Published online Nov 14, 2020. doi: 10.3748/wjg.v26.i42.6626
Peer-review started: April 6, 2020
First decision: April 26, 2020
Revised: July 3, 2020
Accepted: September 28, 2020
Article in press: September 28, 2020
Published online: November 14, 2020
Processing time: 220 Days and 22.8 Hours
Although the association of attention deficit hyperactivity disorder (ADHD) with psychiatric disorders is well known, its association with somatic diseases is unclear. Only few studies have investigated the gastrointestinal (GI) morbidity in adult patients with ADHD.
To measure gastrointestinal comorbidity and its burden on healthcare in young adults with ADHD.
The cohort included subjects aged 17-35 years recruited to the Israel Defense Forces in 2007-2013, 33380 with ADHD and 355652 without (controls). The groups were compared for functional and inflammatory conditions of the gastrointestinal tract and clinic and specialist visits for gastrointestinal symptoms/disease during service (to 2016). Findings were analyzed by generalized linear models adjusted for background variables.
Compared to controls, the ADHD group had more diagnoses of functional gastrointestinal disorders (referred to as FGID), namely, dyspepsia [odds ratio (OR): 1.48, 95% confidence interval (CI): 1.40-1.57, P < 0.001], chronic constipation (OR: 1.64, 95%CI: 1.48-1.81, P < 0.001), and irritable bowel syndrome (OR: 1.67, 95%CI: 1.56-1.80, P < 0.001) but not of organic disorders (inflammatory bowel disease, celiac disease). They had more frequent primary care visits for gastrointestinal symptoms [rate ratio (RR): 1.25, 95%CI: 1.24-1.26, P < 0.001] and referrals to gastrointestinal specialists (RR: 1.96, 95%CI: 1.88-2.03, P < 0.001) and more episodes of recurrent gastrointestinal symptoms (RR: 1.29, 95%CI: 1.21-1.38, P < 0.001). Methylphenidate use increased the risk of dyspepsia (OR: 1.49, 95%CI: 1.28-1.73, P < 0.001) and constipation (OR: 1.42, 95%CI: 1.09-1.84, P = 0.009).
ADHD in young adults is associated with an excess of FGID and increased use of related health services. Research is needed to determine if an integrative approach treating both conditions will benefit these patients and cut costs.
Core Tip: The association of attention deficit hyperactivity disorder (ADHD) with gastrointestinal morbidity and gastrointestinal-associated healthcare burden is unclear. We measured it on a large cohort of young adults, containing 33380 subjects with ADHD and 355652 without. We showed for the first time that ADHD is associated with dyspepsia, chronic constipation, and irritable bowel syndrome but not with inflammatory bowel disease and celiac disease. Furthermore, young adults with ADHD have more frequent primary care visits for gastrointestinal symptoms and referrals to gastrointestinal specialists. ADHD in young adults is associated with an excess of functional gastrointestinal disorders and increased use of related health services.
- Citation: Kedem S, Yust-Katz S, Carter D, Levi Z, Kedem R, Dickstein A, Daher S, Katz LH. Attention deficit hyperactivity disorder and gastrointestinal morbidity in a large cohort of young adults. World J Gastroenterol 2020; 26(42): 6626-6637
- URL: https://www.wjgnet.com/1007-9327/full/v26/i42/6626.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i42.6626
Attention deficit hyperactivity disorder (ADHD) is a chronic condition of inappropriate levels of inattention and/or hyperactivity-impulsiveness that interferes with the quality of social, academic, or occupational functioning. ADHD is one of the most common neuropsychiatric disorders of childhood, with the majority of cases persisting through adulthood[1,2]. The estimated prevalence of ADHD in the 18-44-year age group is 3.4% worldwide[3].
The association of ADHD to psychiatric comorbidity has been well described[4-9], but its association to somatic diseases is less established. According to current literature, ADHD is related to obesity, sleep disorders, and asthma, and may also be associated with otitis media, allergic rhinitis, motor disturbances, urinary symptoms, migraine and celiac disease[10-13].
The literature on gastrointestinal (GI) morbidity in ADHD is scarce in adults. There are more data for children but the findings are inconsistent[14]. A few studies reported an increased prevalence of ADHD in children with GI symptoms, such as encopresis, constipation, chronic diarrhea, and irritable bowel syndrome (IBS)[15,16], and others noted higher rates of abdominal distention, abdominal pain, overweight, and food allergy in children with ADHD[17-20]. Some studies, however, found no association between ADHD and GI symptoms or body mass index (BMI)[21,22].
The aim of this study was to investigate the prevalence and types of gastrointestinal comorbidities in young adults with ADHD and their burden on the healthcare system.
In Israel, one year prior to mandatory recruitment to the Israel Defense Forces (IDF), all eligible men of Jewish, Druze, and Circassian origin, and the majority of women of Jewish origin, undergo a medical assessment which includes review of their primary care medical files, medical history taking, physical examination, and if necessary, referral for further evaluation. The findings are recorded and coded as medical profile. If a major medical problem develops, the profile is adjusted accordingly based on type, duration, and severity[23].
The population of the present study consisted of all young adults of both sexes who were recruited to the IDF between January 2007 and February 2013 and assigned to active duty. This population accounts for about 50% of all Israeli young adult population. The three main groups that are underrepresented in the database are ultra-orthodox men and women, orthodox women and Arabs that are not recruited to active military service. Data were collected retrospectively from the central Medical Corp database for each participant, from recruitment until discharge from military service (mandatory or career) or the end of the study (February 29, 2016).
The study was approved on June 29th 2015 by the institutional review board (IRB) of the IDF Medical Corps in accordance with the Helsinki Declaration. Since it was a database study and participants could not be identified, exemption from informed consent was given by the IRB.
Height and weight were measured by trained personnel during the obligatory medical board examination using a stadiometer and a beam balance scale. BMI was calculated as weight in kilograms divided by height in meters squared. The following sociodemographic data were collected: Year of birth; age at the time of examination; country of birth, categorized as western countries (Europe, America, Australia, South Africa), former Soviet Union, Asia (other than the former Soviet Union; predominantly Western Asia), Africa (other than South Africa; predominantly Maghreb), Ethiopia or Israel; education, categorized as less than 12 years, 12 years, or more than 12 years; and socioeconomic status, ranked on a 10-point Central Bureau of Statistics scale according to place of residence as low (1-4), middle (5-7) or high (8-10).
In Israel, the diagnosis of ADHD in children and adolescents is based on formal questionnaires given to parents and teachers and objective computer-based tests and psychologic tests as needed. All diagnoses are based on DSM and made by MDs or psychologists, expert in this field. The study population was divided by the absence or presence of ADHD using four sources: (1) The medical files of the primary care physicians, reviewed as part of the medical assessment at recruitment to the IDF; (2) The IDF medical profile; (3) Medical records during active duty documenting ICD-9 codes 314.0, 314.00 or 314.01; and (4) The IDF pharmacy database documenting dispensation of methylphenidate. To account for the possibility that ADHD was under-reported before and during IDF service, for the purpose of this study, any patient who met at least one of the four criteria was considered to have ADHD. In some of the analysis, patients who received methylphenidate were analyzed separately from those who did not, because methylphenidate may adversely affect the GI tract. We further divided the ADHD participants who did not receive methylphenidate into two more groups: those who were diagnosed before recruitment to the IDF, did not receive specific medical profile and did not seek for medical help regarding ADHD per-se during their active medical service (mild ADHD); and those who got a specific profile or approached their physician because of their ADHD.
Data on GI symptoms/diseases were collected from the central medical records database of the IDF Medical Corps. Diagnoses of inflammatory bowel diseases (IBDs) and celiac disease were based on the medical profile alone because these are major diseases affecting medical service and therefore would need to be determined very precisely at recruitment. For IBD and celiac disease, the diagnosis was based on endoscopy and histologic findings, and for celiac disease also on serology. Diagnoses of IBS, dyspepsia, and constipation were based on several sources to ensure inclusion of only well-established cases: (1) The IDF medical profile; (2) Medical records during active duty documenting ICD-9 codes 564, 564.1, 564.4, 564.10, 536.9 for IBS, ICD-9 code 536.8 for dyspepsia, or ICD-9 codes 564.0, 564.01, 564.02 for constipation, as assigned by a gastroenterologist expert; or (3) Medical records during active duty documenting these ICD-9 codes assigned by a physician other than a gastroenterologist if the two recordings were separated by an interval of at least 6 mo. Constipation was diagnosed for this study only after hypothyroidism, diabetes and hypercalcemia were ruled out. Functional gastrointestinal disorder (FGID) was defined as the presence of either IBS, dyspepsia, and/or constipation.
GI symptoms besides dyspepsia and constipation were categorized into 12 groups based on ICD-9 codes (Supplementary Table 1).
Recurrent symptoms were defined as any of the GI symptoms recorded more than twice during a period of 3-12 mo.
Outcome measures for the present study were as follows: Diagnosis of IBS, dyspepsia, constipation, IBD, and celiac disease; GI symptoms as the reason for a primary care clinic visit, referral to a GI specialist, and recurrent GI complaints. Independent variables included ADHD and other medical, demographic, and anthropometric data.
The characteristics of the participants are presented as arithmetic mean and standard deviation (± SD) for continuous variables or as number and percentage for categorical variables. The association between ADHD and continuous variables was measured by Student’s t-test and validated by Mann-Whitney test when the distribution of the continuous variables was abnormal. The association of ADHD with categorical variables was measured with chi-square test (χ2) or Fisher’s exact test as appropriate. For regression analysis, we used generalized linear models with ADHD as the independent binary logistic variable. The recruits without ADHD served as the reference group, and the confounders were the sociodemographic and anthropometric variables. Gender and suspected confounders that showed a significant association on univariate analysis at a P level of < 0.10 were entered into the multivariate model. All data were generated with IBM-SPSS software, version 23 (IBM Corp., Armonk, NY, United States).
The cohort included 389032 recruits, 41.3% female, aged 17-35 years, of whom 33380 (8.6%) had ADHD. Table 1 describes the sociodemographic characteristics of the cohort. Data were missing on country of birth for 0.87% of subjects, socioeconomic status for 1.63%, and education for 1.21%. Most ADHD patients (n = 23,138, 69.3%) had mild ADHD, and only 3980 subjects (11.9%) received anti-ADHD drugs during the study period. The ADHD group had a higher percentage of females than the control group (43.3% vs 41.1%, P < 0.001), but this higher proportion occurred only in the mild ADHD group. The ADHD group also had a higher mean socioeconomic class and a higher BMI (P < 0.001 for all).
| ADHD | |||
| No | Yes | ||
| Number of participants | 355652 | 33380 | |
| Gender | Female | 41.10% | 43.30% |
| Male | 58.90% | 56.70% | |
| Socioeconomic status | Low | 26.30% | 14.70% |
| Medium | 53.30% | 50.30% | |
| High | 20.40% | 35.00% | |
| Education | 12 | 94.20% | 93.20% |
| < 12 | 0.30% | 0.10% | |
| > 12 | 5.50% | 6.70% | |
| Comorbidities | None or mild | 68.10% | 63.20% |
| Mild to moderate | 9.20% | 9.80% | |
| Moderate to severe | 22.60% | 27.00% | |
| Country of origin | Western countries | 21.80% | 25.30% |
| Africa | 23.00% | 21.70% | |
| Asia | 20.40% | 25.50% | |
| Former Soviet Union | 20.00% | 13.00% | |
| Ethiopia | 3.50% | 1.10% | |
| Other | 0.50% | 0.60% | |
| Israel | 10.80% | 12.70% | |
| BMI, mean ± SD | Males | 21.93 ± 0.02 | 22.42 ± 0.06 |
| Females | 21.52 ± 0.02 | 21.88 ± 0.07 | |
| Height in cm, mean ± SD | Males | 174.2 ± 0.03 | 174.4 ± 0.10 |
| Females | 162.2 ± 0.03 | 162.2 ± 0.10 | |
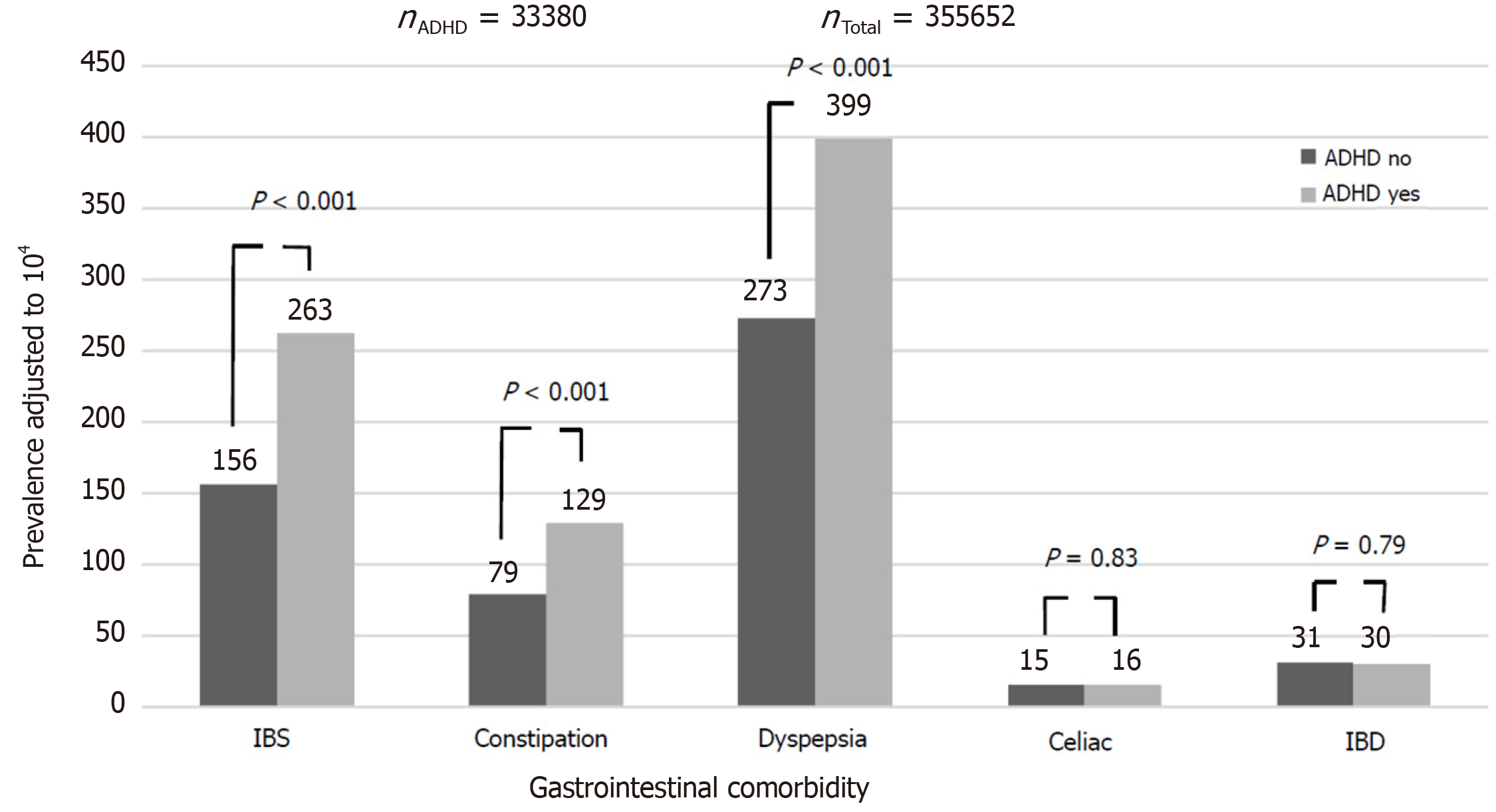
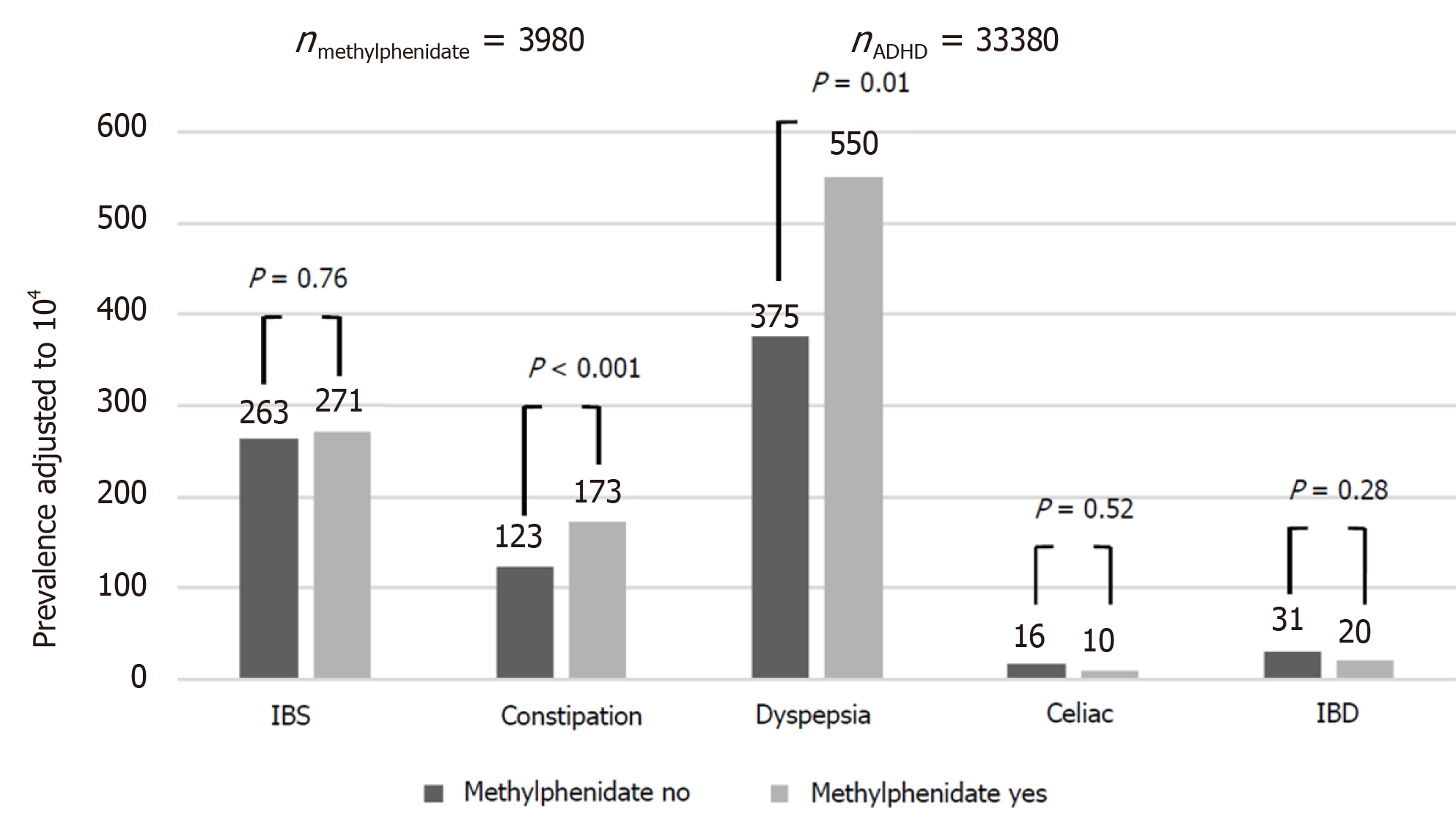
Compared to controls, the ADHD group had a higher rate of dyspepsia [399/104 vs 273/104, odds ratio (OR): 1.48, 95% confidence interval (CI): 1.40-1.57, P < 0.001], constipation (129/104 vs 79/104, OR: 1.64, 95%CI: 1.48-1.81, P < 0.001), IBS (263/104 vs 156/104, OR: 1.67, 95%CI: 1.56-1.80, P < 0.001) and FGID (672/104 vs 449/104, OR: 1.53, 95%CI: 1.47-1.61, P < 0.001). There was no between-group difference in the rate of diagnosis of IBD [30/104 vs 31/104, OR: 0.97, 95%CI: 0.79-1.19, P = not significant (NS)] and celiac disease (16/104 vs 15/104, OR: 1.03, 95%CI: 0.78-1.37, P = NS) (Figure 1). The effect of ADHD on the rate of dyspepsia, constipation, IBS and FGID was larger in females, although still significant in males as well (OR for dyspepsia 1.51 in females and 1.39 in males; for constipation, OR of 1.60 for females and 1.58 for males; for IBS, OR of 1.83 for females and 1.47 for males; and for FGID, the OR was 1.57 for females and 1.43 for males. P < 0.001 for all associations in both genders). Among participants with ADHD, methylphenidate prescription was associated with an increased risk of dyspepsia and constipation, but not of IBS, IBD and celiac disease (Figure 2). These effects were unrelated to the severity of ADHD or the cumulative dose of the drug. On multivariate analysis adjusted for male sex, country of origin, country of birth, socioeconomic status, education and BMI, ADHD was significantly associated with higher rates of dyspepsia, constipation and IBS (Table 2). The association of ADHD with dyspepsia and constipation was more prominent in the subjects taking methylphenidate during the study period. The association of ADHD with IBS remained only in those not taking methylphenidate.
| Univariate model | Multivariate model | ||||||
| Methylphenidate | OR | 95%CI | P value | RR | 95%CI | P value | |
| Celiac | + | 0.66 | 0.25-1.77 | 0.539 | |||
| - | 1.08 | 0.81-1.45 | 0.592 | ||||
| IBD | + | 0.65 | 0.32-1.30 | 0.252 | |||
| - | 1.01 | 0.82-1.25 | 0.919 | ||||
| IBS | + | 1.63 | 1.35-1.98 | C0.001 | 1.02 | 0.81-1.30 | 0.842 |
| - | 1.67 | 1.54-1.80 | < 0.001 | 1.29 | 1.18-1.41 | < 0.001 | |
| Constipation | + | 2.12 | 1.66-2.69 | < 0.001 | 1.6 | 1.21-2.13 | 0.001 |
| - | 1.56 | 1.40-1.74 | < 0.001 | 1.43 | 1.26-1.62 | < 0.001 | |
| Dyspepsia | + | 2.03 | 1.77-2.32 | < 0.001 | 1.75 | 1.49-2.06 | < 0.001 |
| - | 1.4 | 1.31-1.49 | < 0.001 | 1.2 | 1.11-1.29 | < 0.001 | |
| Total complaints | + | 1.82 | 1.55-2.12 | < 0.001 | 1.85 | 1.56-2.18 | < 0.001 |
| - | 1.26 | 1.17-1.35 | < 0.001 | 1.26 | 1.17-1.36 | < 0.001 | |
| Gastroenterologist referrals | + | 2.34 | 2.14-2.57 | < 0.001 | 2.29 | 2.07-2.53 | < 0.001 |
| - | 1.98 | 1.90-2.06 | < 0.001 | 1.99 | 1.90-2.07 | < 0.001 | |
| Visits in a primary care clinic | + | 1.53 | 1.50-1.57 | < 0.001 | 1.56 | 1.52-1.59 | < 0.001 |
| - | 1.23 | 1.22-1.24 | < 0.001 | 1.23 | 1.22-1.24 | < 0.001 | |
In order to assess the risk factors for FGID among participants with ADHD, we compared the characteristics between ADHD with and without FGID and found that in the ADHD group, FGID was associated with female gender, other comorbidities and use of methylphenidate, and was negatively associated with low SES (Supplementary Table 2).
Table 3 summarizes the association between ADHD and referral to a GI specialist according to each GI symptom. Supplementary Tables 3 and 4 summarize the same association for GI-related primary care physician visits and recurrent GI symptoms, respectively. All three tables show a positive association of heartburn and gastroesophageal reflux disease, nausea and vomiting, abdominal pain, and diarrhea with ADHD. On univariate analysis (Table 2), compared to controls, the subjects with ADHD were referred more often to a GI specialist [rate ratio (RR): 1.96, 95%CI: 1.88-2.03, P < 0.001], examined more frequently by a primary care physician for GI symptoms (RR: 1.25, 95%CI: 1.24-1.26, P < 0.001) and had more episodes of recurrent GI symptoms (RR: 1.29, 95%CI: 1.21-1.38, P < 0.001). The association of ADHD with increased use of health resources was independent of methylphenidate prescription, although its magnitude was higher in the subjects taking the drug (Table 2). Among ADHD patients, medical visits due to weight loss were higher only in those who had not received medications. On multivariate analysis adjusted for male sex, country of origin, country of birth, socioeconomic status, education, and BMI, ADHD (with or without medication) was significantly associated with primary care visits for GI symptoms, referrals to a GI specialist, and recurrent GI symptoms (Table 2).
| Gastroenterologist referrals | Gastrointestinal symptom | RR | 95%CI | P value |
| Perianal symptoms | 1.69 | 1.32-2.15 | < 0.001 | |
| Heartburn and GERD | 2.15 | 1.88-2.45 | < 0.001 | |
| Bowel habit changes | 2.04 | 1.36-3.06 | 0.001 | |
| Nausea and vomiting | 2.53 | 2.21-2.89 | < 0.001 | |
| Weight loss | 1.88 | 1.34-2.64 | < 0.001 | |
| Abdominal pain | 1.94 | 1.84-2.04 | < 0.001 | |
| Rectal bleeding and melena | 1.62 | 1.39-1.89 | < 0.001 | |
| Abdominal gas and bloating | 1.58 | 1.13-2.22 | 0.008 | |
| Diarrhea | 2.05 | 1.88-2.23 | < 0.001 | |
| Abdominal mass | 0.98 | 0.30-3.24 | 0.973 | |
| Others | 2.72 | 1.56-4.77 | < 0.001 | |
| Overall | 1.96 | 1.88-2.03 | < 0.001 |
The present study of a large cohort of young adults with ADHD showed that ADHD is associated with an increased rate of comorbid FGID (IBS, constipation, and dyspepsia) but not with somatic immune-mediated GI conditions, such as IBD and celiac disease. In addition, the ADHD group had a significantly increased rate of primary care visits for GI symptoms, referrals to GI specialists, and recurrent GI symptoms than the control group, pointing to the high burden of GI morbidity in individuals with ADHD on healthcare resources. These associations were not related to the use of methylphenidate, although those who received methylphenidate had a higher relative risk of all the measured outcomes except IBS.
The largest study to date on physical comorbidities of ADHD was a symptom-based survey of a nationally representative sample in the United States[20]. The results showed a significant association between ADHD and "serious stomach or bowel problems" which were not specified or categorized by type (inflammatory or functional). Another population-based survey revealed an association between ADHD and recurrent complaints of vomiting and diarrhea within the previous 2 wk or frequent diarrhea and colitis[23].
Psychiatric comorbidities are known to be more common in patients with ADHD, particularly depression, anxiety, and bipolar disorder[4-9,24]. Unfortunately, since young adults with major psychiatric illnesses are not eligible for recruitment to the IDF, we were not able to study the association between FGID and major psychiatric comorbidities in our cohort. The increased utilization of healthcare services by the ADHD population, as shown in our study and in others[25], can be partially explained by the mental stress associated with serving in the army and by these psychiatric comorbidities, respectively. Therefore, patients with ADHD who have GI symptoms might best be treated with an integrative approach by a multidisciplinary team of primary care physician, GI specialist, and psychiatrist.
The association between ADHD and IBS or dyspepsia has not been intensely investigated. There are studies of ADHD and constipation but the results are controversial[7,16,22,23,26]. The present study yielded a positive association between ADHD and constipation that was more prominent in the patients receiving methylphenidate (RR: 1.60 vs 1.43, P < 0.01).
The relatively high prevalence of constipation and FGID in patients with ADHD has several possible explanations. First, it may be attributable to a miscommunication or impaired cross-talk between the central and enteric nervous systems, resulting in altered perceptions of intestinal distension and disordered GI motility[16]. Second, a single neurobiological mechanism may underlie both disorders. This possibility is supported by the known association of ADHD with urinary voiding dysfunction[26]. Third, the behavioral disorders and the high rate of comorbid psychiatric disorders in individuals with ADHD may be related to the pathogenesis of FGID[27], and fourth and most interesting, an evolving hypothesis suggests an important role of the gut-brain axis and intestinal microbiota in modulating ADHD, therefore explaining the overlap between ADHD and FGID[28,29].
In contrast to FGID, immune-mediated conditions such as IBD and celiac disease were not associated with ADHD. A previous small study of 50 children reported a higher prevalence of ADHD among those with IBD[30] but, unlike our study, it did not examine the rate of IBD in patients with ADHD. Likewise, several studies found a higher rate of ADHD among patients with celiac disease[31-33], but whether celiac disease is more prevalent among patients with ADHD is less clear[21,34]. In a recent systematic review of eight studies of ADHD and celiac disease, Ertürk et al[35] concluded that the results were inconsistent, as only three reported a positive correlation. It is worth mentioning that a recently published study from Germany, showed an association between childhood ADHD and immune-mediated diseases, such as type I diabetes, juvenile rheumatoid arthritis and asthma; however, no association was recorded with IBD and celiac disease[36].
Methylphenidate prescriptions were given to 3980 participants (11.92%) during the study period. We considered the receipt of medical treatment a marker of severe disease. However, methylphenidate itself has been associated with adverse GI effects, mainly abdominal pain, decreased or loss of appetite, weight loss, nausea, and vomiting. Indeed, the methylphenidate-treated subjects had a higher relative risk for most of the ADHD-associated outcome measures than the untreated subjects. Moreover, the association of methylphenidate with symptoms of nausea, vomiting, and abdominal pain was high in the assessment of medical visits to either primary care physicians or GI specialists. This finding may have been due either to side effects of the drug or the effects of a more severe form of ADHD.
Since we used a broad definition of ADHD, the rate of ADHD in our population (8.5%) was higher than previously published[3]; the majority of ADHD cases in the study (69.3%) had mild ADHD, and did not consume anti-ADHD drugs or seek help for ADHD symptoms during military service. Nevertheless, ADHD remained associated with FGID (IBS, dyspepsia and constipation) regardless its severity.
The association between ADHD and GI-related functional morbidity may affect clinical decisions and treatment. Attention should be addressed to GI problems in patients with known ADHD, including a careful medical history focused on GI-related morbidity, so as not to miss some of the common GI problems. The presence of ADHD in a patient with GI symptoms, normal laboratory results and no red flags may by itself support the diagnosis of a functional GI disorder. Since FGIDs are now considered disorders of gut-brain interaction and centrally acting neuromodulators are amongst the mainstays of refractory FGIDs, these drugs may be considered in treating patients suffering from both FGID and ADHD.
Previous studies of GI-related comorbidity in ADHD were performed in children; this is the first study to focus on young adults. The main strength of this population-based study is its large size: 389032 participants of whom 33380 had ADHD. Moreover, our control group was well defined and based on a representative sample of the general population. We based the diagnosis of ADHD on medical documentation and not parental or patient reports, which also eliminated the risk of recall bias. Since methylphenidate is associated with substantial GI morbidity, we stratified our data regarding to medication consumption.
The present study has some limitations. We used a broad definition of ADHD, so some of the participants in the ADHD group may have had a mild form of the disease or inactive disease based on childhood medical reports. Our dependence on ICD-9 coding may have allowed for the inclusion of misdiagnoses, and diagnoses that were not strictly based on the ROME criteria; although, our strict criteria for the diagnosis of FGID in terms of duration of symptoms may have helped to overcome this limitation. Also, dyspepsia in this study is mainly uninvestigated dyspepsia, since upper GI endoscopy and Helicobacter pylori testing were not requested. Since the study design was cross-sectional, our results can show only an association between ADHD and GI-related morbidity but not causality. The medication data should be interpreted with caution because it is based on prescriptions and not on confirmed consumption.
In conclusion, ADHD is associated with FGID and a high need for GI-related health services. This study emphasizes the complex interaction between mind and body. Further research is needed to explore the possible combination of treatment of FGID with the neuropsychological therapeutic modalities for ADHD, and to determine if the presence of ADHD can assist in the diagnosis of FGID.
Attention deficit hyperactivity disorder (ADHD) is a very common chronic condition of inappropriate levels of inattention and/or hyperactivity that interferes with the quality of social, academic, or occupational functioning. Although ADHD is associated with some gastrointestinal (GI) symptoms in children, the association of ADHD to GI disorders in adults is not well characterized.
The motivation for the research came from the clinical observation that many young adults attending the GI clinic with functional gastrointestinal disorders (FGID) mention ADHD as a chronic condition they suffer from. Therefore, we decided to conduct a study to confirm this association. Finding an association between ADHD and GI-related functional morbidity might affect clinical decisions and treatment; in such patients who have both ADHD and FGID, treatment should be taken by an integrative approach combined of a multidisciplinary team of primary care physician, GI specialist, and psychiatrist, and centrally acting neuromodulators should be considered in the treatment plan.
The aim of this study was to investigate the prevalence and types of GI comorbidities in young adults with ADHD and their burden on the healthcare system. Indeed, we found an association between ADHD and FGID, such as irritable bowel syndrome (IBS), dyspepsia, and chronic constipation. ADHD was not associated with IBD or celiac disease.
This was a retrospective cohort study, consisting of all young adults of both sexes recruited to the Israeli Defense Forces (IDF) between January 2007 and February 2013 and assigned to active duty. This population accounts for about 50% of the entire Israeli young adult population. Several sources were used to accurately identify ADHD patients as well as to use only well-established diagnoses of IBS, dyspepsia, and constipation. The following sociodemographic data were collected: year of birth; age at the time of examination; country of birth; education; and socioeconomic status. Outcome measures were diagnosis of IBS, dyspepsia, constipation, IBD, and celiac disease, as well as GI symptoms as the reason for a primary care clinic visit, referral to a GI specialist, and recurrent GI complaints.
The cohort included 389032 recruits, 41.3% female, aged 17-35 years, of whom 33380 (8.6%) had ADHD. Most ADHD patients (n = 23138, 69.3%) had mild ADHD, and only 3980 subjects (11.9%) received anti-ADHD drugs during the study period. Compared to controls, the ADHD group had a higher rate of dyspepsia, constipation, IBS and FGID. There was no between-group difference in the rate of diagnosis of IBD and celiac disease. The effect of ADHD on the rate of dyspepsia, constipation, IBS and FGID was larger in females, although still significant in males as well. Among participants with ADHD, methylphenidate prescription was associated with an increased risk of dyspepsia and constipation, but not of IBS, IBD, and celiac disease. Compared to controls, the subjects with ADHD were referred more often to a GI specialist, examined more frequently by a primary care physician for GI symptoms, and had more episodes of recurrent GI symptoms. Participants with ADHD suffered more from recurrent heartburn and gastroesophageal reflux disease, nausea and vomiting, abdominal pain, and diarrhea.
The study contributes to the research in the field since this is the first study to focus on young adults and it is a large size population-based study.
The present study of a large cohort of young adults with ADHD showed that ADHD is associated with an increased rate of comorbid FGID (IBS, constipation, and dyspepsia) but not with somatic immune-mediated GI conditions, such as IBD and celiac disease. In addition, the ADHD group had a significantly increased rate of primary care visits for GI symptoms, referrals to GI specialists, and recurrent GI symptoms than the control group, pointing to the high burden of GI morbidity in individuals with ADHD on healthcare resources. These associations were not related to the use of methylphenidate; although, those who received methylphenidate had a higher relative risk of all the measured outcomes, except IBS. The association between ADHD and GI-related functional morbidity may affect clinical decisions and treatment. Attention should be addressed to GI problems in patients with known ADHD, including a careful medical history focused on GI-related morbidity, so as not to miss some of the common GI problems. The presence of ADHD in a patient with GI symptoms, normal laboratory results and no red flags may by itself support the diagnosis of a functional GI disorder. Since FGIDs are now considered disorders of gut-brain interaction and centrally acting neuromodulators are amongst the mainstays of refractory FGIDs, these drugs may be considered in treating patients suffering from both FGID and ADHD.
ADHD is associated with FGID and a high need for GI-related health services. This study emphasizes the complex interaction between mind and body. Further research is needed to explore the possible combination of treatment of FGID with the neuropsychological therapeutic modalities for ADHD, and to determine if the presence of ADHD can assist in the diagnosis of FGID.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: The Israeli Society of Gastroenterology and Liver Disease.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Israel
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Vasant DH S-Editor: Liu M L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Fayyad J, De Graaf R, Kessler R, Alonso J, Angermeyer M, Demyttenaere K, De Girolamo G, Haro JM, Karam EG, Lara C, Lépine JP, Ormel J, Posada-Villa J, Zaslavsky AM, Jin R. Cross-national prevalence and correlates of adult attention-deficit hyperactivity disorder. Br J Psychiatry. 2007;190:402-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 888] [Cited by in RCA: 842] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 2. | Barkley RA, Fischer M, Smallish L, Fletcher K. The persistence of attention-deficit/hyperactivity disorder into young adulthood as a function of reporting source and definition of disorder. J Abnorm Psychol. 2002;111:279-289. [PubMed] |
| 3. | Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, Demler O, Faraone SV, Greenhill LL, Howes MJ, Secnik K, Spencer T, Ustun TB, Walters EE, Zaslavsky AM. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163:716-723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2318] [Cited by in RCA: 2088] [Article Influence: 109.9] [Reference Citation Analysis (0)] |
| 4. | van Emmerik-van Oortmerssen K, van de Glind G, van den Brink W, Smit F, Crunelle CL, Swets M, Schoevers RA. Prevalence of attention-deficit hyperactivity disorder in substance use disorder patients: a meta-analysis and meta-regression analysis. Drug Alcohol Depend. 2012;122:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 301] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 5. | Green M, Wong M, Atkins D, Taylor J, Feinleib M. Diagnosis of Attention-Deficit/Hyperactivity Disorder. Rockville, MD: Agency for Health Care Policy and Research. 1999. [PubMed] |
| 6. | Larson K, Russ SA, Kahn RS, Halfon N. Patterns of comorbidity, functioning, and service use for US children with ADHD, 2007. Pediatrics. 2011;127:462-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 357] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 7. | Biederman J, Newcorn J, Sprich S. Comorbidity of attention deficit hyperactivity disorder with conduct, depressive, anxiety, and other disorders. Am J Psychiatry. 1991;148:564-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1127] [Cited by in RCA: 948] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 8. | Levin RL, Rawana JS. Attention-deficit/hyperactivity disorder and eating disorders across the lifespan: A systematic review of the literature. Clin Psychol Rev. 2016;50:22-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Piñeiro-Dieguez B, Balanzá-Martínez V, García-García P, Soler-López B; CAT Study Group. Psychiatric Comorbidity at the Time of Diagnosis in Adults With ADHD: The CAT Study. J Atten Disord. 2016;20:1066-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 10. | Adesman AR, Altshuler LA, Lipkin PH, Walco GA. Otitis media in children with learning disabilities and in children with attention deficit disorder with hyperactivity. Pediatrics. 1990;85:442-446. [PubMed] |
| 11. | Brawley A, Silverman B, Kearney S, Guanzon D, Owens M, Bennett H, Schneider A. Allergic rhinitis in children with attention-deficit/hyperactivity disorder. Ann Allergy Asthma Immunol. 2004;92:663-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | van den Heuvel E, Starreveld JS, de Ru M, Krauwer V, Versteegh FG. Somatic and psychiatric co-morbidity in children with attention deficit hyperactivity disorder. Acta Paediatr. 2007;96:454-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Burgu B, Aydogdu O, Gurkan K, Uslu R, Soygur T. Lower urinary tract conditions in children with attention deficit hyperactivity disorder: correlation of symptoms based on validated scoring systems. J Urol. 2011;185:663-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Instanes JT, Klungsøyr K, Halmøy A, Fasmer OB, Haavik J. Adult ADHD and Comorbid Somatic Disease: A Systematic Literature Review. J Atten Disord. 2018;22:203-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 139] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 15. | Johnston BD, Wright JA. Attentional dysfunction in children with encopresis. J Dev Behav Pediatr. 1993;14:381-385. [PubMed] |
| 16. | McKeown C, Hisle-Gorman E, Eide M, Gorman GH, Nylund CM. Association of constipation and fecal incontinence with attention-deficit/hyperactivity disorder. Pediatrics. 2013;132:e1210-e1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 17. | Kaplan BJ, McNicol J, Conte RA, Moghadam HK. Physical signs and symptoms in preschool-age hyperactive and normal children. J Dev Behav Pediatr. 1987;8:305-310. [PubMed] |
| 18. | Hubel R, Jass J, Marcus A, Laessle RG. Overweight and basal metabolic rate in boys with attention-deficit/hyperactivity disorder. Eat Weight Disord. 2006;11:139-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Waring ME, Lapane KL. Overweight in children and adolescents in relation to attention-deficit/hyperactivity disorder: results from a national sample. Pediatrics. 2008;122:e1-e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 204] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 20. | Jameson ND, Sheppard BK, Lateef TM, Vande Voort JL, He JP, Merikangas KR. Medical Comorbidity of Attention-Deficit/Hyperactivity Disorder in US Adolescents. J Child Neurol. 2016;31:1282-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Güngör S, CeliloÄŸlu OS, Ozcan OO, Raif SG, SelimoÄŸlu MA. Frequency of celiac disease in attention-deficit/hyperactivity disorder. J Pediatr Gastroenterol Nutr. 2013;56:211-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Almog M, Gabis LV, Shefer S, Bujanover Y. [Gastrointestinal symptoms in pediatric patients with attention deficit and hyperactivity disorders]. Harefuah. 2010;149:33-36, 62. [PubMed] |
| 23. | Schieve LA, Gonzalez V, Boulet SL, Visser SN, Rice CE, Van Naarden Braun K, Boyle CA. Concurrent medical conditions and health care use and needs among children with learning and behavioral developmental disabilities, National Health Interview Survey, 2006-2010. Res Dev Disabil. 2012;33:467-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 187] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 24. | Sobanski E, Brüggemann D, Alm B, Kern S, Deschner M, Schubert T, Philipsen A, Rietschel M. Psychiatric comorbidity and functional impairment in a clinically referred sample of adults with attention-deficit/hyperactivity disorder (ADHD). Eur Arch Psychiatry Clin Neurosci. 2007;257:371-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 246] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 25. | Hodgkins P, Montejano L, Sasané R, Huse D. Cost of illness and comorbidities in adults diagnosed with attention-deficit/hyperactivity disorder: a retrospective analysis. Prim Care Companion CNS Disord 2011; 13: PCC. 10m01030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Duel BP, Steinberg-Epstein R, Hill M, Lerner M. A survey of voiding dysfunction in children with attention deficit-hyperactivity disorder. J Urol. 2003;170:1521-3; discussion 1523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 81] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 27. | Katzman MA, Bilkey TS, Chokka PR, Fallu A, Klassen LJ. Adult ADHD and comorbid disorders: clinical implications of a dimensional approach. BMC Psychiatry. 2017;17:302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 352] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 28. | Mathee K, Cickovski T, Deoraj A, Stollstorff M, Narasimhan G. The gut microbiome and neuropsychiatric disorders: implications for attention deficit hyperactivity disorder (ADHD). J Med Microbiol. 2020;69:14-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 29. | Dam SA, Mostert JC, Szopinska-Tokov JW, Bloemendaal M, Amato M, Arias-Vasquez A. The Role of the Gut-Brain Axis in Attention-Deficit/Hyperactivity Disorder. Gastroenterol Clin North Am. 2019;48:407-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 30. | Ben-Or O, Zelnik N, Shaoul R, Pacht A, Lerner A. The neurologic profile of children and adolescents with inflammatory bowel disease. J Child Neurol. 2015;30:551-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Butwicka A, Lichtenstein P, Frisén L, Almqvist C, Larsson H, Ludvigsson JF. Celiac Disease Is Associated with Childhood Psychiatric Disorders: A Population-Based Study. J Pediatr 2017; 184: 87-93. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 32. | Diaconu G, Burlea M, Grigore I, Anton DT, Trandafir LM. Celiac disease with neurologic manifestations in children. Rev Med Chir Soc Med Nat Iasi. 2013;117:88-94. [PubMed] |
| 33. | Zelnik N, Pacht A, Obeid R, Lerner A. Range of neurologic disorders in patients with celiac disease. Pediatrics. 2004;113:1672-1676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 173] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 34. | Lahat E, Broide E, Leshem M, Evans S, Scapa E. Prevalence of celiac antibodies in children with neurologic disorders. Pediatr Neurol. 2000;22:393-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Ertürk E, Wouters S, Imeraj L, Lampo A. Association of ADHD and Celiac Disease: What Is the Evidence? J Atten Disord. 2020;24:1371-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 36. | Akmatov MK, Ermakova T, Bätzing J. Psychiatric and Nonpsychiatric Comorbidities Among Children With ADHD: An Exploratory Analysis of Nationwide Claims Data in Germany. J Atten Disord. 2019;Jul 31:1087054719865779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |