Published online Jul 21, 2020. doi: 10.3748/wjg.v26.i27.3963
Peer-review started: March 26, 2020
First decision: April 25, 2020
Revised: May 6, 2020
Accepted: July 4, 2020
Article in press: July 4, 2020
Published online: July 21, 2020
Processing time: 117 Days and 3.7 Hours
The Korea National Cancer Screening Program currently provides screening for colorectal cancer (CRC) for adults older than 50 years with no upper age limit. In general, people are likely to only pay attention to the benefits of cancer screening and to neglect its risks. Most consider the benefits of cancer screening as being far greater than the risks and are unaware that any potential benefits and harms can vary with age.
To report acceptance of an upper age limit for CRC screening and factors associated therewith among cancer-free individuals in Korea.
The present study analyzed data from the Korea National Cancer Screening Survey 2017, a nationally representative random sample of 4500 Korean individuals targeted for screening for the five most common types of cancer. A total of 1922 participants were included in the final analysis. The baseline characteristics of the study population are presented as unweighted numbers and weighted proportions. Both univariate and multivariate logistic regression models were developed to examine factors related with acceptance of an upper age limit for CRC screening; subgroup analysis was also applied.
About 80% (1554/1922) of the respondents agreed that CRC screening should not be offered for individuals older than 80 years. Specifically, those who had never been screened for CRC had the highest acceptance rate (91%). Overall, screening history for CRC [screened by both fecal occult blood test and colonoscopy, adjusted odds ratio (aOR) = 0.33, 95%CI: 0.22-0.50] and other cancers (aOR = 0.55, 95%CI: 0.34-0.87), as well as a family history of cancer (aOR = 0.66, 95%CI: 0.50-0.87), were negatively associated with acceptance of an upper age limit for CRC screening. In contrast, metropolitan residents (aOR = 1.86, 95%CI: 1.29-2.68) and people who exercised regularly (aOR = 1.42, 95%CI: 1.07-1.89) were more likely to accept an upper age limit. After subgrouping, we found gender, marital status, and lifetime smoking history among never-screened individuals and residential region, family history of cancer, and physical activity among never-screened individuals to be associated with acceptance of an upper age limit.
This study describes acceptance of an upper age limit for CRC screening and factors associated with it, and provides perspectives that should be considered, in addition to scientific evidence, when developing population-based cancer screening policies and programs.
Core tip: Although several guidelines recommend setting an upper age for colorectal cancer (CRC) screening, there is a lack of information on perceptions and acceptance thereof. By analyzing data from a national representative survey in South Korea, we were able to determine the acceptance of an upper age limit for CRC screening and factors associated therewith among cancer-free individuals targeted for screening in South Korea.
- Citation: Luu XQ, Lee K, Lee YY, Suh M, Kim Y, Choi KS. Acceptance on colorectal cancer screening upper age limit in South Korea. World J Gastroenterol 2020; 26(27): 3963-3974
- URL: https://www.wjgnet.com/1007-9327/full/v26/i27/3963.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i27.3963
Colorectal cancer (CRC) is one of the most common types of cancer worldwide, with the fourth highest incidence among both sexes and the third highest among men in 2018[1]. In general, CRC is more common in countries with a high Human Development Index score and in Western countries[1]. Meanwhile, screening for CRC is recognized as an effective intervention through which to reduce the numbers of new cancer cases and cancer deaths[2-5]. To reduce the burden of CRC, population-based screening programs with a variety of screening tests, such as colonoscopy, flexible sigmoidoscopy, double-contrast barium enema, and fecal occult blood test (FOBT), have been adopted by many developed countries[6]: The United States Preventive Services Task Force recommends colorectal cancer screening for people aged 50 to 75 years, and for adults aged 76 to 85 years, decisions on CRC screening should reflect the patient’s overall health and their screening history[7]. According to recommendations from the European Colorectal Cancer Screening Guidelines Working Group, FOBT as a primary test in screening programs is effective in reducing mortality among people aged 45-80 years at a screening interval of 2 years; colonoscopy-based screening programs are beneficial for individuals aged 50-74 years[8]. A recently published systematic review showed that the majority of guidelines recommend an age range of 50 to 70 or 75 years as appropriate for CRC screening[9].
Although research on an appropriate age at which to stop CRC screening is limited, a few reasons for setting an upper age limit for CRC screening have been given: One is that older adults involve higher risks of complications due to treatment and screening, particularly colonoscopy, which can be invasive and requires bowel preparation[10-13]. Another is the potential for physiological harm. Parker et al[14] indicated that a positive FOBT test results in a high level of anxiety in patients that returns to normal with negative results. In addition, with increasing age, life expectancy decreases, and this can differ with the presence of comorbidities[15]. Although there is no gold standard for deciding when to stop CRC screening, age is the most common criterion. However, some studies have shown that people are not likely to comply with age-based stoppage recommendations[16-18]. Indeed, about a half of the respondents in a study in England indicated that doctors should never use age as a criterion for deciding when to stop screening, even with a normal colonoscopy test result in previous years[16].
In South Korea, CRC is a major public health concern, as the second most commonly diagnosed cancer type in 2016, increasing in incidence with age up to 85 years[19]. The National Screening Guidelines for CRC in South Korea were developed by a multi-society expert committee in 2015. The committee systematically reviewed the selected screening guidelines and results of randomized controlled trials and considered the incidences of CRC for individual age groups and according to life expectancy. With a life expectancy of 79.9 for men and 85.7 years for women, The Korea National Cancer Center has recommended CRC screening for adults aged 45 to 80 years, as there is insufficient evidence of the benefits of CRC screening after the age of 80 years[20]. Notwithstanding, the Korea National Cancer Screening Program does currently provide biennial FOBT and either colonoscopy or double contrast barium enema as a follow-up test for CRC screening for adults aged over 50 years with no upper limited age[21].
In general, people are likely to only pay attention to the benefits of cancer screening and to neglect its risks. Most consider the benefits of cancer screening as being far greater than the risks and are unaware that any potential benefits and harms can vary with age. Although several CRC screening guidelines recommend setting an upper age, there is a lack of information on perceptions and acceptance of an upper age limit for CRC screening. Accordingly, using a national representative survey of cancer screening in South Korea, our study sought to investigate acceptance of an upper age limit for CRC screening and factors associated therewith among cancer-free individuals targeted for screening in South Korea.
In this study, we used data on 4500 Koreans from the Korean National Cancer Screening Survey (KNCSS) 2017. The data resource profile of the KNCSS has been well described in the literature[21]. In brief, the KNCSS is a nationally represenative, cross-sectional survey covering the five most common types of cancer, including stomach, liver, colorectal, breast, and cervical cancer. Subjects are selected by a multi-stage random sampling method that is stratified by sex, age, and residence area based on annual estimates from the National Statistical Office. The survey focuses on behavioral patterns related to cancer screening. Cancer-free men aged 40 years or over and cancer-free women aged 30 years or over are eligible for inclusion in the KNCSS. In this study, we included only men and women aged 50 years and older who were targeted for CRC screening in the Korea National Cancer Screening Program. A total of 1922 men and women were included in the final analysis. This study was approved by the Institutional Review Board of the National Cancer Center, South Korea (approval number: NCC2019-0233).
Acceptance of an upper age limit for CRC screening was assessed using an informative question that first explained CRC screening recommendations, focusing on an appropriate age for CRC screening, and then asked whether screening should be stopped at an age of 80 years as recommended by the National Cancer Center of Korea. If the participant responded “no,” they were then asked to share their preference for an alternative age at which to stop CRC screening.
Using a structured questionnaire, the participants were asked about their experiences with screening for CRC. The questions included “Have you ever undergone CRC screening?” and, if so, “When did you last undergo CRC screening?” and “What tests did you receive for CRC screening?” Screening status was defined as “screened” for those who had ever undergone FOBT in the past or who ever had a colonoscopy. Otherwise, participants were considered as “non-screened.” Screened individuals were further classified into the following three groups: screened by FOBT only, colonoscopy only, or both. Socio-demographic characteristics, health status, and health-related behaviors were also examined.
The baseline characteristics of the study population are presented as unweighted numbers and weighted proportions. Logistic regression was applied to identify associations between acceptance of an upper age limit and CRC screening history, as well as other factors. First, univariate logistic regression models were used to examine associations for acceptance of an upper age limit with individual factors. Variables with a P value less than 0.05 in the univariate model were included in a multivariable regression model. A final regression model was developed with stratification according to CRC screening history. All statistical analyses were conducted using SAS statistical software (version 9.2, SAS Institute Inc., Cary, NC, United States).
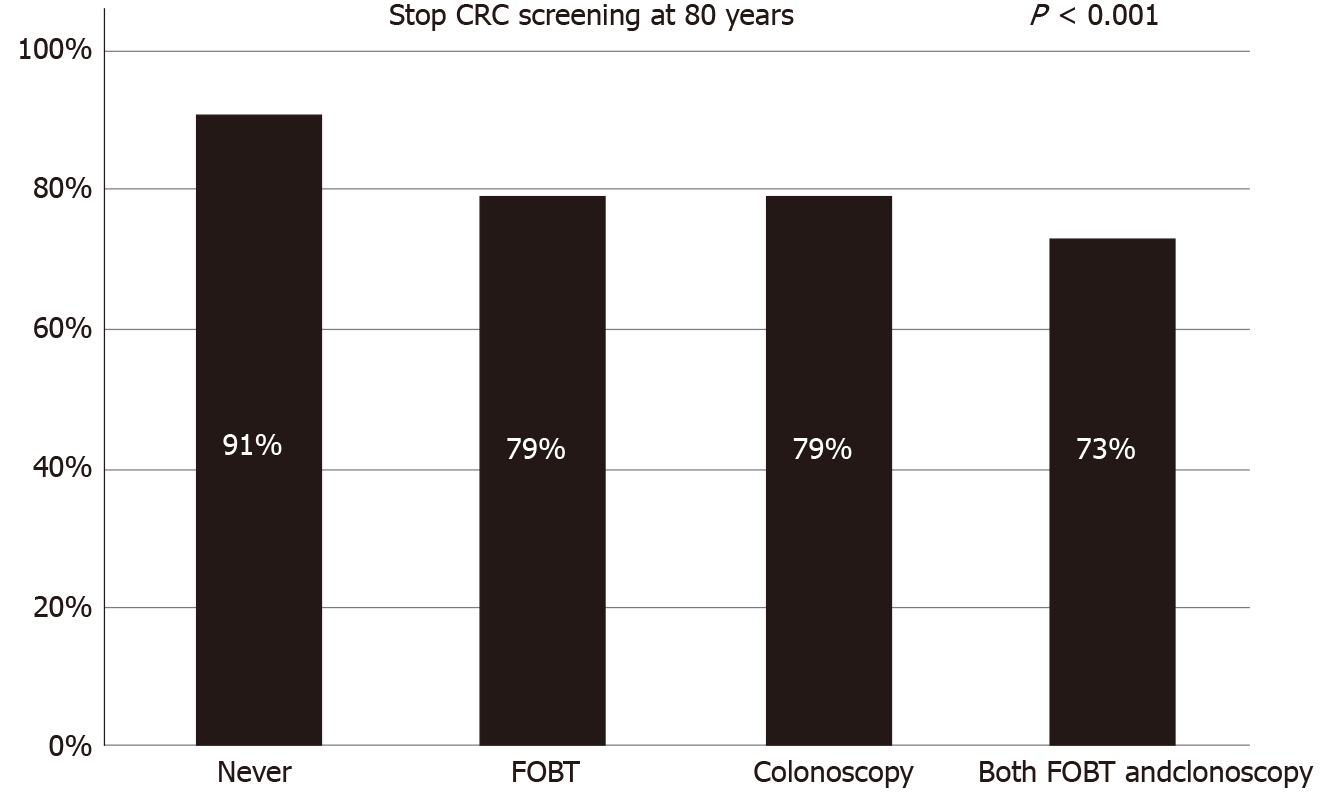
The baseline characteristics of the 1922 participants are presented in Table 1. Overall, 51.8% of the study population was female, and 53% was aged 50-59 years. The majority of the respondents had completed middle school or high school (73.9%), were married (94.7%), and had no family history of cancer (78.5%). About 26% of the participants reported having never been screened for CRC; the histories of CRC screening by FOBT only, colonoscopy only, and both were 35.8%, 15.6%, and 22.4%, respectively. Overall, 80.8% of the respondents agreed with stopping CRC screening at the age of 80 years. Individuals aged 50-59 years, men, metropolitan residents, people living with a spouse, smokers, heavy drinkers, and those who exercised regularly were more in favor of stopping CRC screening at an age of 80 years. Also, those who had never been examined for CRC or any other type of cancer and those without a family history of cancer reported higher acceptance rates for an upper age limit of 80 years. More specifically, those who had never been screened for CRC had the highest acceptance rate (91%), while those who had been examined for CRC through both FOBT and colonoscopy had the lowest acceptance rate (73%) (Figure 1). People who did disagree with an upper age limit at age 80 years reported a preferred alternative age of a mean of 89.8 years (median: 90.0).
| Variables | Total (n = 1922) | Stop CRC screening at 80 years | ||
| No (n = 368) | Yes (n = 1554) | P value | ||
| Age group | ||||
| 50-59 | 1025 (53.3) | 192 (52.2) | 833 (53.6) | 0.002 |
| 60-69 | 675 (35.1) | 115 (31.3) | 560 (36.0) | |
| 70-74 | 222 (11.6) | 61 (16.6) | 161 (10.4) | |
| Sex | ||||
| Male | 927 (48.2) | 153 (41.6) | 774 (49.8) | 0.004 |
| Female | 995 (51.8) | 215 (58.4) | 780 (50.2) | |
| Residential region | ||||
| Rural | 259 (13.5) | 68 (18.5) | 191 (12.3) | < 0.0001 |
| Urban | 779 (40.5) | 170 (46.2) | 629 (40.5) | |
| Metropolitan | 864 (45.0) | 130 (35.3) | 734 (47.2) | |
| Education | ||||
| No formal/Elementary school | 155 (8.1) | 52 (14.1) | 103 (6.6) | < 0.0001 |
| Middle/High school | 1421 (73.9) | 249 (67.7) | 1172 (75.4) | |
| College or more | 346 (18.0) | 67 (18.2) | 279 (18) | |
| Occupation status | ||||
| Employed | 1258 (65.5) | 240 (65.2) | 1018 (65.5) | 0.916 |
| Unemployed/Housewife | 664 (34.6) | 128 (34.8) | 536 (34.5) | |
| Household monthly income (USD) | ||||
| < 3000 | 614 (32) | 135 (36.7) | 479 (30.8) | 0.077 |
| 3000-5000 | 955 (49.7) | 166 (45.1) | 789 (50.8) | |
| > 5000 | 353 (18.4) | 67 (18.2) | 286 (18.4) | |
| Marital status | ||||
| Living without spouse | 102 (5.3) | 28 (7.6) | 74 (4.8) | 0.028 |
| Living with spouse | 1820 (94.7) | 340 (92.4) | 1480 (95.2) | |
| CRC screening experience | ||||
| Never | 504 (26.2) | 45 (12.2) | 459 (29.5) | < 0.0001 |
| FOBT only | 688 (35.8) | 144 (39.1) | 544 (35.0) | |
| Colonoscopy only | 299 (15.6) | 62 (16.9) | 237 (15.3) | |
| Both FOBT and colonoscopy | 431 (22.4) | 117 (31.8) | 314 (20.2) | |
| Other type of cancer screening in NCSP | ||||
| No | 337 (17.5) | 27 (7.3) | 310 (20.0) | < 0.0001 |
| Yes | 1585 (82.5) | 341 (92.7) | 1244 (80.1) | |
| Family history of cancer | ||||
| No | 1508 (78.5) | 263 (71.5) | 1245 (80.1) | < 0.0001 |
| Yes | 414 (21.5) | 105 (28.5) | 309 (19.9) | |
| Lifetime smoking | ||||
| Never or < 5 packs | 1224 (63.7) | 259 (70.4) | 965 (62.1) | 0.003 |
| More than 5 packs | 698 (36.3) | 109 (29.6) | 589 (37.9) | |
| Drinking within 1 yr | ||||
| Never | 499 (26.0) | 115 (31.3) | 384 (24.7) | 0.036 |
| Sometime | 1022 (53.2) | 183 (49.7) | 839 (54.0) | |
| Frequently | 401 (20.9) | 70 (19.0) | 331 (21.3) | |
| Exercise | ||||
| Never | 1044 (54.3) | 223 (60.6) | 821 (52.8) | 0.02 |
| 1-2 d per week | 336 (17.5) | 51 (13.9) | 285 (18.3) | |
| > 3 d per week | 542 (28.2) | 94 (25.5) | 448 (28.8) | |
In both univariate and multivariate regression models, a negative association between CRC screening history and acceptance of an upper age limit for CRC screening at 80 years was observed (Table 2). Those who had been screened for CRC through both FOBT and colonoscopy were less likely to accept the upper age limit [adjusted odds ratio (aOR) = 0.33, 95%CI: 0.22-0.50], compared with those who had never been screened. Similarly, participants who had ever been screened for any other type of cancer (aOR = 0.55, 95%CI: 0.34-0.87) and those with a family history of cancer (aOR = 0.66, 95%CI: 0.50-0.87) were less likely to accept stopping CRC screening at the age of 80 years. In contrast, people who resided in a metropolitan region (aOR = 1.86, 95%CI: 1.29-2.68) and exercised regularly (aOR = 1.42, 95%CI: 1.07-1.89) were more likely to accept the upper age limit.
| Variable | Stop CRC screening at 80 yr | |||
| Univariate | Multivariate | |||
| cOR | 95%CI | aOR | 95%CI | |
| Age group | ||||
| 50-59 | 1.00 | 1.00 | ||
| 60-69 | 1.12 | 0.87-1.45 | 1.22 | 0.92-1.64 |
| 70-74 | 0.61 | 0.44-0.85 | 0.72 | 0.47-1.11 |
| Sex | ||||
| Male | 1.00 | 1.00 | ||
| Female | 0.72 | 0.57-0.9 | 0.86 | 0.59-1.24 |
| Residential region | ||||
| Rural | 1.00 | 1.00 | ||
| Urban | 1.32 | 0.95-1.82 | 1.34 | 0.95-1.91 |
| Metropolitan | 2.01 | 1.44-2.81 | 1.86 | 1.29-2.68 |
| Education | ||||
| No formal/Elementary school | 1.00 | 1.00 | ||
| Middle/High school | 2.38 | 1.66-3.41 | 1.55 | 1.00-2.39 |
| College or more | 2.10 | 1.37-3.22 | 1.28 | 0.74-2.21 |
| Occupation status | ||||
| Employed | 1.00 | |||
| Unemployed/Housewife | 0.99 | 0.78-1.25 | ||
| Household monthly income (USD) | ||||
| < 3000 | 1.00 | 1.00 | ||
| 3000-5000 | 1.34 | 1.04-1.73 | 1.04 | 0.76-1.42 |
| > 5000 | 1.20 | 0.87-1.67 | 0.97 | 0.64-1.47 |
| Marital status | ||||
| Living without spouse | 1.00 | 1.00 | ||
| Living with spouse | 1.65 | 1.05-2.58 | 1.21 | 0.74-1.98 |
| CRC screening experiences | ||||
| Never | 1.00 | 1.00 | ||
| FOBT only | 0.37 | 0.26-0.53 | 0.50 | 0.34-0.75 |
| Colonoscopy only | 0.37 | 0.25-0.57 | 0.49 | 0.31-0.77 |
| Both FOBT and colonoscopy | 0.26 | 0.18-0.38 | 0.33 | 0.22-0.50 |
| Other type of cancer screening in NCSP | ||||
| No | 1.00 | 1.00 | ||
| Yes | 0.32 | 0.21-0.48 | 0.55 | 0.34-0.87 |
| Family history of cancer | ||||
| No | 1.00 | 1.00 | ||
| Yes | 0.62 | 0.48-0.80 | 0.66 | 0.50-0.87 |
| Lifetime smoking | ||||
| Never or < 5 packs | 1.00 | 1.00 | ||
| More than 5 packs | 1.45 | 1.13-1.86 | 1.38 | 0.95-2.02 |
| Drinking within 1 yr | ||||
| Never | 1.00 | 1.00 | ||
| Sometime | 1.37 | 1.06-1.79 | 1.06 | 0.79-1.41 |
| Frequently | 1.42 | 1.02-1.97 | 0.87 | 0.59-1.3 |
| Exercise | ||||
| Never | 1.00 | 1.00 | ||
| 1-2 d per week | 1.52 | 1.09-2.12 | 1.37 | 0.97-1.95 |
| > 3 d per week | 1.29 | 0.99-1.69 | 1.42 | 1.07-1.89 |
Table 3 shows the results of multivariate regression analysis with stratification according to CRC screening experience. Among never-screened individuals, women were less likely to agree with 80 years as a good age at which to stop CRC screening (aOR = 0.25, 95%CI: 0.08-0.82). However, participants living with their spouse (aOR = 3.71, 95%CI: 1.10-12.48) or who had smoked more than five packages in their lifetime (aOR = 7.1, 95%CI: 1.2-40) were more likely to accept the upper age limit. Among ever-screened people, metropolitan residents (aOR = 1.80, 95%CI: 1.23-2.65) and individuals who exercised at least one time per week (one to two days per week aOR = 1.50, 95%CI: 1.02-2.20; more than three days per week (aOR = 1.49, 95%CI: 1.10-2.02) were more likely to accept the upper age limit for CRC screening. Respondents who had a family history of cancer were less likely to accept the CRC screening threshold (aOR = 0.61, 95%CI: 0.46-0.82).
| Variable | Stop CRC screening at 80 yr | |||
| Never-screened (n = 504) | Ever-screened (n = 1418) | |||
| aOR | 95%CI | aOR | 95%CI | |
| Age group | ||||
| 50-59 | 1.00 | 1.00 | ||
| 60-69 | 1.46 | 0.62-3.42 | 1.16 | 0.85-1.59 |
| 70-74 | 0.60 | 0.22-1.66 | 0.71 | 0.44-1.13 |
| Sex | ||||
| Male | 1.00 | 1.00 | ||
| Female | 0.25 | 0.08-0.82 | 1.07 | 0.72-1.59 |
| Residential region | ||||
| Rural | 1.00 | 1.00 | ||
| Urban | 2.25 | 0.69-7.34 | 1.21 | 0.84-1.75 |
| Metropolitan | 2.95 | 0.85-10.21 | 1.80 | 1.23-2.65 |
| Education | ||||
| No formal/Elementary school | 1.00 | 1.00 | 1.00 | |
| Middle/High school | 0.86 | 0.14-5.17 | 1.56 | 1.00-2.46 |
| College or more | 0.66 | 0.09-4.86 | 1.37 | 0.76-2.45 |
| Household monthly income (USD) | ||||
| < 3000 | 1.00 | 1.00 | ||
| 3000-5000 | 1.51 | 0.6-3.84 | 0.95 | 0.68-1.32 |
| > 5000 | 0.77 | 0.25-2.36 | 0.98 | 0.63-1.54 |
| Marital status | ||||
| Living without spouse | 1.00 | 1.00 | ||
| Living with spouse | 3.71 | 1.10-12.48 | 1.02 | 0.60-1.76 |
| Other type of cancer screening in NCSP | ||||
| No | 1.00 | 1.00 | ||
| Yes | 0.81 | 0.39-1.66 | 0.56 | 0.29-1.05 |
| Family history of cancer | ||||
| No | 1.00 | 1.00 | ||
| Yes | 0.90 | 0.36-2.25 | 0.61 | 0.46-0.82 |
| Lifetime smoking | ||||
| Never or < 5 packs | 1.00 | 1.00 | ||
| More than 5 packs | 7.10 | 1.24-40.61 | 1.33 | 0.89-1.99 |
| Drinking within 1 yr | ||||
| Never | 1.00 | 1.00 | ||
| Sometime | 0.72 | 0.3-1.75 | 1.14 | 0.83-1.55 |
| Frequently | 0.31 | 0.09-1.02 | 0.98 | 0.64-1.50 |
| Exercise | ||||
| Never | 1.00 | 1.00 | ||
| 1-2 d per week | 0.83 | 0.34-2.02 | 1.50 | 1.02-2.20 |
| > 3 d per week | 0.87 | 0.37-2.04 | 1.49 | 1.10-2.02 |
In this study, about 80% (1554/1922) of the participants agreed with ceasing CRC at an age of 80 years. This rate is much higher than rates reported in previous studies[16-18]. Pilot results from Lewis et al[17] indicated that 76% of respondents planned to undergo screening for colon cancer as long as they lived and that 64% thought that everyone should get CRC screening as long as they live, although the study was primarily focused on life expectancy and included a small sample size. One study of older adults in England noted that most of the participants disagreed with an age-based stoppage policy, and people wanted to continue to be invited for cancer screening rather than comply with the current stoppage age[18]. Another study of patients’ attitudes toward individualized recommendations to stop CRC screening reported that 64% of respondents found encouraging the use of age to decide when to stop screening as moderately to strongly reasonable[16]. The high rate of acceptance in the current study of setting an upper age limit at 80 years could be considered reasonable and in keeping with these studies, as the life expectancy at birth of the Korean population is very close to 80 years (82.7 years, 2017)[22], which would indicate that the participants want to undergo CRC screening until the end of their life.
Our study highlighted a negative association between CRC screening history and the acceptance of CRC screening stoppage. People who had been examined for CRC screening less favored setting an age limit for CRC screening. This finding is consistent with a study in England in which participants who had undergone CRC screening expressed stronger intentions to seek screening after the proposed upper threshold age than participants who had never undergone CRC screening[18]. One of the possible explanations for this result may be related to the belief that screening will absolutely benefit one’s health. In general, those who have been screened tend to overestimate the benefits of screening and to underestimate the harms caused by screening. Accordingly, they may misunderstand stopping cancer screening as depriving them of its benefits. Also, people who are more health-conscious are more likely to undergo screening and to want to receive screening more frequently[23,24]. Interestingly, in the current study, people with a family history of cancer and those who had ever been screened for other types of cancer were less in favor of setting an upper age limit for CRC screening.
As a golden standard in colorectal screening, colonoscopy is being increasingly used worldwide. Along with that trend, complications associated with colonoscopy are garnering increasing interest among health experts. Complications with colonoscopy can occur both during the procedure, during bowel preparation (e.g., electrolyte imbalance and dehydration), and after the colonoscopy (e.g., infection)[25]. The continuous use of colonoscopy may also pose a greater risk of serious bleeding, perforation, and cardiovascular/pulmonary-related events in older (> 65 years) and much older (> 80 years) adults, especially those who with underlying diseases[26]. Moreover, in previous studies[27,28], a higher colonic polyp prevalence was noted in older patients, for which polypectomy procedures are usually indicated, posing an additional cause of bleeding, pain, and perforation. Indeed, a systematic review and meta-analysis by Day et al[29] demonstrated that much older adults face a 70% higher risk of experiencing a colonoscopy complication overall and a 60% higher risk of perforation in comparison with younger patients. However, with careful assessment of a patient’s overall health condition and age, the risk of colonoscopy-related adverse events is relatively low for almost all age groups[26]. Thus, CRC screening at an older age should be carefully implemented such that its benefits overweighs its harms.
There are several limitations that may affect the interpretation of our results. First, the cross-sectional study design limits the ability to infer causal relationships for the noted associations. Second, information bias could have occurred due to the self-reports of history of lifetime smoking, drinking, and physical activity and the intensity thereof. Finally, we could not document awareness of the benefits and harms of screening, which can be predictors of a person’s attitudes toward screening. Future studies that account for this information in the study design and analyses could be beneficial. Despite all of the above limitations, to the best of our knowledge, this study is the first to address views on the upper threshold of age for CRC screening and associated factors in South Korea. Our study results provide perspectives that should be considered, in addition to scientific evidence, when developing population-based cancer screening policies and programs. It will facilitate the implementation of scientific evidence-based screening programs.
In conclusion, the majority of the participants in this study agreed with the recommendation of the National Cancer Center of Korea to stop CRC screening at the age of 80 years. Nevertheless, CRC screening history was found to be negatively associated with the participants’ acceptance to stop CRC screening at 80 years. In order to reduce unnecessary burden that may arise from cancer screening, it is imperative to explore ways to provide balanced information on the benefits and risks of screening, including setting an upper age limit.
Colorectal cancer (CRC) is one of the most common types of cancer worldwide. Screening for CRC is recognized as an effective intervention through which to reduce the numbers of new cancer cases and cancer deaths. In South Korea, although the Korea National Cancer Center recommends CRC screening for adults aged 45 to 80 years, the Korea National Cancer Screening Program currently provides CRC screening for individuals aged 50 years and older with no upper age limit.
In general, people are likely to only pay attention to the benefits of cancer screening and to neglect its risks. Most consider the benefits of cancer screening as being far greater than the risks and are unaware that any potential benefits and harms can vary with age. Although several CRC screening guidelines recommend setting an upper age, there is a lack of information on perceptions and acceptance of an upper age limit for CRC screening.
In this study, we aimed to investigate acceptance of an upper age limit for CRC screening and factors associated therewith among cancer-free individuals targeted for screening in South Korea.
The present study analyzed data from the Korea National Cancer Screening Survey 2017, a nationally representative survey targeted for cancer screening. A total of 1922 participants were included in the final analysis. The baseline characteristics of the study population are presented as unweighted numbers and weighted proportions. Both univariate and multivariate logistic regression models were developed to examine factors related with acceptance of an upper age limit for CRC screening. Subgroup analysis was also applied.
About 80% of the respondents agreed that CRC screening should not be offered for individuals aged older than 80 years, especially respondents who had never been screened for CRC (91%). Overall, the factors significantly associated with acceptance of an upper limit age among the respondents were residential region, cancer screening history, family history of cancer, and physical activity. By subgroup analysis, we found gender, marital status, and lifetime smoking history among never-screened individuals and residential region, family history of cancer, and physical activity among never-screened individuals to be associated with acceptance of an upper age limit.
The majority of the participants in this study agreed with the recommendation of the National Cancer Center of Korea to stop CRC screening at the age of 80 years. CRC screening history was a strong factor associated with acceptance. In order to reduce unnecessary burden of cancer screening programs, it is recommended to provide balanced information on the benefits and risks of screening.
Our study results provide perspectives that should be considered, in addition to scientific evidence, when developing population-based cancer screening policies and programs. In the future, further research on attitudes and preferences toward cancer screening policies in the general population are required.
We would like to thank the International Cooperation & Education Program (NCCRI·NCCI 52210-52211, 2020) of National Cancer Center, South Korea for supporting the education and training of Xuan Quy Luu.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sporea I S-Editor: Gong ZM L-Editor: A E-Editor: Ma YJ
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55814] [Article Influence: 7973.4] [Reference Citation Analysis (132)] |
| 2. | Kronborg O, Fenger C, Olsen J, Jørgensen OD, Søndergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996;348:1467-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1640] [Cited by in RCA: 1601] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 3. | Scholefield JH, Moss S, Sufi F, Mangham CM, Hardcastle JD. Effect of faecal occult blood screening on mortality from colorectal cancer: results from a randomised controlled trial. Gut. 2002;50:840-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 184] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 4. | Quintero E, Castells A, Bujanda L, Cubiella J, Salas D, Lanas Á, Andreu M, Carballo F, Morillas JD, Hernández C, Jover R, Montalvo I, Arenas J, Laredo E, Hernández V, Iglesias F, Cid E, Zubizarreta R, Sala T, Ponce M, Andrés M, Teruel G, Peris A, Roncales MP, Polo-Tomás M, Bessa X, Ferrer-Armengou O, Grau J, Serradesanferm A, Ono A, Cruzado J, Pérez-Riquelme F, Alonso-Abreu I, de la Vega-Prieto M, Reyes-Melian JM, Cacho G, Díaz-Tasende J, Herreros-de-Tejada A, Poves C, Santander C, González-Navarro A; COLONPREV Study Investigators. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med. 2012;366:697-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 703] [Cited by in RCA: 657] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 5. | Bretthauer M, Kaminski MF, Løberg M, Zauber AG, Regula J, Kuipers EJ, Hernán MA, McFadden E, Sunde A, Kalager M, Dekker E, Lansdorp-Vogelaar I, Garborg K, Rupinski M, Spaander MC, Bugajski M, Høie O, Stefansson T, Hoff G, Adami HO; Nordic-European Initiative on Colorectal Cancer (NordICC) Study Group. Population-Based Colonoscopy Screening for Colorectal Cancer: A Randomized Clinical Trial. JAMA Intern Med. 2016;176:894-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 248] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 6. | Benson VS, Patnick J, Davies AK, Nadel MR, Smith RA, Atkin WS; International Colorectal Cancer Screening Network. Colorectal cancer screening: a comparison of 35 initiatives in 17 countries. Int J Cancer. 2008;122:1357-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 177] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 7. | US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW Jr, García FAR, Gillman MW, Harper DM, Kemper AR, Krist AH, Kurth AE, Landefeld CS, Mangione CM, Owens DK, Phillips WR, Phipps MG, Pignone MP, Siu AL. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;315:2564-2575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1249] [Cited by in RCA: 1387] [Article Influence: 154.1] [Reference Citation Analysis (1)] |
| 8. | Atkin WS, Valori R, Kuipers EJ, Hoff G, Senore C, Segnan N, Jover R, Schmiegel W, Lambert R, Pox C; International Agency for Research on Cancer. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First Edition--Colonoscopic surveillance following adenoma removal. Endoscopy. 2012;44 Suppl 3:SE151-SE163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 9. | Bénard F, Barkun AN, Martel M, von Renteln D. Systematic review of colorectal cancer screening guidelines for average-risk adults: Summarizing the current global recommendations. World J Gastroenterol. 2018;24:124-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 228] [Cited by in RCA: 203] [Article Influence: 29.0] [Reference Citation Analysis (7)] |
| 10. | Church J. Complications of colonoscopy. Gastroenterol Clin North Am. 2013;42:639-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Eckstrom E, Feeny DH, Walter LC, Perdue LA, Whitlock EP. Individualizing cancer screening in older adults: a narrative review and framework for future research. J Gen Intern Med. 2013;28:292-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | Bevan R, Rutter MD. Colorectal Cancer Screening-Who, How, and When? Clin Endosc. 2018;51:37-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (1)] |
| 13. | Lee KT, Harris RP, Schoenborn NL. Individualized Approach to Cancer Screening in Older Adults. Clin Geriatr Med. 2018;34:11-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Parker MA, Robinson MH, Scholefield JH, Hardcastle JD. Psychiatric morbidity and screening for colorectal cancer. J Med Screen. 2002;9:7-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Cho H, Klabunde CN, Yabroff KR, Wang Z, Meekins A, Lansdorp-Vogelaar I, Mariotto AB. Comorbidity-adjusted life expectancy: a new tool to inform recommendations for optimal screening strategies. Ann Intern Med. 2013;159:667-676. [PubMed] [DOI] [Full Text] |
| 16. | Piper MS, Maratt JK, Zikmund-Fisher BJ, Lewis C, Forman J, Vijan S, Metko V, Saini SD. Patient Attitudes Toward Individualized Recommendations to Stop Low-Value Colorectal Cancer Screening. JAMA Netw Open. 2018;1:e185461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Lewis CL, Kistler CE, Amick HR, Watson LC, Bynum DL, Walter LC, Pignone MP. Older adults' attitudes about continuing cancer screening later in life: a pilot study interviewing residents of two continuing care communities. BMC Geriatr. 2006;6:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | von Wagner C, Macedo A, Campbell C, Simon AE, Wardle J, Hammersley V, Weller D, Waller J. Continuing cancer screening later in life: attitudes and intentions among older adults in England. Age Ageing. 2013;42:770-775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Jung KW, Won YJ, Kong HJ, Lee ES. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2016. Cancer Res Treat. 2019;51:417-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 263] [Cited by in RCA: 277] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 20. | Sohn DK, Kim MJ, Park Y, Suh M, Shin A, Lee HY, Im JP, Cho H-M, Hong SP, Kim B-h, Kim Y, Kim JW, Kim H-S, Nam CM, Park DI, Um JW, Oh SN, Lim HS, Chang HJ, Hahm SK, Chung JH, Kim SY, Kim Y, Lee WC, Jeong SY. The Korean guideline for colorectal cancer screening. J Korean Med Assoc. 2015;58:420-432. [RCA] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Kim Y, Jun JK, Choi KS, Lee HY, Park EC. Overview of the National Cancer screening programme and the cancer screening status in Korea. Asian Pac J Cancer Prev. 2011;12:725-730. [PubMed] |
| 22. | Statistics Korea. Life Tables for Korea. 2017 [cited 2019 Dec 22]. Database: Statistics Korea [Internet]. Available from: http://kostat.go.kr/portal/eng/. |
| 23. | Suh M, Choi KS, Lee HY, Hahm MI, Lee YY, Jun JK, Park EC. Socioeconomic Disparities in Colorectal Cancer Screening in Korea: A Nationwide Cross-Sectional Study. Medicine (Baltimore). 2015;94:e1368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Han MA, Choi KS, Jun JK, Kim Y, Park EC, Lee HY. Factors associated with the intention to have colorectal cancer screening in Korean adults. Eur J Cancer Care (Engl). 2011;20:475-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Lin JS, Piper MA, Perdue LA, Rutter CM, Webber EM, O'Connor E, Smith N, Whitlock EP. Screening for Colorectal Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2016;315:2576-2594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 558] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 26. | Lin OS. Performing colonoscopy in elderly and very elderly patients: Risks, costs and benefits. World J Gastrointest Endosc. 2014;6:220-226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | Corley DA, Jensen CD, Marks AR, Zhao WK, de Boer J, Levin TR, Doubeni C, Fireman BH, Quesenberry CP. Variation of adenoma prevalence by age, sex, race, and colon location in a large population: implications for screening and quality programs. Clin Gastroenterol Hepatol. 2013;11:172-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 190] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 28. | Hemmasi G, Sohrabi M, Zamani F, Ajdarkosh H, Rakhshani N, Khoonsari M, Ameli M, Hatami K. Prevalence of colorectal adenoma in an average-risk population aged 40-50 versus 50-60 years. Eur J Cancer Prev. 2015;24:386-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Day LW, Kwon A, Inadomi JM, Walter LC, Somsouk M. Adverse events in older patients undergoing colonoscopy: a systematic review and meta-analysis. Gastrointest Endosc. 2011;74:885-896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 184] [Article Influence: 13.1] [Reference Citation Analysis (1)] |