CRC screening in remote and indigenous populations.
Further research is needed to inform colonoscopy access for remote populations and to optimize screening for indigenous populations. Research in other northern regions is crucial to inform the generalizability of our findings.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Canada
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mudawi HMMY S-Editor: Huang P L-Editor: A P-Editor: Li JH
| 1. | Mendivil J, Appierto M, Aceituno S, Comas M, Rué M. Economic evaluations of screening strategies for the early detection of colorectal cancer in the average-risk population: A systematic literature review. PLoS One. 2019;14:e0227251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Hewitson P, Glasziou P, Watson E, Towler B, Irwig L. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): an update. Am J Gastroenterol. 2008;103:1541-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 720] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 3. | Altobelli E, Lattanzi A, Paduano R, Varassi G, di Orio F. Colorectal cancer prevention in Europe: burden of disease and status of screening programs. Prev Med. 2014;62:132-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 125] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 4. | Champion C, Alvarez GG, Affleck E, Kuziemsky C. A systems perspective on rural and remote colorectal cancer screening access. J Cancer Policy. 2017;14:27-32. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Alasia A, Bedard F, Belanger J, Guimond E, Penney C. Measuring remoteness and accessibility: A set of indices for Canadian communities [Internet]. In: Reports on Special Business Projects. Statistics Canada; 2017 [cited 16 April 2020]. Available from: https://www150.statcan.gc.ca/n1/pub/18-001-x/18-001-x2017002-eng.htm. |
| 6. | Christou A, Katzenellenbogen JM, Thompson SC. Australia's national bowel cancer screening program: does it work for indigenous Australians? BMC Public Health. 2010;10:373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Moore SP, Antoni S, Colquhoun A, Healy B, Ellison-Loschmann L, Potter JD, Garvey G, Bray F. Cancer incidence in indigenous people in Australia, New Zealand, Canada, and the USA: a comparative population-based study. Lancet Oncol. 2015;16:1483-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 8. | Friborg JT, Melbye M. Cancer patterns in Inuit populations. Lancet Oncol. 2008;9:892-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Moore SP, Green AC, Bray F, Coory M, Garvey G, Sabesan S, Valery PC. Colorectal cancer among Indigenous and non-Indigenous people in Queensland, Australia: Toward survival equality. Asia Pac J Clin Oncol. 2016;12:e209-e214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Statistics Canada. Number of new cases and age-standardized rates of primary cancer, by stage at diagnosis, selected cancer type and sex [Internet]. 2017 [cited 23 March 2020] Available from: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1310076201. |
| 11. | Canadian Task Force on Preventive Health Care. Recommendations on screening for colorectal cancer in primary care. CMAJ. 2016;188:340-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 207] [Article Influence: 23.0] [Reference Citation Analysis (1)] |
| 12. | Leddin DJ, Enns R, Hilsden R, Plourde V, Rabeneck L, Sadowski DC, Signh H. Canadian Association of Gastroenterology position statement on screening individuals at average risk for developing colorectal cancer: 2010. Can J Gastroenterol. 2010;24:705-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 13. | Paterson WG, Depew WT, Paré P, Petrunia D, Switzer C, Veldhuyzen van Zanten SJ, Daniels S; Canadian Association of Gastroenterology Wait Time Consensus Group. Canadian consensus on medically acceptable wait times for digestive health care. Can J Gastroenterol. 2006;20:411-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 101] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Health and Social Service Authority. Colorectal Cancer Screening [Internet]. [cited 7 April 2020] Available from: https://www.nthssa.ca/en/services/d%C3%A9pistage-du-cancer/colorectal-cancer-screening. |
| 15. | Population Estimates – NWT. NWT Bureau of Statistics [Internet]. [cited 22 November 2019] Available from: https://www.statsnwt.ca/population/population-estimates/. |
| 16. | Canadian Partnership Against Cancer, Armstrong D, Cheung W, Zhu T, Jalili F, Varner L, Irwin C. Colorectal Cancer Screening in Canada: Monitoring & Evaluation of Quality Indicators [Internet]. Toronto: Canadian Partnership Against Cancer; 2017. Available from: https://s22457.pcdn.co/wp-content/uploads/2019/01/Colorectal-Screening-Monitoring-Report-2014-EN.pdf. |
| 17. | Carethers JM, Doubeni CA. Causes of Socioeconomic Disparities in Colorectal Cancer and Intervention Framework and Strategies. Gastroenterology. 2020;158:354-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 216] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 18. | Cancer Care Ontario. Ontario Cancer Screening Performance Report 2016 [Internet]. Toronto: Cancer Care Ontario; 2016. Available from: https://www.cancercareontario.ca/sites/ccocancercare/files/assets/CCOCancerScreeningPerformanceReport.pdf. |
| 19. | Winawer SJ, Zauber AG, Ho MN, O'Brien MJ, Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3107] [Cited by in RCA: 3128] [Article Influence: 97.8] [Reference Citation Analysis (1)] |
| 20. | Liles EG, Perrin N, Rosales AG, Smith DH, Feldstein AC, Mosen DM, Levin TR. Performance of a quantitative fecal immunochemical test for detecting advanced colorectal neoplasia: a prospective cohort study. BMC Cancer. 2018;18:509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Vaughan-Shaw PG, Cutting J, Borley N, Brooklyn T, Wheeler JM. Two-week wait symptoms are prevalent in screened patients with a positive faecal occult blood test but do not predict cancer. Colorectal Dis. 2014;16:40-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Ahmed S, Leslie A, Thaha MA, Carey FA, Steele RJ. Lower gastrointestinal symptoms are not predictive of colorectal neoplasia in a faecal occult blood screen-positive population. Br J Surg. 2005;92:478-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | de Klerk CM, van der Vlugt M, Bossuyt PM, Dekker E. A large proportion of fecal immunochemical test-positive participants in colorectal cancer screening is symptomatic. United European Gastroenterol J. 2018;6:471-479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Harmston C, Akwei S, Barnes R, Goodyear S, Wong L. Are screen detected colorectal cancers asymptomatic? Colorectal Dis. 2010;12:416-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Olde Bekkink M, McCowan C, Falk GA, Teljeur C, Van de Laar FA, Fahey T. Diagnostic accuracy systematic review of rectal bleeding in combination with other symptoms, signs and tests in relation to colorectal cancer. Br J Cancer. 2010;102:48-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Adelstein BA, Macaskill P, Chan SF, Katelaris PH, Irwig L. Most bowel cancer symptoms do not indicate colorectal cancer and polyps: a systematic review. BMC Gastroenterol. 2011;11:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 27. | Corley DA, Jensen CD, Quinn VP, Doubeni CA, Zauber AG, Lee JK, Schottinger JE, Marks AR, Zhao WK, Ghai NR, Lee AT, Contreras R, Quesenberry CP, Fireman BH, Levin TR. Association Between Time to Colonoscopy After a Positive Fecal Test Result and Risk of Colorectal Cancer and Cancer Stage at Diagnosis. JAMA. 2017;317:1631-1641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 222] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 28. | Flugelman AA, Stein N, Segol O, Lavi I, Keinan-Boker L. Delayed Colonoscopy Following a Positive Fecal Test Result and Cancer Mortality. JNCI Cancer Spectr. 2019;3:pkz024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 29. | Smith H, Brunet N, Tessier A, Boushey R, Kuziemsky C. Barriers to colonoscopy in remote northern Canada: an analysis of cancellations. Int J Circumpolar Health. 2020;79:1816678. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Nader F, Kolahdooz F, Sharma S. Assessing Health Care Access and Use among Indigenous Peoples in Alberta: a Systematic Review. J Health Care Poor Underserved. 2017;28:1286-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Lavoie JG, Kaufert J, Browne AJ, O'Neil JD. Managing Matajoosh: determinants of first Nations' cancer care decisions. BMC Health Serv Res. 2016;16:402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Kolahdooz F, Jang SL, Corriveau A, Gotay C, Johnston N, Sharma S. Knowledge, attitudes, and behaviours towards cancer screening in indigenous populations: a systematic review. Lancet Oncol. 2014;15:e504-e516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 33. | Boardman LA, Lanier AP, French AJ, Schowalter KV, Burgart LJ, Koller KR, McDonnell SK, Schaid DJ, Thibodeau SN. Frequency of defective DNA mismatch repair in colorectal cancer among the Alaska Native people. Cancer Epidemiol Biomarkers Prev. 2007;16:2344-2350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Young TK, Kelly JJ, Friborg J, Soininen L, Wong KO. Cancer among circumpolar populations: an emerging public health concern. Int J Circumpolar Health. 2016;75:29787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 35. | Henrikson NB, Webber EM, Goddard KA, Scrol A, Piper M, Williams MS, Zallen DT, Calonge N, Ganiats TG, Janssens AC, Zauber A, Lansdorp-Vogelaar I, van Ballegooijen M, Whitlock EP. Family history and the natural history of colorectal cancer: systematic review. Genet Med. 2015;17:702-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 36. | Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1367] [Cited by in RCA: 1446] [Article Influence: 111.2] [Reference Citation Analysis (0)] |
| 37. | Gupta S, Lieberman D, Anderson JC, Burke CA, Dominitz JA, Kaltenbach T, Robertson DJ, Shaukat A, Syngal S, Rex DK. Recommendations for Follow-Up After Colonoscopy and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2020; 158: 1131-1153. e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 285] [Article Influence: 57.0] [Reference Citation Analysis (0)] |