Published online Jan 28, 2020. doi: 10.3748/wjg.v26.i4.404
Peer-review started: November 1, 2019
First decision: December 23, 2019
Revised: January 9, 2020
Accepted: January 15, 2020
Article in press: January 15, 2020
Published online: January 28, 2020
Processing time: 77 Days and 22.5 Hours
The incidence of inflammatory bowel disease (IBD) is increasing in Asia. Numerous risk factors associated with IBD development have been investigated.
To investigate trends and environmental risk factors of Crohn’s disease (CD) diagnosed in persons aged ≥ 40 years in South Korea.
Using the National Health Insurance Service database, a total of 14060821 persons aged > 40 years who underwent national health screening in 2009 were followed up until December 2017. Patients with newly diagnosed CD were enrolled and compared with non-CD cohort. CD was identified according to the International Classification of Diseases 10th revision and the rare/intractable disease registration program codes from the National Health Insurance Service database. The mean follow-up periods was 7.39 years. Age, sex, diabetes, hypertension, smoking, alcohol consumption, regular exercise, body mass index, anemia, chronic kidney disease (CKD) and dyslipidemia were adjusted for in the multivariate analysis model.
During the follow-up, 1337 (1.33/100000) patients developed CD. Men in the middle-aged group (40-64 years) had a higher risk than women [adjusted hazard ratio (aHR) 1.46, 95% confidence interval (CI): 1.29-1.66]; however, this difference tended to disappear as the age of onset increases. In the middle-aged group, patients with a history of smoking [aHR 1.46, 95%CI: 1.19-1.79) and anemia (aHR 1.85, 95%CI: 1.55-2.20) had a significantly higher CD risk. In the elderly group (age, ≥ 65 years), ex-smoking and anemia also increased the CD risk (aHR 1.68, 95%CI: 1.22-2.30) and 1.84 (95%CI: 1.47-2.30, respectively). Especially in the middle-aged group, those with CKD had a statistically elevated CD risk (aHR 1.37, 95%CI: 1.05-1.79). Alcohol consumption and higher body mass index showed negative association trend with CD incidence in both of the age groups. [Middle-aged: aHR 0.77 (95%CI: 0.66-0.89) and aHR 0.73 (95%CI: 0.63-0.84), respectively] [Elderly-group: aHR 0.57 (95%CI: 0.42-0.78) and aHR 0.84 (95%CI 0.67-1.04), respectively]. For regular physical activity and dyslipidemia, negative correlation between CD incidences was proved only in the middle-aged group [aHR 0.88 (95%CI: 0.77-0.89) and aHR 0.81 (95%CI: 0.68-0.96), respectively].
History of cigarette smoking, anemia, underweight and CKD are possible risk factors for CD in Asians aged > 40 years.
Core tip: As the population becomes older, clinicians may encounter more cases of older-onset inflammatory bowel disease in the future. We tried to investigate trends and environmental risk factors of Crohn’s disease (CD) diagnosed in persons aged ≥ 40 years in South Korea using nationwide population-based cohort. Along with previously valued risk factors (ex-smoking, anemia and underweight), those with chronic kidney diseases were at risk of developing CD especially in the middle-aged group. Furthermore, alcohol consumption, physical activity and dyslipidemia were demonstrated to be in association with decreased incidence of late-onset Asian CDs.
- Citation: Moon JM, Kang EA, Han K, Hong SW, Soh H, Park S, Lee J, Lee HJ, Im JP, Kim JS. Trends and risk factors of elderly-onset Crohn’s disease: A nationwide cohort study. World J Gastroenterol 2020; 26(4): 404-415
- URL: https://www.wjgnet.com/1007-9327/full/v26/i4/404.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i4.404
Inflammatory bowel disease (IBD), previously considered a Western disease, has recently shown increasing incidence in Asian countries[1]. Previous studies have reported that the incidence of IBD was 3.4 per 100000 in China and 32 per 100000 in South Korea[2,3]. With the increasing incidence, several differences in epidemiology and phenotypes are being observed between Western and Asian patients with IBD[4]. In Asia, the prevalence of ulcerative colitis is approximately twice that of Crohn’s disease (CD)[2]. The use of antibiotics in childhood is known to be a risk factor for the development of IBD; however, this issue is controversial in the East[5]. The differences can be attributed to the complexity of the pathogenesis of IBD. In genetically susceptible hosts, IBD can be caused by a faulty immune response to microbial antigens against environmental stimulus[6]. Although numerous studies have been conducted to clarify the risk factors of IBD, there is still a paucity of evidence and more clinical investigations are needed[7].
The Montreal classification divides CD into three categories according to age at diagnosis: A1 (< 17 years), A2 (17-40 years), and A3 (> 40 years)[8]. More than half of the patients fall into the A2 category. The disease severity and prognosis differ among these groups; however, only a few studies have provided insights into the importance of epidemiological and phenotypical differences in elderly patients. Even less attention has been paid to group A3 as a whole, although elderly patients with CD account for 17%-35% of the total cases[9,10].
Although the pathogenesis of IBD is multifactorial, more emphasis is placed on environmental causes rather than genetic traits in older generations. Previous studies revealed that an older age at diagnosis was less likely to be associated with a family history of IBD[11,12]. This phenomenon is also observed in other autoimmune diseases such as systemic lupus erythematosus[13]. In addition, as the incidence and prevalence of older-onset IBD are rapidly increasing, more extensive clinical data on older adults are needed[14].
Therefore, this study aimed to evaluate the epidemiological features of CD, especially in patients in the A3 category, using data from the mandatory national health check-up in Korea. Furthermore, we attempted to investigate any particular differences in environmental risk factors found in Western and other Asian IBD studies.
The South Korean National Health Insurance Service (NHIS) covers approximately 97% of the population, whereas the rest is covered by Medical Aid[15]. Therefore, the NHIS database is often used as a concrete data source for epidemiological studies. To access the dataset, the review committee of the National Health Insurance Corporation must approve the study.
A registration program of rare and intractable diseases (RIDs) was established in 2006 to provide medical cost benefits for patients with RIDs such as IBD. To be assigned a special code (V code) in this database, the patient needs to meet certain criteria. For CD, the following criteria must be met, with confirmation by a doctor: (1) Clinical manifestation; (2) Endoscopic or radiological findings; and (3) Pathological findings. More detailed information on data approval and access are provided at http://nhiss.nhis.or.kr/bd/ab/bdaba032eng.do.
Individuals who underwent national health examination in 2009 were screened and followed up until December 2017. According to the definition of the V code and International Classification of Diseases, 10th revision (ICD-10) codes, patients with newly diagnosed CD were identified and enrolled as the CD patient group and the others as the control group. The registration code in RID is V130, and the ICD-10 code is K50 for CD.
Subjects diagnosed with IBD before the health examination were excluded at baseline, and those diagnosed during the first year of enrollment were also excluded. The incidence rate was calculated as the number of events per 100000 person-years.
To validate the use of the V code and ICD-10 codes as definitions of IBD, the medical records of all patients with IBD at Seoul National University Hospital, a tertiary teaching hospital in South Korea, were retrospectively reviewed. From January 2010 to December 2013, a total of 330 patients with CD were screened. The diagnostic sensitivity of using the V code and ICD-10 codes as definitions was reported as 94.5% (312/330) for CD[16].
The study population was subdivided into two groups according to age: the middle-aged group (age, 40-64 years) and the elderly group (age, > 65 years). The demographics of the study population were collected from the NHIS database. Information about accompanying diseases was identified using the ICD-10 codes: hypertension (I10-13), diabetes mellitus (E11-14), and dyslipidemia (E78). These definitions of ICD codes have been validated in previous studies[17,18]. Chronic kidney disease (CKD) was defined as an estimated glomerular filtration rate (eGFR) of < 60 mL/min/1.73 m2. Anemia was defined as a serum hemoglobin level of < 12 mg/dL for men and 13 mg/dL for women, in accordance with World Health Organization criteria.
Smoking, alcohol consumption, and physical activity status information were gathered using a self-answered questionnaire. The subjects were categorized into three groups according to smoking behavior: Non-smoker, ex-smoker, and current smoker. Non-smokers were defined as those with a history of smoking < 5 packs. Ex-smokers were those with a history of smoking > 5 packs but had already stopped smoking. Current smokers were those with a history of smoking > 5 packs and were still smoking. The subjects were also divided into three groups according to alcohol consumption level: non-drinker (0 g/d), mild drinker (≤ 30 g/d), and heavy drinker (> 30 g/d). Regular exercise was defined as performing high-intensity exercise for > 3 d/wk for at least 30 min or moderate-intensity exercise for > 5 d/wk for at least 20 min.
Student’s t-test and analysis of variance were used to compare the differences between continuous variables, and the χ2 test was used to compare the differences between categorical variables. Continuous variables are provided as means and standard deviations, whereas categorical variables are shown as numbers and percentages. The Cox proportional hazard model was used for the analysis of CD risk factors, and outcomes are expressed as hazard ratios (HRs) with 95%CIs. Age, sex, diabetes, hypertension, smoking, alcohol consumption, regular exercise, body mass index (BMI), anemia, CKD and dyslipidemia were adjusted for in the multivariate analysis model. A P of < 0.05 was set for defining statistical significance. Statistical analysis was performed using SAS version 9.4 (SAS Institute, Cary, NC, United States) and R version 3.2.3 (The R Foundation for Statistical Computing, Vienna, Austria).
During the mean follow-up period of 7.39 years (7.88 years for the middle-aged group, 6.37 years for the elderly group), a total of 14060821 subjects were screened and 1331 patients with CD were identified (977 in the middle-aged group, 400 in the elderly group).
The baseline demographics of the study population, comparing the CD cohort and the non-CD cohort, are shown in Tables 1 and 2 for the middle-aged group and the elderly group, respectively. The median age of patients with CD in the middle-aged group was 50.0 ± 6.70 years, whereas the median age in the elderly group was 69.92 ± 4.40 years. Male predominance was observed in the middle-aged group. There were statistical differences between the CD cohort and the non-CD cohort in smoking history, dyslipidemia, CKD, BMI, blood pressure, and glucose level in the middle-aged group. In addition to the higher CKD incidence in the CD cohort, subjects in the CD cohort were underweight and less likely to have metabolic disease risks (Table 1). However, in the elderly group, there was no significant sex predominance between the two groups. Subjects in the CD cohort were younger, and only smoking and alcohol consumption status proved to be statistically significant (Table 2).
| Non-CD control | CD patients | P value | |
| (n = 9499756) | (n = 977) | ||
| Age, yr | 50.01 ± 6.73 | 50.00 ± 6.70 | 0.961 |
| Sex | < 0.001 | ||
| Male | 4660907 (49.06) | 570 (58.34) | |
| Smoking | < 0.001 | ||
| Non-smoker | 5945314 (62.58) | 532 (54.45) | |
| Ex-smoker | 1455702 (15.32) | 204 (20.88) | |
| Current smoker | 2098740 (22.09) | 241 (24.67) | |
| Drinking | 0.355 | ||
| 0 | 5258307 (55.35) | 559 (57.22) | |
| 0-30 g | 3599536 (37.89) | 361 (36.95) | |
| > 30 g | 641913 (6.76) | 57 (55.83) | |
| Regular exercise2 | 4974091 (52.36) | 483 (49.44) | 0.067 |
| Lowest income 20%1 | 2446619 (25.75) | 234 (23.95) | 0.197 |
| Diabetes mellitus | 898517 (9.46) | 90 (9.21) | 0.792 |
| Hypertension | 2556964 (26.92) | 258 (26.41) | 0.720 |
| Dyslipidemia | 2041629 (21.49) | 167 (17.09) | 0.001 |
| Chronic kidney disease | 430175 (4.53) | 59 (6.04) | 0.023 |
| Body mass index | 23.97 ± 3.02 | 23.40 ± 3.00 | < 0.001 |
| Waist circumference | 80.54 ± 8.65 | 80.78 ± 8.81 | 0.400 |
| Systolic blood pressure | 122.43 ± 14.88 | 120.9 ± 13.88 | 0.001 |
| Diastolic blood pressure | 76.7 ± 10.19 | 75.79 ± 9.62 | 0.006 |
| Fasting glucose1 | 98.99 ± 24.11 | 96.83 ± 21.55 | 0.005 |
| Non-CD control | CD patients | P value | |
| (n = 4559688) | (n = 400) | ||
| Age, yr | 70.50 ± 5.33 | 69.92 ± 4.40 | 0.029 |
| Sex | |||
| Male | 2031794 (44.56) | 175 (43.75) | 0.745 |
| Smoking | 0.033 | ||
| Non-smoker | 3288608 (72.12) | 278 (69.50) | |
| Ex-smoker | 716415 (15.71) | 81 (20.25) | |
| Current smoker | 554665 (12.16) | 41 (10.25) | |
| Drinking | 0.005 | ||
| 0 | 3444333 (75.54) | 327 (81.75) | |
| 0-30 g | 951250 (20.86) | 57 (14.25) | |
| > 30 g | 164105 (3.60) | 16 (4.00) | |
| Regular exercise2 | 1804398 (39.57) | 158 (39.5) | 0.976 |
| Lowest income 20%1 | 1163622 (25.52) | 111 (27.75) | 0.306 |
| Diabetes mellitus | 976144 (21.41) | 84 (21.00) | 0.8423 |
| Hypertension | 2678260 (58.74) | 245 (61.25) | 0.308 |
| Dyslipidemia | 1578582 (34.62) | 144 (36) | 0.562 |
| Chronic kidney disease | 724332 (15.89) | 59 (14.75) | 0.534 |
| Body mass index | 23.99 ± 3.17 | 23.72 ± 3.24 | 0.084 |
| Waist circumference | 83.14 ± 8.48 | 82.9.6 ± 8.84 | 0.658 |
| Systolic blood pressure | 129.63 ± 15.92 | 126.69 ± 16.05 | 0.940 |
| Diastolic blood pressure | 77.90 ± 9.92 | 77.5 ± 9.87 | 0.421 |
| Fasting glucose1 | 104.19 ± 26.88 | 103.04 ± 28.95 | 0.394 |
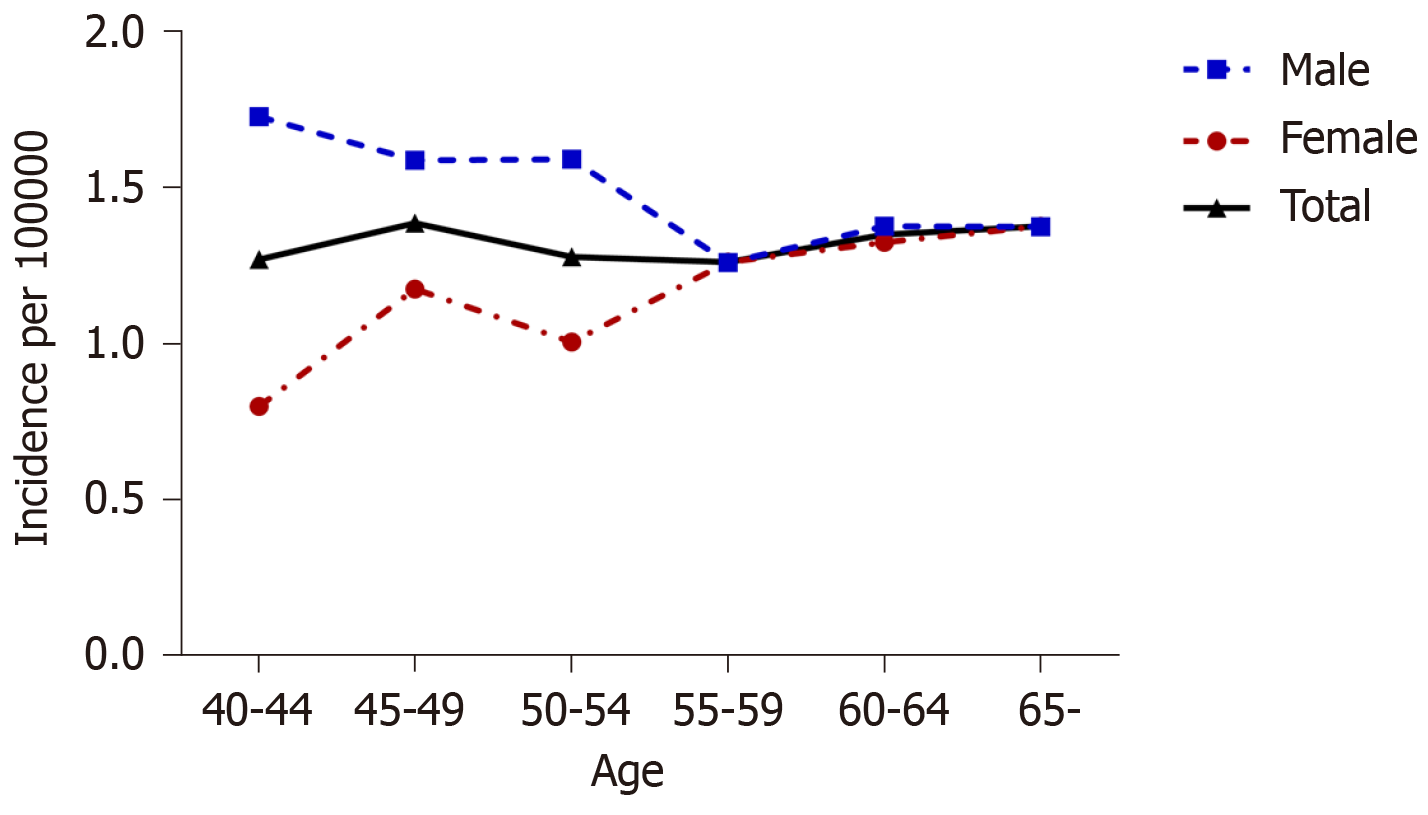
The incidence of CD in the total population was 1.33 per 100000 person-years and that in the elderly group was 1.38 per 100000 person-years (Figure 1). The incidence rate in men varied from 1.73 per 100000 person-years in the 40-44 years age group to 1.38 per 100000 person-years in those older than 65 years. Men in the middle-aged group (40-64 years) had a higher risk than women [adjusted HR (aHR) 1.46, 95%CI: 1.29-1.66]; however, this difference tended to disappear as the age of onset increases.
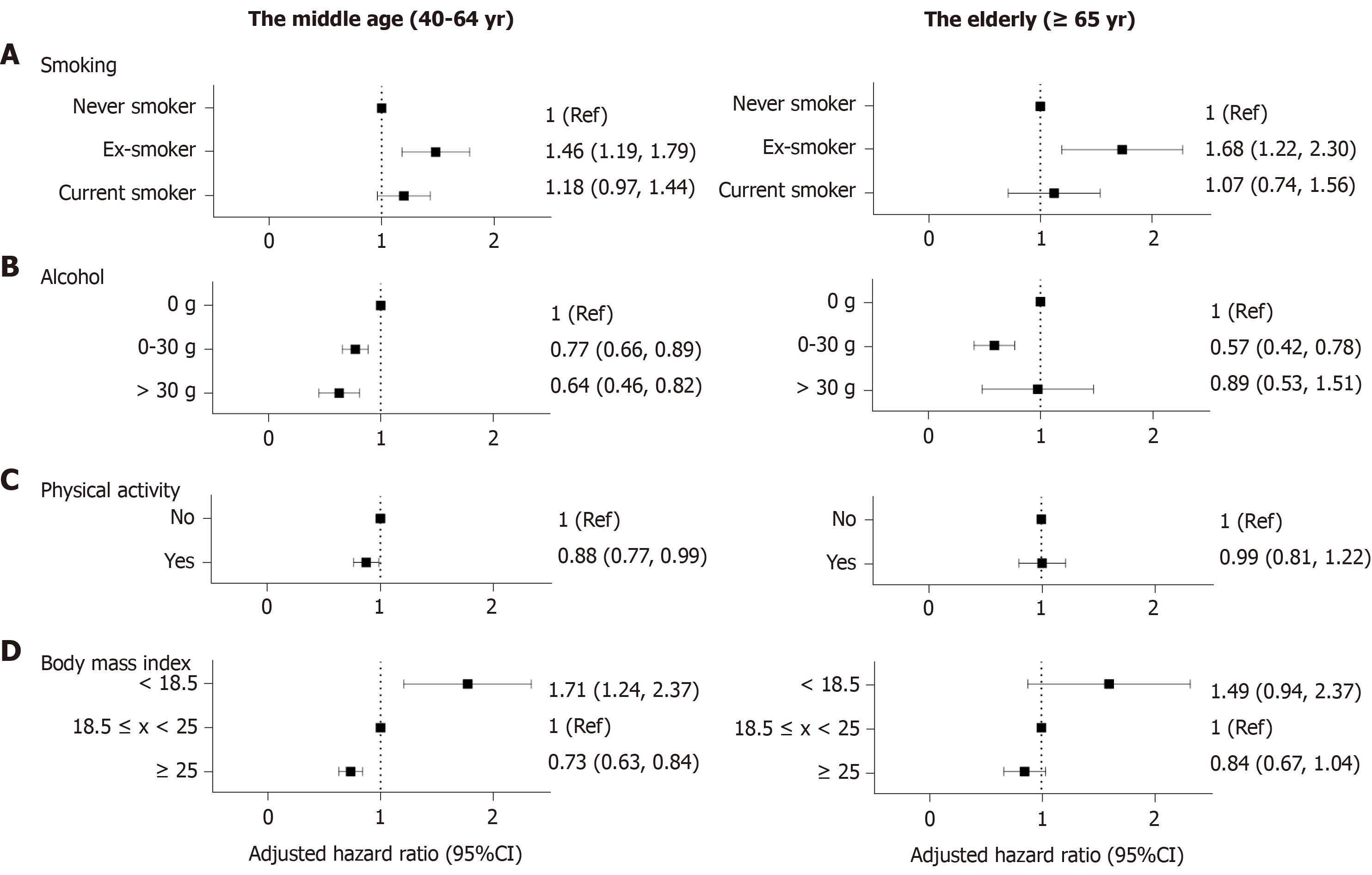
Smoking: Setting non-smoker as the reference, ex-smokers demonstrated an increased risk of CD in both age groups after adjusting for age and sex (middle age: aHR 1.46, 95%CI: 1.19-1.79; elderly: aHR 1.68, 95%CI: 1.22-2.30) However, current smoking failed to show statistical significance (middle age: aHR 1.18, 95%CI: 0.97-1.44; elderly: aHR 1.07, 95%CI: 0.74-1.56) (Figure 2A).
Alcohol consumption: In the middle-aged group, those who consumed more alcohol showed a decreased risk for CD compared with non-drinkers. (aHR 0.77, 95%CI: 0.66-0.89; aHR 0.61, 95%CI: 0.46-0.82) In the elderly, however, mild drinkers (≤ 30 g/d) showed a decreased risk for CD but the status of heavy drinkers (> 30 g) did not show statistical significance (aHR 0.57, 95%CI: 0.42-0.78; aHR 0.89, 95%CI: 0.53-1.51) (Figure 2B).
Physical activity: Regular exercise demonstrated a protective effect against CD in the middle-aged group (aHR 0.88, 95%CI: 0.77-0.99); however, there was no statistical significance in the elderly group (aHR 0.99, 95%CI: 0.81-1.22) (Figure 2C).
BMI: A BMI of > 25 kg/m2 was a protective factor against CD in the population older than 40 years (middle age: aHR 0.73, 95%CI: 0.63-0.84; elderly: aHR 0.84, 95%CI: 0.67-1.04) (Figure 2D). BMI < 18.5 kg/m2 posed a relatively higher risk of CD development than BMI > 25 kg/m2. (middle age: aHR 1.71, 95%CI: 1.24-2.37; elderly: aHR 1.49, 95%CI: 0.94-2.37)
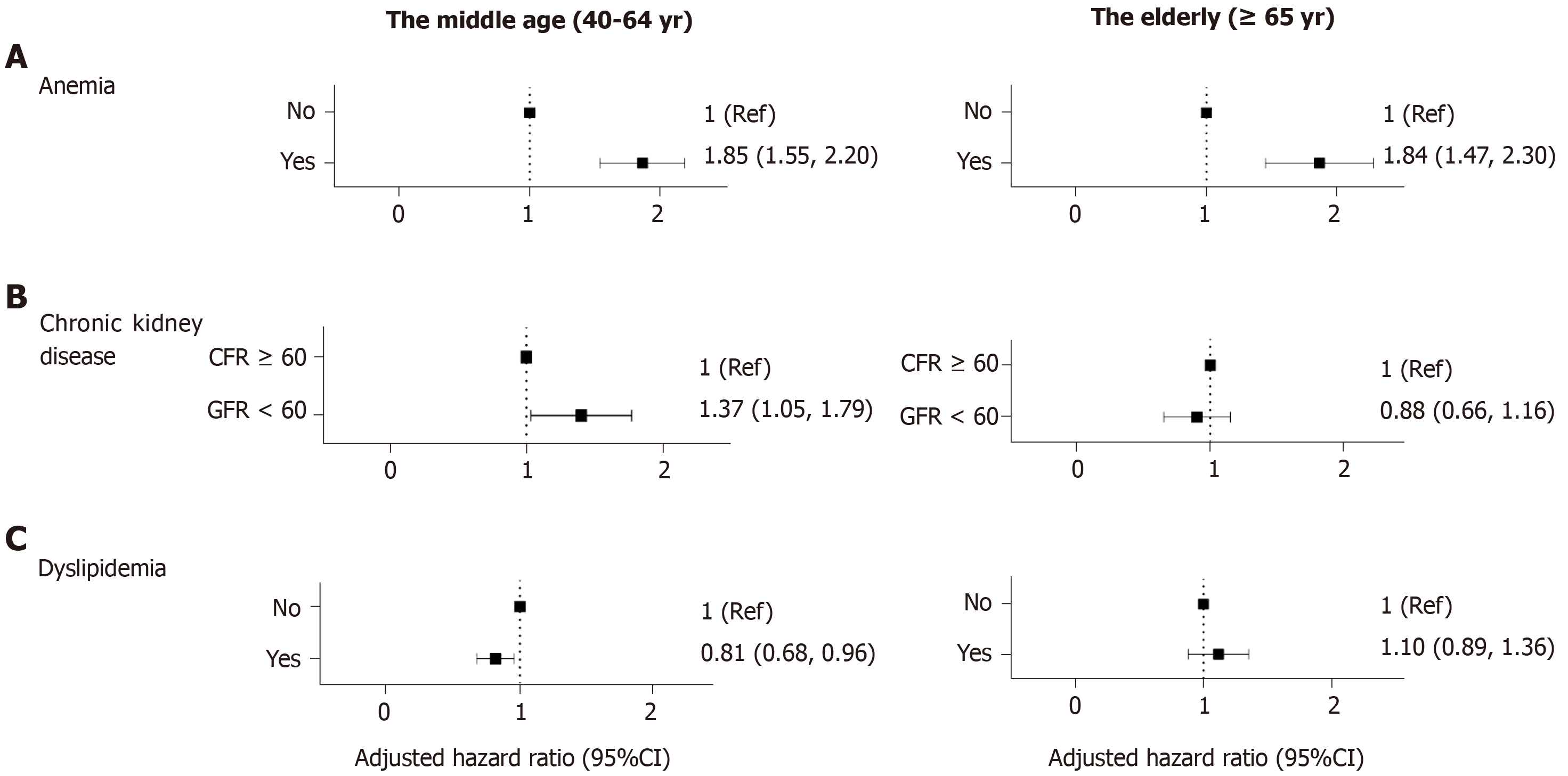
Anemia: Subjects with anemia were at a risk of CD regardless of age (middle age: aHR 1.85, 95%CI: 1.55-2.20; elderly: aHR 1.84, 95%CI: 1.47-2.30). Compared with the other factors analyzed in this study, anemia proved to have the largest effect on the risk of CD (Figure 3A).
CKD: Patients with CKD in the middle-aged group were more likely to be diagnosed with CD (aHR 1.37, 95%CI: 1.05-1.79); however, in the elderly population, CKD did not prove to have statistical meaning (aHR 0.88, 95%CI: 0.66-1.16) (Figure 3B).
Dyslipidemia: Patients diagnosed with dyslipidemia were less likely to develop CD than the control population, among those aged between 40 and 65 years (aHR 0.81, 95%CI: 0.68-0.96) (Figure 3C).
Other factors such as income status, diabetes mellitus, fasting glucose levels, hypertension, serum cholesterol and metabolic syndrome were also analyzed and the results are described in Supplementary Table 1.
This study analyzed the characteristics and possible risk factors of late-onset CD using a large nationwide cohort in South Korea. Numerous studies on patients with very early, early-onset, and pediatric CD were previously published in search for possible risk factors; however, relatively less attention has been paid to patients with older-onset CD[19,20]. Thus, by analyzing patients with CD diagnosed at an age of > 40 years, we aimed to focus on environmental and demographic differences of late-onset CD. Therefore, we arrived at a conclusion that ex-smokers and patients with anemia are at a higher risk of developing CD. On the other hand, alcohol drinking showed a negative association with CD. Particularly for those in the middle-aged group, CKD was a risk factor of CD, whereas obesity, dyslipidemia, and physical activity proved to have a negative correlation with CD.
Previous studies in Korea, China, and Japan showed that CD is more common in men[3,21,22]. This is a feature that is not observed in Western countries. This disparate sex difference in incidence rates in the middle-aged population is diminished in the elderly. This can be partly explained by sex hormonal changes. Considering that estrogen has anti-inflammatory and immune-mediating properties and androgen is considered to be an immune suppressor, androgen reduction in elderly men may be associated with decreased incidence in the elderly[23,24]. Interestingly, whereas other autoimmune diseases such as systemic lupus erythematosus or rheumatoid arthritis have more impact on women of childbearing age, CD has affected more women older than 60 years[24]. A possible explanation for this result is that women of childbearing age may be less affected by CD owing to the protective effect of estrogen. Similarly, the use of oral contraceptives is associated with a reduced risk of IBD[7].
Smoking has been reported to be the best known environmental risk factor for CD; however, the evidence is tangential in Asian populations. A history of smoking was not proven to be a risk factor for CD (odds ratio 1.02) in the Chinese population[21]. Smoking behavior may be more affected by genetic factors and ethnicity[7]. It is interesting to note that in our study, ex-smoking was significantly related to the CD incidence, whereas current smoking failed to show statistical significance. Kondo et al[25] reported that past smoking was a risk factor for CD but active smoking did not demonstrate significance in their case-control study. This may indicate that non-nicotine factors in tobacco smoke pollution are more associated with CD than nicotine itself because the serum nicotine level would be much higher in current smokers than in ex-smokers[25]. Similarly, van der Heide et al[26] observed detrimental effects of passive smoking in the disease course of CD, whereas active smoking had no effect on the outcome. This hypothesis of non-nicotine factors being the culprit should be confirmed in the future by analyzing the multilevel mechanisms of tobacco metabolites.
Previous studies determining the effect of alcohol on IBD reported inconsistent results. Two European cohort studies revealed no association, but a Japanese case-control study demonstrated decreased odds ratio in ever and current alcohol drinkers[25,27,28]. Our study suggests the possibility of a negative association between alcohol consumption and older-onset CD. The results may be confounded by other factors, considering the retrospective nature of the study. Moreover, patients or individuals with susceptible traits may avoid alcohol because it may trigger worsening of symptoms[29]. However, such a negative association with alcohol consumption has been noted in other autoimmune diseases such as autoimmune hypothyroidism and rheumatoid arthritis[30,31]. With respect to the immune system, alcohol acts in a very complicated manner and plays a role in modifying natural killer cell activity and altering cytokine production[30]. Although there is paucity in the understanding of the underlying mechanism, this result of a negative association in older patients with CD may offer a biological plausibility.
The benefits of regular exercise have been studied in several chronic disorders such as dementia, stroke, coronary artery disease, diabetes, skeletal muscle weakening, and psychological illnesses[32,33]. Previous meta-analyses and sensitivity analyses demonstrated its value in preventing the development of CD[7]. Biological plausibility supports this hypothesis through the mechanism of rescuing defective autophagy and reducing proinflammatory cytokines[34,35]. However, it was interesting to note that it failed to show statistical significance in the elderly. This result may be partly due to the scarce CD incidence in the elderly population and also because more subjects in the elderly tend to pursue sedentary lifestyles. It may also indicate that various comorbidities may compromise the interpretation of results in the elderly.
IBD involves chronic inflammation in the gut, and its disease activity involves intestinal blood loss. Thus, such association between anemia and IBD is logical and consistent with previous knowledge. However, whether a causal relationship exists remains unanswered. Anemia may be a possible marker in predicting preclinical stage of the disease. In other words, anemia could reflect continuous gut inflammation in the asymptomatic stage. Indeed, several serologic markers such as perinuclear anti-neutrophil cytoplasmic antibody and Escherichia coli outer membrane porin C antibody precede clinical symptoms of CD[36]. Further studies are needed to clarity the causal relationship, yet we can postulate that individuals with severe unexplained anemia should be dealt with caution with the possibility of preclinical gut inflammation of potential progression.
CKD and IBD share a few characteristics. Systemic inflammatory response may play a role in the pathogenesis of both disease entities[17]. Indeed, elevated inflammatory and pro-inflammatory cytokines function as early predictors of renal insufficiency[37]. However, as decline in eGFR is considered a part of the normal aging process, defining CKD as an eGFR of 60 mL/min/1.73 m2 regardless of age may be misleading[38,39]. Moreover, phenotypical characteristics vary among different age groups: Glomerulonephritis (15.9%-20.2%) and autosomal dominant polycystic kidney disease (5%-12.6%) are more frequent causes of CKD in the population younger than 65 years old, whereas hypertension (13.9%-20.3%) and atherosclerotic reno-vascular disease (14.8%-21.1%) are more common causes in those older than 65 years[40]. This may explain why only those in the middle-aged group with CKD are at a risk of CD, not those in the elderly group. Epidemiological differences exist and support the hypothesis that CKD is a risk factor only in the population more susceptible to autoimmune diseases, such as those with glomerulonephritis and IBD.
Traditionally, weight loss has been considered a main clinical symptom of CD. Body weight change is also one of the main parameters in assessing CD severity. Similarly, we observed that patients with BMI < 18.5 kg/m2 were at a higher risk of developing CD. This implies that being underweight may be a preclinical manifestation of CD[41,42]. To our knowledge, based on a large population based cohort and relatively long follow-up period, we managed to successfully prove decrease in BMI prior to diagnosis in Montreal A3 subjects[42,43]. A growing awareness implies that a prolonged pre-clinical stage to CD precedes symptomatic disease onset[44]. Furthermore, this may indicate that risk factors for metabolic syndrome are likely to present a negative association with CD. Our study revealed a decrease in HR for CD in those with dyslipidemia. This might suggest the possibility that lower levels of cholesterol, although the types were not specified, and/or triglycerides are possible indicators of preclinical CD. Indeed, previous studies have reported that decreased levels of low-density lipoprotein cholesterol and total cholesterol were observed in the CD population compared with healthy subjects, whereas no significant change was reported with respect to high-density lipoprotein cholesterol and triglycerides[45]. With further studies to strengthen the relationship, a particular change in lipid profiles may reflect unrevealed on-going manifestation of preclinical CD[46].
The strength of this study is that we performed an epidemiological analysis using nationwide population cohort of more than 10 million Koreans to investigate the association between CD incidence and a broad range of factors. Yet, our study has a few limitations. Diagnosis based on ICD-10 codes may lead to misclassification and overlook the disease severity. Thus, we attempted to minimize this error by validating the definitions in a previous study[16]. Moreover, this study is retrospective in nature and factors related to CD may show an association rather than a causal relationship. However, previous studies on possible biological mechanisms may suggest a plausible explanation to support the relationship. Furthermore, this study did not evaluate other controversial risk factors, such as medication (non-steroidal anti-inflammatory drugs, oral contraceptive pills, and antibiotics), history of breastfeeding, dietary intake and childhood infection[7]. Moreover, the severity of the CD were not included in the analysis. Since CD is of various phenotypes including penetrating, stricture and so on; further investigation on the disease severity may have revealed further information on the disease phenotype of the Montreal A3 group and the elderly. We also need to mention the relatively long time to disease diagnosis, calling for cautious interpretation in the causality. Future studies are in need to enhance the evidence by investigating the causal relationship, dose-response and other potential risk factors.
As the population becomes older, clinicians may encounter more cases of older-onset IBD in the future. By using a national database, this study demonstrated several potential predictive factors in relation to CD incidence in Asians older than 40 years: Four factors in relation to increased risk (ex-smoking, anemia, CKD, and lower BMI) and three factors in relation to decreased risk (alcohol consumption, physical activity, and dyslipidemia). The association was weaker in the elderly onset CD, except for ex-smoker and anemia. We can assume that these two possess stronger association to CD in A3 group. Considering the ethnic and demographic diversity, this study may pave the way for understanding the pathogenesis of IBD in elderly Asians.
The incidence of inflammatory bowel disease (IBD) is increasing worldwide, including in Asia. Crohn’s disease (CD) has a high incidence at younger ages, but as the life expectancy increases, people in the Montreal classification A3 category (age over 40), especially the elderly-onset CD, receive greater clinical attention.
Disease phenotypes vary by age of onset, but few studies have investigated epidemiological and phenotypic differences in the older people. Given that environmental factors play a pivotal role in the elderly compared with the young, analyzing the nature and environmental risk factors of elderly CD can provide important information in understanding the multifactorial causes of the IBD.
We aimed to assess the trend and risk factors of middle-age and elderly-onset CD in nationwide population-based cohort study, using the claim data of South Korea.
We conducted a retrospective cohort study to evaluate the incidence trends and risk of CD in those over the age of 40 in South Korea. Using both the International Classification of Diseases, 10th revision and rare, intractable disease registration program codes from National Health Insurance Service database, newly diagnosed CD patients were analyzed and compared to non-CD cohort. Hazard ratio was calculated with adjustment to age, sex, diabetes, hypertension, smoking, alcohol consumption, regular exercise, body mass index, anemia, chronic kidney disease (CKD) and dyslipidemia.
Out of 14060821 subjects screened, a total of 1337 cases of newly diagnosed CD was analyzed. During the median follow-up period of 7.4 years, we found that four factors including ex-smoking, anemia, chronic kidney disease and lower body mass index were in relation to increased risk of CD and three factors including alcohol consumption, physical activity and dyslipidemia were in relation to decreased risk of CD.
This study suggests several potential predictive markers associated with CD incidence. Most analyzed factors associated with CD development in the middle-aged and the elderly were found to be less relevant for older people. However, ex-smoking and anemia have proven to be strong associative markers even in the elderly population. We can assume that certain factors interact at the preclinical stage of ongoing inflammation in the IBD pathogenesis, thus careful monitoring of these factors is necessary.
We hypothesized that certain markers could be particularly associated with elderly-onset CD and conducted a large epidemiological study involving more than 10 million Koreans to investigate a wide range of factors. Based on our findings, we can create grounds for future prospective studies to assess the causal relationship, eventually leading to a better understanding about the pathogenesis of elderly IBDs in Asian population.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Xu CF S-Editor: Dou Y L-Editor: A E-Editor: Qi LL
| 1. | Kaplan GG, Ng SC. Understanding and Preventing the Global Increase of Inflammatory Bowel Disease. Gastroenterology. 2017;152:313-321.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 566] [Cited by in RCA: 815] [Article Influence: 101.9] [Reference Citation Analysis (0)] |
| 2. | Ng SC, Tang W, Ching JY, Wong M, Chow CM, Hui AJ, Wong TC, Leung VK, Tsang SW, Yu HH, Li MF, Ng KK, Kamm MA, Studd C, Bell S, Leong R, de Silva HJ, Kasturiratne A, Mufeena MNF, Ling KL, Ooi CJ, Tan PS, Ong D, Goh KL, Hilmi I, Pisespongsa P, Manatsathit S, Rerknimitr R, Aniwan S, Wang YF, Ouyang Q, Zeng Z, Zhu Z, Chen MH, Hu PJ, Wu K, Wang X, Simadibrata M, Abdullah M, Wu JC, Sung JJY, Chan FKL; Asia–Pacific Crohn's and Colitis Epidemiologic Study (ACCESS) Study Group. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn's and colitis epidemiology study. Gastroenterology. 2013;145:158-165.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 597] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 3. | Yang SK, Yun S, Kim JH, Park JY, Kim HY, Kim YH, Chang DK, Kim JS, Song IS, Park JB, Park ER, Kim KJ, Moon G, Yang SH. Epidemiology of inflammatory bowel disease in the Songpa-Kangdong district, Seoul, Korea, 1986-2005: a KASID study. Inflamm Bowel Dis. 2008;14:542-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 381] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 4. | Kim ES, Kim WH. Inflammatory bowel disease in Korea: epidemiological, genomic, clinical, and therapeutic characteristics. Gut Liver. 2010;4:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 5. | Ananthakrishnan AN. Environmental risk factors for inflammatory bowel diseases: a review. Dig Dis Sci. 2015;60:290-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 129] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 6. | Sartor RB. Mechanisms of disease: pathogenesis of Crohn's disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:390-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1158] [Cited by in RCA: 1277] [Article Influence: 67.2] [Reference Citation Analysis (2)] |
| 7. | Piovani D, Danese S, Peyrin-Biroulet L, Nikolopoulos GK, Lytras T, Bonovas S. Environmental Risk Factors for Inflammatory Bowel Diseases: An Umbrella Review of Meta-analyses. Gastroenterology. 2019;157:647-659.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 505] [Article Influence: 84.2] [Reference Citation Analysis (0)] |
| 8. | Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1970] [Cited by in RCA: 2353] [Article Influence: 123.8] [Reference Citation Analysis (2)] |
| 9. | Wei SC, Lin MH, Tung CC, Weng MT, Kuo JS, Shieh MJ, Wang CY, Ho WC, Wong JM, Chen PC. A nationwide population-based study of the inflammatory bowel diseases between 1998 and 2008 in Taiwan. BMC Gastroenterol. 2013;13:166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Sturm A, Maaser C, Mendall M, Karagiannis D, Karatzas P, Ipenburg N, Sebastian S, Rizzello F, Limdi J, Katsanos K, Schmidt C, Jeuring S, Colombo F, Gionchetti P. European Crohn's and Colitis Organisation Topical Review on IBD in the Elderly. J Crohns Colitis. 2017;11:263-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Quezada SM, Steinberger EK, Cross RK. Association of age at diagnosis and Crohn's disease phenotype. Age Ageing. 2013;42:102-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Stepaniuk P, Bernstein CN, Targownik LE, Singh H. Characterization of inflammatory bowel disease in elderly patients: A review of epidemiology, current practices and outcomes of current management strategies. Can J Gastroenterol Hepatol. 2015;29:327-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | MacGregor A, Ollier W, Thomson W, Jawaheer D, Silman A. HLA-DRB1* 0401/0404 genotype and rheumatoid arthritis: increased association in men, young age at onset, and disease severity. J Rheumatol. 1995;22:1032-1036. |
| 14. | Taleban S, Colombel JF, Mohler MJ, Fain MJ. Inflammatory bowel disease and the elderly: a review. J Crohns Colitis. 2015;9:507-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 126] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 15. | Song SO, Jung CH, Song YD, Park CY, Kwon HS, Cha BS, Park JY, Lee KU, Ko KS, Lee BW. Background and data configuration process of a nationwide population-based study using the korean national health insurance system. Diabetes Metab J. 2014;38:395-403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 404] [Cited by in RCA: 539] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 16. | Park S, Chun J, Han KD, Soh H, Kang EA, Lee HJ, Im JP, Kim JS. Dose-response relationship between cigarette smoking and risk of ulcerative colitis: a nationwide population-based study. J Gastroenterol. 2019;54:881-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 17. | Park S, Chun J, Han KD, Soh H, Choi K, Kim JH, Lee J, Lee C, Im JP, Kim JS. Increased end-stage renal disease risk in patients with inflammatory bowel disease: A nationwide population-based study. World J Gastroenterol. 2018;24:4798-4808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 18. | Kang EA, Han K, Chun J, Soh H, Park S, Im JP, Kim JS. Increased Risk of Diabetes in Inflammatory Bowel Disease Patients: A Nationwide Population-based Study in Korea. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 19. | Heyman MB, Kirschner BS, Gold BD, Ferry G, Baldassano R, Cohen SA, Winter HS, Fain P, King C, Smith T, El-Serag HB. Children with early-onset inflammatory bowel disease (IBD): analysis of a pediatric IBD consortium registry. J Pediatr. 2005;146:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 368] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 20. | Ponsonby AL, Catto-Smith AG, Pezic A, Dupuis S, Halliday J, Cameron D, Morley R, Carlin J, Dwyer T. Association between early-life factors and risk of child-onset Crohn's disease among Victorian children born 1983-1998: a birth cohort study. Inflamm Bowel Dis. 2009;15:858-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Leong RW, Lau JY, Sung JJ. The epidemiology and phenotype of Crohn's disease in the Chinese population. Inflamm Bowel Dis. 2004;10:646-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 168] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 22. | Yao T, Matsui T, Hiwatashi N. Crohn's disease in Japan: diagnostic criteria and epidemiology. Dis Colon Rectum. 2000;43:S85-S93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 163] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 23. | Pierdominici M, Maselli A, Varano B, Barbati C, Cesaro P, Spada C, Zullo A, Lorenzetti R, Rosati M, Rainaldi G, Limiti MR, Guidi L, Conti L, Gessani S. Linking estrogen receptor β expression with inflammatory bowel disease activity. Oncotarget. 2015;6:40443-40451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 24. | Cutolo M, Capellino S, Sulli A, Serioli B, Secchi ME, Villaggio B, Straub RH. Estrogens and autoimmune diseases. Ann N Y Acad Sci. 2006;1089:538-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 185] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 25. | Kondo K, Ohfuji S, Watanabe K, Yamagami H, Fukushima W, Ito K, Suzuki Y, Hirota Y; Japanese Case-Control Study Group for Crohn's disease. The association between environmental factors and the development of Crohn's disease with focusing on passive smoking: A multicenter case-control study in Japan. PLoS One. 2019;14:e0216429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | van der Heide F, Dijkstra A, Weersma RK, Albersnagel FA, van der Logt EM, Faber KN, Sluiter WJ, Kleibeuker JH, Dijkstra G. Effects of active and passive smoking on disease course of Crohn's disease and ulcerative colitis. Inflamm Bowel Dis. 2009;15:1199-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 27. | Bergmann MM, Hernandez V, Bernigau W, Boeing H, Chan SS, Luben R, Khaw KT, van Schaik F, Oldenburg B, Bueno-de-Mesquita B, Overvad K, Palli D, Masala G, Carbonnel F, Boutron-Ruault MC, Olsen A, Tjonneland A, Kaaks R, Katzke V, Riboli E, Hart AR. No association of alcohol use and the risk of ulcerative colitis or Crohn's disease: data from a European Prospective cohort study (EPIC). Eur J Clin Nutr. 2017;71:512-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 28. | Salih A, Widbom L, Hultdin J, Karling P. Smoking is associated with risk for developing inflammatory bowel disease including late onset ulcerative colitis: a prospective study. Scand J Gastroenterol. 2018;53:173-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Opstelten JL, de Vries JHM, Wools A, Siersema PD, Oldenburg B, Witteman BJM. Dietary intake of patients with inflammatory bowel disease: A comparison with individuals from a general population and associations with relapse. Clin Nutr. 2019;38:1892-1898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 30. | Carlé A, Pedersen IB, Knudsen N, Perrild H, Ovesen L, Rasmussen LB, Jørgensen T, Laurberg P. Moderate alcohol consumption may protect against overt autoimmune hypothyroidism: a population-based case-control study. Eur J Endocrinol. 2012;167:483-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 31. | Maxwell JR, Gowers IR, Moore DJ, Wilson AG. Alcohol consumption is inversely associated with risk and severity of rheumatoid arthritis. Rheumatology (Oxford). 2010;49:2140-2146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 32. | Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58:498-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 954] [Cited by in RCA: 912] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 33. | Gielen S, Schuler G, Adams V. Cardiovascular effects of exercise training: molecular mechanisms. Circulation. 2010;122:1221-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 291] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 34. | He C, Bassik MC, Moresi V, Sun K, Wei Y, Zou Z, An Z, Loh J, Fisher J, Sun Q, Korsmeyer S, Packer M, May HI, Hill JA, Virgin HW, Gilpin C, Xiao G, Bassel-Duby R, Scherer PE, Levine B. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012;481:511-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 904] [Cited by in RCA: 902] [Article Influence: 69.4] [Reference Citation Analysis (0)] |
| 35. | Nys K, Agostinis P, Vermeire S. Autophagy: a new target or an old strategy for the treatment of Crohn's disease? Nat Rev Gastroenterol Hepatol. 2013;10:395-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 36. | van Schaik FD, Oldenburg B, Hart AR, Siersema PD, Lindgren S, Grip O, Teucher B, Kaaks R, Bergmann MM, Boeing H, Carbonnel F, Jantchou P, Boutron-Ruault MC, Tjønneland A, Olsen A, Crowe FL, Peeters PH, van Oijen MG, Bueno-de-Mesquita HB. Serological markers predict inflammatory bowel disease years before the diagnosis. Gut. 2013;62:683-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 37. | Stenvinkel P. Inflammation in end-stage renal disease: the hidden enemy. Nephrology (Carlton). 2006;11:36-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 142] [Article Influence: 7.5] [Reference Citation Analysis (2)] |
| 38. | O'Sullivan ED, Hughes J, Ferenbach DA. Renal Aging: Causes and Consequences. J Am Soc Nephrol. 2017;28:407-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 331] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 39. | O'Hare AM, Choi AI, Bertenthal D, Bacchetti P, Garg AX, Kaufman JS, Walter LC, Mehta KM, Steinman MA, Allon M, McClellan WM, Landefeld CS. Age affects outcomes in chronic kidney disease. J Am Soc Nephrol. 2007;18:2758-2765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 480] [Cited by in RCA: 501] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 40. | Raman M, Green D, Middleton RJ, Kalra PA. Comparing the impact of older age on outcome in chronic kidney disease of different etiologies: a prospective cohort study. J Nephrol. 2018;31:931-939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 41. | Dong J, Chen Y, Tang Y, Xu F, Yu C, Li Y, Pankaj P, Dai N. Body Mass Index Is Associated with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. PLoS One. 2015;10:e0144872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 42. | Mendall M, Harpsøe MC, Kumar D, Andersson M, Jess T. Relation of body mass index to risk of developing inflammatory bowel disease amongst women in the Danish National Birth Cohort. PLoS One. 2018;13:e0190600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 43. | Khalili H, Ananthakrishnan AN, Konijeti GG, Higuchi LM, Fuchs CS, Richter JM, Chan AT. Measures of obesity and risk of Crohn's disease and ulcerative colitis. Inflamm Bowel Dis. 2015;21:361-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 124] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 44. | Torres J, Colombel JF, Riddle MS. Evidence for Life Before Inflammatory Bowel Disease. Clin Gastroenterol Hepatol. 2016;14:825-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 45. | Agouridis AP, Elisaf M, Milionis HJ. An overview of lipid abnormalities in patients with inflammatory bowel disease. Ann Gastroenterol. 2011;24:181-187. [PubMed] |
| 46. | Soh H, Im JP, Han K, Park S, Hong SW, Moon JM, Kang EA, Chun J, Lee HJ, Kim JS. Crohn's disease and ulcerative colitis are associated with different lipid profile disorders: a nationwide population-based study. Aliment Pharmacol Ther. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |