Published online Jan 21, 2020. doi: 10.3748/wjg.v26.i3.279
Peer-review started: October 15, 2019
First decision: December 4, 2019
Revised: December 17, 2019
Accepted: January 1, 2020
Article in press: January 1, 2020
Published online: January 21, 2020
Processing time: 92 Days and 22.7 Hours
Metabolic disorders are increasingly leading to non-alcoholic fatty liver disease, subsequent steatohepatitis, cirrhosis and hepatocellular carcinoma. Fibroblast growth factors and their receptors play an important role in maintaining metabolic homeostasis also in the liver and disorders in signaling have been identified to contribute to those pathophysiologic conditions leading to hepatic lipid accumulation and chronic inflammation. While specific and well tolerated inhibitors of fibroblast growth factor receptor activity are currently developed for (non-liver) cancer therapy, treatment of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis is still limited. Fibroblast growth factor-mimicking or restoring approaches have recently evolved as a novel therapeutic option and the impact of such interactions with the fibroblast growth factor receptor signaling network during non-alcoholic fatty liver disease/non-alcoholic steatohepatitis development is reviewed here.
Core tip: Non-alcoholic fatty liver disease and non-alcoholic steatohepatitis show globally rising incidences and are expected to become the main reason for liver fibrosis, cirrhosis, liver cancer and end-stage liver disease with need for transplantation. Liver metabolism is, among other factors, regulated by fibroblast growth factors and their receptors. This review highlights the role of these signaling pathways in the context of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis and discusses novel treatment options for these otherwise difficult to treat diseases.
- Citation: Ocker M. Fibroblast growth factor signaling in non-alcoholic fatty liver disease and non-alcoholic steatohepatitis: Paving the way to hepatocellular carcinoma. World J Gastroenterol 2020; 26(3): 279-290
- URL: https://www.wjgnet.com/1007-9327/full/v26/i3/279.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i3.279
Primary liver cancer, hepatocellular carcinoma (HCC) is among the most common cancer related deaths for men and women. HCC commonly develops on the background of various underlying chronic liver diseases and is often called “a disease within a disease”. Besides chronic viral infections and despite the success of vaccination campaigns, its incidence is continuously high, and even increasing, also due to the globally steep increase in metabolic liver diseases leading to non-alcoholic fatty liver disease (NAFLD) and subsequently non-alcoholic steatohepatitis (NASH), fibrosis, cirrhosis and cancer formation[1,2]. With a global prevalence of about 25%, NAFLD and NASH are a major medical burden and linked to an increasing number of patients with end-stage liver disease and transplantation need. A further increase is expected due to growing prevalence of obesity and metabolic syndrome[3].
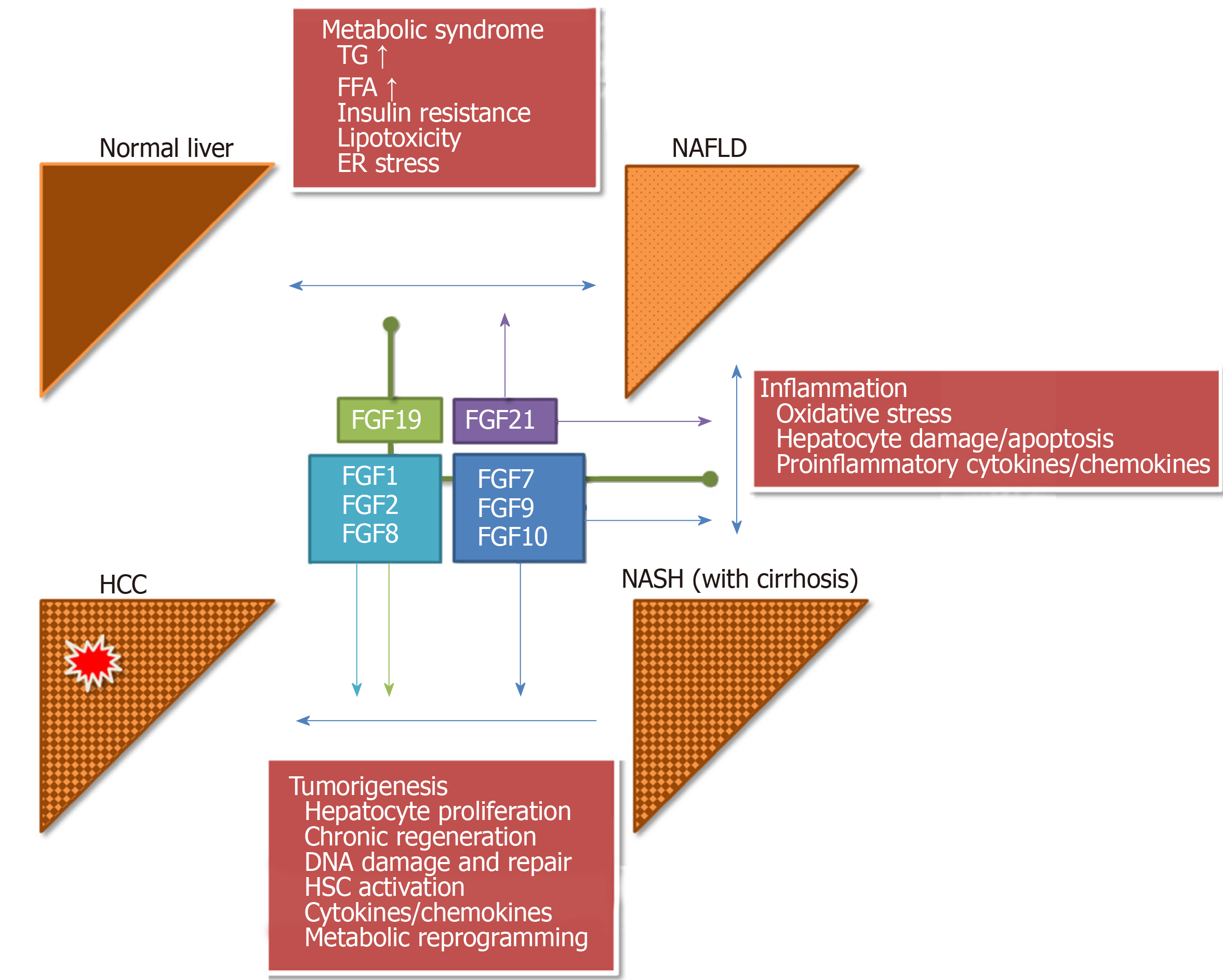
The pathophysiologic mechanisms underlying these processes are still not completely clear. Yet, growth factors like the fibroblast growth factor (FGF) family and fibroblast growth factor receptors (FGFRs) can contribute and drive several of these changes and have been clearly shown to possess oncogenic potential in some circumstances[4,5]. In the liver, esp. FGF19, FGF21 and FGF23 have been shown to physiologically possess endocrine functions in regulating, e.g., homeostasis of bile acids and glucose as well as regulating fasting response, lipid metabolism and other conditions[6-10]. As the dysregulation of these metabolic pathways is considered a key feature of chronic liver diseases leading to obesity, metabolic syndrome, NAFLD, NASH, fibrosis and finally HCC, FGFs and FGFRs could be interesting novel targets for diagnosis, surveillance and treatment of these conditions.
All four FGFRs are transmembrane tyrosine kinase receptors with a single-pass transmembrane domain, an intracellular kinase domain and three extracellular immunoglobulin-like domains which are subject to alternative splicing and thus mediate ligand specificity. Binding of FGFs leads to receptor dimerization and activation of the downstream signaling cascade that mediates processes linked to cellular survival, extracellular matrix and adhesion molecule signaling but also metabolic processes, e.g., via the PI3K/AKT pathway[11,12]. While the expression of FGFR1 (predominantly mesenchymal tissues) and FGFR2 (predominantly mesenchymal and epithelial) is broad, FGFR3 is mostly found in the central nervous system, bone, skin, and to a lesser extend GI tract, kidney and male and female reproductive tissues. FGFR4 is found in endodermal tissues and the somatic myotome, including endocrine, bone marrow, pancreas, lung and liver and gallbladder tissues[5,13]. In summary, all FGFRs are expressed in the liver with higher levels of FGFR3 and FGFR4[14].
In humans, 22 FGFs have been described so far. They can be subclustered into four intracrine (FGF11-14), fifteen paracrine (FGF1-10, 16-18, 20, 22) and three endocrine (FGF19, 21, 23) subfamilies. They consist of 150-300 amino acids and share about 30%-60% sequence homology with different N- and C-terminal parts mediating receptor specificity. Endocrine FGFs need co-receptors of the Klotho family to bind to any of the four FGFRs. Unlike paracrine FGFs, they lack the heparan sulphate binding capacity and can therefore enter circulation and act as hormones[4,15-17]. The general metabolic functions of endocrine FGFs are reviewed elsewhere[4,18] and we will here focus on their role in physiology and pathophysiology of the liver.
FGF1 is expressed in the liver and other tissues, including adipose tissue where it is upregulated upon high-fat diets[19]. It can bind to all FGFRs and can interact with integrins which are mediators of fibrogenesis, too[20,21]. FGF1 and FGF2 are upregulated in chronic liver disease, fibrogenesis and in HCC where these ligands enhance angiogenesis and invasiveness[22,23]. In addition, FGF1 and FGF2 mediate fibrogenesis by activation of hepatic stellate cells which links extracellular matrix modulation and carcinogenesis to NAFLD/NASH[22,24]. Paracrine FGF8 and FGF10 have been shown to play important roles during embryonic liver development and during liver regeneration[25,26]. Esp. FGF10 was shown to regulate hepatoblast function, which links development and repair processes[27]. Upon hepatocyte injury, FGF7 induces progenitor cell proliferation in the liver[28]. The activation of hepatic stellate cells as a response to injury was linked to FGF9, which also induces hepatocyte proliferation in acute liver injury models[29]. Importantly, the activation of hepatic stellate cells as well as the induction of hepatocyte proliferation and recruitment of progenitor cells are key features of acute and chronic liver injury leading to fibrosis, cirrhosis and cancer formation, indicating a central role for FGFs during this process. In human HCC, upregulation of FGF8 family members (FGF8, FGF17 and FGF18) was linked to angiogenesis and enhanced cancer cell survival in 59% of the examined tissue samples. Interestingly, also different FGFRs were upregulated and overall, 82% of cases showed alterations of at least one FGFR and/or FGF[30].
Endocrine FGFs have been shown to control several metabolic pathways in the liver via β-Klotho co-signaling. FGF19 (also called FGF15/19 due to its mouse homologue FGF15 which does not exist in humans) is a key regulator of bile acid metabolism and links gut-liver signaling. The nuclear bile acid receptor FXR induces expression of FGF19 in the ileum which in turn reduces expression of CYP7A1, the rate limiting enzyme for bile acid synthesis in hepatocytes[31]. FGF19 was also shown to control gallbladder volume[32]. Furthermore, FGF19 stimulates protein and glycogen synthesis in hepatocytes independent of insulin and is thus also involved in glucose homeostasis[33].
FGF21 controls a plethora of metabolic pathways in hepatocytes, adipocytes and skeletal muscle[34]. Nutritional stress (e.g., low carbohydrate, high fat ketogenic diets) as well as other means of hepatic injury have confirmed FGF21 as a stress response gene in the liver, e.g., by inducing systemic glucocorticoid levels[35]. Interestingly, FGF21 was also identified to be a key mediator of metabolic effects mediated by gut microbiota. Several studies recently demonstrated a protective effect of probiotic microbiota like Lactobacillus species (esp. L. rhamnosus GG) on energy expenditure, dyslipidemia or steatosis in different animal models, which was shown to be dependent on FGF21 signaling and able to reverse NAFLD[36-39].
Although FGF23 is linked to calcium and phosphate homeostasis in bone and kidney via α-Klotho co-signaling and not considered to play an important role in liver pathophysiology[40], a recent study showed that serum FGF23 was correlated with NAFLD in Chinese patients with type 2 diabetes[41]. Although the exact role of FGF23 in NAFLD pathogenesis is unclear, FGF23 mRNA was detected in the liver and is increased under metabolic stress conditions and chronic liver disease in mice[42]. Yet the observed increase could also be due to the renal pathophysiology of these conditions[43].
Deployment of extracellular matrix material, fibrosis, is the general response of the liver to chronic injury with hepatocyte damage - independent of the causing agent (viral, toxic, metabolic). Chronic hepatocyte damage and cell death leads to persistent inflammation and activation of wound healing and tissue remodeling programs to compensate the loss of functional hepatocytes and to restrict the damaged area by activation of hepatic stellate cells (Figure 1 and Table 1, for more details on general fibrosis mechanisms in the liver please see[44-46]).
| FGFs | NAFLD/NASH | HCC |
| FGF1 | Upregulated in adipose tissue under high fat diet[19] | HSC activation, fibrogenesis, angiogenesis, invasiveness[20,21] |
| FGF2 | No data | HSC activation, fibrogenesis, angiogenesis, invasiveness[22-24] |
| FGF8 family | No data | Proliferation, angiogenesis, matrix modulation[30] |
| FGF19 | Impaired in NAFLD and by insulin resistance, contributes to lipid and bile acid dysbalance[74-76] | Proliferation, invasion, metastasis, inhibition of apoptosis[102-104] |
| FGF21 | Induced by ketogenic diet, reduces insulin sensitivity, mediator of metabolic effects from gut microbiota[35-39,54-56] | Deficiency promotes HCC under obesity conditions[21,61] |
Various factors have been described to contribute to NAFLD/NASH and fibrosis progression, like ROS production or inflammatory cytokine release from adipocytes but also impairment of metabolic pathways in the gut and liver like lipogenesis, cholesterol and insulin signaling[47]. Dietary factors can influence these pathways and esp. high dietary cholesterol, polyunsaturated fatty acids and fructose have been demonstrated to trigger NAFLD/NASH development[48,49]. In absence of insulin, fructose is subjected to liponeogenesis and thus depletes hepatocellular ATP and contributes to mitochondrial damage, ROS production and lipid accumulation, as evidenced by patients with high fructose intake (e.g., soft drinks)[50,51].
Lipid metabolism is considered a key pathogenetic driver of NAFLD progression and fibrosis development. It depends on lipolysis, liponeogenesis and triglyceride oxidation and the overload of the liver with such metabolites can trigger ER stress and autophagy responses[52,53].
FGF21 plays a central role here. It is abundantly expressed at low levels in the liver and can be induced upon fasting[54] and seems to inhibit lipolysis under this condition[34]. Its expression in the liver can be induced by ketogenic (high-fat, low-carbohydrate) diets and it then negatively impacts on insulin sensitivity in adipocytes. Starvation induced hepatic FGF21 expression increases systemic glucocorticoid levels and diminishes physical activity, probably via remote effects in the central nervous system or in adipose tissues[55,56]. Hepatic FGF21 mediates various effects on energy metabolism and insulin sensitivity in the liver and the skeletal muscle via adiponectin[57,58]. In FGF21 deficient mice, a role in glucagon signaling was also described[59]. Upon hepatic injury, FGF21 serves as a protective stress-response regulator and has been shown to ameliorate various hepatotoxic conditions (viral hepatitis, alcohol)[60].
Although high levels of FGF21 are considered to be protective against metabolic stress conditions several studies in mice and humans demonstrated also opposite effects. Deficiencies in FGF21 signaling promote HCC growth in mice on long-term obese diet and restoration of FGF21 improves hepatic steatosis[61,62]. In obese humans, increased hepatic FGF21 was described[9] and linked to prevalence and progression of NAFLD, indicating impaired FGF21 function as was seen in type II diabetes with insulin resistance[63,64]. Elevated expression of FGF21 was also established as a clinical predictive biomarker for steatosis in obese children[65] and a panel consisting of FGF21, BMI, γ-GT and triglycerides was proposed as a biomarker for identification of children with steatosis[66]. In adult patients with HIV, elevated FGF21 was confirmed as a risk factor for steatosis[67] and, overall, combined biomarker panels that include FGF21 have higher predictivity for steatosis also in non-obese patients[68,69]. Interestingly, no data is available on the expression levels of the main receptors for FGF21, FGFR1 and β-Klotho, in NAFLD and NASH.
Maintenance of chronic inflammation is required for the progression of NAFLD to NASH and for fibrosis development. Here, hepatic macrophages, so called Kupffer cells, play a central role in keeping hepatic stellate cells activated. In FGF5 deficient mice, high fat diet lead to severe steatosis and fibrosis via activation of F4/80 macrophages that were positive for CD11b and CD68 and produced TNFα and FasL[70,71].
FGF19 (and its murine homologue FGF15) regulates hepatocyte proliferation. In knockout mice, liver regeneration after partial hepatectomy is impaired and toxic stimuli lead to extensive necrosis and fibrosis, the latter mediated via activation of CTGF signaling[72,73]. In human NAFLD, hepatic response to FGF19 was significantly impaired, esp. under conditions of insulin resistance, and may contribute to lipid and bile acid dysbalance in these patients[74-76]. FGF19 serum levels are reduced in pediatric and adult patients with NAFLD[77,78] while an increase in taurine and glycine metabolizing bacteria like Escherichia or Bilophila was concomitantly observed[79]. The decrease in FGF19 was proposed as a biomarker for steatosis development[77]. In mice, an FGF15-Apo A-I fusion protein (Fibapo) was shown to improve lipid accumulation and metabolic stress under high fat diet conditions in FGF15 knockouts[80], confirming the therapeutic effect of FGF restoration. To overcome potential protumorigenic effects of FGF19 (i.e., induction of cell proliferation), recombinant molecules have been developed and are currently used in human clinical trials. Similar to FGF21, FGF19 was also evaluated as a biomarker for NAFLD and NASH. FGF19 levels were significantly reduced in children with NASH but did not show statistically significant association to the pediatric NAFLD histological score[81]. In a therapeutic study in pediatric patients, FGF19 was significantly increased by lifestyle intervention and a mix containing docosahexaenoic acid, choline, and vitamin E and was therefore proposed as a pharmacodynamic biomarker in this setting[82]. Overall, the predictivity of FGF19 is still under debate[76].
Due to their described hepatoprotective properties and effects on inflammation, metabolism and fibrosis, restoration of FGF19 and FGF21 signaling is considered an attractive novel target for drug development against NAFLD and NASH. In the past, drug development for NAFLD and NASH has been limited by the availability of suitable (surrogate) endpoints and the debate on non-invasive measures is still ongoing[83]. While non-invasive measurements of NASH status and fibrosis have evolved as acceptable endpoints in early stage trials, Phase 3 studies may still require pre- and post-treatment biopsies. Recently, advances in patient selection were achieved that do allow timely readout of e.g., changes in fibrosis, as overall survival may not be achievable pre-cirrhotic patients[53,84].
While several agents are currently in Phase 2 and Phase 3 clinical development for treatment of NASH[53], only few compounds are using FGF signaling as a therapeutic target.
Pegbelfermin (BMS-986036) is a recombinant PEGylated analog of human FGF21. In preclinical models, it improved diabetes, NASH and fibrosis, as demonstrated by increases in adiponectin, improved NAFLD activity score and decreased N-terminal type III collagen propeptide (Pro-C3)[85]. In a Phase 2 study in patients with obesity and type 2 diabetes, pegbelfermin achieved a significant increase in adiponectin and decrease in serum Pro-C3 after 12 wk, comparable to preclinical data. Although effects on HbA1c were not different to placebo, the drug was overall well tolerated and it was concluded that effects on obesity-related diseases like NASH warrant further investigations[86]. This was further analyzed in a randomized, double-blind, placebo-controlled Phase 2 study in patients with confirmed NASH, fibrosis and obesity. Pegbelfermin was administered subcutaneous in a daily (10 mg/d) or a weekly (20 mg/wk) schedule for 16 wk and primary endpoints were changes in magnetic resonance imaging proton density fat fraction (MRI-PDFF), serum Pro-C3, transaminases, and liver stiffness as assessed by MR elastography. A total of 74 patients were enrolled and 68 were eligible after 16 wk of treatment. Here, a significant reduction in MRI-PDFF (-6.8% and -5.2%, respectively) and transaminases (-33.0% and -23.7% for alanine aminotransferase, -30.9% and -26.2% for aspartate aminotransferase, respectively) was detected in both treatment schedules. Furthermore, a significant proportion of patients showed improved serum Pro-C3 and reduced MR elastography liver stiffness, all paralleled by an approximate 15% increase in adiponectin versus placebo. Overall, the drug was also well tolerated in these patients and results from this study suggest clearly a beneficial effect on steatosis, liver injury and fibrosis in NASH patients[87]. Currently, additional studies are ongoing to evaluate pegbelfermin in patients with NASH associated fibrosis, cirrhosis or impaired kidney function.
NGM282 is a recombinant non-tumorigenic variant of FGF19. It was modified at the amino-terminal end to bind to FGFR4/β-Klotho, suppress CYP7A1 expression (the rate limiting step in bile acid synthesis) but does not activate STAT3 signaling which is considered a main driver for hepatocarcinogenesis[88]. Preclinically, NGM282 improved inflammation, hepatocyte damage and fibrosis in models of NASH and of sclerosing cholangitis in mdr2-deficient mice[89,90]. It was well tolerated also in humans and showed signs of reduced CYP7A1 activity, too[91]. In a randomized and placebo-controlled Phase 2 study, NGM282 lead to a rapid reduction in hepatic fat content in more than 70% of the treated patients after 12 wk, paralleled by significant reductions in transaminase and Pro-C3 levels and ELF fibrosis score and increase in low density lipoprotein cholesterol. The overall safety profile was acceptable with predominantly Grade 1 adverse events. As NGM282 is a recombinant peptide, anti-drug antibodies were observed in a small number of patients but were considered not to be neutralizing[92]. After 12 wk, NAFLD activity score was dose-dependently reduced by 2 points in up to 63% of patients without worsening of fibrosis and fibrosis was reduced by one or more stages in up to 42% of patients[93]. Due to its effects on CYP7A1, NGM282 increases low density lipoprotein cholesterol and serum cholesterol. Thus, combination with rosuvastatin was assessed to further improve the cardiovascular risk profile for NASH patients[94].
While these approaches focus on ligands, also FGFRs could be a promising target for NAFLD and NASH drug development. Several small molecule kinase inhibitors targeting FGFRs are currently in clinical development for the treatment of various solid tumors. Yet, in none of the early clinical trials with these multi-kinase inhibitors (usually targeting FGFR1-3), metabolic effects related to FGF signaling other than phosphate increase due to renal FGFR1 inhibition were reported. BLU9931 as well as H3B-6527, two specific inhibitors of FGFR4, suppressed HCC growth in preclinical models but also induced expression of CYP7A1, although no adverse effects on lipid metabolism or steatosis were observed in the investigated rodent models[95,96].
The protective effects of FGF19 via inhibition of endoplasmic reticulum stress, which is commonly observed in metabolic overload during NAFLD and NASH, have been shown to be mediated via the FGFR4/β-Klotho pathway[97]. An upregulation of FGFR4 was recently demonstrated in murine NASH models[98] and in patients developing HCC on fatty liver disease background[99]. To block FGFR4 signaling, a soluble FGFR4 extracellular domain fragment was developed that specifically inhibits FGFR4 activation in vitro and suppressed steatosis and fatty liver development in mice[100], but clinical data in humans is still lacking.
Mimicking FGFR activation is currently investigated with NGM313, a humanized monoclonal antibody binding to an epitope in β-Klotho. It was shown that NGM313 activates FGFR1c signaling, similar to the physiologic role of FGF21. A single dose of NGM313 significantly reduced liver fat content, transaminase levels, HbA1c, triglycerides and low density lipoprotein cholesterol in obese patients with insulin resistance and NAFLD[101].
The above outlined pathways of chronic inflammation and hepatocyte stress conditions commonly involve FGF signaling and various approaches in restoring FGF physiologic conditions have been successful in preventing HCC development in preclinical models. In human HCC, several studies confirmed overexpression of different FGFs and FGFRs, which confirms the attractiveness of targeting FGF signaling also in established HCC[102,103]. FGF19 was recently proposed as a novel diagnostic biomarker with better accuracy than AFP for small HCCs[104]. As many FGFs can have different pro-tumorigenic effects (e.g., by promoting angiogenesis or chronic inflammation), FGFRs seem to be the more attractive here. While esp. inhibition of FGFR4 was shown to reduce growth of experimental HCC models[105,106] and is considered an attractive drug target[107,108], also FGF1, FGF2 and the FGF8 subfamily as well as targeting FGFR1-3 have been shown to possess preclinical anti-HCC activity[103,109,110]. Designing specific inhibitors for FGFR4 was considered challenging and therefore initial approaches used multi-kinase or pan-FGFR inhibitors. While sorafenib was the first multi-kinase inhibitor to achieve an overall survival benefit in HCC[111], other compounds like brivanib or lenvatinib did not achieve this endpoint[103,112]. Preclinically, the specific FGFR4 inhibitors BLU-9931, H3B-6527 or INCB062079 showed promising activities[95,96,113].
Several clinical studies with these and other FGFR4 specific inhibitors are currently ongoing in HCC patients. Phase 1 data for fisogatinib (BLU-554) indicate good target engagement and inhibition of FGFR4 as shown by effects on bile acid and cholesterol synthesis pathways in a dose-dependent manner. With an overall good tolerability, the compound showed signs of clinical efficacy in FGF19-positive HCC patients[114]. FGF401 showed an increase in transaminases and induction of CYP7A1 in preclinical toxicology studies and co-administration of cholestyramine improved the safety profile here[115]. In humans, increase in transaminases was seen, too, but the overall safety profile is considered acceptable and no maximum tolerated dose was reached in a Phase 1/2 study. Patients had to be positive for FGFR4 and β-Klotho expression to be eligible to participation and the overall response rate in the Phase 1 population was about 8%[116].
The high global prevalence of NAFLD and NASH warrants the search for novel treatment options. FGF signaling mediates central metabolic effects in the liver (and other tissues) that are directly linked to the overload of hepatocytes with toxic metabolites and pathogenesis of lipid accumulation and chronic inflammation leading. Several approaches, e.g., FGF-mimicking peptides or receptor-specific targeting agents, have shown promising signs in preclinical and early clinical studies in humans. Yet, due to the plethora of ligands and receptors and a high tissue context dependency, further studies and esp. also long-term studies are urgently needed to fully understand how FGF and FGFR signaling pathways can be fully exploited for the benefit of affected patients.
Specific FGFR4 inhibitors are currently tested in clinical trials in HCC. The positive preclinical results are reflected in encouraging early clinical data from different Phase 1 studies. Yet, the overall efficacy of these compounds needs to be carefully investigated compared to current multi-kinase inhibitors and the emerging immune checkpoint inhibitors.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Abenavoli L, Liu HK, Sitkin S, Tanaka N S-Editor: Ma L L-Editor: A E-Editor: Ma YJ
| 1. | Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C, Al-Raddadi R, Alvis-Guzman N, Amoako Y, Artaman A, Ayele TA, Barac A, Bensenor I, Berhane A, Bhutta Z, Castillo-Rivas J, Chitheer A, Choi JY, Cowie B, Dandona L, Dandona R, Dey S, Dicker D, Phuc H, Ekwueme DU, Zaki MS, Fischer F, Fürst T, Hancock J, Hay SI, Hotez P, Jee SH, Kasaeian A, Khader Y, Khang YH, Kumar A, Kutz M, Larson H, Lopez A, Lunevicius R, Malekzadeh R, McAlinden C, Meier T, Mendoza W, Mokdad A, Moradi-Lakeh M, Nagel G, Nguyen Q, Nguyen G, Ogbo F, Patton G, Pereira DM, Pourmalek F, Qorbani M, Radfar A, Roshandel G, Salomon JA, Sanabria J, Sartorius B, Satpathy M, Sawhney M, Sepanlou S, Shackelford K, Shore H, Sun J, Mengistu DT, Topór-Mądry R, Tran B, Ukwaja KN, Vlassov V, Vollset SE, Vos T, Wakayo T, Weiderpass E, Werdecker A, Yonemoto N, Younis M, Yu C, Zaidi Z, Zhu L, Murray CJL, Naghavi M, Fitzmaurice C. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015. JAMA Oncol. 2017;3:1683-1691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1459] [Cited by in RCA: 1497] [Article Influence: 187.1] [Reference Citation Analysis (0)] |
| 2. | Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4054] [Cited by in RCA: 3788] [Article Influence: 541.1] [Reference Citation Analysis (2)] |
| 3. | Younossi ZM, Henry L, Bush H, Mishra A. Clinical and Economic Burden of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Clin Liver Dis. 2018;22:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 4. | Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov. 2009;8:235-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1242] [Cited by in RCA: 1465] [Article Influence: 91.6] [Reference Citation Analysis (0)] |
| 5. | Heroult M, Ellinghaus P, Ince S, Ocker M. Fibroblast Growth Factor Receptor Signaling in Cancer Biology and Treatment. Curr Sign Trans Ther. 2014;9:15-25. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Fu L, John LM, Adams SH, Yu XX, Tomlinson E, Renz M, Williams PM, Soriano R, Corpuz R, Moffat B, Vandlen R, Simmons L, Foster J, Stephan JP, Tsai SP, Stewart TA. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology. 2004;145:2594-2603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 457] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 7. | Kharitonenkov A. FGFs and metabolism. Curr Opin Pharmacol. 2009;9:805-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 108] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 8. | Lundåsen T, Gälman C, Angelin B, Rudling M. Circulating intestinal fibroblast growth factor 19 has a pronounced diurnal variation and modulates hepatic bile acid synthesis in man. J Intern Med. 2006;260:530-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 324] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 9. | Zhang X, Yeung DC, Karpisek M, Stejskal D, Zhou ZG, Liu F, Wong RL, Chow WS, Tso AW, Lam KS, Xu A. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes. 2008;57:1246-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 658] [Cited by in RCA: 719] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 10. | Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, Vonderfecht S, Hecht R, Li YS, Lindberg RA, Chen JL, Jung DY, Zhang Z, Ko HJ, Kim JK, Véniant MM. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes. 2009;58:250-259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 837] [Cited by in RCA: 950] [Article Influence: 59.4] [Reference Citation Analysis (0)] |
| 11. | Ornitz DM, Itoh N. The Fibroblast Growth Factor signaling pathway. Wiley Interdiscip Rev Dev Biol. 2015;4:215-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1226] [Cited by in RCA: 1484] [Article Influence: 148.4] [Reference Citation Analysis (0)] |
| 12. | Lamothe B, Yamada M, Schaeper U, Birchmeier W, Lax I, Schlessinger J. The docking protein Gab1 is an essential component of an indirect mechanism for fibroblast growth factor stimulation of the phosphatidylinositol 3-kinase/Akt antiapoptotic pathway. Mol Cell Biol. 2004;24:5657-5666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 70] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C, Sjöstedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist PH, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Pontén F. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7696] [Cited by in RCA: 10524] [Article Influence: 1052.4] [Reference Citation Analysis (0)] |
| 14. | Hughes SE. Differential expression of the fibroblast growth factor receptor (FGFR) multigene family in normal human adult tissues. J Histochem Cytochem. 1997;45:1005-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 201] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 15. | Rapraeger AC, Krufka A, Olwin BB. Requirement of heparan sulfate for bFGF-mediated fibroblast growth and myoblast differentiation. Science. 1991;252:1705-1708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1171] [Cited by in RCA: 1196] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 16. | Ding X, Boney-Montoya J, Owen BM, Bookout AL, Coate KC, Mangelsdorf DJ, Kliewer SA. βKlotho is required for fibroblast growth factor 21 effects on growth and metabolism. Cell Metab. 2012;16:387-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 337] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 17. | Kurosu H, Kuro-O M. The Klotho gene family as a regulator of endocrine fibroblast growth factors. Mol Cell Endocrinol. 2009;299:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 142] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 18. | Fernandes-Freitas I, Owen BM. Metabolic roles of endocrine fibroblast growth factors. Curr Opin Pharmacol. 2015;25:30-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Nies VJ, Sancar G, Liu W, van Zutphen T, Struik D, Yu RT, Atkins AR, Evans RM, Jonker JW, Downes MR. Fibroblast Growth Factor Signaling in Metabolic Regulation. Front Endocrinol (Lausanne). 2015;6:193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 20. | Mori S, Wu CY, Yamaji S, Saegusa J, Shi B, Ma Z, Kuwabara Y, Lam KS, Isseroff RR, Takada YK, Takada Y. Direct binding of integrin alphavbeta3 to FGF1 plays a role in FGF1 signaling. J Biol Chem. 2008;283:18066-18075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 118] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 21. | Schuppan D, Ocker M. Integrin-mediated control of cell growth. Hepatology. 2003;38:289-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Yu C, Wang F, Jin C, Huang X, Miller DL, Basilico C, McKeehan WL. Role of fibroblast growth factor type 1 and 2 in carbon tetrachloride-induced hepatic injury and fibrogenesis. Am J Pathol. 2003;163:1653-1662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 131] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 23. | Jin-no K, Tanimizu M, Hyodo I, Kurimoto F, Yamashita T. Plasma level of basic fibroblast growth factor increases with progression of chronic liver disease. J Gastroenterol. 1997;32:119-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Schumacher JD, Guo GL. Regulation of Hepatic Stellate Cells and Fibrogenesis by Fibroblast Growth Factors. Biomed Res Int. 2016;2016:8323747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 25. | Wang J, Rhee S, Palaria A, Tremblay KD. FGF signaling is required for anterior but not posterior specification of the murine liver bud. Dev Dyn. 2015;244:431-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Calmont A, Wandzioch E, Tremblay KD, Minowada G, Kaestner KH, Martin GR, Zaret KS. An FGF response pathway that mediates hepatic gene induction in embryonic endoderm cells. Dev Cell. 2006;11:339-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 108] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 27. | Berg T, Rountree CB, Lee L, Estrada J, Sala FG, Choe A, Veltmaat JM, De Langhe S, Lee R, Tsukamoto H, Crooks GM, Bellusci S, Wang KS. Fibroblast growth factor 10 is critical for liver growth during embryogenesis and controls hepatoblast survival via beta-catenin activation. Hepatology. 2007;46:1187-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 28. | Takase HM, Itoh T, Ino S, Wang T, Koji T, Akira S, Takikawa Y, Miyajima A. FGF7 is a functional niche signal required for stimulation of adult liver progenitor cells that support liver regeneration. Genes Dev. 2013;27:169-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 29. | Antoine M, Wirz W, Tag CG, Gressner AM, Marvituna M, Wycislo M, Hellerbrand C, Kiefer P. Expression and function of fibroblast growth factor (FGF) 9 in hepatic stellate cells and its role in toxic liver injury. Biochem Biophys Res Commun. 2007;361:335-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Gauglhofer C, Sagmeister S, Schrottmaier W, Fischer C, Rodgarkia-Dara C, Mohr T, Stättner S, Bichler C, Kandioler D, Wrba F, Schulte-Hermann R, Holzmann K, Grusch M, Marian B, Berger W, Grasl-Kraupp B. Up-regulation of the fibroblast growth factor 8 subfamily in human hepatocellular carcinoma for cell survival and neoangiogenesis. Hepatology. 2011;53:854-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 31. | Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, Gerard RD, Repa JJ, Mangelsdorf DJ, Kliewer SA. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1297] [Cited by in RCA: 1430] [Article Influence: 71.5] [Reference Citation Analysis (0)] |
| 32. | Choi M, Moschetta A, Bookout AL, Peng L, Umetani M, Holmstrom SR, Suino-Powell K, Xu HE, Richardson JA, Gerard RD, Mangelsdorf DJ, Kliewer SA. Identification of a hormonal basis for gallbladder filling. Nat Med. 2006;12:1253-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 230] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 33. | Kir S, Beddow SA, Samuel VT, Miller P, Previs SF, Suino-Powell K, Xu HE, Shulman GI, Kliewer SA, Mangelsdorf DJ. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science. 2011;331:1621-1624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 513] [Cited by in RCA: 485] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 34. | Hotta Y, Nakamura H, Konishi M, Murata Y, Takagi H, Matsumura S, Inoue K, Fushiki T, Itoh N. Fibroblast growth factor 21 regulates lipolysis in white adipose tissue but is not required for ketogenesis and triglyceride clearance in liver. Endocrinology. 2009;150:4625-4633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 282] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 35. | Cheng X, Vispute SG, Liu J, Cheng C, Kharitonenkov A, Klaassen CD. Fibroblast growth factor (Fgf) 21 is a novel target gene of the aryl hydrocarbon receptor (AhR). Toxicol Appl Pharmacol. 2014;278:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 36. | Ritze Y, Bárdos G, Claus A, Ehrmann V, Bergheim I, Schwiertz A, Bischoff SC. Lactobacillus rhamnosus GG protects against non-alcoholic fatty liver disease in mice. PLoS One. 2014;9:e80169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 221] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 37. | Kim B, Park KY, Ji Y, Park S, Holzapfel W, Hyun CK. Protective effects of Lactobacillus rhamnosus GG against dyslipidemia in high-fat diet-induced obese mice. Biochem Biophys Res Commun. 2016;473:530-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 98] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 38. | Zhao C, Liu L, Liu Q, Li F, Zhang L, Zhu F, Shao T, Barve S, Chen Y, Li X, McClain CJ, Feng W. Fibroblast growth factor 21 is required for the therapeutic effects of Lactobacillus rhamnosus GG against fructose-induced fatty liver in mice. Mol Metab. 2019;29:145-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 39. | Liu Q, Liu Y, Li F, Gu Z, Liu M, Shao T, Zhang L, Zhou G, Pan C, He L, Cai J, Zhang X, Barve S, McClain CJ, Chen Y, Feng W. Probiotic culture supernatant improves metabolic function through FGF21-adiponectin pathway in mice. J Nutr Biochem. 2020;75:108256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 40. | Erben RG. Physiological Actions of Fibroblast Growth Factor-23. Front Endocrinol (Lausanne). 2018;9:267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 113] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 41. | He X, Shen Y, Ma X, Ying L, Peng J, Pan X, Bao Y, Zhou J. The association of serum FGF23 and non-alcoholic fatty liver disease is independent of vitamin D in type 2 diabetes patients. Clin Exp Pharmacol Physiol. 2018;45:668-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 42. | Mirams M, Robinson BG, Mason RS, Nelson AE. Bone as a source of FGF23: regulation by phosphate? Bone. 2004;35:1192-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 159] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 43. | Dokumacioglu E, Iskender H, Musmul A. Effect of hesperidin treatment on α-Klotho/FGF-23 pathway in rats with experimentally-induced diabetes. Biomed Pharmacother. 2019;109:1206-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 44. | Hauff P, Gottwald U, Ocker M. Early to Phase II drugs currently under investigation for the treatment of liver fibrosis. Expert Opin Investig Drugs. 2015;24:309-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 45. | Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1096] [Cited by in RCA: 1380] [Article Influence: 98.6] [Reference Citation Analysis (0)] |
| 46. | Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838-851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1686] [Cited by in RCA: 1565] [Article Influence: 92.1] [Reference Citation Analysis (0)] |
| 47. | Schuppan D, Schattenberg JM. Non-alcoholic steatohepatitis: pathogenesis and novel therapeutic approaches. J Gastroenterol Hepatol. 2013;28 Suppl 1:68-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 202] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 48. | Jeyapal S, Putcha UK, Mullapudi VS, Ghosh S, Sakamuri A, Kona SR, Vadakattu SS, Madakasira C, Ibrahim A. Chronic consumption of fructose in combination with trans fatty acids but not with saturated fatty acids induces nonalcoholic steatohepatitis with fibrosis in rats. Eur J Nutr. 2018;57:2171-2187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 49. | Savard C, Tartaglione EV, Kuver R, Haigh WG, Farrell GC, Subramanian S, Chait A, Yeh MM, Quinn LS, Ioannou GN. Synergistic interaction of dietary cholesterol and dietary fat in inducing experimental steatohepatitis. Hepatology. 2013;57:81-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 228] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 50. | Ter Horst KW, Serlie MJ. Fructose Consumption, Lipogenesis, and Non-Alcoholic Fatty Liver Disease. Nutrients. 2017;9:pii: E981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 215] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 51. | Alwahsh SM, Gebhardt R. Dietary fructose as a risk factor for non-alcoholic fatty liver disease (NAFLD). Arch Toxicol. 2017;91:1545-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 113] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 52. | Neuschwander-Tetri BA. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology. 2010;52:774-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 726] [Cited by in RCA: 841] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 53. | Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1376] [Cited by in RCA: 2916] [Article Influence: 416.6] [Reference Citation Analysis (1)] |
| 54. | Murata Y, Konishi M, Itoh N. FGF21 as an Endocrine Regulator in Lipid Metabolism: From Molecular Evolution to Physiology and Pathophysiology. J Nutr Metab. 2011;2011:981315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 55. | Adams AC, Yang C, Coskun T, Cheng CC, Gimeno RE, Luo Y, Kharitonenkov A. The breadth of FGF21's metabolic actions are governed by FGFR1 in adipose tissue. Mol Metab. 2012;2:31-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 230] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 56. | Bookout AL, de Groot MH, Owen BM, Lee S, Gautron L, Lawrence HL, Ding X, Elmquist JK, Takahashi JS, Mangelsdorf DJ, Kliewer SA. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat Med. 2013;19:1147-1152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 354] [Cited by in RCA: 429] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 57. | Hui X, Feng T, Liu Q, Gao Y, Xu A. The FGF21-adiponectin axis in controlling energy and vascular homeostasis. J Mol Cell Biol. 2016;8:110-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 58. | Lin Z, Tian H, Lam KS, Lin S, Hoo RC, Konishi M, Itoh N, Wang Y, Bornstein SR, Xu A, Li X. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metab. 2013;17:779-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 557] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 59. | Habegger KM, Stemmer K, Cheng C, Müller TD, Heppner KM, Ottaway N, Holland J, Hembree JL, Smiley D, Gelfanov V, Krishna R, Arafat AM, Konkar A, Belli S, Kapps M, Woods SC, Hofmann SM, D'Alessio D, Pfluger PT, Perez-Tilve D, Seeley RJ, Konishi M, Itoh N, Kharitonenkov A, Spranger J, DiMarchi RD, Tschöp MH. Fibroblast growth factor 21 mediates specific glucagon actions. Diabetes. 2013;62:1453-1463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 193] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 60. | Shan Z, Alvarez-Sola G, Uriarte I, Arechederra M, Fernández-Barrena MG, Berasain C, Ju C, Avila MA. Fibroblast growth factors 19 and 21 in acute liver damage. Ann Transl Med. 2018;6:257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 61. | Singhal G, Kumar G, Chan S, Fisher FM, Ma Y, Vardeh HG, Nasser IA, Flier JS, Maratos-Flier E. Deficiency of fibroblast growth factor 21 (FGF21) promotes hepatocellular carcinoma (HCC) in mice on a long term obesogenic diet. Mol Metab. 2018;13:56-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 62. | Bao L, Yin J, Gao W, Wang Q, Yao W, Gao X. A long-acting FGF21 alleviates hepatic steatosis and inflammation in a mouse model of non-alcoholic steatohepatitis partly through an FGF21-adiponectin-IL17A pathway. Br J Pharmacol. 2018;175:3379-3393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 63. | Dushay J, Chui PC, Gopalakrishnan GS, Varela-Rey M, Crawley M, Fisher FM, Badman MK, Martinez-Chantar ML, Maratos-Flier E. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology. 2010;139:456-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 491] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 64. | Yilmaz Y, Eren F, Yonal O, Kurt R, Aktas B, Celikel CA, Ozdogan O, Imeryuz N, Kalayci C, Avsar E. Increased serum FGF21 levels in patients with nonalcoholic fatty liver disease. Eur J Clin Invest. 2010;40:887-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 151] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 65. | Flisiak-Jackiewicz M, Bobrus-Chociej A, Wasilewska N, Tarasow E, Wojtkowska M, Lebensztejn DM. Can hepatokines be regarded as novel non-invasive serum biomarkers of intrahepatic lipid content in obese children? Adv Med Sci. 2019;64:280-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 66. | Hua MC, Huang JL, Hu CC, Yao TC, Lai MW. Including Fibroblast Growth Factor-21 in Combined Biomarker Panels Improves Predictions of Liver Steatosis Severity in Children. Front Pediatr. 2019;7:420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 67. | Praktiknjo M, Djayadi N, Mohr R, Schierwagen R, Bischoff J, Dold L, Pohlmann A, Schwarze-Zander C, Wasmuth JC, Boesecke C, Rockstroh JK, Trebicka J. Fibroblast growth factor 21 is independently associated with severe hepatic steatosis in non-obese HIV-infected patients. Liver Int. 2019;39:1514-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 68. | He L, Deng L, Zhang Q, Guo J, Zhou J, Song W, Yuan F. Diagnostic Value of CK-18, FGF-21, and Related Biomarker Panel in Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Biomed Res Int. 2017;2017:9729107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 69. | Yang M, Xu D, Liu Y, Guo X, Li W, Guo C, Zhang H, Gao Y, Mao Y, Zhao J. Combined Serum Biomarkers in Non-Invasive Diagnosis of Non-Alcoholic Steatohepatitis. PLoS One. 2015;10:e0131664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 70. | Nakashima H, Nakashima M, Kinoshita M, Ikarashi M, Miyazaki H, Hanaka H, Imaki J, Seki S. Activation and increase of radio-sensitive CD11b+ recruited Kupffer cells/macrophages in diet-induced steatohepatitis in FGF5 deficient mice. Sci Rep. 2016;6:34466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 71. | Hanaka H, Hamada T, Ito M, Nakashima H, Tomita K, Seki S, Kobayashi Y, Imaki J. Fibroblast growth factor-5 participates in the progression of hepatic fibrosis. Exp Anim. 2014;63:85-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 72. | Uriarte I, Fernandez-Barrena MG, Monte MJ, Latasa MU, Chang HC, Carotti S, Vespasiani-Gentilucci U, Morini S, Vicente E, Concepcion AR, Medina JF, Marin JJ, Berasain C, Prieto J, Avila MA. Identification of fibroblast growth factor 15 as a novel mediator of liver regeneration and its application in the prevention of post-resection liver failure in mice. Gut. 2013;62:899-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 146] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 73. | Uriarte I, Latasa MU, Carotti S, Fernandez-Barrena MG, Garcia-Irigoyen O, Elizalde M, Urtasun R, Vespasiani-Gentilucci U, Morini S, de Mingo A, Mari M, Corrales FJ, Prieto J, Berasain C, Avila MA. Ileal FGF15 contributes to fibrosis-associated hepatocellular carcinoma development. Int J Cancer. 2015;136:2469-2475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 74. | Schreuder TC, Marsman HA, Lenicek M, van Werven JR, Nederveen AJ, Jansen PL, Schaap FG. The hepatic response to FGF19 is impaired in patients with nonalcoholic fatty liver disease and insulin resistance. Am J Physiol Gastrointest Liver Physiol. 2010;298:G440-G445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 75. | Friedrich D, Marschall HU, Lammert F. Response of fibroblast growth factor 19 and bile acid synthesis after a body weight-adjusted oral fat tolerance test in overweight and obese NAFLD patients: a non-randomized controlled pilot trial. BMC Gastroenterol. 2018;18:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 76. | Liu WY, Xie DM, Zhu GQ, Huang GQ, Lin YQ, Wang LR, Shi KQ, Hu B, Braddock M, Chen YP, Zheng MH. Targeting fibroblast growth factor 19 in liver disease: a potential biomarker and therapeutic target. Expert Opin Ther Targets. 2015;19:675-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 77. | Wojcik M, Janus D, Dolezal-Oltarzewska K, Kalicka-Kasperczyk A, Poplawska K, Drozdz D, Sztefko K, Starzyk JB. A decrease in fasting FGF19 levels is associated with the development of non-alcoholic fatty liver disease in obese adolescents. J Pediatr Endocrinol Metab. 2012;25:1089-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 78. | Eren F, Kurt R, Ermis F, Atug O, Imeryuz N, Yilmaz Y. Preliminary evidence of a reduced serum level of fibroblast growth factor 19 in patients with biopsy-proven nonalcoholic fatty liver disease. Clin Biochem. 2012;45:655-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 79. | Jiao N, Baker SS, Chapa-Rodriguez A, Liu W, Nugent CA, Tsompana M, Mastrandrea L, Buck MJ, Baker RD, Genco RJ, Zhu R, Zhu L. Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut. 2018;67:1881-1891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 515] [Article Influence: 73.6] [Reference Citation Analysis (1)] |
| 80. | Alvarez-Sola G, Uriarte I, Latasa MU, Fernandez-Barrena MG, Urtasun R, Elizalde M, Barcena-Varela M, Jiménez M, Chang HC, Barbero R, Catalán V, Rodríguez A, Frühbeck G, Gallego-Escuredo JM, Gavaldà-Navarro A, Villarroya F, Rodriguez-Ortigosa CM, Corrales FJ, Prieto J, Berraondo P, Berasain C, Avila MA. Fibroblast growth factor 15/19 (FGF15/19) protects from diet-induced hepatic steatosis: development of an FGF19-based chimeric molecule to promote fatty liver regeneration. Gut. 2017;66:1818-1828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 131] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 81. | Nobili V, Alisi A, Mosca A, Della Corte C, Veraldi S, De Vito R, De Stefanis C, D'Oria V, Jahnel J, Zohrer E, Scorletti E, Byrne CD. Hepatic farnesoid X receptor protein level and circulating fibroblast growth factor 19 concentration in children with NAFLD. Liver Int. 2018;38:342-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 82. | Zöhrer E, Alisi A, Jahnel J, Mosca A, Della Corte C, Crudele A, Fauler G, Nobili V. Efficacy of docosahexaenoic acid-choline-vitamin E in paediatric NASH: a randomized controlled clinical trial. Appl Physiol Nutr Metab. 2017;42:948-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 83. | Sanyal AJ, Friedman SL, McCullough AJ, Dimick-Santos L; American Association for the Study of Liver Diseases; United States Food and Drug Administration. Challenges and opportunities in drug and biomarker development for nonalcoholic steatohepatitis: findings and recommendations from an American Association for the Study of Liver Diseases-U.S. Food and Drug Administration Joint Workshop. Hepatology. 2015;61:1392-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 271] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 84. | Younossi ZM, Loomba R, Rinella ME, Bugianesi E, Marchesini G, Neuschwander-Tetri BA, Serfaty L, Negro F, Caldwell SH, Ratziu V, Corey KE, Friedman SL, Abdelmalek MF, Harrison SA, Sanyal AJ, Lavine JE, Mathurin P, Charlton MR, Chalasani NP, Anstee QM, Kowdley KV, George J, Goodman ZD, Lindor K. Current and future therapeutic regimens for nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. 2018;68:361-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 269] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 85. | Luo Y, Krupinski J, Gao S, Charles E, Christian R. BMS-986036, a PEGylated fibroblast growth factor 21 analogue, reduces fibrosis and Pro-C3 in a mouse model of non-alcoholic steatohepatitis. J Hepatol. 2018;68:S396-S397. [DOI] [Full Text] |
| 86. | Charles ED, Neuschwander-Tetri BA, Pablo Frias J, Kundu S, Luo Y, Tirucherai GS, Christian R. Pegbelfermin (BMS-986036), PEGylated FGF21, in Patients with Obesity and Type 2 Diabetes: Results from a Randomized Phase 2 Study. Obesity (Silver Spring). 2019;27:41-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 201] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 87. | Sanyal A, Charles ED, Neuschwander-Tetri BA, Loomba R, Harrison SA, Abdelmalek MF, Lawitz EJ, Halegoua-DeMarzio D, Kundu S, Noviello S, Luo Y, Christian R. Pegbelfermin (BMS-986036), a PEGylated fibroblast growth factor 21 analogue, in patients with non-alcoholic steatohepatitis: a randomised, double-blind, placebo-controlled, phase 2a trial. Lancet. 2019;392:2705-2717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 421] [Article Influence: 70.2] [Reference Citation Analysis (0)] |
| 88. | Zhou M, Wang X, Phung V, Lindhout DA, Mondal K, Hsu JY, Yang H, Humphrey M, Ding X, Arora T, Learned RM, DePaoli AM, Tian H, Ling L. Separating Tumorigenicity from Bile Acid Regulatory Activity for Endocrine Hormone FGF19. Cancer Res. 2014;74:3306-3316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 154] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 89. | Zhou M, Learned RM, Rossi SJ, DePaoli AM, Tian H, Ling L. Engineered FGF19 eliminates bile acid toxicity and lipotoxicity leading to resolution of steatohepatitis and fibrosis in mice. Hepatol Commun. 2017;1:1024-1042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 127] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 90. | Zhou M, Learned RM, Rossi SJ, DePaoli AM, Tian H, Ling L. Engineered fibroblast growth factor 19 reduces liver injury and resolves sclerosing cholangitis in Mdr2-deficient mice. Hepatology. 2016;63:914-929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 107] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 91. | Luo J, Ko B, Elliott M, Zhou M, Lindhout DA, Phung V, To C, Learned RM, Tian H, DePaoli AM, Ling L. A nontumorigenic variant of FGF19 treats cholestatic liver diseases. Sci Transl Med. 2014;6:247ra100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 136] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 92. | Harrison SA, Rinella ME, Abdelmalek MF, Trotter JF, Paredes AH, Arnold HL, Kugelmas M, Bashir MR, Jaros MJ, Ling L, Rossi SJ, DePaoli AM, Loomba R. NGM282 for treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2018;391:1174-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 349] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 93. | Harrison SA, Rossi SJ, Paredes AH, Trotter JF, Bashir MR, Guy CD, Banerjee R, Jaros MJ, Owers S, Baxter BA, Ling L, DePaoli AM. NGM282 Improves Liver Fibrosis and Histology in 12 Weeks in Patients With Nonalcoholic Steatohepatitis. Hepatology. 2019; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 208] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 94. | Rinella ME, Trotter JF, Abdelmalek MF, Paredes AH, Connelly MA, Jaros MJ, Ling L, Rossi SJ, DePaoli AM, Harrison SA. Rosuvastatin improves the FGF19 analogue NGM282-associated lipid changes in patients with non-alcoholic steatohepatitis. J Hepatol. 2019;70:735-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 95. | Hagel M, Miduturu C, Sheets M, Rubin N, Weng W, Stransky N, Bifulco N, Kim JL, Hodous B, Brooijmans N, Shutes A, Winter C, Lengauer C, Kohl NE, Guzi T. First Selective Small Molecule Inhibitor of FGFR4 for the Treatment of Hepatocellular Carcinomas with an Activated FGFR4 Signaling Pathway. Cancer Discov. 2015;5:424-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 254] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 96. | Joshi JJ, Coffey H, Corcoran E, Tsai J, Huang CL, Ichikawa K, Prajapati S, Hao MH, Bailey S, Wu J, Rimkunas V, Karr C, Subramanian V, Kumar P, MacKenzie C, Hurley R, Satoh T, Yu K, Park E, Rioux N, Kim A, Lai WG, Yu L, Zhu P, Buonamici S, Larsen N, Fekkes P, Wang J, Warmuth M, Reynolds DJ, Smith PG, Selvaraj A. H3B-6527 Is a Potent and Selective Inhibitor of FGFR4 in FGF19-Driven Hepatocellular Carcinoma. Cancer Res. 2017;77:6999-7013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 116] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 97. | Teng Y, Zhao H, Gao L, Zhang W, Shull AY, Shay C. FGF19 Protects Hepatocellular Carcinoma Cells against Endoplasmic Reticulum Stress via Activation of FGFR4-GSK3β-Nrf2 Signaling. Cancer Res. 2017;77:6215-6225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 98. | Cui G, Martin RC, Jin H, Liu X, Pandit H, Zhao H, Cai L, Zhang P, Li W, Li Y. Up-regulation of FGF15/19 signaling promotes hepatocellular carcinoma in the background of fatty liver. J Exp Clin Cancer Res. 2018;37:136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 99. | Li Y, Zhang W, Doughtie A, Cui G, Li X, Pandit H, Yang Y, Li S, Martin R. Up-regulation of fibroblast growth factor 19 and its receptor associates with progression from fatty liver to hepatocellular carcinoma. Oncotarget. 2016;7:52329-52339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 100. | Chen Q, Jiang Y, An Y, Zhao N, Zhao Y, Yu C. Soluble FGFR4 extracellular domain inhibits FGF19-induced activation of FGFR4 signaling and prevents nonalcoholic fatty liver disease. Biochem Biophys Res Commun. 2011;409:651-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 101. | Shankar S, Bashir MR, Baxter BA, Phung V, Yan AZ, Rossi SJ, DePaoli AM. NGM313, a novel once-monthly activator of bklotho-FGFR1c, significantly reduces hepatic steatosis and key biomarkers of non-alcoholic steatohepatitis: results of a randomized, active-controlled clamp study in obese insulin-resistant patients with NAFLD. Hepatology. 2018;68:LB-21. [DOI] [Full Text] |
| 102. | Cheng AL, Shen YC, Zhu AX. Targeting fibroblast growth factor receptor signaling in hepatocellular carcinoma. Oncology. 2011;81:372-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 103. | Lee HJ, Kang HJ, Kim KM, Yu ES, Kim KH, Kim SM, Kim TW, Shim JH, Lim YS, Lee HC, Chung YH, Lee YS. Fibroblast growth factor receptor isotype expression and its association with overall survival in patients with hepatocellular carcinoma. Clin Mol Hepatol. 2015;21:60-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 104. | Maeda T, Kanzaki H, Chiba T, Ao J, Kanayama K, Maruta S, Kusakabe Y, Saito T, Kobayashi K, Kiyono S, Nakamura M, Ogasawara S, Suzuki E, Ooka Y, Nakamoto S, Nakagawa R, Muroyama R, Kanda T, Maruyama H, Kato N. Serum fibroblast growth factor 19 serves as a potential novel biomarker for hepatocellular carcinoma. BMC Cancer. 2019;19:1088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 105. | Ho HK, Pok S, Streit S, Ruhe JE, Hart S, Lim KS, Loo HL, Aung MO, Lim SG, Ullrich A. Fibroblast growth factor receptor 4 regulates proliferation, anti-apoptosis and alpha-fetoprotein secretion during hepatocellular carcinoma progression and represents a potential target for therapeutic intervention. J Hepatol. 2009;50:118-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 146] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 106. | French DM, Lin BC, Wang M, Adams C, Shek T, Hötzel K, Bolon B, Ferrando R, Blackmore C, Schroeder K, Rodriguez LA, Hristopoulos M, Venook R, Ashkenazi A, Desnoyers LR. Targeting FGFR4 inhibits hepatocellular carcinoma in preclinical mouse models. PLoS One. 2012;7:e36713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 176] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 107. | Heinzle C, Erdem Z, Paur J, Grasl-Kraupp B, Holzmann K, Grusch M, Berger W, Marian B. Is fibroblast growth factor receptor 4 a suitable target of cancer therapy? Curr Pharm Des. 2014;20:2881-2898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 108. | Raja A, Park I, Haq F, Ahn SM. FGF19-FGFR4 Signaling in Hepatocellular Carcinoma. Cells. 2019;8:pii: E536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 117] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 109. | Hoshi T, Watanabe Miyano S, Watanabe H, Sonobe RMK, Seki Y, Ohta E, Nomoto K, Matsui J, Funahashi Y. Lenvatinib induces death of human hepatocellular carcinoma cells harboring an activated FGF signaling pathway through inhibition of FGFR-MAPK cascades. Biochem Biophys Res Commun. 2019;513:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 110. | Jo JC, Choi EK, Shin JS, Moon JH, Hong SW, Lee HR, Kim SM, Jung SA, Lee DH, Jung SH, Lee SH, Kim JE, Kim KP, Hong YS, Suh YA, Jang SJ, Choi EK, Lee JS, Jin DH, Kim TW. Targeting FGFR Pathway in Human Hepatocellular Carcinoma: Expressing pFGFR and pMET for Antitumor Activity. Mol Cancer Ther. 2015;14:2613-2622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 111. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10265] [Article Influence: 603.8] [Reference Citation Analysis (2)] |
| 112. | Johnson PJ, Qin S, Park JW, Poon RT, Raoul JL, Philip PA, Hsu CH, Hu TH, Heo J, Xu J, Lu L, Chao Y, Boucher E, Han KH, Paik SW, Robles-Aviña J, Kudo M, Yan L, Sobhonslidsuk A, Komov D, Decaens T, Tak WY, Jeng LB, Liu D, Ezzeddine R, Walters I, Cheng AL. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol. 2013;31:3517-3524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 596] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 113. | Ruggeri B, Stubbs M, Yang Y, Juvekar A, Lu L, Condon S, DiMatteo D, Wen X, Collier P, Burn T, Wu L, Wilson D, Yeleswaram S, Roberts A, Yao W, Hollis G, Huber R, Scherle P, Liu PCC. The novel FGFR4-selective inhibitor INCB062079 is efficacious in models of hepatocellular carcinoma harboring FGF19 amplification. Cancer Res. 2017;77:1234. [DOI] [Full Text] |
| 114. | Kim RD, Sarker D, Meyer T, Yau T, Macarulla T, Park JW, Choo SP, Hollebecque A, Sung MW, Lim HY, Mazzaferro V, Trojan J, Zhu AX, Yoon JH, Sharma S, Lin ZZ, Chan SL, Faivre S, Feun LG, Yen CJ, Dufour JF, Palmer DH, Llovet JM, Manoogian M, Tugnait M, Stransky N, Hagel M, Kohl NE, Lengauer C, Sherwin CA, Schmidt-Kittler O, Hoeflich KP, Shi H, Wolf BB, Kang YK. First-in-Human Phase I Study of Fisogatinib (BLU-554) Validates Aberrant FGF19 Signaling as a Driver Event in Hepatocellular Carcinoma. Cancer Discov. 2019;9:1696-1707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 193] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 115. | Schadt HS, Wolf A, Mahl JA, Wuersch K, Couttet P, Schwald M, Fischer A, Lienard M, Emotte C, Teng CH, Skuba E, Richardson TA, Manenti L, Weiss A, Graus Porta D, Fairhurst RA, Kullak-Ublick GA, Chibout SD, Pognan F, Kluwe W, Kinyamu-Akunda J. Bile Acid Sequestration by Cholestyramine Mitigates FGFR4 Inhibition-Induced ALT Elevation. Toxicol Sci. 2018;163:265-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 116. | Chan SL, Schuler M, Lin CC, Choo SP, Weiss K-H, Geier A, Okusaka T, Lim HY, Macarulla T, Zhu AX, Kakizume T, Gu Y, Porta DG, Myers AP, Delord J-P. Ph I/II study of FGF401 in adult pts with HCC or solid tumors characterized by FGFR4/KLB expression. Cancer Res. 2017;77:CT106. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |