Published online Jul 21, 2020. doi: 10.3748/wjg.v26.i27.3889
Peer-review started: March 17, 2020
First decision: April 25, 2020
Revised: May 3, 2020
Accepted: June 17, 2020
Article in press: June 17, 2020
Published online: July 21, 2020
Processing time: 126 Days and 1.4 Hours
Liver transplantation (LT) is currently the only effective treatment option for end-stage liver disease. The importance of animal models in transplantation is widely recognized among researchers. Because of the well-characterized mouse genome and the greater diversity and availability of both genetically modified animals and research reagents, mouse orthotopic LT (MOLT) has become an ideal model for the investigation of liver biology, tissue injury, regulation of alloimmunity and tolerance induction, and the pathogenesis of specific liver diseases. However, due to its complicated and technically demanding procedure, the model has merely been used by only a few research groups in the world for years. For a new learner, training lasting at least a couple of months or even years is required. Most of the investigators have emphasized the importance of elaborate techniques and dedicated instruments in establishing a MOLT model, but some details are often neglected. The nontechnical details are also significant, especially for researchers who have little experience in mouse microsurgery. Here, we review and summarize the crucial technical and nontechnical details in establishing the model of MOLT based on scientific articles and our experience in six aspects: animal selection, anesthesia, perioperative management, organ procurement, back-table preparation, and implantation surgery. We aim to enable research groups to shorten the learning curve and implement the mouse LT procedure with high technical success.
Core tip: As an ideal model for the investigation of basic medical research on liver transplantation (LT), the mouse orthotopic LT (MOLT) model, has been used by only a few research groups worldwide. Most of the investigators attach importance to technical factors in establishing the model. We review and summarize the crucial technical and nontechnical details in establishing the model of MOLT based on the literature and our experience. We aim to enable research groups to shorten the learning curve and implement the mouse LT procedure with high technical success.
- Citation: Li T, Hu Z, Wang L, Lv GY. Details determining the success in establishing a mouse orthotopic liver transplantation model. World J Gastroenterol 2020; 26(27): 3889-3898
- URL: https://www.wjgnet.com/1007-9327/full/v26/i27/3889.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i27.3889
Liver transplantation (LT) is currently the only effective treatment option for end-stage liver disease. The rapid development in basic medical research on LT has benefited from a variety of experiments in small and large animals. Mouse models are particularly attractive because the mouse genome is well characterized and there is greater diversity and availability of genetically modified animals (knockout or transgenic) and research reagents at a relatively low cost[1,2]. The similar anatomy between the mouse model and human LT, including the presence of a gallbladder, and high level of similarity between the mouse H-2 system and human HLA complex have attracted considerable attention[3]. Since it was first described by Qian et al[1] in 1991, mouse orthotopic LT (MOLT) has proved to be a powerful research tool for the investigation of liver biology, tissue injury, regulation of alloimmunity and tolerance induction, and the pathogenesis of specific liver diseases[4]. However, mouse liver vessels are eight times smaller on average than those of adult rats, which makes MOLT a complicated and technically demanding procedure. To achieve consistent success, training lasting at least a couple of months or even years is required, which hinders the application of MOLT and to some extent impedes the basic medical research on LT. Until now, only a handful of transplantation research centers are capable of establishing the MOLT model reliably and reproducibly. Although elaborate techniques and dedicated instruments have been described in recent years[5-7], crucial details related to MOLT are often neglected and rarely mentioned in the literature. Nevertheless, such details regarding animal selection, anesthesia, perioperative management, organ procurement, back-table preparation, and implantation surgery are of importance in the establishment of MOLT, especially for researchers who have little experience in mouse microsurgery. In this paper, we review and summarize the crucial details in establishing the model of MOLT based on scientific articles and our experience, to enable research groups to shorten the learning curve and implement the mouse LT procedure with high technical success.
In mice, liver allografts are accepted indefinitely across major histocompatibility complex barriers without immunosuppressive therapy[8]. Thus, the selection of experimental subjects is based on anatomical/surgical reasons. Almost all investigators choose male mice as their subjects, not only because of their larger body size compared to their peer adult females, but also their necessity to use the penile vein for heparin injection and rehydration therapy[1,2,9]. Due to their widespread application in the field of basic science, wild-type and transgenic C57BL/6 mice are the most commonly used strains. In addition, Balb/c, C3H and other congenic mouse strains are often mentioned in the literature[10,11]. Mouse weight < 23 g is rarely used because stent insertion in the small bile duct is difficult, and the portal vein (PV) and infrahepatic inferior vena cava (IHIVC) may be too short for anastomosis[5]. Mice with a body weight > 33 g are not recommended as donors because they have a large amount of intra-abdominal fat, making the surgical procedures more difficult[12]. According to our experience, mice with a body weight of 25-30 g and donors who are slightly lighter than recipients are optimal for MOLT. In addition, the diameter of the bile duct varies greatly from strain to strain although the mechanism is unknown. The success of biliary reconstruction is the key factor for long-term survival in MOLT. Among the existing strains, C3H mice have wider common bile ducts than any other strains. C57BL/6 and Balb/c mice have small bile ducts, while the diameter of the B10 bile duct is between that of C3H and C57BL/6 mice. Therefore, without influencing the experimental design, investigators should choose a mouse strain with a wider bile duct to facilitate model establishment.
As the most commonly used anesthesia method in rodent experiments, intraperitoneal injection is seldom used in MOLT[12]. Most injectable anesthetics are metabolized in the liver, metabolites can influence hepatic metabolism and can modify hepatic hemodynamics, alter carbohydrate metabolism, which can jeopardize hepatic and cardiopulmonary functions[13]. With the advantage of less cardiovascular depression, low hepatotoxicity, rapid excretion and easy regulation of anesthesia depth, inhalational anesthesia has become the standard anesthesia in MOLT research. With the exception of several studies which used ether to anesthetize the mice[14,15], most investigators prefer isoflurane with a mixture of oxygen[6,10,16,17]. The target inhaled concentration of isoflurane should be 3% to 4% in the induction phase, 2% in the maintenance phase, and < 0.5% in the anhepatic phase[18]. Anesthesia adjustment during the anhepatic phase is crucial for the success of the operation, deep anesthesia can cause peripheral vasodilatation, and accordingly leads to severe hypotension and intraoperative death[19]. However, the effect of anesthesia varies from mouse to mouse. An effective way to monitor depth of anesthesia is to observe the respiration rate of the animal during the operation, a respiration rate of one breath per second is considered to be an ideal state of anesthesia[5].
The mice should be kept in a temperature and light (12/12 h light/dark) controlled facility with free access to food and water before surgery. Deprivation of solid food for 12-24 h prior to surgery can theoretically avoid extension in the gastrointestinal tract and reduce the risk of aspiration under anesthesia[8]; therefore, it has been used by some research groups[8,10,14,20]. However, the impact of fasting has not been examined as mice can maintain a satisfactory long-term survival (> 100 d) without food deprivation[2,16,21]. In our opinion, fasting is unnecessary and such a long period of food deprivation may cause hypoglycemia, which is an adverse factor for intraoperative maintenance and postoperative recovery.
Heat loss during anesthesia increases the risk of cardiac arrhythmia, coagulopathy, and postoperative infection[22,23], which jeopardizes surgical success and postoperative survival rates. Core body temperature decreases suddenly after anesthesia induction and continues to diminish during the course of prolonged general anesthesia. A heating pad and warming lamp are widely used to promote postoperative recovery of MOLT[1,2,16,17,21,24], but have no impact on hypothermia during the operation. We recommend using a warming pad during the entire procedure except for the anhepatic phase to minimize heat loss.
Another significant detail during the perioperative period is that analgesics and antibiotics should be routinely used as indicated in institutional guidelines. The individual distress and suffering of animals must be minimized not only for ethical reasons, but also because of their potentially adverse effects on experimental results[25]. Failure to alleviate acute postoperative pain could result in fluctuations in blood pressure, heart rate, respiratory rate, body temperature, and food and water consumption, which is disadvantageous to early postoperative recovery. As MOLT is performed under clean but not sterile conditions using a surgical microscope, it is critically important to prevent biliary and abdominal infections with the use of local or systemic antibiotics in MOLT, which determines long-term survival.
The main steps in MOLT have reached a consensus among different investigators. After shaving and disinfecting the abdomen, the liver is exposed with the assistance of a wide abdominal incision and retractors. The liver needs to be freed from all ligamentous attachments from its surrounding organs. The PV and IHIVC are skeletonized to the level of the superior mesenteric vein and left renal vein, respectively, to ensure that there will be a sufficient length for cuff preparation and no stenosis in the cuff. The pyloric, splenic, and right renal veins and the right renal artery are ligated and divided to make sure that the right renal vein is separated from the right renal artery at this step, or it will be difficult to divide the posterior wall of the IHIVC. The right adrenal vein and paraesophageal vessel are cauterized or ligated to prevent hemorrhage. Dissection of the hepatic artery depends on the anastomotic methods. The hepatic artery is ligated and divided in the nonarterialized model. When the model involves artery reconstruction, the hepatic artery is dissected to the celiac trunk by ligation and division of the splenic, left gastric, and gastroduodenal arteries for stent anastomosis[6,11,24], the branches of the aorta are also ligated and severed successively to allow preparation of the hepatic–celiac–aortic arterial segment[14,16,21,26] or hepatic–celiac–aortic–mesenteric arterial segment[2,9] for suture anastomosis. For continuous bile flow, the gallbladder is ligated and removed. Operators use their own operating procedures for liver dissection. We prefer a clockwise order. That is, starting from the caudate lobe, followed by the IHIVC, gallbladder, suprahepatic inferior vena cava (SHIVC), paraesophageal vessel, and finally the first porta hepatis. In that order, we leave the bile duct to the last step, to some extent, to reduce biliary ischemic time.
There are three noticeable steps in this part: Heparinization, biliary cannulation and perfusion. Heparin dose varies from 20 to 100 IU. It can be injected through the penile vein as mentioned previously. Other researchers inject heparin through the IHIVC[14,17,20,27] or together with perfusion solution[2,16].
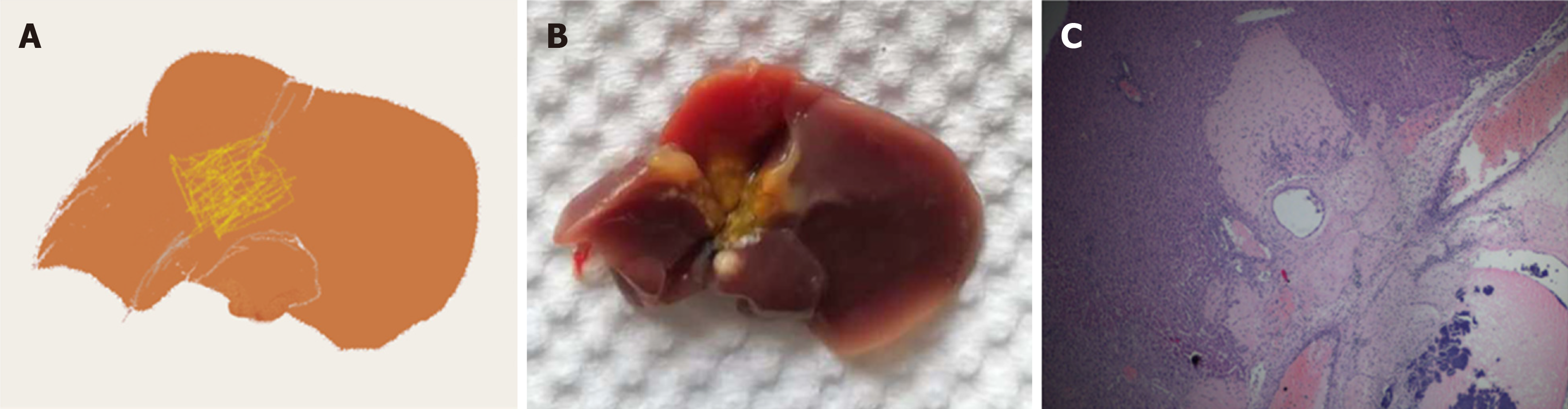
Different materials have been used for bile duct stenting, including polyethylene tube[2,5,9,10,16,21,26,28], Venflon tube[6], Peek TM tube[24], Peek tube[27,29] and epidural catheter[14,17]. Of these, polyethylene tube is the most commonly used material. Almost all these materials need to be stretched to adapt to the small diameter of the bile duct. The length of stent has ranged from 2 mm to 4 mm, except in one study where a 1-cm epidural catheter was chosen. An optimal stent is the key factor for long-term survival. Another important factor is the position where the stent is inserted into the common bile duct. If the stent is inserted too close to the hilum, it may cause obstruction of the bile duct confluence[5]. However, if the insertion position is too far from the hilum, the operative difficulty will be reduced, while the ischemic part of the bile duct is accordingly increased, so there is a risk of leakage or ischemic necrosis of the proximal bile duct[6]. In our experience, necrosis of the hilar region can be observed under these circumstances (Figure 1). Therefore, the bile duct stent should be inserted as close to the hilum without obstructing the bifurcation of the hepatic duct.
The aims of perfusion include flushing out the donor’s residual blood and rapid cooling of the liver[30]. Perfusion solution of 1-10 mL 0.9% NaCl solution Ringer’s solution or University of Wisconsin (UW) solution is injected through the IHIVC, PV and/or abdominal aorta. There is no significant difference between different perfusion solutions based on current literature. However, UW solution must be slowly flushed out the liver graft to prevent hyperkalemia after reperfusion. One thing that needs to be noted is the perfusion pressure. Sinusoid endothelial cell function can be impaired during high-pressure perfusion, whereas low-pressure perfusion may extend the warm ischemic time and increase the incidence of microthrombus formation[7]. Perfusion speed should be limited to 10-30 mL/h to avoid liver cell edema[31].
The donor liver is placed and stored in a container with cold (4°C) preservation solution (same with perfusion solution) until implantation. A two-cuff technique was initially applied in rat OLT in 1979[32]. With the advantage of shortened anhepatic time and improved graft survival, it has developed as the standard anastomotic method for the IHIVC and PV in MOLT. The cuff is prepared before the procedure, and a polyethylene tube[2,24,26,27,29] and intravenous catheter[5,9,17] are the most commonly used cuff materials. The most suitable cuff size is 20 or 18 G for the PV and 18 or 16 G for the IHIVC. Surgical blades are used to mechanically score grooves in the body of the PV and IHIVC cuffs with a surgical microscope, which facilitates more reliable ligation. The cuff-technique of the PV and IHIVC is similar.
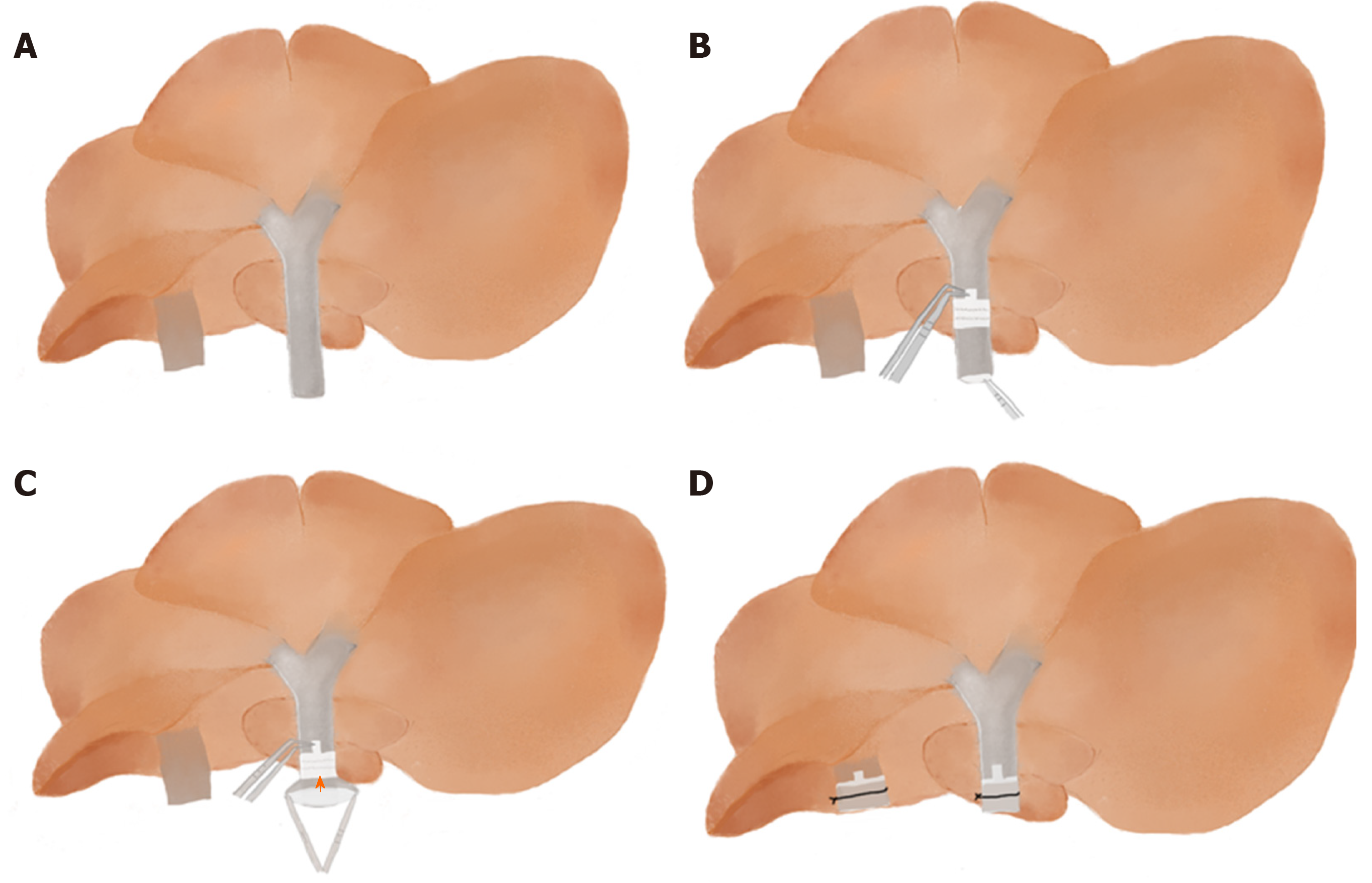
The difficulty in this part is to evert the distal end of vessel over the cuff. After pulling the vessel (IHIVC or PV) through the cuff, Qian et al[1] used a stabilizing clamp to fix the extension handle of the cuff together with the vessel, put the stabilizing clamp on the container, and then everted the vessel wall over the cuff and tied it. Pan et al[17] used forceps instead of the stabilizing clamp to secure the cuff extension (without the PV), then fixed the forceps to the wall of the bath container. Yokota et al[5] clamped the handle of a Weldon miniature bulldog clamp with a mosquito clamp and put soft clay on the handle of the mosquito clamp to fix it in place on the ice, then used the bulldog clamp to hold the extension part of the cuff to fix the cuff. In contrast with other investigators, we have found that there is no need to use additional surgical equipment to clamp the cuff. First of all, make sure the whole donor liver is immersed in water because the vessel wall is naturally opened under water. We carefully rotate the donor liver floating in the dish so that the inferior surface faces upward. We pass forceps through the lumen of the cuff to grasp the vessel so that the cuff is slipped over the vessel. We use forceps with a 45° angle to hold the extension of the cuff and use another curved round handle forceps to fold the end of the vessel over the cuff body to expose the inner endothelial surface and from one side to the other, then secure the IHIVC to the cuff by ligation (Figure 2). This method is simpler than the previous method and a beginner could master this approach within a short time of practice.
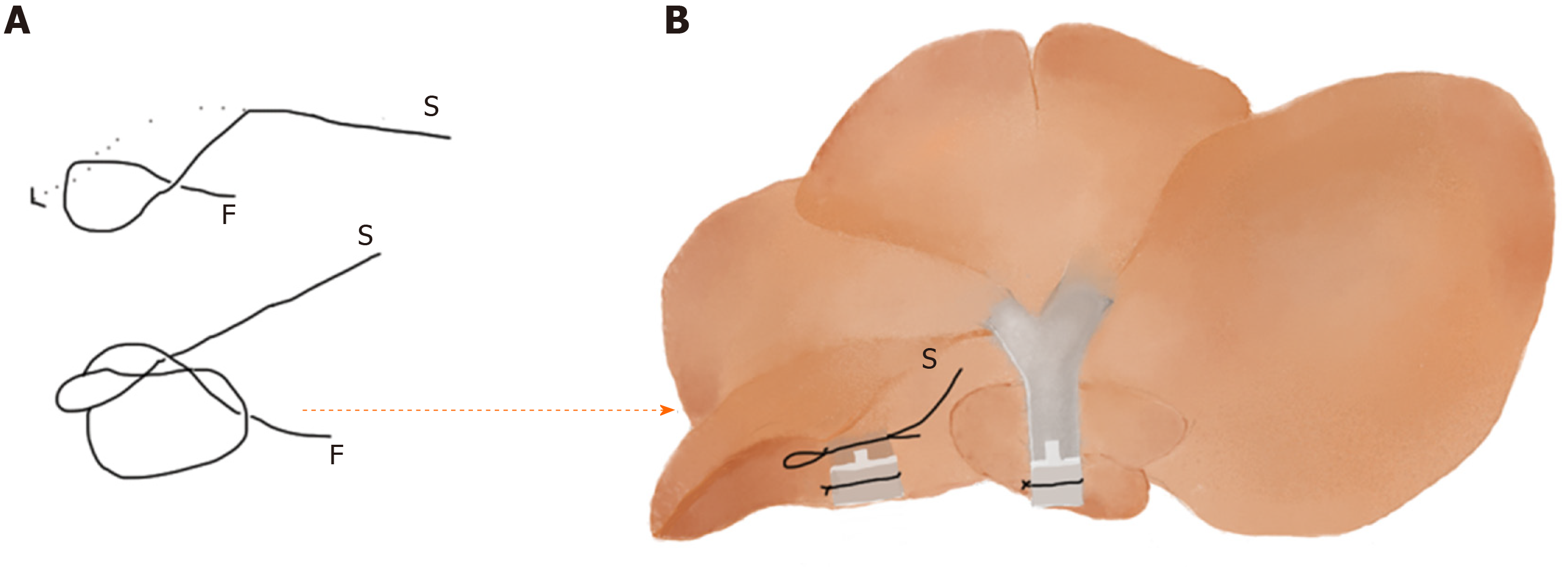
In order to avoid bleeding from the graft IHIVC after portal reperfusion, the donor IHIVC needs to be temporarily occluded at the back-table preparation. A micro clamp positioned on the proximal part of the IHIVC is an effective method in rat OLT[33]. However, in MOLT the donor IHIVC may be too short to be clamped; therefore, a silk tie around the IHIVC between the attached cuff and the right inferior lobe provides a good alternative[5]. However, if the tie is too tight, it could be hard to release after IHIVC anastomosis. On the contrary, if the tie is not tight enough, it may not be effective in preventing bleeding from the IHIVC immediately after PV anastomosis. We recommend the use of a slipknot to eliminate this confusion (Figure 3).
With a midline or a transverse incision and good exposure of the upper abdomen, the liver is separated in a manner similar to that of the donor. One difference is that the PV and IHIVC are dissected to the level of the right renal vein and pyloric vein, respectively. The proper hepatic artery is ligated and divided in the nonarterialized model. In the arterialized model, the proper hepatic artery and the gastroduodenal artery are tied to leave the common hepatic artery for stent insertion, or a segment of the abdominal aorta is separated below the left renal artery for end-to-side artery anastomosis by suturing. Furthermore, extensive dissection of the posterior side of the SHIVC is performed so that a silk suture can pass through for use as liver retraction at the time of SHIVC occlusion.
The IHIVC, PV and SHIVC are clamped in order. Before clamping the PV, a stay suture is placed on the left and right branch of the PV as retraction for PV anastomosis. Occlusion of the SHIVC should include a portion of the diaphragm to prolong the end of the anastomotic vessel and to prevent tearing during anastomosis. The principle of recipient hepatectomy is to leave the recipient vessels as long as possible. Some liver parenchyma is left on the posterior wall of the SHIVC to facilitate posterior wall suture.
The SHIVC is anastomosed with 10-0 nylon suture using a one-suture anastomosis technique. Good exposure of the venous walls, especially in the corners, is directly related to the speed and quality of the anastomosis[34], and the stay suture on the other side of the vessel is of great help. The PV and the IHIVC are reconnected by cuff anastomosis. Reconstruction of the bile flow is achieved by inserting the graft’s stent tube into the recipient’s bile duct and securing it and flushing any air from the vessel cavity to avoid air thrombosis before the anastomosis is complete.
Occlusion of hepatic blood flow causes severe metabolic and hemodynamic disturbances. Cardiac output significantly reduces while systemic vascular resistance accordingly increases during the PV clamping phase, leading to visceral congestion, and accumulation of toxins, tissue ischemia, intestinal edema, bacterial translocation, and impaired renal function[35-37]. Minimizing the duration of PV clamping is one of the significant features of a successful operation. When the MOLT model was first described in 1991, recipient mice with an anhepatic period > 20 min was incompatible with consistent success[1].
Most of the literature has reported a limited anhepatic phase < 20 min since then[2,6,9,10,14,21,24,26]. However, Humar et al[16] reported a series of arterialized MOLT models with a mean anhepatic time of 25.78 min ± 3 min (the longest anhepatic time was 29 min), and achieved a survival rate of 86% at 30 d following surgery. The authors attributed the success partially to hepatic arterial reconstruction. Another group investigated a MOLT model without arterial reconstruction, whose anhepatic time was kept below 25 min, and recorded an excellent (30 d) survival rate of 100%[29]. Similarly, a research group from Pittsburgh suggested a 20-30-min anhepatic phase. They showed that the allogeneic liver grafts survived for > 100 d without the use of immunosuppressive agents[5,38]. In our experience, the mice without arterial reconstruction seem also to tolerate a longer anhepatic phase (< 25 min), which could achieve 100-d survival. One particular case with 31 min before graft reperfusion survived for > 30 d. We believe that the anhepatic phase does not need to be strictly limited as before, and other details also matter in a successful model.
It is well known that hepatic arterial reconstruction is considered to be a necessary step in human and large animal OLT[39]. Whether the MOLT model necessitates hepatic artery reconstruction has been controversial from the beginning. The standard for a successful animal model is long-term survival, which is necessary for further research. In 2002, a comparison of arterialized and nonarterialized OLT in mice showed that eight animals undergoing MOLT with reconstruction of the hepatic artery survived permanently (> 1 mo), whereas only four of the eight control animals receiving a nonarterialized graft survived[2]. At the same time, in another study all recipients of arterialized (n = 6) and nonarterialized (n = 8) syngeneic liver grafts survived > 100 d[26]. With such a difference, various investigators have performed MOLT with or without arterial reconstruction based on their own experience and experimental objectives. One-month or even 100-d survival can be observed in nonarterialized models[1,15,29,38] as well as in arterialized models[2,6,14,16,21,26] (Table 1). Moreover, dissection and anastomosis of the hepatic artery are complicated and time consuming, which is frustrating for beginners. Moreover, due to variation in the origin of the hepatic artery, a few animals have to be excluded from organ donation[7]. In our opinion, arterial reconstruction is unnecessary for the establishment of MOLT for most basic research.
| Year | Donor | Recipient | Anhepatic phase (min) | Artery reconstruction (Y/N) | Anastomotic methods | Anastomotic artery | Survival rate (%) | Ref. | |||
| Suture | Stent | Donor | Recipient | 1-mo | 100 d | ||||||
| 1991 | B6AF1/B6/Balb/C | B6AF1/B6/Balb/C | < 20 | N | 67 | [1] | |||||
| 2002 | Balb/C | Balb/C | < 20 | Y and N | √ | Hepatic-celiac-aortic-mesenteric artery segment | Infrarenal aorta | 100 vs 50 | [2] | ||
| 2003 | C57BL/10 | CBA/Ca | 14.5 ± 1.5 | Y and N | √ | Hepatic-celiac-aortic artery segment | Infrarenal aorta | 100 vs 100 | [26] | ||
| 2003 | C57BL/6 | C57BL/6 | 14-17 | Y | √ | Hepatic–celiac–aortic artery segment | Infrarenal aorta | 100 | [21] | ||
| 2004 | C57BL/6 | C57BL/6 | < 25 | N | 100 | [30] | |||||
| 2007 | C57BL/6 | Balb/C | NA | N | 91 | [15] | |||||
| 2010 | C57BL/6 | C57BL/6 | 15 ± 2 | Y | √ | Hepatic–celiac–aortic artery segment | Infrarenal aorta | 85.7 | [14] | ||
| 2013 | C57BL/6 | C57BL/6 | 25.78 ± 3 | Y | √ | Hepatic–celiac–aortic artery segment | Infrarenal aorta | [16] | |||
| 2014 | C57BL/6 | C3H | NA | N | 100 | [39] | |||||
| 2016 | C57BL/6 | C57BL/6 | 12.5 ± 2 | Y | √ | Celiac trunk | Common hepatic artery | 100 | [6] | ||
The difficulties in the MOLT model make it only available for a few research teams, which largely limits the development of basic research in the field of LT. Although utilizing this model requires the expertise of a microsurgeon, we believe every detail we summarized here would allow a beginner to master this model with a short learning curve that will allow broader dissemination of the technique and favor the use of this clinically important animal OLT model.
The authors thank Dr. Xiao-Kang Li and Dr. Miwa Morita for help in establishing the MOLT model.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bramhall SR S-Editor: Dou Y L-Editor: Webster JR E-Editor: Ma YJ
| 1. | Qian SG, Fung JJ, Demetris AV, Ildstad ST, Starzl TE. Orthotopic liver transplantation in the mouse. Transplantation. 1991;52:562-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 92] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Tian Y, Rüdiger HA, Jochum W, Clavien PA. Comparison of arterialized and nonarterialized orthotopic liver transplantation in mice: prowess or relevant model? Transplantation. 2002;74:1242-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Chen J, Gong W, Ge F, Huang T, Wu D, Liang T. A review of various techniques of mouse liver transplantation. Transplant Proc. 2013;45:2517-2521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Yokota S, Yoshida O, Ono Y, Geller DA, Thomson AW. Liver transplantation in the mouse: Insights into liver immunobiology, tissue injury, and allograft tolerance. Liver Transpl. 2016;22:536-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Yokota S, Ueki S, Ono Y, Kasahara N, Pérez-Gutiérrez A, Kimura S, Yoshida O, Murase N, Yasuda Y, Geller DA, Thomson AW. Orthotopic mouse liver transplantation to study liver biology and allograft tolerance. Nat Protoc. 2016;11:1163-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Oldani G, Lacotte S, Orci LA, Delaune V, Slits F, Gex Q, Morel P, Rubbia-Brandt L, Toso C. Efficient nonarterialized mouse liver transplantation using 3-dimensional-printed instruments. Liver Transpl. 2016;22:1688-1696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Li DY, Shi XJ, Li W, Du XH, Wang GY. Key Points in Establishing a Model of Mouse Liver Transplantation. Transplant Proc. 2015;47:2683-2689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Benseler V, McCaughan GW, Schlitt HJ, Bishop GA, Bowen DG, Bertolino P. The liver: a special case in transplantation tolerance. Semin Liver Dis. 2007;27:194-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 122] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 9. | Shen XD, Gao F, Ke B, Zhai Y, Lassman CR, Tsuchihashi S, Farmer DG, Busuttil RW, Kupiec-Weglinski JW. Inflammatory responses in a new mouse model of prolonged hepatic cold ischemia followed by arterialized orthotopic liver transplantation. Liver Transpl. 2005;11:1273-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Li DY, Xie SL, Wang GY, Dang XW. CD47 blockade alleviates acute rejection of allogeneic mouse liver transplantation by reducing ischemia/reperfusion injury. Biomed Pharmacother. 2020;123:109793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Ono Y, Perez-Gutierrez A, Nakao T, Dai H, Camirand G, Yoshida O, Yokota S, Stolz DB, Ross MA, Morelli AE, Geller DA, Thomson AW. Graft-infiltrating PD-L1hi cross-dressed dendritic cells regulate antidonor T cell responses in mouse liver transplant tolerance. Hepatology. 2018;67:1499-1515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 12. | Zhang H, Zhang Y, Ma F, Bie P, Bai L. Orthotopic transplantation of decellularized liver scaffold in mice. Int J Clin Exp Med. 2015;8:598-606. [PubMed] |
| 13. | Suliburk JW, Gonzalez EA, Kennison SD, Helmer KS, Mercer DW. Differential effects of anesthetics on endotoxin-induced liver injury. J Trauma. 2005;58:711-716; discussion 716-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Huang DR, Wu ZJ, Zhu Y. Modified arterialization of orthotopic liver transplantation in a mouse model. Hepatobiliary Pancreat Dis Int. 2010;9:264-268. [PubMed] |
| 15. | Morita M, Fujino M, Li XK, Kimura H, Nakayama T, Taniguchi M, Sugioka A. Spontaneous tolerance involving natural killer T cells after hepatic grafting in mice. Transpl Immunol. 2007;18:142-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Humar B, Raptis DA, Weber A, Graf R, Clavien PA, Tian Y. Sewed revascularization for arterialized liver transplantation in mice. J Surg Res. 2013;184:e1-e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Pan N, Liu Z, He J, Li S, Lv X, Wang L, Liu Q. Comparison of Methods for the Reconstruction of the Hepatic Artery in Mouse Orthotopic Liver Transplantation. PLoS One. 2015;10:e0133030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | He S, Atkinson C, Qiao F, Chen X, Tomlinson S. Ketamine-xylazine-acepromazine compared with isoflurane for anesthesia during liver transplantation in rodents. J Am Assoc Lab Anim Sci. 2010;49:45-51. [PubMed] |
| 19. | Constantinides C, Mean R, Janssen BJ. Effects of isoflurane anesthesia on the cardiovascular function of the C57BL/6 mouse. ILAR J. 2011;52:e21-e31. [PubMed] |
| 20. | Liu Z, Pan N, Lv X, Li S, Wang L, Liu Q. Orthotopic liver transplantation from cardiac death donors in the mouse: a new model and evaluation of cardiac death time. Iran J Basic Med Sci. 2017;20:683-689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Tian Y, Graf R, Jochum W, Clavien PA. Arterialized partial orthotopic liver transplantation in the mouse: a new model and evaluation of the critical liver mass. Liver Transpl. 2003;9:789-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Devey L, Festing MF, Wigmore SJ. Effect of temperature control upon a mouse model of partial hepatic ischaemia/reperfusion injury. Lab Anim. 2008;42:12-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Sessler DI. Complications and treatment of mild hypothermia. Anesthesiology. 2001;95:531-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 369] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 24. | Zhou S, Palanisamy AP, McGillicuddy JW, Theruvath TP, Emre SH, Chavin KD. New method of stent-facilitated arterial reconstruction for orthotopic mouse liver transplantation. J Surg Res. 2014;187:297-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Gargiulo S, Greco A, Gramanzini M, Esposito S, Affuso A, Brunetti A, Vesce G. Mice anesthesia, analgesia, and care, Part I: anesthetic considerations in preclinical research. ILAR J. 2012;53:E55-E69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 174] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 26. | Steger U, Sawitzki B, Gassel AM, Gassel HJ, Wood KJ. Impact of hepatic rearterialization on reperfusion injury and outcome after mouse liver transplantation. Transplantation. 2003;76:327-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Birsner JH, Wan C, Cheng G, Evans ZP, Polito CC, Fiorini RN, Gilbert G, Haines JK, Schmidt MG, Chavin KD. Steatotic liver transplantation in the mouse: a model of primary nonfunction. J Surg Res. 2004;120:97-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Klein I, Crispe IN. Complete differentiation of CD8+ T cells activated locally within the transplanted liver. J Exp Med. 2006;203:437-447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 85] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 29. | Que X, Debonera F, Xie J, Furth EE, Aldeguer X, Gelman AE, Olthoff KM. Pattern of ischemia reperfusion injury in a mouse orthotopic liver transplant model. J Surg Res. 2004;116:262-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | 't Hart NA, van der Plaats A, Leuvenink HG, Wiersema-Buist J, Olinga P, van Luyn MJ, Verkerke GJ, Rakhorst G, Ploeg RJ. Initial blood washout during organ procurement determines liver injury and function after preservation and reperfusion. Am J Transplant. 2004;4:1836-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Schlegel A, Graf R, Clavien PA, Dutkowski P. Hypothermic oxygenated perfusion (HOPE) protects from biliary injury in a rodent model of DCD liver transplantation. J Hepatol. 2013;59:984-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 138] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 32. | Kamada N, Calne RY. Orthotopic liver transplantation in the rat. Technique using cuff for portal vein anastomosis and biliary drainage. Transplantation. 1979;28:47-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 531] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 33. | Oldani G, Lacotte S, Morel P, Mentha G, Toso C. Orthotopic liver transplantation in rats. J Vis Exp. 2012;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Nakagawa M, Inoue K, Iida T, Asano T. A modified technique of end-to-side microvascular anastomosis for the posterior wall. J Reconstr Microsurg. 2008;24:475-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 35. | Santiago F, Bueno P, Olmedo C, Comino A, Hassan L, Ferrón-Celma I, Muffak K, Serradilla M, Mansilla A, Ramia JM, Villar JM, Garrote D, Ramirez A, Ferrón JA. Time course of intraoperative cytokine levels in liver transplant recipients. Transplant Proc. 2006;38:2492-2494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Pappas G, Palmer WM, Martineau GL, Penn I, Halgrimson CG, Groth CG, Starzl TE. Hemodynamic alterations caused during orthotopic liver transplantation in humans. Surgery. 1971;70:872-875. [PubMed] |
| 37. | Kang YG, Freeman JA, Aggarwal S, DeWolf AM. Hemodynamic instability during liver transplantation. Transplant Proc. 1989;21:3489-3492. [PubMed] |
| 38. | Yoshida O, Kimura S, Dou L, Matta BM, Yokota S, Ross MA, Geller DA, Thomson AW. DAP12 deficiency in liver allografts results in enhanced donor DC migration, augmented effector T cell responses and abrogation of transplant tolerance. Am J Transplant. 2014;14:1791-1805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 39. | Uchida H, Sakamoto S, Matsunami M, Sasaki K, Shigeta T, Kanazawa H, Fukuda A, Nakazawa A, Miyazaki O, Nosaka S, Kasahara M. Hepatic artery reconstruction preserving the pancreaticoduodenal arcade in pediatric liver transplantation with celiac axis compression syndrome: report of a case. Pediatr Transplant. 2014;18:E232-E235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |