Published online Sep 21, 2020. doi: 10.3748/wjg.v26.i35.5362
Peer-review started: March 28, 2020
First decision: April 25, 2020
Revised: May 4, 2020
Accepted: August 22, 2020
Article in press: August 22, 2020
Published online: September 21, 2020
Processing time: 172 Days and 8.2 Hours
The inflammatory bowel diseases (IBD), Crohn’s disease (CD) and ulcerative colitis (UC) are chronic, immune-mediated disorders of the digestive tract. IBD is considered to be a risk factor for developing osteoporosis; however current literature on this matter is inconsistent.
To assess prevalence and development of osteoporosis and low bone mineral density (BMD), and its risk factors, in IBD patients.
Systematic review of population-based studies. Studies were identified by electronic (January 2018) and manual searches (May 2018). Databases searched included EMBASE and PubMed and abstracts from 2014-2018 presented at the United European Gastroenterology Week, the European Crohn’s and Colitis Organisation congress, and Digestive Disease Week were screened. Studies were eligible for inclusion if they investigated either the prevalence of osteoporosis or osteopenia and/or risk factors for osteoporosis or low BMD in IBD patients. Studies on children under the age of 18 were excluded. Only population-based studies were included. All risk factors for osteoporosis and low BMD investigated in any included article were considered. Study quality and the possibility of bias were analysed using the Newcastle-Ottawa scale.
Twelve studies including 3661 IBD patients and 12789 healthy controls were included. Prevalence of osteoporosis varied between 4%-9% in studies including both CD and UC patients; 2%-9% in studies including UC patients, and 7%-15% in studies including CD patients. Among healthy controls, prevalence of osteoporosis was 3% and 10% in two studies. CD diagnosis, lower body mass index (BMI), and lower body weight were risk factors associated with osteoporosis or low BMD. Findings regarding gender showed inconsistent results. CD patients had an increased risk for osteoporosis or low BMD over time, while UC patients did not. Increased age was associated with decreased BMD, and there was a positive association between weight and BMI and BMD over time. Great heterogeneity was found in the included studies in terms of study methodologies, definitions and the assessment of osteoporosis, and only a small number of population-based studies was available.
This systematic review found a possible increase of prevalence of osteoporosis in CD cohorts when compared to UC and cohorts including both disease types. Lower weight and lower BMI were predictors of osteoporosis or low BMD in IBD patients. The results varied considerably between studies.
Core Tip: Being diagnosed with inflammatory bowel disease (IBD) is considered a risk factor for development of osteoporosis, which leads to an increased risk of pathological fractures. This makes osteoporosis associated with great economic and psychological burden. Research made on the relationship between IBD and osteoporosis differs in study design and study populations, and results are inconsistent. The aims with this research are to assess the prevalence of osteoporosis among IBD patients compared to healthy individuals, assess the disease course of osteoporosis or low bone mineral density (BMD) in IBD patients and assess risk factors associated with osteoporosis and low BMD in IBD patients.
- Citation: Kärnsund S, Lo B, Bendtsen F, Holm J, Burisch J. Systematic review of the prevalence and development of osteoporosis or low bone mineral density and its risk factors in patients with inflammatory bowel disease. World J Gastroenterol 2020; 26(35): 5362-5374
- URL: https://www.wjgnet.com/1007-9327/full/v26/i35/5362.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i35.5362
The inflammatory bowel diseases (IBD), encompassing Crohn’s disease (CD) and ulcerative colitis (UC) are chronic, immune-mediated disorders of the digestive tract of unknown aetiology. Being diagnosed with IBD is considered a risk factor for the development of osteoporosis, which leads to an increased risk of pathological fractures[1]. It is hypothesized that the severity and extent of gut inflammation and intestinal malabsorption leading to calcium and vitamin D deficiency in IBD patients might have a detrimental effect on bone[2-4]. Other known risk factors for osteoporosis, that also apply to the population without IBD are female gender, older age, low BMI and smoking[2-5]. As bone-protecting factors, physical activity has been found to have beneficial effects on both bone and cartilage in patients with osteoporosis, whether it’s glucocorticoid-induced or not[6-8].
While osteoporosis is asymptomatic before fractures occur, the development of osteoporotic fractures as a consequence makes osteoporosis associated with great economic and psychological burden[9,10]. Several studies have investigated the relationship between inflammatory bowel diseases and osteoporosis but differences in study design and study populations, as well as inconsistent results and diverging interpretations of them, make it difficult to draw firm conclusions.
With this systematic review, we aimed to assess the prevalence of osteoporosis among IBD patients compared to healthy individuals, as well as the disease course of osteoporosis or low BMD in IBD patients. We also aimed to assess risk factors associated with osteoporosis and low BMD in IBD patients with the intention to find more substantial evidence as to the cause of osteoporosis or low BMD in this patient group.
This systematic review was conducted in accordance with the MOOSE (Meta-analyses Of Observational Studies in Epidemiology) guidelines. Prior to data extraction and analysis, a protocol was registered with PROSPERO (ID CRD42018084259) that has been updated regularly.
Studies were identified through electronic searches and by manually reviewing the reference lists of these studies, as well as relevant review articles. Electronic searches were conducted on January 30, 2018. Databases searched included EMBASE and PubMed. Searches were adjusted where needed for each database. Terms related to “inflammatory bowel disease”, “osteoporosis”, “osteopenia” and “study design” were used. The detailed search strategy is presented in Supplementary Table 1. Prior to title and abstract screening, duplicates and articles written in a language other than English were excluded. Titles and abstracts were screened independently by two of the review’s authors (SK/BL). Disagreement was resolved by consensus. Potentially eligible studies were read in full by the same two authors (SK/BL). Disagreements that could not be resolved by consensus were discussed with a third author (JB) until an agreement was reached. Screening and study selection were made using the review management tool Covidence (http://www.covidence.org).
The search for unpublished articles occurred between May 14-16, 2018, where abstracts from 2014-2018 presented at the United European Gastroenterology Week, the European Crohn’s and Colitis Organisation congress, and Digestive Disease Week were screened. Only the European Crohn’s and Colitis Organisation congress had published abstracts from 2018. The screening was made by searching for the terms “osteoporosis”, “osteopenia”, “bone mineral density” and “inflammatory bowel disease”.
Studies were eligible for inclusion if they investigated either the prevalence of osteoporosis or osteopenia and/or risk factors for osteoporosis or low BMD in IBD patients. Studies on children under the age of 18 were excluded. Only population-based studies were included. All risk factors for osteoporosis and low BMD investigated in any included article were considered.
Quality assessment and risk of bias assessment were performed using the Newcastle-Ottawa scale (NOS), a scale developed for assessment of nonrandomized studies including cohort studies[11]. Stars were given to each article based on criteria in the categories of “selection”, “comparability” and “outcome”. A maximum of nine stars could be allocated to any one study.
From each included study the following information was extracted: (1) Author, year of publication, study period, number of patients included, country of study; (2) Prevalence of osteoporosis in patient groups and, if included in the study, healthy control groups; (3) T, Z and BMD (g/cm2) scores in patient groups and, if included in the study, healthy control groups; (4) Prevalence of osteoporosis and T, Z and BMD (g/cm2) scores in subgroups including gender, type of IBD, age (>/< 50 years), treatment (steroid/non-steroid), previous surgery, and Montreal disease classification; (5) Information regarding changes in rates of osteoporosis over a period of time; and (6) All clinical and socio-demographic risk factors that are investigated to be associated with osteoporosis or lowering of BMD in IBD patients.
Dual-energy X-ray absorptiometry (DXA) is performed on several bone areas and studies were therefore expected to present both overall measurements and/or information on each specific area. Examining risk factors for osteoporosis or low BMD in IBD patients, we considered it an association if at least one measured bone area showed significant association to an investigated risk factor, or if at least one measured bone area showed significantly lower BMD than that same area in a comparison group. If a study only presented the proportion of patients with osteoporosis in each individual bone area, the overall prevalence was defined as that in the bone area in which most patients had osteoporosis. Prevalence of osteopenia was not considered.
If several studies analyzed the same cohort, prevalence estimates and risk factors were only registered once for each cohort. Authors were contacted for possible unpublished data. Studies analyzing CD and UC patients combined are referred to as “IBD studies”, while studies analyzing CD or UC exclusively are described as “CD studies” or “UC studies”, respectively.
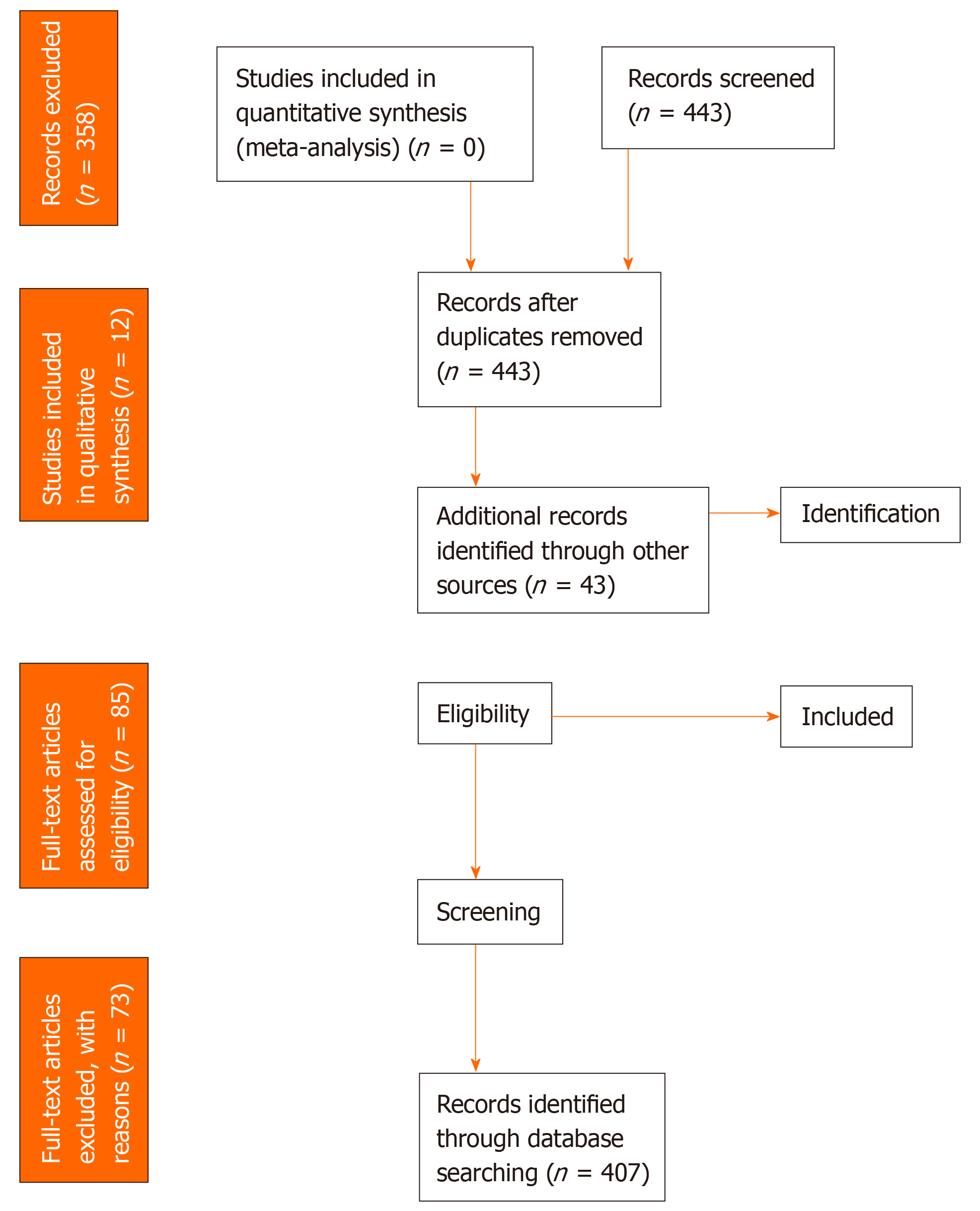
The search identified 449 records. After removing duplicates, non-English language studies and screening abstracts, 84 full-text articles were assessed for eligibility. A total of 12 papers were included in our study, one[12] of which was found via screening of references. An overview of the number of titles, abstracts and full-text articles that were excluded, with justification for their exclusion, can be found in the PRISMA flow diagram, Figure 1. No conference abstracts matched our criteria for inclusion. Authors either declined to provide data or did not respond to our queries in eight cases.
A detailed overview of the characteristics of each study is presented in Table 1. The 12 studies were based on a total of seven cohorts. All twelve studies contributed with unique information despite the fact that some included the same cohort. Information was only registered once for each cohort. Six studies investigated only CD patients[12-17], while the remainder investigated both CD and UC patients. No studies investigated only CD patients. Four studies included a healthy control group[12,15,17,18]. Five were follow up studies[3,12,18-20]. Two studies investigated only premenopausal women[21,22]. Two studies presented only the number of patients that had osteoporosis in each measured bone area but no overall number[13,17]. The total population across all studies was 3661 IBD patients, of which 1833 (50%) were women and 1828 (50%) men. A total of 1546 (42%) patients had UC and 2115 (58%) had CD. The control groups consisted of 12789 healthy individuals.
| Ref. | Publication year | Country of study | Type of study | DXA scan1 | No. of patients | Average age of patients | Cohort | Control group | Follow- up | Comments |
| Andreassen et al[1] | 1998 | Denmark | Cross-sectional study inviting all IBD patients from a well-defined area | Yes | 115 | 37 (16-75); median (range) | CD only | No | No | |
| Andreassen et al[2] | 1999 | Denmark | Cross-sectional case-control study inviting all IBD patients from a well-defined area | Yes | 113 | 37 (16-75); median (range) | CD only | Yes; n = 113 | No | Same cohort used as in Andreassen et al[14] (1998) |
| Bernstein et al[3] | 20032 | United States | Cross-sectional data extracted from population-based Manitoba IBD research registry | Yes | 70; UC: n = 12; CD: n = 58 | 33.0 (7.4); mean (SD) | UC and CD | No | No | Includes only premenopausal women |
| Bernstein et al[4] | 20033 | United States | Cross-sectional data extracted from population-based Manitoba IBD research registry | Yes | 66 (DXA results: n = 70); UC: n = 11; CD: n = 55 | 33.3 (18-44); mean (range) | UC and CD | No | No | Includes only premenopausal women. Same cohort used as in Bernstein (2002) |
| Haugeberg et al[5] | 2001 | Norway | Cross-sectional data from a population-based study. Case control study | Yes | 55 | 38.5 (12.7); mean (SD) | CD only | Yes; n = 52 | No | |
| Jahnsen et al[6] | 1997 | Norway | Cross-sectional case control study | Yes | 60 | 36 (21-75); median (range) | CD only | Yes; n = 60 | No | Includes a cohort of UC patients that is not population-based which was therefore not included |
| Jahnsen et al[7] | 2004 | Norway | Follow-up study | Yes | 60 | 36 (21-75); median (range) | CD only | No | Yes | Includes a cohort of UC patients that is not population-based which was therefore not included |
| 2 yr | Same cohort used as in Jahnsen (1997) | |||||||||
| Leslie et al[8] | 2008 | Canada | Follow-up study with cohort extracted from population-based Manitoba IBD research registry | Yes | 101; UC: n = 45; CD: n = 56 | 46.9 (15.5); mean (SD) | UC and CD | No | Yes; 2.3 ± 0.3 yr | |
| Leslie et al[9] | 2009 | Canada | Follow-up study with cohort extracted from population-based Manitoba IBD research registry | Yes | 101 UC: n = 45 CD: n = 56 | 47 (15); mean (SD) | UC and CD | No | Yes; 2.3 ± 0.3 yr | Same cohort used as in Leslie et al[19] (2008) |
| Schoon et al[10] | 2000 | The Netherlands | Cross-sectional cohort | Yes | 119 | 42 (14); mean (SD) | CD only | No | No | |
| Targownik et al[11] | 2012 | Canada | Follow-up study with data extracted from population-based Manitoba IBD research registry | Yes | 86; UC: n = 32; CD: n = 50; Unclass: n = 4 | 46.7 (14.9); mean (SD); 46 (35-57) median (IQR) | UC and CD | No | Yes; 4.3 ± 0.3 yr | Same cohort used as in Leslie et al[19] (2008) |
| Tsai et al[12] | 2015 | Taiwan | Follow-up case control study with data extracted from population-based registry | No | 3141; UC: n = 1489; CD: n = 1652 | 46.7 (35.6-61.0); median (IQR) | UC and CD | Yes; n = 12564 | Yes; 6.49 ± 3.09 yr | Diagnosis of osteoporosis based on ICD-10 codes |
Table 2 provides detailed quality assessments of the studies according to the NOS. All included studies were allocated stars for the representativeness of the exposed cohort – the average IBD patient – since they are all population based. Osteoporosis was either diagnosed by DXA scan or from ICD-10 codes and hence all studies received at least two stars with regards to selection. With regards to comparability, gender and age were identified to be the most relevant confounding variables. With reference to outcome, 2 years were set as long enough follow-up time for outcomes (osteoporosis or change in BMD) to occur. A maximum of 25% of patients could be lost to follow up in order to receive a star for adequacy of follow up of cohorts.
| Schoon et al[13] (2000) | Jahnsen et al[12] (1997) | Jahnsen et al[16] (2004) | Tsai et al[18] (2015) | Targownik et al[3] (2012) | Leslie et al[20] (2009) | Leslie et al[19] (2008) | Andreassen et al[14] (1998) | Andreassen et al[15] (1999) | Bernstein et al[21] (2003, May) | Bernstein et al[22] (2003, November) | Haugeberg et al[17] (2001) | |
| Selection | ** | *** | ** | **** | ** | ** | ** | ** | *** | ** | ** | *** |
| Comparability | ** | ** | ** | ** | ||||||||
| Outcome | * | * | ** | ** | *** | ** | ** | * | * | * | * | * |
| Total number of stars allocated | 3 | 6 | 4 | 7 | 5 | 4 | 4 | 3 | 6 | 3 | 3 | 6 |
The prevalence of osteoporosis in IBD patients ranged from 2%[18] to 15%[16]. In patients with UC, this range was 2%-9%[18,19], while it was 7%-15%[16,18,19] in CD patients. Two studies provided age- and sex matched controls and in these the prevalence of osteoporosis was 3%[18] and 10%[17], respectively.
Nine studies based on six study cohorts investigated risk factors for osteoporosis or low BMD of which four, based on two study cohorts, investigated both UC and CD patients[19-22] and of which five, based on four study cohorts, investigated only CD patients[12-15,17]. An overview of the most relevant risk factors associated with osteoporosis or low BMD can be found in Table 3, while a detailed list can be found in Supplementary Table 2. Overall, a CD diagnosis, lower body mass index (BMI) and lower body weight were associated with osteoporosis or low BMD. Female gender was found to be associated with lower BMD in a study of both CD and UC patients[20]. In CD cohorts, one study found males to have lower BMD than women[12], one study found no significance[13] and one study found female gender to be predictive for decreased BMD[17]. Use of corticosteroids in any form and with any duration was found to be associated with osteoporosis or low BMD only in studies investigating CD exclusively. Age was associated with osteoporosis or low BMD in studies including only CD patients, where one study found increased age to be a risk factor[15], and one study found patients with reduced BMD to be significantly younger than the patient group without reduced BMD[17].
| Risk factors for reduced BMD | CD | CD + UC | Comments |
| General risk factors | |||
| Gender[5,6,9,10] | +/- | + | Female gender was found to be significantly correlated by Leslie et al[20] (2009) investigating both CD and UC patients. In CD studies, Haugeberg et al[5] found female gender to be a predictive factor for osteoporosis. Jahnsen et al[6] found men to have lower Z-scores than women, whereas Schoon et al[10] found no significant association. |
| Age[2,3,5,9] | + | +/- | Age was significantly associated in the CD studies. However, Haugeberg et al[5] found patients with reduced BMD to be significantly younger than those without reduced BMD. |
| Weight[2,3,5,9] | +, -1 | + | Low weight was found to be a risk factor for low BMD in both CD + UC cohorts. In CD cohorts, Andreassen et al[15] (1999) found a significant positive correlation only in males. Haugeberg et al[17] found a positive correlation between weight and BMD for both genders. |
| BMI[2,5,6,9] | +/- | + | Leslie et al[20] (2009), the only study investigating BMI in CD + UC, found a positive correlation between BMI and BMD. Haugeberg et al[17] found a significant association for CD patients in a bivariate analysis, but not in a multiple linear regression analysis. |
| Steroid treatment[2,3,5,6,9] | +/-2 | - | Multiple risk factors related to steroid usage were investigated. No correlation was found in CD + UC. However, most CD studies did find a correlation. |
| Height[3,5,9] | +/- | +/- | |
| Smoking[3,5,6] | - | - | |
| Vitamin D supplement[3-5] | - | - | |
| Calcium supplement[3-5] | - | - | |
| Serum 25(OH)D[1,5,8] | +/- | +/- | |
| Serum calcium[1,5,8] | - | - | |
| Serum parathyroid hormone[1,5,8] | +/- | + | |
| Disease-specific risk factors | |||
| UC diagnosis[3,9] | Not relevant | - | |
| CD diagnosis[3,6,9] | Not relevant | +/- | |
| Disease location[1,3,5] | - | - | |
| Disease duration[2,3,5,6] | +3,- | - | |
| Surgery[2,3,5,6] | +/- | - |
Five studies based on three cohorts analysed risk factors for a change in BMD over a period of time[3,16,18-20]. Follow-up for the studies varied between 2 years[16] and 6.49 ± 3.09 years[18]. One study included only CD patients[16]. An overview of the most relevant risk factors for change in BMD can be found in Table 4, while a detailed list of all risk factors can be found in Supplementary Table 3. CD patients appeared to have an increased risk of developing lower BMD or osteoporosis over time, while UC patients had no such increased risk. Gender analyses showed contradicting results. An increase in age was found to be associated with a decrease in BMD, whereas an increase (or decrease) in weight and BMI was associated with an increase (or decrease) in BMD.
| Risk factors for change in BMD | CD | CD + UC | Comments |
| General risk factors | |||
| Gender[8,9,11,12] | No data | +/- | No difference was found between genders in one study cohort[8,9,12], whilst another cohort[11] found a greater incidence of osteoporosis in women than in men. |
| Age[8,9,11,12] | No data | +/- | |
| Weight[9,11] | No data | + | |
| BMI[7,9,11] | + | + | |
| Steroid treatment[7-9,11] | - | +/- | |
| Smoking[7] | - | No data | |
| Serum 25-OH D[7,8,11] | + | +/- | |
| Disease-specific risk factors | |||
| Diagnosis[9,11,12] | Not relevant | +1, - | One[13] out of three studies found CD to be associated with an increased risk of osteoporosis. The others found no associations. |
| Disease location[7] | - | No data | |
| Disease activity[11] | No data | - |
This systematic review summarises the prevalence and development of, and risk factors for, osteoporosis or low BMD among patients with IBD. Though not statistically proven to be significant, it seems that CD cohorts have a higher prevalence of osteoporosis as compared to the UC cohorts. We found an association between osteoporosis or low BMD and lower weight and lower BMI in both CD cohorts and cohorts including both CD and UC patients. Two out of four studies investigating gender found female gender to be associated with lower BMD. Age and steroid usage were found to be associated only among CD cohorts. In cohorts that analysed change in BMD over time, increased age was associated with a decrease in BMD and increased weight and BMI were associated with increased BMD. Furthermore, and unlike UC patients, CD patients had an increased risk of osteoporosis or low BMD over time.
The prevalence of osteoporosis among IBD patients ranged from 2%[18] to 15%[16], with a range of 2%[18] to 9%[19] in UC patients and 7%[18,19] to 15%[16] in CD patients. The prevalence among healthy controls was investigated in two studies and was found to be 3%[18] and 10% respectively[17]. Since the data for osteoporosis prevalence in some studies were extracted from measurements of only one bone area, these numbers could be underestimations. The available data did not allow for a meaningful comparison of IBD patients and healthy controls.
When looking at osteoporosis prevalence worldwide in people without IBD, the numbers vary. Approximately 172400 people (around 3%) had osteoporosis in Denmark in 2017 according to the Danish Health Authority[23] and The International Osteoporosis Foundation relies on the estimate that over 200 million people worldwide (around 3%) suffer from it[9]. However, it has been estimated that the actual number of people aged 50 years or older with osteoporosis in Denmark, including undiagnosed inhabitants, is between 146481 and 518272, depending of the calculation method[23]. A nationwide register based Danish study showed that the estimated prevalence of osteoporosis was 40.8% in women and 17.7 % in men, all ≥ 50 years[24]. Due to the wide range of estimates for the prevalence of osteoporosis and the small number of papers included in our study, it is not possible for us to conclude whether its overall prevalence is higher among IBD patients.
The included studies investigated many different risk factors using a variety of methodologies, making it difficult to draw firm, generalized conclusions. We found lower BMI and lower body weight to be associated with lower BMD. Female gender was found to be associated in two out of four studies investigating this risk factor. These are well-documented risk factors for osteoporosis in the background population as well[5].
Surprisingly, use of prednisolone in any dose and duration was found to be associated with decreased BMD in cross-sectional analyses only in CD patients. Steroids are well-recognized bone-resorbing agents[25,26]. Our finding might be explained by the fact that we have considered all forms of prednisolone use with any duration and hence the bones of some patients included in the analyses might have not been exposed enough to prednisolone to be affected[27].
Older age is a well-known risk factor for osteoporosis[5]. Remarkably, age showed significant association with BMD in studies including CD patients only, and one study found the patient group with reduced BMD to be younger than the patients with normal BMD. These studies were small and one of them only included premenopausal women. Hence the analysed population might not be fully representative.
We found that increased age was associated with a decrease in BMD and that increased weight and BMI were associated with an increase in BMD over time. The results of analyses of gender and use of steroids showed no uniformity. These discrepant results are additionally surprising since females are known to be at higher risk of developing osteoporosis[5]. This might again be explained by the lack of large-scale studies and that one study only includes premenopausal women. The only register-based study included in our review concluded that female gender was a risk factor for osteoporosis[18].
The CD cohorts presented the highest range in the prevalence of osteoporosis, though the numbers overlapped. CD, and not UC, appeared to be associated with osteoporosis or low BMD. Only CD patients seemed to have an increased risk for osteoporosis or low BMD over time. Suggested risk factors for developing osteoporosis are small-bowel disease or resection, smoking and corticosteroid treatment[28]. Small-bowel disease and resection are specific to CD and as such could partly explain why the risk of osteoporosis appears to be greater among CD patients. Moreover, CD patients have been shown to have a higher prevalence of smoking than UC patients[29]. Two population-based studies of CD and UC patients, respectively, have shown that on average around 2% of UC patients take corticosteroids at any given time, whereas almost 10% of CD patients do[30,31]. Future analyses ought to be made of UC and CD separately.
Gut inflammation is a disease-specific risk factor that few of the studies included here chose to analyse. According to a synopsis on research evaluating bone disease in patients with IBD from 2014, increased systemic inflammation increases bone resorption[1]. Inflammation serum markers and their role in IBD is an interesting subject that should be researched further.
Current guidelines recommend that high risk IBD patients should be screened for osteoporosis[32,33]. A Danish study found in a cohort of 513 unselected IBD patients with ten years of follow-up that the incidence of osteoporosis was twice as high for IBD patients compared to a control population[34]. This indicates that adequate screening of osteoporosis could benefit IBD patients.
There are several limitations to this systematic review. A systematic review is evidently dependent on the quality of the studies under review. The number of available studies was small, as was the number of patients in most cohorts. Furthermore, the quality of the studies reviewed varied considerably, as did the statistical analyses and use of covariates. This has precluded the possibility of performing a meta-analysis. Also, as all but one study originated in Europe, United States or Canada, their data might not be representative of other parts of the world. Half of the studies investigated only CD patients, while studies analysing both UC and CD patients did not provide stratified information on IBD subtype. Therefore, no data on UC patients exclusively were available. The risk factors discussed above were considered to be associated with osteoporosis if only one measurement (e.g., total hip, femoral neck, etc.) was significant. Therefore, the association between BMD and some risk factors may vary in strength. The follow up time for the included studies varied between two and 6,5 years and hence may have been too short in some studies to identify any change in BMD. One study[18] excluded patients diagnosed with osteoporosis before their IBD diagnosis; to compare this study with other studies that did not exclude this patient group might have affected our results. Finally, analyses conducted during each study were based on different measurements, i.e., T, Z, and BMD scores, and this may also have distorted our analyses.
In conclusion, there seems to be an increased prevalence of osteoporosis among CD, as compared to UC, patients. We found an association between osteoporosis or low BMD and lower weight and lower BMI for CD and UC patients while findings regarding gender were inconsistent across studies. Steroid usage was found to be associated with an increased risk of osteoporosis or low BMD only in patients with CD. Increase in age, decrease in weight and BMI, and diagnosis of CD seem to be associated with a decrease in BMD over time. Firm conclusions are difficult to draw due to considerable heterogeneity in terms of study methodologies, definitions and the assessment of osteoporosis, and the small number of population-based studies. Osteoporosis is a common disease that is associated with great economic and psychological burden worldwide due to the consequences of the disease in terms of osteoporotic fractures, and physicians treating patients with IBD should be aware of the risk for osteoporosis in this patient group. Given the importance of adequate screening and treatment of osteoporosis, there is a need for more prospective population-based research on the relationship between osteoporosis and IBD-patients and subgroups in this population. Any such future studies should assess CD and UC separately, should include healthy subjects as controls, and should assess disease specific risk factors such as gut inflammation markers.
The inflammatory bowel diseases (IBD), encompassing Crohn’s disease (CD) and ulcerative colitis (UC) are chronic, immune-mediated disorders of the digestive tract. Being diagnosed with IBD is considered a risk factor for the development of osteoporosis. The consequence of development of osteoporosis is the increased risk of pathological fractures that in turn are associated with great economic and psychological burden. Several studies have investigated the relationship between inflammatory bowel diseases and osteoporosis but differences in study design and study populations, as well as inconsistent results and diverging interpretations of them, make it difficult to draw firm conclusions.
Considering the severe consequences of osteoporosis, research on risk factors and prevalence of the disease in IBD patients is of great importance in order to conclude how prevailing the disease is in this patient group and its subgroups. It may give clues on how guidelines for screening and treatment of osteoporosis in IBD-patients should be developed, as well pinpointing what areas need more research.
The objectives with this research was to assess the prevalence of osteoporosis among IBD patients compared to healthy individuals, as well as the disease course of osteoporosis or low BMD in IBD patients. We also aimed to assess risk factors associated with osteoporosis and low BMD in IBD patients with the intention to find more substantial evidence as to the cause of osteoporosis or low BMD in this patient group.
For this systematic review, we searched databases including EMBASE and PubMed as well as abstracts from 2014-2018 presented at the United European Gastroenterology Week, the European Crohn’s and Colitis Organisation congress, and Digestive Disease Week were screened. Studies were eligible for inclusion if they investigated either the prevalence of osteoporosis or osteopenia and/or risk factors for osteoporosis or low bone mineral density (BMD in IBD patients. Studies on children under the age of 18 were excluded. Only population-based studies were included. All risk factors for osteoporosis and low BMD investigated in any included article were considered. Study quality and the possibility of bias were analysed using the Newcastle-Ottawa scale.
Twelve studies including 3661 IBD patients and 12789 healthy controls were included. Prevalence of osteoporosis varied between 4%-9% in studies including both CD and UC patients; 2%-9% in studies including UC patients, and 7%-15% in studies including CD patients. Among healthy controls, prevalence of osteoporosis was 3% and 10% in two studies. CD diagnosis, low body mass index (BMI) and low body weight were risk factors associated with osteoporosis or low BMD. Two out of four studies investigating gender found an association between female gender and lower BMD. CD patients had an increased risk for osteoporosis or low BMD over time, while UC patients did not. Increased age was associated with decreased BMD, and there was a positive association between weight and BMI and BMD over time. Great heterogeneity was found in the included studies in terms of study methodologies, definitions and the assessment of osteoporosis, and only a small number of population-based studies was available.
This systematic review found a possible increase of prevalence of osteoporosis in CD cohorts when compared to UC and cohorts including both disease types. Lower weight and lower BMI were predictors of osteoporosis or low BMD in IBD patients. The results varied considerably between studies. Firm conclusions are difficult to draw due to considerable heterogeneity in terms of study methodologies, definitions and the assessment of osteoporosis, and the small number of population-based studies.
Osteoporosis is a common disease that is associated with great economic and psychological burden worldwide due to the consequences of the disease in terms of osteoporotic fractures. Given the importance of adequate screening and treatment of osteoporosis, there is a need for more prospective population-based research on the relationship between osteoporosis and IBD-patients and subgroups in this population. Any such future studies should assess CD and UC separately, should include healthy subjects as controls, and should assess disease specific risk factors such as gut inflammation markers.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Denmark
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Faye AS, Lee JI, Musumeci G S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Targownik LE, Bernstein CN, Leslie WD. Risk factors and management of osteoporosis in inflammatory bowel disease. Curr Opin Gastroenterol. 2014;30:168-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 2. | Siffledeen JS, Fedorak RN, Siminoski K, Jen H, Vaudan E, Abraham N, Seinhart H, Greenberg G. Bones and Crohn's: risk factors associated with low bone mineral density in patients with Crohn's disease. Inflamm Bowel Dis. 2004;10:220-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Targownik LE, Leslie WD, Carr R, Clara I, Miller N, Rogala L, Graff LA, Walker JR, Bernstein CN. Longitudinal change in bone mineral density in a population-based cohort of patients with inflammatory bowel disease. Calcif Tissue Int. 2012;91:356-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Schüle S, Rossel JB, Frey D, Biedermann L, Scharl M, Zeitz J, Freitas-Queiroz N, Pittet V, Vavricka SR, Rogler G, Misselwitz B; Swiss IBD cohort study. Prediction of low bone mineral density in patients with inflammatory bowel diseases. United European Gastroenterol J. 2016;4:669-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | International Osteoporosis Foundation. Who’s at risk. [cited 19 February 2019]. Available from: https://www.iofbonehealth.org/whos-risk. |
| 6. | Castrogiovanni P, Trovato FM, Szychlinska MA, Nsir H, Imbesi R, Musumeci G. The importance of physical activity in osteoporosis. From the molecular pathways to the clinical evidence. Histol Histopathol. 2016;31:1183-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 44] [Reference Citation Analysis (0)] |
| 7. | Pichler K, Loreto C, Leonardi R, Reuber T, Weinberg AM, Musumeci G. RANKL is downregulated in bone cells by physical activity (treadmill and vibration stimulation training) in rat with glucocorticoid-induced osteoporosis. Histol Histopathol. 2013;28:1185-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 8. | Musumeci G, Loreto C, Leonardi R, Castorina S, Giunta S, Carnazza ML, Trovato FM, Pichler K, Weinberg AM. The effects of physical activity on apoptosis and lubricin expression in articular cartilage in rats with glucocorticoid-induced osteoporosis. J Bone Miner Metab. 2013;31:274-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 9. | International Osteoporosis Foundation. Epidemiology. [cited 19 February 2019]. Available from: https://www.iofbonehealth.org/epidemiology. |
| 10. | Targownik LE, Bernstein CN, Leslie WD. Inflammatory bowel disease and the risk of osteoporosis and fracture. Maturitas. 2013;76:315-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Ottawa Hospital Research Institute. [cited 19 February 2019]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. |
| 12. | Jahnsen J, Falch JA, Aadland E, Mowinckel P. Bone mineral density is reduced in patients with Crohn's disease but not in patients with ulcerative colitis: a population based study. Gut. 1997;40:313-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 198] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 13. | Schoon EJ, van Nunen AB, Wouters RS, Stockbrügger RW, Russel MG. Osteopenia and osteoporosis in Crohn's disease: prevalence in a Dutch population-based cohort. Scand J Gastroenterol Suppl. 2000;43-47. [PubMed] |
| 14. | Andreassen H, Rix M, Brot C, Eskildsen P. Regulators of calcium homeostasis and bone mineral density in patients with Crohn's disease. Scand J Gastroenterol. 1998;33:1087-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Andreassen H, Hylander E, Rix M. Gender, age, and body weight are the major predictive factors for bone mineral density in Crohn's disease: a case-control cross-sectional study of 113 patients. Am J Gastroenterol. 1999;94:824-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Jahnsen J, Falch JA, Mowinckel P, Aadland E. Bone mineral density in patients with inflammatory bowel disease: a population-based prospective two-year follow-up study. Scand J Gastroenterol. 2004;39:145-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Haugeberg G, Vetvik K, Stallemo A, Bitter H, Mikkelsen B, Stokkeland M. Bone density reduction in patients with Crohn disease and associations with demographic and disease variables: cross-sectional data from a population-based study. Scand J Gastroenterol. 2001;36:759-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Tsai MS, Lin CL, Tu YK, Lee PH, Kao CH. Risks and predictors of osteoporosis in patients with inflammatory bowel diseases in an Asian population: a nationwide population-based cohort study. Int J Clin Pract. 2015;69:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | Leslie WD, Miller N, Rogala L, Bernstein CN. Vitamin D status and bone density in recently diagnosed inflammatory bowel disease: the Manitoba IBD Cohort Study. Am J Gastroenterol. 2008;103:1451-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 102] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 20. | Leslie WD, Miller N, Rogala L, Bernstein CN. Body mass and composition affect bone density in recently diagnosed inflammatory bowel disease: the Manitoba IBD Cohort Study. Inflamm Bowel Dis. 2009;15:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Bernstein CN, Leslie WD, Taback SP. Bone density in a population-based cohort of premenopausal adult women with early onset inflammatory bowel disease. Am J Gastroenterol. 2003;98:1094-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Bernstein CN, Bector S, Leslie WD. Lack of relationship of calcium and vitamin D intake to bone mineral density in premenopausal women with inflammatory bowel disease. Am J Gastroenterol. 2003;98:2468-2473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Osteoporosis â A disclosure of the collective effort against osteoporosis. [Osteoporose - En afdækning af den samlede indsats mod osteoporose.] [cited 15 April 2019] København: Sundhedsstyrelsen; 2018. Available from: http://www.sst.dk. |
| 24. | Vestergaard P, Rejnmark L, Mosekilde L. Osteoporosis is markedly underdiagnosed: a nationwide study from Denmark. Osteoporos Int. 2005;16:134-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 163] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 25. | Buehring B, Viswanathan R, Binkley N, Busse W. Glucocorticoid-induced osteoporosis: an update on effects and management. J Allergy Clin Immunol. 2013;132:1019-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 26. | Rizzoli R, Biver E. Glucocorticoid-induced osteoporosis: who to treat with what agent? Nat Rev Rheumatol. 2015;11:98-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 123] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 27. | Harslof T, Langdahl B, Vestergaard P, Eiken P, Hermann P, Stilgren L, Grove D, Folkestad L, Jensen T, Brask-Lindemann DD. Glucocorticoid-induced Osteoporosis. [Glukokortikoid-induceret Osteoporose.] [cited 07 December 2019] Dansk Endokrinol Selsk. 2018. Available from: http://www.endocrinology.dk/index.php/3-calcium-og-knoglemetaboliske-sygdomme/4-glukokortikoid-induceret-osteoporose. |
| 28. | Harbord M, Annese V, Vavricka SR, Allez M, Barreiro-de Acosta M, Boberg KM, Burisch J, De Vos M, De Vries AM, Dick AD, Juillerat P, Karlsen TH, Koutroubakis I, Lakatos PL, Orchard T, Papay P, Raine T, Reinshagen M, Thaci D, Tilg H, Carbonnel F; European Crohn’s and Colitis Organisation. The First European Evidence-based Consensus on Extra-intestinal Manifestations in Inflammatory Bowel Disease. J Crohns Colitis. 2016;10:239-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 543] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 29. | Lunney PC, Kariyawasam VC, Wang RR, Middleton KL, Huang T, Selinger CP, Andrews JM, Katelaris PH, Leong RW. Smoking prevalence and its influence on disease course and surgery in Crohn's disease and ulcerative colitis. Aliment Pharmacol Ther. 2015;42:61-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 30. | Burisch J, Kiudelis G, Kupcinskas L, Kievit HAL, Andersen KW, Andersen V, Salupere R, Pedersen N, Kjeldsen J, D'Incà R, Valpiani D, Schwartz D, Odes S, Olsen J, Nielsen KR, Vegh Z, Lakatos PL, Toca A, Turcan S, Katsanos KH, Christodoulou DK, Fumery M, Gower-Rousseau C, Zammit SC, Ellul P, Eriksson C, Halfvarson J, Magro FJ, Duricova D, Bortlik M, Fernandez A, Hernández V, Myers S, Sebastian S, Oksanen P, Collin P, Goldis A, Misra R, Arebi N, Kaimakliotis IP, Nikuina I, Belousova E, Brinar M, Cukovic-Cavka S, Langholz E, Munkholm P; Epi-IBD group. Natural disease course of Crohn's disease during the first 5 years after diagnosis in a European population-based inception cohort: an Epi-IBD study. Gut. 2019;68:423-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 196] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 31. | Burisch J, Katsanos KH, Christodoulou DK, Barros L, Magro F, Pedersen N, Kjeldsen J, Vegh Z, Lakatos PL, Eriksson C, Halfvarson J, Fumery M, Gower-Rousseau C, Brinar M, Cukovic-Cavka S, Nikulina I, Belousova E, Myers S, Sebastian S, Kiudelis G, Kupcinskas L, Schwartz D, Odes S, Kaimakliotis IP, Valpiani D, D'Incà R, Salupere R, Chetcuti Zammit S, Ellul P, Duricova D, Bortlik M, Goldis A, Kievit HAL, Toca A, Turcan S, Midjord J, Nielsen KR, Andersen KW, Andersen V, Misra R, Arebi N, Oksanen P, Collin P, de Castro L, Hernandez V, Langholz E, Munkholm P; Epi-IBD Group. Natural Disease Course of Ulcerative Colitis During the First Five Years of Follow-up in a European Population-based Inception Cohort-An Epi-IBD Study. J Crohns Colitis. 2019;13:198-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 115] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 32. | Kornbluth A, Hayes M, Feldman S, Hunt M, Fried-Boxt E, Lichtiger S, Legnani P, George J, Young J. Do guidelines matter? Implementation of the ACG and AGA osteoporosis screening guidelines in inflammatory bowel disease (IBD) patients who meet the guidelines' criteria. Am J Gastroenterol. 2006;101:1546-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 33. | Farraye FA, Melmed GY, Lichtenstein GR, Kane SV. ACG Clinical Guideline: Preventive Care in Inflammatory Bowel Disease. Am J Gastroenterol. 2017;112:241-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 354] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 34. | Lo B, Holm JP, Vester-Andersen MK, Bendtsen F, Vind I, Burisch J. Incidence, Risk Factors and Evaluation of Osteoporosis in Patients With Inflammatory Bowel Disease: A Danish Population-Based Inception Cohort With 10 Years of Follow-Up. J Crohns Colitis. 2020;14:904-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |