Published online Dec 21, 2020. doi: 10.3748/wjg.v26.i47.7528
Peer-review started: September 30, 2020
First decision: November 13, 2020
Revised: November 18, 2020
Accepted: November 29, 2020
Article in press: November 29, 2020
Published online: December 21, 2020
Processing time: 79 Days and 19.2 Hours
In recent years, an increasing prevalence of obesity in inflammatory bowel disease (IBD) has been observed. Obesity, moreover, has been directly correlated with a more severe clinical course and loss of response to treatment.
To assess the prevalence and associated factors of obesity in IBD.
We collected data about IBD disease pattern and activity, drugs and laboratory investigations in our center. Anthropometric measures were retrieved and obesity defined as a body mass index (BMI) > 30. Then, we compared characteristics of obese vs non obese patients, and Chi-squared test and Student’s t test were used for discrete and continuous variables, respectively, at univariate analysis. For multivariate analysis, we used binomial logistic regression and estimated odd ratios (OR) and 95% confidence intervals (CI) to ascertain factors associated with obesity.
We enrolled 807 patients with IBD, either ulcerative colitis (UC) or Crohn’s disease (CD). Four hundred seventy-four patients were male (58.7%); the average age was 46.2 ± 13.2 years; 438 (54.2%) patients had CD and 369 (45.8%) UC. We enrolled 378 controls, who were comparable to IBD group for age, sex, BMI, obesity, diabetes and abdominal circumference, while more smokers and more subjects with hypertension were observed among controls. The prevalence of obesity was 6.9% in IBD and 7.9% in controls (not statistically different; P = 0.38). In the comparison of obese IBD patients and obese controls, we did not find any difference regarding diabetes and hypertension prevalence, nor in sex or smoking habits. Obese IBD patients were younger than obese controls (51.2 ± 14.9 years vs 60.7 ± 12.1 years, P = 0.03). At univariate analysis, obese IBD were older than normal weight ones (51.2 ± 14.9 vs 44.5 ± 15.8, P = 0.002). IBD onset age was earlier in obese population (44.8 ± 13.6 vs 35.6 ± 15.6, P = 0.004). We did not detect any difference in disease extension. Obese subjects had consumed more frequently long course of systemic steroids (66.6% vs 12.5%, P = 0.02) as well as antibiotics such as metronidazole or ciprofloxacin (71.4% vs 54.7%, P = 0.05). No difference about other drugs (biologics, mesalazine or thiopurines) was observed. Disease activity was similar between obese and non obese subjects both for UC and CD. Obese IBD patients suffered more frequently from arterial hypertension, type 2 diabetes, non-alcoholic fatty liver disease. Regarding laboratory investigations, obese IBD patients had higher levels of triglyceridemia, fasting blood glucose, gamma-glutamyl-transpeptidase. On multivariate analysis, however, the only factor that appeared to be independently linked to obesity in IBD was the high abdominal circumference (OR = 16.3, 95%CI: 1.03-250, P = 0.04).
Obese IBD patients seem to have features similar to general obese population, and there is no disease-specific factor (disease activity, extension or therapy) that may foster obesity in IBD.
Core Tip: Obesity in inflammatory bowel disease (IBD) may be correlated with a more severe clinical course and loss of response to treatment. We did not find any peculiar difference between obese IBD patients and controls. On the other hand, it is possible that some drugs, such as steroids or antibiotics may contribute to the development of obesity in IBD, despite our results suggest that a more complex interaction of several factors could be more likely.
- Citation: Losurdo G, La Fortezza RF, Iannone A, Contaldo A, Barone M, Ierardi E, Di Leo A, Principi M. Prevalence and associated factors of obesity in inflammatory bowel disease: A case-control study. World J Gastroenterol 2020; 26(47): 7528-7537
- URL: https://www.wjgnet.com/1007-9327/full/v26/i47/7528.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i47.7528
Obesity is a growing problem in developed countries, since it is going to become the leading cause for mortality due to cardiovascular events[1]. In Italy, it is estimated that about the 18% of population is suffering from obesity[2]. The World Health Organization (WHO) defines obesity by a value of body mass index (BMI) above 30 kg/m2, but obesity underlies as well an excessive visceral fat distribution, with several alterations at hormonal, inflammatory and endothelial level[3]. Inflammatory bowel disease (IBD) is a group of chronic inflammatory autoimmune disorders of gastrointestinal tract, mainly represented by Crohn’s disease (CD) and ulcerative colitis (UC). The problem of obesity is spreading in the context of IBD, since, in the past, it has been rarely recognized for the frequent association between IBD and malnutrition. However, nowadays, novel and more effective drugs are able to stop the progression of the disease, thus preventing malnutrition[4]. Indeed, a study performed in 2002 found a prevalence of obesity in CD of about 3%[5]; one decade later, however, in another study a prevalence of 31.5% was recorded[6]. Co-occurrence of obesity and IBD is not just a casual phenomenon and it has been emphasized that obesity may lead to a higher risk of perianal complications, higher hospitalization rates and greater risk of disease flares[5]. Moreover, obese patients with IBD on azathioprine were more likely to need courses of systemic corticosteroids and had higher recurrence rates after stopping the drug[7]. Furthermore, it has been estimated that an increase of one unit of BMI may increase the risk of therapeutic failure of 4%[8] and, in particular, a high BMI was an independent predictor of adalimumab therapy failure[9]. Considering that IBD per se could increase the risk of endothelial dysfunction and cardiovascular risk[10,11], the association between obesity and IBD may represent a very important issue.
Therefore, we aimed, in a case-control study, to investigate the prevalence of obesity in IBD patients and detect possible factors associated to this condition.
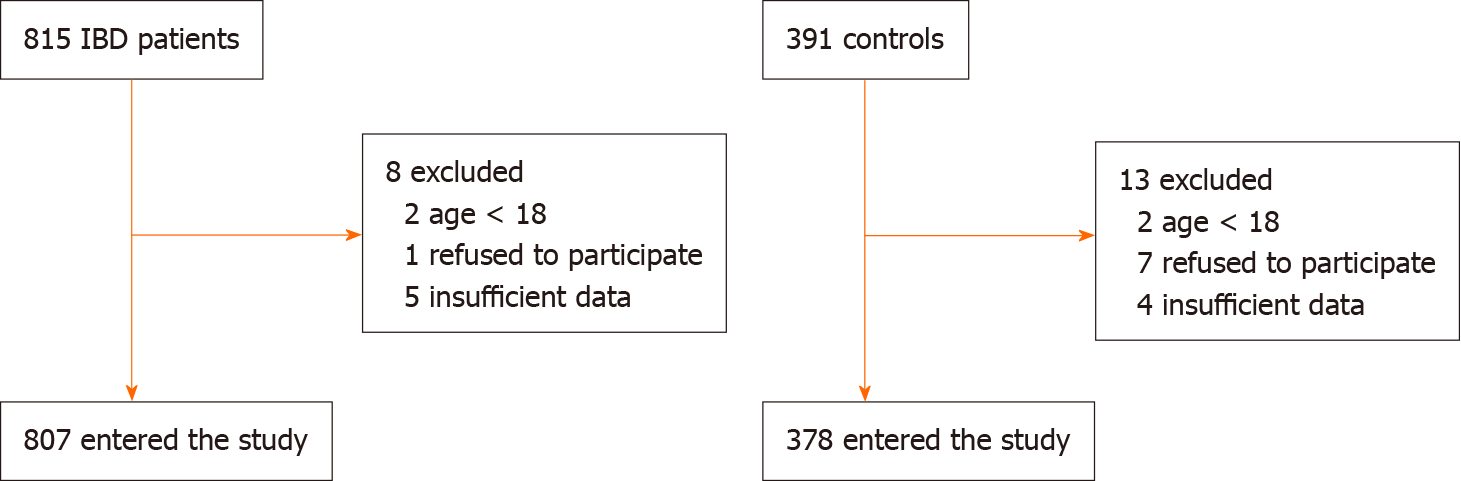
We consecutively recruited IBD patients referred to our outpatient tertiary Gastroenterology Unit (University Hospital Policlinico, Bari, Italy) in the period October 2016-October 2017. We only excluded patients aging < 18, doubtful IBD diagnosis and those who refused to participate in the study. Outpatients with functional gastrointestinal disorders constituted the control group. The study was approved by the independent Ethics Committee of the Policlinico di Bari (protocol No. 4862) and was performed according to the Helsinki declaration 1975 statements.
For each patient, age, sex, abdominal circumference, weight and BMI, smoking habits and relevant comorbidities were collected. Obesity was diagnosed when BMI > 30[3]. For IBD patients, the diagnosis was achieved by a combination of endoscopy, histology (in all cases) and, for all CD patients, a transmural evaluation by magnetic resonance enterography. Then, we collected data about IBD staging (according to Montreal classification), clinical disease activity [partial Mayo for UC and Harvey-Bradshaw index (HBI) for CD] and specific therapies. For IBD patients we recorded data about laboratory investigations, in particular full blood count, erythrocyte sedimentation rate (ESR), C reactive protein (CRP), parameters of liver function, glucose and fat homeostasis. Liver steatosis was diagnosed by abdominal ultrasound, according to known criteria and already described in a previous experience[12,13].
Controls underwent only anthropometric and clinical history assessment, because all examinations that have been performed for IBD are not indicated nor refunded by Italian Health Service.
At univariate analysis, we compared IBD patients with and without obesity. Student's t test was used for continuous variables, while the chi-square test was used for discrete variables. Correlation was analyzed by Pearson’s r. Significant factors in univariate analysis were analyzed at multivariate analysis by binary logistic regression, considering obesity as an independent variable. Odds ratios (ORs) and respective 95% confidence intervals (CI) were calculated. All analyses were two-tailed; P values < 0.05 were considered statistically significant. The analysis was carried out using SPSS.21 software for Windows.
We enrolled 807 patients with IBD. The process of patients selection is reported in Figure 1. Four hundred seventy-four patients were male (58.7%); the average age was 46.2 ± 13.2 years. The average age of onset of IBD was 20 ± 9.4 years and we did not find any difference of age onset between UC and CD (19.8 ± 6.8 vs 20.4 ± 6.2; P = 0.19). Of these, 438 (54.2%) patients had CD and 369 (45.8%) UC.
The majority of CD patients had an inflammatory behavior (54.1%) and an ileal localization (45.2%); perianal involvement was reported only in 3 patients (0.68%). The 24.9% of patients with CD had undergone previous surgical treatment. The average clinical disease activity detected at enrollment and assessed by Harvey Bradshaw Index was 1.3 ± 2.4.
Regarding UC, 164 (44.4%) patients had proctitis. 30 patients (8.1%) had undergone colectomy. The mean clinical activity of disease detected at the time of enrollment, assessed by Mayo partial Score, was 1.7 ± 1. Further details about baseline features of IBD population are reported in Table 1.
| Parameter | mean ± SD or n (%) |
| Age (yr) | 46.2 ± 13.2 |
| Sex | |
| Male | 474 (58.7) |
| Female | 333 (41.3) |
| Smokers | 132 (16.4) |
| BMI (kg/m2) | 24.5 ± 5.0 |
| CD | 438 (54.2) |
| UC | 369 (45.8) |
| IBD age onset | 20.0 ± 9.4 |
| HBI | 1.3 ± 2.4 |
| CD behavior | |
| Inflammatory B1 | 237 (54.1) |
| Stricturing B2 | 128 (29.2) |
| Penetrating B3 | 60 (16.7) |
| CD extension | |
| L1 Ileal | 198 (45.2) |
| L2 colic | 44 (10.0) |
| L3 ileocolic | 164 (37.4) |
| Perianal | 3 (0.68) |
| L4 upper GI | 16 (3.6) |
| UC extension | |
| Proctitis E1 | 164 (44.4) |
| Left colitis E2 | 55 (14.9) |
| Pancolitis E3 | 123 (40.7) |
| Mayo Score | 1.7 ± 1 |
| CD previous surgery | 109 (24.9) |
| Colectomy for UC | 30 (8.1) |
| Azathioprine | 300 (37.2) |
| Systemic corticosteroids | |
| < 3 courses/yr | 471 (58.4) |
| > 3 courses/yr | 101 (12.5) |
| Topical corticosteroids | |
| In course | 94 (11.6) |
| Previously taken | 190 (23.5) |
| Infliximab | 147 (18.2) |
| Adalimumab | 131 (16.2) |
| Golimumab | 28 (3.5) |
| Vedolizumab | 35 (4.3) |
| Antibiotics | 451 (55.9) |
| Diabetes | 39 (4.8) |
| Hypertension | 79 (9.8) |
| Waist circumference (> 102 cm males, > 88 females) | 138 (17.1) |
| Liver steatosis | 232 (28.7) |
We enrolled 378 controls, who were comparable to IBD group for age and sex, as shown in Table 2, reporting the main characteristics of IBD patients and controls.
| Variable | IBD (n = 807) | Controls (n = 378) | P value |
| Age | 46.2 ± 13.2 | 45.9 ± 17.7 | 0.66 |
| Male sex | 474 (58.7%) | 210 (55.5%) | 0.30 |
| BMI | 24.5 ± 5.0 | 24.4 ± 4.4 | 0.74 |
| Obesity | 56 (6.9%) | 30 (7.9%) | 0.38 |
| Diabetes | 39 (4.8%) | 14 (3.7%) | 0.77 |
| Smokers | 132 (16.4%) | 90 (23.8%) | 0.002 |
| Hypertension | 79 (9.8%) | 82 (21.7%) | < 0.001 |
| Abdominal circumference > 102 in males, > 88 in females | 138 (17.1%) | 76 (20.1%) | 0.21 |
The prevalence of obesity was 6.9% in IBD and 7.9% in controls (not statistically different; P = 0.38). Values of BMI were comparable between the two groups (24.5 ± 5.0 vs 24.4 ± 4.4, P = 0.74). More patients with hypertension and more smokers were observed in control group. Obesity rate did not differ between CD and UC (respectively 7.3% and 6.5%, P = 0.65).
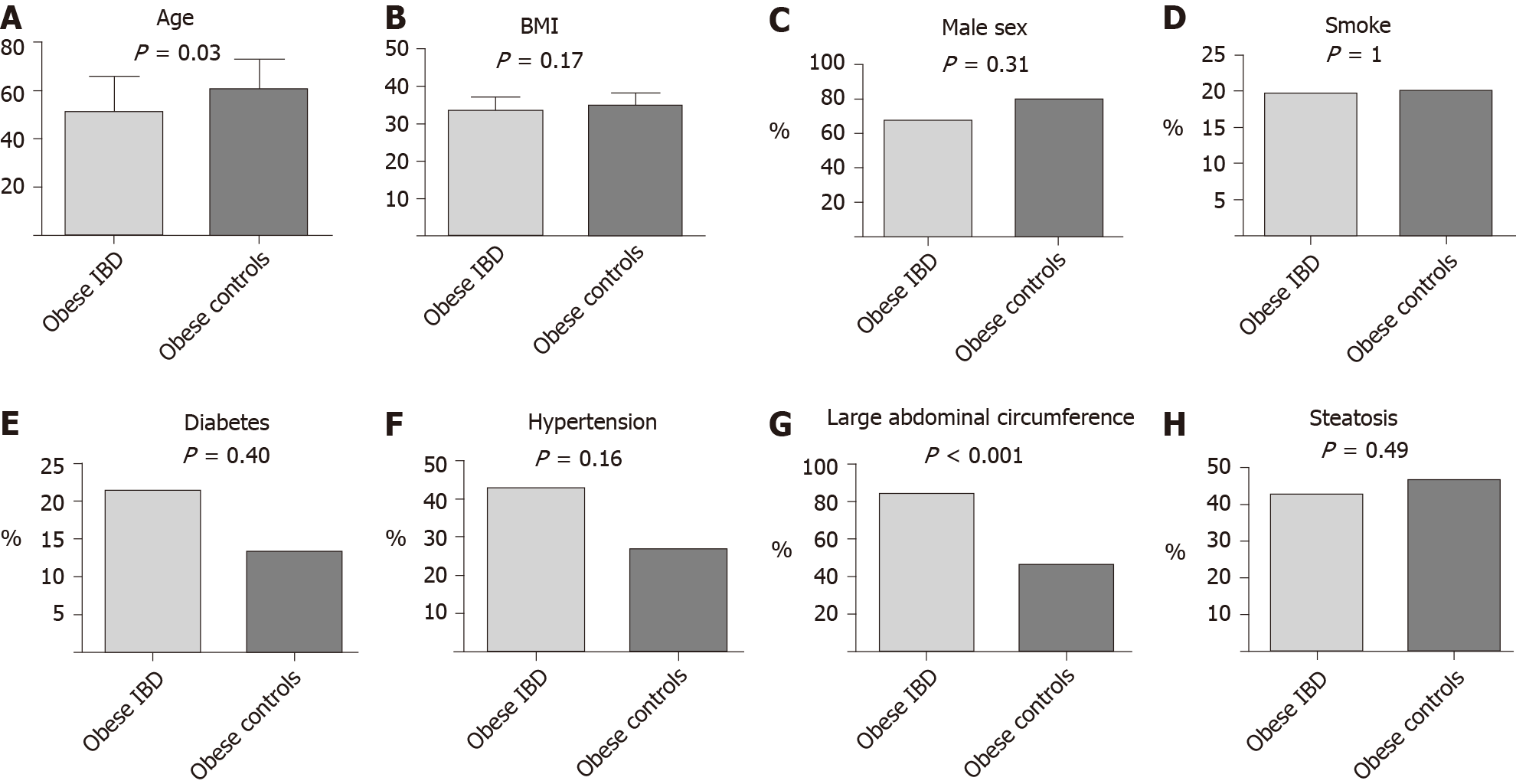
In the comparison of obese IBD patients and obese controls, we did not find any difference in main comorbidities (diabetes and hypertension). No differences in sex or smoking habits were observed. Obese IBD patients were younger than obese controls (51.2 ± 14.9 years vs 60.7 ± 12.1 years, P = 0.03). Additionally, an abdominal circumference > 102 cm in males and > 88 cm in females was observed more frequently in obese IBD group (83.9% vs 46.7%, P < 0.001). The results of such analyses are graphically represented in Figure 2.
At univariate analysis, we compared IBD obese vs IBD non obese patients. We observed that obese ones were older than normal weight subjects (51.2 ± 14.9 vs 44.5 ± 15.8, P = 0.002). IBD onset age was earlier in obese population (44.8 ± 13.6 vs 35.6 ± 15.6, P = 0.004). We did not detect any difference regarding other considered characteristics of IBD, such as disease location according to Montreal classification. When the drugs used for IBD therapy were taken into account, obese subjects had consumed more frequently long courses of systemic steroids (66.6% vs 12.5%, P = 0.02) as well as antibiotics such as metronidazole or ciprofloxacin (71.4% vs 54.7%, P = 0.05). No difference about other drugs (biologics, mesalazine or thiopurines) was observed.
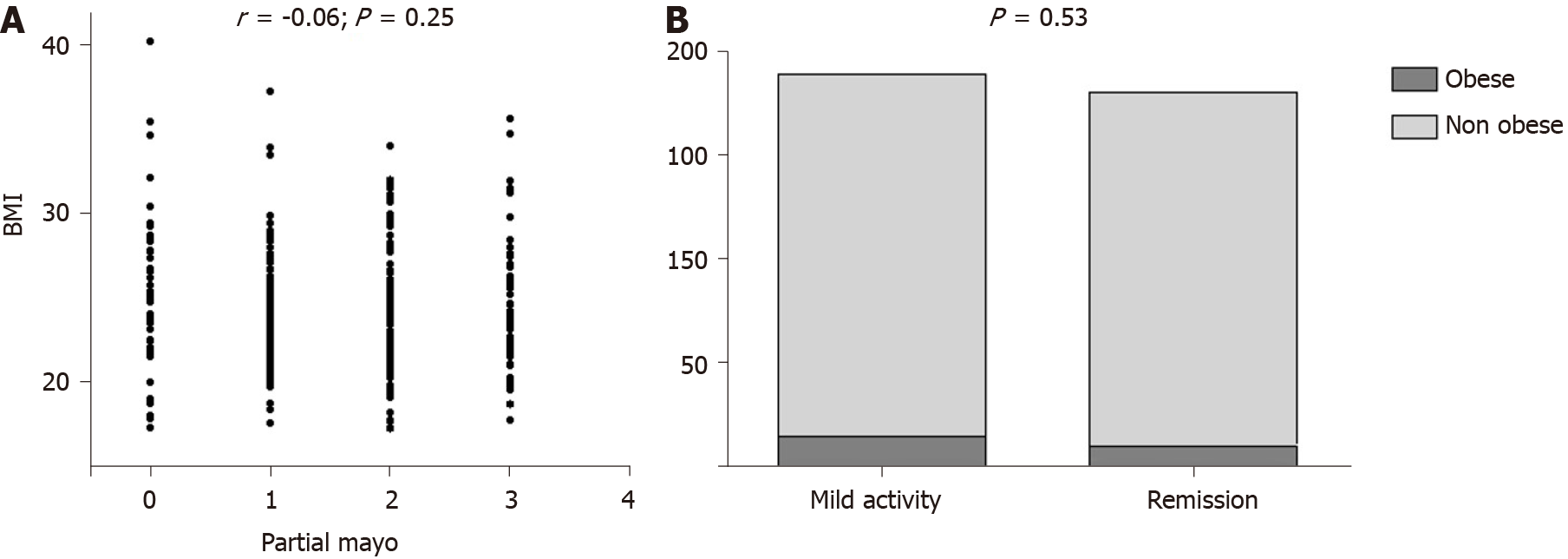
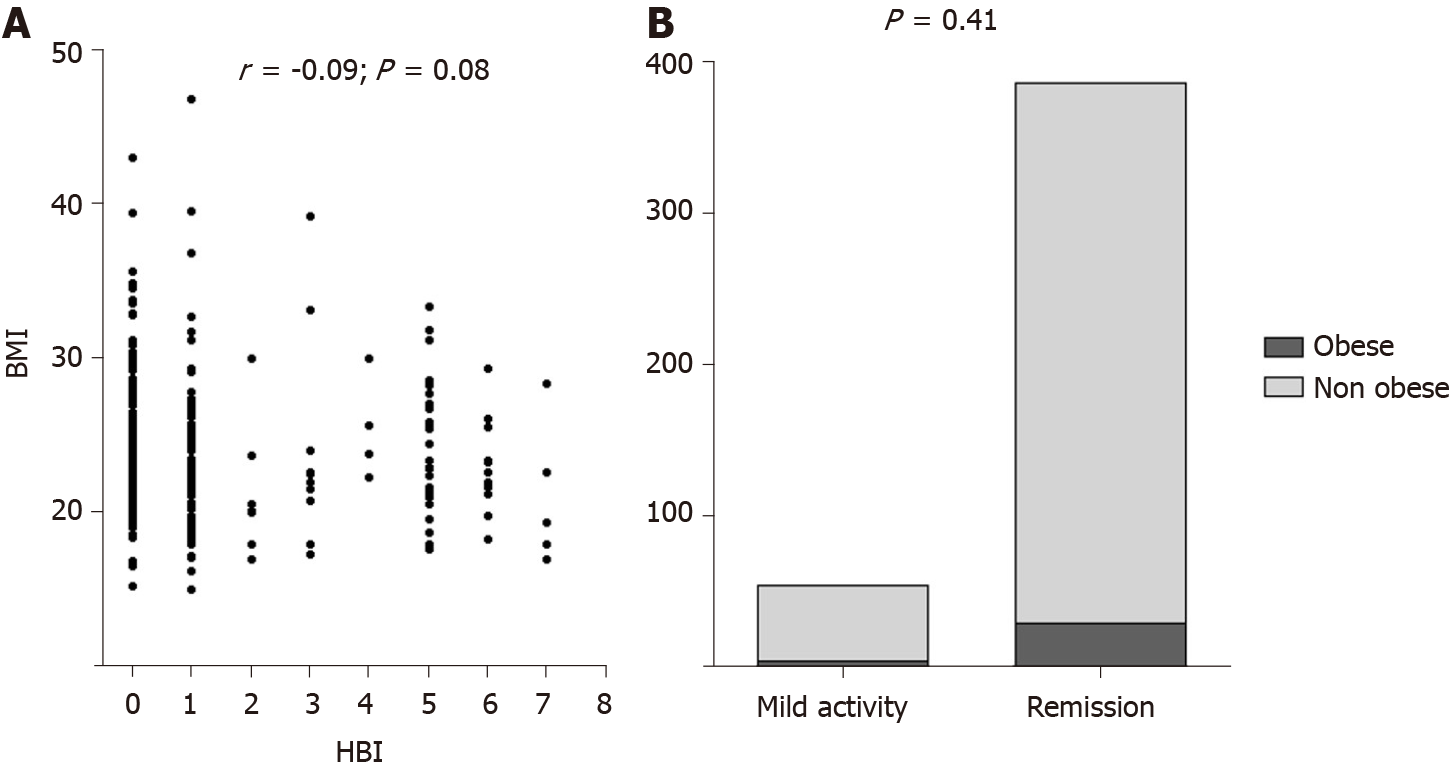
Disease activity was similar between obese and non obese subjects both for UC and CD. Indeed, among UC patients, we did not find any correlation between BMI and partial Mayo subscore (r = -0.06; P = 0.25), as illustrated in Figure 3A. Clinical remission phase was observed respectively in the 41.6% and 49.3% of obese and non obese UC patients (P = 0.53; Figure 3B). Among CD patients, we similarly did not detect any correlation between BMI and HBI (r = -0.09; P = 0.08), as reported in Figure 4A. Clinical remission phase was observed respectively in the 93.7% and 87.7% of obese and non obese CD patients (P = 0.41; Figure 4B).
Obese IBD patients suffered more frequently from arterial hypertension (42.8% vs 15.3%, P < 0.001), type 2 diabetes (21.4% vs 3.7%, P < 0.001), liver steatosis (76.8% vs 25.2%, P < 0.001), and had a significantly higher value of abdominal circumference equal to or greater than the cut-offs of central obesity (83.9% vs 17.3%, P < 0.001). Regarding laboratory investigations, obese IBD patients had higher levels of triglyceridemia (161 ± 71 vs 107 ± 55, P < 0.001), fasting blood glucose (113 ± 46 vs 89 ± 18, P < 0.001) , gamma-glutamyl-transpeptidase (0.89 ± 0.99 vs 0.55 ± 0.91, P = 0.04), and low blood levels of HDL Cholesterol (47 ± 12 vs 56 ± 19, P = 0.001).
On multivariate analysis, however, the only factor that appeared to be independently linked to obesity in IBD was the high abdominal circumference (OR = 16.3, 95%CI: 1.03-250, P = 0.04).
The prevalence of obesity in IBD, a hot topic at the moment, has been investigated in several studies, showing highly variable values ranging from 5% to 30%[5,6]. A Scottish study based on a population of 489 IBD patients showed that 18% of patients had the features of obesity (compared to 23% of the general population); obese patients with CD were 18%, while obese patients with UC were 17.5%[14]. In the present study, we found a similar prevalence between controls and IBD which seems to confirm data from literature. The obesity rate in our cohort (6.9%) was only slightly lower than the 10.8% in the general population reported according to the European Eurostat survey[15]. Our results, additionally, underline that obesity in IBD patients has some peculiar features in comparison with obese controls. In detail, obese IBD subjects are younger than control counterpart, and this could be explained by the fact that, during the history of the disease, some factors such as steroid consumption could have favored weight gain. However, only at univariate analysis, steroid use was a predictor of obesity in IBD even if we were able to evaluate simply the exposure, and not its amount, and this could be considered as a limitation in our study. Additionally, the number of relapses occurred during the clinical history of patients was not collected from medical records, and this could be another limitation. Another feature of obese IBD patients is that they tend to have less frequently a large abdominal circumference than obese controls (Figure 2G), thus suggesting the possibility that fat distribution could be also localized in areas different than waist, such as hip or limbs. Indeed, some studies evidenced that in IBD the ratio between visceral and subcutaneous fat is altered[16] compared to healthy population, and this may explain our finding. Unfortunately, we did not take other anthropometric measurements, therefore we were unable to confirm this hypothesis.
Furthermore, we did not find any correlation between disease activity and BMI. This could be in disagreement with some literature data, showing that visceral fat[17,18] and high BMI[19] are associated with a dismal prognosis. However, our study was cross-sectional, therefore we could not evaluate the evolution of the disease during a follow up period. This could be acknowledged as another limitation. Nevertheless, some other studies did not find a strong association between BMI and disease activity and prognosis[20-23], and this underlines how this topic is still debated and with conflicting evidences.
Another important finding was that only the abdominal circumference was independently associated with obesity in IBD. We are aware that this could be an obvious result, but if we consider that it was not very common in our cohort (less than 50%), it is noteworthy to be underlined. Systemic steroids were associated with obesity only at univariate analysis, and this is an easily comprehensible link that has been already elucidated in literature[24]. Another interesting result was the most extensive use of antibiotics in obese IBD subjects. It is presumable that they could promote a dysbiosis, which in turn might facilitate the development of obesity, as already suggested by many clinical and basic science studies[25]. Finally, the high risk of diabetes, hypertension and liver steatosis is a well know phenomenon that seems to be related to obesity rather than to IBD itself[26].
In conclusion, our study may lay the foundation for some additional speculations. Since epidemiologically IBD are increasing in developed countries, the pathogenetic role and influence on the outcome of disease played by the diet should not be underestimated and must be further investigated. In this regard, it has been already demonstrated that IBD patients, even in remission phase, tend to have a high lipid and low fiber intake[27]. Furthermore, It may be useful to plan new clinical studies aimed at evaluating clinical, laboratory and endoscopic parameters at the baseline and following BMI changes induced by dietary regimens, since this topic is still very poorly investigated.
In recent years, an increasing prevalence of obesity in inflammatory bowel disease (IBD) has been observed.
To investigate the relationship between obesity and IBD.
To evaluate the prevalence of obesity in IBD and associated factors.
We collected data about IBD disease pattern and activity, drugs and laboratory investigations in our center. Anthropometric measures were retrieved and obesity defined as a body mass index (BMI) > 30. Then, we compared characteristics of obese vs non obese patients, and Chi-squared test and Student’s t test were used for discrete and continuous variables, respectively, at univariate analysis. For multivariate analysis, we used binomial logistic regression and estimated odd ratios and 95% confidence intervals to ascertain factors associated with obesity.
The prevalence of obesity was 6.9% in IBD and 7.9% in controls (not statistically different; P = 0.38). Obese IBD were older than normal weight ones. IBD onset age was earlier in obese population. Obese subjects had consumed more frequently long course of systemic steroids as well as antibiotics such as metronidazole or ciprofloxacin. Obese IBD patients suffered more frequently from arterial hypertension, type 2 diabetes, non-alcoholic fatty liver disease. On multivariate analysis, however, the only factor that appeared to be independently linked to obesity in IBD was the high abdominal circumference.
Obese IBD patients seem to have features similar to general obese population, and there is no disease-specific factor (disease activity, extension or therapy) that may foster obesity in IBD.
Dietary interventions to explore whether BMI variation may have some benefit on IBD course are warranted.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li C, Szilagyi A S-Editor: Gao CC L-Editor: A P-Editor: Liu JH
| 1. | Yoon KH, Lee JH, Kim JW, Cho JH, Choi YH, Ko SH, Zimmet P, Son HY. Epidemic obesity and type 2 diabetes in Asia. Lancet. 2006;368:1681-1688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1114] [Cited by in RCA: 1134] [Article Influence: 59.7] [Reference Citation Analysis (0)] |
| 2. | Seravalle G, Grassi G. Obesity and hypertension. Pharmacol Res. 2017;122:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 491] [Article Influence: 61.4] [Reference Citation Analysis (0)] |
| 3. | World Health Organization. Obesity. [cited April 13, 2020]. Available from: https://www.who.int/topics/obesity/en/. |
| 4. | Vadan R, Gheorghe LS, Constantinescu A, Gheorghe C. The prevalence of malnutrition and the evolution of nutritional status in patients with moderate to severe forms of Crohn's disease treated with Infliximab. Clin Nutr. 2011;30:86-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Blain A, Cattan S, Beaugerie L, Carbonnel F, Gendre JP, Cosnes J. Crohn's disease clinical course and severity in obese patients. Clin Nutr. 2002;21:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 175] [Article Influence: 7.6] [Reference Citation Analysis (1)] |
| 6. | Seminerio JL, Koutroubakis IE, Ramos-Rivers C, Hashash JG, Dudekula A, Regueiro M, Baidoo L, Barrie A, Swoger J, Schwartz M, Weyant K, Dunn MA, Binion DG. Impact of Obesity on the Management and Clinical Course of Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2015;21:2857-2863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 134] [Article Influence: 13.4] [Reference Citation Analysis (1)] |
| 7. | Holtmann MH, Krummenauer F, Claas C, Kremeyer K, Lorenz D, Rainer O, Vogel I, Böcker U, Böhm S, Büning C, Duchmann R, Gerken G, Herfarth H, Lügering N, Kruis W, Reinshagen M, Schmidt J, Stallmach A, Stein J, Sturm A, Galle PR, Hommes DW, D'Haens G, Rutgeerts P, Neurath MF. Significant differences between Crohn's disease and ulcerative colitis regarding the impact of body mass index and initial disease activity on responsiveness to azathioprine: results from a European multicenter study in 1,176 patients. Dig Dis Sci. 2010;55:1066-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Kurnool S, Nguyen NH, Proudfoot J, Dulai PS, Boland BS, Vande Casteele N, Evans E, Grunvald EL, Zarrinpar A, Sandborn WJ, Singh S. High body mass index is associated with increased risk of treatment failure and surgery in biologic-treated patients with ulcerative colitis. Aliment Pharmacol Ther. 2018;47:1472-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 9. | Chiu YL, Rubin DT, Vermeire S, Louis E, Robinson AM, Lomax KG, Pollack PF, Paulson SK. Serum adalimumab concentration and clinical remission in patients with Crohn's disease. Inflamm Bowel Dis. 2013;19:1112-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 10. | Principi M, Mastrolonardo M, Scicchitano P, Gesualdo M, Sassara M, Guida P, Bucci A, Zito A, Caputo P, Albano F, Ierardi E, Di Leo A, Ciccone MM. Endothelial function and cardiovascular risk in active inflammatory bowel diseases. J Crohns Colitis. 2013;7:e427-e433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Principi M, Montenegro L, Losurdo G, Zito A, Devito F, Bulzis G, Carbonara R, Ierardi E, Di Leo A, Ciccone MM. Endothelial function and cardiovascular risk in patients with inflammatory bowel disease in remission phase. Scand J Gastroenterol. 2016;51:253-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Losurdo G, Iannone A, Contaldo A, Barone M, Ierardi E, Di Leo A, Principi M. Chronic Viral Hepatitis in a Cohort of Inflammatory Bowel Disease Patients from Southern Italy: A Case-Control Study. Pathogens. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | European Association for the Study of the Liver (EASL). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 3176] [Article Influence: 352.9] [Reference Citation Analysis (4)] |
| 14. | Steed H, Walsh S, Reynolds N. A brief report of the epidemiology of obesity in the inflammatory bowel disease population of Tayside, Scotland. Obes Facts. 2009;2:370-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 123] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 15. | European Commission. European health interview survey (EHIS). [cited April 21, 2020]. Available from: https://ec.europa.eu/eurostat/web/microdata/european-health-interview-survey. |
| 16. | Bryant RV, Schultz CG, Ooi S, Goess C, Costello SP, Vincent AD, Schoeman S, Lim A, Bartholomeusz FD, Travis SPL, Andrews JM. Visceral Adipose Tissue Is Associated With Stricturing Crohn's Disease Behavior, Fecal Calprotectin, and Quality of Life. Inflamm Bowel Dis. 2019;25:592-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (1)] |
| 17. | Wei Y, Zhu F, Gong J, Yang J, Zhang T, Gu L, Zhu W, Guo Z, Li Y, Li N, Li J. High Visceral to Subcutaneous Fat Ratio Is Associated with Increased Postoperative Inflammatory Response after Colorectal Resection in Inflammatory Bowel Disease. Gastroenterol Res Pract. 2018;2018:6270514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Stidham RW, Waljee AK, Day NM, Bergmans CL, Zahn KM, Higgins PD, Wang SC, Su GL. Body fat composition assessment using analytic morphomics predicts infectious complications after bowel resection in Crohn's disease. Inflamm Bowel Dis. 2015;21:1306-1313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Yerushalmy-Feler A, Ben-Tov A, Weintraub Y, Amir A, Galai T, Moran-Lev H, Cohen S. High and low body mass index may predict severe disease course in children with inflammatory bowel disease. Scand J Gastroenterol. 2018;53:708-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Mentella MC, Scaldaferri F, Pizzoferrato M, Gasbarrini A, Miggiano GAD. The Association of Disease Activity, BMI and Phase Angle with Vitamin D Deficiency in Patients with IBD. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Connelly TM, Juza RM, Sangster W, Sehgal R, Tappouni RF, Messaris E. Volumetric fat ratio and not body mass index is predictive of ileocolectomy outcomes in Crohn's disease patients. Dig Surg. 2014;31:219-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 22. | Singh S, Proudfoot J, Xu R, Sandborn WJ. Obesity and Response to Infliximab in Patients with Inflammatory Bowel Diseases: Pooled Analysis of Individual Participant Data from Clinical Trials. Am J Gastroenterol. 2018;113:883-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 23. | Singh S, Proudfoot J, Xu R, Sandborn WJ. Impact of Obesity on Short- and Intermediate-Term Outcomes in Inflammatory Bowel Diseases: Pooled Analysis of Placebo Arms of Infliximab Clinical Trials. Inflamm Bowel Dis. 2018;24:2278-2284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Jahnsen J, Falch JA, Mowinckel P, Aadland E. Body composition in patients with inflammatory bowel disease: a population-based study. Am J Gastroenterol. 2003;98:1556-1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 114] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 25. | Cammarota G, Ianiro G, Cianci R, Bibbò S, Gasbarrini A, Currò D. The involvement of gut microbiota in inflammatory bowel disease pathogenesis: potential for therapy. Pharmacol Ther. 2015;149:191-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 137] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 26. | Principi M, Iannone A, Losurdo G, Mangia M, Shahini E, Albano F, Rizzi SF, La Fortezza RF, Lovero R, Contaldo A, Barone M, Leandro G, Ierardi E, Di Leo A. Nonalcoholic Fatty Liver Disease in Inflammatory Bowel Disease: Prevalence and Risk Factors. Inflamm Bowel Dis. 2018;24:1589-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 27. | Principi M, Losurdo G, Iannone A, Contaldo A, Deflorio V, Ranaldo N, Pisani A, Ierardi E, Di Leo A, Barone M. Differences in dietary habits between patients with inflammatory bowel disease in clinical remission and a healthy population. Ann Gastroenterol. 2018;31:469-473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |