Published online Nov 14, 2020. doi: 10.3748/wjg.v26.i42.6599
Peer-review started: June 26, 2020
First decision: August 22, 2020
Revised: August 27, 2020
Accepted: September 23, 2020
Article in press: September 23, 2020
Published online: November 14, 2020
Processing time: 139 Days and 22.8 Hours
The Hippo signaling pathway regulates organ size by regulating cell proliferation and apoptosis with terminal effectors including Yes-associated protein-1 (YAP-1). Dysregulation in Hippo pathway has been proposed as one of the therapeutic targets in hepatocarcinogenesis. The levels of reactive oxygen species (ROS) increase during the progression from early to advanced hepatocellular carcinoma (HCC).
To study the activation of YAP-1 by ROS-induced damage in HCC and the involved signaling pathway.
The expression of YAP-1 in HCC cells (Huh-7, HepG2, and SNU-761) was quantified using real-time polymerase chain reaction and immunoblotting. Human HCC cells were treated with H2O2, which is a major component of ROS in living organisms, and with either YAP-1 small interfering RNA (siRNA) or control siRNA. To investigate the role of YAP-1 in HCC cells under oxidative stress, MTS assays were performed. Immunoblotting was performed to evaluate the signaling pathway responsible for the activation of YAP-1. Eighty-eight surgically resected frozen HCC tissue samples and 88 nontumor liver tissue samples were used for gene expression analyses.
H2O2 treatment increased the mRNA and protein expression of YAP-1 in HCC cells (Huh-7, HepG2, and SNU-761). Suppression of YAP-1 using siRNA transfection resulted in a significant decrease in tumor proliferation during H2O2 treatment both in vitro and in vivo (both P < 0.05). The oncogenic action of YAP-1 occurred via the activation of the c-Myc pathway, leading to the upregulation of components of the unfolded protein response (UPR), including 78-kDa glucose-regulated protein and activating transcription factor-6 (ATF-6). The YAP-1 mRNA levels in human HCC tissues were upregulated by 2.6-fold compared with those in nontumor tissues (P < 0.05) and were positively correlated with the ATF-6 Levels (Pearson’s coefficient = 0.299; P < 0.05).
This study shows a novel connection between YAP-1 and the UPR through the c-Myc pathway during oxidative stress in HCC. The ROS-induced activation of YAP-1 via the c-Myc pathway, which leads to the activation of the UPR pathway, might be a therapeutic target in HCC.
Core Tip: We found a novel connection between Yes-associated protein-1 (YAP-1) and the unfolded protein response (UPR) through the c-Myc pathway during oxidative stress in hepatocellular carcinoma (HCC). As the Hippo pathway and c-Myc pathway share many important functions, including the regulation of growth, death and survival in cells and the regulation of stress resistance and life spans in organisms, we speculate that the interaction between YAP-1 and c-Myc is a point of convergence that allows HCC proliferation. The reactive oxygen species-induced activation of YAP-1 via the c-Myc pathway, which leads to the activation of the UPR pathway, might be a therapeutic target in HCC.
- Citation: Cho Y, Park MJ, Kim K, Kim SW, Kim W, Oh S, Lee JH. Reactive oxygen species-induced activation of Yes-associated protein-1 through the c-Myc pathway is a therapeutic target in hepatocellular carcinoma. World J Gastroenterol 2020; 26(42): 6599-6613
- URL: https://www.wjgnet.com/1007-9327/full/v26/i42/6599.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i42.6599
Reactive oxygen species (ROS), such as H2O2, superoxide radicals, and hydroxyl radicals, contribute to tumor progression by enhancing DNA damage and altering cell signaling pathways[1,2]. It has been recently suggested that ROS are involved in tumor metastasis, which is a complex process that includes angiogenesis, epithelial-to-mesenchymal transition, invasion, and migration within the tumor microenvironment[3]. ROS also control the expression of matrix metalloproteinases and mitogen-activated protein kinases (MAPKs), the activation of the Ras pathway, and the downregulation of E-cadherin expression[4].
Hepatocellular carcinoma (HCC) is one of the common fatal malignancies which results in approximately one million worldwide deaths every year[5]. Oxidative stress is known to be the most important factor of HCC development[6,7]. The major etiologies of HCC, including chronic hepatitis B or C, alcohol-related liver disease, and nonalcoholic fatty liver disease, increase ROS levels[8,9]. ROS levels are also positively correlated with HCC progression[10,11].
The Hippo signaling pathway regulates organ size by regulating both cell proliferation and apoptosis with terminal effectors such as yes-associated protein (YAP)[12,13]. The key components of the Hippo pathway include sterile 20-like kinases (Mst1 and Mst2; homologues of D. hippo), large tumor suppressors (Lats1 and Lats2; homologues of warts), YAP, its paralog protein transcriptional coactivator with PDZ-binding motif (TAZ), transcriptional coactivators, and homologues of yorkie. Inactivation of the Hippo pathway leads to uncontrolled cell proliferation in epithelial cells and stem cells[14,15] and oncogenic transformation[16], both of which are mediated by the upregulation of YAP. Dysregulation of the Hippo pathway has been proposed as one of the therapeutic targets in hepatocarcinogenesis[17-19]. A previous study showed that YAP is an independent predictive marker for the overall survival and disease-free survival of HCC patients and that it is associated with tumor differentiation[20]. The Hippo pathway, which regulates tumorigenesis, also has an important role in mediating oxidative stress[21]. Shao et al[13] suggested the involvement of YAP in causing cardiomyocyte survival during oxidative stress[13].
Thus, the activation of YAP-1 by ROS-induced damage has been hypothesized to exacerbate the progression of HCC, but it remains unclear which signaling pathway is involved. Here, we investigated ROS-induced YAP-1 activation in HCC and the associated signaling pathway.
Human HCC cell lines including Huh-7 and HepG2, which are well-differentiated HCC cell lines, and SNU-761, which is a poorly differentiated HCC cell line were used in this study. We used Dulbecco’s modified Eagle medium (DMEM; Huh-7 and HepG2) or in RPMI 1640 (SNU-761) supplemented with 10% fetal bovine serum (FBS), 100000 U/L penicillin, and 100 mg/L streptomycin, with or without 100 nmol/L insulin for cell culture.
HCC cell proliferation was measured with the Cell Titer 96 Aqueous One Solution cell proliferation assay (Promega, Madison, WI, United States), on the basis of the cellular conversion of the colorimetric reagent3, 4-(5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazoliumsalt (MTS) into soluble formazan by the dehydrogenase enzyme found in metabolically proliferating cells. Following each treatment, 20 μL of the dye solution was added to each well of a 96-wellplate and incubated for 2 h. Then, the absorbance was recorded at a wavelength of 490 nm using an enzyme-linked immunosorbent assay plate reader (Molecular Devices, Sunnyvale, CA, United States).
Cells were seeded in a 6-well culture plate (2 × 105 cells per well) in 2 mL antibiotic-free medium supplemented with 10% FBS. Once the cells reached 60%-80% confluence, they were transfected with small interfering RNA (siRNA) using the siRNA Transfection Reagent (Santa Cruz Biotechnology Inc., Santa Cruz, CA, United States) according to the manufacturer’s instructions. The cells were treated with siRNA for 6 h at 37 °C, and then, growth medium containing 20% FBS and antibiotics was added. After 18 h, the medium was replaced with fresh medium containing 10% FBS and antibiotics. Twenty-four hours after transfection, the cells were used in further experiments.
Briefly, H2O2 (100 μmol/L)-treated MH134 cells (5 × 107 cells per mouse) were subcutaneously transplanted into the flanks of C3H mice in the control group (n = 10). The tumor volume was measured using a Vernier caliper and calculated as [length × (width)2]/2. YAP-1 siRNA transfected MH134 cells were subcutaneously implanted on the flank of mice in YAP siRNA group, and control siRNA transfected MH134 cells were implanted in control siRNA group. The maximal diameter of each nodule was measured every day for 13 d.
The cells were lysed for 20 min on ice with lysis buffer and centrifuged at 14000 g for 10 min at 4 °C. The samples were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes, blotted with the appropriate primary antibodies at a dilution of 1:1000, and treated with peroxidase-conjugated secondary antibodies (Biosource International, Camarillo, CA, United States). The bound antibodies were visualized using a chemiluminescent substrate (ECL; Amersham, Arlington Heights, IL, United States) and exposed to Kodak X-OMAT film (Kodak, New Haven, CT, United States). The primary antibodies, including rabbit anti-phospho-p42/44 MAPK, anti-phosphorylated-Akt, and rabbit anti-c-Myc, were purchased from Cell Signaling Technology (Danvers, MA, United States). The goat anti-β-actin antibody was purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, United States). The densitometric analyses were performed with Image J software (National Institutes of Health, Bethesda, MD, United States).
The total ribonucleic acids (RNAs) were extracted from Huh-7, HepG2, and SNU-761 cells using TRIzol Reagent (Invitrogen, Carlsbad, CA, United States). The complementary deoxyribonucleic acid (cDNA) templates were prepared using oligo (dT) random primers and Moloney Murine Leukemia Virus (MoMLV) reverse transcriptase. After the reverse transcription reaction, the cDNA template was amplified by polymerase chain reaction (PCR) using Taq polymerase (Invitrogen). YAP-1 mRNA expression was quantified by real-time PCR (Light Cycler; Roche Molecular Biochemicals, Mannheim, Germany) using SYBR green as the fluorophore (Molecular Probes, Eugene, OR, United States). The primers for YAP-1 were as follows: Forward: 5′-TGAACAAACGTCCAGCAAGATAC-3′; and reverse: 5′-CAGCCCCCAAAATGAACAGTAG-3′. The primers for c-Myc were as follows: Forward: 5′- CCCGCTTCTCTGAAAGGCTCTC-3′; and reverse: 5′- CTCTGCTGCTGCTGCTGCTGGTAG-3′. For the unfolded protein response (UPR) markers, the following primers were used: Glucose-regulated protein 78 (GRP78), forward: 5′-GACGGGCAAAGATGTCAGGAA-3′ and reverse: 5′-TCATAGTAGACCGGAACAGATCCA-3′; XBP1, forward: 5′-TTGTCACCCCTCCAGAACATC-3′ and reverse: 5′-TCCAGAATGCCCAACAGGAT-3′; activating transcription factor-6 (ATF-6), forward: 5′-TTGGCATTTATAATACTGAACTATGGA-3′ and reverse: 5′-TTTGATTTGCAGGGCTCAC-3′. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene expression was used as a control. The level of YAP-1 mRNA expression was calculated as the relative intensity of the PCR product band compared with that of the GAPDH gene using the 2–ΔΔCt method. All the PCR experiments were performed in triplicate.
The statistical analyses were performed using PASW version 21.0 (SPSS Inc., Chicago, IL, United States). All the experimental results were obtained from three independent experiments using cells from three separate isolations and are presented as the mean ± standard deviation (SD). For comparisons between groups, the data were analyzed by the Mann–Whitney U test or one-way ANOVA. For all the tests, P < 0.05 was regarded as statistically significant.
Ethical approval was obtained from the ethics committee at CHA University. We carried out this study in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The in vivo study protocol was approved by the Institutional Animal Care and Use Committee (IACUC-180027) of CHA University. All the in vivo surgical procedures were performed under anesthesia with 2, 2, 2-tribromoethanol, and all efforts were made to minimize suffering.
All the experiments using human tissues were approved by the Bundang CHA Medical Center Institutional Review Board (CHAMC 2018-02-037). All the human tissues were provided by the Bundang CHA Biobank of Bundang CHA Medical Center. For the gene expression analyses, 88 surgically resected frozen HCC tissue samples and 88 nontumor liver tissue samples were analyzed. Cases were prospectively and consecutively identified at Bundang CHA Medical Center between 2012 and 2018.
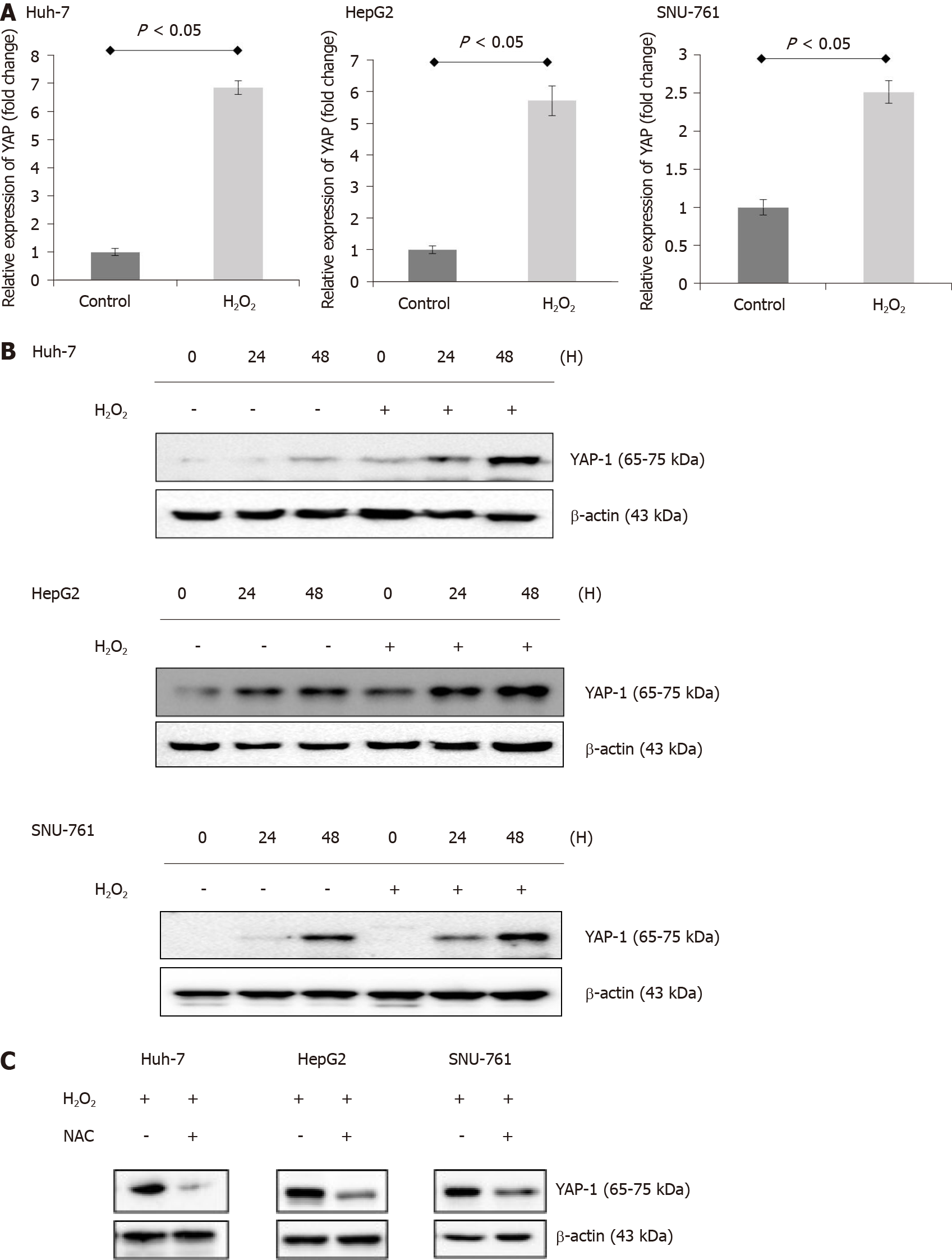
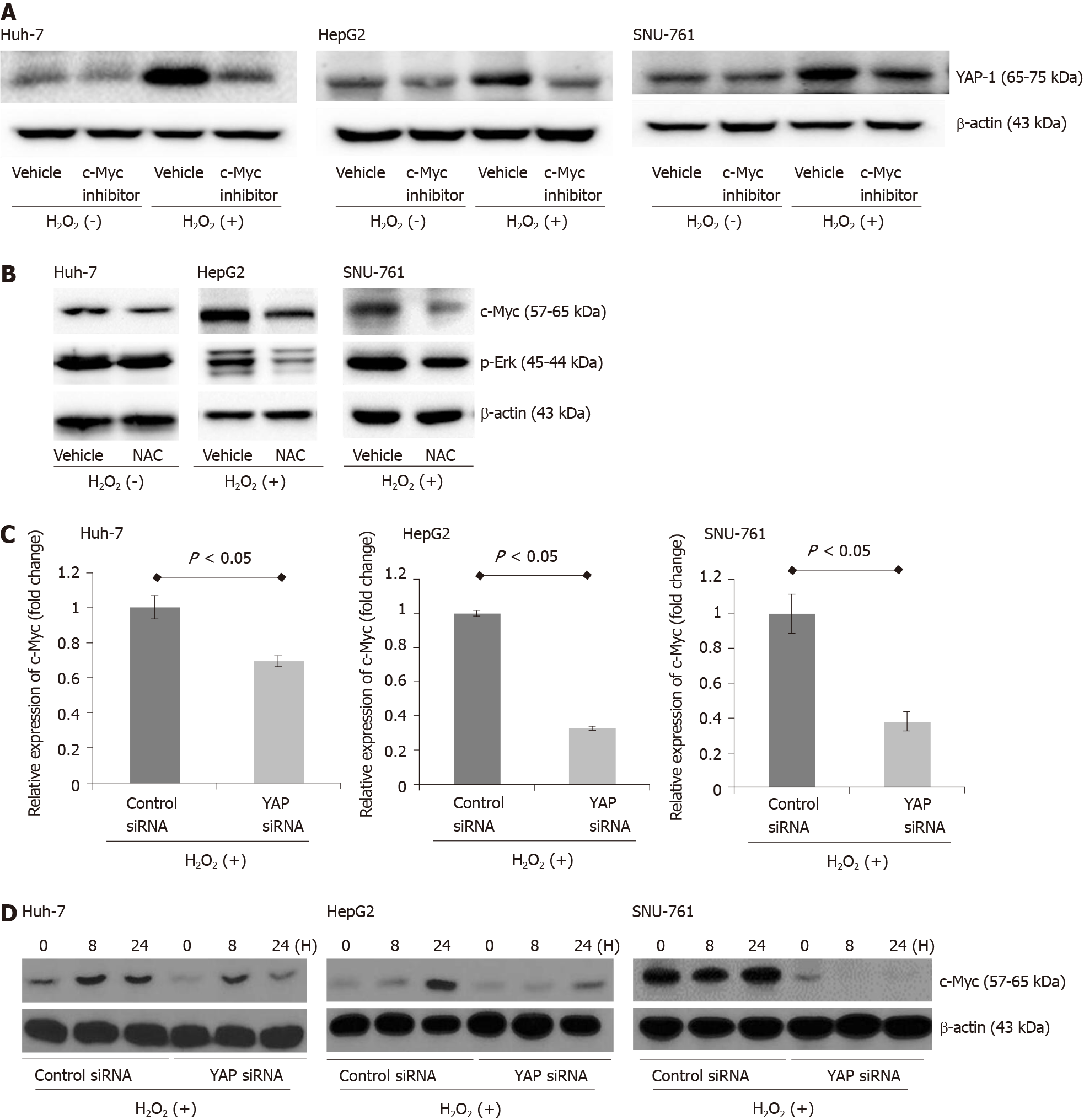
To analyze the potential ROS-induced changes in YAP-1 expression in HCC cells, we treated human HCC cells (Huh-7, HepG2, and SNU-761 cells) with 150 μmol/L H2O2. Real-time PCR and immunoblot analyses indicated that H2O2 treatment increased the mRNA (Figure 1A) and protein (Figure 1B) expression of YAP-1 in the HCC cells. These effects were inhibited following treatment of the cells with the antioxidant N-acetylcysteine (NAC) (Figure 1C). The antioxidant treatment significantly suppressed the protein expressions of YAP-1 in HCC cells.
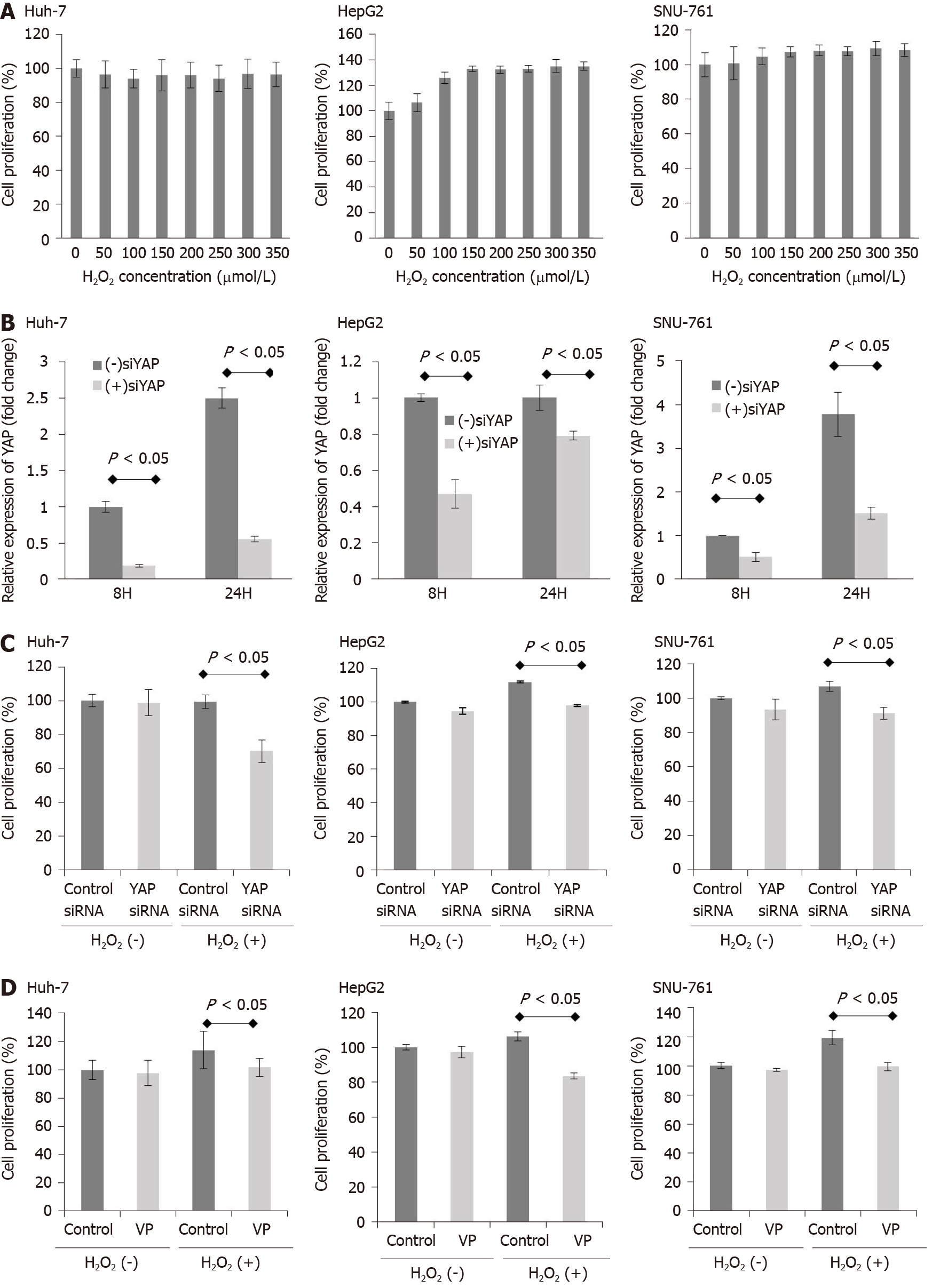
Next, to investigate whether exposure to H2O2 impacts HCC cell survival, HCC cells were treated with H2O2 (0-350 μmol/L), and the ROS levels were increased by intervals of 50 μmol/L. As shown in Figure 2A, exposure to H2O2 (0-350 μmol/L) did not reduce HCC cell survival. Then, we examined the efficacy YAP-1 siRNA transfection with real-time PCR. YAP-1 siRNA transfection significantly suppressed YAP-1 mRNA expression compared to control siRNA transfection in HCC cells (Figure 2B; P < 0.05). Next, we performed an MTS assay to evaluate whether YAP-1 modulates HCC cell proliferation. Suppression of YAP-1 using siRNA transfection or verteporfin treatment (YAP-1 inhibitor) resulted in a significant decrease in tumor proliferation during exposure 150 μmol/L H2O2 in vitro (Figure 2C and D; both P < 0.05).
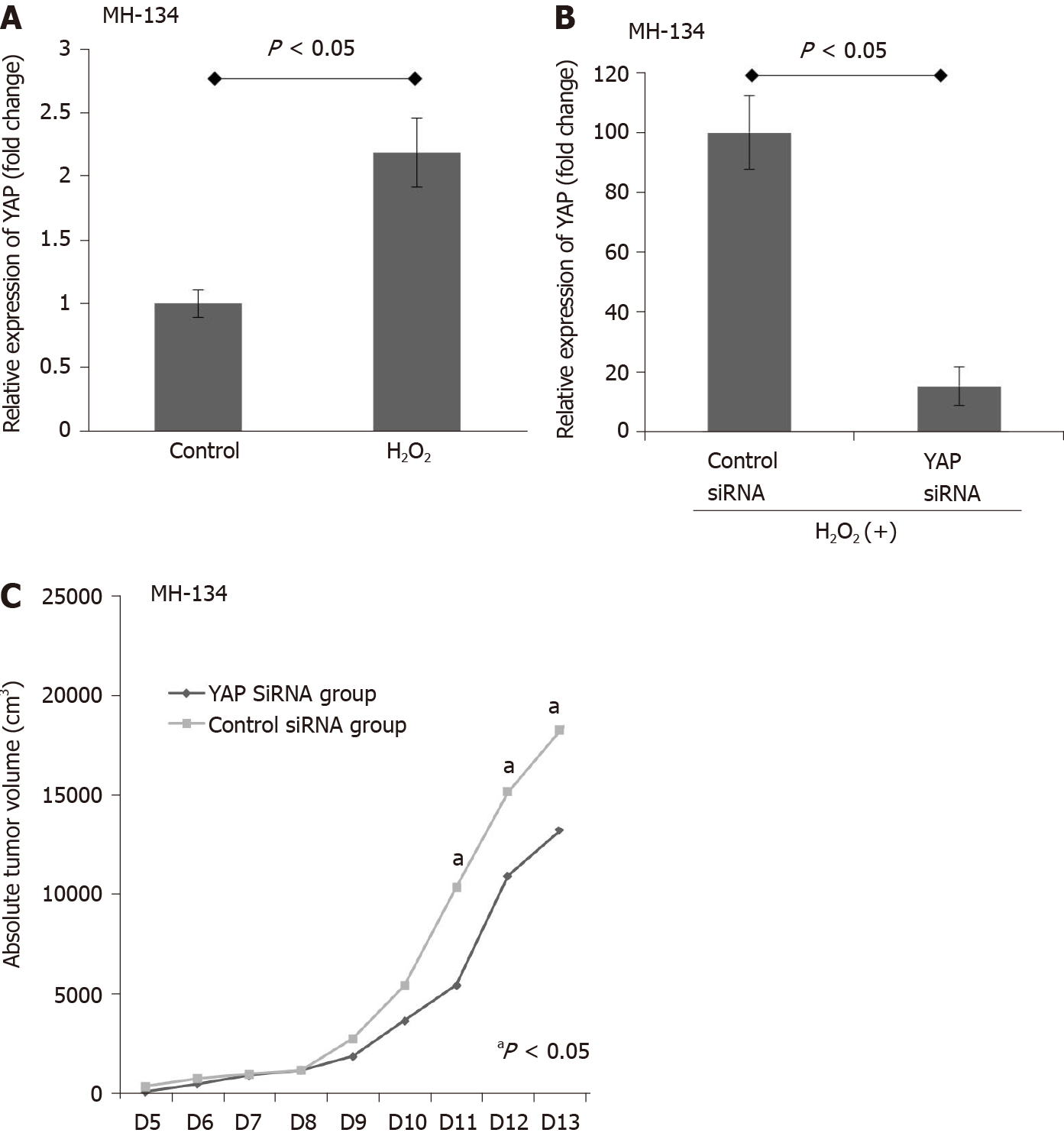
The antitumor effects of YAP-1 siRNA were examined using an in vivo xenograft model. First, we evaluated whether exposure to ROS changes the expression of YAP-1 in the murine HCC cell line MH134. H2O2 treatment significantly increased the proliferation of the MH134 cells (Figure 3A; P < 0.05). We also confirmed that suppression of YAP-1 using siRNA transfection resulted in significantly decreased mRNA expression of YAP-1 in the MH134 cells treated with 150 μmol/L H2O2 (Figure 3B). In the xenograft tumor model, the YAP-1 siRNA group showed significantly suppressed tumor growth compared to the control siRNA group at days 11, 12, and 13 after tumor budding (Figure 3C; all P < 0.05).
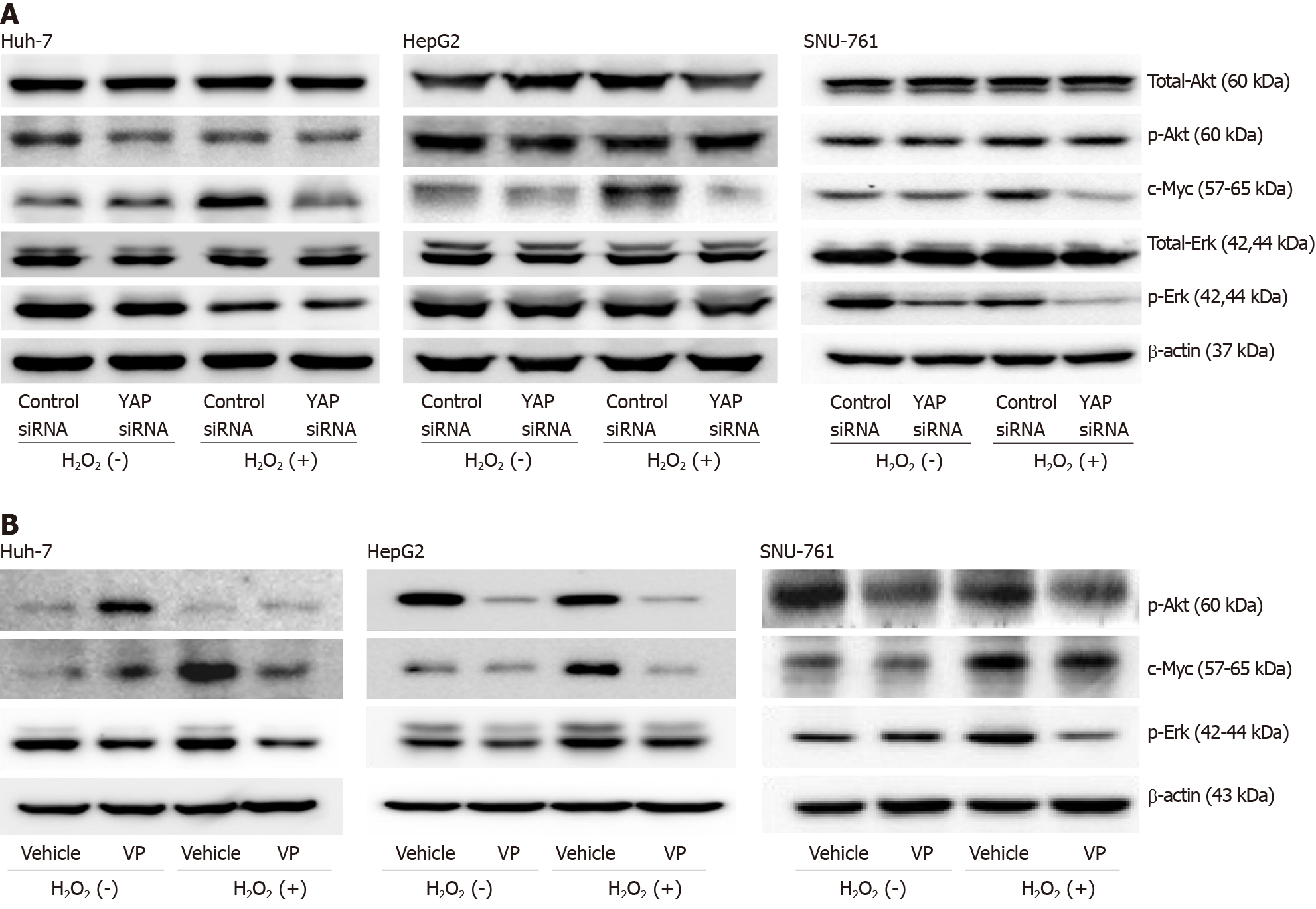
The immunoblot assay results showed that the downregulation of YAP-1 caused by siRNA transfection or verteporfin treatment decreased the protein expression of c-Myc in the ROS-exposed HCC cell lines (Figure 4A and B). When the ROS-exposed HCC cells were treated with a c-Myc inhibitor (10058-F4, 60 μmol/L), the protein expression of YAP-1 was significantly decreased compared with that in the control-treated cells (Figure 5A). Moreover, treatment with the antioxidant NAC downregulated the expression of c-Myc in the ROS-exposed HCC cell lines (Figure 5B). We also performed real-time PCR and immunoblot analyses to evaluate whether up-regulation of the c-Myc pathway was dependent on YAP-1 expressions. YAP-1 siRNA transfection significantly suppressed c-Myc mRNA expression compared to control siRNA transfection in ROS-exposed HCC cells (Figure 5C; all P < 0.05). Immunoblot analyses of c-Myc also revealed that ROS-exposed HCC cells transfected with YAP-1 siRNA showed suppressed protein expression of c-Myc as compared to those transfected with control siRNA (Figure 5D).
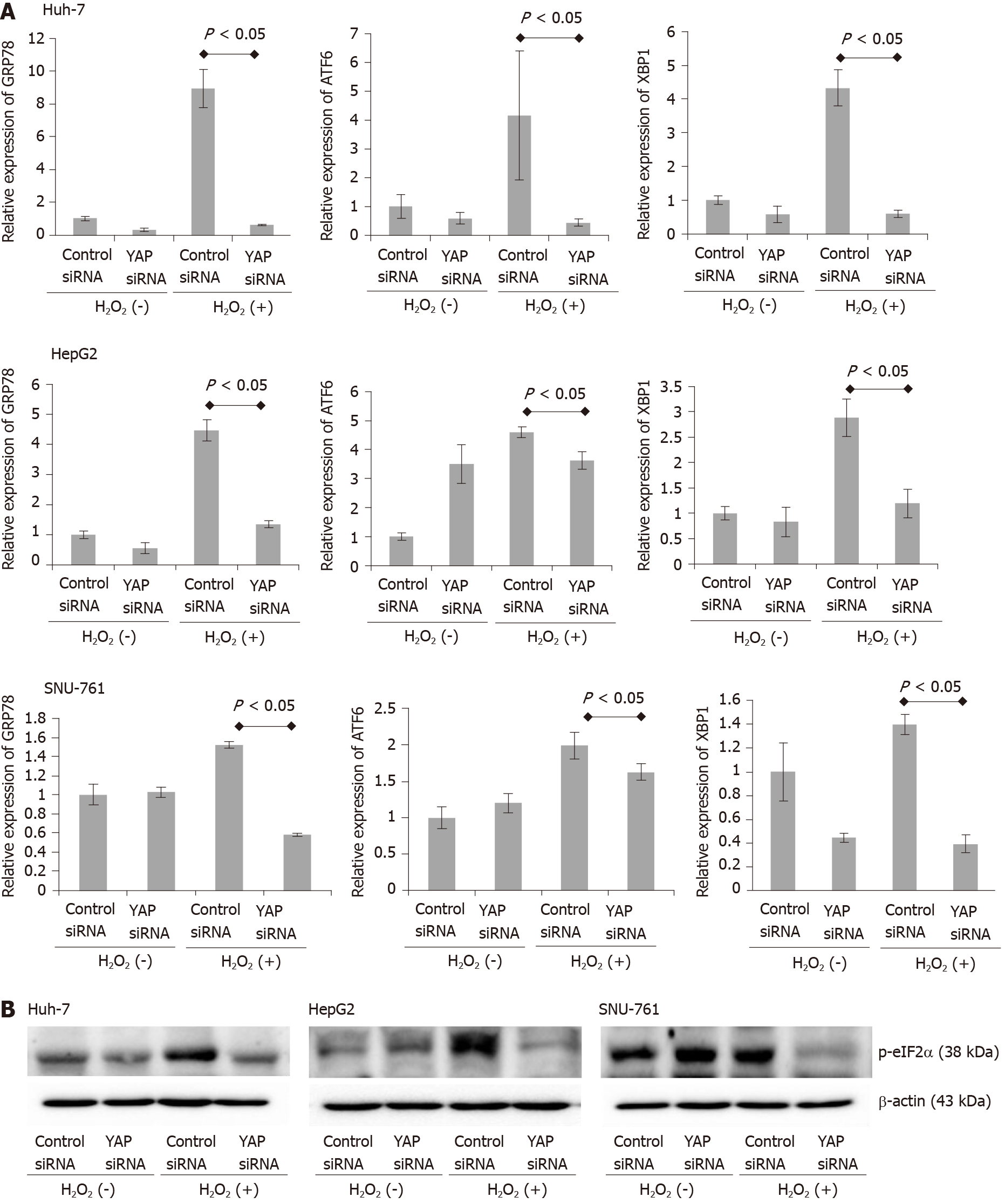
To determine whether the oncogenic action of YAP-1, which occurs via the activation of the c-Myc pathway, leads to the upregulation of components of the UPR, we performed real-time PCR on cells treated with or without H2O2 for 78-kDa GRP78/BiP, ATF-6, and XBP1 (Figure 6A). ROS exposure significantly enhanced the mRNA expression of GRP78, ATF-6, and XBP1 in the HCC cell lines. The downregulation of YAP-1 by siRNA transfection also significantly suppressed the expression of the UPR markers compared to control siRNA transfection. We also performed immunoblot analysis to evaluate the endoplasmic reticulum (ER) stress marker phosphorylated eIF-2α (Figure 6B); the results revealed that the transfection of YAP-1 siRNA attenuated the protein expression of phosphorylated eIF-2α compared to control siRNA transfection.
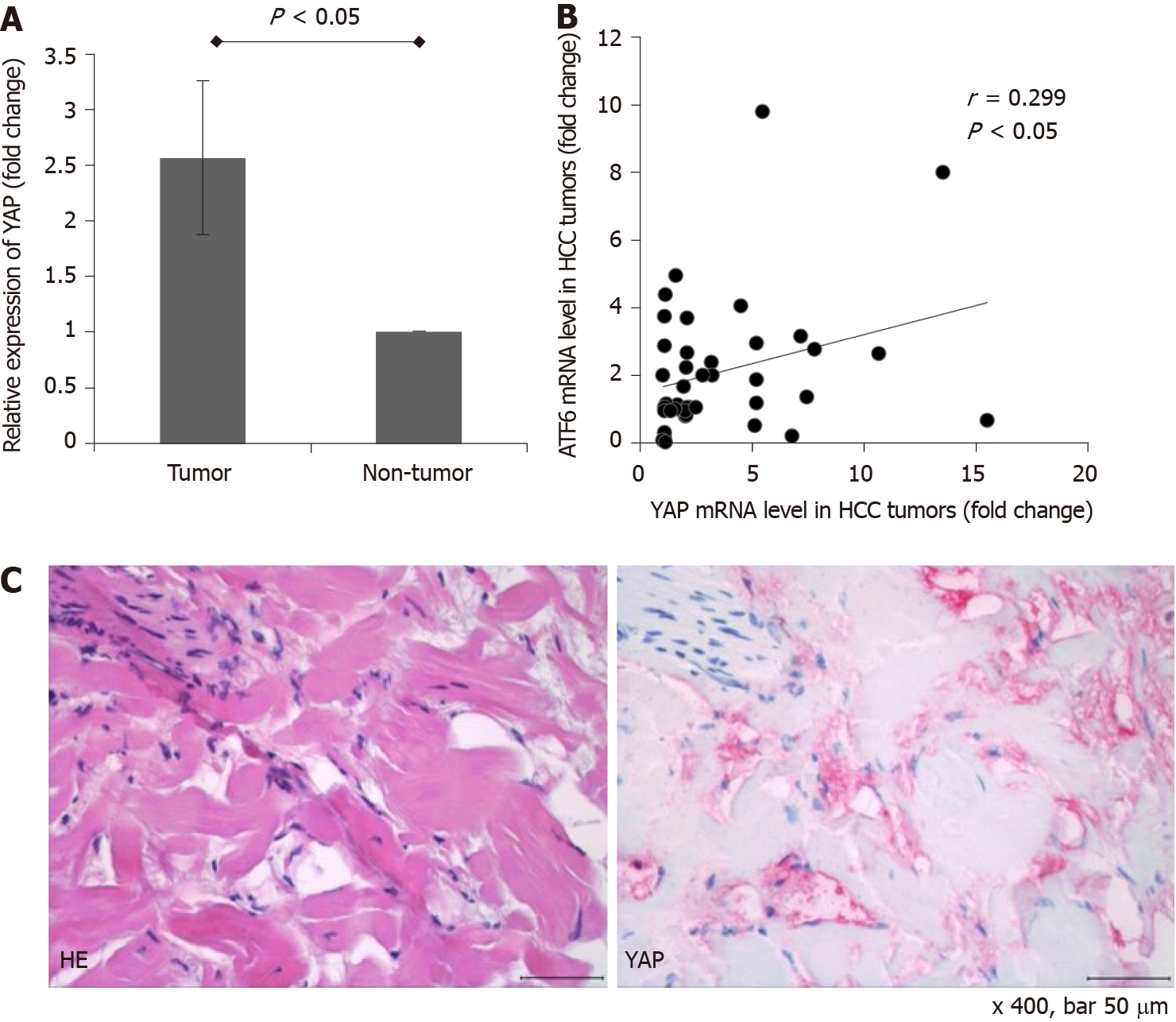
For the gene expression analyses, 88 surgically resected frozen HCC tumor tissue samples and 88 paired nontumor liver tissue samples were evaluated. The majority of the patients (n = 71, 80.7%) had stage I HCC according to the American Joint Commission on Cancer 8th edition HCC staging system. 11 patients (12.5%) and 6 patients (6.8%) had stage II and stage III HCC, respectively. No patient had major vascular invasion or lymph node/distant metastasis. The expression of YAP-1 was further determined in the resected HCC tissues and adjacent nontumor tissues using real-time PCR. The mean mRNA expression of YAP-1 was upregulated by 2.6-fold in the HCC tissues compared with the nontumor tissues (Figure 7A; P < 0.05). Among the 88 HCC tumor tissues, YAP-1 RNA expression was upregulated in 42 samples (47.7%) compared to the nontumor tissues, and YAP-1 expression was positively correlated with ATF-6 expression (Figure 7B; Pearson’s coefficient = 0.299; P < 0.05). For one patient whose YAP-1 expression in HCC tissue was 15.5-fold higher than that in nontumor tissue, we performed immunohistochemical staining for YAP-1 with HCC tissue, which is shown in Figure 7C.
This study revealed that the ROS-induced activation of YAP-1 via the c-Myc pathway, which leads to the activation of the UPR, might be a therapeutic target in HCC. We have elucidated the molecular mechanism by which YAP-1 mediates the survival of HCC cells under oxidative stress.
Carcinogenesis leads to the accumulation of misfolded proteins in the ER[22]. Then, the UPR is activated to restore normal cellular function by degrading the misfolded proteins and activating the production of chaperones, such as GRP78. However, under pathological conditions, prolonged UPR activation can promote apoptosis, leading to cell death. Overall, if ER stress is too severe, the UPR leads to translational arrest and induces specific factors for cell survival or cell death. In several cancers, the expression of UPR components is enhanced, indicating the dependency of these cancers on the UPR[23]. Thus, there is a possibility that modification of the UPR might have anticancer effects.
Hypoxia is one of the major mediators of UPR-inducing pathways. Human fibrosarcoma and lung carcinoma cells upregulated GRP78 expression and XBP1 splicing under hypoxic conditions in vitro[24]. Tumor formation with aberrant microcirculation might lead to hypoxic conditions, which induce the UPR. Gradually, the UPR increases cell survival and tumor proliferation, which thereby increases hypoxia in the core of the tumor. After the sequestration of GRP78 by misfolded proteins, ATF-6, inositol requiring protein 1, and protein kinase RNA-like endoplasmic reticulum kinase (PERK) act as transducers to transmit the ER stress signal to the cytosol and nucleus. Activated ATF-6 translocates to the Golgi, where proteases cleave it and release its fragments into the cytosol[25]. Indeed, enhanced nuclear translocation of the ATF-6 fragment is observed in various cancers, including HCC. In this study, we identified the potential of ATF-6 to act as an effector of HCC under oxidative stress.
The c-Myc pathway undergoes chromosomal translocation and gene amplification in many cancers, including HCC. Activated c-Myc pathway upregulates oncogenes which are involved in ribosome biogenesis. Previous studies reported that elevated protein synthesis due to increased c-Myc expression in cancer cells lead to UPR activation[26,27]. Activation of UPR signaling promotes autophagy in tumor cells under conditions of hypoxia, oxidative stress, and nutrient limitation. Our findings suggest a key link between YAP-1-mediated oncogenic transformation and HCC cell survival via the c-Myc-mediated UPR under oxidative stress.
There are increasing lines of evidence suggesting that the loss-of-function mutations in components of the Hippo pathway and hyperactivation of YAP-1 have been observed in many cancers. Thus, we speculate that the regulating the YAP-1-c-Myc pathway might be a crucial mechanism through which the Hippo pathway regulates hepatocarcinogenesis.
Several multikinase inhibitors that have been approved for advanced HCC, including sorafenib, regorafenib, and lenvatinib, have shown modest survival advantages[28,29]. Recent evidence suggests that long-term treatment of HCC leads to hypoxia-mediated sorafenib resistance in patients with HCC because tumor-driving pathways, including YAP-1, become activated[30-32]. However, the molecular mechanism of sorafenib resistance is unclear. Here, we found that ROS are the primary triggers of YAP-1-c-Myc-UPR signaling hyperactivation during oxidative stress, and this phenomenon is also observed in human HCC tissues.
In conclusion, our study shows a novel connection between YAP-1 and the UPR through the c-Myc pathway during oxidative stress in HCC. As the Hippo pathway and c-Myc pathway share many important functions, including the regulation of growth, death and survival in cells and the regulation of stress resistance and life spans in organisms, we speculate that the interaction between YAP-1 and c-Myc is a point of convergence that allows HCC proliferation. The ROS-induced activation of YAP-1 via the c-Myc pathway, which leads to the activation of the UPR pathway, might be a therapeutic target in HCC.
Reactive oxygen species (ROS) contribute to tumor progression by promoting DNA damage and altering cell signaling pathways. It has been recently suggested that ROS are involved in tumor metastasis, which is a complex process that includes epithelial-to-mesenchymal transition, migration, invasion, and angiogenesis within the tumor microenvironment.
Oxidative stress is the most important causative factor of hepatocellular carcinoma (HCC). The major etiologies of HCC, including chronic hepatitis B or C, alcohol-related liver disease, and nonalcoholic fatty liver disease, increase ROS levels. Thus, the activation of yes-associated protein-1 (YAP-1) by ROS-induced damage has been hypothesized to exacerbate the progression of HCC.
We investigated the activation of YAP-1 by ROS-induced damage in HCC and the involved signaling pathway.
The expression of YAP-1 was quantified using real-time PCR and immunoblotting. Human HCC cells were treated with H2O2, and with either YAP-1 small interfering RNA (siRNA) or control siRNA. MTS assays were performed to evaluate HCC cell proliferation. To investigate the signaling pathway, immunoblotting was performed. Eighty-eight surgically resected frozen HCC tissues and 88 nontumor paired liver tissues were used for gene expression analyses.
H2O2 treatment increased the mRNA and protein expression of YAP-1 in HCC cells. Suppression of YAP-1 resulted in a significant decrease in tumor proliferation during H2O2 treatment both in vitro and in vivo. The oncogenic action of YAP-1 occurred via the activation of the c-Myc pathway, leading to the upregulation of components of the unfolded protein response, including 78-kDa glucose-regulated protein and activating transcription factor-6 (ATF-6). The YAP-1 mRNA levels in human HCC tissues were upregulated by 2.6-fold compared with those in nontumor tissues and were positively correlated with the ATF-6 Levels.
This study shows a novel connection between YAP-1 and the unfolded protein response (UPR) through the c-Myc pathway during oxidative stress in HCC. We speculate that the interaction between YAP-1 and c-Myc is a point of convergence that allows HCC proliferation.
The ROS-induced activation of YAP-1 via the c-Myc pathway, which leads to the activation of the UPR pathway, might be a therapeutic target in HCC.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Uhlmann D, Yao D S-Editor: Yan JP L-Editor: A P-Editor: Liu JH
| 1. | Gupta A, Butts B, Kwei KA, Dvorakova K, Stratton SP, Briehl MM, Bowden GT. Attenuation of catalase activity in the malignant phenotype plays a functional role in an in vitro model for tumor progression. Cancer Lett. 2001;173:115-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Liu YN, Lee WW, Wang CY, Chao TH, Chen Y, Chen JH. Regulatory mechanisms controlling human E-cadherin gene expression. Oncogene. 2005;24:8277-8290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 156] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 3. | Wu WS. The signaling mechanism of ROS in tumor progression. Cancer Metastasis Rev. 2006;25:695-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 565] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 4. | Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, Leake D, Godden EL, Albertson DG, Nieto MA, Werb Z, Bissell MJ. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436:123-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 940] [Cited by in RCA: 989] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 5. | Hanouneh IA, Alkhouri N, Singal AG. Hepatocellular carcinoma surveillance in the 21st century: Saving lives or causing harm? Clin Mol Hepatol. 2019;25:264-269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 6. | Sasaki Y. Does oxidative stress participate in the development of hepatocellular carcinoma? J Gastroenterol. 2006;41:1135-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Lee JS. The mutational landscape of hepatocellular carcinoma. Clin Mol Hepatol. 2015;21:220-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 8. | El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557-2576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3846] [Cited by in RCA: 4260] [Article Influence: 236.7] [Reference Citation Analysis (2)] |
| 9. | Cichoż-Lach H, Michalak A. Oxidative stress as a crucial factor in liver diseases. World J Gastroenterol. 2014;20:8082-8091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 910] [Cited by in RCA: 787] [Article Influence: 71.5] [Reference Citation Analysis (8)] |
| 10. | Lim SO, Gu JM, Kim MS, Kim HS, Park YN, Park CK, Cho JW, Park YM, Jung G. Epigenetic changes induced by reactive oxygen species in hepatocellular carcinoma: methylation of the E-cadherin promoter. Gastroenterology 2008; 135: 2128-2140, 2140.e1-2140. e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 284] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 11. | Ko E, Seo HW, Jung G. Telomere length and reactive oxygen species levels are positively associated with a high risk of mortality and recurrence in hepatocellular carcinoma. Hepatology. 2018;67:1378-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 12. | Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, Gayyed MF, Anders RA, Maitra A, Pan D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120-1133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2037] [Cited by in RCA: 1921] [Article Influence: 106.7] [Reference Citation Analysis (0)] |
| 13. | Shao D, Zhai P, Del Re DP, Sciarretta S, Yabuta N, Nojima H, Lim DS, Pan D, Sadoshima J. A functional interaction between Hippo-YAP signalling and FoxO1 mediates the oxidative stress response. Nat Commun. 2014;5:3315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 224] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 14. | Zhou D, Conrad C, Xia F, Park JS, Payer B, Yin Y, Lauwers GY, Thasler W, Lee JT, Avruch J, Bardeesy N. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell. 2009;16:425-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 754] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 15. | Zhou D, Zhang Y, Wu H, Barry E, Yin Y, Lawrence E, Dawson D, Willis JE, Markowitz SD, Camargo FD, Avruch J. Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (Yap) overabundance. Proc Natl Acad Sci USA. 2011;108:E1312-E1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 389] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 16. | Tapon N, Harvey KF, Bell DW, Wahrer DC, Schiripo TA, Haber D, Hariharan IK. salvador Promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell. 2002;110:467-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 656] [Cited by in RCA: 702] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 17. | Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19:491-505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1948] [Cited by in RCA: 1877] [Article Influence: 125.1] [Reference Citation Analysis (0)] |
| 18. | Li H, Wolfe A, Septer S, Edwards G, Zhong X, Abdulkarim AB, Ranganathan S, Apte U. Deregulation of Hippo kinase signalling in human hepatic malignancies. Liver Int. 2012;32:38-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 19. | Perra A, Kowalik MA, Ghiso E, Ledda-Columbano GM, Di Tommaso L, Angioni MM, Raschioni C, Testore E, Roncalli M, Giordano S, Columbano A. YAP activation is an early event and a potential therapeutic target in liver cancer development. J Hepatol. 2014;61:1088-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 184] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 20. | Xu MZ, Yao TJ, Lee NP, Ng IO, Chan YT, Zender L, Lowe SW, Poon RT, Luk JM. Yes-associated protein is an independent prognostic marker in hepatocellular carcinoma. Cancer. 2009;115:4576-4585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 402] [Cited by in RCA: 421] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 21. | Lehtinen MK, Yuan Z, Boag PR, Yang Y, Villén J, Becker EB, DiBacco S, de la Iglesia N, Gygi S, Blackwell TK, Bonni A. A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell. 2006;125:987-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 679] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 22. | Nagelkerke A, Bussink J, Sweep FC, Span PN. The unfolded protein response as a target for cancer therapy. Biochim Biophys Acta. 2014;1846:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Backer MV, Backer JM, Chinnaiyan P. Targeting the unfolded protein response in cancer therapy. Methods Enzymol. 2011;491:37-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Mahadevan NR, Zanetti M. Tumor stress inside out: cell-extrinsic effects of the unfolded protein response in tumor cells modulate the immunological landscape of the tumor microenvironment. J Immunol. 2011;187:4403-4409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Schewe DM, Aguirre-Ghiso JA. ATF6alpha-Rheb-mTOR signaling promotes survival of dormant tumor cells in vivo. Proc Natl Acad Sci USA. 2008;105:10519-10524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 274] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 26. | Dey S, Tameire F, Koumenis C. PERK-ing up autophagy during MYC-induced tumorigenesis. Autophagy. 2013;9:612-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 27. | Hart LS, Cunningham JT, Datta T, Dey S, Tameire F, Lehman SL, Qiu B, Zhang H, Cerniglia G, Bi M, Li Y, Gao Y, Liu H, Li C, Maity A, Thomas-Tikhonenko A, Perl AE, Koong A, Fuchs SY, Diehl JA, Mills IG, Ruggero D, Koumenis C. ER stress-mediated autophagy promotes Myc-dependent transformation and tumor growth. J Clin Invest. 2012;122:4621-4634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 336] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 28. | Greten TF, Lai CW, Li G, Staveley-O'Carroll KF. Targeted and Immune-Based Therapies for Hepatocellular Carcinoma. Gastroenterology. 2019;156:510-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 199] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 29. | Kim TH, Kim SY, Tang A, Lee JM. Comparison of international guidelines for noninvasive diagnosis of hepatocellular carcinoma: 2018 update. Clin Mol Hepatol. 2019;25:245-263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 158] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 30. | Gao J, Rong Y, Huang Y, Shi P, Wang X, Meng X, Dong J, Wu C. Cirrhotic stiffness affects the migration of hepatocellular carcinoma cells and induces sorafenib resistance through YAP. J Cell Physiol. 2019;234:2639-2648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 31. | Zhou TY, Zhuang LH, Hu Y, Zhou YL, Lin WK, Wang DD, Wan ZQ, Chang LL, Chen Y, Ying MD, Chen ZB, Ye S, Lou JS, He QJ, Zhu H, Yang B. Inactivation of hypoxia-induced YAP by statins overcomes hypoxic resistance tosorafenib in hepatocellular carcinoma cells. Sci Rep. 2016;6:30483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 32. | Makol A, Kaur H, Sharma S, Kanthaje S, Kaur R, Chakraborti A. Vimentin as a potential therapeutic target in sorafenib resistant HepG2, a HCC model cell line. Clin Mol Hepatol. 2020;26:45-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |