Published online Jul 14, 2020. doi: 10.3748/wjg.v26.i26.3792
Peer-review started: November 19, 2020
First decision: April 2, 2020
Revised: May 9, 2020
Accepted: June 20, 2020
Article in press: June 20, 2020
Published online: July 14, 2020
Processing time: 238 Days and 15.4 Hours
Blastocystis hominis (B. hominis) and Dientamoeba fragilis (D. fragilis) are two protozoan parasites of human bowel that are found throughout the world. There is still debate about the pathogenicity of these protozoans, despite them being commonly associated with gastrointestinal symptoms and can cause health issue in both children and adults. These parasites are usually transmitted through faecal-oral contact particularly under poor hygiene conditions or food/water contamination. Once a person is infected, the parasites live in the large intestine and are passed in the faeces.
To investigate the effect of triple antibiotic therapy using enema infusion in the treatment of B. hominis and D. fragilis infections.
This retrospective longitudinal study was conducted in a single medical centre, which included fifty-four patients (≥ 18 years) who were positive for D. fragilis, B. hominis or both between 2017 and 2018. The treatment consisted of triple antibiotics that were infused over two consecutive days through rectal enema. Faecal samples were collected from participants pre- and post-treatment and were tested for parasites using microscopy and polymerase chain reaction. Patients’ symptoms were recorded prior and after the treatment as well as patient demographic data.
Patients (n = 54), were either positive for B. hominis (37%), D. fragilis (35%) or both (28%). All patients completed the two-day treatment and no serious adverse effect was reported. The most common side effect experienced by the patients during the treatment was urine discolouration which was cleared by six weeks of follow-up. Common symptoms reported prior to treatment were diarrhoea, abdominal pain, constipation and fatigue. Other symptoms included abdominal discomfort, dizziness and blood in the stool. Eighty-nine percent of patients completed a final stool test post-treatment. At six weeks post-treatment, 79% of patients cleared the parasites from their faeces. Symptoms such as abdominal discomfort, dizziness and blood in the stool decreased significantly at both seven days and six weeks post-treatment (P < 0.040). The enema retention time, bowel preparation, previous antibiotic treatment or previous gastrointestinal problems had no significant effect on parasite eradication.
Overall, eradication of parasites and improvement of clinical outcomes were observed in treated patients, showing the efficacy of this combination to eradicate the parasites and provide positive clinical outcome.
Core tip: Intestinal parasitic infections caused by Blastocystis hominis (B. hominis) and Dientamoeba fragilis (D. fragilis) have the ability to cause illness. This study investigated the effect of a triple antibiotic therapy using 2-d enema infusion for treatment of patients who were positive to B. hominis, D. fragilis or both. A significant reduction in major symptoms as well as parasite eradication were observed post-treatment. Larger clinical trials should further investigate improvements of such therapy using larger volume enemas and alternative delivery routes.
- Citation: Roshan N, Clancy A, Gunaratne AW, LeBusque A, Pilarinos D, Borody TJ. Two-day enema antibiotic therapy for parasite eradication and resolution of symptoms. World J Gastroenterol 2020; 26(26): 3792-3799
- URL: https://www.wjgnet.com/1007-9327/full/v26/i26/3792.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i26.3792
The two anaerobic protozoa, Blastocystis hominis (B. hominis) and Dientamoeba fragilis (D. fragilis) are described as “neglected parasites”, which are found in the intestinal tract of humans[1]. Intestinal protozoan infections are a global problem in both developed and developing countries and can cause considerable morbidity especially in children[1]. In many low-income countries, poverty and poor sanitation are the main causal factors for transmission. Both parasites are generally transmitted through faecal-oral contact or water/food contamination and are often detected in the stool of patients with gastrointestinal problems[2,3]. These symptoms are comparable with those experienced with irritable bowel syndrome (IBS) and inflammatory bowel disease (IBD). As such, a possible link between these infections, IBD and IBS has been suggested[3]. Thus, the eradication of these parasitic infections may be necessary when they are the only infectious agents detected in symptomatic patients.
Blastocystis species (spp) are unicellular enteric parasites present in most species of animals and have extensive genetic diversity. B. hominis is the only member of Stramenopiles that infects humans and exists in four different forms; cyst, ameboid, granular and vacuolar[4]. Blastocystis spp have a global distribution. Its prevalence in humans, in developing countries is estimated to be around 76%-100% compared to 5% in developed countries[5]. Symptoms associated with Blastocystis infection include abdominal pain, diarrhoea, nausea, bloating and fatigue[5].
The other protozoan, D. fragilis, was described for the first time by Jepps and Dobell in England in 1918[6]. It was initially misclassified as an amoeba, and was later re-classified as a unicellular flagellate despite lacking a flagellum[7]. The global distribution of D. fragilis is reported to range between 0.4% to 71%, with developed countries showing a higher prevalence. D. fragilis has been associated with symptoms such as abdominal pain, diarrhoea and loose stools[5,8].
Although, these parasites have a well-documented pathogenic potential, there is still debate on whether these infections should be treated and there are few studies that have investigated the treatment options for these infections. In addition, these parasites are relatively difficult to eradicate and there are often side effects associated with oral antibiotic therapy.
Metronidazole as an oral antiprotozoal antibiotic was first developed in 1962 and was originally used for management of trichomoniasis[9]. Some studies have reported the success of metronidazole in the treatment of D. fragilis and B. hominis infections[10], while others showed treatment failure[11]. In a study investigating the treatment of D. fragilis with metronidazole or tetracycline, a microbiological response was observed in 60% of patients and relief of symptoms was shown in only 30% of patients treated with metronidazole, while tetracycline had no effect[9]. These findings suggested that a substantial number of patients were insufficiently treated with metronidazole and more efficient drugs were needed for treating this infection. Some studies combined several anti-parasite medications to achieve higher efficacy[12,13].
Other studies have combined anti-parasitic antibiotics to be used intra-luminally for treating resistant parasitic infections. Triple antibiotic therapy using nitazoxanide, secnidazole and furazolidone through colonic infusion may be an effective method for eradicating D. fragilis and B. hominis infections for those who fail oral antibiotic therapy[14]. Nitazoxanide was first described as a human cestocidal drug in 1984[9]. Clinical trials have demonstrated relief of metronidazole-resistant parasite diarrhoea following treatment with nitazoxanide. Adverse effects associated with nitazoxanide are uncommon and the incidence is reported to be lower than metronidazole[9,15]. The other antibiotic that has been considered for the treatment of these parasites is secnidazole, which has a longer half-life compared to metronidazole. It has been used for treatment of trichomoniasis, giardiasis and amoebiasis, with a cure rate of 80%-100%. Mild nausea is one of the side effects reported in a few patients. Another antibiotic with a broader antibacterial and antiprotozoal activity is furazolidone, which is a synthetic nitrofuran derivative[9]. It has shown activity particularly against Entamoeba histolytica and Giardia lamblia and is suggested as an alternative drug in the case of treatment failure with first line agents. There has not been any studies assessing the efficacy of furazolidone specifically in the treatment of D. fragilis or B. hominis infections[9]. Our study aimed to investigate the effect of combined antibiotics administered via enema infusion to eradicate D. fragilis and B. hominis and improve symptoms.
This retrospective, single centre longitudinal study was conducted between January 2017 and December 2018. Patients 18 years or older, who were positive for D. fragilis, B. hominis or both were invited to participate in this study. The treatment consisted of triple antibiotics (furazolidone 0.9 g, nitazoxanide 3 g and secnidazole 3.6 g) infused over two consecutive days through rectal enema. The antibiotic enema was administered via enema bag (gravity fed) containing the medication diluted in 300 ml of normal saline and delivered via rectum into the bowel. In the case of allergy, the culprit drug was replaced by paromomycin 4.5 g or diloxanide furoate 4.5 g. Faecal specimens were collected from study participants at baseline and six weeks after completion of treatment. Stool microscopy and polymerase chain reaction were used for parasite detection. Symptoms were recorded by the patients on a standard questionnaire prior to treatment and subsequently at three days, seven days and six weeks after the treatment. Patient demographic data (age, gender, previous treatment, concomitant medications and relevant medical history) were also collected to identify any confounding variables within the study population.
Statistical analysis was conducted using GraphPad Prism v.8 (La Jolla, CA, United States) software. Statistical differences between three or more sets of data were analysed using one-way analysis of variance and nonparametric technique, followed by Tukey’s multiple comparison post-test if the P value was significant. Wilcoxon matched-pairs signed rank test was used to compare the differences between two sets of data. The association between categorical variables were determined using Chi-square and Fisher’s exact test. P values of < 0.05 were considered significant. The study was approved by the institutions Ethics Committee (CDD19/C02).
Fifty-four (16 males) patients with an age range of 21-81 years (median age 49 years) participated in the study and all the patients completed the treatment. Twenty-three patients had received prior antibiotic therapy (including diloxanide furoate, metronidazole, trimethoprim/sulfamethoxazole (bactrim), secnidazole, tinidazole, furazolidone, doxycycline, paromomycin and nitazoxanide) and 18 patients had history of gastrointestinal symptoms (Table 1). Of the 54 patients, 37% were positive to B. hominis, 35% to D. fragilis and 28% to both. Only one patient was asymptomatic prior to the treatment; 83% of the patients had five or more of the symptoms listed in Table 1. The most common symptoms were diarrhoea, constipation, abdominal pain and fatigue. All patients completed the two days of antibiotic enema infusions administered in a volume of 300 mL. The median retention time for the enema among the patients was 3 h (ranging from 0.5 h to 12 h) and 39% of the patients had bowel preparation prior to the treatment. No serious adverse event was reported. The most common side effect experienced by the patients undergoing the treatment was urine discolouration, which cleared by six weeks of follow-up. Out of 54 patients, 48 completed a final stool test for investigation of parasite eradication at six weeks post-treatment. Overall 79% of patients cleared the parasites from their faeces at six weeks (Table 2).
| Gender (n) | Female (38), male (16) |
| Age mean (range), yr | 49 (21-81) |
| Bowel preparation (n) | Yes (21), no (33) |
| Parasite present (n) | BH only (20), DF only (19), both (15) |
| Prior antibiotic treatment for parasite eradication (n) | 23 |
| History of gastrointestinal problems (n) | 18 |
| Symptoms n) | |
| Diarrhoea (36) | Fatigue (38) |
| Constipation (27) | Lethargy (12) |
| Bloating (39) | Malaise (15) |
| Flatulence (37) | Blood in the stool (10) |
| Trapped wind/gurgling (14) | Low mood (22) |
| Loss of appetite (18) | Anxiety (13) |
| Dizziness/light headed (26) | Reflux/heartburn (15) |
| Headaches (22) | Muscular weakness (22) |
| Nausea (21) | Itchy skin (15) |
| Vomiting (7) | Skin rash (7) |
| Anal itching (21) | Discoloured urine (11) |
| Metallic taste (14) | Abdominal discomfort/pain (38) |
| Photo-sensitivity (9) |
| Parasite | ||||
| BH only | DFonly | Both | Total | |
| Number of patients | 20 | 19 | 15 | 54 |
| Number tested at 6 wk | 19 | 16 | 13 | 48 |
| Number cleared at 6 wk | 14 (29) | 13 (27) | 11 (23) | 38 (79) |
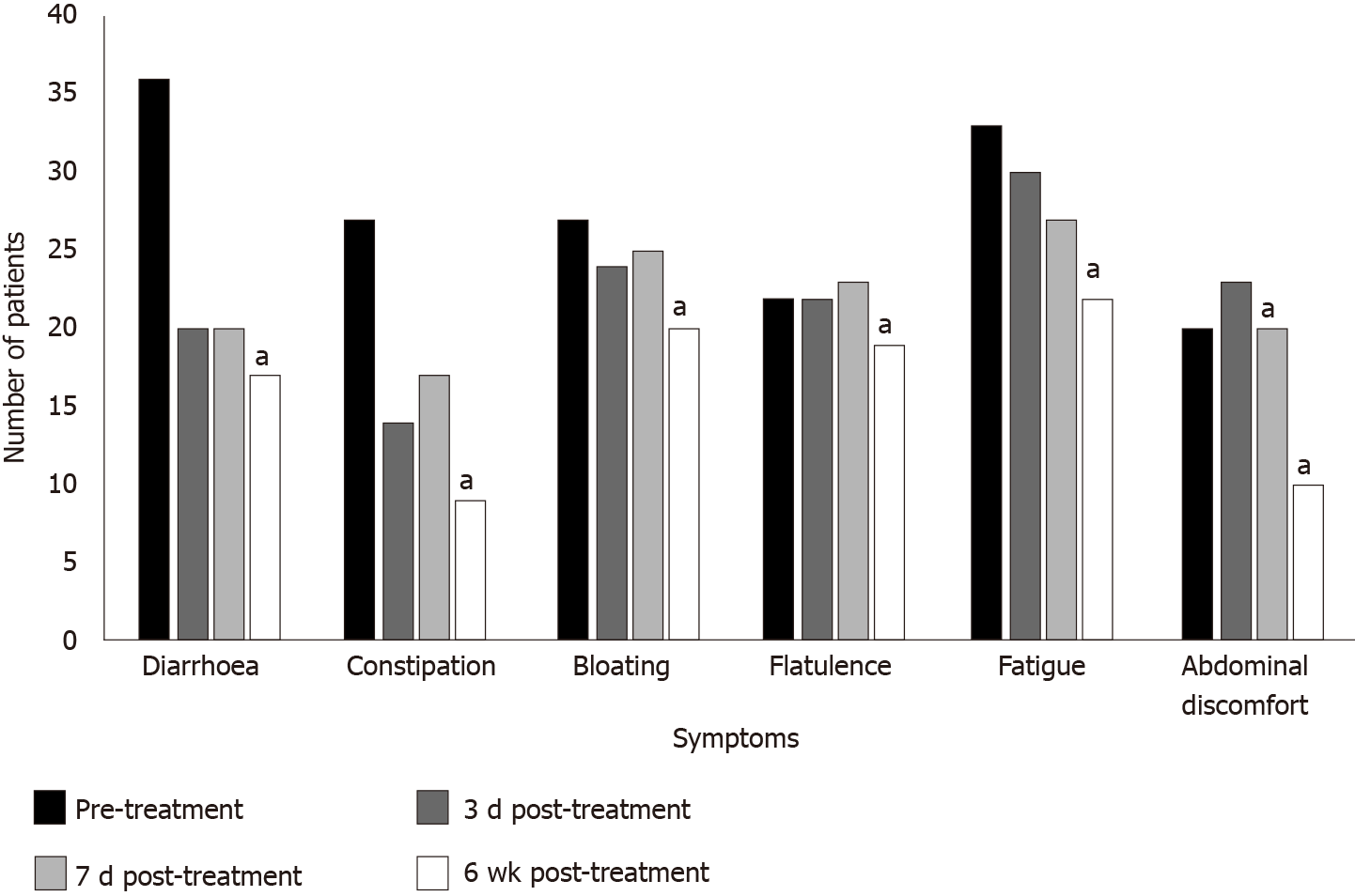
There was a significant reduction in abdominal discomfort, dizziness and blood in the stool at both seven days and six weeks post-treatment (P < 0.040) (Figure 1). Symptoms such as diarrhoea, constipation, bloating, flatulence, nausea, vomiting, anal itching, muscular weakness, itchy skin and skin rash showed significant improvement at six weeks post-treatment (P < 0.040). A significant improvement in patients’ mood was shown at all time-points, three days, seven days and six weeks post-treatment (P < 0.001). There was no significant association between the enema retention time, bowel preparation, previous antibiotic treatment or previous gastrointestinal problems and eradication of parasites.
Statistical significance: aP < 0.05.
In this study, successful eradication of B. hominis and D. fragilis infections occurred in 79% of patients at six weeks post-treatment with 2-d enema infusion using triple antibiotic therapy. An improvement in major clinical symptoms, diarrhoea and abdominal pain was also observed six weeks after the treatment. To our knowledge, this is the first study to investigate the efficacy of three novel antibiotics through a different route of delivery - enema infusion - in patients seeking treatment for B. hominis and D. fragilis infections.
B. hominis and D. fragilis have a worldwide distribution and are more commonly found than both G. lamblia and Cryptosporidium spp[5]. Exploring combinations of antibiotics and alternative methods of delivery is important to improve the clearance rate of B. hominis and D. fragilis infections and to eradicate the resistant strains. Therefore, this study examined the effect of a new combination of drugs infused via enema in a small group of patients seeking treatment for parasite infection. This showed a successful eradication of parasites and improvement of clinical outcomes. Although, treatment did not work for approximately 20% of patients, it still showed a higher achievement compared to monotherapy with conventional drugs such as metronidazole. The success and tolerability of this therapy may be due to the delivery of a high concentration of drugs to the colon where B. hominis and D. fragilis usually reside, with insignificant systemic absorption. Using an enema as the mode of delivery may be beneficial by bypassing the systemic absorption seen in oral dosing and hence, minimising the side effects.
Previously, a small number of studies have reported the effect of different antibiotic treatments in treating these parasitic infections. In a placebo-controlled trial, the efficacy of metronidazole in inducing remission and eradicating the parasites was investigated[16]. The study showed a higher eradication rate, one month following the treatment. However, the six months follow-up showed a high rate of recrudescence in patients treated with metronidazole. Therefore, it was concluded that metronidazole may be ineffective in achieving complete eradication of B. hominis, mainly due to drug resistance[5]. Another longitudinal, prospective case study on 11 symptomatic patients positive for Blastocystis showed that metronidazole failed to cure any of the patients[17]. Resistance to metronidazole was first reported in 1991, which brought doubt about its value as a first line treatment[18]. Metronidazole might be an effective treatment for certain patients, but does not necessarily provide complete eradication in patients with severe infection[17]. The variation in treatment response can be due to existence of resistance subtypes of parasite[9], which question the use of metronidazole as the single and first line of therapy.
In another longitudinal, prospective case study, 10 patients with diarrhoea-predominant IBS who were positive to B. hominis were treated with oral triple antibiotic therapy including diloxanide furoate, trimethoprim/sulfamethoxazole and secnidazole[14]. The parasite was eradicated in 60% of patients with IBS, which showed an improvement over conventional monotherapy such has metronidazole[14]. Similarly, in a retrospective cohort study from the Netherlands, 93 symptomatic patients were treated with paromomycin, along with other antibiotics such as, clioquinol and metronidazole[19]. Paromomycin showed a higher eradication rate of 98% compared to clioquinol (83%) and metronidazole (57%)[19]. While a number of antibiotics have shown to be effective in treating either B. hominis or D. fragilis infection and are recommended as therapeutic options, these recommendations are mainly based on a small number of non-randomised studies[9]. Moreover, there is little in vitro susceptibility data for these parasites. Further, prospective randomised studies are required to better understand the effectiveness of antibiotic combinations and modes of administration in B. hominis or D. fragilis infection eradication.
B. hominis and D. fragilis establish in the anaerobic environment of the human colon and thrive in presence of bacteria and in some circumstances can invade the intestinal mucosa[20]. Although, these two parasites are considered harmless, they are associated with a range of symptoms such as diarrhoea and abdominal pain[21], and have been associated with IBD and IBS[18]. It is suggested that they may contribute to symptoms via the disruption of gut microbiota, resulting in a decrease in beneficial bacteria such as Bifidobacterium and Lactobacillus spp. In this study, patients reported post-treatment symptoms at three different time points. Some of the symptoms such as abdominal discomfort and blood in the stools were resolved as early as seven days post-treatment. A few other studies have followed-up the possible adverse effects and symptoms post-treatment; however, those treatments were based on a mono-therapy[8,16]. In a study by Nigro et al[16], the six months follow-up on patients treated with metronidazole for B. hominis infection showed parasite recrudescence with return of symptoms.
This study reported both parasite eradication and pre- and post-treatment symptom comparisons. Other variables such as enema retention time were measured to investigate the possible factors contributing to efficacy. There are a number of limitations of this study, such as the relatively small sample size and lack of control group. Also, patients were followed-up for duration of six weeks while a longer follow-up period may give us information of late symptom return. Moreover, further studies with larger sample size and a control group could provide broader understanding of the treatment efficacy.
Overall, this study showed a significant achievement in both parasitic eradication and improvement of clinical outcomes which points to the use of combination therapies with an alternative delivery as first line of therapy. Larger scale randomised controlled trials could help improve efficacy and so symptom resolution.
Blastocystis hominis (B. hominis) and Dientamoeba fragilis (D. fragilis) are the two anaerobic protozoa which can be found in the intestinal tract of humans and can be transmitted via faecal-oral contact. Patients infected with these parasites can experience symptoms similar to those with irritable bowel syndrome such as abdominal pain, constipation, bloating or diarrhoea. The infections caused by the intestinal protozoa are global problems in both developed and developing countries and can cause considerable morbidity in the younger population.
A high failure rate has been observed with use of single drugs such as metronidazole in the treatment of B. hominis and D. fragilis parasitic infections, thus this has led to the development of novel combination therapies.
This study aimed to investigate the effect of combined antibiotics administered via enema infusion to eradicate D. fragilis and B. hominis and to determine their effect on resolution of symptoms.
A retrospective, single centre longitudinal study was conducted between 2017-2018 on patients 18 years or older, who were positive for D. fragilis, B. hominis or both. Triple antibiotics were infused over two days through rectal enema. Faecal specimens were screened for parasites from patients at baseline and six weeks after completion of treatment. Symptoms were recorded at three days, seven days and six weeks after the treatment. Patient demographic data were also collected to identify any confounding variables within the study population.
The results showed that the majority of patients (79%) showed complete clearance of parasites post-antibiotic enema infusion treatment. Improvement was observed in major clinical symptoms such as abdominal pain post-treatment. The most common side effects experienced were urine discolouration, which improved following the treatment.
A significant achievement in both parasitic eradication and improvement of clinical outcomes were observed, with minimal side effects. These points to the use of combination therapies via enema as a potential first line of therapy for parasite eradication.
In order to better understand the effect of antibiotic combinations and mode of administration in eradication of B. hominis or D. fragilis infections, further prospective randomised studies are required.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: García-Elorriaga G, Manenti AS-Editor: Liu M L-Editor: A E-Editor: Ma YJ
| 1. | Ibrahim AN, Al-Ashkar AM, Nazeer JT. Additional Glance on the Role of Dientamoeba fragilis & Blastocystis hominis in Patients with Irritable Bowel Syndrome. Iran J Parasitol. 2018;13:100-107. [PubMed] |
| 2. | Coyle CM, Varughese J, Weiss LM, Tanowitz HB. Blastocystis: to treat or not to treat. Clin Infect Dis. 2012;54:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 3. | Stark D, van Hal S, Marriott D, Ellis J, Harkness J. Irritable bowel syndrome: a review on the role of intestinal protozoa and the importance of their detection and diagnosis. Int J Parasitol. 2007;37:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 119] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Stensvold CR, Smith HV, Nagel R, Olsen KE, Traub RJ. Eradication of Blastocystis carriage with antimicrobials: reality or delusion? J Clin Gastroenterol. 2010;44:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 5. | Rostami A, Riahi SM, Haghighi A, Saber V, Armon B, Seyyedtabaei SJ. The role of Blastocystis sp. and Dientamoeba fragilis in irritable bowel syndrome: a systematic review and meta-analysis. Parasitol Res. 2017;116:2361-2371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 6. | Windsor JJ, Macfarlane L, Hughes-Thapa G, Jones SK, Whiteside TM. Detection of Dientamoeba fragilis by culture. Br J Biomed Sci. 2003;60:79-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Garcia LS. Dientamoeba fragilis, One of the Neglected Intestinal Protozoa. J Clin Microbiol. 2016;54:2243-2250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 8. | Girginkardeşler N, Coşkun S, Cüneyt Balcioğlu I, Ertan P, Ok UZ. Dientamoeba fragilis, a neglected cause of diarrhea, successfully treated with secnidazole. Clin Microbiol Infect. 2003;9:110-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Nagata N, Marriott D, Harkness J, Ellis JT, Stark D. Current treatment options for Dientamoeba fragilis infections. Int J Parasitol Drugs Drug Resist. 2012;2:204-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Stark D, Barratt J, Roberts T, Marriott D, Harkness J, Ellis J. A review of the clinical presentation of dientamoebiasis. Am J Trop Med Hyg. 2010;82:614-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 11. | Roberts T, Stark D, Harkness J, Ellis J. Update on the pathogenic potential and treatment options for Blastocystis sp. Gut Pathog. 2014;6:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 12. | Hui W, Li T, Liu W, Zhou C, Gao F. Fecal microbiota transplantation for treatment of recurrent C. difficile infection: An updated randomized controlled trial meta-analysis. PLoS One. 2019;14:e0210016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 111] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 13. | Borody TJ, Grippi D, Le Busque A, Gadalla S, Dawson V, Jaworski A. Improved eradication protocol for Blastocystis hominis. Am J Gatroenterol. 2015;110:S590. [DOI] [Full Text] |
| 14. | Nagel R, Bielefeldt-Ohmann H, Traub R. Clinical pilot study: efficacy of triple antibiotic therapy in Blastocystis positive irritable bowel syndrome patients. Gut Pathog. 2014;6:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (2)] |
| 15. | Fox LM, Saravolatz LD. Nitazoxanide: a new thiazolide antiparasitic agent. Clin Infect Dis. 2005;40:1173-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 289] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 16. | Nigro L, Larocca L, Massarelli L, Patamia I, Minniti S, Palermo F, Cacopardo B. A placebo-controlled treatment trial of Blastocystis hominis infection with metronidazole. J Travel Med. 2003;10:128-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Nagel R, Cuttell L, Stensvold CR, Mills PC, Bielefeldt-Ohmann H, Traub RJ. Blastocystis subtypes in symptomatic and asymptomatic family members and pets and response to therapy. Intern Med J. 2012;42:1187-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 18. | Sekar U, Shanthi M. Blastocystis: Consensus of treatment and controversies. Trop Parasitol. 2013;3:35-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 19. | van Hellemond JJ, Molhoek N, Koelewijn R, Wismans PJ, van Genderen PJ. Is paromomycin the drug of choice for eradication of Dientamoeba fragilis in adults? Int J Parasitol Drugs Drug Resist. 2012;2:162-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Yason JA, Liang YR, Png CW, Zhang Y, Tan KSW. Interactions between a pathogenic Blastocystis subtype and gut microbiota: in vitro and in vivo studies. Microbiome. 2019;7:30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 111] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 21. | Kurt Ö, Doğruman Al F, Tanyüksel M. Eradication of Blastocystis in humans: Really necessary for all? Parasitol Int. 2016;65:797-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |