Published online Jan 28, 2020. doi: 10.3748/wjg.v26.i4.383
Peer-review started: November 14, 2019
First decision: December 12, 2019
Revised: January 7, 2020
Accepted: January 11, 2020
Article in press: January 11, 2020
Published online: January 28, 2020
Processing time: 65 Days and 3.5 Hours
Gastrostomy tube is an effective and safe long-term feeding access that is well-tolerated by patients. The typical placement routes include surgical, endoscopic and interventional radiologic placement. In particular, percutaneous interventional radiologic gastrostomy (PIRG) has increasingly become the preferred method of choice in many practices. Although many PIRG techniques have been developed since the 1980s, there is still a paucity of evidence supporting the choice of a most-optimal PIRG technique. Hence, there is a large variation in institutional approach to PIRG. We are a large, quaternary academic institution with an extensive experience in PIRG. Therefore, we aim to present the “push” PIRG technique utilized in our institution, to review the current literature, to discuss the optimal choice of PIRG technique and to generate further interests in comparison studies.
Core tip: Percutaneous interventional radiologic gastrostomy has become a popular choice for gastrostomy placement. However, there hasn't been an established most-optimal percutaneous interventional radiologic gastrostomy method. Therefore, we would like to present what we believe is the best approach and hope to spark readers' interests in pursuing further direct comparison studies.
- Citation: Partovi S, Li X, Moon E, Thompson D. Image guided percutaneous gastrostomy catheter placement: How we do it safely and efficiently. World J Gastroenterol 2020; 26(4): 383-392
- URL: https://www.wjgnet.com/1007-9327/full/v26/i4/383.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i4.383
Gastrostomy catheters provide alternative feeding access for patients who cannot tolerate oral intake. Common feeding indications include head and neck tumors, complex postsurgical head and neck reconstructions, esophageal tumors, esophageal motility disorders and neurological disorders leading to dysphagia with a high risk of aspiration (e.g., amyotrophic lateral sclerosis, multiple sclerosis, stroke with residual impairment)[1]. Other conditions that may warrant gastrostomy catheters include severe gastroparesis and small bowel obstruction. For these indications, gastrostomy catheters are used to provide gastric decompression and palliation.
Gastrostomy catheters can be placed in a variety of ways. The three primary modalities include surgical, endoscopic and interventional radiologic placement. Each technique may be preferred under specific circumstances and is based on local expertise. There is ongoing debate with regard to the most optimal modality[2,3]. Invasive surgical gastrostomy was first described in the 1800s and has a 100% technical success rate[4]. It is typically pursued through a left sagittal incision followed by a direct cut-down to the gastric body for access. A minimally invasive alternative is percutaneous endoscopic gastrostomy (PEG) which requires moderate conscious sedation and local anesthetics. Thereby, it minimizes the risk of cardiopulmonary compromise that is associated with general anesthesia. In comparison to surgical gastrostomy, PEG is much quicker and is performed in the endoscopy suites. Thus, the endoscopic approach negates the need for an expensive operating room. Further, in comparison to surgical gastrostomy, PEG has been reported to have decreased overall morbidity and mortality[2,5]. However, there have been reports documenting inadvertent tumor metastasis to gastrostomy placement sites[6]. This phenomenon can be a result of tumor seeding from passing the rigid endoscope through malignancy located along the advancement path. With the advent of image guided techniques, percutaneous interventional radiological gastrostomy (PIRG) has become an evolving and attractive alternative. It circumvents some limitations of surgical and PEG approaches, such as tumor seeding, potential disruption of the postsurgical head and neck reconstruction zone or complications associated with invasive surgeries. In addition, PIRG can identify and assess for the location of surrounding structures using image guidance and colonic opacification. The use of these techniques decreases the risk of accidental organ injury and ultimately increases patient safety. On the other side, in comparison to PEG, PIRG required a dedicated fluoroscopy suite, which may not be readily available at all institutions. Further, institutional expertise in either PIRG or PEG may dictate the preference for either techniques due to complication rates and duration of the procedure.
Similar to PEG, PIRG can also be performed in either a “pull” or “push” technique[7,8]. The “pull” PIRG technique is performed by advancing a needle into the air-insufflated stomach. A catheter is then advanced through the esophagus into the oral cavity via a retrograde approach. A guidewire is advanced through the catheter gaining through-and-through access. The gastrostomy catheter is fastened to the guidewire, and pulled antegrade through the mouth, esophagus, and abdominal wall. It is typically secured to the anterior abdominal wall utilizing a mushroom-retaining method. In our institution, we prefer the “push” PIRG technique. The purpose of this article is to present our institutional approach to “push” PIRG technique and the perioperative care of this patient population in a stepwise fashion.
Prior to the procedure, a thorough pre-procedural assessment should be completed. If available, a recent computed tomography (CT) within the last two to four months should be reviewed to assess the gastric anatomy and the suitability of gastrostomy catheter placement. While evaluating the anatomy, it should be considered that after air insufflation, the anatomy can change markedly such that a previously inaccessible gastric body may become accessible. Therefore, if a prior CT is not available, it is not advisable to repeat the scan before the procedure. Instead a cone-beam CT can be performed after gastric insufflation to assess the anatomy as well as the chance of obtaining percutaneous access. Pre-procedure complete blood count and coagulation labs should be checked. Based on our institutional guidelines, platelets should be above 50 × 109/L and international normalized ratio should be lower than 1.5. Other contraindications include uncorrectable coagulopathy, massive hepatosplenomegaly or anteriorly overlying colon preventing a safe access to the stomach, peritonitis, and bowel ischemia. Due to the risk of bleeding, access through omental implants should be avoided. Cone beam CT or available prior CT imaging is helpful to determine a safe direct access to the gastric body without intervening omental implants. If ascites is seen between the stomach and abdominal wall, a paracentesis should be performed at the day of procedure.
In the vast majority of cases, gastrostomy catheter placement can be pursued using moderate conscious sedation with intravenous fentanyl and midazolam administration. If the patient is unable to cooperate or is agitated during the procedure, general anesthesia may be considered.
Ideally a nasogastric tube (NGT) should be in place before the patient comes to the procedure room. However, in cases with challenging NGT placement, such as those with complex postsurgical head and neck reconstructions, the NGT can be placed under fluoroscopy guidance. A 4-French Kumpe or 5-French MPA catheter can be inserted through one of the nostrils over a hydrophilic guidewire (typically a 0.035-inch Glidewire) into the stomach. The guidewire is then removed and the 4-French Kumpe or 5-French MPA catheter remains in place to inflate the stomach with air. The Kumpe or MPA catheter needs to be threaded very cautiously in postsurgical head and neck patients. It should be placed under continuous fluoroscopy imaging to avoid damages to the post-surgical area, such as a complex reconstructed flap. Next, in the procedure suite a focused pre-procedural ultrasound must be performed to mark the edge of the left liver lobe and the location of the epigastric arteries. Both structures need to be avoided to decrease the risk of significant bleeding. Two g of intravenous cefazolin will be given to the patient immediately prior to the procedure as antibiotic prophylaxis. In cases of penicillin allergy, 1 g intravenous vancomycin or 600 mg intravenous clindamycin can be administered alternatively[9].
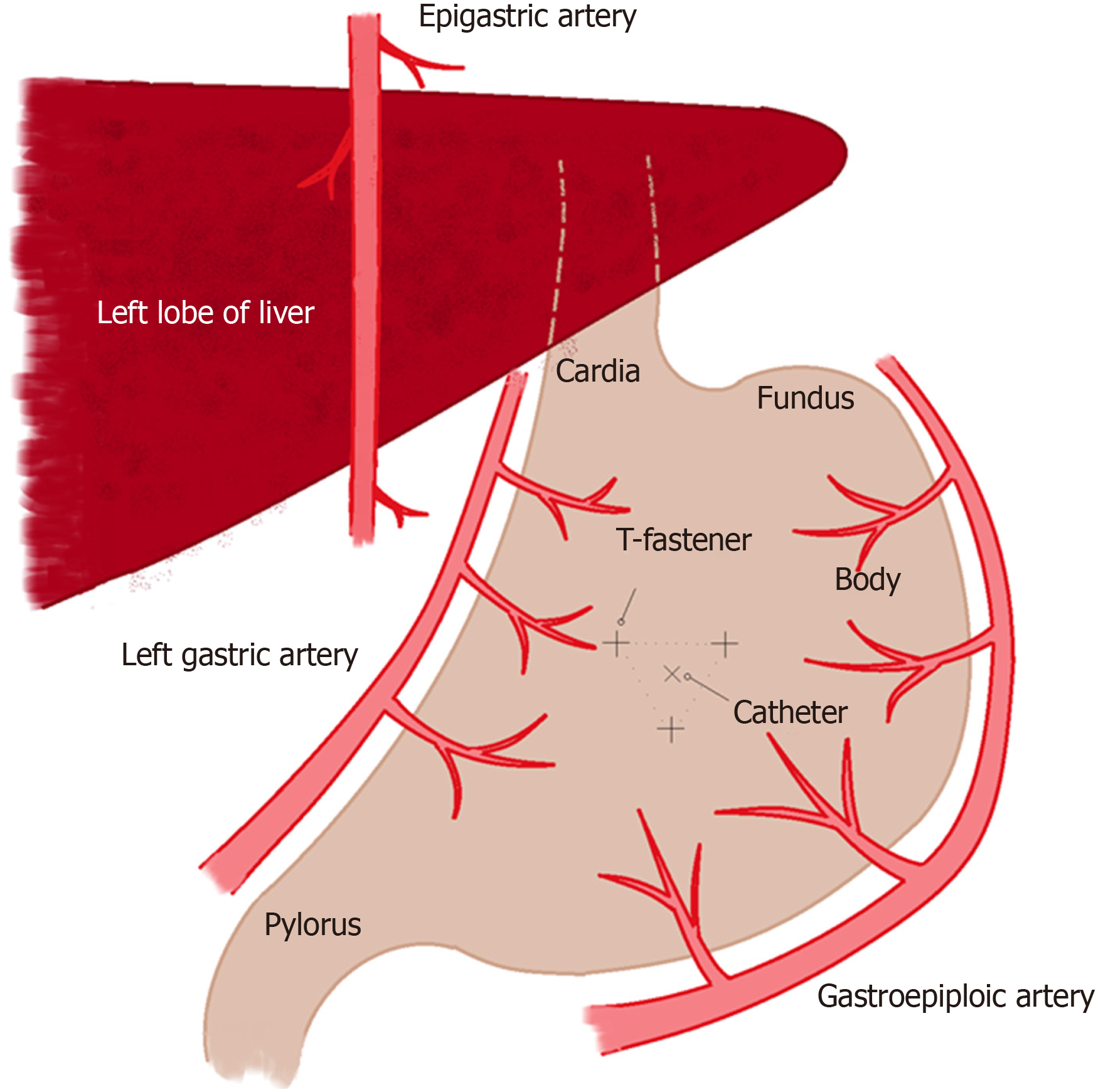
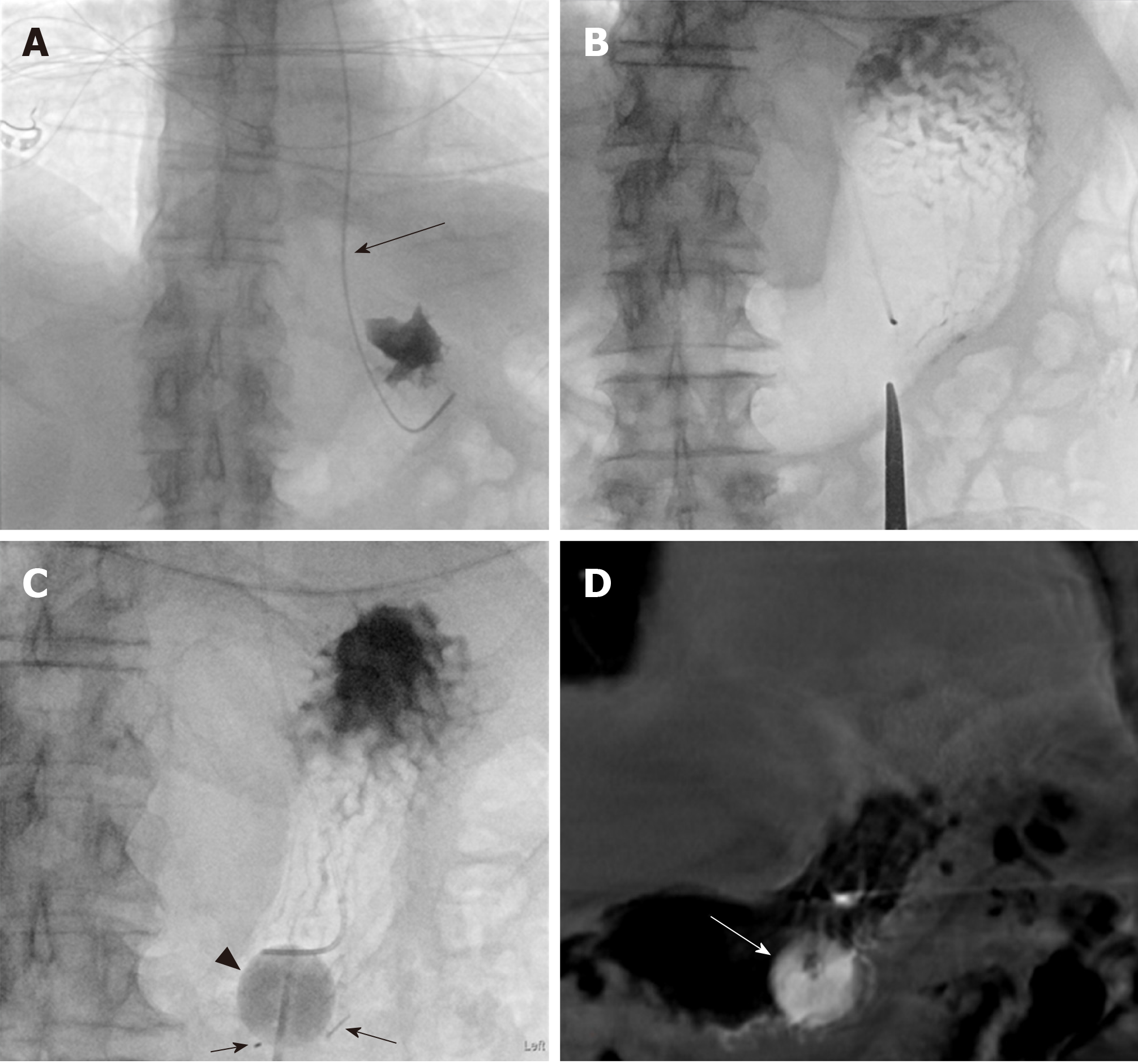
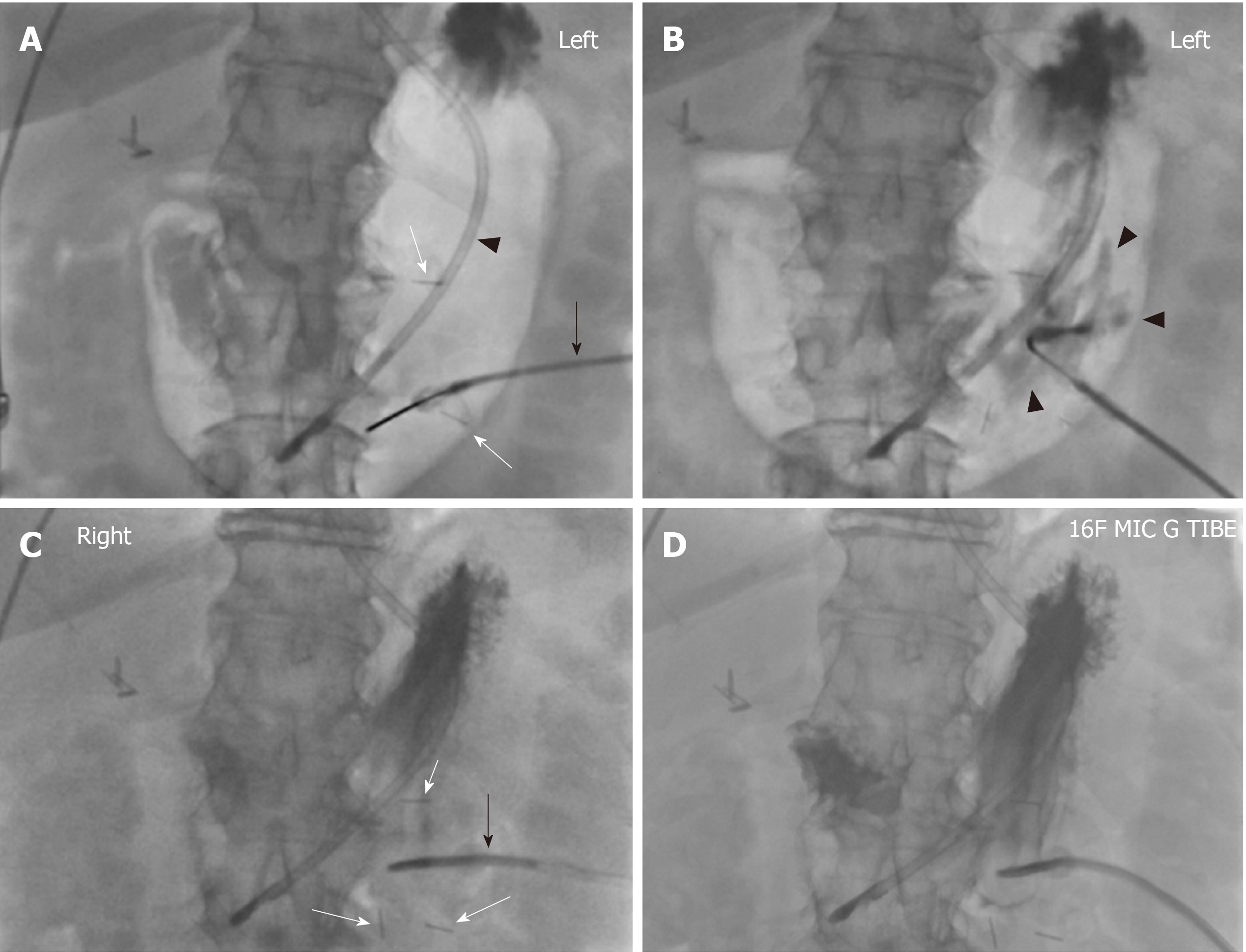
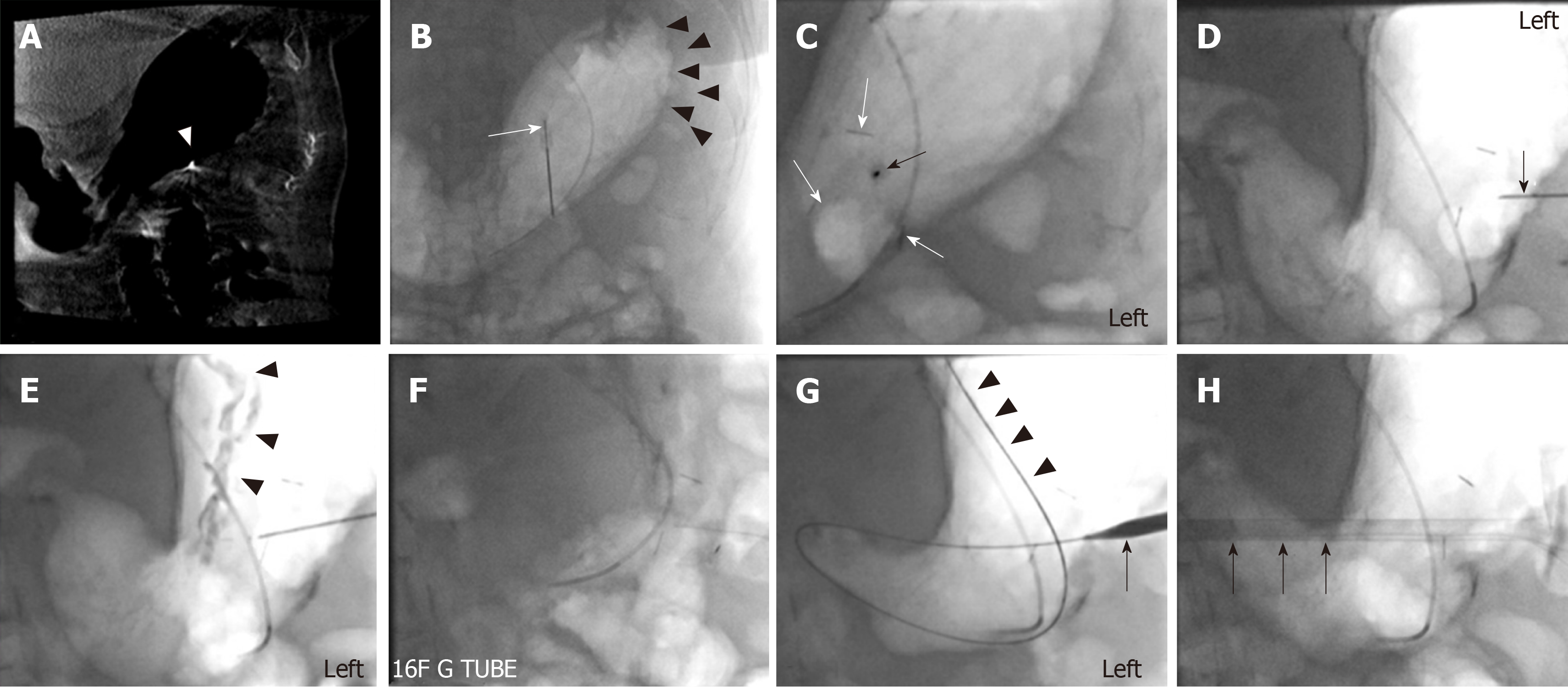
At the beginning of the procedure, the abdomen is prepared in the usual sterile fashion. The stomach is manually inflated with air to pitch the gastric body against the abdominal wall. If the stomach decompresses rapidly through peristalsis as evidenced on fluoroscopy, 1 mg of glucagon is given intravenously to decrease the peristaltic motion and thereby maintain the gastric insufflation. Optimal gastric insufflation is of the utmost importance to facilitate safe access, to reduce the risk of gastric tube misplacement, and to decrease the risk of accidental organ injuries. If there is any doubt about the safety of percutaneous access based on fluoroscopy, cone-beam CT can be performed. The access site is chosen under imaging guidance and should be half way between the greater and lesser gastric curvatures overlying the gastric body and should be angled towards the pylorus. The angled approach is advisable since it facilitates conversion to a gastrojejunostomy tube later if clinically warranted. Once the stomach is sufficiently inflated and the access site has been selected, the gastropexy is performed by using three T-bar fasteners in a triangular fashion around the selected percutaneous access site (Figure 1). The first T-bar fastener is lined up with the beam in the left anterior oblique view projecting over the gastric body. The T-bar fastener is advanced slowly under the fluoroscopic guidance until resistance is met. The prior CT or intra-procedural cone beam CT can help assess the distance needed to be traversed towards the gastric wall. The C-arm is then rotated to the right anterior oblique view to visualize the gastric wall tenting in real time and to assess the exact depth of the T-bar fastener. While tenting the gastric wall, the T-bar fastener is connected to an empty syringe and is advanced with continuous suction until air is aspirated into the syringe. Iodinated contrast is then injected to ascertain the intragastric location of the T-bar fastener, which is then released. This process is repeated with the second and third T-bar fastener.
Next, the actual percutaneous gastrostomy is performed. First an 18-gauge needle is lined up with the beam in the left anterior oblique view projecting over the gastric body at the selected entry site. The needle is angled towards to the antrum and pylorus and is advanced slowly under the fluoroscopic guidance until resistance is met. Again, the needle advancement distance to the gastric wall can be assessed on prior CT or intra-procedural cone-beam CT. The C-arm is then rotated to the right anterior oblique view to visualize gastric wall tenting in real-time and to assess the exact depth of the needle. While tenting the gastric wall the needle is connected to an empty syringe and is advanced under continuous mild suction until air is aspirated into the syringe. Iodinated contrast is then injected to ascertain the intragastric location of the needle. At this time, a 0.035-inch Amplatz stiff wire is advanced through the 18-gauge needle into the stomach. Subsequently, the needle is removed over the wire. After sequential dilation of the access site by stiff dilators, the peel-away sheath is advanced over the guidewire. This is followed by gastrostomy catheter placement over the Amplatz wire through the peel-away sheath. The peel-away sheath is four French larger than the gastrostomy catheter. We typically place a 16-French gastrostomy catheter which requires a 20-French peel-away sheath or an 18-French gastrostomy catheter which requires a 22-French peel-away sheath. As gastrostomy catheter we utilize a balloon-type silicone catheter with a large lumen instead of the smaller caliber pigtail catheter. Next, the balloon of the gastrostomy catheter is inflated with the maximal recommended amount of sterile water (which is usually 5 cc and 10 cc for the 16- and 18-French gastrostomy catheters. The gastrostomy catheter is pulled back until resistance is met and this essentially secures the intraluminal balloon against the lining of the near gastric wall. Finally, the Amplatz wire is removed and iodinated contrast is injected through the freshly placed gastrostomy catheter to ascertain location within the gastric body. The gastrostomy catheter is connected to a drainage bag (we typically use a Foley bag) to enable gravity drainage for 24 h before starting to use the gastrostomy catheter for enteral feeding. The main reason for 24-h passive drainage is to remove retained gastric products (potentially mixed with hemorrhagic products from the procedure).
Alternatively, the tract can be dilated with an angioplasty technique using balloons. When applying this technique, we typically use a 6 mm × 40 mm or 7 mm × 40 mm Mustang balloon (Boston Scientific, Natick, MA) to place a 16-French tube. After tract dilation the gastrostomy catheter can be advanced into the gastric body while deflating the angioplasty balloon. This method may result in a tighter gastrostomy catheter access tract thereby potentially preventing leakage in the future.
Special attention should be given when performing PIRG in patients with massive ascites. A paracentesis should be performed prior to the gastrostomy procedure to provide a favorable safe access. Once the gastrostomy catheter is placed, the tract should be allowed to mature for approximately 6 to 8 wk prior to any further tube manipulation as premature tube changes or gastrojejunostomy tube conversion is associated with a significant risk of losing access when ascites is present.
At 24-h post-procedure, a focused abdominal exam is performed to assess for peritoneal signs and associated potential malposition of the gastrostomy catheter on all patients. Potential hematoma formation is also assessed. Post-procedural complete blood count, such as hemoglobin, hematocrit and white blood cell, should be analyzed for potential bleeding and infection. If the abdominal exam is completely benign and the patient is asymptomatic, post-procedural complete blood count may not be required. A mild increase of the white blood cell count post procedure is frequently encountered and is not concerning. At this point, if the patient does not exhibit any signs of leak, infection or bleeding, a 2-h sterile water trial should be conducted. A 25 cc sterile water bolus is given in the first hour, followed by another 25 cc bolus in the second hour. If the patient tolerates the sterile water trial without any signs of peritoneal irritation or leakage, tube feeding can be started slowly. The procedure is tolerated well and typically no dedicated post-procedural pain management is needed.
The T-bar fasteners usually falls off after 2 to 3 wk. A follow-up appointment in the interventional radiology outpatient clinic is scheduled 3 wk post procedure to assess for gastrostomy catheter function and potential complaints. At that time, it is again checked whether all three T-bar fasteners fell off. If there is retained material it will be removed during the clinic visit. Newer T-bar fasteners are falling off without removal requirement. Seldom there is retained material which can be removed during the outpatient clinic visit. If gastrostomy catheter leakage is noticed during follow-up the gastrostomy catheter can be upsized to a larger caliber. Conservative alternatives for treatment of leakage are slowing down the tube feeds to a “trickle” fashion or placing the gastrostomy intermittently to suction to decompress the stomach. Further, in case of leakage it is important to ensure that the retention balloon is inflated at its maximum capacity and is firmly placed against the anterior abdominal wall. If the tract has matured for 6 to 8 weeks and leakage persists a conversion of the gastrostomy catheter to the gastrojejunostomy tube is recommended. In a gastrojejunostomy tube the jejunostomy lumen can be used for feeding purposes while the gastrostomy lumen is used for venting purposes and placed on suction to decompress the stomach.
Based on our experience the procedure is usually performed in less than 30 min with moderate conscious sedation under fluoroscopy guidance and less than 45 min under both fluoroscopy and cone beam CT guidance. The typical fluoroscopic time is between 5 to 7 min. Representative PIRG placement cases are shown in Figures 2-4 to demonstrate the said steps with fluoroscopy and procedure time.
Certain disease conditions require an alternative enteral feeding access. NGT can provide short-term access, but its long-term use is limited by patient discomfort and higher malposition and aspiration risk. Further, the postsurgical head and neck patient is not a candidate for NGT placement due to the risk of damaging the surgical field, such as a complex reconstruction flap. For patients with a functional gastrointestinal tract, total parenteral nutrition is not ideal due to a lack of bowel stimulation leading to a compromised gut defense barrier and high rates of infection[10]. Further, parenteral nutrition is costly. In certain patients the gastrostomy catheter can provide a durable solution for long-term feeding.
Since its inception in 1981, the PIRG technique has gradually improved and has become a safe and effective alternative to the traditional surgical or endoscopic gastrostomy catheter placements. It requires moderate conscious sedation and local anesthetics, thus minimizing the risks of cardiorespiratory compromise. Further, it is a relatively quick procedure that can be performed in the interventional radiology suite, which negates the need for an expensive operating room. The average cost of PIRG is comparable to or slightly cheaper than PEG and lower than surgical gastrostomy. Barkmeier et al[11] reported that the cost of PIRG to be $1985 while that of PEG to be $1862 in an United States institution while Galaski et al[12] reported $407 for PIRG and $591 for PEG in Canadian dollars. In addition, since it can be performed in a timely manner (the vast majority of PIRG procedures are below 30 min at our institution), scheduling is often more flexible as well.
Clinically, there are a limited number of absolute contraindications for PIRG, including active peritonitis, uncorrectable coagulopathy, and acute bowel ischemia (if the gastrostomy catheter is used for feeding purposes)[13]. Modifications and improvement of the current practice can avoid much of the technical difficulties. The use of image guided direct transabdominal access can avoid an obstructing neoplastic mass involving the pharynx, larynx, esophagus and stomach. The use of cone-beam CT in addition to fluoroscopic guidance in challenging cases can effectively identify the insufflated stomach and avoid needle punctures to the adjacent organs such as the colon, liver and spleen. In the past, colonic interposition was an absolute contraindication[1]. However, with fluoroscopic guidance, an infracolonic approach is feasible.
The technical success rate of PIRG is high. PIRG success rate ranges between 98%-100%[14-17]. In comparison, surgical gastrostomy has a 100% success rate due to the nature of the open approach[2]. PEG has a success rate ranging between 84%-96%[12,18,19]. The technical difficulties associated with PEG are largely due to inability to adequately transilluminate the anterior abdominal wall, such as in the case of morbidly obese patients. Local anatomical anomalies such as large hiatal hernia and obstructing oropharyngeal cancer are limiting the use of PEG. PIRG can be successfully performed in patients who have failed PEG attempts. Thornton et al[20] have reported that PIRG was attempted in forty-two patients who have failed PEG trial due to gastrointestinal obstruction, suboptimal transillumination, and cardiorespiratory decompensation. In this population forty-one patients underwent successful PIRG placement.
In addition to the high technical success rate, PIRG has low procedure-related mortality rates that is comparable to or better than other modalities. It is worth emphasizing that patients referred to PIRG are often ill and sometimes have failed previous PEG attempts. Bell et al[21] reported the 30-d mortality rate to be 17.1% in 416 patients, although only 2 of these patient deaths were procedure-related. Similarly, Ahmed et al[14] reported a 19.7% one-year mortality in 305 patients. Among those, only one procedure-related death occured. Further, Perona et al[15] reported a 0.2% procedure-related mortality rate over twelve years. Wollman et al[2] reported a procedure-related mortality rate of 0.3% for PIRG and 0.53% for PEG. A British meta-analysis has reported 1.8% and 2.2% of procedure-mortality for PIRG and PEG groups respectively in 2379 patients[22]. PIRG associated complications are generally divided into major and minor categories: major complications being abscess formation, bowel perforation, peritonitis, and hemorrhage while minor complications include superficial infection, tube dislodgement, tube occlusion and leakage. de Baere et al[17] reported 1.4% and 5.4% major and minor complications rates in five hundred patients. Similarly, Ahmed et al[14] reported 2% and 5% major and minor complication rates in 300 patients. Further, PEG and PIRG groups have comparable complication rates that are markedly lower than that of the surgical gastrostomy group. In an earlier meta-analysis, the surgical gastrostomy catheter group had a 29% overall complication rate while PEG and PIRG groups had 15.4% and 13.3%, respectively[2]. Barkmeier et al[12] reported five minor complications in forty-two PIRG patients and sixteen in forty-five PEG patients.
The most common major complications associated with PIRG are peritonitis and hemorrhage. Peritonitis occurs in approximately 1.3% of the patients[23]. Peritonitis can occur as a result of chemical irritation due to bowel content leakage from tube dislodgement, peritoneal catheterization, peritoneal iodinated contrast injection, colonic perforation or leakage around the puncture sites. Peritonitis can also occur due to bacterial infection from skin flora invasion through the catheterization track or from bowel content. Therefore, antibiotics prophylaxis is recommended. It is worth noting that peritonitis can potentially cause significant morbidity and mortality as this patient population tends to be frail[24,25]. The newly placed gastrostomy catheter must be allowed to passive drainage for 24 h prior to the initiation of tube feeding to decompress the gastric body and minimize the risk of leakage. If per oral medication administration is required for certain patient populations a nasogatric tube should be used in the first 24 h. While giving the per oral medication the freshly placed gastrostomy catheter can be clamped for 30 to 60 min to enable absorption of the administered per oral medication before placing the gastrostomy catheter to passive drainage again. Specific attention must be given to assess for post-procedure peritonitis. Signs of peritonitis include fever, chills, tachycardia, hypotension, elevated white blood cell count, abdominal pain, and guarding/rebound tenderness on post procedure physical exam. Hemorrhage occurs in approximately 1.4% of all patients[23]. Large volume hemorrhage is rare unless the epigastric or gastroepiploic vessels are injured during the procedure. Therefore, it is important to assess the bowel and vascular anatomy with pre-procedural CT and ultrasound. Furthermore, it is critical to choose the initial gastrostomy site near the upper third of the abdomen to avoid the epigastric vessels. In addition, access site should be directed at the mid-section of the stomach equidistant from the greater and lesser curvatures to avoid the gastric and gastroepiploic vessels.
There has been a paucity of data comparing the two different PIRG techniques: the so-called “push” and “pull” techniques. A review of literature shows comparable major complication rates across both groups and either comparable or slightly lower minor complication rates in the “pull” group as compared to the “push” group[24,26-28]. A detailed look into the studies has shown that the gastrostomy catheter used in the “push” group is consistently smaller in caliber and often exclusively of the pigtail-retaining mechanism. Laasch et al[27] used 10.5-French or 12-French “push” gastrostomy catheters and 20-French “pull” gastrostomy catheters in their study. A more recent study used 14.5-French mean-sized “push” gastrostomy catheters and 21-French mean-sized “pull” gastrostomy catheters[28]. Multiple studies have shown that smaller caliber tubes are more prone to either tubal occlusion or failure to meet feeding goals[24]. In addition, Funaki et al[29] reported a much more significant tubal complication rate in pigtail-retaining catheters in the “push” group than mushroom-retaining tubes in the “pull” group (36% vs 2%). It has been documented in the literature that larger bore “push” type catheters (20 to 24-French) can be successfully placed via the “push” technique. Particularly with moderate conscious sedation the "push" technique leads to less patient discomfort compared to the "pull" technique during which per oral advancement and manipulation is required. Further, the "pull" technique is not ideal for many patients requiring enteral feeding, such as those with head and neck or esophageal tumors as well as postsurgical head and neck patients. In our practice we typically place a 16- or 18-French non-pigtail gastrostomy catheters using the "push" technique. It is yet to be determined whether the 16 to 18-French balloon-retaining “push” non-pigtail gastrostomy catheters used at our institution may perform comparably to the mushroom-retained “pull” gastrostomy catheters in those patients who qualify for the "pull" technique.
Based our experience the "push" technique can be performed faster, is safer in the head and neck patient population, and requires less sedation. Further, it is associated with less patient discomfort as the gastrostomy catheter does not need to be pulled through the mouth. In addition, there is a decreased risk of infection due to a lack of per-oral catheterization with the transition of bacteria from the oral cavity into the gastrointestinal flora or vice versa[30]. Further, the “push” technique minimizes the risks of tumor seeding through the track as opposed to “pull” type gastrostomy catheter placement[26]. Therefore, we prefer to use “push” technique at our institution, although there is still ongoing debate over the most optimal method.
Since its inception in 1981, PIRG has gained popularity and has become a viable alternative to the traditional surgical gastrostomy catheter placement. Its technical success and complication rates are comparable to that of PEG and lower than that of surgical placement. The two main types of PIRG include the “push” and “pull” gastrostomy catheter placement techniques. Although both techniques have been established since the late 1990s, there exists a paucity of studies comparing the two. Based on our institutional experience the “push” technique is faster, safer and better tolerated by patients. Its complication rates are comparable to that of the “pull” technique and may be even lower when using larger-bore, balloon-retaining non-pigtail catheters. In this paper we have described in a stepwise fashion our procedural technique and have discussed the perioperative care.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kotzampassi K, Jinga M S-Editor: Dou Y L-Editor: A E-Editor: Ma YJ
| 1. | Ho SG, Marchinkow LO, Legiehn GM, Munk PL, Lee MJ. Radiological percutaneous gastrostomy. Clin Radiol. 2001;56:902-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (2)] |
| 2. | Wollman B, D'Agostino HB, Walus-Wigle JR, Easter DW, Beale A. Radiologic, endoscopic, and surgical gastrostomy: an institutional evaluation and meta-analysis of the literature. Radiology. 1995;197:699-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 294] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 3. | Rustom IK, Jebreel A, Tayyab M, England RJ, Stafford ND. Percutaneous endoscopic, radiological and surgical gastrostomy tubes: a comparison study in head and neck cancer patients. J Laryngol Otol. 2006;120:463-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 87] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Lyon SM, Pascoe DM. Percutaneous gastrostomy and gastrojejunostomy. Semin Intervent Radiol. 2004;21:181-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 5. | Miller RE, Kummer BA, Tiszenkel HI, Kotler DP. Percutaneous endoscopic gastrostomy. Procedure of choice. Ann Surg. 1986;204:543-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Cruz I, Mamel JJ, Brady PG, Cass-Garcia M. Incidence of abdominal wall metastasis complicating PEG tube placement in untreated head and neck cancer. Gastrointest Endosc. 2005;62:708-11; quiz 752, 753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Szymski GX, Albazzaz AN, Funaki B, Rosenblum JD, Hackworth CA, Zernich BW, Leef JA. Radiologically guided placement of pull-type gastrostomy tubes. Radiology. 1997;205:669-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Preshaw RM. A percutaneous method for inserting a feeding gastrostomy tube. Surg Gynecol Obstet. 1981;152:658-660. [PubMed] |
| 9. | Venkatesan AM, Kundu S, Sacks D, Wallace MJ, Wojak JC, Rose SC, Clark TW, d'Othee BJ, Itkin M, Jones RS, Miller DL, Owens CA, Rajan DK, Stokes LS, Swan TL, Towbin RB, Cardella JF; Society of Interventional Radiology Standards of Practice Committee. Practice guidelines for adult antibiotic prophylaxis during vascular and interventional radiology procedures. Written by the Standards of Practice Committee for the Society of Interventional Radiology and Endorsed by the Cardiovascular Interventional Radiological Society of Europe and Canadian Interventional Radiology Association [corrected]. J Vasc Interv Radiol. 2010;21:1611-30; quiz 1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 185] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 10. | Ponsky JL, Gauderer MW. Percutaneous endoscopic gastrostomy: indications, limitations, techniques, and results. World J Surg. 1989;13:165-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 92] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Barkmeier JM, Trerotola SO, Wiebke EA, Sherman S, Harris VJ, Snidow JJ, Johnson MS, Rogers WJ, Zhou XH. Percutaneous radiologic, surgical endoscopic, and percutaneous endoscopic gastrostomy/gastrojejunostomy: comparative study and cost analysis. Cardiovasc Intervent Radiol. 1998;21:324-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 90] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Galaski A, Peng WW, Ellis M, Darling P, Common A, Tucker E. Gastrostomy tube placement by radiological versus endoscopic methods in an acute care setting: a retrospective review of frequency, indications, complications and outcomes. Can J Gastroenterol. 2009;23:109-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Sutcliffe J, Wigham A, Mceniff N, Dvorak P, Crocetti L, Uberoi R. CIRSE Standards of Practice Guidelines on Gastrostomy. Cardiovasc Intervent Radiol. 2016;39:973-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Ahmed O, Jilani D, Sheth S, Giger M, Funaki B. Radiologically Guided Placement of Mushroom-retained Gastrostomy Catheters: Long-term Outcomes of Use in 300 Patients at a Single Center. Radiology. 2015;276:588-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Perona F, Castellazzi G, De Iuliis A, Rizzo L. Percutaneous radiologic gastrostomy: a 12-year series. Gut Liver. 2010;4 Suppl 1:S44-S49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 16. | Shin JH, Park AW. Updates on percutaneous radiologic gastrostomy/gastrojejunostomy and jejunostomy. Gut Liver. 2010;4 Suppl 1:S25-S31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | de Baere T, Chapot R, Kuoch V, Chevallier P, Delille JP, Domenge C, Schwaab G, Roche A. Percutaneous gastrostomy with fluoroscopic guidance: single-center experience in 500 consecutive cancer patients. Radiology. 1999;210:651-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 108] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Blondet A, Lebigot J, Nicolas G, Boursier J, Person B, Laccoureye L, Aubé C. Radiologic versus endoscopic placement of percutaneous gastrostomy in amyotrophic lateral sclerosis: multivariate analysis of tolerance, efficacy, and survival. J Vasc Interv Radiol. 2010;21:527-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Hoffer EK, Cosgrove JM, Levin DQ, Herskowitz MM, Sclafani SJ. Radiologic gastrojejunostomy and percutaneous endoscopic gastrostomy: a prospective, randomized comparison. J Vasc Interv Radiol. 1999;10:413-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Thornton FJ, Varghese JC, Haslam PJ, McGrath FP, Keeling F, Lee MJ. Percutaneous gastrostomy in patients who fail or are unsuitable for endoscopic gastrostomy. Cardiovasc Intervent Radiol. 2000;23:279-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Bell SD, Carmody EA, Yeung EY, Thurston WA, Simons ME, Ho CS. Percutaneous gastrostomy and gastrojejunostomy: additional experience in 519 procedures. Radiology. 1995;194:817-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 79] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Grant DG, Bradley PT, Pothier DD, Bailey D, Caldera S, Baldwin DL, Birchall MA. Complications following gastrostomy tube insertion in patients with head and neck cancer: a prospective multi-institution study, systematic review and meta-analysis. Clin Otolaryngol. 2009;34:103-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 164] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 23. | Covarrubias DA, O'Connor OJ, McDermott S, Arellano RS. Radiologic percutaneous gastrostomy: review of potential complications and approach to managing the unexpected outcome. AJR Am J Roentgenol. 2013;200:921-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Kuo YC, Shlansky-Goldberg RD, Mondschein JI, Stavropoulos SW, Patel AA, Solomon JA, Soulen MC, Kwak A, Itkin M, Chittams JL, Trerotola SO. Large or small bore, push or pull: a comparison of three classes of percutaneous fluoroscopic gastrostomy catheters. J Vasc Interv Radiol. 2008;19:557-63; quiz 564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Kahriman G, Ozcan N, Donmez H. Fluoroscopy-guided placement of pull-type mushroom-retained gastrostomy tubes in 102 patients. Diagn Interv Imaging. 2017;98:715-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Yang Y, Schneider J, Düber C, Pitton MB. Comparison of fluoroscopy-guided Pull-type percutaneous radiological gastrostomy (Pull-type-PRG) with conventional percutaneous radiological gastrostomy (Push-type-PRG): clinical results in 253 patients. Eur Radiol. 2011;21:2354-2361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Laasch HU, Wilbraham L, Bullen K, Marriott A, Lawrance JA, Johnson RJ, Lee SH, England RE, Gamble GE, Martin DF. Gastrostomy insertion: comparing the options--PEG, RIG or PIG? Clin Radiol. 2003;58:398-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 81] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Currie BM, Getrajdman GI, Covey AM, Alago W, Erinjeri JP, Maybody M, Boas FE. Push versus pull gastrostomy in cancer patients: A single center retrospective analysis of complications and technical success rates. Diagn Interv Imaging. 2018;99:547-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Funaki B, Zaleski GX, Lorenz J, Menocci PB, Funaki AN, Rosenblum JD, Straus C, Leef JA. Radiologic gastrostomy placement: pigtail- versus mushroom-retained catheters. AJR Am J Roentgenol. 2000;175:375-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Maetani I, Tada T, Ukita T, Inoue H, Sakai Y, Yoshikawa M. PEG with introducer or pull method: a prospective randomized comparison. Gastrointest Endosc. 2003;57:837-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |