Published online Sep 7, 2020. doi: 10.3748/wjg.v26.i33.4996
Peer-review started: May 29, 2020
First decision: June 12, 2020
Revised: June 14, 2020
Accepted: August 1, 2020
Article in press: August 1, 2020
Published online: September 7, 2020
Processing time: 97 Days and 14.4 Hours
Liver fat accumulation is associated with increased cholesterol synthesis and hypersecretion of biliary cholesterol, which may be related to the development of cholelithiasis.
To investigate whether liver fat accumulation measured by high-speed T2-corrected multi-echo magnetic resonance spectroscopy (MRS) is a risk factor for cholelithiasis.
Forty patients with cholelithiasis and thirty-one healthy controls were retrospectively enrolled. The participants underwent high-speed T2-corrected multi-echo single-voxel MRS of the liver at a 3T MR scanner. The proton density fat fraction (PDFF) and R2 value were calculated. Serum parameters and waist circumference (WC) were recorded. Spearman’s correlation analysis was used to analyze the relationship between PDFF, R2, and WC values. Multivariate logistic regression analysis was carried out to determine the significant predictors of the risk of cholelithiasis. Receiver operating characteristic curve (ROC) analysis was used to evaluate the discriminative performance of significant predictors.
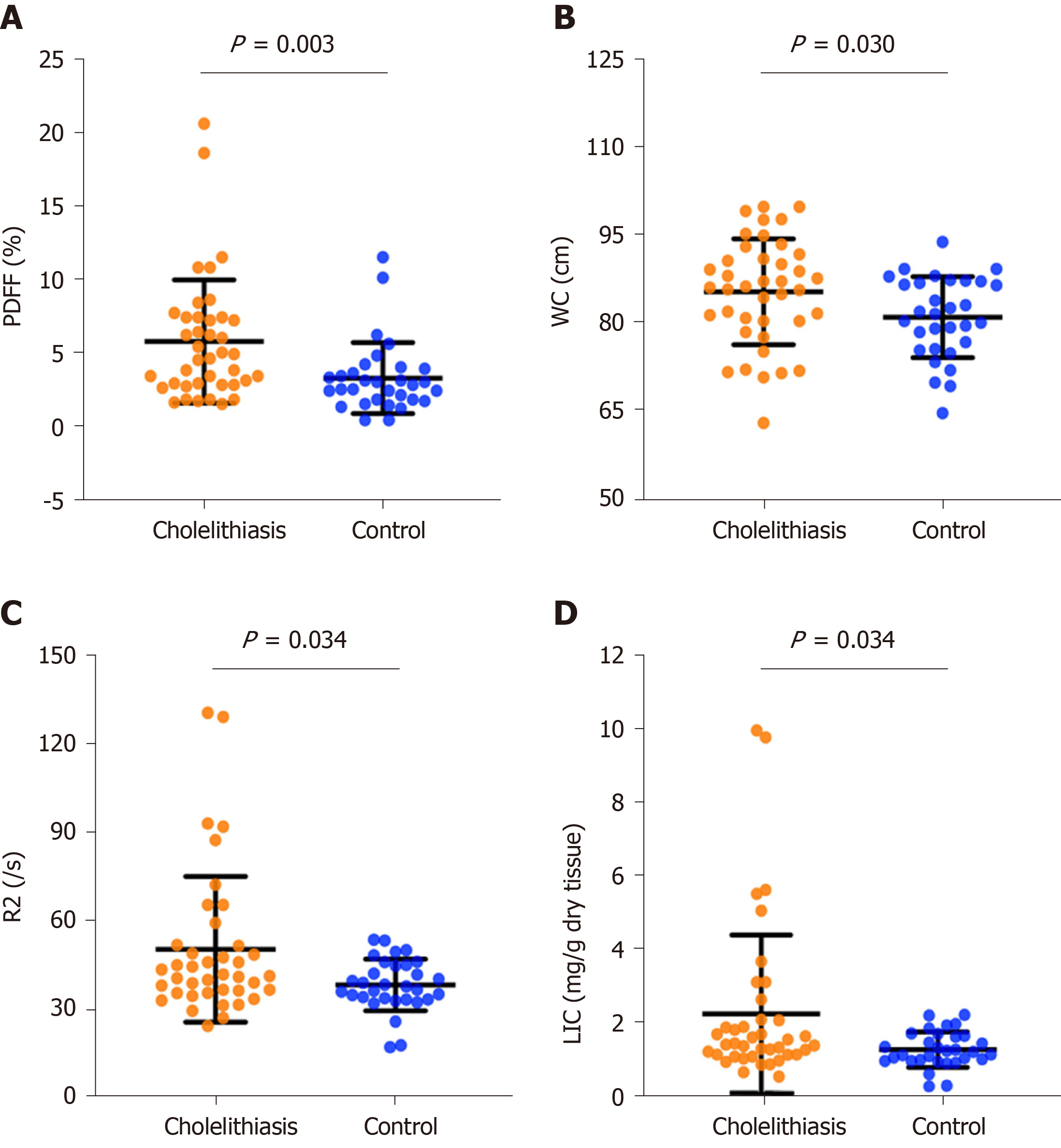
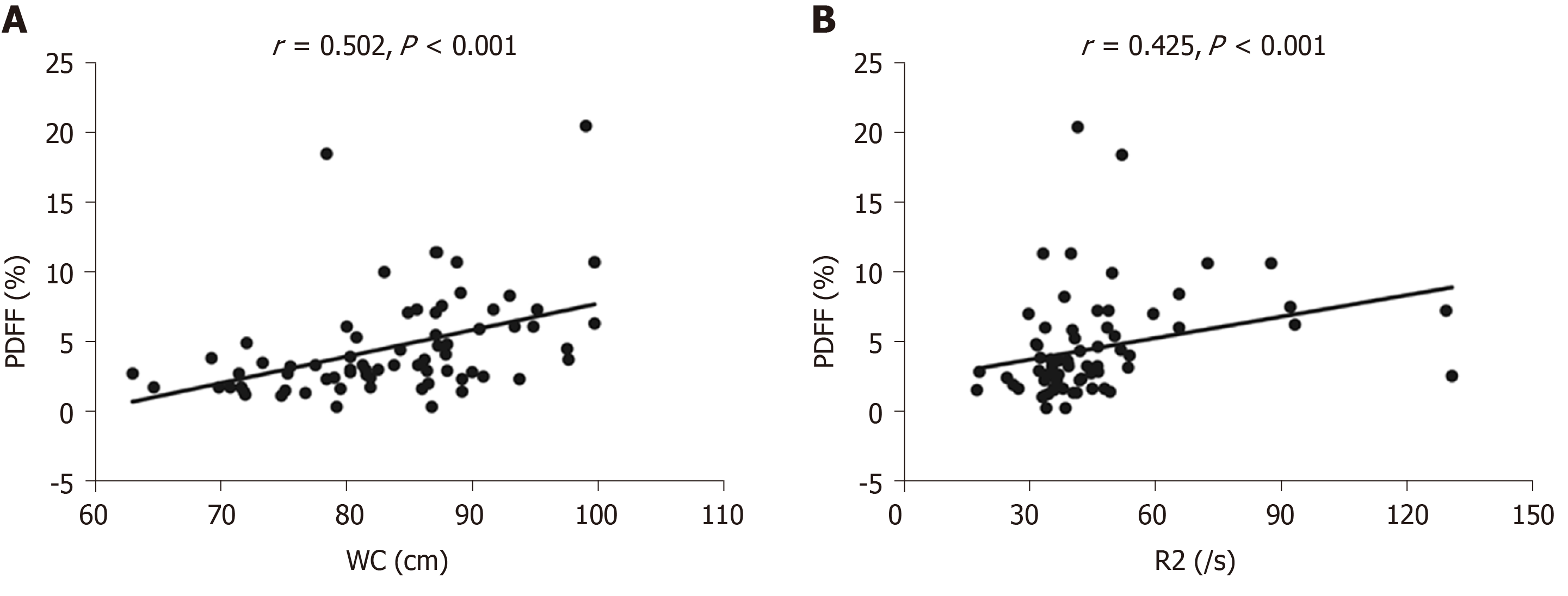
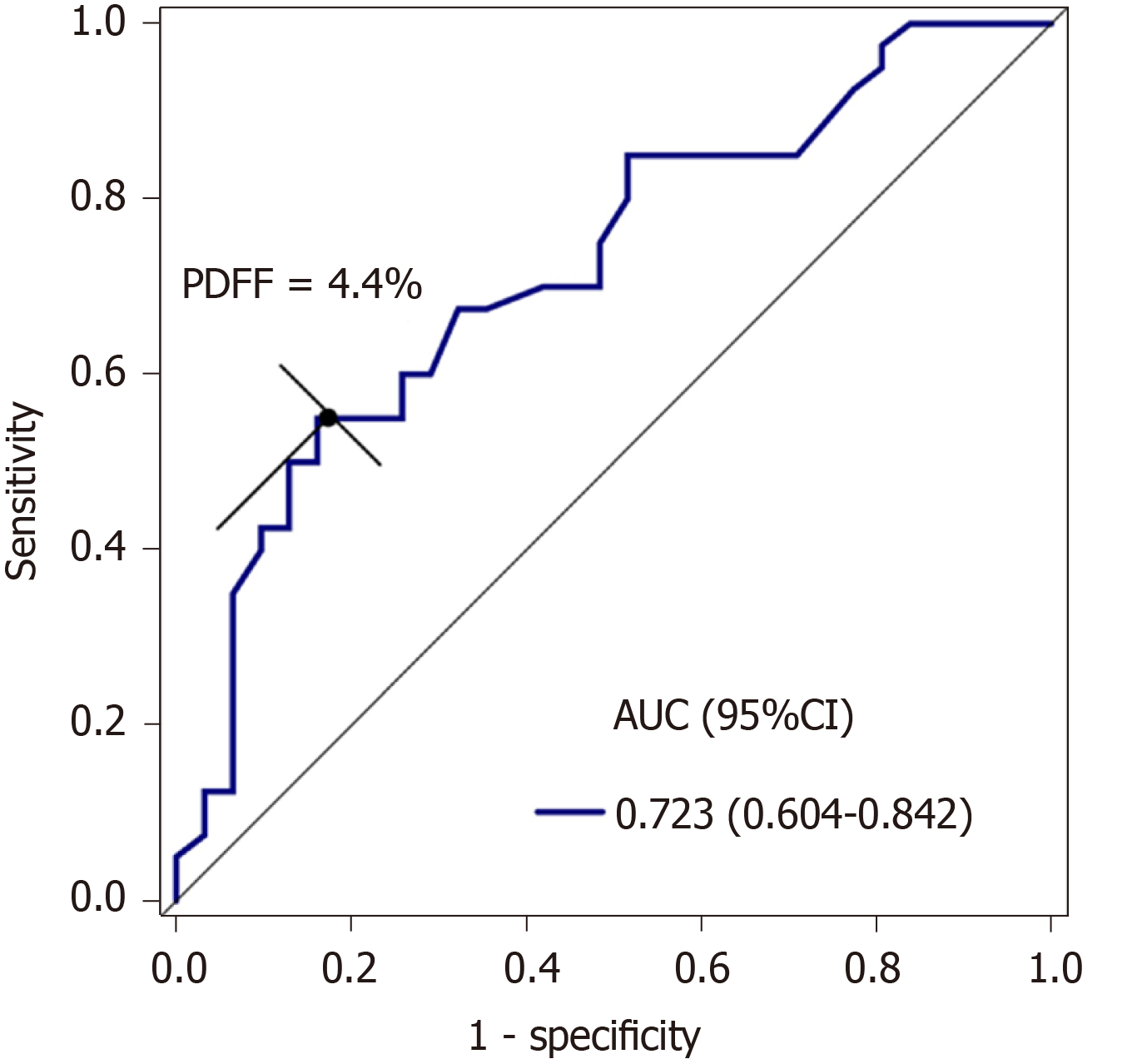
Patients with cholelithiasis had higher PDFF, R2, and WC values compared with healthy controls (5.8% ± 4.2% vs 3.3% ± 2.4%, P = 0.001; 50.4 ± 24.8/s vs 38.3 ± 8.8/s, P = 0.034; 85.3 ± 9.0 cm vs 81.0 ± 6.9 cm, P = 0.030; respectively). Liver iron concentration extrapolated from R2 values was significantly higher in the cholelithiasis group (2.21 ± 2.17 mg/g dry tissue vs 1.22 ± 0.49 mg/g dry tissue, P = 0.034) than in the healthy group. PDFF was positively correlated with WC (r = 0.502, P < 0.001) and R2 (r = 0.425, P < 0.001). Multivariate logistic regression analysis showed that only PDFF was an independent risk factor for cholelithiasis (odds ratio = 1.79, 95%CI: 1.22-2.62, P = 0.003). ROC analysis showed that the area under the curve of PDFF was 0.723 for discriminating cholelithiasis from healthy controls, with a sensitivity of 55.0% and specificity of 83.9% when the cut-off value of PDFF was 4.4%.
PDFF derived from high speed T2-corrected multi-echo MRS can predict the risk of cholelithiasis.
Core tip: The increase in total cholesterol synthesis can cause tissues to be overloaded with fatty acids, resulting in more lithogenic bile due to overproduction of hepatic cholesterol. Our study showed that patients with cholelithiasis had higher liver fat content and R2 value as assessed by high speed T2-corrected multi-echo magnetic resonance spectroscopy (MRS). MRS can be used for quantitative detection of mild liver steatosis, and proton density fat fraction (PDFF) derived from MRS can predict the risk of cholelithiasis. PDFF measured by MRS can quantitatively detect liver steatosis in a simple breath-hold of 15 s, which holds a good potential for clinical application.
- Citation: Chen H, Zeng WK, Shi GZ, Gao M, Wang MZ, Shen J. Liver fat accumulation measured by high-speed T2-corrected multi-echo magnetic resonance spectroscopy can predict risk of cholelithiasis. World J Gastroenterol 2020; 26(33): 4996-5007
- URL: https://www.wjgnet.com/1007-9327/full/v26/i33/4996.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i33.4996
Cholelithiasis is one of the most common and substantial health problems of the digestive system worldwide[1-3]. Although the prognosis of cholelithiasis has improved with advances in surgical and imaging technology, it still constitutes one of the common causes of hospitalization and can lead to cholecystitis, obstructive jaundice, acute pancreatitis, cholangitis, and gallbladder cancer[4]. Clinically, the diagnosis of cholelithiasis is not difficult. However, there has been a considerable effort to identify a surrogate marker to predict the risk of cholelithiasis. Previously, abdominal visceral fat accumulation[5-7] and excessive heme iron consumption[8] were found to be related to a higher risk of gallstone disease. Liver fat accumulation associated with increased body cholesterol synthesis and hypersecretion of biliary cholesterol may also be related to cholelithiasis development[7], and nonalcoholic fatty liver disease (NAFLD) is an independent risk factor for cholelithiasis development[9-11] as well as gallstone-related diseases with an odds ratio (OR) of 3.6[12].
Currently, liver biopsy is the gold standard for evaluating liver fat accumulation. However, biopsy is an invasive procedure limited by sampling bias and variability among observers and intra-observers; moreover, it may also lead to complications such as bleeding and pseudoaneurysms[13]. Computed tomography (CT)[14] and ultrasonography[15] have been previously used to detect liver steatosis. CT measures liver fat content based on the ratio of Hounsfield units of the liver and kidney parenchyma and detects liver iron overload by showing increased attenuation[16]. However, both CT and ultrasonography have limited accuracy. Moreover, patients undergoing CT examination have the risk of radiation exposure. Recently, magnetic resonance imaging (MRI) has become a noninvasive method for quantifying liver fat content based on the difference between water and fat resonance frequencies[17]. Chemical shift imaging based on the two-point Dixon method has been widely used to analyze fat content by a simple algebraic operation of in-phase and opposed-phase images[18]. However, this method is susceptible to the effect of magnetic field inhomogeneity. The latest modified Dixon technique with six echoes and T2* correction (multi-echo Dixon) can obtain high-quality whole liver 3D proton density fat fraction (PDFF) and R2* maps[19]. However, confounding factors, such as T1 bias and T2* decay, require correction for more accurate quantification[20].
Advanced magnetic resonance spectroscopy (MRS) has been shown to be a robust method for quantifying liver fat and iron accumulation[21-24]. The multi-echo single-voxel stimulated echo acquisition mode (STEAM) spectroscopy sequence minimizes the influence of T1 relaxation and corrects the T2 effect of different echo times (TEs), and can achieve satisfactory quantification of liver fat and iron[25]. This technique measures the PDFF by the exponential fit of five echoes to infer the area under the fat and water peak at TE = 0 ms, the fraction of liver proton density attributable to liver fat, which is a basic tissue characteristic and a direct method for measuring liver fat content. It can quantify liver fat in a single breath-hold of 15 s and can accurately quantify liver iron with R2 values[22,26]. Nowadays, multi-echo MRS is regarded as the non-invasive gold standard for quantifying liver fat and iron content[27,28]. However, whether liver fat and iron content as assessed by multi-echo MRS are predictive of cholelithiasis remains to be determined.
In the present study, high-speed T2-corrected multi-echo MRS was performed in patients with cholelithiasis to quantify liver fat and iron contents. The purpose of this study was to investigate whether liver fat content measured by PDFF derived from high-speed T2-corrected multi-echo MRS can predict the risk of cholelithiasis.
The study was approved by the Institutional Review Board of our hospital (approval No. SYSEC-KY-KS-2020-047). Informed consent from each participant was waived because of the retrospective nature of the study. Patients with cholelithiasis including gallstones or cholangiolithiasis between March 2019 and November 2019 were identified from our hospital database. Patients were included if they had cholelithiasis confirmed by ultrasonography, and underwent liver MRI examination including high-speed T2-corrected multi-echo MRS. The exclusion criteria were as follows: Age < 18 years, alcohol consumption of more than 30 g per day within the preceding 10 years or more than 10 g per day in the previous year, liver cirrhosis caused by drugs or hepatitis virus, chronic liver disease, liver inflammation, liver tumors, history of liver surgery, history of diabetes, medication for hypertension, and malignant tumors of other organs. The age-matched healthy adults were included as controls. The inclusion criteria for the control group were as follows: Age > 18 years and no history of gallbladder disease, liver tumor, liver inflammation, cirrhosis, or liver surgery. The exclusion criteria were: Unavailable information on alcohol consumption and history of advanced cancer, diabetes, or hypertension. Finally, 40 patients with cholelithiasis (aged 54.8 ± 14.6 years, 23 men and 17 women) and 31 healthy controls (aged 50.6 ± 14.3 years, 21 men and 10 women) were included in the study.
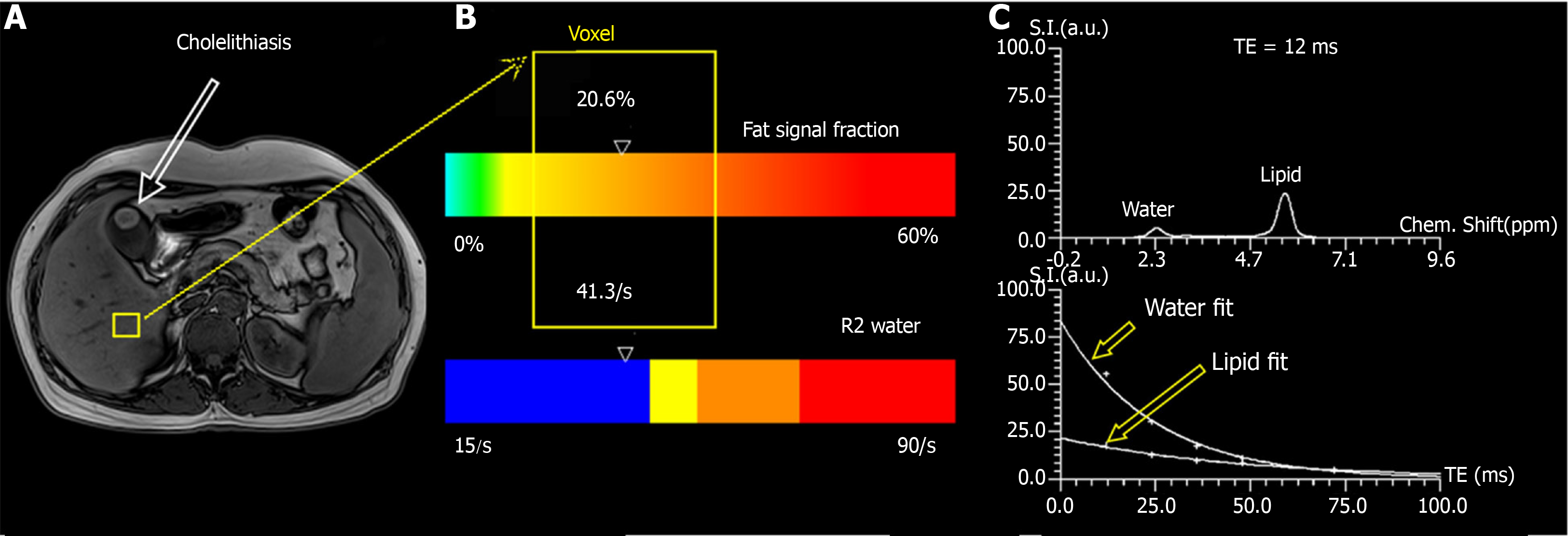
MRI was performed on a 3T MR scanner (MAGNETOM Skyra; Siemens Healthcare, Erlangen, Germany), using an 18-channel phased-array surface coil. MRI sequences included conventional MRI and high-speed T2-corrected multi-echo single-voxel 1H-MRS. The participants underwent conventional MRI prior to MRS acquisition to position the voxel. This sequence was performed in a single breath-hold of 15 s. The acquisition parameters were as follows: Echo time (TE) = 12, 24, 36, 48, and 72 ms, repetition time (TR) = 3000 ms, and flip angle = 90°. STEAM was used because of its inherently short TE[13] and can provide more consistent fat fraction estimates due to J-coupling effects compared with the point-resolved spectroscopy (PRESS) model[23]. A total of 1024 sampling points were acquired at a bandwidth of 1200 Hz. The voxel size was 30 mm × 30 mm × 30 mm. An experienced MRI technologist placed the voxel in the right lobe of the liver, avoiding blood vessels, bile ducts, chemical displacement artifacts, and fluctuations. This technique uses signal integrals from water and lipid spectrum fits to estimate T2 decay and assess the equilibrium signal at TE of 0 ms[22]. The obtained MRS data were post-processed inline by using the prototype software package (Siemens Healthcare, Erlangen, Germany), and a spectrum with T2 correction, a table report with the quantitative information (fat fraction, and R2 value of water and fit error), PDFF, and R2 map were obtained automatically[19] (Figure 1).
Serum alanine aminotransferase, gamma glutamyl transpeptidase (GGT), aspartate aminotransferase, total bilirubin, alkaline phosphatase, serum uric acid, serum uric glucose, total cholesterol, triglycerides, serum iron, serum iron saturation, serum ferritin, transferrin, and hemoglobin were measured using standard reagents. Waist circumference (WC) was measured horizontally at the level of the umbilicus on the MR images for each participant. The time between laboratory examination and MR examination was within one week.
Numerical variables are presented as the mean ± SD. Fisher’s exact test was used for comparing categorical variables. Data distributions were tested for normality by Shapiro-Wilk test. Mean values were compared between the cholelithiasis group and control group by either Student’s t-test (when data were normally distributed) or non-parametric Mann-Whitney U-test (when data were not normally distributed). Spearman’s correlation was used to analyze the relationship between MRI-based PDFF, R2, and WC. Univariate logistic regression analysis, followed by multivariate logistic regression analysis, was performed to determine independent predictors of cholelithiasis. Receiver operating characteristic curve (ROC) analysis was used to evaluate the performance of significant parameters in discriminating cholelithiasis from healthy controls. The optimal cut-off value was determined according to the Youden index. A P value < 0.05 indicated statistical significance (two tailed). All statistical analyses were performed using SPSS (Chicago, IL, United States, version 23.0).
The demographic and clinical characteristics of the study population are shown in Table 1. In the cholelithiasis group, mean WC ranged from 62.9 cm to 99.1 cm. In the healthy group, WC ranged from 64.6 cm to 93.8 cm. WC was significantly higher in the cholelithiasis group than in the healthy group (85.3 ± 9.0 cm vs 81.0 ± 6.9 cm, P = 0.030). Mean GGT level was significantly higher in patients with cholelithiasis than in the healthy group (246.3 ± 317.8 U/L vs 103.8 ± 146.4 U/L, P = 0.037). The normal range of GGT level is 45-125 U/L. There were no significant differences in other biochemical findings between the cholelithiasis group and healthy controls.
| Characteristic | Cholelithiasis (n = 40) | Control (n = 31) | P value |
| Demographic | |||
| Age (yr) | 54.8 ± 14.6 | 50.6 ± 14.3 | 0.235 |
| Women | 42.5% (17/40) | 32.3% (10/31) | 0.463 |
| Anthropometric | |||
| Waist circumference (cm) | 85.3 ± 9.0 | 81.0 ± 6.9 | 0.030a |
| Biochemical | |||
| ALT (U/L) | 84.6 ± 115.9 | 69.5 ± 156.2 | 0.2261 |
| AST (U/L) | 61.0 ± 94.9 | 52.9 ± 75.5 | 0.3301 |
| TBIL (µmol/L) | 29.4 ± 56.6 | 19.5 ± 17.6 | 0.9211 |
| GGT (U/L) | 246.3 ± 317.8 | 103.8 ± 146.4 | 0.0371, a |
| ALP (U/L) | 146.3 ± 122.8 | 125.2 ± 92.1 | 0.3821 |
| Serum uric acid (µmol/L) | 332.0 ± 121.0 | 375.9 ± 107.4 | 0.1901 |
| Serum uric glucose (mmol/L) | 5.6 ± 1.7 | 6.3 ± 3.6 | 0.6061 |
| Total cholesterol (mg/L) | 5.2 ± 1.5 | 5.1 ± 1.2 | 0.6621 |
| Triglycerides (mmol/L) | 2.0 ± 1.7 | 1.2 ± 0.5 | 0.0741 |
| Serum iron (µmol/L) | 13.8 ± 6.2 | 18.0 ± 6.5 | 0.068 |
| Serum iron saturation (%) | 28.1 ± 12.0 | 35.4 ± 11.3 | 0.085 |
| Serum ferritin (ug/L) | 496.3 ± 335.0 | 328.5 ± 302.3 | 0.0741 |
| Transferrin (g/L) | 2.0 ± 0.4 | 2.1 ± 0.5 | 0.698 |
| Hemoglobin (g/L) | 127.8 ± 19.6 | 128.5 ± 19.4 | 0.886 |
| MRI | |||
| PDFF (%) | 5.8 ± 4.2 | 3.3 ± 2.4 | 0.0011, a |
| R2 (/s) | 50.4 ± 24.8 | 38.3 ± 8.8 | 0.0341, a |
In the cholelithiasis group, PDFF ranged from 1.5% to 20.6%, and R2 values ranged from 24.4/s to 129.4/s. In the healthy group, PDFF ranged from 0.4% to 11.5%, and R2 values ranged from 17.2/s to 53.7/s. Mean PDFF (5.8% ± 4.2% vs 3.3% ± 2.4%, P = 0.001) and mean R2 (50.4 ± 24.8/s vs 38.3 ± 8.8/s, P = 0.034) values were significantly higher in the cholelithiasis group than in the healthy group. The values of liver iron concentration (LIC) extrapolated from R2 values were calculated based on the known iron calibration equation[29,30]. In the cholelithiasis group, LIC ranged from 0.48 mg/g to 10.01 mg/g dry tissue. In the healthy group, LIC ranged from 0.21 mg/g to 2.18 mg/g dry tissue. Mean LIC values were significantly higher in the cholelithiasis group (2.21 ± 2.17 mg/g dry tissue vs 1.22 ± 0.49 mg/g dry tissue, P = 0.034) than in the healthy group (Figure 2).
Correlation analysis showed that there was a positive correlation between PDFF and WC (r = 0.502, P < 0.001) and PDFF was positively correlated with R2 (r = 0.425, P < 0.001) (Figure 3 and Table 2). Univariate logistic regression analysis showed that PDFF, R2, WC, and GGT were significantly associated with cholelithiasis (P < 0.05). PDFF, R2, WC, and GGT were chosen for multivariate logistic regression analysis and only PDFF was a significant independent risk factor for predicting cholelithiasis (P = 0.003, OR: 1.79, 95%CI: 1.22-2.62).
| Variable | Univariate analysis | Multivariate analysis | ||
| Odds ratio (95%CI) | P value | Odds ratio (95%CI) | P value | |
| PDFF | 1.33 (1.07-1.65) | < 0.01 | 1.79 (1.22-2.62) | 0.003a |
| R2 | 1.05 (1.01-1.10) | < 0.05 | - | - |
| WC | 1.07 (1.01-1.14) | < 0.05 | - | - |
| GGT | 1.01 (1.00-.1.01) | < 0.05 | - | - |
ROC analysis showed that the area under the curve (AUC) of PDFF was 0.723. With an optimal threshold of 4.4%, PDFF had a sensitivity and specificity of 55.0% and 83.9%, respectively, for discriminating cholelithiasis from healthy controls (Figure 4).
Our study showed that patients with cholelithiasis had a higher liver fat content and R2 value as assessed by high speed T2-corrected multi-echo MRS. Liver fat fraction rather than R2 or WC was a significant independent risk factor for cholelithiasis. To our knowledge, this is the first study to show that liver fat accumulation is a risk factor for cholelithiasis using quantitative MRS.
There is a high prevalence of liver steatosis in cholelithiasis patients. Roldan-Valadez et al[3] found that approximately half of patients with symptomatic cholelithiasis have liver steatosis histologically, when a cut-off value > 5% in total lipid content of liver biopsies is defined as liver steatosis[31]. It is known that an increase in total cholesterol synthesis in the body can cause tissues to be overloaded with fatty acids, resulting in more lithogenic bile by the overproduction of hepatic cholesterol[10]. The increase in cholesterol synthesis and the high secretion of biliary cholesterol might cause the accumulation of liver fat, and may be related to the occurrence of cholelithiasis[6]. Thus, it is important to monitor liver fat content longitudinally in patients with liver fat accumulation. Previously, GGT was shown to be a surrogate indicator for liver fat accumulation in middle-aged adults in China[32]. A large-scale longitudinal cohort study using ultrasound imaging in 283446 Korean adults demonstrated a bidirectional association between liver steatosis and gallstones[33]. Furthermore, Koller et al[34] demonstrated that NAFLD may represent a pathogenic association between metabolic syndrome and cholelithiasis[34]. Our results showed that patients with cholelithiasis had an average PDFF of 5.8%, which was higher than that in healthy controls. This is consistent with the results of previous findings[3,10,11]. Compared with traditional ultrasound and CT examination, MRS can be used for quantitative detection of mild liver steatosis[17], especially in patients with grade 1 steatosis. PDFF measured by MRS could quantitatively detect liver steatosis in a simple breath-hold of 15 s, which showed a good potential for clinical application.
Abdominal visceral fat accumulation is a commonly used measure to predict the risk of cholelithiasis. A multi-ethnic study of atherosclerosis previously showed that WC could provide the best discrimination for NAFLD in the total population[35]. WC has exhibited potential use in monitoring changes in visceral adipose tissue deposits, which convey the greatest health risks[36]. Similarly, previous studies have also shown that WC predicts the risk of developing gallstones in United States men[37] and Chinese adults[5], and was strongly associated with liver fat in women in a white German population[38]. Cholelithiasis and liver steatosis may share a common risk factor such as obesity, which can be assessed quantitatively by WC. In our study, patients with cholelithiasis also had higher WC, and PDFF was positively correlated with WC. Moreover, our study demonstrated that only PDFF was an independent predictor of cholelithiasis by multiple logistic regression analysis with an OR of 1.79 and an AUC of 0.723 in discriminating cholelithiasis from healthy controls. Notably, although WC is an easily achievable indicator of obesity, it is insensitive in the detection of mild liver steatosis. Ding et al[32] also found no significant correlation between WC and the risk of coronary stenosis after multivariable adjustment and liver fat accumulation may be more important in predicting subclinical coronary atherosclerosis than general and abdominal fat accumulation. Previous studies have demonstrated that PDFF derived from MRS had an accuracy of 100% in assessing fat concentration in patients with symptomatic cholelithiasis histologically[3] and could be used for the quantification of varying degrees of hepatic steatosis in histology[39,40]. Taken together, these results highlight the superiority of MRS over morphometric assessment when assessing and monitoring mild liver steatosis in cholelithiasis.
In our study, patients with cholelithiasis had a mild magnitude of increase in PDFF and LIC than normal controls. Iron overload has the ability to synergistically upregulate the level of ferritin and fat accumulation in an intact organism, e.g., Caenorhabditis elegans, thus providing experimental evidence supporting the link between iron and obesity[41]. In addition, a previous study has also demonstrated that liver iron load influences hepatic fat fraction in dialysis patients who routinely received erythropoiesis-stimulating agents and iron therapy, and iron overload induced by iron therapy may aggravate or trigger NAFLD in dialysis patients[42]. This study confirmed the ability of iatrogenic iron overload in dialysis patients to induce an increase of liver fat fraction and its regression with the normalization of LIC. Taken together, iron products may have an adverse effect on the pathophysiology of NAFLD.
In our study, patients with cholelithiasis also showed higher R2 values. Both R2 relaxometry and R2* relaxometry can be used to determine LIC. R2 relaxometry shows an excellent correlation with the LIC but requires a long imaging time (approximately 20 min). The R2* relaxation method shows excellent reproducibility, but the fact that sequence parameters and image analysis programs differ in many studies has been described as a disadvantage[43]. Liver iron plays an important role in the development of non-alcoholic steatohepatitis (NASH)[38]. Iron overload itself can cause liver cell injury, and any hepatocyte injury can also lead to iron accumulation[44]. The co-existence of increased liver fat can further increase the risk of NASH development, and even promoted the development of liver fibrosis and hepatocellular carcinoma[29,45]. Our study demonstrated a positive correlation between PDFF and R2 value. Similarly, Mamidipalli et al[46] found an association between R2* and PDFF. In their study, a chemical shift-based imaging approach, other than multi-echo T2-corrected MRS, was used to analyze liver fat content. Karlsson et al[26] also demonstrated a positive linear correlation between PDFF and R2* in chronic liver disease after excluding data from patients who had elevated iron levels. However, we measured PDFF using the STEAM model, whereas Karlsson et al[26] measured PDFF using the PRESS sequence[26]. Compared with PRESS, the STEAM model adds a damage gradient to eliminate the residual magnetization vector before each 90° pulse, thereby reducing approximately half of the TE value to obtain the attenuation curve of water and fat. This sequence allows for clearer separation of water and lipid in the spectrum signal[23,47] and provides a more consistent fat fraction estimate compared to PRESS[47]. A previous study demonstrated that liver fat content was an influential covariate of liver R2* value in a population with NAFLD, regardless of field strength[48]. T2-corrected MRS has been shown to more accurately simulate the variable T2 effects as evaluated using eight lipid phantoms doped with iron and is considered an accurate and repeatable method for noninvasive liver lipid quantification[22]. Collectively, MRS can be used as a quantitative tool to simultaneously monitor the liver fat content and hepatic iron overload[29].
Our study has some limitations. First, the sample size was relatively small as patients were recruited from one institution. A large population from multiple centers is needed for further validation of our findings. Second, liver biopsy was not performed in our study. Previous studies have shown that there is a correlation between MRS-PDFF and lipid content, and MRS-PDFF can reliably replace liver biopsy to evaluate liver fat content[3]. Although MRS cannot determine the size of fat droplets, it does determine the total amount of liver fat. Moreover, liver biopsy is unsafe and unethical in healthy participants. Third, MRS assesses liver PDFF and R2 in a single voxel. Measurement of fat and iron content from the entire liver might be suitable for heterogeneous liver steatosis. In such circumstances, multiple region of interests of MRS placed in different lobes of the liver can be adopted as a potential solution.
In conclusion, our study has demonstrated that PDFF measured by high speed T2-corrected multi-echo MRS is associated with cholelithiasis and is an independent risk factor for cholelithiasis. High speed T2-corrected multi-echo MRS can be used to detect liver fat accumulation to predict the risk of cholelithiasis development.
Cholelithiasis is one of the most common and substantial health problems of the digestive system worldwide. Liver biopsy is the gold standard for evaluating liver fat accumulation, however, biopsy is an invasive procedure limited by sampling bias and variability among observers and intra-observers. Computed tomography (CT) and ultrasonography have been previously used to detect liver steatosis. However, both CT and ultrasonography have limited accuracy. Advanced magnetic resonance spectroscopy (MRS) has been shown to be a robust method for quantifying liver fat and iron accumulation. Whether liver fat and iron content as assessed by multi-echo MRS are predictive of cholelithiasis remains to be determined.
The increase in total cholesterol synthesis can cause tissues to be overloaded with fatty acids, resulting in more lithogenic bile due to overproduction of hepatic cholesterol. The multi-echo single-voxel stimulated echo acquisition mode (STEAM) spectroscopy sequence minimizes the influence of T1 relaxation and corrects the T2 effect of different echo times, and can achieve satisfactory quantification of liver fat and iron, which may be helpful to diagnosis mild liver steatosis in patients with cholelithiasis.
To investigate whether liver fat accumulation measured by high-speed T2-corrected multi-echo MRS is a risk factor for cholelithiasis. The findings obtained can provide information for predicting the risk of cholelithiasis by using MRS.
This study retrospectively enrolled 40 patients with cholelithiasis (mean age: 54.8 ± 14.6 years) and 31 healthy controls (mean age: 50.6 ± 14.3 years) who underwent high-speed T2-corrected multi-echo single-voxel MRS. Magnetic resonance imaging was performed on a 3T MR scanner (MAGNETOM Skyra; Siemens Healthcare, Erlangen, Germany). The proton density fat fraction (PDFF), R2 value, and waist circumference (WC) were calculated. Spearman’s correlation analysis was used to analyze the relationship between PDFF, R2 and WC values. Significant predictors of the risk of cholelithiasis were determined by multivariate logistic regression analysis. Receiver operating characteristic curve analysis was used to evaluate the discriminative performance of significant predictors.
Patients with cholelithiasis had higher PDFF (5.8% ± 4.2% vs 3.3% ± 2.4%, P = 0.001), R2 value (50.4 ± 24.8/s vs 38.3 ± 8.8/s, P = 0.034), and WC value (85.3 ± 9.0 cm vs 81.0 ± 6.9 cm, P = 0.030) compared with healthy controls. PDFF was positively correlated with WC (r = 0.502, P < 0.001) and R2 (r = 0.425, P < 0.001). Only PDFF was an independent risk factor for cholelithiasis (P = 0.003, OR: 1.79, 95%CI: 1.22-2.62), and the area under the curve of PDFF was 0.723 for discriminating cholelithiasis from healthy controls at the cut-off value of PDFF was 4.4%. However, due to the relatively small sample size, a large population from multiple centers is needed for further validation of our findings.
PDFF measured by high speed T2-corrected multi-echo MRS is associated with cholelithiasis. MRS can be used as a quantitative tool to simultaneously monitor the liver fat content and hepatic iron overload.
This study describes that PDFF derived from high speed T2-corrected multi-echo MRS can predict the risk of cholelithiasis. MRS can quantitatively detect liver steatosis in a simple breath-hold of 15s, which holds a good potential for clinical application.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rostoker G S-Editor: Yan JP L-Editor: Wang TQ P-Editor: Ma YJ
| 1. | Matos C. Will Dual-Energy CT Become the Reference Standard to Evaluate Gallstone Disease? Radiology. 2019;292:407-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Khan M, Kazi TG, Afridi HI, Sirajuddin, Bilal M, Akhtar A, Khan S, Kadar S. Variation of calcium, copper and iron levels in serum, bile and stone samples of patients having different types of gallstone: A comparative study. Clin Chim Acta. 2017;471:254-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Roldan-Valadez E, Favila R, Martínez-López M, Uribe M, Ríos C, Méndez-Sánchez N. In vivo 3T spectroscopic quantification of liver fat content in nonalcoholic fatty liver disease: Correlation with biochemical method and morphometry. J Hepatol. 2010;53:732-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | He H, Tan C, Wu J, Dai N, Hu W, Zhang Y, Laine L, Scheiman J, Kim JJ. Accuracy of ASGE high-risk criteria in evaluation of patients with suspected common bile duct stones. Gastrointest Endosc. 2017;86:525-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 5. | Stinton LM, Shaffer EA. Epidemiology of gallbladder disease: cholelithiasis and cancer. Gut Liver. 2012;6:172-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 556] [Cited by in RCA: 737] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 6. | Sekine K, Nagata N, Sakamoto K, Arai T, Shimbo T, Shinozaki M, Okubo H, Watanabe K, Imbe K, Mikami S, Nozaki Y, Sakurai T, Yokoi C, Kojima Y, Kobayakawa M, Yanase M, Akiyama J, Noda M, Uemura N. Abdominal visceral fat accumulation measured by computed tomography associated with an increased risk of gallstone disease. J Gastroenterol Hepatol. 2015;30:1325-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Stender S, Nordestgaard BG, Tybjaerg-Hansen A. Elevated body mass index as a causal risk factor for symptomatic gallstone disease: a Mendelian randomization study. Hepatology. 2013;58:2133-2141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 8. | Tsai CJ, Leitzmann MF, Willett WC, Giovannucci EL. Heme and non-heme iron consumption and risk of gallstone disease in men. Am J Clin Nutr. 2007;85:518-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Alvaro D. Gallstones: Bad Company for the Steatotic Liver. Gastroenterology. 2017;152:1284-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Jaruvongvanich V, Sanguankeo A, Upala S. Significant Association Between Gallstone Disease and Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Dig Dis Sci. 2016;61:2389-2396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 11. | Liu J, Lin H, Zhang C, Wang L, Wu S, Zhang D, Tang F, Xue F, Liu Y. Non-alcoholic fatty liver disease associated with gallstones in females rather than males: a longitudinal cohort study in Chinese urban population. BMC Gastroenterol. 2014;14:213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Reddy SK, Zhan M, Alexander HR, El-Kamary SS. Nonalcoholic fatty liver disease is associated with benign gastrointestinal disorders. World J Gastroenterol. 2013;19:8301-8311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Zhan C, Olsen S, Zhang HC, Kannengiesser S, Chandarana H, Shanbhogue KP. Detection of hepatic steatosis and iron content at 3 Tesla: comparison of two-point Dixon, quantitative multi-echo Dixon, and MR spectroscopy. Abdom Radiol (NY). 2019;44:3040-3048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Idilman IS, Akata D, Hazirolan T, Doganay Erdogan B, Aytemir K, Karcaaltincaba M. Nonalcoholic fatty liver disease is associated with significant coronary artery disease in type 2 diabetic patients: a computed tomography angiography study 2. J Diabetes. 2015;7:279-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Kim BJ, Cheong ES, Kang JG, Kim BS, Kang JH. Relationship of epicardial fat thickness and nonalcoholic fatty liver disease to coronary artery calcification: From the CAESAR study. J Clin Lipidol. 2016;10:619-626.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Labranche R, Gilbert G, Cerny M, Vu KN, Soulières D, Olivié D, Billiard JS, Yokoo T, Tang A. Liver Iron Quantification with MR Imaging: A Primer for Radiologists. Radiographics. 2018;38:392-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 119] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 17. | Zhao YZ, Gan YG, Zhou JL, Liu JQ, Cao WG, Cheng SM, Bai DM, Wang MZ, Gao FQ, Zhou SM. Accuracy of multi-echo Dixon sequence in quantification of hepatic steatosis in Chinese children and adolescents. World J Gastroenterol. 2019;25:1513-1523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 18. | Satkunasingham J, Besa C, Bane O, Shah A, de Oliveira A, Gilson WD, Kannengiesser S, Taouli B. Liver fat quantification: Comparison of dual-echo and triple-echo chemical shift MRI to MR spectroscopy. Eur J Radiol. 2015;84:1452-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Hetterich H, Bayerl C, Peters A, Heier M, Linkohr B, Meisinger C, Auweter S, Kannengießer SA, Kramer H, Ertl-Wagner B, Bamberg F. Feasibility of a three-step magnetic resonance imaging approach for the assessment of hepatic steatosis in an asymptomatic study population. Eur Radiol. 2016;26:1895-1904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Yoo H, Lee JM, Yoon JH, Kang HJ, Lee SM, Yang HK, Han JK. T2* Mapping from Multi-Echo Dixon Sequence on Gadoxetic Acid-Enhanced Magnetic Resonance Imaging for the Hepatic Fat Quantification: Can It Be Used for Hepatic Function Assessment? Korean J Radiol. 2017;18:682-690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Henninger B, Zoller H, Kannengiesser S, Zhong X, Jaschke W, Kremser C. 3D Multiecho Dixon for the Evaluation of Hepatic Iron and Fat in a Clinical Setting. J Magn Reson Imaging. 2017;46:793-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Pineda N, Sharma P, Xu Q, Hu X, Vos M, Martin DR. Measurement of hepatic lipid: high-speed T2-corrected multiecho acquisition at 1H MR spectroscopy--a rapid and accurate technique. Radiology. 2009;252:568-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 123] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 23. | Meisamy S, Hines CD, Hamilton G, Sirlin CB, McKenzie CA, Yu H, Brittain JH, Reeder SB. Quantification of hepatic steatosis with T1-independent, T2-corrected MR imaging with spectral modeling of fat: blinded comparison with MR spectroscopy. Radiology. 2011;258:767-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 317] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 24. | Zhong X, Nickel MD, Kannengiesser SA, Dale BM, Kiefer B, Bashir MR. Liver fat quantification using a multi-step adaptive fitting approach with multi-echo GRE imaging. Magn Reson Med. 2014;72:1353-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 175] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 25. | Boudinaud C, Abergel A, Joubert-Zakeyh J, Fontarensky M, Pereira B, Chauveau B, Garcier JM, Chabrot P, Boyer L, Magnin B. Quantification of steatosis in alcoholic and nonalcoholic fatty liver disease: Evaluation of four MR techniques versus biopsy. Eur J Radiol. 2019;118:169-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Karlsson M, Ekstedt M, Dahlström N, Forsgren MF, Ignatova S, Norén B, Dahlqvist Leinhard O, Kechagias S, Lundberg P. Liver R2* is affected by both iron and fat: A dual biopsy-validated study of chronic liver disease. J Magn Reson Imaging. 2019;50:325-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Uhrig M, Mueller J, Longerich T, Straub BK, Buschle LR, Schlemmer HP, Mueller S, Ziener CH. Susceptibility based multiparametric quantification of liver disease: Non-invasive evaluation of steatosis and iron overload. Magn Reson Imaging. 2019;63:114-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Begovatz P, Koliaki C, Weber K, Strassburger K, Nowotny B, Nowotny P, Müssig K, Bunke J, Pacini G, Szendrödi J, Roden M. Pancreatic adipose tissue infiltration, parenchymal steatosis and beta cell function in humans. Diabetologia. 2015;58:1646-1655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 29. | Lin H, Fu C, Kannengiesser S, Cheng S, Shen J, Dong H, Yan F. Quantitative analysis of hepatic iron in patients suspected of coexisting iron overload and steatosis using multi-echo single-voxel magnetic resonance spectroscopy: Comparison with fat-saturated multi-echo gradient echo sequence. J Magn Reson Imaging. 2018;48:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | St Pierre TG, Clark PR, Chua-anusorn W, Fleming AJ, Jeffrey GP, Olynyk JK, Pootrakul P, Robins E, Lindeman R. Noninvasive measurement and imaging of liver iron concentrations using proton magnetic resonance. Blood. 2005;105:855-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 663] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 31. | Lupsor M, Badea R. Imaging diagnosis and quantification of hepatic steatosis: is it an accepted alternative to needle biopsy? Rom J Gastroenterol. 2005;14:419-425. [PubMed] |
| 32. | Ding L, Peng K, Lin L, Li M, Wang T, Dai M, Zhao Z, Xu M, Lu J, Chen Y, Wang W, Bi Y, Xu Y, Ning G. The impact of fat distribution on subclinical coronary atherosclerosis in middle-aged Chinese adults. Int J Cardiol. 2017;235:118-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Chang Y, Noh YH, Suh BS, Kim Y, Sung E, Jung HS, Kim CW, Kwon MJ, Yun KE, Noh JW, Shin H, Cho YK, Ryu S. Bidirectional Association between Nonalcoholic Fatty Liver Disease and Gallstone Disease: A Cohort Study. J Clin Med. 2018;7:458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 34. | Koller T, Kollerova J, Hlavaty T, Huorka M, Payer J. Cholelithiasis and markers of nonalcoholic fatty liver disease in patients with metabolic risk factors. Scand J Gastroenterol. 2012;47:197-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 35. | Tison GH, Blaha MJ, Nasir K, Blumenthal RS, Szklo M, Ding J, Budoff MJ. Relation of Anthropometric Obesity and Computed Tomography Measured Nonalcoholic Fatty Liver Disease (from the Multiethnic Study of Atherosclerosis). Am J Cardiol. 2015;116:541-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Ross R, Rissanen J, Hudson R. Sensitivity associated with the identification of visceral adipose tissue levels using waist circumference in men and women: effects of weight loss. Int J Obes Relat Metab Disord. 1996;20:533-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Tsai CJ, Leitzmann MF, Willett WC, Giovannucci EL. Prospective study of abdominal adiposity and gallstone disease in US men. Am J Clin Nutr. 2004;80:38-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 117] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 38. | Kühn JP, Meffert P, Heske C, Kromrey ML, Schmidt CO, Mensel B, Völzke H, Lerch MM, Hernando D, Mayerle J, Reeder SB. Prevalence of Fatty Liver Disease and Hepatic Iron Overload in a Northeastern German Population by Using Quantitative MR Imaging. Radiology. 2017;284:706-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 39. | Hu F, Yang R, Huang Z, Wang M, Yuan F, Xia C, Wei Y, Song B. 3D Multi-Echo Dixon technique for simultaneous assessment of liver steatosis and iron overload in patients with chronic liver diseases: a feasibility study. Quant Imaging Med Surg. 2019;9:1014-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 40. | Wu Q, Fu X, Zhuo Z, Zhao M, Ni H. The application value of ultra-short echo time MRI in the quantification of liver iron overload in a rat model. Quant Imaging Med Surg. 2019;9:180-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 41. | Wang H, Jiang X, Wu J, Zhang L, Huang J, Zhang Y, Zou X, Liang B. Iron Overload Coordinately Promotes Ferritin Expression and Fat Accumulation in Caenorhabditis elegans. Genetics. 2016;203:241-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 42. | Rostoker G, Loridon C, Griuncelli M, Rabaté C, Lepeytre F, Ureña-Torres P, Issad B, Ghali N, Cohen Y. Liver Iron Load Influences Hepatic Fat Fraction in End-Stage Renal Disease Patients on Dialysis: A Proof of Concept Study. EBioMedicine. 2019;39:461-471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 43. | Henninger B, Alustiza J, Garbowski M, Gandon Y. Practical guide to quantification of hepatic iron with MRI. Eur Radiol. 2020;30:383-393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 44. | Uraz S, Aygun C, Sonsuz A, Ozbay G. Serum iron levels and hepatic iron overload in nonalcoholic steatohepatitis and chronic viral hepatitis. Dig Dis Sci. 2005;50:964-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 45. | Powell EE, Ali A, Clouston AD, Dixon JL, Lincoln DJ, Purdie DM, Fletcher LM, Powell LW, Jonsson JR. Steatosis is a cofactor in liver injury in hemochromatosis. Gastroenterology. 2005;129:1937-1943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 117] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 46. | Mamidipalli A, Hamilton G, Manning P, Hong CW, Park CC, Wolfson T, Hooker J, Heba E, Schlein A, Gamst A, Durelle J, Paiz M, Middleton MS, Schwimmer JB, Sirlin CB. Cross-sectional correlation between hepatic R2* and proton density fat fraction (PDFF) in children with hepatic steatosis. J Magn Reson Imaging. 2018;47:418-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 47. | Hamilton G, Middleton MS, Bydder M, Yokoo T, Schwimmer JB, Kono Y, Patton HM, Lavine JE, Sirlin CB. Effect of PRESS and STEAM sequences on magnetic resonance spectroscopic liver fat quantification. J Magn Reson Imaging. 2009;30:145-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 174] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 48. | Bashir MR, Wolfson T, Gamst AC, Fowler KJ, Ohliger M, Shah SN, Alazraki A, Trout AT, Behling C, Allende DS, Loomba R, Sanyal A, Schwimmer J, Lavine JE, Shen W, Tonascia J, Van Natta ML, Mamidipalli A, Hooker J, Kowdley KV, Middleton MS, Sirlin CB; NASH Clinical Research Network (NASH CRN). Hepatic R2* is more strongly associated with proton density fat fraction than histologic liver iron scores in patients with nonalcoholic fatty liver disease. J Magn Reson Imaging. 2019;49:1456-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |