Published online Sep 14, 2020. doi: 10.3748/wjg.v26.i34.5118
Peer-review started: May 19, 2020
First decision: June 20, 2020
Revised: June 30, 2020
Accepted: August 14, 2020
Article in press: August 14, 2020
Published online: September 14, 2020
Processing time: 113 Days and 1.6 Hours
Non-alcoholic fatty liver disease (NAFLD), in which abnormal lipid metabolism plays an important role in disease progression, has become a pandemic. Abnormal lipid metabolism, for example an increased fat intake, has been thought to be an initial factor leading to NAFLD. The small intestine is the main site of dietary lipid absorption. A number of clinical trials have shown that acupuncture has positive effects in the regulation of lipid metabolism, which is closely associated with the progression of NAFLD. We therefore hypothesized that, acupuncture can improve the conditions of NAFLD by regulating intestinal absorption of lipid.
To study the role of acupuncture treatment in the improvement of metabolic syndrome secondary to NAFLD by mouse model.
8-wk-old male C57BL/6J mice were fed a methionine- and choline-deficient diet for 3 wk. Then, all mice were separated randomly into acupoints group (AG) or non-acupoints group (NG) with high fat diet feeding. Needling treatment was performed at Zu san li, Guan yuan and Yong quan acupoints as acupuncture treatment to AG mice while non-acupoints place to NG mice. Finally, mice were anesthetized with an injection of ketamine-medetomidine and euthanized by exsanguination.
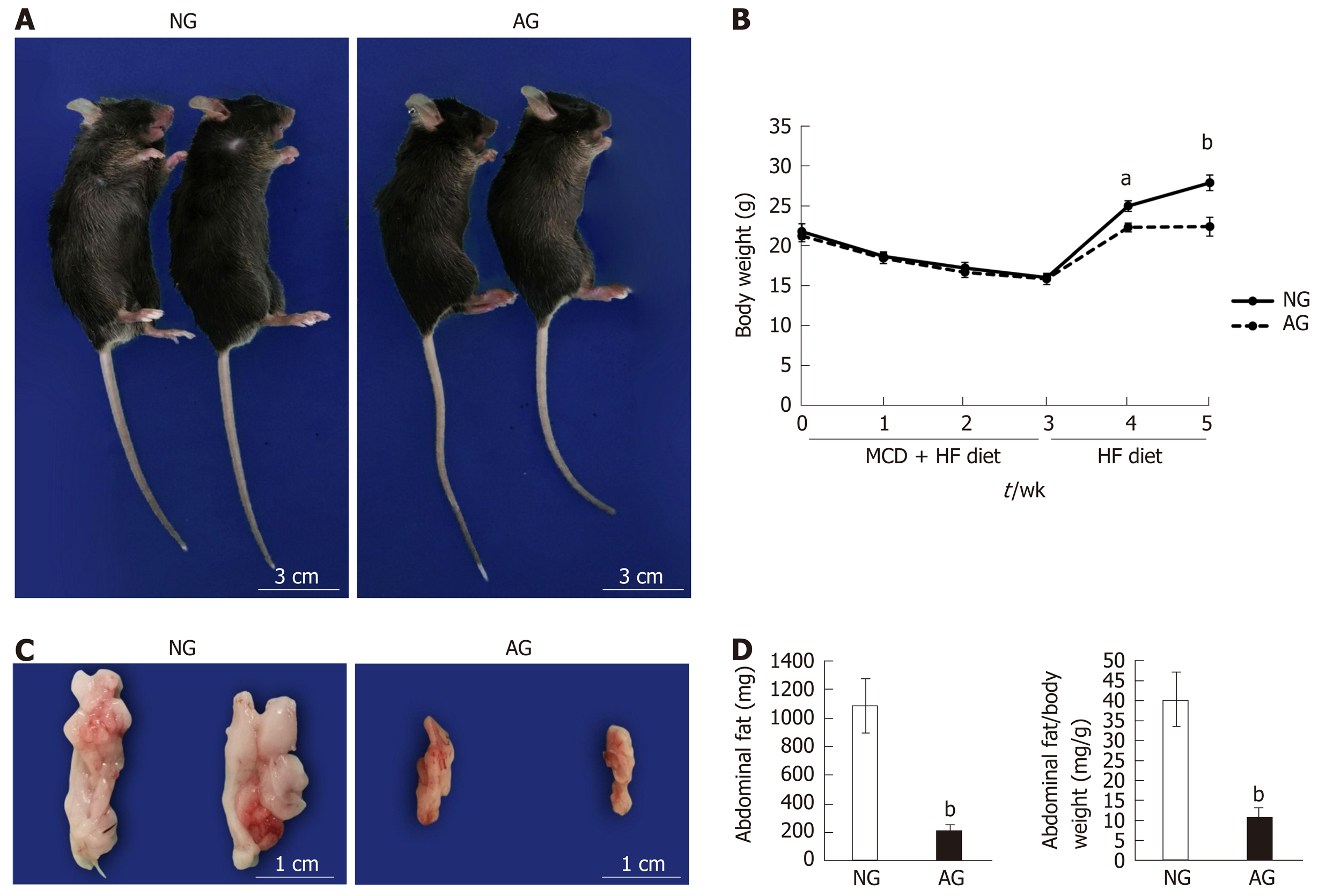
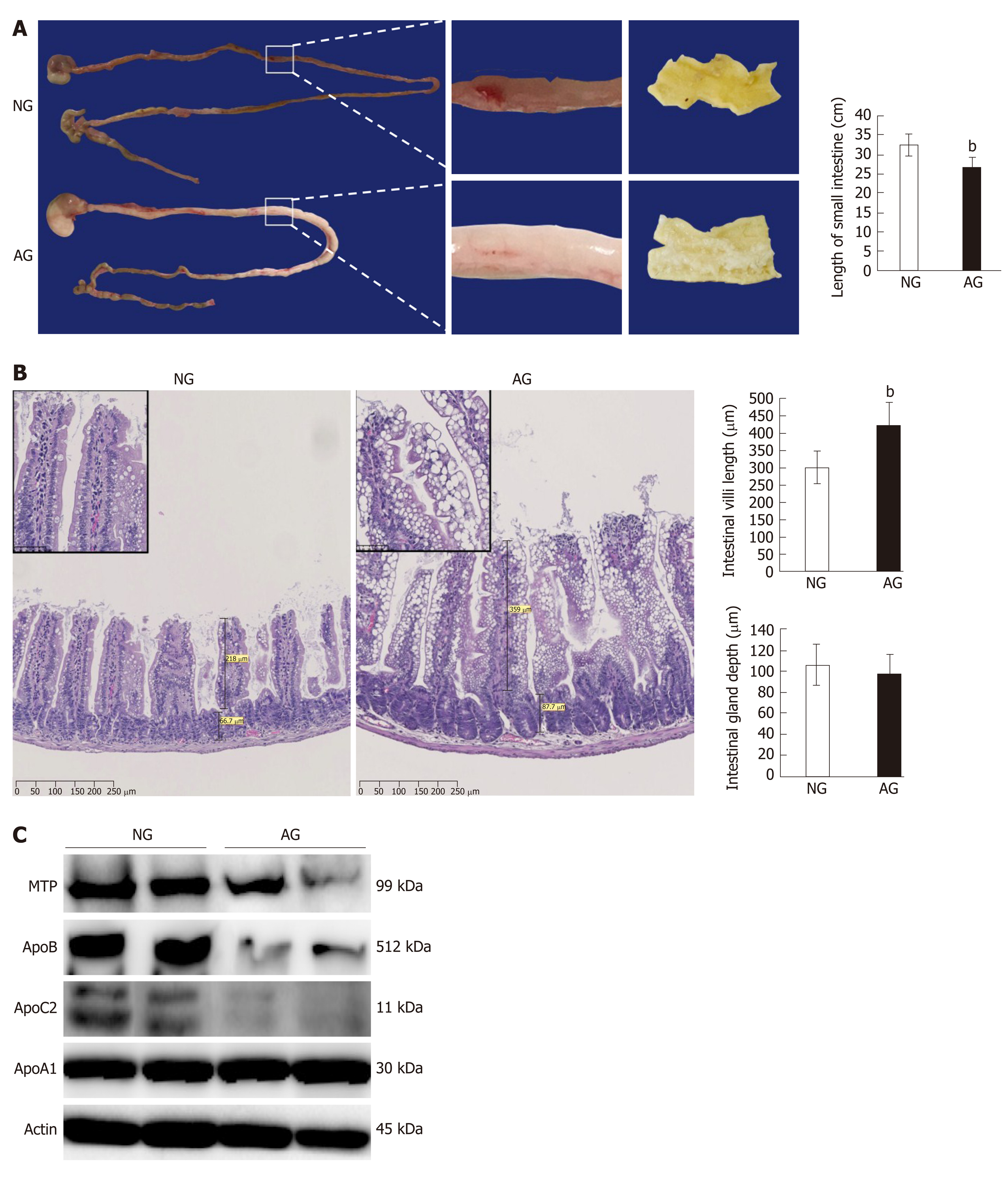
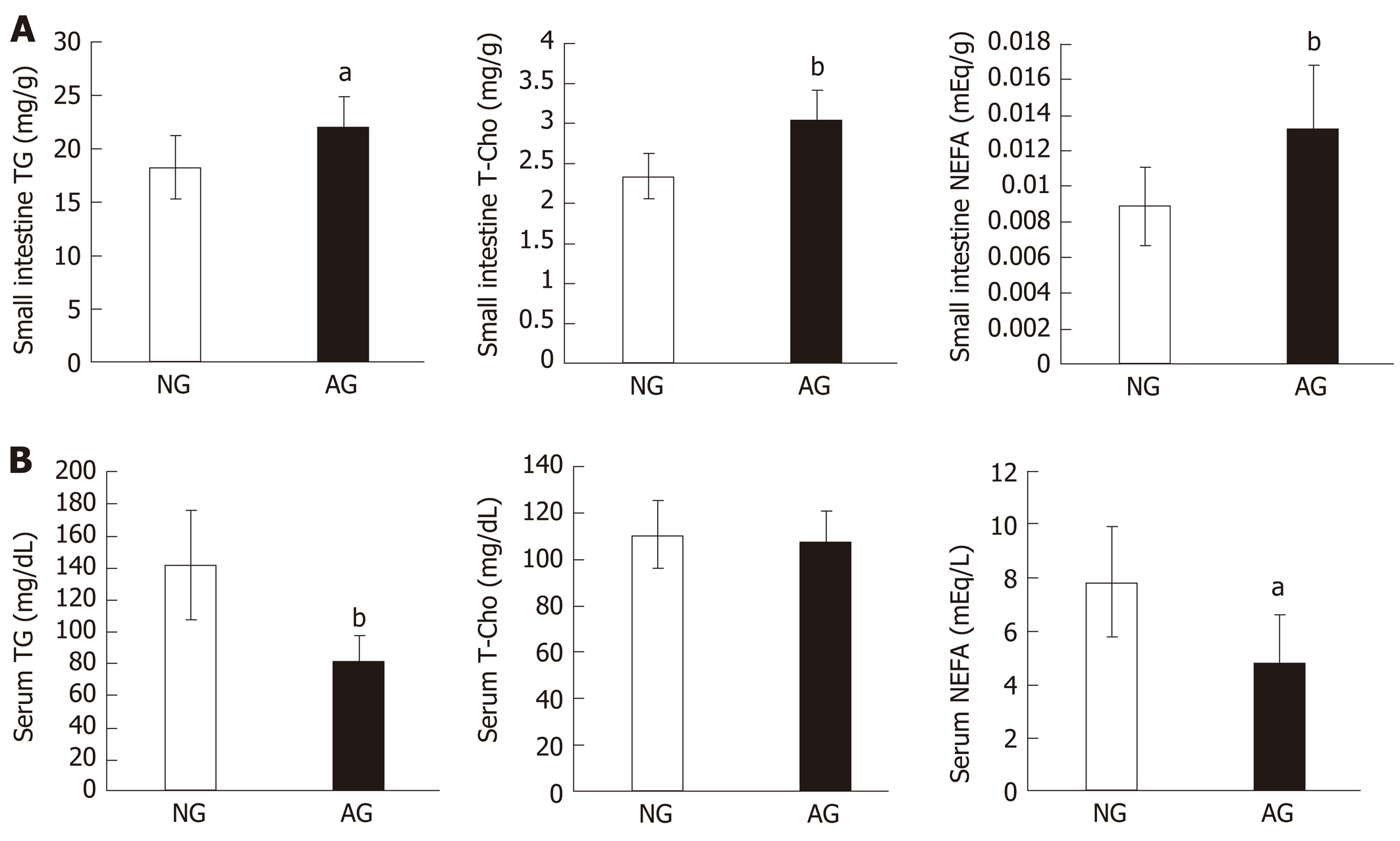
An apparent improvement of obesity was found in AG mice after acupuncture treatment. In AG mice, the body weight was much lower (22.6 ± 1.2 g vs 28.1 ± 1.0 g, P < 0.005) in comparison to NG mice. The length of small intestine in AG mice was significantly shorter (26.7 ± 2.3 cm vs 32.7 ± 2.7 cm, P < 0.005). A large amount of chyme was observed in the lumen of the AG small intestine. The expression of microsomal triglyceride transfer protein, apolipoprotein B and apolipoprotein C2 was downregulated. Triacylglycerols (TGs), total cholesterol and nonesterified fatty acid (NEFA) levels of the small intestinal tissue were significantly higher in AG mice, but the serum TGs and NEFA levels were reduced in AG mice.
These results indicate that acupuncture at Zu san li, Guan yuan and Yong quan suppressed lipid absorption by downregulating the expression of apolipoproteins in the small intestine.
Core Tip: In the present study, our experimental data showed that acupuncture treatment suppressed high-fat-induced body weight gain, reduced the accumulation of intra-abdominal fat, inhibited lipid absorption in the small intestine and downregulated the blood lipid level in mice with non-alcoholic fatty liver disease, suggesting that acupuncture potentially had variable beneficial effects in improving the lipid metabolism in the presence of abnormal liver metabolism. Furthermore, we also found more fat deposition in the intestinal epithelium with the decreased expression of some intestinal apolipoproteins in acupuncture-treated mice, suggesting that acupuncture may suppress lipid absorption by downregulating the expression of apolipoproteins in the small intestine.
- Citation: Han J, Guo X, Meng XJ, Zhang J, Yamaguchi R, Motoo Y, Yamada S. Acupuncture improved lipid metabolism by regulating intestinal absorption in mice. World J Gastroenterol 2020; 26(34): 5118-5129
- URL: https://www.wjgnet.com/1007-9327/full/v26/i34/5118.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i34.5118
Non-alcoholic fatty liver disease (NAFLD) has become a pandemic liver disease in the twenty-first century, and currently affects approximately one billion individuals worldwide. The prevalence of NAFLD is still increasing each year, with the high incidence of metabolic syndromes, such as obesity and dyslipidemia, especially hypertriglyceridemia[1]. Although the molecular mechanism of NAFLD is not fully understood, a considerable proportion of NAFLD patients are found to have metabolic syndrome[2]. Abnormal lipid metabolism, for example an increased fat intake, has been thought to be an initial factor leading to NAFLD[3].
Lipids are known to be one of major sources of food energy, and play a crucial role in systemic metabolism, especially in patients with chronic liver diseases like NAFLD[4]. The small intestine is the main site of dietary lipid absorption in the body. Dietary lipids are hydrolyzed and digested in the lumen of small intestine. These products are then taken up by enterocytes, and are transported, re-synthesized, assembled and secreted into circulation with lipoproteins, such as very low-density lipoprotein (VLDL), high density lipoprotein (HDL) or chylomicrons (CMs), for utilization in peripheral tissues. Apolipoproteins, including apolipoprotein A (ApoA), apolipoprotein B (ApoB), apolipoprotein C (ApoC) and microsomal triglyceride transfer protein (MTP), play important roles during this process. Triacylglycerol (TG) and nonesterified fatty acid (NEFA) are packaged into large, spherical CMs and secreted into the lymphatic system; this process mainly relies on the activity of MTP and ApoB[5,6]. However, in addition to CMs, cholesterol can be secreted in HDL. In HDL, MTP showed no effect on the secretion of cholesterol, while the deficiency of ApoA1 can specifically decrease the quantities of cholesterol secreted with HDL[5,7]. The improvement of lipid metabolism helps to inhibit the progress of NAFLD.
Acupuncture is one of the most important external interventions in traditional Chinese medicine (TCM). It has a long history of more than 2000 years as a treatment for many diseases on a basic of the Yellow Emperor’s Classic of Internal Medicine[8]. According to the TCM theory, all diseases were caused by a stagnation of “qi” and “blood” which is a result of the imbalance between “Yin” and “Yang”[9]. In recent years, due to its low cost and simplicity, the role of acupuncture in disease prevention and treatment has attracted increased attention from researchers, and there is a great deal of evidence to show that acupuncture can have pathophysiological consequences and alleviate symptoms in multiple organ systems, such as diseases of digestive system[10], diseases of nerve[11], as well as diseases of skin[12,13]. A number of clinical trials have shown that acupuncture has positive effects in therapy for simple obesity and that it can significantly reduce plasma lipid levels, indicating that acupuncture treatment may have important roles in the regulation of lipid metabolism, which is closely associated with the progression of NAFLD[14]. However, few modern medical studies have reported the exact mechanisms by which regulate lipid metabolism.
In a recent study, using a mouse model of methionine- and choline-deficient (MCD) diet-induced NAFLD, we investigated the effects of needling treatment at three acupoints, Zu san li (ST36), Guan yuan (CV4) and Yong quan (KI1). According to the TCM theory, most of the acupoints are located on different meridians which correspond to different systems and have different functions[8]. ST36 is located on the stomach meridian of foot-Yangming, which has a function in generating stomach qi to regulate digestion and absorption. While KI1 is located on kidney meridian of foot-Shaoyin, which is an important acupoint in regulating the motion of both qi and blood. CV4 is front Mu point of small intestine which is a key acupoint in regulating the motion of qi belonging to conception vessel. Our results demonstrated that acupuncture treatment suppressed the progression of NAFLD by controlling the intrahepatic pathological process[15]. According to TCM theory, the three acupoints are located at very important sites of the body. They have roles in the regulation of various metabolic activities, including gastrointestinal motility, glandular secretion and nerve conduction[9,10,14,16]. We therefore hypothesized that, in addition to local effects, acupuncture on ST36, KI1 and CV4 can also improve the conditions of NAFLD by regulating systemic metabolism.
In this study, we investigated the role of acupuncture treatment in the improvement of metabolic syndrome secondary to NAFLD by employing the above-described mouse model. Furthermore, we focused on the major organ responsible for lipid absorption, the small intestine, and observed its morphological and functional changes. Our results suggest that acupuncture may provide an alternative approach for improving metabolic syndromes in patients with NAFLD and to prevent the progression of the disease.
The animals used in this study were 8-wk-old male C57BL/6J mice that weighed approximately 20 g, purchased from Sankyo Labo Service Corporation, INC. Japan. The animals were maintained at a temperature of 21-25 °C with a 12-h light–dark cycle and ad libitum access to drinking water. All mice were fed with an MCD + high fat (HF) diet (60% fat; KBT Oriental Corporation, Saga, Japan) for 3 wk to induce NAFLD. After that, MCD diet stopped. They were then fed an HF (60% fat; KBT Oriental Corporation, Saga, Japan) diet for 2 wk to maintain their hyperlipidemia. When HF diet started, they were separated randomly into two groups: The acupoints group (AG, n = 10) and the non-acupoints group (NG, n = 10) for the needling treatment. After two weeks’ treatment, mice were anesthetized with an injection of ketamine-medetomidine and euthanized by exsanguination[17]. Whole blood samples from axillary vessels were kept at room temperature until coagulation, then all blood cells were removed by centrifugation and serum samples were frozen with liquid nitrogen for use in further experiments. The intestine was removed, photographed, and the length was measured. Then, the small intestine was cut into pieces, frozen with liquid nitrogen, or fixed in 10% neutral-buffered formalin for using in further experiments.
The mice were randomly separated into AG and NG. Needling treatment started as well as HF diet started. As described previously[15], three acupoints, ST36, KI1 and CV4, or corresponding non-acupoints were needled with 13-mm needles (Suzhou Medical Appliance Factory 0.25 mm × 13 mm), which were rotated slowly at 60 rotations per minute for 2 min, without retaining the needle. All mice were needled without anesthesia in a mouse retainer. The location of ST36 in mouse is 1.5 mm below the anterior tibial tubercle of both sides’ hind limbs. KI1 is located in the centre of both sides’ hind soles. CV4 is 10 mm below the mouse’s navel.
The experimental protocols were approved by the Ethics Committee of Animal Care and Experimentation, Kanazawa Medical University, Japan. Our project code was 2019-21 (registered on July 3, 2019). All experiments were implemented in compliance with the Institutional Guidelines for Animal Experiments and the Law (No. 105) and Notification (No. 6) of the Japanese government. The number of animals used in this experiment was minimized and we attempted to minimize the animals' suffering.
After 24 h of fixation in 10% neutral-buffered formalin, we selected the same place of the mouse jejunum, embedded the specimen in paraffin, cut the specimen into sequential sections of 4 μm in thickness and performed hematoxylin and eosin (HE) staining. Images of the sections were captured with the Nano Zoomer Digital Pathology Virtual Slide Viewer software program (Hamamatsu Photonics Corp, Hamamatsu, Japan) and the villi lengths and gland depths were measured.
Small intestine samples were lysed with radio immunoprecipitation assay (RIPA, SIGMA) lysis buffer containing protease inhibitor cocktail and denatured with 2 × Laemmli sample buffer (Bio-Rad Laboratories, Inc.) at 95°C for 5 min. Then, the protein samples were electrophoretically loaded onto 7.5% or 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels and transferred onto Immun-Blot PVDF membranes (Bio-Rad Laboratories, K.K., Tokyo, Japan). The membranes were then incubated overnight at 4°C with anti-β-actin monoclonal antibody (Cell Signaling Technology, Inc., 1:1000), MTTP antibody (Abcam PLC., 1:500), ApoA1 antibody (Abcam PLC., 1:500), ApoB antibody (Santa Cruz Biotechnology, Inc., 1:500), and ApoC2 antibody (Bioss Inc., 1:500) diluted in self-made TBS-T buffer. After washing with TBS-T, the membranes were incubated with secondary antibody at room temperature for 1 h. The secondary antibodies used were horseradish peroxidase-conjugated anti-rabbit antibody and anti-mouse antibody (Cell Signaling Technology, Inc., 1:5000). After visualization by an enhanced chemi-luminescence kit (Bio-Rad Laboratories, Inc.), the protein bands were checked and acquired by luminescent image analyzer (FUJIFILM, LAS400).
To check the serum and small intestine lipids profiles, the TG, NEFA and total cholesterol (T-Cho) levels were determined using commercial assay kits (Wako Pure Chemical Co.). For the small intestine, frozen tissues were weighed and homogenized. Lipids were extracted as described previously[15] with chloroform-methanol (2/1 v/v) and dried and solubilized in 2-propanol.
Quantitative Real-time polymerase chain reaction was used to analyze the gene expression in tissue specimens. Total RNA was extracted from the fat and small intestine with a ReliaPrep™ RNA Tissue Miniprep kit (Promega). The whole process was carrying out under RNase-free conditions in order to prevent degradation. Custom primers and a TaqMan probe for the amplification reaction were purchased from Life Technologies. The mRNA expression levels were analyzed. The relative expression levels were normalized to 18S ribosomal RNA and the fold-change in AG mice was calculated in comparison to NG mice.
All data are expressed as the mean ± SD. Statistical significance was analyzed with a 2-sided Student's t-test or Welch’s t-test, as appropriate. Statistical analyses were performed using Microsoft Excel. P values of < 0.05 were considered to indicate statistical significance.
After 2 wk of needling treatment, an apparent improvement in obesity was observed in AG mice in comparison to NG mice. General observation revealed that the AG mouse body was obviously thinner and smaller in comparison to the NG mouse body (Figure 1A). After acupuncture treatment, the body weight of the AG mice 22.6 ± 1.2 g was much lower than that of the NG mice 28.1 ± 1.0 g, indicating that acupuncture decelerated the body weight increase induced by HF feeding (Figure 1B). Moreover, the intra-abdominal fat of AG mice 213.8 ± 43.5 mg was clearly reduced in comparison to NG mice 1088.3 ± 193.0 mg (Figure 1C and D). A similar trend was also observed in the ratio of abdominal adipose tissue weight to body weight, which was significantly decreased (NG vs AG: 40.3 ± 6.8 vs 11.0 ± 1.9, Figure 1D). In concrete terms, the average abdominal fat weight of AG mice was only 20% of that in NG mice, while the ratio of abdominal fat weight to body weight in AG mice was one-fourth of that in NG mice.
The small intestine is closely associated with lipid absorption and metabolism. Grossly, the length of small intestine in AG mice 26.7 ± 2.3 cm was significantly shorter than that in NG mice 32.7 ± 2.7 cm, meanwhile, large amounts of chyme was observed in the lumen of the AG small intestine (Figure 2A). The average length of the AG mouse small intestine was approximately 6 cm shorter than that of the NG mice small intestine. HE staining of the small intestine revealed the accumulation of a huge number of adipose droplets in the intestinal epithelium of AG mice (Figure 2B). Nevertheless, the length of small intestinal villi of AG mice was nearly 1.4 times the length in NG mice (AG mice vs NG mice: 421.3 ± 67.1 μm vs 302.1 ± 47.7 μm, Figure 2B). However, the depth of the small intestinal gland in NG 105.9 ± 20.0 μm and AG 98.2 ± 17.4 μm mice did not differ to a statistically significant extent (Figure 2B). The expression levels of some important apolipoproteins related to intestinal lipid absorption, including MTP, ApoB and ApoC2, were downregulated in AG mice in comparison to NG mice, while the expression of ApoA1 did not differ to a statistically significant extent (Figure 2C). The expression levels of other factors related to embolism, such as GLUT1, 3, 4, and 5, lipa, and LDLR, did not differ between the two groups to a statistically significant extent (Supplementary Figure 1).
The TG, T-Cho and NEFA levels of small intestinal tissue in AG mice (TG 22.1 ± 2.8 mg/g, T-Cho 3.0 ± 0.4 mg/g, NEFA 0.01 ± 0.004 mEq/g) were significantly higher in comparison to NG mice (TG 18.2 ± 2.8 mg/g, T-Cho 2.3 ± 0.3 mg/g, NEFA 0.009 ± 0.002 mEq/g, Figure 3A). However, the serum lipid levels did not change in the same way. Furthermore, the serum TG and NEFA levels in AG mice (TG 81.5 ± 16.6 mg/dL, NEFA 4.8 ± 1.9 mEq/L) were reduced in comparison to NG mice (TG 141.4 ± 34.3mg/dL, NEFA 7.8 ± 2.0 mEq/L). The serum T-Cho levels in the two groups did not differ to a statistically significant extent (AG mice vs NG mice: 106.8 ± 12.9 mg/dL vs 110.5 ± 14.4 mg/dL, Figure 3B).
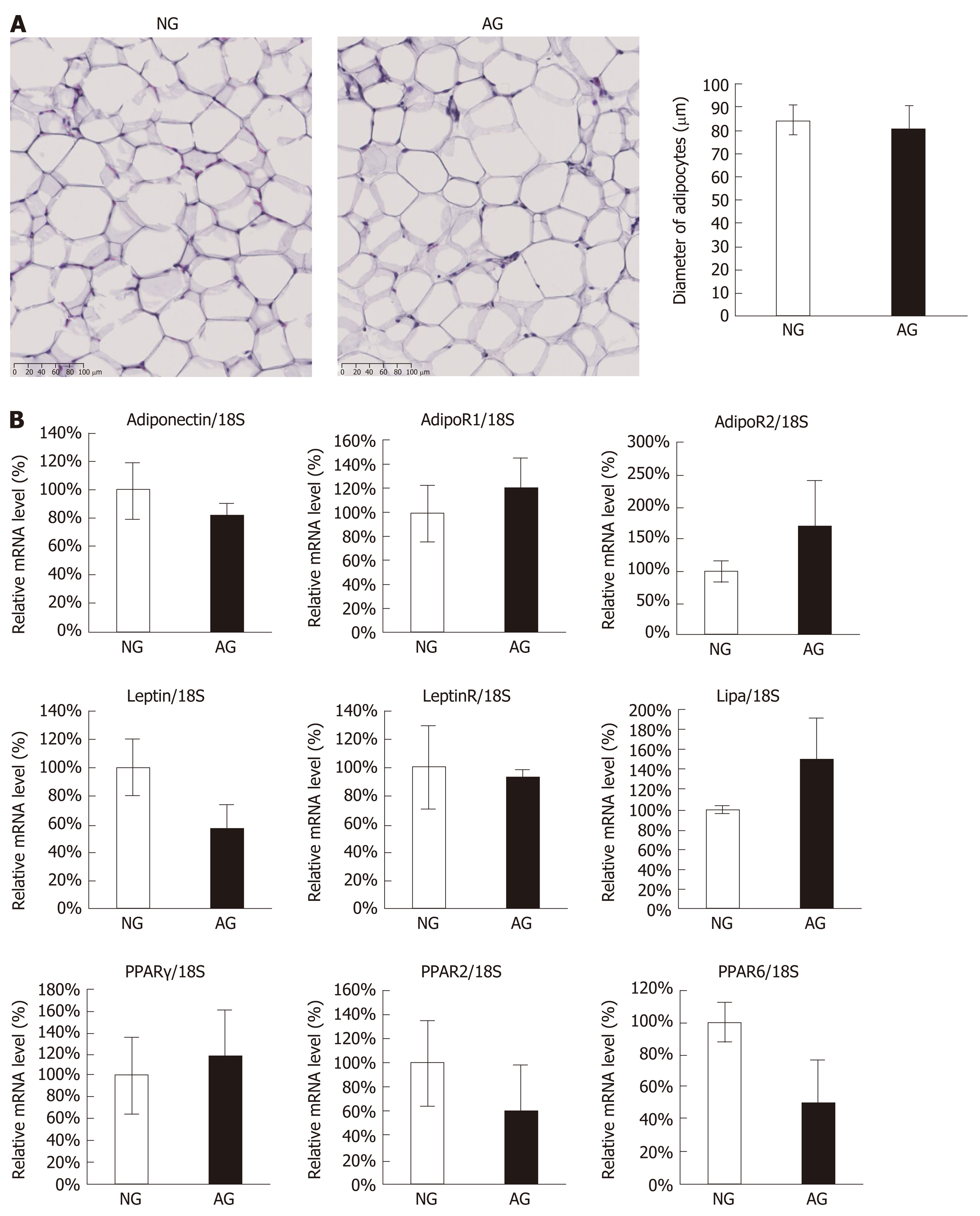
In order to explain the changes that occurred in AG mice, we firstly focused on adipose tissue itself. However, we found no significant differences in the tissue morphology of the two groups (Figure 4A) or in the expression levels of crucial genes related to the lipid metabolism of adipocytes (Figure 4B).
In the present study, our experimental data showed that acupuncture treatment suppressed HF-induced body weight gain, reduced the accumulation of intra-abdominal fat, inhibited lipid absorption in the small intestine and downregulated the blood lipid level in mice with NAFLD, suggesting that acupuncture potentially had variable beneficial effects in improving the lipid metabolism in the presence of abnormal liver metabolism.
Metabolic syndrome, which includes conditions such as obesity, dyslipidemia and insulin resistance, is closely related to the progression of NAFLD; conversely, chronic liver disease can further aggravate systemic metabolism abnormality[18,19]. Obesity, especially excess abdominal adiposity, is a high-risk factor for this disease and weight control is considered to improve the liver condition of patients with NAFLD[20]. Generally, improvements of diet and lifestyle are recommended; however, the effect on weight loss is often unsatisfactory. A large body of clinical and experimental evidence shows that acupuncture is effective for the treatment of obesity, and that acupuncture treatment is accompanied by a reduction in obesity-related com-plications, such as hyperlipidemia[21,22]. Indeed, in this study, the AG mice were observed to have lower body weight and less abdominal adiposity with an improved blood lipid status, in comparison to NG mice. In a recent study, we also reported that acupuncture significantly inhibited the progression of NAFLD, where reduced inflammatory responses and oxidative stress in the liver and enhanced hepatic metabolism were observed in mice with modest NAFLD after acupuncture treatment[15]. From the present results, in addition to the direct effects on the liver, the lower body weight and serum lipid levels produced by acupuncture treatment also played an important role in the improvement of the liver function, indicating that the beneficial effects of acupuncture on NAFLD not only rely on the control of the intrahepatic pathological process but also depend on the regulation of the systemic metabolism of the body.
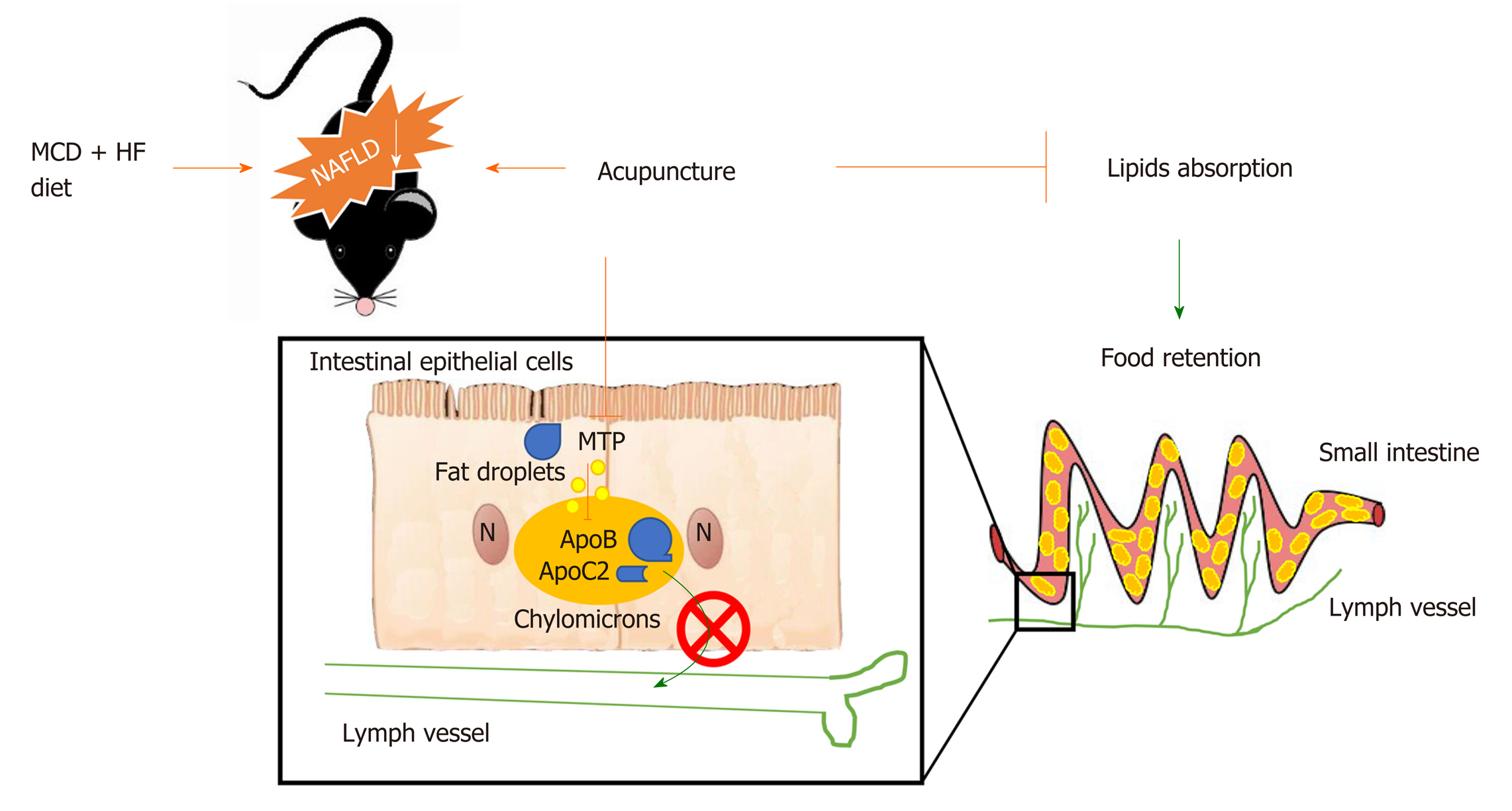
Furthermore, we also found more fat deposition in the intestinal epithelium with the decreased expression of some intestinal apolipoproteins in AG mice, suggesting that acupuncture may suppress lipid absorption by downregulating the expression of apolipoproteins in the small intestine, as we showed in Figure 5. At present, the mechanism underlying the effects of acupuncture in the treatment of obesity is still not fully understood, and although some related mechanisms have been reported in other studies[23,24], we suggest-at least in some situations-that the inhibition of intestinal lipid absorption plays important roles in the effects of acupuncture in the treatment of obesity. In fact, acupuncture has been widely used in the treatment of obesity in the clinical setting; thus, in view of the reduced lipid absorption, proper nutritional supplementation should be considered throughout the treatment process.
After dietary fat is digested by the intestinal lipase system in the lumen of the small intestine[25], the predominant lipids cross the apical membrane and enter the enterocytes in a protein-independent or -dependent manner[26,27]. When the lipid concentrations in the lumen are much higher than the intracellular levels, the passive uptake becomes the main way for enterocytes to absorb lipids[28]. In this study, an HF diet resulted in a high lipid concentration in the lumen, permitting the uptake of a large amounts of lipids by the enterocytes. However, when these lipids cannot be secreted into the lymphatic system or circulation, they are stored as cytosolic lipid droplets in the enterocytes[29]. Monoacylglycerol and free fatty acids are packaged into ApoB-rich chylomicrons for secretion into lymphatic vessels, in which MTP deliver lipids to the nascent ApoB and assist in folding[30,31]. The inhibition of MTP can lead to the degradation of ApoB, which causes the downregulation of chylomicron secretion[32,33]. The decreased expression of MTP and ApoB in the AG intestine may result in the accumulation of lipids by reducing chylomicron secretion in the enterocytes. In addition to chylomicron, cholesterol is secreted in MTP- and ApoB-independent HDL pathways, with ApoA1 playing a pivotal role in HDL secretion[34]. The expression of ApoA1 in the NG and AG groups did not differ to a statistically significant extent (Figure 2C). Thus, less abdominal adipose tissue and lower plasma TG and NEFA levels were found in AG mice; however, the cholesterol levels in plasma (Figure 3) and liver tissue[15] did not differ to a significantly extent between the two groups.
The central and enteric nervous systems play important roles in gastrointestinal motility and gastric emptying is closely associated with the synthesis of intestinal apolipoproteins[35,36]. Many studies have confirmed that acupuncture has a regulating effect on gastrointestinal motility through the pathways of the nervous system[37]. Food retention was clearly observed in the AG intestine (Figure 2A), suggesting that gastrointestinal motility may be relatively repressed in these mice. Thus, decreased expressions of intestinal MTP and ApoB in AG intestines might be a response to the activation of an ileo-jejunal negative feedback loop caused by weaken gastrointestinal motility[36].
The present study also demonstrated some changes in intestinal morphology. Overloading of nutrients can induce intestinal epithelial cell proliferation[38]; thus, food retention in the lumen and lipid accumulation in the enterocytes may increase the burden of small intestinal metabolism, which contributed to the longer villi in AG mice. Furthermore, intestinal enlargement is often found in obese and/or diabetic animals, which may result from hunger perception-induced neural stimulation[39]. The improvement of metabolic syndrome after acupuncture treatment may normalize the size of the intestine in AG mice. The specific mechanism needs to be confirmed by further studies in the future.
The present study was associated with some limitations. Firstly, the present study did not employ metabolic cage or pair feeding; thus, the quantities of feed and feces were not monitored. Consequently, in addition to the reduced absorption of the small intestine, the positive effect of acupuncture on weight loss may also have been associated with restrained feeding or the promotion of fecal excretion. Secondly, the metabolism of other nutrients also affects the progression of NAFLD, but only lipid-related indicators were observed; thus, the roles of acupuncture in glucose and energy metabolism may also directly or indirectly inhibit intestinal lipid absorption. Finally, the treatment of NAFLD by acupuncture was found to have a short-term (only 2 wk) effect, which led to a dramatic difference in body weight between the two groups. Rapid weight loss may also exacerbate conditions associated with this disease, such as portal fibrosis or necroinflammation, the side effects of acupuncture as a treatment to promote weight control need to be investigated during long-term treatment.
In conclusion, the results of the present study suggest that acupuncture inhibited lipid absorption in the small intestine by downregulating the expression of intestinal apolipoproteins, causing some changes in the intestinal morphology. We suggest that, in addition to the control of the intrahepatic pathological process, acupuncture may improve the outcomes of patients with NAFLD by regulating the systemic metabolism of the body.
Non-alcoholic fatty liver disease (NAFLD) has become a pandemic liver disease in the twenty-first century, and the prevalence of NAFLD is still increasing each year. However, there are still no effective drugs for the therapy of this disease.
Acupuncture is one of the most important external interventions in traditional Chinese medicine (TCM), which has been applied in the treatment of various diseases and symptoms. However, the mechanisms underlying its action remain unclear, which limits its development.
In previous study, we found that acupuncture treatment significantly inhibited the progression of NAFLD by controlling the intrahepatic pathological process. In this study, we investigated the role of acupuncture treatment in the improvement of metabolic syndrome secondary to NAFLD.
Since laboratory experiments cannot be easily carried out on the human body without need, we established a mouse model of NAFLD by administering a classic diet inducing NAFLD. Animal experiments are of great value and can provide a lot of convenience for signal pathways study. According to TCM, we for the first time selected the three acupoints of Zu san li (ST36), Yong quan (KI1) and Guan yuan (CV4) for needling mice in order to the effect of acupuncture treatment in treating NAFLD.
Acupuncture treatment can suppress intestinal lipid absorption by downregulating the expression of apolipoproteins in the small intestine, and then improve obesity and hyperlipidemia. In fact, acupuncture has been widely used in the treatment of obesity in the clinical setting; thus, in view of the reduced lipid absorption, proper nutritional supplementation should be considered throughout the treatment process. Acupuncture treatment normalized the size of the intestine and contributed to the longer villi. The specific mechanism needs to be confirmed by further studies in the future.
We suggest that, in addition to the control of the intrahepatic pathological process, acupuncture may improve the outcomes of patients with NAFLD by regulating the systemic metabolism of the body.
In future study, the quantities of feed and feces need to be monitored by using metabolic cage. In addition to lipid, the role of acupuncture in another nutrients metabolism should be investigated. The side effects of long-term treatment need to be further observed.
We would like to thank Yuka Hiramatsu, Yonenaga Chikako, Yamabe Yukie, Tanaka Asari and Manabu Yamashita for their expert technical assistance and Professor Yasuo Iida from Department of Mathematics for his biostatistics review certificate.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Isik A, Hann HW S-Editor: Zhang H L-Editor: A P-Editor: Zhang YL
| 1. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7517] [Article Influence: 835.2] [Reference Citation Analysis (0)] |
| 2. | Eslam M, Valenti L, Romeo S. Genetics and epigenetics of NAFLD and NASH: Clinical impact. J Hepatol. 2018;68:268-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 732] [Cited by in RCA: 689] [Article Influence: 98.4] [Reference Citation Analysis (0)] |
| 3. | Watt MJ, Miotto PM, De Nardo W, Montgomery MK. The Liver as an Endocrine Organ-Linking NAFLD and Insulin Resistance. Endocr Rev. 2019;40:1367-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 411] [Article Influence: 68.5] [Reference Citation Analysis (2)] |
| 4. | Chen YS, Liu HM, Lee TY. Ursodeoxycholic Acid Regulates Hepatic Energy Homeostasis and White Adipose Tissue Macrophages Polarization in Leptin-Deficiency Obese Mice. Cells. 2019;8:253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 5. | Ko CW, Qu J, Black DD, Tso P. Regulation of intestinal lipid metabolism: current concepts and relevance to disease. Nat Rev Gastroenterol Hepatol. 2020;17:169-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 310] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 6. | Mielenz M. Invited review: nutrient-sensing receptors for free fatty acids and hydroxycarboxylic acids in farm animals. Animal. 2017;11:1008-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Yu XH, Zhang DW, Zheng XL, Tang CK. Cholesterol transport system: An integrated cholesterol transport model involved in atherosclerosis. Prog Lipid Res. 2019;73:65-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 152] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 8. | Zhou W, Benharash P. Effects and mechanisms of acupuncture based on the principle of meridians. J Acupunct Meridian Stud. 2014;7:190-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 9. | Gao Y, Chen R, Liang F. [Mechanisms of acupuncture for non-alcoholic fatty liver disease: researches progress and prospects]. Zhong guo Zhen Jiu. 2018;38:109-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 10. | Wang HY, Liang CM, Cui JW, Pan L, Hu H, Fang HJ. [Acupuncture improves hepatic lipid metabolism by suppressing oxidative stress in obese nonalcoholic fatty liver disease rats]. Zhen Ci Yan Jiu. 2019;44:189-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 11. | Dimitrova A, Murchison C, Oken B. Acupuncture for the Treatment of Peripheral Neuropathy: A Systematic Review and Meta-Analysis. J Altern Complement Med. 2017;23:164-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 105] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 12. | Isik A, Ramanathan R. Approaches to the treatment of pilonidal sinus disease, clinical practice in 2019. Int Wound J. 2020;17:508-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Isik A, Karavas E, Firat D. Spontaneous milk fistula from an axillary accessory breast. Breast J. 2019;25:154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Zhang Y, Li J, Mo G, Liu J, Yang H, Chen X, Liu H, Cai T, Zhang X, Tian X, Zhou Z, Huang W. Acupuncture and Related Therapies for Obesity: A Network Meta-Analysis. Evid Based Complement Alternat Med. 2018;2018:9569685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Meng X, Guo X, Zhang J, Moriya J, Kobayashi J, Yamaguchi R, Yamada S. Acupuncture on ST36, CV4 and KI1 Suppresses the Progression of Methionine- and Choline-Deficient Diet-Induced Nonalcoholic Fatty Liver Disease in Mice. Metabolites. 2019;9:299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 16. | Lai HC, Chang QY, Hsieh CL. Signal Transduction Pathways of Acupuncture for Treating Some Nervous System Diseases. Evid Based Complement Alternat Med. 2019;2019:2909632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Nawata A, Noguchi H, Mazaki Y, Kurahashi T, Izumi H, Wang KY, Guo X, Uramoto H, Kohno K, Taniguchi H, Tanaka Y, Fujii J, Sasaguri Y, Tanimoto A, Nakayama T, Yamada S. Overexpression of Peroxiredoxin 4 Affects Intestinal Function in a Dietary Mouse Model of Nonalcoholic Fatty Liver Disease. PLoS One. 2016;11:e0152549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Yoon HJ, Cha BS. Pathogenesis and therapeutic approaches for non-alcoholic fatty liver disease. World J Hepatol. 2014;6:800-811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 19. | Kim KH, Lee MS. Pathogenesis of Nonalcoholic Steatohepatitis and Hormone-Based Therapeutic Approaches. Front Endocrinol (Lausanne). 2018;9:485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 20. | Ueno T, Sugawara H, Sujaku K, Hashimoto O, Tsuji R, Tamaki S, Torimura T, Inuzuka S, Sata M, Tanikawa K. Therapeutic effects of restricted diet and exercise in obese patients with fatty liver. J Hepatol. 1997;27:103-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 404] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 21. | Zhong YM, Luo XC, Chen Y, Lai DL, Lu WT, Shang YN, Zhang LL, Zhou HY. Acupuncture versus sham acupuncture for simple obesity: a systematic review and meta-analysis. Postgrad Med J. 2020;96:221-227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 22. | Mazidi M, Abbasi-Parizad P, Abdi H, Zhao B, Rahsepar AA, Tavallaie S, Parizadeh SM, Rezaie P, Safariyan M, Nematy M, Mohammadi M, Darbandi M, Darbandi S, Ghayour-Mobarhan M, Ferns GA. The effect of electro-acupuncture on pro-oxidant antioxidant balance values in overweight and obese subjects: a randomized controlled trial study. J Complement Integr Med. 2017;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Shu Q, Chen L, Wu S, Li J, Liu J, Xiao L, Chen R, Liang F. Acupuncture Targeting SIRT1 in the Hypothalamic Arcuate Nucleus Can Improve Obesity in High-Fat-Diet-Induced Rats with Insulin Resistance via an Anorectic Effect. Obes Facts. 2020;13:40-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 24. | Lu M, He Y, Gong M, Li Q, Tang Q, Wang X, Wang Y, Yuan M, Yu Z, Xu B. Role of Neuro-Immune Cross-Talk in the Anti-obesity Effect of Electro-Acupuncture. Front Neurosci. 2020;14:151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | Mu H, Høy CE. The digestion of dietary triacylglycerols. Prog Lipid Res. 2004;43:105-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 415] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 26. | Mansbach CM 2nd, Gorelick F. Development and physiological regulation of intestinal lipid absorption. II. Dietary lipid absorption, complex lipid synthesis, and the intracellular packaging and secretion of chylomicrons. Am J Physiol Gastrointest Liver Physiol. 2007;293:G645-G650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 27. | Chen M, Yang Y, Braunstein E, Georgeson KE, Harmon CM. Gut expression and regulation of FAT/CD36: possible role in fatty acid transport in rat enterocytes. Am J Physiol Endocrinol Metab. 2001;281:E916-E923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 97] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Hennessy AA, Ross RP, Fitzgerald GF, Caplice N, Stanton C. Role of the gut in modulating lipoprotein metabolism. Curr Cardiol Rep. 2014;16:515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Demignot S, Beilstein F, Morel E. Triglyceride-rich lipoproteins and cytosolic lipid droplets in enterocytes: key players in intestinal physiology and metabolic disorders. Biochimie. 2014;96:48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 30. | Hussain MM, Rava P, Walsh M, Rana M, Iqbal J. Multiple functions of microsomal triglyceride transfer protein. Nutr Metab (Lond). 2012;9:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 194] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 31. | Jiang ZG, Liu Y, Hussain MM, Atkinson D, McKnight CJ. Reconstituting initial events during the assembly of apolipoprotein B-containing lipoproteins in a cell-free system. J Mol Biol. 2008;383:1181-1194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Liao W, Chan L. Apolipoprotein B, a paradigm for proteins regulated by intracellular degradation, does not undergo intracellular degradation in CaCo2 cells. J Biol Chem. 2000;275:3950-3956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | Bertolotti A, Wang X, Novoa I, Jungreis R, Schlessinger K, Cho JH, West AB, Ron D. Increased sensitivity to dextran sodium sulfate colitis in IRE1beta-deficient mice. J Clin Invest. 2001;107:585-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 334] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 34. | Iqbal J, Hussain MM. Evidence for multiple complementary pathways for efficient cholesterol absorption in mice. J Lipid Res. 2005;46:1491-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 35. | Glatzle J, Kalogeris TJ, Zittel TT, Guerrini S, Tso P, Raybould HE. Chylomicron components mediate intestinal lipid-induced inhibition of gastric motor function. Am J Physiol Gastrointest Liver Physiol. 2002;282:G86-G91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Mourad FH, Saadé NE. Neural regulation of intestinal nutrient absorption. Prog Neurobiol. 2011;95:149-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 37. | Li H, He T, Xu Q, Li Z, Liu Y, Li F, Yang BF, Liu CZ. Acupuncture and regulation of gastrointestinal function. World J Gastroenterol. 2015;21:8304-8313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 114] [Cited by in RCA: 117] [Article Influence: 11.7] [Reference Citation Analysis (3)] |
| 38. | Mao J, Hu X, Xiao Y, Yang C, Ding Y, Hou N, Wang J, Cheng H, Zhang X. Overnutrition stimulates intestinal epithelium proliferation through β-catenin signaling in obese mice. Diabetes. 2013;62:3736-3746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 39. | Hvid H, Jensen SR, Witgen BM, Fledelius C, Damgaard J, Pyke C, Rasmussen TB. Diabetic Phenotype in the Small Intestine of Zucker Diabetic Fatty Rats. Digestion. 2016;94:199-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |