Published online Aug 14, 2020. doi: 10.3748/wjg.v26.i30.4465
Peer-review started: March 14, 2020
First decision: April 12, 2020
Revised: April 25, 2020
Accepted: July 4, 2020
Article in press: July 4, 2020
Published online: August 14, 2020
Processing time: 152 Days and 20.2 Hours
Lenvatinib has become an indispensable part of treatment regimens for patients with advanced hepatocellular carcinoma (aHCC). Several recent real-world studies appear to have confirmed this; however, there are etiological differences. This necessitates further real-world studies of lenvatinib across diverse populations, such as in China.
To investigate the efficacy and safety of lenvatinib in a Chinese HCC patient population under real-world conditions.
This is a retrospective and multiregional study involving patients with aHCC receiving lenvatinib monotherapy. Efficacy was assessed using the Response Evaluation Criteria in Solid Tumors version 1.1. Baseline characteristics and adverse events (AEs) were recorded throughout the entire study.
In total, 54 HCC patients treated with lenvatinib monotherapy were included for final analysis. The objective response rate was 22% (n = 12) with a progression-free survival (PFS) of 168 d; however, AEs occurred in 92.8% of patients. Multivariate analysis showed that the Barcelona Clinic Liver Cancer stage [hazard ratio (HR) 0.465; 95%CI: 0.23-0.93; P = 0.031], portal vein tumor thrombus (HR 0.38; 95%CI: 0.15-0.94; P = 0.037) and Child-Pugh classifications (HR 0.468; 95%CI: 0.22-0.97; P = 0.042) were significant factors affecting PFS. The sensitivity (56.7%) and specificity (83.3%) of decreasing serum biomarkers including alpha-fetoprotein were calculated in order to predict tumor size reduction. Gene sequencing also provided insights into potential gene mutation signatures related to the effect of lenvatinib.
Our findings confirm previous evidence from the phase III REFLECT study. The majority of patients in this Chinese sample were suffering from concomitant hepatitis B virus-related HCC. However, further analysis suggested that baseline characteristics, changes in serum biomarkers and gene sequencing may hold the key for predicting lenvatinib responses. Further large-scale prospective studies that incorporate more basic medical science measures should be conducted.
Core tip: This is a real-world study of advanced hepatocellular carcinoma patients treated with lenvatinib monotherapy in China. The majority of patients in this study presented with hepatitis B virus infection. Our analysis of the safety and efficacy of this intervention confirms previous evidence from the phase III REFLECT study. A multivariate analysis of participant characteristics with changes in serum biomarkers and gene sequencing provides a more comprehensive understanding of lenvatinib responses. Although based on a small sample, this new knowledge has clinical implications and necessitates further research.
- Citation: Wang DX, Yang X, Lin JZ, Bai Y, Long JY, Yang XB, Seery S, Zhao HT. Efficacy and safety of lenvatinib for patients with advanced hepatocellular carcinoma: A retrospective, real-world study conducted in China. World J Gastroenterol 2020; 26(30): 4465-4478
- URL: https://www.wjgnet.com/1007-9327/full/v26/i30/4465.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i30.4465
Primary liver cancer, which is predominantly hepatocellular carcinoma (HCC), remains one of the most common malignant tumors with approximately 841000 new cases and 782000 deaths annually[1]. Over the past decade, sorafenib, a multikinase inhibitor, has been considered the only first-line treatment for patients with advanced HCC (aHCC). Systemic therapies for patients with aHCC are rapidly changing, with some new agents showing clinical efficacy in phase III trials[2]. The REFLECT trial compared sorafenib to lenvatinib and, having setting noninferiority criteria as analytical endpoints, found that the overall survival (OS) for those administered lenvatinib was similar to that for those administered sorafenib[3]. Further subgroup analysis found that lenvatinib significantly improved all secondary endpoints including the objective response rate (ORR), progression-free survival (PFS) and time-to-progression (TTP), especially in the Asian-Pacific subgroup. Based on these findings, lenvatinib has been approved worldwide and has become an alternative first-line treatment for patients with aHCC[4].
Results from randomized controlled trials (RCTs) have tended to conflict with real-world studies, perhaps because of the nature of experimental controls and constraints. Therefore, lenvatinib monotherapy should be confirmed as efficacious in clinical practice. To date, Obi et al[5] found that the early therapeutic response rate to lenvatinib reached 40% across a small sample of 16 patients. A further multicenter study conducted in Japan involving 37 participants appears to have confirmed these findings with an ORR of 32.4% and a disease control rate (DCR) of 70.3% at 12 wk[6,7].
However, striving to maximize efficiency while avoiding side effects is proving difficult. More recently, in 2019, Sasaki et al[8] suggested that lenvatinib should be administered to patients with relatively good hepatic functions because these patients are more capable of receiving a sufficient relative dose intensity, which then significantly influences objective responses. Lenvatinib doses are generally determined by a patient’s weight, and in a further related study, Eso et al[9] found that the delivered dose: Intensity/body surface area ratio at 60 d can be an important factor for treatment intensity. In addition, the response to lenvatinib monotherapy has been found to be similar to that of transarterial chemoembolization (TACE), and the therapeutic action of lenvatinib in normalizing blood vessels may be more conducive to the treatment of TACE[10,11]. Therefore, TACE and lenvatinib combined may yield more favorable results for patients with aHCC.
The aforementioned studies focused predominantly on Japanese populations; however, there are a number of not so subtle differences between populations. For example, more than 50% of the global burden of HCC occurs in China, with 76% of these patients having been infected with hepatitis B virus (HBV)[12,13]. In the REFLECT study, researchers have also found that lenvatinib efficacy is not identical between etiological subgroups. Therefore, the massive HCC patient population with concomitant conditions in China must be examined to compare differences before developing guidelines. Lenvatinib was formally approved in China in September 2018; however, research focusing specifically on this population under real-world conditions is not readily available. It is well known that HCC patients in Japan generally also suffer concomitant HCV infection, although this is clearly not the case in the Chinese population[13].
In this study, we investigate the efficacy and safety of lenvatinib across a Chinese HCC patient population under real-world conditions. We also attempt to develop predictions using baseline characteristics, tumor biomarkers and gene mutations, thereby incorporating basic medical research with higher levels of evidence. This novel approach was designed to develop an evidence base to guide clinicians and to gain insight into lenvatinib responses.
This is a retrospective and multiregional study involving Chinese patients diagnosed with aHCC. Participants were routinely attending multidisciplinary team consultations. All patients were fully informed about the objectives of this study and provided formal consent. Data were collected from patients during lenvatinib interventions for a period of one year from December 2018 to December 2019. The study protocol was compliant with the principles of the Declaration of Helsinki and was approved by the Institutional Review Board and Ethics Committee at Peking Union Medical College Hospital.
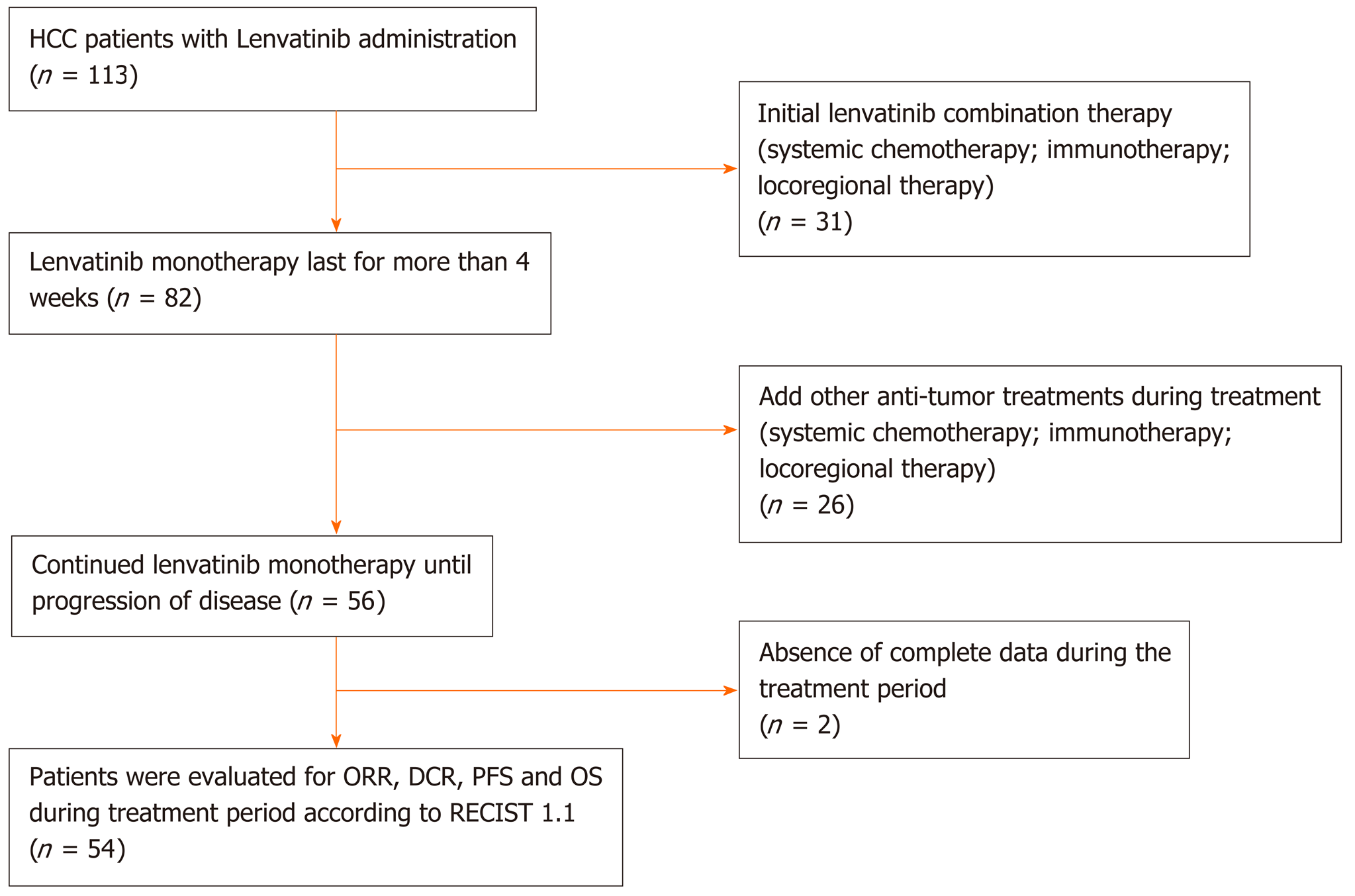
A total of 113 patients were initially deemed eligible. Each of these participants had received a confirmed HCC diagnosis using pathological assessment methods or through specific HCC imaging. The initial sample included participants who had not been recommended for hepatic resection, liver transplantation or any other radical ablation. Patients with Barcelona Clinic Liver Cancer (BCLC) stage B (not applicable for TACE or progressed on locoregional therapy) or BCLC stage C, a Child-Pugh score of A-B, and an Eastern Cooperative Oncology Group performance status (ECOG-PS) of 0-2 were included (please see Supplementary material for details). Following these criteria, we excluded 31 patients who had been treated with lenvatinib combination therapies at the beginning of treatment. Twenty-six patients were also excluded because they had received an additional antitumor therapy including systematic or locoregional therapy while receiving lenvatinib during this study.
Adverse events (AEs) were analyzed across the 56 remaining patients, of whom 54 patients provided complete information for further analysis. All 54 patients included were administered lenvatinib monotherapy until disease progression or until encountering an intolerant adverse event. The study design flow diagram is shown in Figure 1.
Initial lenvatinib doses were consistent with guidelines, and were administered orally at 8 mg/d when an individual patient weighed < 60 kg and 12 mg/d for those weighing ≥ 60 kg. Regimens may have been interrupted and even discontinued with the occurrence of unacceptable or serious AEs or when tumor progression was not inhibited.
Imaging examinations were conducted using enhanced computed tomography, magnetic resonance imaging or other available imaging technologies every 4-8 wk after initiation of lenvatinib treatment. Changes in tumor size were assessed by two independent specialists using RECIST 1.1 and were categorized as a complete response (CR), partial response (PR), stable disease (SD) or progressive disease (PD).
During the observation period, AEs were collected in detail and assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE version 4.0). According to the instructions, when grade 3 or more severe AEs occurred, dose reduction took place, or a temporary interruption was commenced until symptoms subsided to pharmaceutically manageable grades 1 or 2.
Baseline characteristics were systematically collected and included age, gender, serum biochemistry, extrahepatic spread (EHS), tumor occupation, portal vein thrombus (PVT), history of treatment and size of the target lesion. We also recorded combined characteristics including the ECOG-PS, albumin-bilirubin stage (ALBI), Child-Pugh class and BCLC stage by reviewing histories or through calculations using the available evidence. Utilizing these enabled us to analyze potential factors affecting ORR and PFS.
The patients were divided into different subgroups and stratified according to previous treatments, liver occupation, portal vein invasion, HBV and ALBI grades. Concomitant HBV was confirmed by HBV surface antigen testing. ALBI scores were calculated using the following formula: [log10 bilirubin (μmol/L) × 0.66] + [albumin (g/L) × -0.085], and ALBI grade was determined as Grade I = ≤ -2.60, Grade II = > -2.60 to -1.39, and Grade III > -1.39.
Patients with stable disease were categorized into three subgroups, which included the following: Diminished tumor size that did not reach the partial response standard (SS), stable disease without any significant tumor size change (ST) and stable disease with a tumor size increase that did not reach the progression standard (SP). SS and PR statuses were clustered into a “shrinking” group in which tumors were contracting in response to treatment. ST, SP and PD statuses were clustered into an “unshrinking” group in which participants were evidently not responding to treatment.
Recording of alpha-fetoprotein (AFP) values before and after administration of lenvatinib within 4 wk was conducted to develop response predictions in both the shrinking and unshrinking groups. Gene mutation information was collected from those who had provided samples for next generation sequencing. Genes needed to appear at least twice to be considered for further analysis. Differences in information reflecting gene mutations were calculated for both groups and compared.
Baseline data included continuous and categorical variables, which were calculated and presented as the means with corresponding standard deviations or as simple numbers and percentages. Statistical analyses of the differences between variables were conducted using the χ2 or Fisher’s exact tests. Two tailed P values of less than 0.05 were considered indicative of statistical significance. Five patient characteristics that may have affected ORRs were analyzed using a multivariate logistic regression model. The Kaplan-Meier method was applied to generate PFS curves, and a log-rank test was used to compare PFS curves for different subgroups.
The variables associated with PFS were analyzed using multivariate Cox proportional hazard analysis. The results of the multivariate analysis are presented as odds ratios (ORs) or hazard ratios (HRs) with corresponding 95%CI and P values. The sensitivity and specificity of diagnostics were calculated to assess their predictive capabilities for tumor changes using AFP values. Mutated gene frequencies were used to construct a gene mutation map of patients with different responses to lenvatinib. All statistical analyses were performed using SPSS 22 and R software (version 3.6.1).
A total of 56 patients were treated with lenvatinib monotherapy until progression of disease. A further two participants were excluded due to a lack of baseline data. Complete analysis was performed using data from 54 patients. Twenty-five patients were diagnosed by the method of specific imaging. The average age was 59 (± 12) years, and 85% (n = 46) were male. Of this total number, 40 patients were HBV positive. The proportion of patients with cirrhosis or portal hypertension was 72% (n = 39) and 54% (n = 29), respectively. Combining serum biochemistry and baseline characteristics resulted in proportions of Child-Pugh class A and B of 81% (n = 44) and 19% (n = 10), respectively (Table 1).
| All (n = 54) | HBV-related HCC (n = 40) | |
| Age, yr | 58.94 ± 12.10 | 57.49 ± 12.03 |
| Gender (male:female) | 46:8 | 35:5 |
| Height, cm | 172.04 ± 7.65 | 171.72 ± 7.24 |
| Weight, kg | 70.47 ± 13.72 | 69.21 ± 12.35 |
| Etiology (HBV:HCV:Others) | 40:5:9 | 40 |
| Total bilirubin, mg/dL | 29.81 ± 31.35 | 32.40 ± 33.17 |
| Albumin, g/dL | 38.53 ± 5.64 | 38.73 ± 5.01 |
| Prothrombin time, positive, % | 14 (26%) | 10 (25%) |
| Extrahepatic spread | 18 (33.3%) | 12 (30%) |
| Lymphatic metastasis | 33 (61%) | 28 (70%) |
| Liver occupation (< 50%: > 50%) | 38:15 | 27:13 |
| Portal vein thrombus | 21 (39%) | 16 (40%) |
| Baseline AFP (ng/mL)(≥ 200: < 200) | 32:22 | 22:18 |
| Native: Recurrence | 28:26 | 19:21 |
| History of treatment (TACE: RFA: Targeted therapy) | 37:21:17 | 29:14:14 |
| History of Radiotherapy | 11 (20%) | 7 (18%) |
| Initial dose of LEN (8 mg: 12 mg) | 26:28 | 21:19 |
| Diagnostic method (Image: Pathology) | 25:29 | 21:19 |
| Size of target lesion, cm | 6.93 ± 4.75 | 7.25 ± 4.36 |
In 28% (n = 15), liver occupation was greater than 50%. Approximately 39% (n = 21) had a PVT, and 33% (n = 18) showed EHS. In terms of treatment history, 11 patients had previously received radiotherapy, 69% (n = 37) received TACE, 39% (n = 21) received radiofrequency ablation (RFA), and 31% (n = 17) received another targeted therapy. The tumor size across all patients was 6.93 cm (± 4.75), and the number of patients receiving doses of 8 mg and 12 mg was 26 and 28, respectively. Approximately 33% (n = 18) were considered to be in stage B, and 67% (n = 36) in stage C, according to the BCLC criteria. In addition, 27 patients were ALBI grade I, 25 were grade II, and two patients were grade III (Table 2).
| All (n = 54) | HBV-related HCC (n = 40) | |
| ECOG PS (0: 1: 2) | 11:38:5 | 9:28:3 |
| Child-Pugh score (5: 6: 7: 8) | 29:15:7:3 | 21:13:4:2 |
| ALBI grade (1: 2: 3) | 27:25:2 | 20:19:1 |
| BCLC stage (B: C) | 18:36 | 12:28 |
| TNM stage (IIIA: IIIB: IVA: IVB) | 8:7:18:21 | 3:6:16:15 |
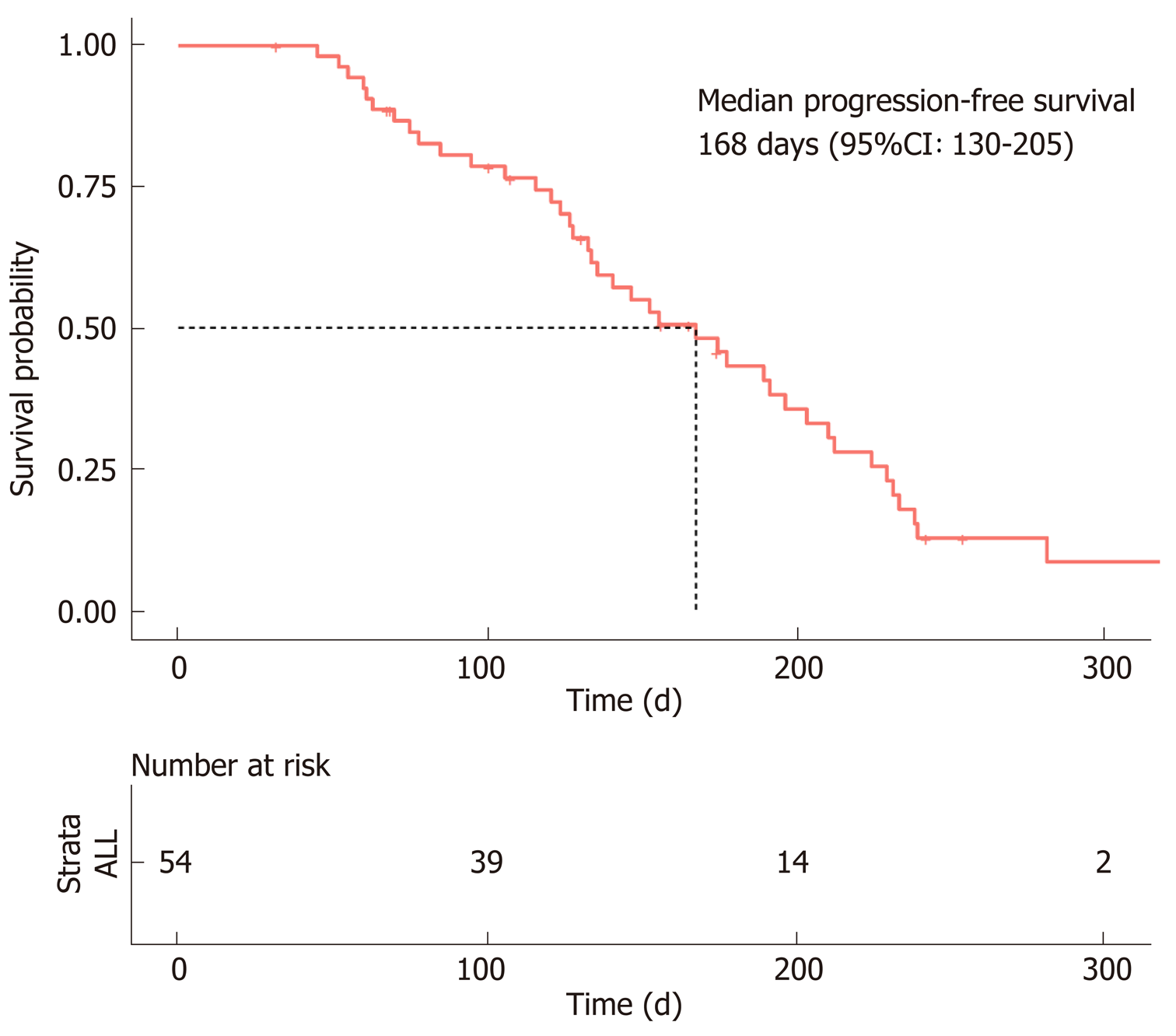
In accordance with the RECIST 1.1 criteria, no patients achieved a CR, a PR was observed in only 12 patients, SD was observed in 36 patients, and PD was observed in six patients. The ORR was 22% (n = 12), and the DCR was 88% (n = 48). The median PFS was estimated to be 5.6 mo (95%CI: 4.3-6.8), and the TTP was 5.1 mo (95%CI: 3.8-6.3) (Figure 2).
Overall survival could not be calculated due to the death rate. Of the patients with concomitant HBV, the number with PR and SD was 10 and 26, respectively, giving an ORR of 25% and a DCR of 90%. The median PFS was 5.8 mo (95%CI: 4.1–7.5), and the TTP was 5.2 mo (95%CI: 4.2-6.2) (Table 3).
| Investigator review according to RECIST 1.1 | ALL (n = 54) | HBV-related HCC (n = 40) | P value |
| Progression-free survival (d, 95%CI) | 168 (130-205) | 175 (124-226) | 0.250 |
| Time to progression (d, 95%CI) | 153 (116-189) | 156 (126-186) | 0.520 |
| Objective response | 22% | 25% | 0.753 |
| Complete response | 0 | 0 | - |
| Partial response | 12 | 10 | - |
| Stable disease | 36 | 26 | 0.866 |
| Progressive disease | 6 | 4 | - |
| Disease control rate | 88% | 90% | 0.863 |
| Decreased AFP predicts tumor reduction | |||
| Se | 56.7% | 53.8% | - |
| Sp | 83.3% | 85.7% | - |
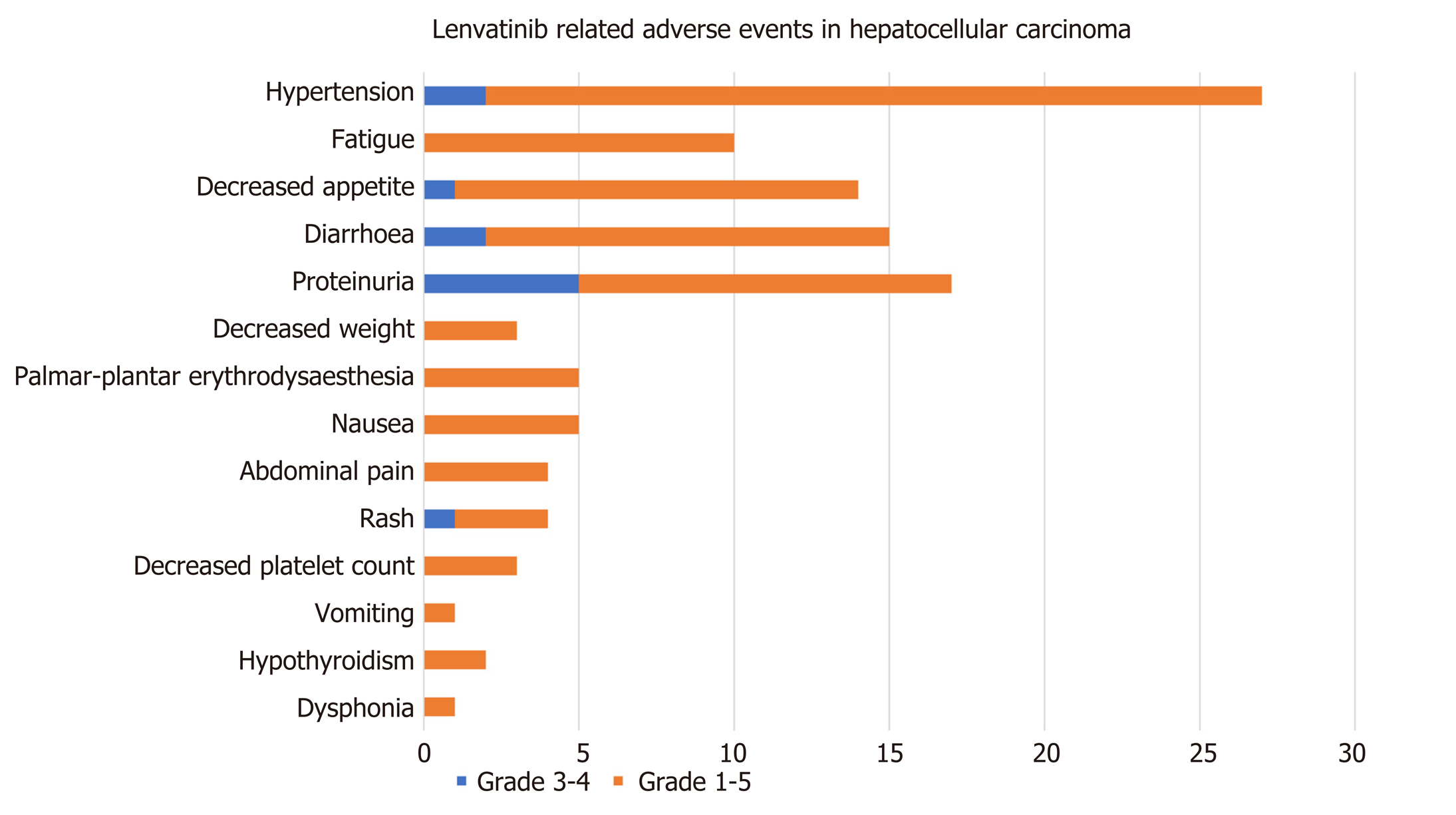
Of the 56 patients who continued to be treated with lenvatinib monotherapy, 92.86% (n = 52) developed AEs, and the incidence of grade 3-4 AEs was 21.15% (n = 11). There were no grade 5 AEs. The most common AEs encountered were hypertension in 44.64% (n = 25), decreased appetite in 23.21% (n = 13) and diarrhea in 23.21% (n = 13). Proteinuria was encountered by 21.43% (n = 12) and fatigue by 17.86% (n = 10), followed by hand–foot skin reaction (n = 6), nausea (n = 5), abdominal pain (n = 4), rash (n = 4), decreased weight (n = 3), decreased platelet count (n = 3), hypothyroidism (n = 2), dysphonia (n = 1) and vomiting (n = 1). Complete AE data with percentages are shown in Figure 3.
Among the grade 3-4 AEs, the incidence of proteinuria was the highest, reaching 9.6%, followed by diarrhea (n = 2), hypertension (n = 2), decreased appetite (n = 1) and rash (n = 1) (Table 4).
| Event | Hepatocellular carcinoma (n = 56) | |
| Any grade | Grade 3-4 | |
| Any adverse event | 52 (92.9) | 11 (21.2) |
| Hypertension | 25 (44.6) | 2 (3.8) |
| Fatigue | 10 (17.9) | 0 |
| Decreased appetite | 13 (23.2) | 1 (1.9) |
| Diarrhea | 13 (23.2) | 2 (3.8) |
| Proteinuria | 12 (21.4) | 5 (9.6) |
| Decreased weight | 3 (5.4) | 0 |
| Hand-foot skin reaction | 6 (10.7) | 0 |
| Nausea | 5 (8.9) | 0 |
| Abdominal pain | 4 (7.1) | 0 |
| Rash | 4 (7.1) | 1 (1.9) |
| Decreased platelet count | 3 (5.4) | 0 |
| Vomiting | 1 (1.8) | 0 |
| Hypothyroidism | 2 (3.6) | 0 |
| Dysphonia | 1 (1.8) | 0 |
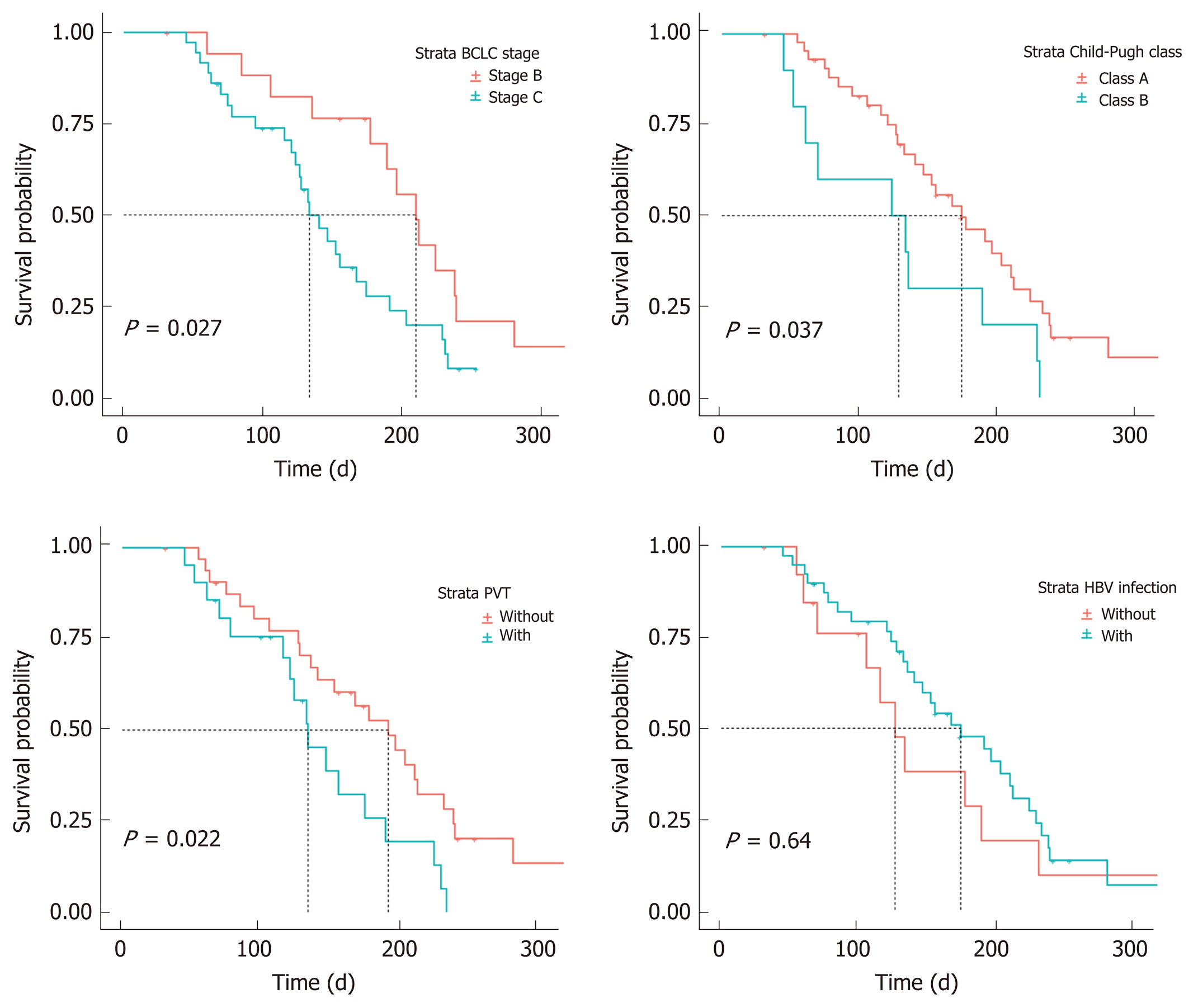
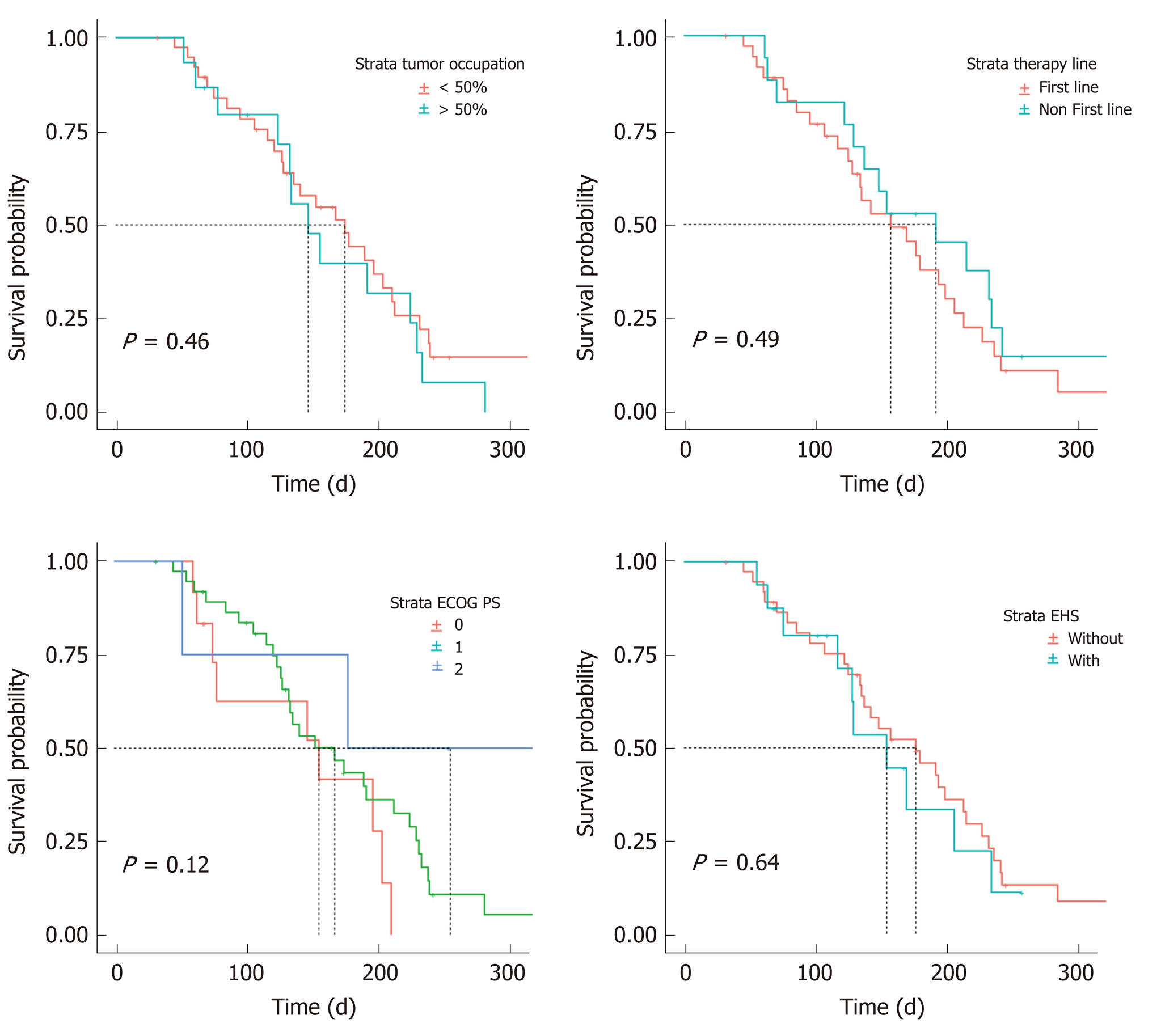
There did not appear to be a significant relationship between the ORR and the factors analyzed, which were age, gender, HBV infection, first-line therapy, EHS, tumor occupation, PVT, and history of TACE. However, Cox regression analysis suggested that age (HR: 0.95, CI: 0.92-0.99, P < 0.01) and PVT (HR: 0.38, CI: 0.15-0.94, P < 0.037) were significant factors affecting PFS. The median PFS was estimated to be 6.4 mo (95%CI: 4.9-7.8) in 33 patients without PVT and 4.4 mo (95%CI: 3.5-5.3) in 21 patients with PVT (Table 5).
| Clinical factors | Category | Analysis of ORR | Analysis of PFS | ||
| P value | OR, 95%CI | P value | HR, 95%CI | ||
| Age (yr) | < 58.8 | 0.489 | 0.978 (0.919-1.041) | 0.010 | 0.959 (0.929-0.990) |
| Gender | Male | 0.571 | 1.828 (0.227-14.724) | 0.606 | 1.137 (0.698-1.850) |
| HBV infection | HBV | 0.371 | 2.300 (0.370-14.290) | 0.151 | 1.844 (0.799-4.257) |
| First-line therapy | Sorafenib | 0.212 | 0.324 (0.055-1.901) | 0.167 | 1.724 (0.796-3.734) |
| Extrahepatic spread | Without | 0.604 | 0.600 (0.088-4.118) | 0.443 | 0.675 (0.247-1.844) |
| Tumor occupation | < 50% | 0.937 | 1.080 (0.162-7.178) | 0.169 | 2.043 (0.738-5.654) |
| Portal vein thrombus | Without | 0.987 | 0.985 (0.167-5.817) | 0.037 | 0.381 (0.154-0.944) |
| History of TACE | With | 0.396 | 2.229 (0.350-14.186) | 0.776 | 0.875 (0.348-2.197) |
| Combination factors | |||||
| ECOG-PS score | 0 | 0.066 | 3.571 (0.876-14.564) | 0.05 | 4.9 (0.998-24.193) |
| ALBI stage | 1 | 0.651 | - | 0.462 | 0.574 (0.130-2.524) |
| Child-Pugh class | A | 0.061 | - | 0.042 | 0.468 (0.225-0.973) |
| BCLC stage | B | 0.487 | 1.593 (0.425-5.971) | 0.031 | 0.465 (0.232-0.931) |
According to our analysis of combined factors, the ORR did not appear to have a significant relationship with ECOG-PS scores, ALBI stages, Child-Pugh classes or BCLC stages. However, changes in PFS were significantly related to patients with Child-Pugh class A or B disease (HR: 0.468; 95%CI: 0.22-0.97; P = 0.042) and BCLC stage B or C disease (HR: 0.465; 95%CI: 0.23-0.93; P = 0.031). The median PFS was 7.0 mo (95%CI: 6.0-8.0 mo) in 18 patients with BCLC stage B disease, 4.4 mo (95%CI: 3.6-5.2) in 36 patients with BCLC stage C disease, 5.8 mo (95%CI: 4.3-7.3) in 44 patients with Child-Pugh class A, and 4.1 mo (95%CI: 0.8-7.4) in 10 patients with Child-Pugh class B (Figure 4 and Figure 5).
As previously described, the “shrinking” group consisted of 21 patients, and the “unshrinking” group consisted of 33 patients. AFP serum concentrations in 56% of patients (n = 30) decreased after treatment. Using this decrease in AFP concentration to predict a reduction in tumor volume, the sensitivity and specificity were calculated to be 56.7% and 83.3%, respectively (Table 3).
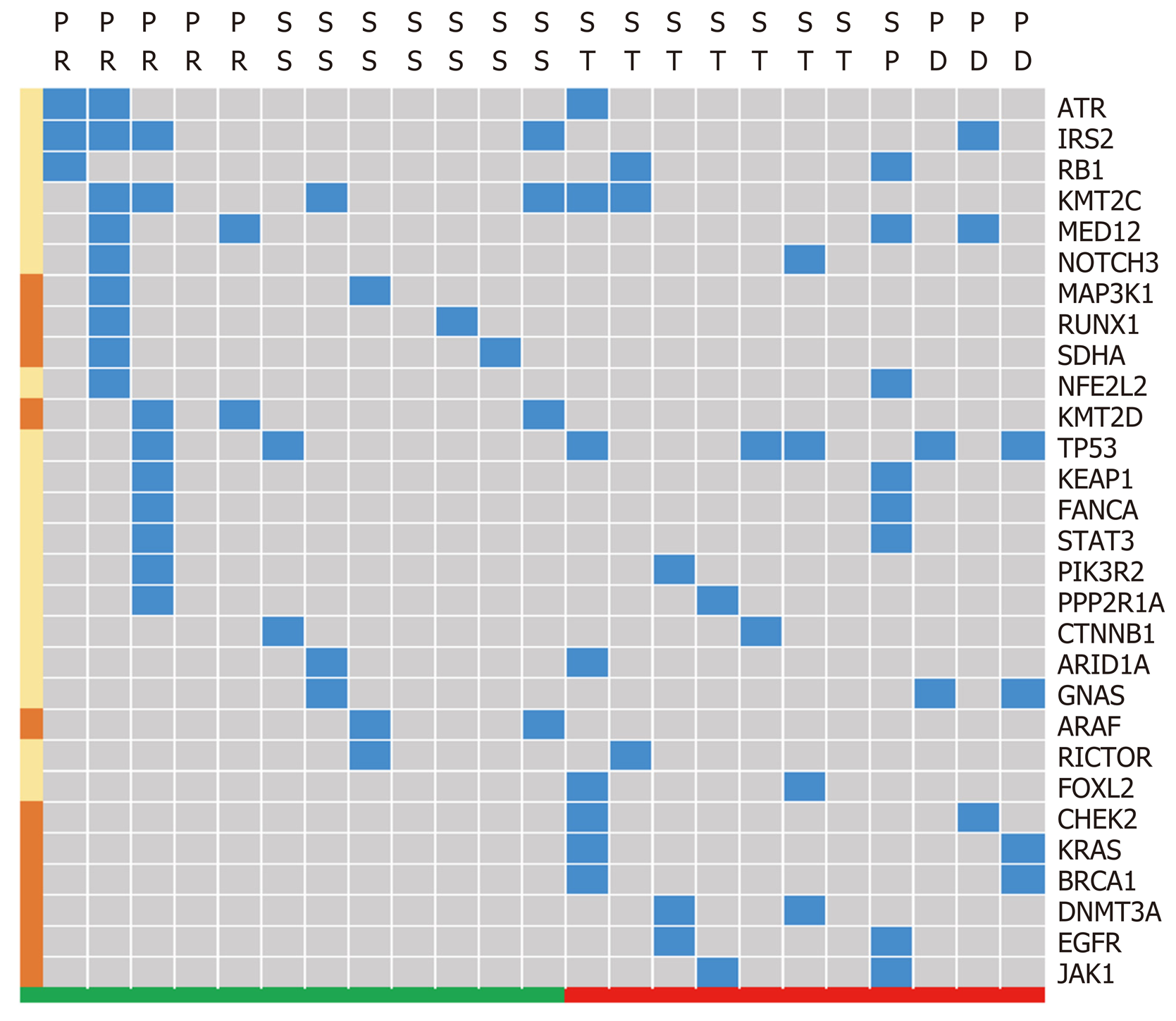
Gene sequence data were only collected from 23 patients, including 13 patients with a reduced tumor size and 11 patients without notable reduction. The high frequency mutations detected were KMT2C, TP53, and IRS2. Subgroup analysis demonstrated that variations in the CHEK2, KRAS, BRCA1, DNMT3A, and JAK1 genes were relatively concentrated in patients without tumor reduction, while SKHA, RUNX1, MAP3K1, KMT2D and ARAF gene variations appeared relatively concentrated in patients with tumor reduction (Figure 6).
The systematic treatment for hepatocellular carcinoma has dramatically changed over the past two years. To date, sorafenib and lenvatinib have been approved as first-line treatments for HCC; however, in the near future, the TA regimen (i.e., atezolizumab plus bevacizumab), which has a positive effect, will also play an important role in first-line treatments[14]. However, with this novel study design, we hoped to analyze the efficacy and safety of lenvatinib from a variety of aspects. The objective was to develop a more comprehensive understanding of its real-world effectiveness.
By contrast, sorafenib provides an ORR of less than 10%, whereas lenvatinib appears to almost double this rate according to the REFLECT trial (18.8%) and similar real-world studies that have observed ORRs ranging from 20-40% based on the mRECIST criteria[15]. However, to date, few studies have attempted to describe the efficacy of lenvatinib monotherapy in a Chinese population. Furthermore, the apparent differences between HBV and non-HBV cases within this population raise a number of interesting questions. The aforementioned results in our study suggest that HCC carcinoma patients in China, most of whom have HBV infection (40/54), respond positively to lenvatinib (ORR, 22%; PFS, 5.6 mo). However, comparative differences between patients with and without HBV infection are not readily available due to the very limited number of non-HBV infected patients[14]. This result may be consistent with the findings of a meta-analysis conducted by Casadei et al[16] who highlighted a clear trend favoring lenvatinib over sorafenib (HR, 0.82; 95%CI: 0.60–1.15) in HBV-positive patients.
For patients with BCLC stage B disease participating in the REFLECT trial, Kudo et al[11] found an ORR for lenvatinib of 61.3% with a PFS of 9.1 mo, which are higher than those achieved with any other known molecular targeted agent offered to HCC patients. Interestingly, of the patients with BCLC stage B disease, most were intolerant of chemoembolization or progressed despite previous TACE therapy. This means that most patients had good liver function and were therefore more likely to receive sustained lenvatinib treatment, which is also associated with a more favorable prognosis. However, our study appears to confirm that the mPFS for patients with BCLC stage B disease is significantly prolonged in contrast to patients with stage C. While this appears to provide valuable insight, the outcomes of the multivariate analysis in this study should be interpreted cautiously due to the small sample.
Currently, second-line therapy after sorafenib is increasingly being investigated as the majority of patients who initially receive sorafenib also require a second-line or possibly a combined intervention. Hiraoka et al[6,7] found that there was no significant difference in the ORR or DCR between patients who had (or had not) previously received sorafenib. This early evidence perhaps suggests that lenvatinib provides a beneficial therapeutic response not only as a first-line treatment but also as a potential second-line intervention. Seventeen patients were treated with sorafenib in our study, and the multivariate analysis suggested that there was no significant difference in either the ORR (HR: 0.324; 95%CI: 0.055-1.901; P = 0.212) or PFS (HR: 1.724; 95%CI: 0.796-3.734; P = 0.167). These results appear to support the notion that lenvatinib can be used as an alternative second-line therapy; however, confirmatory studies are required.
Patients with ≥ 50% liver occupation and portal vein invasion at the main portal branch were excluded in the REFLECT trial; however, in clinical practice, a considerable number of patients who meet these criteria are treated with lenvatinib. Therefore, to accurately analyze efficacy and identify patients who are most likely to benefit from lenvatinib, we conducted a further multivariate analysis of potentially influential factors. Stratified analysis demonstrated that liver occupation was not a significant factor affecting the ORR (HR: 1.409; 95%CI: 0.353-5.620; P = 0.627) or PFS (HR: 0.779; 95%CI: 0.401-1.514; P = 0.462). In contrast, portal vein invasion potentially affects PFS, although this finding is not completely consistent with a previous study. Hatanaka et al[17] found that a PS of 0 and the presence of both macrovascular invasion and EHS were significant factors affecting overall PFS. The factors affecting both the ORR and PFS are not identical across studies, although these results all indicate that liver function and malignancy are strongly related to patient prognosis. Therefore, protecting liver function to avoid interrupting treatments due to AEs appears to be important for prolonging survival.
In this study, we utilized descriptive statistics rather than correlation analysis between AEs and clinical characteristics because there are a number of factors that could confound our interpretation. For example, Ueshima et al[18] found that using a Child–Pugh score of 5 and ALBI grade I predict higher response rates and lower treatment discontinuation. However, the attributed ALBI scores were constantly changing during the treatment period, and there was a significant decline in ALBI scores from the baseline, which was observed at 4 and 12 wk after the start of treatment[7]. It is worth mentioning that hypertension, diarrhea, fatigue and decreased appetite were the main side effects in the present study, which are subtly different from those highlighted in the study by Hiraoka et al[6]. The side effects observed here are generally more tolerable than the side effects encountered with sorafenib, which enables clinicians to prolong regimens, thereby increasing the opportunity for patients to respond positively. In addition, we found that albuminuria is particularly apparent in patients with HCC, with a rate of 10% for grade 3-4. This side effect can potentially weaken the patient's PS and cause treatment interruptions. Fortunately, this may well be manageable, if clinicians can preempt imbalanced urinary protein levels and adjust medications in a timely fashion.
AFP levels represent the activity of tumors under certain circumstances, and clinicians usually interpret AFP changes to assist in understanding treatment effects[19,20]. The results of this study suggest that the downward trend in AFP levels from baseline after introducing lenvatinib is a direct response. Upon further analysis, we found AFP to be a potential biomarker for predicting a reduction in tumor volume. In practice, clinicians may be able to adjust lenvatinib treatments by observing changes in tumor sizes in accordance with decreasing tumor markers. However, it is important to be tentative in order to avoid false progression predictions.
Gene sequencing has been used to guide treatment planning in the field of HCC for many years, but identifying predictive genetic markers for lenvatinib treatment is frontier research and has not yet been widely considered[21]. Even though lenvatinib is a multitarget anti-angiogenic tyrosine kinase inhibitor that can be administered without previously established gene guidelines, this certainly appears to be the next logical step for enhancing the treatment effect of lenvatinib. The inhibitory potential of fibroblast growth factor receptor (FGFR) 1-4 in lenvatinib is different to that in sorafenib and is possibly the reason for the observed improvement in the overall effect[22]. In this study, the results of the gene mutation analysis were consistent with the published mutational landscape of HCC[23,24].
For example, in 2017, Finn et al[25] performed a study that focused on tumor gene expression clustering analysis in patients treated with lenvatinib. Patients were divided into three groups by clustering using expression levels of 36 genes involved in angiogenic and/or growth factor pathways. They found that for patients treated with lenvatinib, improvement in overall survival was seen in the group with higher vascular endothelial growth factor and FGF expression[25]. Likewise, we found that mutations associated with the lenvatinib target, particularly FGFRs1-4, were less frequent, which may confirm previous findings[26]; however, further research is required. An issue preventing us from carrying out correlative statistical analyses was the small number of archived tumor samples, but this study suggests some genes (and potentially intervention-related mutations) that might be used to prompt the use of lenvatinib.
While this study had a number of advantages and certainly adds to the current evidence base, it also had some limitations. Even though the research design embedded strict eligibility criteria and patients were from diverse regions of China, this was a retrospective, small-scale study with a limited number of observations, which meant there was a lack of OS data. The results of the multivariate analysis and effectiveness of predictive biomarkers, including AFP values and gene mutations, should be interpreted cautiously. In general, most participants in this study were suffering from HBV-related HCC, and lenvatinib appears effective to some degree, which confirms findings from the phase III REFLECT study. However, further analysis suggests that patients with reasonably good hepatic function may benefit more from lenvatinib treatment. Changes in AFP values and gene sequences may hold the potential to predict responses to lenvatinib during the therapeutic process although further exploratory studies are necessary.
In conclusion, the majority of this Chinese sample suffered from concomitant HBV-related HCC. Lenvatinib appears effective, which confirms previous findings from the phase III REFLECT study. However, further analysis suggests baseline characteristics, changes in serum biomarkers and gene sequencing may hold the key for predicting responses to lenvatinib. Further large-scale prospective studies that incorporate the collection and analysis of more basic medical science measures are necessary.
Lenvatinib has become an indispensable part of regimens for patients with advanced hepatocellular carcinoma (aHCC). Recently, several real-world studies appear to have confirmed this.
Ethnicity appears to, at least partially, cause etiological differences, which necessitates further real-world studies of lenvatinib across diverse populations, such as in China.
To develop a more comprehensive understanding of the real-world effectiveness of lenvatinib by analyzing its efficacy and safety from a variety of aspects.
This is a retrospective and multiregional study involving patients from across China who were diagnosed with aHCC and received lenvatinib monotherapy. Data were collected from patients during lenvatinib interventions from December 2018 to December 2019. After strict eligibility criteria were applied, efficacy was assessed using the RECIST 1.1 criteria. Baseline characteristics and adverse events (AEs) were recorded throughout the entire study.
In total, 54 HCC patients treated with lenvatinib monotherapy were included for final analysis. The majority of patients in this Chinese sample were suffering from concomitant HBV-related HCC. The objective response rate was 22% with a progression-free survival (PFS) of 168 d; however, AEs occurred in 92.8% of patients. The multivariate analysis showed that the Barcelona Clinic Liver Cancer stage, portal vein tumor thrombus and Child-Pugh classifications were significant factors affecting PFS. The sensitivity and specificity were calculated for decreasing serum biomarkers including alpha-fetoprotein in order to predict tumor size reduction. Gene sequencing also provided insights into potential gene mutation signatures related to the lenvatinib effect.
Our findings confirm previous evidence from the phase III REFLECT study. Further analysis suggests that baseline characteristics, changes in serum biomarkers and gene sequencing may hold the key for predicting lenvatinib responses.
Randomized controlled studies and real-world studies consistently report the beneficial effect of lenvatinib, but its application in HCC patients has only recently begun. Future research should focus on screening patients to ensure that we can identify those who will benefit most from lenvatinib and how the side effects can be effectively managed.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Abdelsameea E, Cheng JT, Kuroda H S-Editor: Wang JL L-Editor: Webster JR P-Editor: Zhang YL
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55843] [Article Influence: 7977.6] [Reference Citation Analysis (132)] |
| 2. | Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2066] [Cited by in RCA: 3176] [Article Influence: 529.3] [Reference Citation Analysis (37)] |
| 3. | Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3128] [Cited by in RCA: 3831] [Article Influence: 547.3] [Reference Citation Analysis (1)] |
| 4. | Marquardt JU, Saborowski A, Czauderna C, Vogel A. The Changing Landscape of Systemic Treatment of Advanced Hepatocellular Carcinoma: New Targeted Agents and Immunotherapies. Target Oncol. 2019;14:115-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Obi S, Sato T, Sato S, Kanda M, Tokudome Y, Kojima Y, Suzuki Y, Hosoda K, Kawai T, Kondo Y, Isomura Y, Ohyama H, Nakagomi K, Ashizawa H, Miura Y, Amano H, Mochizuki H, Omata M. The efficacy and safety of lenvatinib for advanced hepatocellular carcinoma in a real-world setting. Hepatol Int. 2019;13:199-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Hiraoka A, Kumada T, Kariyama K, Takaguchi K, Itobayashi E, Shimada N, Tajiri K, Tsuji K, Ishikawa T, Ochi H, Hirooka M, Tsutsui A, Shibata H, Tada T, Toyoda H, Nouso K, Joko K, Hiasa Y, Michitaka K; Real-life Practice Experts for HCC (RELPEC) Study Group and the HCC 48 Group (hepatocellular carcinoma experts from 48 clinics in Japan). Therapeutic potential of lenvatinib for unresectable hepatocellular carcinoma in clinical practice: Multicenter analysis. Hepatol Res. 2019;49:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 7. | Hiraoka A, Kumada T, Kariyama K, Takaguchi K, Atsukawa M, Itobayashi E, Tsuji K, Tajiri K, Hirooka M, Shimada N, Shibata H, Ishikawa T, Ochi H, Tada T, Toyoda H, Nouso K, Tsutsui A, Itokawa N, Imai M, Joko K, Hiasa Y, Michitaka K; Real-life Practice Experts for HCC (RELPEC) Study Group, HCC 48 Group (hepatocellular carcinoma experts from 48 clinics in Japan). Clinical features of lenvatinib for unresectable hepatocellular carcinoma in real-world conditions: Multicenter analysis. Cancer Med. 2019;8:137-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 109] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 8. | Sasaki R, Fukushima M, Haraguchi M, Miuma S, Miyaaki H, Hidaka M, Eguchi S, Matsuo S, Tajima K, Matsuzaki T, Hashimoto S, Ooba K, Kugiyama Y, Yatsuhashi H, Motoyoshi Y, Shigeno M, Kinoshita N, Nakao K. Response to Lenvatinib Is Associated with Optimal RelativeDose Intensity in Hepatocellular Carcinoma: Experience in Clinical Settings. Cancers (Basel). 2019;11:1769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 9. | Eso Y, Nakano S, Mishima M, Arasawa S, Iguchi E, Nakamura F, Takeda H, Takai A, Takahashi K, Taura K, Seno H. Dose Intensity/Body Surface Area Ratio is a Novel Marker Useful for Predicting Response to Lenvatinib against Hepatocellular Carcinoma. Cancers (Basel). 2019;12:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Kudo M, Han G, Finn RS, Poon RT, Blanc JF, Yan L, Yang J, Lu L, Tak WY, Yu X, Lee JH, Lin SM, Wu C, Tanwandee T, Shao G, Walters IB, Dela Cruz C, Poulart V, Wang JH. Brivanib as adjuvant therapy to transarterial chemoembolization in patients with hepatocellular carcinoma: A randomized phase III trial. Hepatology. 2014;60:1697-1707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 285] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 11. | Kudo M. Extremely High Objective Response Rate of Lenvatinib: Its Clinical Relevance and Changing the Treatment Paradigm in Hepatocellular Carcinoma. Liver Cancer. 2018;7:215-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 12. | Zheng R, Qu C, Zhang S, Zeng H, Sun K, Gu X, Xia C, Yang Z, Li H, Wei W, Chen W, He J. Liver cancer incidence and mortality in China: Temporal trends and projections to 2030. Chin J Cancer Res. 2018;30:571-579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 241] [Article Influence: 34.4] [Reference Citation Analysis (1)] |
| 13. | de Martel C, Maucort-Boulch D, Plummer M, Franceschi S. World-wide relative contribution of hepatitis B and C viruses in hepatocellular carcinoma. Hepatology. 2015;62:1190-1200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 348] [Cited by in RCA: 382] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 14. | Faivre S, Rimassa L, Finn RS. Molecular therapies for HCC: Looking outside the box. J Hepatol. 2020;72:342-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 266] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 15. | Zhu XD, Sun HC. Emerging agents and regimens for hepatocellular carcinoma. J Hematol Oncol. 2019;12:110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 85] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 16. | Casadei Gardini A, Puzzoni M, Montagnani F, Marisi G, Tamburini E, Cucchetti A, Solaini L, Foschi FG, Conti F, Ercolani G, Cascinu S, Scartozzi M. Profile of lenvatinib in the treatment of hepatocellular carcinoma: design, development, potential place in therapy and network meta-analysis of hepatitis B and hepatitis C in all Phase III trials. Onco Targets Ther. 2019;12:2981-2988. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Hatanaka T, Kakizaki S, Nagashima T, Namikawa M, Tojima H, Shimada Y, Takizawa D, Naganuma A, Arai H, Sato K, Harimoto N, Shirabe K, Uraoka T. Analyses of objective response rate, progression-free survival, and adverse events in hepatocellular carcinoma patients treated with lenvatinib: A multicenter retrospective study. Hepatol Res. 2020;50:382-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 18. | Ueshima K, Nishida N, Hagiwara S, Aoki T, Minami T, Chishina H, Takita M, Minami Y, Ida H, Takenaka M, Sakurai T, Watanabe T, Morita M, Ogawa C, Hiraoka A, Johnson P, Kudo M. Impact of Baseline ALBI Grade on the Outcomes of Hepatocellular Carcinoma Patients Treated with Lenvatinib: A Multicenter Study. Cancers (Basel). 2019;11:952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 117] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 19. | Snyder A, Morrissey MP, Hellmann MD. Use of Circulating Tumor DNA for Cancer Immunotherapy. Clin Cancer Res. 2019;25:6909-6915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 20. | Kuzuya T, Asahina Y, Tsuchiya K, Tanaka K, Suzuki Y, Hoshioka T, Tamaki S, Kato T, Yasui Y, Hosokawa T, Ueda K, Nakanishi H, Itakura J, Takahashi Y, Kurosaki M, Izumi N. Early decrease in α-fetoprotein, but not des-γ-carboxy prothrombin, predicts sorafenib efficacy in patients with advanced hepatocellular carcinoma. Oncology. 2011;81:251-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 21. | Valle JW, Lamarca A, Goyal L, Barriuso J, Zhu AX. New Horizons for Precision Medicine in Biliary Tract Cancers. Cancer Discov. 2017;7:943-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 457] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 22. | Finn R, Kudo M, Cheng A, Wyrwicz L, Ngan R, Blanc J, Baron A, Vogel A, Ikeda M, Piscaglia F. 59PD Final analysis of serum biomarkers in patients (pts) from the phase III study of lenvatinib (LEN) vs sorafenib (SOR) in unresectable hepatocellular carcinoma (uHCC)[REFLECT]. Ann Oncol. 2018;29:mdy269.057. |
| 23. | Li N, Li L, Chen Y. The Identification of Core Gene Expression Signature in Hepatocellular Carcinoma. Oxid Med Cell Longev. 2018;2018:3478305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 24. | Müller M, Bird TG, Nault JC. The landscape of gene mutations in cirrhosis and hepatocellular carcinoma. J Hepatol. 2020;72:990-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 114] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 25. | Finn R, Kudo M, Cheng A, Wyrwicz L, Ngan R, Blanc J, Baron A, Vogel A, Ikeda M, Piscaglia FJAoO. LBA30Analysis of serum biomarkers (BM) in patients (pts) from a phase 3 study of lenvatinib (LEN) vs sorafenib (SOR) as first-line treatment for unresectable hepatocellular carcinoma (uHCC). Ann Oncol. 2017;28. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Matsuki M, Hoshi T, Yamamoto Y, Ikemori-Kawada M, Minoshima Y, Funahashi Y, Matsui J. Lenvatinib inhibits angiogenesis and tumor fibroblast growth factor signaling pathways in human hepatocellular carcinoma models. Cancer Med. 2018;7:2641-2653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 186] [Article Influence: 26.6] [Reference Citation Analysis (1)] |