Published online Nov 14, 2020. doi: 10.3748/wjg.v26.i42.6638
Peer-review started: August 18, 2020
First decision: September 12, 2020
Revised: September 15, 2020
Accepted: September 25, 2020
Article in press: September 25, 2020
Published online: November 14, 2020
Processing time: 87 Days and 1 Hours
Colorectal cancer is a common digestive cancer worldwide. As a comprehensive treatment for locally advanced rectal cancer (LARC), neoadjuvant therapy (NT) has been increasingly used as the standard treatment for clinical stage II/III rectal cancer. However, few patients achieve a complete pathological response, and most patients require surgical resection and adjuvant therapy. Therefore, identifying risk factors and developing accurate models to predict the prognosis of LARC patients are of great clinical significance.
To establish effective prognostic nomograms and risk score prediction models to predict overall survival (OS) and disease-free survival (DFS) for LARC treated with NT.
Nomograms and risk factor score prediction models were based on patients who received NT at the Cancer Hospital from 2015 to 2017. The least absolute shrinkage and selection operator regression model were utilized to screen for prognostic risk factors, which were validated by the Cox regression method. Assessment of the performance of the two prediction models was conducted using receiver operating characteristic curves, and that of the two nomograms was conducted by calculating the concordance index (C-index) and calibration curves. The results were validated in a cohort of 65 patients from 2015 to 2017.
Seven features were significantly associated with OS and were included in the OS prediction nomogram and prediction model: Vascular_tumors_bolt, cancer nodules, yN, body mass index, matchmouth distance from the edge, nerve aggression and postoperative carcinoembryonic antigen. The nomogram showed good predictive value for OS, with a C-index of 0.91 (95%CI: 0.85, 0.97) and good calibration. In the validation cohort, the C-index was 0.69 (95%CI: 0.53, 0.84). The risk factor prediction model showed good predictive value. The areas under the curve for 3- and 5-year survival were 0.811 and 0.782. The nomogram for predicting DFS included ypTNM and nerve aggression and showed good calibration and a C-index of 0.77 (95%CI: 0.69, 0.85). In the validation cohort, the C-index was 0.71 (95%CI: 0.61, 0.81). The prediction model for DFS also had good predictive value, with an AUC for 3-year survival of 0.784 and an AUC for 5-year survival of 0.754.
We established accurate nomograms and prediction models for predicting OS and DFS in patients with LARC after undergoing NT.
Core Tip: The manuscript focuses on the risk factors after administration of neoadjuvant therapy for locally advanced rectal cancer. We utilized the least absolute shrinkage and selection operator and Cox regression to identify risk factors for overall survival and disease-free survival and explore their prognostic value. Based on the factors, we built two nomograms and two risk factor score prediction models to predict survival time. The nomograms were validated by calibration and the concordance index, and the prediction model was validated with receiver operating characteristic curves. The risk factors included in the model and nomograms are associated with survival and recurrence and can aid physicians to improve patient survival.
- Citation: Wei FZ, Mei SW, Chen JN, Wang ZJ, Shen HY, Li J, Zhao FQ, Liu Z, Liu Q. Nomograms and risk score models for predicting survival in rectal cancer patients with neoadjuvant therapy. World J Gastroenterol 2020; 26(42): 6638-6657
- URL: https://www.wjgnet.com/1007-9327/full/v26/i42/6638.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i42.6638
In recent years, neoadjuvant therapy (NT) has been increasingly implemented because it can reduce the risk of local recurrence and toxicity[1,2]. Numerous international guidelines recommend NT as the standard treatment for locally advanced rectal cancer (LARC)[3]. Because of the different sensitivities to adjuvant therapy, approximately 15%-27% of patients achieve a pathological complete response (pCR), and the majority of patients with stage II/III rectal cancer require surgery or adjuvant therapy[4]. Therefore, achieving a pCR is closely related to the need for subsequent treatment. Unlike patients who directly undergo surgical resection, those who first receive NT have more vulnerable immune systems, which can affect surgical outcomes[5] and influence overall survival (OS) and disease-free survival (DFS).
Global studies have reported that colorectal cancer accounts for approximately 1 of 10 newly diagnosed cancer cases and cancer-related deaths, and approximately one-third of colorectal cancer cases are rectal cancer[6,7]. Identifying prognostic factors and accurately predicting OS and DFS can provide individualized treatments for patients and improve their quality of life.
Previous studies have revealed that the number of lymph nodes, response to NT, neoadjuvant rectal score (NAR score), ypTNM stage, and family history[3,8-10] are related to OS and DFS. However, few modules or nomograms use clinical features to predict OS and DFS for LARC after NT. Therefore, identifying clinical features that can serve as prognostic factors and developing accurate models to predict OS and DFS could easily determine clinical treatments and improve the prognosis of patients who have received NT.
In this study, we screened preoperative and postoperative clinical features and constructed a nomogram and risk factor prediction model to predict OS and DFS. To the best of our knowledge, this study is the first attempt to build a nomogram to predict OS and DFS by screening risk factors using least absolute shrinkage and selection operator (LASSO) regression.
We analyzed 220 patients who were clinically diagnosed with LARC and divided them into two groups: 165 patients in the primary cohort and 65 patients in the validation cohort. All patients were admitted to the Colorectal Surgery Department of the National Cancer Hospital from 2015 to 2017 and were administered preoperative NT followed by laparoscopic total mesorectal excision (TME).
We collected available demographic and clinical characteristics before NT and after TME surgery as follows: Age, sex, body mass index (BMI), clinical T (cT) and N stages (cN), pathological T (yT) and N stages (yN), ypTNM, total number of lymph nodes, positive lymph node status, preoperative chemotherapy cycle, radiotherapy cycle, distance of the tumor from the anal verge before NT and after NT, pathological response, preoperative chemotherapy regimen, radiotherapy dose, operating time, matchmouth distance from the edge, surgical procedure, preoperative carcino-embryonic antigen (CEA), postoperative CEA, and follow-up data.
This study was approved by the ethics committee at our institution. The clinical information and characteristics were recorded and analyzed after consent was obtained from the patients and their families.
Regarding preoperative radiotherapy, the long-course regimen radiation dose ranged from 45.0-50.5 Gy; for patients who received the short-course regimen, the total dose was 25 Gy. Radiation was delivered to the pelvic cavity and tumor bed at 10 MV. All patients received TME approximately 2-60 weeks after NT based on their physical conditions. For patients who had received adjuvant therapy, three chemotherapeutic regimens were completed following radiotherapy: XELOX, capecitabine or 5-fluorouracil (5-FU) alone and capecitabin or 5-FU combined with other medicine.
Clinical data were obtained from follow-up visits conducted by the outpatient clinic and by telephone or email. For patients who visited the outpatient clinic, the medical history was collected, and a complete physical examination was carried out. Serum tumor marker CEA measurements and enhanced CT examinations of the pelvis were performed to detect and monitor recurrence and physical condition[3,8-10]. A colonoscopy was performed every 6 months for the first two years and once a year after two years. All patients were followed up every three months after surgery, and the last follow-up month was March 2020. DFS was defined as the time from the date of surgery to the time of recurrence or death, whereas OS was defined as the time from the date of surgery to the time of death or the last date of follow-up.
LASSO regression and nomogram construction were conducted with R software (version 3.6.1). The prognostic factors were initially screened via LASSO regression through the R packages “survival” and “glmnet”. We utilized Cox regression to validate the prognostic factors. Then, the Kaplan-Meier (K-M) prognosis curves were drawn using the online tool Sanger box. Continuous variables were analyzed by Cox regression, and the R package “survival” was utilized to analyze variables. Each sample was categorized, and the differences in the K-M prognosis curves between the two groups were analyzed. Then, the cycle was repeated, and the P value of each sample was calculated and assessed using the log-rank test. The nomograms were established based on the key factors screened by the LASSO regression R package “rms”. The C-index and calibration curves of the nomograms for OS and DFS reflect the accuracy between the predicted and observed results. Risk factor prediction models were built using the R package “survival”, and ROC curves were constructed with the R package “survivalROC”. LASSO regression, Cox regression, K-M curves and prediction models were based on 220 patients, and nomograms were built according to the primary cohort and validated using the validation cohort.
Figure 1 shows the workflow of our study. All patients underwent TME surgery. In the primary cohort, 99 (63.9%) patients were men, and 56 (36.1%) were women; 30 patients experienced recurrence, while 18 died. In the validation cohort, 53 (81.5%) patients were men, and 12 (18.5%) were women;, and 17 patients experienced recurrence, and 15 died (Tables 1-4). The median follow-up time was 41 months, and the median OS was 40.73 months (range, 2 to 62 mo). The 1-year, 3-year, and 5-year OS rates were 99.35%, 67.74%, and 4.52%, respectively. The median DFS was 38.54 (range, 2 to 62 mo), and the 1-year, 3-year, and 5-year DFS rates were 92.26%, 61.29%, and 3.23%, respectively.
| Variable | Primary cohort (n = 155) | Validation cohort (n = 65) | ||
| No. of patients | % | No. of patients | % | |
| Age | ||||
| Median | 60.00 | 61.00 | ||
| Range | 52.00-66.00 | 51.00-65.50 | ||
| BMI | ||||
| Median | 24.13 | 23.44 | ||
| Range | 21.78-26.50 | 21.80-25.27 | ||
| Death | ||||
| Yes | 18 | 11.6 | 15 | 23.1 |
| No | 137 | 88.4 | 50 | 76.9 |
| Her-2 | ||||
| 1 | 34 | 21.9 | 18 | 27.7 |
| 2 | 17 | 11 | 6 | 9.2 |
| 3 | 3 | 1.9 | 2 | 3.1 |
| 4 | 1 | 0.6 | ||
| 5 | 100 | 64.5 | 39 | 60 |
| BRAF-V600E | ||||
| 1 | 105 | 67.7 | 45 | 69.2 |
| 2 | 6 | 3.9 | ||
| 3 | 1 | 0.6 | ||
| 4 | 1 | 0.6 | 1 | 1.5 |
| 5 | 42 | 27.1 | 19 | 29.2 |
| P53 | ||||
| 1 | 11 | 7.1 | 2 | 3.1 |
| 2 | 7 | 4.5 | 1 | 1.5 |
| 3 | 1 | 0.6 | 2 | 3.1 |
| 4 | 15 | 9.7 | 7 | 10.8 |
| 5 | 121 | 78.1 | 53 | 81.5 |
| ASA | ||||
| 1 | 3 | 1.9 | 3 | 4.6 |
| 2 | 122 | 78.7 | 48 | 73.8 |
| 3 | 30 | 19.4 | 14 | 21.5 |
| Sex | ||||
| Male | 99 | 63.9 | 53 | 81.5 |
| Female | 56 | 36.1 | 12 | 18.5 |
| Variable | Primary cohort (n = 155) | Validation cohort (n = 65) | ||
| No. of patients | % | No. of patients | % | |
| Preoperative chemotherapy cycle | ||||
| Median | 2.00 | 3.00 | ||
| Range | 2.00-3.00 | 2.00-3.00 | ||
| Surgery a few weeks after radiotherapy | ||||
| Median | 8.00 | 9.00 | ||
| Range | 7.00-11.00 | 7.00-15.00 | ||
| Distance from margin before NT | ||||
| Median | 5.00 | 5.00 | ||
| Range | 3.00-7.00 | 3.00-7.00 | ||
| Distance from margin after NT | ||||
| Median | 5.00 | 5.00 | ||
| Range | 3.00-7.00 | 3.00-7.00 | ||
| Preoperative CEA | ||||
| Median | 2.85 | 3.35 | ||
| Range | 1.60-4.73 | 1.52-6.21 | ||
| cT | ||||
| 2 | 1 | 1.5 | ||
| 3 | 120 | 77.4 | 52 | 80 |
| 4 | 35 | 22.6 | 12 | 18.5 |
| cN | ||||
| 0 | 54 | 34.8 | 21 | 32.3 |
| 1 | 73 | 47.1 | 33 | 50.8 |
| 2 | 28 | 18.1 | 11 | 16.9 |
| cM | ||||
| 0 | 146 | 94.2 | 62 | 95.4 |
| 1 | 9 | 5.8 | 3 | 4.6 |
| cTNM | ||||
| 2 | 52 | 33.5 | 21 | 32.3 |
| 3 | 94 | 60.6 | 41 | 63.1 |
| 4 | 9 | 5.8 | 3 | 4.6 |
| yT | ||||
| 0 | 22 | 14.2 | 8 | 12.3 |
| 1 | 4 | 26 | 1 | 1.5 |
| 2 | 34 | 21.9 | 15 | 23.1 |
| 3 | 84 | 54.2 | 35 | 53.8 |
| 4 | 11 | 7.1 | 6 | 9.2 |
| yN | ||||
| 0 | 88 | 56.8 | 33 | 50.8 |
| 1 | 47 | 30.3 | 23 | 35.4 |
| 2 | 19 | 12.3 | 9 | 13.8 |
| 3 | 1 | 0.6 | ||
| yM | ||||
| 0 | 146 | 94.2 | 62 | 95.4 |
| 1 | 9 | 5.8 | 3 | 4.6 |
| ypTNM | ||||
| 0 | 21 | 13.5 | 8 | 12.3 |
| 1 | 28 | 18.1 | 12 | 18.5 |
| 2 | 39 | 25.2 | 15 | 23.1 |
| 3 | 58 | 37.4 | 27 | 41.5 |
| 4 | 9 | 5.8 | 3 | 4.6 |
| Pathological changes after treatment | ||||
| 1 | 85 | 54.8 | 38 | 58.5 |
| 2 | 48 | 31 | 19 | 29.2 |
| 3 | 22 | 14.2 | 8 | 12.3 |
| TRG | ||||
| 0 | 3 | 1.9 | 2 | 3.1 |
| 1 | 27 | 17.4 | 14 | 21.5 |
| 2 | 62 | 40 | 26 | 40.0 |
| 3 | 41 | 26.5 | 15 | 23.1 |
| 4 | 22 | 14.2 | 8 | 12.3 |
| Preoperative simultaneous chemotherapy | ||||
| Yes | 126 | 81.3 | 51 | 78.5 |
| No | 29 | 18.7 | 14 | 21.5 |
| Preoperative radiotherapy | ||||
| Yes | 3 | 1.9 | 4 | 6.2 |
| No | 152 | 98.1 | 61 | 93.8 |
| Preoperative chemotherapy | ||||
| Yes | 26 | 16.8 | 10 | 15.4 |
| No | 129 | 83.2 | 55 | 84.6 |
| Variable | Primary cohort (n = 155) | Validation cohort (n = 65) | ||
| No. of patients | % | No. of patients | % | |
| Total number of lymph nodes | ||||
| Median | 16.00 | 17.00 | ||
| Range | 12.00-22.00 | 11.00-22.00 | ||
| Positive lymph node status | ||||
| Median | 0.00 | 0.00 | ||
| Range | 0.00-1.00 | 0.00-2.00 | ||
| Operating time | ||||
| Median | 193.00 | 209.00 | ||
| Range | 158.00-237.00 | 148.00-257.00 | ||
| Matchmouth distance from the edge | ||||
| Median | 3.00 | 2.00 | ||
| Range | 0.00-4.00 | 1.00-4.00 | ||
| Amount of bleeding during surgery | ||||
| Median | 50.00 | 50.00 | ||
| Range | 20.00-100.00 | 20.00-60.00 | ||
| Joint organ cut | ||||
| Yes | 8 | 5.2 | 3 | 4.6 |
| No | 147 | 94.8 | 62 | 95.4 |
| Side-side lymph node sweep | ||||
| Yes | 5 | 3.2 | 3 | 4.6 |
| No | 150 | 96.8 | 62 | 95.4 |
| Preventive mouth-building | ||||
| Yes | 35 | 22.6 | 15 | 23.1 |
| No | 120 | 77.4 | 50 | 76.9 |
| Retention of the left colon artery | ||||
| Yes | 9 | 5.8 | 6 | 9.2 |
| No | 146 | 94.2 | 59 | 90.8 |
| Postoperative pathology | ||||
| 1 | 3 | 1.9 | 1 | 1.5 |
| 2 | 128 | 82.6 | 52 | 80 |
| 3 | 19 | 12.3 | 11 | 16.9 |
| 4 | 1 | 0.6 | 1 | 1.5 |
| 5 | 4 | 2.6 | ||
| Cancer nodules | ||||
| Yes | 17 | 11 | 11 | 16.9 |
| No | 138 | 89 | 54 | 83.1 |
| Nerve aggression | ||||
| Yes | 30 | 19.4 | 23 | 35.4 |
| No | 125 | 80.6 | 42 | 64.6 |
| Vascular_tumors_bolt | ||||
| Yes | 17 | 11 | 9 | 13.8 |
| No | 138 | 89 | 56 | 86.2 |
| Variable | Primary cohort (n = 155) | Validation cohort (n = 65) | ||
| No. of patients | % | No. of patients | % | |
| Number of cycles of postoperative chemotherapy regimens | ||||
| Median | 4.00 | 4.00 | ||
| Range | 0.00-6.00 | 0.00-6.00 | ||
| Postoperative exhaust | ||||
| Median | 3.00 | 3.00 | ||
| Range | 3.00-5.00 | 3.00-5.00 | ||
| Postoperative defecation | ||||
| Median | 5.00 | 5.00 | ||
| Range | 3.00-6.00 | 4.00-6.00 | ||
| Postoperative ureter removal time | ||||
| Median | 4.00 | 4.00 | ||
| Range | 4.00-5.00 | 3.00-5.00 | ||
| Postoperative CEA | ||||
| Median | 2.41 | 2.55 | ||
| Range | 1.59-3.705 | 1.70-3.41 | ||
| Postoperative adjuvant therapy | ||||
| Yes | 101 | 65.2 | 45 | 69.2 |
| No | 54 | 34.8 | 20 | 30.8 |
| Postoperative bleeding | ||||
| Yes | 2 | 1.3 | ||
| No | 153 | 98.7 | 65 | 100 |
| Postoperative intestinal fistula | ||||
| Yes | 5 | 3.2 | ||
| No | 150 | 96.8 | 65 | 100 |
| Intestinal obstruction after surgery | ||||
| Yes | 1 | 1.5 | ||
| No | 155 | 100 | 64 | 98.5 |
| Unplanned postoperative surgery | ||||
| Yes | 3 | 1.9 | ||
| No | 152 | 98.1 | 65 | 100 |
| Cardiovascular accidents | ||||
| Yes | 1 | 0.6 | ||
| No | 154 | 99.4 | 65 | 100 |
| Postoperative complications | ||||
| Yes | 7 | 4.5 | 1 | 1.5 |
| No | 148 | 95.5 | 64 | 98.5 |
| Recurrence | ||||
| Yes | 30 | 19.4 | 17 | 26.2 |
| No | 125 | 80.6 | 48 | 73.8 |
Based on the clinical data, there were 10 potential prognostic factors in the LASSO regression model for OS selected out of 50 clinical features: Vascular_tumors_bolt, cancer nodules, yN, cT, ypTNM, BMI, matchmouth distance from the edge, nerve aggression, postoperative CEA and operation time (Figure 2A and B). We utilized Cox regression to validate the prognostic value. Among the factors, there were three factors with a value of P > 0.05: Operation time, cT and ypTNM (Table 5).
| Variable | P value | OR | 95%CI |
| yN | 0.003 | ||
| 1 vs 0 | 0.947 | 576.353 | 0.000-4.138E+84 |
| 2 vs 0 | 0.935 | 2450.459 | 0.000-1.758E+85 |
| 3 vs 0 | 0.934 | 2902.876 | 0.000-2.084E+85 |
| Cancer nodules | 0.003 | 3.278 | 1.506-7.134 |
| Nerve aggression | < 0.0001 | 3.446 | 1.726-6.882 |
| Vascular_tumors_bolt | 0.009 | 2.924 | 1.309-6.531 |
| ypTNM | 0.112 | ||
| 1 vs 0 | 0.110 | 0.267 | 0.053-1.346 |
| 2 vs 0 | 0.962 | 0.000 | 0.000-2.397E+244 |
| 3 vs 0 | 0.102 | 0.299 | 0.071-1.268 |
| 4 vs 0 | 0.801 | 0.856 | 0.254-2.886 |
| cT | 0.057 | ||
| 3 vs 2 | 0.018 | 14.337 | 1.585-129.724 |
| 4 vs 2 | 0.192 | 2.011 | 0.705-5.735 |
| Matchmouth distance from the edge | 0.012 | 0.805 | 0.679-0.953 |
| Postoperative CEA | 0.037 | 1.017 | 1.001-1.034 |
| BMI | 0.031 | 1.113 | 1.010-1.226 |
| Operation time | 0.068 | 1.004 | 1.000-1.008 |
There were two potential prognostic factors for DFS in the LASSO regression model based on 50 clinical features: ypTNM and nerve aggression (Figure 3A and B). We utilized Cox regression to validate the two factors, which were shown to have a good prognostic value for DFS (Table 6).
| Variable | P value | OR | 95%CI |
| ypTNM | 0.001 | ||
| 1 vs 0 | 0.003 | 0.089 | 0.018-0.445 |
| 2 vs 0 | 0.001 | 0.032 | 0.004-0.266 |
| 3 vs 0 | 0.017 | 0.291 | 0.105-0.805 |
| 4 vs 0 | 0.198 | 0.558 | 0.230-1.355 |
| Nerve aggression | < 0.0001 | 3.01 | 1.681-5.388 |
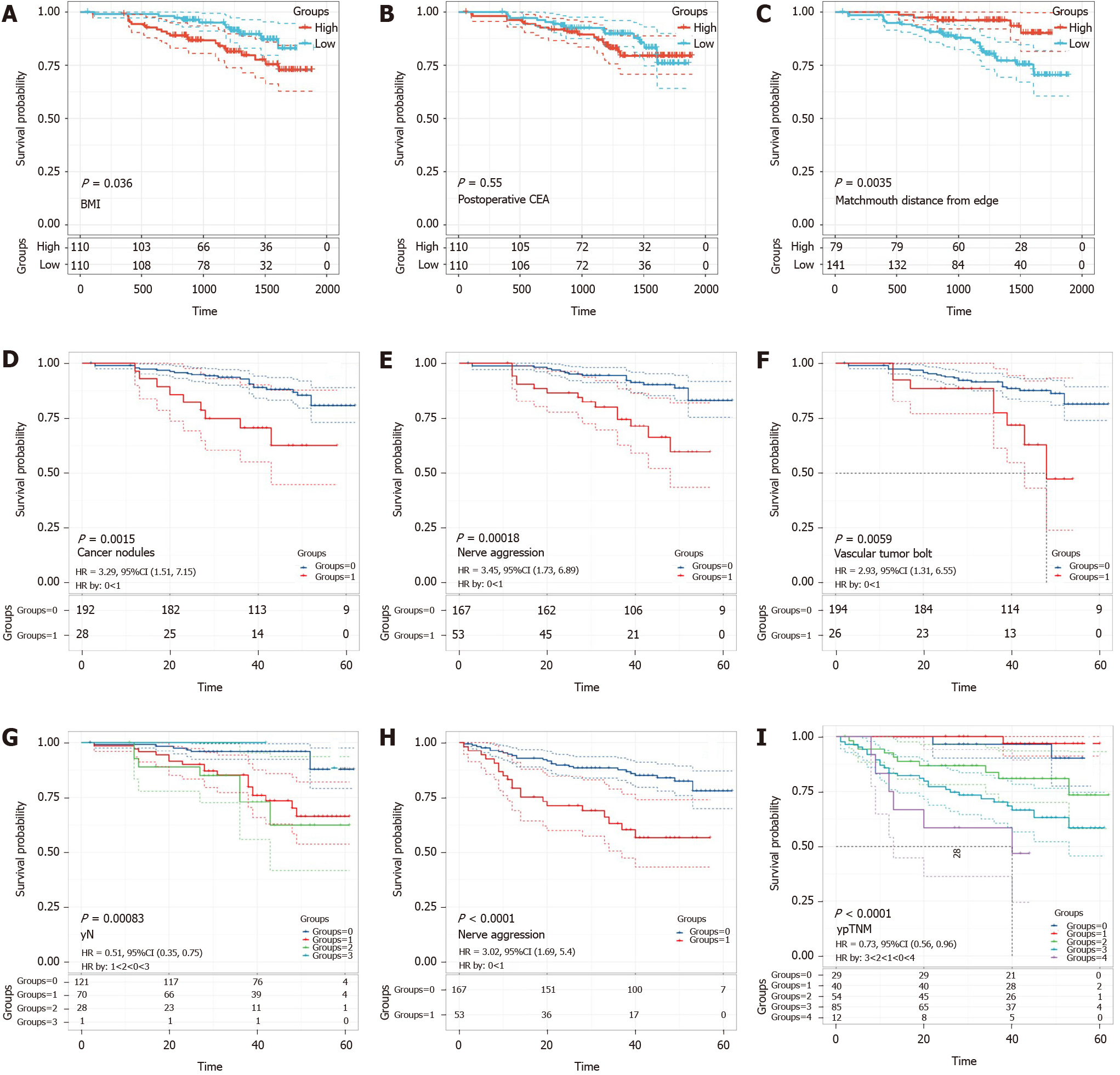
As shown in Figure 4A-C, all continuous variables were grouped into high expression and low expression groups. The K-M curve of the prognosis difference between the two groups for each variable was analyzed to determine which prognostic factors were associated with a good prognosis of LARC patients treated with NT. K-M curves of classified variables are also shown to highlight the prognostic value (Figure 4D-I). The result of Kaplan-Meier curves for the prognostic factors of OS and DFS are shown in Tables 7 and 8.
| Variable | P value | HR | 95%CI |
| yN | 0.00083 | 0.51 | 0.35-0.75 |
| Cancer nodules | 0.0015 | 3.29 | 1.51-7.15 |
| Nerve aggression | 0.00018 | 3.45 | 1.73-6.89 |
| Vascular tumors bolt | 0.0059 | 2.93 | 1.31-6.55 |
| Matchmouth distance from edge | 0.0035 | 0.80 | 0.67-0.95 |
| Postoperative CEA | 0.55 | 1.02 | 1.00-1.03 |
| BMI | 0.036 | 1.12 | 1.02-1.23 |
| Variable | P value | HR | 95%CI | |
| ypTNM | < 0.0001 | 0.73 | 0.56-0.96 | |
| Nerve aggression | < 0.0001 | 3.02 | 1.69-5.4 |
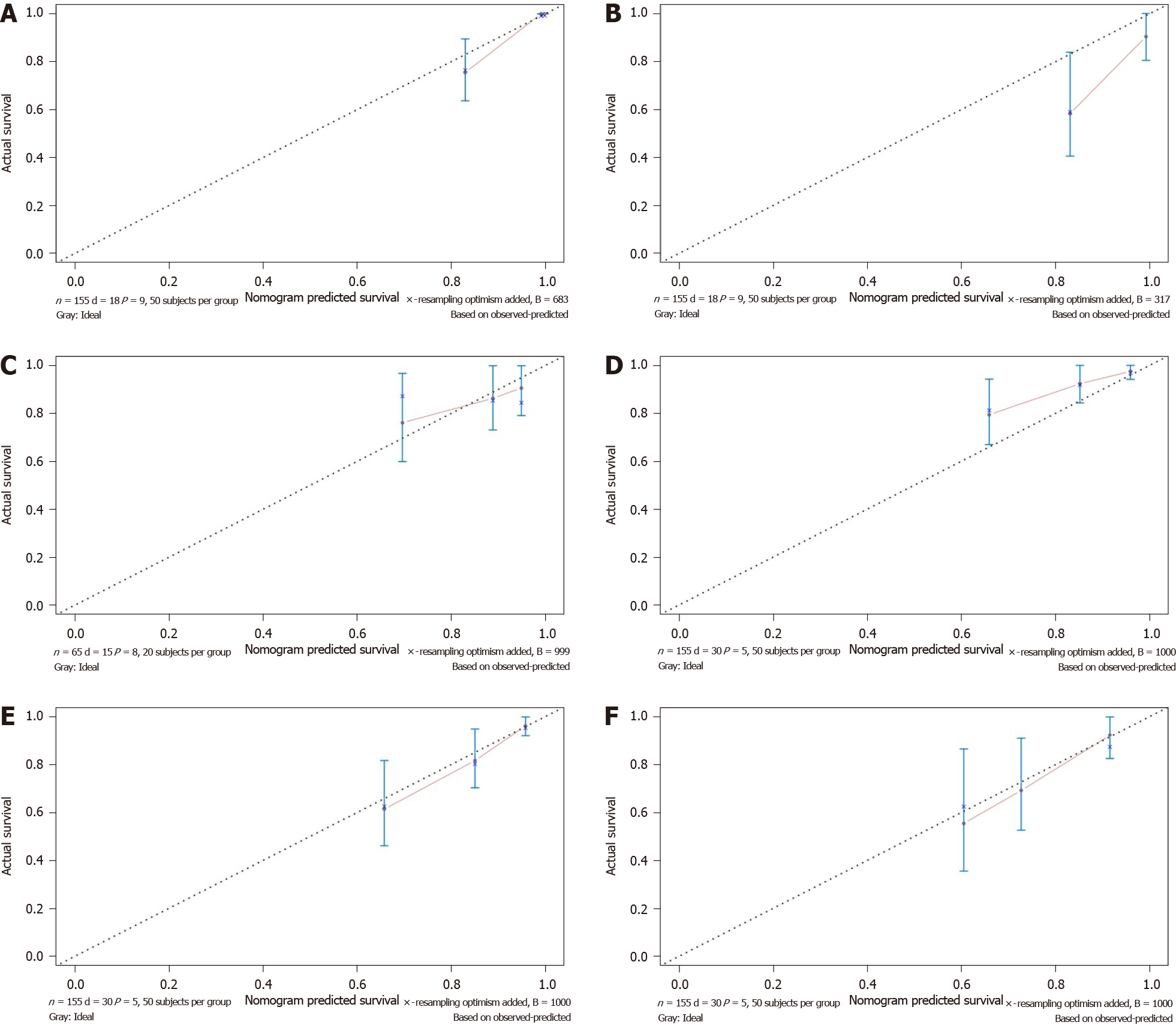
The nomogram integrated all of the prognostic factors for OS and DFS as shown in Figure 5A and B; these factors were screened by LASSO regression. The C-index for prediction of OS was 0.91 (95%CI: 0.85-0.97), and that for DFS prediction was 0.77 (95%CI: 0.69-0.85).
The effectiveness of the nomograms was tested in the validation cohort, and the C-index and calibration plot revealed the prognostic value of these models for OS and DFS. The C-index for prediction of OS was 0.69 (95%CI: 0.53-0.84), and that for prediction of DFS was 0.71 (95%CI: 0.61-0.81). Therefore, the established nomograms were well calibrated and showed good predictive value for OS and DFS (Figure 6).
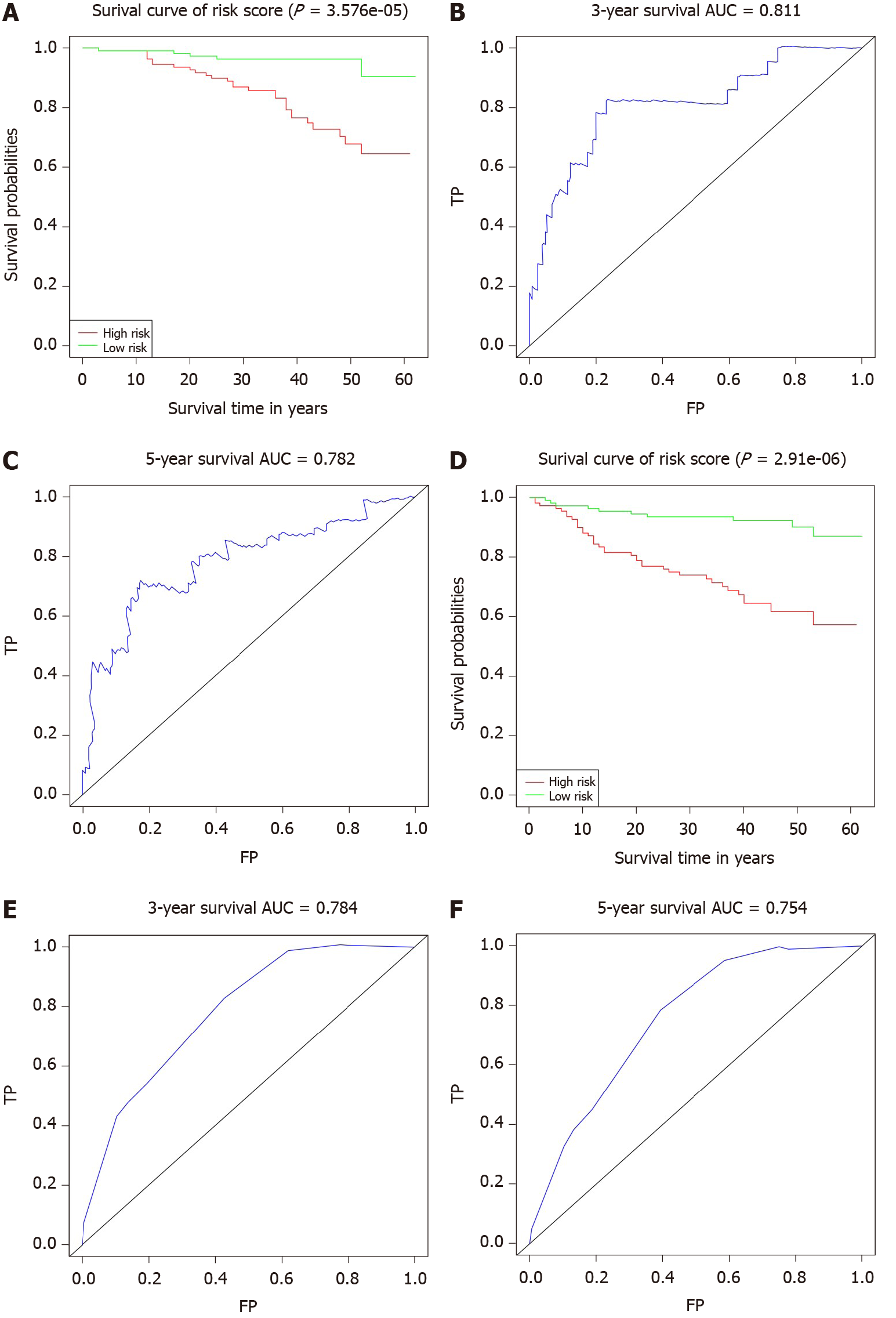
We utilized Cox proportional hazards regression analysis of the clinical characteristics to develop the prognostic models (Figure 7A-F). According to the prognostic risk score, all patients were divided into a low-risk and a high-risk group. The risk scores reflected the 3-year and 5-year survival rates of the patients. K-M curves were used to show the relationship of the risk score with OS and DFS in the low-risk and high-risk groups, and these curves verified that a low risk score had a stronger positive association with OS and DFS (OS: P = 3.576e-05; DFS: P = 2.91e-06; Figure 7A and D). The AUCs of ROC curves for 3-year and 5-year OS were 0.811 and 0.782 (Figure 7B and C). The AUC for 3-year DFS was 0.784, and that for 5-year DFS was 0.754, as shown in Figure 7D and F.
Recently, NT has emerged as the standard treatment for LARC patients[11-14]. Patients who cannot achieve a pCR usually undergo surgery and receive adjuvant therapy. Compared to patients who undergo traditional surgery and adjuvant therapy without NT, patients who receive NT have a more complex physical condition because of the influence of NT[15,16]. Additionally, the prognostic factors for OS and DFS also change. Thus, exploring the prognostic factors that can predict OS and DFS has become necessary.
Many studies have revealed that lymph node metastasis, low BRCA2 expression and other variables can be prognostic factors for patients administered NT. In our study, we developed and validated risk score prediction models and nomograms for OS and DFS based on clinical characteristics. Preliminary screening of potential factors by LASSO regression can reduce the number of features included and screen only critical factors[17,18]. Cox regression and K-M curves can further verify the prognostic value of key factors. The followings were included in the nomogram for OS: Vascular_tumors_bolt, cancer nodules, yN, BMI, matchmouth distance from the edge, nerve aggression and postoperative CEA. The nomogram of DFS included the following variables: ypTNM and nerve aggression. The risk factor score prediction models included the same risk factors as the nomograms. The AUCs for the prediction models for both OS and DFS were high and showed that a low risk score had a strong positive association with the years of survival, indicating that the risk factor and prognostic models had good prognostic value for LARC.
Regarding the prognostic factors of OS, 50 candidate clinical features were reduced to 10 potential predictors, and through Cox regression analysis, three factors could be eliminated: Operation time, cT and ypTNM. The P values of operation time, cT and ypTNM were higher than 0.05. The distance of the tumor from the anal margin is closely related to operation time and other important factors[19-21] because if the tumor is close to the anus, anal preservation will be prioritized. However, removing the anus or preserving the lower anus can be a lengthy procedure; therefore, the operation time may be related to the tumor location after NT. In addition to the distance from the margin after NT, the matchmouth distance from the edge can more comprehensively reflect the tumor type. Changes in the size of the tumor can influence the type of surgery, which will also affect the distance of the matchmouth from the edge. Changes in tumor size before and after NT were related to the tumor response to treatment. Therefore, although the operation time and ypTNM can reflect the different statuses, they also have a close relationship with the matchmouth distance from the edge, thus we excluded the two variables. Regarding the distance from the margin to the anus, a shorter distance from the matchmouth to the anus corresponds to shorter survival time.
Laparoscopic surgery for colorectal cancer has a shorter postoperative exhaust time than conventional left hemicolectomy[22]. Postoperative exhaust time is an important postoperative indicator that is closely related to obstructive colorectal cancer[23,24]. In our cohort, only one patient presented with obstruction; therefore, the prognostic value of postoperative exhaust time was not screened out by the LASSO regression analysis.
The appearance of cancer nodules is an important factor associated with primary tumor metastasis and has been suggested to reflect the effects of adjuvant therapy. With the development of UICC/AJCC staging standard, the definition and staging of cancerous nodules have gradually improved, and the prognostic value of nodules in colon cancer is also increasing. In previous studies, cancer nodules were thought to significantly increase the rates of local recurrence and metastasis in colorectal cancer[25]. Cancer nodules had the lowest contribution to our nomogram for OS; if patients have cancer nodules, the nomogram score will increase, and OS will decrease.
yN was evaluated after surgery. For tumors located in or near the rectum, the N stage significantly more frequently either remained stable or progressed, but treatment with surgery and adjuvant therapy could also have an effect. yN is a good prognostic factor for DFS and cancer-specific survival[26-28]. Pathological examination is very important for patients who receive NT because it can ensure the appropriate staging and treatment. In our study, both LASSO regression and the K-M curves revealed that yN had good prognostic value; thus, we included this variable to ensure that our nomogram fully reflects the condition after adjuvant therapy. Regarding yN, in the nomogram, as the N stage progresses, the nomogram score increases and survival decreases. Of note, yN3, which is to the left of yN0 and yN1, may be due to lymph node changes after NT, which was found at a high rate by the surgeon performing the resection.
BMI reflects the patients’ weight and height. As a risk factor for colorectal cancer[29,30], the BMI value is an important prognostic indicator. Patients with a higher BMI tend to be more obese and have shorter survival based on our nomogram. We also explored the level of the serum tumor marker CEA because it is an important and strong diagnostic biomarker both before therapy and after surgery[31]. In our nomogram, a higher CEA level indicates shorter survival.
LARC poses several challenges, including recurrence[32]. Tumor recurrence is an important factor affecting the prognosis and survival of tumor patients[33]. A lower probability of recurrence leads to a higher survival rate. In previous studies, recurrence has been linked with biomarkers such as BRAF-6000E, RAS and CD8-positive T-cells[11,34,35], and an early diagnosis[25] can take advantage of the patients’ clinical information. In identifying predictive factors of DFS, 50 clinical features were reduced to 2 potential predictors of DFS. The DFS nomogram included ypTNM and nerve aggression. Pathologic TNM (ypTNM) has been considered a good prognostic factor in many studies. Utilizing ypTNM, our study also confirmed that ypTNM is a strong predictor for DFS[36-38]. Nerve aggression was also an important predictive factor in our study. A higher ypTNM or presence of nerve aggression corresponds to a shorter survival time.
There are limitations to our study. The data included here were all from a single network of tumor hospitals, thus lacking representation of the general population. Additionally, our research in the field of molecular target design is poorly established.
Recurrence, cancer nodules, yN, positive lymph node status, BMI, matchmouth distance from the edge, distance from the margin after NT and postoperative CEA were prognostic factors for OS, and ypTNM and nerve aggression were prognostic value for DFS. We created and validated nomograms and prediction models that can objectively and accurately predict OS and DFS in LARC patients.
Neoadjuvant therapy (NT) has been increasingly used as the standard treatment for clinical stage II/III rectal cancer. Risk factors after administration of neoadjuvant therapy for locally advanced rectal cancer (LARC) are still under debate.
There is a lack of consensus concerning the risk factors after administration of neoadjuvant therapy for LARC. Nomograms and risk prediction models for survival can help clinicians to choose therapy according to patient's individual risk.
The main aim of this study was to explore the prognostic factors and establish effective prognostic nomograms and risk score prediction models to predict overall survival (OS) and disease-free survival (DFS) for LARC treated with NT.
Nomograms and risk factor score prediction models were based on patients who received NT. LASSO regression was utilized to screen for prognostic risk factors, which were validated by the Cox regression. ROC curves, C-index and calibration curves were performed to evaluate the prediction models and nomograms.
Seven features, including vascular_tumors_bolt, cancer nodules, yN, body mass index (BMI), matchmouth distance from the edge, nerve aggression and postoperative carcinoembryonic antigen (CEA), were significantly associated with OS. The nomogram for predicting DFS included ypTNM and nerve aggression. The primary and validate cohort showed good predictive value. The prediction model for OS and DFS had good predictive value.
We established accurate nomograms and prediction models for predicting OS and DFS in patients with LARC after undergoing NT.
Larger prospective multicenter clinical studies need to be performed to validate the nomograms and risk score prediction models of OS and DFS.
We would like to thank the National Cancer Center/National Sciences Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: García-Flórez LJ, Velenik V S-Editor: Zhang H L-Editor: MedE-Ma JY P-Editor: Liu JH
| 1. | Ren DL, Li J, Yu HC, Peng SY, Lin WD, Wang XL, Ghoorun RA, Luo YX. Nomograms for predicting pathological response to neoadjuvant treatments in patients with rectal cancer. World J Gastroenterol. 2019;25:118-137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 2. | Oronsky B, Reid T, Larson C, Knox SJ. Locally advanced rectal cancer: The past, present, and future. Semin Oncol. 2020;47:85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 3. | Bengulescu I, Radu P, Iorga C, Bratucu M, Pasnicu C, Garofil D, Popa F, Strambu V. The Value of Endoscopy as a Predictive Factor when Evaluating the Clinical Response to Neoadjuvant Chemoradiotherapy for Patients with Rectal Cancer. Chirurgia (Bucur). 2020;115:373-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Xiao L, Yu X, Deng W, Feng H, Chang H, Xiao W, Zhang H, Xi S, Liu M, Zhu Y, Gao Y. Pathological Assessment of Rectal Cancer after Neoadjuvant Chemoradiotherapy: Distribution of Residual Cancer Cells and Accuracy of Biopsy. Sci Rep. 2016;6:34923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Zhang F, Yao S, Li Z, Liang C, Zhao K, Huang Y, Gao Y, Qu J, Li Z, Liu Z. Predicting treatment response to neoadjuvant chemoradiotherapy in local advanced rectal cancer by biopsy digital pathology image features. Clin Transl Med. 2020;Online ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Bengulescu I, Radu P, Iorga C, Bratucu M, Pasnicu C, Garofil D, Popa F, Strambu V. Parameters for Predicting Tumour Response Following Neoadjuvant Chemoradiotherapy for Patients with Rectal Cancer. Chirurgia (Bucur). 2020;115:365-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55631] [Article Influence: 7947.3] [Reference Citation Analysis (131)] |
| 8. | Li Destri G, Maugeri A, Ramistella A, La Greca G, Conti P, Trombatore G, Vecchio GM, Magro GG, Barchitta M, Agodi A. The prognostic impact of neoadjuvant chemoradiotherapy on lymph node sampling in patients with locally advanced rectal cancer. Updates Surg. 2020;72:793-800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Baek JH, Baek DW, Kang BW, Kim HJ, Park SY, Park JS, Choi GS, Kim JG. Prognostic Impact of the Neoadjuvant Rectal Score as Compared With the Tumor Regression Grade and Yield Pathologic TNM Stage in Patients With Locally Advanced Rectal Cancer After Neoadjuvant Chemoradiotherapy. In Vivo. 2020;34:1993-1999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Parisi A, Cortellini A, Venditti O, Santo V, Sidoni T, Cannita K, Ficorella C, Porzio G. Family History of Cancer as Potential Prognostic Factor in Stage III Colorectal Cancer: a Retrospective Monoinstitutional Study. J Gastrointest Cancer. 2020;51:1094-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Farchoukh L, Hartman DJ, Ma C, Celebrezze J, Medich D, Bahary N, Frank M, Pantanowitz L, Pai RK. Intratumoral budding and automated CD8-positive T-cell density in pretreatment biopsies can predict response to neoadjuvant therapy in rectal adenocarcinoma. Mod Pathol. 2020;Online ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Malekzadeh Moghani M, Alahyari S, Moradi A, Nasiri M. Pathological Predictors of Response to Neoadjuvant Treatment in Rectal Carcinoma. J Gastrointest Cancer. 2020; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Giesen LJX, Borstlap WAA, Bemelman WA, Tanis PJ, Verhoef C, Olthof PB, DUTCH SNAPSHOT RESEARCH GROUP. Effect of understaging on local recurrence of rectal cancer. J Surg Oncol. 2020; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Toomey S, Gunther J, Carr A, Weksberg DC, Thomas V, Salvucci M, Bacon O, Sherif EM, Fay J, Kay EW, Sheehan KM, McNamara DA, Sanders KL, Mathew G, Breathnach OS, Grogan L, Morris PG, Foo WC, You YN, Prehn JH, O'Neill B, Krishnan S, Hennessy BT, Furney SJ. Genomic and Transcriptomic Characterisation of Response to Neoadjuvant Chemoradiotherapy in Locally Advanced Rectal Cancer. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Nie K, Shi L, Chen Q, Hu X, Jabbour SK, Yue N, Niu T, Sun X. Rectal Cancer: Assessment of Neoadjuvant Chemoradiation Outcome based on Radiomics of Multiparametric MRI. Clin Cancer Res. 2016;22:5256-5264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 306] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 16. | Song C, Chung JH, Kang SB, Kim DW, Oh HK, Lee HS, Kim JW, Lee KW, Kim JH, Kim JS. Impact of Tumor Regression Grade as a Major Prognostic Factor in Locally Advanced Rectal Cancer after Neoadjuvant Chemoradiotherapy: A Proposal for a Modified Staging System. Cancers (Basel). 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 17. | Shu Z, Fang S, Ding Z, Mao D, Cai R, Chen Y, Pang P, Gong X. MRI-based Radiomics nomogram to detect primary rectal cancer with synchronous liver metastases. Sci Rep. 2019;9:3374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 18. | Cui Y, Yang X, Shi Z, Yang Z, Du X, Zhao Z, Cheng X. Radiomics analysis of multiparametric MRI for prediction of pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Eur Radiol. 2019;29:1211-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 169] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 19. | Clermonts SHEM, Köeter T, Pottel H, Stassen LPS, Wasowicz DK, Zimmerman DDE. Outcomes of completion total mesorectal excision are not compromised by prior transanal minimally invasive surgery. Colorectal Dis. 2020;22:790-798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Kang L, Chen YG, Zhang H, Zhang HY, Lin GL, Yang YC, Chen WH, Luo SL, Chen N, Tong WD, Shen ZL, Xiong DH, Xiao Y, Zhang ZT, Wang JP. Transanal total mesorectal excision for rectal cancer: a multicentric cohort study. Gastroenterol Rep (Oxf). 2019;8:36-41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 21. | Bullock M, Nasir IUI, Hemandas A, Qureshi T, Figueiredo N, Heald R, Parvaiz A. Standardised approach to laparoscopic total mesorectal excision for rectal cancer: a prospective multi-centre analysis. Langenbecks Arch Surg. 2019;404:547-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Cui W, Zhu G, Zhou T, Mao X, Wang X, Chen Y. Laparoscopic and conventional left hemicolectomy in colon cancer. J BUON. 2020;25:240-247. [PubMed] |
| 23. | Yang L, Ma W, Wang M, Zhang R, Bi T, Zhou S. Efficacy of intestinal obstruction stent combined with laparoscopic surgery and neoadjuvant chemotherapy in patients with obstructive colorectal cancer. Oncol Lett. 2019;18:1931-1937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Li W, Jin X, Liang G. The efficacy of endoscopic stenting combined with laparoscopy in the treatment of left colon cancer with obstruction. J Cancer Res Ther. 2019;15:375-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 25. | Stephensen BD, Reid F, Shaikh S, Carroll R, Smith SR, Pockney P; on behalf of the PREDICT Study Group collaborators. C-reactive protein trajectory to predict colorectal anastomotic leak: PREDICT Study. Br J Surg. 2020;Online ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 26. | Reggiani Bonetti L, Lionti S, Domati F, Barresi V. Do pathological variables have prognostic significance in rectal adenocarcinoma treated with neoadjuvant chemoradiotherapy and surgery? World J Gastroenterol. 2017;23:1412-1423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | Swellengrebel HA, Bosch SL, Cats A, Vincent AD, Dewit LG, Verwaal VJ, Nagtegaal ID, Marijnen CA. Tumour regression grading after chemoradiotherapy for locally advanced rectal cancer: a near pathologic complete response does not translate into good clinical outcome. Radiother Oncol. 2014;112:44-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | van Heeswijk MM, Lambregts DM, Palm WM, Hendriks BM, Maas M, Beets GL, Beets-Tan RG. DWI for Assessment of Rectal Cancer Nodes After Chemoradiotherapy: Is the Absence of Nodes at DWI Proof of a Negative Nodal Status? AJR Am J Roentgenol. 2017;208:W79-W84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 29. | Ochs-Balcom HM, Kanth P, Farnham JM, Abdelrahman S, Cannon-Albright LA. Colorectal cancer risk based on extended family history and body mass index. Genet Epidemiol. 2020;Online ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Mohamed Sad L, Elsaka AM, Abdelmonem Zamzam Y, Gharib Khairallah F. Phase angle, body mass index and KRAS status of metastatic colorectal cancer in response to chemotherapy with and without target therapy: clinical impact and survival. J BUON. 2020;25:914-926. [PubMed] |
| 31. | Huang CS, Chen CY, Huang LK, Wang WS, Yang SH. Prognostic value of postoperative serum carcinoembryonic antigen levels in colorectal cancer patients who smoke. PLoS One. 2020;15:e0233687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 32. | Tan G, Wong J. Surgical management and hyperthermic intraperitoneal chemotherapy for locally advanced colorectal cancer. J Gastrointest Oncol. 2020;11:508-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Uttam S, Stern AM, Sevinsky CJ, Furman S, Pullara F, Spagnolo D, Nguyen L, Gough A, Ginty F, Lansing Taylor D, Chakra Chennubhotla S. Spatial domain analysis predicts risk of colorectal cancer recurrence and infers associated tumor microenvironment networks. Nat Commun. 2020;11:3515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 34. | Kobayashi S, Takahashi S, Takahashi N, Masuishi T, Shoji H, Shinozaki E, Yamaguchi T, Kojima M, Gotohda N, Nomura S, Yoshino T, Taniguchi H. Survival Outcomes of Resected BRAF V600E Mutant Colorectal Liver Metastases: A Multicenter Retrospective Cohort Study in Japan. Ann Surg Oncol. 2020;27:3307-3315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 35. | Ebi H, Bando H, Taniguchi H, Sunakawa Y, Okugawa Y, Hatanaka Y, Hosoda W, Kumamoto K, Nakatani K, Yamazaki K. Japanese Society of Medical Oncology Clinical Guidelines: Molecular Testing for Colorectal Cancer Treatment, 4th edition. Cancer Sci. 2020; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 36. | Rödel C, Martus P, Papadoupolos T, Füzesi L, Klimpfinger M, Fietkau R, Liersch T, Hohenberger W, Raab R, Sauer R, Wittekind C. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2005;23:8688-8696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 918] [Cited by in RCA: 949] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 37. | Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, Kuo LJ, Calvo FA, García-Aguilar J, Glynne-Jones R, Haustermans K, Mohiuddin M, Pucciarelli S, Small W, Suárez J, Theodoropoulos G, Biondo S, Beets-Tan RG, Beets GL. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11:835-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1189] [Cited by in RCA: 1449] [Article Influence: 96.6] [Reference Citation Analysis (0)] |
| 38. | Marco MR, Zhou L, Patil S, Marcet JE, Varma MG, Oommen S, Cataldo PA, Hunt SR, Kumar A, Herzig DO, Fichera A, Polite BN, Hyman NH, Ternent CA, Stamos MJ, Pigazzi A, Dietz D, Yakunina Y, Pelossof R, Garcia-Aguilar J; Timing of Rectal Cancer Response to Chemoradiation Consortium. Consolidation mFOLFOX6 Chemotherapy After Chemoradiotherapy Improves Survival in Patients With Locally Advanced Rectal Cancer: Final Results of a Multicenter Phase II Trial. Dis Colon Rectum. 2018;61:1146-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 116] [Article Influence: 16.6] [Reference Citation Analysis (0)] |