Published online Jul 28, 2020. doi: 10.3748/wjg.v26.i28.4055
Peer-review started: February 3, 2020
First decision: February 27, 2020
Revised: May 24, 2020
Accepted: June 18, 2020
Article in press: June 18, 2020
Published online: July 28, 2020
Processing time: 175 Days and 15 Hours
In 2018, the pan-Janus kinase (JAK) inhibitor tofacitinib was launched for the treatment of ulcerative colitis (UC). Although tofacitinib has proven efficacious in patients with active UC, it failed in patients with Crohn’s disease (CD). This finding strongly hints at a different contribution of JAK signaling in both entities. Here, we review the current knowledge on the interplay between the JAK/signal transducer and activator of transcription (STAT) pathway and inflammatory bowel diseases (IBD). In particular, we provide a detailed overview of the differences and similarities of JAK/STAT-signaling in UC and CD, highlight the impact of the JAK/STAT pathway in experimental colitis models and summarize the published evidence on JAK/STAT-signaling in immune cells of IBD as well as the genetic association between the JAK/STAT pathway and IBD. Finally, we describe novel treatment strategies targeting JAK/STAT inhibition in UC and CD and comment on the limitations and challenges of the new drug class.
Core tip: The pan-Janus kinase (JAK)-inhibitor tofacitinib is efficacious in patients with active ulcerative colitis (UC) but not Crohn’s disease (CD), which hints at different contributions of JAK-signaling in both entities. In this review, available data on differential JAK/signal transducer and activator of transcription (STAT)-signaling in UC and CD were analyzed. The literature review identified differential cell-subset specific JAK/STAT-signaling including increased T-cell-associated STAT1 signaling in CD and STAT6 signaling in UC, while in myeloid cells inflammatory STAT1 was increased in UC compared with CD indicating a less inflammatory role of myeloid cells in CD. Development of JAK/STAT-inhibitors with specific targeting of associated inflammatory pathways might further improve the efficacy and safety profiles of this drug class.
- Citation: Cordes F, Foell D, Ding JN, Varga G, Bettenworth D. Differential regulation of JAK/STAT-signaling in patients with ulcerative colitis and Crohn’s disease. World J Gastroenterol 2020; 26(28): 4055-4075
- URL: https://www.wjgnet.com/1007-9327/full/v26/i28/4055.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i28.4055
Inflammatory bowel diseases (IBD) comprise the entities ulcerative colitis (UC) and Crohn`s disease (CD) as well as unclassified IBD, which are chronic remitting diseases characterized by intestinal inflammation and the risk of uncontrolled disease activity which may lead to severe complications such as fistulas and strictures in CD, and colorectal neoplasia in both entities[1-5]. Despite the plethora of available medical treatment options for IBD, treatment of patients is still complex and often challenging due to loss of response as well as adverse events including opportunistic infections[6-8]. Population based studies report that 46% of patients with CD and 14% of patients with UC are still being treated with systemic corticosteroids for more than 6 months to achieve remission[9,10].
The introduction of biological therapy has improved the spectrum of anti-inflammatory treatment. Nevertheless, the induction and maintenance of remission can still be challenging due to primary non response and secondary loss of response to biological therapy. Indeed, approximately one third of patients with CD and UC were classified as primary non-responders, and up to 50% of patients with IBD had a secondary loss of response to biological therapy or had to stop treatment due to severe side effects[6,11]. Anti-tumor necrosis factor (TNF) therapy has the risk of immunogenicity including provocation of an immune response with occurrence of neutralizing antibodies towards anti-TNFα resulting in secondary loss of response. These observations clearly underline the need for new therapeutic agents with improvement of tolerability and long-term efficacy[12].
Targeting cytokine signaling is already a proven therapeutic strategy as blockade of TNFα has been shown to have good efficacy in the treatment of IBD. However, aside from TNFα, a wide range of cytokines are crucially involved in maintaining intestinal inflammation and reveal a major role in the pathogenesis of IBD[13]. Thus, modulation of several IBD-associated cytokines simultaneously appears to be a promising therapeutic target in IBD[14-16]. Janus kinases (JAKs) mediate intracellular signaling of various cytokines and growth factors[17-20].
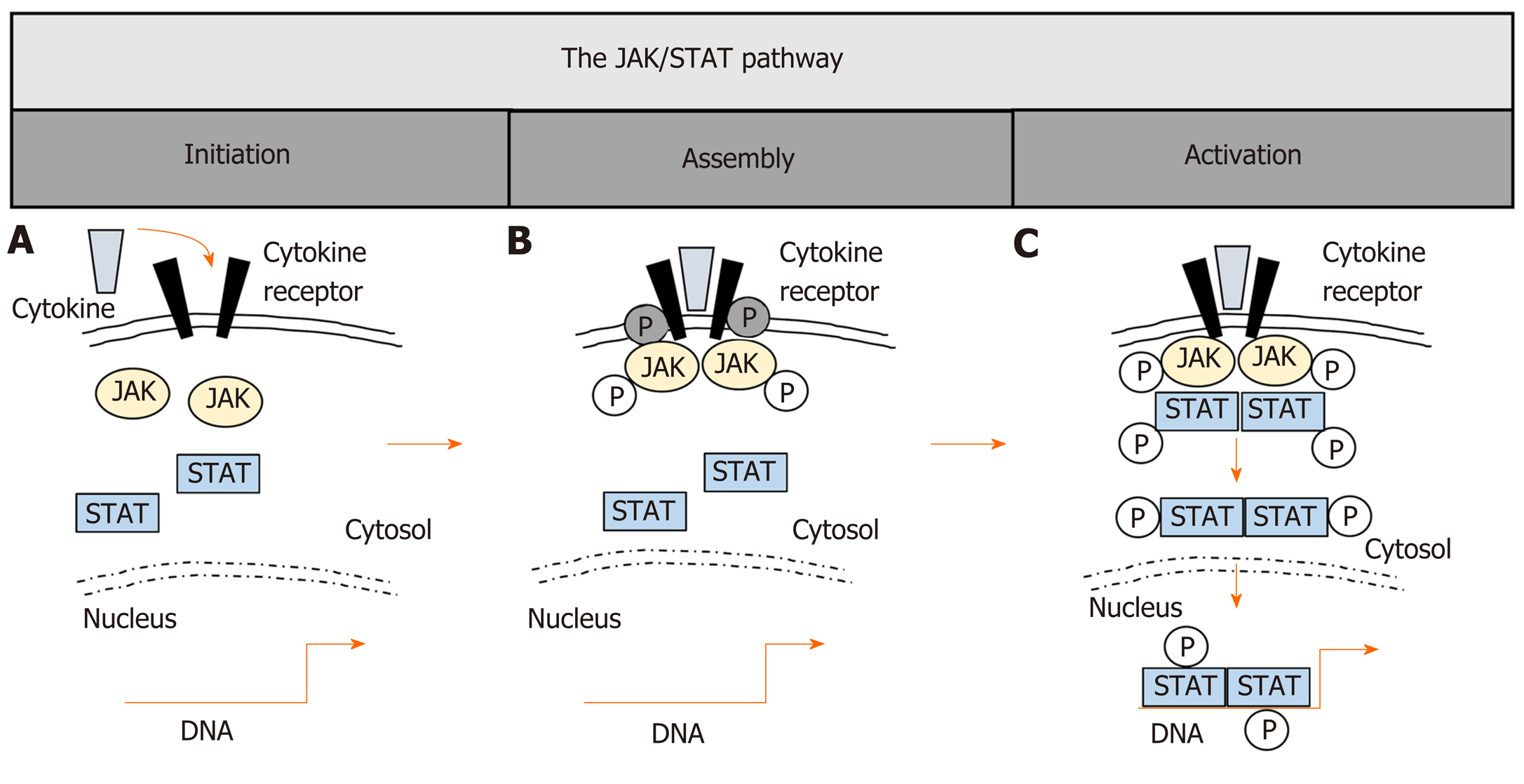
JAKs are potent therapeutic targets and blockade can potentially interfere with more than 50 cytokines[17,18]. JAKs are constitutively bound to cytokine receptors and are crucial in biological responses, mediating signals via signal transducers and activators of transcription (STATs)[21-23]. Four different JAK molecules (JAK1, JAK2, JAK3 and tyrosine kinase (TYK) 2) and seven members of the STAT family (STAT1, 2, 3, 4, 5a, 5b, 6) are known[18,24-27]. STAT5a and STAT5b represent two proteins with almost identical amino acids but are encoded by different genes[28]. Binding of cytokines to their receptors mainly activates specific JAKs and subsequent STATs as final initiators of JAK signaling and can lead to specific cellular responses[29-31]. The general mechanisms of JAK/STAT-signaling are summarized in Figure 1. Due to the fact that JAK/STAT-signaling is utilized by various cytokines, these pathways have become prominent targets for simultaneous inhibition of multiple pro-inflammatory cytokines[32-34]. Nevertheless, targeting JAK/STAT-signaling is highly complex as overlapping JAK/STAT activation by various cytokines with induction of more than one specific downstream signaling pathway is known[35-37]. In detail, similar JAK/STAT components can be activated by varying cytokines of related receptor families, which include cytokines of the interferon (IFN) receptor family i.e., type I/II/III IFNs, IL-10, IL-19, IL-20, IL-22), the common γ-chain receptor family (i.e., IL-2, IL-4 IL-7, IL-9, IL-15, IL-21), the gp130 receptor family (i.e., IL-6, IL-11, IL-12, IL-23), the common β-chain receptors (i.e., GM-CSF, IL-3, IL-5) and the single chain receptor family (i.e., Epo, GH)[29,30,38]. Besides these classical canonical pathways, which include subsequent JAK/STAT activation, non-canonical pathways with independent activation of either JAKs or STATs for signal transduction have been described[39-41]. Furthermore, JAKs can also activate other downstream targets separate to the classical STATs[42]. It is incompletely understood how specific signaling can be achieved regarding the complexity of JAK/STAT activation, but a cell subtype-associated specificity of JAK/STAT-signaling has been suggested[31] as activation of the same STATs can lead to partially opposing effects in cells of the innate compared to the adaptive immune system. For instance, STAT3 mediates regulatory signaling in epithelial or myeloid cells[43], while in lymphocytes, STAT3 activation results in predominantly pro-inflammatory responses such as Th17 differentiation and inhibition of regulatory T-cells[44-46], which further underlines the great plasticity and complexity of JAK/STAT-signaling in regulatory or inflammatory responses of different cell compartments.
The JAK/STAT pathway has been associated with several immune-mediated diseases besides its important impact in cellular signaling[47-50]. The pan-JAK inhibitor tofacitinib results in predominant inhibition of JAK1/JAK3 at adequate dosage[51,52], leading to inhibited JAK-associated intracellular signaling of various cytokines and growth factors[17,18] and was first developed and approved as a synthetic disease-modifying anti-rheumatic drug (DMARD)[53-56] for the treatment of moderate to severe rheumatoid arthritis (RA) in 2017 throughout Europe[53-55,57]. Due to its clinical efficacy in RA, tofacitinib was subsequently investigated as a treatment option for IBD[58-61].
In a recently published phase III RCT, tofacitinib demonstrated efficacy for induction and maintenance of remission in patients with UC[61], which led to its approval for treatment of moderate-to-severe UC[62]. More specifically, 40.6% of patients treated with 10 mg tofacitinib twice daily achieved remission at week 52 as the primary endpoint as compared to 11.1% of patients treated with placebo[61]. Of note, in patients with CD, tofacitinib did not reach its primary endpoints including clinical response or remission at week 26 of maintenance in a phase IIb clinical trial[58]. In detail, clinical response or remission rates for treatment with 10 mg twice daily were 55.8% compared to 38.1% with placebo treatment, which was not significantly different. The lack of clinical response to tofacitinib treatment in patients with CD was further confirmed by the observation of worsening disease activity in 19.3% of patients treated with 10 mg twice daily in an open-label 48-week extension phase II clinical trial[63] which led to premature termination of the trial. However, in the phase IIb trial, biomarkers of inflammation such as C-reactive protein were different in both cohorts with increasing levels in the placebo group and stable levels in the treatment group indicating a likely treatment effect of tofacitinib even in patients with CD[58]. There is ongoing debate regarding the possibility of lack of therapeutic efficacy being explained by an unusually high placebo rate and the small sample size of the study. On the other hand, the lack of superiority of tofacitinib over placebo in patients with CD in clinical trials might also reflect different pathogenic roles of JAK/STAT-signaling in UC and CD[64,65].
Previous data from our own group demonstrated that a more regulatory monocyte phenotype was induced by tofacitinib at adequate dosage under inflammatory conditions[66]. In line with the observations from clinical trials, the regulatory impact of tofacitinib was stronger in monocytes derived from patients with UC as compared to CD[66]. These observations led to the hypothesis that the JAK/STAT pathway might be differentially activated in UC, mediating stronger inflammatory responses compared to CD. Therefore, we summarize and discuss the available evidence on JAK/STAT-signaling in patients with UC and CD in different cell compartments as well as the genetic association and further discuss the clinical implications.
The JAK/STAT pathway has a crucial impact on the regulation of T-cell differentiation. Additionally, dysregulated JAK/STAT-signaling leading to aberrant T-cell differentiation as well as defective regulatory T-cell activity has been suggested as important in IBD pathogenesis[43,67-70]. Excessive T-cell activation and infiltration of the colonic mucosa has been observed in both CD and UC[70,71], but the precise role of T-cells in both phenotypes is still a matter of debate. Historically, aberrant Th1 differentiation and associated cytokines (like IFNγ and IL-2) were predominantly associated with the pathogenesis of CD[72] and Th2-cell-associated cytokines with UC[73]. However, this concept has partially been abandoned as the contribution of different T-cell subsets infiltrating the intestinal mucosa is far more complex and has been demonstrated as a core player in the pathogenesis of both entities[69,74,75]. Differences in the JAK/STAT-signaling in T-cells obtained from UC and CD have also been described, which may lead to similar but incongruent T-cell differentiation in both entities.
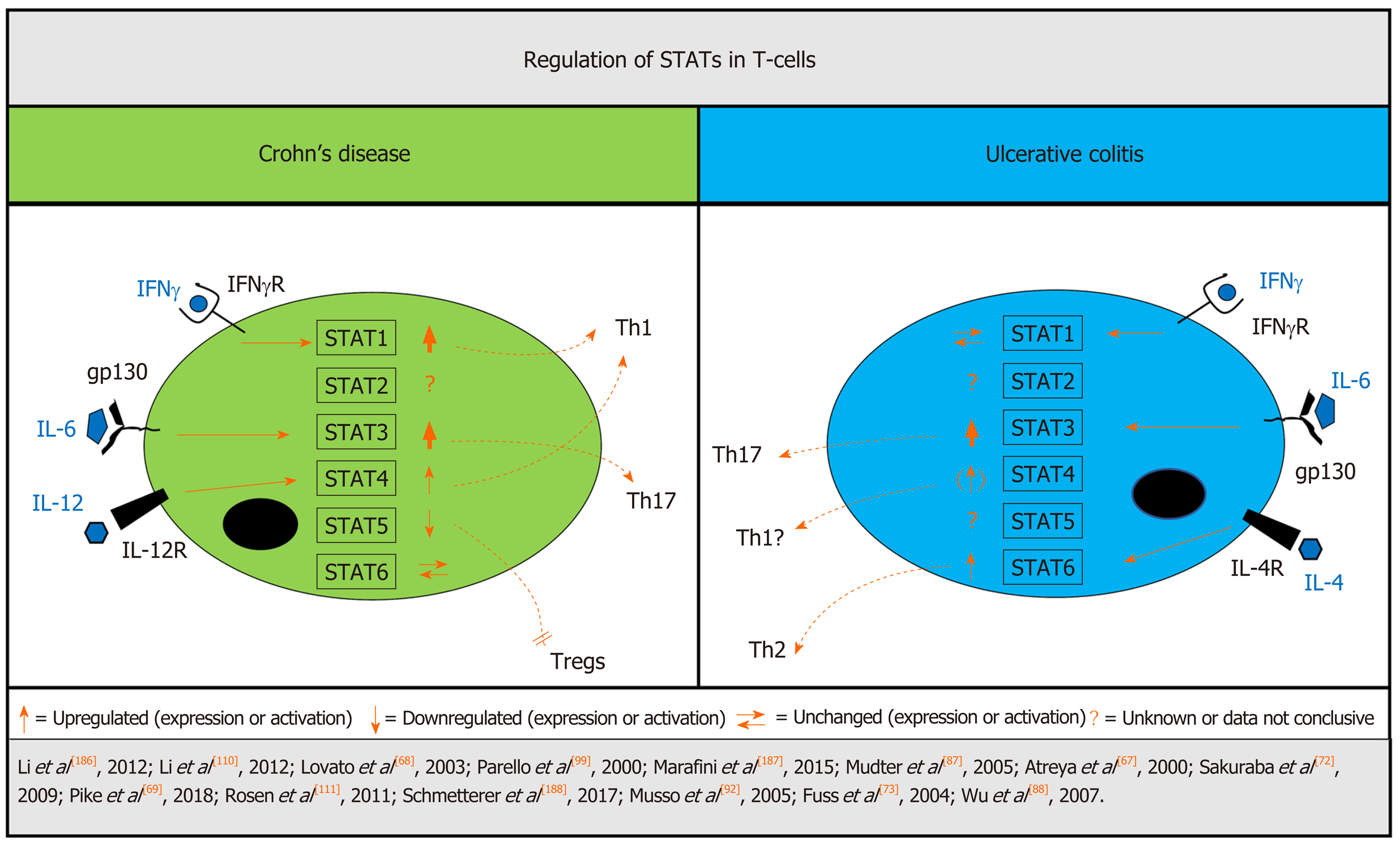
Most studies assess STAT-signaling as a surrogate marker for JAKs. Before we focus on IBD-related aberrant STAT-signaling in the T-cell compartment, we summarize the impact of JAK/STAT-signaling on T-cells in experimental colitis. Available data on T-cell attributed STAT expression/activation in UC and CD has also been summarized in Figure 2.
T-cell-associated IFNγ induced a reduction in epithelial integrity and Paneth cells in mouse crypt enteroids, and led to exacerbation of radiation colitis[76]. This effect could be abolished by JAK1,2 inhibition indicating the important impact of the JAK/STAT pathway in T-cell-mediated impairment of the intestinal barrier. Furthermore, several anti-inflammatory molecules ameliorating disease activity in experimental colitis are associated with modulation of JAK/STAT-signaling in lymphocytes. In detail, decreased expression or activation of JAK1 and JAK2[77], STAT1[77], STAT3[77-79] and STAT4[77,79] as well as enhancement of STAT5[78] phosphorylation in T-cells, have been linked to effective pharmacological treatment of DSS-induced murine colitis and TNBS-induced rat colitis. Additionally, inflammatory activity was reduced by general STAT1 knockout in DSS-treated STAT1-null mice[80] and by specific inhibition of STAT1 in T-cells in TNBS-induced colitis[81]. Furthermore, knockdown of TYK2 ameliorated DSS-induced and TNBS-induced murine colitis possibly due to reduced Th1 and Th17 activity[82]. In addition, in murine colitis, STAT6/Th2-linked cytokine IL-4 is associated with reduced Treg induction[83,84]. In contrast to this, STAT6-deficient mice were shown to be protected against experimental colitis, partially due to reduced Th2 cytokines[85].
STAT1 is closely linked to IFNγ-receptor signaling and has an important involvement in Th1 differentiation[86]. Mudter et al[87] found increased IFNγ-induced STAT1 but not phospho-STAT1 in lamina propria T-cells of CD compared to patients with UC. Similarly, elevated gene expression of STAT1 as well as that of other IFNγ-Th1-related genes was detected by Wu et al[88] in colonic biopsies of patients with CD but not UC. Of note, the latter study did not provide cell specific investigations or analysis of STAT1 on protein level. Taken together, these data confirm an important role for STAT1-associated Th1 response in CD.
STAT2 is associated with type I IFN signaling[89]. So far, only a trend towards decreased STAT2 gene expression in lamina propria mononuclear cells (LPMC) from patients with active UC and CD has been reported[87] without further available data on STAT2 signaling in IBD.
In contrast, the impact of STAT3 in T-cell differentiation has been widely investigated. JAK1, JAK2 and TYK2 are involved in the activation of STAT3[29] and increased STAT3 activation and signaling contributes to pathogenic Th17 differentiation and promotion of inflammation[45], and is involved in the pathogenesis of UC and CD[74,75]. However, STAT3 has also been linked to IL-10-dependent regulatory features of Tregs[90,91] and the definitive role of STAT3 in T-cell regulation and its contribution to IBD has not been finally elucidated. Various studies on T-cell-associated STAT3 signaling have demonstrated increased levels of STAT3 and phospho-STAT3 in mucosal tissue samples from patients with UC and CD[67,68,87,92]. Furthermore, Jin et al[93] performed transcriptomic and proteomic analyses of colonic biopsies and demonstrated that the inflammatory response of the IL-6-JAK/STAT3 signaling pathway was positively enriched in CD and UC samples. T-cells represent a large population of immune cells in the intestinal mucosa[94] but whether these data could be attributed to only T-cells is not clear, as no cell compartment specific analysis was performed. Specific analyses of STAT3 signaling in CD-derived intestinal T-cells revealed constitutive STAT3 activation[68] with IL-6-attributed induction of downstream anti-apoptotic genes[67]. Furthermore, impaired STAT3-regulating mechanism has been identified in IBD. In a healthy environment, the phosphatase DUSP2 is induced by activation of T-cells and inhibits STAT3. Impaired DUSP2 expression is associated with enhanced STAT3 signaling and Th17 induction[45]. It is noteworthy that DUSP2 has been demonstrated to be decreased in peripheral blood samples derived from patients with UC and active disease flare[45]. However, no direct comparison with CD patients has been performed to date and no studies have investigated the role of DUSP2 in CD to date. Another STAT3-regulating factor is the protein tyrosine phosphatase PTPN2, which is linked to prevention of auto-reactivity in the context of T-cell homeostasis by STAT3 dephosphorylation[95]. Deficiency of PTPN2 is associated with autoimmunity by increased lymphocyte activation[96] and PTPN2 genetic risk locus with loss of function has been linked to CD[95]. Taken together, impaired direct or indirect STAT3 regulation leading to increased STAT3 activation seems to be involved in both UC and CD pathogenesis, even though underlying mechanisms might partially differ.
The role of STAT4 in T-cells has been well described and various studies demonstrated an IL-12-triggered STAT4-dependent development of IFNγ-secreting Th1-cells[97,98]. Available data on STAT4 in colonic tissue samples from patients with IBD are controversial; while increased IL-12-dependent expression and activation of STAT4 in T-cells from colonic mucosa was found in CD[68] associated with IL-12-dependent Th1 polarization[99], no increased T-cell-associated STAT4 signaling was detected in patients with UC[99]. In contrast to these studies, increased STAT4 was found in the mucosa of adult[100] and pediatric patients with UC but not in patients with CD[101]. However, subtype analyses of intestinal cells were not performed in the latter studies, thus data can only speculatively be linked to STAT4 signaling in T-cells. In summary, published evidence of increased T-cell-associated STAT4 signaling in CD is strong, emphasizing the important role of Th1 response in CD pathogenesis. In UC, increased STAT4 signaling was also detected, but direct linkage to T-cells was not investigated: Thus, Th1 response may also play a distinct role in intestinal inflammation in UC but still remains a matter of debate.
STAT5 is mainly linked to induction of regulatory FoxP3+T-cells (Tregs)[102] and limitation of Th17 differentiation via IL-2[103], while Treg development is negatively controlled by STAT3[104] and thus disruption of the STAT3/STAT5 balance might shift T-cell differentiation towards Th17 development[46]. Tregs can limit autoimmunity, but in IBD, Tregs are not able to control inflammation due to increased induction and differentiation of effector T-cells[105,106]. Direct data on STAT5 in IBD T-cells are scarce. In CD, α4β7+CD4+cells, which are strongly associated with gut-homing lymphocytes, show decreased induction of pSTAT5 in response to IL-2, while pSTAT3+cells were increased after specific stimulation[107]. However, data on T-cell-associated STAT5 signaling in UC are still missing.
STAT6 is associated with Th2 differentiation[108,109]. Although Mudter et al[87] found no increased STAT6 expression in LPMCs in IBD patients, there was increased activation of STAT6 in colonic tissue of inactive UC[110] as well as specifically in LPMCs in UC, in contrast to lower levels in CD[111]. It is noteworthy that T-cells represent the largest immune cell population of LPMCs; however, no specific T-cell analysis was performed. Nevertheless, both studies point to the overactivation of STAT6 in UC, which further underlines the importance of the Th2 response in UC pathogenesis, while in CD the role of STAT6 remains unclear.
Monocytes are central to our health as uncontrolled and sustained inflammation can lead to auto-inflammatory syndromes and sometimes to autoimmune diseases. Monocytes can be a driving force in such diseases when their ability to also contribute to the resolution of inflammation is impaired. Therefore, anti-inflammatory mechanisms of monocytes, are of vast importance for downregulation and resolution of inflammation[112-115]. As an example, we recently demonstrated that GM-CSF-activated monocytes also have regulatory capabilities such as in the induction of Tregs from naïve T–cells in in vitro co-cultures thus leading to amelioration of experimental colitis in mice in vivo[116,117]. Furthermore, in IBD, monocytes are key players and function as effectors of inflammation[118-120], presumably because most intestinal macrophages are replenished by peripheral monocytes from the circulation[121,122]. In the intestine, recruited monocytes immediately adapt to the local environment. When they replenish intestinal monocytes during homeostasis, they become tolerogenic, but they differentiate into inflammatory drivers in the presence of intestinal inflammation[123] which turns monocytes into important targets for treatment.
JAK/STAT-signaling does play decisive roles in monocyte-differentiation and regulation of monocyte-activation[66,124]. For example, de Vries et al[124] demonstrated that JAK1/STAT1 inhibition resulted in a prominent switch of macrophages from pro-inflammatory M1-like to a more regulatory M2-like phenotype, which leads to an enhanced recovery in acute rescue DSS-colitis. Similarly, JAK1/3 inhibition dose-dependently leads to a predominant M2-like phenotype shift of human monocytes derived from healthy volunteers and patients with IBD accompanied by an increased anti-inflammatory potential reflected by enhanced Treg induction[66].
In monocytes, STAT1 activation via i.e., IFNγR is believed to be strongly inflammatory[125,126]. Schreiber et al[127] showed that patients with active UC had significantly higher levels of STAT1 expression and activation in colonic tissue compared to active CD, which could be predominantly attributed to infiltrating peripheral neutrophils and monocytes/macrophages. Among other regulatory mechanisms, STAT activation is inhibited by proteins of the suppressor of cytokine signaling (SOCS) family[128-131] and SOCS3 and SOCS1 are involved in STAT1 regulation. Indeed, Schreiber and colleagues[127] further detected that increased STAT1 activation was associated with distinctly lower mucosal SOCS3 protein levels in UC compared to patients with CD. In contrast, Soendergaard et al[132] found SOCS1 and SOCS3 mucosal RNA level to be upregulated in an inflammation-dependent manner in UC compared to controls; however, no patients with CD were included in this study and direct comparison is missing.
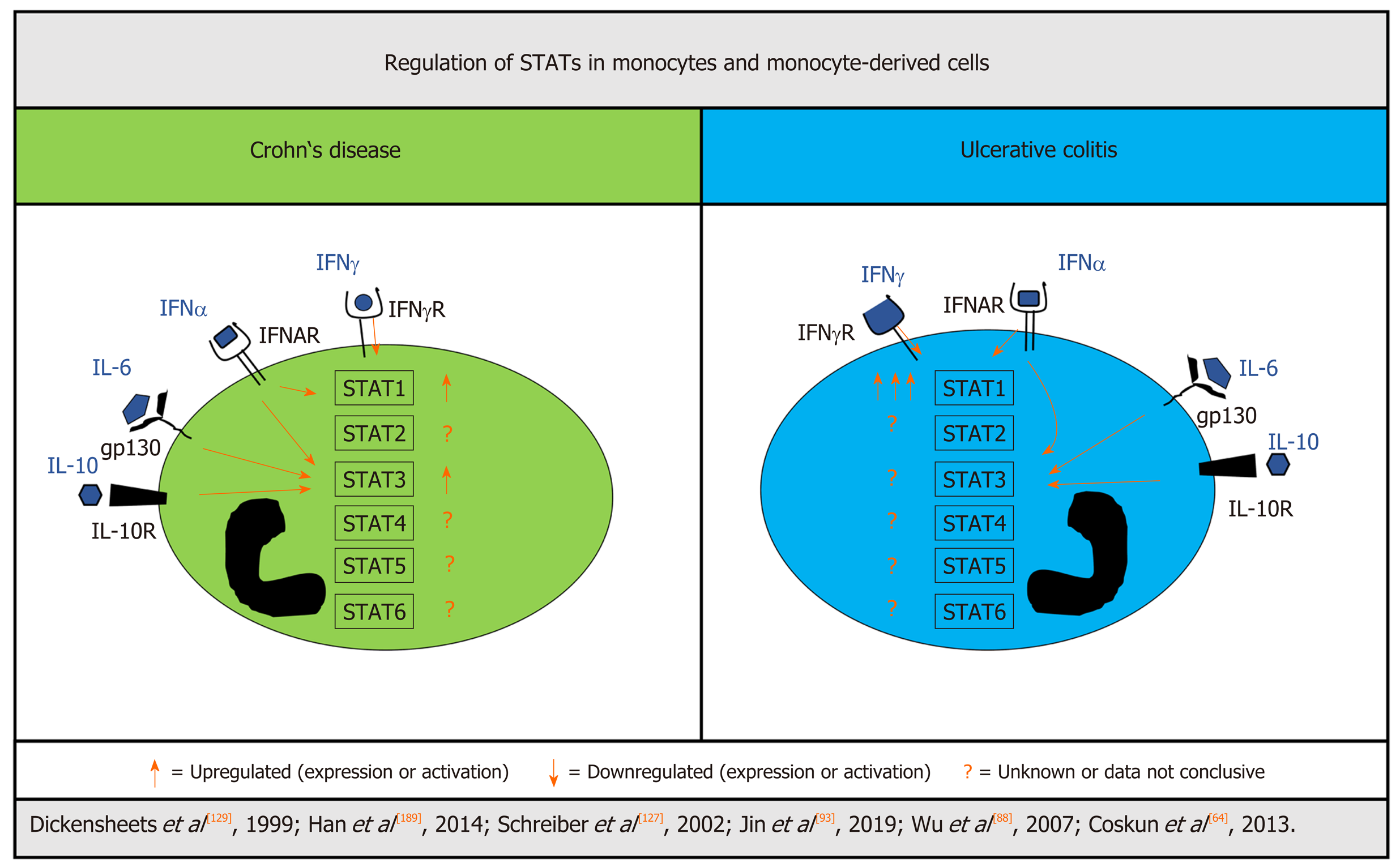
Opposite to STAT1, STAT3 is associated with a more protective role in myeloid cells against inflammation and seems to be important for mucosal homeostasis[43,133,134]. In their mouse study, Takeda et al[43] genetically disrupted STAT3 in macrophages and neutrophils resulting in chronic enterocolitis induced by high levels of pro-inflammatory cytokines. Furthermore, the suppressive effects of IL-10 on macrophages and neutrophils were completely abolished in mice lacking STAT3 in these cells, further emphasizing the importance of STAT3 signaling in the regulation of inflammation[43]. Depending on the environmental stimuli, monocytes, when leaving the bloodstream, can differentiate into macrophages and dendritic cells (DCs). An analysis of STAT activation in subsets of DCs from patients with CD revealed enhanced IL-10-induced STAT3-signaling in myeloid DCs[135]. The study did not include patients with UC, so the role of STAT3 in UC-derived DCs remains unclear. Monocyte-specific expression of other STAT members including STAT2, STAT4, STAT5 and STAT6 has not yet been studied in IBD in detail. Available data on STAT expression/activation in monocytes and –derived cells in UC and CD are summarized in Figure 3.
Taken together, the contribution of JAK/STAT in IBD-derived myeloid cells in driving inflammation cannot be elucidated yet due to the scarcity of available data. However, STAT1 seems to be particularly increased in monocytes/macrophages in UC but not CD, which is possibly linked to an insufficient mechanism to regulate STAT1 signaling, and hints at a more pro-inflammatory phenotype of monocy-tes/macrophages in UC compared to CD.
For IBD, more than 250 genome susceptibility loci could be specified, of which some reveal an association with the JAK/STAT pathway[136-139]. However, systematic analysis and comparison of the JAK/STAT components and associated genetic risk loci in UC and CD are scarce. We therefore summarize the available studies on JAK/STAT-associated susceptibility genes in IBD and elucidate the similarities and differences in UC vs CD in the next paragraphs and in Table 1[136,138-158].
| Ulcerative colitis | Crohn’s disease | |
| JAK1 | No association found to date | No association found to date |
| JAK2 | Yang et al[140], 2011 | Yang et al[140], 2011 |
| Barrett et al[136], 2008 | Barrett et al[136], 2008 | |
| Wellcome trust case control | Wellcome trust case control | |
| Consortium[138], 2007 | Consortium[138], 2007 | |
| Anderson et al[141], 2009 | Anderson et al[141], 2009 | |
| Zhang et al[142], 2014 | Zhang et al[142], 2014 | |
| Hedl et al[143], 2016 | Hedl et al[143], 2016: N/A | |
| Cleynen et al[144], 2013: N/A | Cleynen et al[144], 2013 | |
| Ferguson et al[145], 2010: N/A | Ferguson et al[145], 2010 | |
| Prager et al[146], 2014: No association | Prager et al[146], 2014 | |
| JAK3 | No association found to date | No association found to date |
| TYK2 | Can et al[147], 2015 | Can et al[147], 2015 |
| Lian et al[148], 2013 | Lian et al[148], 2013: No association | |
| Sato et al[149], 2009: No association | Sato et al[149], 2009 | |
| STAT1 | No association found to date | No association found to date |
| STAT2 | No association found to date | No association found to date |
| STAT3 | Barrett et al[136], 2008 | Barrett et al[136], 2008 |
| Wellcome Trust Case Control | Wellcome Trust Case Control | |
| Consortium[138], 2007 | Consortium[138], 2007 | |
| Anderson et al[150], 2011 | Anderson et al[150], 2011 | |
| Willson et al[151], 2012: N/A | Willson et al[151], 2012 (pediatric patients) | |
| Zhang et al[142], 2014 | Zhang et al[142], 2014 | |
| Prager et al[146], 2014: No association | Prager et al[146], 2014 | |
| STAT4 | Glas et al[152], 2010: No association | Glas et al[152], 2010 |
| Diaz-Gallo et al[153], 2010 | Diaz-Gallo et al[153], 2010: No association | |
| Liu et al[139], 2015 | Liu et al[139], 2015: No association | |
| STAT5 | Huang et al[154], 2015 (STAT5A/STAT3 haplotypes): No association | Huang et al[154], 2015 (STAT5A/STAT3 haplotypes) |
| STAT6 | Klein et al[155], 2015: No association | Klein et al[155], 2015 |
| Xia et al[156], 2003: No association | Xia et al[156], 2003: No association | |
| Chua et al[157], 2016: No association | Chua et al[157], 2016: No association | |
| de Jong et al[158], 2003: No association | de Jong et al[158], 2003: No association |
To date, the genetic risk loci associated with JAK1 have not been identified in IBD. This appears reasonable, as JAK1-deficient mice are non-viable[159,160], which underlines the fundamental involvement of JAK1 in cell signaling as essential for survival[160,161]. Similarly, JAK3-linked genetic risk loci have not been detected in IBD so far. Patients suffering from genetic defects in JAK3 develop severe combined immunodeficiency without specificity for IBD[161,162].
In contrast, an association of JAK2 gene variants with both UC and CD has been clearly shown in various studies[136,138,140]. Especially the IBD risk locus rs10758669 within the JAK2 region has been widely investigated. Alongside associations with both UC and CD[136,141,142], gain of function of this gene variant including increased JAK2 signaling in macrophages of patients with UC and healthy donors carrying the rs10758669 CC genotype was shown[143]. Furthermore, in CD, the JAK2 variant rs10758669 was associated with a more complicated disease course and shorter time interval to stenosis occurrence[144,145]. Increased intestinal permeability was detected in patients with CD with the C risk allele within this JAK2 variant, which might hint at JAK2 being involved in increasing permeability in IBD as a possible pathomechanism[146].
The available data of TYK2 gene polymorphism and IBD association are rare and divergent. Although TYK2 has been identified as a susceptibility gene for both UC and CD in the Turkish population[147], in Malaysian patients no association between CD and TYK2 genes has been detected[148]. Conversely, in the Japanese population, a strong association of TYK2 with CD, but not UC was found[149].
Contrary to STAT1/STAT2, for which no genetic association for UC or CD has been found to date, STAT3 gene variants have been distinctly associated with susceptibility for both, CD and UC[136,138,150,163]. Willson et al[151] analyzed STAT3 genetic variant rs744166 and demonstrated that pediatric patients with CD carrying STAT3 “A” risk allele revealed enhanced STAT3 activation in intestinal tissue and increased signaling linked to intestinal leukocyte homing. A meta-analysis including 10298 patients with CD and 4244 patients with UC, which further evaluated this STAT3 variant, confirmed the (A) allele to increase susceptibility for both UC and CD[164]. Of note, Caucasian carriers of the (A) allele were more susceptible to UC and CD as compared to other ethnicities[164].
Available data on STAT4 genetic variants linked with UC or CD are divergent. Jostins et al[137] detected an increased association of STAT4 polymorphisms with IBD in general without specific association with either UC or CD. Glas et al[152] investigated STAT4 genetic variant rs7574865 and found an association with colonic disease manifestation and early onset of disease in CD without increased UC susceptibility. Conversely, two studies identified an association of rs7574865 with increased risk for UC but not CD[139,153].
Specific polymorphisms in the STAT5 gene locus have not been detected for IBD to date. However, Huang and colleagues[154] identified that STAT5A/STAT3 haplotypes were generally linked to IBD and to CD.
The data on STAT6 genetic variants and IBD susceptibility are also rare: In a small study, comprising 243 patients with CD and 100 patients with UC, Klein et al[155] detected a link between STAT6 gene risk locus G2964A (rs324015) and the subgroup of patients with CD, which revealed no variation in the CARD15 gene. However, the association of CD with STAT6 polymorphism rs324015 could not be confirmed by other studies[156-158], which suggests that STAT6 gene variants may not have an important involvement in IBD susceptibility.
A distinct genetic association has been shown for specific protein tyrosine phosphatases (PTPs) which are involved in regulation of JAK/STAT-signaling. Among those, PTPN22, which is linked to the regulation of STAT1 and Th1[69,165-167], has been detected as a risk gene for CD susceptibility without an association to UC[137,150]. Surprisingly, PTPN22 variant rs33996649 was identified as protective against CD, while it is associated with an increased risk for other autoimmune diseases[136,153,168]. Similarly, genetic variants of PTPN2, which is associated with regulation of STAT1 and STAT3 phosphorylation, were found to be linked to CD but not UC[138]. In detail, patients with CD carrying PTPN2 loss-of-function variant rs1893217 showed increased Th1- and Th17-related transcription factors and cytokines. Similarly, STAT1/3 activation, which is associated with Th1 and Th17 differentiation, was increased in PTPN2-/-CD4 T-cells[95]. However, in a large meta-analysis, associations between PTPN2 gene polymorphisms and both CD and UC were found, which emphasizes the importance of PTPN2 and associated regulation of STAT1/3-linked Th1 and Th17 differentiation in both entities[169]. For PTPN11, regulating STAT1 signaling[81,170], a genetic association with UC but not CD was found[171]. Furthermore, in DSS-induced colitis, conditional PTPN11 knockout in T-cells increased colitis severity[172] stressing the important role of PTPN11 in colitis pathogenesis. Additionally, several studies have identified a marked association between the cytokine receptor IL-23R and IL-12R genes, which are both linked to Th17 differentiation, in both UC and CD[137,141,150,173]. The relevance of IL-23 and IL-12 signaling on IBD pathogenesis is further confirmed by the therapeutic efficacy of the IL-12/IL-23 blocker ustekinumab in both entities.
Regarding the summarized data, it becomes evident that aberrant signaling of various direct and indirect components of the JAK/STAT pathway are critically involved in initiation and/or perpetuation of inflammation in both UC and CD. Dysregulated JAK/STAT-signaling is possibly linked to specific JAK/STAT-associated genetic predispositions and is partially cell subset specific with different signaling aberrations in the innate vs adaptive immunity. With the regulatory drug approval of the pan-JAK inhibitor tofacitinib, therapeutic inhibition of the JAK/STAT pathway is available for the clinical management of patients with UC. While the OCTAVE study program has proven the efficacy of tofacitinib for UC, some questions regarding its safety in terms of herpes zoster infection[61] and pulmonary embolism have occurred recently as severe adverse events in patients treated twice daily with 10 mg tofacitinib were observed[174]. Furthermore, tofacitinib revealed no effective response in CD compared to placebo in a phase II RCT[58]. These data underline that adequate selection of patients with UC for tofacitinib treatment is key.
Targeting JAK/STAT components more precisely rather than inhibiting the complete pathway appears promising to limit adverse events and potentially improve clinical response in CD. Various compounds associated with selective JAK inhibition, including filgotinib and upadacitinib as predominantly JAK1-selective inhibitors, are currently under clinical investigation for IBD, while upadacitinib has most recently been approved for the treatment of RA[175,176]. Filgotinib has entered a phase III RCT due to promising results in moderate-to-severe CD including induction of clinical remission and mucosal healing[177]. Results from the phase II RCT with upadacitinib also show dose-dependent favorable outcomes with 27% clinical remission rates in patients with CD treated with 6 mg twice daily[178] and phase III studies in CD and UC are ongoing (NCT03006068, NCT03345836). Furthermore, the JAK3-selective inhibitor Pf-06651600 and dual JAK1/TYK2 inhibitor Pf-06700841 are currently being investigated in phase II RCTs for CD (NCT03395184) and UC (NCT02958865). Additionally, the agent BMS-986165 is a selective TYK2 inhibitor leading to blockade of IL-12-, IL-23-, and type I IFN signaling[179] and is being investigated in moderate-to-severe CD in a phase II RCT (NCT03934216). The intestinally restricted pan-JAK inhibitor TD-1473 is also under investigation in a phase II RCT in CD (NCT03635112) and a phase II study is planned for UC (NCT03920254).
However, since all JAKs are involved in complex biological processes, which control a wide range of cellular responses, inhibition of a specific JAK may lead to imprecise outcomes including both inhibition of regulatory and inflammatory pathways as well as unwanted side effects. Thus, specific inhibition of STATs as downstream effectors of JAK/STAT-signaling might be the next step in using the JAK/STAT pathway as a therapeutic target. However, therapeutic agents investigated in IBD still target JAKs and to our knowledge, no STAT inhibitor has been established for IBD treatment to date, which might be due to certain challenges: STATs lack catalytic activity and pharmacological targeting is complex compared to the kinase domains of JAK, which represent distinct targets for therapeutic inhibition[31]. Nevertheless, STAT inhibitors are currently under investigation in several oncologic diseases such as acute myeloid leukemia or recurrent malignant glioma[180]; thus, the investigation of pharmacological STAT targeting might also be prospectively possible in IBD.
Of note, in considering JAK/STAT-signaling as a therapeutic target, one should take into account that STATs are associated with partially counter-directional functions in different cell compartments. Namely, STAT3 has different function in monocytes and monocyte-derived cells (innate immune system) with rather regulatory features i.e., IL-10 release, while in T-cells (adaptive immune system) STAT3 is strongly pro-inflammatory involved in Th17 differentiation. It needs to be emphasized that an important regulatory impact of the innate immune system was suggested for CD[143]. This is supported by the fact that in myeloid cells of patients with CD, STAT3 reveals strong activation[135], while pro-inflammatory STAT1 is significantly lower in its expression and activation in myeloid cells of intestinal tissue of active CD compared to UC[127]. Furthermore, our own observations demonstrate higher levels of pro-inflammatory cytokines including TNFα and IL-6 in a pro-inflammatory setting and a stronger susceptibility to regulatory tofacitinib treatment in UC compared to CD-derived monocytes[66]. These data underline the suggestion of myeloid cells as part of the innate immune system which possibly plays a less inflammatory and more regulatory role in CD compared to UC. The pan-JAK inhibitor tofacitinib revealed no significant efficacy in CD and a possible explanation might be the broad JAK/STAT inhibition which targets T-cell associated inflammatory signaling[17] but might simultaneously inhibit important regulatory responses in myeloid cells in CD. Indeed, the impact of JAK inhibition specifically on myeloid cells is controversial: Some studies describe an effective inhibition of pro-inflammatory responses by JAK blockade[17,181], while conversely, upregulated pro-inflammatory signaling has also been described[182,183]. During intestinal injury, peripheral monocyte recruitment to intestinal tissues is increased, which are pro-inflammatory and primed in an inflammatory environment to contribute to inflammatory outcomes[66,184,185]. Thus, induction of pro-inflammatory myeloid cells by broad JAK inhibition might contribute to its sustained impact on intestinal inflammation. With regard to that, cell subset-specific JAK/STAT targeting has been suggested as a future therapeutic strategy in IBD to improve efficacy[143], especially in CD.
Last but not least, data on JAK/STAT components as genetic risk loci clearly indicate that patients with IBD reveal specific overlap and differences such as JAK2, STAT3 as common and STAT regulating PTP22 and PTPN11 as potential different genetic risk loci. In terms of an individualized therapy, genotypic adaptation of medical treatment or individualized drug dosing, which is already established for thiopurine treatment in patients with IBD by assessing TMPT genotypes, would be an elegant future clinical target for different JAK/STAT-associated genotypes. Hedl and coworker[143] already demonstrated that monocyte-derived macrophages (MDMs) of patients with the JAK2 rs10758669 CC risk gene revealed enhanced JAK2 signaling and increased pro- and anti-inflammatory cytokines. In rs10758669 AA disease carrier-derived MDMs compared to C carrier-derived MDMs, lower doses of JAK2 inhibitor and tofacitinib changed decreasing to increasing inflammatory cytokines. These data further underline the need to adapt dosage or treatment to JAK-associated genotype. In addition to an improved individualized therapy, JAK-genotyping could be of use to anticipate disease course as was already shown for the JAK2 genetic variant rs10758669 and STAT4 genetic variant rs7574865, which were both associated with a complicated disease course in CD[144,145,152].
A clear assignment of JAK/STAT components to CD or UC pathogenesis is difficult due to pleiotropic and partially overlapping dysregulation of JAK/STAT molecules or its regulators. Nevertheless, some general conclusions can be drawn:
First, in T-cells, STAT3 is associated with a critical role in both UC and CD pathogenesis and over-activation is linked to increased Th17 response and intestinal inflammation. Aberrant T-cell linked STAT1 expression is predominantly detected in CD and STAT6 expression predominantly in UC, while there is evidence for dysregulation of STAT4 in both entities. These data indicate a partial confirmation of the Th1/CD and Th2/UC concept but also hint at a certain impact of Th1 in UC.
Secondly, the overlap of both UC and CD-associated risk genes STAT3, JAK2, IL23R and IL12R, all being involved in Th17-cell differentiation, further strongly underlines the contribution of dysregulated Th17 response to both UC and CD. Furthermore, risk loci of genes associated with Th1 differentiation were found in both UC and CD such as STAT4 in both entities and STAT1-regulating PTP22 in CD and PTP11 in UC, respectively. These data further underline the importance of Th1 response in CD but again emphasize an overlap of Th1-cells which also contributes to UC and weaken the hypothesis of the specific role of Th1 in CD.
Thirdly, in contrast to lymphocytes, the JAK/STAT pathway has a more regulatory impact on myeloid cells in IBD. Only STAT1, mainly increased in UC-derived myeloid cells, seems to be associated with rather inflammatory features which points to a more inflammatory role of the JAK/STAT pathway in UC than CD and might partially explain the clinical response to tofacitinib in UC compared to patients with CD.
To conclude, the summarized data strongly indicate that development of sophisticated JAK/STAT inhibitors might further improve the efficacy and safety profiles of this drug class and improve their positioning in the therapeutic algorithm. This might be achieved by specific targeting of JAK/STAT components or cell subset specific JAK/STAT inhibition in T-cells to minimize the effects of JAK/STAT blockade on myeloid cells. Finally, assessment of specific genotypes as part of a personalized treatment approach might not only increase safety and efficacy of JAK/STAT inhibition including optimized dosing, but could also serve as a predictive marker for disease course in CD.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Day AS, Gangl A, Nielsen OH, Serban ED S-Editor: Gong ZM L-Editor: Webster JR E-Editor: Zhang YL
| 1. | Bettenworth D, Lopez R, Hindryckx P, Levesque BG, Rieder F. Heterogeneity in endoscopic treatment of Crohn's disease-associated strictures: An international inflammatory bowel disease specialist survey. J Gastroenterol. 2016;51:939-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 2. | Molendijk I, Nuij VJ, van der Meulen-de Jong AE, van der Woude CJ. Disappointing durable remission rates in complex Crohn's disease fistula. Inflamm Bowel Dis. 2014;20:2022-2028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 3. | Mooiweer E, van der Meulen-de Jong AE, Ponsioen CY, van der Woude CJ, van Bodegraven AA, Jansen JM, Mahmmod N, Kremer W, Siersema PD, Oldenburg B; Dutch Initiative on Crohn’s and Colitis. Incidence of Interval Colorectal Cancer Among Inflammatory Bowel Disease Patients Undergoing Regular Colonoscopic Surveillance. Clin Gastroenterol Hepatol. 2015;13:1656-1661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Pariente B, Hu S, Bettenworth D, Speca S, Desreumaux P, Meuwis MA, Danese S, Rieder F, Louis E. Treatments for Crohn's Disease-Associated Bowel Damage: A Systematic Review. Clin Gastroenterol Hepatol. 2019;17:847-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Zhu Z, Mei Z, Guo Y, Wang G, Wu T, Cui X, Huang Z, Zhu Y, Wen D, Song J, He H, Xu W, Cui L, Liu C. Reduced Risk of Inflammatory Bowel Disease-associated Colorectal Neoplasia with Use of Thiopurines: a Systematic Review and Meta-analysis. J Crohns Colitis. 2018;12:546-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 6. | Ben-Horin S, Chowers Y. Review article: loss of response to anti-TNF treatments in Crohn's disease. Aliment Pharmacol Ther. 2011;33:987-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 471] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 7. | Kirchgesner J, Lemaitre M, Carrat F, Zureik M, Carbonnel F, Dray-Spira R. Risk of Serious and Opportunistic Infections Associated With Treatment of Inflammatory Bowel Diseases. Gastroenterology. 2018;155:337-346.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 414] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 8. | Wong DJ, Roth EM, Feuerstein JD, Poylin VY. Surgery in the age of biologics. Gastroenterol Rep (Oxf). 2019;7:77-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 9. | Burisch J, Katsanos KH, Christodoulou DK, Barros L, Magro F, Pedersen N, Kjeldsen J, Vegh Z, Lakatos PL, Eriksson C, Halfvarson J, Fumery M, Gower-Rousseau C, Brinar M, Cukovic-Cavka S, Nikulina I, Belousova E, Myers S, Sebastian S, Kiudelis G, Kupcinskas L, Schwartz D, Odes S, Kaimakliotis IP, Valpiani D, D'Incà R, Salupere R, Chetcuti Zammit S, Ellul P, Duricova D, Bortlik M, Goldis A, Kievit HAL, Toca A, Turcan S, Midjord J, Nielsen KR, Andersen KW, Andersen V, Misra R, Arebi N, Oksanen P, Collin P, de Castro L, Hernandez V, Langholz E, Munkholm P; Epi-IBD Group. Natural Disease Course of Ulcerative Colitis During the First Five Years of Follow-up in a European Population-based Inception Cohort-An Epi-IBD Study. J Crohns Colitis. 2019;13:198-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 113] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 10. | Burisch J, Kiudelis G, Kupcinskas L, Kievit HAL, Andersen KW, Andersen V, Salupere R, Pedersen N, Kjeldsen J, D'Incà R, Valpiani D, Schwartz D, Odes S, Olsen J, Nielsen KR, Vegh Z, Lakatos PL, Toca A, Turcan S, Katsanos KH, Christodoulou DK, Fumery M, Gower-Rousseau C, Zammit SC, Ellul P, Eriksson C, Halfvarson J, Magro FJ, Duricova D, Bortlik M, Fernandez A, Hernández V, Myers S, Sebastian S, Oksanen P, Collin P, Goldis A, Misra R, Arebi N, Kaimakliotis IP, Nikuina I, Belousova E, Brinar M, Cukovic-Cavka S, Langholz E, Munkholm P; Epi-IBD group. Natural disease course of Crohn's disease during the first 5 years after diagnosis in a European population-based inception cohort: an Epi-IBD study. Gut. 2019;68:423-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 187] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 11. | Papamichael K, Vande Casteele N, Ferrante M, Gils A, Cheifetz AS. Therapeutic Drug Monitoring During Induction of Anti-Tumor Necrosis Factor Therapy in Inflammatory Bowel Disease: Defining a Therapeutic Drug Window. Inflamm Bowel Dis. 2017;23:1510-1515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 12. | Peyrin-Biroulet L, Lémann M. Review article: remission rates achievable by current therapies for inflammatory bowel disease. Aliment Pharmacol Ther. 2011;33:870-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 146] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 13. | Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002;20:495-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1030] [Cited by in RCA: 1024] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 14. | Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14:329-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1545] [Cited by in RCA: 1967] [Article Influence: 178.8] [Reference Citation Analysis (1)] |
| 15. | Neurath MF, Finotto S, Glimcher LH. The role of Th1/Th2 polarization in mucosal immunity. Nat Med. 2002;8:567-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 451] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 16. | Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 888] [Cited by in RCA: 863] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 17. | Ghoreschi K, Jesson MI, Li X, Lee JL, Ghosh S, Alsup JW, Warner JD, Tanaka M, Steward-Tharp SM, Gadina M, Thomas CJ, Minnerly JC, Storer CE, LaBranche TP, Radi ZA, Dowty ME, Head RD, Meyer DM, Kishore N, O'Shea JJ. Modulation of innate and adaptive immune responses by tofacitinib (CP-690,550). J Immunol. 2011;186:4234-4243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 532] [Cited by in RCA: 499] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 18. | Ghoreschi K, Laurence A, O'Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228:273-287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 944] [Cited by in RCA: 883] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 19. | O'Shea JJ, Pesu M, Borie DC, Changelian PS. A new modality for immunosuppression: targeting the JAK/STAT pathway. Nat Rev Drug Discov. 2004;3:555-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 256] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 20. | Baker SJ, Rane SG, Reddy EP. Hematopoietic cytokine receptor signaling. Oncogene. 2007;26:6724-6737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 184] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 21. | Shuai K, Liu B. Regulation of JAK-STAT signalling in the immune system. Nat Rev Immunol. 2003;3:900-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 990] [Cited by in RCA: 1050] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 22. | Stark GR, Darnell JE. The JAK-STAT pathway at twenty. Immunity. 2012;36:503-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1154] [Cited by in RCA: 1176] [Article Influence: 90.5] [Reference Citation Analysis (0)] |
| 23. | Zundler S, Neurath MF. Integrating Immunologic Signaling Networks: The JAK/STAT Pathway in Colitis and Colitis-Associated Cancer. Vaccines (Basel). 2016;4:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 24. | Darnell JE, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4322] [Cited by in RCA: 4586] [Article Influence: 147.9] [Reference Citation Analysis (0)] |
| 25. | Leonard WJ, O'Shea JJ. Jaks and STATs: biological implications. Annu Rev Immunol. 1998;16:293-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1357] [Cited by in RCA: 1385] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 26. | Levy DE, Darnell JE. Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2306] [Cited by in RCA: 2452] [Article Influence: 106.6] [Reference Citation Analysis (0)] |
| 27. | O'Shea JJ, Murray PJ. Cytokine signaling modules in inflammatory responses. Immunity. 2008;28:477-487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 614] [Cited by in RCA: 576] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 28. | Grimley PM, Dong F, Rui H. Stat5a and Stat5b: fraternal twins of signal transduction and transcriptional activation. Cytokine Growth Factor Rev. 1999;10:131-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 181] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 29. | Kisseleva T, Bhattacharya S, Braunstein J, Schindler CW. Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene. 2002;285:1-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 793] [Cited by in RCA: 814] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 30. | Schindler C, Strehlow I. Cytokines and STAT signaling. Adv Pharmacol. 2000;47:113-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 89] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Villarino AV, Kanno Y, Ferdinand JR, O'Shea JJ. Mechanisms of Jak/STAT signaling in immunity and disease. J Immunol. 2015;194:21-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 405] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 32. | Moodley D, Yoshida H, Mostafavi S, Asinovski N, Ortiz-Lopez A, Symanowicz P, Telliez JB, Hegen M, Clark JD, Mathis D, Benoist C. Network pharmacology of JAK inhibitors. Proc Natl Acad Sci USA. 2016;113:9852-9857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 33. | Soendergaard C, Bergenheim FH, Bjerrum JT, Nielsen OH. Targeting JAK-STAT signal transduction in IBD. Pharmacol Ther. 2018;192:100-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 34. | Villarino AV, Kanno Y, O'Shea JJ. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat Immunol. 2017;18:374-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 863] [Cited by in RCA: 868] [Article Influence: 108.5] [Reference Citation Analysis (0)] |
| 35. | Lischke A, Moriggl R, Brändlein S, Berchtold S, Kammer W, Sebald W, Groner B, Liu X, Hennighausen L, Friedrich K. The interleukin-4 receptor activates STAT5 by a mechanism that relies upon common gamma-chain. J Biol Chem. 1998;273:31222-31229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 36. | Nguyen KB, Watford WT, Salomon R, Hofmann SR, Pien GC, Morinobu A, Gadina M, O'Shea JJ, Biron CA. Critical role for STAT4 activation by type 1 interferons in the interferon-gamma response to viral infection. Science. 2002;297:2063-2066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 407] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 37. | Villarino A, Hibbert L, Lieberman L, Wilson E, Mak T, Yoshida H, Kastelein RA, Saris C, Hunter CA. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity. 2003;19:645-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 391] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 38. | Broughton SE, Dhagat U, Hercus TR, Nero TL, Grimbaldeston MA, Bonder CS, Lopez AF, Parker MW. The GM-CSF/IL-3/IL-5 cytokine receptor family: from ligand recognition to initiation of signaling. Immunol Rev. 2012;250:277-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 188] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 39. | Nan Y, Wu C, Zhang YJ. Interferon Independent Non-Canonical STAT Activation and Virus Induced Inflammation. Viruses. 2018;10:196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 40. | Pfeffer SR, Fan M, Du Z, Yang CH, Pfeffer LM. Unphosphorylated STAT3 regulates the antiproliferative, antiviral, and gene-inducing actions of type I interferons. Biochem Biophys Res Commun. 2017;490:739-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 41. | Majoros A, Platanitis E, Kernbauer-Hölzl E, Rosebrock F, Müller M, Decker T. Canonical and Non-Canonical Aspects of JAK-STAT Signaling: Lessons from Interferons for Cytokine Responses. Front Immunol. 2017;8:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 239] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 42. | Dawson MA, Bannister AJ, Göttgens B, Foster SD, Bartke T, Green AR, Kouzarides T. JAK2 phosphorylates histone H3Y41 and excludes HP1alpha from chromatin. Nature. 2009;461:819-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 482] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 43. | Takeda K, Clausen BE, Kaisho T, Tsujimura T, Terada N, Förster I, Akira S. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10:39-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 943] [Cited by in RCA: 942] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 44. | Durant L, Watford WT, Ramos HL, Laurence A, Vahedi G, Wei L, Takahashi H, Sun HW, Kanno Y, Powrie F, O'Shea JJ. Diverse targets of the transcription factor STAT3 contribute to T cell pathogenicity and homeostasis. Immunity. 2010;32:605-615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 590] [Cited by in RCA: 568] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 45. | Lu D, Liu L, Ji X, Gao Y, Chen X, Liu Y, Liu Y, Zhao X, Li Y, Li Y, Jin Y, Zhang Y, McNutt MA, Yin Y. The phosphatase DUSP2 controls the activity of the transcription activator STAT3 and regulates TH17 differentiation. Nat Immunol. 2015;16:1263-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 46. | Yang XP, Ghoreschi K, Steward-Tharp SM, Rodriguez-Canales J, Zhu J, Grainger JR, Hirahara K, Sun HW, Wei L, Vahedi G, Kanno Y, O'Shea JJ, Laurence A. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat Immunol. 2011;12:247-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 502] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 47. | Casanova JL, Holland SM, Notarangelo LD. Inborn errors of human JAKs and STATs. Immunity. 2012;36:515-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 252] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 48. | Gao B, Wang H, Lafdil F, Feng D. STAT proteins - key regulators of anti-viral responses, inflammation, and tumorigenesis in the liver. J Hepatol. 2012;57:430-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 146] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 49. | O'Shea JJ, Plenge R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity. 2012;36:542-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 692] [Cited by in RCA: 874] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 50. | Seavey MM, Dobrzanski P. The many faces of Janus kinase. Biochem Pharmacol. 2012;83:1136-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 51. | Changelian PS, Flanagan ME, Ball DJ, Kent CR, Magnuson KS, Martin WH, Rizzuti BJ, Sawyer PS, Perry BD, Brissette WH, McCurdy SP, Kudlacz EM, Conklyn MJ, Elliott EA, Koslov ER, Fisher MB, Strelevitz TJ, Yoon K, Whipple DA, Sun J, Munchhof MJ, Doty JL, Casavant JM, Blumenkopf TA, Hines M, Brown MF, Lillie BM, Subramanyam C, Shang-Poa C, Milici AJ, Beckius GE, Moyer JD, Su C, Woodworth TG, Gaweco AS, Beals CR, Littman BH, Fisher DA, Smith JF, Zagouras P, Magna HA, Saltarelli MJ, Johnson KS, Nelms LF, Des Etages SG, Hayes LS, Kawabata TT, Finco-Kent D, Baker DL, Larson M, Si MS, Paniagua R, Higgins J, Holm B, Reitz B, Zhou YJ, Morris RE, O'Shea JJ, Borie DC. Prevention of organ allograft rejection by a specific Janus kinase 3 inhibitor. Science. 2003;302:875-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 541] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 52. | Meyer DM, Jesson MI, Li X, Elrick MM, Funckes-Shippy CL, Warner JD, Gross CJ, Dowty ME, Ramaiah SK, Hirsch JL, Saabye MJ, Barks JL, Kishore N, Morris DL. Anti-inflammatory activity and neutrophil reductions mediated by the JAK1/JAK3 inhibitor, CP-690,550, in rat adjuvant-induced arthritis. J Inflamm (Lond). 2010;7:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 322] [Cited by in RCA: 377] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 53. | Kremer JM, Cohen S, Wilkinson BE, Connell CA, French JL, Gomez-Reino J, Gruben D, Kanik KS, Krishnaswami S, Pascual-Ramos V, Wallenstein G, Zwillich SH. A phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) versus placebo in combination with background methotrexate in patients with active rheumatoid arthritis and an inadequate response to methotrexate alone. Arthritis Rheum. 2012;64:970-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 271] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 54. | Singh JA, Hossain A, Tanjong Ghogomu E, Kotb A, Christensen R, Mudano AS, Maxwell LJ, Shah NP, Tugwell P, Wells GA. Biologics or tofacitinib for rheumatoid arthritis in incomplete responders to methotrexate or other traditional disease-modifying anti-rheumatic drugs: a systematic review and network meta-analysis. Cochrane Database Syst Rev. 2016;CD012183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 55. | Singh JA, Saag KG, Bridges SL, Akl EA, Bannuru RR, Sullivan MC, Vaysbrot E, McNaughton C, Osani M, Shmerling RH, Curtis JR, Furst DE, Parks D, Kavanaugh A, O'Dell J, King C, Leong A, Matteson EL, Schousboe JT, Drevlow B, Ginsberg S, Grober J, St Clair EW, Tindall E, Miller AS, McAlindon T. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol. 2016;68:1-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1062] [Cited by in RCA: 1350] [Article Influence: 135.0] [Reference Citation Analysis (0)] |
| 56. | Tanaka Y, Suzuki M, Nakamura H, Toyoizumi S, Zwillich SH; Tofacitinib Study Investigators. Phase II study of tofacitinib (CP-690,550) combined with methotrexate in patients with rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Care Res (Hoboken). 2011;63:1150-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 239] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 57. | European Medicines Agency. Xeljanz: EPAR - Public assessment report. In: Ema.europa.eu [Internet]. Accessed 2020 Jan. Available from: https://www.ema.europa.eu/en/documents/assessment-report/xeljanz-epar-public-assessment-report_en.pdf . |
| 58. | Panés J, Sandborn WJ, Schreiber S, Sands BE, Vermeire S, D'Haens G, Panaccione R, Higgins PDR, Colombel JF, Feagan BG, Chan G, Moscariello M, Wang W, Niezychowski W, Marren A, Healey P, Maller E. Tofacitinib for induction and maintenance therapy of Crohn's disease: results of two phase IIb randomised placebo-controlled trials. Gut. 2017;66:1049-1059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 285] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 59. | Panés J, Su C, Bushmakin AG, Cappelleri JC, Healey P. Direct and Indirect Effects of Tofacitinib on Treatment Satisfaction in Patients with Ulcerative Colitis. J Crohns Colitis. 2016;10:1310-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 60. | Sandborn WJ, Ghosh S, Panes J, Vranic I, Su C, Rousell S, Niezychowski W; Study A3921063 Investigators. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med. 2012;367:616-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 585] [Cited by in RCA: 631] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 61. | Sandborn WJ, Su C, Sands BE, D'Haens GR, Vermeire S, Schreiber S, Danese S, Feagan BG, Reinisch W, Niezychowski W, Friedman G, Lawendy N, Yu D, Woodworth D, Mukherjee A, Zhang H, Healey P, Panés J; OCTAVE Induction 1, OCTAVE Induction 2, and OCTAVE Sustain Investigators. Tofacitinib as Induction and Maintenance Therapy for Ulcerative Colitis. N Engl J Med. 2017;376:1723-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 896] [Cited by in RCA: 1184] [Article Influence: 148.0] [Reference Citation Analysis (0)] |
| 62. | European Medicines Agency. Xeljanz-H-C-4214-X-0005: EPAR - Assessment Report - Variation. In: Ema.europa.eu [Internet]. Accessed 2020 Jan. Available from: https://www.ema.europa.eu/en/documents/variation-report/xeljanz-h-c-4214-x-0005-epar-assessment-report-variation_en.pdf. |
| 63. | Panés J, D'Haens GR, Higgins PDR, Mele L, Moscariello M, Chan G, Wang W, Niezychowski W, Su C, Maller E. Long-term safety and tolerability of oral tofacitinib in patients with Crohn's disease: results from a phase 2, open-label, 48-week extension study. Aliment Pharmacol Ther. 2019;49:265-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 64. | Coskun M, Salem M, Pedersen J, Nielsen OH. Involvement of JAK/STAT signaling in the pathogenesis of inflammatory bowel disease. Pharmacol Res. 2013;76:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 257] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 65. | Feagan B. Update on Tofacitinib for Inflammatory Bowel Disease. Gastroenterol Hepatol (NY). 2016;12:572-574. [PubMed] |
| 66. | Cordes F, Lenker E, Spille LJ, Weinhage T, Bettenworth D, Kessel C, Schmidt HH, Foell D, Varga G. Tofacitinib Reprograms Human Monocytes of IBD Patients and Healthy Controls Toward a More Regulatory Phenotype. Inflamm Bowel Dis. 2020;26:391-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 67. | Atreya R, Mudter J, Finotto S, Müllberg J, Jostock T, Wirtz S, Schütz M, Bartsch B, Holtmann M, Becker C, Strand D, Czaja J, Schlaak JF, Lehr HA, Autschbach F, Schürmann G, Nishimoto N, Yoshizaki K, Ito H, Kishimoto T, Galle PR, Rose-John S, Neurath MF. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nat Med. 2000;6:583-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 945] [Cited by in RCA: 1026] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 68. | Lovato P, Brender C, Agnholt J, Kelsen J, Kaltoft K, Svejgaard A, Eriksen KW, Woetmann A, Ødum N. Constitutive STAT3 activation in intestinal T cells from patients with Crohn's disease. J Biol Chem. 2003;278:16777-16781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 145] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 69. | Pike KA, Tremblay ML. Protein Tyrosine Phosphatases: Regulators of CD4 T Cells in Inflammatory Bowel Disease. Front Immunol. 2018;9:2504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 70. | Globig AM, Hennecke N, Martin B, Seidl M, Ruf G, Hasselblatt P, Thimme R, Bengsch B. Comprehensive intestinal T helper cell profiling reveals specific accumulation of IFN-γ+IL-17+coproducing CD4+ T cells in active inflammatory bowel disease. Inflamm Bowel Dis. 2014;20:2321-2329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 114] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 71. | Mencarelli A, Vacca M, Khameneh HJ, Acerbi E, Tay A, Zolezzi F, Poidinger M, Mortellaro A. Calcineurin B in CD4+ T Cells Prevents Autoimmune Colitis by Negatively Regulating the JAK/STAT Pathway. Front Immunol. 2018;9:261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 72. | Sakuraba A, Sato T, Kamada N, Kitazume M, Sugita A, Hibi T. Th1/Th17 immune response is induced by mesenteric lymph node dendritic cells in Crohn's disease. Gastroenterology. 2009;137:1736-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 182] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 73. | Fuss IJ, Heller F, Boirivant M, Leon F, Yoshida M, Fichtner-Feigl S, Yang Z, Exley M, Kitani A, Blumberg RS, Mannon P, Strober W. Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis. J Clin Invest. 2004;113:1490-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 516] [Cited by in RCA: 576] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 74. | Hovhannisyan Z, Treatman J, Littman DR, Mayer L. Characterization of interleukin-17-producing regulatory T cells in inflamed intestinal mucosa from patients with inflammatory bowel diseases. Gastroenterology. 2011;140:957-965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 285] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 75. | Ueno A, Jijon H, Chan R, Ford K, Hirota C, Kaplan GG, Beck PL, Iacucci M, Fort Gasia M, Barkema HW, Panaccione R, Ghosh S. Increased prevalence of circulating novel IL-17 secreting Foxp3 expressing CD4+ T cells and defective suppressive function of circulating Foxp3+ regulatory cells support plasticity between Th17 and regulatory T cells in inflammatory bowel disease patients. Inflamm Bowel Dis. 2013;19:2522-2534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 172] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 76. | Eriguchi Y, Nakamura K, Yokoi Y, Sugimoto R, Takahashi S, Hashimoto D, Teshima T, Ayabe T, Selsted ME, Ouellette AJ. Essential role of IFN-γ in T cell-associated intestinal inflammation. JCI Insight. 2018;3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 77. | Acharya S, Timilshina M, Jiang L, Neupane S, Choi DY, Park SW, Lee SY, Jeong BS, Kim JA, Nam TG, Chang JH. Amelioration of Experimental autoimmune encephalomyelitis and DSS induced colitis by NTG-A-009 through the inhibition of Th1 and Th17 cells differentiation. Sci Rep. 2018;8:7799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 78. | Long Y, Li S, Qin J, Xie L, Gan L, Jie F, Wu Y, Li Y, Du Q. Kuijieling regulates the differentiation of Treg and Th17 cells to ameliorate experimental colitis in rats. Biomed Pharmacother. 2018;105:781-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 79. | Xu J, Liu J, Yue G, Sun M, Li J, Xiu X, Gao Z. Therapeutic effect of the natural compounds baicalein and baicalin on autoimmune diseases. Mol Med Rep. 2018;18:1149-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 80. | Bandyopadhyay SK, de la Motte CA, Kessler SP, Hascall VC, Hill DR, Strong SA. Hyaluronan-mediated leukocyte adhesion and dextran sulfate sodium-induced colitis are attenuated in the absence of signal transducer and activator of transcription 1. Am J Pathol. 2008;173:1361-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 81. | Wu X, Guo W, Wu L, Gu Y, Gu L, Xu S, Wu X, Shen Y, Ke Y, Tan R, Sun Y, Xu Q. Selective sequestration of STAT1 in the cytoplasm via phosphorylated SHP-2 ameliorates murine experimental colitis. J Immunol. 2012;189:3497-3507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 82. | Ishizaki M, Akimoto T, Muromoto R, Yokoyama M, Ohshiro Y, Sekine Y, Maeda H, Shimoda K, Oritani K, Matsuda T. Involvement of tyrosine kinase-2 in both the IL-12/Th1 and IL-23/Th17 axes in vivo. J Immunol. 2011;187:181-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 83. | Van Kampen C, Gauldie J, Collins SM. Proinflammatory properties of IL-4 in the intestinal microenvironment. Am J Physiol Gastrointest Liver Physiol. 2005;288:G111-G117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 84. | Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, Mitsdoerffer M, Strom TB, Elyaman W, Ho IC, Khoury S, Oukka M, Kuchroo VK. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(-) effector T cells. Nat Immunol. 2008;9:1347-1355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 914] [Cited by in RCA: 877] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 85. | Rosen MJ, Chaturvedi R, Washington MK, Kuhnhein LA, Moore PD, Coggeshall SS, McDonough EM, Weitkamp JH, Singh AB, Coburn LA, Williams CS, Yan F, Van Kaer L, Peebles RS, Wilson KT. STAT6 deficiency ameliorates severity of oxazolone colitis by decreasing expression of claudin-2 and Th2-inducing cytokines. J Immunol. 2013;190:1849-1858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 86. | Schulz EG, Mariani L, Radbruch A, Höfer T. Sequential polarization and imprinting of type 1 T helper lymphocytes by interferon-gamma and interleukin-12. Immunity. 2009;30:673-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 229] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 87. | Mudter J, Weigmann B, Bartsch B, Kiesslich R, Strand D, Galle PR, Lehr HA, Schmidt J, Neurath MF. Activation pattern of signal transducers and activators of transcription (STAT) factors in inflammatory bowel diseases. Am J Gastroenterol. 2005;100:64-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 189] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 88. | Wu F, Dassopoulos T, Cope L, Maitra A, Brant SR, Harris ML, Bayless TM, Parmigiani G, Chakravarti S. Genome-wide gene expression differences in Crohn's disease and ulcerative colitis from endoscopic pinch biopsies: insights into distinctive pathogenesis. Inflamm Bowel Dis. 2007;13:807-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 217] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 89. | Steen HC, Gamero AM. STAT2 phosphorylation and signaling. JAKSTAT. 2013;2:e25790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 90. | Hossain DM, Panda AK, Manna A, Mohanty S, Bhattacharjee P, Bhattacharyya S, Saha T, Chakraborty S, Kar RK, Das T, Chatterjee S, Sa G. FoxP3 acts as a cotranscription factor with STAT3 in tumor-induced regulatory T cells. Immunity. 2013;39:1057-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 91. | Schmetterer KG, Neunkirchner A, Wojta-Stremayr D, Leitner J, Steinberger P, Pickl WF. STAT3 governs hyporesponsiveness and granzyme B-dependent suppressive capacity in human CD4+ T cells. FASEB J. 2015;29:759-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 92. | Musso A, Dentelli P, Carlino A, Chiusa L, Repici A, Sturm A, Fiocchi C, Rizzetto M, Pegoraro L, Sategna-Guidetti C, Brizzi MF. Signal transducers and activators of transcription 3 signaling pathway: an essential mediator of inflammatory bowel disease and other forms of intestinal inflammation. Inflamm Bowel Dis. 2005;11:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 86] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 93. | Jin L, Li L, Hu C, Paez-Cortez J, Bi Y, Macoritto M, Cao S, Tian Y. Integrative Analysis of Transcriptomic and Proteomic Profiling in Inflammatory Bowel Disease Colon Biopsies. Inflamm Bowel Dis. 2019;25:1906-1918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 94. | van Wijk F, Cheroutre H. Mucosal T cells in gut homeostasis and inflammation. Expert Rev Clin Immunol. 2010;6:559-566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 95. | Spalinger MR, Kasper S, Chassard C, Raselli T, Frey-Wagner I, Gottier C, Lang S, Atrott K, Vavricka SR, Mair F, Becher B, Lacroix C, Fried M, Rogler G, Scharl M. PTPN2 controls differentiation of CD4⁺ T cells and limits intestinal inflammation and intestinal dysbiosis. Mucosal Immunol. 2015;8:918-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 96. | Wiede F, Sacirbegovic F, Leong YA, Yu D, Tiganis T. PTPN2-deficiency exacerbates T follicular helper cell and B cell responses and promotes the development of autoimmunity. J Autoimmun. 2017;76:85-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 97. | Kaplan MH, Sun YL, Hoey T, Grusby MJ. Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature. 1996;382:174-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 937] [Cited by in RCA: 936] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 98. | Thierfelder WE, van Deursen JM, Yamamoto K, Tripp RA, Sarawar SR, Carson RT, Sangster MY, Vignali DA, Doherty PC, Grosveld GC, Ihle JN. Requirement for Stat4 in interleukin-12-mediated responses of natural killer and T cells. Nature. 1996;382:171-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 877] [Cited by in RCA: 882] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 99. | Parrello T, Monteleone G, Cucchiara S, Monteleone I, Sebkova L, Doldo P, Luzza F, Pallone F. Up-regulation of the IL-12 receptor beta 2 chain in Crohn's disease. J Immunol. 2000;165:7234-7239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 89] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 100. | Pang YH, Zheng CQ, Yang XZ, Zhang WJ. Increased expression and activation of IL-12-induced Stat4 signaling in the mucosa of ulcerative colitis patients. Cell Immunol. 2007;248:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 101. | Ohtani K, Ohtsuka Y, Ikuse T, Baba Y, Yamakawa Y, Aoyagi Y, Fujii T, Kudo T, Nagata S, Shimizu T. Increased mucosal expression of GATA-3 and STAT-4 in pediatric ulcerative colitis. Pediatr Int. 2010;52:584-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 102. | Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol. 2007;178:280-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 589] [Cited by in RCA: 645] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 103. | Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, Shevach EM, O'shea JJ. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1238] [Cited by in RCA: 1216] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 104. | Yao Z, Kanno Y, Kerenyi M, Stephens G, Durant L, Watford WT, Laurence A, Robinson GW, Shevach EM, Moriggl R, Hennighausen L, Wu C, O'Shea JJ. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood. 2007;109:4368-4375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 458] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 105. | Fantini MC, Becker C, Tubbe I, Nikolaev A, Lehr HA, Galle P, Neurath MF. Transforming growth factor beta induced FoxP3+ regulatory T cells suppress Th1 mediated experimental colitis. Gut. 2006;55:671-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 193] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 106. | Maul J, Loddenkemper C, Mundt P, Berg E, Giese T, Stallmach A, Zeitz M, Duchmann R. Peripheral and intestinal regulatory CD4+ CD25(high) T cells in inflammatory bowel disease. Gastroenterology. 2005;128:1868-1878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 497] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 107. | Lord JD, Long SA, Shows DM, Thorpe J, Schwedhelm K, Chen J, Kita M, Buckner JH. Circulating integrin alpha4/beta7+ lymphocytes targeted by vedolizumab have a pro-inflammatory phenotype. Clin Immunol. 2018;193:24-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 108. | Yagi R, Zhu J, Paul WE. An updated view on transcription factor GATA3-mediated regulation of Th1 and Th2 cell differentiation. Int Immunol. 2011;23:415-420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 183] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 109. | Zundler S, Neurath MF. Immunopathogenesis of inflammatory bowel diseases: functional role of T cells and T cell homing. Clin Exp Rheumatol. 2015;33:S19-S28. [PubMed] |
| 110. | Li Y, Deuring J, Peppelenbosch MP, Kuipers EJ, de Haar C, van der Woude CJ. STAT1, STAT6 and adenosine 3',5'-cyclic monophosphate (cAMP) signaling drive SOCS3 expression in inactive ulcerative colitis. Mol Med. 2012;18:1412-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 111. | Rosen MJ, Frey MR, Washington MK, Chaturvedi R, Kuhnhein LA, Matta P, Revetta FL, Wilson KT, Polk DB. STAT6 activation in ulcerative colitis: a new target for prevention of IL-13-induced colon epithelial cell dysfunction. Inflamm Bowel Dis. 2011;17:2224-2234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 112. | Geissmann F, Auffray C, Palframan R, Wirrig C, Ciocca A, Campisi L, Narni-Mancinelli E, Lauvau G. Blood monocytes: distinct subsets, how they relate to dendritic cells, and their possible roles in the regulation of T-cell responses. Immunol Cell Biol. 2008;86:398-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 277] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 113. | Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2322] [Cited by in RCA: 2245] [Article Influence: 149.7] [Reference Citation Analysis (0)] |
| 114. | Murray PJ. Immune regulation by monocytes. Semin Immunol. 2018;35:12-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 115. | Varga G, Foell D. Anti-inflammatory monocytes-interplay of innate and adaptive immunity. Mol Cell Pediatr. 2018;5:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 116. | Däbritz J, Weinhage T, Varga G, Wirth T, Walscheid K, Brockhausen A, Schwarzmaier D, Brückner M, Ross M, Bettenworth D, Roth J, Ehrchen JM, Foell D. Reprogramming of monocytes by GM-CSF contributes to regulatory immune functions during intestinal inflammation. J Immunol. 2015;194:2424-2438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 117. | Weinhage T, Däbritz J, Brockhausen A, Wirth T, Brückner M, Belz M, Foell D, Varga G. Granulocyte Macrophage Colony-Stimulating Factor-Activated CD39+/CD73+ Murine Monocytes Modulate Intestinal Inflammation via Induction of Regulatory T Cells. Cell Mol Gastroenterol Hepatol. 2015;1:433-449.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 118. | Bain CC, Mowat AM. Macrophages in intestinal homeostasis and inflammation. Immunol Rev. 2014;260:102-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 119. | Liu H, Dasgupta S, Fu Y, Bailey B, Roy C, Lightcap E, Faustin B. Subsets of mononuclear phagocytes are enriched in the inflamed colons of patients with IBD. BMC Immunol. 2019;20:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 120. | Wallace KL, Zheng LB, Kanazawa Y, Shih DQ. Immunopathology of inflammatory bowel disease. World J Gastroenterol. 2014;20:6-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 356] [Cited by in RCA: 419] [Article Influence: 38.1] [Reference Citation Analysis (2)] |
| 121. | Bain CC, Mowat AM. The monocyte-macrophage axis in the intestine. Cell Immunol. 2014;291:41-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 122. | Gren ST, Grip O. Role of Monocytes and Intestinal Macrophages in Crohn's Disease and Ulcerative Colitis. Inflamm Bowel Dis. 2016;22:1992-1998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 141] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 123. | Desalegn G, Pabst O. Inflammation triggers immediate rather than progressive changes in monocyte differentiation in the small intestine. Nat Commun. 2019;10:3229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 78] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 124. | De Vries LCS, Duarte JM, De Krijger M, Welting O, Van Hamersveld PHP, Van Leeuwen-Hilbers FWM, Moerland PD, Jongejan A, D'Haens GR, De Jonge WJ, Wildenberg ME. A JAK1 Selective Kinase Inhibitor and Tofacitinib Affect Macrophage Activation and Function. Inflamm Bowel Dis. 2019;25:647-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 125. | Dufour A, Bellac CL, Eckhard U, Solis N, Klein T, Kappelhoff R, Fortelny N, Jobin P, Rozmus J, Mark J, Pavlidis P, Dive V, Barbour SJ, Overall CM. C-terminal truncation of IFN-γ inhibits proinflammatory macrophage responses and is deficient in autoimmune disease. Nat Commun. 2018;9:2416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 126. | Platanitis E, Decker T. Regulatory Networks Involving STATs, IRFs, and NFκB in Inflammation. Front Immunol. 2018;9:2542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 159] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 127. | Schreiber S, Rosenstiel P, Hampe J, Nikolaus S, Groessner B, Schottelius A, Kühbacher T, Hämling J, Fölsch UR, Seegert D. Activation of signal transducer and activator of transcription (STAT) 1 in human chronic inflammatory bowel disease. Gut. 2002;51:379-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 171] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 128. | Starr R, Willson TA, Viney EM, Murray LJ, Rayner JR, Jenkins BJ, Gonda TJ, Alexander WS, Metcalf D, Nicola NA, Hilton DJ. A family of cytokine-inducible inhibitors of signalling. Nature. 1997;387:917-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1594] [Cited by in RCA: 1622] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 129. | Dickensheets HL, Venkataraman C, Schindler U, Donnelly RP. Interferons inhibit activation of STAT6 by interleukin 4 in human monocytes by inducing SOCS-1 gene expression. Proc Natl Acad Sci USA. 1999;96:10800-10805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 140] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 130. | Posselt G, Schwarz H, Duschl A, Horejs-Hoeck J. Suppressor of cytokine signaling 2 is a feedback inhibitor of TLR-induced activation in human monocyte-derived dendritic cells. J Immunol. 2011;187:2875-2884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 131. | Pupjalis D, Goetsch J, Kottas DJ, Gerke V, Rescher U. Annexin A1 released from apoptotic cells acts through formyl peptide receptors to dampen inflammatory monocyte activation via JAK/STAT/SOCS signalling. EMBO Mol Med. 2011;3:102-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 132. | Soendergaard C, Kvist PH, Thygesen P, Reslow M, Nielsen OH, Kopchick JJ, Holm TL. Characterization of Growth Hormone Resistance in Experimental and Ulcerative Colitis. Int J Mol Sci. 2017;18:2046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 133. | Alonzi T, Newton IP, Bryce PJ, Di Carlo E, Lattanzio G, Tripodi M, Musiani P, Poli V. Induced somatic inactivation of STAT3 in mice triggers the development of a fulminant form of enterocolitis. Cytokine. 2004;26:45-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 134. | Welte T, Zhang SS, Wang T, Zhang Z, Hesslein DG, Yin Z, Kano A, Iwamoto Y, Li E, Craft JE, Bothwell AL, Fikrig E, Koni PA, Flavell RA, Fu XY. STAT3 deletion during hematopoiesis causes Crohn's disease-like pathogenesis and lethality: a critical role of STAT3 in innate immunity. Proc Natl Acad Sci USA. 2003;100:1879-1884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 314] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 135. | Nieminen JK, Niemi M, Sipponen T, Salo HM, Klemetti P, Färkkilä M, Vakkila J, Vaarala O. Dendritic cells from Crohn's disease patients show aberrant STAT1 and STAT3 signaling. PLoS One. 2013;8:e70738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 136. | Barrett JC, Hansoul S, Nicolae DL, Cho JH, Duerr RH, Rioux JD, Brant SR, Silverberg MS, Taylor KD, Barmada MM, Bitton A, Dassopoulos T, Datta LW, Green T, Griffiths AM, Kistner EO, Murtha MT, Regueiro MD, Rotter JI, Schumm LP, Steinhart AH, Targan SR, Xavier RJ; NIDDK IBD Genetics Consortium, Libioulle C, Sandor C, Lathrop M, Belaiche J, Dewit O, Gut I, Heath S, Laukens D, Mni M, Rutgeerts P, Van Gossum A, Zelenika D, Franchimont D, Hugot JP, de Vos M, Vermeire S, Louis E; Belgian-French IBD Consortium; Wellcome Trust Case Control Consortium, Cardon LR, Anderson CA, Drummond H, Nimmo E, Ahmad T, Prescott NJ, Onnie CM, Fisher SA, Marchini J, Ghori J, Bumpstead S, Gwilliam R, Tremelling M, Deloukas P, Mansfield J, Jewell D, Satsangi J, Mathew CG, Parkes M, Georges M, Daly MJ. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn's disease. Nat Genet. 2008;40:955-962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2114] [Cited by in RCA: 2044] [Article Influence: 120.2] [Reference Citation Analysis (0)] |
| 137. | Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA, Essers J, Mitrovic M, Ning K, Cleynen I, Theatre E, Spain SL, Raychaudhuri S, Goyette P, Wei Z, Abraham C, Achkar JP, Ahmad T, Amininejad L, Ananthakrishnan AN, Andersen V, Andrews JM, Baidoo L, Balschun T, Bampton PA, Bitton A, Boucher G, Brand S, Büning C, Cohain A, Cichon S, D'Amato M, De Jong D, Devaney KL, Dubinsky M, Edwards C, Ellinghaus D, Ferguson LR, Franchimont D, Fransen K, Gearry R, Georges M, Gieger C, Glas J, Haritunians T, Hart A, Hawkey C, Hedl M, Hu X, Karlsen TH, Kupcinskas L, Kugathasan S, Latiano A, Laukens D, Lawrance IC, Lees CW, Louis E, Mahy G, Mansfield J, Morgan AR, Mowat C, Newman W, Palmieri O, Ponsioen CY, Potocnik U, Prescott NJ, Regueiro M, Rotter JI, Russell RK, Sanderson JD, Sans M, Satsangi J, Schreiber S, Simms LA, Sventoraityte J, Targan SR, Taylor KD, Tremelling M, Verspaget HW, De Vos M, Wijmenga C, Wilson DC, Winkelmann J, Xavier RJ, Zeissig S, Zhang B, Zhang CK, Zhao H; International IBD Genetics Consortium (IIBDGC), Silverberg MS, Annese V, Hakonarson H, Brant SR, Radford-Smith G, Mathew CG, Rioux JD, Schadt EE, Daly MJ, Franke A, Parkes M, Vermeire S, Barrett JC, Cho JH. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3979] [Cited by in RCA: 3594] [Article Influence: 276.5] [Reference Citation Analysis (0)] |
| 138. | Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661-678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7741] [Cited by in RCA: 7152] [Article Influence: 397.3] [Reference Citation Analysis (0)] |
| 139. | Liu QF, Li Y, Zhao QH, Wang ZY, Hu S, Yang CQ, Ye K, Li L. Association of STAT4 rs7574865 polymorphism with susceptibility to inflammatory bowel disease: A systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2015;39:627-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 140. | Yang SK, Jung Y, Kim H, Hong M, Ye BD, Song K. Association of FCGR2A, JAK2 or HNF4A variants with ulcerative colitis in Koreans. Dig Liver Dis. 2011;43:856-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 141. | Anderson CA, Massey DC, Barrett JC, Prescott NJ, Tremelling M, Fisher SA, Gwilliam R, Jacob J, Nimmo ER, Drummond H, Lees CW, Onnie CM, Hanson C, Blaszczyk K, Ravindrarajah R, Hunt S, Varma D, Hammond N, Lewis G, Attlesey H, Watkins N, Ouwehand W, Strachan D, McArdle W, Lewis CM; Wellcome Trust Case Control Consortium, Lobo A, Sanderson J, Jewell DP, Deloukas P, Mansfield JC, Mathew CG, Satsangi J, Parkes M. Investigation of Crohn's disease risk loci in ulcerative colitis further defines their molecular relationship. Gastroenterology. 2009;136:523-529.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 152] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 142. | Zhang JX, Song J, Wang J, Dong WG. JAK2 rs10758669 polymorphisms and susceptibility to ulcerative colitis and Crohn's disease: a meta-analysis. Inflammation. 2014;37:793-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 143. | Hedl M, Proctor DD, Abraham C. JAK2 Disease-Risk Variants Are Gain of Function and JAK Signaling Threshold Determines Innate Receptor-Induced Proinflammatory Cytokine Secretion in Macrophages. J Immunol. 2016;197:3695-3704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 144. | Cleynen I, González JR, Figueroa C, Franke A, McGovern D, Bortlík M, Crusius BJ, Vecchi M, Artieda M, Szczypiorska M, Bethge J, Arteta D, Ayala E, Danese S, van Hogezand RA, Panés J, Peña SA, Lukas M, Jewell DP, Schreiber S, Vermeire S, Sans M. Genetic factors conferring an increased susceptibility to develop Crohn's disease also influence disease phenotype: results from the IBDchip European Project. Gut. 2013;62:1556-1565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 215] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 145. | Ferguson LR, Han DY, Fraser AG, Huebner C, Lam WJ, Morgan AR, Duan H, Karunasinghe N. Genetic factors in chronic inflammation: single nucleotide polymorphisms in the STAT-JAK pathway, susceptibility to DNA damage and Crohn's disease in a New Zealand population. Mutat Res. 2010;690:108-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 146. | Prager M, Büttner J, Haas V, Baumgart DC, Sturm A, Zeitz M, Büning C. The JAK2 variant rs10758669 in Crohn's disease: altering the intestinal barrier as one mechanism of action. Int J Colorectal Dis. 2012;27:565-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 147. | Can G, Tezel A, Gürkan H, Can H, Yılmaz B, Ünsal G, Soylu AR, Ümit HC. Tyrosine kinase-2 gene polymorphisms are associated with ulcerative colitis and Crohn's disease in Turkish Population. Clin Res Hepatol Gastroenterol. 2015;39:489-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 148. | Lian LH, Lau TP, Lee VL, Lee WS, Hilmi I, Goh KL, Chua KH. Lack of association between TYK2 and STAT3 genes and Crohn's disease in the Malaysian population. Genet Mol Res. 2013;12:167-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 149. | Sato K, Shiota M, Fukuda S, Iwamoto E, Machida H, Inamine T, Kondo S, Yanagihara K, Isomoto H, Mizuta Y, Kohno S, Tsukamoto K. Strong evidence of a combination polymorphism of the tyrosine kinase 2 gene and the signal transducer and activator of transcription 3 gene as a DNA-based biomarker for susceptibility to Crohn's disease in the Japanese population. J Clin Immunol. 2009;29:815-825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 150. | Anderson CA, Boucher G, Lees CW, Franke A, D'Amato M, Taylor KD, Lee JC, Goyette P, Imielinski M, Latiano A, Lagacé C, Scott R, Amininejad L, Bumpstead S, Baidoo L, Baldassano RN, Barclay M, Bayless TM, Brand S, Büning C, Colombel JF, Denson LA, De Vos M, Dubinsky M, Edwards C, Ellinghaus D, Fehrmann RS, Floyd JA, Florin T, Franchimont D, Franke L, Georges M, Glas J, Glazer NL, Guthery SL, Haritunians T, Hayward NK, Hugot JP, Jobin G, Laukens D, Lawrance I, Lémann M, Levine A, Libioulle C, Louis E, McGovern DP, Milla M, Montgomery GW, Morley KI, Mowat C, Ng A, Newman W, Ophoff RA, Papi L, Palmieri O, Peyrin-Biroulet L, Panés J, Phillips A, Prescott NJ, Proctor DD, Roberts R, Russell R, Rutgeerts P, Sanderson J, Sans M, Schumm P, Seibold F, Sharma Y, Simms LA, Seielstad M, Steinhart AH, Targan SR, van den Berg LH, Vatn M, Verspaget H, Walters T, Wijmenga C, Wilson DC, Westra HJ, Xavier RJ, Zhao ZZ, Ponsioen CY, Andersen V, Torkvist L, Gazouli M, Anagnou NP, Karlsen TH, Kupcinskas L, Sventoraityte J, Mansfield JC, Kugathasan S, Silverberg MS, Halfvarson J, Rotter JI, Mathew CG, Griffiths AM, Gearry R, Ahmad T, Brant SR, Chamaillard M, Satsangi J, Cho JH, Schreiber S, Daly MJ, Barrett JC, Parkes M, Annese V, Hakonarson H, Radford-Smith G, Duerr RH, Vermeire S, Weersma RK, Rioux JD. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat Genet. 2011;43:246-252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1095] [Cited by in RCA: 1044] [Article Influence: 74.6] [Reference Citation Analysis (1)] |
| 151. | Willson TA, Kuhn BR, Jurickova I, Gerad S, Moon D, Bonkowski E, Carey R, Collins MH, Xu H, Jegga AG, Guthery SL, Denson LA. STAT3 genotypic variation and cellular STAT3 activation and colon leukocyte recruitment in pediatric Crohn disease. J Pediatr Gastroenterol Nutr. 2012;55:32-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 152. | Glas J, Seiderer J, Nagy M, Fries C, Beigel F, Weidinger M, Pfennig S, Klein W, Epplen JT, Lohse P, Folwaczny M, Göke B, Ochsenkühn T, Diegelmann J, Müller-Myhsok B, Roeske D, Brand S. Evidence for STAT4 as a common autoimmune gene: rs7574865 is associated with colonic Crohn's disease and early disease onset. PLoS One. 2010;5:e10373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 153. | Diaz-Gallo LM, Palomino-Morales RJ, Gómez-García M, Cardeña C, Rodrigo L, Nieto A, Alcain G, Cueto I, López-Nevot MA, Martin J. STAT4 gene influences genetic predisposition to ulcerative colitis but not Crohn's disease in the Spanish population: a replication study. Hum Immunol. 2010;71:515-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 154. | Huang C, Haritunians T, Okou DT, Cutler DJ, Zwick ME, Taylor KD, Datta LW, Maranville JC, Liu Z, Ellis S, Chopra P, Alexander JS, Baldassano RN, Cross RK, Dassopoulos T, Dhere TA, Duerr RH, Hanson JS, Hou JK, Hussain SZ, Isaacs KL, Kachelries KE, Kader H, Kappelman MD, Katz J, Kellermayer R, Kirschner BS, Kuemmerle JF, Kumar A, Kwon JH, Lazarev M, Mannon P, Moulton DE, Osuntokun BO, Patel A, Rioux JD, Rotter JI, Saeed S, Scherl EJ, Silverberg MS, Silverman A, Targan SR, Valentine JF, Wang MH, Simpson CL, Bridges SL, Kimberly RP, Rich SS, Cho JH, Rienzo AD, Kao LWH, McGovern DPB, Brant SR, Kugathasan S. Characterization of genetic loci that affect susceptibility to inflammatory bowel diseases in African Americans. Gastroenterology. 2015;149:1575-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 155. | Klein W, Tromm A, Folwaczny C, Hagedorn M, Duerig N, Epplen J, Schmiegel W, Griga T. The G2964A polymorphism of the STAT6 gene in inflammatory bowel disease. Dig Liver Dis. 2005;37:159-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 156. | Xia B, Crusius JB, Wu J, Zwiers A, van Bodegraven AA, Peña AS. Signal transducer and activator of transcription 6 gene G2964A polymorphism and inflammatory bowel disease. Clin Exp Immunol. 2003;131:446-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 157. | Chua KH, Ng JG, Ng CC, Hilmi I, Goh KL, Kee BP. Association of NOD1, CXCL16, STAT6 and TLR4 gene polymorphisms with Malaysian patients with Crohn's disease. PeerJ. 2016;4:e1843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 158. | de Jong DJ, Franke B, Naber AH, Willemen JJ, Heister AJ, Brunner HG, de Kovel CG, Hol FA. No evidence for involvement of IL-4R and CD11B from the IBD1 region and STAT6 in the IBD2 region in Crohn's disease. Eur J Hum Genet. 2003;11:884-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 159. | Rodig SJ, Meraz MA, White JM, Lampe PA, Riley JK, Arthur CD, King KL, Sheehan KC, Yin L, Pennica D, Johnson EM, Schreiber RD. Disruption of the Jak1 gene demonstrates obligatory and nonredundant roles of the Jaks in cytokine-induced biologic responses. Cell. 1998;93:373-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 622] [Cited by in RCA: 636] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 160. | Sakamoto K, Wehde BL, Rädler PD, Triplett AA, Wagner KU. Generation of Janus kinase 1 (JAK1) conditional knockout mice. Genesis. 2016;54:582-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 161. | Aringer M, Cheng A, Nelson JW, Chen M, Sudarshan C, Zhou YJ, O'Shea JJ. Janus kinases and their role in growth and disease. Life Sci. 1999;64:2173-2186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 162. | Baird AM, Thomis DC, Berg LJ. T cell development and activation in Jak3-deficient mice. J Leukoc Biol. 1998;63:669-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 163. | Rossin EJ, Lage K, Raychaudhuri S, Xavier RJ, Tatar D, Benita Y; International Inflammatory Bowel Disease Genetics Constortium, Cotsapas C, Daly MJ. Proteins encoded in genomic regions associated with immune-mediated disease physically interact and suggest underlying biology. PLoS Genet. 2011;7:e1001273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 412] [Cited by in RCA: 378] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 164. | Zhang J, Wu J, Peng X, Song J, Wang J, Dong W. Associations between STAT3 rs744166 polymorphisms and susceptibility to ulcerative colitis and Crohn's disease: a meta-analysis. PLoS One. 2014;9:e109625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 165. | Brownlie RJ, Miosge LA, Vassilakos D, Svensson LM, Cope A, Zamoyska R. Lack of the phosphatase PTPN22 increases adhesion of murine regulatory T cells to improve their immunosuppressive function. Sci Signal. 2012;5:ra87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 166. | Fousteri G, Jofra T, Debernardis I, Stanford SM, Laurenzi A, Bottini N, Battaglia M. The protein tyrosine phosphatase PTPN22 controls forkhead box protein 3 T regulatory cell induction but is dispensable for T helper type 1 cell polarization. Clin Exp Immunol. 2014;178:178-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 167. | Sanchez-Blanco C, Clarke F, Cornish GH, Depoil D, Thompson SJ, Dai X, Rawlings DJ, Dustin ML, Zamoyska R, Cope AP, Purvis HA. Protein tyrosine phosphatase PTPN22 regulates LFA-1 dependent Th1 responses. J Autoimmun. 2018;94:45-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 168. | Diaz-Gallo LM, Espino-Paisán L, Fransen K, Gómez-García M, van Sommeren S, Cardeña C, Rodrigo L, Mendoza JL, Taxonera C, Nieto A, Alcain G, Cueto I, López-Nevot MA, Bottini N, Barclay ML, Crusius JB, van Bodegraven AA, Wijmenga C, Ponsioen CY, Gearry RB, Roberts RL, Weersma RK, Urcelay E, Merriman TR, Alizadeh BZ, Martin J. Differential association of two PTPN22 coding variants with Crohn's disease and ulcerative colitis. Inflamm Bowel Dis. 2011;17:2287-2294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 169. | Zhang JX, He JH, Wang J, Song J, Lei HB, Wang J, Dong WG. Associations between PTPN2 polymorphisms and susceptibility to ulcerative colitis and Crohn's disease: a meta-analysis. Inflamm Res. 2014;63:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 170. | Takagi K, Takagi M, Kanangat S, Warrington KJ, Shigemitsu H, Postlethwaite AE. Modulation of TNF-alpha gene expression by IFN-gamma and pamidronate in murine macrophages: regulation by STAT1-dependent pathways. J Immunol. 2005;174:1801-1810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 171. | Narumi Y, Isomoto H, Shiota M, Sato K, Kondo S, Machida H, Yanagihara K, Mizuta Y, Kohno S, Tsukamoto K. Polymorphisms of PTPN11 coding SHP-2 as biomarkers for ulcerative colitis susceptibility in the Japanese population. J Clin Immunol. 2009;29:303-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 172. | Liu W, Guo W, Shen L, Chen Z, Luo Q, Luo X, Feng G, Shu Y, Gu Y, Xu Q, Sun Y. T lymphocyte SHP2-deficiency triggers anti-tumor immunity to inhibit colitis-associated cancer in mice. Oncotarget. 2017;8:7586-7597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 173. | Pidasheva S, Trifari S, Phillips A, Hackney JA, Ma Y, Smith A, Sohn SJ, Spits H, Little RD, Behrens TW, Honigberg L, Ghilardi N, Clark HF. Functional studies on the IBD susceptibility gene IL23R implicate reduced receptor function in the protective genetic variant R381Q. PLoS One. 2011;6:e25038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 174. | U.S. Food Drug Administration (FDA). FDA approves Boxed Warning about increased risk of blood clots and death with higher dose of arthritis and ulcerative colitis medicine tofacitinib (Xeljanz, Xeljanz XR). In: Fda.gov [Internet]. Accessed 2020 Jan. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-approves-boxed-warning-about-increased-risk-blood-clots-and-death-higher-dose-arthritis. |
| 175. | European Medicines Agency. Rinvoq: EPAR - Medicine overview. In: Ema.europa.eu [Internet]. Accessed 2020 Jan 20. Available from: https://www.ema.europa.eu/en/documents/overview/rinvoq-epar-medicine-overview_en.pdf . |
| 176. | Duggan S, Keam SJ. Upadacitinib: First Approval. Drugs. 2019;79:1819-1828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 177. | Vermeire S, Schreiber S, Petryka R, Kuehbacher T, Hebuterne X, Roblin X, Klopocka M, Goldis A, Wisniewska-Jarosinska M, Baranovsky A, Sike R, Stoyanova K, Tasset C, Van der Aa A, Harrison P. Clinical remission in patients with moderate-to-severe Crohn's disease treated with filgotinib (the FITZROY study): results from a phase 2, double-blind, randomised, placebo-controlled trial. Lancet. 2017;389:266-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 336] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 178. | Sandborn WJ, Feagan BG, Panes J, D’Haens GR, Colombel JF, Zhou Q, Huang B, Enejosa JV, Pangan AL, Lacerda AP. Safety and Efficacy of ABT-494 (Upadacitinib), an Oral Jak1 Inhibitor, as Induction Therapy in Patients with Crohn's Disease: Results from Celest. Gastroenterology. 2017;152:S1308–S1309. [DOI] [Full Text] |
| 179. | Xie JH, Gillooly K, Zhang Y, Yang X, Zupa-Fernandez A, Cheng L, Strnad J, Heimrich E, Zhou X, Chen J, Chaudhry C, Li S, Moslin R, Wrobleski S, Burke J. 349 – BMS-986165 Is a Highly Potent and Selective Allosteric Inhibitor of TYK2, Blocks IL-12, IL-23 and Type I Interferon Signaling and Provides for Robust Efficacy in Preclinical Models of Inflammatory Bowel Disease. Gastroenterology. 2018;154:S-1357. [DOI] [Full Text] |
| 180. | ClinicalTrials. STAT inhibitor clinical trials. In: ClinicalTrials.gov [Internet]. Accessed 2020 Jan. Available from: https://clinicaltrials.gov/ct2/results?cond=term=intr=STAT+inhibitorcntry=state=city=dist=Search=Searchtype=Intr . |
| 181. | Yarilina A, Xu K, Chan C, Ivashkiv LB. Regulation of inflammatory responses in tumor necrosis factor-activated and rheumatoid arthritis synovial macrophages by JAK inhibitors. Arthritis Rheum. 2012;64:3856-3866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 182. | Pattison MJ, Mackenzie KF, Arthur JS. Inhibition of JAKs in macrophages increases lipopolysaccharide-induced cytokine production by blocking IL-10-mediated feedback. J Immunol. 2012;189:2784-2792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 183. | Wang H, Brown J, Gao S, Liang S, Jotwani R, Zhou H, Suttles J, Scott DA, Lamont RJ. The role of JAK-3 in regulating TLR-mediated inflammatory cytokine production in innate immune cells. J Immunol. 2013;191:1164-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 184. | Bain CC, Bravo-Blas A, Scott CL, Perdiguero EG, Geissmann F, Henri S, Malissen B, Osborne LC, Artis D, Mowat AM. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat Immunol. 2014;15:929-937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 905] [Cited by in RCA: 863] [Article Influence: 78.5] [Reference Citation Analysis (0)] |
| 185. | Rivollier A, He J, Kole A, Valatas V, Kelsall BL. Inflammation switches the differentiation program of Ly6Chi monocytes from antiinflammatory macrophages to inflammatory dendritic cells in the colon. J Exp Med. 2012;209:139-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 415] [Cited by in RCA: 448] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 186. | Li Y, de Haar C, Peppelenbosch MP, van der Woude CJ. New insights into the role of STAT3 in IBD. Inflamm Bowel Dis. 2012;18:1177-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 187. | Marafini I, Angelucci E, Pallone F, Monteleone G. The IL-12/23/STAT Axis as a Therapeutic Target in Inflammatory Bowel Disease: Mechanisms and Evidence in Man. Dig Dis. 2015;33 Suppl 1:113-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 188. | Schmetterer KG, Pickl WF. The IL-10/STAT3 axis: Contributions to immune tolerance by thymus and peripherally derived regulatory T-cells. Eur J Immunol. 2017;47:1256-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 189. | Han J, Theiss AL. Stat3: friend or foe in colitis and colitis-associated cancer? Inflamm Bowel Dis. 2014;20:2405-2411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |