Published online Nov 14, 2020. doi: 10.3748/wjg.v26.i42.6689
Peer-review started: August 28, 2020
First decision: September 30, 2020
Revised: October 11, 2020
Accepted: October 26, 2020
Article in press: October 26, 2020
Published online: November 14, 2020
Processing time: 76 Days and 15.1 Hours
Hereditary diffuse gastric cancer (HDGC) is a familial cancer syndrome often associated with germline mutations in the CDH1 gene. However, the frequency of CDH1 mutations is low in patients with HDGC in East Asian countries. Herein, we report three cases of HDGC harboring a missense CDH1 variant, c.1679C>G, from a single Japanese family.
A 26-year-old female (Case 1) and a 51-year-old male (father of Case 1), who had a strong family history of gastric cancer, were diagnosed with advanced diffuse gastric cancer. After genetic counselling, a 25-year-old younger brother of Case 1 underwent surveillance esophagogastroduodenoscopy that detected small signet ring cell carcinoma foci as multiple pale lesions in the gastric mucosa. Genetic analysis revealed a CDH1 c.1679C>G variant in all three patients.
It is important for individuals suspected of having HDGC to be actively offered genetics evaluation. This report will contribute to an increased awareness of HDGC.
Core Tip: Hereditary diffuse gastric cancer (HDGC) has rarely been reported in East Asian countries. We report a Japanese HDGC family with a missense CDH1 variant, c.1679C>G (p.T560R). We clearly detected early signet ring cell carcinoma foci by esophagogastroduodenoscopy with white light imaging, non-magnifying narrow band imaging (NBI) and magnifying NBI. In this family, active genetics evaluation and intensive endoscopic surveillance resulted in early diagnosis and treatment of HDGC.
- Citation: Hirakawa M, Takada K, Sato M, Fujita C, Hayasaka N, Nobuoka T, Sugita S, Ishikawa A, Mizukami M, Ohnuma H, Murase K, Miyanishi K, Kobune M, Takemasa I, Hasegawa T, Sakurai A, Kato J. Case series of three patients with hereditary diffuse gastric cancer in a single family: Three case reports and review of literature. World J Gastroenterol 2020; 26(42): 6689-6697
- URL: https://www.wjgnet.com/1007-9327/full/v26/i42/6689.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i42.6689
Gastric cancer (GC) is the fifth most common neoplasm and the third most deadly cancer worldwide, with an estimated 783000 deaths per year[1]. Although most instances of GC are sporadic, approximately 1%-3% of cases arise as a result of inherited cancer syndromes[2]. Hereditary diffuse gastric cancer (HDGC) is an autosomal dominant cancer syndrome. The relationship between HDGC and germline mutation of CDH1, encoding the tumor-suppressor protein E-cadherin, was first identified in New Zealand families[3]. To date, over 155 germline CDH1 mutations, of which the majority are pathogenic and a number of variants are unclassified, have been described[2]. However, the detection rate of CDH1 germline mutations in patients with HDGC is low and few cases have been reported in East Asian countries[4-10]. In the current report, we present the clinical courses of three cases with HDGC harboring a germline pathogenic variant of CDH1, c.1679C>G, from a single family.
Cases 1-3: Unremarkable.
Case 1: The proband is a 26-year-old female. She was referred to our hospital for screening esophagogastroduodenoscopy (EGD) because her older brother died of GC 3 years ago at another hospital.
Case 2: A 51-year-old male (father of Case 1) visited our hospital for screening EGD because he had a strong family history of gastric cancer.
Case 3: As a result of taking the detailed family history, we noted that Cases 1 and 2 had several family members with GC. We suspected HDGC and performed genetic counselling for a 25-year-old younger brother of Case 1.
Cases 1-3: The patients had a free previous medical history.
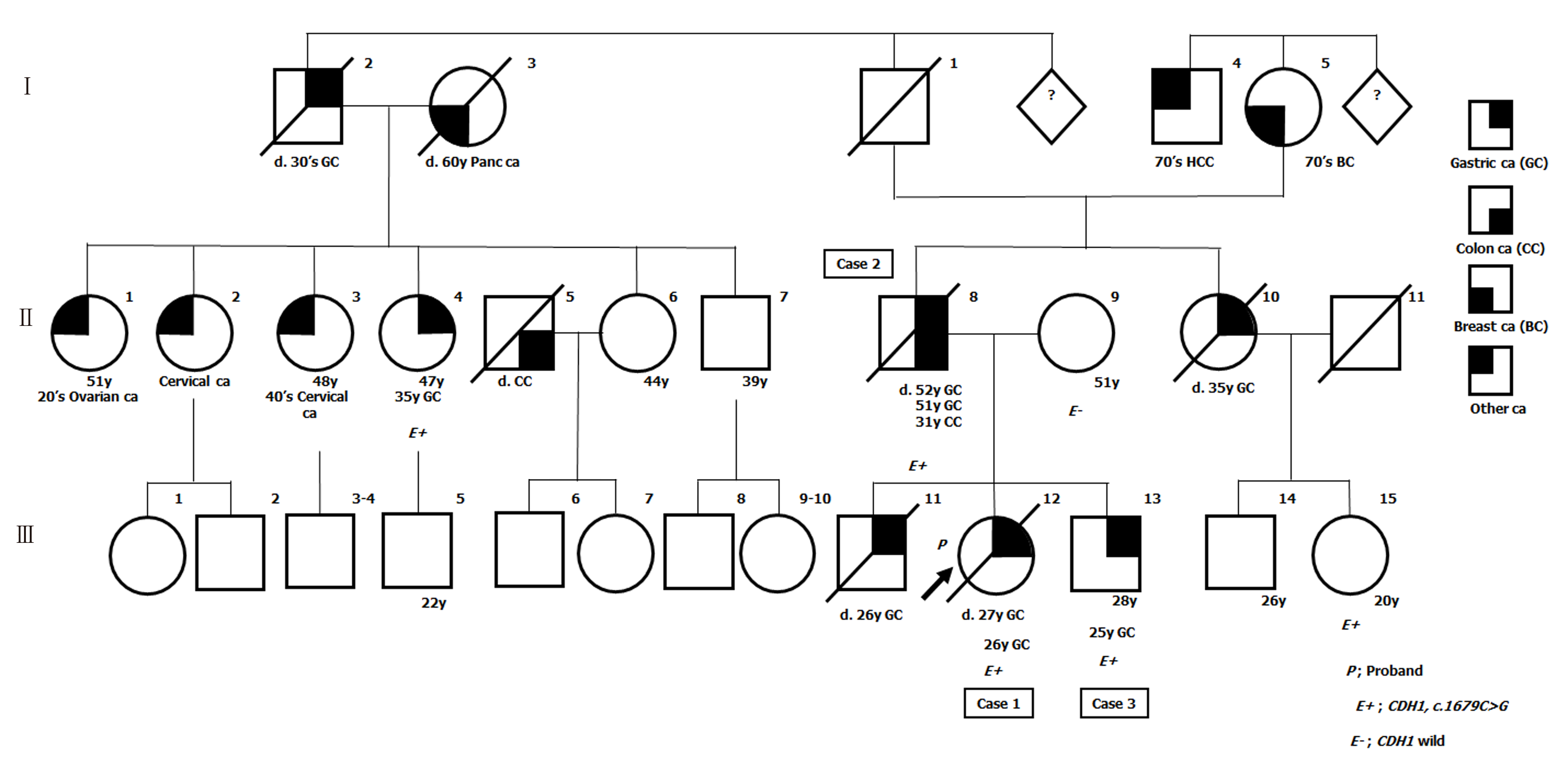
Cases 1-3 had several family members with GC. Pedigree of this family is shown in Figure 1.
Cases 1-3: Unremarkable.
Cases 1-3: The serum levels of CEA and CA 19-9 were within normal limits.
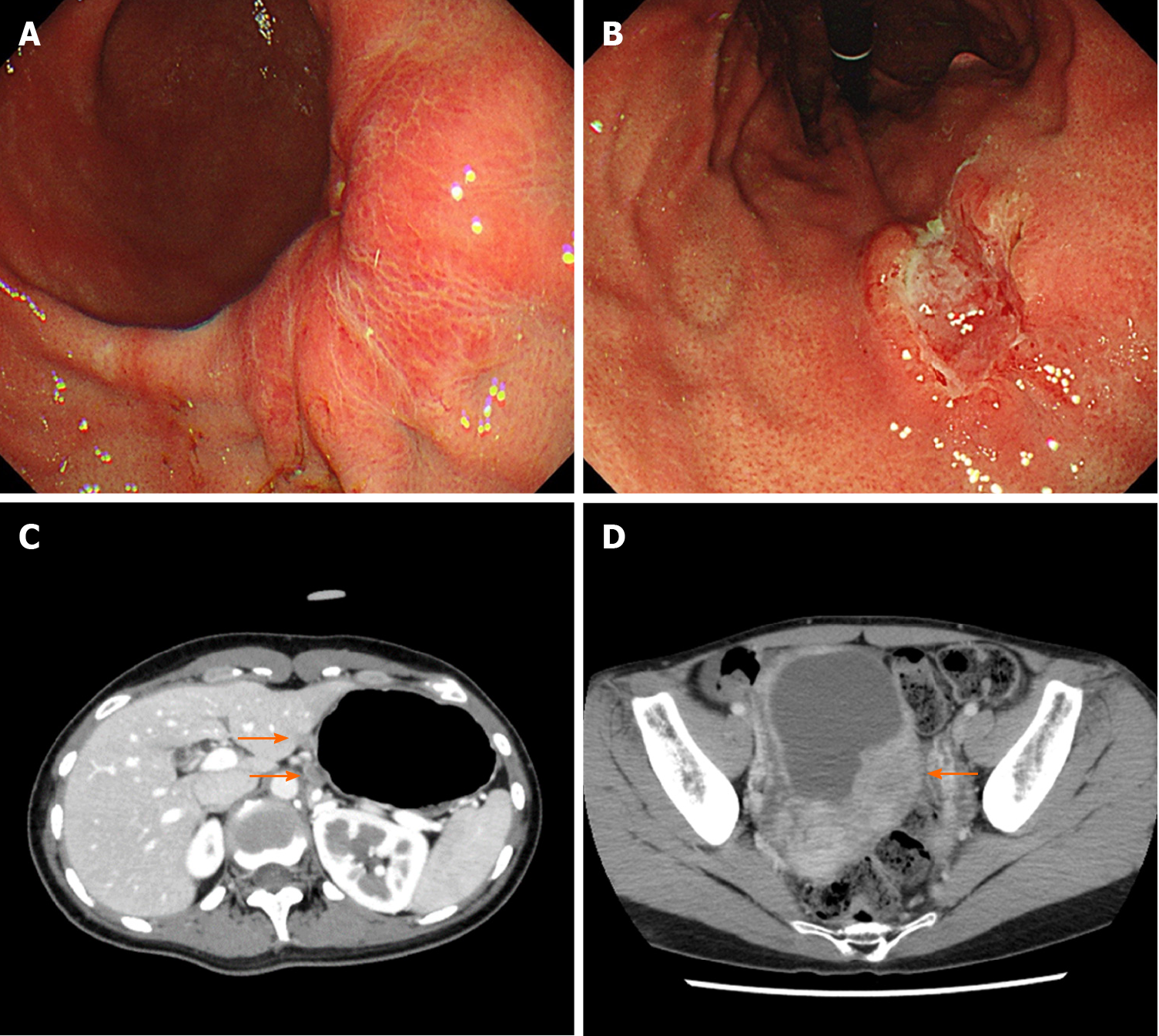
Case 1: EGD revealed advanced GC at the lower and middle body of the stomach on a background of non-atrophic gastric mucosa (Figure 2A and B). The biopsy specimens demonstrated diffuse type adenocarcinoma without Helicobacter pylori co-infection. Computed tomography (CT) revealed lymph node metastases along the lesser curvature of the stomach (Figure 2C).
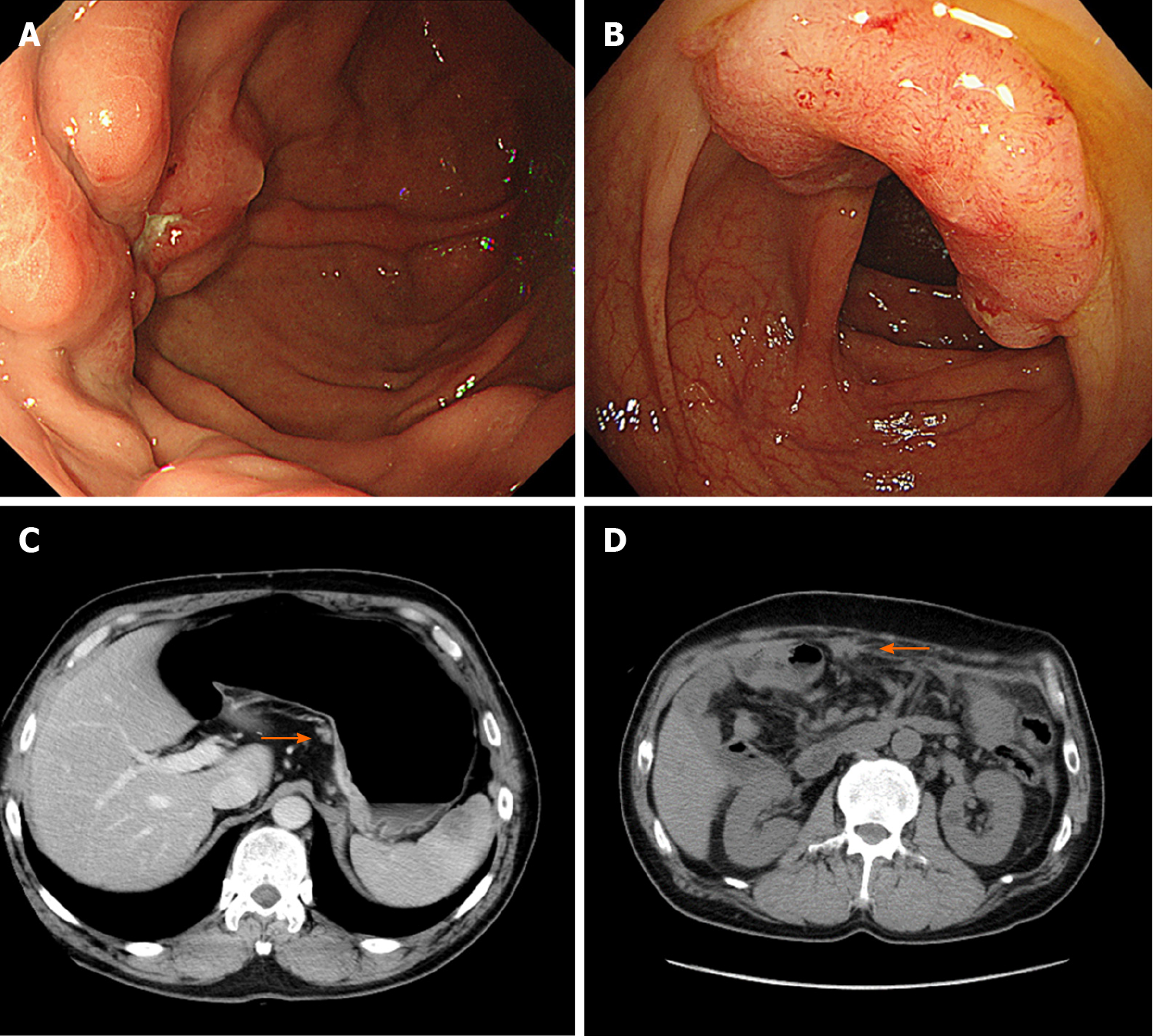
Case 2: The patient had surveillance EGD that showed a Borrmann type 3 tumor at the fundus on a background of non-atrophic gastric mucosa (Figure 3A). A histopathological examination of the biopsy specimens revealed diffuse type adenocarcinoma without Helicobacter pylori co-infection. Furthermore, advanced colon cancer at the ascending colon was also detected by screening colonoscopy, although histopathological analysis indicated this was an intestinal adenocarcinoma (Figure 3B). No distant metastases were identified by CT (Figure 3C).
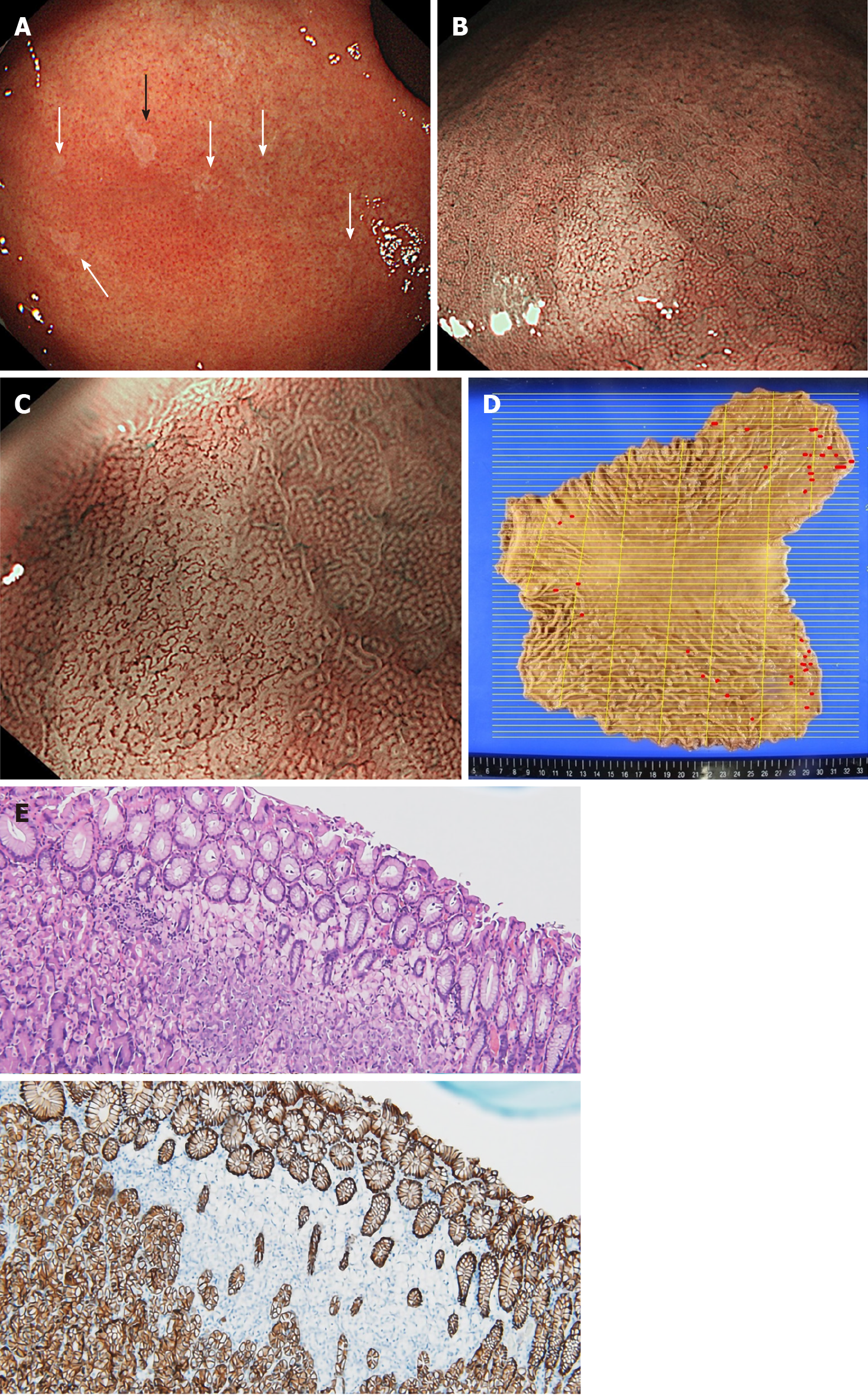
Case 3: He received surveillance EGD that detected multiple small pale lesions, mainly in the greater curvature of the stomach (Figure 4A). Narrow band imaging (NBI) without magnification showed clearly isolated whitish areas, and NBI with magnification detected “wavy” microvessels, indicating diffuse type GC, in these lesions (Figure 4B and C). We took 6 targeted biopsies from these lesions, which revealed signet ring cell carcinoma (SRCC) in all the specimens.
The presence of germline CDH1 c.1679C>G (p.T560R) variant: As the three patients (Cases 1, 2 and 3) fulfilled the International Gastric Cancer Linkage Consortium (IGCLC) criteria for HDGC[2], we tested all of them for germline CDH1 mutation. This genetic testing revealed a CDH1 c.1679C>G (p.T560R) variant in all three patients.
The final diagnosis of Case 1 is HDGC.
The final diagnosis of Case 2 is HDGC and colon cancer.
The final diagnosis of Case 3 is HDGC.
The patient underwent total gastrectomy with D2 Lymphadenectomy (pT4aN1M0, Stage IIIA).
The patient underwent total gastrectomy with D2 Lymphadenectomy (pT4aN3aM0, Stage IIIB) and right hemicolectomy with D3 Lymphadenectomy (pT2N0M0, Stage I).
Total gastrectomy with D1 Lymphadenectomy was performed (pT1N0M0, Stage IA). A total of 36 SRCC foci were observed by histological examination of the entire gastric mucosa (Figure 4D). Immunohistochemistry revealed loss of E-cadherin expression in areas corresponding to SRCC foci, which was compatible with the findings in HGDC (Figure 4E)[3].
Ovarian metastasis was detected by CT during the adjuvant chemotherapy (Figure 2D). Although systemic chemotherapy was continued, the patient died two years after the diagnosis.
The GC was treated with adjuvant chemotherapy. Despite treatment, the disease progressed due to peritoneal carcinomatosis during the adjuvant chemotherapy (Figure 3D), and the patient died one year after the diagnosis.
No evidence of GC recurrence has been observed in the 3 years after diagnosis.
Based on the result of genetic analysis, we further performed genetic counselling and genetic testing for their relatives to the extent that this was possible, and detected this variant in two of them (Figure 1). As the two p.T560R variant carriers refused prophylactic gastrectomy, we are currently continuing endoscopic surveillance for them.
Here we present an HDGC family with a missense CDH1 substitution variant, c.1679C>G (p.T560R). The p.T560R variant had been reported three times in patients with HDGC[11-13]. Yelskaya et al[12] reported that the p.T560R mutation created a novel 5¢ splice donor site that led to truncation of E-cadherin. Furthermore, Pena-Couso et al[13] performed functional analyses, which revealed that the p.T560R mutation causes an abnormal pattern of E-cadherin expression in the cytoplasm, disrupts cell-cell adhesion and promotes cellular invasion. Consistent with these reports, loss of E-cadherin expression at SRCC foci was observed in Case 3. Furthermore, we observed early recurrence and rapid progression of GC after radical resection in Cases 1 and 2. E-cadherin is a member of the cadherin family and mediates calcium-dependent cell-cell adhesion[14]. Reduction of E-cadherin expression promotes invasion and metastasis in various cancer types through initiation of the epithelial-mesenchymal transition[15]. Indeed, HDGC patients with germline CDH1 mutations have shorter survival times compared to those without germline CDH1 mutations[16]. On the other hand, the loss of E-cadherin may not be sufficient for the development of invasive gastric adenocarcinoma, because signet ring-like cells are observed in gastric mucosa of E-cadherin-deficient mice, but this does not lead to development of carcinomas that invade the submucosa[17]. In addition to the loss of E-cadherin, other genes, such as Smad4 and p53, may play important roles in tumorigenesis and metastasis in HDGC[18].
With respect to gastric endoscopic findings, multiple small pale lesions were observed with white light imaging in Case 3 and all biopsy specimens from the pale lesions revealed SRCC. Pale lesions in HDGC patients possibly reflect microscopic foci of early SRCC, although their presence is not diagnostic for this disease[2,7,10,19]. On the other hand, Hüneburg and colleagues[20] reported that combining targeted biopsies from abnormal findings (including pale lesions) with random biopsies did not improve detection of SRCC foci in CDH1 mutation-positive HDGC patients. Currently, the IGCLC guidelines for endoscopic surveillance of HDGC recommend that all endoscopically visible lesions (including pale areas) are biopsied, and after sampling of all visible lesions, five random biopsies should be taken from each of the following anatomical zones: prepyloric, antrum, transitional zone, body, fundus and cardia[18]. Nevertheless, the rate at which SRCC foci are detected in CDH1 mutation carriers following endoscopy is 45%-60%, which is relatively low[19,21-23]. Further studies are needed to improve the accuracy of endoscopic diagnosis of HDGC. Additionally, we recognized the SRCC foci as clearly isolated whitish areas by NBI and observed wavy microvessels inside the lesions by magnifying NBI. NBI has not previously been validated as a method for diagnosis of patients with HDGC[19,23]. Interestingly, the NBI findings that we observed in Case 3 are similar to those previously reported in studies of early SRCC patients[24-27]. Although the detection of small intramucosal SRCC foci is not easy because most of them are covered by a normal foveolar epithelium, the endoscopic findings that we observed in Case 3 are informative for the detection of early SRCC foci in CDH1 mutation-positive HDGC patients.
Lastly, it is well known that germline CDH1 mutations increase the lifetime risk of developing lobular breast cancer. Although we performed breast cancer screening for Case 1, no breast cancer was detected. In contrast, coexistence of colon cancer was revealed in Case 2. Currently, it is unclear whether CDH1 germline mutations also increase the risk of colorectal cancer. There are several case reports of colorectal SRCCs in germline CDH1 mutation carriers[28-31]. However, as the histopathology of colon cancer in Case 2 indicated intestinal adenocarcinoma, the relationship between CDH1 mutation and development of colon cancer in Case 2 is not certain. Interestingly, Salahshor et al[32] reported that the colorectal cancer subtype associated with HDGC can be intestinal adenocarcinoma. Further studies are needed to clarify whether germline CDH1 mutations cause colorectal carcinogenesis.
We report an HDGC family with a missense CDH1 variant, c.1679C>G (p.T560R), where active genetics evaluation and intensive endoscopic surveillance in Case 3 resulted in early diagnosis and treatment of HDGC. HDGC has rarely been reported in East Asian countries. However, the rarity of HDGC in East Asian Countries may be related to insufficient surveillance or overlooked cases and may not reflect the actual prevalence. We therefore recommend that individuals suspected of having HDGC (e.g., fulfilling the IGCLC criteria for HDGC, existence of multiple SRCC foci) should be offered genetic counselling and mutation analysis in cooperation with cancer genetics professionals. The present report will contribute to an increased awareness of HDGC and will improve the performance of endoscopic diagnosis for early SRCC foci in HDGC patients harboring a CDH1 mutation.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jiang QP S-Editor: Gao CC L-Editor: A P-Editor: Ma YJ
| 1. | Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, Znaor A, Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3585] [Cited by in RCA: 4863] [Article Influence: 694.7] [Reference Citation Analysis (1)] |
| 2. | van der Post RS, Vogelaar IP, Carneiro F, Guilford P, Huntsman D, Hoogerbrugge N, Caldas C, Schreiber KE, Hardwick RH, Ausems MG, Bardram L, Benusiglio PR, Bisseling TM, Blair V, Bleiker E, Boussioutas A, Cats A, Coit D, DeGregorio L, Figueiredo J, Ford JM, Heijkoop E, Hermens R, Humar B, Kaurah P, Keller G, Lai J, Ligtenberg MJ, O'Donovan M, Oliveira C, Pinheiro H, Ragunath K, Rasenberg E, Richardson S, Roviello F, Schackert H, Seruca R, Taylor A, Ter Huurne A, Tischkowitz M, Joe ST, van Dijck B, van Grieken NC, van Hillegersberg R, van Sandick JW, Vehof R, van Krieken JH, Fitzgerald RC. Hereditary diffuse gastric cancer: updated clinical guidelines with an emphasis on germline CDH1 mutation carriers. J Med Genet. 2015;52:361-374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 448] [Cited by in RCA: 394] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 3. | Guilford P, Hopkins J, Harraway J, McLeod M, McLeod N, Harawira P, Taite H, Scoular R, Miller A, Reeve AE. E-cadherin germline mutations in familial gastric cancer. Nature. 1998;392:402-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1135] [Cited by in RCA: 1136] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 4. | Kim HC, Wheeler JM, Kim JC, Ilyas M, Beck NE, Kim BS, Park KC, Bodmer WF. The E-cadherin gene (CDH1) variants T340A and L599V in gastric and colorectal cancer patients in Korea. Gut. 2000;47:262-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Wang Y, Song JP, Ikeda M, Shinmura K, Yokota J, Sugimura H. Ile-Leu substitution (I415L) in germline E-cadherin gene (CDH1) in Japanese familial gastric cancer. Jpn J Clin Oncol. 2003;33:17-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Yamada H, Shinmura K, Ito H, Kasami M, Sasaki N, Shima H, Ikeda M, Tao H, Goto M, Ozawa T, Tsuneyoshi T, Tanioka F, Sugimura H. Germline alterations in the CDH1 gene in familial gastric cancer in the Japanese population. Cancer Sci. 2011;102:1782-1788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Yamada M, Fukagawa T, Nakajima T, Asada K, Sekine S, Yamashita S, Okochi-Takada E, Taniguchi H, Kushima R, Oda I, Saito Y, Ushijima T, Katai H. Hereditary diffuse gastric cancer in a Japanese family with a large deletion involving CDH1. Gastric Cancer. 2014;17:750-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 8. | Funakoshi T, Miyamoto S, Kakiuchi N, Nikaido M, Setoyama T, Yokoyama A, Horimatsu T, Yamada A, Torishima M, Kosugi S, Yamada H, Sugimura H, Haga H, Sakai Y, Ogawa S, Seno H, Muto M, Chiba T. Genetic analysis of a case of Helicobacter pylori-uninfected intramucosal gastric cancer in a family with hereditary diffuse gastric cancer. Gastric Cancer. 2019;22:892-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Sugimoto S, Yamada H, Takahashi M, Morohoshi Y, Yamaguchi N, Tsunoda Y, Hayashi H, Sugimura H, Komatsu H. Early-onset diffuse gastric cancer associated with a de novo large genomic deletion of CDH1 gene. Gastric Cancer. 2014;17:745-749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Iwaizumi M, Yamada H, Fukue M, Maruyama Y, Sonoda A, Sugimoto M, Koda K, Kushima R, Maekawa M, Sugimura H. Two independent families with strongly suspected hereditary diffuse gastric cancer based on the probands' endoscopic findings. Clin J Gastroenterol. 2020;13:754-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Benusiglio PR, Malka D, Rouleau E, De Pauw A, Buecher B, Noguès C, Fourme E, Colas C, Coulet F, Warcoin M, Grandjouan S, Sezeur A, Laurent-Puig P, Molière D, Tlemsani C, Di Maria M, Byrde V, Delaloge S, Blayau M, Caron O. CDH1 germline mutations and the hereditary diffuse gastric and lobular breast cancer syndrome: a multicentre study. J Med Genet. 2013;50:486-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 119] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 12. | Yelskaya Z, Bacares R, Salo-Mullen E, Somar J, Lehrich DA, Fasaye GA, Coit DG, Tang LH, Stadler ZK, Zhang L. CDH1 Missense Variant c.1679C>G (p.T560R) Completely Disrupts Normal Splicing through Creation of a Novel 5' Splice Site. PLoS One. 2016;11:e0165654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Pena-Couso L, Perea J, Melo S, Mercadillo F, Figueiredo J, Sanches JM, Sánchez-Ruiz A, Robles L, Seruca R, Urioste M. Clinical and functional characterization of the CDH1 germline variant c.1679C>G in three unrelated families with hereditary diffuse gastric cancer. Eur J Hum Genet. 2018;26:1348-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;251:1451-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2396] [Cited by in RCA: 2421] [Article Influence: 71.2] [Reference Citation Analysis (0)] |
| 15. | Luo W, Fedda F, Lynch P, Tan D. CDH1 Gene and Hereditary Diffuse Gastric Cancer Syndrome: Molecular and Histological Alterations and Implications for Diagnosis And Treatment. Front Pharmacol. 2018;9:1421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 16. | van der Post RS, Vogelaar IP, Manders P, van der Kolk LE, Cats A, van Hest LP, Sijmons R, Aalfs CM, Ausems MG, Gómez García EB, Wagner A, Hes FJ, Arts N, Mensenkamp AR, van Krieken JH, Hoogerbrugge N, Ligtenberg MJ. Accuracy of Hereditary Diffuse Gastric Cancer Testing Criteria and Outcomes in Patients With a Germline Mutation in CDH1. Gastroenterology 2015; 149: 897-906. e19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 17. | Mimata A, Fukamachi H, Eishi Y, Yuasa Y. Loss of E-cadherin in mouse gastric epithelial cells induces signet ring-like cells, a possible precursor lesion of diffuse gastric cancer. Cancer Sci. 2011;102:942-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Park JW, Jang SH, Park DM, Lim NJ, Deng C, Kim DY, Green JE, Kim HK. Cooperativity of E-cadherin and Smad4 Loss to promote diffuse-type gastric adenocarcinoma and metastasis. Mol Cancer Res. 2014;12:1088-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Shaw D, Blair V, Framp A, Harawira P, McLeod M, Guilford P, Parry S, Charlton A, Martin I. Chromoendoscopic surveillance in hereditary diffuse gastric cancer: an alternative to prophylactic gastrectomy? Gut. 2005;54:461-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 117] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 20. | Hüneburg R, Marwitz T, van Heteren P, Weismüller TJ, Trebicka J, Adam R, Aretz S, Perez Bouza A, Pantelis D, Kalff JC, Nattermann J, Strassburg CP. Chromoendoscopy in combination with random biopsies does not improve detection of gastric cancer foci in CDH1 mutation positive patients. Endosc Int Open. 2016;4:E1305-E1310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Mi EZ, Mi EZ, di Pietro M, O'Donovan M, Hardwick RH, Richardson S, Ziauddeen H, Fletcher PC, Caldas C, Tischkowitz M, Ragunath K, Fitzgerald RC. Comparative study of endoscopic surveillance in hereditary diffuse gastric cancer according to CDH1 mutation status. Gastrointest Endosc. 2018;87:408-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 22. | Moslim MA, Heald B, Tu C, Burke CA, Walsh RM. Early genetic counseling and detection of CDH1 mutation in asymptomatic carriers improves survival in hereditary diffuse gastric cancer. Surgery. 2018;164:754-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Lim YC, di Pietro M, O'Donovan M, Richardson S, Debiram I, Dwerryhouse S, Hardwick RH, Tischkowitz M, Caldas C, Ragunath K, Fitzgerald RC. Prospective cohort study assessing outcomes of patients from families fulfilling criteria for hereditary diffuse gastric cancer undergoing endoscopic surveillance. Gastrointest Endosc. 2014;80:78-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 24. | Nakayoshi T, Tajiri H, Matsuda K, Kaise M, Ikegami M, Sasaki H. Magnifying endoscopy combined with narrow band imaging system for early gastric cancer: correlation of vascular pattern with histopathology (including video). Endoscopy. 2004;36:1080-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 335] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 25. | Nagahama T, Yao K, Maki S, Yasaka M, Takaki Y, Matsui T, Tanabe H, Iwashita A, Ota A. Usefulness of magnifying endoscopy with narrow-band imaging for determining the horizontal extent of early gastric cancer when there is an unclear margin by chromoendoscopy (with video). Gastrointest Endosc. 2011;74:1259-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 145] [Article Influence: 10.4] [Reference Citation Analysis (1)] |
| 26. | Okada K, Fujisaki J, Kasuga A, Omae M, Hirasawa T, Ishiyama A, Inamori M, Chino A, Yamamoto Y, Tsuchida T, Nakajima A, Hoshino E, Igarashi M. Diagnosis of undifferentiated type early gastric cancers by magnification endoscopy with narrow-band imaging. J Gastroenterol Hepatol. 2011;26:1262-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 27. | Watari J, Tomita T, Ikehara H, Taki M, Ogawa T, Yamasaki T, Kondo T, Toyoshima F, Sakurai J, Kono T, Tozawa K, Ohda Y, Oshima T, Fukui H, Hirota S, Miwa H. Diagnosis of small intramucosal signet ring cell carcinoma of the stomach by non-magnifying narrow-band imaging: A pilot study. World J Gastrointest Endosc. 2015;7:1070-1077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Pharoah PD, Guilford P, Caldas C; International Gastric Cancer Linkage Consortium. Incidence of gastric cancer and breast cancer in CDH1 (E-cadherin) mutation carriers from hereditary diffuse gastric cancer families. Gastroenterology. 2001;121:1348-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 424] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 29. | Richards FM, McKee SA, Rajpar MH, Cole TR, Evans DG, Jankowski JA, McKeown C, Sanders DS, Maher ER. Germline E-cadherin gene (CDH1) mutations predispose to familial gastric cancer and colorectal cancer. Hum Mol Genet. 1999;8:607-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 234] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 30. | Brooks-Wilson AR, Kaurah P, Suriano G, Leach S, Senz J, Grehan N, Butterfield YS, Jeyes J, Schinas J, Bacani J, Kelsey M, Ferreira P, MacGillivray B, MacLeod P, Micek M, Ford J, Foulkes W, Australie K, Greenberg C, LaPointe M, Gilpin C, Nikkel S, Gilchrist D, Hughes R, Jackson CE, Monaghan KG, Oliveira MJ, Seruca R, Gallinger S, Caldas C, Huntsman D. Germline E-cadherin mutations in hereditary diffuse gastric cancer: assessment of 42 new families and review of genetic screening criteria. J Med Genet. 2004;41:508-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 265] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 31. | Oliveira C, Bordin MC, Grehan N, Huntsman D, Suriano G, Machado JC, Kiviluoto T, Aaltonen L, Jackson CE, Seruca R, Caldas C. Screening E-cadherin in gastric cancer families reveals germline mutations only in hereditary diffuse gastric cancer kindred. Hum Mutat. 2002;19:510-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 113] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 32. | Salahshor S, Hou H, Diep CB, Loukola A, Zhang H, Liu T, Chen J, Iselius L, Rubio C, Lothe RA, Aaltonen L, Sun XF, Lindmark G, Lindblom A. A germline E-cadherin mutation in a family with gastric and colon cancer. Int J Mol Med. 2001;8:439-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |