Published online Jul 21, 2020. doi: 10.3748/wjg.v26.i27.3917
Peer-review started: December 15, 2019
First decision: April 1, 2020
Revised: May 15, 2020
Accepted: July 1, 2020
Article in press: July 1, 2020
Published online: July 21, 2020
Processing time: 219 Days and 7.2 Hours
Chronic hepatitis B virus (HBV) infection is a leading cause of liver morbidity and mortality worldwide. Liver fibrosis resulting from viral infection-associated inflammation and direct liver damage plays an important role in disease management and prognostication. The mechanisms underlying the contribution of the liver microenvironment to fibrosis in HBV patients are not fully understood. There is an absence of effective clinical treatments for liver fibrosis progression; thus, establishing a suitable in vitro microenvironment in order to design novel therapeutics and identify molecular biomarkers to stratify patients is urgently required.
To examine a subset of pre-selected microenvironment factors of chronic HBV patients that may underlie fibrosis, with a focus on fibroblast activation.
We examined the gene expression of key microenvironment factors in liver samples from patients with more advanced fibrosis compared with those with less severe fibrosis. We also used the human stellate cell line LX-2 in the in vitro study. Using different recombinant cytokines and growth factors or their combination, we studied how these factors interacted with LX-2 cells and pinpointed the cross-talk between the aforementioned factors and screened the most important factors.
Of the secreted factors examined, transforming growth factor (TGF)-β1, interleukin (IL)-1β and tumor necrosis factor (TNF)-α were increased in patients with advanced fibrosis. We found that besides TGF-β1, IL-1β can also induce a profibrotic cascade by stimulating the expression of connective tissue growth factor and platelet-derived growth factor (PDGF) in LX-2 cells. Furthermore, the proinflammatory response can be elicited in LX-2 cells following treatment with IL-1β and TNF-α, suggesting that stellate cells can respond to proinflammatory stimuli. By combining IL-1β and TGF-β1, we observed not only fibroblast activation as shown by αlpha-smooth muscle actin and PDGF induction, but also the inflammatory response as shown by increased expression of IL-1β.
Collectively, our data from HBV patients and in vitro studies demonstrate that the hepatic microenvironment plays an important role in mediating the crosstalk between profibrotic and proinflammatory responses and modulating fibrosis in chronic HBV patients. For the establishment of a suitable in vitro microenvironment for HBV-induced liver fibrosis, not only TGF-β1 but also IL-1β should be considered as a necessary environmental factor.
Core tip: We partially profiled the microenvironment factors in hepatitis B virus (HBV) infected patients. In vitro, we stimulated the hepatic stellate cell line LX-2 with different growth factors, cytokines or their combination. This study demonstrated that the hepatic microenvironment, which involves the crosstalk between profibrotic and proinflammatory factors, underlies fibrosis in hepatitis patients. The treatment of stellate cells with interleukin-1β combined with transforming growth factor-β1 may serve as an in vitro model for fibrotic HBV infected patients, which better represents the liver microenvironment.
- Citation: Yao QY, Feng YD, Han P, Yang F, Song GQ. Hepatic microenvironment underlies fibrosis in chronic hepatitis B patients. World J Gastroenterol 2020; 26(27): 3917-3928
- URL: https://www.wjgnet.com/1007-9327/full/v26/i27/3917.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i27.3917
Chronic hepatitis B virus (HBV) infection is a leading cause of liver morbidity and mortality worldwide, primarily due to the complications of end-stage liver disease such as decompensated cirrhosis and hepatocellular carcinoma[1,2]. Continuous viral infection-associated inflammation and direct liver damage caused by viral components are accompanied by the transformation of hepatic stellate cells (HSCs) into activated myofibroblasts, fibroblast propagation, and deposition of extracellular matrix, resulting in fibrosis and in the most severe form, liver cirrhosis[3,4]. Staging hepatic fibrosis in chronic liver disease is a key indicator in judging the condition, deciding on the treatment strategy and evaluating the treatment effect. With the development of non-invasive approaches, especially imaging technology, the accuracy of the assessment of liver fibrosis has been improved[5-10]. Assessing fibrosis stage plays an important role in disease management and prognostication for individual HBV patients considering that the fibrosis degree and the time to progression to cirrhosis are heterogenous[10,11]. There is an absence of effective clinical treatments for liver fibrosis progression; thus, establishing a suitable in vitro microenvironment in order to design novel therapeutics and identify molecular biomarkers to stratify patients is urgently required.
Due to the lack of HBV receptors in mice, the in vivo study of HBV-induced liver fibrosis is time consuming and costly. For an in vitro study, neither cell lines nor 3D liver organoids can correctly simulate the patient's viral hepatitis internal environment. Recent studies have shown that various factors such as metabolic factors, growth factors, inflammatory factors, and microRNA all have an impact on the progression of fibrosis[10,12-17]. Therefore, determining the roles that the hepatic environment plays in patients will greatly facilitate the establishment of a suitable in vitro environment, which will represent specific pathogenic factors in hepatitis patients. Such microenvironment factors are important for developing in vitro fibrosis assays which can be used for identifying novel drug targets and drug screening. The hepatic microenvironment consists of hepatocytes, liver sinusoidal endothelial cells, resident Kupffer cells, HSCs, as well as infiltrating immune cells and a variety of secreted factors including growth factors, cytokines and chemokines, and extracellular matrix. Among the various non-parenchymal cells, HSCs play central roles in liver fibrosis following their activation into profibrotic myofibroblast-like cells in diseases such as chronic alcohol intake, hepatitis B and C, fatty liver disease, obesity and diabetes[18,19]. We set out to screen a subset of preselected microenvironment factors in chronic HBV patients with different degrees of fibrosis, and used HSCs to study how these factors interact to modulate fibrogenesis and progression of fibrosis in chronic hepatitis B patients.
Human liver specimens were collected from patients who had undergone liver biopsy due to chronic hepatitis B from January 2016 to June 2017 at the Department of Gastroenterology, Zhongshan Hospital, Fudan University (Shanghai, China). The tissues were fixed in formalin and embedded in paraffin for hematoxylin-eosin and silver impregnation staining as described previously[10]. The scores for inflammation (recorded as G0-4) and fibrosis (recorded as S0-4) were examined in a blinded fashion by an experienced gastrointestinal pathologist. The clinicopathologic characteristics of all samples are shown in Table 1. This study was approved by the Clinical Research Ethics Committee of Zhongshan Hospital Fudan University, and written informed consent was obtained from each patient.
| Parameters | Chronic hepatitis B | P value1 | |
| No or mild fibrosis | Advanced fibrosis | ||
| (S0-1, n = 16) | (S3-4, n = 16) | ||
| Gender | 0.716 | ||
| Male | 11 | 9 | |
| Female | 5 | 7 | |
| Age (yr) | 0.479 | ||
| < 45 | 9 | 6 | |
| ≥ 45 | 7 | 10 | |
| ALT (U/L) | 0.704 | ||
| < 50 | 12 | 10 | |
| ≥ 50 | 4 | 6 | |
| HBV-DNA (copies/mL) | 0.479 | ||
| < 105 | 10 | 7 | |
| ≥ 105 | 6 | 9 | |
| Inflammation | 0.037 | ||
| G1-2 | 15 | 9 | |
| G3-4 | 1 | 7 | |
Total RNA was extracted from liver tissue embedded in paraffin using the RNeasy FFPE Kit (Qiagen, Germany) according to the manufacturer’s protocol. Briefly after paraffin was removed from the samples, they were treated with lysis buffer containing proteinase K for 15 min. After lysis, the samples were incubated at 80ºC for another 15 min to reverse formalin crosslinking. Genomic DNA was then removed using DNase and DNase Booster Buffer. Finally, concentrated RNA was purified using RNeasy MinElute spin columns.
LX-2 cells (Merck, Germany) were cultured following the manufacturer’s instructions. Briefly, the cells were thawed in high glucose DMEM supplements with 10% HI-FBS (Gibco, heat inactivated, Australia). Treatment was conducted in the same medium. The cells were treated with cytokines or growth factors for 16 h or 48 h and then collected for subsequent analysis. Human recombinant cytokines or growth factors used in this study were from R&D System as follows: Transforming growth factor (TGF)-β1, epidermal growth factor (EGF), fibroblast growth factor (FGF) (1:1 mixture of FGF1 and FGF2), platelet-derived growth factor (PDGF), interleukin (IL)-1β and tumor necrosis factor (TNF)-α. The final concentration used for all growth factors and cytokines was 10 ng/mL.
Reverse transcription–quantitative PCR (RT–qPCR) analyses were performed using PrimeScriptTM RT Master Mix (TaKaRa) and THUNDERBIRDTM SYBR qPCR Mix (TOYOBO) kits following the manufacturers’ instructions. Well-known fibrosis marker genes, including fibroblast activation protein (FAP), αlpha-smooth muscle actin (α-SMA), tissue inhibitor of metalloproteinase 1 (TIMP1), collagen type 1 alpha (COL1A) and toll-like receptor (TLR) family genes were chosen to assess the degree of fibrosis. EGF, TGF-β1, PDGF, connective tissue growth factor (CTGF) and FGF family genes were chosen to assess the expression of growth factors. IL-1β, IL-6, TNF-α, and Nod-like receptor protein 3 (NLRP3) were chosen to assess the expression of inflammatory factors. The primer sequences are listed in Supplementary Table 1. RT-qPCR reactions were performed with the ABI thermal cycler, and the primer sets were determined to be quantitative. Threshold cycles and melting curve measurements were performed using software. P values were calculated by the Student-t test.
Values presented are expressed as the means ± SE. For all the in vitro studies, statistical comparisons were performed using the non-parametric Mann–Whitney–Wilcoxon two-tail test. To compare the human clinical characteristics (Table 1), the Chi-square test was performed. P < 0.05 was considered significant. a: 0.01 < P < 0.05; b: 0.001 < P < 0.01; c: P < 0.001. All statistical analyses were performed using SPSS 26.0 software (IBM, NY, US).
To examine the hepatic microenvironment underlying fibrosis, a group of 32 chronic HBV patients who had not received antiviral treatment were selected. Liver biopsy was conducted in these patients to determine if they met the criteria for antiviral treatment. Fibrosis stage was assessed by experienced pathologists using silver impregnation staining, and the fibrotic tissue area greatly increased as fibrosis progressed[10,20,21] (Supplementary Figure 1). To pinpoint which hepatic micro-environment factors are critical for fibrosis in chronic HBV patients, the patients were divided into two groups according to fibrosis stage: no or mild fibrosis (S0-1), and advanced fibrosis (S3-4). The patients in both groups showed comparable demographics, including gender and age (P = 0.465 and P = 0.288, respectively, Table 1). Liver function in the two groups of patients was also comparable indicated by alanine aminotransferase (ALT) level (P = 0.446, Table 1). Interestingly, inflammation level in the two groups of patients showed a strong trend towards a positive correlation (χ2 = 4.167, P = 0.041) (Supplementary Figure 1 and Table 1), indicating a more pro-inflammatory microenvironment in patients with advanced fibrosis although their viral parameters and other clinical characteristics were comparable.
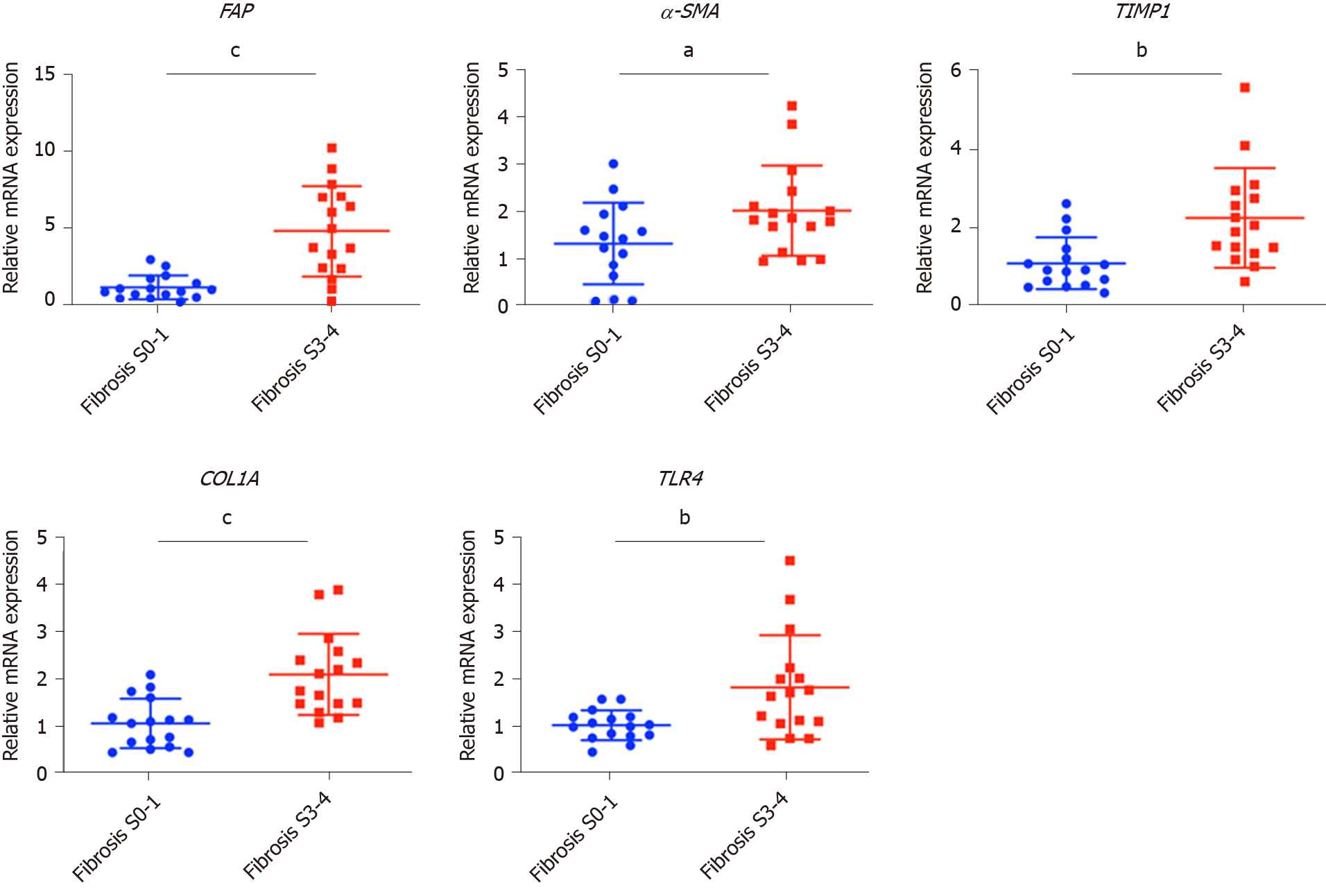
Fibrosis in chronic HBV patients involved various extracellular factors, including growth factors, cytokines and other factors, altogether laying the foundation of the pathological microenvironment. To identify the most critical components, quantitative gene expression of these factors in the HBV patients’ liver samples was carried out (Table 1). As expected, the gene expression of FAP, a-SMA, TIMP1 and COL1A1, markers of fibrosis and extracellular matrix deposition[22,23], were up-regulated in patients with advanced fibrosis (Figure 1). TLR4 was significantly up-regulated and further confirmed that several pathways related to fibrosis, were more active in advanced fibrosis patients (Figure 1)[24], while other TLRs showed comparable levels in the two groups of patients (Supplementary Figure 2).
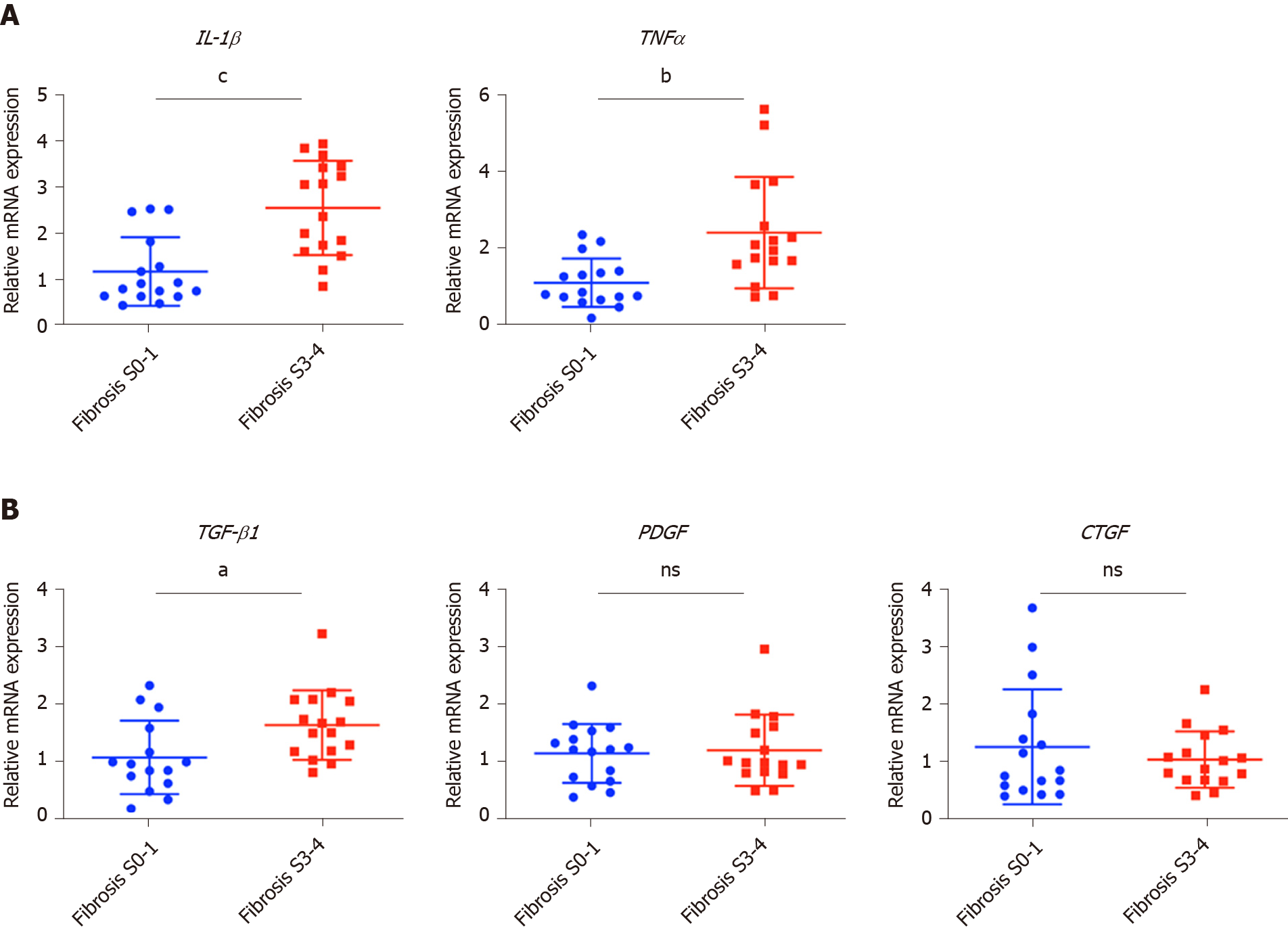
To confirm the clinicopathological results, we quantitated the expression levels of inflammatory factors, including IL-1β, IL6, NLRP3 and TNF-α, which contribute to fibrosis directly or indirectly[25-31]. Of the cytokines profiled, IL-1β and TNF-α, but not IL6 and NLRP3 were up-regulated in patients with advanced fibrosis, correlating with the more severe inflammation found in these patients (Figure 2A, Table 1 and Supplementary Figure 3A). This indicated that IL-1β and TNF-α may progress fibrosis in HBV patients.
With regard to the growth factors, we quantitated the gene expression of TGF-β1, EGF, FGF1, FGF2, FGF7, PDGF and CTGF. Only TGF-β1 showed increased expression in patients with advanced fibrosis (Figure 2B), suggesting its critical function in activated myofibroblasts[32]. The FGFs showed comparable levels in the two groups of patients indicating that they were not critical factors in the progression of fibrosis (Supplementary Figure 3B). Interestingly, PDGF which can cause liver fibrosis independent of the TGF-β pathway, and CTGF a TGF-β downstream modulator which can amplify profibrogenic action, were not increased in patients with advanced fibrosis (Figure 2B)[33,34]. Altogether, TGF-β1, IL-1β and TNF-α were identified as critical components of the hepatic microenvironment and may underlie the advancement of fibrosis in HBV patients.
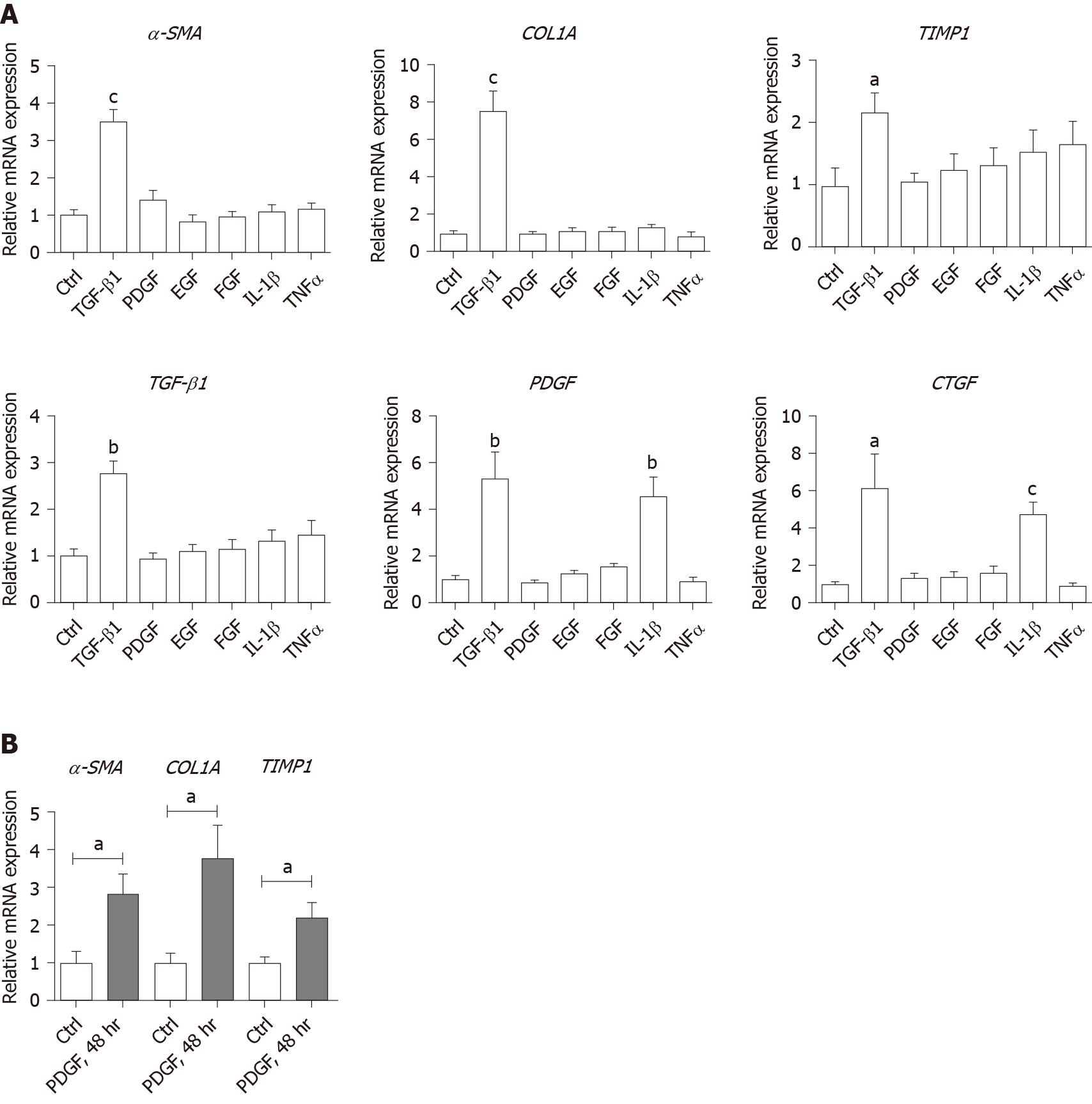
In order to verify whether growth factors and inflammatory factors can be used to simulate the hepatic microenvironment of HBV hepatitis patients in vitro, LX-2 an immortalized human HSCs cell line was used to study how various microenvironment factors impact fibrogenesis and propagate the pathologic process. Four growth factors and two inflammatory factors, including TGF-β1, PDGF, EGF, FGF, IL-1β, and TNF-α, were selected for the in vitro study. Of these factors, consistent with the well-established function of TGF-β1 in activating HSCs, TGF-β1 increased the expression of α-SMA, COL1A1, and TIMP1 16 h post-treatment (Figure 3A). TGF-β1 also induced the expression of growth factors including itself, PDGF and CTGF. IL-1β and TNF-α, both up-regulated in HBV patients with advanced fibrosis had no impact on activating HSCs (Figure 3A). Interestingly, IL-1β but not TNF-α treatment led to up-regulation of PDGF and CTGF, suggesting its potential impact on modulating fibrosis (Figure 3A). It is noteworthy that at 48 h, but not 16 h post-treatment, PDGF also led to moderate induction of α-SMA, COL1A1, and TIMP1 (Figure 3B), suggesting that IL-1β can potentially activate LX-2 cells by upregulating these factors although in a delayed manner.
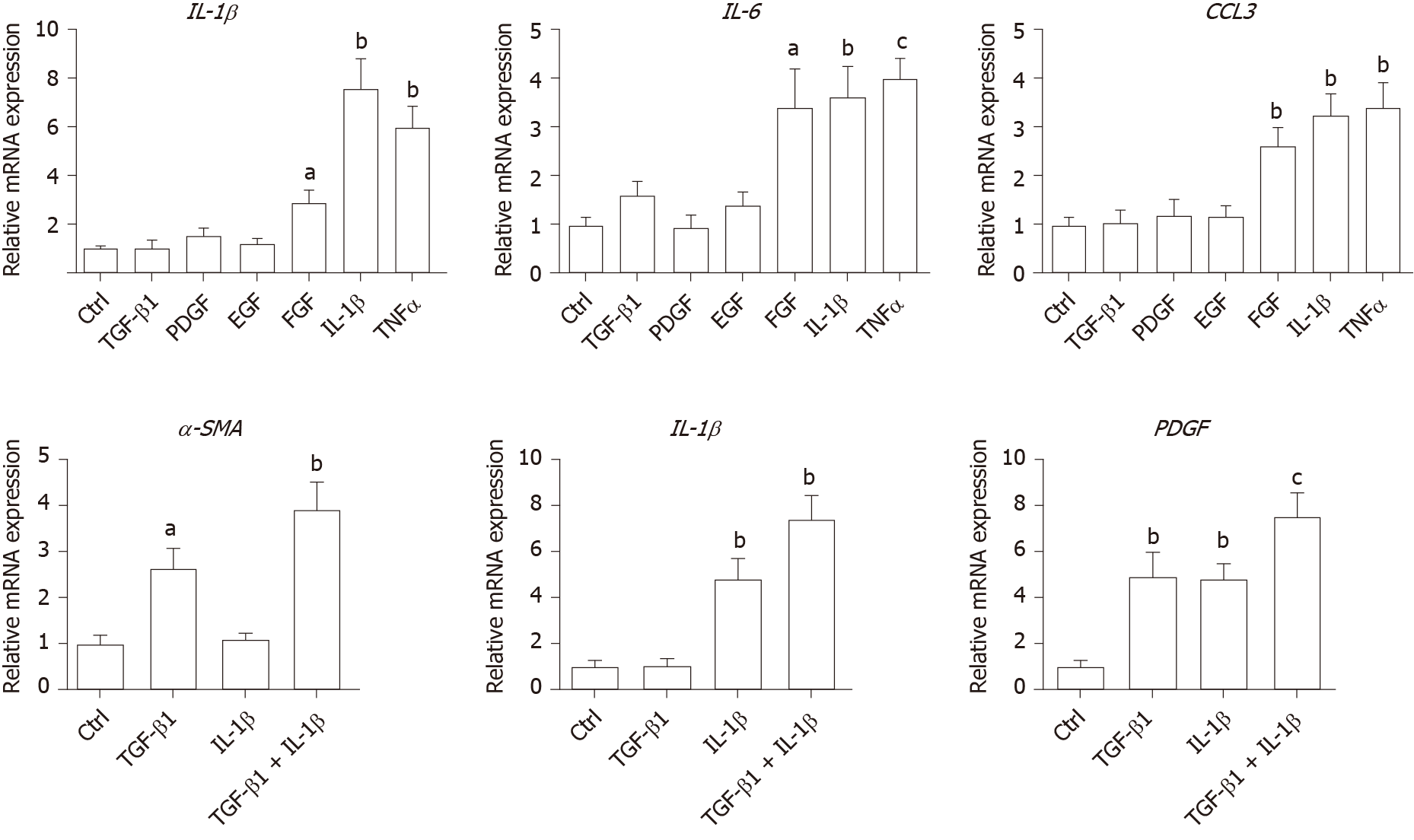
As inflammation is a key player during fibrosis in HBV patients, we determined the gene expression of a few cytokine factors following treatment with the aforementioned factors. Although most growth factors generally had a minimum impact, FGF as well as IL-1β and TNF-α, increased gene expression of the cytokine factors (Figure 4A). These data suggest that not only HSCs can respond to cytokines by inducing PDGF and CTGF to activate HSCs, but they can also propagate the inflammatory process. Such crosstalk between the profibrotic and proinflammatory pathways plays important roles in regulating fibrosis. We then treated LX-2 cells with IL-1β combined with TGF-β1, and found that this treatment induced α-SMA, IL-1β as well as PDGF, suggesting both fibroblast activation and the proinflammatory response (Figure 4B). Although PDGF induction occurs in a synergistic manner, no statistical significance was noted between either monotreatment or combination treatment (Figure 4B). Taken together, our data suggest that as a central player, HSCs mediate fibrosis by responding to various microenvironment factors and exert pleiotropic functions including not only activating hepatic fibrosis but also propagating the proinflammatory response.
Our findings indicate that the inflammatory factor IL-1β is a central player in fibrogenesis and progression of fibrosis in chronic hepatitis B patients. IL-1β may activate HSCs via PDGF, and synergize with TGF-β1 in the progression of fibrosis. To establish a suitable in vitro microenvironment for HBV-induced liver fibrosis, not only TGF-β1 but also IL-1β should be considered as a necessary environmental factor. In our previous study, we demonstrated in vivo that the hepatic microenvironment strongly supported hepatic trans-programming, suggesting the importance of the microenvironment in determining cell fate[35]. For chronic HBV patients, the hepatic microenvironment consists of a variety of resident and infiltrating host immune cells, secreted factors and extracellular matrix proteins. How the microenvironment in HBV patients contributes to liver fibrosis is not well characterized. Currently, an effective clinical therapy to suppress the progression of liver fibrosis is still unavailable. 3D organoid technology, which will provide an effective tool for fundamental mechanism research and drug screening[36], still lacks a suitable culture system to simulate the patient's viral hepatitis internal environment. Hence, the development of novel drugs to treat fibrosis urgently requires accurate in vitro models. Identifying critical microenvironment factors and determining how these factors interact with each other to modulate fibrosis is important in order to design novel therapeutics and identify molecular biomarkers to stratify patients.
Among the secreted factors we profiled, TGF-β1, IL-1β and TNF-α were increased in patients with advanced fibrosis. TGF-β1 is a well-known fibrosis activating factor, and our results confirmed this. Interestingly, compared with previous studies[12,14], we found that IL-1β, but not IL-6, NLRP3, or TNF-α is a central player in fibrogenesis and the progression of fibrosis in chronic HBV patients. Moreover, our results showed that the expression of PDGF, a known HSCs activation factor[15,16], did not increase with progression of fibrosis. When human stellate cells (LX-2) were treated with cytokines in vitro, we found that either IL-1β or TGF-β1 induced the expression of PDGF. These results indicated that IL-1β may play a critical role in the progression of fibrosis in chronic HBV patients. IL-1β, a dominant IL-1 secreted isoform produced by various cells including fibroblasts and myeloid-originated immune cells play key roles in both acute and chronic inflammation[37,38]. Circulating IL-1β is elevated in patients with chronic liver diseases, including alcoholic liver disease, chronic hepatitis B and C, and primary biliary cirrhosis[39-42]. Our study further demonstrated that in HBV patients with advanced fibrosis, elevated intrahepatic IL-1β expression may mediate inflammation and tissue damage, and propagate the profibrotic cascade. In fact, consistent with our data, in another independent study, HSCs were shown to respond to IL-1β by inducing IL-1β, IL1-Ra, and MMP-9[43]. However, it is unclear whether IL-1β is a driver or a consequence of fibrosis, and whether elevated IL-1β may have protective effects in liver regeneration given the fact that abolishing IL-1Ra encoding the antagonist of the IL-1 receptor delays regeneration after partial hepatectomy[44]. In order to further reveal whether this phenomenon is caused by HBV infection, it is necessary to further analyze samples from non-HBV patients and chronic HBV patients receiving antiviral treatments in future studies.
In the current study, HBV patients with advanced fibrosis also showed a high degree of inflammation despite viral parameters among these patients being comparable (Table 1). Therefore, the microenvironment in hepatitis patients provides the possibility for cross-talk between profibrotic and proinflammatory signals[45]. Interestingly, the proinflammatory response can be elicited in the “fibroblast” cell line LX-2 following treatment with IL-1β and TNF-α, providing a positive feedback loop to exacerbate inflammation. Finally, by combining IL-1β and TGF-β1, we observed not only fibroblast activation but also the inflammatory response, which may serve as a model to determine further aspects of the pathogenesis of liver fibrosis in HBV patients. In summary, our findings suggest that IL-1β is a central player in the hepatic microenvironment of viral hepatitis patients and plays a critical role in modulating fibrosis and the cross-talk between profibrotic and proinflammatory stimuli, and may converge on stellate cells to propagate these key pathologic events.
Chronic hepatitis B virus (HBV) infection is a leading cause of liver morbidity and mortality worldwide. Liver fibrosis resulting from viral infection-associated inflammation and direct liver damage plays an important role in disease management and prognostication. The mechanisms underlying the contribution of the liver microenvironment to fibrosis in HBV patients are not fully understood. There is an absence of effective clinical treatments for liver fibrosis progression; therefore, establishing a suitable in vitro microenvironment is urgently required in order to design novel therapeutics and identify molecular biomarkers to stratify patients.
Due to the lack of HBV receptors in mice, the in vivo study of HBV-induced liver fibrosis is time consuming and costly. For in vitro study, neither cell lines nor 3D liver organoids can correctly simulate the patient's viral hepatitis internal environment. Therefore, determining the roles the hepatic environment play in the patient will greatly facilitate the establishment of a suitable in vitro environment to reflect more hepatitis patient-specific pathogenic factors. Such factors are important for developing in vitro fibrosis assays which can be used to identify novel drug targets and drug screening.
In this study, we set out to screen a subset of preselected microenvironment factors, including growth and inflammatory factors, in chronic HBV patients with different degrees of fibrosis. In addition, hepatic stellate cells (HSCs) were used to study how these factors interact to modulate fibrogenesis and progression of fibrosis in chronic hepatitis B patients.
We examined the gene expression of key microenvironment factors using liver samples from patients with more advanced fibrosis compared to those with less severe fibrosis. We also carried out an in vitro study using the human stellate cell line LX-2. Different recombinant cytokines and growth factors or their combination were used to study how these factors interacted with LX-2 cells and to pinpoint the cross-talk between the aforementioned factors and screen the most important factors.
Of the secreted factors examined, transforming growth factor (TGF)-β1, interleukin-1β and tumor necrosis factor (TNF)-α were increased in patients with advanced fibrosis. We found that besides TGF-β1, IL-1β can also induce a profibrotic cascade by stimulating the expression of connective tissue growth factor and platelet-derived growth factor (PDGF) in LX-2 cells. Furthermore, the proinflammatory response can be elicited in LX-2 cells during treatment with IL-1β and TNF-α, suggesting that stellate cells can respond to proinflammatory stimuli. When IL-1β and TGF-β1 were combined, we observed not only fibroblast activation as shown by α-SMA and PDGF induction, but also the inflammatory response as shown by increased expression of IL-1β.
Collectively, our data from HBV patients and in vitro studies demonstrate that the hepatic microenvironment plays an important role in mediating the crosstalk between profibrotic and proinflammatory responses and modulating fibrosis in chronic HBV patients. Our findings indicate that the inflammatory factor IL-1β is a central player in fibrogenesis and progression of fibrosis in chronic hepatitis B patients. IL-1β may activate HSCs via PDGF, and synergize with TGF-β1 in fibrosis progression. To establish a suitable in vitro microenvironment for HBV-induced liver fibrosis, not only TGF-β1 but also IL-1β should be considered as a necessary environmental factor.
Our study demonstrated that the hepatic microenvironment involves crosstalk between profibrotic and proinflammatory factors in hepatitis patients and underlies fibrosis. The treatment of stellate cells with IL-1β combined with TGF-β1 may serve as an in vitro model for fibrotic HBV infected patients and can reflect the liver microenvironment.
We acknowledge Yan-Ting Zou and Shu-Yu Li for their excellent laboratory assistance.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Abd El-Razek A, Schwabl P S-Editor: Liu M L-Editor: Webster JR E-Editor: Ma YJ
| 1. | MacLachlan JH, Cowie BC. Hepatitis B virus epidemiology. Cold Spring Harb Perspect Med. 2015;5:a021410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 199] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 2. | Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3:383-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1260] [Cited by in RCA: 1210] [Article Influence: 172.9] [Reference Citation Analysis (2)] |
| 3. | Suhail M, Abdel-Hafiz H, Ali A, Fatima K, Damanhouri GA, Azhar E, Chaudhary AG, Qadri I. Potential mechanisms of hepatitis B virus induced liver injury. World J Gastroenterol. 2014;20:12462-12472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Ryder SD, Irving WL, Jones DA, Neal KR, Underwood JC; Trent Hepatitis C Study Group. Progression of hepatic fibrosis in patients with hepatitis C: a prospective repeat liver biopsy study. Gut. 2004;53:451-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 196] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 5. | Razek AA, Massoud SM, Azziz MR, El-Bendary MM, Zalata K, Motawea EM. Prediction of esophageal varices in cirrhotic patients with apparent diffusion coefficient of the spleen. Abdom Imaging. 2015;40:1465-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Besheer T, El-Bendary M, Elalfy H, Abd El-Maksoud M, Salah M, Zalata K, Elkashef W, Elshahawy H, Raafat D, Elemshaty W, Almashad N, Zaghloul H, El-Gilany AH, Abdel Razek AA, Abd Elwahab M. Prediction of Fibrosis Progression Rate in Patients with Chronic Hepatitis C Genotype 4: Role of Cirrhosis Risk Score and Host Factors. J Interferon Cytokine Res. 2017;37:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Besheer T, Elalfy H, Abd El-Maksoud M, Abd El-Razek A, Taman S, Zalata K, Elkashef W, Zaghloul H, Elshahawy H, Raafat D, Elemshaty W, Elsayed E, El-Gilany AH, El-Bendary M. Diffusion-weighted magnetic resonance imaging and micro-RNA in the diagnosis of hepatic fibrosis in chronic hepatitis C virus. World J Gastroenterol. 2019;25:1366-1377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Besheer T, Arafa M, El-Maksoud MA, Elalfy H, Hasson A, Zalata K, Elkashef W, Elshahawy H, Raafat D, Elemshaty W, Elsayed E, Zaghloul H, Razek AA, El-Bendary M. Diagnosis of cirrhosis in patients with chronic hepatitis C genotype 4: Role of ABCB11 genotype polymorphism and plasma bile acid levels. Turk J Gastroenterol. 2018;29:299-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Mikolasevic I, Orlic L, Franjic N, Hauser G, Stimac D, Milic S. Transient elastography (FibroScan(®)) with controlled attenuation parameter in the assessment of liver steatosis and fibrosis in patients with nonalcoholic fatty liver disease - Where do we stand? World J Gastroenterol. 2016;22:7236-7251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 205] [Cited by in RCA: 199] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 10. | Parikh P, Ryan JD, Tsochatzis EA. Fibrosis assessment in patients with chronic hepatitis B virus (HBV) infection. Ann Transl Med. 2017;5:40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 11. | Jieanu CF, Ungureanu BS, Săndulescu DL, Gheonea IA, Tudorașcu DR, Ciurea ME, Purcărea VL. Quantification of liver fibrosis in chronic hepatitis B virus infection. J Med Life. 2015;8:285-290. [PubMed] |
| 12. | Wick G, Grundtman C, Mayerl C, Wimpissinger TF, Feichtinger J, Zelger B, Sgonc R, Wolfram D. The immunology of fibrosis. Annu Rev Immunol. 2013;31:107-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 261] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 13. | Yilmaz B, Koklu S, Buyukbayram H, Yalçin K, Korkmaz U, Posul E, Can G, Kurt M. Chronic hepatitis B associated with hepatic steatosis, insulin resistance, necroinflammation and fibrosis. Afr Health Sci. 2015;15:714-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Deng YQ, Zhao H, Ma AL, Zhou JY, Xie SB, Zhang XQ, Zhang DZ, Xie Q, Zhang G, Shang J, Cheng J, Zhao WF, Zou ZQ, Zhang MX, Wang GQ; China HepB Related Fibrosis Assessment Research Group. Selected Cytokines Serve as Potential Biomarkers for Predicting Liver Inflammation and Fibrosis in Chronic Hepatitis B Patients With Normal to Mildly Elevated Aminotransferases. Medicine (Baltimore). 2015;94:e2003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Ying HZ, Chen Q, Zhang WY, Zhang HH, Ma Y, Zhang SZ, Fang J, Yu CH. PDGF signaling pathway in hepatic fibrosis pathogenesis and therapeutics (Review). Mol Med Rep. 2017;16:7879-7889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 170] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 16. | Bai Q, An J, Wu X, You H, Ma H, Liu T, Gao N, Jia J. HBV promotes the proliferation of hepatic stellate cells via the PDGF-B/PDGFR-β signaling pathway in vitro. Int J Mol Med. 2012;30:1443-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Tsay HC, Yuan Q, Balakrishnan A, Kaiser M, Möbus S, Kozdrowska E, Farid M, Tegtmeyer PK, Borst K, Vondran FWR, Kalinke U, Kispert A, Manns MP, Ott M, Sharma AD. Hepatocyte-specific suppression of microRNA-221-3p mitigates liver fibrosis. J Hepatol. 2019;70:722-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14:397-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1221] [Cited by in RCA: 1972] [Article Influence: 246.5] [Reference Citation Analysis (0)] |
| 19. | Yin C, Evason KJ, Asahina K, Stainier DY. Hepatic stellate cells in liver development, regeneration, and cancer. J Clin Invest. 2013;123:1902-1910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 570] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 20. | Feldmann G. Critical analysis of the methods used to morphologically quantify hepatic fibrosis. J Hepatol. 1995;22:49-54. [PubMed] |
| 21. | Standish RA, Cholongitas E, Dhillon A, Burroughs AK, Dhillon AP. An appraisal of the histopathological assessment of liver fibrosis. Gut. 2006;55:569-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 324] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 22. | Baiocchini A, Montaldo C, Conigliaro A, Grimaldi A, Correani V, Mura F, Ciccosanti F, Rotiroti N, Brenna A, Montalbano M, D'Offizi G, Capobianchi MR, Alessandro R, Piacentini M, Schininà ME, Maras B, Del Nonno F, Tripodi M, Mancone C. Extracellular Matrix Molecular Remodeling in Human Liver Fibrosis Evolution. PLoS One. 2016;11:e0151736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 168] [Article Influence: 18.7] [Reference Citation Analysis (1)] |
| 23. | Lay AJ, Zhang HE, McCaughan GW, Gorrell MD. Fibroblast activation protein in liver fibrosis. Front Biosci (Landmark Ed). 2019;24:1-17. [PubMed] |
| 24. | Yang L, Seki E. Toll-like receptors in liver fibrosis: cellular crosstalk and mechanisms. Front Physiol. 2012;3:138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 25. | Yu C, Wang F, Jin C, Huang X, Miller DL, Basilico C, McKeehan WL. Role of fibroblast growth factor type 1 and 2 in carbon tetrachloride-induced hepatic injury and fibrogenesis. Am J Pathol. 2003;163:1653-1662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 131] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 26. | Yu C, Wang F, Jin C, Wu X, Chan WK, McKeehan WL. Increased carbon tetrachloride-induced liver injury and fibrosis in FGFR4-deficient mice. Am J Pathol. 2002;161:2003-2010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 97] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 27. | Cheng AL, Shen YC, Zhu AX. Targeting fibroblast growth factor receptor signaling in hepatocellular carcinoma. Oncology. 2011;81:372-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Fuchs BC, Hoshida Y, Fujii T, Wei L, Yamada S, Lauwers GY, McGinn CM, DePeralta DK, Chen X, Kuroda T, Lanuti M, Schmitt AD, Gupta S, Crenshaw A, Onofrio R, Taylor B, Winckler W, Bardeesy N, Caravan P, Golub TR, Tanabe KK. Epidermal growth factor receptor inhibition attenuates liver fibrosis and development of hepatocellular carcinoma. Hepatology. 2014;59:1577-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 284] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 29. | Heldin CH. Targeting the PDGF signaling pathway in the treatment of non-malignant diseases. J Neuroimmune Pharmacol. 2014;9:69-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 30. | Weiskirchen R. Hepatoprotective and Anti-fibrotic Agents: It's Time to Take the Next Step. Front Pharmacol. 2015;6:303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 31. | Ihn H. Pathogenesis of fibrosis: role of TGF-beta and CTGF. Curr Opin Rheumatol. 2002;14:681-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 260] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 32. | Dooley S, ten Dijke P. TGF-β in progression of liver disease. Cell Tissue Res. 2012;347:245-256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 468] [Cited by in RCA: 590] [Article Influence: 42.1] [Reference Citation Analysis (1)] |
| 33. | Czochra P, Klopcic B, Meyer E, Herkel J, Garcia-Lazaro JF, Thieringer F, Schirmacher P, Biesterfeld S, Galle PR, Lohse AW, Kanzler S. Liver fibrosis induced by hepatic overexpression of PDGF-B in transgenic mice. J Hepatol. 2006;45:419-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 195] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 34. | Brigstock DR. Connective tissue growth factor (CCN2, CTGF) and organ fibrosis: lessons from transgenic animals. J Cell Commun Signal. 2010;4:1-4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 35. | Song G, Pacher M, Balakrishnan A, Yuan Q, Tsay HC, Yang D, Reetz J, Brandes S, Dai Z, Pützer BM, Araúzo-Bravo MJ, Steinemann D, Luedde T, Schwabe RF, Manns MP, Schöler HR, Schambach A, Cantz T, Ott M, Sharma AD. Direct Reprogramming of Hepatic Myofibroblasts into Hepatocytes In Vivo Attenuates Liver Fibrosis. Cell Stem Cell. 2016;18:797-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 163] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 36. | Nantasanti S, de Bruin A, Rothuizen J, Penning LC, Schotanus BA. Concise Review: Organoids Are a Powerful Tool for the Study of Liver Disease and Personalized Treatment Design in Humans and Animals. Stem Cells Transl Med. 2016;5:325-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 37. | Dinarello CA. Interleukin-1beta and the autoinflammatory diseases. N Engl J Med. 2009;360:2467-2470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 162] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 38. | Gabay C, Lamacchia C, Palmer G. IL-1 pathways in inflammation and human diseases. Nat Rev Rheumatol. 2010;6:232-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 651] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 39. | Ludwiczek O, Vannier E, Moschen A, Salazar-Montes A, Borggraefe I, Gabay C, Enrich B, Kaser A, Siegmund B, Dinarello C, Tilg H. Impaired counter-regulation of interleukin-1 by the soluble IL-1 receptor type II in patients with chronic liver disease. Scand J Gastroenterol. 2008;43:1360-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 40. | McClain CJ, Cohen DA, Dinarello CA, Cannon JG, Shedlofsky SI, Kaplan AM. Serum interleukin-1 (IL-1) activity in alcoholic hepatitis. Life Sci. 1986;39:1479-1485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 90] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 41. | Tilg H, Vogel W, Wiedermann CJ, Shapiro L, Herold M, Judmaier G, Dinarello CA. Circulating interleukin-1 and tumor necrosis factor antagonists in liver disease. Hepatology. 1993;18:1132-1138. [PubMed] |
| 42. | Tilg H, Wilmer A, Vogel W, Herold M, Nölchen B, Judmaier G, Huber C. Serum levels of cytokines in chronic liver diseases. Gastroenterology. 1992;103:264-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 546] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 43. | Meier RPH, Meyer J, Montanari E, Lacotte S, Balaphas A, Muller YD, Clément S, Negro F, Toso C, Morel P, Buhler LH. Interleukin-1 Receptor Antagonist Modulates Liver Inflammation and Fibrosis in Mice in a Model-Dependent Manner. Int J Mol Sci. 2019;20:1295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 44. | Sgroi A, Gonelle-Gispert C, Morel P, Baertschiger RM, Niclauss N, Mentha G, Majno P, Serre-Beinier V, Buhler L. Interleukin-1 receptor antagonist modulates the early phase of liver regeneration after partial hepatectomy in mice. PLoS One. 2011;6:e25442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 45. | Seki E, Schwabe RF. Hepatic inflammation and fibrosis: functional links and key pathways. Hepatology. 2015;61:1066-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 738] [Article Influence: 73.8] [Reference Citation Analysis (0)] |