Published online Aug 7, 2020. doi: 10.3748/wjg.v26.i29.4327
Peer-review started: March 11, 2020
First decision: April 8, 2020
Revised: April 24, 2020
Accepted: July 14, 2020
Article in press: July 14, 2020
Published online: August 7, 2020
Processing time: 148 Days and 18.2 Hours
Hepatocellular carcinoma (HCC) is the sixth most common type of cancer and the fourth leading cause of cancer-related death worldwide. Sarcomatoid HCC, which contains poorly differentiated carcinomatous and sarcomatous components, is a rare histological subtype of HCC that differs from conventional HCC. It is highly aggressive and has a poor prognosis. Its clinicopathological characteristics, surgical outcomes and underlying mechanisms of its highly aggressive nature have not been fully elucidated.
To examine the clinicopathological characteristics and surgical outcomes of sarcomatoid HCC and explore the histogenesis of sarcomatoid HCC.
In total, 196 patients [41 sarcomatoid HCC and 155 high-grade (Edmondson-Steiner grade III or IV) HCC] who underwent surgical resection between 2007 and 2017 were retrospectively reviewed. The characteristics and surgical outcomes of sarcomatoid HCC were compared with those of patients with high-grade HCC. The histological composition of invasive and metastatic sarcomatoid HCCs was evaluated.
Sarcomatoid HCC was more frequently diagnosed at an advanced stage with a larger tumor and higher rates of nonspecific symptom, adjacent organ invasion and lymph node metastasis than high-grade HCC (all P < 0.05). Compared with high-grade HCC patients, sarcomatoid HCC patients are less likely to have typical dynamic imaging features of HCC (44.4% vs 72.7%, P = 0.001) and elevated serum alpha-fetoprotein levels (> 20 ng/mL; 36.6% vs 78.7%, P < 0.001). The sarcomatoid group had a significantly shorter median recurrence-free survival (5.6 mo vs 16.4 mo, log-rank P < 0.0001) and overall survival (10.5 mo vs 48.1 mo, log-rank P < 0.0001) than the high-grade group. After controlling for confounding factors, the sarcomatoid subtype was identified as an independent predictor of poor prognosis. Pathological analyses indicated that invasive and metastatic lesions were mainly composed of carcinomatous components.
Sarcomatoid HCC was associated with a more advanced stage, atypical dynamic imaging, lower serum alpha-fetoprotein levels and a worse prognosis. The highly aggressive nature of sarcomatoid HCC is perhaps mediated by carcinomatous components.
Core tip: Sarcomatoid hepatocellular carcinoma is a rare malignancy. Patients usually present with an advanced stage of the disease and have a poor prognosis. Histopathological examination plays an important role in diagnosis because serologic and radiologic examinations cannot help in distinguishing this disease from conventional hepatocellular carcinoma or other intrahepatic masses. Most patients have a history of chronic liver disease; thus, regular ultrasound screening of such patients can help detect tumors at an early stage and reduce the risk of death. Moreover, we discovered that carcinomatous components occupied the predominant proportion of invasions and metastases, and we developed a hypothesis regarding the occurrence of the disease.
- Citation: Wang JP, Yao ZG, Sun YW, Liu XH, Sun FK, Lin CH, Ren FX, Lv BB, Zhang SJ, Wang Y, Meng FY, Zheng SZ, Gong W, Liu J. Clinicopathological characteristics and surgical outcomes of sarcomatoid hepatocellular carcinoma. World J Gastroenterol 2020; 26(29): 4327-4342
- URL: https://www.wjgnet.com/1007-9327/full/v26/i29/4327.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i29.4327
Hepatocellular carcinoma (HCC) is the sixth most common type of cancer and the fourth leading cause of cancer-related death worldwide[1]. Sarcomatoid HCC is a histological subtype of HCC that differs from conventional HCC and presents with an unusual characteristic: sarcomatoid HCCs contain variable proportions of sarcomatous and carcinomatous components, wherein the sarcomatous component usually consists of spindle-shaped cells that form interlacing bundles and show a partial storiform pattern, and the carcinomatous component commonly comprises poorly differentiated [Edmondson-Steiner (ES) grade III or IV] conventional HCC cells[2,3]. Previous studies have indicated that certain anticancer therapies, such as transcatheter arterial chemoembolization (TACE), can lead to more frequent sarcomatous changes in HCC[4,5]. However, an increasing number of sarcomatoid HCC cases without previous anticancer therapy have been reported[6-8]. To date, the pathogenesis of the sarcomatous change has not been elucidated.
Sarcomatoid HCC is a rare malignancy, with an incidence of 1.7%-1.9% among surgically resected HCC cases and 3.9%-9.4% among autopsied HCC patients[3,4,8-11]. A few studies have reported that sarcomatoid HCC is associated with a higher recurrence rate, more frequent metastasis and poorer survival than conventional HCC[2,3,8,12]. However, these studies did not further stratify conventional HCC into low- (ES grade I and II) and high-grade (ES grade III and IV) HCC; in particular, high-grade HCC is considered similar to sarcomatoid HCC in terms of histological differentiation, more aggressive nature and poor prognosis[2,3,13,14]. In addition, although sarcomatoid HCC has a high incidence of adjacent organ invasion and metastasis[8], the underlying mechanisms remain unknown. One previous study reported that most portal venous invasions and metastases had sarcomatous components, indicating that the sarcomatous component is responsible for metastasis[3].
In this study, we comprehensively compared the clinicopathological characteristics and surgical outcomes of sarcomatoid HCC and high-grade HCC patients. We also analyzed the histological composition of metastatic and invasive sarcomatoid HCCs to study the relative importance of sarcomatous and carcinomatous components in highly aggressive behavior.
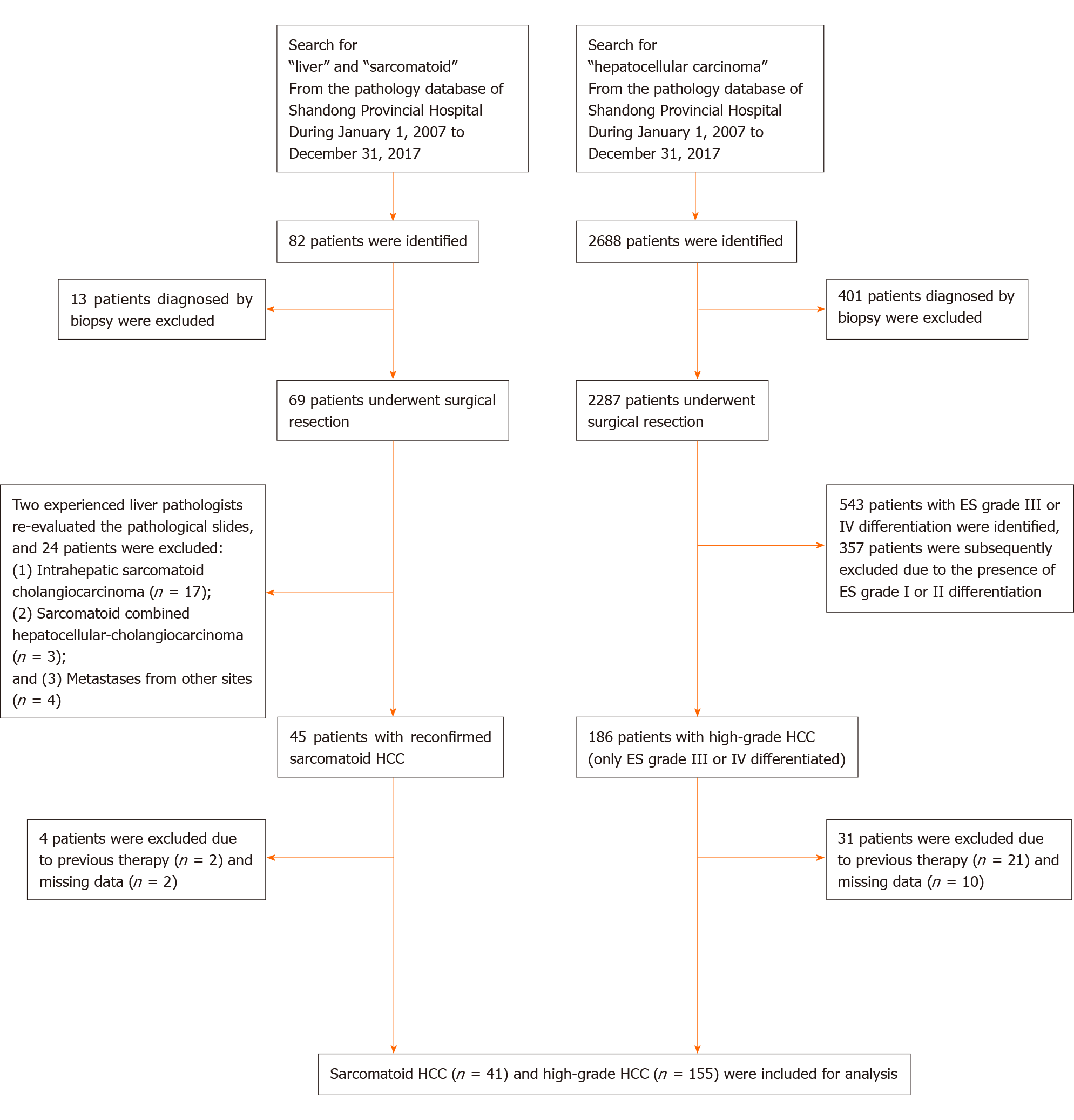
From January 2007 to December 2017, a total of 2287 patients underwent surgical resection for HCC at Shandong Provincial Hospital. We retrospectively reviewed the pathological records of these patients, of which 186 were diagnosed with high-grade HCC defined as grade III or IV differentiated HCC according to the ES classification[15]. In addition, 45 patients who underwent surgical resection for sarcomatoid HCC were identified from the pathology database of Shandong Provincial Hospital. We excluded patients who had previous interventions (including TACE, radiofrequency ablation and previous surgical resection) or missing data. A total of 41 sarcomatoid HCC and 155 high-grade HCC patients were included in the final analysis. The flowchart of patient selection is shown in Figure 1.
The medical records of the included patients were retrospectively reviewed. A standardized record form was used to collect clinical information, including age, sex, symptoms, alcoholism, hepatitis virus B or C infection, liver cirrhosis, laboratory test results, Child-Pugh classification and tumor-specific characteristics, such as tumor size, tumor number and macrovascular invasion. Tumor staging was classified according to the Barcelona Clinic Liver Cancer staging system and the American Joint Committee on Cancer (AJCC) staging system (8th edition, 2017). Computed tomography (CT) and magnetic resonance imaging (MRI) images were also reviewed.
Routine follow-ups were conducted during the 1st and 3rd mo after resection and subsequently every 2 to 3 mo during the first postoperative year and every 3 to 6 mo thereafter. At each follow-up, serum alpha-fetoprotein (AFP) levels and liver function were assessed, and abdominal ultrasonography was completed. An abdominal CT or MRI was performed at an interval of 6 to 12 mo depending on the postoperative time. If recurrence was suspected, an additional CT or MRI scan was performed immediately. Overall survival (OS) was defined as the time from the date of surgery to the date of death or last follow-up. Recurrence was defined as the appearance of a new lesion as confirmed by CT or MRI during follow-up. Recurrence-free survival (RFS) was calculated from the date of surgery to the date of the first documented recurrence, death or last follow-up.
Numerical data are presented as the median (range) or mean ± standard deviation. Differences between groups were compared using Pearson’s χ2 test or the two-tailed Fisher’s exact test for categorical data and the Mann-Whitney U test for numerical data. OS and RFS were determined using the Kaplan-Meier method, and differences between groups were assessed by the log-rank test. To evaluate further the impact of histological subtype on prognosis, univariate analyses of prognostic factors were performed using univariate Cox regression analysis. Among the parameters with P < 0.1 in the univariate analyses, age, serum AFP level, AJCC stage, differentiation grade of the carcinomatous component and histological subtype were used to build a basic multivariate Cox proportional hazard model since they reflect multiple aspects of a patient’s condition. We also adjusted for variables that changed the matched hazard ratio of the histological subtype by at least 5% upon addition into the model[16]. A P value < 0.05 was considered to indicate statistical significance. Kaplan-Meier curves were generated and analyzed using GraphPad Prism 7 (GraphPad Software Inc., San Diego, CA, United States). All other statistical analyses were performed using IBM SPSS 23.0 software (SPSS Inc., Armonk, NY, United States).
A total of 196 patients, including 41 sarcomatoid and 155 high-grade HCC patients, were included in this study, and the clinical and laboratory characteristics are shown in Table 1. In both the sarcomatoid and high-grade HCC groups, the majority of the patients were men (80.5% and 86.5%, P = 0.339), with a median age of 54 years and 55 years (P = 0.942), respectively. During the first hospital visit, sarcomatoid HCC patients had a higher incidence of concomitant symptoms than high-grade HCC patients (78.0% vs 55.5%, P = 0.009), including symptoms of epigastric discomfort (63.4% vs 43.2%), weight loss (34.1% vs 18.1%) and fever (22% vs 2.6%). The etiology of hepatopathy, presence of cirrhosis, Child-Pugh classification and laboratory test results were comparable between the two groups (all P > 0.05). However, serum AFP levels were significantly lower in the sarcomatoid group than in the high-grade group (5.8 ng/mL vs 348.0 ng/mL, P < 0.001).
| Variables | Sarcomatoid HCC, n = 41 | High-grade HCC, n = 155 | P value |
| Age at diagnosis, median (range), yr | 54 (15-74) | 55 (28-81) | 0.942 |
| Sex (male/female) | 33/8 | 134/21 | 0.339 |
| Follow-up duration, median (range), mo | 10.5 (1.7-81.7) | 27.7 (2.1-138.5) | < 0.001 |
| Symptom at diagnosis | 32 (78.0) | 86 (55.5) | 0.009 |
| Alcoholism | 10 (24.4) | 38 (24.5) | 0.987 |
| HBsAg(+) | 29 (70.7) | 124 (80.0) | 0.202 |
| HCV-Ab(+) | 3 (7.3) | 5 (3.2) | 0.368 |
| Liver cirrhosis | 32 (78.0) | 133 (85.8) | 0.226 |
| Child-Pugh class, A/B | 36/5 | 143/12 | 0.359 |
| AFP, median (range), ng/mL | 5.8 (1.0-16660.0) | 348.0 (2.0-63208.5) | < 0.001 |
| AFP level | < 0.001 | ||
| ≤ 20 ng/mL | 26 (63.4) | 33 (21.3) | |
| > 20, < 400 ng/mL | 10 (24.4) | 47 (30.3) | |
| ≥ 400 ng/mL | 5 (12.2) | 75 (48.4) | |
| Creatinine, median (range), mg/dL | 0.77 (0.50-1.11) | 0.76 (0.49-1.58) | 0.475 |
| ALT, median (range), U/L | 26 (10-145) | 32 (8-301) | 0.204 |
| Total bilirubin, median (range), mg/dL | 0.85 (0.33-2.45) | 0.95 (0.33-10.29) | 0.095 |
| Albumin, mean ± SD, g/dL | 3.9 ± 0.5 | 4.0 ± 0.5 | 0.082 |
| INR, median (range) | 1.11 (0.96-1.53) | 1.08 (0.84-1.50) | 0.066 |
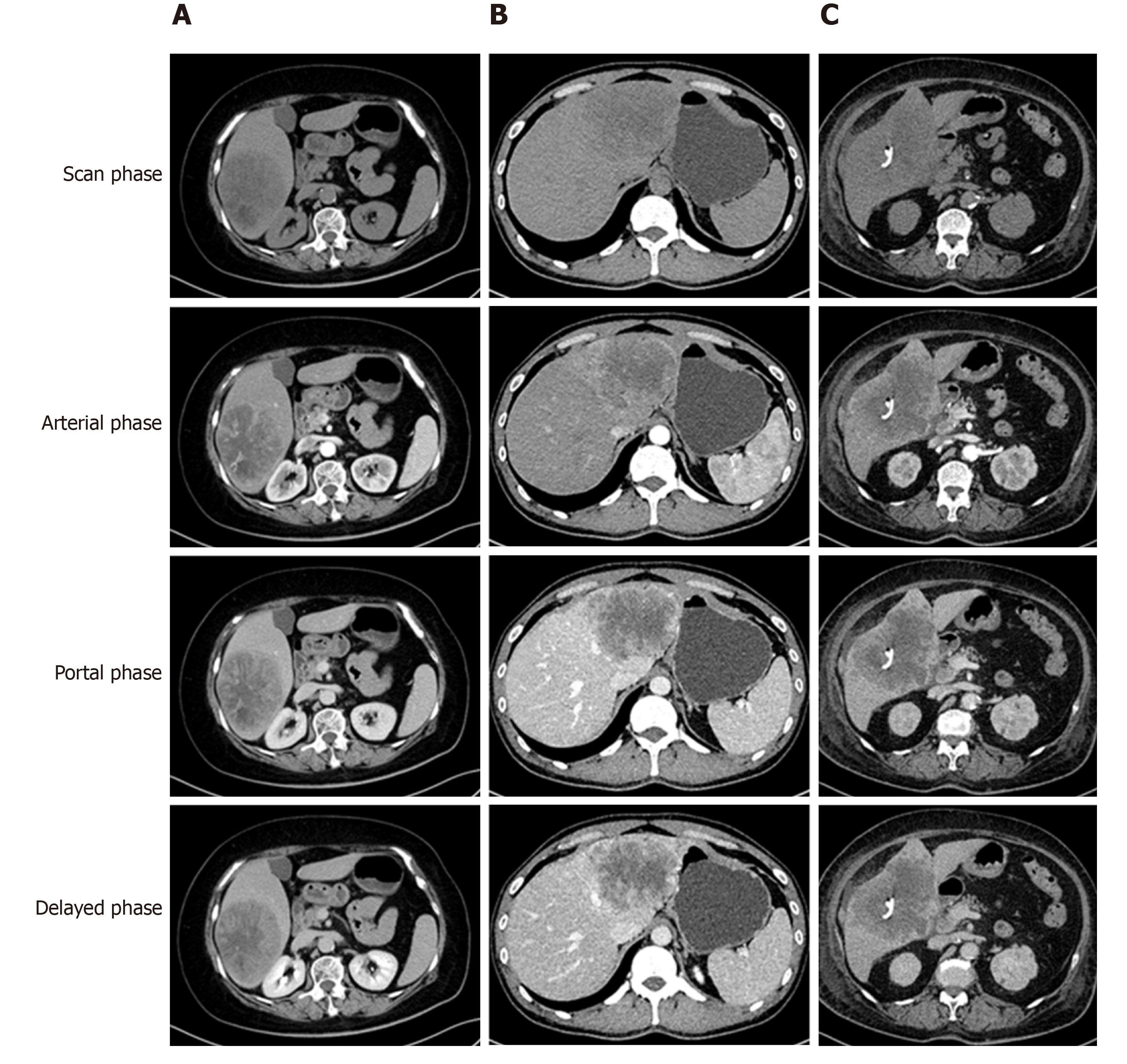
The tumor-specific characteristics are shown in Table 2. At the time of diagnosis, the frequency of sarcomatoid HCC patients presenting with typical dynamic image patterns (arterial phase enhancement and portal and delayed phase washout) was significantly lower than that of patients with high-grade HCC (44.4% vs 72.7%, P = 0.001). Some sarcomatoid HCCs might be misdiagnosed as intrahepatic cholangiocarcinoma (iCCA) or a hepatic abscess (Figure 2). Compared to high-grade HCC patients, sarcomatoid HCC patients had larger tumors, a lower incidence of tumor encapsulation and higher frequencies of tumor necrosis, adjacent organ invasion, lymph node metastasis and advanced AJCC stage (all P < 0.05). In addition, the two groups showed significant differences in the differentiation grades of carcinomatous components (P < 0.05). Patients in the sarcomatoid HCC group showed a trend towards a higher postoperative recurrence rate than those in the high-grade HCC group, but the difference was not significant (P = 0.091).
| Variables | Sarcomatoid HCC, n = 41 | High-grade HCC, n = 155 | P value |
| Tumor size, median (range), cm | 8.6 (1.5-24.0) | 5.5 (1.3-19.0) | 0.007 |
| Tumor number, single/multiple | 25/16 | 108/47 | 0.443 |
| Tumor location | 0.549 | ||
| Left lobe | 11 (26.8) | 34 (21.9) | |
| Right lobe | 28 (68.3) | 106 (68.4) | |
| Both lobes | 2 (4.9) | 15 (9.7) | |
| Tumor encapsulation | 11 (26.8) | 77 (49.7) | 0.009 |
| Tumor necrosis | 35 (85.4) | 53 (34.2) | < 0.001 |
| Spontaneous rupture | 4 (9.8) | 9 (5.8) | 0.477 |
| Adjacent organ invasion | 9 (22.0) | 13 (8.4) | 0.024 |
| Satellite nodules | 6 (14.6) | 25 (16.1) | 0.816 |
| Macrovascular invasion | 7 (17.1) | 34 (21.9) | 0.496 |
| Microvascular invasion | 16 (39.0) | 63 (40.6) | 0.851 |
| Lymph node metastasis | 9 (22.0) | 9 (5.8) | 0.004 |
| Differentiation grade of the carcinomatous component | < 0.001 | ||
| II | 3 (7.3) | 0 (0) | |
| III | 19 (46.4) | 116 (74.8) | |
| IV | 8 (19.5) | 39 (25.2) | |
| NA1 | 11 (26.8) | 0 (0) | |
| AJCC stage | 0.006 | ||
| Stage I | 10 (24.4) | 68 (43.9) | |
| Stage II | 7 (17.0) | 25 (16.1) | |
| Stage III | 15 (36.6) | 53 (34.2) | |
| Stage IV | 9 (22.0) | 9 (5.8) | |
| BCLC stage | 0.543 | ||
| 0 | 0 (0) | 2 (1.3) | |
| A | 19 (46.4) | 95 (61.3) | |
| B | 6 (14.6) | 15 (9.7) | |
| C | 16 (39.0) | 43 (27.7) | |
| Recurrence | 34 (82.9) | 108 (69.7) | 0.091 |
| Recurrence pattern | 34 | 108 | 0.246 |
| Intrahepatic | 14 (41.2) | 62 (57.4) | |
| Extrahepatic | 8 (23.5) | 17 (15.7) | |
| Both | 12 (35.3) | 29 (26.9) | |
| Imaging data at diagnosis | 36 | 139 | |
| Typical dynamic image patterns2 | 16 (44.4) | 101 (72.7) | 0.001 |
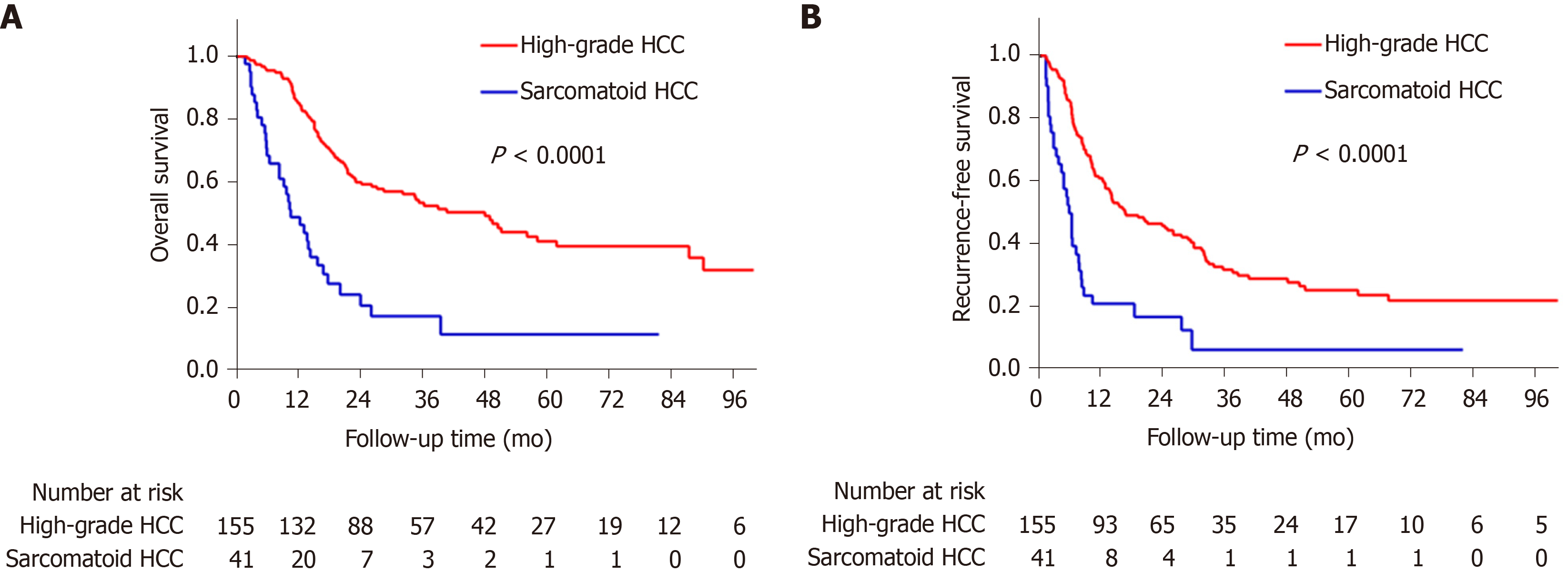
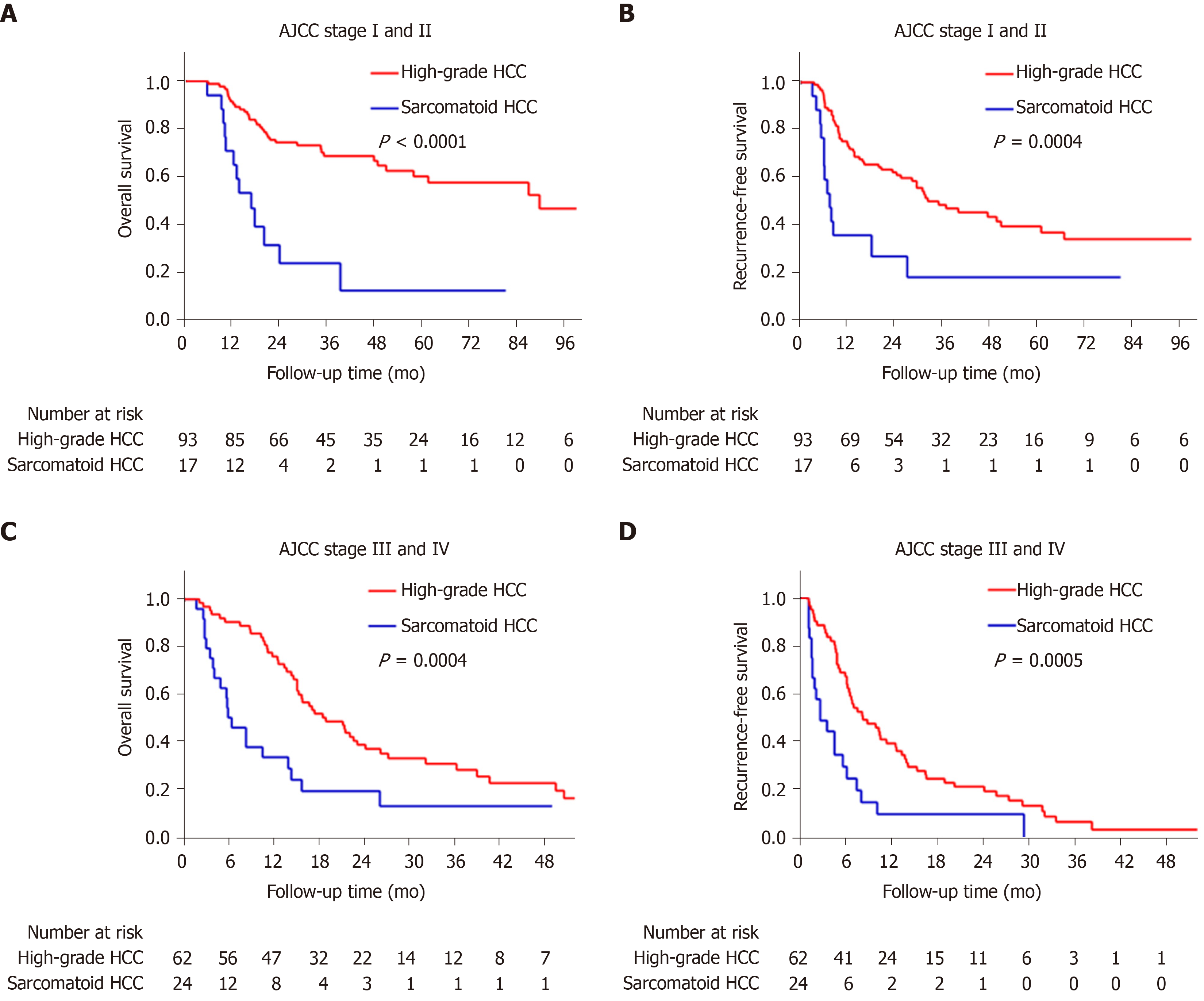
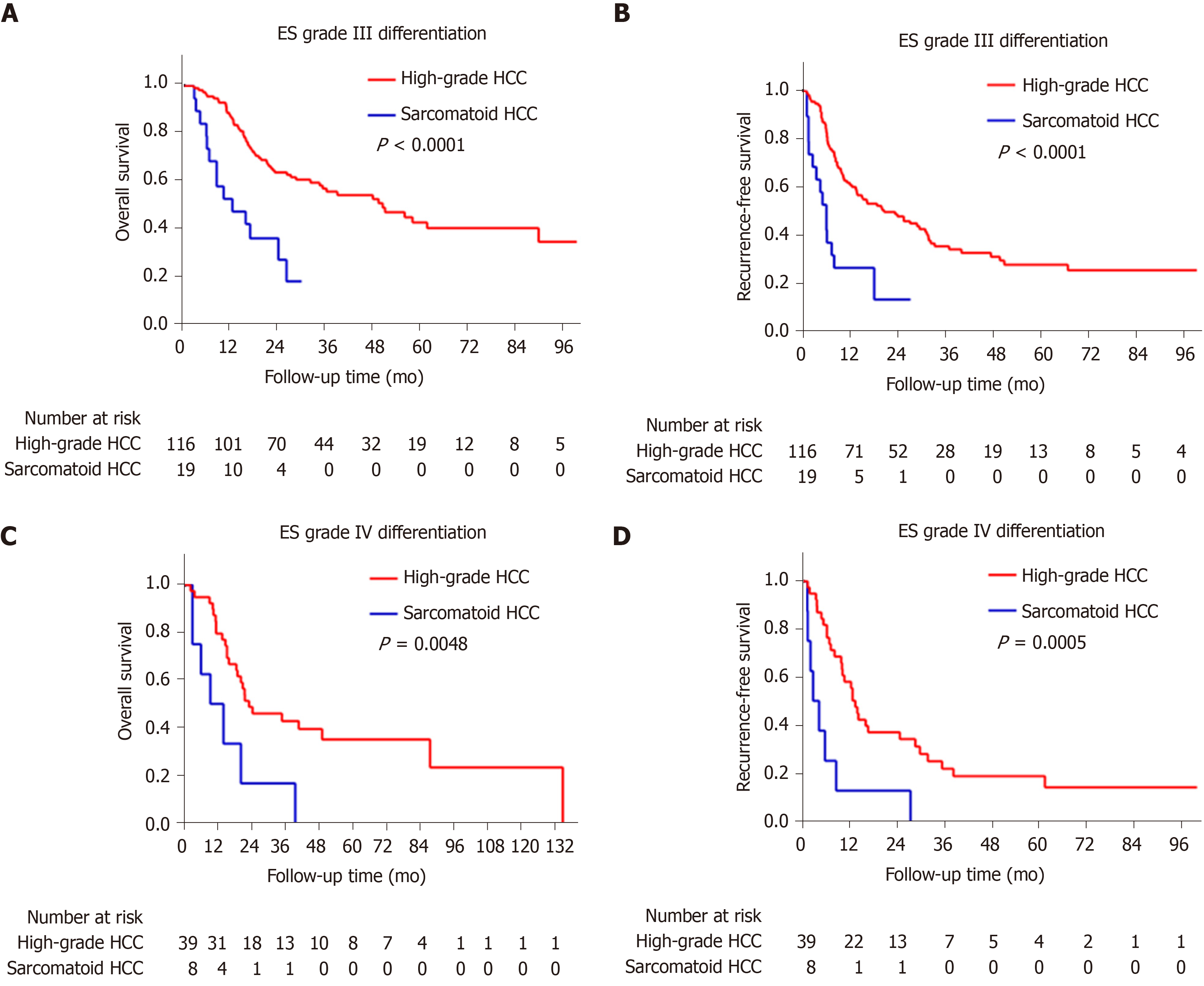
The sarcomatoid HCC patients had a shorter median OS than high-grade HCC patients (10.5 mo vs 48.1 mo, P < 0.0001, Figure 3A). The 1-, 3- and 5-year OS rates were 48.8%, 17.3% and 11.5% for the sarcomatoid group and 85.2%, 53.4% and 41.1% for the high-grade group, respectively. Moreover, the sarcomatoid HCC group had a shorter median RFS than the high-grade HCC group (5.6 mo vs 16.4 mo, P < 0.0001, Figure 3B). The RFS rates post resection were 49.7% and 83.1% at 6 mo, 20.9% and 60.2% at 1 year and 6.3% and 31.8% at 3 years for the sarcomatoid and high-grade groups, respectively. Even after stratification by AJCC stage (Figure 4) or differentiation grade of the carcinomatous component (Figure 5), the sarcomatoid HCC patients still had worse OS and shorter RFS than the high-grade HCC patients in each subgroup (all P < 0.05).
Cox regression analysis was used to verify whether the sarcomatoid type is an independent prognostic factor for HCC patients. Our univariate analysis showed that larger tumors, multiple tumors, a lack of tumor encapsulation, tumor necrosis, macro- and microvascular invasion, adjacent organ invasion, lymph node metastasis, advanced AJCC stage, poorer differentiation of the carcinomatous component and sarcomatoid subtype were significantly associated with increased mortality (Table 3) and recurrence (Table 4) in the total population (all P < 0.05). In addition, age < 55 years, Child-Pugh B and lower serum AFP levels were significantly related to increased mortality (all P < 0.05, Table 3). After controlling for confounding factors, sarcomatoid subtype was identified as an independent predictor of poorer OS and RFS in the multivariable analysis (all P < 0.05, Table 3 and Table 4).
| Variables | HR (95%CI) | P value | HR (95%CI) | P value |
| Age, ≥ 55 yr vs < 55 yr | 0.687 (0.477-0.991) | 0.044 | 0.977 (0.654-1.460) | 0.910 |
| Sex, male vs female | 0.972 (0.579-1.630) | 0.913 | ||
| Hepatitis virus infection, yes vs no | 1.140 (0.718-1.811) | 0.579 | ||
| Alcoholism, yes vs no | 1.101 (0.723-1.677) | 0.653 | ||
| Liver cirrhosis, yes vs no | 1.027 (0.620-1.703) | 0.917 | ||
| Child-Pugh, B vs A | 2.287 (1.281-4.083) | 0.005 | Excluded1 | Excluded |
| AFP, > 20 ng/mL vs ≤ 20 ng/mL | 0.560 (0.386-0.813) | 0.002 | 0.555 (0.377-0.817) | 0.003 |
| Tumor size, > 5 cm vs ≤ 5 cm | 2.718 (1.806-4.089) | < 0.001 | 1.871 (1.146-3.054) | 0.012 |
| Tumor number, multiple vs single | 1.647 (1.129-2.402) | 0.010 | Excluded | Excluded |
| Tumor encapsulation, yes vs no | 0.443 (0.302-0.651) | < 0.001 | Excluded | Excluded |
| Tumor necrosis, yes vs no | 2.237 (1.548-3.233) | < 0.001 | 0.973 (0.622-1.523) | 0.904 |
| Macrovascular invasion, yes vs no | 3.403 (2.256-5.133) | < 0.001 | 2.308 (1.438-3.702) | 0.001 |
| Microvascular invasion, yes vs no | 1.615 (1.115-2.338) | 0.011 | Excluded | Excluded |
| Adjacent organ invasion, yes vs no | 2.329 (1.407-3.857) | 0.001 | Excluded | Excluded |
| Lymph node metastasis, yes vs no | 2.413 (1.371-4.249) | 0.002 | Excluded | Excluded |
| AJCC stage, III + IV vs I + II | 3.221 (2.200-4.715) | < 0.001 | 1.763 (1.085-2.864) | 0.022 |
| Histological subtype, sHCC vs hgHCC | 3.460 (2.283-5.243) | < 0.001 | 3.140 (2.032-4.851) | < 0.001 |
| Differentiation grade of carcinomatous component | Ptrend < 0.001 | Ptrend = 0.421 | ||
| NA2 | 1 (reference) | 1 (reference) | ||
| II | 0.383 (0.083-1.758) | 0.217 | 0.297 (0.062-1.422) | 0.129 |
| III | 0.226 (0.115-0.444) | < 0.001 | 0.644 (0.281-1.475) | 0.298 |
| IV | 0.327 (0.159-0.670) | 0.002 | 0.739 (0.311-1.755) | 0.494 |
| Variables | HR (95% CI) | P value | HR (95% CI) | P value |
| Age, ≥ 55 yr vs < 55 yr | 0.743 (0.533-1.035) | 0.079 | 0.857 (0.605-1.214) | 0.386 |
| Sex, male vs female | 0.771 (0.493-1.206) | 0.254 | ||
| Hepatitis virus infection, yes vs no | 1.369 (0.879-2.133) | 0.165 | ||
| Alcoholism, yes vs no | 0.940 (0.634-1.394) | 0.759 | ||
| Liver cirrhosis, yes vs no | 1.283 (0.790-2.084) | 0.314 | ||
| Child-Pugh, B vs A | 1.699 (0.938-3.078) | 0.081 | Excluded1 | Excluded |
| AFP, > 20 ng/mL vs ≤ 20 ng/mL | 0.710 (0.501-1.006) | 0.054 | 0.680 (0.469-0.986) | 0.042 |
| Tumor size, > 5 cm vs ≤ 5 cm | 2.398 (1.685-3.413) | < 0.001 | 1.680 (0.469-0.986) | 0.015 |
| Tumor number, multiple vs single | 2.058 (1.460-2.902) | < 0.001 | 1.210 (0.824-1.777) | 0.331 |
| Tumor encapsulation, yes vs no | 0.561 (0.400-0.786) | 0.001 | Excluded | Excluded |
| Tumor necrosis, yes vs no | 1.726 (1.239-2.404) | 0.001 | Excluded | Excluded |
| Macrovascular invasion, yes vs no | 2.922 (1.961-4.354) | < 0.001 | Excluded | Excluded |
| Microvascular invasion, yes vs no | 1.646 (1.178-2.300) | 0.003 | Excluded | Excluded |
| Adjacent organ invasion, yes vs no | 2.329 (1.445-3.753) | 0.001 | Excluded | Excluded |
| Lymph node metastasis, yes vs no | 2.327 (1.356-3.992) | 0.002 | Excluded | Excluded |
| AJCC stage, III + IV vs I + II | 3.416 (2.420-4.821) | < 0.001 | 2.752 (1.879-4.032) | < 0.001 |
| Histological subtype, sHCC vs hgHCC | 2.842 (1.913-4.222) | < 0.001 | 2.355 (1.564-3.546) | < 0.001 |
| Differentiation grade of carcinomatous component | Ptrend = 0.036 | Ptrend = 0.194 | ||
| NA2 | 1 (reference) | 1 (reference) | ||
| II | 1.001 (0.264-3.790) | 0.999 | 0.650 (0.162-2.603) | 0.543 |
| III | 0.445 (0.215-0.922) | 0.029 | 1.605 (0.672-3.832) | 0.287 |
| IV | 0.642 (0.298-1.380) | 0.256 | 2.025 (0.822-4.988) | 0.125 |
Sarcomatoid HCC is composed of both sarcomatous and carcinomatous components (Figure 6); however, it remains unknown which component is the primary contributor to the highly aggressive nature of sarcomatoid HCC. To explore further this, the histological composition of lymph node metastases, macrovascular invasions, bile duct invasions and multiple liver tumor lesions of sarcomatoid HCCs were analyzed. Nine patients showed a total of 33 lymph node metastases. Of these, 26 (78.8%) metastases contained purely carcinomatous components, two (6.1%) were purely sarcomatous and five (15.1%) had mixed carcinomatous and sarcomatous components. Seven macrovascular invasions and two bile duct invasions were observed in eight patients (one patient had both macrovascular and bile duct invasion). Six of these invasions were evaluated by pathological examination, and only two (33.3%) had sarcomatous components. A total of 16 patients were confirmed to have multiple liver tumors (10 patients with multinodular HCC and six with satellite nodules). Of the 10 patients with multinodular HCC, only two (20.0%) had concurrent sarcomatoid HCC, whereas the others had simultaneous sarcomatoid and conventional HCC. In addition, of the six patients with satellite nodules, sarcomatous changes were found in only two patients (33.3%).
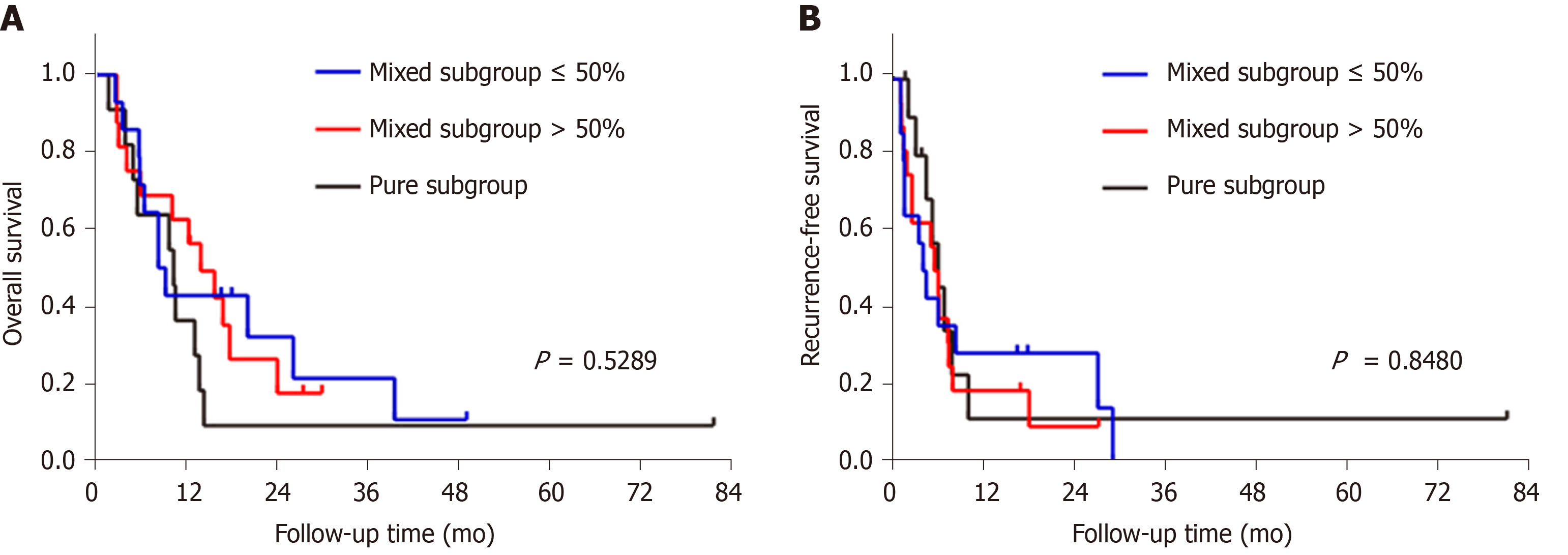
Next, we divided the sarcomatoid HCC patients into three subgroups according to the proportion of sarcomatous components in the tumor: (1) Mixed subgroup ≤ 50% (n = 14 patients); (2) Mixed subgroup > 50% (n = 16); and (3) Pure subgroup (n = 11). The OS and RFS were similar among these subgroups (Figure 7).
Sarcomatoid HCC is a rare histological subtype of HCC, with an incidence of approximately 2% of surgically resected cases[3,8]. A few studies have reported that sarcomatoid HCC is more aggressive than conventional HCC and associated with a worse prognosis[2,3,8,12]. However, sarcomatoid HCC usually has a worse differentiation grade[12], which is regarded as a prognostic factor for HCC, and this fact can lead to inaccurate comparisons between sarcomatoid HCC and conventional HCC. In this study, high-grade HCC, which is thought to be similar to sarcomatoid HCC in terms of histological differentiation, was used as the control in a detailed examination of the clinicopathological characteristics and surgical outcomes of sarcomatoid HCC. Moreover, this study analyzed the histological composition of metastatic and invasive sarcomatoid HCCs.
Our results demonstrate that sarcomatoid HCC is more frequently diagnosed at an advanced AJCC stage with relatively larger tumors and higher rates of adjacent organ invasion and lymph node metastasis. During the first visit to the hospital, sarcomatoid HCC patients have a higher incidence of epigastric discomfort, weight loss and fever than high-grade HCC patients. In particular, the incidence of fever was nearly 10 times higher in the sarcomatoid HCC group than in the high-grade HCC group, perhaps due to the higher frequency of tumor necrosis caused by the relatively larger size and faster progression of sarcomatoid HCC tumors. Imaging plays a critical role in HCC diagnosis. There are typical dynamic image patterns considered specific for HCC[17]. However, more than 60% of sarcomatoid HCCs show MRI features more similar to iCCA than to HCC[18]. In our study, only 44.4% of sarcomatoid HCC patients presented with typical dynamic image patterns of HCC, and some patients with sarcomatoid HCC may be misdiagnosed with iCCA or a hepatic abscess. Consistent with the results of previous studies[8,12], our findings show that the majority of patients with sarcomatoid HCC had a history of chronic viral hepatitis and liver cirrhosis, which are key risk factors for the development of HCC[19]. Regular ultrasound screening of these high-risk patients could help detect tumors at an early stage and reduce the risk of death[19,20]. Of note, only 36.6% of sarcomatoid HCC patients had serum AFP levels > 20 ng/mL in the current study. Therefore, serum AFP tests may have no use for the early detection of sarcomatoid HCC. Moreover, both serologic and radiologic examinations could not help in distinguishing the sarcomatoid subtype from conventional HCC or other intrahepatic masses, and this might be problematic when selecting a potential recipient of liver transplantation because the sarcomatoid subtype usually predicts a poor prognosis[2].
Previous studies have demonstrated that patients with resected sarcomatoid HCC have a worse RFS than those with resected conventional HCC[2,8]. Similar to the findings of previous studies, we found that the median RFS of sarcomatoid HCC patients was significantly shorter than that of high-grade HCC patients (5.6 mo vs 16.4 mo, P < 0.0001). A previous study from Taiwan indicated that sarcomatoid HCC is more prone to extrahepatic metastasis than conventional HCC after curative therapy[12]. Nevertheless, in our study, there was no difference in the recurrence pattern between the sarcomatoid and high-grade HCC groups, perhaps due to their similar histological differentiation. Several previous studies have also indicated that the OS of sarcomatoid HCC patients is significantly worse than that of conventional HCC patients, with a 3-year OS rate ranging from 8.0% to 17.5%[2,8,12,21]. Consistent with previous studies, the current study showed that patients with resected sarcomatoid HCC had an elevated risk of death, with an abysmal 3-year OS compared with the prognosis of resected high-grade HCC patients (17.3% vs 53.4%, P < 0.0001). Even after controlling for confounding factors, the association between sarcomatoid HCC and worse RFS and OS persisted. These results strongly suggest that sarcomatoid HCC is more aggressive than high-grade HCC, although they are similar in terms of histological differentiation.
The histogenesis of sarcomatous tissue in cancers, including HCC, has not yet been elucidated. The most widely accepted theory is the conversion theory, which postulates that the sarcomatous element derives from the carcinoma during tumor evolution[3,9,22-25]. Several recent studies indicate that epithelial-mesenchymal transition is an important mechanism underlying the sarcomatous change, which further confirms the conversion theory[26-28]. Sarcomatoid HCCs comprise variable proportions of sarcomatous and carcinomatous components. A previous study reported no survival difference between mixed and pure sarcomatoid HCC patients[12]. However, the proportion of sarcomatous tissue in some patients could not be precisely evaluated because of pathological diagnosis by biopsy[12]. In our study, all patients with sarcomatoid HCC underwent surgical resection and were divided into three subgroups according to the proportion of the sarcomatous component. Our results indicated that the OS and RFS were similar among the three subgroups, suggesting that the proportion of the sarcomatoid component is not associated with prognosis.
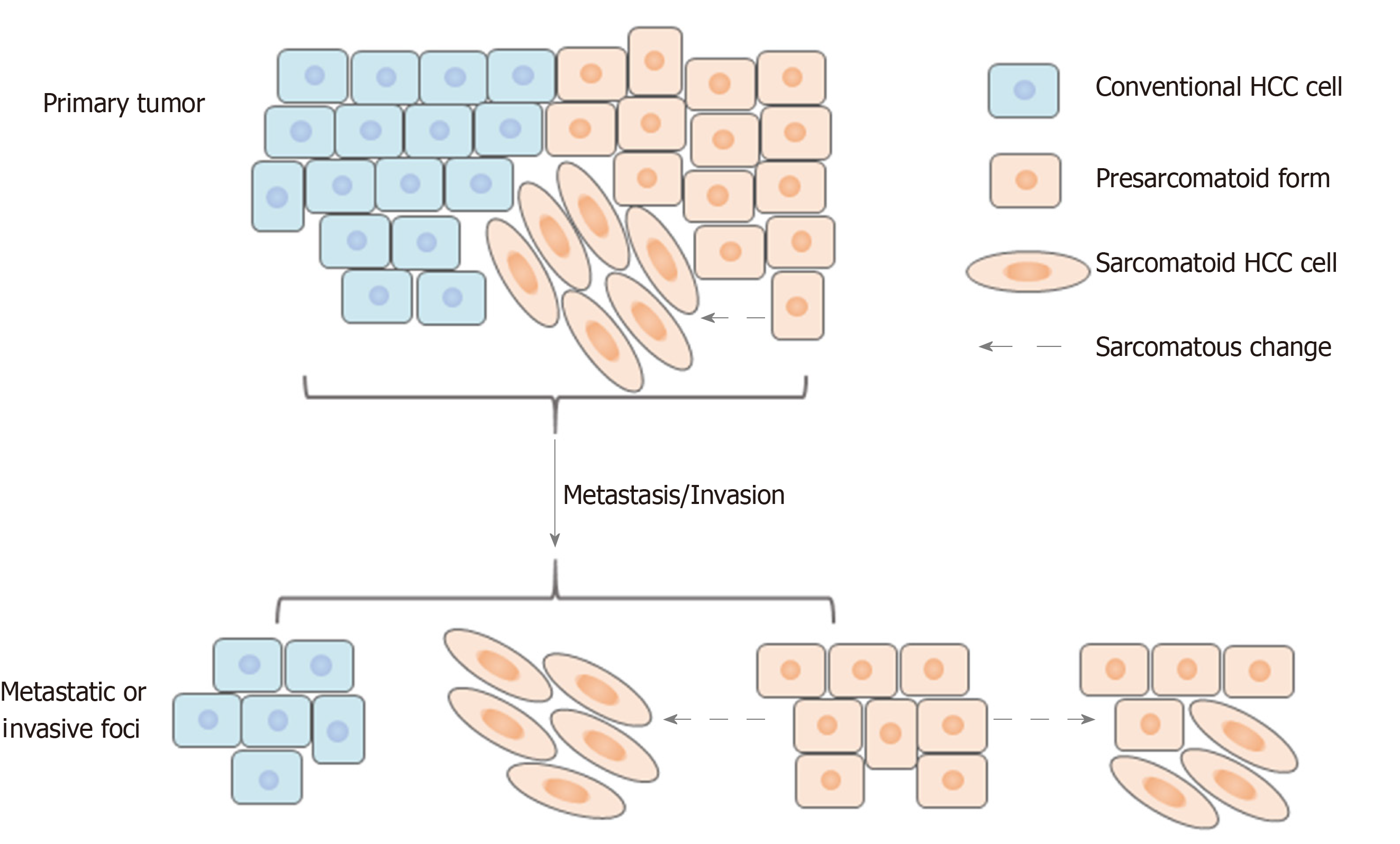
Several previous studies demonstrated that sarcomatoid HCC is associated with a high frequency of portal venous invasion and metastasis[3,11,29,30]; our study showed a higher rate of lymph node metastasis but not of venous invasion. However, few studies have explored the related mechanisms, and only Maeda et al[3] have reported the composition of portal venous invasions and metastases. They studied 13 cases of sarcomatoid HCC treated by surgical resection and found that most portal venous invasions and metastases had sarcomatous components, which were postulated to be responsible for metastasis. Contrary to their results, our study demonstrated that the majority of lymph node metastases, macrovascular/bile duct invasions and satellite nodules were composed of carcinomatous components. This apparent inconsistency may be because in Maeda’s study, five (38%) patients underwent preoperative treatment, such as TACE, and their analysis included an autopsied case with extensive postoperative metastases comprising sarcomatoid components[3]. In line with our findings, however, they showed that the metastatic lymph nodes in two patients comprised carcinomatous components. In addition, some invasions and metastases had mixed carcinomatous and sarcomatous components. Based on these findings, we speculate that the carcinomatous component might be the dominant factor mediating the highly aggressive nature of sarcomatoid HCC. Furthermore, the carcinomatous components might exist in a “presarcomatoid” state[31], in which the tumor cells have cellular morphology typical of conventional HCC but greatly enhanced invasive ability. As a result, sarcomatous changes might occur after invasion or metastasis in some cases (Figure 8). This could also explain the phenomenon of sarcomatoid HCC being associated with more frequent lymph node metastasis and poorer prognosis than high-grade HCC even when they are similar in terms of histological differentiation. Further studies focusing on the underlying molecular pathogenesis of sarcomatoid HCC are urgently needed to understand better its highly aggressive biological features.
There are limitations of our study. First, this was a retrospective study conducted in a single center, and randomized studies should be performed in multiple centers. Second, although this study presented an analysis of one of the largest series of sarcomatoid HCC cases, the relatively small number of patients may have influenced the accuracy of the results, and additional studies with more cases should be performed. Third, in this study, we discovered that carcinomatous components occupied the predominant proportion of invasions and metastases and developed a hypothesis regarding the occurrence of sarcomatoid HCC. The related mechanisms were not further explored, and future studies should address these issues.
In conclusion, compared with high-grade HCC, sarcomatoid HCC is associated with more advanced AJCC stage, an atypical dynamic image pattern and lower serum AFP levels. Patients with sarcomatoid HCC have significantly worse RFS and OS than those with high-grade HCC. Furthermore, the highly aggressive nature of sarcomatoid HCC seems to be mediated by its carcinomatous components.
Hepatocellular carcinoma (HCC) is the sixth most common type of cancer and the fourth leading cause of cancer-related death worldwide. Sarcomatoid HCC, which contains poorly differentiated carcinomatous and sarcomatous components, is a rare histological subtype of HCC that differs from conventional HCC. It is highly aggressive and has a poor prognosis. Its clinicopathological characteristics, surgical outcomes and underlying mechanisms of its highly aggressive nature have not been fully elucidated.
A few studies have reported that sarcomatoid HCC is associated with a higher recurrence rate, more frequent metastasis and poorer survival than conventional HCC. However, these studies did not further stratify conventional HCC into low- [Edmondson-Steiner (ES) grade I and II] and high-grade (ES grade III and IV) HCC; in particular, high-grade HCC is considered similar to sarcomatoid HCC in terms of histological differentiation, more aggressive nature and poor prognosis. In addition, although sarcomatoid HCC has a high incidence of adjacent organ invasion and metastasis, the underlying mechanisms remain unknown. One previous study reported that most portal venous invasions and metastases had sarcomatous components, indicating that the sarcomatous component is responsible for metastasis. However, in the study, five (38%) patients underwent preoperative treatment, such as TACE, and their analysis included an autopsied case with extensive postoperative metastases comprising sarcomatoid components. Therefore, the results might be biased. In view of the deficiencies of previous studies, we will conduct further studies on sarcomatoid HCC.
To examine the clinicopathological characteristics and surgical outcomes of sarcomatoid HCC and explore the histogenesis of sarcomatoid HCC.
In total, 196 patients [41 sarcomatoid HCC and 155 high-grade (ES grade III or IV) HCC] who underwent surgical resection between 2007 and 2017 were retrospectively reviewed. The characteristics and surgical outcomes of sarcomatoid HCC were compared with those of patients with high-grade HCC. The histological composition of invasive and metastatic sarcomatoid HCCs was evaluated.
Sarcomatoid HCC was more frequently diagnosed at an advanced stage with a larger tumor and higher rates of nonspecific symptom, adjacent organ invasion and lymph node metastasis than high-grade HCC (all P < 0.05). Compared with high-grade HCC patients, sarcomatoid HCC patients are less likely to have typical dynamic imaging features of HCC (44.4% vs 72.7%, P = 0.001) and elevated serum alpha-fetoprotein levels (> 20 ng/mL; 36.6% vs 78.7%, P < 0.001). The sarcomatoid group had a significantly shorter median recurrence-free survival (5.6 mo vs 16.4 mo, log-rank P < 0.0001) and overall survival (10.5 mo vs 48.1 mo, log-rank P < 0.0001) than the high-grade group. After controlling for confounding factors, the sarcomatoid subtype was identified as an independent predictor of poor prognosis. Pathological analyses indicated that invasive and metastatic lesions were mainly composed of carcinomatous components.
Sarcomatoid HCC was associated with a more advanced stage, atypical dynamic imaging, lower serum alpha-fetoprotein levels and a worse prognosis. The highly aggressive nature of sarcomatoid HCC is perhaps mediated by carcinomatous components.
Studies focusing on the underlying molecular pathogenesis of sarcomatoid HCC are urgently needed to understand better its highly aggressive biological features.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rauchfuss F, Guerra DL S-Editor: Wang JL L-Editor: Filipodia E-Editor: Ma YJ
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55814] [Article Influence: 7973.4] [Reference Citation Analysis (132)] |
| 2. | Hwang S, Lee SG, Lee YJ, Ahn CS, Kim KH, Park KM, Moon KM, Moon DB, Ha TY, Yu ES, Choi GW. Prognostic impact of sarcomatous change of hepatocellular carcinoma in patients undergoing liver resection and liver transplantation. J Gastrointest Surg. 2008;12:718-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Maeda T, Adachi E, Kajiyama K, Takenaka K, Sugimachi K, Tsuneyoshi M. Spindle cell hepatocellular carcinoma. A clinicopathologic and immunohistochemical analysis of 15 cases. Cancer. 1996;77:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 4. | Kojiro M, Sugihara S, Kakizoe S, Nakashima O, Kiyomatsu K. Hepatocellular carcinoma with sarcomatous change: a special reference to the relationship with anticancer therapy. Cancer Chemother Pharmacol. 1989;23 Suppl:S4-S8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 100] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Koda M, Maeda Y, Matsunaga Y, Mimura K, Murawaki Y, Horie Y. Hepatocellular carcinoma with sarcomatous change arising after radiofrequency ablation for well-differentiated hepatocellular carcinoma. Hepatol Res. 2003;27:163-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Lu J, Zhang J, Xiong XZ, Li FY, Ye H, Cheng Y, Zhou RX, Lin YX, Cheng NS. Primary hepatic sarcomatoid carcinoma: clinical features and prognosis of 28 resected cases. J Cancer Res Clin Oncol. 2014;140:1027-1035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Yu Y, Zhong Y, Wang J, Wu D. Sarcomatoid hepatocellular carcinoma (SHC): a case report. World J Surg Oncol. 2017;15:219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Lu J, Xiong XZ, Li FY, Ye H, Lin YX, Zhou RX, Cai YL, Jin YW, Cheng NS. Prognostic Significance of Sarcomatous Change in Patients with Hepatocellular Carcinoma After Surgical Resection. Ann Surg Oncol. 2015;22 Suppl 3:S1048-S1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Kakizoe S, Kojiro M, Nakashima T. Hepatocellular carcinoma with sarcomatous change. Clinicopathologic and immunohistochemical studies of 14 autopsy cases. Cancer. 1987;59:310-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 10. | Yamaguchi R, Nakashima O, Yano H, Kutami R, Kusaba A, Kojiro M. Hepatocellular carcinoma with sarcomatous change. Oncol Rep. 1997;4:525-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Watanabe J, Nakashima O, Kojiro M. Clinicopathologic study on lymph node metastasis of hepatocellular carcinoma: a retrospective study of 660 consecutive autopsy cases. Jpn J Clin Oncol. 1994;24:37-41. [PubMed] |
| 12. | Liao SH, Su TH, Jeng YM, Liang PC, Chen DS, Chen CH, Kao JH. Clinical Manifestations and Outcomes of Patients with Sarcomatoid Hepatocellular Carcinoma. Hepatology. 2019;69:209-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 13. | Han DH, Choi GH, Kim KS, Choi JS, Park YN, Kim SU, Park JY, Ahn SH, Han KH. Prognostic significance of the worst grade in hepatocellular carcinoma with heterogeneous histologic grades of differentiation. J Gastroenterol Hepatol. 2013;28:1384-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Martins-Filho SN, Paiva C, Azevedo RS, Alves VAF. Histological Grading of Hepatocellular Carcinoma-A Systematic Review of Literature. Front Med (Lausanne). 2017;4:193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 113] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 15. | EDMONDSON HA, STEINER PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7:462-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 16. | Kernan WN, Viscoli CM, Brass LM, Broderick JP, Brott T, Feldmann E, Morgenstern LB, Wilterdink JL, Horwitz RI. Phenylpropanolamine and the risk of hemorrhagic stroke. N Engl J Med. 2000;343:1826-1832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 457] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 17. | de Lope CR, Tremosini S, Forner A, Reig M, Bruix J. Management of HCC. J Hepatol. 2012;56 Suppl 1:S75-S87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 484] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 18. | Seo N, Kim MJ, Rhee H. Hepatic sarcomatoid carcinoma: magnetic resonance imaging evaluation by using the liver imaging reporting and data system. Eur Radiol. 2019;29:3761-3771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 3025] [Article Influence: 432.1] [Reference Citation Analysis (3)] |
| 20. | Choi DT, Kum HC, Park S, Ohsfeldt RL, Shen Y, Parikh ND, Singal AG. Hepatocellular Carcinoma Screening Is Associated With Increased Survival of Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2019;17:976-987.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 21. | Wu L, Tsilimigras DI, Farooq A, Hyer JM, Merath K, Paredes AZ, Mehta R, Sahara K, Shen F, Pawlik TM. Management and outcomes among patients with sarcomatoid hepatocellular carcinoma: A population-based analysis. Cancer. 2019;125:3767-3775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 22. | Shin P, Ohmi S, Sakurai M. Hepatocellular carcinoma combined with hepatic sarcoma. Acta Pathol Jpn. 1981;31:815-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | McCluggage WG. Malignant biphasic uterine tumours: carcinosarcomas or metaplastic carcinomas? J Clin Pathol. 2002;55:321-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 253] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 24. | Lester TR, Hunt KK, Nayeemuddin KM, Bassett RL, Gonzalez-Angulo AM, Feig BW, Huo L, Rourke LL, Davis WG, Valero V, Gilcrease MZ. Metaplastic sarcomatoid carcinoma of the breast appears more aggressive than other triple receptor-negative breast cancers. Breast Cancer Res Treat. 2012;131:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Kim DK, Kim BR, Jeong JS, Baek YH. Analysis of intrahepatic sarcomatoid cholangiocarcinoma: Experience from 11 cases within 17 years. World J Gastroenterol. 2019;25:608-621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Kim HM, Kim H, Park YN. Sarcomatoid cholangiocarcinoma with osteoclast-like giant cells associated with hepatolithiasis: A case report. Clin Mol Hepatol. 2015;21:309-313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Sung CO, Choi H, Lee KW, Kim SH. Sarcomatoid carcinoma represents a complete phenotype with various pathways of epithelial mesenchymal transition. J Clin Pathol. 2013;66:601-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Yen CH, Lai CC, Liao CC, Wang SF, Liao YJ, Tung CY, Hung JH, Huang SF, Chen YA. Characterization of a new murine cell line of sarcomatoid hepatocellular carcinoma and its application for biomarker/therapy development. Sci Rep. 2017;7:3052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Eriguchi N, Aoyagi S, Okuda K, Hara M, Fukuda S, Tamae T, Ohdo M, Kanazawa N, Kawabata M, Kodama T, Nishimura K, Hamada S. Unusual liver carcinomas with sarcomatous features: analysis of four cases. Surg Today. 2001;31:530-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Honda H, Hayashi T, Yoshida K, Takenaka K, Kaneko K, Fukuya T, Tateshi Y, Ro T, Maeda T, Masuda K. Hepatocellular carcinoma with sarcomatous change: characteristic findings of two-phased incremental CT. Abdom Imaging. 1996;21:37-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Kuroda N, Tamura M, Hes O, Michal M, Shuin T, Toi M, Hayashi Y, Lee GH. Chromophobe renal cell carcinoma with prominent lymph node metastasis and polysomy of chromosome 21: poorly differentiated form or "presarcomatoid" form? Med Mol Morphol. 2011;44:168-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |