Published online Sep 7, 2020. doi: 10.3748/wjg.v26.i33.4933
Peer-review started: March 28, 2020
First decision: May 21, 2020
Revised: July 21, 2020
Accepted: August 12, 2020
Article in press: August 12, 2020
Published online: September 7, 2020
Processing time: 159 Days and 18.8 Hours
End-stage liver disease caused by non-alcoholic steatohepatitis (NASH) is the second leading indication for liver transplantation. To date, only moderately effective pharmacotherapies exist to treat NASH. Understanding the pathogenesis of NASH is therefore crucial for the development of new therapies. The inflammatory cytokine tumor necrosis factor alpha (TNF-α) is important for the progression of liver disease. TNF signaling via TNF receptor 1 (TNFR1) has been hypothesized to be important for the development of NASH and hepatocellular carcinoma in whole-body knockout animal models.
To investigate the role of TNFR1 signaling in hepatocytes for steatohepatitis development in a mouse model of diet-induced NASH.
NASH was induced by a western-style fast-food diet in mice deficient for TNFR1 in hepatocytes (TNFR1ΔHEP) and their wild-type littermates (TNFR1fl/fl). Glucose tolerance was assessed after 18 wk and insulin resistance after 19 wk of feeding. After 20 wk mice were assessed for features of NASH and the metabolic syndrome such as liver weight, liver steatosis, liver fibrosis and markers of liver inflammation.
Obesity, liver injury, inflammation, steatosis and fibrosis was not different between TNFR1ΔHEP and TNFR1fl/fl mice. However, Tnfr1 deficiency in hepatocytes protected against glucose intolerance and insulin resistance.
Our results indicate that deficiency of TNFR1 signaling in hepatocytes does not protect from diet-induced NASH. However, improved insulin resistance in this model strengthens the role of the liver in glucose homeostasis.
Core tip: We investigated the role of hepatocellular tumor necrosis factor receptor 1 (TNFR1) signaling in diet-induced non-alcoholic steatohepatitis in mice with a deficiency for TNFR1 solely in hepatocytes. In contrast to most whole-body knock-out models, diet-induced non-alcoholic steatohepatitis is not aggravated by hepatocellular TNFR1 deficiency in our study. However, insulin resistance was markedly improved, which strengthens the role of the liver in glucose homeostasis.
- Citation: Bluemel S, Wang Y, Lee S, Schnabl B. Tumor necrosis factor alpha receptor 1 deficiency in hepatocytes does not protect from non-alcoholic steatohepatitis, but attenuates insulin resistance in mice. World J Gastroenterol 2020; 26(33): 4933-4944
- URL: https://www.wjgnet.com/1007-9327/full/v26/i33/4933.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i33.4933
Complications of the obesity epidemic are increasing globally. Over 70% of obese adults have non-alcoholic fatty liver disease (NAFLD)[1]. Disease progression to non-alcoholic steatohepatitis (NASH) and cirrhosis – often complicated by hepatocellular carcinoma (HCC) – worsens the prognosis. Therefore, NASH and its complications are becoming the second leading indication for liver transplantation[2,3]. Besides weight loss, only moderately effective therapies exist to reverse NASH[4]. Understanding the pathogenesis of NASH is therefore crucial for the development of novel treatment strategies.
The development of NAFLD and NASH is triggered by intestinal dysbiosis[5-13]. This is underlined by the transmission of the disease through co-housing experiments in mice and microbiota transplantation to germ-free mice[14,15]. Poor nutrition and dysbiosis can damage the intestinal epithelial barrier and facilitate endotoxemia[16]. Lipopolysaccharide (LPS), which is a component of the outer membrane of Gram-negative bacteria, acts as an endotoxin. LPS activates toll-like receptor 4 (TLR-4). TLR-4 expression is elevated in liver biopsies of patients with NASH compared with NAFLD[17]. Downstream signals of TLR activation, such as release of tumor necrosis factor alpha (TNF-α), contribute to liver inflammation and fibrosis[18-20].
TNF is a pro-inflammatory cytokine thought to be substantially involved in the progression of liver disease[18-20]. TNF signal transduction is mediated by TNF receptor 1 (TNFR1) and TNFR2. Whereas TNFR1 activation is thought to drive inflammation and metabolic alterations, TNFR2 regulates regeneration and immune response in a protective manner[21,22]. In obesity, adipocytes are a major systemic source of TNF, while in the liver TNF is mainly released from Kupffer cells[23,24]. Obese patients have higher serum levels of TNF, and patients with NASH have higher hepatic expression of TNF compared with NAFLD[25,26]. In a mouse model of NAFLD, TNF was suggested to be a driver of HCC development and tumor-associated inflammation[27,28]. Conversely, obese mice with whole-body knockout of Tnfr1 have lower hepatic lipid accumulation, lower level of the pro-inflammatory cytokine IL-6, and lower hepatic accumulation of neutrophils and macrophages[27]. Moreover, inducers of hepatocellular endoplasmic reticulum (ER) stress, which promotes NASH progression, are lower in Tnfr1-deficient mice[28,29]. This suggests that TNF signaling via TNFR1 is important for the development of steatohepatitis. However, results are conflicting, with other studies showing more hepatic steatosis as well as fibrosis and higher inflammatory markers in mouse livers of high-fat diet-induced NAFLD and dysfunctional TNFR1[30]. Therefore, the role of TNF and TNFR1 signaling in NAFLD and NASH remains unclear. The abovementioned studies on TNFR1 involvement in hepatocellular ER stress suggest that TNFR1 signaling in hepatocytes is crucial for NASH progression (rather than TNFR1 activation in other liver-homed cells, such as Kupffer cells or stellate cells).
To address this question, we investigated the role of TNFR1 signaling in hepatocytes on hepatic steatosis and steatohepatitis in a mouse model of diet-induced NASH.
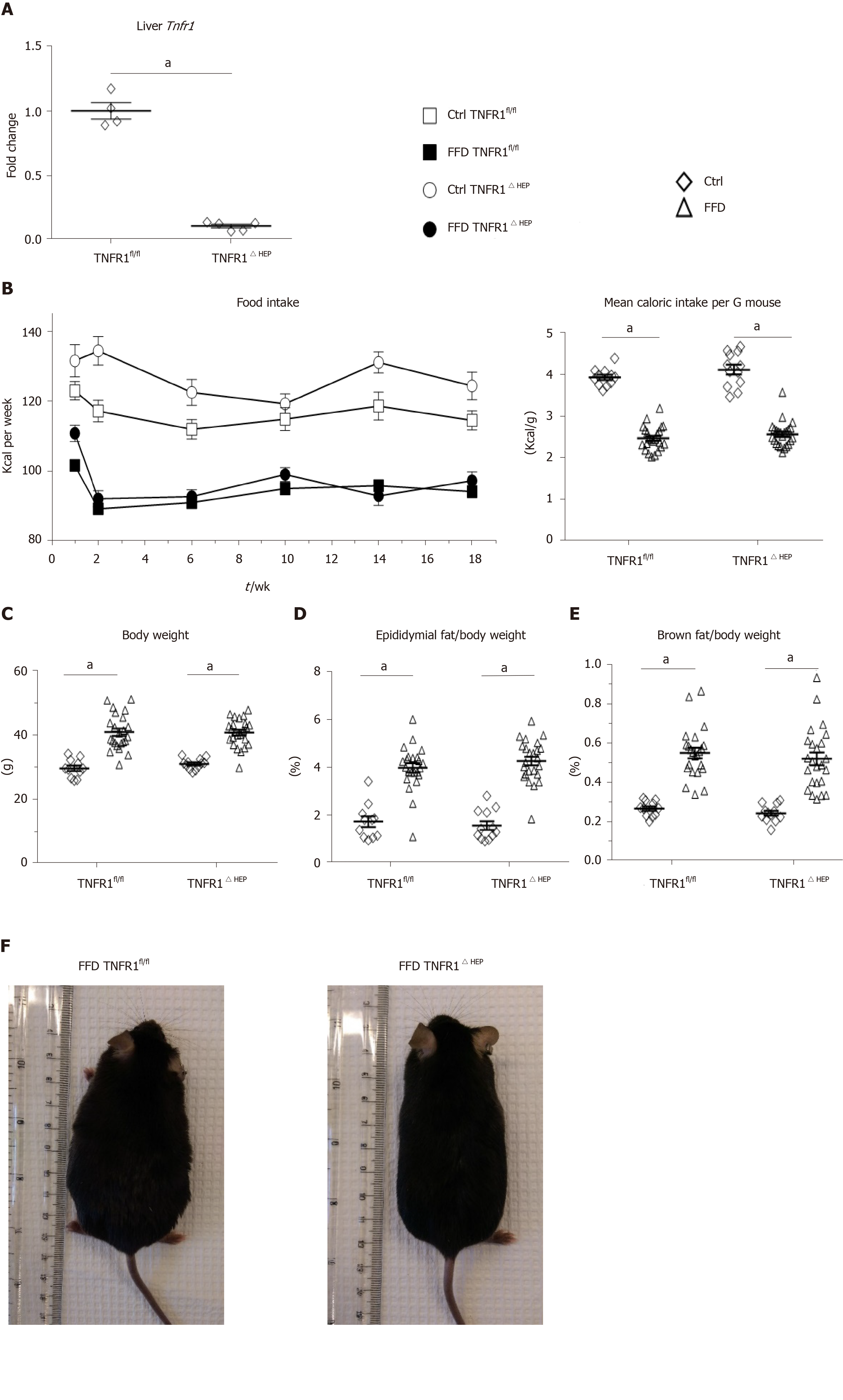
Mice with loxP sites inserted in the tumor necrosis factor receptor 1 gene (Tnfr1flxneo/flxneo) were generated after receiving sperm from the European Mouse Mutant Archive[31]. These mice have exons 2 to 5 flanked with loxP sites and were crossed with albumin-Cre transgenic mice (The Jackson Laboratory, Sacramento, CA) to create mice with a deficiency of Tnfr1 (Tnfrsf1a) specifically in hepatocytes (TNFR1ΔHEP). Albumin-Cre negative Tnfr1flxneo/flxneo littermates were used as controls (TNFR1fl/fl). All mice were on a C57BL/6 background, and were maintained on a 12:12-h light-dark cycle. After weaning, male mice were housed with littermates of the same genotype for experiments. At age 8 wk, mice were started on a fast food diet (FFD) consisting of irradiated western-style diet (AIN-76A; TestDiet, St. Louis, MO, United States) for 20 wk[32]. Drinking water was supplemented with 23.1 g/L fructose (F0127; Sigma Aldrich, St. Louis, MO, United States) and 18.9 g/L glucose (G8270; Sigma Aldrich) to mimic high-fructose corn syrup containing soft drinks[3]. Control mice (Ctrl) received autoclaved tap water and irradiated standard chow (5053 PicoLab Rodent Diet; LabDiet, St. Louis, MO, United States). Mice had free access to food and water.
A glucose tolerance test (GTT) was performed after 18 wk of feeding by injecting 1 µg glucose/g body weight intraperitoneally[32]. After 19 wk of feeding, an insulin tolerance test (ITT) was performed by intraperitoneal injection of 0.5 mU/g body weight insulin (Novolin N NPH; Novo Nordisk Inc, Princeton, NJ, United States). Prior to both tests, mice were fasted for 6 h. Blood glucose levels were assessed from tail vein blood before injection (t = 0 min) and at t = 15, 30, 60, 90, and 120 min following injection.
All animal studies were reviewed and approved by the Institutional Animal Care and Use Committee of the University of California, San Diego.
Biochemical analyses were done according to the manufacturers’ protocols. Alanine amino transferase (ALT) was measured from plasma obtained from inferior vena cava using a kinetic assay (TR71121; Thermo Fisher Scientific, Waltham, MA, United States). Triglyceride levels were measured with a colorimetric endpoint assay (T7532; Pointe Scientific, Canton, MI, United States) after tissue homogenization in phosphate-buffered saline, and precipitation of lipids using methanol and chloroform. Liver hydroxyproline was extracted from 150-250 mg of mixed liver specimens from the right and left liver lobes[32]. The tissue was homogenized in 6N HCl (3750-32; USABlueBook, Forest Park, GA, United States) using lysing matrix C tubes (MP116912; MP Biomedicals, Santa Ana, CA, United States) and a Mini-BeadBeater-96 (GlenMills, Clifton, NJ, United States)[32,33]. The homogenate was incubated at 110 °C for 18 h and subsequently filtered using Whatman® filter paper grade 595 1/2 (WHA10311644; Sigma Aldrich). The lysate was incubated with chloramine T- (C9887; Sigma Aldrich) as well as Ehrlich’s perchloric acid solution (AC168760250; Thermo Fisher Scientific). Triplicates were measured at 558 nm (SpectraMax 190 Microplate Reader; Molecular Devices LLC, Sunnyvale, CA, United States)[33,34].
At harvesting, the median liver lobe including the gall bladder was fixed in 10% formalin (HT501128; Sigma Aldrich) for 24 to 48 h, and then transferred to 70% ethanol and embedded in paraffin[32]. Five µm paraffin sections were stained with hematoxylin and eosin (H&E) (38015 and 380161 SelecTech; Leica Biosystems Inc., Buffalo Grove, IL, United States) or 0.1% picro Sirius red (color index 35780, 365548; Sigma-Aldrich), respectively.
Liver RNA was extracted using Ambion Trizol Reagent (15596; Thermo Fisher Scientific). RNA was treated with RQ1 RNase-Free DNase (M6101; Promega), and was reverse-transcribed using the Applied Biosystems High-Capacity cDNA Reverse Transcription Kit (43688; Thermo Fisher Scientific). Real-time qPCR was performed on the Applied Biosystems StepOnePlus Thermocycler (4376600; Thermo Fisher Scientific) using iTaq Universal SYBR Green Supermix (Bio-Rad, Hercules, CA, United States). Primer sequences were obtained from the Harvard PrimerBank[35,36]. The primer used for proving the absence of Tnfr1 was generated using NCBI primer blast; the sequences were: ACCGTGACAATCCCCTGTAA (Fwd) and CTCTTTGACA GGCACGGGAT (Rev). These primer amplified mRNA from exon 3 to exon 7. Gene expression was normalized to mouse TATA-Box binding protein gene and expressed relative to Ctrl-fed TNFR1fl/fl mice.
Numbers of biological replicates for each experiment are given in the respective figure legends. The area under the curve was used to compare blood glucose levels from ITT. The area over baseline (AOB) was calculated to compare blood glucose levels from GTT. Groups were compared by two-way analysis of variance with Tukey’s post hoc test. All results are expressed as mean + standard error of the mean. Analyses and data plots were done with GraphPad Prism 6.01 (GraphPad Software, Inc., La Jolla, CA, United States). Significant differences are marked with (a) if P < 0.05.
TNFR1ΔHEP mice were fed for 20 wk with a western-style diet (FFD) to evaluate, if the absence of TNFR1 in hepatocytes is protective against diet-induced NASH.
Lower Tnfr1 expression in liver samples from mice with a deficiency of TNFR1 in hepatocytes is shown in Figure 1A. Food intake is given in Figure 1B. FFD led to an increase in body weight and adipose tissue (Figure 1C-F). However, FFD-fed TNFR1ΔHEP mice did not differ from their TNFR1fl/fl littermates in terms of body weight, epididymal fat weight and brown fat weight (Figure 1C-E). Taken together, western-style diet-fed TNFR1ΔHEP mice develop similar features of obesity compared with their TNFR1fl/fl littermates.
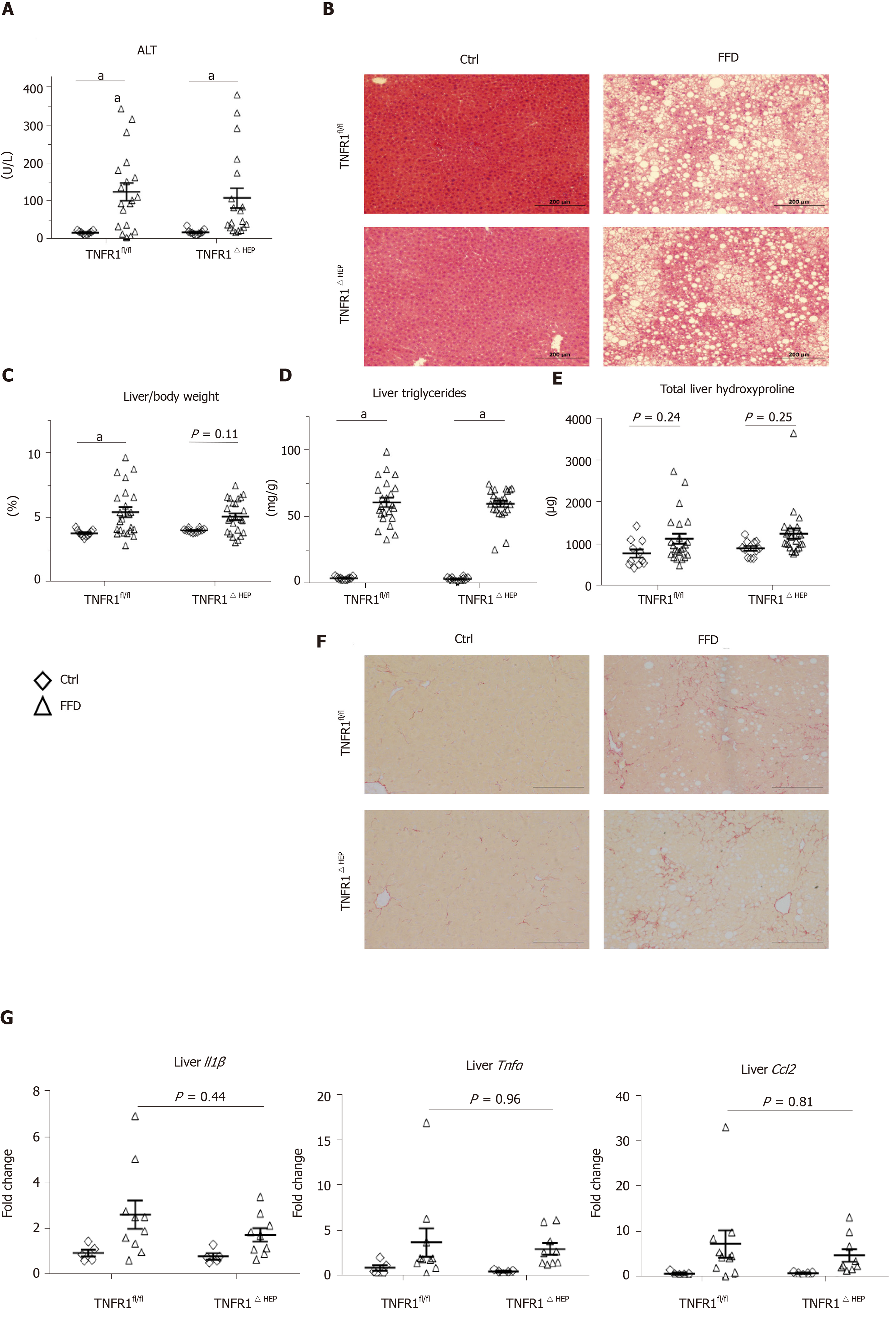
To determine the significance of TNFR1 in hepatocytes for the development of NASH, parameters of liver injury, steatosis and fibrosis were investigated. Hepatic injury, expressed as plasma ALT, was not significantly different between FFD-fed TNFR1ΔHEP mice and their TNFR1fl/fl littermates (Figure 2A and B). Similarly, hepatic steatosis, expressed as total liver triglycerides and relative liver weight, did not differ between FFD-fed TNFR1ΔHEP mice and their TNFR1fl/fl littermates (Figure 2C and D). Representative liver sections are given in Figure 2B. Fibrosis, expressed as total liver hydroxyproline, was higher in FFD-fed mice, but not significantly different from control-fed mice in TNFR1ΔHEP as well as TNFR1fl/fl mice (Figure 2E and F). Liver inflammation, assessed by gene expression for Il1β, Tnf, and Ccl2, did not differ between FFD-fed TNFR1ΔHEP mice and TNFR1fl/fl littermates (Figure 2G). Taken together, western-style diet-fed TNFR1ΔHEP mice develop similar signs of liver inflammation and fibrosis compared with TNFR1fl/fl littermates.
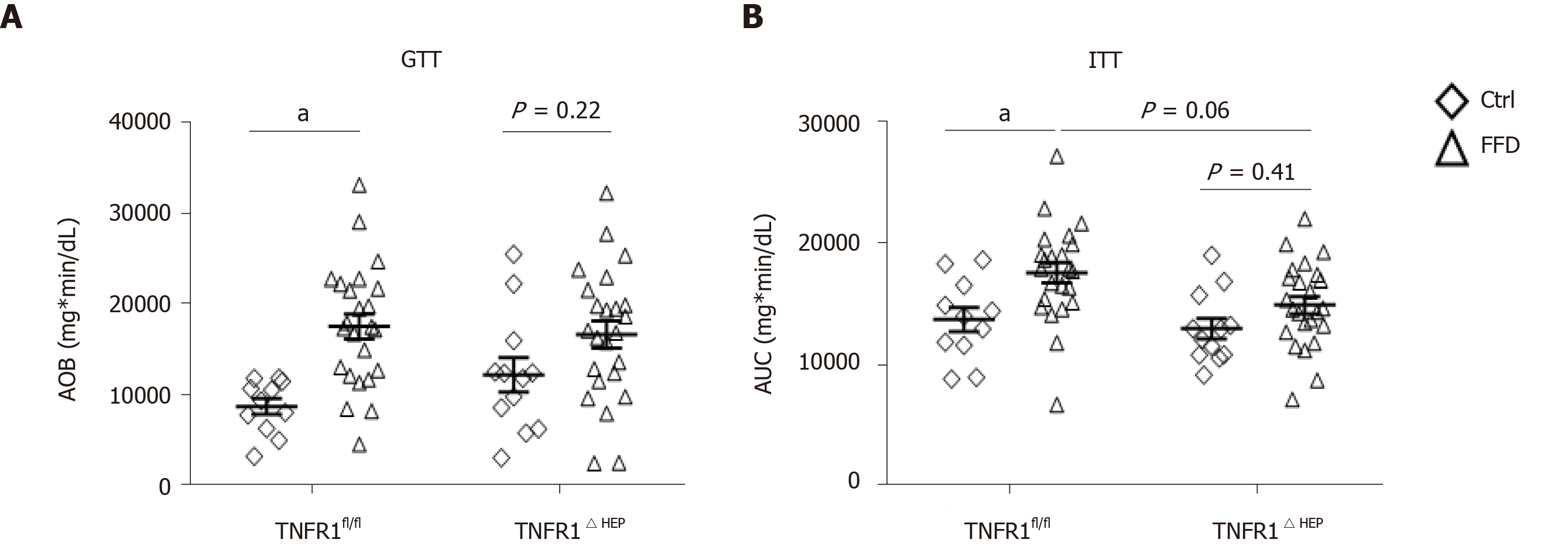
To assess the metabolic phenotype, TNFR1ΔHEP mice and their TNFR1fl/fl littermates were subjected to a glucose and an insulin tolerance test. The FFD-fed TNFR1ΔHEP mice did not develop glucose intolerance compared to their Ctrl-fed littermates (Figure 3A). The result was confirmed with the insulin tolerance test that showed a higher drop of blood glucose levels after insulin injections in FFD-fed TNFR1ΔHEP mice compared with TNFR1fl/fl littermates (Figure 3B). Taken together, although western-style diet equally induced obesity in TNFR1ΔHEP and TNFR1fl/fl littermate mice, interestingly, TNFR1ΔHEP mice were resistant to the development of diet-induced glucose intolerance and insulin resistance.
The role of the TNF receptor 1 in hepatocytes for diet-induced NASH was investigated in mice with a hepatocyte-specific deficiency of TNFR1 (TNFR1ΔHEP). Obese mice with deficiency of TNFR1 in hepatocytes did not develop less liver disease than their TNFR1fl/fl littermates. However, TNFR1ΔHEP mice were protected from glucose intolerance and insulin resistance.
Previous studies, using Tnfr1 whole-body knockout animals, concluded that TNFR1 signaling is a driver for NASH. Constitutional activation of TNFR1 in mice promoted the progression of NASH, but protected from insulin resistance[37], in contrast to our findings and studies showing the importance of TNFR1 signaling in diabetes development[38-40] (discussed in more detail below). On the other hand, deficiency of Tnfr1 was used to prove the contribution of TNFR1 signaling for downstream activation of STAT3 or the induction of ER stress both to promote NASH progression and HCC development[27,28]. In contrast to these studies, in our study Tnfr1 was deleted in hepatocytes only. Kupffer cells are the primary source of hepatic TNF release[24]. Moreover, TNF acts on Kupffer cells in an autocrine manner[41]. Therefore, it can be speculated that the expected protective effect of hepatocyte-specific TNFR1 deficiency was reduced by increased TNFR1 signaling in Kupffer cells or recruited immune cells. These immune cells might have caused the release of different inflammation mediators, such as IL-1β or IL-6, which in turn resulted in activation of inflammatory pathways in hepatocytes that are independent from TNFR1 activation. This hypothesis is supported by a study that used mice with a whole-body knock out of Tnfr1 and also found increased NASH features[30]. In this study by Lambertucci et al[30], Tnfr1 deficiency resulted in increased numbers of both resident (i.e. Kupffer cells) and recruited macrophages into the liver, as well as up-regulation of IL-1β and IL-6 in the liver along with increased release into the plasma. IL-1β promotes alcoholic and non-alcoholic liver disease[42,43]. In the present study, we did not detect differences in IL-1β expression between obese mice with a hepatocyte-specific TNFR1 deficiency and their wild-type littermates.
Another possible explanation for our finding might be an enhanced signal transduction via TNFR2. TNFR2 is a mediator of systemic inflammation and enhances cytotoxicity of monocytes[44-46]. Reduced expression of adhesion molecules VCAM-1 and ICAM-1, which both facilitate leukocyte infiltration, was described in a double-knockout model of Tnfr1 and Tnfr2, along with a reduction of hepatic steatosis and fibrosis[47]. However, a more prominent role of TNFR2 would have occurred in the whole-body knockout models as well. Higher serum level of soluble TNFR2 were detected in patients with HCC and hepatitis C[48]. The role of TNFR2 for NASH development is not conclusive, but it is thought to be protective[49].
The increase in hepatic triglyceride content in NAFLD is associated with hepatic insulin resistance[50]. This is mediated by the insulin receptor substrate IRS-2 and protein kinase-Cε. We did not detect a difference in hepatic triglycerides between obese Tnfr1 deficient mice and their Tnfr1fl/fl littermates. However, compared to lean controls, only Tnfr1fl/fl mice had high blood glucose level after glucose and insulin challenge. Our observation that mice with a deficiency of Tnfr1 in hepatocytes are protected from insulin resistance, strengthens previous findings of Tnfr1 deficiency and improved insulin resistance[38,49]. TNF-mediated insulin resistance occurs via phosphorylation of the insulin receptor substrate IRS-1, which inhibits insulin-action in hepatocytes, and therefore promotes insulin resistance[39,40]. Phosphorylation of IRS-1 was reduced in mice with a whole-body deficiency of Tnfr1, thus enhancing insulin-uptake into hepatocytes[38]. A similar mechanism occurs in brown adipose tissue and myocytes – alternative major sites of insulin action[51]. The effect seen in our study emphasizes the important role of hepatocytes for glucose homeostasis during obesity development.
Our findings were limited by the selective deficiency of TNFR1 in hepatocytes. As mentioned above, selective deficiency of TNFR1 in Kupffer cells might have clarified the cell type specific role for the pathogenesis of NASH. Another limitation was that we could only speculate about the compensating role of TNFR2. As TNFR2 signaling is expected to be protective, a selective manipulation of TNFR2 would be an interesting target for future studies. Furthermore, we could not investigate the mechanisms leading to insulin resistance in more details, because samples were not taken from starved animals, as usually done in metabolic studies.
In conclusion, our results do not indicate that loss of TNFR1 in hepatocytes can protect from diet-induced NASH. However, improved insulin resistance in this model confirms the important role of the liver for glucose homeostasis during obesity.
Understanding the pathogenesis of non-alcoholic steatohepatitis (NASH) is crucial for the development of new therapies. The inflammatory cytokine tumor necrosis factor alpha (TNF-α) is important for the progression of liver disease. It binds to two receptors, TNF receptor 1 (TNFR1) and TNFR2.
TNF signaling via TNFR1 has been hypothesized to be important for the development of NASH and hepatocellular carcinoma in whole-body knockout animal models.
The aim of our study was to investigate the hepatocyte specific role of TNFR1 signaling for diet-induced steatohepatitis.
NASH was induced by a 20-wk western-style fast-food diet in mice deficient of TNFR1 in hepatocytes (TNFR1ΔHEP) and their wild-type littermates (TNFR1fl/fl). Features of NASH as well as glucose tolerance and insulin resistance were assessed.
Obesity, liver injury, inflammation, steatosis, and fibrosis was not different between TNFR1ΔHEP and TNFR1fl/fl mice. However, Tnfr1 deficiency in hepatocytes protected mice against glucose intolerance and insulin resistance.
Our results do not indicate that inhibition of TNFR1 signaling in hepatocytes can protect from diet-induced NASH. However, improved insulin resistance in this model confirms the important role of the liver for glucose homeostasis during obesity.
Compensatory mechanisms, possibly occurring via TNFR2 signaling, need to be investigated in future studies.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Cheng JT, Jamali R, Williams RS, Zhang L S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Wang LL
| 1. | Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3655] [Cited by in RCA: 3718] [Article Influence: 161.7] [Reference Citation Analysis (2)] |
| 2. | Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2405] [Cited by in RCA: 2292] [Article Influence: 163.7] [Reference Citation Analysis (0)] |
| 3. | Charlton M, Krishnan A, Viker K, Sanderson S, Cazanave S, McConico A, Masuoko H, Gores G. Fast food diet mouse: novel small animal model of NASH with ballooning, progressive fibrosis, and high physiological fidelity to the human condition. Am J Physiol Gastrointest Liver Physiol. 2011;301:G825-G834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 350] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 4. | Patel SS, Siddiqui MS. Current and Emerging Therapies for Non-alcoholic Fatty Liver Disease. Drugs. 2019;79:75-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Abdou RM, Zhu L, Baker RD, Baker SS. Gut Microbiota of Nonalcoholic Fatty Liver Disease. Dig Dis Sci. 2016;61:1268-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Hartmann P, Chen WC, Schnabl B. The intestinal microbiome and the leaky gut as therapeutic targets in alcoholic liver disease. Front Physiol. 2012;3:402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Hartmann P, Seebauer CT, Schnabl B. Alcoholic liver disease: the gut microbiome and liver cross talk. Alcohol Clin Exp Res. 2015;39:763-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 222] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 8. | Llorente C, Schnabl B. The gut microbiota and liver disease. Cell Mol Gastroenterol Hepatol. 2015;1:275-284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 133] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 9. | Wieland A, Frank DN, Harnke B, Bambha K. Systematic review: microbial dysbiosis and nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2015;42:1051-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 164] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 10. | Yan AW, Schnabl B. Bacterial translocation and changes in the intestinal microbiome associated with alcoholic liver disease. World J Hepatol. 2012;4:110-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 11. | Chen Y, Yang F, Lu H, Wang B, Chen Y, Lei D, Wang Y, Zhu B, Li L. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology. 2011;54:562-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 674] [Cited by in RCA: 797] [Article Influence: 56.9] [Reference Citation Analysis (3)] |
| 12. | Llopis M, Cassard AM, Wrzosek L, Boschat L, Bruneau A, Ferrere G, Puchois V, Martin JC, Lepage P, Le Roy T, Lefèvre L, Langelier B, Cailleux F, González-Castro AM, Rabot S, Gaudin F, Agostini H, Prévot S, Berrebi D, Ciocan D, Jousse C, Naveau S, Gérard P, Perlemuter G. Intestinal microbiota contributes to individual susceptibility to alcoholic liver disease. Gut. 2016;65:830-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 423] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 13. | Boursier J, Mueller O, Barret M, Machado M, Fizanne L, Araujo-Perez F, Guy CD, Seed PC, Rawls JF, David LA, Hunault G, Oberti F, Calès P, Diehl AM. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. 2016;63:764-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 763] [Cited by in RCA: 1049] [Article Influence: 116.6] [Reference Citation Analysis (0)] |
| 14. | Le Roy T, Llopis M, Lepage P, Bruneau A, Rabot S, Bevilacqua C, Martin P, Philippe C, Walker F, Bado A, Perlemuter G, Cassard-Doulcier AM, Gérard P. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut. 2013;62:1787-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 715] [Article Influence: 59.6] [Reference Citation Analysis (0)] |
| 15. | Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ, Camporez JP, Shulman GI, Gordon JI, Hoffman HM, Flavell RA. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179-185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1620] [Cited by in RCA: 1878] [Article Influence: 144.5] [Reference Citation Analysis (0)] |
| 16. | Bluemel S, Williams B, Knight R, Schnabl B. Precision medicine in alcoholic and nonalcoholic fatty liver disease via modulating the gut microbiota. Am J Physiol Gastrointest Liver Physiol. 2016;311:G1018-G1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 17. | Sharifnia T, Antoun J, Verriere TG, Suarez G, Wattacheril J, Wilson KT, Peek RM, Abumrad NN, Flynn CR. Hepatic TLR4 signaling in obese NAFLD. Am J Physiol Gastrointest Liver Physiol. 2015;309:G270-G278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 194] [Article Influence: 19.4] [Reference Citation Analysis (1)] |
| 18. | Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1361] [Cited by in RCA: 1555] [Article Influence: 86.4] [Reference Citation Analysis (1)] |
| 19. | Su GL. Lipopolysaccharides in liver injury: molecular mechanisms of Kupffer cell activation. Am J Physiol Gastrointest Liver Physiol. 2002;283:G256-G265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 354] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 20. | Leist M, Gantner F, Jilg S, Wendel A. Activation of the 55 kDa TNF receptor is necessary and sufficient for TNF-induced liver failure, hepatocyte apoptosis, and nitrite release. J Immunol. 1995;154:1307-1316. [PubMed] |
| 21. | Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1944] [Cited by in RCA: 2037] [Article Influence: 92.6] [Reference Citation Analysis (0)] |
| 22. | Dong Y, Fischer R, Naudé PJ, Maier O, Nyakas C, Duffey M, Van der Zee EA, Dekens D, Douwenga W, Herrmann A, Guenzi E, Kontermann RE, Pfizenmaier K, Eisel UL. Essential protective role of tumor necrosis factor receptor 2 in neurodegeneration. Proc Natl Acad Sci USA. 2016;113:12304-12309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 125] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 23. | Sun B, Karin M. Obesity, inflammation, and liver cancer. J Hepatol. 2012;56:704-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 396] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 24. | Luster MI, Germolec DR, Yoshida T, Kayama F, Thompson M. Endotoxin-induced cytokine gene expression and excretion in the liver. Hepatology. 1994;19:480-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 131] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | Ruiz AG, Casafont F, Crespo J, Cayón A, Mayorga M, Estebanez A, Fernadez-Escalante JC, Pons-Romero F. Lipopolysaccharide-binding protein plasma levels and liver TNF-alpha gene expression in obese patients: evidence for the potential role of endotoxin in the pathogenesis of non-alcoholic steatohepatitis. Obes Surg. 2007;17:1374-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 205] [Article Influence: 11.4] [Reference Citation Analysis (1)] |
| 26. | Crespo J, Cayón A, Fernández-Gil P, Hernández-Guerra M, Mayorga M, Domínguez-Díez A, Fernández-Escalante JC, Pons-Romero F. Gene expression of tumor necrosis factor alpha and TNF-receptors, p55 and p75, in nonalcoholic steatohepatitis patients. Hepatology. 2001;34:1158-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 503] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 27. | Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, Osterreicher CH, Takahashi H, Karin M. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1403] [Cited by in RCA: 1368] [Article Influence: 91.2] [Reference Citation Analysis (1)] |
| 28. | Nakagawa H, Umemura A, Taniguchi K, Font-Burgada J, Dhar D, Ogata H, Zhong Z, Valasek MA, Seki E, Hidalgo J, Koike K, Kaufman RJ, Karin M. ER stress cooperates with hypernutrition to trigger TNF-dependent spontaneous HCC development. Cancer Cell. 2014;26:331-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 415] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 29. | Kim JY, Garcia-Carbonell R, Yamachika S, Zhao P, Dhar D, Loomba R, Kaufman RJ, Saltiel AR, Karin M. ER Stress Drives Lipogenesis and Steatohepatitis via Caspase-2 Activation of S1P. Cell. 2018;175:133-145.e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 251] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 30. | Lambertucci F, Arboatti A, Sedlmeier MG, Motiño O, Alvarez ML, Ceballos MP, Villar SR, Roggero E, Monti JA, Pisani G, Quiroga AD, Martín-Sanz P, Carnovale CE, Francés DE, Ronco MT. Disruption of tumor necrosis factor alpha receptor 1 signaling accelerates NAFLD progression in mice upon a high-fat diet. J Nutr Biochem. 2018;58:17-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 31. | Van Hauwermeiren F, Armaka M, Karagianni N, Kranidioti K, Vandenbroucke RE, Loges S, Van Roy M, Staelens J, Puimège L, Palagani A, Berghe WV, Victoratos P, Carmeliet P, Libert C, Kollias G. Safe TNF-based antitumor therapy following p55TNFR reduction in intestinal epithelium. J Clin Invest. 2013;123:2590-2603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 32. | Bluemel S, Wang L, Martino C, Lee S, Wang Y, Williams B, Horvath A, Stadlbauer V, Zengler K, Schnabl B. The Role of Intestinal C-type Regenerating Islet Derived-3 Lectins for Nonalcoholic Steatohepatitis. Hepatol Commun. 2018;2:393-406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 33. | Iwaisako K, Haimerl M, Paik YH, Taura K, Kodama Y, Sirlin C, Yu E, Yu RT, Downes M, Evans RM, Brenner DA, Schnabl B. Protection from liver fibrosis by a peroxisome proliferator-activated receptor δ agonist. Proc Natl Acad Sci USA. 2012;109:E1369-E1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 139] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 34. | Jamall IS, Finelli VN, Que Hee SS. A simple method to determine nanogram levels of 4-hydroxyproline in biological tissues. Anal Biochem. 1981;112:70-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 421] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 35. | Spandidos A, Wang X, Wang H, Dragnev S, Thurber T, Seed B. A comprehensive collection of experimentally validated primers for Polymerase Chain Reaction quantitation of murine transcript abundance. BMC Genomics. 2008;9:633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 206] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 36. | Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8442] [Cited by in RCA: 10166] [Article Influence: 242.0] [Reference Citation Analysis (0)] |
| 37. | Aparicio-Vergara M, Hommelberg PP, Schreurs M, Gruben N, Stienstra R, Shiri-Sverdlov R, Kloosterhuis NJ, de Bruin A, van de Sluis B, Koonen DP, Hofker MH. Tumor necrosis factor receptor 1 gain-of-function mutation aggravates nonalcoholic fatty liver disease but does not cause insulin resistance in a murine model. Hepatology. 2013;57:566-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 38. | Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389:610-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1684] [Cited by in RCA: 1659] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 39. | Kanety H, Feinstein R, Papa MZ, Hemi R, Karasik A. Tumor necrosis factor alpha-induced phosphorylation of insulin receptor substrate-1 (IRS-1). Possible mechanism for suppression of insulin-stimulated tyrosine phosphorylation of IRS-1. J Biol Chem. 1995;270:23780-23784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 319] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 40. | Hotamisligil GS, Murray DL, Choy LN, Spiegelman BM. Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proc Natl Acad Sci USA. 1994;91:4854-4858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 829] [Cited by in RCA: 850] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 41. | Kitamura K, Nakamoto Y, Akiyama M, Fujii C, Kondo T, Kobayashi K, Kaneko S, Mukaida N. Pathogenic roles of tumor necrosis factor receptor p55-mediated signals in dimethylnitrosamine-induced murine liver fibrosis. Lab Invest. 2002;82:571-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 42. | Llorente C, Jepsen P, Inamine T, Wang L, Bluemel S, Wang HJ, Loomba R, Bajaj JS, Schubert ML, Sikaroodi M, Gillevet PM, Xu J, Kisseleva T, Ho SB, DePew J, Du X, Sørensen HT, Vilstrup H, Nelson KE, Brenner DA, Fouts DE, Schnabl B. Gastric acid suppression promotes alcoholic liver disease by inducing overgrowth of intestinal Enterococcus. Nat Commun. 2017;8:837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 182] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 43. | Yang AM, Inamine T, Hochrath K, Chen P, Wang L, Llorente C, Bluemel S, Hartmann P, Xu J, Koyama Y, Kisseleva T, Torralba MG, Moncera K, Beeri K, Chen CS, Freese K, Hellerbrand C, Lee SM, Hoffman HM, Mehal WZ, Garcia-Tsao G, Mutlu EA, Keshavarzian A, Brown GD, Ho SB, Bataller R, Stärkel P, Fouts DE, Schnabl B. Intestinal fungi contribute to development of alcoholic liver disease. J Clin Invest. 2017;127:2829-2841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 361] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 44. | Douni E, Kollias G. A critical role of the p75 tumor necrosis factor receptor (p75TNF-R) in organ inflammation independent of TNF, lymphotoxin alpha, or the p55TNF-R. J Exp Med. 1998;188:1343-1352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 90] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 45. | Riches DW, Chan ED, Zahradka EA, Winston BW, Remigio LK, Lake FR. Cooperative signaling by tumor necrosis factor receptors CD120a (p55) and CD120b (p75) in the expression of nitric oxide and inducible nitric oxide synthase by mouse macrophages. J Biol Chem. 1998;273:22800-22806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 46. | Wajant H, Siegmund D. TNFR1 and TNFR2 in the Control of the Life and Death Balance of Macrophages. Front Cell Dev Biol. 2019;7:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 282] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 47. | Tomita K, Tamiya G, Ando S, Ohsumi K, Chiyo T, Mizutani A, Kitamura N, Toda K, Kaneko T, Horie Y, Han JY, Kato S, Shimoda M, Oike Y, Tomizawa M, Makino S, Ohkura T, Saito H, Kumagai N, Nagata H, Ishii H, Hibi T. Tumour necrosis factor alpha signalling through activation of Kupffer cells plays an essential role in liver fibrosis of non-alcoholic steatohepatitis in mice. Gut. 2006;55:415-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 352] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 48. | Bastard JP, Fellahi S, Audureau É, Layese R, Roudot-Thoraval F, Cagnot C, Mahuas-Bourcier V, Sutton A, Ziol M, Capeau J, Nahon P, ANRS CO12 CirVir group. Elevated adiponectin and sTNFRII serum levels can predict progression to hepatocellular carcinoma in patients with compensated HCV1 cirrhosis. Eur Cytokine Netw. 2018;29:112-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 49. | Wandrer F, Liebig S, Marhenke S, Vogel A, John K, Manns MP, Teufel A, Itzel T, Longerich T, Maier O, Fischer R, Kontermann RE, Pfizenmaier K, Schulze-Osthoff K, Bantel H. TNF-Receptor-1 inhibition reduces liver steatosis, hepatocellular injury and fibrosis in NAFLD mice. Cell Death Dis. 2020;11:212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 50. | Perry RJ, Samuel VT, Petersen KF, Shulman GI. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature. 2014;510:84-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 733] [Cited by in RCA: 858] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 51. | Nieto-Vazquez I, Fernández-Veledo S, Krämer DK, Vila-Bedmar R, Garcia-Guerra L, Lorenzo M. Insulin resistance associated to obesity: the link TNF-alpha. Arch Physiol Biochem. 2008;114:183-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 329] [Article Influence: 19.4] [Reference Citation Analysis (0)] |