Published online Oct 14, 2020. doi: 10.3748/wjg.v26.i38.5863
Peer-review started: June 4, 2020
First decision: July 29, 2020
Revised: August 13, 2020
Accepted: September 10, 2020
Article in press: September 10, 2020
Published online: October 14, 2020
Processing time: 132 Days and 8.1 Hours
People with achalasia typically have a thick lower esophageal muscularis propria (LEMP), and peroral endoscopic myotomy (POEM) has been effective in treating most patients. LEMP thickness may be associated with the outcomes and prognosis after POEM. However, more evidence is needed regarding the relationship between LEMP thickness and patient prognosis after POEM.
To assess the association between LEMP thickness, measured using endoscopic ultrasound (EUS), and long-term prognosis, especially relapse, after POEM for achalasia.
All medical records, including EUS data, of patients who underwent POEM to treat achalasia at Shengjing Hospital of China Medical University from January 2012 to September 2018 were retrospectively reviewed. LEMP thickness was measured by EUS, and a thickness of ≥ 3 mm was defined as thickened. The severity of patient symptoms was evaluated using the Eckardt score. Relapse was defined as a 3-point rise in the Eckardt score after a period of clinical remission. The relationship between patient characteristics, muscle thickness, and recurrence was analyzed.
Eighty-two patients (32 males and 50 females, aged 17-78 years) and 85 POEM procedures were included. In total, 76.8% (63/82 patients) of patients had a thickened muscularis propria. Older age and longer disease course were associated with muscularis propria thickening (P < 0.05). The mean postoperative follow-up time was 35.4 ± 17.2 mo (range, 8-87.5 mo) in 60 patients. Five patients with Eckardt scores > 3 refused further management after their symptoms were relieved. The relapse rate was 12.73% (7/55 cases). Five patients, four of whom had muscularis propria thickening, had disease recurrence within 12 mo after the procedure. Achalasia relapsed in one patient who had a thickened muscularis propria after 24 mo and in another patient who did not have a thickened muscularis propria after 30 mo. Patients with recurrence were typically younger and had a shorter disease course (P < 0.05). The relapse rate in patients with a non-thickened muscularis propria tended to be higher (18.2%, 2/11 patients) than that in patients with a thickened muscularis propria (11.4%, 5/44 patients), although no significant difference was found. Age (hazard ratio = 0.92; 95% confidence interval: 0.865-0.979; P < 0.05) and being male (hazard ratio = 7.173; 95% confidence interval: 1.277-40.286; P < 0.05) were identified as risk factors for symptomatic recurrence by multivariable analysis using the Cox model.
Patients with a thickened muscularis are typically older and have a longer disease course. Younger age and the male sex are associated with increased recurrence. Patients with a thin muscularis propria may be prone to relapse, although further validation is needed.
Core Tip: This study investigated the clinical significance of lower esophageal muscularis propria (LEMP) thickness in achalasia patients. We retrospectively enrolled 82 patients who underwent peroral endoscopic myotomy (POEM) at our center. The results showed that endoscopic ultrasound-measured LEMP thickness may help diagnose achalasia and predict long-term prognosis after POEM. Patients with a thickened muscularis were typically older and had a longer disease course. Younger age and male gender were risk factors for recurrence. Achalasia was more likely to relapse after POEM in patients with a thin LEMP, although further validation is needed.
- Citation: Liao Y, Xiao TY, Wu YF, Zhang JJ, Zhang BZ, Wang YD, Wang S, Liu X, Sun SY, Guo JT. Endoscopic ultrasound-measured muscular thickness of the lower esophageal sphincter and long-term prognosis after peroral endoscopic myotomy for achalasia. World J Gastroenterol 2020; 26(38): 5863-5873
- URL: https://www.wjgnet.com/1007-9327/full/v26/i38/5863.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i38.5863
Achalasia is currently the most common primary esophageal motility disease[1]. While its etiology remains uncertain, achalasia is characterized by the destruction of inhibitory ganglion cells in the myenteric plexus, causing severe myopathy in smooth muscles and leading to aperistalsis and impaired relaxation of the lower esophageal sphincter (LES)[1,2]. In addition, thickened lower esophageal muscularis propria (LEMP) has been observed in individuals with achalasia in vivo and during autopsies[3-7]. LEMP hypertrophy may be a response to esophageal functional obstruction or a primary lesion[2]. Hence, LEMP thickness is thought to be associated with the disease state.
Existing treatments for achalasia, including pharmacotherapy, clostridium botulinum injection, endoscopic balloon catheter dilation, and Heller myotomy, target the compulsory release of the stenosis segment of the LES. Peroral endoscopic myotomy (POEM) has emerged as an increasingly utilized treatment approach, especially in patients with multiple comorbidities who cannot undergo laparoscopic or open surgical interventions[8,9]. POEM is considered safe for patients as young as 3 years old, with no upper age limit, according to several efficacy studies with a maximum follow-up period of 3 years[10-13].
Despite its safety, patients who undergo POEM are at risk for disease recurrence[14-16], although there is no recognized predictor. However, treatment history, type of mucosal damage, and reflux symptoms were included in a newly developed risk prediction scoring system[17]. Considering that the POEM procedure is performed directly on the muscularis propria and LEMP thickness is associated with the postprocedural balloon catheter dilation outcome[7], LEMP thickness is thought to be associated with POEM outcome. However, no correlation between muscle thickness and prognosis at 1 year after POEM has been reported[18,19], and few studies with longer follow-up periods include assessment of the relationship between LEMP thickness and POEM outcomes.
Therefore, this study aimed to examine the relationship between LEMP thickness, measured by endoscopic ultrasound (EUS), and patient characteristics including long-term prognosis after POEM for achalasia at our health center in the past 7 years.
All medical records of patients who underwent POEM to treat achalasia at Shengjing Hospital of China Medical University from January 2012 to September 2018 were retrospectively reviewed. The inclusion criteria were diagnosis of achalasia and record of LEMP thickness measured by EUS examination before POEM. The exclusion criteria were as follows: (1) History of gastrointestinal open surgery; (2) Gastrointestinal malignancy; (3) Coagulopathy or other systemic disorders that precluded safe use of general anesthesia; and (4) Unwillingness to provide informed consent.
From the patient medical charts, demographic information, disease duration defined by time of symptom onset to POEM procedure, symptoms, Eckardt scores[20], degree of esophageal dilation based on the upper gastrointestinal tract X-ray, endoscopy findings, thickness of LEMP according to EUS findings, and treatment outcomes were collected for analysis.
This study was approved by the Institutional Review Board and the Ethics Committee of China Medical University. All patients voluntarily chose their therapeutic course and provided written informed consent for the POEM procedure. Written informed consent was obtained from the parents or guardians of patients younger than 18 years of age.
Clinical symptoms of all patients were evaluated according to the Eckardt score before and after the procedure. The Eckardt score is the sum of the symptom scores for dysphagia, regurgitation, retrosternal pain (with a score of 0 indicating the absence of symptoms, 1 indicating occasional symptoms, 2 indicating daily symptoms, and 3 indicating symptoms at each meal), and weight loss (with 0 indicating no weight loss, 1 indicating a loss of < 5 kg, 2 indicating a loss of 5-10 kg, and 3 indicating a loss of > 10 kg)[20]. Therefore, scores could range from 0 to 12, with 0 representing no symptoms.
All EUS evaluations were performed by experienced technicians using a 360° Radial-Array Ultrasound Gastroscope (EG-3870URK; PENTAX Medical, Tokyo, Japan) and an ultrasound scanner (EUB 6500; Hitachi, Tokyo, Japan). The thickness of the muscularis propria at the esophagogastric junction (EGJ) was assessed before POEM, and a thickness of ≥ 3 mm was considered thickened. The cutoff value for the muscular thickness was determined according to the ordered sample cluster method based on age, disease duration, preoperative Eckardt score, and postprocedural outcome. The completeness of the myotomy was verified by EUS after POEM.
POEM procedures were performed by three experienced therapeutic endoscopists. Patients fasted for 24-48 h and were forbidden from drinking water for 4-6 h before the procedure. If gastroscopy or EUS revealed any liquid or food residue in the esophagus, a decompression tube was indwelled for at least 24 h before POEM, and nutrients were introduced intravenously. During the procedure, patients were placed in the left recumbent position under general anesthesia with tracheal intubation. The POEM procedure was performed as follows: (1) A submucosal injection (Boston Scientific, United States) of a mixture of saline and methylene blue was administered into the esophageal wall at 12-15 cm above the EGJ; (2) A submucosal tunnel passing over the EGJ was created using a hook knife (KD-620LR; Olympus Corp., Tokyo, Japan) or a triangle tipped knife (KD-640L; Olympus) extending 3 cm into the proximal stomach; (3) Inner circular myotomy began 2-3 cm below the tunnel entry and ended at the cardia; and (4) After careful hemostasis using hemostatic clips (FD-410LR; Olympus), several metal clips [ROOC-D-26-195; Micro-Tech (Nanjing) Co., Ltd., Jiangsu, China] were applied to close the mucosal entry. CO2 was used as the endoscope air supply. Prophylactic antibiotics and proton pump inhibitors were administered intravenously for at least 2 d after the procedure.
Follow-up for all patients was conducted by telephone calls or clinic visits, and clinical symptoms were assessed using the Eckardt score. The procedure was considered effective, and the patient was in clinical remission if the postoperative Eckardt score was ≤ 3. Relapse was defined as a rise in the Eckardt score to > 3 after a period of clinical remission[15]. For patients who underwent the procedure twice, the first set of data was used to analyze the disease recurrence rate.
Statistical analyses were performed using SPSS version 25.0 software (IBM, Armonk, NY, United States). Normally distributed continuous variables are expressed as the mean ± SD and were compared using a t-test. Categorical data were compared using the chi-square test and are expressed as numbers (percentages). The association between age and disease duration was analyzed by univariate logistic regression analysis. Recurrence-free survival was investigated using the Kaplan–Meier estimate of time-to-event and compared using a log-rank test. After confirming that the possible risk factors satisfied the proportional hazard hypothesis, the Cox proportional hazards model was used to evaluate risk factors for clinical recurrence. P < 0.05 was considered statistically significant. The ordered sample cluster analysis was performed using DPS version 7.05 software (Zhejiang University, Hangzhou, China)[21].
Eighty-two patients (32 males and 50 females aged 17-78 year with a mean age of 46.5 ± 14.9 year) and a total of 85 procedures were included. The average disease duration was 102.4 ± 127.2 mo (range, 1-516 mo). All patients denied having a previous diagnosis of esophageal disease. Six patients had a history of an endoscopic procedure. Of all patients, 63 (76.8%) had a thickened LEMP.
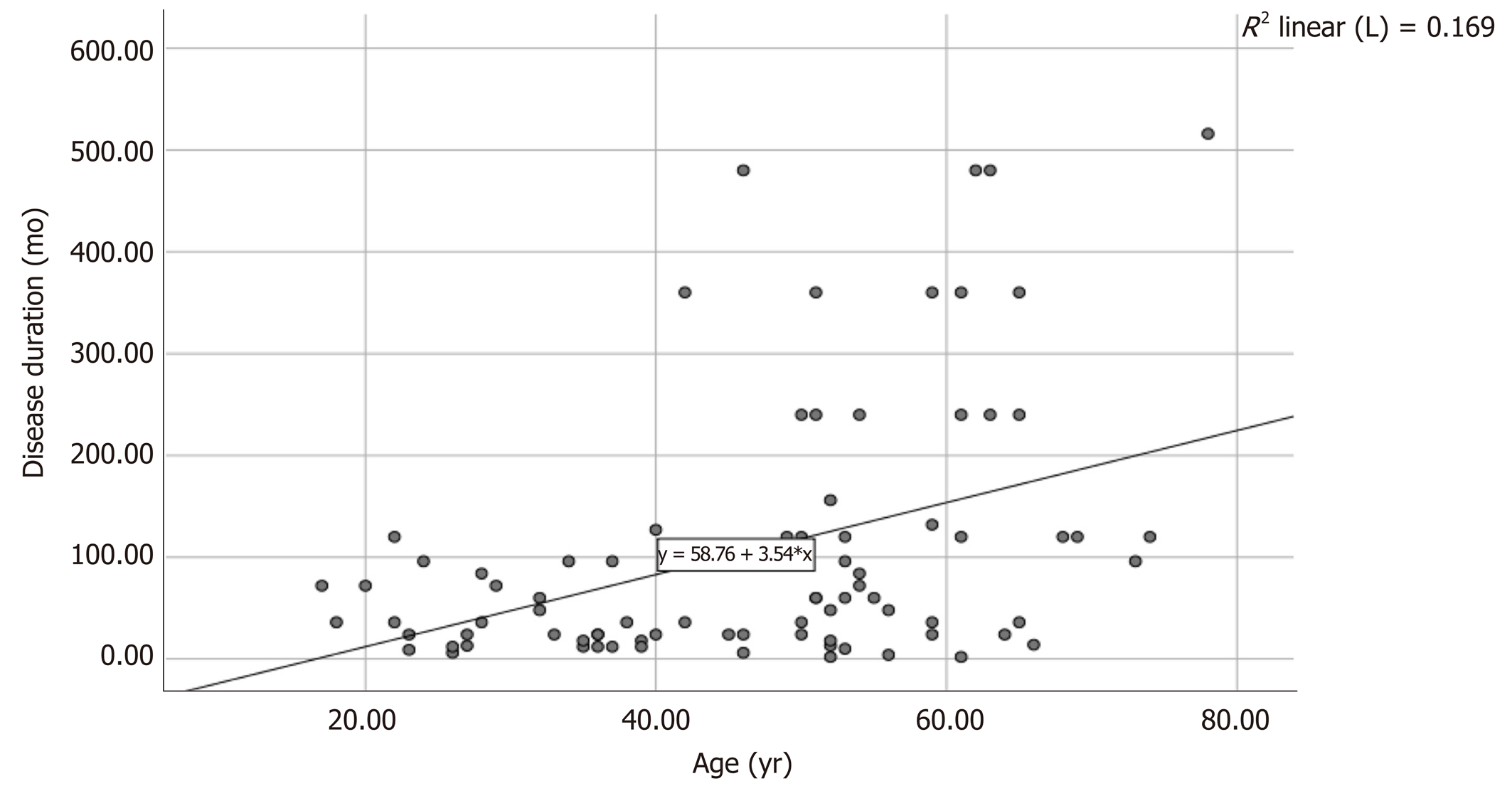
Data on the correlation between patient background and LEMP thickness are shown in Table 1. Significant differences in age (48.4 ± 14.4 years vs 40.4 ± 15.2 years, P < 0.05) and disease duration (126.63 ± 138.49 mo vs 37.79 ± 35.52 mo, P < 0.05) were observed between the thickened and non-thickened LEMP groups, and both variables were associated with thickened LEMP. Specifically, older patients with longer disease duration were more likely to have a thickened LEMP. In addition, there was a positive correlation between patient age and duration of the disease (Figure 1).
| Thickness of LEMP | P value | ||
| < 3 mm (n = 19) | ≥ 3 mm (n = 63) | ||
| Age (yr) | 40.4 ± 15.2 | 48.4 ± 14.4 | 0.04 |
| Sex (male/female) | 6:13 | 26:37 | 0.448 |
| Disease duration (mo) | 37.79 ± 35.52 | 126.63 ± 138.49 | < 0.001 |
| History of endoscopic surgery (+/-) | 1:18 | 5:58 | 0.619 |
| Dilation grade (I/II/III) | 6:4:1 (11) | 15:20:7 (42) | 0.667 |
| Preoperative Eckardt score | 6.53 ± 2.04 | 6.68 ± 1.90 | 0.758 |
The degree of esophageal dilation in 53 patients was evaluated, while the rest of the patients underwent barium esophagraphy at other medical centers and were not included. Of these, 21 (39.6%), 24 (45.3%), and 8 (15.1%) patients exhibited degrees I, II, and III dilations, respectively.
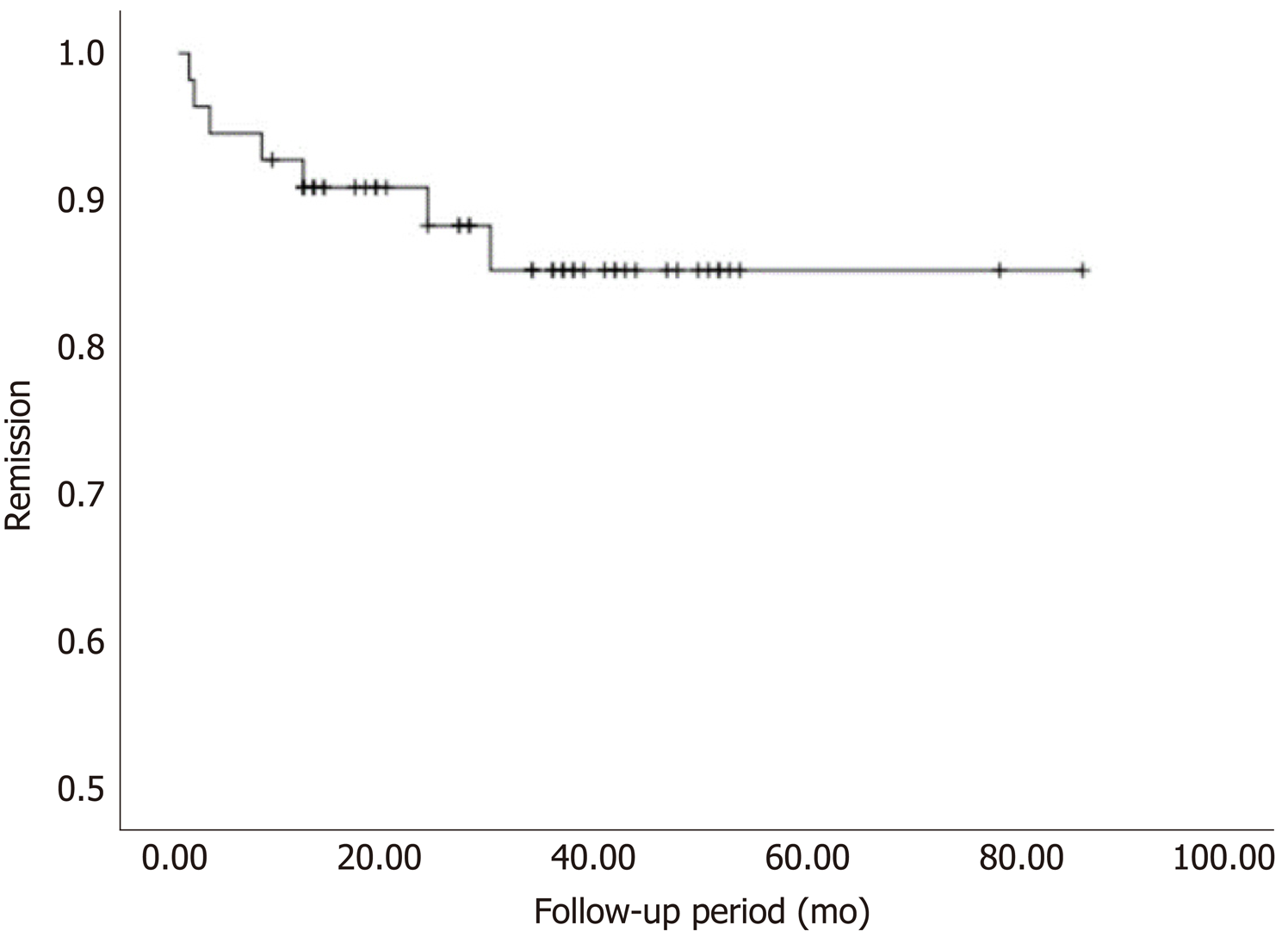
The overall POEM success rate was 94.12% (80/85 procedures). There was only one reported case of post-procedure infection, which was resolved using conservative treatment. The EUS data confirmed complete myotomy after POEM in 62 cases. Sixty patients had follow-ups between 8 and 87.5 mo (mean: 35.4 ± 17.2 mo) after the procedure. Five patients with Eckardt scores > 3 refused further follow-up after their symptoms were relieved. There were no significant differences in age, sex, disease duration, preoperative Eckardt score, or LEMP thickness between the effective (n = 55) and ineffective (n = 5) groups. The relapse rate was 12.73% (7/55 cases). Five patients, four of whom had a thickened LEMP, had symptom recurrence within 12 mo after the procedure. One patient who had a thickened LEMP relapsed after 24 mo while another, who did not have a thickened LEMP, relapsed after 30 mo. Overall, the effectiveness (absence of relapse) of POEM was 87.3% up to 87 mo postoperatively (Figure 2).
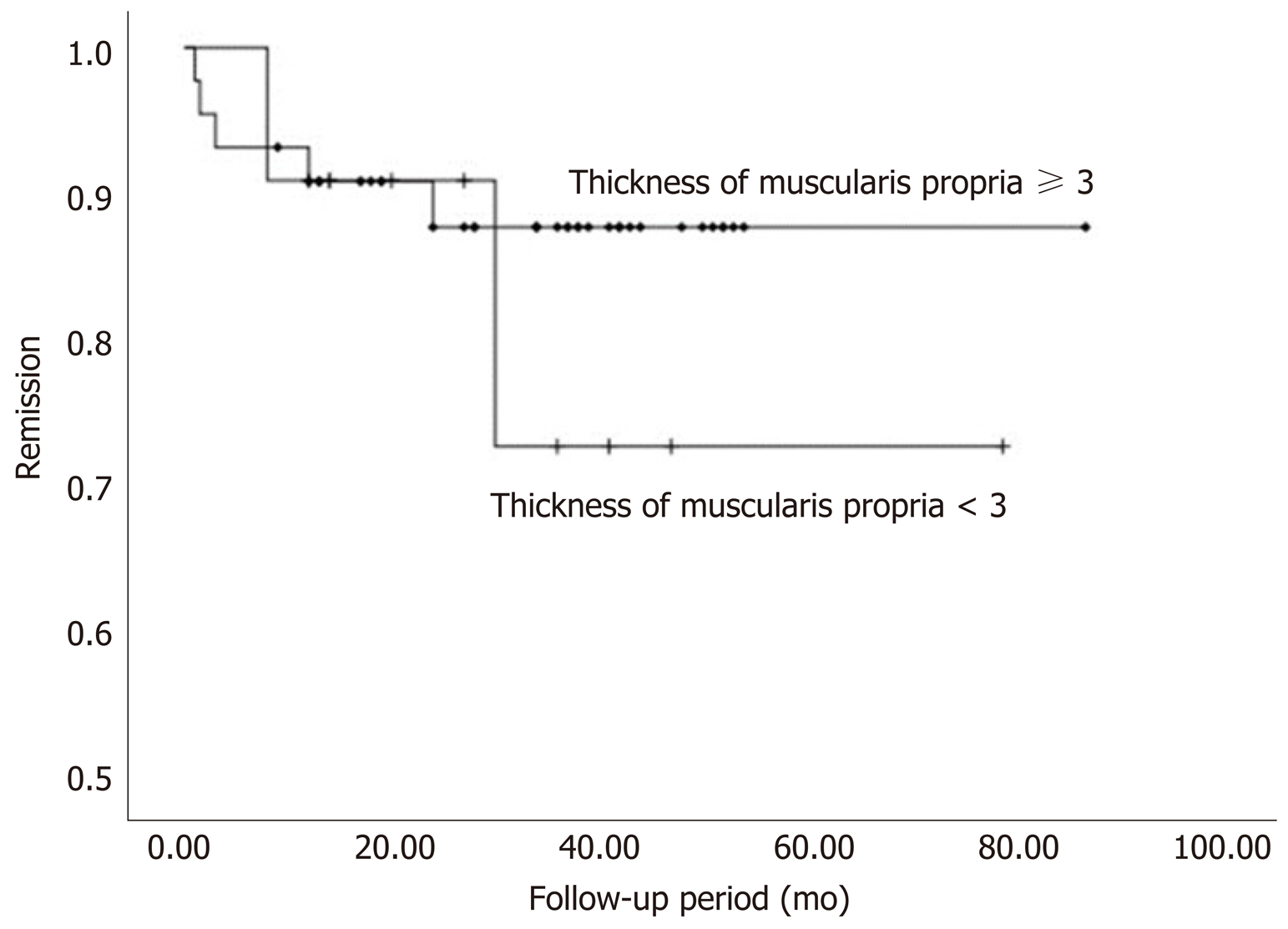
Patients with symptomatic recurrence were younger and had shorter disease duration. The relapse rate in patients without a thickened LEMP tended to be higher than patients with a thickened LEMP (2/11 patients vs 5/44 patients). However, no significant difference was found. Data on patient characteristics according to POEM outcomes are shown in Table 2 and Figure 3. Younger age (hazard ratio = 0.92; 95% confidence interval: 0.865-0.979; P < 0.05) and male gender (hazard ratio = 7.173; 95% confidence interval: 1.277-40.286; P < 0.05) were found to be associated with recurrence according to Cox regression analysis (Table 3).
| Not effective (n = 5) | Clinical success/effective (n = 55) | P value | ||
| No relapse (n = 48) | Relapse (n = 7) | |||
| Age (yr) | 33.8 ± 11.97 | 48.02 ± 14.73 | 34.00 ± 13.05 | 0.021a |
| Sex (male/female) | 3:2 | 16:32 | 4:3 | 0.280 |
| Disease duration (mo) | 82.8 ± 60.36 | 117.29 ± 131.41 | 35.57 ± 30.42 | 0.001a |
| Preoperative Eckardt score | 6.60 ± 2.40 | 6.65 ± 1.74 | 7.57 ± 2.23 | 0.209a |
| Verification of myotomy by EUS (Yes/No)1 | 3:2 | 39:11 | 6:1 | 0.539 |
| LEMP (thickened/ non-thickened) | 4:1 | 39:9 | 5:2 | 0.832 |
| Variable | Univariate | Multivariate | ||
| Hazard ratio (95%CI) | P value | Hazard ratio (95%CI) | P value | |
| Age | 0.943 (0.893-0.995) | 0.033 | 0.920 (0.865-0.979) | 0.008 |
| Male sex | 2.829 (0.629-12.712) | 0.175 | 7.173 (1.277-40.286) | 0.025 |
| Disease duration | 0.984 (0.963-1.006) | 0.144 | NA | 0.402 |
| Preoperative Eckardt score | 1.296 (0.855-1.965) | 0.222 | - | - |
| LEMP ≥ 3 mm | 0.606 (0.117-3.128) | 0.550 | - | - |
Although several previous studies have demonstrated that LEMP thickness is associated with POEM procedure duration and outcomes, the present study adds to the literature by specifically examining the relationship between LEMP thickness and long-term outcomes, including disease relapse after POEM. The results from the present retrospective chart review and statistical analysis suggest that non-thickened LEMP may be associated with increased disease recurrence. In addition, there is a correlation between older age, longer disease duration, and thickened LEMP.
In previous reports, no significant correlation has been found between the severity of achalasia evaluated by Eckardt score and LEMP thickness[19,22]. However, age, pneumatic dilation history, and male sex have been reported as predictors of a thickened LEMP in several studies[6,19,22,23]. Similarly, the present study showed a positive association between age and muscle thickness. In addition, the present analysis found an association between disease duration and LEMP thickness, as patients with a thickened LEMP had a significantly longer disease course. The results may be attributed to differences in patient characteristics among the studies as we also found a positive association between age and disease duration. Thus, the thickened LEMP could be due to prolonged exposure to reactive hyperplasia since longer disease duration means longer stimulation time. The reversibility of the LES hypertrophy after POEM provides supporting evidence for this theory[18].
The thickness of the LEMP may be associated with procedural outcomes and postoperative prognosis. We were not able to statistically evaluate the relationship between POEM-related complications and muscle thickness due to the small sample population. Previous studies have shown, without statistical significance, that there tended to be a positive association between muscle thickness, procedure duration, and complication rate[24]. As for postoperative prognosis, symptomatic recurrence has been reported to occur between 2-5 years after POEM[14]. A medium-term recurrence rate of 18% was reported in a 2-year follow-up study. Although no explanations for recurrence were given, there was an association between younger age and increased rate of relapse[16]. Watanabe et al[18] reported no significant difference in clinical outcomes between patients with thick and those with thin LEMP in a 1-year follow-up study, observing no cases of recurrence. Li et al[15] reported a cumulative recurrence rate of 13.7% at 5 years post-POEM that correlated with disease duration and interventional treatment history. In the same study, age and symptom severity showed no correlation with achalasia relapse.
Since the analysis of the present study confirmed a relationship between patient characteristics and LEMP thickness, the effect on recurrence was also analyzed. Although there was no statistical significance, there was a slightly higher relapse rate in patients with non-thickened LEMP. The lack of statistical significance may be due to the treatment of the muscle thickness measurements as discontinuous data. Patients with a younger age and shorter disease duration had an increased rate of relapse. This may be explained by the better healing ability in younger individuals and the fact that patients with shorter disease duration did not have adequate reactive hyperplasia of the LES after esophageal functional obstruction. Our results may suggest that POEM in patients with a certain LEMP thickness caused by reactive hyperplasia has a lower disease recurrence rate.
Cox analysis showed that disease duration was not associated with recurrence, whereas age remained an effective predictor. This may be explained by the co-linear relationship between age and disease duration. Male sex was also a risk factor for recurrence, although the large confidence interval may indicate that this was a result of a sampling error. Besides, the extent of myotomy was not associated with POEM prognosis[25]. Therefore, our result is still reliable, although we are not able to discuss the effect of the degree of myotomy on prognosis after POEM as all patients enrolled underwent myotomy of the same degree in this study. Our result, which shows that younger age is associated with increased recurrence, concurs with the findings of Werner et al[16] but contradicts those of Li et al[15,16]. Therefore, the association between age, disease duration, and relapse after POEM remains unclear, and further large-scale validation studies are needed.
The Chicago classification, based on high-resolution manometry, is a predictor of achalasia treatment outcomes. Subtype II has the best prognosis, whereas the prognosis of subtype I is slightly poorer and subtype III can be difficult to treat[26]. Patients within the achalasia subtypes tend to have similar LEMP thicknesses[19,24]. The difference in prognostic efficacy between LEMP thickness and subtype is still unclear. However, some participants in our study were not available for manometry. To include a larger sample size in the present study, we did not evaluate the relationship between Chicago classification and POEM prognosis, nor did we compare the subtypes with LEMP thickness. We expect future studies to reveal the relationship between achalasia subtypes, LEMP thickness, and POEM prognosis.
LEMP thickness may help in diagnosing achalasia. A thickened LEMP in patients with achalasia was first observed in an autopsy[4] and with the development of EUS, measurement of the intrinsic muscle layer thickness in vivo is feasible[8,27-30]. Using a 7.5-MHz ultrasound endoscope, Devière et al[3] reported a thickened LEMP in individuals with achalasia compared with healthy controls. Conversely, Ponsot et al[31] argued that those results may have been attributable to puckering of the EGJ and found that the LEMP was not thicker in patients with achalasia than in healthy controls. In the 30 years since these studies, the number of studies that use different EUS techniques and include more participants have increased. Our results agree with many of these studies, where most of the individuals in the present study had a thickened LEMP[5-7]. This suggests that LEMP thickness may be of value in achalasia diagnosis. However, because the identification of the differences in LEMP structure between patients with achalasia and healthy individuals is not easy, the use of muscle thickness in diagnosing achalasia is still controversial.
Our study has several limitations. First, it was done retrospectively and without a healthy control group for comparison of LEMP thickness according to age and other patient characteristics. Second, there was no protocol for measuring the esophageal wall thickness and variations in measurements have been noted in previous studies. Third, evaluation of disease severity and recurrence using the Eckardt score can be highly subjective where physicians may tend to report more marked symptom improvement than patients[32]. Fourth, the cross-sectional area (CSA) of the muscularis propria may be a better indicator to estimate the degree of hypertrophy since a disassociation between muscle thickness and the CSA in some individuals with a dilated esophagus was observed. Patients with a dilated and distended esophagus may have a thin LEMP but a high CSA because of distension of the esophageal wall[5]. Fifth, although the normal value of LEMP thickness is unknown, the cutoff of 3 mm is higher than that in previous studies[3,23,24]. This suggests that our patient population may not be the best representative of Achalasia patients in general, a fact that might undermine the external validity of the results. However, considering the difference of age and disease duration in patients with different LEMP thicknesses, we believed that the 3 mm cutoff in this study is reasonable. In addition, the pathophysiology of achalasia is not fully understood and the role of muscular features in the progression and prognosis of the disease is still undetermined. Therefore, future studies should focus on clarifying the etiology of the disease to explain the mechanisms associated with its pathophysiology.
Most patients with achalasia have a thickened muscularis propria determined by EUS measurement. Therefore, it is likely that EUS can help diagnose achalasia. Patients with a thickened muscularis are typically older and had a longer disease course. Younger age and male gender are associated with recurrence in the present study. Patients with a thin muscularis propria may have a higher risk of symptomatic recurrence, but validation through further large-scale studies is needed.
Peroral endoscopic myotomy (POEM) procedure is an effective treatment for achalasia as it cuts open the lower esophageal muscularis propria (LEMP) directly. LEMP thickness of achalasia patients may be associated with the outcomes and prognosis after POEM.
Patients who undergo POEM are at risk for disease recurrence. Several predictors have been reported while the relationship between LEMP thickness and long-term prognosis after POEM is unclear.
In this retrospective study, we analyzed the relationship between LEMP thickness, patient characteristics, and long-term prognosis after POEM in individuals who underwent POEM for achalasia at our health center in the past seven years.
All medical records, including EUS data, of patients who underwent POEM to treat achalasia at Shengjing Hospital of China Medical University from January 2012 to September 2018 were retrospectively reviewed. The severity of patient symptoms was evaluated using the Eckardt score. Relapse was defined as a 3-point rise in the Eckardt score after a period of clinical remission. The relationship between patient characteristics, muscle thickness, and recurrence was analyzed.
Older age and longer disease course were associated with muscularis propria thickening (P < 0.05). Patients with recurrence were typically younger and had a shorter disease course (P < 0.05). The relapse rate in patients with a non-thickened muscularis propria tended to be higher (18.2%, 2/11 patients) than patients with a thickened muscularis propria (11.4%, 5/44 patients), although no significant difference was found. Age (hazard ratio = 0.92; 95% confidence interval: 0.865-0.979; P < 0.05) and male sex (hazard ratio = 7.173; 95% confidence interval: 1.277-40.286; P < 0.05) were identified as risk factors for symptomatic recurrence by multivariable analysis using the Cox model.
Patients with a thickened muscularis are typically older and have a longer disease course than those without a thickened muscularis. Younger age and male sex are associated with increased recurrence. Patients with a thin muscularis propria may be prone to relapse, although further validation is needed.
A large-scale prospective study should be conducted to gain more evidence for the relationship between achalasia subtypes, LEMP thickness, and POEM prognosis.
We thank Professor Si-Yu Sun, the co-corresponding author of this article, for his support and guidance to this study, as well as all other doctors who participated in this study.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gobejishvili L, Morling JR, Musquer N S-Editor: Zhang L L-Editor: Wang TQ P-Editor: Zhang YL
| 1. | Familiari P, Greco S, Volkanovska A, Gigante G, Cali A, Boškoski I, Costamagna G. Achalasia: current treatment options. Expert Rev Gastroenterol Hepatol. 2015;9:1101-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Park W, Vaezi MF. Etiology and pathogenesis of achalasia: the current understanding. Am J Gastroenterol. 2005;100:1404-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 255] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 3. | Devière J, Dunham F, Rickaert F, Bourgeois N, Cremer M. Endoscopic ultrasonography in achalasia. Gastroenterology. 1989;96:1210-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 54] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Ferguson TB, Woodbury JD, Roper CL, Burford TH. Giant muscular hypertrophy of the esophagus. Ann Thorac Surg. 1969;8:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Mittal RK, Kassab G, Puckett JL, Liu J. Hypertrophy of the muscularis propria of the lower esophageal sphincter and the body of the esophagus in patients with primary motility disorders of the esophagus. Am J Gastroenterol. 2003;98:1705-1712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Dogan I, Puckett JL, Padda BS, Mittal RK. Prevalence of increased esophageal muscle thickness in patients with esophageal symptoms. Am J Gastroenterol. 2007;102:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Li SW, Tseng PH, Chen CC, Liao WC, Liu KL, Lee JM, Lee YC, Chuah SK, Wu MS, Wang HP. Muscular thickness of lower esophageal sphincter and therapeutic outcomes in achalasia: A prospective study using high-frequency endoscopic ultrasound. J Gastroenterol Hepatol. 2018;33:240-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Wang S, Liu X, Ge N, Wang G, Guo J, Liu W, Hu J, Sun S. The relationship between the interruption of the lower esophageal sphincter and relief of dysphagia after per-oral endoscopic myotomy for achalasia. Endosc Ultrasound. 2020;9:252-258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (1)] |
| 9. | Tan S, Zhong C, Ren Y, Luo X, Xu J, Fu X, Peng Y, Tang X. Efficacy and Safety of Peroral Endoscopic Myotomy in Achalasia Patients with Failed Previous Intervention: A Systematic Review and Meta-Analysis. Gut Liver. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Inoue H, Sato H, Ikeda H, Onimaru M, Sato C, Minami H, Yokomichi H, Kobayashi Y, Grimes KL, Kudo SE. Per-Oral Endoscopic Myotomy: A Series of 500 Patients. J Am Coll Surg. 2015;221:256-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 349] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 11. | Li CJ, Tan YY, Wang XH, Liu DL. Peroral endoscopic myotomy for achalasia in patients aged ≥ 65 years. World J Gastroenterol. 2015;21:9175-9181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Chen X, Li QP, Ji GZ, Ge XX, Zhang XH, Zhao XY, Miao L. Two-year follow-up for 45 patients with achalasia who underwent peroral endoscopic myotomy. Eur J Cardiothorac Surg. 2015;47:890-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Chen WF, Li QL, Zhou PH, Yao LQ, Xu MD, Zhang YQ, Zhong YS, Ma LL, Qin WZ, Hu JW, Cai MY, He MJ, Cui Z. Long-term outcomes of peroral endoscopic myotomy for achalasia in pediatric patients: a prospective, single-center study. Gastrointest Endosc. 2015;81:91-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 14. | Teitelbaum EN, Dunst CM, Reavis KM, Sharata AM, Ward MA, DeMeester SR, Swanström LL. Clinical outcomes five years after POEM for treatment of primary esophageal motility disorders. Surg Endosc. 2018;32:421-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 102] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 15. | Li QL, Wu QN, Zhang XC, Xu MD, Zhang W, Chen SY, Zhong YS, Zhang YQ, Chen WF, Qin WZ, Hu JW, Cai MY, Yao LQ, Zhou PH. Outcomes of per-oral endoscopic myotomy for treatment of esophageal achalasia with a median follow-up of 49 months. Gastrointest Endosc. 2018;87:1405-1412.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 16. | Werner YB, Costamagna G, Swanström LL, von Renteln D, Familiari P, Sharata AM, Noder T, Schachschal G, Kersten JF, Rösch T. Clinical response to peroral endoscopic myotomy in patients with idiopathic achalasia at a minimum follow-up of 2 years. Gut. 2016;65:899-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 168] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 17. | Liu XY, Cheng J, Chen WF, Liu ZQ, Wang Y, Xu MD, Chen SY, Zhong YS, Zhang YQ, Yao LQ, Zhou PH, Li QL. A risk-scoring system to predict clinical failure for patients with achalasia after peroral endoscopic myotomy. Gastrointest Endosc. 2020;91:33-40.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 18. | Watanabe D, Tanaka S, Kawara F, Abe H, Ariyoshi R, Nakano Y, Takao T, Morita Y, Toyonaga T, Umegaki E, Kodama Y. Clinical impact of peroral endoscopic myotomy for esophageal motility disorders on esophageal muscle layer thickness. Endosc Int Open. 2019;7:E525-E532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Oumrani S, Barret M, Roseau G, Brieau B, Leblanc S, Coriat R, Prat F, Chaussade S. Do we need endoscopic ultrasonography for the workup of patients with esophageal motility disorder? Clin Res Hepatol Gastroenterol. 2019;43:608-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Eckardt VF, Aignherr C, Bernhard G. Predictors of outcome in patients with achalasia treated by pneumatic dilation. Gastroenterology. 1992;103:1732-1738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 523] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 21. | Tang QY, Zhang CX. Data Processing System (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect Sci. 2013;20:254-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 697] [Cited by in RCA: 591] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 22. | Sotoudehmanesh R, Mikaeli J, Daneshpajooh M, Modirzadeh A, Mehrabi N. Endoscopic ultrasonography findings in patients with achalasia. Esophagus. 2011;8:187-190. [DOI] [Full Text] |
| 23. | Minami H, Inoue H, Isomoto H, Urabe S, Nakao K. Clinical application of endoscopic ultrasonography for esophageal achalasia. Dig Endosc. 2015;27 Suppl 1:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Watanabe D, Tanaka S, Ariyoshi R, Abe H, Kawara F, Toyonaga T. Muscle layer thickness affects the peroral endoscopic myotomy procedure complexity. Dis Esophagus. 2018;31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | He C, Li M, Lu B, Ying X, Gao C, Wang S, Ma C, Jin C. Long-Term Efficacy of Peroral Endoscopic Myotomy for Patients with Achalasia: Outcomes with a Median Follow-Up of 36 Months. Dig Dis Sci. 2019;64:803-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 26. | Vaezi MF, Pandolfino JE, Vela MF. ACG clinical guideline: diagnosis and management of achalasia. Am J Gastroenterol. 2013;108:1238-49; quiz 1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 357] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 27. | Sahai AV. EUS is trending! Endosc Ultrasound. 2018;7:353-355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Sun S, Wang C, Wang S. Remember, interventional EUS is performed using an elevator-containing scope as well. Endosc Ultrasound. 2018;7:73-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 29. | Pesenti C, Bories E, Caillol F, Ratone JP, Godat S, Monges G, Poizat F, Raoul JL, Ries P, Giovannini M. Characterization of subepithelial lesions of the stomach and esophagus by contrast-enhanced EUS: A retrospective study. Endosc Ultrasound. 2019;8:43-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 30. | Hu J, Ge N, Wang S, Liu X, Guo J, Wang G, Sun S. The Role of Endoscopic Ultrasound and Endoscopic Resection for Gastric Glomus: A Case Series and Literature Review. J Transl Int Med. 2019;7:149-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Ponsot P, Chaussade S, Palazzo L, Amouyal P, Gaudric M, Couturier D, Paolaggi JA. Endoscopic ultrasonography in achalasia. Gastroenterology. 1990;98:253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Larssen L, Medhus AW, Hjermstad MJ, Körner H, Glomsaker T, Søberg T, Gleditsch D, Hovde O, Nesbakken A, Tholfsen JK, Skreden K, Hauge T. Patient-reported outcomes in palliative gastrointestinal stenting: a Norwegian multicenter study. Surg Endosc. 2011;25:3162-3169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |