Published online Dec 14, 2020. doi: 10.3748/wjg.v26.i46.7312
Peer-review started: August 27, 2020
First decision: September 30, 2020
Revised: October 6, 2020
Accepted: November 2, 2020
Article in press: November 2, 2020
Published online: December 14, 2020
Processing time: 108 Days and 15.3 Hours
Extrahepatic biliary duct injury (BDI) remains a complicated issue for surgeons. Although several approaches have been explored to address this problem, the high incidence of complications affects postoperative recovery. As a nonimmunogenic scaffold, an animal-derived artificial bile duct (ada-BD) could replace the defect, providing good physiological conditions for the regeneration of autologous bile duct structures without changing the original anatomical and physiologic conditions.
To evaluate the long-term feasibility of a novel heterogenous ada-BD for treating extrahepatic BDI in pigs.
Eight pigs were randomly divided into two groups in the study. The animal injury model was developed with an approximately 2 cm segmental defect of various parts of the common bile duct (CBD) for all pigs. A 2 cm long novel heterogenous animal-derived bile duct was used to repair this segmental defect (group A, ada-BD-to-duodenum anastomosis to repair the distal CBD defect; group B, ada-BD-to-CBD anastomosis to repair the intermedial CBD defect). The endpoint for observation was 6 mo (group A) and 12 mo (group B) after the operation. Liver function was regularly tested. Animals were euthanized at the above endpoints. Histological analysis was carried out to assess the efficacy of the repair.
The median operative time was 2.45 h (2-3 h), with a median anastomosis time of 60.5 min (55-73 min). All experimental animals survived until the endpoints for observation. The liver function was almost regular. Histologic analysis indicated a marked biliary epithelial layer covering the neo-bile duct and regeneration of the submucosal connective tissue and smooth muscle without significant signs of immune rejection. In comparison, the submucosal connective tissue was more regular and thicker in group B than in group A, and there was superior integrity of the regeneration of the biliary epithelial layer. Despite the advantages of the regeneration of the bile duct smooth muscle observed in group A, the effect on the patency of the ada-BD grafts in group B was not confirmed by macroscopic assessment and cholangiography.
This approach appears to be feasible for repairing a CBD defect with an ada-BD. A large sample study is needed to confirm the durability and safety of these preliminary results.
Core Tip: Extrahepatic biliary duct injury (BDI) remains a complicated issue for surgeons. Although several materials have been explored to repair extrahepatic biliary duct defects, the long-term efficacy is still unclear. This study evaluated the long-term feasibility of a novel heterogenous animal-derived artificial bile duct (ada-BD) for treating extrahepatic BDI in pigs. Eight pigs were randomly divided into two groups according to the various types of defects of the common bile duct. The results indicated that ada-BD safely served as a nonimmunogenic scaffold to replace the defect without changing the original anatomical and physiological conditions.
- Citation: Shang H, Zeng JP, Wang SY, Xiao Y, Yang JH, Yu SQ, Liu XC, Jiang N, Shi XL, Jin S. Extrahepatic bile duct reconstruction in pigs with heterogenous animal-derived artificial bile ducts: A preliminary experience. World J Gastroenterol 2020; 26(46): 7312-7324
- URL: https://www.wjgnet.com/1007-9327/full/v26/i46/7312.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i46.7312
With the development of laparoscopic biliary surgery, the incidence of biliary duct injury (BDI) is markedly increasing[1]. Multi-institutional studies have shown that approximately 34%-49% of hepatobiliary surgeons have experienced one to two cases of BDI[2,3]. In the United States, cholecystectomy alone could induce 7500 cases of BDI a year[4]. The present approaches to treat BDI are as follows: End-to-end anastomosis, hepatectomy and choledochojejunostomy. The end-to-end anastomosis of the bile duct (BD), regarded as the optimal approach to deal with BDI, has a high incidence of postoperative biliary stricture due to factors such as the high tension of the anastomosis caused by defects of the BD tissue[5]. Choledochojejunostomy is the most common method to repair BDI. However, 20% of patients suffer recurrent BD infection, and some require liver transplantation due to postoperative secondary liver abscess or biliary cirrhosis.
In long-term treatment, the risk of mortality in BDI patients is 2- to 3-times higher that of normal rehabilitation[6,7]. Moreover, the quality of life is worse in BDI patients than in patients with normal rehabilitation or healthy people, even if BDI patients achieve long-term survival[8]. The artificial BD is a novel material for repairing BD tissue loss by replacing the injured BD, which could avoid the excessive tension of anastomosis and postoperative reflux cholangitis by preserving the Oddis sphincter[9-11]. Nevertheless, due to limitations by the immunocompatibility and difficulties in matching the tube diameter, studies of industrial and synthetic artificial BDs are still in the stage of animal experiments. In 2016, Cheng et al[12] reported the application of decellularized ureteral grafts in BD defect repair in a homologous porcine model. However, this research was limited to 3 mo of observation and showed that a T-tube or a silicone stent was needed to maintain the patency of the graft.
In the present study, a novel heterogeneous animal-derived artificial bile duct (ada-BD) was developed to treat extrahepatic BDI in a pig model to observe its long-term efficacy and safety. The ada-BD was obtained through a series of patented chemical modification processes, such as removing immunogenicity, strengthening crosslinking, anticalcification, etc. with the bovine ureter. In this study, the ada-BD was used as a replacement to repair a segmental defect of the extrahepatic BD in a pig model to assess its efficacy and safety for biliary regeneration.
A total of eight Guangxi Bama minipigs of either sex weighing 25-35 kg were obtained from Beijing Shichuang Century Miniature Pig Breeding Base (Beijing, China). All the experimental pigs were housed and fed for 2 wk prior to the operation at the Experimental Animal Center of Beijing Kuibu Shichuang Biotechnology Co., Ltd. to become acclimatized to the new environment. All procedures were performed under anesthesia to minimize pain or discomfort. All the experimental animals were sacrificed with intravenous 10% potassium chloride. Autopsy was conducted to determine the cause of death when unexpected death occurred.
A fresh bovine ureter was obtained under sterile conditions and served as the raw material after removing the appendant connective tissues surrounding the ureter. First, obtained ureters were cut into pieces 5.0 cm to 10.0 cm in length. Second, cells or cell fragments as well as tissue protein, nucleic acid and lipid molecules in the ureter were removed by decellularization with detergent. Third, the tissue preservation solution (0.25-0.7 wt% glutaraldehyde solution or Hank’s solution) was perfused into the tube to enhance the histocompatibility without influencing the cell growth of the epithelium. Subsequently, a shaping mold was used to shape the decellularized ureter to the appropriate diameter and thickness. Finally, the decellularized ureteral graft underwent tissue crosslinking and anticalcification treatment in 0.2-2.0 wt% glutaraldehyde solution at 10-60 °C and pH 2.5-8.5 for 2-48 h to increase the shrinkage temperature of the tissue up to 82 °C.
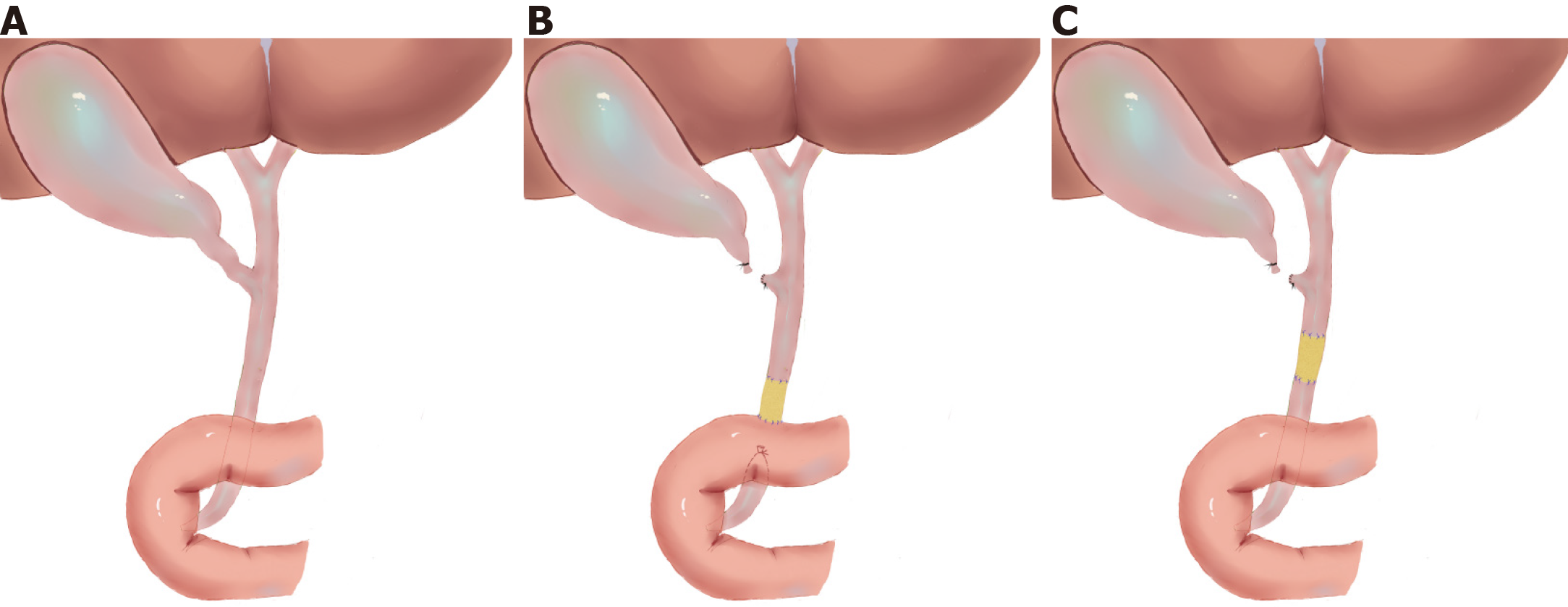
All the pigs were categorized into two groups according to various parts of the common bile duct (CBD) defect: Group A (distal CBD defect) and group B (intermediate CBD defect). The time points of observation were set to 6 mo after the operation in group A and 12 mo after the operation in group B. A midline incision was made to expose the CBD. The CBD defect model was generated by resecting the CBD after cholecystectomy: 2 cm in length located in the distal CBD (group A) and the intermediate CBD (group B).
Preoperative fasting for 12 h with free access to water was administered for all the experimental animals, which were administered general anesthesia with mechanical ventilation and premedication with intramuscular ketamine (20 mg/kg) followed by maintenance with a continuous infusion of 0.2 mg/(kg·min) of propofol. Antibiotic prophylaxis was routinely used during the perioperative period.
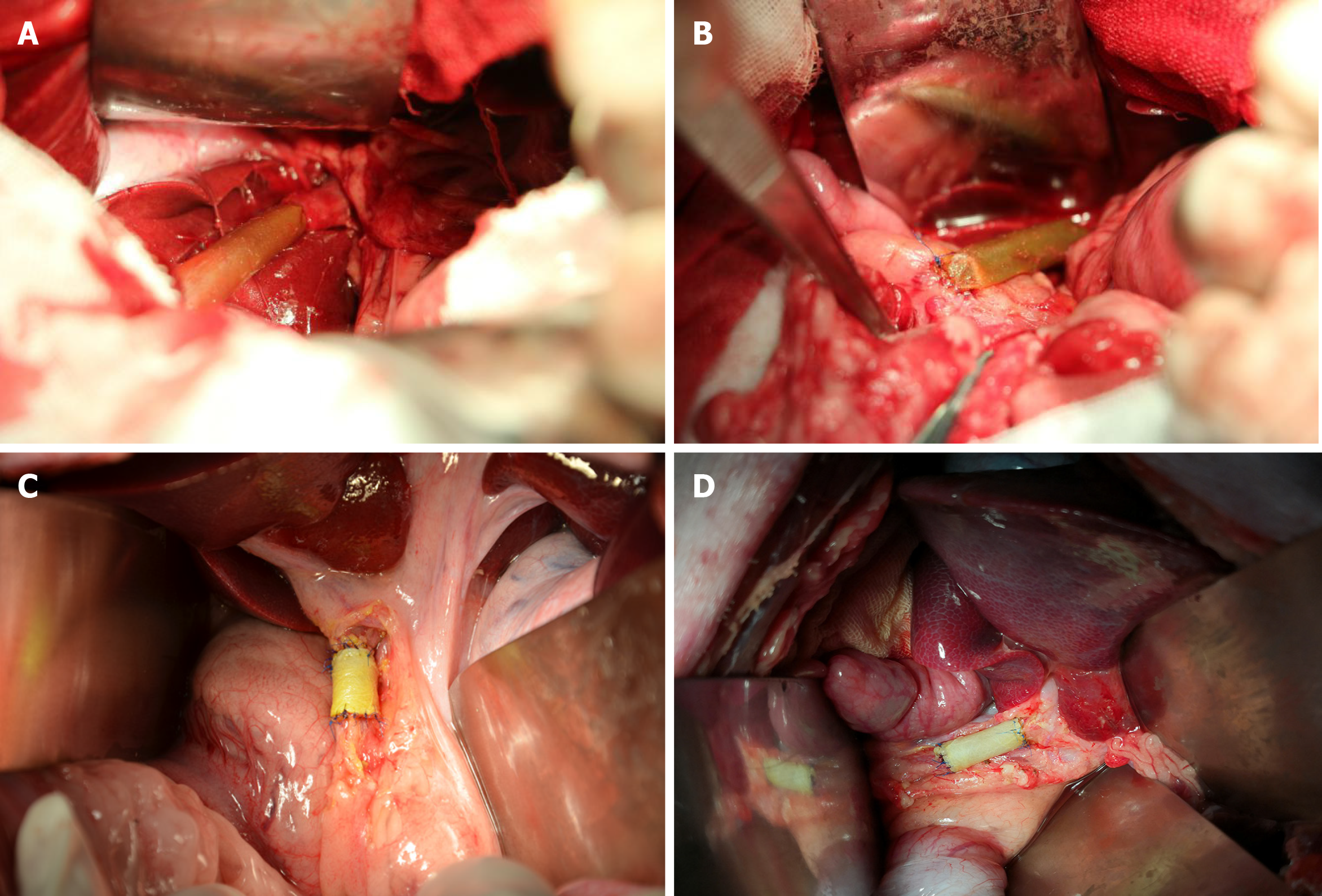
After the establishment of the CBD injury model, an ada-BD (2 cm in length) was used to reconstruct the CBD (Figure 1). In group A, an end-to-end anastomosis with interrupted 5-0 Prolene sutures was performed by everting mucosa to mucosa sutures between the proximal edge of the incised CBD stump and the ada-BD as well as an end-to-side anastomosis between the distal edge of the ada-BD and duodenum (Figure 2A and 2B). Unlike group A, end-to-end intermittent eversion sutures were carried out in both anastomoses between the stumps of the incised BD and the ada-BD in group B (Figure 2C and D). When the anastomoses were done, a silica gel drainage tube was positioned at the inferior margin of the liver to drain the ascites and to observe biliary leakage after the operation. Then, the greater omentum was brought up to wrap the replaced area. The animals were given intravenous cefazolinoral (1 g dissolved in 100 mL of 154 mmol/L saline) for 3 d after the operation. Drinking water and standard diet were allowed at 12 h and 24 h after the operation, respectively. All animal experiments were carried out by an experienced hepatobiliary surgery team.
Jaundice, appetite and mental status were observed to evaluate postoperative complications. The result of autopsy was the gold standard of complications of bile leakage, jaundice, lithogenesis and postoperative infection.
Alanine aminotransferase (ALT), aspartate aminotransferase, alkaline phosphatase, gamma-glutamyl transferase, total bilirubin (TBil), and TBil of the drainage were monitored by an automatic biochemistry analyzer to evaluate liver function, jaundice and bile leakage before the operation and before sacrifice.
The animals were sacrificed at 6 mo and 12 mo. Autopsy was conducted by the procurement of the en bloc liver associated with the entire extrahepatic BD, including the partial duodenum and pancreas. Subsequent cholangiography was performed via distal anastomosis catheterization (group A) or duodenal papilla (group B).
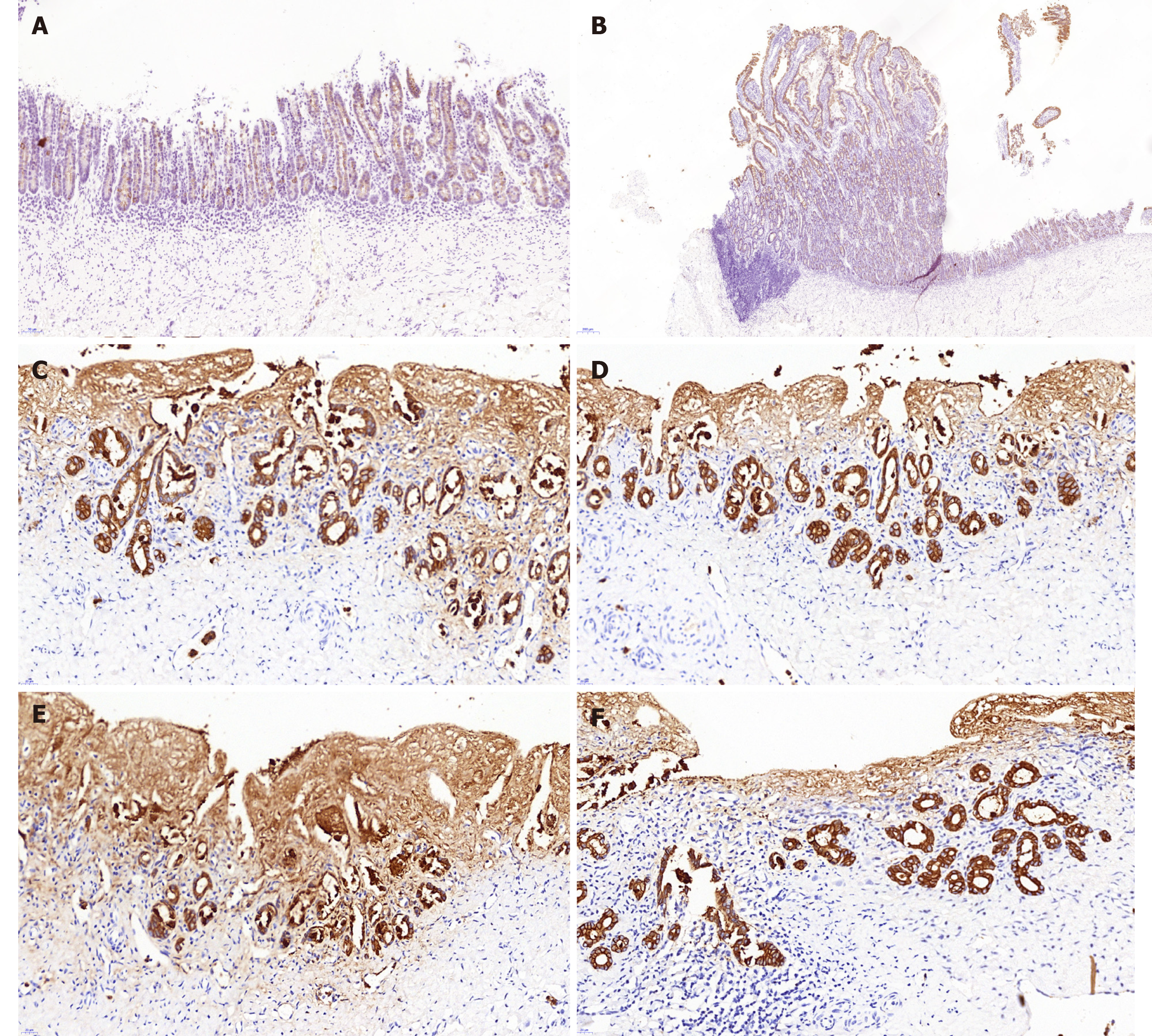
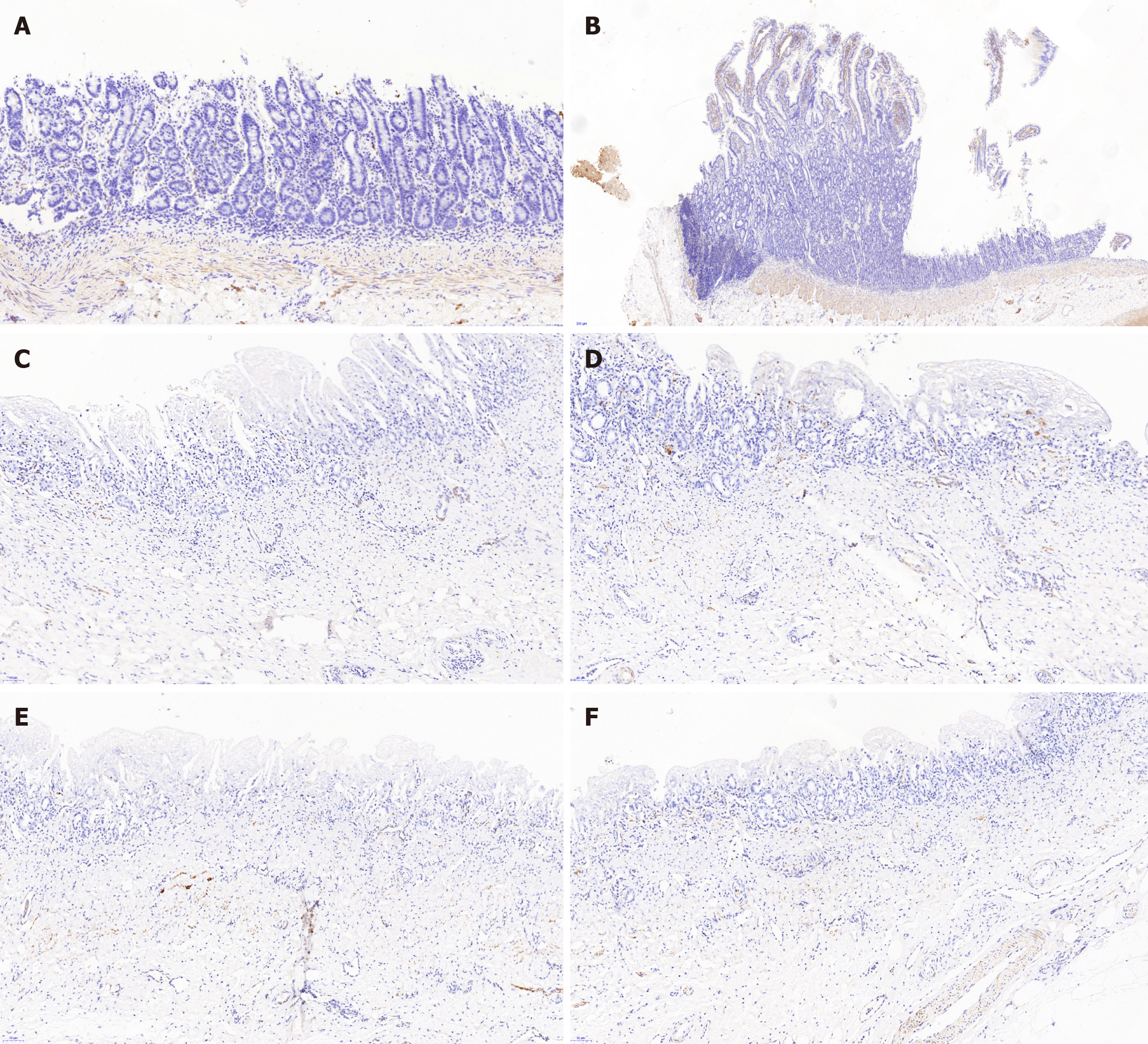
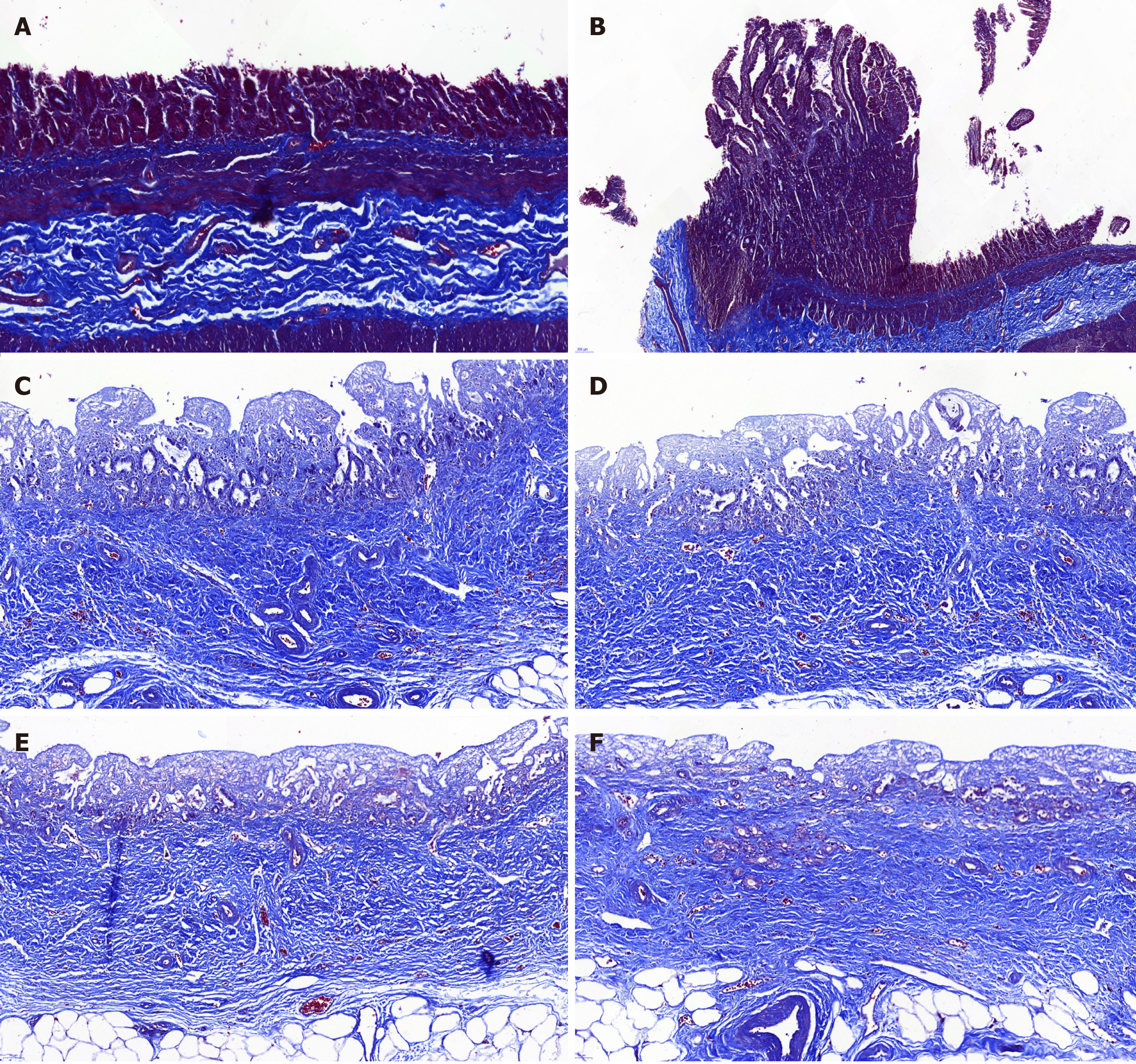
All the experimental animals were sacrificed with intravenous 10% potassium chloride. Then, the extrahepatic biliary tree including the gallbladder was resected en bloc from the animal and fixed in 10% neutral buffered formalin. Then, the tissues were delivered to the histopathology laboratory for sectioning. Following the observation of the gross morphology of the reconstructed BD, microscopic analyses including hematoxylin/eosin staining, cytokeratin 19 immunostaining, desmin immunostaining and Masson’s trichrome staining were performed, which were used to observe the epithelial regeneration in vivo, tissue regeneration in the smooth muscle layer and connective tissue regeneration in the submucosal layer.
The relevant data were analyzed by SPSS 25.0 (IBM Corporation; Armonk, NY, United States). Continuous variables are expressed as the median (range). An independent-samples Mann-Whitney U test was used to analyze various continuous variables. All tests were two sided, and P values less than 0.05 indicate a statistically significant difference.
Eight animals were enrolled in the study. The median operative time was 2.45 h (2-3 h), with a median anastomosis time of 60.5 min (55-73 min). For group A, one animal developed bile leakage on postoperative day (PoD) 4, which was confirmed by drainage examination. The standard diagnosis for postoperative bile leakage was the same as that in humans (a bilirubin concentration of the drainage at least 3-times higher than the serum bilirubin concentration measured at the same time)[13]. Fortunately, this pig spontaneously recovered with adequate drainage and oral antibiotic administration (norfloxacin, 10 mg/kg) and was subsequently sacrificed at 6 mo after the initial operation according to the predetermined time points. In addition, another three animals in group A were sacrificed as planned at the same time. All experimental animals were euthanized with intravenous 10% potassium chloride. The autopsy of the pig with bile leakage showed a severe inflamed encapsulated adhesion surrounding the hepatic hilar caused by bile leakage. After separating the adhesion, we found that the graft was patent, in place and completely covered by the visceral layer of the peritoneum, similar to the other three pigs. Of these three pigs, one animal suffered an incision infection and recovered well after thorough debridement and daily disinfection with iodophor.
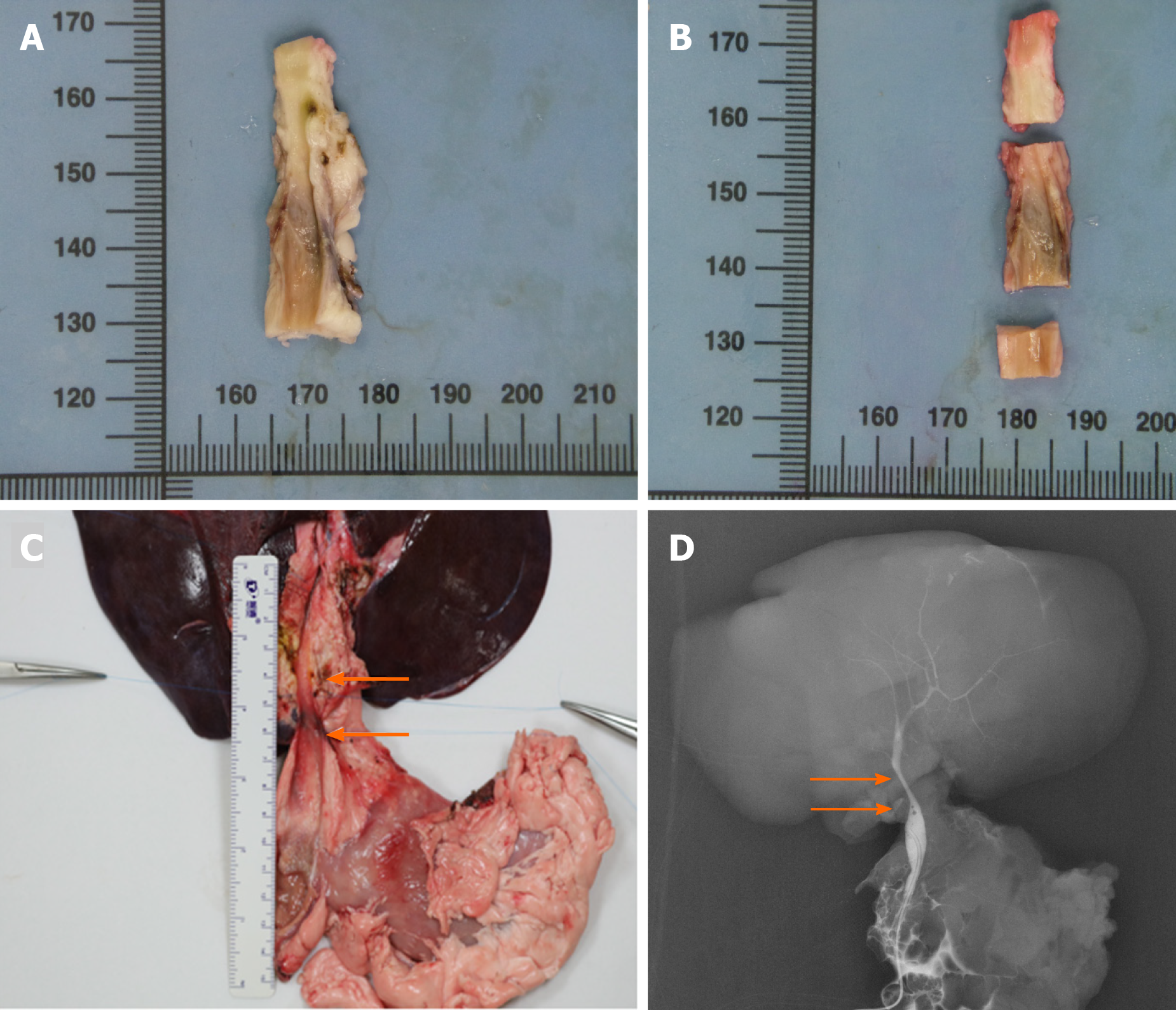
The remaining four animals in group B survived to the predetermined date of 12 mo postoperatively without any complications or weight loss. All of them were sacrificed at 12 mo to conduct a long-term observation. The macroscopic assessment of the entire extrahepatic BD, especially for interposition grafts, was performed. The results showed that the inner walls of the anastomoses were smooth without scars or stricture (Figure 3A and 3B).
The results of liver function tests of all the experimental animals were within the normal range except for the pig with bile leakage in group A. In contrast, only the levels ofALT on PoD 7 were significantly increased in group A (Table 1).
| Normal range | PoD 1 | PoD 7 | 6 mo after operation | 12 mo after operation | |
| AST in U/L | 0-97.5 | 39.30 (29.0-40.8) | 33.85 (27.0-50.0) | 29.55 (21.0-41.0) | 31.45 (22.0-36.6) |
| ALT in U/L | 0-86.7 | 54.00 (35.0-77.0) | 44.25 (35.1-106.0)a | 33.50 (21.0-37.0) | 30.55 (27.0-35.5) |
| ALP in U/L | 0-200.2 | 60.50 (15.0-78.0) | 69.65 (48.0-212.0) | 62.50 (46.0-102.1) | 60.00 (53.3-61.0) |
| GGT in U/L | 0-88.0 | 40.30 (21.0-52.0) | 51.05 (35.0-78.0) | 38.20 (35.0-48.6) | 35.60 (32.0-44.3) |
| TBil in mg/dL | 0-5.55 | 0.835 (0.30-1.30) | 0.905 (0.40-3.20) | 0.680 (0.52-1.50) | 0.655 (0.53-0.80) |
Cholangiography was performed via distal anastomosis catheterization (group A) or duodenal papilla catheterization (group B) after the procurement of the en bloc liver associated with the entire extrahepatic BD, including the partial duodenum and pancreas. The results indicated that all ada-BDs remained patent without any signs of stricture or obstruction (Figure 3C and 3D).
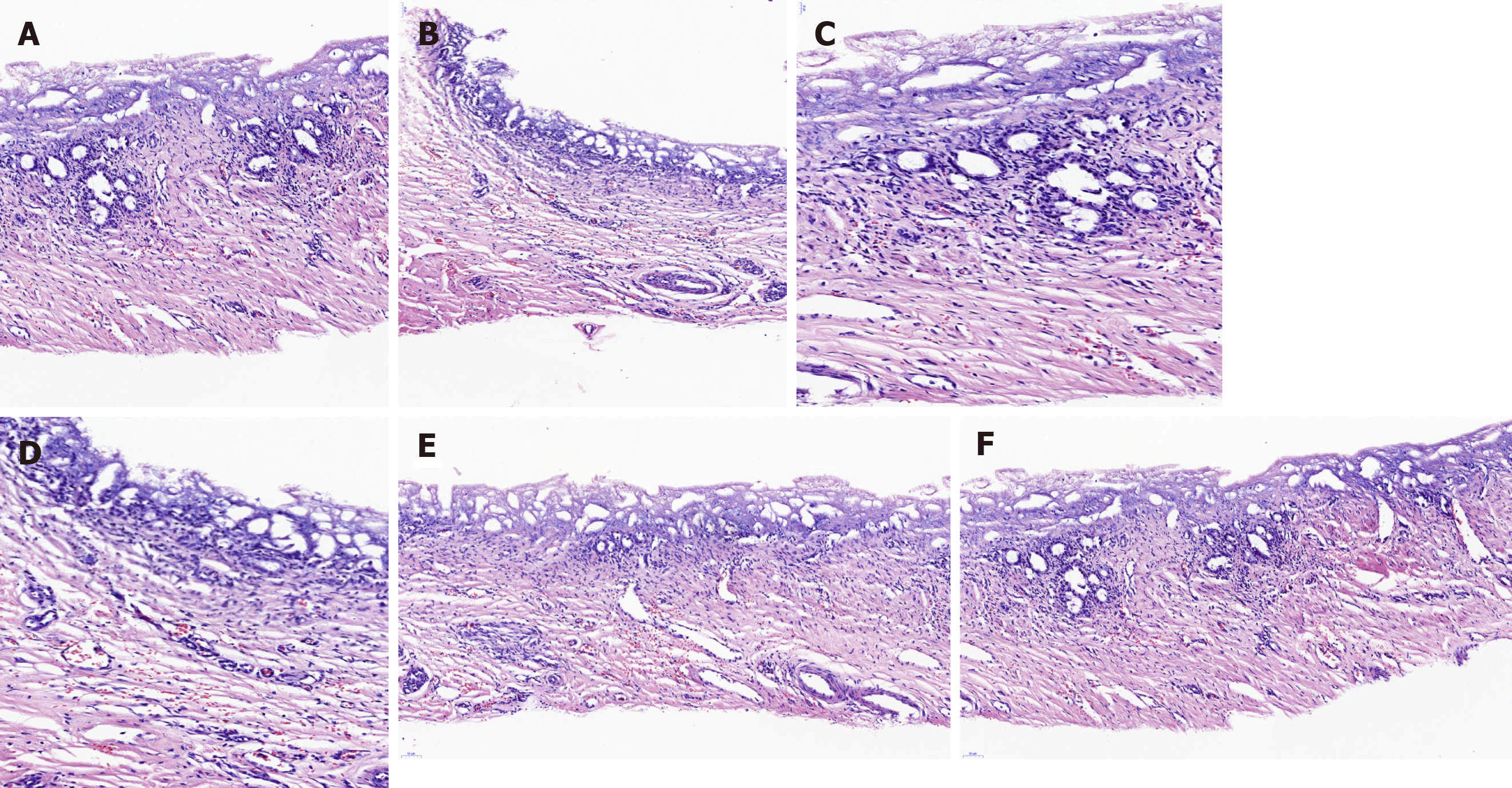
Histopathology analysis of the neo-extrahepatic BD in group A and group B showed re-epithelialized tall columnar cells overlaying the surface of the inner wall without marked transmural neutrophil and lymphoplasma cell infiltrate (Figure 4). Meanwhile, marked neovascularization was observed in the regenerated regions (Figure 4E and 4F). Cytokeratin 19 immunostaining indicated that the graft was covered by the regeneration of the BD epithelium and accessory glands on the inner wall. Superior integrity of the regeneration of the biliary epithelial layer was observed in group B compared to that in group A. In group B, it appeared that the BD showed no significant differences from a normal benign BD (Figure 5). Smooth muscle regeneration was confirmed by detecting the desmin-positive area, which was significantly larger in group A than in group B (Figure 6). Masson’s trichrome staining was performed to investigate the regeneration of the submucosal layer and collagen. Regenerative connective tissue was observed in group A and gradually became thicker and more regular in group B (Figure 7).
In 1985, Erich Mühe[14] initially described a novel surgical technique, laparoscopic cholecystectomy, as a less invasive procedure to cure symptomatic cholelithiasis and cholecystitis. Through decades of practice and research, laparoscopic cholecystectomy has become the preferred option for treating symptomatic cholelithiasis and cholecystitis[15,16]. However, accompanying the development of laparoscopic cholecystectomy, the incidence of BDI of the laparoscopic technique seemed to be higher than that of the open technique[17]. Current studies claimed that the laparoscopic technique for biliary disease was associated with an increased number of BDIs[1,4]. The end-to-end anastomosis of the BD is regarded as the optimal approach for treating BDI. Nevertheless, there remains a high incidence of reconstructive failure due to unrepairable BD tissue defects. Thus, as an alternative option, Roux-en-Y choledochojejunostomy has been widely used to address the above conditions by effectively reconstructing the bilioenteric anatomy. However, the option has only improved the repair of the pathology of BDIs, which still shows a certain incidence of complications caused by the reflux of intestinal contents into the biliary tree, including cholangitis, strictures, bile leaks and a relatively high risk of cholangiocarcinoma[6]. Repairing the BDI while preserving the function of the Oddis sphincter remains a challenge for hepatobiliary surgeons.
In recent years, the development of novel materials has brought a new era for extrahepatic BDI. An extensive review of the literature shows that several materials have been used as scaffolds to treat BDIs. In 2002, Gómez et al[18] found that thin-walled FEP-ringed Gore-Tex vascular grafts were useful in the repair of BD injuries, which was the first report of an artificial BD in the field of BD repair. After that, several synthetic materials were applied to the manufacture of artificial BDs. However, the further application of the artificial BD in clinical trials was limited by the mechanical strength of the original materials as well as the molding technique. A report from Li et al[19] demonstrated a novel exploration of biosynthetic material by the experimental repair of BD defects in pigs using collagen scaffolds loaded with human collagen-binding bFGF. The short-term experimental results showed good immunocompatibility. Nevertheless, factors including a complicated manufacturing process and difficulty in making tubes may limit the application of this material to clinical trials.
In addition, several studies have performed animal research in which defects of the BD were patched with autogenous tissue in an attempt to avoid immunological rejection. The autogenous tissues included autogenous veins, single-layer small intestinal submucosa, etc. Although definite superiority in regard to compliance and immunocompatibility was observed, their further clinical utility was restricted by the mismatch of the diameter and thickness as well as the complicated procedure of the acquisition and preparation of autogenous materials[11,20]. From a pathophysiological point of view, animal-derived materials are similar in compliance and structure compared with those of humans. Thus, theoretically, animal-derived materials could present improved immunocompatibility following decellularization by removing cellular antigens. The similarity in diameter, thickness, flexibility and strength leads to advantages in the ease of anastomosis and the matching of different tissues as well as in the preservation of the Oddis sphincter function in preventing reflux cholangitis. A homologous animal-derived decellularized ureteral graft was explored in a BD defect porcine model[12]. However, only 3 mo of observation was carried out accompanied by unsatisfying results of the patency of the graft without T-tube or a silicone stent inserted. In addition, the strict requirement of homology also limits further exploration in clinical applications. Thus, the long-term observation of the efficacy and safety of heterogenous animal-derived materials in repairing BD remains unclear.
In the present study, we investigated the long-term replacement effect of ada-BD for two types of extrahepatic BD segmental injury models in the pig model. We chose ada-BD because it showed a high similarity between the bovine ureter and BD in the following ways: (1) A similar pathological structure (mucosa, muscular layer and outer membrane); (2) A similar sterile environment (with only slight differences in pH value and composition); (3) A similar physiological pressure (ureteral internal pressure 7-13 cmH2O, physiological pressure of the CBD 12 cmH2O); and (4) Similar in diameter, thickness and compliance. The result of immunological rejection is the primary cause of the induction of graft loss after transplantation[21,22]. Thus, the immunogenicity of grafts is an urgent problem that must be resolved. In this work, the immunogenicity of the bovine ureter was effectively removed via the technique of Triton-X-100/phosphate buffered saline decellularization. Subsequently, strengthening crosslinking associated with anticalcification was carried out with a 0.20-2.0 wt% glutaraldehyde solution to improve the mechanical properties, thermal stability and anticalcification feature. Finally, an animal-derived BD scaffold was manufactured for use in this study.
Overall, the duration of the operation and the BD anastomosis were basically the same as in human surgery. Cheng et al[12] established an animal BD damage and repair model by resecting a 1 cm long CBD followed by bridging the defect using a 2 cm long decellularized ureteral graft without tension. However, this animal experiment only considered the tension of anastomosis without considering the increased risk of biliary stricture caused by the fold of the redundant BD after the operation.
Different from previous studies, the present damage and repair model was developed with a 2 cm-to-2 cm replacement using the ada-BD to avoid longitudinal anastomotic tension and redundant BD tissue. The initial four animals in group A underwent ada-BD-to-duodenum anastomosis to repair the distal defect of the CBD and were sacrificed at the predetermined date of 6 mo postoperatively. Among these animals, the main complications, including bile leakage (1/8) and incision infection (1/8), were cured by conservative treatment without weight loss. The resulting increase in ALT on PoD 7 in group A was associated with liver function damage caused by bile leakage. Macroscopic assessments indicated that the ada-BD appeared to induce the covering of the visceral layer of the peritoneum and the regeneration of the biliary mucous membrane without the formation of any scars or strictures. As supplementary evidence, histological analysis (hematoxylin/eosin staining and cytokeratin 19 immunostaining) showed that the inner wall of the graft was covered by regenerated BD epithelium without marked inflammatory cell infiltration.
The above observations suggest that no obvious post transplantation rejection or biliary infection occurred in the experimental animals. With regard to the extrahepatic BD, the smooth muscle in the submucosal layer is a vital component that maintains the structural integrity, ensures the rigid structure and participates in the process of bile secretion. The desmin-positive area detected revealed the significant regeneration of smooth muscle in group A. Simultaneously, the remarkable regeneration of connective tissue in the submucosal layer was confirmed by Masson’s trichrome staining.
In comparison, the remaining four long-term observation animals recovered uneventfully after the operation. In addition, the histological analysis suggested the superiority in group B compared to group A regarding the regeneration of the BD epithelium and connective tissue. Notably, improved regeneration of the BD smooth muscle was observed in group A compared with group B. This phenomenon indicated that ada-BD-to-duodenum anastomosis showed a better induction effect on the regeneration of the BD smooth muscle compared with ada-BD-to-CBD anastomosis. No effect on the patency of the extrahepatic BD was found according to the macroscopic assessment and cholangiography. Thus, a prolonged investigation is needed to observe the long-term patency of the ada-BD graft. The enhancement of the regeneration of connective tissue in the submucosal layer in group B was confirmed by Masson’s trichrome staining. Both the macroscopic and microscopic assessment of the neo-extrahepatic BD showed no significant differences from a normal duct at 12 mo. Apparently, based on the results above, the ada-BD, as a nonimmunogenic scaffold, could provide good physiological conditions for the regeneration of autologous BD structures. This would suggest that the ada-BD was successfully used for the repair of BD segmental defects and achieved a similar effect as autologous BD end-to-end anastomosis.
This study evaluated an ada-BD for the reconstruction of a segmental extrahepatic BD defect in pigs. Extrahepatic BD reconstruction using this novel animal-derived BD scaffold as an interposition tube graft was safe and feasible. The long-term observation indicated that the reconstructed BD could serve as a native BD for bile drainage. An additional large sample study is needed to confirm the durability and safety of these preliminary results.
Several approaches have been explored to address extrahepatic biliary duct injury (BDI). The high incidence of complications affects postoperative recovery. Animal-derived artificial bile duct (ada-BD), a nonimmunogenic scaffold, could replace the defect via providing good physiological conditions for the regeneration of autologous bile duct structures without changing the original anatomical and physiologic conditions.
To assess the safety and feasibility of a novel animal derived material for treating extrahepatic BDI in pigs.
To evaluate and confirm the long-term feasibility of a novel heterogenous ada-BD for treating extrahepatic BDI.
Eight pigs were randomly divided into two groups. Surgery was performed to develop a bile duct injury and repair model via a 2 cm long novel heterogenous ada-BD to repair an approximately 2 cm segmental defect of various parts of the common bile duct (CBD) for all pigs. Group A received ada-BD-to-duodenum anastomosis to repair the distal CBD defect, and group B received ada-BD-to-CBD anastomosis to repair the intermedial CBD defect. The endpoint for observation was 6 mo (group A) and 12 mo (group B) after the operation.
All experimental animals survived until the endpoints for observation. The liver function was almost regular. Histologic analysis indicated a marked biliary epithelial layer covering the neo-bile duct and regeneration of the submucosal connective tissue and smooth muscle without significant signs of immune rejection. In comparison, the submucosal connective tissue was more regular and thicker in group B than in group A, and there was superior integrity of the regeneration of the biliary epithelial layer. Despite the advantages of the regeneration of the bile duct smooth muscle observed in group A, the effect on the patency of the ada-BD grafts in group B was not confirmed by macroscopic assessment and cholangiography.
Repairing a CBD defect with an ada-BD appears to be a feasible and safe approach. A large sample study is needed to confirm the durability and safety of these preliminary results.
Ada-BD showed a high similarity between the bovine ureter and bile duct. The decellularized bovine ureter provided an optimal pathophysiological condition and a novel material to avoid immunological rejection. All the encouraging results bring a new hope for bile duct repairment.
We thank Yang LX for assistance in the development of the article.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gupta N S-Editor: Chen XF L-Editor: Filipodia P-Editor: Wang LL
| 1. | Halbert C, Pagkratis S, Yang J, Meng Z, Altieri MS, Parikh P, Pryor A, Talamini M, Telem DA. Beyond the learning curve: incidence of bile duct injuries following laparoscopic cholecystectomy normalize to open in the modern era. Surg Endosc. 2016;30:2239-2243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 2. | Ismael HN, Cox S, Cooper A, Narula N, Aloia T. The morbidity and mortality of hepaticojejunostomies for complex bile duct injuries: a multi-institutional analysis of risk factors and outcomes using NSQIP. HPB (Oxford). 2017;19:352-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 3. | Iannelli A, Paineau J, Hamy A, Schneck AS, Schaaf C, Gugenheim J. Primary versus delayed repair for bile duct injuries sustained during cholecystectomy: results of a survey of the Association Francaise de Chirurgie. HPB (Oxford). 2013;15:611-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Sicklick JK, Camp MS, Lillemoe KD, Melton GB, Yeo CJ, Campbell KA, Talamini MA, Pitt HA, Coleman J, Sauter PA, Cameron JL. Surgical management of bile duct injuries sustained during laparoscopic cholecystectomy: perioperative results in 200 patients. Ann Surg. 2005;241:786-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 294] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 5. | Jabłonska B. End-to-end ductal anastomosis in biliary reconstruction: indications and limitations. Can J Surg. 2014;57:271-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Stilling NM, Fristrup C, Wettergren A, Ugianskis A, Nygaard J, Holte K, Bardram L, Sall M, Mortensen MB. Long-term outcome after early repair of iatrogenic bile duct injury. A national Danish multicentre study. HPB. 17:394-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 7. | Adamsen S, Hansen OH, Funch-Jensen P, Schulze S, Stage JG, Wara P. Bile duct injury during laparoscopic cholecystectomy: a prospective nationwide series. J Am Coll Surg. 1997;184:571-578. [PubMed] |
| 8. | Andersson R, Eriksson K, Blind PJ, Tingstedt B. Iatrogenic bile duct injury--a cost analysis. HPB. 10:416-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Heistermann HP, Palmes D, Stratmann U, Hohlbach G, Hierlemann H, Langer M, Spiegel HU. A new technique for reconstruction of the common bile duct by an autologous vein graft and a biodegradable endoluminal stent. J Invest Surg. 2006;19:57-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Miyazawa M, Torii T, Toshimitsu Y, Okada K, Koyama I, Ikada Y. A tissue-engineered artificial bile duct grown to resemble the native bile duct. Am J Transplant. 2005;5:1541-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Rosen M, Ponsky J, Petras R, Fanning A, Brody F, Duperier F. Small intestinal submucosa as a bioscaffold for biliary tract regeneration. Surgery. 2002;132:480-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Cheng Y, Xiong XZ, Zhou RX, Deng YL, Jin YW, Lu J, Li FY, Cheng NS. Repair of a common bile duct defect with a decellularized ureteral graft. World J Gastroenterol. 2016;22:10575-10583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (2)] |
| 13. | Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L, Fan ST, Yokoyama Y, Crawford M, Makuuchi M, Christophi C, Banting S, Brooke-Smith M, Usatoff V, Nagino M, Maddern G, Hugh TJ, Vauthey JN, Greig P, Rees M, Nimura Y, Figueras J, DeMatteo RP, Büchler MW, Weitz J. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011;149:680-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 948] [Cited by in RCA: 1408] [Article Influence: 100.6] [Reference Citation Analysis (0)] |
| 15. | Tang CL, Schlich T. Surgical Innovation and the Multiple Meanings of Randomized Controlled Trials: The First RCT on Minimally Invasive Cholecystectomy (1980-2000). J Hist Med Allied Sci. 2017;72:117-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Gupta V, Jain G. Safe laparoscopic cholecystectomy: Adoption of universal culture of safety in cholecystectomy. World J Gastrointest Surg. 2019;11:62-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 136] [Cited by in RCA: 112] [Article Influence: 18.7] [Reference Citation Analysis (16)] |
| 17. | Peng C, Ling Y, Ma C, Ma X, Fan W, Niu W, Niu J. Safety Outcomes of NOTES Cholecystectomy Versus Laparoscopic Cholecystectomy: A Systematic Review and Meta-Analysis. Surg Laparosc Endosc Percutan Tech. 2016;26:347-353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Gómez NA, Alvarez LR, Mite A, Andrade JP, Alvarez JR, Vargas PE, Tomalá NE, Vivas AF, Zapatier JA. Repair of bile duct injuries with Gore-Tex vascular grafts: experimental study in dogs. J Gastrointest Surg. 2002;6:116-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Li Q, Tao L, Chen B, Ren H, Hou X, Zhou S, Zhou J, Sun X, Dai J, Ding Y. Extrahepatic bile duct regeneration in pigs using collagen scaffolds loaded with human collagen-binding bFGF. Biomaterials. 2012;33:4298-4308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Normand C, Linde C, Bogale N, Blomström-Lundqvist C, Auricchio A, Stellbrink C, Witte KK, Mullens W, Sticherling C, Marinskis G, Sciaraffia E, Papiashvili G, Iovev S, Dickstein K. Cardiac resynchronization therapy pacemaker or cardiac resynchronization therapy defibrillator: what determines the choice? Europace. 2019;21:918-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Lim EJ, Chin R, Nachbur U, Silke J, Jia Z, Angus PW, Torresi J. Effect of Immunosuppressive Agents on Hepatocyte Apoptosis Post-Liver Transplantation. PLoS One. 2015;10:e0138522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Perry I, Neuberger J. Immunosuppression: towards a logical approach in liver transplantation. Clin Exp Immunol. 2005;139:2-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |