Published online Sep 7, 2020. doi: 10.3748/wjg.v26.i33.4900
Peer-review started: April 30, 2020
First decision: June 13, 2020
Revised: June 24, 2020
Accepted: August 20, 2020
Article in press: August 20, 2020
Published online: September 7, 2020
Processing time: 126 Days and 20.8 Hours
In the last years, several studies have been focused on elucidate the role of tumor microenvironment (TME) in cancer development and progression. Within TME, cells from adaptive and innate immune system are one of the main abundant components. The dynamic interactions between immune and cancer cells lead to the activation of complex molecular mechanisms that sustain tumor growth. This important cross-talk has been elucidate for several kind of tumors and occurs also in patients with liver cancer, such as hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (iCCA). Liver is well-known to be an important immunological organ with unique microenvironment. Here, in normal conditions, the rich immune-infiltrating cells cooperate with non-parenchymal cells, such as liver sinusoidal endothelial cells and Kupffer cells, favoring self-tolerance against gut antigens. The presence of underling liver immunosuppressive microenvironment highlights the importance to dissect the interaction between HCC and iCCA cells with immune infiltrating cells, in order to understand how this cross-talk promotes tumor growth. Deeper attention is, in fact, focused on immune-based therapy for these tumors, as promising approach to counteract the intrinsic anti-tumor activity of this microenvironment. In this review, we will examine the key pathways underlying TME cell-cell communications, with deeper focus on the role of natural killer cells in primary liver tumors, such as HCC and iCCA, as new opportunities for immune-based therapeutic strategies.
Core tip: Natural killer (NK) cells are an important innate immune cell type with high cytotoxic activity, mainly involved in the clearance of virus-infected and tumor cells. Due to their potential anti-tumor activity, NK cells are gaining a deeper attention as a promising strategy for immune-based cancer therapy. Several studies reveal that both in hepatocellular carcinoma and intrahepatic cholangiocarcinoma, NK cells infiltrate within tumors and their high frequency was found to be related with a favorable overall survival in these patients. In this review, the authors summarize the current literature on NK cells and their role in primary liver tumors.
- Citation: Polidoro MA, Mikulak J, Cazzetta V, Lleo A, Mavilio D, Torzilli G, Donadon M. Tumor microenvironment in primary liver tumors: A challenging role of natural killer cells. World J Gastroenterol 2020; 26(33): 4900-4918
- URL: https://www.wjgnet.com/1007-9327/full/v26/i33/4900.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i33.4900
Tumor microenvironment (TME) has emerged as a pivotal factor in driving tumor development and progression[1]. Cells from both adaptive and innate immune system are the main components of TME, which establishes dynamic interactions with cancer cells. The resultant cross-talk leads to activation of complex molecular mechanisms that finally foster tumor growth by inhibition of anti-tumor activity of immune cells[2]. This phenomenon occurs also in patients with liver tumors, such as hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (iCCA). Being, the liver an important organ, in which the rich immune-infiltrating cells with non-parenchymal cells, such as liver sinusoidal endothelial cells and Kupffer cells (KCs) cooperate to maintain the immunosuppressive microenvironment favoring self-tolerance against gut antigens, it is of paramount importance to know how HCC and iCCA cells interact with immune infiltrating cells to promote the pro-inflammatory and immu-nosuppressive environment that finally foster tumor growth. In this narrative review, we explore the key pathways involved in TME cell-cell communications, with particular focus on the emerging role of natural killer cells (NK) as new opportunities for immune-based therapeutic strategies. For doing that, a comprehensive literature search was conducted using PubMed to identify relevant articles published between 2000 and 2020. The search was limited to articles in English and it was further broadened by extensive cross-checking of all the references in the articles retrieved to identify eventual additional non-indexed literature.
In physiological conditions, the liver has a unique microenvironment in which a delicate balance between cells of the innate and adaptive immune systems is required to maintain a strong immunosuppressive microenvironment[3,4].
Daily, about 80% of liver blood flow derives from the gastrointestinal tract through the portal vein carrying high concentrations of pathogen-derived molecules. Due to this high load of bacterial antigen, liver immunosurveillance plays a crucial role in maintaining self-tolerance, thus avoiding a severe immune self-response[5,6].
There are many cell populations involved in the hepatic tolerogenic process. The first line of defense is represented by liver sinusoidal endothelial cells (LSECs), the most abundant non-parenchymal liver cells with scavenger and immunologic functions. These unique cells have a high expression of several scavenger receptors, such as mannose receptors, major histocompatibility complex (MHC) class I (MHC-I) and MHCII[7]. These surface receptors allow for internalization of the antigens of pathogens, presenting them directly to T lymphocytes. Their role as antigen presenting cells (APCs) together with the increasing expression of co-inhibitory molecules, such as programmed death-ligand 1 (PD-L1) on LSECs, after recognition of antigens drives CD8+ T cell tolerance. Moreover, LSECs have been shown to influence APC functions of dendritic cells (DCs), leading to a reduction in their ability to activate T cells[8,9].
KCs are tissue resident macrophages located throughout the liver sinusoids. KCs have phagocytic and cytokine secretion activities, thus eliminating circulating molecules and releasing IL-10 and transforming growth factor beta (TGFβ), which leads to suppression of T cell activity[10,11].
Furthermore, the liver is rich with innate immune cells, including NK and NK T cells, and cells from the adaptive immune system, such as T and B lymphocytes. Under a steady-state condition, the balance of these cell functions is crucial for preventing the acute immune response within the liver against common gut pathogens.
In the last two decades, tumorigenesis has been recognized as a complex and dynamic process orchestrated by multiple different cell types, with each one of them playing a key role in tumoral development and progression[12].
Despite cancer cells, which have developed as a consequence of genetic mutations, holding the main role in driving carcinogenesis, an increasing interest has been aimed to TME, pointed out as a contributor to progression and metastatization of several tumors[13,14].
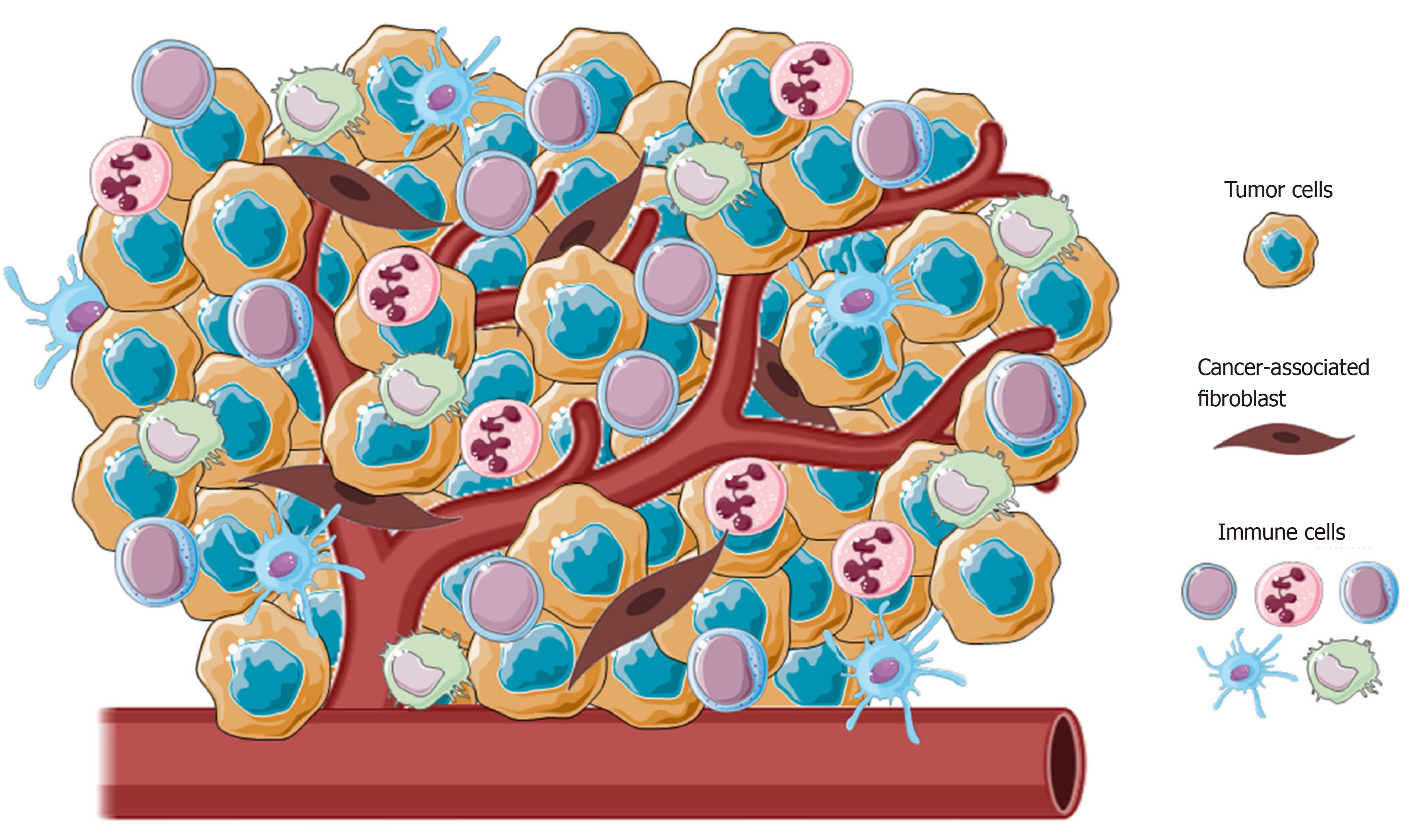
Cancer-associated fibroblasts (CAFs), blood and lymphatic vascular network, extracellular matrix and immune cells from both innate and adaptive immunity are principal components of TME (Figure 1)[15]. In normal conditions, the main role of immune surveillance is protection against pathogens, maintenance of tissue homeostasis and eradication of incipient cancer cells[16,17]. In contrast, in sites of chronic inflammation, as in neoplasia, immune inflammatory cells could persist and display an aberrant effect on cancer cells.
Immune cells could be present at any sites of tumor, from the center to the invasive margin, and their location or density have been largely demonstrated having a chief regulatory effect in promoting tumor progression[18,19]. These immune cells sculpt the TME through the secretion of several molecules, as growth factors, cytokines and chemokines, which in turn sustain and augment this inflammatory state, stimulating cancer cell proliferation, tumor angiogenesis and spreading[20-22].
Furthermore, the TME has been described to be a prognostic factor for several tumors and involved in the response of tumors to conventional therapies[22,23]. This recent comprehension of a tumor as a multi-cellular complex has allowed us to understand the mechanism underlying the crosstalk between cancer and tumor-infiltrating immune cells to design immune-based therapy[24].
Most of the research on therapies targeting the TME have been extensively focused on enhancing T cell cytotoxic activities. Immune checkpoint inhibitors, as antagonists of programmed cell death protein 1 (PD-1), PDL-1 and cytotoxic T lymphocyte antigen 4 (CTLA4), have been promising and efficacious in ameliorating patient prognosis with different solid tumors and hematological malignancies[25-27].
On the other hand, from innate immunity, NK cells are gaining more attention. These cells have been recognized to have a role in immune surveillance by excreting cytotoxic substances that eliminate malignant cells[28]. Studies highlighted the positive correlation between NK cell infiltration in tumors and a better prognosis[29,30]. Despite that, in tumors, they exhibit low to no cytotoxic activity, due to the immuno-suppressive environment of the TME[31,32].
Efforts in identifying of mechanism aimed to restore the anti-tumoral effect of NK cells could represent the basis for developing new immune-based therapeutic strategies, leading to more effective treatments in combination with conventional therapies[33-35].
Since their discovery, NK cells have been valued for their rapid recognition and clearance of tumor cells without previous stimulation and antigenic specificity[31,36].
NK cells are able to kill tumor cells through several mechanisms, including exocytosis of cytotoxic perforin and granzymes; tumor necrosis factor (TNF) family death receptors, such as fas ligand (FasL) or TNF-related apoptosis-inducing ligand (TRAIL); antibody-dependent cellular cytotoxicity; and pro-inflammatory cytokine release, such as interferon (IFN)-γ[37]. In healthy adults, NK cells represent about 10%-15% of circulating lymphocytes that are subdivided into two distinct subsets defined on the basis of the cellular membrane expression of CD56 and CD16, namely, CD56bright (CD56brightCD16neg) and CD56dim (CD56dimCD16pos)[37,38]. The two CD56bright and CD56dim NK cell subsets are distinct in their tissue distribution and their roles in immunity. Mainly represented in the blood (up to 90%), the CD56dim NK subset primarily acts through their high cytotoxic activity, although growing evidence shows their capability to also produce IFN-γ[39]. On the other hand, the CD56bright NK subset is less cytotoxic but exerts important immune-regulatory functions through secretion of chemokines and pro-inflammatory cytokines (i.e., IFN-γ and TNF-a) in response to different stimuli (i.e., IL-1β, IL-2, IL-12, IL-15 and/or IL-18) delivered by surrounding cells at tissue sites (i.e., macrophages, DCs and T lymphocytes)[36,40,41]. In fact, the CD56bright subset represents only 5-10 % of circulating NK cells and mainly resides in peripheral tissues such as liver, and gastrointestinal and female reproductive tracts. Although it is still being debated, CD56bright NK cells are largely accepted as precursors of more differentiated CD56dim NK cells[42].
The activation of NK cells is controlled by an array of inhibitory and activating NK cell receptors (iNKRs and aNKRs, respectively) differently expressed at their cell surface[43]. In resting conditions, NK cell cytotoxic activity is repressed due to the inhibitory receptors, including inhibitory killer Ig-like receptors (KIRs) and the C-type lectin receptor NKG2A, recognizing alleles of MHCI. Based on the “missing self-hypothesis”[44], the absence of MHC-I on target/tumor cells gives “license” to NK cell killing through aNKRs, such as natural cytotoxicity receptors (NCRs; NKp30, NKp46, and NKp44), the C-type lectin receptors NKG2D and NKG2C, DNAX accessory molecule-1 (DNAM-1) and activating KIRs (aKIRs) that bind their putative ligands on stressed, viral infected or tumor cells[45].
Human liver resident NK (lr-NK) cells were described for the first time in the late 1970s and defined as highly cytotoxic NK cells resident in the hepatic sinusoids[46,47]. Differently to peripheral blood, CD56dim and CD56bright NK cells are present at similar frequencies in human liver[48,49]. Recently, the specific phenotype of human CD56bright lr-NK cells has been described with the constitutive expression of the chemokine receptors CXCR6 and CCR5, along with the tissue-residency marker CD69[48,50,51]. The mechanisms involved in the recruitment of NK cells at the liver site are still unclear, although the interaction of NK cells with sinusoidal endothelial cells certainly plays a key role. Additionally, high expression of CXCR3, CXCR6 and CCR5 on lr-NK cells may play an important role in their liver retention, since their cognate ligands (i.e., CCL3, CCL5 and CXCL16) are highly produced by cholangiocytes, sinusoidal endothelial cells, hepatocytes and KCs[48]. Lr-NK cells are characterized by strong cytotoxic activity, high constitutive expression of TRAIL and FasL and secretion of IFN-γ, TNF-a, granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-2[52-54]. On the other hand, the human liver has developed a high degree of immune tolerance[55]. In order to maintain a tolerogenic liver environment, KCs produce high doses of IL-10 that are critical in the control of NK cell-mediated alloreactivity[56,57]. Moreover, the interplay between lr-NK cells and DCs induces the expansion of tolerogenic T cells (T regs) via the engagement of the inhibitory NKG2A receptor[58]. Lr-NK cells are also important in liver regeneration after tissue damage[59,60]. The interaction of NK cells with KCs, fibroblasts and stem cells induces the secretion of several soluble factors able to induce the proliferation of hepatic cells[59,61], although overstimulation of lr-NK cells can inhibit, rather than promote, liver regeneration[62].
Interestingly, in the liver, lr-NK cells have been identified to be endowed with a unique so-called “memory-like” NK (ml-NK) phenotype[63]. Human ml-NK cells have been described in cytomegalovirus (CMV) infection, resulting in the specific NKG2C+ phenotype able to produce a higher amount of IFN-γ upon being re-challenged with the same virus[64,65]. However, the existence of these ml-NK cells in other human virus infections, such as hepatitis, have not been reported yet. Thus, the impact of the liver in generation of highly heterogenous lr-NK cell subsets reflect either their cytotoxic or tolerogenic profiles.
HCC is the first liver tumor and the third leading cause of cancer-related death worldwide[66,67]. The majority of HCC occurs in patients with underlying liver disease. Patients with chronic liver disease have sustained hepatic inflammation, fibrosis and aberrant hepatocyte regeneration[68]. Specifically, there are some clearly defined agents related to cancer development, including HBV, HCV, metabolic causes (non-alcoholic fatty liver disease and nonalcoholic steatohepatitis) and external factors (e.g., aflatoxin)[69-71]. Timing of HCC diagnosis is crucial for patient life expectancy[72]. At the early stage, surgical resection, transplantation or local ablation have been demonstrated to improve clinical outcomes in patients with HCC, despite a high recurrence rate of about 70% at 5 years[73-76].
Unfortunately, more than 50% of newly diagnosed patients already have advanced or unresectable disease. For these patients, prognosis and treatment are very challenging, in particular when underlying liver dysfunction could limit most of the available therapeutic options.
Outcomes with traditional chemotherapies have been investigated in several clinical trials with no statistically significative improvement in OS[77-79]. Lack of response is notoriously described for HCC and may be due to the chemo-refractoriness of hepatocytes, which are able to express a variety of multi-drug resistance genes and p53 mutations[80,81]. Moreover, cytotoxic therapies may be limited in the setting of advanced stage disease if underlying liver cirrhosis is present[82]. This highlights that the development of new therapies is fundamental for the management of patients with HCC. In the last few year, increasing attention has been focused on target therapy to develop a more effective treatment for patients with HCC[83].
In 2007, sorafenib was approved as a first-line treatment for patients with advanced or metastatic HCC, showing an increase in the OS of patients compared to standard treatment[84-87]. Sorafenib, is a multi-kinase inhibitor with anti-proliferative and anti-angiogenic effects, increases HCC cell apoptosis by blocking several molecular targets, including Raf/MEK/ERK pathway, vascular endothelial growth factor receptor (VEGFR)-2, VEGFR-3, platelet-derived growth factor receptor beta (PDGFR-β) and many other tyrosine kinases[88,89]. Despite such efforts, the median life expectancy of patients with HCC treated with sorafenib is about 1 year[90].
In these patients, an adverse scenario, immunotherapy, is gaining a relevant role as a potential tool for new immune target strategies focused on counteracting the immunosuppressive HCC microenvironment.
HCC has been recognized to be an immunogenic cancer, arising from chronic liver inflammation, as a result of viral infection or toxin[91,92]. Several studies have shown that this tumoral milieu, which is also enriched in several pro-inflammatory chemokines released by tumoral and non-tumoral cells, enhances the immunosuppressive physiological microenvironment contributing to HCC pro-gression[93,94].
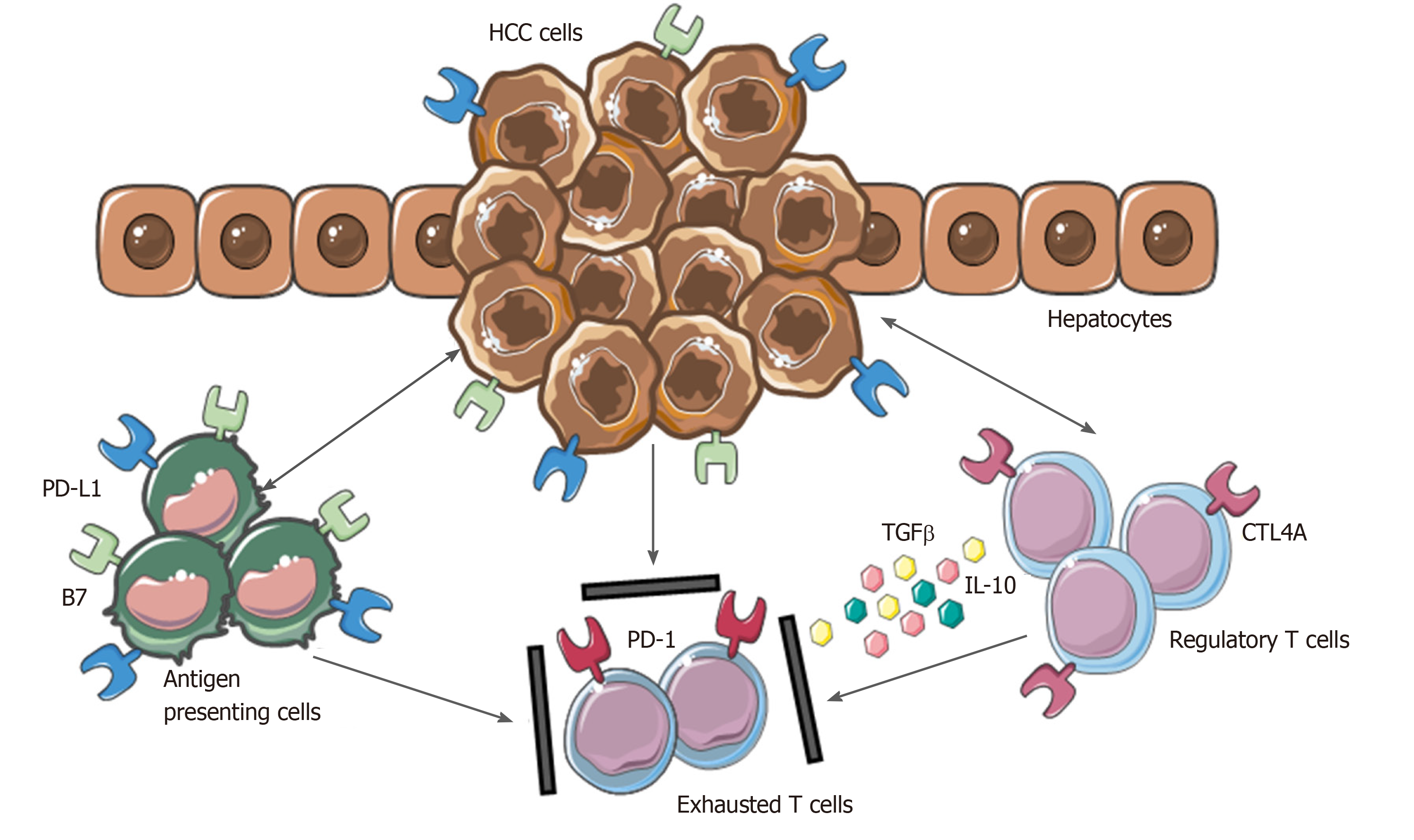
As a result of the persistent inflammatory state, HCC has a rich immune infiltrate, in which tumor-infiltrating lymphocytes (TILs) represent one of the most abundant populations within the TME[95]. Tumor-associated Treg cells have been shown to have a detrimental impact, favoring tumor immune evasion by markedly reducing the activity of effector cells through secretion of both IL-10 and TGFβ and cell-cell interaction[96].
Moreover, the inhibitory PD-L1 molecule was found to be highly expressed in patients with HCC from KCs, tumor cells and LSECs correlating with PD-1 upregulation on CD8+ cytotoxic cells. These mechanisms lead to the dysfunctional activity of CD8+ cells resulting in an exhausted phenotype (Figure 2).
Of note, the balance between Treg and cytotoxic T cells infiltration has been found to correlate with the prognosis. A higher presence of CD4+ cells in tumoral areas correlate with increased recurrence risk and worse OS[97-99]. In this regard, the overexpression of immune checkpoints in cancer and TME cells and their related pivotal role in HCC may represent a promising therapeutic strategy for counteracting anti-tumor immunity.
To date, several clinical trials are ongoing to investigate the efficacy of blockade of three immune checkpoints (anti-CTLA-4 and anti-PD-1/PD-L1) as a single agent or in combination with standard therapies for the treatment of advanced HCC[100]. Besides the promising results observed in these clinical trials, increasing attention has been focused on NK cells to develop a new immune strategy. Their role, localization and future perspective in HCC are discussed in detail below.
Hepatic NK cells are thought to play an important role in the immunological protection against different liver cancers, including HCC[52,53,101,102]. However, several numerical and phenotypic changes have been described in NK cells during the development of HCC. Patients with HCC at various stages of disease show both reduced frequencies and absolute number of peripheral blood NK cells. In particular, the specific CD56dim NK cell subsets displayed a dramatic reduction in patients with HCC[103,104]. Studies focused on intrahepatic NK cells in patients with HCC also observed the reduction in the frequency of tumor-infiltrating NK cells compared to tumor-adjacent lr-NK cells, which was mainly related to the reduced number of the CD56dim NK cell subset[103,105]. However, a higher number of total CD56+ (CD56dim and CD56bright) tumor- infiltrating NK cells predict a better outcome with regard to OS in patients with HCC[106,107].
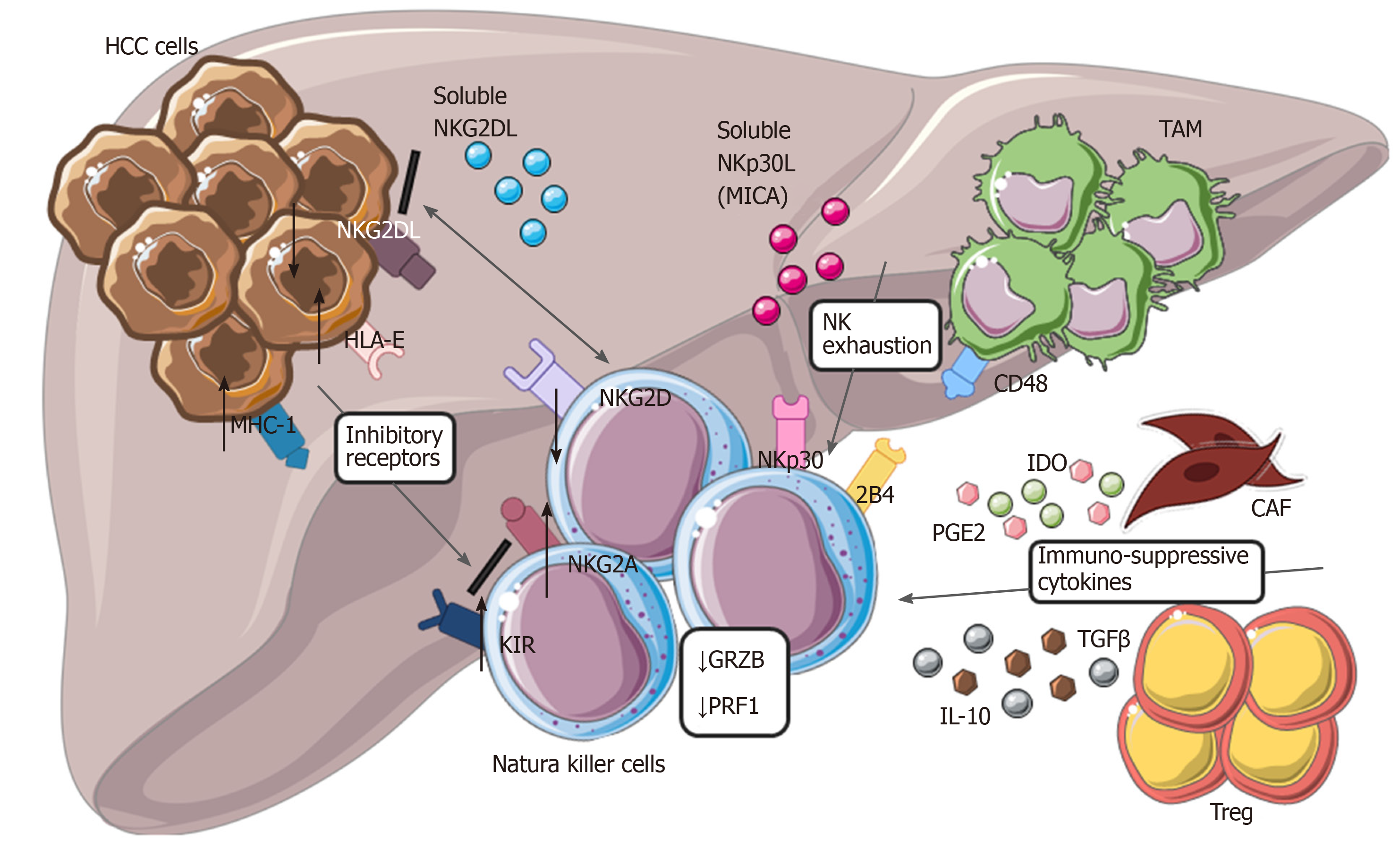
In addition, some studies have reported the increased frequency of specific NK cell subsets associated with slower HCC progression, as was observed for CD11b-/CD27- NK cells[108]. Regarding the specific molecular mechanism that boosts anti-tumor NK cell activity, the engagement of NKG2D activating receptor has been shown to enhance NK cell cytotoxicity against HCC[109]. However, this potent anti-tumor NK cell effector-function against HCC seems to be more effective in the early stages of HCC and decreases as soon as the tumor progresses. In fact, the reduced frequency of both circulating and intrahepatic NK cells was particularly noticeable in patients with advanced stages of HCC[110]. Moreover, both CD56dim and CD56bright NK cells in patients with end-stage HCC exhibited anergic effector functions in the peripheral blood and at the tumor site[103]. Specifically, reduced NK cell cytotoxic activity (i.e., lower production of granzymes and cytotoxic perforin) and lower secretion of cytokines (i.e., TNF-a and IFN-γ) associated with the progression and invasion of HCC were reported[111,112]. Various mechanisms are involved in the functional impairment of NK cells in advanced HCC[113]. For instance, down-modulation of NKG2D results in defective NK cell activation and recognition of tumor cells[109,114,115].
On the other hand, excessive stimulation of numerous inhibitory receptors expressed on NK cells negatively control their anti-tumor response. In particular, expression of the specific inhibitory NKp30 splice-variant along with higher levels of its soluble ligand (NKp30L) B7-H6 were found in patients with the late stages of HCC[115]. The aberrant engagement of the NKp30 pathway and CD48/2B4 interaction with tumor-infiltrating macrophages also induce rapid NK cell exhaustion[110,111,116]. Moreover, the specific immunogenetic profile of KIR/HLA affects the prognosis of patients with HCC[117]. Likely, high expression of HLA-E molecule in HCC triggers the inhibitory NKG2A receptor[118]. Albeit KIRs and NKG2A, several other immune checkpoints, including PD-1, Tim-3 and CD96, can inhibit the activity of NK cells in HCC[119]. Poor clinical outcomes for patients with HCC correlates with expression of PD-1 and CD96 on tumor-infiltrating NK cells[120,121] (Figure 3).
Additionally, the increased expression of PD-1 and Tim-3 on NK cells was found to significantly increase during chronic HBV and HCV infections[97,122]. Another mechanism contributing to NK cell impairment in HCC relies on the expansion of CD4+/CD25+ T regs and increased secretion of the immunosuppressive cytokines, including IL-10 and TGFβ[110,111,123]. The variations in the cytokine milieu able to inhibit cytotoxic activity and secretion of cytokines by NK cells in HCC also include soluble immunomodulators, such as TGFβ, prostaglandin E2 (PGE2) and indoleamine 2,3-dioxygenase (IDO)[124,125].
Numerus strategies employed by HCC to evade NK cell immunosurveillance in later stages of the disease are used for HCC treatment[112,113]. Molecular target drugs such as sorafenib, a multikinase inhibitor, and bortezomib, a proteasome inhibitor, trigger hepatic NK cell antitumor responses, resulting in higher cytotoxicity and IFN-γ production[126]. In addition, histone deacetylase inhibitors (HDACis) promote MICA or MICB expression on hepatoma cells, thus increasing the susceptibility of hepatoma cells to NK cell-mediated lysis[127,128]. Recently, therapeutic strategies have been focused on targeting NK cell checkpoints, such as NKG2A, KIRs, PD-1 and CTLA4 to boost activation and reverse dysfunction in NK cells[129]. In addition, several clinical studies have demonstrated the efficacy of allogenic NK cells in adoptive immunotherapy of HCC treatment[130].
CCA accounts for about 15% of all primary liver malignancies and is second after HCC in number of cases[131,132]. Indeed, CCA is a group of heterogeneous tumors arising at different levels of the biliary tree.
The most recent classification describes three different CCA subtypes looking at different anatomical regions: iCCA, peri-hilar CCA (pCCA) and distal CCA (dCCA)[133,134]. Several studies in the last two decades revealed that the incidence of these subtypes is vary. iCCA incidence has been increasing in contrast with the decrease of pCCA and dCCA incidence[135-137].
Different risk factors and survival rates seem to be related to each of them. Despite the etiologies remaining unclear in most cases of CCA, some risk factors are well established. For example, liver flukes (Opistorchis viverrini and Clonorchis sinensis) have been clearly associated with iCCA in East Asia. On the other hand, especially in Europe, primary sclerosing cholangitis (PSC) is a demonstrated risk factor for CCA, specifically correlated to pCCA variant. Viral hepatitis (HBV and HCV) has been identified as definitive risk factors more associated with iCCA than pCCA. Emerging role have been given to metabolic syndrome, alcohol and smoking[138-140].
Diagnosis is usually tardive due to its vague symptoms. Patients with iCCA are generally asymptomatic (20%-25% incidental finding), appearing tardively cachectic, with abdominal pain and fatigue. In contrast, pCCA most frequently manifests as painless jaundice. No proper biomarkers are available and diagnosis is a combination of clinical, radiological and unspecific histologic-biochemical markers. It has long been argued that a staging system for CCA needs to be found[133,141,142].
iCCA treatment is very dismal due to the well-known lack of response to the conventional chemotherapy[143,144]. Nowadays, surgical resection together with liver transplantation, for highly selected patients, are the only potentially curative treatments for iCCA, with median disease-free survival (DFS) duration of 12–36 mo. For advanced stage and unresectable iCCA, transarterial chemoembolization (TACE) is a treatment option considered to prolong OS in patients[145,146].
For patients with iCCA, due to the lack of effective curative strategies, understanding the molecular mechanism together with the TME interactions involved in tumoral progression and chemoresistance could open the possibility of developing new potential target therapies.
CCA is characterized by a dense desmoplastic TME composed of high stromal cell infiltration together with immune cells from the adaptive and innate immune systems[147]. In the last years, several studies have been focused on understanding the mechanism underlying the interplay between stromal cells and neoplastic cells in CCA.
In the liver, CCA CAFs have been shown to have multiple sources of origin, including from hepatic stellate cells, circulating bone marrow-derived precursor cells and portal fibroblasts[148,149].
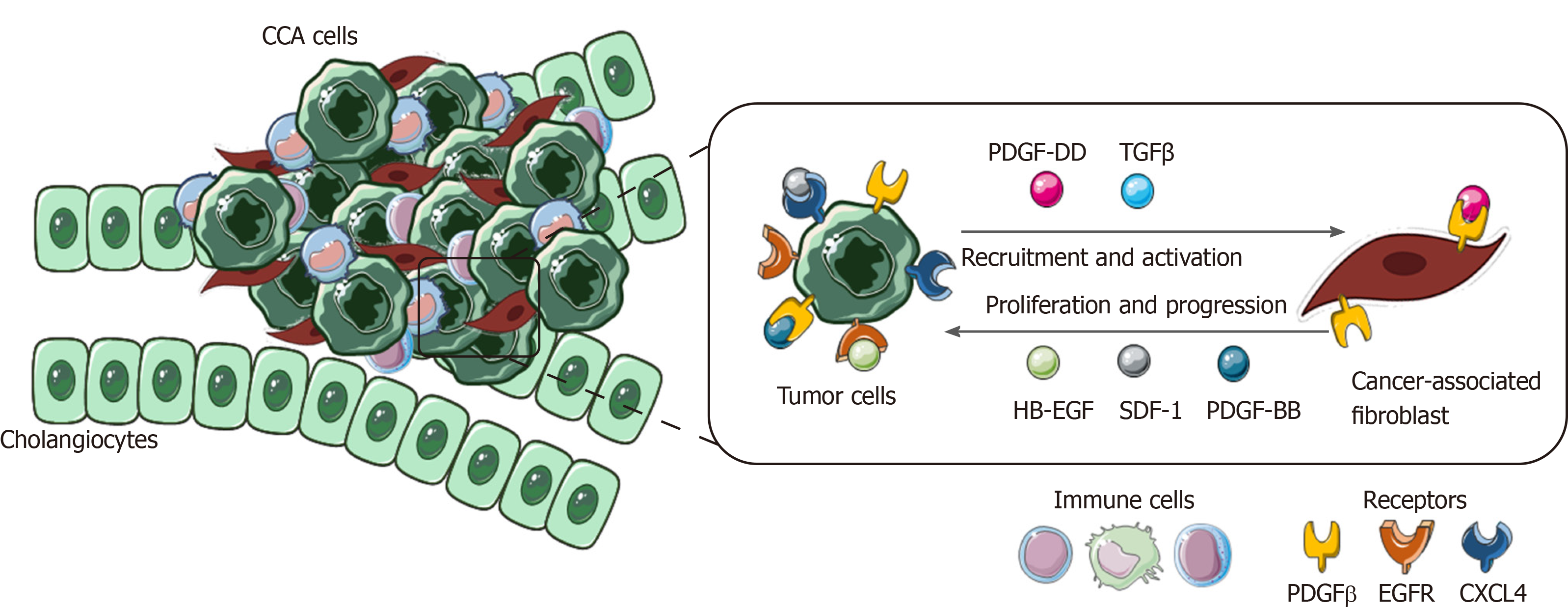
In the tumoral milieu, CCA cells have been reported to be the main cellular component secreting PDGF-DD, which acts by binding to its receptor PDGFR-β expressed on CAFs, leading to their recruitment in the tumor site, together with other factors, such as TGFβ[150,151].
Once recruited and activated within the tumor, CAFs are able to enhance CCA growth and progression through the secretion of tumor matrix, providing the scaffold and the release of various soluble factors[152-154].
PDGF-BB is an important paracrine survival signal released by CAFs that influence tumor malignant phenotype. By binding to its cognate receptor, PDGFR-β on CCA cells, it leads to an intracellular signaling cascade able to protect tumor cells from TRAIL-induced apoptosis by activating Hedgehog signaling and sustaining TGFβ secretion[155,156].
Among cytokines, chemokines and growth factors released by CAFs, stromal cell-derived factor-1 (SDF)-1 and heparin-binding EGF-like growth factor (HB-EGF) have key roles in promoting proliferation, migration and invasion of CCA cells expressing their related receptors, CXCL4 and EGFR. In the meantime, the secretion of HB-EGF by CAFs is sustained by a TGFβ feedback release from CCA cells, upon EGFR activation[157-159].
This highlights the presence of multiple paracrine signals exchanged between CCA cells and CAFs. The latter promotes tumor cell proliferation and invasion and in turn actively recruited and activated by CCA in a self-perpetuating loop (Figure 4).
Due to key role of stromal cells in CCA TME, in the last few years, several studies have been focused on targeting CAFs and their released factors[160-162]. The most recent study investigated the role of an FDA-approved anti-fibrotic drug called nintedanib. This tyrosine kinase inhibitor is able to inhibit fibroblast growth factor receptor (FGFR) and PDGFR, showing promising results in reduction of CCA growth and aggressiveness both in vitro and in vivo[163]. The emerging important role of CAFs could be an attractive target to ameliorate the treatment of patients with CCA.
Together with CAFs, cells from the innate and adaptive immune systems have been found to infiltrate the TME and significantly sustain CCA malignant transformation. Among them, tumor-associated macrophages (TAMs) represent the most relevant primary immune cells.
Macrophages could be resident (KCs) or recruited from circulating monocytes via soluble factors, such as monocyte chemoattractant protein 1 (MCP-1/CCL2), released in the tumor milieu[151,164]. Once in the liver, they differentiate into tissue macrophages, acquiring specific subset and activation status according to the exposition of multiple signals in the TME[165].
Normally, macrophages could be polarized in two possible phenotypes: M1 (anti-tumorigenesis), which is activated by INF-g, and M2 (pro-tumorigenesis) in response to anti-inflammatory cytokines, such as IL-10, TGFβ and IL-4[166,167].
In CCA TME, studies revealed that either CAF or CCA cells are involved in TAM recruitment and inducement of a M2 phenotype via the STAT3 pathway. TAMs polarized as pro-tumorigenic, the most representative TAM in TME, have been shown to promote tumor progression and modulation of the surrounding microenvironment through the subsequent secretion of pro-inflammatory and tumor-promoting mediators (IL-6, IL-1, TGFβ, VEGF and PDGF)[147,168,169]. Indeed, increasing evidence has shown that, in iCCA, the higher number of M2 TAMs correlated with a poor prognosis[170-172].
In the last year, more attention has been paid to neutrophils, the most abundant of the white blood cells, which represent one of first lines of defense against invading pathogens. Different studies have revealed that neutrophils are recruited within tumors driven by CXCL5, a chemotactic cytokine. Tumor-associated neutrophils (TANs) and elevated pre-operative neutrophil to lymphocyte ratio (NLR) are associated with a poor prognosis in patients with iCCA[173-176].
Further studies are needed to deeper elucidate the role of TAMs and TANs in iCCA, in terms of possible use as predictive markers for patients undergoing surgical resection. As described for HCC, iCCA arises in a chronically inflamed liver as a consequence of bile duct injury and the related TME contributes to the development of an anti-tumor immune milieu.
CCA cells with CAFs and TAMs are able to secrete various factors, such as CCL2, recruiting and stimulating T regs. These release back IL-10 and TGFβ that inhibit cytotoxic T cells, suppressing the immune response[177,178]. Such mechanisms are similar and have already been described for HCC. Despite this, not much literature is present on TILs in iCCA, focusing mainly on immunohistochemistry analysis in terms of number and presence of CD4+ and CD8+ cells.
Briefly, CD4+ cells have been found to infiltrate tumor specimens. On the contrary, CD8+ cells are present at the tumor margin, correlating with prognosis. Patients with iCCA with low infiltration of cytotoxic T cells show a worse prognosis[179,180]. The presence of both TIL and PDL-1/PD-1 expression makes iCCA possibly suitable for immunological target therapies[181,182].
Less efforts have been made to unveil the specific role of NK cells in iCCA[151]. However, several preclinical and clinical studies have assessed the activity of NK cells against iCCA. The use of in vitro cytokine-activated NK cells in combination with cetuximab, the mAb against EGFR, has shown benefits in a higher antibody-dependent cellular cytotoxicity response against human iCCA cell lines such as HuCCT-1 and OZ[183]. Moreover, the multiple infusions of ex vivo-expanded human NK cells into iCCA xenograft mice (HuCCT-1 tumor-bearing nude mice) resulted in NK cell-mediated cytolytic response with inhibition of tumor growth[184].
Recently, an elevated intra-tumoral expression of CXCL9, an IFN-γ inducible chemokine, was associated with a large number of tumor-infiltrating NK cells, leading to favorable postoperative survival in patients with iCCA[185]. Additionally, elevated expression of NKG2D ligands in human iCCA correlate with improved DFS and OS in patients[186]. Although these findings hold promise, further studies are needed to investigate the role of NK cells in the pathogenesis of iCCA. In fact, similar to HCC, strategies with the aim of evading NK cell immunosurveillance in CCA have been reported. For instance, iCCA cells are able to induce apoptosis in NK cells, via the Fas/FasL pathway, and escape the inflammatory response by upregulating the antiapoptotic c-FLIP system[187]. On the other hand, several nucleotide polymorphisms (SNPs) located within the NKG2D receptor gene (KLRK1) have been linked to impaired NK cell effector functions and higher risk of cancer[188].
Specifically, the development of CCA in patients with PSC have been associated with polymorphisms in the NKG2D gene, thus patients who are homozygous for the NKG2D alleles are likely to develop CCA. These data clearly support different roles and clinical impacts of NK cells in iCCA disease. However, it is still not clear how these activities are related to the specific blood circulating and liver resident NK cells.
The recent advances in the understanding the important cross-talk between cancer cells and cell infiltrating TME allowed to identify various mechanisms underlying tumor development and progression. The pathways beyond this cells-cells cooperation have been demonstrated to have harmful role in impaired immune cells activation and also in therapeutic response. In particular, NK cells have been reported to have a prominent role in maintaining the homeostasis in the liver even in case of liver tumors. Yet, new therapies based on targeting NK cells with the aim to restore their impaired cytotoxic activity within tumor are gaining attention. In the era of precision medicine, this challenging research area could open the possibility to develop new potential therapeutic strategies in combination with conventional therapies for the treatment of HCC and iCCA patients.
In this review, we have examined the key pathways underlying TME cell-cell communications, with deeper focus on the role of natural killer cells in primary liver tumors, such as HCC and iCCA, as new opportunities for immune-based therapeutic strategies.
The authors thank Dr. Soldani C, Dr. Franceschini B and Dr. Costa G from the Hepatobiliary Immunopathology Laboratory, Humanitas Clinical and Research Center – IRCCS, Rozzano, Milan (Italy) for their contribution in the reviewing the pertinent literature.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Manfredi S S-Editor: Yan JP L-Editor: A P-Editor: Ma YJ
| 1. | Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904-5912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1334] [Cited by in RCA: 1728] [Article Influence: 101.6] [Reference Citation Analysis (0)] |
| 2. | Wang M, Zhao J, Zhang L, Wei F, Lian Y, Wu Y, Gong Z, Zhang S, Zhou J, Cao K, Li X, Xiong W, Li G, Zeng Z, Guo C. Role of tumor microenvironment in tumorigenesis. J Cancer. 2017;8:761-773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1071] [Cited by in RCA: 955] [Article Influence: 119.4] [Reference Citation Analysis (0)] |
| 3. | Jenne CN, Kubes P. Immune surveillance by the liver. Nat Immunol. 2013;14:996-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 780] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 4. | Horst AK, Neumann K, Diehl L, Tiegs G. Modulation of liver tolerance by conventional and nonconventional antigen-presenting cells and regulatory immune cells. Cell Mol Immunol. 2016;13:277-292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 203] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 5. | Son G, Kremer M, Hines IN. Contribution of gut bacteria to liver pathobiology. Gastroenterol Res Pract. 2010;2010:453563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 6. | Trivedi PJ, Adams DH. Gut-liver immunity. J Hepatol. 2016;64:1187-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 7. | Wohlleber D, Knolle PA. The role of liver sinusoidal cells in local hepatic immune surveillance. Clin Transl Immunology. 2016;5:e117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | Thomson AW, Knolle PA. Antigen-presenting cell function in the tolerogenic liver environment. Nat Rev Immunol. 2010;10:753-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 598] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 9. | Shetty S, Lalor PF, Adams DH. Liver sinusoidal endothelial cells - gatekeepers of hepatic immunity. Nat Rev Gastroenterol Hepatol. 2018;15:555-567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 336] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 10. | Bilzer M, Roggel F, Gerbes AL. Role of Kupffer cells in host defense and liver disease. Liver Int. 2006;26:1175-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 586] [Cited by in RCA: 617] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 11. | Dixon LJ, Barnes M, Tang H, Pritchard MT, Nagy LE. Kupffer cells in the liver. Compr Physiol. 2013;3:785-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 461] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 12. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 47087] [Article Influence: 3363.4] [Reference Citation Analysis (5)] |
| 13. | Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH, Wang XZ, Zhao YW, Wei YQ. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS One. 2012;7:e50946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 677] [Cited by in RCA: 803] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 14. | Galdiero MR, Garlanda C, Jaillon S, Marone G, Mantovani A. Tumor associated macrophages and neutrophils in tumor progression. J Cell Physiol. 2013;228:1404-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 322] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 15. | Chen F, Zhuang X, Lin L, Yu P, Wang Y, Shi Y, Hu G, Sun Y. New horizons in tumor microenvironment biology: challenges and opportunities. BMC Med. 2015;13:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 555] [Cited by in RCA: 525] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 16. | Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883-899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8437] [Cited by in RCA: 8174] [Article Influence: 544.9] [Reference Citation Analysis (0)] |
| 17. | Newton K, Dixit VM. Signaling in innate immunity and inflammation. Cold Spring Harb Perspect Biol. 2012;4:a006049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1055] [Cited by in RCA: 1174] [Article Influence: 90.3] [Reference Citation Analysis (0)] |
| 18. | DeNardo DG, Andreu P, Coussens LM. Interactions between lymphocytes and myeloid cells regulate pro- versus anti-tumor immunity. Cancer Metastasis Rev. 2010;29:309-316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 330] [Cited by in RCA: 382] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 19. | Gonzalez H, Hagerling C, Werb Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev. 2018;32:1267-1284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 765] [Cited by in RCA: 1426] [Article Influence: 203.7] [Reference Citation Analysis (0)] |
| 20. | Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1802] [Cited by in RCA: 2084] [Article Influence: 130.3] [Reference Citation Analysis (1)] |
| 21. | Ricciardi M, Zanotto M, Malpeli G, Bassi G, Perbellini O, Chilosi M, Bifari F, Krampera M. Epithelial-to-mesenchymal transition (EMT) induced by inflammatory priming elicits mesenchymal stromal cell-like immune-modulatory properties in cancer cells. Br J Cancer. 2015;112:1067-1075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 138] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 22. | Pottier C, Wheatherspoon A, Roncarati P, Longuespée R, Herfs M, Duray A, Delvenne P, Quatresooz P. The importance of the tumor microenvironment in the therapeutic management of cancer. Expert Rev Anticancer Ther. 2015;15:943-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 23. | Galon J, Pagès F, Marincola FM, Angell HK, Thurin M, Lugli A, Zlobec I, Berger A, Bifulco C, Botti G, Tatangelo F, Britten CM, Kreiter S, Chouchane L, Delrio P, Arndt H, Asslaber M, Maio M, Masucci GV, Mihm M, Vidal-Vanaclocha F, Allison JP, Gnjatic S, Hakansson L, Huber C, Singh-Jasuja H, Ottensmeier C, Zwierzina H, Laghi L, Grizzi F, Ohashi PS, Shaw PA, Clarke BA, Wouters BG, Kawakami Y, Hazama S, Okuno K, Wang E, O'Donnell-Tormey J, Lagorce C, Pawelec G, Nishimura MI, Hawkins R, Lapointe R, Lundqvist A, Khleif SN, Ogino S, Gibbs P, Waring P, Sato N, Torigoe T, Itoh K, Patel PS, Shukla SN, Palmqvist R, Nagtegaal ID, Wang Y, D'Arrigo C, Kopetz S, Sinicrope FA, Trinchieri G, Gajewski TF, Ascierto PA, Fox BA. Cancer classification using the Immunoscore: a worldwide task force. J Transl Med. 2012;10:205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 632] [Cited by in RCA: 634] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 24. | Kruger S, Ilmer M, Kobold S, Cadilha BL, Endres S, Ormanns S, Schuebbe G, Renz BW, D'Haese JG, Schloesser H, Heinemann V, Subklewe M, Boeck S, Werner J, von Bergwelt-Baildon M. Advances in cancer immunotherapy 2019 - latest trends. J Exp Clin Cancer Res. 2019;38:268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 399] [Cited by in RCA: 389] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 25. | Buchbinder EI, Desai A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am J Clin Oncol. 2016;39:98-106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1427] [Cited by in RCA: 1704] [Article Influence: 189.3] [Reference Citation Analysis (0)] |
| 26. | Rotte A. Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J Exp Clin Cancer Res. 2019;38:255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 345] [Cited by in RCA: 670] [Article Influence: 111.7] [Reference Citation Analysis (0)] |
| 27. | Topalian SL, Taube JM, Pardoll DM. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science. 2020;367:eaax0182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 702] [Article Influence: 140.4] [Reference Citation Analysis (0)] |
| 28. | Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2338] [Cited by in RCA: 2879] [Article Influence: 169.4] [Reference Citation Analysis (0)] |
| 29. | Xu B, Chen L, Li J, Zheng X, Shi L, Wu C, Jiang J. Prognostic value of tumor infiltrating NK cells and macrophages in stage II+III esophageal cancer patients. Oncotarget. 2016;7:74904-74916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 30. | Cursons J, Souza-Fonseca-Guimaraes F, Foroutan M, Anderson A, Hollande F, Hediyeh-Zadeh S, Behren A, Huntington ND, Davis MJ. A Gene Signature Predicting Natural Killer Cell Infiltration and Improved Survival in Melanoma Patients. Cancer Immunol Res. 2019;7:1162-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 199] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 31. | Pahl J, Cerwenka A. Tricking the balance: NK cells in anti-cancer immunity. Immunobiology. 2017;222:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 149] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 32. | Rezvani K, Rouce R, Liu E, Shpall E. Engineering Natural Killer Cells for Cancer Immunotherapy. Mol Ther. 2017;25:1769-1781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 325] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 33. | Veluchamy JP, Kok N, van der Vliet HJ, Verheul HMW, de Gruijl TD, Spanholtz J. The Rise of Allogeneic Natural Killer Cells As a Platform for Cancer Immunotherapy: Recent Innovations and Future Developments. Front Immunol. 2017;8:631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 143] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 34. | Hinshaw DC, Shevde LA. The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Res. 2019;79:4557-4566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 750] [Cited by in RCA: 2138] [Article Influence: 356.3] [Reference Citation Analysis (2)] |
| 35. | Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2103] [Cited by in RCA: 2012] [Article Influence: 143.7] [Reference Citation Analysis (0)] |
| 36. | Caligiuri MA. Human natural killer cells. Blood. 2008;112:461-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1216] [Cited by in RCA: 1409] [Article Influence: 82.9] [Reference Citation Analysis (0)] |
| 37. | Cichicki F, Schlums H, Theorell J, Tesi B, Miller JS, Ljunggren HG, Bryceson YT. Diversification and Functional Specialization of Human NK Cell Subsets. Curr Top Microbiol Immunol. 2016;395:63-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 38. | De Maria A, Bozzano F, Cantoni C, Moretta L. Revisiting human natural killer cell subset function revealed cytolytic CD56(dim)CD16+ NK cells as rapid producers of abundant IFN-gamma on activation. Proc Natl Acad Sci USA. 2011;108:728-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 282] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 39. | Fehniger TA, Cooper MA, Nuovo GJ, Cella M, Facchetti F, Colonna M, Caligiuri MA. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood. 2003;101:3052-3057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 637] [Cited by in RCA: 650] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 40. | Ferlazzo G, Pack M, Thomas D, Paludan C, Schmid D, Strowig T, Bougras G, Muller WA, Moretta L, Münz C. Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs. Proc Natl Acad Sci USA. 2004;101:16606-16611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 433] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 41. | Di Vito C, Mikulak J, Mavilio D. On the Way to Become a Natural Killer Cell. Front Immunol. 2019;10:1812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 42. | Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9:495-502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1343] [Cited by in RCA: 1269] [Article Influence: 74.6] [Reference Citation Analysis (0)] |
| 43. | Kärre K. Natural killer cell recognition of missing self. Nat Immunol. 2008;9:477-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 206] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 44. | Long EO, Kim HS, Liu D, Peterson ME, Rajagopalan S. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu Rev Immunol. 2013;31:227-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 967] [Cited by in RCA: 943] [Article Influence: 78.6] [Reference Citation Analysis (0)] |
| 45. | Luo DZ, Vermijlen D, Ahishali B, Triantis V, Plakoutsi G, Braet F, Vanderkerken K, Wisse E. On the cell biology of pit cells, the liver-specific NK cells. World J Gastroenterol. 2000;6:1-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 46. | Nakatani K, Kaneda K, Seki S, Nakajima Y. Pit cells as liver-associated natural killer cells: morphology and function. Med Electron Microsc. 2004;37:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 47. | Hudspeth K, Donadon M, Cimino M, Pontarini E, Tentorio P, Preti M, Hong M, Bertoletti A, Bicciato S, Invernizzi P, Lugli E, Torzilli G, Gershwin ME, Mavilio D. Human liver-resident CD56(bright)/CD16(neg) NK cells are retained within hepatic sinusoids via the engagement of CCR5 and CXCR6 pathways. J Autoimmun. 2016;66:40-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 201] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 48. | Mikulak J, Bruni E, Oriolo F, Di Vito C, Mavilio D. Hepatic Natural Killer Cells: Organ-Specific Sentinels of Liver Immune Homeostasis and Physiopathology. Front Immunol. 2019;10:946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 49. | Harmon C, Robinson MW, Fahey R, Whelan S, Houlihan DD, Geoghegan J, O'Farrelly C. Tissue-resident Eomes(hi) T-bet(lo) CD56(bright) NK cells with reduced proinflammatory potential are enriched in the adult human liver. Eur J Immunol. 2016;46:2111-2120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 113] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 50. | Stegmann KA, Robertson F, Hansi N, Gill U, Pallant C, Christophides T, Pallett LJ, Peppa D, Dunn C, Fusai G, Male V, Davidson BR, Kennedy P, Maini MK. CXCR6 marks a novel subset of T-bet(lo)Eomes(hi) natural killer cells residing in human liver. Sci Rep. 2016;6:26157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 193] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 51. | Li N, Puga Yung GL, Pradier A, Toso C, Giostra E, Morard I, Spahr L, Seebach JD. NK cell isolation from liver biopsies: phenotypic and functional analysis of low cell numbers by flow cytometry. Front Immunol. 2013;4:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 52. | Hudspeth K, Pontarini E, Tentorio P, Cimino M, Donadon M, Torzilli G, Lugli E, Della Bella S, Gershwin ME, Mavilio D. The role of natural killer cells in autoimmune liver disease: a comprehensive review. J Autoimmun. 2013;46:55-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 53. | Tang L, Peng H, Zhou J, Chen Y, Wei H, Sun R, Yokoyama WM, Tian Z. Differential phenotypic and functional properties of liver-resident NK cells and mucosal ILC1s. J Autoimmun. 2016;67:29-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 54. | Cunningham EC, Sharland AF, Bishop GA. Liver transplant tolerance and its application to the clinic: can we exploit the high dose effect? Clin Dev Immunol. 2013;2013:419692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 55. | Chen Y, Sun R, Jiang W, Wei H, Tian Z. Liver-specific HBsAg transgenic mice are over-sensitive to Poly(I:C)-induced liver injury in NK cell- and IFN-gamma-dependent manner. J Hepatol. 2007;47:183-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 56. | Lassen MG, Lukens JR, Dolina JS, Brown MG, Hahn YS. Intrahepatic IL-10 maintains NKG2A+Ly49- liver NK cells in a functionally hyporesponsive state. J Immunol. 2010;184:2693-2701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 57. | Jinushi M, Takehara T, Tatsumi T, Yamaguchi S, Sakamori R, Hiramatsu N, Kanto T, Ohkawa K, Hayashi N. Natural killer cell and hepatic cell interaction via NKG2A leads to dendritic cell-mediated induction of CD4 CD25 T cells with PD-1-dependent regulatory activities. Immunology. 2007;120:73-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 58. | Tosello-Trampont A, Surette FA, Ewald SE, Hahn YS. Immunoregulatory Role of NK Cells in Tissue Inflammation and Regeneration. Front Immunol. 2017;8:301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 59. | Li N, Hua J. Immune cells in liver regeneration. Oncotarget. 2017;8:3628-3639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 60. | Mattiola I, Pesant M, Tentorio PF, Molgora M, Marcenaro E, Lugli E, Locati M, Mavilio D. Priming of Human Resting NK Cells by Autologous M1 Macrophages via the Engagement of IL-1β, IFN-β, and IL-15 Pathways. J Immunol. 2015;195:2818-2828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 61. | Bi J, Zheng X, Chen Y, Wei H, Sun R, Tian Z. TIGIT safeguards liver regeneration through regulating natural killer cell-hepatocyte crosstalk. Hepatology. 2014;60:1389-1398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 62. | Majewska-Szczepanik M, Paust S, von Andrian UH, Askenase PW, Szczepanik M. Natural killer cell-mediated contact sensitivity develops rapidly and depends on interferon-α, interferon-γ and interleukin-12. Immunology. 2013;140:98-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 63. | Reeves RK, Li H, Jost S, Blass E, Li H, Schafer JL, Varner V, Manickam C, Eslamizar L, Altfeld M, von Andrian UH, Barouch DH. Antigen-specific NK cell memory in rhesus macaques. Nat Immunol. 2015;16:927-932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 224] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 64. | Hydes T, Abuhilal M, Armstrong T, Primrose J, Takhar A, Khakoo S. Natural killer cell maturation markers in the human liver and expansion of an NKG2C+KIR+ population. Lancet. 2015;385 Suppl 1:S45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 65. | Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913-2921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5379] [Cited by in RCA: 5134] [Article Influence: 466.7] [Reference Citation Analysis (0)] |
| 66. | Bruix J, Sherman M; Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4333] [Cited by in RCA: 4507] [Article Influence: 225.4] [Reference Citation Analysis (0)] |
| 67. | Yang JD, Roberts LR. Hepatocellular carcinoma: A global view. Nat Rev Gastroenterol Hepatol. 2010;7:448-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 979] [Cited by in RCA: 1060] [Article Influence: 70.7] [Reference Citation Analysis (0)] |
| 68. | Raza A, Sood GK. Hepatocellular carcinoma review: current treatment, and evidence-based medicine. World J Gastroenterol. 2014;20:4115-4127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 316] [Cited by in RCA: 340] [Article Influence: 30.9] [Reference Citation Analysis (4)] |
| 69. | Gomes MA, Priolli DG, Tralhão JG, Botelho MF. Hepatocellular carcinoma: epidemiology, biology, diagnosis, and therapies. Rev Assoc Med Bras (1992). 2013;59:514-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 70. | Tunissiolli NM, Castanhole-Nunes MMU, Biselli-Chicote PM, Pavarino EC, da Silva RF, da Silva RC, Goloni-Bertollo EM. Hepatocellular Carcinoma: a Comprehensive Review of Biomarkers, Clinical Aspects, and Therapy. Asian Pac J Cancer Prev. 2017;18:863-872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 60] [Reference Citation Analysis (0)] |
| 71. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4097] [Article Influence: 585.3] [Reference Citation Analysis (6)] |
| 72. | Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A; OLT for HCC Consensus Group. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13:e11-e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 761] [Cited by in RCA: 785] [Article Influence: 60.4] [Reference Citation Analysis (1)] |
| 73. | Roayaie S, Jibara G, Tabrizian P, Park JW, Yang J, Yan L, Schwartz M, Han G, Izzo F, Chen M, Blanc JF, Johnson P, Kudo M, Roberts LR, Sherman M. The role of hepatic resection in the treatment of hepatocellular cancer. Hepatology. 2015;62:440-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 321] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 74. | Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg. 2015;261:947-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 658] [Article Influence: 65.8] [Reference Citation Analysis (0)] |
| 75. | Kim S, Shin J, Kim DY, Choi GH, Kim MJ, Choi JY. Postoperative Recurrence of Hepatocellular Carcinoma: The Importance of Distinguishing between Intrahepatic Metastasis and Multicentric Occurrence-Response. Clin Cancer Res. 2019;25:5427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 76. | Nowak AK, Stockler MR, Chow PK, Findlay M. Use of tamoxifen in advanced-stage hepatocellular carcinoma. A systematic review. Cancer. 2005;103:1408-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 77. | Louafi S, Boige V, Ducreux M, Bonyhay L, Mansourbakht T, de Baere T, Asnacios A, Hannoun L, Poynard T, Taïeb J. Gemcitabine plus oxaliplatin (GEMOX) in patients with advanced hepatocellular carcinoma (HCC): results of a phase II study. Cancer. 2007;109:1384-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 139] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 78. | Cox J, Weinman S. Mechanisms of doxorubicin resistance in hepatocellular carcinoma. Hepat Oncol. 2016;3:57-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 120] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 79. | Hussain SP, Schwank J, Staib F, Wang XW, Harris CC. TP53 mutations and hepatocellular carcinoma: insights into the etiology and pathogenesis of liver cancer. Oncogene. 2007;26:2166-2176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 439] [Article Influence: 24.4] [Reference Citation Analysis (1)] |
| 80. | Meng X, Franklin DA, Dong J, Zhang Y. MDM2-p53 pathway in hepatocellular carcinoma. Cancer Res. 2014;74:7161-7167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 172] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 81. | Eatrides J, Wang E, Kothari N, Kim R. Role of Systemic Therapy and Future Directions for Hepatocellular Carcinoma. Cancer Control. 2017;24:1073274817729243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 82. | Chen S, Cao Q, Wen W, Wang H. Targeted therapy for hepatocellular carcinoma: Challenges and opportunities. Cancer Lett. 2019;460:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 177] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 83. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10261] [Article Influence: 603.6] [Reference Citation Analysis (2)] |
| 84. | Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3128] [Cited by in RCA: 3818] [Article Influence: 545.4] [Reference Citation Analysis (1)] |
| 85. | Longo L, de Freitas LBR, Santos D, Grivicich I, Álvares-da-Silva MR. Sorafenib for Advanced Hepatocellular Carcinoma: A Real-Life Experience. Dig Dis. 2018;36:377-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 86. | Personeni N, Pressiani T, Rimassa L. Lenvatinib for the treatment of unresectable hepatocellular carcinoma: evidence to date. J Hepatocell Carcinoma. 2019;6:31-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 87. | Cervello M, Bachvarov D, Lampiasi N, Cusimano A, Azzolina A, McCubrey JA, Montalto G. Molecular mechanisms of sorafenib action in liver cancer cells. Cell Cycle. 2012;11:2843-2855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 88. | Ziogas IA, Tsoulfas G. Evolving role of Sorafenib in the management of hepatocellular carcinoma. World J Clin Oncol. 2017;8:203-213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 89. | Llovet JM, Hernandez-Gea V. Hepatocellular carcinoma: reasons for phase III failure and novel perspectives on trial design. Clin Cancer Res. 2014;20:2072-2079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 323] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 90. | Hernandez-Gea V, Toffanin S, Friedman SL, Llovet JM. Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology. 2013;144:512-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 593] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 91. | Buonaguro L, Mauriello A, Cavalluzzo B, Petrizzo A, Tagliamonte M. Immunotherapy in hepatocellular carcinoma. Ann Hepatol. 2019;18:291-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 92. | Keenan BP, Fong L, Kelley RK. Immunotherapy in hepatocellular carcinoma: the complex interface between inflammation, fibrosis, and the immune response. J Immunother Cancer. 2019;7:267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 155] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 93. | Tahmasebi Birgani M, Carloni V. Tumor Microenvironment, a Paradigm in Hepatocellular Carcinoma Progression and Therapy. Int J Mol Sci. 2017;18:405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 138] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 94. | Lu C, Rong D, Zhang B, Zheng W, Wang X, Chen Z, Tang W. Current perspectives on the immunosuppressive tumor microenvironment in hepatocellular carcinoma: challenges and opportunities. Mol Cancer. 2019;18:130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 303] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 95. | Tu JF, Ding YH, Ying XH, Wu FZ, Zhou XM, Zhang DK, Zou H, Ji JS. Regulatory T cells, especially ICOS+ FOXP3+ regulatory T cells, are increased in the hepatocellular carcinoma microenvironment and predict reduced survival. Sci Rep. 2016;6:35056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 103] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 96. | Li H, Wu K, Tao K, Chen L, Zheng Q, Lu X, Liu J, Shi L, Liu C, Wang G, Zou W. Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma. Hepatology. 2012;56:1342-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 394] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 97. | Sideras K, Biermann K, Verheij J, Takkenberg BR, Mancham S, Hansen BE, Schutz HM, de Man RA, Sprengers D, Buschow SI, Verseput MC, Boor PP, Pan Q, van Gulik TM, Terkivatan T, Ijzermans JN, Beuers UH, Sleijfer S, Bruno MJ, Kwekkeboom J. PD-L1, Galectin-9 and CD8+ tumor-infiltrating lymphocytes are associated with survival in hepatocellular carcinoma. Oncoimmunology. 2017;6:e1273309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 116] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 98. | Dai X, Xue J, Hu J, Yang SL, Chen GG, Lai PBS, Yu C, Zeng C, Fang X, Pan X, Zhang T. Positive Expression of Programmed Death Ligand 1 in Peritumoral Liver Tissue is Associated with Poor Survival after Curative Resection of Hepatocellular Carcinoma. Transl Oncol. 2017;10:511-517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 99. | Kudo M. Immuno-Oncology Therapy for Hepatocellular Carcinoma: Current Status and Ongoing Trials. Liver Cancer. 2019;8:221-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 100. | Donadon M, Hudspeth K, Cimino M, Di Tommaso L, Preti M, Tentorio P, Roncalli M, Mavilio D, Torzilli G. Increased Infiltration of Natural Killer and T Cells in Colorectal Liver Metastases Improves Patient Overall Survival. J Gastrointest Surg. 2017;21:1226-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 101. | Peng H, Wisse E, Tian Z. Liver natural killer cells: subsets and roles in liver immunity. Cell Mol Immunol. 2016;13:328-336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 132] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 102. | Cai L, Zhang Z, Zhou L, Wang H, Fu J, Zhang S, Shi M, Zhang H, Yang Y, Wu H, Tien P, Wang FS. Functional impairment in circulating and intrahepatic NK cells and relative mechanism in hepatocellular carcinoma patients. Clin Immunol. 2008;129:428-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 229] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 103. | Fathy A, Eldin MM, Metwally L, Eida M, Abdel-Rehim M. Diminished absolute counts of CD56dim and CD56bright natural killer cells in peripheral blood from Egyptian patients with hepatocellular carcinoma. Egypt J Immunol. 2009;16:17-25. [PubMed] |
| 104. | Guo CL, Yang HC, Yang XH, Cheng W, Dong TX, Zhu WJ, Xu Z, Zhao L. Associations between infiltrating lymphocyte subsets and hepatocellular carcinoma. Asian Pac J Cancer Prev. 2012;13:5909-5913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 105. | Chew V, Tow C, Teo M, Wong HL, Chan J, Gehring A, Loh M, Bolze A, Quek R, Lee VK, Lee KH, Abastado JP, Toh HC, Nardin A. Inflammatory tumour microenvironment is associated with superior survival in hepatocellular carcinoma patients. J Hepatol. 2010;52:370-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 205] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 106. | Chew V, Chen J, Lee D, Loh E, Lee J, Lim KH, Weber A, Slankamenac K, Poon RT, Yang H, Ooi LL, Toh HC, Heikenwalder M, Ng IO, Nardin A, Abastado JP. Chemokine-driven lymphocyte infiltration: an early intratumoural event determining long-term survival in resectable hepatocellular carcinoma. Gut. 2012;61:427-438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 229] [Cited by in RCA: 278] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 107. | Zhang QF, Yin WW, Xia Y, Yi YY, He QF, Wang X, Ren H, Zhang DZ. Liver-infiltrating CD11b-CD27- NK subsets account for NK-cell dysfunction in patients with hepatocellular carcinoma and are associated with tumor progression. Cell Mol Immunol. 2017;14:819-829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 117] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 108. | Chu PS, Nakamoto N, Taniki N, Ojiro K, Amiya T, Makita Y, Murata H, Yamaguchi A, Shiba S, Miyake R, Katayama T, Ugamura A, Ikura A, Takeda K, Ebinuma H, Saito H, Kanai T. On-treatment decrease of NKG2D correlates to early emergence of clinically evident hepatocellular carcinoma after interferon-free therapy for chronic hepatitis C. PLoS One. 2017;12:e0179096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 109. | Wu Y, Kuang DM, Pan WD, Wan YL, Lao XM, Wang D, Li XF, Zheng L. Monocyte/macrophage-elicited natural killer cell dysfunction in hepatocellular carcinoma is mediated by CD48/2B4 interactions. Hepatology. 2013;57:1107-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 215] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 110. | Hoechst B, Voigtlaender T, Ormandy L, Gamrekelashvili J, Zhao F, Wedemeyer H, Lehner F, Manns MP, Greten TF, Korangy F. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology. 2009;50:799-807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 533] [Cited by in RCA: 526] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 111. | Sun C, Sun HY, Xiao WH, Zhang C, Tian ZG. Natural killer cell dysfunction in hepatocellular carcinoma and NK cell-based immunotherapy. Acta Pharmacol Sin. 2015;36:1191-1199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 153] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 112. | Sung PS, Jang JW. Natural Killer Cell Dysfunction in Hepatocellular Carcinoma: Pathogenesis and Clinical Implications. Int J Mol Sci. 2018;19:3648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 113. | Easom NJW, Stegmann KA, Swadling L, Pallett LJ, Burton AR, Odera D, Schmidt N, Huang WC, Fusai G, Davidson B, Maini MK. IL-15 Overcomes Hepatocellular Carcinoma-Induced NK Cell Dysfunction. Front Immunol. 2018;9:1009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 114. | Mantovani S, Oliviero B, Lombardi A, Varchetta S, Mele D, Sangiovanni A, Rossi G, Donadon M, Torzilli G, Soldani C, Porta C, Pedrazzoli P, Chiellino S, Santambrogio R, Opocher E, Maestri M, Bernuzzi S, Rossello A, Clément S, De Vito C, Rubbia-Brandt L, Negro F, Mondelli MU. Deficient Natural Killer Cell NKp30-Mediated Function and Altered NCR3 Splice Variants in Hepatocellular Carcinoma. Hepatology. 2019;69:1165-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 115. | Sprinzl MF, Reisinger F, Puschnik A, Ringelhan M, Ackermann K, Hartmann D, Schiemann M, Weinmann A, Galle PR, Schuchmann M, Friess H, Otto G, Heikenwalder M, Protzer U. Sorafenib perpetuates cellular anticancer effector functions by modulating the crosstalk between macrophages and natural killer cells. Hepatology. 2013;57:2358-2368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 139] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 116. | Cariani E, Pilli M, Zerbini A, Rota C, Olivani A, Zanelli P, Zanetti A, Trenti T, Ferrari C, Missale G. HLA and killer immunoglobulin-like receptor genes as outcome predictors of hepatitis C virus-related hepatocellular carcinoma. Clin Cancer Res. 2013;19:5465-5473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 117. | Chen A, Shen Y, Xia M, Xu L, Pan N, Yin Y, Miao F, Shen C, Xie W, Zhang J. Expression of the nonclassical HLA class I and MICA/B molecules in human hepatocellular carcinoma. Neoplasma. 2011;58:371-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 118. | Khan M, Arooj S, Wang H. NK Cell-Based Immune Checkpoint Inhibition. Front Immunol. 2020;11:167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 254] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 119. | Liu Y, Cheng Y, Xu Y, Wang Z, Du X, Li C, Peng J, Gao L, Liang X, Ma C. Increased expression of programmed cell death protein 1 on NK cells inhibits NK-cell-mediated anti-tumor function and indicates poor prognosis in digestive cancers. Oncogene. 2017;36:6143-6153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 206] [Cited by in RCA: 285] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 120. | Sun H, Huang Q, Huang M, Wen H, Lin R, Zheng M, Qu K, Li K, Wei H, Xiao W, Sun R, Tian Z, Sun C. Human CD96 Correlates to Natural Killer Cell Exhaustion and Predicts the Prognosis of Human Hepatocellular Carcinoma. Hepatology. 2019;70:168-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 203] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 121. | Ju Y, Hou N, Meng J, Wang X, Zhang X, Zhao D, Liu Y, Zhu F, Zhang L, Sun W, Liang X, Gao L, Ma C. T cell immunoglobulin- and mucin-domain-containing molecule-3 (Tim-3) mediates natural killer cell suppression in chronic hepatitis B. J Hepatol. 2010;52:322-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 193] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 122. | Langhans B, Alwan AW, Krämer B, Glässner A, Lutz P, Strassburg CP, Nattermann J, Spengler U. Regulatory CD4+ T cells modulate the interaction between NK cells and hepatic stellate cells by acting on either cell type. J Hepatol. 2015;62:398-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 123. | Li T, Yang Y, Hua X, Wang G, Liu W, Jia C, Tai Y, Zhang Q, Chen G. Hepatocellular carcinoma-associated fibroblasts trigger NK cell dysfunction via PGE2 and IDO. Cancer Lett. 2012;318:154-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 294] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 124. | Shen Y, Wei Y, Wang Z, Jing Y, He H, Yuan J, Li R, Zhao Q, Wei L, Yang T, Lu J. TGF-β regulates hepatocellular carcinoma progression by inducing Treg cell polarization. Cell Physiol Biochem. 2015;35:1623-1632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 125. | Kohga K, Takehara T, Tatsumi T, Ishida H, Miyagi T, Hosui A, Hayashi N. Sorafenib inhibits the shedding of major histocompatibility complex class I-related chain A on hepatocellular carcinoma cells by down-regulating a disintegrin and metalloproteinase 9. Hepatology. 2010;51:1264-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 126. | Armeanu S, Bitzer M, Lauer UM, Venturelli S, Pathil A, Krusch M, Kaiser S, Jobst J, Smirnow I, Wagner A, Steinle A, Salih HR. Natural killer cell-mediated lysis of hepatoma cells via specific induction of NKG2D ligands by the histone deacetylase inhibitor sodium valproate. Cancer Res. 2005;65:6321-6329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 292] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 127. | Yang H, Lan P, Hou Z, Guan Y, Zhang J, Xu W, Tian Z, Zhang C. Histone deacetylase inhibitor SAHA epigenetically regulates miR-17-92 cluster and MCM7 to upregulate MICA expression in hepatoma. Br J Cancer. 2015;112:112-121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 128. | Muntasell A, Ochoa MC, Cordeiro L, Berraondo P, López-Díaz de Cerio A, Cabo M, López-Botet M, Melero I. Targeting NK-cell checkpoints for cancer immunotherapy. Curr Opin Immunol. 2017;45:73-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 144] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 129. | Lim O, Jung MY, Hwang YK, Shin EC. Present and Future of Allogeneic Natural Killer Cell Therapy. Front Immunol. 2015;6:286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 130. | Hoyos S, Navas MC, Restrepo JC, Botero RC. Current controversies in cholangiocarcinoma. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1461-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 131. | Blechacz B. Cholangiocarcinoma: Current Knowledge and New Developments. Gut Liver. 2017;11:13-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 347] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 132. | Blechacz B, Komuta M, Roskams T, Gores GJ. Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2011;8:512-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 533] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 133. | Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, Lind GE, Folseraas T, Forbes SJ, Fouassier L, Geier A, Calvisi DF, Mertens JC, Trauner M, Benedetti A, Maroni L, Vaquero J, Macias RI, Raggi C, Perugorria MJ, Gaudio E, Boberg KM, Marin JJ, Alvaro D. Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol. 2016;13:261-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 731] [Cited by in RCA: 969] [Article Influence: 107.7] [Reference Citation Analysis (0)] |
| 134. | Lim JH. Cholangiocarcinoma: morphologic classification according to growth pattern and imaging findings. AJR Am J Roentgenol. 2003;181:819-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 215] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 135. | Chung YE, Kim MJ, Park YN, Choi JY, Pyo JY, Kim YC, Cho HJ, Kim KA, Choi SY. Varying appearances of cholangiocarcinoma: radiologic-pathologic correlation. Radiographics. 2009;29:683-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 276] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 136. | Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, Pawlik TM, Gores GJ. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;60:1268-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 862] [Cited by in RCA: 1073] [Article Influence: 97.5] [Reference Citation Analysis (0)] |
| 137. | Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology. 2011;54:173-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 722] [Cited by in RCA: 689] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 138. | Gupta A, Dixon E. Epidemiology and risk factors: intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr. 2017;6:101-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 133] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 139. | Khan SA, Tavolari S, Brandi G. Cholangiocarcinoma: Epidemiology and risk factors. Liver Int. 2019;39 Suppl 1:19-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 498] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 140. | Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145:1215-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 923] [Cited by in RCA: 966] [Article Influence: 80.5] [Reference Citation Analysis (0)] |
| 141. | Banales JM, Cardinale V, Macias RIR, Andersen JB, Braconi C, Carpino G, Alvaro D, Calvisi DF. Cholangiocarcinoma: State-of-the-art knowledge and challenges. Liver Int. 2019;39 Suppl 1:5-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 142. | Chun YS, Javle M. Systemic and Adjuvant Therapies for Intrahepatic Cholangiocarcinoma. Cancer Control. 2017;24:1073274817729241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 143. | Marin JJG, Lozano E, Herraez E, Asensio M, Di Giacomo S, Romero MR, Briz O, Serrano MA, Efferth T, Macias RIR. Chemoresistance and chemosensitization in cholangiocarcinoma. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1444-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 144. | Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15:95-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1052] [Cited by in RCA: 1141] [Article Influence: 163.0] [Reference Citation Analysis (0)] |
| 145. | El-Diwany R, Pawlik TM, Ejaz A. Intrahepatic Cholangiocarcinoma. Surg Oncol Clin N Am. 2019;28:587-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 132] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 146. | Høgdall D, Lewinska M, Andersen JB. Desmoplastic Tumor Microenvironment and Immunotherapy in Cholangiocarcinoma. Trends Cancer. 2018;4:239-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 105] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 147. | Chuaysri C, Thuwajit P, Paupairoj A, Chau-In S, Suthiphongchai T, Thuwajit C. Alpha-smooth muscle actin-positive fibroblasts promote biliary cell proliferation and correlate with poor survival in cholangiocarcinoma. Oncol Rep. 2009;21:957-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 148. | Chen Z, Guo P, Xie X, Yu H, Wang Y, Chen G. The role of tumour microenvironment: a new vision for cholangiocarcinoma. J Cell Mol Med. 2019;23:59-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 149. | Cadamuro M, Morton SD, Strazzabosco M, Fabris L. Unveiling the role of tumor reactive stroma in cholangiocarcinoma: an opportunity for new therapeutic strategies. Transl Gastrointest Cancer. 2013;2:130-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 150. | Fabris L, Perugorria MJ, Mertens J, Björkström NK, Cramer T, Lleo A, Solinas A, Sänger H, Lukacs-Kornek V, Moncsek A, Siebenhüner A, Strazzabosco M. The tumour microenvironment and immune milieu of cholangiocarcinoma. Liver Int. 2019;39 Suppl 1:63-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 116] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 151. | Zhang XF, Dong M, Pan YH, Chen JN, Huang XQ, Jin Y, Shao CK. Expression pattern of cancer-associated fibroblast and its clinical relevance in intrahepatic cholangiocarcinoma. Hum Pathol. 2017;65:92-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 152. | Sha M, Jeong S, Qiu BJ, Tong Y, Xia L, Xu N, Zhang JJ, Xia Q. Isolation of cancer-associated fibroblasts and its promotion to the progression of intrahepatic cholangiocarcinoma. Cancer Med. 2018;7:4665-4677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 153. | Roy S, Glaser S, Chakraborty S. Inflammation and Progression of Cholangiocarcinoma: Role of Angiogenic and Lymphangiogenic Mechanisms. Front Med (Lausanne). 2019;6:293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 154. | Fingas CD, Bronk SF, Werneburg NW, Mott JL, Guicciardi ME, Cazanave SC, Mertens JC, Sirica AE, Gores GJ. Myofibroblast-derived PDGF-BB promotes Hedgehog survival signaling in cholangiocarcinoma cells. Hepatology. 2011;54:2076-2088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 155. | Cadamuro M, Nardo G, Indraccolo S, Dall'olmo L, Sambado L, Moserle L, Franceschet I, Colledan M, Massani M, Stecca T, Bassi N, Morton S, Spirli C, Fiorotto R, Fabris L, Strazzabosco M. Platelet-derived growth factor-D and Rho GTPases regulate recruitment of cancer-associated fibroblasts in cholangiocarcinoma. Hepatology. 2013;58:1042-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 132] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 156. | Ohira S, Sasaki M, Harada K, Sato Y, Zen Y, Isse K, Kozaka K, Ishikawa A, Oda K, Nimura Y, Nakanuma Y. Possible regulation of migration of intrahepatic cholangiocarcinoma cells by interaction of CXCR4 expressed in carcinoma cells with tumor necrosis factor-alpha and stromal-derived factor-1 released in stroma. Am J Pathol. 2006;168:1155-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 100] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 157. | Clapéron A, Mergey M, Aoudjehane L, Ho-Bouldoires TH, Wendum D, Prignon A, Merabtene F, Firrincieli D, Desbois-Mouthon C, Scatton O, Conti F, Housset C, Fouassier L. Hepatic myofibroblasts promote the progression of human cholangiocarcinoma through activation of epidermal growth factor receptor. Hepatology. 2013;58:2001-2011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 158. | Brivio S, Cadamuro M, Strazzabosco M, Fabris L. Tumor reactive stroma in cholangiocarcinoma: The fuel behind cancer aggressiveness. World J Hepatol. 2017;9:455-468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (1)] |
| 159. | Mertens JC, Fingas CD, Christensen JD, Smoot RL, Bronk SF, Werneburg NW, Gustafson MP, Dietz AB, Roberts LR, Sirica AE, Gores GJ. Therapeutic effects of deleting cancer-associated fibroblasts in cholangiocarcinoma. Cancer Res. 2013;73:897-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 159] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 160. | Heits N, Heinze T, Bernsmeier A, Kerber J, Hauser C, Becker T, Kalthoff H, Egberts JH, Braun F. Influence of mTOR-inhibitors and mycophenolic acid on human cholangiocellular carcinoma and cancer associated fibroblasts. BMC Cancer. 2016;16:322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 161. | Thongchot S, Ferraresi A, Vidoni C, Loilome W, Yongvanit P, Namwat N, Isidoro C. Resveratrol interrupts the pro-invasive communication between cancer associated fibroblasts and cholangiocarcinoma cells. Cancer Lett. 2018;430:160-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 162. | Yamanaka T, Harimoto N, Yokobori T, Muranushi R, Hoshino K, Hagiwara K, Gantumur D, Handa T, Ishii N, Tsukagoshi M, Igarashi T, Tanaka H, Watanabe A, Kubo N, Araki K, Shirabe K. Nintedanib inhibits intrahepatic cholangiocarcinoma aggressiveness via suppression of cytokines extracted from activated cancer-associated fibroblasts. Br J Cancer. 2020;122:986-994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 163. | Bansal R, van Baarlen J, Storm G, Prakash J. The interplay of the Notch signaling in hepatic stellate cells and macrophages determines the fate of liver fibrogenesis. Sci Rep. 2015;5:18272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 164. | Subimerb C, Pinlaor S, Lulitanond V, Khuntikeo N, Okada S, McGrath MS, Wongkham S. Circulating CD14(+) CD16(+) monocyte levels predict tissue invasive character of cholangiocarcinoma. Clin Exp Immunol. 2010;161:471-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 165. | Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1025] [Cited by in RCA: 1124] [Article Influence: 74.9] [Reference Citation Analysis (0)] |
| 166. | Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, Seifi B, Mohammadi A, Afshari JT, Sahebkar A. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233:6425-6440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1445] [Cited by in RCA: 3260] [Article Influence: 465.7] [Reference Citation Analysis (0)] |
| 167. | Subimerb C, Pinlaor S, Khuntikeo N, Leelayuwat C, Morris A, McGrath MS, Wongkham S. Tissue invasive macrophage density is correlated with prognosis in cholangiocarcinoma. Mol Med Rep. 2010;3:597-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 168. | Boulter L, Guest RV, Kendall TJ, Wilson DH, Wojtacha D, Robson AJ, Ridgway RA, Samuel K, Van Rooijen N, Barry ST, Wigmore SJ, Sansom OJ, Forbes SJ. WNT signaling drives cholangiocarcinoma growth and can be pharmacologically inhibited. J Clin Invest. 2015;125:1269-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 215] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 169. | Hasita H, Komohara Y, Okabe H, Masuda T, Ohnishi K, Lei XF, Beppu T, Baba H, Takeya M. Significance of alternatively activated macrophages in patients with intrahepatic cholangiocarcinoma. Cancer Sci. 2010;101:1913-1919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 223] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 170. | Thanee M, Loilome W, Techasen A, Namwat N, Boonmars T, Pairojkul C, Yongvanit P. Quantitative changes in tumor-associated M2 macrophages characterize cholangiocarcinoma and their association with metastasis. Asian Pac J Cancer Prev. 2015;16:3043-3050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 171. | Sun D, Luo T, Dong P, Zhang N, Chen J, Zhang S, Liu L, Dong L, Zhang S. CD86+/CD206+ tumor-associated macrophages predict prognosis of patients with intrahepatic cholangiocarcinoma. PeerJ. 2020;8:e8458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 172. | Zhou SL, Dai Z, Zhou ZJ, Chen Q, Wang Z, Xiao YS, Hu ZQ, Huang XY, Yang GH, Shi YH, Qiu SJ, Fan J, Zhou J. CXCL5 contributes to tumor metastasis and recurrence of intrahepatic cholangiocarcinoma by recruiting infiltrative intratumoral neutrophils. Carcinogenesis. 2014;35:597-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 173. | Mao ZY, Zhu GQ, Xiong M, Ren L, Bai L. Prognostic value of neutrophil distribution in cholangiocarcinoma. World J Gastroenterol. 2015;21:4961-4968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 174. | Omichi K, Cloyd JM, Yamashita S, Tzeng CD, Conrad C, Chun YS, Aloia TA, Vauthey JN. Neutrophil-to-lymphocyte ratio predicts prognosis after neoadjuvant chemotherapy and resection of intrahepatic cholangiocarcinoma. Surgery. 2017;162:752-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 175. | Buettner S, Spolverato G, Kimbrough CW, Alexandrescu S, Marques HP, Lamelas J, Aldrighetti L, Gamblin TC, Maithel SK, Pulitano C, Weiss M, Bauer TW, Shen F, Poultsides GA, Marsh JW, IJzermans JNM, Koerkamp BG, Pawlik TM. The impact of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio among patients with intrahepatic cholangiocarcinoma. Surgery. 2018;164:411-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 176. | Chen Y, Ma L, He Q, Zhang S, Zhang C, Jia W. TGF-β1 expression is associated with invasion and metastasis of intrahepatic cholangiocarcinoma. Biol Res. 2015;48:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 177. | Loeuillard E, Conboy CB, Gores GJ, Rizvi S. Immunobiology of cholangiocarcinoma. JHEP Rep. 2019;1:297-311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 178. | Yothagaree T, Sa-Ngiamwibool P, Koonmee S, Pairojkul C. Tumor-infiltrating CD8+ lymphocytes of as a prognostic factor of intrahepatic cholangiocarcinoma. Hepato Biliary Surg Nutr. 2019;8 Suppl 1:AB038. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 179. | Asahi Y, Hatanaka KC, Hatanaka Y, Kamiyama T, Orimo T, Shimada S, Nagatsu A, Sakamoto Y, Kamachi H, Kobayashi N, Fukai M, Taketomi A. Prognostic impact of CD8+ T cell distribution and its association with the HLA class I expression in intrahepatic cholangiocarcinoma. Surg Today. 2020;50:931-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 180. | Ma K, Wei X, Dong D, Wu Y, Geng Q, Li E. PD-L1 and PD-1 expression correlate with prognosis in extrahepatic cholangiocarcinoma. Oncol Lett. 2017;14:250-256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 181. | Zhu Y, Wang XY, Zhang Y, Xu D, Dong J, Zhang Z, Yi CH, Jia HL, Yang X. Programmed death ligand 1 expression in human intrahepatic cholangiocarcinoma and its association with prognosis and CD8+ T-cell immune responses. Cancer Manag Res. 2018;10:4113-4123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 182. | Morisaki T, Umebayashi M, Kiyota A, Koya N, Tanaka H, Onishi H, Katano M. Combining cetuximab with killer lymphocytes synergistically inhibits human cholangiocarcinoma cells in vitro. Anticancer Res. 2012;32:2249-2256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 183. | Jung IH, Kim DH, Yoo DK, Baek SY, Jeong SH, Jung DE, Park SW, Chung YY. In Vivo Study of Natural Killer (NK) Cell Cytotoxicity Against Cholangiocarcinoma in a Nude Mouse Model. In Vivo. 2018;32:771-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 184. | Fukuda Y, Asaoka T, Eguchi H, Yokota Y, Kubo M, Kinoshita M, Urakawa S, Iwagami Y, Tomimaru Y, Akita H, Noda T, Gotoh K, Kobayashi S, Hirata M, Wada H, Mori M, Doki Y. Endogenous CXCL9 affects prognosis by regulating tumor-infiltrating natural killer cells in intrahepatic cholangiocarcinoma. Cancer Sci. 2020;111:323-333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 185. | Tsukagoshi M, Wada S, Yokobori T, Altan B, Ishii N, Watanabe A, Kubo N, Saito F, Araki K, Suzuki H, Hosouchi Y, Kuwano H. Overexpression of natural killer group 2 member D ligands predicts favorable prognosis in cholangiocarcinoma. Cancer Sci. 2016;107:116-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 186. | Carnevale G, Carpino G, Cardinale V, Pisciotta A, Riccio M, Bertoni L, Gibellini L, De Biasi S, Nevi L, Costantini D, Overi D, Cossarizza A, de Pol A, Gaudio E, Alvaro D. Activation of Fas/FasL pathway and the role of c-FLIP in primary culture of human cholangiocarcinoma cells. Sci Rep. 2017;7:14419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 187. | Hayashi T, Imai K, Morishita Y, Hayashi I, Kusunoki Y, Nakachi K. Identification of the NKG2D haplotypes associated with natural cytotoxic activity of peripheral blood lymphocytes and cancer immunosurveillance. Cancer Res. 2006;66:563-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 107] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 188. | Melum E, Karlsen TH, Schrumpf E, Bergquist A, Thorsby E, Boberg KM, Lie BA. Cholangiocarcinoma in primary sclerosing cholangitis is associated with NKG2D polymorphisms. Hepatology. 2008;47:90-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 94] [Article Influence: 5.5] [Reference Citation Analysis (0)] |