Published online Oct 14, 2020. doi: 10.3748/wjg.v26.i38.5874
Peer-review started: June 9, 2020
First decision: July 29, 2020
Revised: August 12, 2020
Accepted: September 17, 2020
Article in press: September 17, 2020
Published online: October 14, 2020
Processing time: 127 Days and 1.5 Hours
Direct-acting antivirals (DAAs) are recommended for the treatment of hepatitis C virus (HCV) infection in patients treated with methadone or buprenorphine.
To assess HCV treatment rates in an Opioid Treatment Program (OTP).
This longitudinal study included 501 patients (81.4% men, median age: 45 years; interquartile range: 39-50 years) enrolled in an OTP between October 2015 and September 2017. Patients were followed until September 2019. Data on socio-demographics, substance use, HCV infection, human immunodeficiency virus (HIV) infection and laboratory parameters were collected at entry. We analyzed medical records to evaluate HCV treatment. Kaplan-Meier methods and Cox regression models were used to analyze the DAA treatment uptake and to identify treatment predictors.
Prevalence of HCV and HIV infection was 70% and 34%, respectively. Among anti-HCV-positive (n = 336) patients, 47.2%, 41.3%, and 31.9% used alcohol, cannabis, and cocaine, respectively. HCV-RNA tests were positive in 233 (69.3%) patients. Twentyeight patients (8.3%) cleared the infection, and 59/308 (19.1%) had received interferon-based treatment regimens before 2015. Among 249 patients eligible, 111 (44.6%) received DAAs. Treatment rates significantly increased over time from 7.8/100 person-years (p-y) (95%CI: 5.0-12.3) in 2015 to 18.9/100 p-y (95%CI: 11.7-30.3) in 2019. In a multivariate analysis, patients with HIV co-infection were twice as likely to receive DAAs (HR = 1.94, 95%CI: 1.21-3.12) than patients with HCV mono-infection. Current drug use was an independent risk factor for not receiving treatment against infection (HR = 0.48, 95%CI: 0.29-0.80).
HCV treatment is evolving in patients with HCV-HIV co-infection. Ongoing drug use while in an OTP might negatively impact the readiness to treat infection.
Core Tip: Longitudinal study carried out in the only Opioid Treatment Program authorized for the provision of methadone or buprenorphine in a large urban area of 360000 inhabitants. Results indicate that hepatitis C virus treatment rates are increasing since the introduction of direct antiviral agents and identifies gaps and challenges on the readiness to treat infection.
- Citation: Sanvisens A, Rivas I, Faure E, Espinach N, Hernandez-Rubio A, Majó X, Colom J, Muga R. Monitoring hepatitis C virus treatment rates in an Opioid Treatment Program: A longitudinal study. World J Gastroenterol 2020; 26(38): 5874-5883
- URL: https://www.wjgnet.com/1007-9327/full/v26/i38/5874.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i38.5874
It is estimated that 10 million people with substance use disorder (SUD) have hepatitis C virus (HCV) infection[1]. In addition, it is believed that a proportion of HCV infections remain undiagnosed in individuals with SUD. According to the World Health Organization (WHO), 23% of new HCV infections occur in patients with SUD[2]. In the United States and western Europe, two out of every three new HCV infections are believed to be associated with substance use[2].
The introduction of direct-acting antiviral agents (DAAs) in 2013 caused substantial changes in the clinical outcomes of HCV infection. Pharmacotherapy for HCV infection is administered for shorter periods of time (i.e., 8-12 wk) and sustained virological responses (SVR) are achieved in over 90% of patients, irrespective of the HCV genotype. Several studies have revealed that DAAs showed efficacy in difficult-to-treat populations, including individuals with SUD[3-8].
The WHO aims to eliminate HCV infection by 2030. The defining features of that goal are to achieve a 90% reduction in new cases, diagnose 90% of all individuals infected with HCV, treat 80% of those eligible, and reduce death by 65%. In this context, individuals with SUD have been recognized as a target population for improving the identification of HCV-related disease and for implementing HCV micro-elimination strategies[9-11]. The strategy is to promote a cascade of care, or a continuum of services that should be provided to cure HCV in persons living with hepatitis[2].
Current guidelines for HCV care and treatment are provided, among others, by the American Association for the Study of the Liver (AASLD), the European Association for the Study of the Liver (EASL), and the WHO[12-14]. All of these organizations recommend DAAs for treating HCV infection, including in individuals with SUD. Indeed, several studies have indicated that SUDs did not affect adherence to treatment or imply worse response rates[15-17].
More than 120000 people have been treated with DAAs since the Strategic Plan for Tackling Hepatitis C was implemented by the Spanish National Health System in 2015[18]. At the same time, up to 60000 patients are regularly treated with opioid agonist therapy (i.e., methadone) in Spain. Individuals treated with methadone might have a history of injected drug use, and consequently, they might have acquired blood-borne infections, like HCV, after they began injecting drugs[19]. A previous study on individuals that participated in Opioid Treatment Programs (OTPs) in Catalonia, Spain, showed that the prevalences of HCV and human immunodeficiency virus (HIV) infections were 74% and 54%, respectively[20].
We hypothesized that in the context of the changes made in the provision of HCV care, OTP sites might be experiencing increasing proportions of patients that are eligible for HCV treatment. Therefore, we studied OTP participants to analyze assessment of infection, treatment rates, and predictors of treatment with DAAs.
This longitudinal study included ex-heroin users enrolled in an OTP between October 2015 and September 2017. The OTP operates in a municipal outpatient clinic specialized in the treatment of SUDs in Badalona (240000 inhabitants) and Santa Coloma de Gramenet (120000 inhabitants), Spain. The selection process of the study population was conducted in the only addiction clinic for the provision of methadone in both cities during the study period.
In the OTP, methadone is dispensed on site, via a mobile unit (Intercity Methadone Bus), and in five community pharmacies. In addition, the outpatient clinic conduct harm reduction programs, which include needle exchanges, condom distribution, and psychosocial interventions[21].
For OTP inclusion, patients had to be over age 18 years and they had to have an opioid dependence diagnosis, based on the Diagnostic and Statistical Manual of Mental Disorders, 4th edition criteria[22]. Additional details have been described previously[20,21]. The municipal clinic was affiliated with primary care centers and nearby hospitals, where patients were referred for confirmatory tests (e.g., HCV-RNA), radiology (e.g., ultrasound), and consultations with specialists (e.g., hepatologists). Physicians at the OTP clinic did not evaluate liver disease or treat HCV infection; those patients were referred to the hospital, where hepatologists and/or internist treated HCV infection. The Spanish health system provided universal access to DAAs, but these drugs were only dispensed in hospital pharmacies.
Patients were informed of the objective of the study, and all patients provided written consent. The study was approved by the Ethics Committee of the Hospital Universitari Germans Trias i Pujol (PI-15-100). This study was compliant with ethical standards for medical research and good clinical practice principles, and it was performed in accordance with the World Medical Association's Declaration of Helsinki.
We collected data on socio-demographic variables (education level, employment, and prior imprisonment), opioid use (age at first drug use, main route of administration), biochemistry and hematological parameters, including liver function tests (aspartate aminotransferase, alanine aminotransferase, gamma glutamyl transferase, and total bilirubin). We also ascertained the presence of HIV and HCV infections and HCV-RNA, the genotype, and any antecedent of HCV treatment with interferon-based regimens.
Patients that tested anti-HCV positive and had not previously received IFN/RBV treatment regimens were followed-up until September 30, 2019. Specifically, we reviewed clinical charts to ascertain data on HCV-RNA, the genotype, and DAA treatments, including the date of initiation, type, duration, and clinical outcome (i.e., SVR). In addition, we checked the national death registry for all patients.
We performed a descriptive analysis of the data. Continuous variables are presented as the median and interquartile range (IQR); categorical variables are presented as the relative frequency. We performed Chi-square tests, Fisher’s exact tests, Student’s t-tests, and Mann Whitney U tests, when appropriate, to detect statistically significant differences between groups. To analyze treatment rates and predictors of treatment with DAAs, we excluded patients treated with IFN/RBV from the analysis. Patient follow-up was evaluated from January 2015 (when DAAs were introduced in Spain) until death or the end of the study, on September 30, 2019. Patient follow-up data were calculated in terms of person-years (p-y). Rates in p-y were defined as the quotient of the number of events observed during the study period (in the numerator) and the sum of all the individual follow-up times (in the denominator). We used Kaplan-Meier methods to estimate the cumulative incidence of treatment with DAAs. Cox regression models were used to analyze predictors of DAA treatment administration. All covariates that were significant in the univariate analysis were included in a multivariate analysis. P values < 0.05 were considered statistically significant. All statistical analyses were performed with Stata software (version 11.0; College Station, TX, United States).
Between October 2015 and September 2017, 501 patients (81.4% men) were enrolled in the OTP. The median age at study entry was 45 years (IQR: 39-50 years), 88% were Spanish-born and 96% of patients had been on opioid agonist therapy for more than 10 years (on average, since 2006; IQR: 2000-2014). The majority of patients (98.5%) was treated with methadone, 70% were unemployed, 49.5% had a history of incarceration and 65% had used injected drugs.
A total of 336 (67%) patients tested positive for anti-HCV antibodies (83% men; median age 46 years, IQR: 41-51 years); these patients had been taking opioid agonist therapy for a median of 15.3 years (IQR: 5.6-19.2 years). The prevalence of alcohol, cannabis, and cocaine use at study entry was 47.2%, 41.3%, and 31.9%, respectively. The prevalence of HIV co-infection was 47.6% (160/336). The characteristics of anti-HCV positive patients are shown in Table 1.
| Anti-HCV positive, n = 336, n (%) | |
| Female, n (%) | 57 (17.0) |
| Age (yr), median (IQR) | 46 (41-50) |
| Time in OTP (yr), median (IQR) | 15.3 (5.6-19.2) |
| Opiate agonists | |
| Methadone | 331 (98.5) |
| Buprenorphine | 5 (1.5) |
| Antecedent of injection drug use (n = 326) | 282 (86.5) |
| History of incarceration (n = 291) | 158 (54.3) |
| Current substance use (last month) (n = 213), n (%) | |
| Alcohol | 101 (47.2) |
| Cannabis | 88 (41.3) |
| Cocaine | 68 (31.9) |
| Heroin | 49 (23.0) |
| Blood parameters | |
| Leucocyte (× 109/L) (n = 298) | 6.7 (5.3-8.6) |
| Lymphocyte (× 109/L) (n = 296) | 2.2 (1.5-2.8) |
| Platelets (× 109/L) (n = 295) | 181 (138-232) |
| Hemoglobin (mg/dL) (n = 296) | 14.3 (13-15.1) |
| AST (U/L) (n = 284) | 31 (21-52) |
| ALT (U/L) (n = 252) | 30 (18-52.5) |
| GGT (U/L) (n = 242) | 44.5 (25-89) |
| Total bilirubin (mg/dL) (n = 238) | 0.5 (0.4-0.7) |
| Total cholesterol (mg/dL) (n = 210) | 168.5 (144-194) |
| HIV infection, n (%) (n = 334) | 160 (47.9) |
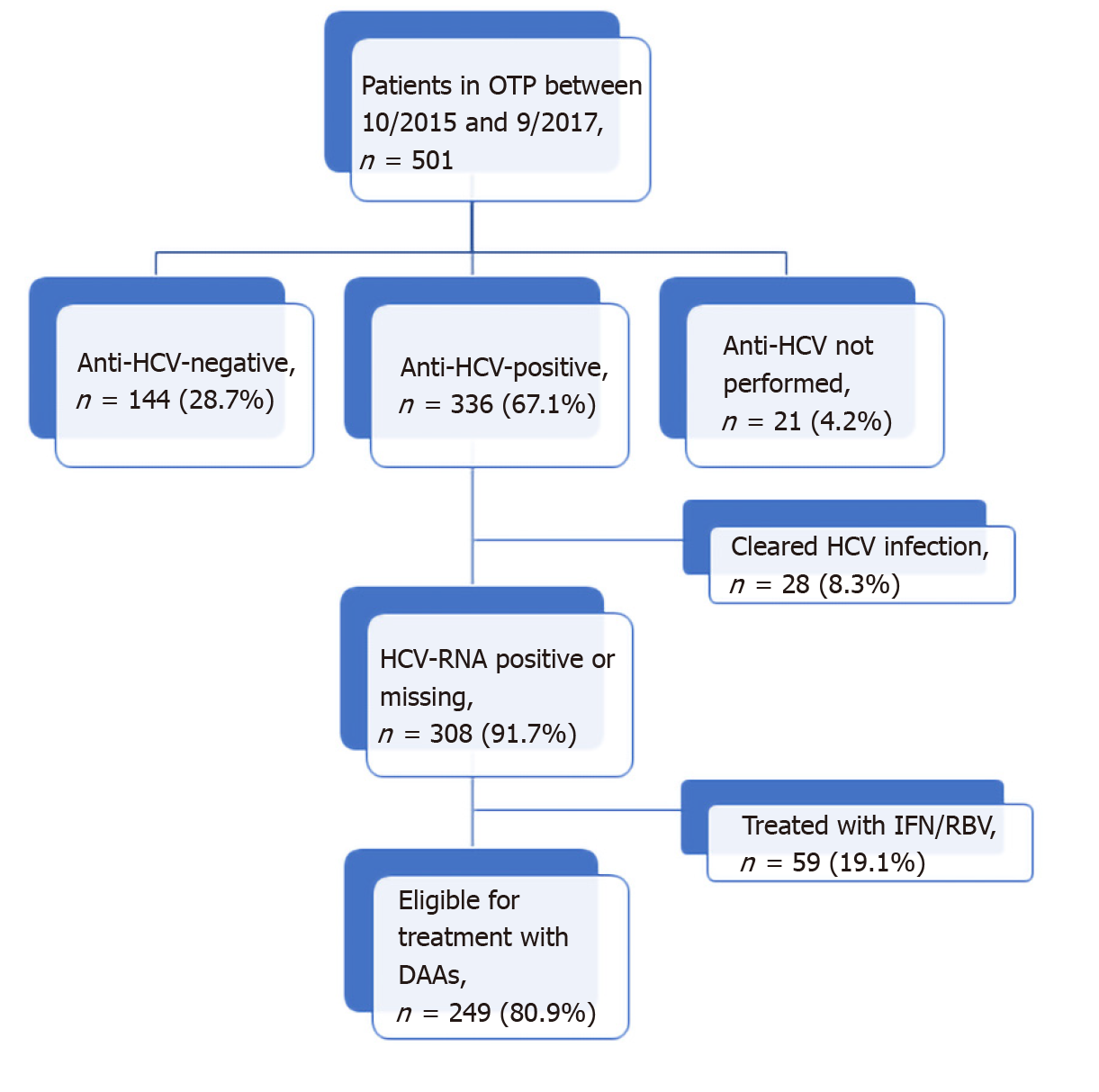
Of the 336 anti-HCV positive patients, 233 (69.3%) had positive results on an HCV-RNA test. The median of HCV-RNA was 11.4 (IQR: 1.5-44.8) × 105 IU/mL and the majority (59.2%) of cases were genotype 1a/1b. Only 28 (8.3%) patients had cleared the infection, and 59/308 (19.1%) had been previously treated with IFN/RBV. HCV-RNA was not determined in 75 patients that were anti-HCV positive (22.3%). The distribution of patients according to HCV infection status is shown in Figure 1.
As of September 2019, among the 249 patients eligible (Figure 1) for DAA treatment, 111 (44.6%) were treated. Of those, 90% achieved SVR. The most frequent DAA combinations were sofosbuvir/ledipasvir, sofosbuvir/velpatasvir, and glecaprevir /pibrentasvir.
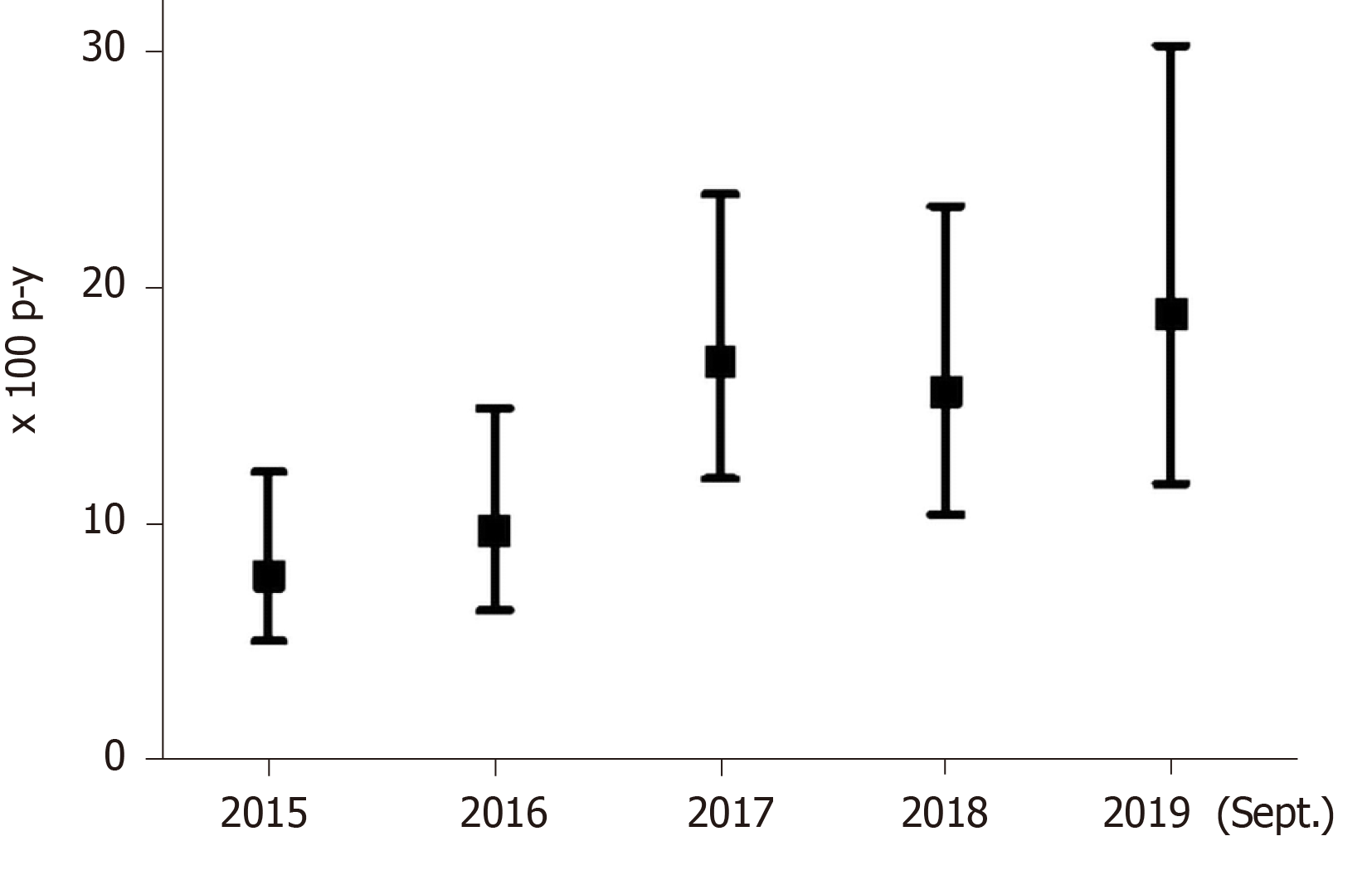
The 249 patients eligible for DAA treatment were followed-up for a median of 4.3 years (IQR: 2.4-4.7 years; total follow-up 879.3 p-y). The overall DAA treatment rate was 12.6/100 p-y (95%CI: 10.5-15.2) and treatment rates increased from 7.8/100 p-y (95%CI: 5.0-12.3), in 2015, to 18.9/100 p-y (95%CI: 11.7-30.3), in 2019.
Figure 2 shows treatment rates with DAAs since 2015. Patients with HCV-HIV co-infection had a treatment rate of 18.0/100 p-y (95%CI: 14.2-22.8); in contrast, patients with HCV mono-infection had a treatment rate of 8.6/100 p-y (95%CI: 6.4-11.7), (P < 0.001). Thus, the incidence rate ratio was 2.09 (95%CI: 1.4-3.1).
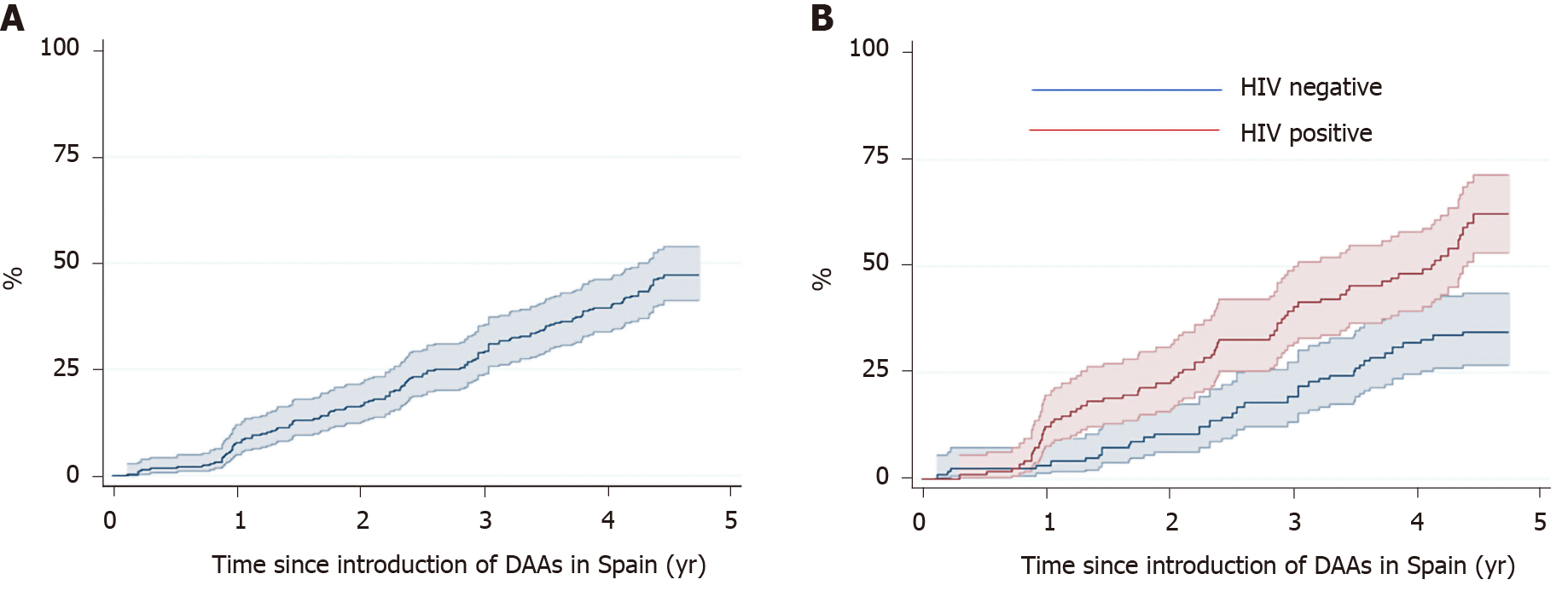
Figure 3 shows the Kaplan-Meier estimates of receiving treatment with DAAs. After four years, the probability of receiving DAA treatment was 39.5% (95CI%: 33.6-46.0) overall, 32% (95CI%: 24.4-41.0) in the HCV mono-infected patients, and 48.1% (95%CI: 39.3-57.8) in the HIV co-infected patients (P < 0.001).
The Cox regression models showed that HIV co-infected patients were twice as likely to receive HCV treatment, compared to those with HCV mono-infection (HR = 1.94, 95%CI: 1.21-3.12, P = 0.006). In addition, patients with ongoing drug use while in the OTP were 2.1-fold less likely to receive DAAs (HR = 0.48, 95%CI: 0.29-0.80) compared to those who do not used drugs (P = 0.004). The Cox regression models on predictors of treatment are shown in Table 2.
| Unadjusted HR (95%CI) | Adjusted HR (95%CI) | |
| Female | 0.79 (0.46-1.34) | |
| Age: 5 years increase | 1.17 (1.06-1.30) | 0.98 (0.81-1.18) |
| OTP and substance use related variables | ||
| Time in OTP (yr) | 1.03 (1.01-1.06) | 1.02 (0.99-1.05) |
| Alcohol use (last month) | 0.58 (0.37-0.92) | 0.72 (0.45-1.17) |
| Substance use1 (last month) | 0.47 (0.30-0.74) | 0.48 (0.29-0.80) |
| Antecedent of injection drug use | 1.35 (0.72-2.51) | |
| History of incarceration | 1.10 (0.75-1.63) | |
| Co-morbidity | ||
| HIV infection | 2.23 (1.52-3.28) | 1.94 (1.21-3.12) |
This study provides a snapshot of the access to curative HCV treatment in patients treated with methadone. Furthermore, it shows that after the introduction of DAAs in Spain, nearly 50% of patients with an anti-HCV positive test were treatment naive. Moreover, we observed significantly lower rates of treatment among patients with HCV mono-infection than among patients with HCV-HIV co-infection.
Few studies in Spain have analyzed DAA treatment rates among patients enrolled in an OTP. In contrast, a European study showed that, after the introduction of DAAs, HCV treatment rates were 23/100 p-y among individuals that injected drugs and had HCV-HIV co-infection[23], which was twice the rate observed in our study. However, it is interesting to note that, since 2015, the proportion of patients that received treatments against HCV infection has increased and that DAA treatment showed efficacy in this difficult to treat population. In fact, the HCV treatment guidelines provided by the AASLD, EASL, and WHO have recommended individualized treatments for patients in the OTP[12-14].
In this study, HIV co-infection and ongoing drug use while in OTP were two independent predictors of whether a person received HCV treatment. The probability of being treated against infection was significantly higher in the co-infected group compared to the HCV mono-infection group. This finding might be related to differences in the continuum of care in the HCV mono-infected and the HIV co-infected. In Spain, HCV mono-infected patients receive regular care and treatment in hospital-based Hepatology units while HCV-HIV co-infected patients are managed in HIV/Aids units having integrated services, psychosocial support and flexible time-slots for visits.
In this cohort, current drug use was associated with a lower probability of receiving HCV treatment. In this sense, health care professionals may perceive current drug use as a barrier to prescribe HCV treatment, despite international guidelines that recommend treatment of infection[12-14]. In patients with SUD, treating HCV infection has been considered a preventive intervention aimed to halt the transmission[24,25]. A recent clinical trial used electronic blisters to monitor adherence to DAA treatment among patients that used drugs and were in an OTP; they showed that 97% of participants completed the treatment, and 94% achieved SVR[5].
This study had some limitations. First, the external validity of the results might have been limited due to the single-center study design. However, the OTP studied was the largest operating in metropolitan Barcelona, Spain, and only authorized to provide methadone or buprenorphine in a large urban area. Second, data related to the dose of methadone were not available and we also lacked data on treatment adherence and potential pharmacological interactions that might have led to DAA discontinuation. However, few studies have reported significant pharmacological interactions between DAAs and methadone[26,27]. Although some DAAs can increase the methadone or buprenorphine concentrations in blood, dose adjustments are not required, and monitoring withdrawal symptoms is merely recommended[27,28]. Third, we could have underestimated the HCV treatment rate with DAAs because some anti HCV-positive patients were considered treatment eligible without having a confirmatory RNA-HCV test.
In contrast, our study population is anchored in an OTP with a large number of patients and real-world conditions which is relevant to generate evidence in a population difficult to treat and retain.
In conclusion, this study highlights the challenges of measuring the continuum of HCV care while in an OTP. The goal of HCV elimination requires more targeted interventions to rapidly identifying those out of care.
The introduction of direct-acting antiviral agents (DAAs) is associated with substantial changes in clinical outcomes of hepatitis C virus (HCV) infection. In this context, individuals with substance use disorder (SUD) have been recognized as a target population for the treatment of HCV infection.
Retention in treatment of SUD is key for the assessment and cure of HCV. In HCV infection, up to 80% of persons who inject drugs are infected but only a proportion is on treatment. In this sense, it is important to know real-life data in drug use populations. The Opioid Treatment Program (OTP) in metropolitan Barcelona, Spain, reports an increasing proportion of patients that are eligible for HCV treatment with DAAs.
Our main objective was to assess HCV infection status and treatment rates in a population primarily admitted for the treatment of SUD. Given the longitudinal nature of the study we aimed to identify gaps and challenges in using DAAs. In doing so we hypothesized on potential barriers that difficult the access to treatment in this population.
We specifically analyzed annual treatment rates with DAAs in the context of HCV mono-infection and human immunodeficiency virus (HIV) co-infection. In addition, we estimated the cumulative incidence and main predictors of HCV treatment.
Results confirm a high prevalence of HCV infection in the OTP (67%) and the increasing rates of treatment over time. Almost 50% of HCV-positive patients were treatment naive (as of September 2019) in a health care system without restrictions in terms of insurance coverage. Patients with ongoing drug use and those with HCV mono-infection were less likely to be treated with respect to those with HIV co-infection.
To the best of our knowledge this is the first study in Spain reporting on HCV treatment rates with DAAs in an OTP. We conclude that treatment rates increase over time and that higher rates are observed in the HIV-coinfected. The observed differences may be related to the lack of integrated care services for the HCV mono-infected. In addition, current drug use has an impact on the readiness to treat HCV infection.
The goal of HCV elimination requires targeted interventions to identify those out of care and to implement strategies focused on traditional and local barriers. Surmounting barriers is necessary to eradicate HCV infection in people seeking treatment of SUD. The integrated management of liver disease with hepatologists, infectious diseases and addiction specialists may have an impact in reducing end stage liver disease.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: Spanish Society of Internal Medicine, CBA007583.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Marasco G, Mazzarelli C S-Editor: Huang P L-Editor: A P-Editor: Ma YJ
| 1. | Nelson PK, Mathers BM, Cowie B, Hagan H, Des Jarlais D, Horyniak D, Degenhardt L. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet. 2011;378:571-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 949] [Cited by in RCA: 973] [Article Influence: 69.5] [Reference Citation Analysis (0)] |
| 2. | World Health Organization. Global Hepatitis Report, 2017. Available from: http://apps.who.int/iris/bitstream/handle/10665/255016/9789241565455-eng.pdf;jsessionid=DF62E9980474A159A2A3220713D6E50D?sequence=1. |
| 3. | Christensen S, Buggisch P, Mauss S, Böker KHW, Schott E, Klinker H, Zimmermann T, Weber B, Reimer J, Serfert Y, Wedemeyer H. Direct-acting antiviral treatment of chronic HCV-infected patients on opioid substitution therapy: Still a concern in clinical practice? Addiction. 2018;113:868-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 4. | Dore GJ, Altice F, Litwin AH, Dalgard O, Gane EJ, Shibolet O, Luetkemeyer A, Nahass R, Peng CY, Conway B, Grebely J, Howe AY, Gendrano IN, Chen E, Huang HC, Dutko FJ, Nickle DC, Nguyen BY, Wahl J, Barr E, Robertson MN, Platt HL; C-EDGE CO-STAR Study Group. Elbasvir-Grazoprevir to Treat Hepatitis C Virus Infection in Persons Receiving Opioid Agonist Therapy: A Randomized Trial. Ann Intern Med. 2016;165:625-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 302] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 5. | Grebely J, Dalgard O, Conway B, Cunningham EB, Bruggmann P, Hajarizadeh B, Amin J, Bruneau J, Hellard M, Litwin AH, Marks P, Quiene S, Siriragavan S, Applegate TL, Swan T, Byrne J, Lacalamita M, Dunlop A, Matthews GV, Powis J, Shaw D, Thurnheer MC, Weltman M, Kronborg I, Cooper C, Feld JJ, Fraser C, Dillon JF, Read P, Gane E, Dore GJ; SIMPLIFY Study Group. Sofosbuvir and velpatasvir for hepatitis C virus infection in people with recent injection drug use (SIMPLIFY): an open-label, single-arm, phase 4, multicentre trial. Lancet Gastroenterol Hepatol. 2018;3:153-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 231] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 6. | Grebely J, Mauss S, Brown A, Bronowicki JP, Puoti M, Wyles D, Natha M, Zhu Y, Yang J, Kreter B, Brainard DM, Yun C, Carr V, Dore GJ. Efficacy and Safety of Ledipasvir/Sofosbuvir With and Without Ribavirin in Patients With Chronic HCV Genotype 1 Infection Receiving Opioid Substitution Therapy: Analysis of Phase 3 ION Trials. Clin Infect Dis. 2016;63:1405-1411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 7. | Macías J, Morano LE, Téllez F, Granados R, Rivero-Juárez A, Palacios R, Ríos M, Merino D, Pérez-Pérez M, Collado A, Figueruela B, Morano A, Freyre-Carrillo C, Martín JM, Rivero A, García F, Pineda JA; HEPAVIR group from the Sociedad Andaluza de Enfermedades Infecciosas (SAEI) and the GEHEP group from the Sociedad Española de Enfermedades Infecciosas y Microbiología (SEIMC). Response to direct-acting antiviral therapy among ongoing drug users and people receiving opioid substitution therapy. J Hepatol. 2019;71:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 8. | Selfridge M, Cunningham EB, Milne R, Drost A, Barnett T, Lundgren K, Guarasci K, Grebely J, Fraser C. Direct-acting antiviral treatment for hepatitis C, reinfection and mortality among people attending an inner-city community health centre in Victoria, Canada. Int J Drug Policy. 2019;72:106-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Lazarus JV, Pericàs JM, Colombo M, Ninburg M, Wiktor S, Thursz M. Viral hepatitis: "E" is for equitable elimination. J Hepatol. 2018;69:762-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Lazarus JV, Safreed-Harmon K, Thursz MR, Dillon JF, El-Sayed MH, Elsharkawy AM, Hatzakis A, Jadoul M, Prestileo T, Razavi H, Rockstroh JK, Wiktor SZ, Colombo M. The Micro-Elimination Approach to Eliminating Hepatitis C: Strategic and Operational Considerations. Semin Liver Dis. 2018;38:181-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 181] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 11. | Kranidioti H, Chatzievagelinou C, Protopapas A, Papatheodoridi M, Zisimopoulos K, Evangelidou E, Antonakaki P, Vlachogiannakos J, Triantos C, Elefsiniotis I, Goulis J, Mela M, Anagnostou O, Tsoulas C, Deutsch M, Papatheodoridis G, Manolakopoulos S. Clinical and epidemiological characteristics of hepatitis C virus-infected people who inject drugs: a Greek descriptive analysis. Ann Gastroenterol. 2018;31:598-603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62:932-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 912] [Cited by in RCA: 992] [Article Influence: 99.2] [Reference Citation Analysis (0)] |
| 13. | European Association for the Study of the Liver. European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2018. J Hepatol. 2018;69:461-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1281] [Cited by in RCA: 1212] [Article Influence: 173.1] [Reference Citation Analysis (0)] |
| 14. | World Health Organization. Guidelines for the care and treatment of persons diagnosed with chronic hepatitis C virus infection. 2018. Available from: http://apps.who.int/iris/bitstream/handle/10665/273174/9789241550345-eng.pdf?ua=1. |
| 15. | Grebely J, deVlaming S, Duncan F, Viljoen M, Conway B. Current approaches to HCV infection in current and former injection drug users. J Addict Dis. 2008;27:25-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Kramer JR, Kanwal F, Richardson P, Giordano TP, Petersen LA, El-Serag HB. Importance of patient, provider, and facility predictors of hepatitis C virus treatment in veterans: a national study. Am J Gastroenterol. 2011;106:483-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Aspinall EJ, Corson S, Doyle JS, Grebely J, Hutchinson SJ, Dore GJ, Goldberg DJ, Hellard ME. Treatment of hepatitis C virus infection among people who are actively injecting drugs: a systematic review and meta-analysis. Clin Infect Dis. 2013;57 Suppl 2:S80-S89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 261] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 18. | Ministerio de Sanidad Consumo y Bienestar Social. Plan Estratégico para el Abordaje de la Hepatitis C en el Sistema Nacional de Salud (PEAHC). 2018. Available from: https://www.mscbs.gob.es/ciudadanos/enfLesiones/enfTransmisibles/hepatitisC/PlanEstrategicoHEPATITISC/docs/Plan_Estrategico_Abordaje_Hepatitis_C_%28PEAHC%29.pdf. |
| 19. | Thomas DL, Vlahov D, Solomon L, Cohn S, Taylor E, Garfein R, Nelson KE. Correlates of hepatitis C virus infections among injection drug users. Medicine (Baltimore). 1995;74:212-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 285] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 20. | Muga R, Rivas I, Faure E, Fuster D, Zuluaga P, Rubio M, Muñoz T, Torrens M, Tor J, Sanvisens A. Sex-specific disease outcomes of HIV-positive and HIV-negative drug users admitted to an opioid substitution therapy program in Spain: a cohort study. BMC Infect Dis. 2014;14:504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Sanvisens A, Rivas I, Faure E, Muñoz T, Rubio M, Fuster D, Tor J, Muga R. [Characteristics of heroin dependent patients admitted to a methadone treatment program]. Med Clin (Barc). 2014;142:53-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th Ed. (DSM IV-TR). Arlington, VA: 2000. |
| 23. | Peters L, Laut K, Resnati C, Del Campo S, Leen C, Falconer K, Trofimova T, Paduta D, Gatell J, Rauch A, Lacombe K, Domingo P, Chkhartishvili N, Zangerle R, Matulionyte R, Mitsura V, Benfield T, Zilmer K, Khromova I, Lundgren J, Rockstroh J, Mocroft A; EuroSIDA Study Group. Uptake of hepatitis C virus treatment in HIV/hepatitis C virus-coinfected patients across Europe in the era of direct-acting antivirals. AIDS. 2018;32:1995-2004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Leask JD, Dillon JF. Review article: treatment as prevention - targeting people who inject drugs as a pathway towards hepatitis C eradication. Aliment Pharmacol Ther. 2016;44:145-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Martin NK, Foster GR, Vilar J, Ryder S, Cramp ME, Gordon F, Dillon JF, Craine N, Busse H, Clements A, Hutchinson SJ, Ustianowski A, Ramsay M, Goldberg DJ, Irving W, Hope V, De Angelis D, Lyons M, Vickerman P, Hickman M. HCV treatment rates and sustained viral response among people who inject drugs in seven UK sites: real world results and modelling of treatment impact. J Viral Hepat. 2015;22:399-408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 26. | Badri PS, Dutta S, Wang H, Podsadecki TJ, Polepally AR, Khatri A, Zha J, Chiu YL, Awni WM, Menon RM. Drug Interactions with the Direct-Acting Antiviral Combination of Ombitasvir and Paritaprevir-Ritonavir. Antimicrob Agents Chemother. 2016;60:105-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 27. | Ogbuagu O, Friedland G, Bruce RD. Drug interactions between buprenorphine, methadone and hepatitis C therapeutics. Expert Opin Drug Metab Toxicol. 2016;12:721-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Hulskotte EG, Bruce RD, Feng HP, Webster LR, Xuan F, Lin WH, O'Mara E, Wagner JA, Butterton JR. Pharmacokinetic interaction between HCV protease inhibitor boceprevir and methadone or buprenorphine in subjects on stable maintenance therapy. Eur J Clin Pharmacol. 2015;71:303-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |