Published online Aug 14, 2020. doi: 10.3748/wjg.v26.i30.4501
Peer-review started: March 25, 2020
First decision: April 25, 2020
Revised: May 29, 2020
Accepted: July 22, 2020
Article in press: July 22, 2020
Published online: August 14, 2020
Processing time: 142 Days and 2.9 Hours
No guideline recommends antiviral therapy for hepatitis B e antigen (HBeAg)-positive chronic hepatitis B patients with persistently normal alanine aminotransferase levels and a high hepatitis B virus (HBV) DNA viral load.
To evaluate the feasibility and safety of a Chinese herbal formula as a therapeutic option for chronic HBV infection.
In total, 395 patients (30–65 years old) with confirmed HBeAg-positive chronic hepatitis B infection and persistently normal alanine aminotransferase were randomized to receive either Chinese herbal formula or placebo for 96 wk. Endpoints to evaluate therapeutic efficacy included: (1) HBV DNA levels decreased to less than 4 log10 IU/mL at weeks 48 and 96; and (2) HBeAg clearance and seroconversion rates at weeks 48 and 96.
HBV DNA levels ≤ 4 log10 IU/mL were 10.05% at week 48 and 18.59% at week 96 in the treatment group. The HBeAg clearance and conversion rates were 8.54% and 8.04% at week 48 and 16.08% and 14.57% at week 96, respectively. However, HBV DNA levels ≤ 4 log10 IU/mL were 2.55% and 2.55% at weeks 48 and 96, respectively, and the HBeAg clearance rates were 3.06% and 5.61% at weeks 48 and 96, respectively, in the control group. The quantitative hepatitis B surface antigen and HBeAg levels at baseline and changes during the treatment period as well as the alanine aminotransferase elevation at weeks 12 and 24 were strong predictors of HBeAg clearance.
High rates of HBV DNA reduction, HBeAg clearance and seroconversion could be achieved with Chinese herbal formula treatments, and the treatments were relatively safe for HBeAg-positive chronic hepatitis B-infected patients with persistently normal alanine aminotransferase. The ability of the compound to modulate host immune function probably contributed to this effect.
Core tip: Hepatitis B e antigen-positive chronic hepatitis B patients with persistently normal alanine aminotransferase levels and a high hepatitis B virus DNA viral load may progress to cirrhosis or hepatocellular carcinoma. However, no guideline recommends antiviral therapy for it because of poor efficacy. In the present study we report the feasibility and safety profiles of a Chinese herbal formula as a therapeutic option for chronic hepatitis B virus infection.
- Citation: Xing YF, Wei CS, Zhou TR, Huang DP, Zhong WC, Chen B, Jin H, Hu XY, Yang ZY, He Q, Jiang KP, Jiang JM, Hu ZB, Deng X, Yang F, Li FY, Zhao G, Wang LC, Mi YQ, Gong ZJ, Guo P, Wu JH, Shi WQ, Yang HZ, Zhou DQ, Tong GD. Efficacy of a Chinese herbal formula on hepatitis B e antigen-positive chronic hepatitis B patients. World J Gastroenterol 2020; 26(30): 4501-4522
- URL: https://www.wjgnet.com/1007-9327/full/v26/i30/4501.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i30.4501
Most patients with hepatitis B e antigen (HBeAg)-positive chronic hepatitis B virus (HBV) infection with basically normal alanine aminotransferase (ALT) levels and high viral load have no obvious clinical symptoms[1-4]. Antiviral therapy is not recommended for these patients by any authoritative guidelines. Despite long-term normal ALT levels, a high HBV DNA viral load persists, and liver lesions progress unrecognized and advance gradually. Some patients may even progress to cirrhosis or hepatocellular carcinoma (HCC), and the risk increases with age, especially after the age of 30. Even if a chronic HBV carrier shows minimal or no necroinflammation or fibrosis in the liver (previously termed the “immune tolerant” phase), a high level of HBV DNA integration and clonal hepatocyte expansion suggests that hepa-tocarcinogenesis could be already underway in this early phase of the infection[5].
Previous studies have shown that liver injury in chronic hepatitis B (CHB) patients with normal ALT levels was always mild, and the long-term clinical outcomes were not serious[6,7]. A long-term follow-up study in Taiwan of China, which enrolled 240 HBeAg-positive patients with normal ALT levels and had a median follow-up period of 6.8 years (1-17 years) and a mean age at entry of 27 years, showed that 85% of the patients had HBeAg seroconversion and sustained remission. The cumulative incidence of cirrhosis in 17 years was 12.5%, and the cumulative incidence of HCC was 0%[8]. However, for patients over 35 years old, another study in Taiwan (REVEAL) reported a median follow-up of 7 years, HBeAg clearance of 187 (43.4%) and an annual incidence of only 6.2[9]. As accumulating research data show, age is proportional to the progression of CHB. The REVEAL study also showed that HBV DNA was an independent predictor of hepatitis B progression in patients over 35 years old, and the incidence of cirrhosis increased with HBV DNA level (300 copies/mL-106 copies/mL), which was 4.5%-36.2%. In this study, the corresponding cumulative incidence of hepatocellular carcinoma was 1.3% and 14.9% in patients with HBV DNA < 300 copies/mL and HBV DNA > 106 copies/mL, respectively[10,11]. HBV DNA level was independent of HBeAg status, ALT level and other risk factors[12]. Another study concerning HBeAg seroconversion showed that in CHB patients with e antigen seroconversion before age 40, only 4.1% would progress to cirrhosis, while with e antigen seroconversion after age 40 and 50, the incidence of cirrhosis was 27.3% and 33.3%, respectively[13]. Liver biopsy also indicated a gradient relationship between fibrosis severity and age[14]. Therefore, in recent years, Chinese guidelines and European Association for the Study of the Liver guidelines have lowered the age for monitoring antiviral therapy in CHB patients with normal ALT from 40 years old to 30 years old[1,4].
For HBeAg-positive CHB patients with normal ALT, antiviral therapy was not recommended by various authoritative guidelines, mainly due to poor efficacy. The results of a small sample study of pegylated interferon for the treatment of HBV carriers reported that the seroconversion rate was below 10%[15]. However, another lamivudine study showed that the seroconversion rate was only 2% in HBV carriers[16]. In recent years, clinical trials of vaccines against HBV have all ended in failure[17]. With the advent of new potent antiviral drugs, the recently published 192 wk study of patients with CHB in the immune tolerant stage of tenofovir therapy showed that 5% of patients achieved e antigen seroconversion. Although more than 50% of patients had reached HBV DNA clearance during the treatment, they all relapsed 6 mo after drug withdrawal, suggesting that the efficacy of antiviral therapy for such patients was unsatisfactory[18].
We have more than 20 years of clinical experiences in treating CHB infection and chronic carriers with invigorating kidney and clearing away the heat and expelling superficial evils (ICE) formula. In our previous studies, we recruited 62 patients with CHB and treated with ICE. Results showed that the HBeAg clearance and virologic response of the treated group were significantly better than that of the control group[19]. Preliminary clinical multicenter research of short courses of treatment during the national 11th five-year period project indicated that the decrease of HBV DNA was greater than 2 log by 17.5%, and the decrease of hepatitis B surface antigen (HBsAg) by 1 log was about 10% after 52 wk of ICE intervention[20-22]. In addition, liver histological results showed significant improvements in liver fibrosis, and immunohistochemistry showed significant decreases in the expression of HBsAg and hepatitis B core antigen (HBcAg) in responding patients. These studies suggest that ICE has a better effect on interfering with chronic HBV carriers[23].
Through this study, the effects of the ICE formula on patients with HBeAg-positive CHB with normal ALT who were over 30 were evaluated, and serological indexes, HBV DNA changes and related factors were analyzed. This study provided clinical evidence for traditional Chinese medicine (TCM) treatment for chronic HBV carriers, especially chronic HBV carriers over 30 years old with a higher risk of disease progression.
HBeAg-positive patients with chronic HBV infections were recruited from May 2013 to May 2014 at 20 different hospitals and medical centers for this study (Table 1). A total of 395 patients were enrolled. The inclusion and exclusion criteria used for patient selection are shown in Table 2.
| Name | Location (city and province) |
| Shanghai Shuguang hospital, Shanghai University of Traditional Chinese Medicine | Shanghai |
| The Second Hospital Affiliated with Zhejiang University of Traditional Chinese Medicine | Hangzhou, Zhejiang |
| Xiamen Hospital of Traditional Chinese Medicine | Xiamen, Fujian |
| Shenzhen Hospital Affiliated with Guangzhou University of Chinese Medicine | Shenzhen, Guangdong |
| Foshan Hospital of Traditional Chinese Medicine | Foshan, Guangdong |
| The Third People’s Hospital of Shenzhen | Shenzhen, Guangdong |
| Guangdong Hospital of Traditional Chinese Medicine | Guangzhou, Guangdong |
| The Third Affiliated Hospital of Sun Yat-sen University | Guangzhou, Guangdong |
| Ruikang Hospital of Guangxi College of Traditional Chinese Medicine | Nanning, Guangxi |
| The First Affiliated Hospital of Guangxi College of Traditional Chinese Medicine | Nanning, Guangxi |
| Attached Hospital of Chengdu University of Traditional Chinese Medicine | Chengdu, Sichuan |
| West China Hospital, West China School of Medicine, Sichuan University | Chengdu, Sichuan |
| Beijing Ditan Hospital, Capital Medical University | Beijing |
| Xiyuan Hospital, China Academy of Traditional Chinese Medicine | Beijing |
| 302 Military Hospital of China | Beijing |
| Tianjin Infectious Disease Hospital | Tianjin |
| Beijing Youan Hospital, Capital Medical University | Beijing |
| Hubei Provincial Hospital of TCM | Wuhan, Hubei |
| People's Hospital of Wuhan University | Wuhan, Hubei |
| The First Hospital of Hunan University of Chinese Medicine | Changsha, Hunan |
| Inclusion criteria | Exclusion criteria |
| Conform with the diagnostic criteria of HBeAg (+) chronic hepatitis B | Inactive HBsAg (+) carriers |
| Conform with the pathogenesis and syndromes of kidney deficiency | Serum a-fetoprotein abnormal |
| Age 30-65 yr | Pregnancy or breast feeding |
| ALT ≤ 40 IU/L | Coinfection with HIV, HCV, HDV |
| HBsAg > 10 IU/mL and < 105 IU/mL HBV DNA (105-109 IU/mL) | Histologic evidence of cirrhosis; Evidence of any other chronic liver disease |
| Liver biopsy: Liver histology showed Knodell HAI > 4, Ishak fibrosis score > 3 were also included | Mental illness or any other serious systemic illness |
| Voluntary | Interferon-γ within 6 mo; Antivirus treatment with nucleoside |
| Abuse alcohol or illegal drugs; Allergic to the drug ingredients |
The study was approved by the Ethics Committees at Shenzhen Hospital affiliated with the Fourth Clinical Medical College of Guangzhou University of TCM and was conducted in accordance with the ethical guidelines of the 1975 Declaration of Helsinki and the Good Clinical Practice Guidelines. All enrolled patients gave written informed consent before enrollment. The clinical trial registration identifier is ChiCTR-TPR-17011944 (http://www.chictr.org.cn/index.aspx).
The Chinese herbal formula (ICE granules) was composed of Phyllanthus urinaria Linn, Radix et caulis acanthopanacis senticosi, Herba Epimedii, and so forth, which are listed in Table 3. The placebo was composed of water-soluble starch, glucosum anhydricum, edible chocolate brown pigment and lyochromes. Both were made into drug granules in Shenzhen Sanjiu Medical & Pharmaceutical Co., Ltd., China, a renowned good manufactory practice-certified state-level manufacturer of concentrated herbal extracts (its products can be purchased in China). The whole production process, from validating the raw materials to the final products, strictly complied with the standards of good manufactory practice and Chinese pharmacopoeia[24]. Decoction and extraction of each dried medicinal herb was performed in a single batch. After extraction, the herbal preparation was separated, concentrated and spray dried into the form of a granule. The chemical compositions of the final products were analyzed, while all the herbal preparations were tested to ensure safety for human consumption, including heavy metals, microorganism contamination and insecticides. Finally, the different kinds of granules were mixed in accordance with their proportion in the Chinese herbal formula and packed in sealed plastic sachets. The composition of a sachet of granules (32.67 g) was the same as that of 190 g raw herbs, which was the daily dose of each patient. The placebo was similar to the herbal granules in shape, color, taste and packaging.
| Chinese name | Latin name | Parts of plant used | Dose of dryplant (grams) | Dose afterextraction (grams) |
| Ye xia zhu | Phyllanthus urinaria Linn | Whole plant | 30 | 12.00 |
| Ci wu jia | Radix et caulis acanthopanacis senticosi | Root and rhizome | 10 | 0.50 |
| Xian ling pi | Herba Epimedii | Overground part | 30 | 1.50 |
| Nv zhen zi | Fructus ligustri lucidi | Mature fruit | 15 | 1.50 |
| Han lian cao | Herba ecliptae | Overground part | 15 | 1.50 |
| Chai hu | Radix bupleuri | Root | 10 | 1.67 |
| Bai shao | Radix paeoniae alba | Root | 10 | 1.00 |
| Zhi shi | Fructus aurantii immaturus | Fruitlet | 10 | 1.67 |
| Tao ren | Semen persicae | Nuts | 10 | 0.50 |
| Gan cao | Radix glycyrrhizae | Root and rhizome | 5 | 0.83 |
| Hu zhang | Rhizoma polygoni cuspidati | Root and rhizome | 15 | 1.00 |
| Xi huang cao | Herba rabdosiae serrae | Whole plant | 30 | 9.00 |
| Total | 190 | 32.67 |
The study was a multicenter, randomized, double-blinded and placebo-controlled clinical trial of the Chinese herbal formula versus placebo at a ratio of 1:1 for 96 wk. Each patient was instructed to dissolve a sachet of granules (32.67 g, either study drug or placebo) in 200 mL of warm water in a cup and to take 100 mL of the solution in the morning and the rest in the afternoon every day.
Randomization was performed within one month after the screening had been completed using a voice interactive random assortment system[25]. Tests were carried out at week 0, 4 and 12 and then every 12 wk thereafter through week 96. At each clinic visit, laboratory tests were performed to evaluate liver function and determine the safety of treatment and possible adverse events. Serum was assayed for HBV DNA, HBsAg, antibody to HBsAg, HBeAg and antibody to HBeAg at baseline and at weeks 24 and 48. Serum helper T1 cell and helper T2 cell cytokine levels, including interleukin (IL)-2, IL-4, IL-10 and interferon-γ (IFN-γ), were detected at baseline and at weeks 48 and 96. Patients were withdrawn from the study for any of the following reasons: Occurrence of intolerable or worsening adverse events and failure to comply with the protocol or withdrawal of consent.
All subjects undergoing blood testing were uniformly assayed in the central laboratory of Shanghai Amidikang Medical Laboratory, China. All subjects underwent complete blood counts and serum biochemistry detections, including ALT, aspartate transaminase, platelet, γ-glutamyltransferase, blood urea nitrogen and creatinine tests with the Cobas ISE 800 chemistry analyzer (Roche Diagnostics, Holliston, MA, United States)[26]. HBsAg, hepatitis B surface antibody, HBeAg, hepatitis B envelope antibody and hepatitis B core antibody were measured with the Architect i2000 assay (Abbott Laboratories, Philippines)[27]. The HBsAg titer in serum was quantified according to the manufacturer’s instructions. An initial manual dilution of 1:100 was performed on all samples. Samples with HBsAg titers of greater than 250 IU/mL were manually diluted to 1:500 to bring the reading within the linear range. Samples with HBsAg levels of less than 0.05 IU/mL at 1:100 dilution were retested undiluted. Serum cytokine levels of IL-2, IL-4, IL-10 and IFN-γ were detected by ELISA kits (Pharmingen, San Diego, CA, United States) according to the manufacturer’s instructions. Serum HBV DNA levels were quantified using the Cobas TaqMan assay (Roche Diagnostics, Branchburg, NJ, the United States) with the lowest detection limit at 20 IU/mL.
All subjects underwent percutaneous liver biopsy guided by ultrasonography[28]. Liver biopsy was performed using 16-G Tru-Cut biopsy needles (Menghini, Bard Company of the United States). A minimum of 1.5 cm of liver tissue with at least six portal tracts was required for appropriate diagnosis. The specimens were immediately fixed, paraffin-embedded, stained with hematoxylin-eosin and sent to the Department of Pathology at the Shenzhen Traditional Chinese Medicine Hospital. The Knodell histological activity index (HAI)[29] and Ishak's system[30,31] were used by two experienced pathologists who were blinded to the clinical information of the subjects to grade the collected samples. The Knodell HAI was used to describe the hepatocellular necroinflammation activity with grades of 0 ± 4, while liver fibrosis was semiquantitatively assessed according to Ishak's system and was graded from stage 0 to stage 6.
The primary efficacy end point was the proportion of patients with a virologic response at weeks 48 and 96 (including HBV DNA levels decreasing at least 2 log10 units and less than 4 log10 IU/mL). Secondary efficacy end points were the proportion of patients with HBeAg loss or seroconversion to anti-HBe at weeks 48 and 96. In addition, adverse events including symptoms, signs and clinical laboratory abnormalities within 96 wk were documented, and discontinuation of therapy was recorded.
Multicenter randomized double-blind control, ICE group: the placebo group was randomized 1:1, the viral response rate (viral load decreased by 2 log after treatment) was the main effect index, and the sample content was estimated by SPSS 22.0 according to the 11th five-year “national special program for major infectious diseases” research data. The TCM treatment group 2-year virologic response rate was 25%, that of the placebo control group was 5%, and the research on the basis of the optimized treatment plan chooses a better response rate crowd (10 e5-10 e9). Two years is expected to make the TCM group virologic response rate of 30%, and the control group was 5%. According to the rate difference between the two groups, P1 = 5%, P2 = 30%, alpha = 0.05, beta = 0.20, with an estimated total of 278 cases. According to our previous data of the 11th five-year “national special program for major infectious diseases,” the empirical sample shedding rate was < 10%, and the adjusted sample content was 306 cases. Therefore, we chose to randomly enroll 400 cases in total or 200:200 cases (experimental group:control group).
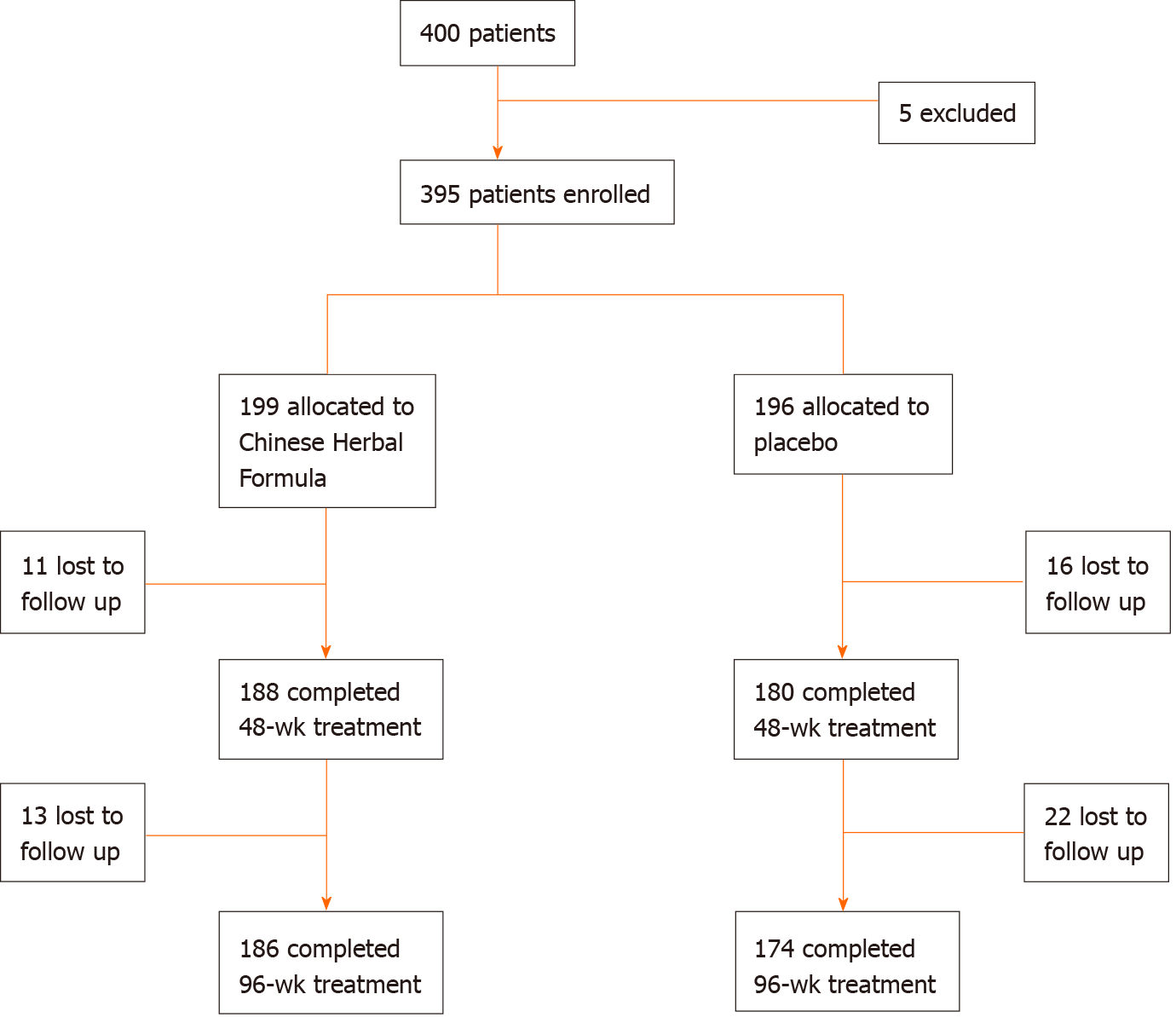
Of the 400 patients initially screened, 5 were excluded, and a total of 395 patients were included in the treatment group (199 cases) and the control group (196 cases). During 96 wk of follow-up, 13 cases and 22 cases dropped out, respectively. The dropout rate was 6.5% and 11.2%, respectively, meeting the criteria of lost to follow-up (Figure 1).
The intention-to-treat analysis included all patients who were randomly allocated to one of the two groups. A last observation carried forward analysis was conducted for any missing data on primary or secondary outcomes. Analysis of safety included data for all patients who had taken at least one dose of study medication after randomization. SPSS 22.0 package (SPSS Inc., Chicago, IL, United States) was used to perform the analysis. Continuous variables were expressed as the mean ± standard deviation. An independent samples t-test was used to compare differences between the two groups. A paired samples t-test was performed to calculate differences between prior and after treatment in one group. Categorical variables were expressed as absolute and relative frequencies. The Chi-square test or Fisher’s exact test were used to compare the differences in proportions between the two groups. Univariable and multivariable logistic regression analyses were conducted to evaluate the magnitude and significance of the association. A two-sided P value < 0.05 was considered statistically significant.
Four hundred patients were planned to be enrolled in this project, while 395 patients were actually enrolled, conforming to the inclusion criteria. Each group was balanced. The two main visit time nodes for statistical analysis were at weeks 48 and 96. All the data were statistically analyzed by the Capital Medical University School of Public Health. The project team received blinded results from the clinical evaluation center of Chinese Academy of TCM on March 13, 2015, which is group A: ICE, Group C: the placebo control. The relevant main index data are described in Table 4.
| Variable | Treatment (ICE) group (n = 199) | Control group(n = 196) | χ2/t/ Z | P value |
| Age (mean ± SD), yr | 38.51 ± 7.63 | 38.90 ± 7.54 | -0.904 | 0.366a |
| Range | 30-65 | 30-63 | ||
| Sex, n (%) | 0.013 | 0.910b | ||
| Male | 128 (64.3) | 125 (63.8) | ||
| Female | 71 (35.7) | 71 (36.2) | ||
| Regions (N) | 2.796 | 0.593b | ||
| Eastern | 30 (14.42) | 28 (14.97) | ||
| Western | 22 (10.58) | 18 (9.63) | ||
| Southern | 89 (42.79) | 69 (36.90) | ||
| Northern | 40 (19.23) | 48 (25.67) | ||
| Central | 27 (12.98) | 24 (12.83) | ||
| Smoking | 0.491 | 0.484b | ||
| Yes | 57 (28.64) | 50 (25.51) | ||
| No | 142 (71.36) | 146 (74.49) | ||
| Alcohol consumption | 3.626 | 0.057b | ||
| Yes | 18 (9.05) | 30 (15.31) | ||
| No | 181 (90.95) | 166 (84.69) | ||
| Genotype | 0.522 | 0.770b | ||
| B | 99 (49.75) | 93 (47.45) | ||
| C | 90 (45.23) | 95 (48.47) | ||
| D | 10 (5.02) | 8 (4.08) | ||
| Genealogy of hepatocellular carcinoma | 2 (1.00) | 3 (1.53) | 0.000 | 0.986b |
| Clinical course (mean ± SD) week | 90.21 ± 22.40 | 86.69 ± 27.20 | -1.607 | 0.108a |
| Liver function (mean ± SD) | ||||
| ALT, IU/L | 29.29 ± 8.19 | 30.12 ± 6.32 | 1.126 | 0.261c |
| AST, IU/L | 24.86 ± 7.53 | 25.79 ± 6.19 | 1.340 | 0.181c |
| TB, μmol/L | 14.34 ± 3.25 | 13.98 ± 4.15 | 0.961 | 0.337c |
| HBV DNA baseline level, (%) | 0.154 | 0.695b | ||
| 2 to < 5 log10 IU/mL | 0 (0) | 0 (0) | ||
| 5 to < 7 log10 IU/mL | 14 (2.01) | 12 (1.02) | ||
| 7to < 9 log10 IU/mL | 185 (97.99) | 184 (98.98) | ||
| HBsAg (mean ± SD), log10 IU/mL | 3.86 ± 0.52 | 3.89 ± 0.42 | -0.758 | 0.449a |
| HBeAg (mean ± SD), SCO/mL | 1138.18 ± 423.99 | 1158.40 ± 401.86 | -0.393 | 0.695a |
| HBeAb (mean ± SD), SCO/mL | 38.43 ± 14.28 | 40.01 ± 12.15 | -0.904 | 0.366a |
| Histological scores | ||||
| Knodell (HAI), n (%) | 168 (84.40) | 156 (79.60) | 0.010 | 0.922b |
| ≥ 4 | 60 (35.71) | 55(35.26) | ||
| < 4 | 108 (64.29) | 101 (64.74) | ||
| Ishak (FIB), n (%) | 0.035 | 0.851b | ||
| ≥ 2 | 67 (39.90) | 60 (38.46) | ||
| < 2 | 101 (60.10) | 96 (61.54) |
Virologic response: The proportion of patients with reduced HBV DNA levels of > 2 log10 IU/mL was 15.08% (30/199) at week 48 and 30.15% (60/199) at week 96 for the treatment group compared to 6.63% (13/196, P = 0.007) and 6.12% (12/196, P = 0.000), respectively, for the control group. The percentages of patients with HBV DNA levels ≤ 4 log10 IU/mL were 10.05% (20/199) at week 48 and 18.59% (37/199) at week 96 for the treatment group compared to 2.55% (5/196, P = 0.002) and 3.06% (6/196, P = 0.00), respectively, for the control group. Among patients in the treatment group, serum HBV DNA was undetectable in 1.01% (2/199) at week 48 and 2.01% (4/199) at week 96; in the control group, it was in 0% (0/196) and 0% (0/196), respectively. There was no significant difference between the two groups (P = 0.159 and P = 0.136, respectively, Table 5).
| Treatment response | Treatment (ICE) group (n = 199) | Control group ( n = 196) | χ2/Z | P value |
| 48 wk | ||||
| Patients with HBV DNA level decline > 2 log10 IU/mL, n (%) | 30 (15.08) | 13 (6.63) | 7.255 | 0.007 |
| Patients with HBV DNA level ≤ 4 log10 IU/mL, n (%) | 20 (10.05) | 5 (2.55) | 9.367 | 0.002 |
| Patients with undetectable HBV DNA (≤ 20 IU/mL), n (%) | 2 (1.01) | 0 (0.00) | 1.980 | 0.159 |
| 96 wk | ||||
| Patients with HBV DNA level decline > 2 log10 IU/mL, n (%) | 60 (30.15) | 12 (6.12) | 38.249 | 0.000 |
| Patients with HBV DNA level ≤ 4 log10 IU/mL, n (%) | 37 (18.59) | 6 (3.06) | 24.555 | 0.000 |
| Patients with undetectable HBV DNA (≤ 20 IU/mL), n (%) | 4 (2.01) | 0 (0.00) | 2.227 | 0.136 |
Serological response: The proportion of patients with a decline ≥ 0.5 log10 in HBsAg levels in the treatment group was 24.62% (49/199) at week 48 and 41.71% (83/199) at week 96 compared to the control group, which was 9.69% (19/196 P = 0.000) and 20.92% (41/196, P = 0.000), respectively. The percentages of patients with a decline ≥ 1 log10 in HBsAg levels in the treatment group were 14.57% (29/199) at week 48 and 31.66% (63/199) at week 96 compared to the control group, which was 5.61% (11/196, P = 0.003) and 11.22% (22/196, P = 0.000), respectively. Furthermore, the proportion of patients with a decline ≥ 2 log10 in HBsAg level in the treatment group was 3.52% (7/199) at week 48 and 8.54% (17/199) at week 96 compared to the control group, which was 1.02% (2/196, P = 0.185) and 0.51% (1/196, P = 0.008), respectively. Neither group had patients with HBsAg ≤ 0.05 at weeks 48 and 96 (Table 6).
| Treatment response | Treatment (ICE) group (n = 199) | Control group (n = 196) | χ2/Z | P value |
| 48 wk | ||||
| Patients with HBsAg level decline ≥ 0.5 log10 IU/mL, n (%) | 49 (24.62) | 19 (9.69) | 15.443 | 0.000 |
| Patients with HBsAg level decline ≥ 1 log10 IU/mL, n (%) | 29 (14.57) | 11 (5.61) | 8.712 | 0.003 |
| Patients with HBsAg level decline ≥ 2 log10 IU/mL, n (%) | 7 (3.52) | 2 (1.02) | 1.758 | 0.185 |
| Patients with undetectable HBsAg (≤ 0.05 IU/mL), n (%) | 0 (0.00) | 0 (0.00) | 0.000 | 1 |
| Patients with HBeAg level decline ≥ 1 log10 S/CO), n (%) | 45 (22.61) | 5 (2.55) | 35.947 | 0.000 |
| Patients with undetectable HBeAg (≤ 1.00 S/CO), n (%) | 17 (8.54) | 5 (2.55) | 6.740 | 0.009 |
| Seroconversion rates of HBeAga, n (%) | 16 (8.04) | 4 (2.04) | 7.394 | 0.007 |
| 96 wk | ||||
| Patients with HBsAg level decline ≥ 0.5 log10 IU/mL, n (%) | 83 (41.71) | 41 (20.92) | 19.817 | 0.000 |
| Patients with HBsAg level decline ≥ 1 log10 IU/mL, n (%) | 63 (31.66) | 22 (11.22) | 24.413 | 0.000 |
| Patients with HBsAg level decline ≥ 2 log10 IU/mL, n (%) | 17 (8.54) | 1 (0.51) | 7.129 | 0.008 |
| Patients with undetectable HBsAg (≤ 0.05 IU/mL), n (%) | 0 (0) | 0 (0) | 0.000 | 1 |
| Patients with HBeAg level decline ≥ 1 log10 S/CO), n (%) | 51 (25.63) | 9 (4.59) | 33.919 | 0.000 |
| Patients with undetectable HBeAg (≤ 1.00 S/CO), n (%) | 32 (16.08) | 11 (5.61) | 11.154 | 0.001 |
| Seroconversion rates of HBeAga, n (%) | 29 (14.57) | 9 (4.46) | 11.962 | 0.001 |
The percentage of patients with a decline ≥ 1 log10 in HBeAg levels in the treatment group was 22.61% (45/199) at week 48 and 25.63% (51/199) at week 96 compared to that of the control group, which was 2.55% (5/196, P = 0.000) and 4.59% (9/196, P = 0.000), respectively. In the treatment group, 8.54% of patients (17/199) at week 48 and 16.08% (32/199) at week 96 demonstrated HBeAg clearance compared to the control group, which was 2.55% (5/196, P = 0.009) and 5.61% (11/196, P = 0.001), respectively. Seroconversion rates of HBeAg in the treatment group were 8.04% (16/199) at week 48 and 14.57% (29/199) at week 96 compared to the control group, which was 2.04% (4/196, P = 0.007) and 4.46% (9/196, P = 0.001), respectively (Table 6). In both groups, patients exhibited declines in HBsAg and HBeAg levels (Figure 2).
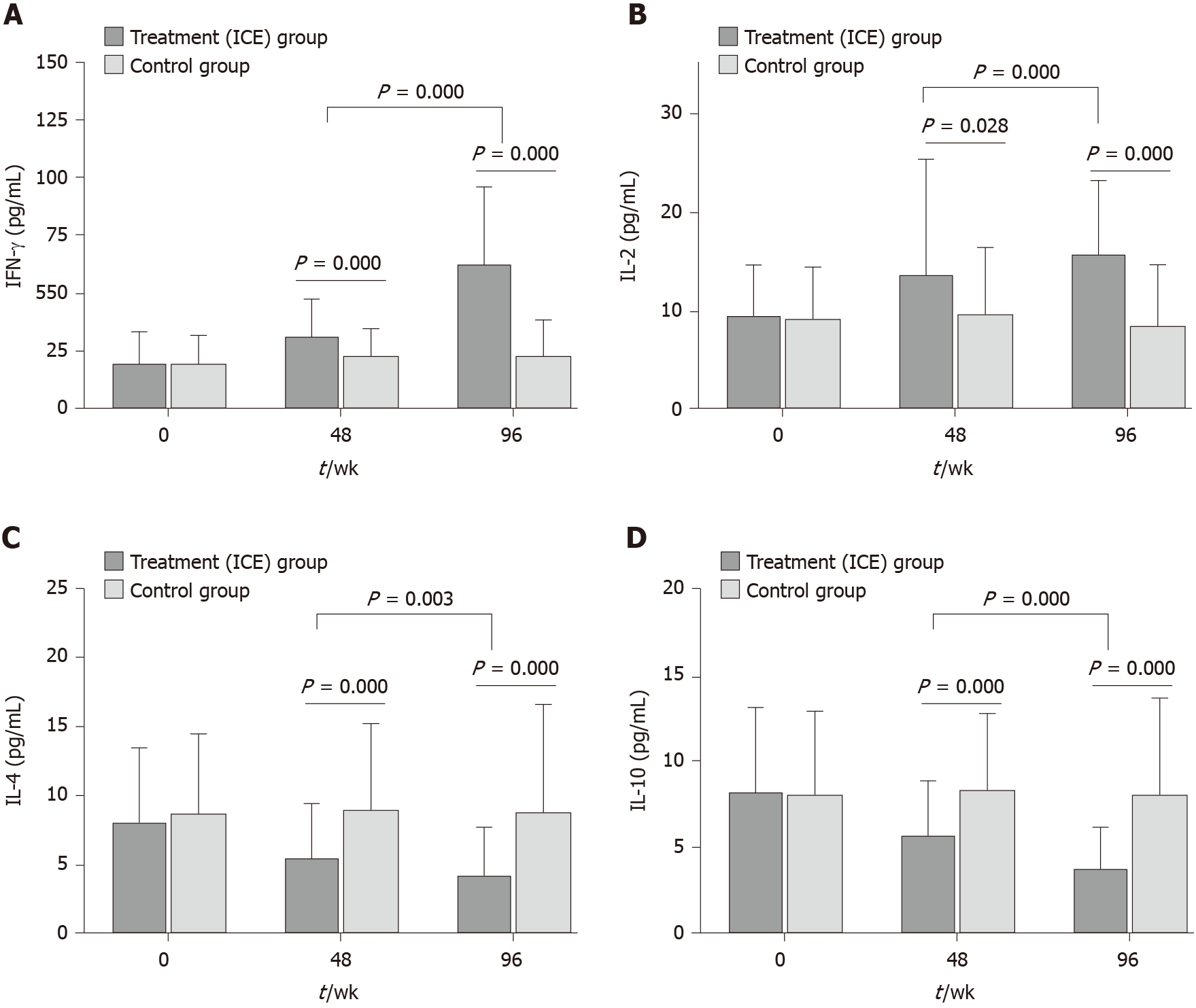
Serum cytokine levels: After 48 wk of ICE treatment, patients showed a significant increase in the mean levels of serum IFN-γ and IL-2 compared to the levels of these cytokines determined prior to treatment (27.80 ± 20.26 vs 19.90 ± 14.69, P = 0.000; 13.76 ± 11.74 vs 9.97 ± 6.52, P = 0.028, respectively). At week 96, in the ICE group, IFN-γ and IL-2 levels increased (57.54 ± 38.62 vs 20.08 ± 18.54, P = 0.000; 15.92 ± 7.54 vs 8.59 ± 6.21, P = 0.000, respectively). IFN-γ and IL-2 levels were not changed in the control group (P > 0.05). In addition, there was a marked decrease in the mean serum levels of IL-4 and IL-10 at week 48 (5.61 ± 3.83 vs 9.07 ± 6.27, P = 0.000; 5.85 ± 3.14 vs 8.57 ± 4.33, P = 0.000, respectively) and at week 96 (4.41 ± 3.37 vs 8.97 ± 7.75, P = 0.000; 3.92 ± 2.31 vs 8.27 ± 5.49, P = 0.000, respectively). In contrast, there were no differences in the levels of serum IL-4 and IL-10 before and after treatment with placebo in the control group (P > 0.05, Figure 3).
Moreover, among the ICE group patients, IFN-γ and IL-2 levels increased significantly (P = 0.000; P = 0.000, respectively), while IL-4 and IL-10 levels decreased significantly (P = 0.003; P = 0.000, respectively) at week 96 compared with week 48 (Figure 3).
At weeks 12 and 24, 15.58% (31/199) and 18.09% (36/199), respectively of the subjects in the treatment group showed an elevated ALT level (> 50 IU/L) with a maximum of 594 IU/L, and total bilirubin levels were all < 35 mmol/L. To assess the effects of the quantitative HBeAg and HBsAg levels and changes during the early period of treatment, we assessed the HBeAg and HBsAg levels at baseline, week 24 change from baseline and week 36 change from baseline using univariable logistic regression analysis. The results showed that baseline HBeAg [odds ratio (OR), 1.653, P = 0.03] and HBsAg (OR, 2.431, P = 0.004), week 24 HBeAg change from baseline (OR, 2.762, P < 0.001), week 36 HBeAg change from baseline (OR, 3.411, P < 0.01), week 24 HBsAg change from baseline (OR, 4.458, P < 0.001), week 36 HBsAg change from baseline (OR, 5.371, P < 0.001), week 12 ALT elevation (OR, 2.676, P = 0.016), week 24 ALT elevation (OR, 3.373, P = 0.003), week 48 IFN-γ elevation (OR, 2.735, P = 0.002) and week 48 IL-2 week 2 clearance (OR, 2.003, P = 0.008) were strong predictors for HBeAg clearance at week 96. Baseline sex, age and HBV DNA level were not statistically significant (Table 7).
| Variables | Univariable analysis | Multivariable analysis | ||||
| OR | 95%CI | P | OR | 95%CI | P | |
| Sex | 0.788 | (0.367-1.986) | 0.453 | |||
| Age | 0.947 | (0.886-1.198) | 0.715 | |||
| HBV DNA | 0.633 | (0.574-1.393) | 0.214 | |||
| IFN-γ | 0.915 | (0.837-2.131) | 0.656 | |||
| IL-2 | 0.773 | (0.512-1.318) | 0.375 | |||
| Baseline HBeAg | 1.653 | (1.332-2.257) | 0.030 | 1.027 | (1.145-1. 908) | 0.047 |
| Baseline HBsAg | 2.431 | (1.236-3.915) | 0.004 | 1.339 | (1.131-1.862) | 0.009 |
| Week 24 HBeAg change from baseline | 2.762 | (1.562-4.256) | < 0.001 | 2.338 | (1.636-4.863) | < 0.001 |
| Week 36 HBeAg change from baseline | 3.411 | (1.976-4.526) | < 0.001 | 3.185 | (1.977-5.466) | < 0.001 |
| Week 24 HBsAg change from baseline | 4.458 | (2.153-10.198) | < 0.001 | 3.273 | (1.375-5.216) | < 0.001 |
| Week 36 HBsAg change from baseline | 5.371 | (3.239-6.392) | < 0.001 | 5.788 | (2.726-10.612) | < 0.001 |
| Week 12 ALT elevation | 2.676 | (1.133-5.432) | 0.016 | 2.049 | (1.363-9.198) | 0.006 |
| Week 24 ALT elevation | 3.373 | (2.637-7.568) | 0.003 | 3.788 | (2.728-7.687) | 0.003 |
| Week 48 IFN-γ elevation | 2.735 | (1.317-6.682) | 0.002 | 2.171 | (1.163-2.961) | 0.007 |
| Week 48 IL-2 elevation | 2.133 | (1.171-8.616) | 0.008 | 1.882 | (1.026-2.613) | 0.020 |
To further evaluate baseline and changes in HBeAg and HBsAg in early treatment in predicting HBeAg clearance, multivariable logistic regressions were conducted for HBeAg and HBsAg levels at weeks 24 and 36 and HBsAg change from baseline adjusted for age, sex, HBV DNA and an increase at weeks 12 and 24 ALT. Similar to the univariable regression analysis results, all were significantly related to HBeAg clearance. The ORs of ALT elevation at week 12 (OR, 2.049, P = 0.006) and week 24 (OR 3.788, P = 0.003) as well as week 48 IFN-γ elevation (OR, 2.171, P = 0.007) and week 48 IL-2 elevation (OR, 1.882, P = 0.020) were adjusted for age, sex and HBV DNA (Table 7).
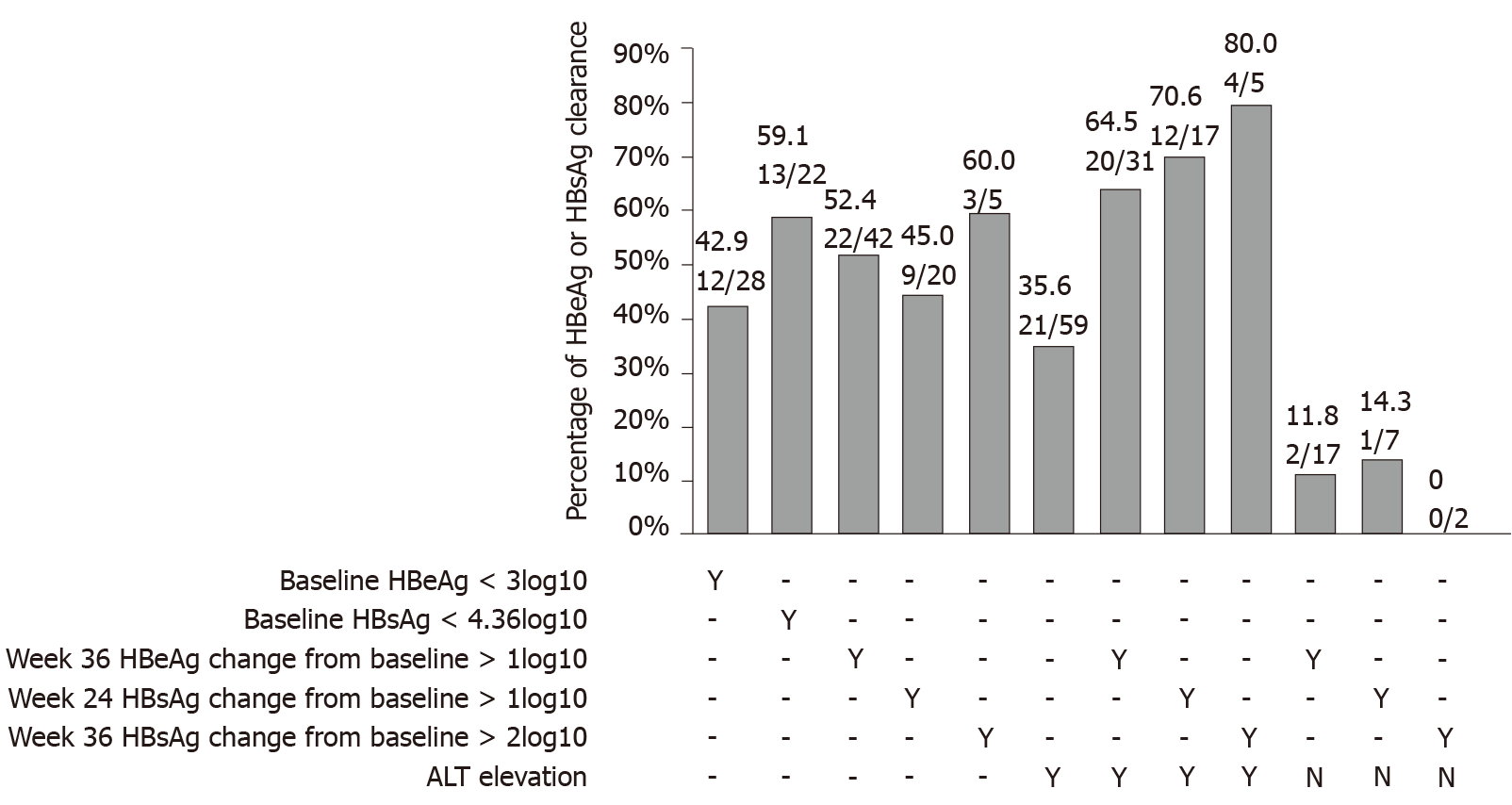
Based on the optimal cutoff values, in our data set, the rates of HBeAg clearance were 42.9% (12/28), 52.0% (13/22), 52.4% (22/42), 45.0% (9/20), 60.0% (3/5) and 35.6% (21/59) for patients with baseline HBeAg < 3 log10, baseline HBsAg < 4.36 log10 IU/mL, week 36 HBeAg change from baseline 1 log week, 24 HBsAg change from baseline >1 log10 IU/mL, week 36 HBsAg change from baseline > 2 log10 IU/mL and ALT elevation, respectively (Figure 4). We combined HBeAg, HBsAg decrease and ALT elevation together. Patients with a week 36 HBeAg change from baseline > 1 log10 SCO/mL and ALT elevation had an HBeAg clearance rate of 64.5% (20/31), and HBsAg change from baseline > 1 log10 IU/mL, > 2 log10 IU/mL with ALT elevation had an HBeAg clearance rate of 70.6% (12/17), 80.0% (4/5), respectively. Only 11.8% (2/17) of patients with week 36 cleared HBeAg showed > 1 log10 SCO/ml HBeAg change from baseline with no ALT elevation. A total of 14.3% (1/7) of patients who cleared HBeAg were among those with week 24 HBsAg change from baseline 1 log10 IU/mL and no ALT elevation, and 0% (0/2) of patients had week 36 HBsAg change from baseline 2 log10 IU/mL and no ALT elevation (Figure 4).
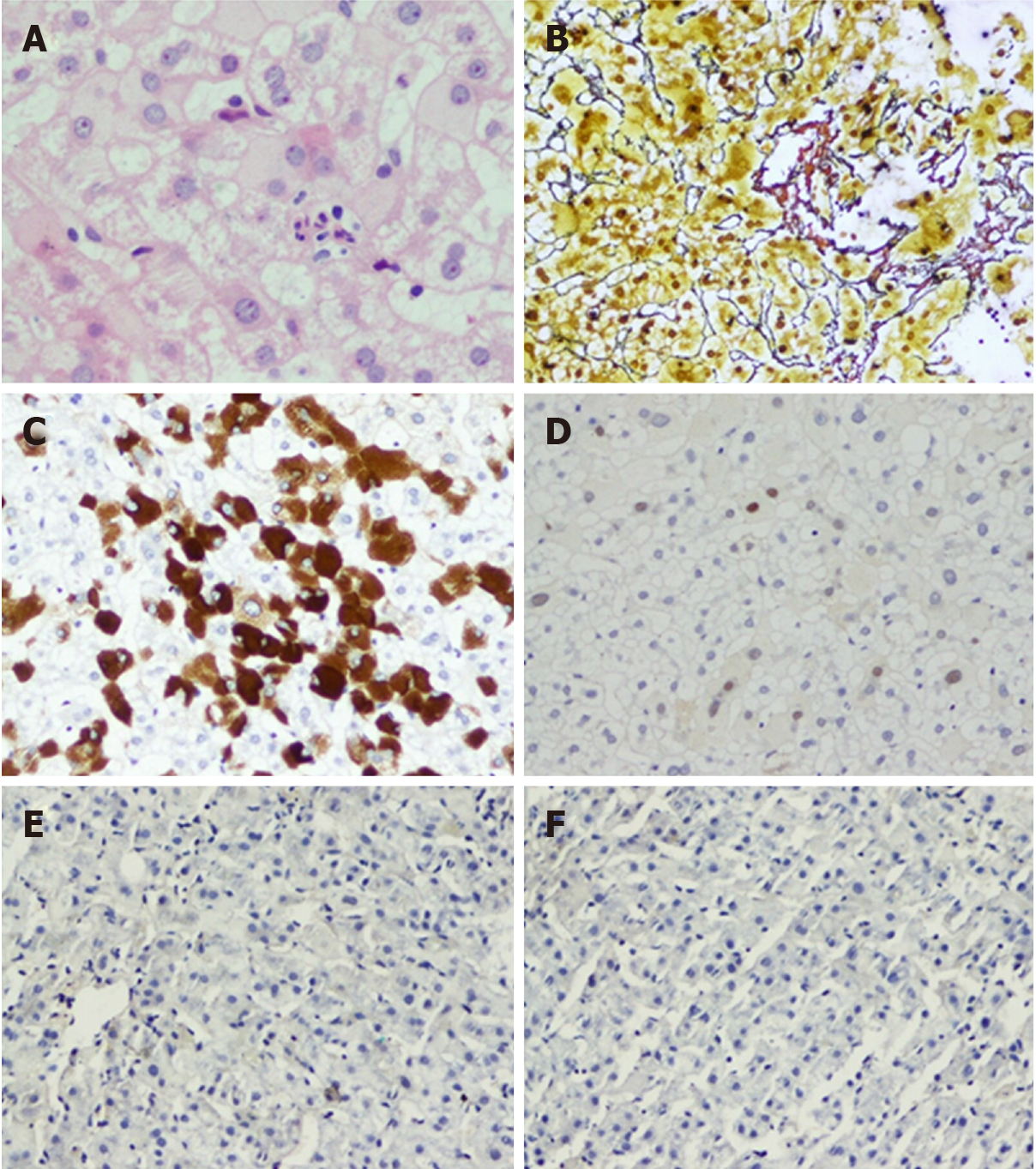
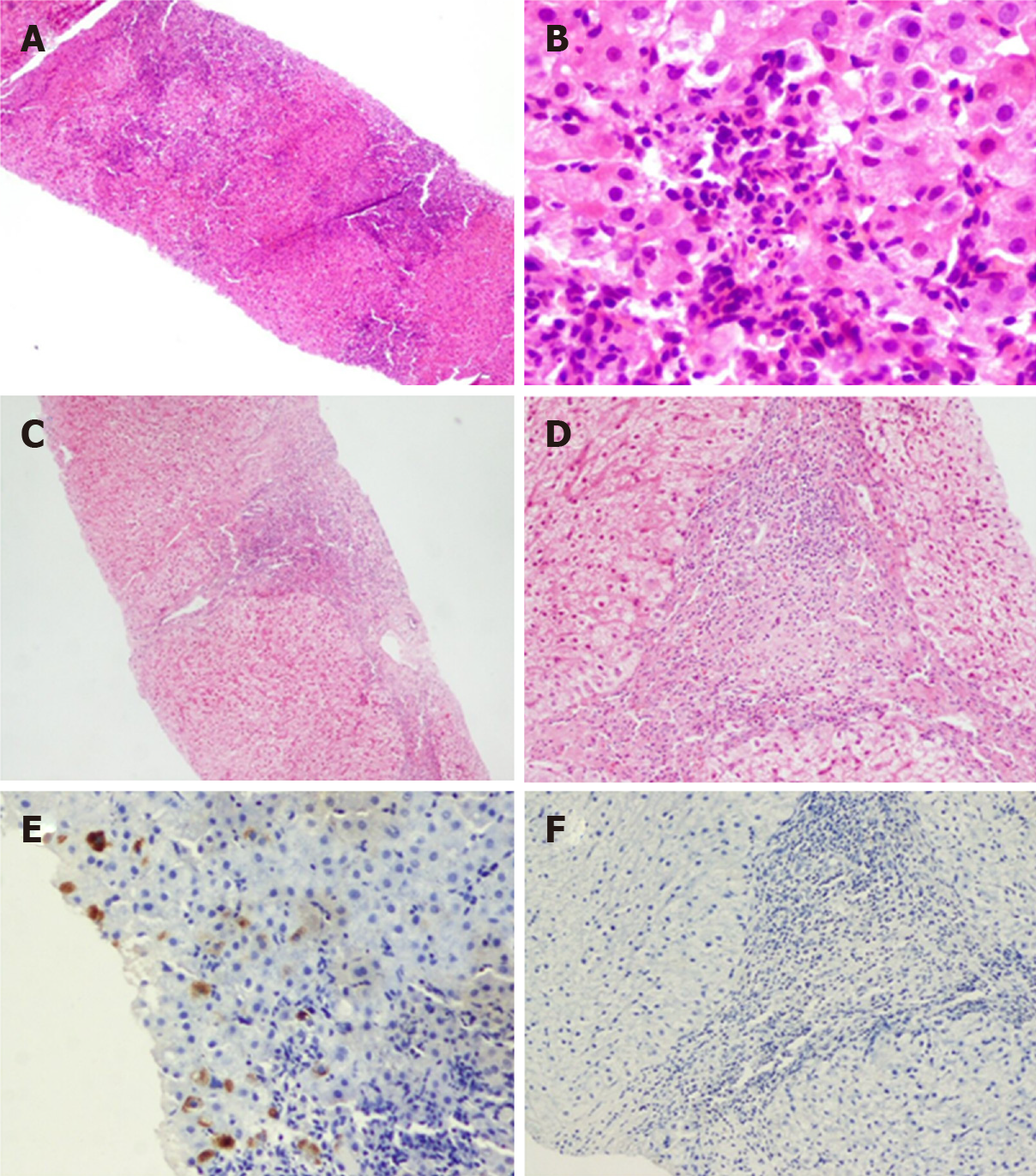
The present study included liver biopsies from 324 patients with chronic HBV infection, including 168 patients in the ICE group and 156 patients in the control group. A total of 138 (42.6%) patients underwent paired biopsy twice, including 72 patients in the ICE group and 66 patients in the control group. Images of two typical cases in the ICE group for these conditions are shown in Figures 5 and 6.
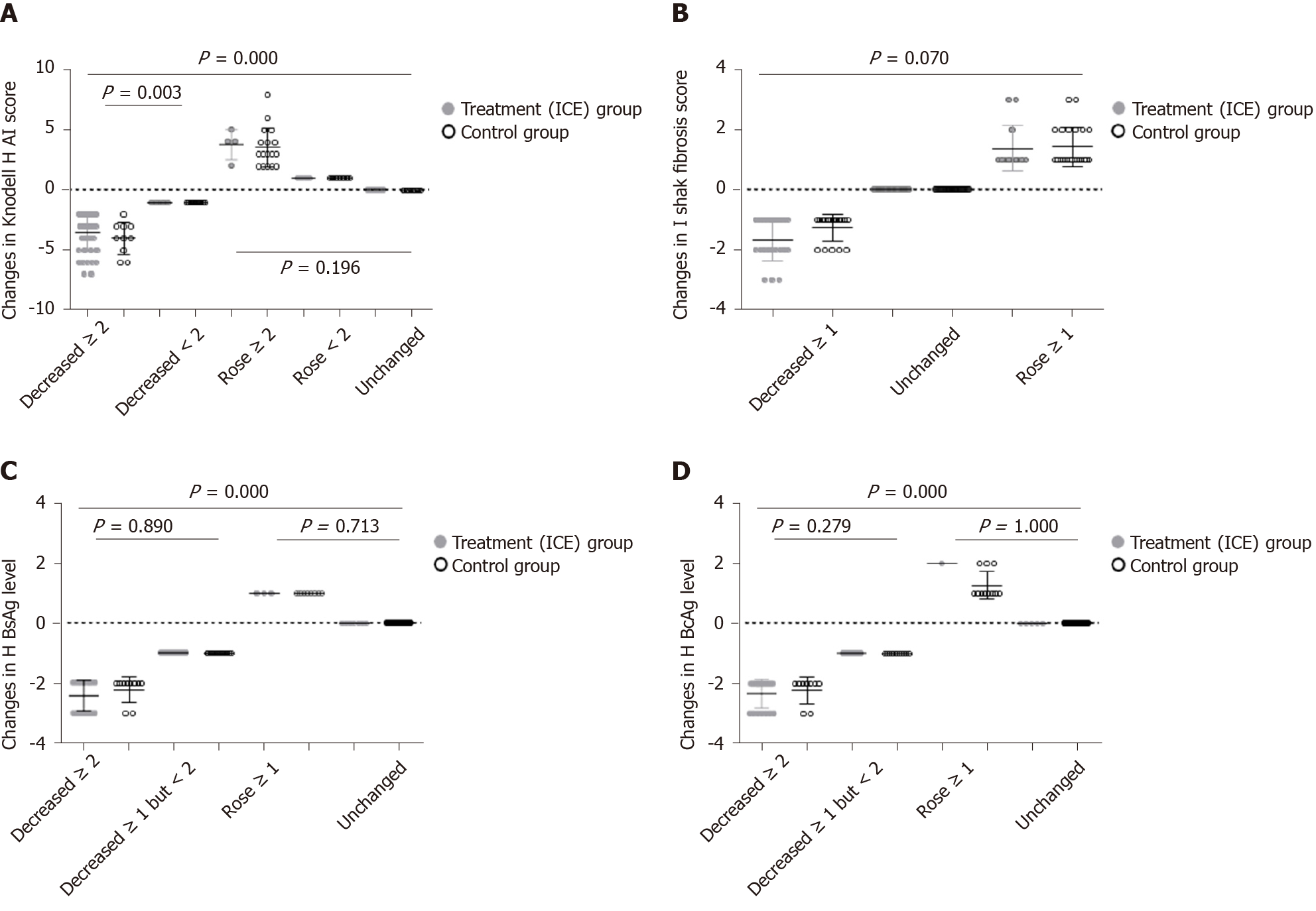
Changes in Knodell HAI score at week 96: As shown in Figure 7, 138 patients underwent liver biopsies twice. A total of 73 patients showed a decrease in the Knodell HAI score at 96 week, including 51 patients in the ICE group and 22 patients in the control group. There were 41 patients in the ICE group and 10 patients in the control group whose scores decreased by ≥ 2 points, and there were 10 patients in the ICE group and 12 patients in the control group whose scores decreased by < 2 points. The difference between the two groups was statistically significant (P = 0.003). A total of 65 patients in the ICE group and the control group showed no improvement or even deterioration in the Knodell HAI score, and 4 patients in the ICE group and 17 patients in the control group increased ≥ 2 points. The Knodell HAI scores of 6 patients in the ICE group and 13 patients in the control group rose < 2 points, and 11 patients in the ICE group and 14 patients in the control group showed an unchanged Knodell HAI score. There was no significant difference between the two groups (P = 0.196).
Changes in Ishak fibrosis score at week 96: As shown in Figure 7, a total of 42 patients had decreased Ishak fibrosis scores at week 96. There were 23 patients in the ICE group and 19 patients in the control group whose scores decreased by ≥ 1 point.
After 96 wk of administration, a total of 96 patients in the ICE group and the control group showed no improvement or even deterioration in Ishak fibrosis score with 13 patients in the ICE group and 23 patients in the control group increasing ≥ 1 point, respectively. The Ishak fibrosis scores of 36 patients in the ICE group and 24 patients in the control group showed an unchanged Ishak fibrosis score. There was no significant difference between the two groups (P = 0.070).
Changes in liver HBsAg levels at week 96: There are five levels of liver HBsAg, HBcAg: “-,” “+,” “+ +,” “+ + +” and “+ + + +,” which are converted to scores of 0, 1, 2, 3 and 4, respectively. After 96 wk of administration, there were a total of 85 patients in the ICE group and the control group whose liver HBsAg level decreased. There were 28 patients in the ICE group and 10 patients in the control group whose scores decreased by ≥ 2 points, and there were 34 patients in the ICE group and 13 patients in the control group whose scores decreased by ≥ 1 but < 2 points. There was no significant difference between the two groups (P = 0.890). After 96 wk of administration, a total of 53 patients in the ICE group and the control group showed no improvement or even deterioration in liver HBsAg levels with 3 patients in the ICE group and 8 patients in the control increasing ≥ 1 point. Seven patients in the ICE group and 35 patients in the control group showed unchanged liver HBsAg levels. There was no significant difference between the two groups (P = 0.713); however, there was a significant difference in liver HBsAg levels between the two groups (P = 0.000, Figure 7).
Changes in liver HBcAg levels at week 96: After 96 wk of administration, there were a total of 86 patients in the ICE group and the control group whose liver HBcAg level decreased. There were 21 patients in the ICE group and 9 patients in the control group whose scores decreased by ≥ 2 points, and there were 45 patients in the ICE group and 11 patients in the control group whose scores decreased by ≥ 1 but < 2 points. There was no significant difference between the two groups (P = 0.279).
After 96 wk of administration, a total of 52 patients in the ICE group and the control group showed no improvement or even deterioration in liver HBcAg levels with 1 patient in the ICE group and 11 patients in the control group increasing ≥ 1 point. Eleven patients in the ICE group and 35 patients in the control group showed unchanged liver HBcAg levels. There was no significant difference between the two groups (P = 1.000). However, there was a significant difference in liver HBcAg levels between the two groups (P = 0.000, Figure 7).
Adverse events: During follow-up, there were five adverse events, including two cases of diarrhea (one in each group), one case of dizziness (control group) and one case of nausea (control group). Patients continued to take the medicine after symptom relief. One case was terminated due to the discovery of hepatocellular carcinoma (control group).
Drug combination: Six cases (two in the ICE group, four in the control group, bicyclol, wuzhi tablets, glycyrrhizic acid preparations, etc.) were treated with drugs with hepatoprotective effects due to abnormal liver function. One case (ICE group) was treated with contac due to cold. One case (control group) was treated with TCM due to leg injury. One case (control group) was treated with Euthyrox due to abnormal thyroid function.
In the treatment (ICE) group, 52 subjects were followed for 48 wk, and 43 subjects were followed for 24 wk. All subjects with cleared HBeAg maintained HBeAg clearance during the treatment, while five patients showed delayed HBeAg clearance.
However, in the control group, 23 subjects were followed for 48 wk and 36 for 24 wk; only one patient had HBeAg clearance during the period of follow-up.
In the treatment group, there was no obvious increase in HBV DNA levels after drug withdrawal, ALT levels were in the normal range, and no aggravations were observed.
Previous studies have shown that in CHB patients, liver lesions progressed with age. After the age of 30, the severity of hepatic inflammatory activity and fibrosis was significantly higher in CHB patients than in those under 30 years of age[35-37]. To further confirm the liver histopathology of CHB patients with persistent normal ALT at enrollment, 82.0% (324/395) of patients underwent liver biopsy. A total of 35.5% (115/324) of patients with normal serum ALT had a ≥ 4 inflammation score of liver histopathology, and 39.2% (127/324) of patients had a ≥ 2 fibrosis score. Our liver biopsy data indicated that chronic hepatitis can be diagnosed in nearly 40% of HBV carriers as a serological diagnosis. Consistent with a previous study, our results further validated that the risk of liver inflammation and fibrosis increased with age in chronic HBV carriers over 30 years old[23], which is the reason that this study defined the age of ALT-normal HBeAg-positive chronic HBV carriers as above 30 years old. The new edition of China’s 2015 guidelines and the 2017 European Association for the Study of the Liver guidelines have adjusted the age of observation in the indications of antiviral therapy, which was reduced from > 40 years old to > 30 years old. This change is consistent with the research criteria we set[1,4].
Despite recent advances in the treatment of CHB, including multiple nucleoside/nucleotide analogs, no treatment is suitable for chronic HBV carriers with normal ALT or patients in the immune-tolerance phase. Because these patients over 30 years of age are at an increased risk of disease progression with age, most of them desperately need effective and safe treatment to reduce persistently high levels of HBV DNA and prevent progression to cirrhosis and HCC[9-11]. Attempts and efforts have been made by many researchers in this regard, including tenofovir therapy[14-16]. For CHB patients with normal ALT, antiviral therapy is less effective even if the histological examination indicates chronic hepatitis. A recent study showed that after treatment with interferon, 17.54% of patients with normal ALT but significant liver inflammation or fibrosis (G4, S3) demonstrated a sustained virologic response, which is significantly lower than those patients with elevated ALT (28.57%)[38]. Our study modified the TCM compound therapy from our previous national science and technology major project during the 11th five-year plan period[20] and extended the treatment course to 96 wk.
In this study, a total of 22 patients were lost to follow-up in the placebo group, and 13 patients were lost to follow-up in the ICE-treated group. After a 96-wk treatment, 18.59% (37/199) of CHB patients had HBV DNA levels ≤ 4 log10 IU/mL, and the HBeAg clearance and conversion rates were 16.08% (32/199) and 14.57% (29/199), respectively. HBeAg clearance persisted in the treatment group patients at 24 or 48 wk after drug withdrawal. Extended HBeAg clearance was observed in five cases, while one case was observed in the control group. Furthermore, serum HBsAg levels and liver HBsAg expression were decreased in the ICE treatment group. In addition, 35.6% (59/199) of patients in the ICE-treated group had increased ALT levels after the 12- or 24-wk therapy in comparison with 5.1% (10/196) of patients in the control group. The mean serum bilirubin level was < 35 mmol/L, which may be related to the immune activation and anti-HBV response during ICE therapy. Paired biopsies showed a significant difference between the treatment group and the control group after 96 wk of treatment regarding the Knodell HAI score, indicating inflammation in the liver. Although the fibrosis score decreased ≥ 1 subgroup, there was no difference between the two groups (P = 0.08). In the no improvement subgroup, the treatment group was significantly better than the control group (P = 0.001), and there was also a significant difference between the two groups in the total fibrosis score (P = 0.000). In this study, except for four patients with diarrhea, dizziness or nausea after initiation of the medication, no other serious adverse effects occurred, indicating that ICE treatment is relatively safe. No deterioration of liver function occurred in this study. Therefore, the reduction in HBV DNA, clearance and conversion rates of HBeAg in this study were significantly higher than those reported in previous antiviral therapies.
In ancient China, there was no unambiguous description of CHB. In recent years, according to the natural history of chronic HBV infection, clinical manifestations and the theory of TCM epidemiology[39], we proposed that “kidney asthenia and hepatic blood prostrated by dampness-heat” is the pathogenesis of CHB. During the course of infection, dampness-heat was considered the initial pathogenic factor, deficiency of kidney qi was its underlying factor, and stagnation of the liver channel was a critical link in its pathology. In this context, the main principles of treating chronic HBV infection are invigorating the kidney, clearing away the heat evil, expelling superficial evils, activating blood and eliminating dampness. According to the above data and clinical experience, we propose the experiential effective recipe for ICE. Radix et caulis acanthopanacis senticosi, Herba Epimedii, Fructus ligustri lucidi and Herba ecliptae are the main drugs in this formula. Modern studies have proven that these herbs have liver-protective and immunity-enhancing effects[40]. In TCM theory, Phyllanthus urinaria Linn has the effects of clearing heat, removing toxicity and softening and resolving hard mass. The anti-HBV effect of this herb has been demonstrated[41-48]. Rhizoma polygoni cuspidati clears heat and removes toxicity in TCM theory and has an antiviral effect in modern medicine[49,50]. Radix bupleuri, Radix paeoniae alba, Fructus aurantii immaturus and Radix glycyrrhizae are minister drugs and have effects of eliminating pathogenic factors[51]. Semen persicae is the assistant drug and promotes blood circulation to remove blood stasis and antiliver fibrosis following CHB. Radix glycyrrhizae is an envoy drug that removes toxicity and moderates the properties of herbs. The ICE formula exerts therapeutic effects through the concerted application of monarch, minister, assistant and envoy by tonifying, clearing, expelling and activating methods.
After 96 wk of treatment of HBeAg-positive chronic HBV carriers with normal ALT, more than 80% of patients still could not achieve HBV DNA reduction to ≤ 4 log10 IU/mL or HBeAg clearance. Therefore, selection of the benefit population is essential for therapy outcome prediction. Quantitative determination of HBsAg and HBeAg is of great significance for antiviral therapy because it can be used as an immune control indicator to predict serum HBeAg clearance rate, HBsAg clearance rate and long-term prognosis[52-55]. In addition, HBsAg and HBeAg level reduction at 24 and 36 wk during the treatment period were important predictors of the sustained response of ICE. Univariate logistic regression analysis showed that HBsAg levels decreased significantly at 24 and 36 wk after treatment compared with baseline. At 36 wk after treatment, the HBeAg level decreased significantly as well. Notably, the ALT level increased significantly at 12 and 24 wk. In addition, HBeAg clearance was statistically significant at baseline. As mentioned above, the changes in ALT and HBsAg were related to HBeAg clearance. CHB patients with elevated ALT and a decline > 1 log10 IU/mL in HBsAg level from baseline at week 24 achieved an HBeAg clearance rate of 70.6% (12/17), and for those in whom HBsAg changed from baseline > 2 log10 IU/mL at week 36, the rate was 80% (4/5). CHB patients with elevated ALT and a decline > 1 log10 IU/mL in HBeAg from baseline at week 36 achieved an HBeAg clearance rate of 64.5% (20/31). Therefore, in clinical practice, increased ALT, decreased HBsAg at week 12 and 24 and decreased HBeAg at week 24 and 36 are potential indicators for the early prediction of HBeAg clearance in TCM therapy, and this response was relatively extended compared with interferon[56].
We further investigated the changes in immune function of patients before and after treatment. Elevated IFN-γ and IL-2 levels at week 48 were statistically significant in predicting serological clearance of HBeAg[42,57]. At weeks 48 and 96, serum IFN-γ and IL-2 increased significantly in the ICE group compared with the control group. It has been demonstrated in several studies that IL-2 as well as IFN-γ production therapy may amplify the immune response by regulating T lymphocytes and natural killer cells, which showed clinical efficacy in Caucasian HBV DNA- and HBeAg-positive patients. Another pilot study showed that IL-2 combined with IFN-γ treatment induced HBV-specific CD4+ T cell proliferative responses, which could be important in controlling viremia in chronic HBV carriers. Therefore, ICE induced antiviral immune responses mainly through IL-2 and IFN-γ expression, which might lead to consequent viral elimination.
Although our study suggests that HBV DNA, HBsAg and HBeAg decreased significantly after TCM therapy, long-term prognosis, including long-term changes in HBsAg and long-term benefits in reducing the risks of cirrhosis and HCC are still unclear. However, in this study, we tried to use TCM to treat patients with unsatisfactory antiviral efficacy, controversial therapies or risks of disease progression. We provided a safe and effective therapy in this study.
In conclusion, in patients with HBeAg-positive chronic HBV infection with normal ALT, ICE therapy can achieve reduced HBV replication, increased HBeAg clearance rate and serum conversion rate and significant liver histology improvement. ICE therapy is safe and effective, and the effects may be related to host immune status modulation.
No guideline recommends antiviral therapy for hepatitis B e antigen (HBeAg)-positive chronic hepatitis B patients with persistently normal alanine aminotransferase (ALT) levels and a high hepatitis B virus (HBV) DNA viral load. Despite long-term normal ALT levels, a high HBV DNA viral load persists, and liver lesions progress unrecognized and advance gradually.
The purpose of this study was to provide clinical evidence for traditional Chinese medicine treatment for chronic HBV carriers, especially chronic HBV carriers over 30 years old with a higher risk of disease progression.
To evaluate the feasibility and safety of a Chinese herbal formula as a therapeutic option for chronic HBV infection.
The 395 patients (30–65 years old) with confirmed HBeAg-positive chronic hepatitis B infection and persistently normal ALT were randomized to receive either the Chinese herbal formula or placebo for 96 wk. Endpoints to evaluate therapeutic efficacy included: (1) HBV DNA levels decreased to less than 4 log10 IU/mL at weeks 48 and 96; and (2) HBeAg clearance and seroconversion rates at weeks 48 and 96.
HBV DNA levels ≤ 4 log10 IU/mL were 10.05% at week 48 and 18.59% at week 96 in the treatment group. The HBeAg clearance and conversion rates were 8.54% and 8.04 at week 48 and 16.08% and 14.57% at week 96, respectively. However, HBV DNA levels ≤ 4 log10 IU/mL were 2.55% and 2.55% at weeks 48 and 96, respectively, and the HBeAg clearance rates were 3.06% and 5.61% at weeks 48 and 96, respectively, in the control group. The quantitative hepatitis B surface antigen and HBeAg levels at baseline and changes during the treatment period as well as the ALT elevation at weeks 12 and 24 were strong predictors of HBeAg clearance.
High rates of HBV DNA reduction, HBeAg clearance and seroconversion could be achieved with Chinese herbal formula treatments, and the treatments were relatively safe for HBeAg-positive chronic hepatitis B -infected patients with persistently normal ALT. The ability of the compound to modulate host immune function probably contributed to this effect.
We provided a safe and effective therapy in treating patients with unsatisfactory antiviral efficacy, controversial therapies or risks of disease progression. Traditional Chinese medicine treatment may be a therapeutic option for chronic HBV infection.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dogan U, Thomopoulos K S-Editor: Zhang H L-Editor: Filipodia P-Editor: Ma YJ
| 1. | European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3745] [Cited by in RCA: 3802] [Article Influence: 475.3] [Reference Citation Analysis (1)] |
| 2. | Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS, Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 2846] [Article Influence: 406.6] [Reference Citation Analysis (0)] |
| 3. | Shiha G, Ibrahim A, Helmy A, Sarin SK, Omata M, Kumar A, Bernstien D, Maruyama H, Saraswat V, Chawla Y, Hamid S, Abbas Z, Bedossa P, Sakhuja P, Elmahatab M, Lim SG, Lesmana L, Sollano J, Jia JD, Abbas B, Omar A, Sharma B, Payawal D, Abdallah A, Serwah A, Hamed A, Elsayed A, AbdelMaqsod A, Hassanein T, Ihab A, GHaziuan H, Zein N, Kumar M. Asian-Pacific Association for the Study of the Liver (APASL) consensus guidelines on invasive and non-invasive assessment of hepatic fibrosis: a 2016 update. Hepatol Int. 2017;11:1-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 170] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 4. | Chinese Society of Hepatology, Chinese Medical Association. Chinese Society of Infectious Diseases, Chinese Medical Association, Hou JL, lai W. [The guideline of prevention and treatment for chronic hepatitis B: a 2015 update]. Zhonghua Gan Zang Bing Za Zhi. 2015;23:888-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 89] [Reference Citation Analysis (0)] |
| 5. | Mason WS, Gill US, Litwin S, Zhou Y, Peri S, Pop O, Hong ML, Naik S, Quaglia A, Bertoletti A, Kennedy PT. HBV DNA Integration and Clonal Hepatocyte Expansion in Chronic Hepatitis B Patients Considered Immune Tolerant. Gastroenterology. 2016;151:986-998.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 319] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 6. | Hui CK, Leung N, Yuen ST, Zhang HY, Leung KW, Lu L, Cheung SK, Wong WM, Lau GK; Hong Kong Liver Fibrosis Study Group. Natural history and disease progression in Chinese chronic hepatitis B patients in immune-tolerant phase. Hepatology. 2007;46:395-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 209] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 7. | Andreani T, Serfaty L, Mohand D, Dernaika S, Wendum D, Chazouillères O, Poupon R. Chronic hepatitis B virus carriers in the immunotolerant phase of infection: histologic findings and outcome. Clin Gastroenterol Hepatol. 2007;5:636-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Chu CM, Hung SJ, Lin J, Tai DI, Liaw YF. Natural history of hepatitis B e antigen to antibody seroconversion in patients with normal serum aminotransferase levels. Am J Med. 2004;116:829-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 229] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 9. | Liu J, Yang HI, Lee MH, Batrla-Utermann R, Jen CL, Lu SN, Wang LY, You SL, Hsiao CK, Chen CJ; REVEAL-HBV Study Group. Distinct seromarkers predict different milestones of chronic hepatitis B progression. Hepatology. 2014;60:77-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Tseng TC, Liu CJ, Yang HC, Su TH, Wang CC, Chen CL, Kuo SF, Liu CH, Chen PJ, Chen DS, Kao JH. High levels of hepatitis B surface antigen increase risk of hepatocellular carcinoma in patients with low HBV load. Gastroenterology. 2012;142:1140-1149.e3; quiz e13-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 437] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 11. | Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ; Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer-In HBV (the REVEAL-HBV) Study Group. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;130:678-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1164] [Cited by in RCA: 1174] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 12. | Chen CF, Lee WC, Yang HI, Chang HC, Jen CL, Iloeje UH, Su J, Hsiao CK, Wang LY, You SL, Lu SN, Chen CJ; Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer in HBV (REVEAL–HBV) Study Group. Changes in serum levels of HBV DNA and alanine aminotransferase determine risk for hepatocellular carcinoma. Gastroenterology. 2011;141:1240-1248, 1248.e1-1248.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 204] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 13. | Chu CM, Liaw YF. Chronic hepatitis B virus infection acquired in childhood: special emphasis on prognostic and therapeutic implication of delayed HBeAg seroconversion. J Viral Hepat. 2007;14:147-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 127] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 14. | Hui CK, Leung N, Shek TW, Yao H, Lee WK, Lai JY, Lai ST, Wong WM, Lai LS, Poon RT, Lo CM, Fan ST, Lau GK; Hong Kong Liver Fibrosis Study Group. Sustained disease remission after spontaneous HBeAg seroconversion is associated with reduction in fibrosis progression in chronic hepatitis B Chinese patients. Hepatology. 2007;46:690-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Carey I, D'Antiga L, Bansal S, Longhi MS, Ma Y, Mesa IR, Mieli-Vergani G, Vergani D. Immune and viral profile from tolerance to hepatitis B surface antigen clearance: a longitudinal study of vertically hepatitis B virus-infected children on combined therapy. J Virol. 2011;85:2416-2428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | D'Antiga L, Aw M, Atkins M, Moorat A, Vergani D, Mieli-Vergani G. Combined lamivudine/interferon-alpha treatment in "immunotolerant" children perinatally infected with hepatitis B: a pilot study. J Pediatr. 2006;148:228-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Xiao Y, Zeng Y, Alexander E, Mehta S, Joshi SB, Buchman GW, Volkin DB, Middaugh CR, Isaacs SN. Adsorption of recombinant poxvirus L1-protein to aluminum hydroxide/CpG vaccine adjuvants enhances immune responses and protection of mice from vaccinia virus challenge. Vaccine. 2013;31:319-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Chan HL, Chan CK, Hui AJ, Chan S, Poordad F, Chang TT, Mathurin P, Flaherty JF, Lin L, Corsa A, Gaggar A, Subramanian GM, McHutchison JG, Lau G, Lee S, Gane EJ. Effects of tenofovir disoproxil fumarate in hepatitis B e antigen-positive patients with normal levels of alanine aminotransferase and high levels of hepatitis B virus DNA. Gastroenterology. 2014;146:1240-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 19. | Tong GD, Liu YM, Liu ZZ. 62 Cases of Chronic Hepatitis B Treated by Bushen Qingtou Prescription. Journal of Anhui Traditional Chinese Medical College. 2001;20:20-22. |
| 20. | He J, Zhou D, Tong G, Xing Y, Chen Y, Zhang X, Zhan B, Gao H, Zhou X, Xiong Y, Liu X, Peng L, Qiu M, Zheng Y. Efficacy and safety of a chinese herbal formula (invigorating kidney and strengthening spleen) in chronic hepatitis B virus carrier: results from a multicenter, randomized, double-blind, and placebo-controlled trial. Evid Based Complement Alternat Med. 2013;2013:961926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Zhang P, Du HB, Tong GD, Li XK, Sun XH, Chi XL, Xing YF, Zhou ZH, Li Q, Chen B, Wang H, Wang L, Jin H, Mao DW, Wang XB, Wu QK, Li FP, Hu XY, Lu BJ, Yang ZY, Zhang MX, Shi WB, He Q, Li Y, Jiang KP, Xue JD, Li XD, Jiang JM, Lu W, Tian GJ, Hu ZB, Guo JC, Li CZ, Deng X, Luo XL, Li FY, Zhang XW, Zheng YJ, Zhao G, Wang LC, Wu JH, Guo H, Mi YQ, Gong ZJ, Wang CB, Jiang F, Guo P, Yang XZ, Shi WQ, Yang HZ, Zhou Y, Sun NN, Jiao YT, Gao YQ, Zhou DQ, Ye YA. Serum hepatitis B surface antigen correlates with fibrosis and necroinflammation: A multicentre perspective in China. J Viral Hepat. 2018;25:1017-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 22. | Chen YJ, Li HZ, Tong GD, He JS, Xing YF, Gao H, Zhou XZ, Qiu M, Zheng YJ, Xu WJ, Xu SM, Chen L, Tang HH, Zhang L, Zhan BL, Ma WF, Sun XF, Li Q, Zhang XH, Zhou DQ. Antiviral Therapeutic Effects of Bushen Qingtou Prescription to Hepatitis B Virus Carriers with Positive e Antigen. Chin J Exp Tradit Med Formulae. 2013;19:283-288. |
| 23. | Xing YF, Zhou DQ, He JS, Wei CS, Zhong WC, Han ZY, Peng DT, Shao MM, Sham TT, Mok DK, Chan CO, Tong GD. Clinical and histopathological features of chronic hepatitis B virus infected patients with high HBV-DNA viral load and normal alanine aminotransferase level: A multicentre-based study in China. PLoS One. 2018;13:e0203220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China. Beijing: People’s Medical Publishing House, 2005. |
| 25. | Lam MY, Lee H, Bright R, Korzenik JR, Sands BE. Validation of interactive voice response system administration of the Short Inflammatory Bowel Disease Questionnaire. Inflamm Bowel Dis. 2009;15:599-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Horowitz GL, Zaman Z, Blanckaert NJ, Chan DW, Dubois JA, Golaz O, Mensi N, Keller F, Stolz H, Klingler K, Marocchi A, Prencipe L, McLawhon RW, Nilsen OL, Oellerich M, Luthe H, Orsonneau JL, Richeux G, Recio F, Roldan E, Rymo L, Wicktorsson AC, Welch SL, Wieland H, Grawitz AB, Mitsumaki H, McGovern M, Ng K, Stockmann W. MODULAR ANALYTICS: A New Approach to Automation in the Clinical Laboratory. J Autom Methods Manag Chem. 2005;2005:8-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Liu TW, Yeh ML, Huang CF, Lin IL, Huang JF, Dai CY, Chen YL, Chuang WL, Yu ML. Clinical performance of a new hepatitis B surface antigen quantitative assay with automatic dilution. Kaohsiung J Med Sci. 2015;31:26-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 28. | Szadowska A, Lasota J, Pertyński T, Juszyński A. Percutaneous fine needle aspiration biopsy of tumorous lesions of the liver and the pancreas guided by ultrasonography. Patol Pol. 1988;39:73-82. [PubMed] |
| 29. | Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW, Wollman J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:431-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2558] [Cited by in RCA: 2509] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 30. | Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3521] [Cited by in RCA: 3784] [Article Influence: 126.1] [Reference Citation Analysis (1)] |
| 31. | Brunt EM. Grading and staging the histopathological lesions of chronic hepatitis: the Knodell histology activity index and beyond. Hepatology. 2000;31:241-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 339] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 32. | Tran TT. Immune tolerant hepatitis B: a clinical dilemma. Gastroenterol Hepatol (N Y). 2011;7:511-516. [PubMed] |
| 33. | Perrillo RP, Lai CL, Liaw YF, Dienstag JL, Schiff ER, Schalm SW, Heathcote EJ, Brown NA, Atkins M, Woessner M, Gardner SD. Predictors of HBeAg loss after lamivudine treatment for chronic hepatitis B. Hepatology. 2002;36:186-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 226] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 34. | Yuen MF, Yuan HJ, Hui CK, Wong DK, Wong WM, Chan AO, Wong BC, Lai CL. A large population study of spontaneous HBeAg seroconversion and acute exacerbation of chronic hepatitis B infection: implications for antiviral therapy. Gut. 2003;52:416-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 107] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 35. | Tan Y, Ye Y, Zhou X, Chen L, Wen D. Age as a predictor of significant fibrosis features in HBeAg-negative chronic hepatitis B virus infection with persistently normal alanine aminotransferase. PLoS One. 2015;10:e0123452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 36. | Cheng JL, Wang XL, Yang SG, Zhao H, Wu JJ, Li LJ. Non-ALT biomarkers for markedly abnormal liver histology among Chinese persistently normal alanine aminotransferase-chronic hepatitis B patients. World J Gastroenterol. 2017;23:2802-2810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Lai M, Hyatt BJ, Nasser I, Curry M, Afdhal NH. The clinical significance of persistently normal ALT in chronic hepatitis B infection. J Hepatol. 2007;47:760-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 263] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 38. | Chen J, Xu CR, Xi M, Hu WW, Tang ZH, Zang GQ. Predictors of liver histological changes and a sustained virological response to peginterferon among chronic hepatitis B e antigen-positive patients with normal or minimally elevated alanine aminotransferase levels. J Viral Hepat. 2017;24:573-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 39. | Tong G, Peng S. Disscussion on the treatment of chronic hepatitis B based on the Liu's theory of kidney deficiency and incubative pathogen. J Tradit Chin Med. 2004;45:726-728. [DOI] [Full Text] |
| 40. | Yang Y, Nian H, Tang X, Wang X, Liu R. Effects of the combined Herba Epimedii and Fructus Ligustri Lucidi on bone turnover and TGF-β1/Smads pathway in GIOP rats. J Ethnopharmacol. 2017;201:91-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 41. | Deng W, Zheng M, Zhang J, Huang C, Zhang Y. [Immunologic functions of total flavone of Epimedium of two species in Guizhou]. Zhongguo Zhong Yao Za Zhi. 2011;36:511-513. [PubMed] |
| 42. | Zhou D, Tong G, Chen Y, Gao H, Qiu M, Zheng Y, Chen L, Zhan B, Xing Y. A randomized double-blind placebo-controlled clinical trial on a Chinese herbal formula (invigorating the kidney and the spleen gelatin capsule) in the treatment of chronic hepatitis B virus carriers. Chin J Exp Tradit Med Formulae. 2010;16:246-249. [DOI] [Full Text] |
| 43. | Chan HL, Sung JJ, Fong WF, Chim AM, Yung PP, Hui AY, Fung KP, Leung PC. Double-blinded placebo-controlled study of Phyllanthus urinaris for the treatment of chronic hepatitis B. Aliment Pharmacol Ther. 2003;18:339-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 44. | Liu S, Wei W, Li Y, Lin X, Shi K, Cao X, Zhou M. In vitro and in vivo anti-hepatitis B virus activities of the lignan nirtetralin B isolated from Phyllanthus niruri L. J Ethnopharmacol. 2014;157:62-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 45. | Li Y, Jiang M, Li M, Chen Y, Wei C, Peng L, Liu X, Liu Z, Tong G, Zhou D, He J. Compound Phyllanthus urinaria L Inhibits HBV-Related HCC through HBx-SHH Pathway Axis Inactivation. Evid Based Complement Alternat Med. 2019;2019:1635837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 46. | Liu S, Wei W, Shi K, Cao X, Zhou M, Liu Z. In vitro and in vivo anti-hepatitis B virus activities of the lignan niranthin isolated from Phyllanthus niruri L. J Ethnopharmacol. 2014;155:1061-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 47. | Mohan M, James P, Valsalan R, Nazeem PA. Molecular docking studies of phytochemicals from Phyllanthus niruri against Hepatitis B DNA Polymerase. Bioinformation. 2015;11:426-431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 48. | Wu Y, Lu Y, Li SY, Song YH, Hao Y, Wang Q. Extract from Phyllanthus urinaria L. inhibits hepatitis B virus replication and expression in hepatitis B virus transfection model in vitro. Chin J Integr Med. 2015;21:938-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 49. | Fan HT, Ding SL, Lin HS. [Pharmacological of Polygoni cuspidati rhizoma]. Zhongguo Zhong Yao Za Zhi. 2013;38:2545-2548. [PubMed] |
| 50. | Zhou YX, Chen J, Li JP, Wang YL, Jin XD. Chinese medicinal herbs in treating model rats with hepatic fibrosis. Afr J Tradit Complement Altern Med. 2009;7:104-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 51. | Yang F, Dong X, Yin X, Wang W, You L, Ni J. Radix Bupleuri: A Review of Traditional Uses, Botany, Phytochemistry, Pharmacology, and Toxicology. Biomed Res Int. 2017;2017:7597596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 52. | Ahn SH, Chan HL, Chen PJ, Cheng J, Goenka MK, Hou J, Lim SG, Omata M, Piratvisuth T, Xie Q, Yim HJ, Yuen MF; APPROACH Working Group. Chronic hepatitis B: whom to treat and for how long? Propositions, challenges, and future directions. Hepatol Int. 2010;4:386-395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 53. | Marcellin P, Bonino F, Lau GK, Farci P, Yurdaydin C, Piratvisuth T, Jin R, Gurel S, Lu ZM, Wu J, Popescu M, Hadziyannis S; Peginterferon alfa-2a in HBeAg-negative Chronic Hepatitis B Study Group. Sustained response of hepatitis B e antigen-negative patients 3 years after treatment with peginterferon alpha-2a. Gastroenterology. 2009;136:2169-2179.e1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 260] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 54. | Sonneveld MJ, Rijckborst V, Boucher CA, Hansen BE, Janssen HL. Prediction of sustained response to peginterferon alfa-2b for hepatitis B e antigen-positive chronic hepatitis B using on-treatment hepatitis B surface antigen decline. Hepatology. 2010;52:1251-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 226] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 55. | Brunetto MR, Moriconi F, Bonino F, Lau GK, Farci P, Yurdaydin C, Piratvisuth T, Luo K, Wang Y, Hadziyannis S, Wolf E, McCloud P, Batrla R, Marcellin P. Hepatitis B virus surface antigen levels: a guide to sustained response to peginterferon alfa-2a in HBeAg-negative chronic hepatitis B. Hepatology. 2009;49:1141-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 358] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 56. | Cao Z, Liu Y, Ma L, Lu J, Jin Y, Ren S, He Z, Shen C, Chen X. A potent hepatitis B surface antigen response in subjects with inactive hepatitis B surface antigen carrier treated with pegylated-interferon alpha. Hepatology. 2017;66:1058-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 57. | Yang F, Yu X, Zhou C, Mao R, Zhu M, Zhu H, Ma Z, Mitra B, Zhao G, Huang Y, Guo H, Wang B, Zhang J. Hepatitis B e antigen induces the expansion of monocytic myeloid-derived suppressor cells to dampen T-cell function in chronic hepatitis B virus infection. PLoS Pathog. 2019;15:e1007690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |