Published online Nov 21, 2020. doi: 10.3748/wjg.v26.i43.6891
Peer-review started: June 19, 2020
First decision: August 22, 2020
Revised: September 4, 2020
Accepted: October 20, 2020
Article in press: October 20, 2020
Published online: November 21, 2020
Processing time: 153 Days and 10.2 Hours
While Crohn’s disease has been studied extensively in high-income countries, its epidemiology and care in low and lower-middle income countries (LLMICs) is not well established due to a lack of disease registries and diagnostic capacity.
To describe the published burden, diagnostic/treatment capacity, service utilization, challenges/barriers to individuals with Crohn’s in LLMICs and their providers.
We conducted a scoping review utilizing a full search strategy was developed and conducted in PubMed, Embase and World Health Organization Global Index Medicus. Two independent reviewers screened the titles and abstracts of all of the publications found in this search, reviewed selected publications, and extracted relevant data, which underwent descriptive review and was analyzed in Excel.
The database search yielded 4486 publications, 216 of which were determined to be relevant to the research questions. Of all 79 LLMICs, only 21 (26.6%) have publications describing individuals with Crohn’s. Overall, the highest number of studies came from India, followed by Tunisia, and Egypt. The mean number of Crohn’s patients reported per study is 57.84 and the median is 22, with a wide range from one to 980.
This scoping review has shown that, although there is a severe lack of population-based data about Crohn’s in LLMICs, there is a signal of Crohn’s in these settings around the world.
Core Tip: This scoping review demonstrates the lack of epidemiologic data on Crohn’s disease in low and lower-middle income countries, but that it does exist in these settings and presents unique challenges. There is a need for population-based research to fully understand the its burden among the world’s poorest people.
- Citation: Rajbhandari R, Blakemore S, Gupta N, Adler AJ, Noble CA, Mannan S, Nikolli K, Yih A, Joshi S, Bukhman G. Crohn’s disease in low and lower-middle income countries: A scoping review. World J Gastroenterol 2020; 26(43): 6891-6908
- URL: https://www.wjgnet.com/1007-9327/full/v26/i43/6891.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i43.6891
Crohn’s disease is a chronic gastrointestinal disease which appears to be only moderately heritable[1,2], and multiple possible environmental and behavioral causes have been in invoked[3], including insufficient contact with infectious diseases in childhood (the hygiene hypothesis), antibiotic exposure, tobacco use, and consumption of highly processed foods. Crohn’s has historically been regarded as a “lifestyle” disease of industrialized countries[4,5]. First described in the United States in 1932, Crohn’s was increasingly diagnosed in Europe and North America during the 20th century, where around 0.5% of the population is now thought to be affected[6,7]. More recently, Crohn’s has been recognized in the rapidly developing upper-middle income countries of East Asia[8] and South America[9], with prevalence rates as high as 24 per 100000 in Brazil[10], and 11 per 100000 in South Korea[11].
In contrast, previous systematic reviews of the published literature on Crohn’s epidemiology have found few studies on either the prevalence or incidence of Crohn’s coming from the low- and lower-middle income countries (LLMICs)[12,13]. A review of population-based studies that were published between 1990 and 2016 found data from only four LLMICs[14]. These LLMIC studies were all in Asia (the Gampaha district of Sri Lanka, the Hyderabad district of India, Manilla city in the Phillippines, and Central Jakarata in Indonesia), had all come out of the prospective Asia-Pacific Crohn’s and Colitis Epidemiologic Study[14-17], and were all focused on urban areas. An earlier systematic review of incidence and prevalence studies (both population and facility-based) published between 1950 and 2010 only identified data from three countries that were classified as LLMICs at the time the research was conducted[18]. These countries were Sri Lanka (prospective study of Columbo and Gampaha districts)[19], Panama (restrospective review of hospital data from the Colon district)[20], and China (review of published reports from all hospitals)[21]. Currently, two of these countries—China and Panama are classified as upper-middle income and high income respectively. As a result, the 2017 Global Burden of Disease study largely based its inflammatory bowel disease (IBD) rate estimates for LLMICs on global trends[22].
The vast majority of the world’s poorest billion people live in the rural areas of LLMICs in sub-Saharan Africa and South Asia[23]. In the absence of primary population data regarding Crohn’s in these countries, there has been a perception that the burden of Crohn’s remains low among the global poor. Prior systematic reviews on Crohn’s have been limited, however, by narrow inclusion criteria focused on epidemiology (incidence and prevalence). Furthermore, the lack of reports regarding Crohn’s in LLMICs may be due to limitations in access to diagnosis and treatment for Crohn’s rather than the absence of disease. The correct diagnosis of Crohn’s requires a complex chain of events beginning with patients seeking care and ending with colonoscopy and histology. A break in any part of this chain resulting from gaps in financing, education, equipment, or supplies on the part of the patient or provider can result in a missed diagnosis. Even the pathological diagnosis of Crohn’s may be confused with intestinal tuberculosis in the absence of experienced healthworkers[24]. Crohn’s may also have an impact on patients living in extreme poverty that is out of proportion with the disease prevalence. Follow-up care for Crohn’s, like many other chronic diseases, requires frequent visits to health facilities, a steady supply of medications (including biologics), and often surgery[24]. The absence of the services could result in a high rates of disability and death among the poor affected by Crohn’s in LLMICs.
As part of an effort to understand the non-communicable burden of disease among the world’s poorest, we have conducted a scoping review with broad inclusion criteria focused on the experience with Crohn’s in LLMICs[25]. Specifically, this review seeks to answer the following research questions: (1) What is the published evidence regarding the burden of Crohn’s disease in communities and health facilities in LLMICs? Is underdiagnosis a problem? (2) What is the diagnostic and treatment capacity for Crohn’s disease in LLMICs? What services, equipment, and medications are used to diagnose and manage Crohn’s in LLMICs? (3) What challenges and barriers are there to providers and patients with Crohn’s disease in LLMICs in terms of diagnosis, treatment, and long-term management? And (4) What is known from the published literature regarding the social and demographic characteristics of patients with Crohn’s disease in LLMICs?
This review considered studies that describe cases of individuals with Crohn’s disease in an LLMIC as defined by The World Bank[17]. The World Bank categorizes the world’s countries into four income groups based on gross national income per capita: low-income countries, lower-middle income countries, upper-middle income countries, and high-income countries. The group of interest, LLMIC, includes both low-income countries and lower-middle income countries, or countries with a gross national income per capita of United Staes $3895 or less. To capture possible undiagnosed or misdiagnosed cases of Crohn’s in LLMICs that do not have any published Crohn’s data, as well as to understand their diagnostic and treatment capacity for Crohn’s, studies that mention the use of diagnostics (i.e., colonoscopy, small bowel follow-through, stool calprotectin), findings (i.e., skip lesions, cobblestone, small bowel obstruction), and treatments (i.e., colectomy, small bowel resection, infliximab) utilized in managing Crohn’s disease were also included. See Supplementary Table 1 for a full list of search terms. Publications that do not describe cases of individuals diagnosed with Crohn’s disease were excluded from this review. Relevant secondary sources (i.e., reviews, editorials, and commentaries) were excluded and used as background information. Studies that are based on cases of Crohn’s in middle, upper-middle, or high-income countries were excluded. Studies that are published in a language other than English were excluded. Studies describing non-human animals were excluded.
The search strategy aimed to locate published studies from all years. An initial limited search of PubMed was undertaken to identify articles on the topic. The text words contained in the titles and abstracts of relevant articles, and the index terms used to describe the articles were used to develop a full search strategy for PubMed in collaboration with an experienced medical librarian (see Supplementary Table 1 for full search strategy). The search strategy, including all identified keywords and index terms, was adapted for Embase and World Health Organization (WHO) Global Index Medicus. The team used free text and Medical Subject Headings, when applicable. Searches were conducted on publications in English for all years.
The information sources for this review were the databases PubMed, Embase, and WHO Global Index Medicus, which includes AMRO (Africa), IMEMR (Eastern Mediterranean), IMSEAR (South East Asia), LILACS (Americas), and WPRIM (Western Pacific), as well as MEDLINE and SciELO.
Following the search on May 14, 2019, all identified citations were collated and uploaded into EndNote X9 2018 (Clarivate Analytics, PA, United States) and duplicates removed. The study selection process consisted of two parts. First, two independent reviewers (SB and SM) screened the title and abstracts of all of the initially selected publications and included all of the studies that indicated a signal of Crohn’s disease or any related Crohn’s diagnostics or Crohn’s treatment in an LLMIC. Studies that met or could potentially meet the inclusion criteria were saved for full text review in EndNote. Any disagreements that arise between the reviewers were resolved through discussion, or with a third reviewer.
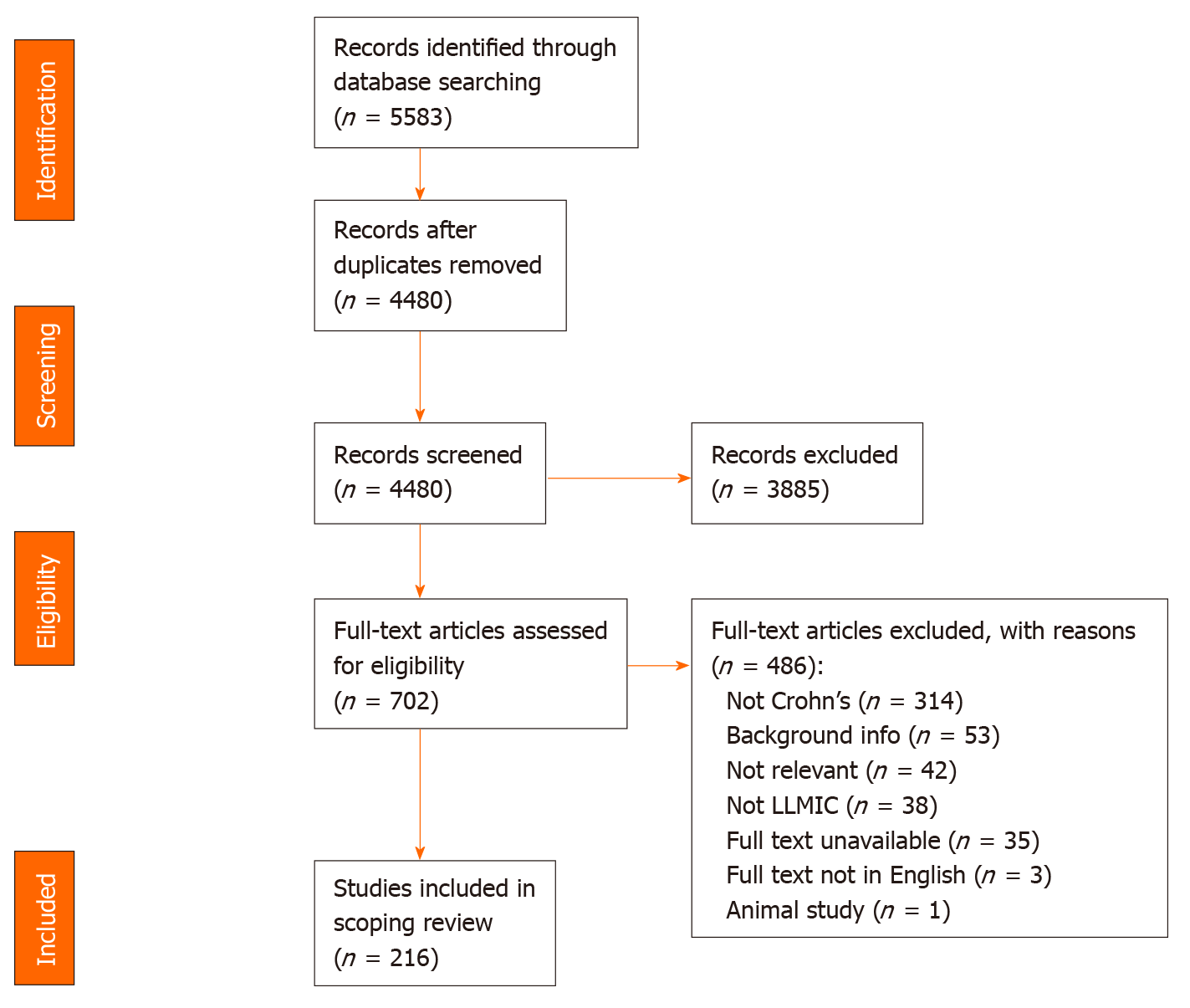
Next, a full text review was conducted to categorize the studies based on country and relevance to the research question. Two independent reviewers (KN and SM) assessed the full text of selected citations in detail against the inclusion criteria. Reasons for exclusion of full text studies that did not meet the inclusion criteria were recorded and are reported in a Preferred Reporting Items for Systematic Reviews and Meta-analyses flow diagram (Figure 1). Any disagreements that arose between the reviewers were resolved through discussion, or with a third reviewer (SB).
Full text of the publications selected were reviewed by two independent researchers (KN and SM) and data was extracted using a pre-structured and tested data collection form in Microsoft Excel. The data extracted includes specific details about the state of Crohn’s disease burden and care in LLMICs according to the review questions and specific objectives of the study. Any disagreements that arose between the reviewers were resolved through discussion, or with a third reviewer (SB). Authors of papers were contacted to request missing or additional data, where required.
A charting table was developed (see Supplementary Table 2, data extraction instrument) to record key information of the source, including the title, author, journal, date of publication, country, study years, study design, and results relevant to the review questions. In relation to burden, we collected data on number of cases reported, prevalence, incidence, odds, mortality rate, disability-adjusted life year rate, and average disease duration. Patient characteristics include both sociodemographic characteristics (age, residency, socioeconomic status, insurance coverage, out of pocket expenses) and clinical features of their disease (age at diagnosis, sex, risk factors, disease severity, disease behavior, and disease location, Crohn’s Disease Activity Index, extraintestinal manifestations, comorbidities, and disease outcomes). Qualitative information about disease diagnosis, management, long-term and follow-up care, and complications was documented to understand care pathways. Availability of diagnostic and treatment services included blood tests, stool tests, tissue pathology, Tuberculosis (TB) testing, endoscopy, radiology/imaging, other equipment, providers, and financing. Qualitative information about provider challenges (diagnostic and management) and patient barriers (access and financial) were collected in the table.
The descriptive findings extracted from the studies identified were charted to summarize the results of the research objectives of this review. The charted data then underwent a narrative review and descriptive analysis to identify emerging themes found in the data in terms of Crohn’s care pathways and availability of diagnostic and treatment services. Quantitative data regarding the burden of Crohn’s were analyzed in Excel.
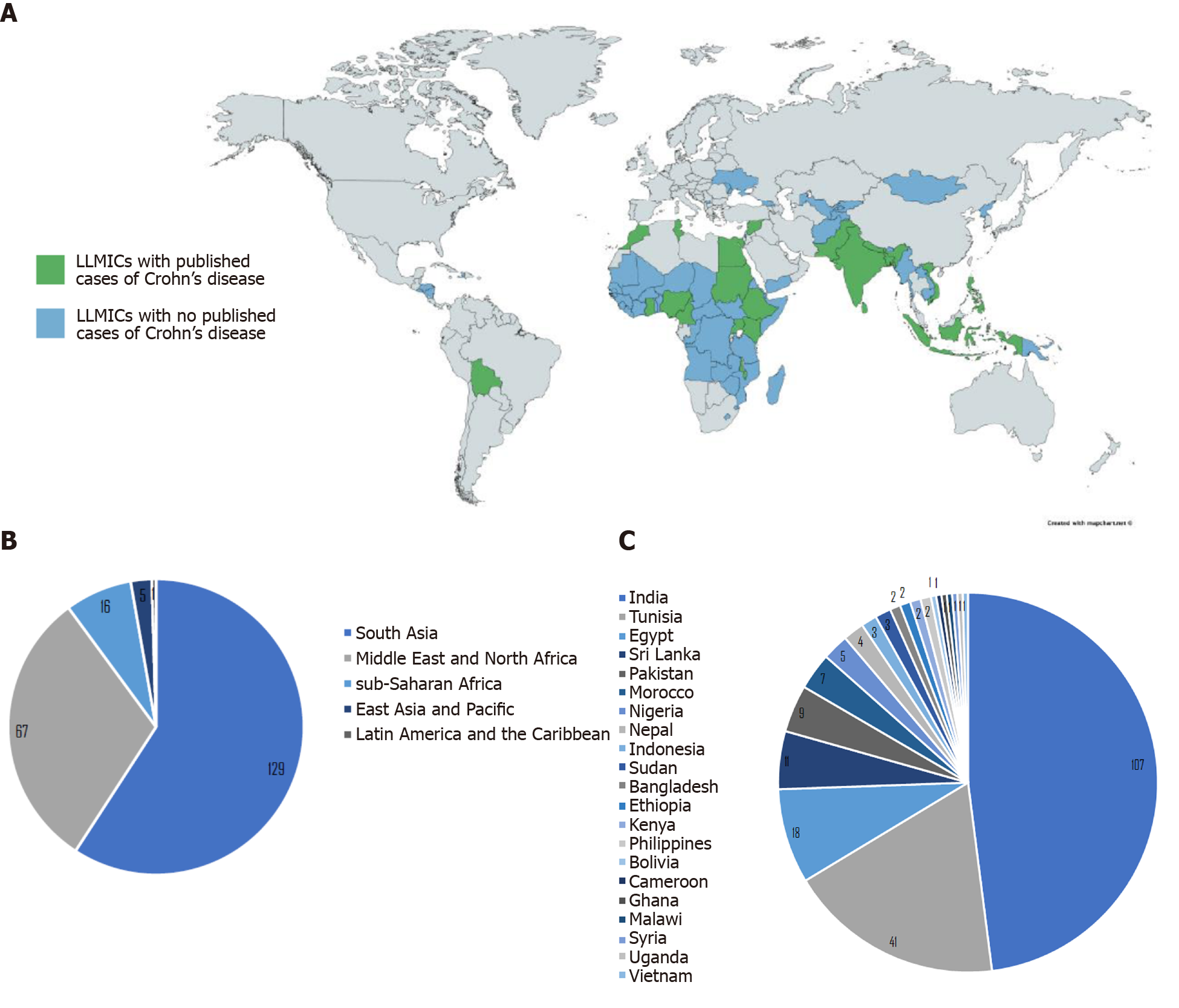
The initial database search of studies that describe Crohn’s disease and related diagnostics and findings in LLMICs found 4480 publications after removing duplicates, 702 of which were kept after title and abstract screening (Figure 1). Of those 702 publications, 216 were relevant to the research questions, 208 (96.3%) of which were based in lower-middle income countries and 8 (3.7%) of which were based in low income countries (see Supplementary Table 3 for a summary of all studies included in review by country). Of all 79 LLMICs, we only found 21 (26.6%) with studies describing individuals with Crohn’s disease. Most (73.4%) of the LLMICs do not have any studies describing individuals with Crohn’s disease identified through our search (Figure 2A).
Of the relevant articles, 129 (59.7%) were based in LLMICs in South Asia, 67 (31.0%) were from the Middle East and North Africa, 16 (7.4%) were from sub-Saharan Africa, 5 (2.3%) were from East Asia and Pacific, and 1 (0.5%) was from Latin America (see Supplementary Table 3, Figure 2B). The majority of Crohn’s studies identified are from India (49.5%), followed by Tunisia (19.0%), Egypt (8.3%), and Sri Lanka (5.1%). Bolivia, Cameroon, Ghana, Malawi, the Philippines, Syria, Uganda, and Vietnam each had one Crohn’s disease study (0.5%) (see Supplementary Table 3, Figure 2C).
Overall, the mean number of cases of Crohn’s disease reported per study is 57.84 and the median is 22, but ranges widely from single-patient case studies to cohorts of as many as 980 individuals with Crohn’s, and varies from country to country (Table 1). Countries in South Asia (63.42%) and the Middle East and North Africa (61.73%) regions reported substantially higher mean numbers of Crohn’s cases per study compared to sub-Saharan Africa (3.25%) and East Asia and Pacific (2.67%). Syria (106) and Morocco (102) reported the highest mean number of cases per study, while Bangladesh, Cameroon, Ghana, Malawi, and Uganda, each only have one single-patient case study (Table 1).
| Region/Country | n1 | Total cases | Mean | Median | Range |
| Overall | 2201 | 12725 | 57.84 | 22.00 | 1-980 |
| South Asia | 131 | 8485 | 64.77 | 17.00 | 1-980 |
| India | 107 | 8054 | 75.27 | 22.00 | 1-980 |
| Sri Lanka | 10 | 332 | 33.20 | 6.00 | 1-153 |
| Pakistan | 9 | 82 | 9.11 | 3.00 | 1-52 |
| Nepal | 4 | 16 | 4.00 | 2.00 | 1-11 |
| Bangladesh | 1 | 1 | 1.00 | 1.00 | 1 |
| Middle East and North Africa | 67 | 4165 | 62.16 | 39.00 | 1-226 |
| Tunisia | 41 | 2984 | 72.78 | 45.00 | 1-226 |
| Egypt | 18 | 361 | 20.06 | 12.50 | 1-100 |
| Morocco | 7 | 714 | 102.00 | 101.00 | 68-136 |
| Syria | 1 | 106 | 106.00 | 106.00 | 106 |
| sub-Saharan Africa | 16 | 52 | 3.25 | 1.00 | 1-17 |
| Nigeria | 5 | 15 | 3.00 | 1.00 | 1-8 |
| Sudan | 3 | 23 | 7.67 | 8.00 | 3-12 |
| Ethiopia | 2 | 8 | 4.00 | 4.00 | 1-7 |
| Kenya | 2 | 2 | 1.00 | 1.00 | 1-1 |
| Cameroon | 1 | 1 | 1.00 | 1.00 | 1 |
| Ghana | 1 | 1 | 1.00 | 1.00 | 1 |
| Malawi | 1 | 1 | 1.00 | 1.00 | 1 |
| Uganda | 1 | 1 | 1.00 | 1.00 | 1 |
| East Asia and Pacific | 5 | 15 | 3.00 | 8.00 | 1-6 |
| Indonesia | 2 | 6 | 3.00 | 3.00 | 1-5 |
| Philippines | 2 | 3 | 1.50 | 1.50 | 1-2 |
| Vietnam | 1 | 6 | 6.00 | 6.00 | 6 |
| Latin America and the Caribbean | 1 | 8 | 8.00 | 8.00 | 8 |
| Bolivia | 1 | 8 | 8.00 | 8.00 | 8 |
Of the 21 LLMICs included in the full-text review, only two countries had studies estimating prevalence of Crohn’s, India and Sri Lanka (Table 2). Two studies reported prevalence of Crohn’s in Sri Lanka, ranging from 1.2 per 100000 in the Colombo and Gampaha Districts in 2010[19], to 2.33 per 100000 in the Central Province in 2018[26]. Four of the 21 included countries countries—India, Indonesia, Sri Lanka, and the Philippines—reported incidence of Crohn’s disease, with most of these data coming from one multi-country study published in 2019[14] (Table 2). This study reported annual incidence of Crohn’s ranging from 0.14 per 100000 in the Philippines to 3.91 per 100000 in India[14]. The 2010 study from Sri Lanka reported an annual incidence of Crohn’s disease of 0.09 per 100000 in the Colombo and Gampha Districts[19].
| Country | Prevalence | Incidence |
| India | - | 3.91 per 100000 (Ng et al[14], 2019) |
| Sri Lanka | 1.2 per 100000 (Niriella et al[19], 2010) | 0.52 per 100000 (Ng et al[14], 2019) |
| 2.33 per 100000 (Kalubowila et al[25], 2018) | 0.09 per 100000 (Niriella et al[19], 2010) | |
| Indonesia | - | 0.27 per 100000 (Ng et al[14], 2019) |
| Philippines | - | 0.14 per 100000 (Ng et al[14], 2019) |
Of the 216 studies included in the review, 112 discussed the utilization of diagnostic and treatment services, all of which include cases that were confirmed via both colonoscopy and histology (Table 3). Of the 21 LLMICs included, all but Bolivia and Syria had at least one study discussing the utilization of of Crohn’s diagnostic services: Blood testing, stool testing, TB testing, radiology/imaging, endoscopy, and pathology services. South Asian countries reported the highest utilization of diagnostic services, with only Nepal lacking studies mentioning stool testing and TB testing (Table 3, see Supplementary Table 4 for numbers of studies reporting utilization of Crohn’s disease diagnostic and treatment services from each country). Included LLMICs in the Middle East and North Africa all had multiple studies reporting the utilization of endoscopy, radiology, and stool testing, however only one study from Egypt mentioned TB testing (see Supplementary Table 4)[27]. Studies from sub-Saharan Africa also mentioned TB testing as a tool for diagnosing Crohn’s less frequently compared to those from South Asia, with only two of the 16 included sub-Saharan African countries having studies discussing it in this context[28,29]. Twelve studies (five from India[30-34], two from Tunisia[35,36], and one each from Egypt[37], Nepal[38], Sudan[28], Ethiopia[39], and Uganda[40]) described Crohn’s being diagnosed surgically or on autopsy. Two studies, one a multi-country study from Asia and one from Nigeria, mentioned that only a clinical diagnosis of Crohn’s disease was made without endoscopic and pathologic investigation unless multiple diseases were suspected. In India, a failed trial of anti-tubercular therapy was mentioned in 11 studies as an important part of diagnosing Crohn’s disease. Two studies from Pakistan, and one study each from Ethiopia and Malawi, also discussed first treating their patients with anti-tubercular therapy to aid in Crohn’s disease diagnosis. It is important to note that many countries only have one or two included studies, so the absence of diagnostics mentioned might reflect a lack of academic research rather than a true lack of diagnostic capacity in those countries (see Supplementary Table 4).
| Country/ Region | Diagnostics | Medical | Surgical | ||||||||||||||||
| Endo-scopy | Path-ology | Radio-logy | Blood Test-ing | Stool Test-ing | Trial of ATT | Surgical/ autopsy diagnosis | Clinical diagnosis only | TB Test-ing | Cortico-steroids | Amino-salicyclates | Immuno-modulators | Biologic agents | Nutritional therapy | Colect-omy | Ost-omy | Small bowel resection | Ileoanal pouch | Stricture-plasty | |
| Overall (n = 216) | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| South Asia (n = 129) | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| India (n = 107) | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |
| Sri Lanka (n = 11) | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||
| Pakistan (n = 9) | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||
| Nepal (n = 3) | X | X | X | X | X | X | X | X | X | X | X | ||||||||
| Bangladesh (n = 2) | X | X | X | X | X | ||||||||||||||
| Middle East and North Africa (n = 67) | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||
| Tunisia (n = 41) | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||
| Egypt (n = 18) | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||
| Morocco (n = 7) | X | X | X | X | X | ||||||||||||||
| sub-Saharan Africa (n = 16) | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||
| Nigeria (n = 5) | X | X | X | X | X | X | X | X | X | ||||||||||
| Sudan (n = 3) | X | X | X | X | X | X | X | X | X | ||||||||||
| Ethiopia (n = 2) | X | X | X | X | X | X | X | X | X | X | X | ||||||||
| Kenya (n = 2) | X | X | |||||||||||||||||
| Uganda (n = 1) | X | X | X | X | X | ||||||||||||||
| Ghana (n = 1) | X | X | X | X | X | ||||||||||||||
| Cameroon (n = 1) | X | X | X | X | X | X | X | ||||||||||||
| Malawi (n = 1) | X | X | X | X | X | X | X | X | X | ||||||||||
| East Asia and Pacific (n = 5) | X | X | X | X | X | X | X | ||||||||||||
| Indonesia (n = 3) | X | X | X | X | X | ||||||||||||||
| Philippines (n = 2) | X | X | X | ||||||||||||||||
| Vietnam (n = 1) | X | X | X | ||||||||||||||||
Of the 21 LLMICs included, all but Bolivia, Ghana, and Syria had at least one study discussing the utilization of one or more Crohn’s medications or surgical treatments (Table 3). Corticosteroids, aminosalicyclates, and immunomodulators are the most frequently reported medications overall, while biologic agents are the least available (see Supplementary Table 4). Studies from India, Pakistan, Sri Lanka, Tunisia, and Egypt, report the use of medications in all major Crohn’s medication categories, while those from Bangladesh, Morocco, Kenya, Uganda, and Vietnam did not mention any Crohn’s medications (Table 3). The use of biologics were only discussed in 26 of the 216 studies, and were not mentioned in any studies from sub-Saharan Africa or East Asia and Pacific (see Supplementary Table 4). Nutritional therapy was also scaresly mentioned, with only six studies discussing dietary changes as a treatment for IBD: four from India[41-44], one from Egypt[45], and one from Malawi[46] (see Supplementary Table 4).
The most frequently discussed Crohn’s surgery overall is colectomy, followed closely by small bowel resection (see Supplementary Table 4). Ileoanal pouches were not specifically described in any of the included studies, but several studies described other or unspecified anal surgery for Crohn’s disease. Studies from Ghana, Malawi, and the Philippines did not mention any Crohn’s surgeries, and those from Bangladesh, Morocco, Sudan, and Kenya discussed surgery but did not specify which types (see Supplementary Table 4). Again, it is important to note that those countries with higher numbers of included studies also report the greatest use of diagnostics, medications, and surgeries.
Of the 216 studies in 21 countries included in this review, only 29 studies in 11 countries discussed patient geographic, socioeconomic, or cost information (see Supplementary Table 5 for a summary of patient geographic residency, socioeconomic characteristics, insurance coverage, and out of pocket costs). India had the most information due to the large number of available publications. All eight[14,32,47-52] of the studies from India that discussed patients’ geography reported that more individuals with Crohn’s resided in urban areas compared to rural areas (see Supplementary Table). Similarly, studies from Egypt[53], Ethiopia[54], and Indonesia[14,55] report individuals with Crohn’s coming from from cities more frequently. Sri Lanka, on the other hand, has a recent study reporting more cases among rural communities (73.9%) than urban (26.1%)[53]. Two older studies from Steury in 1975[54] and Bhatt in 1980[55] also describe more rural Crohn’s patients than urban.
Only eight studies from three of the included countries reported on socioeconomic characteristics, either income level, education level, or employment status: India, Tunisia, and Sudan (see Supplementary Table 5). Four of the five Indian studies[49,51,56,57], as well as the one from Sudan[41], reported that most individuals with Crohn’s disease belong to the middle or upper class and have relatively high level of education. In contrast, one study from India reported that the majority of individuals with Crohn’s are non-graduates (62.6%), are unemployed or unskilled workers (62.3%), and have an annual family income of less than 1000000 INR (approximately $14K) (83.8%)[52,58]. One Tunisian study from 2014 discussing socioeconomic characteristics reported that over half of the patients had a university education[59], whereas another in 2017 reported that 22.2% had a university education[60]. This study also reported that 34.3% of patients had “bad” socioeconomic conditions, 49% had “good” socioeconomic conditions, and 16.7 had “well” socioeconomic conditions[60].
Three included studies from India and two from Nigeria describe out of pocket costs and insurance coverage of individuals with Crohn’s disease (see Supplementary Table 5). Both of the studies from Nigeria reported that national health insurance programs are available but that coverage is limited, so all treatments were paid for out of pocket by patients[61,62]. Studies from India were more variable, with one from 2009 explaining that cost of medications was not a factor[56], another in 2017 reporting that 60% of patients were covered by private insurance[63], and most recently in 2019 where 14.3% of patients discontinued Adalimumab due to high cost.
Of the 21 LLMICs included in this review, 14 hypothesized at least one specific diagnostic, management, access, or financial challenge or barrier to individuals with Crohn’s disease and providers (see Supplementary Table 6 for a summary of diagnostic, management, access, and financial challenges and barriers to individuals with Crohn’s and providers in LLMICs). The most commonly reported provider challenge is differentiating between Crohn’s and intestinal tuberculosis (ITB), due to the high prevalence of TB in LLMICs and its overlap of symptoms and endoscopic features. This can result in long delays in disease diagnosis and thus appropriate treatment. A total of 36 studies in 10 countries in the review included distinguishing between Crohn’s and ITB as a diagnostic challenge to providers (Table 4). This was followed by diagnostic delays due to perceived rarity of IBD and lack of clinical awareness among providers, which was mentioned in 17 studies from eight countries, and lack of quality diagnostic facilities, which was mentioned in 14 studies from eight countries. Management challenges to Crohn’s providers were discussed less frequently than diagnostic challenges, with three studies reporting limited use of biologics due to cost, and one reporting risk of TB reactivation on biologics.
| Provider diagnostic challenges | Number of countries | Number of studies |
| Difficulty differentiating between Crohn’s and ITB | 10 | 36 |
| Low disease index of suspicion/clinical awareness due to perceived rarity of Crohn’s leads to underdiagnosis | 8 | 17 |
| Lack of quality diagnostic facilities and investigational modalities | 8 | 14 |
| Difficulty differentiating between Crohn’s and other infectious diseases | 7 | 16 |
| Difficulty differentiating between Crohn’s and UC | 5 | 7 |
| Diagnosis of Crohn’s made on histological exam of resected colon | 2 | 3 |
| Lack of reliable TB testing modalities | 2 | 2 |
| Provider Management Challenges | ||
| Use of biologics is limited due to cost | 1 | 3 |
| High risk of TB infection reactivation in patients treated with biologics | 1 | 1 |
| Patient Access Barriers | ||
| Lack of access to high quality health care services | 4 | 9 |
| Lack of education/knowledge about disease | 3 | 3 |
| Lack of access to Crohn’s medications | 1 | 1 |
| Patient Financial Barriers | ||
| Patients unable to afford treatment in general (medications and surgeries) | 6 | 9 |
| High cost of diagnostic testing | 3 | 4 |
| Lack of insurance coverage | 2 | 4 |
| Patients unable to afford biologics | 1 | 3 |
The most frequently reported patient barrier was cost of Crohn’s surgeries and medications, particularly biologics. Patients’ inability to afford the costs of their treatment in general was mentioned in nine studies, and high cost of biologics specifically in three studies (Table 4). Lack of access to high quality health care facilities was another common patient barrier, with nine studies describing access to care as a patient barrier.
The majority of publications describing Crohn’s disease in low and lower-middle income countries are from South Asia, with nearly half of the studies included in this review taking place in India. However, 73% of LLMICs worldwide don’t have any published studies on Crohn’s, highlighting a major lack of published data. There is an even more severe lack of population-based epidemiologic data about Crohn’s in LLMICs, with only four LLMICs reporting incidence or prevalence data-- India, Indonesia, Sri Lanka, and the Philippines, all of which are in Asia. Given the numerous diagnostic challenges facing gastroenterologists in diagnosing Crohn’s in LLMICs, this gap in knowledge might be reflective of providers’ inability to diagnose the disease due to these challenges, or simply a lack of resources to publish the data, rather than a true absence of IBD in these populations. It is quite telling that a majority (211) of the studies included in this review are from large tertiary facilities in capitol cities, which have the capacity to diagnose Crohn’s disease (see Supplementary Table 7 for a summary of all facilities included in the review).
The primary diagnostic challenges facing Crohn’s disease providers in LLMIC are: difficulty differentiating between Crohn’s and ITB, limited clinical awareness and low index of suspicion, and a lack of quality diagnostic facilities and investigational modalities. Distinguishing between Crohn’s and ITB is a serious issue for gastroenterologists in countries where TB is prevalent. Crohn’s and ITB overlap substantially in terms of their clinical symptoms, as well as their endoscopic, pathologic, and radiologic findings, but have entirely different treatment methods. Many providers in LLMICs treat TB empirically before even considering Crohn’s disease, which can delay diagnosis by months or years. Algorithms for accurately diagnosing Crohn’s and TB is an important area of gastroenterology research in LLMICs.
This review is unique from prior global studies of IBD because it is the first to be focused specifically on Crohn’s disease in LLMICs. Its scoping nature has also allowed us to adapt our research questions and inclusion criteria throughout the review process, thus identifying a larger number of LLMICs reporting individuals with Crohn’s disease compared to prior reviews. These countries are: Bangladesh, Bolivia, Cameroon, Egypt, Ethiopia, Ghana, India, Indonesia, Kenya, Malawi, Morocco, Nepal, Nigeria, Pakistan, the Philippines, Sri Lanka, Sudan, Syria, Tunisia, Uganda, and Vietnam. This review also documents diagnostic barriers that suggest that current incidence and prevalence estimates for these countries are likely too low.
Crohn’s disease research in general is very important in LLMICs, especially in those countries where there is no or very little data. What little data is published from LLMICs is generated primarily from large tertiary hospital and likely does not reflect the status of Crohn’s in the rest of the country. This lack of published data has contributed to a perceived rarity of Crohn’s by gastroenterologists in LLMICs, reducing their awareness of the disease and thus likelihood of accurately diagnosing it. It is critical to study and publish data on Crohn’s in LLMICs, even if they are single facility-based or case-studies, and to set up clinical data registries so that population-based epidemiologic research can shed light on the true burden of Crohn’s in these settings. A survey of gastroenterology providers in LLMICs, including in-depth interviews, would also be useful to more comprehensively capture the nuances of diagnosing and treating Crohn’s disease in low resource settings. Future studies should also collect and report data on the geography of where patients’ reside, as well as socioeconomic information such as income-level or employment.
Crohn’s disease is a time-consuming and expensive disease to diagnose and treat, and thus cost is the most significant barrier faced by Crohn’s patients in LLMICs. Biologics are especially expensive, and in some countries such as Nigeria, are only available through a special application to the government. This is further exacerbated by a general lack of insurance coverage among these patients. Although Crohn’s has historically been documented primarily among wealthier populations living in urban centers, this review does give some indication of Crohn’s in patients living in rural areas in India, Sri Lanka, Egypt, Tunisia, Ethiopia, Kenya, Indonesia, the Philippines, and Bolivia. Education of both gastroenterologists and other providers in LLMICs about the symptoms and findings of Crohn’s, especially at rural lower-level facilities, is also essential to improving disease awareness among providers and thus accurate diagnosis. Diagnosis is further delayed by a lack of reliable diagnostic facilities in remote areas of LLMICs, particularly endoscopy and pathology. Decentralizing Crohn’s care by training providers at lower-level care facilities in endoscopy and colonoscopy can potentially reduce underdiagnosis of Crohn’s in these areas. This may also be an opportunity to utilize telemedicine and telepathology technologies.
This scoping review has shown that Crohn’s disease does indeed exist in LLMICs in South Asia, the Middle East and North Africa, sub-Saharan Africa, East Asia and Pacific, and Latin America and the Caribbean, sometimes in large numbers. There is a pressing need to study the population epidemiology of Crohns’ disease in LLMICs to fully understand the burden of Crohn’s disease among the world’s poorest people. This is particularly important in those countries where it has not been studied at all, and in rural areas, where there is likely the most significant underdiagnosis and lack of access to medical care.
Crohn’s disease is a chronic gastrointestinal disease that has been recognized in the rapidly developing upper-middle income countries of East Asia, and South America. In contrast, previous systematic reviews of published literature have found few studies on either the prevalence or incidence of Crohn’s coming from the low- and lower-middle income countries (LLMICs). In the absence of primary population data regarding Crohn’s in these countries, there has been a perception that the burden of Crohn’s remains low among the global poor. As part of an effort to understand the non-communicable burden of disease among the world’s poorest, we have conducted a scoping review with broad inclusion criteria focused on the experience with Crohn’s in LLMICs.
The correct diagnosis of Crohn’s requires a complex chain of events beginning with patients seeking care and ending with colonoscopy and histology. A break in any part of this chain resulting from gaps in financing, education, equipment, or supplies on the part of the patient or provider can result in a missed diagnosis. Crohn’s may also have an impact on patients living in extreme poverty that is out of proportion with the disease prevalence. Follow-up care for Crohn’s, like many other chronic diseases, requires frequent visits to health facilities, a steady supply of medications (including biologics), and often surgery. The absence of the services could result in a high rates of disability and death among the poor affected by Crohn’s in LLMICs.
As part of an effort to understand the non-communicable burden of disease among the world’s poorest, we have conducted a scoping review with broad inclusion criteria focused on the experience with Crohn’s in LLMICs. Specifically, this review seeks to answer the following research questions: (1) What is the published evidence regarding the burden of Crohn’s disease in communities and health facilities in LLMICs? Is underdiagnosis a problem? (2) What is the diagnostic and treatment capacity for Crohn’s disease in LLMICs? What services, equipment, and medications are used to diagnose and manage Crohn’s in LLMICs? (3) What challenges and barriers are there to providers and patients with Crohn’s disease in LLMICs in terms of diagnosis, treatment, and long-term management? And (4) What is known from the published literature regarding the social and demographic characteristics of patients with Crohn’s disease in LLMICs?
The authors conducted a scoping review utilizing a full search strategy of studies that describe cases of individuals with Crohn’s disease in an LLMIC as defined by The World Bank that was developed and conducted in PubMed, Embase and World Health Organization Global Index Medicus. To capture possible undiagnosed or misdiagnosed cases of Crohn’s in LLMICs that do not have any published Crohn’s data, as well as to understand their diagnostic and treatment capacity for Crohn’s, studies that mention the use of diagnostics (i.e. colonoscopy, small bowel follow-through, stool calprotectin), findings (i.e. skip lesions, cobblestone, small bowel obstruction), and treatments (i.e. colectomy, small bowel resection, infliximab) utilized in managing Crohn’s disease were also included. Two independent reviewers screened the titles and abstracts of all of the publications found in this search, reviewed selected publications, and extracted relevant data, which underwent descriptive review and was analyzed in Excel.
The database search yielded 4486 publications, 216 of which were determined to be relevant to the research questions. Of all 79 LLMICs, only 21 (26.6%) have publications describing individuals with Crohn’s. The majority of Crohn’s studies identified are from India (49.5%), followed by Tunisia (19.0%), Egypt (8.3%), and Sri Lanka (5.1%). The mean number of Crohn’s patients reported per study is 56.84 and the median is 22, with a wide range from one to 980. Of the 21 LLMICs included in the review, only two countries (India and Sri Lanka) had studies estimating prevalence and only four countries (India, Indonesia, Sri Lanka and Philippines) had studies reporting incidence of Crohn’s disease with most of the data coming from one multi-country study.
Of the 216 studies included in the review, 112 discussed the utilization of diagnostic and treatment services, all of which include cases that were confirmed via both colonoscopy and histology. Of the 21 LLMICs included, all but Bolivia and Syria had at least one study discussing the utilization of Crohn’s diagnostic services: Blood testing, stool testing, TB testing, radiology/imaging, endoscopy, and pathology services. Corticosteroids, aminosalicyclates, and immunomodulators are the most frequently reported medications overall, while biologic agents are the least available. The most frequently discussed Crohn’s surgery overall is colectomy, followed closely by small bowel resection. Ileoanal pouches were not specifically described in any of the included studies, but several studies described other or unspecified anal surgery for Crohn’s disease. Of the 216 studies in 21 countries included in this review, only 29 studies in 11 countries discussed patient geographic, socioeconomic, or cost information.
Of the 21 LLMICs included in this review, 14 hypothesized at least one specific diagnostic, management, access, or financial challenge or barrier to individuals with Crohn’s disease and providers. The most commonly reported provider challenge is differentiating between Crohn’s and intestinal tuberculosis (ITB), due to the high prevalence of TB in LLMICs and its overlap of symptoms and endoscopic features. This can result in long delays in disease diagnosis and thus appropriate treatment. This was followed by diagnostic delays due to perceived rarity of IBD and lack of clinical awareness among providers, which was mentioned in 17 studies from eight countries, and lack of quality diagnostic facilities, which was mentioned in 14 studies from eight countries. The most frequently reported patient barrier was the cost of Crohn’s surgeries and medications, particularly biologics. Lack of access to high quality health care facilities was another common patient barrier, with nine studies describing access to care as a patient barrier.
A lack of published data has contributed to a perceived rarity of Crohn’s by gastroenterologists in LLMICs, reducing their awareness of the disease and thus likelihood of accurately diagnosing it. It is critical to study and publish data on Crohn’s in LLMICs, even if they are single facility-based or case-studies, and to set up clinical data registries so that population-based epidemiologic research can shed light on the true burden of Crohn’s in these settings.
This scoping review has shown that Crohn’s disease does indeed exist in LLMICs in South Asia, the Middle East and North Africa, sub-Saharan Africa, East Asia and Pacific, and Latin America and the Caribbean, sometimes in large numbers.
This scoping review demonstrates that although there is a lack of rigorous epidemiologic data on Crohn’s disease in LLMICs, it actually does exist, sometimes in large numbers, in these settings and presents unique challenges that need to be addressed to advance non-communicable diseases care in low resource settings.
There is a pressing need to study the population epidemiology of Crohns’ disease in LLMICs to fully understand the burden of Crohn’s disease among the world’s poorest people. This is particularly important in those countries where it has not been studied at all, and in rural areas, where there is likely the most significant underdiagnosis and lack of access to medical care.
Patients with Crohn’s disease do indeed exist in low resource settings and there are existing models of service delivery that could be learned from and adapted to meet the unique challenges of management. For example, this study has found that one of the primary diagnostic challenges facing Crohn’s disease providers in LLMIC are: difficulty differentiating between Crohn’s and ITB. Crohn’s and ITB overlap substantially in terms of their clinical symptoms, as well as their endoscopic, pathologic, and radiologic findings, but have entirely different treatment methods. Many providers in LLMICs treat TB empirically before even considering Crohn’s disease, which can delay diagnosis by months or years.
Decentralizing Crohn’s care by training providers at lower-level care facilities in endoscopy and colonoscopy can potentially reduce underdiagnosis of Crohn’s in these areas. This may also be an opportunity to utilize telemedicine and telepathology technologies.
This study utilized broad inclusion criteria to map the landscape of studies showing where and how services for the management of Crohn’s disease are provided and organized.
If there are existing models of service delivery for patients with Crohn’s disease in low resource settings, there can also be valuable lessons in how these models can be adapted and translated to similar areas still in need of services.
This review seeks to answer the following research questions: (1) What is the published evidence regarding the burden of Crohn’s disease in communities and health facilities in LLMICs? Is underdiagnosis a problem? (2) What is the diagnostic and treatment capacity for Crohn’s disease in LLMICs? What services, equipment, and medications are used to diagnose and manage Crohn’s in LLMICs? (3) What challenges and barriers are there to providers and patients with Crohn’s disease in LLMICs in terms of diagnosis, treatment, and long-term management? And (4) What is known from the published literature regarding the social and demographic characteristics of patients with Crohn’s disease in LLMICs?
Education of both gastroenterologists and other providers in LLMICs about the symptoms and findings of Crohn’s, especially at rural lower-level facilities, is essential to improving disease awareness among providers and thus accurate diagnosis. Decentralizing Crohn’s care by training providers at lower-level care facilities in endoscopy and colonoscopy can potentially reduce underdiagnosis of Crohn’s in these areas. This may also be an opportunity to utilize telemedicine and telepathology technologies.
Crohn’s disease research in general is very important in LLMICs, especially in those countries where there is no or very little data. What little data is published from LLMICs is generated primarily from large tertiary hospitals and likely does not reflect the status of Crohn’s in the rest of the country.
It is critical to study and publish data on Crohn’s in LLMICs, even if they are single facility-based or case-studies, and to set up clinical data registries so that population-based epidemiologic research can shed light on the true burden of Crohn’s in these settings.
A survey of gastroenterology providers in LLMICs, including in-depth interviews, would also be useful to more comprehensively capture the nuances of diagnosing and treating Crohn’s disease in low resource settings. Future studies should also collect and report data on the geography of where patients’ reside, as well as socioeconomic information such as income-level or employment. In addition, algorithms for accurately diagnosing Crohn’s and TB is an important area of gastroenterology research in LLMICs.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: Massachusetts Medical Society; American Society for Gastrointestinal Endoscopy; and American Association for the Study of Liver Diseases.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: El-Hussuna A, Odes S, Pang Z S-Editor: Zhang L L-Editor: A P-Editor: Ma YJ
| 1. | Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn's disease. Lancet. 2017;389:1741-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1121] [Cited by in RCA: 1806] [Article Influence: 225.8] [Reference Citation Analysis (111)] |
| 2. | Gordon H, Trier Moller F, Andersen V, Harbord M. Heritability in inflammatory bowel disease: from the first twin study to genome-wide association studies. Inflamm Bowel Dis. 2015;21:1428-1434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 3. | Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1299] [Cited by in RCA: 1512] [Article Influence: 84.0] [Reference Citation Analysis (2)] |
| 4. | Cook GC. Tropical gastroenterology. Oxford, UK: Oxford University Press; 1980.. [DOI] [Full Text] |
| 5. | Bernstein CN, Shanahan F. Disorders of a modern lifestyle: reconciling the epidemiology of inflammatory bowel diseases. Gut. 2008;57:1185-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 201] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 6. | CROHN BB, GINZBURG L, OPPENHEIMER GD. Regional ileitis; a pathologic and clinical entity. Am J Med. 1952;13:583-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 84] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12:720-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1157] [Cited by in RCA: 1874] [Article Influence: 187.4] [Reference Citation Analysis (1)] |
| 8. | Prideaux L, Kamm MA, De Cruz PP, Chan FK, Ng SC. Inflammatory bowel disease in Asia: a systematic review. J Gastroenterol Hepatol. 2012;27:1266-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 265] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 9. | Kotze PG, Underwood FE, Damião AOMC, Ferraz JGP, Saad-Hossne R, Toro M, Iade B, Bosques-Padilla F, Teixeira FV, Juliao-Banos F, Simian D, Ghosh S, Panaccione R, Ng SC, Kaplan GG. Progression of Inflammatory Bowel Diseases Throughout Latin America and the Caribbean: A Systematic Review. Clin Gastroenterol Hepatol. 2020;18:304-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 125] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 10. | Lima Martins A, Volpato RA, Zago-Gomes MDP. The prevalence and phenotype in Brazilian patients with inflammatory bowel disease. BMC Gastroenterol. 2018;18:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 11. | Yang SK, Yun S, Kim JH, Park JY, Kim HY, Kim YH, Chang DK, Kim JS, Song IS, Park JB, Park ER, Kim KJ, Moon G, Yang SH. Epidemiology of inflammatory bowel disease in the Songpa-Kangdong district, Seoul, Korea, 1986-2005: a KASID study. Inflamm Bowel Dis. 2008;14:542-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 381] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 12. | GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:17-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1466] [Cited by in RCA: 1458] [Article Influence: 291.6] [Reference Citation Analysis (0)] |
| 13. | Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL, Sung JJY, Kaplan GG. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018;390:2769-2778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2677] [Cited by in RCA: 4111] [Article Influence: 513.9] [Reference Citation Analysis (110)] |
| 14. | Ng SC, Kaplan GG, Tang W, Banerjee R, Adigopula B, Underwood FE, Tanyingoh D, Wei SC, Lin WC, Lin HH, Li J, Bell S, Niewiadomski O, Kamm MA, Zeng Z, Chen M, Hu P, Ong D, Ooi CJ, Ling KL, Miao Y, Miao J, Janaka de Silva H, Niriella M, Aniwan S, Limsrivilai J, Pisespongsa P, Wu K, Yang H, Ng KK, Yu HH, Wang Y, Ouyang Q, Abdullah M, Simadibrata M, Gunawan J, Hilmi I, Lee Goh K, Cao Q, Sheng H, Ong-Go A, Chong VH, Ching JYL, Wu JCY, Chan FKL, Sung JJY. Population Density and Risk of Inflammatory Bowel Disease: A Prospective Population-Based Study in 13 Countries or Regions in Asia-Pacific. Am J Gastroenterol. 2019;114:107-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 188] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 15. | Ng SC, Tang W, Ching JY, Wong M, Chow CM, Hui AJ, Wong TC, Leung VK, Tsang SW, Yu HH, Li MF, Ng KK, Kamm MA, Studd C, Bell S, Leong R, de Silva HJ, Kasturiratne A, Mufeena MNF, Ling KL, Ooi CJ, Tan PS, Ong D, Goh KL, Hilmi I, Pisespongsa P, Manatsathit S, Rerknimitr R, Aniwan S, Wang YF, Ouyang Q, Zeng Z, Zhu Z, Chen MH, Hu PJ, Wu K, Wang X, Simadibrata M, Abdullah M, Wu JC, Sung JJY, Chan FKL; Asia–Pacific Crohn's and Colitis Epidemiologic Study (ACCESS) Study Group. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn's and colitis epidemiology study. Gastroenterology. 2013;145:158-165.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 597] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 16. | Kasturiratne A, Mufeena MN, Mettananda KC, Fernandopulle N, Rajindrajith S, Waraketiya PR, Weerasinghe SK, Ranaweera A, Hewavisenthi SJ, de Silva AP, de Silva HJ. Incidence of inflammatory bowel disease in Gampaha district: details of the Sri Lankan component of the Asia-Pacific Crohn's and colitis epidemiology study. Ceylon Med J. 2014;59:16-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | World Bank Country and Lending Groups: Historical Classification by Income. Washington, DC: World Bank; 2020. Available from: URL: http://databank.worldbank.org/data/download/site-content/OGHIST.xls Accessed January 29, 2020. |
| 18. | Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, Kaplan GG. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46-54.e42; quiz e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3789] [Cited by in RCA: 3527] [Article Influence: 271.3] [Reference Citation Analysis (5)] |
| 19. | Niriella MA, De Silva AP, Dayaratne AH, Ariyasinghe MH, Navarathne MM, Peiris RS, Samarasekara DN, Satharasinghe RL, Rajindrajith S, Dassanayake AS, Wickramasinghe AR, de Silva HJ. Prevalence of inflammatory bowel disease in two districts of Sri Lanka: a hospital based survey. BMC Gastroenterol. 2010;10:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Linares de la Cal JA, Cantón C, Hermida C, Pérez-Miranda M, Maté-Jiménez J. Estimated incidence of inflammatory bowel disease in Argentina and Panama (1987-1993). Rev Esp Enferm Dig. 1999;91:277-286. [PubMed] |
| 21. | Zheng JJ, Zhu XS, Huangfu Z, Shi XH, Guo ZR. Prevalence and incidence rates of Crohn's disease in mainland China: a meta-analysis of 55 years of research. J Dig Dis. 2010;11:161-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Robles Aguilar G, Sumner A. Who Are the World's Poor? A New Profile of Global Multidimensional Poverty. Working Paper 499. Washington, DC: Center for Global Development; 2019: 1-39. Available from: URL: https://www.cgdev.org/sites/default/files/who-are-worlds-poor-new-profile-global-multidimensional-poverty.pdf. |
| 23. | Hu PJ. Inflammatory Bowel Disease in Asia: The Challenges and Opportunities. Intest Res. 2015;13:188-190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Bukhman G, Mocumbi AO, Horton R. Reframing NCDs and injuries for the poorest billion: a Lancet Commission. Lancet. 2015;386:1221-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Kalubowila U, Liyanaarachchi T, Galketiya KB, Rathnayaka P, Piyasena INAP, Tennakoon S, Perera KMP, Pathirana SDMU, Wettewa DB, Ratnayake WRANMP, Raayiz RM, Dissanayaka DMIU. Epidemiology and clinical course of inflammatory bowel disease in the Central Province of Sri Lanka: A hospital-based study. JGH Open. 2018;2:129-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Fawzy A, Prince A, Hassan AA, Fayed A, Zschock M, Naga M, Omar M, Salam M, El-Sayed A. Epidemiological studies on Johne’s disease in ruminants and Crohn’s disease in humans in Egypt. Int J Vet Sci Med. 2013;1:79-86. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Masri SH, Satir AA. Crohn's disease in three Sudanese patients. East Afr Med J. 1975;52:284-293. [PubMed] |
| 28. | Alegbeleye BJ. Crohn's disease in a developing African mission hospital: a case report. J Med Case Rep. 2019;13:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Amarapurkar DN, Patel ND, Rane PS. Diagnosis of Crohn's disease in India where tuberculosis is widely prevalent. World J Gastroenterol. 2008;14:741-746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 98] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 30. | Mahurkar S, Banerjee R, Rani VS, Thakur N, Rao GV, Reddy DN, Chandak GR. Common variants in NOD2 and IL23R are not associated with inflammatory bowel disease in Indians. J Gastroenterol Hepatol. 2011;26:694-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Makharia GK, Ramakrishna BS, Abraham P, Choudhuri G, Misra SP, Ahuja V, Bhatia SJ, Bhasin DK, Dadhich S, Dhali GK, Desai DC, Ghoshal UC, Goswami BD, Issar SK, Jain AK, Jayanthi V, Loganathan G, Pai CG, Puri AS, Rana SS, Ray G, Singh SP, Sood A; Indian Society of Gastroenterology Task Force on Inflammatory Bowel Disease. Survey of inflammatory bowel diseases in India. Indian J Gastroenterol. 2012;31:299-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 32. | Kalaria R, Desai D, Abraham P, Joshi A, Gupta T, Shah S. Temporal Change in Phenotypic Behaviour in Patients with Crohn's Disease: Do Indian Patients Behave Differently from Western and Other Asian Patients? J Crohns Colitis. 2016;10:255-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 33. | Chauhan V, Goel V, Jain M, Gupta G, Pokharna R, Sharma SS, Nijhawan S. Capsule endoscopy for obscure gastrointestinal bleeding: A single-center experience. J Dig Endosc. 2018;9:168-75. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 34. | Feki S, Bouzid D, Abida O, Chtourou L, Elloumi N, Toumi A, Hachicha H, Amouri A, Tahri N, Masmoudi H. Genetic association and phenotypic correlation of TLR4 but not NOD2 variants with Tunisian inflammatory bowel disease. J Dig Dis. 2017;18:625-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Makni A, Magherbi H, El Heni A, Haddad A, Daghfouss A, Rebai W, Chebbi F, Ksantini R, Fterich F, Jouini M, Kacem M, Ben Mami N, Filali A, Ben Safta Z. Surgical management of primary crohn's disease. descriptive study about 226 patients. Tunis Med. 2017;95:185-191. [PubMed] |
| 36. | Hussein HM and Muhammad EMS. Inflammatory bowel disease: Diagnostic and therapeutic modalities. 2006; 10: 175-187. Available from: https://www.researchgate.net/publication/260055166_Inflammatory_bowel_diseases_Diagnostic_and_therapeutic_modalities#fullTextFileContent. |
| 37. | Karki S, Karak AK, Sinha AK, Kumar B, Upadhyaya P, Pandey SR, Regmi R. Crohn disease in Nepal: true rarity or gross underdiagnosis? BMJ Case Rep. 2009;2009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 38. | Mengesha B, Johnson O, Taye M, Gemetchu T. Crohn's disease: report of seven cases from Ethiopia. East Afr Med J. 1997;74:397-399. [PubMed] |
| 39. | KIBAYA AK. Possible case of Crohn's disease in a Ruanda native. East Afr Med J. 1946;23:317-320. [PubMed] |
| 40. | Ganesh R, Suresh N, Ezhilarasi S, Rajajee S, Sathiyasekaran M. Crohn's disease presenting as palatal ulcer. Indian J Pediatr. 2006;73:229-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 41. | Benjamin J, Makharia G, Ahuja V, Joshi YK. Body composition in Indian patients with Crohn's disease during active and remission phase. Trop Gastroenterol. 2011;32:285-291. [PubMed] |
| 42. | Kochhar R, Gupta V, Dutta U, Singh K, Kochhar R. Infliximab induced endophthalmitis in a patient of fistulizing Crohn's disease. Indian J Gastroenterol. 2011;30:241-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 43. | Sonavane AD, Sonawane P, Amarapurkar DN. Inflammatory Bowel Disease Across the Age Continuum: Similarity and Disparity. Indian J Pediatr. 2018;85:989-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 44. | Elhusseiny MH, Amine AK, Salem OE, Tayel DI, Elsayed EA. Low FODMAP diet in Egyptian patients with Crohn's disease in remission phase with functional gastrointestinal symptoms. JGH Open. 2018;2:15-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 45. | Manda G, Finch P, Mponda K. Pyoderma gangrenosum associated with Crohn's disease in a Malawian teenage boy: case report and review of literature. Trop Doct. 2018;48:43-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 46. | Singh AV, Singh SV, Sohal JS, Singh PK. Genotype profiles of Mycobacterium avium subspecies paratuberculosis recovered from suspected and Crohn's disease patients in India. J Commun Dis. 2010;42:91-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 47. | Balasubramanian LK, Kuppanan S, Vellingiri B, Keshavro S, Leelakrishnan V. Efficacy of differential non-invasive approaches in determining the clinical course in patients with Crohn’s disease. Asian Biomed. 2011;5:625-633. [DOI] [Full Text] |
| 48. | Pugazhendhi S, Sahu MK, Subramanian V, Pulimood A, Ramakrishna BS. Environmental factors associated with Crohn's disease in India. Indian J Gastroenterol. 2011;30:264-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 49. | Goel A, Dutta AK, Pulimood AB, Eapen A, Chacko A. Clinical profile and predictors of disease behavior and surgery in Indian patients with Crohn's disease. Indian J Gastroenterol. 2013;32:184-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 50. | Larsson G, Shenoy T, Ramasubramanian R, Balakumaran LK, Småstuen MC, Bjune GA, Moum BA. Routine diagnosis of intestinal tuberculosis and Crohn's disease in Southern India. World J Gastroenterol. 2014;20:5017-5024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 51. | Amarapurkar AD, Amarapurkar DN, Rathi P, Sawant P, Patel N, Kamani P, Rawal K, Baijal R, Sonawane A, Narawane N, Kolekar S, Totla N. Risk factors for inflammatory bowel disease: A prospective multi-center study. Indian J Gastroenterol. 2018;37:189-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 52. | Subasinghe D, Wijekoon NS, Nawarathne NM, Samarasekera DN. Disease-related knowledge in inflammatory bowel disease: experience of a tertiary care centre in a developing country in South Asia. Singapore Med J. 2010;51:484-489. [PubMed] |
| 53. | Rios-Dalenz J, Smith LB, Thompson TF. Diseases of the colon and rectum in Bolivia. Am J Surg. 1975;129:661-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 54. | Steury EM, Templeton AC. Crohn's disease in Africa. A case report and review. Trop Geogr Med. 1980;32:172-173. [PubMed] |
| 55. | Bhatt J, Patil S, Joshi A, Abraham P, Desai D. Self-reported treatment adherence in inflammatory bowel disease in Indian patients. Indian J Gastroenterol. 2009;28:143-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 56. | Tomar SK, Kedia S, Upadhyay AD, Bopanna S, Yadav DP, Goyal S, Jain S, Makharia G, Ahuja V, Singh N. Impact of dietary beliefs and practices on patients with inflammatory bowel disease: An observational study from India. JGH Open. 2017;1:15-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 57. | Khalifa SE, Mudawi HM, Fedail SS. Presentation and management outcome of inflammatory bowel disease in Sudan. Trop Gastroenterol. 2005;26:194-196. [PubMed] |
| 58. | Ennaifer R, Elleuch N, Cheikh M, Hefaiedh R, Romdhane H, Ben Nejma H, Belhadj N. Risk factors of psychological disorders in inflammatory bowel disease in a tunisian survey. Results of a cross-sectional study. Tunis Med. 2014;92:723-726. [PubMed] |
| 59. | Mrabet S, Ksiaa M, Elleuch N, Jaziri H, Ben Mansour I, Braham A, Ajmi S, Ben Slama A, Jmaa A. Quality of life in inflammatory bowel disease in Tunisian patients. Tunis Med. 2017;95:229-235. [PubMed] |
| 60. | Alatise OI, Otegbayo JA, Nwosu MN, Lawal OO, Ola SO, Anyanwu SN, Ndububa DA, Akere A, Odike MA, Agbakwuru EA, Soyemi OM, Okonkwo UC. Characteristics of inflammatory bowel disease in three tertiary health centers in southern Nigeria. West Afr J Med. 2012;31:28-33. [PubMed] |
| 61. | Ekwunife CN, Nweke IG, Achusi IB, Ekwunife CU. Ulcerative Colitis Prone to Delayed Diagnosis in a Nigerian Population: Case Series. Ann Med Health Sci Res. 2015;5:311-313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 62. | Kamat N, Ganesh Pai C, Surulivel Rajan M, Kamath A. Cost of Illness in Inflammatory Bowel Disease. Dig Dis Sci. 2017;62:2318-2326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 63. | Kamat N, Kedia S, Ghoshal UC, Nehra A, Makharia G, Sood A, Midha V, Gupta V, Choudhuri G, Ahuja V. Effectiveness and safety of adalimumab biosimilar in inflammatory bowel disease: A multicenter study. Indian J Gastroenterol. 2019;38:44-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |