Published online Oct 28, 2020. doi: 10.3748/wjg.v26.i40.6111
Peer-review started: July 31, 2020
First decision: August 22, 2020
Revised: August 28, 2020
Accepted: September 17, 2020
Article in press: September 17, 2020
Published online: October 28, 2020
Processing time: 89 Days and 4.2 Hours
Portal hypertension and bleeding from gastroesophageal varices is the major cause of morbidity and mortality in patients with cirrhosis. Portal hypertension is initiated by increased intrahepatic vascular resistance and a hyperdynamic circulatory state. The latter is characterized by a high cardiac output, increased total blood volume and splanchnic vasodilatation, resulting in increased mesenteric blood flow. Pharmacological manipulation of cirrhotic portal hypertension targets both the splanchnic and hepatic vascular beds. Drugs such as angiotensin converting enzyme inhibitors and angiotensin II type receptor 1 blockers, which target the components of the classical renin angiotensin system (RAS), are expected to reduce intrahepatic vascular tone by reducing extracellular matrix deposition and vasoactivity of contractile cells and thereby improve portal hypertension. However, these drugs have been shown to produce significant off-target effects such as systemic hypotension and renal failure. Therefore, the current pharmacological mainstay in clinical practice to prevent variceal bleeding and improving patient survival by reducing portal pressure is non-selective -blockers (NSBBs). These NSBBs work by reducing cardiac output and splanchnic vasodilatation but most patients do not achieve an optimal therapeutic response and a significant proportion of patients are unable to tolerate these drugs. Although statins, used alone or in combination with NSBBs, have been shown to improve portal pressure and overall mortality in cirrhotic patients, further randomized clinical trials are warranted involving larger patient populations with clear clinical end points. On the other hand, recent findings from studies that have investigated the potential use of the blockers of the components of the alternate RAS provided compelling evidence that could lead to the development of drugs targeting the splanchnic vascular bed to inhibit splanchnic vasodilatation in portal hypertension. This review outlines the mechanisms related to the pathogenesis of portal hypertension and attempts to provide an update on currently available therapeutic approaches in the management of portal hypertension with special emphasis on how the alternate RAS could be manipulated in our search for development of safe, specific and effective novel therapies to treat portal hypertension in cirrhosis.
Core Tip: Cirrhosis is a major cause of death and affects over 300 million people worldwide. Portal hypertension is a life-threatening complication of cirrhosis, characterized by splanchnic vasodilatation, the formation of varices and variceal bleeding, which account for much of the mortality and morbidity in cirrhotic patients. Current “gold standard” clinical treatment is non-selective -blockers; however, their efficacy and tolerability are suboptimal. Recent findings from the author’s laboratory and others suggest that the alternate renin angiotensin system is a potential target to design and develop novel therapeutics to inhibit splanchnic vasodilatation in cirrhotic portal hypertension.
- Citation: Gunarathne LS, Rajapaksha H, Shackel N, Angus PW, Herath CB. Cirrhotic portal hypertension: From pathophysiology to novel therapeutics. World J Gastroenterol 2020; 26(40): 6111-6140
- URL: https://www.wjgnet.com/1007-9327/full/v26/i40/6111.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i40.6111
Cirrhosis and its complications are responsible for a large number of deaths worldwide annually. The Global Burden of Disease study in 2017 reported over 1.32 million cirrhosis-related deaths globally, which was approximately 2.4% of all deaths worldwide[1]. Almost 90% of the patients with cirrhosis eventually develop portal hypertension and this condition is a prequel to the majority of deaths in cirrhotic patients[2]. Portal hypertension can also develop in the absence of liver cirrhosis and this is termed non-cirrhotic portal hypertension[3].
The majority of complications that occur in patients with decompensated cirrhosis including the development of gastro-esophageal varices, ascites, spontaneous bacterial peritonitis (SBP), hepato-renal syndrome (HRS), hepato-pulmonary syndrome (HPS), hyper-splenism, and even hepato-cellular carcinoma, are related to the evolution of portal hypertension[4-7]. Discussion on these complications other than portal hypertension and variceal bleeding is beyond the scope of this review and the reader is referred to excellent reviews published elsewhere[8-12]. Gastroesophageal varices are the most clinically significant outcome, contributing to 25%-50% of mortality in patients because of their tendency for rupture and haemorrhage[2,13,14].
During the past few decades, many advances have been made in the understanding of the pathophysiology of portal hypertension in cirrhosis. Initially it was suggested that portal hypertension is simply a consequence of the distortion of normal tissue architecture and function in the cirrhotic liver[15,16]. However, it was subsequently shown that increased portal inflow caused by splanchnic vasodilatation also plays an important role[15,17,18].
These advances in understanding have led to the development of new therapeutic strategies. However, these new treatment options, including the use of non-selective -blockers (NSBBs), are not effective in all patients highlighting the importance of developing novel therapies that can precisely target portal hypertension in cirrhosis. Recent research suggests that the renin angiotensin system (RAS) is an important physiological system that contributes to portal hypertension and it is therefore a potential target for future pharmacotherapies[19-21]. Supporting this, many studies have shown that drugs which modulate the RAS reduce portal pressure in cirrhotic animal models and human patients with portal hypertension[22-26].
This review outlines the pathophysiological mechanisms related to the development of portal hypertension in cirrhosis and current therapeutic approaches in the management of this condition. Based on the evidence from recent advances in RAS research, in particular work on the role of the “alternate axis” of the RAS, in this review we specifically emphasize the contribution of the RAS to the pathogenesis of cirrhotic portal hypertension and how this system may be manipulated to treat this condition.
Changes in the pressure along a blood vessel (ΔP) result from the interaction of blood flow (Q) and the resistance (R) opposed to that flow. Accordingly, changes in the vascular resistance and/or blood flow will translate into the changes in the pressure. The same physical properties can be used to determine the factors contributing to portal hypertension. In the portal circulation, Q represents the portal venous inflow and R represents the portal vascular resistance opposed to that flow. The development of cirrhotic portal hypertension is a cumulative effect of increased portal venous resistance to the incoming venous flow due to anatomical and functional changes in the intrahepatic circulation and increased portal venous flow as a result of splanchnic vasodilatation and increased cardiac output[27,28].
Increased hepatic vascular resistance in the cirrhotic liver is a result of both the fixed obstruction created by hepatic structural changes, and alteration of hepatic vascular tone. Vascular obliteration by the formation of scar tissue and regenerative nodules in the cirrhotic liver during tissue remodelling and scarring contributes to approximately 70% of the increase in hepatic resistance[29,30]. Activated hepatic stellate cells (HSCs) responsible for excessive production of extracellular matrix (ECM) promote scar tissue formation, thereby replacing the functional liver tissue with fibrous matrix[19,31,32]. The remaining 30% increase in hepatic resistance is governed by the contraction of activated HSCs and vascular smooth muscle cells (VSMCs) which is modulated by a range of vasoconstrictors[24,25]. The level of angiotensin II (Ang II), a potent vasoconstrictor peptide of the RAS, is elevated in the cirrhotic liver, augmenting HSC proliferation and sinusoidal vasoconstriction via increased expression of Ang II type 1 receptor (AT1R) on HSCs[33-35]. Herath and colleagues[24] have shown that the upregulated AT1R in the cirrhotic rat liver responded to Ang II with an increased vasoconstrictive response compared to perfused control livers. As liver disease progresses, the activated HSCs augment the production of another potent vasoconstrictor peptide, endothelin-1 (ET-1). The HSCs become an autocrine target to ET-1 and acting via endothelin receptors, ETRA and ETRB2, and increases HSC proliferation and contraction, thereby increasing hepatic vascular tone and resistance[36,37]. Moreover, cyclooxygenase -derived prostaglandins (prostaglandin H2) and thromboxane A2, and lipoxygenase-derived leukotrienes are also over-produced in the cirrhotic livers modulating hepatic vascular tone[38,39].
The elevated intrahepatic vascular tone is further affected by endothelial dysfunction which is characterised by a reduced intrahepatic bioavailability of vasodilators such as nitric oxide (NO), together with an increased release of vasoconstrictors[29,39,40]. Reduced activity of endothelial nitric oxide synthases (eNOS) is responsible for decreased hepatic NO production and impaired intrahepatic vasodilatation[41,42]. Reduced eNOS activity is mainly due to the binding of eNOS to the inhibitory protein caveolin-1 in venous and sinusoidal endothelial cells of the cirrhotic liver[43]. However, the contribution of many other factors has also been described, such as dysregulation of eNOS phosphorylation due to abnormal signaling by the regulator protein kinase B, reduced expression of eNOS activator G-protein coupled receptor kinase interactor-1, deficiency of eNOS co-factor tetrahydrobiopterin and activation of RhoA/Rho-kinase[44-47].
As outlined above, splanchnic vasodilation leading to increased portal blood flow also contributes to the pathogenesis of portal hypertension. In contrast to the presence of endothelial dysfunction to vasodilators in hepatic vasculature, in cirrhotic splanchnic vessels, vasodilatation is promoted by local over-production of vasodilators, which, along with intrinsic vascular hypocontractility allow increased blood flow through the splanchnic vessels.
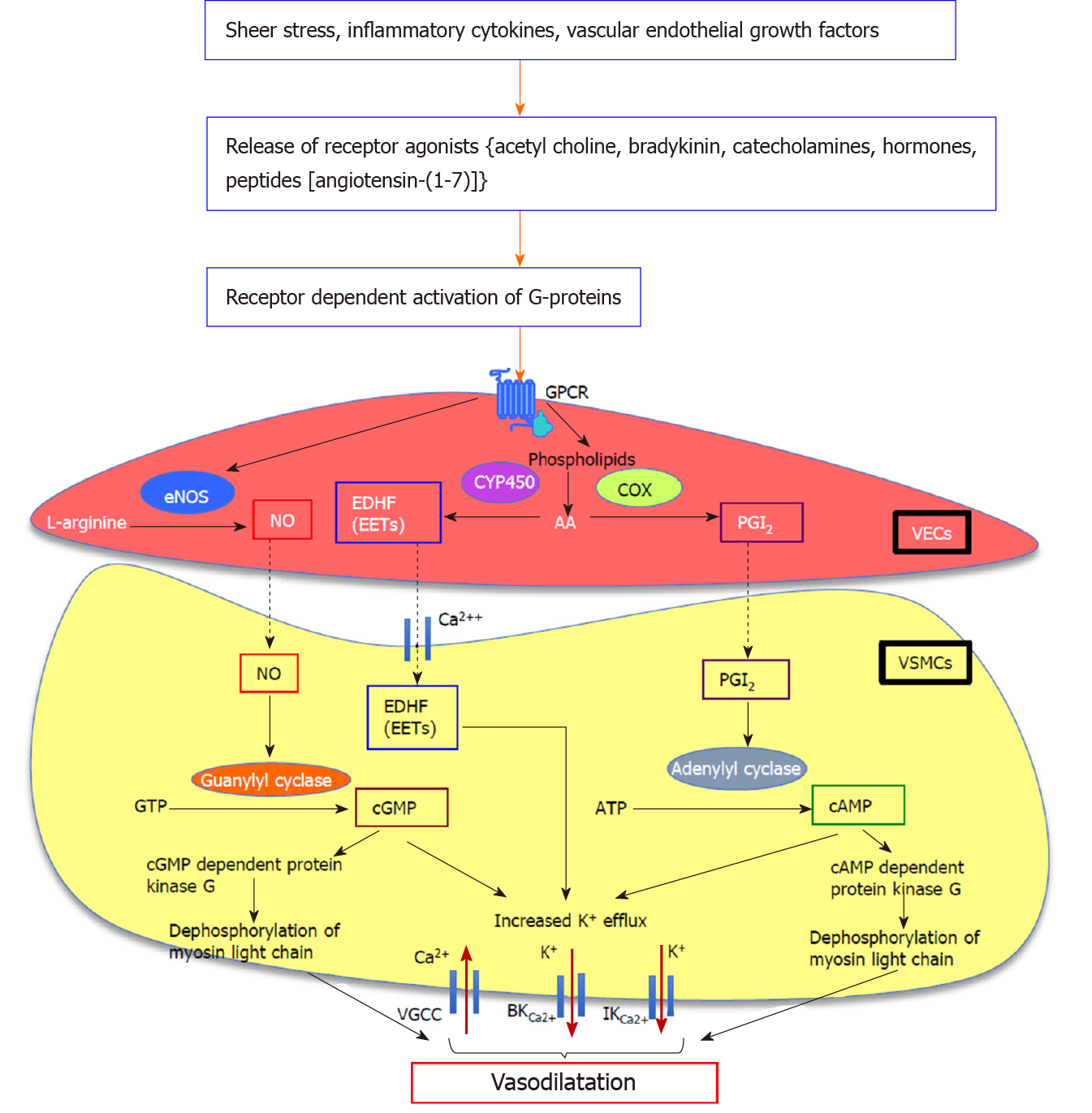
Hyperactive splanchnic vascular endothelial cells over-produce NO in response to different stimuli such as shear stress, inflammatory cytokines and vascular endothelial growth factors (VEGF)[39,40,48,49]. The main isomer responsible for splanchnic vascular over-production of NO is eNOS. When released from the endothelial cells, NO diffuses into VSMCs, where it directly stimulates membrane-bound soluble guanylate cyclase to release cyclic guanosine monophosphate (cGMP) from guanosine triphosphate. Subsequently, cGMP induces K+ efflux increasing intracellular Ca2+ concentration and activates cGMP dependent protein kinase to dephosphorylate myosin light chain kinase, inducing vasodilatation[38,40] (Figure 1). Vasodilatory molecules such as angiotensin-(1-7) [Ang-(1-7)][22], endogenous cannabinoids (ECs) and carbon monoxide (CO)[50] have been shown to regulate the vasodilatory pathways via eNOS/NO dependent mechanisms.
Several lines of research evidence support the augmented role of eNOS in increased NO over-production in the splanchnic vascular bed in cirrhosis. It has been reported that there is an elevated eNOS activity and nitrite levels in portal circulation of human cirrhotic patients undergoing liver transplantation[51]. In agreement with this, elevated eNOS expression and activity has been reported in the splanchnic vessels of portal hypertensive rats[52-55]. A number of in-vivo and in-vitro studies have shown that eNOS inhibition with non-selective NOS inhibitor (iNOS), N(G)-nitro-L-arginine methyl ester, reduced vascular hypocontractility and plasma volume, and increased perfusion pressure in portal hypertensive rats[25,56-59], providing evidence for the importance of eNOS/NO system in splanchnic and systemic vasodilatation in cirrhosis. Supporting this, denudation of vessels to remove the endothelium ameliorated vascular hypocontractility, suggesting that over-production of NO is endothelium dependent[60,61].
However, NOS/NO is not the only system involved in altering splanchnic hemodynamics in cirrhosis. This fact is proven by the studies in which inhibition of NO or denudation of cirrhotic rat vessels failed to completely normalize the splanchnic vascular hypocontractility[56,58,62,63]. Moreover, eNOS and/or iNOS gene knockout mice still developed hyperdynamic circulation after the induction of portal hypertension[64]. This suggests that in addition to NO, other paracrine/autocrine vasodilators such as prostaglandin I2 (PGI2), endothelium-derived hyperpolarizing factor(s) (EDHFs) including epoxyeicosatrienoic acids, hydrogen sulphide (H2S), CO, and ECs may contribute to the pathogenesis of hyperdynamic circulation and splanchnic vasodilatation in cirrhosis[40,65]. It is shown that molecules such as PGI2[66-68] and ECs[69] activate adenyl cyclase to increase cyclic adenosine monophosphate (cAMP) release in response to mechanical/humoral stimuli, thereby inducing the relaxation of VSMCs. The vasodilatory mechanism of H2S has been shown to be regulated via opening of K+-ATP channels[70]. In the absence of NO, EDHFs also act as prominent vasodilatory molecules, to induce vasodilatation via arachidonic acid metabolism and activation of K+ channels in the VSMCs[40,71,72]. Whilst both eNOS/NO system and EDHFs may participate in vasodilatory responses in the same vasculature, evidence also points to the possibility that the contribution of each system may depend on the size of the vessels[73]. This was based on the observations that different regions of rat superior mesenteric artery responded differently to acetylcholine-induced endothelium-dependent relaxation, and in comparison with large conduit vessels such as the aorta, it has been shown that the contribution of NO was most prominent in the aorta with highest expression of eNOS, whereas that of EDHF was most prominent in the distal mesenteric arteries accompanying the least eNOS expression[73-75].
It should be noted that this vasodilated hyperdynamic circulatory state of cirrhosis persists despite the activation of powerful vasoconstrictor systems in the systemic circulation such as the RAS, endothelin system and sympathetic tone. It appears that this resistance to the vasoconstrictor agents is likely due to the presence of an intrinsic vascular hypo-responsiveness of the splanchnic/systemic vessels[28,30,76]. A number of human and animal studies have demonstrated a splanchnic vascular hypo-responsiveness to various vasoconstrictors including ET-1, Ang II, adrenoceptor agonists, neuropeptide Y and vasopressin in cirrhosis. Supporting this, a reduced level of portal ET-1 was found to be accompanied by an increased expression of the vasodilatory ETB receptor in both mesenteric vascular endothelial and smooth muscle cells of cirrhotic rats[77]. A marked increase in norepinephrine concentrations has been detected in portal circulation of cirrhotic humans and rats, however it is proposed that the sustained sympathetic over-activation with norepinephrine may lead to vascular desensitization, thereby aggravating splanchnic vasodilatation in cirrhosis[78,79]. Similarly, Ang II plasma concentration is also elevated in cirrhosis[80], however it is speculated that the binding of receptor desensitizing proteins such as arrestin-2 and GRK2 to the AT1R may be responsible for vascular hyporeactivity to Ang II[81]. It has also been suggested that despite the attenuated reactivity to vasoconstrictors, expression and affinity of some receptors to their endogenous vasoconstrictors are not altered, and therefore, alterations in downstream signal transduction pathways is likely to be primarily responsible for this vascular hypo-reactivity[81-83]. However, this phenomenon of hypo-contractility of cirrhotic splanchnic vessels was questioned by a recent study published by our laboratory, demonstrating that pressor response of splanchnic vessels obtained from liver transplant recipients to Ang II was similar to that of control splanchnic vessels[84]. Taken together, these results imply more work is needed to clarify splanchnic vascular hypo-responsiveness to various vasoconstrictors in portal hypertension.
The development of a venous vascular network of porto-systemic collaterals also accompanies the development of portal hypertension in cirrhosis. Formation of collaterals by activation of angiogenesis is modulated by several growth factors such as VEGF, placental growth factor, pigment epithelial derived factor and platelet derived growth factor (PDGF)[29,38,49,85]. Blocking VEGF and PDGF receptors with sorafenib administration for one month has been shown to reduce the extent of collateralization and portal pressure in cirrhotic rats[86,87], and reduced portal venous flow in patients with hepatocellular carcinoma[88]. Some studies also suggested that up-regulated NO in splanchnic vasculature may also play a role in collateral formation[48]. Nicotinamide adenine dinucleotide phosphate oxidase has also been shown to play an important role in portal hypertension by modulating splanchnic angiogenesis and the formation of portosystemic collaterals[89].
Portal hypertension is defined as a sustained increase in the pressure gradient between portal and systemic circulation. The most common parameter used to measure portal pressure is the hepatic venous pressure gradient (HVPG), the pressure gradient between the portal vein (PV) and inferior vena cava (IVC)[12]. In healthy individuals, the normal HVPG is 1-5 mmHg. In compensated cirrhosis, a HVPG between 6-10 mmHg portends mild subclinical portal hypertension, whilst clinically significant complications of portal hypertension develop when it exceeds 10 mmHg. Decompensated cirrhosis is characterised by an HVPG above 12 mmHg, which is manifested by the development of porto-systemic shunting, variceal rupture and bleeding[90,91]. Moreover, it has been reported that the probability of survival in cirrhotic patients with HVPG above 16 mmHg was below 70% of those patients who have an HVPG below this level and that this poor survival rate was related to Child-Pugh class[92]. The HVPG is a quantitative assessment but there are other indicators of PHT including the presence of varices on endoscopy, physical exam findings of splenomegaly, ascites and caput medusa as well as classical imaging findings on ultrasound, computer tomography and magnetic resonance imaging studies.
Portal pressure can be measured in experimental animals by direct cannulation of the PV or its tributary, the superior mesenteric vein. However, this is not feasible in the clinical setting and therefore, HVPG measurement has been adopted as the most common method for the monitoring of portal pressure. In this technique, a French balloon tipped catheter is inserted into the right internal jugular vein and advanced through right atrium and IVC into the hepatic vein (HV) under fluoroscopic guidance. Free hepatic vein pressure (HVP) is measured when the tip of the catheter is free in the HV. The balloon is then inflated occluding flow and creating a proximal static column of blood, which transmits the pressure from the hepatic sinusoids to the catheter. To obtain accurate and reliable pressure measurements, the readings are permanently traced and at least 3 pressure readings are obtained after HV occlusion to calculate the wedged hepatic venous pressure (WHVP)[93]. HVPG is the difference between WHVP and the HVP, and is a measure of sinusoidal pressure which is closely correlated with portal pressure[94]. In the normal liver, the HVPG is slightly lower than portal pressure, owing to pressure equilibrium through the interconnected sinusoids. However, in the cirrhotic liver the static column of blood created by occluding the hepatic vein cannot be decompressed at the sinusoidal level due to the disrupted connections between sinusoids, therefore, the HVPG gives a good approximation of the portal pressure in cirrhosis[90,93,94]. However, it should be noted that HVPG is a measure of sinusoidal pressure and does not accurately assess pre-sinusoidal portal hypertension that occurs in pre-cirrhotic primary biliary cholangitis.
Although HVPG is considered to be the gold standard in the measurement of portal pressure, this technique is an invasive and its reliability depends on the experience of the physician performing the procedure. Therefore, methods to evaluate the severity of liver fibrosis and stiffness such as liver transient elastography, ultrasound elastography, doppler and contrast-enhanced ultrasonography and serum tests, have been developed and validated as tools for the prediction of the presence of clinically significant portal hypertension and gastroesophageal varices using the assessment of cirrhosis extent as a predictor of the extent of portal hypertension[95,96].
Recent studies have focused on integrating artificial intelligence in the diagnosis of liver disease and portal hypertension. Such studies use computer-based programs to determine whether there is a correlation between different diagnostic markers and clinical outcomes. These computer programs use non-linear statistical analyses in order to establish a relationship between different input and output variables. A recent study using artificial neural networks (ANNs) compared the diagnostic performance of seven input variables (transient elastography and six serum tests) in predicting the presence of cirrhosis, clinically significant portal hypertension and oesophageal varices in patients[95]. This study revealed that liver stiffness measured by transient elastography could be used to detect portal hypertension and varices with > 80% accuracy via ANNs. Another study showed the effectiveness of a data mining software in establishing the relationships between various input variables, including clinical parameters, serum markers, liver ultrasonography and transient elastography, compared with portal pressure measured by HVPG[97]. This software used different classification meta-algorithms on selected datasets to show that there is a relationship between the above input variables and measured portal hypertension, suggesting this meta-algorithm analysis could be used as a substitute for the measurement of portal pressure by HVPG. Whilst these methods work well in advanced cirrhosis with established portal hypertension, they perform less well in earlier stages of liver disease.
Most events that occur during the evolution of chronic liver disease are the consequences of portal hypertension. Clinical manifestations of decompensated cirrhosis including the formation of varices and variceal bleeding, ascites, HRS and HPS have been directly related to the development of a hyperdynamic circulation, splanchnic vasodilatation and portal hypertension in cirrhosis. It should be noted that in 2016, HRS types 1 and 2 were reclassified as HRS-AKI and HRS-NAKI respectively, with the difference between the two dependent upon the trajectory of the creatinine rise above baseline[98,99]. The development of decompensation events is associated with a reduction in the median survival of a patient to less than 2 years, from 12 years in a cirrhotic patient without these complications[91].
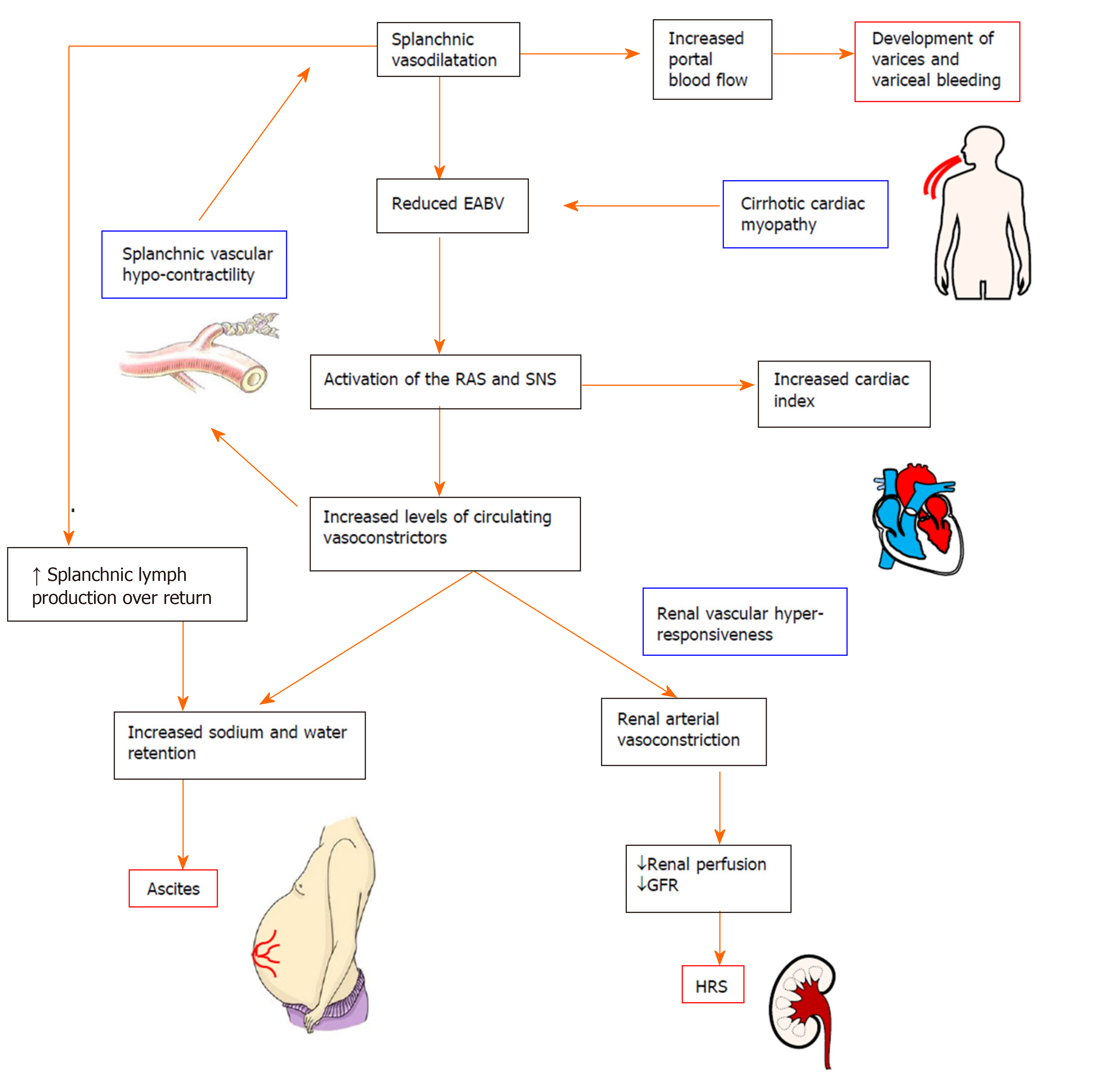
The hyperdynamic circulation in cirrhosis is characterized by systemic and splanchnic vasodilatation, plasma volume expansion and increased cardiac index. Splanchnic vasodilatation results in an excessive blood volume in the splanchnic vascular compartment, leading to a reduction in effective arterial blood volume. Subsequently, endogenous neuro-hormonal systems such as the RAS and sympathetic nervous system are activated in an attempt to restore normal blood volume via increasing sodium and water retention and by promoting vasoconstriction and increased cardiac output[11] (Figure 2). However, in decompensated cirrhosis, these compensatory mechanisms are ineffective due to the intrinsic splanchnic vascular hypocontractility. Patients with advanced cirrhosis may also develop cirrhotic cardiomyopathy which limits the ability of the heart to increase cardiac output[26].
Development of gastroesophageal varices and associated massive upper gastrointestinal bleeding is the leading cause of death in cirrhotic patients. Varices are dilated collateral venous communications formed between portal and systemic circulations, mainly in the upper gastrointestinal region. Varices can divert 90% or more of the elevated portal flow back into heart. Eventually they remodel and progressively enlarge to accommodate increasing blood flow producing a risk of rupture and bleeding[12].
In cirrhotic patients, the collateral circulation begins to develop when HVPG rises above 10 mmHg. When HVPG is elevated above 12 mmHg, there is a significant risk of variceal rupture and bleeding[90,91]. By far the most clinically significant and relevant of these are the gastroesophageal varices, although they can also occur within stomach, rectum, omentum and elsewhere in the gastrointestinal tract. Gastroesophageal varices are more prone to bleed because of the lack of external tissue support and high trans-luminal pressure gradient due to negative oesophageal luminal pressure during inspiration[100,101].
Upon diagnosis of cirrhosis, varices are detected in about 30% and 60% of compensated and decompensated patients, respectively[102-104]. Patients without varices develop them at a rate of 8% per year. Long term follow-up studies revealed up to 90% of the cirrhotic patients will eventually develop varices. There is a 10%-30% risk for variceal rupture within the first year of diagnosis[105]. Despite the advances in the management of variceal bleeding in the past few decades, average 6-wk in-hospital mortality of first variceal bleeding still approaches 20%[100,106,107].
Over the past few decades, a number of advances have been made in the development of novel treatment strategies to treat portal hypertension and its complications such as variceal bleeding. These therapies aim at reducing the risk of bleeding from varices by dropping the HVPG at least by 20% from baseline pressure or to less than 12 mmHg. However, the effectiveness of the currently available drugs including NSBBs in the treatment of variceal bleeding is limited, therefore there is an ongoing need for investigation and development of new therapies.
In 1981, the NSBBs were first introduced as a treatment for recurrent gastrointestinal bleeding in patients with cirrhosis[108]. Later, they were shown to be similarly effective in primary prophylaxis for variceal bleeding[109]. Since then, the effectiveness of NSBBs as first line pharmacotherapy in variceal bleeding has been documented by many clinical trials[110-114]. NSBBs lower HVPG by two principal mechanisms; firstly, by reducing cardiac output which leads to a subsequent reduction in splanchnic blood flow (cardiac 1-adrenergic blockade) and secondly by increasing splanchnic vascular resistance by allowing unopposed vasoconstrictive alpha-adrenergic activity thereby reducing portal blood flow (splanchnic 2-adrenergic blockade)[26,115-117].
American Association for the Study of Liver Disease guidelines, British Society of Gastroenterology guidelines and the Baveno VI consensus recommend the use of NSBBs in both primary and secondary prophylaxis of variceal bleeding[118-120], and their effectiveness has been demonstrated by a number of clinical trials. In patients with existing small varices, NSBBs slowed the progression of small varices into large varices (11% and 37%, NSBB vs placebo control, respectively) and decreased the incidence of first variceal hemorrhage (12% and 22%, NSBB vs placebo control, respectively)[121]. In patients with medium or large varices and at a high risk of bleeding, they reduced the risk of bleeding to 14% from 30% in control subjects[122]. The effectiveness of NSBBs in secondary prophylaxis has been shown by studies which demonstrated that at one year of treatment with propranolol, the drug reduced recurrent bleeding of cirrhotic patients with gastrointestinal bleeding[108,111]. However, NSBBs are not similarly effective in preventing the development of new varices in early cirrhotic patients with mild subclinical portal hypertension[117]. Moreover, these drugs are contraindicated in patients at the time of acute variceal bleeding due to their systemic hypotensive and cardiac side effects[123].
Despite their proven clinical effectiveness, traditional NSBBs such as propranolol and nadolol are contraindicated or poorly tolerated in up to 15%-20% of patients and up to 60% of patients do not achieve any therapeutically useful fall in HVPG with these NSBBs[105,124,125]. NSBBs produce a number of cardiac and non-cardiac adverse effects, such as headaches, fatigue, asthma and shortness of breath, which led to the discontinuation of treatment in about 15% of the patients in clinical trials[126,127].
The 1-adrenergic effects of NSBBs in reducing cardiac output has detrimental side effects in patients with advanced cirrhosis. In end stage cirrhotic patients with refractory ascites and/or SBP, NSBBs blunt the ability to maintain an adequate systemic arterial blood pressure, and defective renal perfusion may lead to the development of HRS[128,129], and in end stage cirrhosis NSBBs have been associated with increased mortality[130].The use of NSBBs is also contraindicated in around 15% of the cirrhotic patients who have asthma, chronic obstructive pulmonary diseases poor cardiac function or low blood pressure[91].
The traditional and most widely used NSBBs in clinical practice are propranolol and nadolol. Many clinical trials suggest that both drugs have a similar effectiveness in primary prophylaxis of variceal bleeding. However, some studies showed that the side effects produced by nadolol (9%-13%) were lower compared to those of propranolol (12%-31%), although direct comparison between the two groups has not been performed[131]. Moreover, compared to propranolol, nadolol has a longer half-life in the circulation attributed to its low lipid solubility and hepatic metabolism, thus patients can be dosed less frequently[132].
Similar to propranolol, the NSBB timolol is effective in reducing HVPG, particularly in early cirrhosis (12% and 13% reduction in timolol and propranolol, respectively)[133,134]. Other NSBBs used as antihypertensive drugs including sotalol, pindolol and penbutalol may also be effective in the treatment of portal hypertension; however, the efficacy of these drugs has not yet been tested in patients with oesophageal varices.
The NSBB, carvedilol, has been shown to be more effective than propranolol in reducing portal pressure[135]. In one clinical trial, carvedilol and propranolol resulted in a HVPG reduction of more than 20% from baseline values, or to less than 12 mmHg, in 64% and 14% of cirrhotic patients, respectively[136]. In addition to its potent -blocker activity on reducing splanchnic vasodilatation, the effectiveness of carvedilol is likely due to its potential anti-1 adrenergic activity, which is about one-tenth of its -blocker activity, thus reducing intrahepatic vascular resistance[137]. Thus, whilst carvedilol decreases cardiac output with concomitant increase in splanchnic resistance by blocking the receptors, its anti-1 receptor activity helps decrease hepatic vascular tone and hepatic resistance[138]. Moreover, carvedilol is also known to have antioxidant, antifibrotic and anti-inflammatory properties, which might potentially benefit patients with advanced cirrhosis[132]. However, the vasodilating anti-1 effect of carvedilol has potential to enhance systemic hypotension, leading to a reduction in renal perfusion and therefore, its clinical application is limited, especially in cirrhotic patients with refractory ascites and in patients with advanced, decompensated cirrhosis[136,139]. However, labetalol, another NSBB with similar anti-1 adrenergic activity failed to reduce HVPG in patients with cirrhosis[140]. Although individual studies demonstrated that carvedilol may be more effective than traditional NSBBs such as propranolol in preventing variceal bleeding in cirrhotic patients, a recent meta-analysis which systematically analysed ten randomized clinical trials using carvedilol, propranolol and nadolol, concluded that carvedilol is not as effective as traditional NSBBs in reducing mortality, variceal bleeding and serious adverse events[141]. Thus, the authors concluded that additional evidence is required from adequately powered, long-term, double-blind, randomised clinical trials, evaluating both clinical and haemodynamic outcomes. Importantly, in the United Kingdom, there are adequately powered randomized clinical trials in progress comparing the effectiveness of carvedilol vs placebo[142] and carvedilol vs endoscopic variceal ligation therapy[143] in cirrhotic patients and the outcomes of these studies are yet to be published. These are the first trials investigating the effectiveness of carvedilol on variceal bleeding in cirrhotic patients.
Selective 1 receptor blockers such as atenolol and metoprolol also reduce HVPG in cirrhotic patients. The reduction of HVPG achieved with these selective blockers is related to their 1 receptor blockade-mediated effect on cardiac index, which contrasts with the cumulative cardiac (1) and splanchnic (2) effects of NSBBs such as propranolol. It was reported that the efficacy of atenolol in reducing HVPG and variceal bleeding was less and not sustained in cirrhotic patients compared to propranolol[144,145]. Similarly, treatment with metoprolol also was associated with higher rate of recurrent bleeding[146]. Based on these findings and also due to their profound cardiac side effects, selective 1 receptor blockers are not therefore recommended in the prophylaxis of variceal hemorrhage[125].
Splanchnic vasoconstrictors such as vasopressin, somatostatin and their analogues are used alone or in combination with endoscopic therapy to treat patients with acute variceal bleeding[118].
Similar to NSBBs, vasopressin reduces portal pressure by producing splanchnic vasoconstriction and thereby decreasing portal venous blood flow. However, due to its potent vasoconstrictive ability vasopressin has been shown to produce multiple side effects in the heart and systemic circulation including hypertension, ventricular arrhythmias, cardiac and peripheral ischemia, and bowel ischemia[131]. Combination therapy of vasodilator nitroglycerine has been shown to counteract the cardiac side effects of vasopressin in cirrhotic patients and experimental animals[147]. The synthetic vasopressin analogue terlipressin which is selective for the V1 vasopressin receptor has significantly fewer side effects and therefore, it is recommended for patients with acute variceal bleeding[148,149]. However, terlipressin is not available in some countries including the United States and Canada.
Treatment with somatostatin and its analogues such as octreotide and vapreotide is also recommended for the treatment of acute variceal haemorrhage[150]. Among these, only octreotide is readily available in many countries including the United States. Although these drugs were previously shown to promote splanchnic vasoconstriction via inhibiting the release of vasodilatory peptide glucagon, it is now known that the local adrenergic 1 receptor-mediated vasoconstrictive effects of somatostatins are responsible for counteracting the splanchnic vasodilatation. A recent meta-analysis showed that the efficacy of somatostatin/octreotide on variceal re-bleeding was similar to that of vasopressin/terlipressin[151]. In current guidelines, however, they are recommended for acute variceal bleeding[152], although they may have side-effects such as tachyphylaxis, bradycardia and hypertension[153,154].
Reduction of intrahepatic and/or porto-collateral resistance using vasodilatory agents such as nitrates could also be an ideal therapeutic approach in the treatment of portal hypertension in cirrhosis. These agents such as isosorbide-5-mononitrate (ISMN), are ineffective when given as monotherapy but when given as a combined treatment with an NSSB improve the efficacy of NSBBs in the reduction of HVPG, and may be very useful in secondary prophylaxis for variceal bleeding[155] The combined treatment, has been shown to be effective for those patients who are non-responders to standard NSBB treatment alone[121,156].
Selective 1 adrenergic receptor blockers such as prazosin are another experimental therapy for the modification of intrahepatic vascular tone. A study in 12 patients with oesophageal varices demonstrated that short-term and long-term administration of prazosin reduced HVPG by 25% and 19% of the baseline, respectively[157]. However, prazosin produced long term deleterious side effects in cirrhotic patients by promoting peripheral vasodilatation and enhancing sodium and water retention, leading to the development of ascites. A study comparing the effects of combination therapy of propranolol with ISMN or prazosin showed that propranolol plus prazosin treatment reduced HVPG in cirrhotic patients more than propranolol plus ISMN treatment[158]. However, systemic hypotension was more evident with combination therapy of prazosin compared to ISMN.
Statins are another category of therapy that has been widely studied in the modulation of intrahepatic resistance and vascular tone. These drugs have been shown to selectively enhance eNOS activity in cirrhotic livers, promoting the production of NO in hepatic sinusoidal endothelial cells, thus they appear to behave as “true liver-selective vasodilators” in cirrhosis[47,159,160]. In the cirrhotic liver, statins also reduce the contraction and proliferation of myofibroblastic HSCs by blocking RhoA-dependent non-canonical hedgehog pathway[161]. In addition, statins have been shown to reduce neoangiogenesis and collateral flow by blunting the non-canonical hedgehog signalling pathway in experimental cirrhosis[161].
A number of clinical studies have highlighted the potential importance of the use of statins such as simvastatin and atorvastatin in the treatment of portal hypertension in cirrhosis. Recently, a systematic review and meta-analysis summarized the results of 13 studies that used statins in patients with chronic liver disease, to show that these drugs significantly reduce the progression of chronic liver disease into decompensated cirrhosis by 46%, patient mortality by 46%, and the risk of variceal bleeding and progression of portal hypertension by 26% in cirrhotic patients[162]. An important multicenter double blind randomized controlled trial demonstrated that the treatment with simvastatin significantly reduced of HVPG (by 8.3%) in cirrhotic patients with portal hypertension, without inducing arterial hypotension, supporting the argument that these drugs are ‘true liver-selective vasodilators’[163]. Simvastatin also produced an additive effect on reducing HVPG in the patients receiving NSBB treatment (by 11%), possibly related to increased bioavailability of NO in the hepatic sinusoidal circulation[116]. In another recent clinical trial, addition of simvastatin to the NSBB treatment decreased overall mortality in patients although it failed to reduce the rate of re-bleeding from varices[164]. Whilst this study showed that 3% of the patients treated with simvastatin developed severe adverse side effects such as rhabdomyolysis, a more recent clinical trial suggested that statins-associated risks appear to be dose-related[165]. The above clinical evidence suggests that statins possess a clear potential for the treatment of portal hypertension in cirrhotic patients, however, further randomized clinical trials are warranted involving larger patient populations with clear clinical end points.
A recent study in experimental animal models suggests that statins may have divergent effects in cirrhotic and non-cirrhotic portal hypertension. Uschner and colleagues[161] showed that in a non-cirrhotic portal hypertensive rat model, statins worsened the portosystemic shunt flow and portal hypertension, via increasing extrahepatic angiogenesis through the canonical hedgehog pathway, suggesting that in a clinical setting, the use of statins could possibly be limited to cirrhotic patients with portal hypertension.
Selective hepatic delivery of vasodilators such as NO has also been identified as an ideal strategy in the modification of intrahepatic vascular tone. Unexpectedly, delivery of liver specific NO donor NCX-100 failed to decrease HVPG in a phase II clinical trial[166]. In addition, antioxidants such as ascorbic acid, caffeine and dark chocolate are proposed as therapies that improve endothelial dysfunction in cirrhosis[167-169]. Experimental evidence also suggests that multikinase inhibitors such as sorafenib could be used to inhibit collateral formation and/or neoangiogenesis by blocking both VEGF and PDGF receptors. However, the challenge with this therapy is the inability of the drug to differentiate pathological neoangiogenesis from physiological neoan-giogenesis such as the cyclic changes of female reproductive tract[86,87,170].
The selective 2 adrenergic receptor agonist clonidine is another potential therapy for the treatment of portal hypertension in cirrhosis. 2 receptor agonists diminish sympathetic activity and reduce cardiac output and increase portal venous tone, thus they are expected to reduce portal venous inflow. Studies have shown that clonidine reduced HVPG by > 10% from baseline in around 87% of alcoholic cirrhotic patients[171]. However, the usefulness of clonidine as a treatment for portal hypertension is limited by deleterious systemic and renal side effects[172].
The RAS has long been recognized as a simple enzymatic cascade responsible for the regulation of cardiovascular and fluid homeostasis. However, understanding of this system has dramatically changed over the last few decades. It is now known that besides its physiological role, the RAS also mediates complex regulatory functions involved in disease progression and wound healing[173,174].
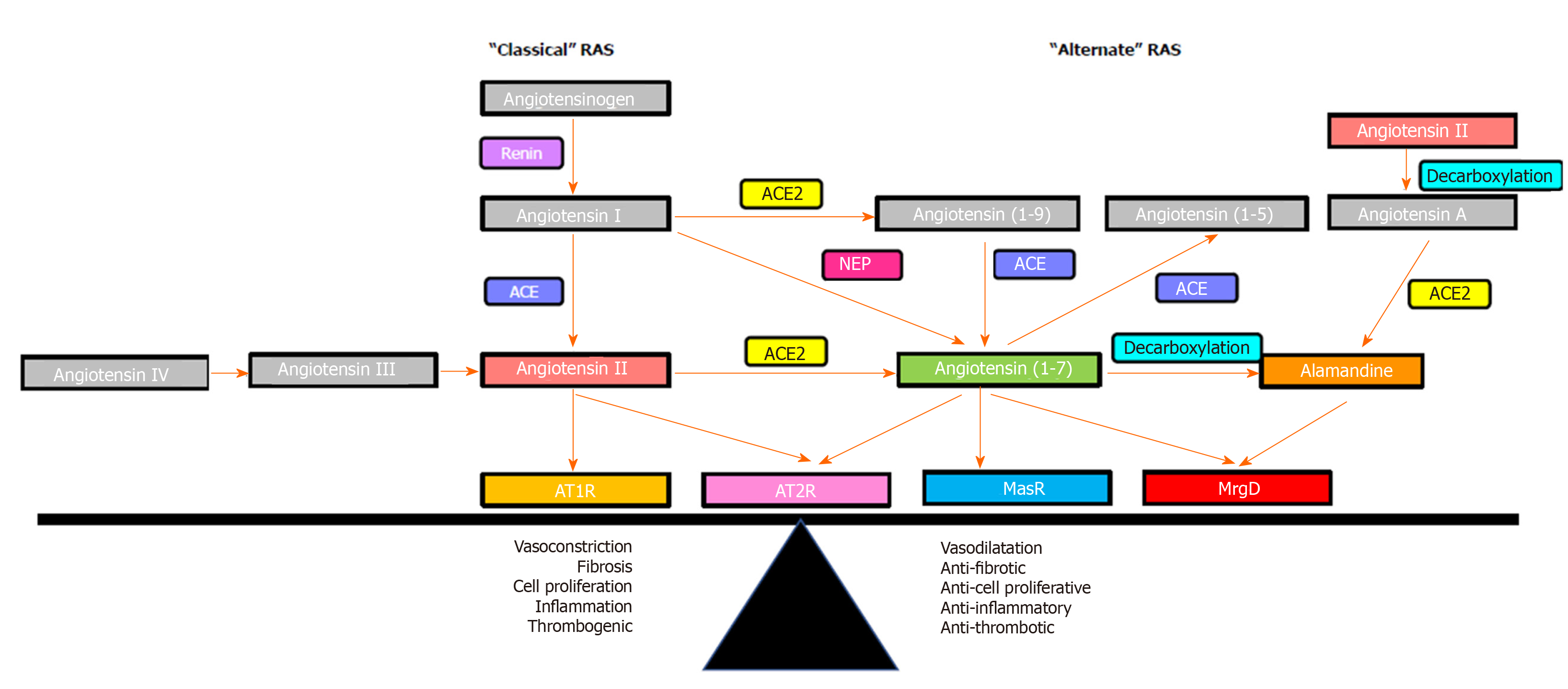
The RAS is comprised of two axes that function interdependently (Figure 3). The “classical axis” of the RAS comprises angiotensin converting enzyme (ACE), the vasoconstrictive peptide Ang II and its receptor AT1R. ACE cleaves the biologically inactive decapeptide angiotensin I (Ang I) to the octapeptide Ang II. Ang II is a potent vasoconstrictor that acts via the AT1R in regulating blood pressure, and fluid and electrolyte homeostasis[53,175]. In addition to its vasoactive role, Ang II plays an important roles in the wound healing responses via cell proliferation, tissue inflammation and fibrogenesis[176-180].
It is now known there is an “alternate axis” of the RAS comprising its active carboxypeptidase of angiotensin converting enzyme 2 (ACE2, a structural homologue of ACE), the effector heptapeptide Ang-(1-7) and its receptor Mas. ACE2 cleaves a single C-terminal residue from Ang II and Ang I to produce Ang-(1-7) and Ang-(1-9), respectively[181-183]. In comparison with ACE, the affinity of ACE2 for cleaving Ang I is low; however, ACE2 has more than 400-fold greater affinity to cleave Ang II to produce Ang-(1-7)[24,184-187]. Ang-(1-7) can also be formed from Ang I and Ang-(1-9) by the enzymatic action of neutral endopeptidase neprilysin and ACE, respectively[24,187,188].
In 1988, Schiavone and colleagues[189] published the first report of a biological action of Ang-(1-7) characterized in vitro, which was immediately followed by a report showing an in vivo action of the peptide[190]. Following these discoveries, a large body of research proved that it is an active peptide which has opposing effects to Ang II, including vasodilatation[25,191-194]. In addition, Ang-(1-7) also has anti-inflammatory, anti-fibrotic and anti-proliferative properties, thus attenuating disease progression, including chronic liver[80,195] cardiac[196-199] and renal[200-204] diseases. Available evidence suggests that the vasodilatory effects of Ang-(1-7) are regulated through increased production of NO[205-209] via activation of eNOS in endothelial cells[25,209,210]. It has been shown that Ang-(1-7) regulates Ser1177/Thr495 phosphorylation to increase eNOS activity and NO production via a P13K/Akt sensitive pathway[209]. It has also been shown that Ang-(1-7) mediates vasodilatation by potentiating bradykinin, the effector peptide of the kallikrein-kinin system, to release NO and vasodilatory prostacyclins. This suggests that kallikrein-kinin system and eNOS both contribute to Ang-(1-7)-mediated vasodilatation[205,208,211-213].
Identification of Ang-(1-7) as a functional peptide of the alternate RAS provided impetus to further investigate its mechanism of action. Evidence for the presence of a specific Ang-(1-7) receptor came in a study which showed that in vivo and in vitro functional responses to Ang (1-7) were abolished by the synthetic Ang-(1-7) analogue D-Ala7-Ang-(1-7) (A779), whereas the effect of Ang-II could not be blocked by A779[214]. Subsequently, radioligand binding studies in bovine aortic endothelial cells demonstrated that both A779, and a non-peptide Ang-(1-7) agonist AVE0991 competed for binding with Ang-(1-7), which led to the speculation that Ang-(1-7) should have a specific receptor[215,216]. In 2003, Santos and colleagues[217] discovered the functional receptor for Ang-(1-7) and named it as the Mas receptor (MasR), which is a G protein-coupled receptor encoded by the Mas protooncogene. Specificity of MasR to Ang-(1-7) was demonstrated by Ang-(1-7) failing to bind to the kidneys of MasR knockout mice (MasR-KO), and Ang-(1-7) failing to exert a vasodilatory response in aortic rings isolated from these mice. Moreover, mesenteric arterial vessels isolated from MasR-KO mice showed a blunted vasodilatory response to acetylcholine, suggesting an endothelial dysfunction in Mas-KO mice, which confirmed the role of MasR in regulating the vascular tone[218,219]. Confirming the role of MasR in modulating vascular tone, Xu and colleagues[220] have shown that MasR deletion results in increased blood pressure and endothelial dysfunction. In other studies, MasR deletion altered ventricular function, vascular resistance and increased the profibrotic profile and Ca2+ regulation in cardiomyocytes, further implying the functional significance of the Ang-(1-7)/MasR axis[221,222].
However, more recent studies have speculated on the existence of a second receptor for Ang-(1-7) which appears to play a leading role in controlling vascular tone. Initial evidence for this came from a study which showed that vasodilatory effects of Ang-(1-7) in rat aorta could not be blocked by MasR blocker A779, but were completely abolished by a synthetic Ang-(1-7) antagonist D-Pro7-Ang-(1-7) (D-Pro)[223]. Subsequently, work from our laboratory showed that in the cirrhotic perfused rat liver, the perfusion pressure reduction effect of Ang-(1-7) was blocked by D-Pro but not by A779, suggesting the existence of a receptor subpopulation other than MasR that is sensitive to D-Pro[25]. Lautner and colleagues[224] were able to characterize a novel receptor, which they identified as the Mas-related G protein-coupled receptor type-D (MrgD), that is activated upon binding to the newly discovered vasodilatory RAS peptide alamandine. Importantly, they found that the vasodilatory effects of alamandine were blocked by D-Pro, but not by A779. This confirmed previous reports that COS cells over-expressed with either MasR or MrgD released arachidonic acid in response to Ang-(1-7) stimulation, suggesting that both the MasR and MrgD mediate Ang-(1-7) action by releasing arachidonic acid, a precursor molecule of prominent intracellular vasodilating signaling pathways[225] (Figure 1). More recently, another study strongly supported the concept that MrgD is a second receptor for Ang-(1-7) by showing that in the absence of either MasR or MrgD activation, mesangial cells failed to increase cAMP release in response to Ang-(1-7), and both A779 and D-Pro abolished cAMP release by HEK293 cells transfected with MasR or MrgD[226]. This study also showed that MrgD-KO mice had an impaired hemodynamic response to Ang-(1-7) administration. These findings collectively confirmed that MrgD is a functional receptor mediating the vasodilatory effects of Ang-(1-7).
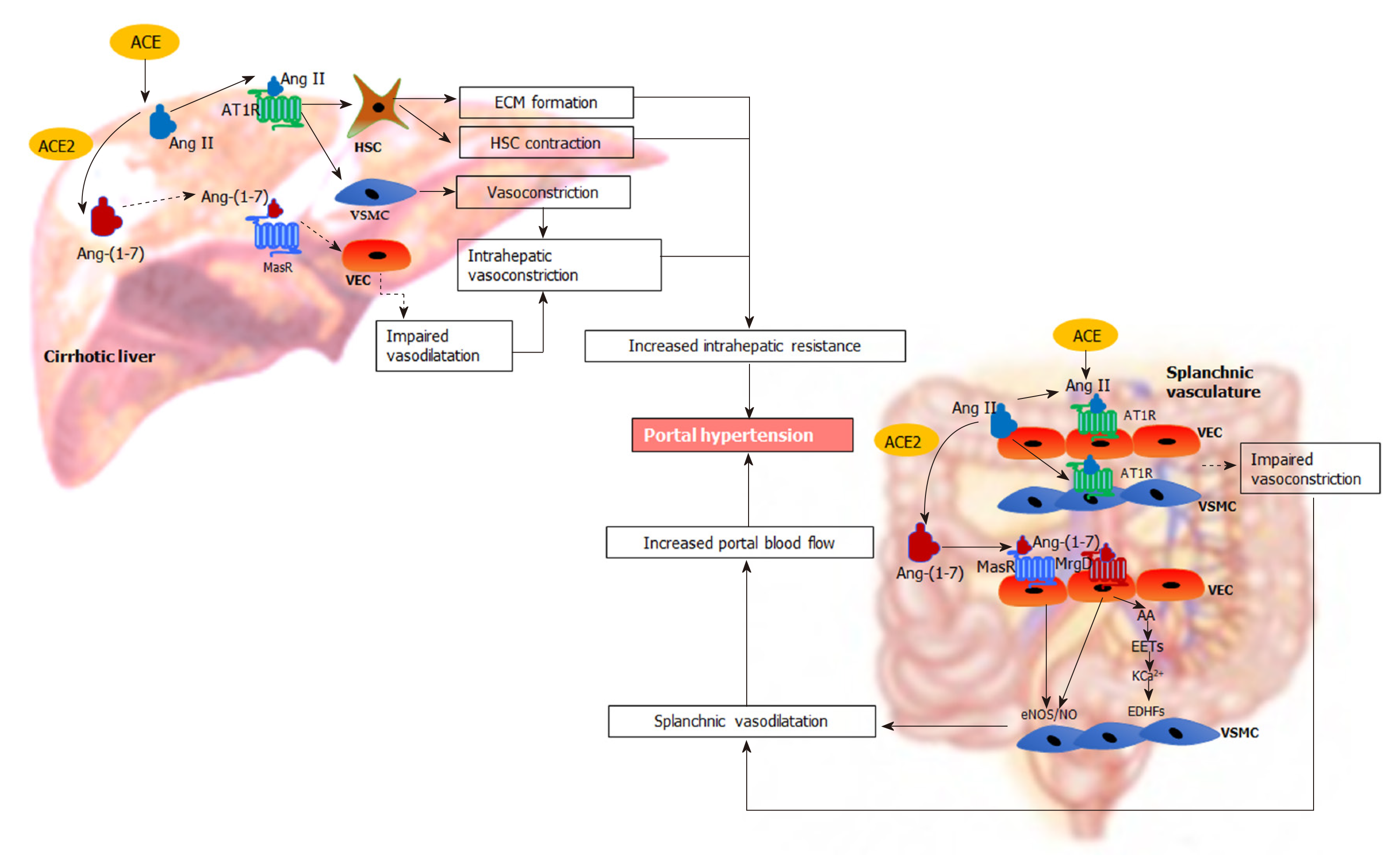
It is well known that the RAS is activated in cirrhosis and plays a major role in the development of portal hypertension[4,173,227,228] (Figure 4). In cirrhotic patients and animal models of cirrhosis, the components of both classical and alternate axes of the RAS are markedly upregulated[34,53,80]. Work from our laboratory and others showed that the components of the classical RAS, in particular ACE and Ang II are significantly elevated in the circulation and the liver of cirrhotic animals, contributing to liver fibrosis and increased intrahepatic vascular tone[25,35,229-231]. Consistent with this, blockade of the classical RAS has been shown to improve experimental liver fibrosis and intrahepatic vascular resistance[24,229,232-234]. Recent discoveries demonstrate that the components of the alternate RAS including ACE2 and Ang-(1-7) are also upregulated in the circulation and the liver of cirrhotic animals[35,80,230]. Importantly, these RAS components are also increased in the splanchnic vascular bed of cirrhotic animals and have been suggested to play a pivotal role in splanchnic vasodilatation and the development of portal hypertension[22,23]. Therefore, manipulation of the RAS, particularly the alternate RAS, may provide a novel approach to treat portal hypertension in cirrhosis.
In cirrhotic livers, Ang II levels are markedly increased, promoting HSC proliferation and ECM production[19,235], which increases intrahepatic resistance to the portal flow. Inhibition of Ang II action via ACE inhibitors (ACEi) and/or angiotensin receptor blockers (AT1R blockers) (ARBs), has therefore been shown not only to attenuate hepatic fibrosis but also to alleviate intrahepatic resistance in animal models of cirrhosis[229,236,237].
In addition, the activated contractile phenotype of HSCs express increased AT1R and contract in response to Ang II and thereby increase sinusoidal vasoconstriction, leading to increased intrahepatic vascular resistance[33,175]. Supporting this phenomenon, work from our laboratory showed that the vasoconstrictive response to Ang II administration is increased in the perfused cirrhotic rat liver preparation compared to that in healthy liver, presumably via the effects of Ang II on upregulated AT1R in VSMCs cells and sinusoidal myofibroblastic HSCs[24]. This study also demonstrated that hepatic production of Ang II is increased in cirrhosis, suggesting that locally produced Ang II contributes to increased intrahepatic vascular resistance and highlighting the importance of the hepatic RAS in the regulation of portal hypertension.
It has been suggested that increased local expression of the components of the alternate RAS may be effective in counteracting the detrimental effects of the classical RAS in the cirrhotic liver[53]. Supporting this concept, work published by our laboratory demonstrated that liver-specific sustained over-expression of ACE2 ameliorated liver fibrosis and improved hepatic perfusion by reducing hepatic levels of profibrotic and vasoconstrictor peptide Ang II with concomitant increase in antifibrotic and vasodilator peptide Ang-(1-7)[238]. These findings supported earlier work published by our laboratory that infusion of Ang-(1-7) into cirrhotic rats significantly improved hepatic fibrosis by suppressing HSC activation, although portal pressure was not measured in this study[80]. Direct support for a vasodilatory action of Ang-(1-7) in the cirrhotic liver comes from work performed in our laboratory which showed that in situ perfused cirrhotic rat liver preparations pre-constricted with Ang II or methoxamine underwent a marked endothelium-dependent, and AT1R/AT2R independent, vasodilatory response to the infusion of Ang-(1-7)[25]. Moreover, work by our laboratory also demonstrated that administration of Ang-(1-7) or the non-peptide MasR agonist AVE0991 reduced the activation of primary rat HSCs in culture whereas the MasR blocker A779 increased their activation, as reflected by increased -smooth muscle actin expression. In other words, the antifibrotic and vasodilatory effects that can be produced by components of the alternate RAS would be expected to improve portal hypertension by reducing intrahepatic vascular resistance.
In contrast to the effect of vasoactive molecules in the intrahepatic vasculature, the systemic and splanchnic vessels are hyporesponsive to circulatory vasoconstrictors including Ang II and thus, these vessels remain dilated in cirrhosis[239-241].
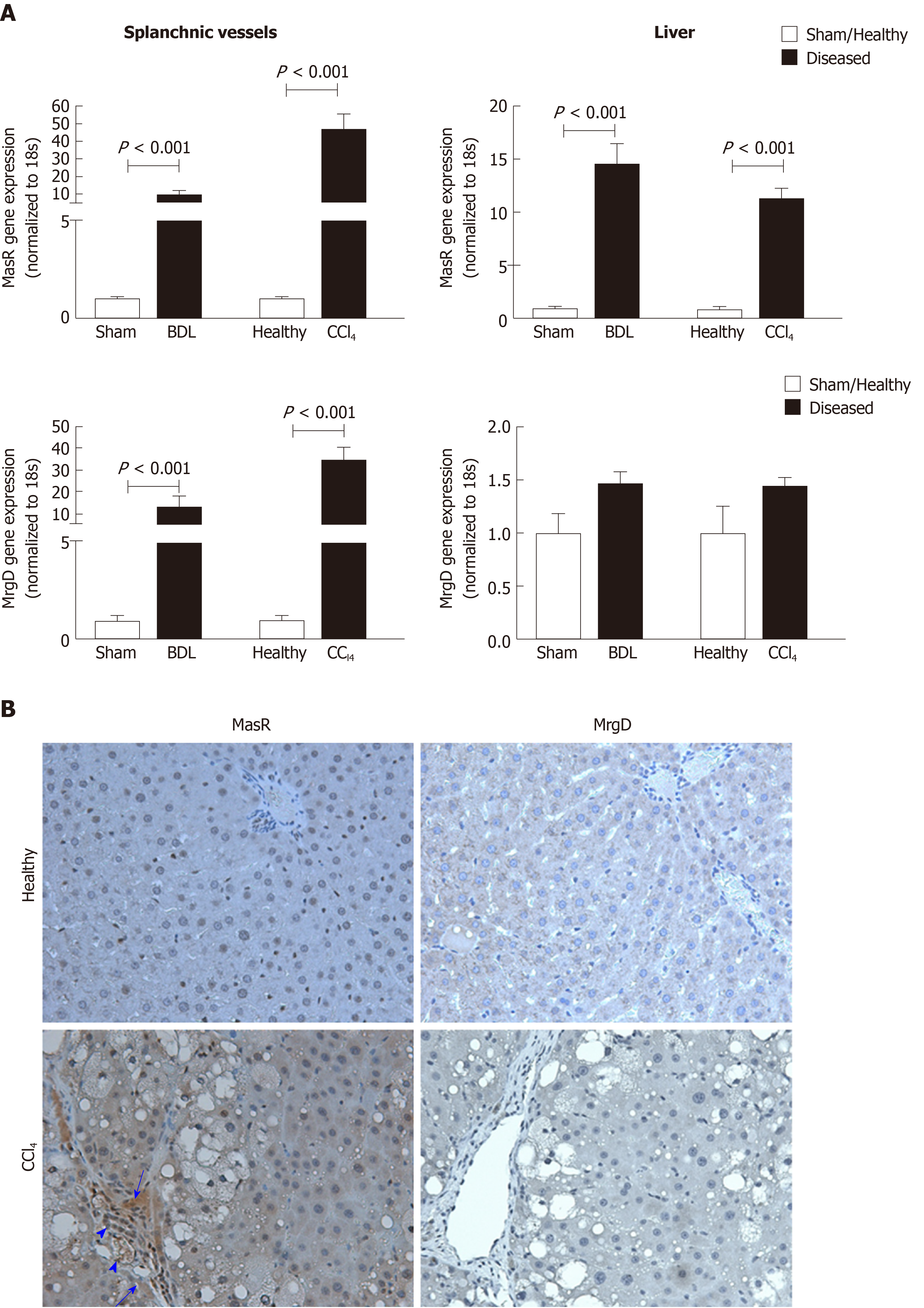
ACE2 and Ang-(1-7) production are increased in the splanchnic vasculature of cirrhotic rats and cirrhotic patients compared to healthy controls, suggesting a potential role of Ang-(1-7) in splanchnic vasodilatation in cirrhosis[22]. Interestingly, in the systemic circulation Ang-(1-7) levels are increased with the progression of liver disease but Ang II rises only after the establishment of portal hypertension[35,80,242]. In cirrhotic patients at liver transplant, the Ang-(1-7)/Ang II ratio is elevated in the splanchnic circulation compared to systemic circulation, and is negatively correlated with the systemic vascular resistance, suggesting that there is an augmented activity of local Ang-(1-7) in the splanchnic vascular compartment[242]. Functional evidence for this association comes from work published by our laboratory that infusion of Ang-(1-7) reduced splanchnic vascular resistance in cirrhotic rats to a much greater degree than in controls, suggesting that Ang-(1-7) is a key mediator of splanchnic vasodilatation in cirrhosis[22]. Consistent with enhanced Ang-(1-7) activity, MasR is also upregulated in both human and rat cirrhotic splanchnic vessels, and the MasR blocker A779 blocked the vasodilatory effects of Ang-(1-7) and greatly reduced splanchnic vasodilation in cirrhotic rats in vivo[22]. Moreover, we have recently demonstrated for the first time that in addition to MasR, the alternate receptor for Ang-(1-7), MrgD, is also upregulated in the splanchnic vessels of the cirrhotic rats[23]. This study demonstrated that both MasR and MrgD blockade improved portal hypertension, likely via the inhibition of Ang-(1-7) mediated splanchnic vasodilatation[23].
Dilatation of the splanchnic vascular bed has also been linked to intrinsic vascular hyporesponsive to vasoconstrictors including Ang II in cirrhosis[243-245]. Supporting this in vitro vessel work, vasoconstriction measured by evaluating forearm blood flow responses to intra-arterial administration of Ang II was found to be lower in cirrhotic patients compared to the healthy controls[246]. Mechanistic insights into this was provided by studies which demonstrated that AT1R expression was either normal or upregulated in splanchnic vessels from cirrhotic patients[81,83], suggesting that vascular hyporesponsive to vasopressors is likely due to the changes that occur downstream of AT1R[82,247]. In support of this phenomenon, Ferlitsch and colleagues[240] demonstrated that a reduced response of forearm blood flow to intra-arterial administration of vasopressors including Ang II in cirrhotic patients was completely restored to that in control subjects by coadministration with vitamin C, suggesting that generation of intracellular reactive oxygen species, which is closely linked to the activation of AT1R, may be responsible for hyporesponsive to Ang II. These findings were however not in agreement with earlier findings which showed that AT1R-independent G-protein stimulation with AlCl3/NaF induced an intact contractile response of hepatic artery which is similar in magnitude to that of the control vessels obtained from organ donors and suggested that hyporesponsive to Ang II may be related to a receptor-specific phenomenon localized upstream from the G-protein level[243]. In marked contrast to these findings however, recent work published by our laboratory using splanchnic arterial vessels isolated from liver transplant recipients demonstrated that they were not hyporesponsive to the pressor effect of Ang II[84]. Thus, there is conflicting literature data regarding the vasoactivity of splanchnic arteries[84,243,245] and forearm arteries[240,246] to vasopressors such as Ang II, implying more work is needed to clarify the vasoactivity of mesenteric arterial vessels in portal hypertension as not only different vascular beds may respond differently to vasopressors but also differences in vasoactive responses may be due to the compounding effects of different experimental conditions[248].
It is clear that the RAS contributes to the pathogenesis of portal hypertension. Whilst inhibition of classical RAS is expected to reduce intrahepatic vascular tone, inhibition of the alternate RAS in splanchnic vasculature would be expected to improve portal hypertension by increasing splanchnic vascular resistance. Thus, both the classical and the alternate RAS are potential targets that can be manipulated in portal hypertension.
Since the primary contribution of classical RAS to increased intrahepatic resistance in cirrhosis was identified through the evidence from studies in experimental cirrhosis, the effect of inhibition of the classical RAS has been studied in a number of clinical trials. The anti-portal hypertensive effect of ACEi such as captopril and enalapril, and ARBs such as losartan, candesartan and irbesartan have been studied. These drugs would be expected to reduce portal pressure by reducing the Ang II mediated increase in intrahepatic resistance in cirrhosis. In addition to reducing Ang II production from Ang I, ACE inhibition also prevents the degradation of vasodilatory Ang-(1-7), and would be expected to increase intrahepatic Ang-(1-7) concentration. Although ACEi and ARBs are widely used in clinical practice to treat systemic hypertension, relatively limited and variable data are available to assess and compare their effectiveness in liver disease and portal hypertension[20,249-252].
A comprehensive meta-analysis by Tandon and colleagues[26] summarized the findings of nineteen clinical trials that included three ACEi and nine ARB studies performed up until 2009. This analysis concluded that ACEi and/or ARBs were equally effective as NSBBs in early Child Pugh A cirrhosis where ACEi/ARBs showed a similar reduction in HVPG (17%) to NSBBs (21%). However, in advanced Child Pugh B or C cirrhosis, ACEi/ARB’s were not effective in reducing HVPG and showed only a 3% reduction whereas NSBBs were just as effective as in Child Pugh A disease. This study concluded that in early stages of cirrhosis, the RAS is a major player in increased intrahepatic vascular resistance. However, in advanced cirrhosis, ACEi/ARBs are ineffective in reducing portal pressure likely due in part to the activation of other vasoconstrictive pathways such as sympathetic tone and the endothelin system that increase intrahepatic vascular tone[26,241,253]. Furthermore, it is known that treatment with ACEi/ARBs is associated with a significant hypotensive effect and renal impairment in patients with advanced cirrhosis because a baseline activation of the RAS is vital to maintain adequate arterial pressure and renal perfusion[26,254,255].
In addition, the effects of ACEi/ARBs as antifibrotic therapy have been studied in a number of clinical trials. In addition to any effects of these drugs on intrahepatic tone, attenuation of fibrosis would be expected to lead to improvement of intrahepatic vascular resistance to the portal blood flow. An open label randomized clinical trial reported that ARB candesartan significantly improved hepatic fibrosis in patients with compensated alcoholic cirrhosis with small but significant decrease in mean arterial pressure[256]. A recent systematic meta-analysis, which was performed on four randomized controlled trials published up until 2014, summarized the antifibrotic effects of ACEi/ARBs and suggested efficacy of these drugs in alleviating hepatic fibrosis but no data were available on the effects on portal pressure[257].
As outlined above, there is considerable evidence that the components of the alternate RAS are upregulated in the hepatic, systemic and splanchnic circulations in cirrhosis[24,35,80,230]. It has been shown that vasodilatory actions of Ang-(1-7) is more prominent in the splanchnic vascular bed compared to the systemic vascular beds[258]. Indeed, in animal models, Ang-(1-7), the effector peptide of the alternate RAS, contributes to portal hypertension by reducing splanchnic vascular resistance, resulting in increased splanchnic blood flow[22]. The effects of Ang-(1-7) in splanchnic vascular bed appears to be mediated at least in part via the MasR, because the specific MasR blocker A779 increased splanchnic vascular resistance, thereby reducing splanchnic blood flow in cirrhotic rat models[22]. However, this treatment produced only a modest effect on portal hypertension, particularly in one animal model where A779 also increased intrahepatic vascular resistance.
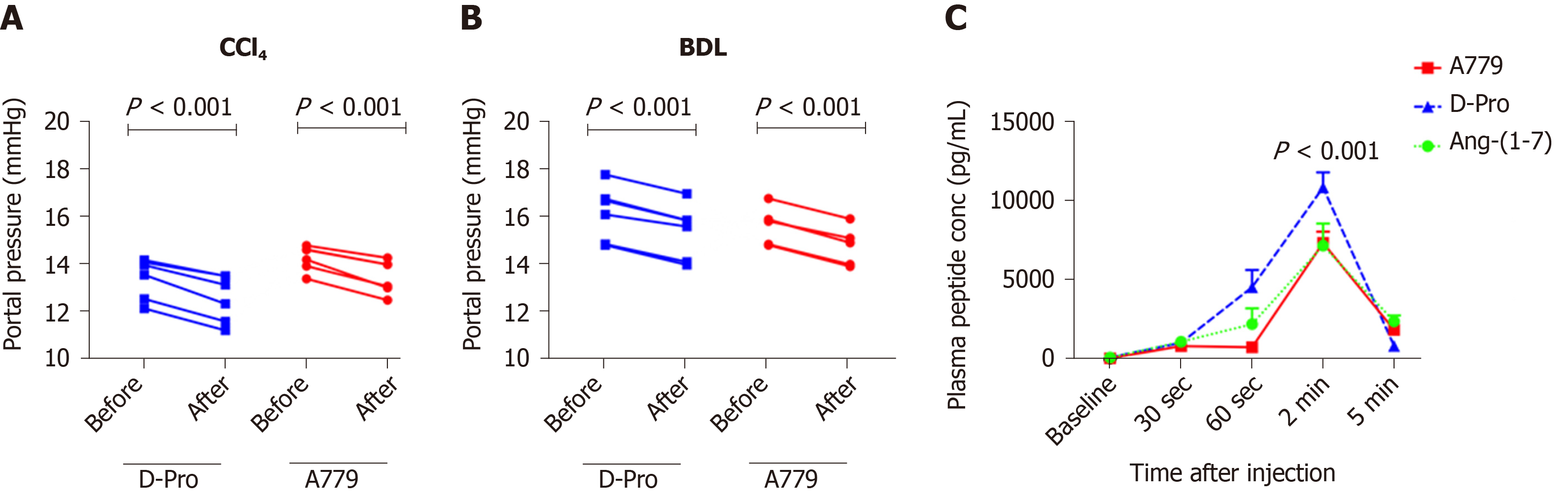
In addition to MasR, vasodilatory effects of Ang-(1-7) could also be mediated by the newly characterized MrgD[226]. Recent work by our laboratory demonstrated for the first time that similar to MasR, MrgD is also upregulated in the cirrhotic splanchnic vessels[23]. Moreover, a bolus injection of MrgD blocker D-Pro significantly reduced portal pressure in cirrhotic rat models (Figure 5A and B), and this reduction was similar to that of the MasR blockade. However, we noted that both MasR or MrgD blockers did not produce a clinically meaningful reduction in portal pressure (i.e., < 20% from the baseline) and the pressure lowering effect was only sustained for up to 20-25 min. This was possibly due to the rapid metabolism of these peptide blockers in the rat circulation (Figure 5C). Therefore, this study warranted further experiments in which continuous infusions of the receptor blockers should be employed to detect whether they have a clinically significant and meaningful effect on portal pressure in experimental models. Nevertheless, we made an important discovery in this study that unlike MasR, MrgD is not upregulated in the hepatic vascular bed of cirrhotic animals (Figure 6), suggesting that vasodilatory effects of MrgD may be confined to the splanchnic vascular bed in cirrhosis. This finding, if proven, is of central importance because MasR blockade with A779 did not lower portal pressure in the bile duct ligated cirrhotic model since the effect of A779 on hepatic MasR compromises the overall effect on portal pressure[22]. Thus, knowledge of the tissue-specific expression of MrgD is important since it could provide a guide for the development of novel therapies that specifically target splanchnic vasodilatation with minimal off-target effects.
In cirrhosis, portal hypertension is initiated by increased intrahepatic vascular resistance due to fixed obstruction of the portal vascular bed resulting from tissue fibrosis and other contractile cells. It is also associated with a hyperdynamic circulatory state characterized by a high cardiac output, increased total blood volume and splanchnic vasodilatation, resulting in increased mesenteric blood flow and this further increases portal pressure. Despite major advances in understanding the pathophysiology of portal hypertension, treatment options for this condition are limited. At present, NSBBs are the most widely accepted pharmacotherapy in clinical practice to prevent variceal bleeding. However, a significant proportion of cirrhotic patients are intolerant of NSBBs and fail to achieve an optimal therapeutic response. Therefore, there is an unmet major need to develop more safe, specific and effective pharmacotherapies for the treatment of portal hypertension.
Recent developments in understating the complex mechanisms of the pathogenesis of portal hypertension have opened up avenues for the development of novel therapies. These emerging therapeutic options target both the increased splanchnic vasodilatation and elevated intrahepatic vascular resistance. It is well known that the RAS is an important pathological system upregulated in both hepatic and splanchnic vascular beds in cirrhosis. Whilst the blockers of the classical RAS such as ACEi and ARBs can have a therapeutic effect by improving intrahepatic vascular tone via reduced hepatic fibrosis and vasoconstriction, they also cause adverse off-target effects such as systemic hypotension and renal failure. On the other hand, recent findings from studies that have investigated the potential use of the blockers of the components of the alternate RAS provided compelling evidence to justify the development of drugs targeting the splanchnic vascular bed to inhibit splanchnic vasodilatation in portal hypertension. In view of this, it is now evident that splanchnic vascular bed-specific inhibition of the alternate RAS may provide a better therapeutic option than manipulating the classical RAS in portal hypertension.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tripathi D S-Editor: Yan JP L-Editor: A P-Editor: Ma YJ
| 1. | GBD 2017 Cirrhosis Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:245-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1080] [Cited by in RCA: 1008] [Article Influence: 201.6] [Reference Citation Analysis (4)] |
| 2. | de Franchis R, Primignani M. Natural history of portal hypertension in patients with cirrhosis. Clin Liver Dis. 2001;5:645-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 231] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 3. | Khanna R, Sarin SK. Non-cirrhotic portal hypertension - diagnosis and management. J Hepatol. 2014;60:421-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 264] [Article Influence: 24.0] [Reference Citation Analysis (2)] |
| 4. | Bosch J, García-Pagán JC. Complications of cirrhosis. I. Portal hypertension. J Hepatol. 2000;32:141-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 338] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 5. | Tandon P, Garcia-Tsao G. Portal hypertension and hepatocellular carcinoma: prognosis and beyond. Clin Gastroenterol Hepatol. 2006;4:1318-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Abraldes JG, Bosch J. Clinical Features and Natural History of Variceal Hemorrhage. In: Sanyal AJ, Shah VH. Portal Hypertension Clinical Gastroenterology. Totwa, NJ: Humana Press Inc., 2005: 3-15. |
| 7. | Ripoll C, Groszmann R, Garcia-Tsao G, Grace N, Burroughs A, Planas R, Escorsell A, Garcia-Pagan JC, Makuch R, Patch D, Matloff DS, Bosch J; Portal Hypertension Collaborative Group. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133:481-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 747] [Cited by in RCA: 810] [Article Influence: 45.0] [Reference Citation Analysis (1)] |
| 8. | Rodríguez-Roisin R, Krowka MJ. Hepatopulmonary syndrome--a liver-induced lung vascular disorder. N Engl J Med. 2008;358:2378-2387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 422] [Article Influence: 24.8] [Reference Citation Analysis (1)] |
| 9. | Lange PA, Stoller JK. The hepatopulmonary syndrome. Ann Intern Med. 1995;122:521-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 214] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 10. | Salerno F, Gerbes A, Ginès P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56:1310-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 356] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 11. | Schrier RW, Arroyo V, Bernardi M, Epstein M, Henriksen JH, Rodés J. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988;8:1151-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1131] [Cited by in RCA: 1022] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 12. | Sanyal AJ, Bosch J, Blei A, Arroyo V. Portal hypertension and its complications. Gastroenterology. 2008;134:1715-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 240] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 13. | Grace ND, Groszmann RJ, Garcia-Tsao G, Burroughs AK, Pagliaro L, Makuch RW, Bosch J, Stiegmann GV, Henderson JM, de Franchis R, Wagner JL, Conn HO, Rodes J. Portal hypertension and variceal bleeding: an AASLD single topic symposium. Hepatology. 1998;28:868-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 259] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 14. | Garcia-Tsao G, Bosch J, Groszmann RJ. Portal hypertension and variceal bleeding--unresolved issues. Summary of an American Association for the study of liver diseases and European Association for the study of the liver single-topic conference. Hepatology. 2008;47:1764-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 172] [Article Influence: 10.1] [Reference Citation Analysis (1)] |
| 15. | Benoit JN, Womack WA, Hernandez L, Granger DN. "Forward" and "backward" flow mechanisms of portal hypertension. Relative contributions in the rat model of portal vein stenosis. Gastroenterology. 1985;89:1092-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 101] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Whipple AO. The Problem of Portal Hypertension in Relation to the Hepatosplenopathies. Ann Surg. 1945;122:449-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 236] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 17. | ATKINSON M, LOSOWSKY MS. The mechanism of ascites formation in chronic liver disease. Q J Med. 1961;30:153-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 18. | SHERLOCK S, SHALDON S. The aetiology and management of ascites in patients with hepatic cirrhosis: a review. Gut. 1963;4:95-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3381] [Cited by in RCA: 4119] [Article Influence: 206.0] [Reference Citation Analysis (3)] |
| 20. | Töx U, Steffen HM. Impact of inhibitors of the Renin-Angiotensin-aldosterone system on liver fibrosis and portal hypertension. Curr Med Chem. 2006;13:3649-3661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Herath CB, Grace JA, Angus PW. Therapeutic potential of targeting the renin angiotensin system in portal hypertension. World J Gastrointest Pathophysiol. 2013;4:1-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 22. | Grace JA, Klein S, Herath CB, Granzow M, Schierwagen R, Masing N, Walther T, Sauerbruch T, Burrell LM, Angus PW, Trebicka J. Activation of the MAS receptor by angiotensin-(1-7) in the renin-angiotensin system mediates mesenteric vasodilatation in cirrhosis. Gastroenterology 2013; 145: 874-884. e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 23. | Gunarathne LS, Angus PW, Herath CB. Blockade of Mas Receptor or Mas-Related G-Protein Coupled Receptor Type D Reduces Portal Pressure in Cirrhotic but Not in Non-cirrhotic Portal Hypertensive Rats. Front Physiol. 2019;10:1169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Herath CB, Lubel JS, Jia Z, Velkoska E, Casley D, Brown L, Tikellis C, Burrell LM, Angus PW. Portal pressure responses and angiotensin peptide production in rat liver are determined by relative activity of ACE and ACE2. Am J Physiol Gastrointest Liver Physiol. 2009;297:G98-G106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Herath CB, Mak K, Burrell LM, Angus PW. Angiotensin-(1-7) reduces the perfusion pressure response to angiotensin II and methoxamine via an endothelial nitric oxide-mediated pathway in cirrhotic rat liver. Am J Physiol Gastrointest Liver Physiol. 2013;304:G99-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Tandon P, Abraldes JG, Berzigotti A, Garcia-Pagan JC, Bosch J. Renin-angiotensin-aldosterone inhibitors in the reduction of portal pressure: a systematic review and meta-analysis. J Hepatol. 2010;53:273-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 27. | Martell M, Coll M, Ezkurdia N, Raurell I, Genescà J. Physiopathology of splanchnic vasodilation in portal hypertension. World J Hepatol. 2010;2:208-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 28. | Groszmann RJ, Abraldes JG. Portal hypertension: from bedside to bench. J Clin Gastroenterol. 2005;39:S125-S130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 29. | Bosch J, Groszmann RJ, Shah VH. Evolution in the understanding of the pathophysiological basis of portal hypertension: How changes in paradigm are leading to successful new treatments. J Hepatol. 2015;62:S121-S130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 171] [Article Influence: 17.1] [Reference Citation Analysis (1)] |
| 30. | Wiest R, Groszmann RJ. The paradox of nitric oxide in cirrhosis and portal hypertension: too much, not enough. Hepatology. 2002;35:478-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 308] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 31. | Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1096] [Cited by in RCA: 1380] [Article Influence: 98.6] [Reference Citation Analysis (0)] |
| 32. | Ramachandran P, Iredale JP. Reversibility of liver fibrosis. Ann Hepatol. 2009;8:283-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 33. | Bataller R, Ginès P, Nicolás JM, Görbig MN, Garcia-Ramallo E, Gasull X, Bosch J, Arroyo V, Rodés J. Angiotensin II induces contraction and proliferation of human hepatic stellate cells. Gastroenterology. 2000;118:1149-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 350] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 34. | Paizis G, Cooper ME, Schembri JM, Tikellis C, Burrell LM, Angus PW. Up-regulation of components of the renin-angiotensin system in the bile duct-ligated rat liver. Gastroenterology. 2002;123:1667-1676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 145] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 35. | Herath CB, Warner FJ, Lubel JS, Dean RG, Jia Z, Lew RA, Smith AI, Burrell LM, Angus PW. Upregulation of hepatic angiotensin-converting enzyme 2 (ACE2) and angiotensin-(1-7) levels in experimental biliary fibrosis. J Hepatol. 2007;47:387-395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 127] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 36. | Kowalczyk A, Kleniewska P, Kolodziejczyk M, Skibska B, Goraca A. The role of endothelin-1 and endothelin receptor antagonists in inflammatory response and sepsis. Arch Immunol Ther Exp (Warsz). 2015;63:41-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 207] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 37. | Pinzani M, Milani S, De Franco R, Grappone C, Caligiuri A, Gentilini A, Tosti-Guerra C, Maggi M, Failli P, Ruocco C, Gentilini P. Endothelin 1 is overexpressed in human cirrhotic liver and exerts multiple effects on activated hepatic stellate cells. Gastroenterology. 1996;110:534-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 264] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 38. | Fernandez M. Molecular pathophysiology of portal hypertension. Hepatology. 2015;61:1406-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 39. | Iwakiri Y. Endothelial dysfunction in the regulation of cirrhosis and portal hypertension. Liver Int. 2012;32:199-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 40. | Vorobioff JD, Groszmann RJ. Portal Hypertension – Molecular Mechanisms. In: Muriel P. Liver Pathophysiology- Therapies and Antioxidants. 1st ed. United Kingdom: Academic Press, 2017: 435-449. [DOI] [Full Text] |
| 41. | Rockey DC. Vasoactive agents in intrahepatic portal hypertension and fibrogenesis: implications for therapy. Gastroenterology. 2000;118:1261-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 42. | Rockey DC, Chung JJ. Reduced nitric oxide production by endothelial cells in cirrhotic rat liver: endothelial dysfunction in portal hypertension. Gastroenterology. 1998;114:344-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 268] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 43. | Shah V, Toruner M, Haddad F, Cadelina G, Papapetropoulos A, Choo K, Sessa WC, Groszmann RJ. Impaired endothelial nitric oxide synthase activity associated with enhanced caveolin binding in experimental cirrhosis in the rat. Gastroenterology. 1999;117:1222-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 233] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 44. | Liu S, Premont RT, Kontos CD, Zhu S, Rockey DC. A crucial role for GRK2 in regulation of endothelial cell nitric oxide synthase function in portal hypertension. Nat Med. 2005;11:952-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 195] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 45. | Morales-Ruiz M, Cejudo-Martín P, Fernández-Varo G, Tugues S, Ros J, Angeli P, Rivera F, Arroyo V, Rodés J, Sessa WC, Jiménez W. Transduction of the liver with activated Akt normalizes portal pressure in cirrhotic rats. Gastroenterology. 2003;125:522-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 46. | Matei V, Rodríguez-Vilarrupla A, Deulofeu R, Colomer D, Fernández M, Bosch J, Garcia-Pagán JC. The eNOS cofactor tetrahydrobiopterin improves endothelial dysfunction in livers of rats with CCl4 cirrhosis. Hepatology. 2006;44:44-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 88] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 47. | Trebicka J, Hennenberg M, Laleman W, Shelest N, Biecker E, Schepke M, Nevens F, Sauerbruch T, Heller J. Atorvastatin lowers portal pressure in cirrhotic rats by inhibition of RhoA/Rho-kinase and activation of endothelial nitric oxide synthase. Hepatology. 2007;46:242-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 238] [Article Influence: 13.2] [Reference Citation Analysis (2)] |
| 48. | Sumanovski LT, Battegay E, Stumm M, van der Kooij M, Sieber CC. Increased angiogenesis in portal hypertensive rats: role of nitric oxide. Hepatology. 1999;29:1044-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 73] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 49. | Abraldes JG, Iwakiri Y, Loureiro-Silva M, Haq O, Sessa WC, Groszmann RJ. Mild increases in portal pressure upregulate vascular endothelial growth factor and endothelial nitric oxide synthase in the intestinal microcirculatory bed, leading to a hyperdynamic state. Am J Physiol Gastrointest Liver Physiol. 2006;290:G980-G987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 139] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 50. | De las Heras D, Fernández J, Ginès P, Cárdenas A, Ortega R, Navasa M, Barberá JA, Calahorra B, Guevara M, Bataller R, Jiménez W, Arroyo V, Rodés J. Increased carbon monoxide production in patients with cirrhosis with and without spontaneous bacterial peritonitis. Hepatology. 2003;38:452-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 51. | Sarela AI, Mihaimeed FM, Batten JJ, Davidson BR, Mathie RT. Hepatic and splanchnic nitric oxide activity in patients with cirrhosis. Gut. 1999;44:749-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 80] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 52. | Niederberger M, Ginés P, Martin PY, Tsai P, Morris K, McMurtry I, Schrier RW. Comparison of vascular nitric oxide production and systemic hemodynamics in cirrhosis versus prehepatic portal hypertension in rats. Hepatology. 1996;24:947-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 53. | Grace JA, Herath CB, Mak KY, Burrell LM, Angus PW. Update on new aspects of the renin-angiotensin system in liver disease: clinical implications and new therapeutic options. Clin Sci. 123:225-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 54. | Cahill PA, Redmond EM, Hodges R, Zhang S, Sitzmann JV. Increased endothelial nitric oxide synthase activity in the hyperemic vessels of portal hypertensive rats. J Hepatol. 1996;25:370-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 100] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 55. | Hori N, Wiest R, Groszmann RJ. Enhanced release of nitric oxide in response to changes in flow and shear stress in the superior mesenteric arteries of portal hypertensive rats. Hepatology. 1998;28:1467-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 93] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 56. | Lee FY, Colombato LA, Albillos A, Groszmann RJ. N omega-nitro-L-arginine administration corrects peripheral vasodilation and systemic capillary hypotension and ameliorates plasma volume expansion and sodium retention in portal hypertensive rats. Hepatology. 1993;17:84-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 68] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 57. | Serna E, Mauricio MD, Lluch P, Segarra G, Cortina B, Lluch S, Medina P. Basal release of nitric oxide in the mesenteric artery in portal hypertension and cirrhosis: role of dimethylarginine dimethylaminohydrolase. J Gastroenterol Hepatol. 2013;28:880-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 58. | Angeli P, Fernández-Varo G, Dalla Libera V, Fasolato S, Galioto A, Arroyo V, Sticca A, Guarda S, Gatta A, Jiménez W. The role of nitric oxide in the pathogenesis of systemic and splanchnic vasodilation in cirrhotic rats before and after the onset of ascites. Liver Int. 2005;25:429-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 59. | Sieber CC, Lopez-Talavera JC, Groszmann RJ. Role of nitric oxide in the in vitro splanchnic vascular hyporeactivity in ascitic cirrhotic rats. Gastroenterology. 1993;104:1750-1754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 138] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 60. | Atucha NM, Shah V, García-Cardeña G, Sessa WE, Groszmann RJ. Role of endothelium in the abnormal response of mesenteric vessels in rats with portal hypertension and liver cirrhosis. Gastroenterology. 1996;111:1627-1632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 61. | Ros J, Jiménez W, Lamas S, Clària J, Arroyo V, Rivera F, Rodés J. Nitric oxide production in arterial vessels of cirrhotic rats. Hepatology. 1995;21:554-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 62. | Bolognesi M, Sacerdoti D, Di Pascoli M, Angeli P, Quarta S, Sticca A, Pontisso P, Merkel C, Gatta A. Haeme oxygenase mediates hyporeactivity to phenylephrine in the mesenteric vessels of cirrhotic rats with ascites. Gut. 2005;54:1630-1636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 63. | Mathie RT, Ralevic V, Moore KP, Burnstock G. Mesenteric vasodilator responses in cirrhotic rats: a role for nitric oxide? Hepatology. 1996;23:130-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 64. | Iwakiri Y, Cadelina G, Sessa WC, Groszmann RJ. Mice with targeted deletion of eNOS develop hyperdynamic circulation associated with portal hypertension. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1074-G1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 65. | Di Pascoli M, Sacerdoti D, Pontisso P, Angeli P, Bolognesi M. Molecular Mechanisms Leading to Splanchnic Vasodilation in Liver Cirrhosis. J Vasc Res. 2017;54:92-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 66. | Hamilton G, Phing RC, Hutton RA, Dandona P, Hobbs KE. The relationship between prostacyclin activity and pressure in the portal vein. Hepatology. 1982;2:236-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 67. | Fernández M, García-Pagán JC, Casadevall M, Mourelle MI, Piqué JM, Bosch J, Rodés J. Acute and chronic cyclooxygenase blockage in portal-hypertensive rats: influence in nitric oxide biosynthesis. Gastroenterology. 1996;110:1529-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 72] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 68. | Oberti F, Sogni P, Cailmail S, Moreau R, Pipy B, Lebrec D. Role of prostacyclin in hemodynamic alterations in conscious rats with extrahepatic or intrahepatic portal hypertension. Hepatology. 1993;18:621-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 60] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 69. | Bátkai S, Mukhopadhyay P, Harvey-White J, Kechrid R, Pacher P, Kunos G. Endocannabinoids acting at CB1 receptors mediate the cardiac contractile dysfunction in vivo in cirrhotic rats. Am J Physiol Heart Circ Physiol. 2007;293:H1689-H1695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 70. | Fiorucci S, Antonelli E, Mencarelli A, Orlandi S, Renga B, Rizzo G, Distrutti E, Shah V, Morelli A. The third gas: H2S regulates perfusion pressure in both the isolated and perfused normal rat liver and in cirrhosis. Hepatology. 2005;42:539-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 420] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 71. | Bauersachs J, Popp R, Hecker M, Sauer E, Fleming I, Busse R. Nitric oxide attenuates the release of endothelium-derived hyperpolarizing factor. Circulation. 1996;94:3341-3347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 305] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 72. | Adeagbo AS, Henzel MK. Calcium-dependent phospholipase A2 mediates the production of endothelium-derived hyperpolarizing factor in perfused rat mesenteric prearteriolar bed. J Vasc Res. 1998;35:27-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 73. | Shimokawa H, Yasutake H, Fujii K, Owada MK, Nakaike R, Fukumoto Y, Takayanagi T, Nagao T, Egashira K, Fujishima M, Takeshita A. The importance of the hyperpolarizing mechanism increases as the vessel size decreases in endothelium-dependent relaxations in rat mesenteric circulation. J Cardiovasc Pharmacol. 1996;28:703-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 520] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 74. | Quilley J, Fulton D, McGiff JC. Hyperpolarizing factors. Biochem Pharmacol. 1997;54:1059-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 114] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 75. | Sacerdoti D, Gatta A, McGiff JC. Role of cytochrome P450-dependent arachidonic acid metabolites in liver physiology and pathophysiology. Prostaglandins Other Lipid Mediat. 2003;72:51-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 76. | Iwakiri Y, Groszmann RJ. The hyperdynamic circulation of chronic liver diseases: from the patient to the molecule. Hepatology. 2006;43:S121-S131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 404] [Article Influence: 21.3] [Reference Citation Analysis (14)] |
| 77. | Du QH, Han L, Jiang JJ, Li PT, Wang XY, Jia X. Increased endothelin receptor B and G protein coupled kinase-2 in the mesentery of portal hypertensive rats. World J Gastroenterol. 2013;19:2065-2072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 78. | SHALDON C, PEACOCK JH, WALKER RM, PALMER DB, BADRICK FE. The portal venous content of adrenaline and noradrenaline in portal hypertension. Lancet. 1961;1:957-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 79. | Gaudin C, Ruget G, Braillon A, Selz F, Cuche JL, Lebrec D. Portal and arterial free and conjugated noradrenaline in two models of portal hypertension in rats. Life Sci. 1989;45:1333-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 80. | Lubel JS, Herath CB, Tchongue J, Grace J, Jia Z, Spencer K, Casley D, Crowley P, Sievert W, Burrell LM, Angus PW. Angiotensin-(1-7), an alternative metabolite of the renin-angiotensin system, is up-regulated in human liver disease and has antifibrotic activity in the bile-duct-ligated rat. Clin Sci. 117:375-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 81. | Hennenberg M, Trebicka J, Biecker E, Schepke M, Sauerbruch T, Heller J. Vascular dysfunction in human and rat cirrhosis: role of receptor-desensitizing and calcium-sensitizing proteins. Hepatology. 2007;45:495-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 82. | Hennenberg M, Biecker E, Trebicka J, Jochem K, Zhou Q, Schmidt M, Jakobs KH, Sauerbruch T, Heller J. Defective RhoA/Rho-kinase signaling contributes to vascular hypocontractility and vasodilation in cirrhotic rats. Gastroenterology. 2006;130:838-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 83. | Neef M, Biecker E, Heller J, Schepke M, Nischalke HD, Wolff M, Spengler U, Reichen J, Sauerbruch T. Portal hypertension is associated with increased mRNA levels of vasopressor G-protein-coupled receptors in human hepatic arteries. Eur J Clin Invest. 2003;33:249-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 84. | Casey S, Herath C, Rajapaksha I, Jones R, Angus P. Effects of angiotensin-(1-7) and angiotensin II on vascular tone in human cirrhotic splanchnic vessels. Peptides. 2018;108:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 85. | Fernández M, Semela D, Bruix J, Colle I, Pinzani M, Bosch J. Angiogenesis in liver disease. J Hepatol. 2009;50:604-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 445] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 86. | Mejias M, Garcia-Pras E, Tiani C, Miquel R, Bosch J, Fernandez M. Beneficial effects of sorafenib on splanchnic, intrahepatic, and portocollateral circulations in portal hypertensive and cirrhotic rats. Hepatology. 2009;49:1245-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 247] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 87. | Reiberger T, Angermayr B, Schwabl P, Rohr-Udilova N, Mitterhauser M, Gangl A, Peck-Radosavljevic M. Sorafenib attenuates the portal hypertensive syndrome in partial portal vein ligated rats. J Hepatol. 2009;51:865-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 88. | Coriat R, Gouya H, Mir O, Ropert S, Vignaux O, Chaussade S, Sogni P, Pol S, Blanchet B, Legmann P, Goldwasser F. Reversible decrease of portal venous flow in cirrhotic patients: a positive side effect of sorafenib. PLoS One. 2011;6:e16978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 89. | Angermayr B, Fernandez M, Mejias M, Gracia-Sancho J, Garcia-Pagan JC, Bosch J. NAD(P)H oxidase modulates angiogenesis and the development of portosystemic collaterals and splanchnic hyperaemia in portal hypertensive rats. Gut. 2007;56:560-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 90. | Kumar A, Sharma P, Sarin SK. Hepatic venous pressure gradient measurement: time to learn! Indian J Gastroenterol. 2008;27:74-80. [PubMed] |
| 91. | Garcia-Tsao G. The Use of Nonselective Beta Blockers for Treatment of Portal Hypertension. Gastroenterol Hepatol. 13:617-619. [PubMed] |
| 92. | Merkel C, Bolognesi M, Sacerdoti D, Bombonato G, Bellini B, Bighin R, Gatta A. The hemodynamic response to medical treatment of portal hypertension as a predictor of clinical effectiveness in the primary prophylaxis of variceal bleeding in cirrhosis. Hepatology. 2000;32:930-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 165] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 93. | Groszmann RJ, Wongcharatrawee S. The hepatic venous pressure gradient: anything worth doing should be done right. Hepatology. 2004;39:280-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 390] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 94. | Bouhassira D, Sabaté JM, Coffin B, Le Bars D, Willer JC, Jian R. Effects of rectal distensions on nociceptive flexion reflexes in humans. Am J Physiol. 1998;275:G410-G417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 95. | Procopet B, Cristea VM, Robic MA, Grigorescu M, Agachi PS, Metivier S, Peron JM, Selves J, Stefanescu H, Berzigotti A, Vinel JP, Bureau C. Serum tests, liver stiffness and artificial neural networks for diagnosing cirrhosis and portal hypertension. Dig Liver Dis. 2015;47:411-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 96. | Berzigotti A, Ashkenazi E, Reverter E, Abraldes JG, Bosch J. Non-invasive diagnostic and prognostic evaluation of liver cirrhosis and portal hypertension. Dis Markers. 2011;31:129-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 97. | Marozas M, Zykus R, Sakalauskas A, Kupčinskas L, Lukoševičius A. Noninvasive Evaluation of Portal Hypertension Using a Supervised Learning Technique. J Healthc Eng. 2017;2017:6183714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 98. | Angeli P, Ginès P, Wong F, Bernardi M, Boyer TD, Gerbes A, Moreau R, Jalan R, Sarin SK, Piano S, Moore K, Lee SS, Durand F, Salerno F, Caraceni P, Kim WR, Arroyo V, Garcia-Tsao G. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. J Hepatol. 2015;62:968-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 527] [Article Influence: 52.7] [Reference Citation Analysis (1)] |
| 99. | Wong F, Angeli P. New diagnostic criteria and management of acute kidney injury. J Hepatol. 2017;66:860-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 100. | Garcia-Tsao G, Sanyal AJ, Grace ND, Carey WD; Practice Guidelines Committee of American Association for Study of Liver Diseases; Practice Parameters Committee of American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Am J Gastroenterol. 2007;102:2086-2102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 256] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 101. | Dell'era A, Bosch J. Review article: the relevance of portal pressure and other risk factors in acute gastro-oesophageal variceal bleeding. Aliment Pharmacol Ther. 2004;20 Suppl 3:8-15; discussion 16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 102. | Zipprich A, Garcia-Tsao G, Rogowski S, Fleig WE, Seufferlein T, Dollinger MM. Prognostic indicators of survival in patients with compensated and decompensated cirrhosis. Liver Int. 2012;32:1407-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 197] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 103. | Haq I, Tripathi D. Recent advances in the management of variceal bleeding. Gastroenterol Rep (Oxf). 5:113-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 104. | Graham DY, Smith JL. The course of patients after variceal hemorrhage. Gastroenterology. 1981;80:800-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 601] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 105. | D'Amico G, Pagliaro L, Bosch J. The treatment of portal hypertension: a meta-analytic review. Hepatology. 1995;22:332-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 467] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 106. | Hernández-Gea V, Berzigotti A. Clinical Evaluation and Prognosis. Dig Dis. 2015;33:515-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 107. | Chalasani N, Kahi C, Francois F, Pinto A, Marathe A, Bini EJ, Pandya P, Sitaraman S, Shen J. Improved patient survival after acute variceal bleeding: a multicenter, cohort study. Am J Gastroenterol. 2003;98:653-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 108. | Lebrec D, Poynard T, Hillon P, Benhamou JP. Propranolol for prevention of recurrent gastrointestinal bleeding in patients with cirrhosis: a controlled study. N Engl J Med. 1981;305:1371-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 345] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 109. | Pascal JP, Cales P. Propranolol in the prevention of first upper gastrointestinal tract hemorrhage in patients with cirrhosis of the liver and esophageal varices. N Engl J Med. 1987;317:856-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 220] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 110. | Poynard T, Calès P, Pasta L, Ideo G, Pascal JP, Pagliaro L, Lebrec D. Beta-adrenergic-antagonist drugs in the prevention of gastrointestinal bleeding in patients with cirrhosis and esophageal varices. An analysis of data and prognostic factors in 589 patients from four randomized clinical trials. Franco-Italian Multicenter Study Group. N Engl J Med. 1991;324:1532-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 312] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 111. | Poynard T, Lebrec D, Hillon P, Sayegh R, Bernuau J, Naveau S, Chaput JC, Klepping C, Rueff B, Benhamou JP. Propranolol for prevention of recurrent gastrointestinal bleeding in patients with cirrhosis: a prospective study of factors associated with rebleeding. Hepatology. 1987;7:447-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 70] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 112. | Idéo G, Bellati G, Fesce E, Grimoldi D. Nadolol can prevent the first gastrointestinal bleeding in cirrhotics: a prospective, randomized study. Hepatology. 1988;8:6-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 106] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 113. | Pagliaro L, D'Amico G, Sörensen TI, Lebrec D, Burroughs AK, Morabito A, Tiné F, Politi F, Traina M. Prevention of first bleeding in cirrhosis. A meta-analysis of randomized trials of nonsurgical treatment. Ann Intern Med. 1992;117:59-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 222] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 114. | Sarin SK, Groszmann RJ, Mosca PG, Rojkind M, Stadecker MJ, Bhatnagar R, Reuben A, Dayal Y. Propranolol ameliorates the development of portal-systemic shunting in a chronic murine schistosomiasis model of portal hypertension. J Clin Invest. 1991;87:1032-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 67] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 115. | Ohnishi K, Nakayama T, Saito M, Hatano H, Tsukamoto T, Terabayashi H, Sugita S, Wada K, Nomura F, Koen H. Effects of propranolol on portal hemodynamics in patients with chronic liver disease. Am J Gastroenterol. 1985;80:132-135. [PubMed] |
| 116. | Mastai R, Bosch J, Navasa M, Kravetz D, Bruix J, Viola C, Rodés J. Effects of alpha-adrenergic stimulation and beta-adrenergic blockade on azygos blood flow and splanchnic haemodynamics in patients with cirrhosis. J Hepatol. 1987;4:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 117. | Groszmann RJ, Garcia-Tsao G, Bosch J, Grace ND, Burroughs AK, Planas R, Escorsell A, Garcia-Pagan JC, Patch D, Matloff DS, Gao H, Makuch R; Portal Hypertension Collaborative Group. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med. 2005;353:2254-2261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 714] [Cited by in RCA: 653] [Article Influence: 32.7] [Reference Citation Analysis (1)] |
| 118. | de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2011] [Cited by in RCA: 2293] [Article Influence: 229.3] [Reference Citation Analysis (3)] |
| 119. | Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1439] [Article Influence: 179.9] [Reference Citation Analysis (3)] |
| 120. | Tripathi D, Stanley AJ, Hayes PC, Patch D, Millson C, Mehrzad H, Austin A, Ferguson JW, Olliff SP, Hudson M, Christie JM; Clinical Services and Standards Committee of the British Society of Gastroenterology. U.K. guidelines on the management of variceal haemorrhage in cirrhotic patients. Gut. 2015;64:1680-1704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 454] [Cited by in RCA: 416] [Article Influence: 41.6] [Reference Citation Analysis (2)] |
| 121. | Merkel C, Sacerdoti D, Bolognesi M, Buonamico P, Sticca A, Amodio P, Angeli P, Micotti L, Gatta A. Effect of chronic treatment with nadolol plus isosorbide mononitrate on liver blood flow and liver metabolic activity in cirrhosis. Eur J Gastroenterol Hepatol. 1999;11:1221-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 122. | D'Amico G, Pagliaro L, Bosch J. Pharmacological treatment of portal hypertension: an evidence-based approach. Semin Liver Dis. 1999;19:475-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 600] [Cited by in RCA: 484] [Article Influence: 18.6] [Reference Citation Analysis (1)] |
| 123. | Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W; Practice Guidelines Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1229] [Cited by in RCA: 1210] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 124. | García-Pagán JC, Villanueva C, Vila MC, Albillos A, Genescà J, Ruiz-Del-Arbol L, Planas R, Rodriguez M, Calleja JL, González A, Solà R, Balanzó J, Bosch J; MOVE Group. Mononitrato Varices Esofágicas. Isosorbide mononitrate in the prevention of first variceal bleed in patients who cannot receive beta-blockers. Gastroenterology. 2001;121:908-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 76] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 125. | Ge PS, Runyon BA. The changing role of beta-blocker therapy in patients with cirrhosis. J Hepatol. 2014;60:643-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 126. | Garcia-Tsao G, Lim JK; Members of Veterans Affairs Hepatitis C Resource Center Program. Management and treatment of patients with cirrhosis and portal hypertension: recommendations from the Department of Veterans Affairs Hepatitis C Resource Center Program and the National Hepatitis C Program. Am J Gastroenterol. 2009;104:1802-1829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 173] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 127. | López-Méndez E, Uribe M. Beta blockers in portal hypertension. Are they really a good option? Ann Hepatol. 2006;5:86-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 128. | Sersté T, Melot C, Francoz C, Durand F, Rautou PE, Valla D, Moreau R, Lebrec D. Deleterious effects of beta-blockers on survival in patients with cirrhosis and refractory ascites. Hepatology. 2010;52:1017-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 376] [Article Influence: 25.1] [Reference Citation Analysis (1)] |
| 129. | Mandorfer M, Bota S, Schwabl P, Bucsics T, Pfisterer N, Kruzik M, Hagmann M, Blacky A, Ferlitsch A, Sieghart W, Trauner M, Peck-Radosavljevic M, Reiberger T. Nonselective β blockers increase risk for hepatorenal syndrome and death in patients with cirrhosis and spontaneous bacterial peritonitis. Gastroenterology 2014; 146: 1680-90. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 280] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 130. | Blasco-Algora S, Masegosa-Ataz J, Alonso S, Gutiérrez ML, Fernández-Rodriguez C. Non-selective β-blockers in advanced cirrhosis: a critical review of the effects on overall survival and renal function. BMJ Open Gastroenterol. 2016;3:e000104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 131. | Bolognesi M, Balducci G, Garcia-Tsao G, Gatta A, Gines P, Merli M, Rodes J, Stiegmann GV. Complications in the medical treatment of portal hypertension. In: de Franchis R. Portal Hypertension III: Proceedings of the Third Baverno International Consensus Workshop on Definitions, Methodology and Therapeutic Strategies. Oxford: Blackwell Science, 2001. [DOI] [Full Text] |
| 132. | Tripathi D, Hayes PC. Beta-blockers in portal hypertension: new developments and controversies. Liver Int. 2014;34:655-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 133. | Escorsell A, Ferayorni L, Bosch J, García-Pagán JC, García-Tsao G, Grace ND, Rodés J, Groszmann RJ. The portal pressure response to beta-blockade is greater in cirrhotic patients without varices than in those with varices. Gastroenterology. 1997;112:2012-2016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 134. | Groszmann RJ, Bosch J, Grace ND, Conn HO, Garcia-Tsao G, Navasa M, Alberts J, Rodes J, Fischer R, Bermann M. Hemodynamic events in a prospective randomized trial of propranolol versus placebo in the prevention of a first variceal hemorrhage. Gastroenterology. 1990;99:1401-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 452] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 135. | Sinagra E, Perricone G, D'Amico M, Tinè F, D'Amico G. Systematic review with meta-analysis: the haemodynamic effects of carvedilol compared with propranolol for portal hypertension in cirrhosis. Aliment Pharmacol Ther. 2014;39:557-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 136. | Bañares R, Moitinho E, Piqueras B, Casado M, García-Pagán JC, de Diego A, Bosch J. Carvedilol, a new nonselective beta-blocker with intrinsic anti- Alpha1-adrenergic activity, has a greater portal hypotensive effect than propranolol in patients with cirrhosis. Hepatology. 1999;30:79-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 134] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 137. | Bosch J. Carvedilol for portal hypertension in patients with cirrhosis. Hepatology. 2010;51:2214-2218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 138. | Bosch J. Carvedilol: the β-blocker of choice for portal hypertension? Gut. 2013;62:1529-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 139. | Bañares R, Moitinho E, Matilla A, García-Pagán JC, Lampreave JL, Piera C, Abraldes JG, De Diego A, Albillos A, Bosch J. Randomized comparison of long-term carvedilol and propranolol administration in the treatment of portal hypertension in cirrhosis. Hepatology. 2002;36:1367-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 75] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 140. | Freeman JG, Barton JR, Record CO. Effect of isosorbide dinitrate, verapamil, and labetalol on portal pressure in cirrhosis. Br Med J (Clin Res Ed). 1985;291:561-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 57] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 141. | Zacharias AP, Jeyaraj R, Hobolth L, Bendtsen F, Gluud LL, Morgan MY. Carvedilol versus traditional, non-selective beta-blockers for adults with cirrhosis and gastroesophageal varices. Cochrane Database Syst Rev. 2018;10:CD011510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (18)] |
| 142. | Trust KsCHN. Beta-blockers for Oesophageal Varices (BOPPP). [accessed 2018 Dec 17]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT03776955 ClinicalTrials.gov Identifier: NCT03776955. |
| 143. | Tripathi D, Hayes PC, Richardson P, Rowe I, Ferguson J, Devine P, Mathers J, Poyner C, Jowett S, Handley K, Grant M, Slinn G, Ahmed K, Brocklehurst P. Study protocol for a randomised controlled trial of carvedilol versus variceal band ligation in primary prevention of variceal bleeding in liver cirrhosis (CALIBRE trial). BMJ Open Gastroenterol. 2019;6:e000290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 144. | Mills PR, Rae AP, Farah DA, Russell RI, Lorimer AR, Carter DC. Comparison of three adrenoreceptor blocking agents in patients with cirrhosis and portal hypertension. Gut. 1984;25:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 81] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 145. | Hillon P, Lebrec D, Muńoz C, Jungers M, Goldfarb G, Benhamou JP. Comparison of the effects of a cardioselective and a nonselective beta-blocker on portal hypertension in patients with cirrhosis. Hepatology. 1982;2:528-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 100] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 146. | Westaby D, Melia WM, Macdougall BR, Hegarty JE, Gimson AE, Williams R. B1 selective adrenoreceptor blockade for the long term management of variceal bleeding. A prospective randomised trial to compare oral metoprolol with injection sclerotherapy in cirrhosis. Gut. 1985;26:421-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 147. | Groszmann RJ, Kravetz D, Bosch J, Glickman M, Bruix J, Bredfeldt J, Conn HO, Rodes J, Storer EH. Nitroglycerin improves the hemodynamic response to vasopressin in portal hypertension. Hepatology. 1982;2:757-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 167] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 148. | Ioannou G, Doust J, Rockey DC. Terlipressin for acute esophageal variceal hemorrhage. Cochrane Database Syst Rev. 2003;: CD002147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 149. | Walker S, Stiehl A, Raedsch R, Kommerell B. Terlipressin in bleeding esophageal varices: a placebo-controlled, double-blind study. Hepatology. 1986;6:112-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 36] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 150. | Fortune BE, Jackson J, Leonard J, Trotter JF. Vapreotide: a somatostatin analog for the treatment of acute variceal bleeding. Expert Opin Pharmacother. 2009;10:2337-2342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 151. | Wang C, Han J, Xiao L, Jin CE, Li DJ, Yang Z. Efficacy of vasopressin/terlipressin and somatostatin/octreotide for the prevention of early variceal rebleeding after the initial control of bleeding: a systematic review and meta-analysis. Hepatol Int. 2015;9:120-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 152. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1777] [Cited by in RCA: 1816] [Article Influence: 259.4] [Reference Citation Analysis (2)] |
| 153. | Bari K, Garcia-Tsao G. Treatment of portal hypertension. World J Gastroenterol. 2012;18:1166-1175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 95] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (1)] |
| 154. | Escorsell A, Bandi JC, Andreu V, Moitinho E, García-Pagán JC, Bosch J, Rodés J. Desensitization to the effects of intravenous octreotide in cirrhotic patients with portal hypertension. Gastroenterology. 2001;120:161-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 132] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 155. | Bureau C, Péron JM, Alric L, Morales J, Sanchez J, Barange K, Payen JL, Vinel JP. "A La Carte" treatment of portal hypertension: Adapting medical therapy to hemodynamic response for the prevention of bleeding. Hepatology. 2002;36:1361-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 156. | García-Pagán JC, Feu F, Bosch J, Rodés J. Propranolol compared with propranolol plus isosorbide-5-mononitrate for portal hypertension in cirrhosis. A randomized controlled study. Ann Intern Med. 1991;114:869-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 172] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 157. | Albillos A, Lledó JL, Bañares R, Rossi I, Iborra J, Calleja JL, Garrido A, Escartin P, Bosch J. Hemodynamic effects of alpha-adrenergic blockade with prazosin in cirrhotic patients with portal hypertension. Hepatology. 1994;20:611-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 158. | Albillos A, García-Pagán JC, Iborra J, Bandi JC, Cacho G, Pérez-Paramo M, Escorsell A, Calleja JL, Escartín P, Bosch J. Propranolol plus prazosin compared with propranolol plus isosorbide-5-mononitrate in the treatment of portal hypertension. Gastroenterology. 1998;115:116-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 72] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 159. | Trebicka J, Hennenberg M, Odenthal M, Shir K, Klein S, Granzow M, Vogt A, Dienes HP, Lammert F, Reichen J, Heller J, Sauerbruch T. Atorvastatin attenuates hepatic fibrosis in rats after bile duct ligation via decreased turnover of hepatic stellate cells. J Hepatol. 2010;53:702-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 185] [Article Influence: 12.3] [Reference Citation Analysis (1)] |
| 160. | Abraldes JG, Rodríguez-Vilarrupla A, Graupera M, Zafra C, García-Calderó H, García-Pagán JC, Bosch J. Simvastatin treatment improves liver sinusoidal endothelial dysfunction in CCl4 cirrhotic rats. J Hepatol. 2007;46:1040-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 178] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 161. | Uschner FE, Ranabhat G, Choi SS, Granzow M, Klein S, Schierwagen R, Raskopf E, Gautsch S, van der Ven PF, Fürst DO, Strassburg CP, Sauerbruch T, Diehl AM, Trebicka J. Statins activate the canonical hedgehog-signaling and aggravate non-cirrhotic portal hypertension, but inhibit the non-canonical hedgehog signaling and cirrhotic portal hypertension. Sci Rep. 2015;5:14573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 162. | Kim RG, Loomba R, Prokop LJ, Singh S. Statin Use and Risk of Cirrhosis and Related Complications in Patients With Chronic Liver Diseases: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2017; 15: 1521-1530. e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 131] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 163. | Abraldes JG, Albillos A, Bañares R, Turnes J, González R, García-Pagán JC, Bosch J. Simvastatin lowers portal pressure in patients with cirrhosis and portal hypertension: a randomized controlled trial. Gastroenterology. 2009;136:1651-1658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 324] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 164. | Abraldes JG, Villanueva C, Aracil C, Turnes J, Hernandez-Guerra M, Genesca J, Rodriguez M, Castellote J, García-Pagán JC, Torres F, Calleja JL, Albillos A, Bosch J; BLEPS Study Group. Addition of Simvastatin to Standard Therapy for the Prevention of Variceal Rebleeding Does Not Reduce Rebleeding but Increases Survival in Patients With Cirrhosis. Gastroenterology 2016; 150: 1160-1170. e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 223] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 165. | Pose E, Napoleone L, Amin A, Campion D, Jimenez C, Piano S, Roux O, Uschner FE, de Wit K, Zaccherini G, Alessandria C, Angeli P, Bernardi M, Beuers U, Caraceni P, Durand F, Mookerjee RP, Trebicka J, Vargas V, Andrade RJ, Carol M, Pich J, Ferrero J, Domenech G, Llopis M, Torres F, Kamath PS, Abraldes JG, Solà E, Ginès P. Safety of two different doses of simvastatin plus rifaximin in decompensated cirrhosis (LIVERHOPE-SAFETY): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Gastroenterol Hepatol. 2020;5:31-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (1)] |
| 166. | Berzigotti A, Bellot P, De Gottardi A, Garcia-Pagan JC, Gagnon C, Spénard J, Bosch J. NCX-1000, a nitric oxide-releasing derivative of UDCA, does not decrease portal pressure in patients with cirrhosis: results of a randomized, double-blind, dose-escalating study. Am J Gastroenterol. 2010;105:1094-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 167. | Hernández-Guerra M, García-Pagán JC, Turnes J, Bellot P, Deulofeu R, Abraldes JG, Bosch J. Ascorbic acid improves the intrahepatic endothelial dysfunction of patients with cirrhosis and portal hypertension. Hepatology. 2006;43:485-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 168. | Hsu SJ, Lee FY, Wang SS, Hsin IF, Lin TY, Huang HC, Chang CC, Chuang CL, Ho HL, Lin HC, Lee SD. Caffeine ameliorates hemodynamic derangements and portosystemic collaterals in cirrhotic rats. Hepatology. 2015;61:1672-1684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 169. | De Gottardi A, Berzigotti A, Seijo S, D'Amico M, Thormann W, Abraldes JG, García-Pagán JC, Bosch J. Postprandial effects of dark chocolate on portal hypertension in patients with cirrhosis: results of a phase 2, double-blind, randomized controlled trial. Am J Clin Nutr. 2012;96:584-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 170. | Bosch J, Abraldes JG, Fernández M, García-Pagán JC. Hepatic endothelial dysfunction and abnormal angiogenesis: new targets in the treatment of portal hypertension. J Hepatol. 2010;53:558-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 152] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 171. | Albillos A, Bañares R, Barrios C, Clemente G, Rossi I, Escartin P, Bosch J. Oral administration of clonidine in patients with alcoholic cirrhosis. Hemodynamic and liver function effects. Gastroenterology. 1992;102:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 172. | Blendis LM. Clonidine for portal hypertension: a sympathetic solution? Ann Intern Med. 1992;116:515-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 173. | Simões E Silva AC, Miranda AS, Rocha NP, Teixeira AL. Renin angiotensin system in liver diseases: Friend or foe? World J Gastroenterol. 2017;23:3396-3406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 81] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (1)] |
| 174. | Ferrario CM. New physiological concepts of the renin-angiotensin system from the investigation of precursors and products of angiotensin I metabolism. Hypertension. 2010;55:445-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 175. | Lubel JS, Herath CB, Burrell LM, Angus PW. Liver disease and the renin-angiotensin system: recent discoveries and clinical implications. J Gastroenterol Hepatol. 2008;23:1327-1338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 176. | Campbell DJ. Clinical relevance of local Renin Angiotensin systems. Front Endocrinol. 5:113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 177. | Bataller R, Gäbele E, Schoonhoven R, Morris T, Lehnert M, Yang L, Brenner DA, Rippe RA. Prolonged infusion of angiotensin II into normal rats induces stellate cell activation and proinflammatory events in liver. Am J Physiol Gastrointest Liver Physiol. 2003;285:G642-G651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 105] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 178. | Kagami S, Border WA, Miller DE, Noble NA. Angiotensin II stimulates extracellular matrix protein synthesis through induction of transforming growth factor-beta expression in rat glomerular mesangial cells. J Clin Invest. 1994;93:2431-2437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 750] [Cited by in RCA: 765] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 179. | Mezzano SA, Ruiz-Ortega M, Egido J. Angiotensin II and renal fibrosis. Hypertension. 2001;38:635-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 378] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 180. | Ruiz-Ortega M, Rupérez M, Esteban V, Rodríguez-Vita J, Sánchez-López E, Carvajal G, Egido J. Angiotensin II: a key factor in the inflammatory and fibrotic response in kidney diseases. Nephrol Dial Transplant. 2006;21:16-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 249] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 181. | Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, Breitbart RE, Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000;87:E1-E9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2045] [Cited by in RCA: 2188] [Article Influence: 87.5] [Reference Citation Analysis (0)] |
| 182. | Oudit GY, Crackower MA, Backx PH, Penninger JM. The role of ACE2 in cardiovascular physiology. Trends Cardiovasc Med. 2003;13:93-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 197] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 183. | Burrell LM, Johnston CI, Tikellis C, Cooper ME. ACE2, a new regulator of the renin-angiotensin system. Trends Endocrinol Metab. 2004;15:166-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 247] [Cited by in RCA: 269] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 184. | Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, Godbout K, Parsons T, Baronas E, Hsieh F, Acton S, Patane M, Nichols A, Tummino P. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem. 2002;277:14838-14843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1073] [Cited by in RCA: 1119] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 185. | Guy JL, Jackson RM, Acharya KR, Sturrock ED, Hooper NM, Turner AJ. Angiotensin-converting enzyme-2 (ACE2): comparative modeling of the active site, specificity requirements, and chloride dependence. Biochemistry. 2003;42:13185-13192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 148] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 186. | Zisman LS, Meixell GE, Bristow MR, Canver CC. Angiotensin-(1-7) formation in the intact human heart: in vivo dependence on angiotensin II as substrate. Circulation. 2003;108:1679-1681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 87] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 187. | Rice GI, Thomas DA, Grant PJ, Turner AJ, Hooper NM. Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochem J. 2004;383:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 467] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 188. | Yamamoto K, Chappell MC, Brosnihan KB, Ferrario CM. In vivo metabolism of angiotensin I by neutral endopeptidase (EC 3.4.24.11) in spontaneously hypertensive rats. Hypertension. 1992;19:692-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 145] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 189. | Schiavone MT, Santos RA, Brosnihan KB, Khosla MC, Ferrario CM. Release of vasopressin from the rat hypothalamo-neurohypophysial system by angiotensin-(1-7) heptapeptide. Proc Natl Acad Sci USA. 1988;85:4095-4098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 213] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 190. | Chappell MC, Brosnihan KB, Diz DI, Ferrario CM. Identification of angiotensin-(1-7) in rat brain. Evidence for differential processing of angiotensin peptides. J Biol Chem. 1989;264:16518-16523. [PubMed] |
| 191. | Ferrario CM, Brosnihan KB, Diz DI, Jaiswal N, Khosla MC, Milsted A, Tallant EA. Angiotensin-(1-7): a new hormone of the angiotensin system. Hypertension. 1991;18:III126-III133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 96] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 192. | Mahon JM, Carr RD, Nicol AK, Henderson IW. Angiotensin(1-7) is an antagonist at the type 1 angiotensin II receptor. J Hypertens. 1994;12:1377-1381. [PubMed] |
| 193. | Benter IF, Ferrario CM, Morris M, Diz DI. Antihypertensive actions of angiotensin-(1-7) in spontaneously hypertensive rats. Am J Physiol. 1995;269:H313-H319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 101] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 194. | Iyer SN, Ferrario CM, Chappell MC. Angiotensin-(1-7) contributes to the antihypertensive effects of blockade of the renin-angiotensin system. Hypertension. 1998;31:356-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 162] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 195. | Rajapaksha IG, Gunarathne LS, Asadi K, Cunningham SC, Sharland A, Alexander IE, Angus PW, Herath CB. Liver-Targeted Angiotensin Converting Enzyme 2 Therapy Inhibits Chronic Biliary Fibrosis in Multiple Drug-Resistant Gene 2-Knockout Mice. Hepatol Commun. 2019;3:1656-1673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 196. | Loot AE, Roks AJ, Henning RH, Tio RA, Suurmeijer AJ, Boomsma F, van Gilst WH. Angiotensin-(1-7) attenuates the development of heart failure after myocardial infarction in rats. Circulation. 2002;105:1548-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 252] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 197. | Grobe JL, Mecca AP, Mao H, Katovich MJ. Chronic angiotensin-(1-7) prevents cardiac fibrosis in DOCA-salt model of hypertension. Am J Physiol Heart Circ Physiol. 2006;290:H2417-H2423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 167] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 198. | Gava E, de Castro CH, Ferreira AJ, Colleta H, Melo MB, Alenina N, Bader M, Oliveira LA, Santos RA, Kitten GT. Angiotensin-(1-7) receptor Mas is an essential modulator of extracellular matrix protein expression in the heart. Regul Pept. 2012;175:30-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 199. | Wang J, He W, Guo L, Zhang Y, Li H, Han S, Shen D. The ACE2-Ang (1-7)-Mas receptor axis attenuates cardiac remodeling and fibrosis in post-myocardial infarction. Mol Med Rep. 2017;16:1973-1981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 200. | DelliPizzi AM, Hilchey SD, Bell-Quilley CP. Natriuretic action of angiotensin(1-7). Br J Pharmacol. 1994;111:1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 121] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 201. | Santos RA, Baracho NC. Angiotensin-(1-7) is a potent antidiuretic peptide in rats. Braz J Med Biol Res. 1992;25:651-654. [PubMed] |
| 202. | Su Z, Zimpelmann J, Burns KD. Angiotensin-(1-7) inhibits angiotensin II-stimulated phosphorylation of MAP kinases in proximal tubular cells. Kidney Int. 2006;69:2212-2218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 150] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 203. | Zimpelmann J, Burns KD. Angiotensin-(1-7) activates growth-stimulatory pathways in human mesangial cells. Am J Physiol Renal Physiol. 2009;296:F337-F346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 204. | Benter IF, Yousif MH, Dhaunsi GS, Kaur J, Chappell MC, Diz DI. Angiotensin-(1-7) prevents activation of NADPH oxidase and renal vascular dysfunction in diabetic hypertensive rats. Am J Nephrol. 2008;28:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 148] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 205. | Li P, Chappell MC, Ferrario CM, Brosnihan KB. Angiotensin-(1-7) augments bradykinin-induced vasodilation by competing with ACE and releasing nitric oxide. Hypertension. 1997;29:394-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 175] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 206. | Brosnihan KB, Li P, Ferrario CM. Angiotensin-(1-7) dilates canine coronary arteries through kinins and nitric oxide. Hypertension. 1996;27:523-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 263] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 207. | Oliveira MA, Fortes ZB, Santos RA, Kosla MC, De Carvalho MH. Synergistic effect of angiotensin-(1-7) on bradykinin arteriolar dilation in vivo. Peptides. 1999;20:1195-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 86] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 208. | Heitsch H, Brovkovych S, Malinski T, Wiemer G. Angiotensin-(1-7)-Stimulated Nitric Oxide and Superoxide Release From Endothelial Cells. Hypertension. 2001;37:72-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 139] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 209. | Sampaio WO, Souza dos Santos RA, Faria-Silva R, da Mata Machado LT, Schiffrin EL, Touyz RM. Angiotensin-(1-7) through receptor Mas mediates endothelial nitric oxide synthase activation via Akt-dependent pathways. Hypertension. 2007;49:185-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 417] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 210. | Tesanovic S, Vinh A, Gaspari TA, Casley D, Widdop RE. Vasoprotective and atheroprotective effects of angiotensin (1-7) in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2010;30:1606-1613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 126] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 211. | Paula RD, Lima CV, Khosla MC, Santos RA. Angiotensin-(1-7) potentiates the hypotensive effect of bradykinin in conscious rats. Hypertension. 1995;26:1154-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 96] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 212. | Oliveira MA, Carvalho MH, Nigro D, Passaglia Rde C, Fortes ZB. Angiotensin-(1-7) and bradykinin interaction in diabetes mellitus: in vivo study. Peptides. 2002;23:1449-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 213. | Greco AJ, Master RG, Fokin A Jr, Baber SR, Kadowitz PJ. Angiotensin-(1-7) potentiates responses to bradykinin but does not change responses to angiotensin I. Can J Physiol Pharmacol. 2006;84:1163-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 214. | Santos RA, Campagnole-Santos MJ, Baracho NC, Fontes MA, Silva LC, Neves LA, Oliveira DR, Caligiorne SM, Rodrigues AR, Gropen Júnior C. Characterization of a new angiotensin antagonist selective for angiotensin-(1-7): evidence that the actions of angiotensin-(1-7) are mediated by specific angiotensin receptors. Brain Res Bull. 1994;35:293-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 236] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 215. | Tallant EA, Lu X, Weiss RB, Chappell MC, Ferrario CM. Bovine aortic endothelial cells contain an angiotensin-(1-7) receptor. Hypertension. 1997;29:388-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 86] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 216. | Wiemer G, Dobrucki LW, Louka FR, Malinski T, Heitsch H. AVE 0991, a nonpeptide mimic of the effects of angiotensin-(1-7) on the endothelium. Hypertension. 2002;40:847-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 144] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 217. | Santos RA, Simoes e Silva AC, Maric C, Silva DM, Machado RP, de Buhr I, Heringer-Walther S, Pinheiro SV, Lopes MT, Bader M, Mendes EP, Lemos VS, Campagnole-Santos MJ, Schultheiss HP, Speth R, Walther T. Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci USA. 2003;100:8258-8263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1319] [Cited by in RCA: 1368] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 218. | Peiró C, Vallejo S, Gembardt F, Azcutia V, Heringer-Walther S, Rodríguez-Mañas L, Schultheiss HP, Sánchez-Ferrer CF, Walther T. Endothelial dysfunction through genetic deletion or inhibition of the G protein-coupled receptor Mas: a new target to improve endothelial function. J Hypertens. 2007;25:2421-2425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 219. | Rabelo LA, Xu P, Todiras M, Sampaio WO, Buttgereit J, Bader M, Santos RA, Alenina N. Ablation of angiotensin (1-7) receptor Mas in C57Bl/6 mice causes endothelial dysfunction. J Am Soc Hypertens. 2008;2:418-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 220. | Xu P, Costa-Goncalves AC, Todiras M, Rabelo LA, Sampaio WO, Moura MM, Santos SS, Luft FC, Bader M, Gross V, Alenina N, Santos RA. Endothelial dysfunction and elevated blood pressure in MAS gene-deleted mice. Hypertension. 2008;51:574-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 152] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 221. | Santos RA, Castro CH, Gava E, Pinheiro SV, Almeida AP, Paula RD, Cruz JS, Ramos AS, Rosa KT, Irigoyen MC, Bader M, Alenina N, Kitten GT, Ferreira AJ. Impairment of in vitro and in vivo heart function in angiotensin-(1-7) receptor MAS knockout mice. Hypertension. 2006;47:996-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 177] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 222. | Dias-Peixoto MF, Santos RA, Gomes ER, Alves MN, Almeida PW, Greco L, Rosa M, Fauler B, Bader M, Alenina N, Guatimosim S. Molecular mechanisms involved in the angiotensin-(1-7)/Mas signaling pathway in cardiomyocytes. Hypertension. 2008;52:542-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 126] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 223. | Silva DM, Vianna HR, Cortes SF, Campagnole-Santos MJ, Santos RA, Lemos VS. Evidence for a new angiotensin-(1-7) receptor subtype in the aorta of Sprague-Dawley rats. Peptides. 2007;28:702-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 224. | Lautner RQ, Villela DC, Fraga-Silva RA, Silva N, Verano-Braga T, Costa-Fraga F, Jankowski J, Jankowski V, Sousa F, Alzamora A, Soares E, Barbosa C, Kjeldsen F, Oliveira A, Braga J, Savergnini S, Maia G, Peluso AB, Passos-Silva D, Ferreira A, Alves F, Martins A, Raizada M, Paula R, Motta-Santos D, Klempin F, Pimenta A, Alenina N, Sinisterra R, Bader M, Campagnole-Santos MJ, Santos RA. Discovery and characterization of alamandine: a novel component of the renin-angiotensin system. Circ Res. 2013;112:1104-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 292] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 225. | Gembardt F, Grajewski S, Vahl M, Schultheiss HP, Walther T. Angiotensin metabolites can stimulate receptors of the Mas-related genes family. Mol Cell Biochem. 2008;319:115-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 226. | Tetzner A, Gebolys K, Meinert C, Klein S, Uhlich A, Trebicka J, Villacañas Ó, Walther T. G-Protein-Coupled Receptor MrgD Is a Receptor for Angiotensin-(1-7) Involving Adenylyl Cyclase, cAMP, and Phosphokinase A. Hypertension. 2016;68:185-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 116] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 227. | Hennenberg M, Trebicka J, Sauerbruch T, Heller J. Mechanisms of extrahepatic vasodilation in portal hypertension. Gut. 2008;57:1300-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 124] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 228. | Lugo-Baruqui A, Muñoz-Valle JF, Arévalo-Gallegos S, Armendáriz-Borunda J. Role of angiotensin II in liver fibrosis-induced portal hypertension and therapeutic implications. Hepatol Res. 2010;40:95-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 229. | Paizis G, Gilbert RE, Cooper ME, Murthi P, Schembri JM, Wu LL, Rumble JR, Kelly DJ, Tikellis C, Cox A, Smallwood RA, Angus PW. Effect of angiotensin II type 1 receptor blockade on experimental hepatic fibrogenesis. J Hepatol. 2001;35:376-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 126] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 230. | Paizis G, Tikellis C, Cooper ME, Schembri JM, Lew RA, Smith AI, Shaw T, Warner FJ, Zuilli A, Burrell LM, Angus PW. Chronic liver injury in rats and humans upregulates the novel enzyme angiotensin converting enzyme 2. Gut. 2005;54:1790-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 281] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 231. | Bataller R, Gäbele E, Parsons CJ, Morris T, Yang L, Schoonhoven R, Brenner DA, Rippe RA. Systemic infusion of angiotensin II exacerbates liver fibrosis in bile duct-ligated rats. Hepatology. 2005;41:1046-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 135] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 232. | Jonsson JR, Clouston AD, Ando Y, Kelemen LI, Horn MJ, Adamson MD, Purdie DM, Powell EE. Angiotensin-converting enzyme inhibition attenuates the progression of rat hepatic fibrosis. Gastroenterology. 2001;121:148-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 226] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 233. | Yoshiji H, Kuriyama S, Fukui H. Angiotensin-I-converting enzyme inhibitors may be an alternative anti-angiogenic strategy in the treatment of liver fibrosis and hepatocellular carcinoma. Possible role of vascular endothelial growth factor. Tumour Biol. 2002;23:348-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 87] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 234. | Yoshiji H, Kuriyama S, Noguchi R, Ikenaka Y, Yoshii J, Yanase K, Namisaki T, Kitade M, Yamazaki M, Asada K, Akahane T, Tsujimoto T, Uemura M, Fukui H. Amelioration of liver fibrogenesis by dual inhibition of PDGF and TGF-beta with a combination of imatinib mesylate and ACE inhibitor in rats. Int J Mol Med. 2006;17:899-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 235. | Bataller R, Schwabe RF, Choi YH, Yang L, Paik YH, Lindquist J, Qian T, Schoonhoven R, Hagedorn CH, Lemasters JJ, Brenner DA. NADPH oxidase signal transduces angiotensin II in hepatic stellate cells and is critical in hepatic fibrosis. J Clin Invest. 2003;112:1383-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 426] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 236. | Yoshiji H, Yoshii J, Ikenaka Y, Noguchi R, Tsujinoue H, Nakatani T, Imazu H, Yanase K, Kuriyama S, Fukui H. Inhibition of renin-angiotensin system attenuates liver enzyme-altered preneoplastic lesions and fibrosis development in rats. J Hepatol. 2002;37:22-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 237. | Wei HS, Lu HM, Li DG, Zhan YT, Wang ZR, Huang X, Cheng JL, Xu QF. The regulatory role of AT 1 receptor on activated HSCs in hepatic fibrogenesis:effects of RAS inhibitors on hepatic fibrosis induced by CCl(4). World J Gastroenterol. 2000;6:824-828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 74] [Cited by in RCA: 85] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 238. | Mak KY, Chin R, Cunningham SC, Habib MR, Torresi J, Sharland AF, Alexander IE, Angus PW, Herath CB. ACE2 Therapy Using Adeno-associated Viral Vector Inhibits Liver Fibrosis in Mice. Mol Ther. 2015;23:1434-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 239. | Macgilchrist AJ, Howes LG, Hawksby C, Reid JL. Plasma noradrenaline in cirrhosis: a study of kinetics and temporal relationship to ascites formation. Eur J Clin Invest. 1991;21:238-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 240. | Ferlitsch A, Pleiner J, Mittermayer F, Schaller G, Homoncik M, Peck-Radosavljevic M, Wolzt M. Vasoconstrictor hyporeactivity can be reversed by antioxidants in patients with advanced alcoholic cirrhosis of the liver and ascites. Crit Care Med. 2005;33:2028-2033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 241. | Angus PW. Role of endothelin in systemic and portal resistance in cirrhosis. Gut. 2006;55:1230-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 242. | Vilas-Boas WW, Ribeiro-Oliveira A Jr, Pereira RM, Ribeiro Rda C, Almeida J, Nadu AP, Simões e Silva AC, dos Santos RA. Relationship between angiotensin-(1-7) and angiotensin II correlates with hemodynamic changes in human liver cirrhosis. World J Gastroenterol. 2009;15:2512-2519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 243. | Schepke M, Heller J, Paschke S, Thomas J, Wolff M, Neef M, Malago M, Molderings GJ, Spengler U, Sauerbruch T. Contractile hyporesponsiveness of hepatic arteries in humans with cirrhosis: evidence for a receptor-specific mechanism. Hepatology. 2001;34:884-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 244. | Miñano C, Garcia-Tsao G. Clinical pharmacology of portal hypertension. Gastroenterol Clin North Am. 2010;39:681-695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 245. | Sieber CC, Groszmann RJ. In vitro hyporeactivity to methoxamine in portal hypertensive rats: reversal by nitric oxide blockade. Am J Physiol. 1992;262:G996-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 246. | Newby DE, Jalan R, Masumori S, Hayes PC, Boon NA, Webb DJ. Peripheral vascular tone in patients with cirrhosis: role of the renin-angiotensin and sympathetic nervous systems. Cardiovasc Res. 1998;38:221-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 247. | Hennenberg M, Trebicka J, Kohistani AZ, Heller J, Sauerbruch T. Vascular hyporesponsiveness to angiotensin II in rats with CCl(4)-induced liver cirrhosis. Eur J Clin Invest. 2009;39:906-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 248. | Hill CE, Phillips JK, Sandow SL. Heterogeneous control of blood flow amongst different vascular beds. Med Res Rev. 2001;21:1-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 249. | Eriksson LS, Kågedal B, Wahren J. Effects of captopril on hepatic venous pressure and blood flow in patients with liver cirrhosis. Am J Med. 1984;76:66-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 250. | Baik SK, Park DH, Kim MY, Choi YJ, Kim HS, Lee DK, Kwon SO, Kim YJ, Park JW, Chang SJ. Captopril reduces portal pressure effectively in portal hypertensive patients with low portal venous velocity. J Gastroenterol. 2003;38:1150-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 251. | Chiang HT, Cheng JS, Lin M, Tseng WS, Chang JM, Lai KH. Haemodynamic effects of enalaprilat on portal hypertension in patients with HBsAg-positive cirrhosis. J Gastroenterol Hepatol. 1995;10:256-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 252. | Svoboda P, Ochmann J, Kantorová I. Effect of enalapril treatment and sclerotherapy of esophageal varices on hepatic hemodynamics in portal hypertension. Hepatogastroenterology. 1992;39:549-552. [PubMed] |
| 253. | Gracia-Sancho J, Laviña B, Rodríguez-Vilarrupla A, García-Calderó H, Bosch J, García-Pagán JC. Enhanced vasoconstrictor prostanoid production by sinusoidal endothelial cells increases portal perfusion pressure in cirrhotic rat livers. J Hepatol. 2007;47:220-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 254. | Schepke M, Wiest R, Flacke S, Heller J, Stoffel-Wagner B, Herold T, Ghauri M, Sauerbruch T. Irbesartan plus low-dose propranolol versus low-dose propranolol alone in cirrhosis: a placebo-controlled, double-blind study. Am J Gastroenterol. 2008;103:1152-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 255. | Schepke M, Werner E, Biecker E, Schiedermaier P, Heller J, Neef M, Stoffel-Wagner B, Hofer U, Caselmann WH, Sauerbruch T. Hemodynamic effects of the angiotensin II receptor antagonist irbesartan in patients with cirrhosis and portal hypertension. Gastroenterology. 2001;121:389-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 89] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 256. | Kim MY, Cho MY, Baik SK, Jeong PH, Suk KT, Jang YO, Yea CJ, Kim JW, Kim HS, Kwon SO, Yoo BS, Kim JY, Eom MS, Cha SH, Chang SJ. Beneficial effects of candesartan, an angiotensin-blocking agent, on compensated alcoholic liver fibrosis - a randomized open-label controlled study. Liver Int. 2012;32:977-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 257. | Zhu Q, Li N, Li F, Zhou Z, Han Q, Lv Y, Sang J, Liu Z. Therapeutic effect of renin angiotensin system inhibitors on liver fibrosis. J Renin Angiotensin Aldosterone Syst. 2016;17:1470320316628717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 258. | Sampaio WO, Nascimento AA, Santos RA. Systemic and regional hemodynamic effects of angiotensin-(1-7) in rats. Am J Physiol Heart Circ Physiol. 2003;284:H1985-H1994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 130] [Article Influence: 5.9] [Reference Citation Analysis (0)] |