Published online Jul 14, 2020. doi: 10.3748/wjg.v26.i26.3737
Peer-review started: January 19, 2020
First decision: May 1, 2019
Revised: June 2, 2020
Accepted: June 20, 2020
Article in press: June 20, 2020
Published online: July 14, 2020
Processing time: 177 Days and 4.5 Hours
Immunotherapy targeting programmed death-1 (PD-1) or programmed death-ligand-1 (PD-L1) has been shown to be effective in a variety of malignancies but has poor efficacy in pancreatic ductal adenocarcinoma (PDAC). Studies have shown that PD-L1 expression in tumors is an important indicator of the efficacy of immunotherapy. Tumor cells usually evade chemotherapy and host immune surveillance by epigenetic changes. Protein arginine methylation is a common posttranslational modification. Protein arginine methyltransferase (PRMT) 1 is deregulated in a wide variety of cancer types, whose biological role in tumor immunity is undefined.
To investigate the combined effects and underlying mechanisms of anti-PD-L1 and type I PRMT inhibitor in pancreatic cancer in vivo.
PT1001B is a novel type I PRMT inhibitor with strong activity and good selectivity. A mouse model of subcutaneous Panc02-derived tumors was used to evaluate drug efficacy, toxic and side effects, and tumor growth in vivo. By flow cytometry, we determined the expression of key immune checkpoint proteins, detected the apoptosis in tumor tissues, and analyzed the immune cells. Immunohistochemistry staining for cellular proliferation-associated nuclear protein Ki67, TUNEL assay, and PRMT1/PD-L1 immunofluorescence were used to elucidate the underlying molecular mechanism of the antitumor effect.
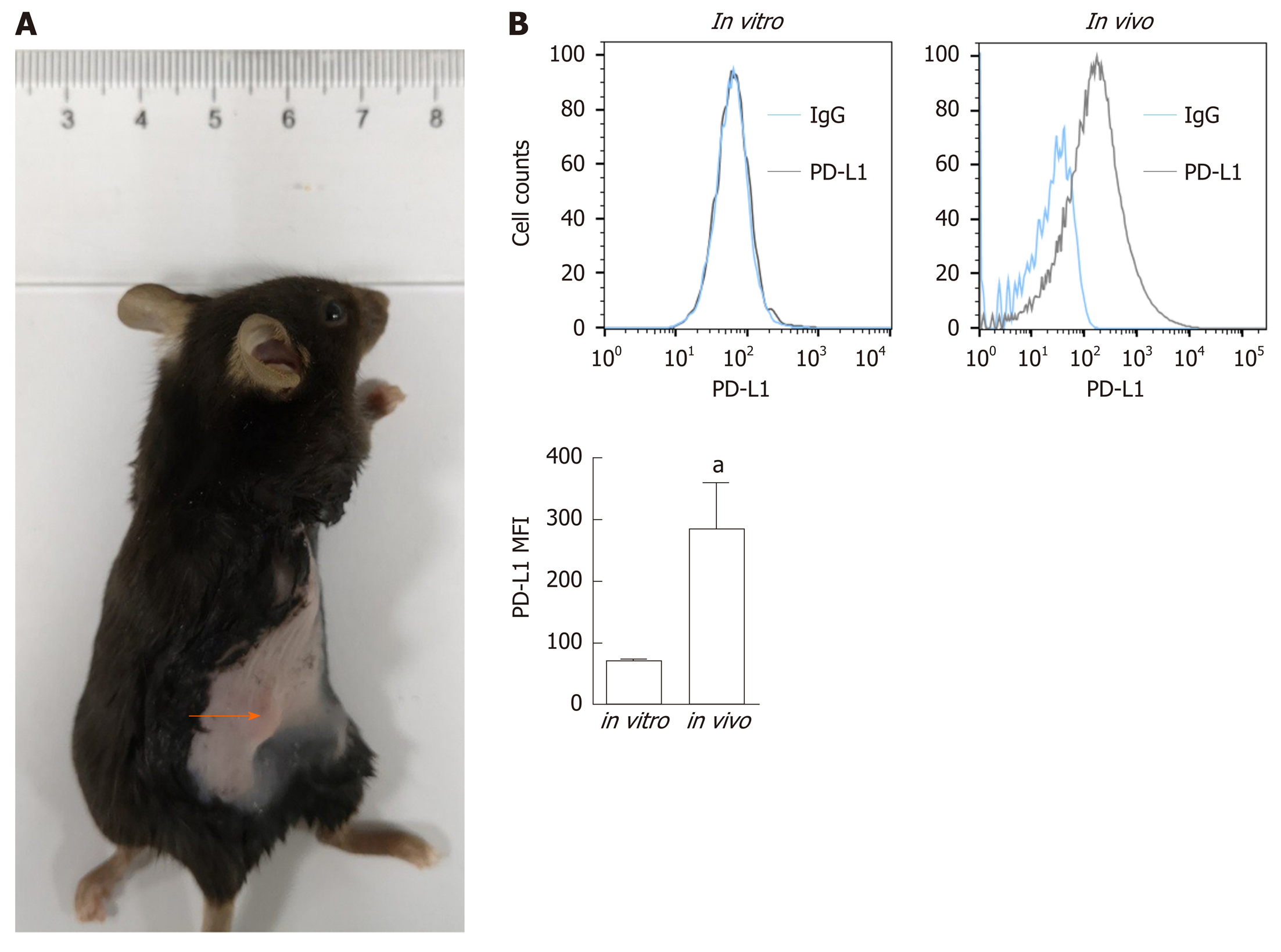
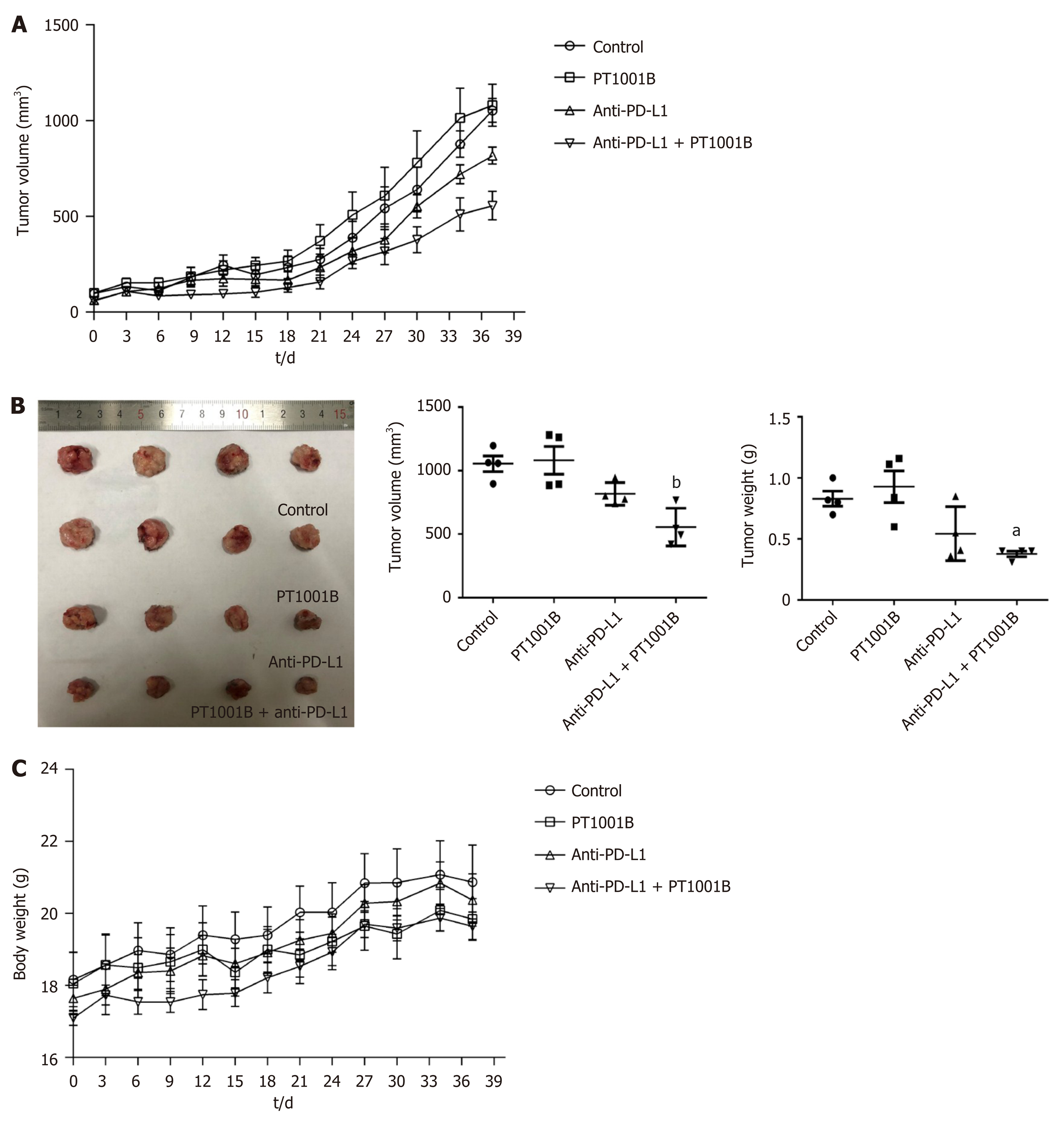
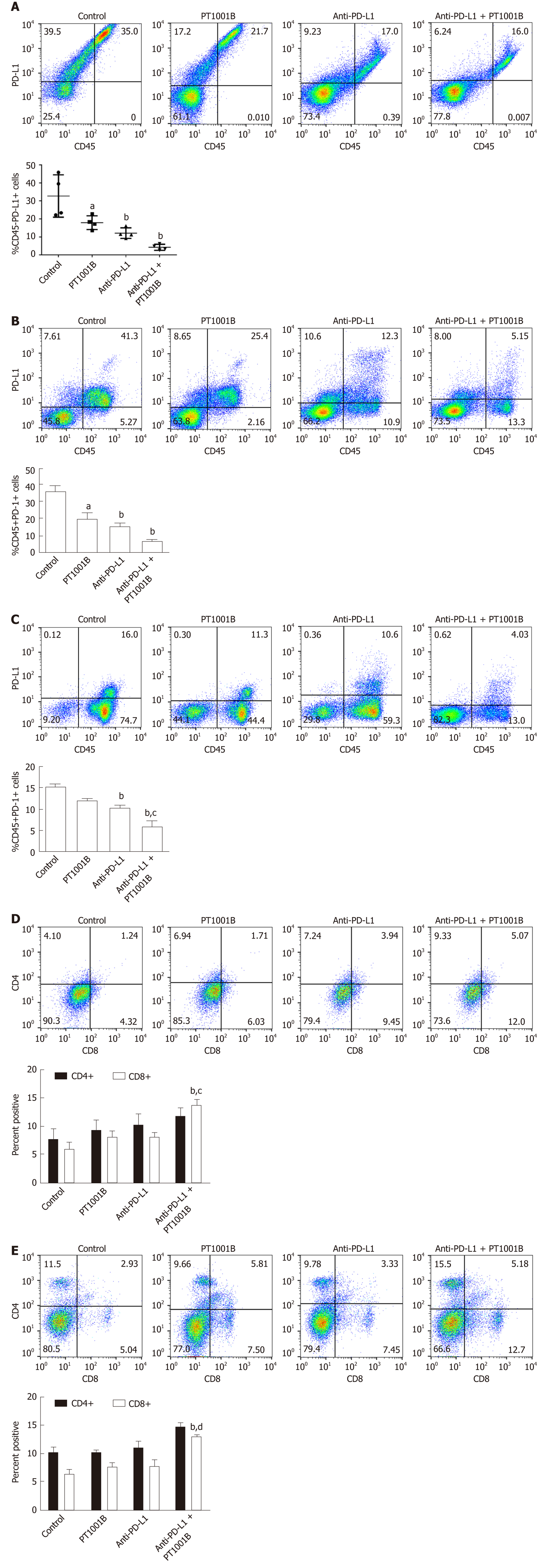
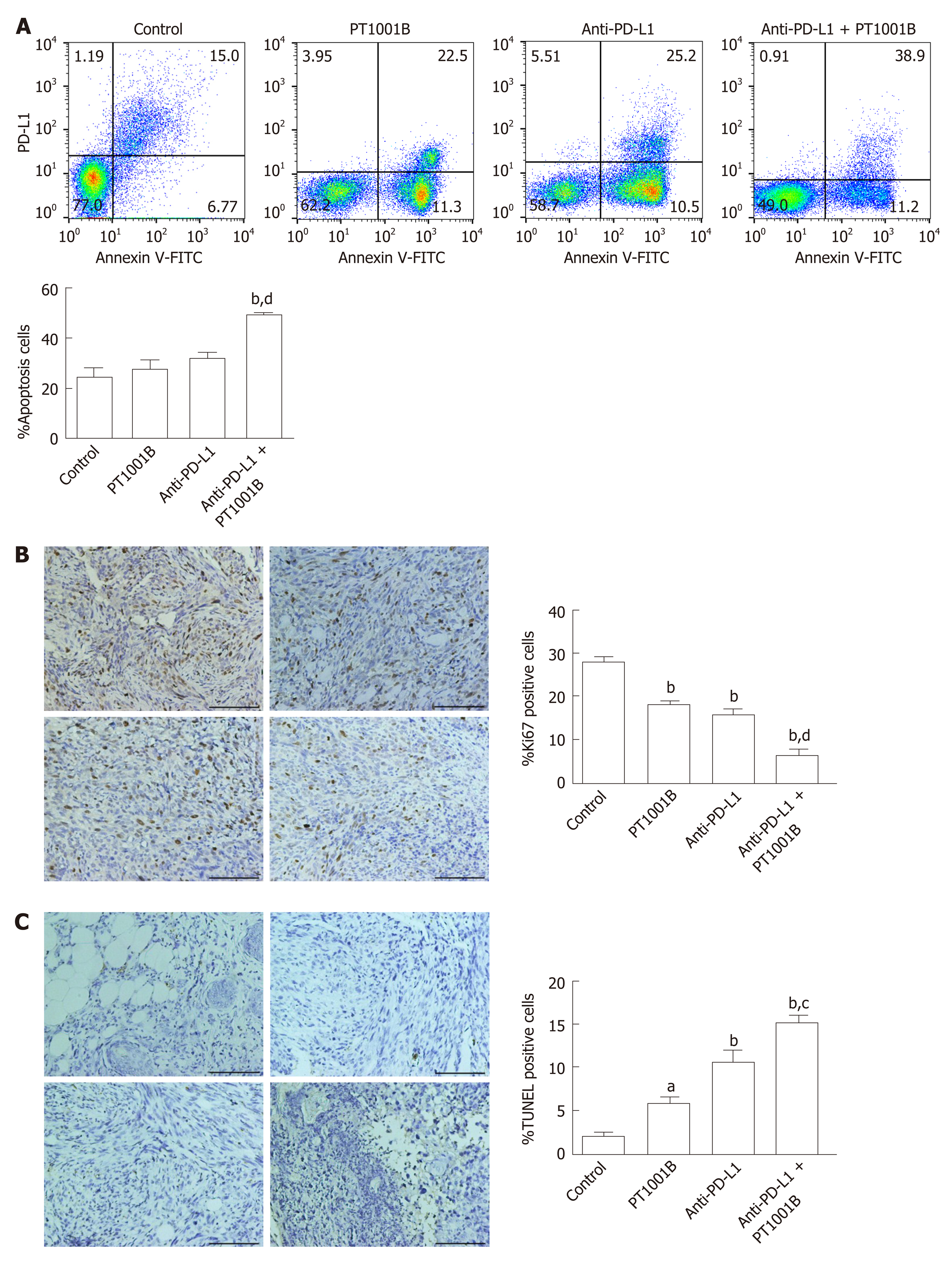
Cultured Panc02 cells did not express PD-L1 in vitro, but tumor cells derived from Panc02 transplanted tumors expressed PD-L1. The therapeutic efficacy of anti-PD-L1 mAb was significantly enhanced by the addition of PT1001B as measured by tumor volume (1054.00 ± 61.37 mm3vs 555.80 ± 74.42 mm3, P < 0.01) and tumor weight (0.83 ± 0.06 g vs 0.38 ± 0.02 g, P < 0.05). PT1001B improved antitumor immunity by inhibiting PD-L1 expression on tumor cells (32.74% ± 5.89% vs 17.95% ± 1.92%, P < 0.05). The combination therapy upregulated tumor-infiltrating CD8+ T lymphocytes (23.75% ± 3.20% vs 73.34% ± 4.35%, P < 0.01) and decreased PD-1+ leukocytes (35.77% ± 3.30% vs 6.48% ± 1.08%, P < 0.001) in tumor tissue compared to the control. In addition, PT1001B amplified the inhibitory effect of anti-PD-L1 on tumor cell proliferation and enhanced the induction of tumor cell apoptosis. PRMT1 downregulation was correlated with PD-L1 downregulation.
PT1001B enhances antitumor immunity and combining it with anti-PD-L1 checkpoint inhibitors provides a potential strategy to overcome anti-PD-L1 resistance in PDAC.
Core tip: Pancreatic ductal adenocarcinoma exhibits marginal responses to immune checkpoint inhibitors targeting programmed death-ligand-1 (PD-L1), and the underlying mechanism remains poorly understood. PT1001B, an inhibitor of type I protein arginine methyltransferases (PRMTs), significantly enhanced the therapeutic efficacy of anti-PD-L1 mAb. PT1001B improved antitumor immunity by inhibiting PD-L1 expression on tumor cells, upregulating tumor infiltrating CD8+ T lymphocytes, and decreasing PD-1+ leukocytes. In addition, PT1001B amplified the inhibitory effect of anti-PD-L1 on tumor cell proliferation and enhanced the induction of tumor cell apoptosis. PRMT1 downregulation was correlated with PD-L1 downregulation. Thus, PRMT1 is a potential therapeutic target.
- Citation: Zheng NN, Zhou M, Sun F, Huai MX, Zhang Y, Qu CY, Shen F, Xu LM. Combining protein arginine methyltransferase inhibitor and anti-programmed death-ligand-1 inhibits pancreatic cancer progression. World J Gastroenterol 2020; 26(26): 3737-3749
- URL: https://www.wjgnet.com/1007-9327/full/v26/i26/3737.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i26.3737
Pancreatic ductal adenocarcinoma (PDAC) is the seventh leading cause of cancer-related death worldwide and the fourth leading cause of cancer-related death in the United States[1,2]. Most cases of PDAC are diagnosed at the metastatic stage, and the median survival of these patients is less than one year[2]. Many factors are associated with the poor survival of PDAC patients and the treatment challenges, including the lack of early detection, high risk of relapse after curative surgery, and poor response to chemotherapy, radiation, molecular targeted therapy, and immunotherapy[3]. Programmed death-ligand-1 (PD-L1), also known as B7-H1, is a cell surface protein and one of two ligands for programmed death-1 (PD-1), a costimulatory molecule that negatively regulates T cell responses[4]. Ligation of PD-L1 on cancer cells to PD-1 on T cells suppresses T cell activation and proliferation. While immunotherapy using anti-PD-1/PD-L1 antibodies has been shown to be effective for many types of malignancies[5,6], its activity is limited in PDAC[7]. The mechanism underlying pancreatic cancer resistance to anti-PD-1/PD-L1 immunotherapy is poorly understood, but it has been suggested that PD-L1 expression in tumors is an important indicator of checkpoint immunotherapy efficacy[8,9].
Protein arginine methylation is a common posttranslational modification that plays a role in multiple pathways, including cell cycle control, RNA processing, and DNA replication[10]. Protein arginine methyltransferases (PRMTs) are enzymes that catalyze the transfer of a methyl group from S-adenosylmethionine to arginine[10]. PRMT family members are classified into PRMT types I, II, and III based on the nature of the catalyzed methylation reaction[11]. PRMT1 is the predominant type I methyltransferase responsible for approximately 85% of all cellular arginine methylation events. PRMT1 is deregulated in a wide variety of cancer types, e.g., pancreatic adenocarcinoma[12,13], gastric cancer[14], and lung cancer[15]. This enzyme controls epithelial-mesenchymal transition in cancer cells[16]. PRMT1 can catalyze arginine methylation on histones and other proteins, such as Axin and epidermal growth factor receptor[17,18]. PRMT1 is upregulated in pancreatic cancer and exerts an oncogenic role by regulating the β-catenin protein level[13].
The aim of the present study was to evaluate the effect of type I PRMT inhibitor against PDAC in mice and to investigate the influence of PRMT1 on PD-L1 expression.
The murine PDAC cell line Panc02, which is syngeneic to C57BL/6 mice, was obtained from the cell bank of the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). Panc02 cells were cultured in DMEM (Gibco, Grand Island, NY, United States) with 10% fetal bovine serum (Gibco) at 37°C in a 5% CO2 atmosphere.
Female C57BL/6 mice (specific pathogen-free grade) aged 5 wk were obtained from Shanghai Jihui Experimental Animal Feeding Co., Ltd and housed in a specific pathogen-free facility (23°C, 12 h/12 h light/dark cycle, 50% humidity, and ad libitum access to food and water). All experimental procedures were approved by the ethics committee of Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, and the protocols adhered to approved institutional protocols set by the China Association of Laboratory Animal Care. Panc02 cells (5 × 106) suspended in 100 μL cold PBS were subcutaneously injected into the lower back region of each mouse. The tumor volume was calculated using the following formula: 0.52 × length × width2. When the tumors reached approximately 100 mm3, the tumor-bearing mice were randomly divided into four groups (n = 4) that were treated with control solvent (PBS, once daily), PT1001B (30 mg/kg, once daily, synthesized by Wang et al[19]), anti-PD-L1 mAb (200 μg/mouse, every 2 d for 5 intervals, Clone No. 10F.9G2, BioXcell), or PT1001B + anti-PD-L1 mAb via intraperitoneal injection. Mice were sacrificed at 37 d following the initial injection, and tumors were removed and weighed.
Immunophenotypic analyses of splenocytes and single-cell suspensions from tumors were assessed by flow cytometry (FCM). All primary antibodies used in this study were purchased from BioLegend (CA, United States). Cells were stained with antibodies specific for CD45-APC-Cy7 (Clone 30-F11), CD4-PE-Cy7 (Clone RM4-4), CD8-FITC (Clone 53-6.7), PD-L1-APC (Clone 10F.9G2), and PD-1-PE (Clone RMP1-30). For the apoptosis analysis, the cells were stained with annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) (BD apoptosis assay kit, BD Pharmingen, CA, United States) according to the manufacturer’s protocol. Fresh PDAC tumor tissues from the mouse model were minced into small pieces and then digested with collagenase type IV to generate a single-cell suspension. After being filtered and washed with cold PBS, the cells were incubated with the primary antibodies on ice for 30 min, washed, fixed in PBS containing 1% formalin, and analyzed on a flow cytometer (CyAn ADP, Beckman). Data were visualized using FlowJo software.
The tumor tissues isolated from sacrificed mice were immediately fixed in 4% paraformaldehyde for 24 h and embedded in paraffin. The embedded sections were sliced into 5-μm sections for staining. The deparaffinized and rehydrated sections were boiled in a high-pressure pot with sodium citrate antigen retrieval solution for 3 min. After three washes in PBS, the sections were incubated with 3% hydrogen peroxide in methanol for 15 min to inhibit endogenous peroxidase activity. After nonspecific reactions were blocked with 10% normal rabbit serum, the sections were incubated overnight at 4°C with rabbit polyclonal antibodies specific to Ki67 (GB13030-2, 1:200, Servicebio). Then, the sections were washed, incubated at room temperature for 50 min with horseradish peroxidase-conjugated secondary antibody (GB23303, 1:200, Servicebio), and counterstained with hematoxylin.
The TUNEL assay was performed according to the kit protocol (11684817910, Roche). Briefly, tumor tissue sections were deparaffinized in xylene, rehydrated in PBS, and incubated with proteinase K working solution for 25 min at 37°C. After three washes in PBS, the sections were incubated in permeabilization working solution for 20 min. Then, TdT and dUTP were mixed at a 1:9 ratio; the tissue samples were incubated with the resulting mixture in a flat wet box at 37°C for 3-4 h. After three washes in PBS, the slides were immersed in 3% H2O2 at room temperature for 15 min in the dark. After three washes in PBS, the specimens were covered with converter-POD for 30 min and then washed three times in PBS. The slides were visualized with the DAB substrate and observed by microscopy (OLYMPUS, Japan). TUNEL-positive cells were counted with ImageJ, and the apoptotic index was calculated as the ratio of apoptotic cells to total cells in each field.
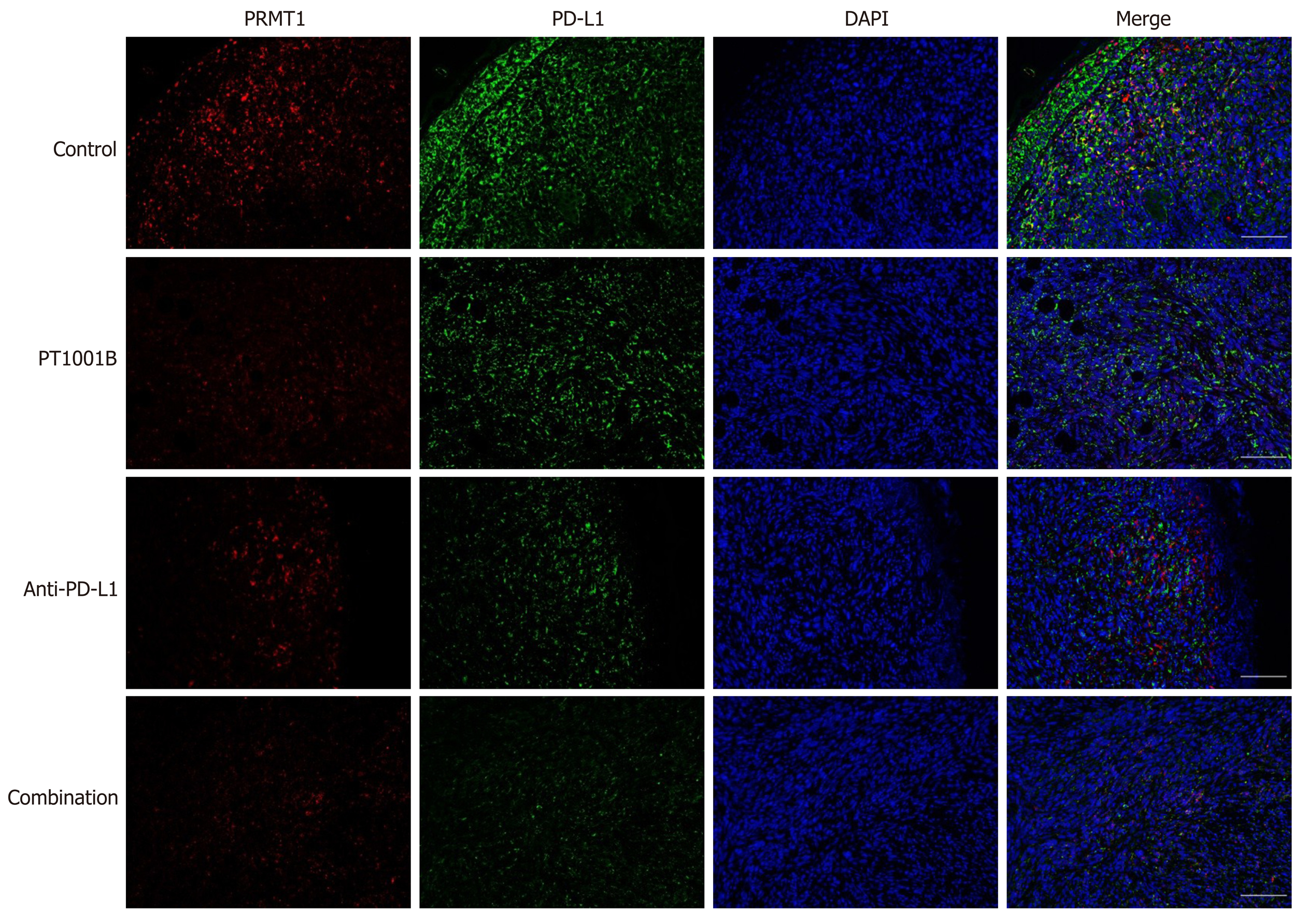
After antigen retrieval, nonspecific binding was blocked with 1% bovine serum albumin for 30 min, and the tissue sections were incubated overnight at 4°C with a primary antibody against PD-L1 (GB11339, 1:200, Servicebio). Thereafter, the sections were incubated with Alexa Fluor®488-conjugated goat anti-mouse IgG (GB25301, 1:400, Servicebio) for 50 min at room temperature. After incubation with CY3 reagent for 10 min, the sections were heated in a microwave to remove antibodies bound to the tissue. After nonspecific binding was blocked, the samples were incubated overnight at 4°C with a primary antibody against PRMT1 (sc-166963, 1:1000, Santa Cruz). Thereafter, the sections were incubated with horseradish peroxidase-labeled goat anti-mouse IgG (GB23301, 1:500, Servicebio) secondary antibody for 50 min at room temperature. Nuclei were stained with DAPI for 5 min at room temperature. Immunopositive cells were analyzed using a fluorescence microscope (Eclipse ci, NIKON, Japan).
All data are shown as the mean ± SE. All data were assessed by t-tests or one-way ANOVA (GraphPad Prism 6.0). P values < 0.05 were considered to indicate statistical significance.
To determine PD-L1 expression in tumor-bearing mice, we transplanted Panc02 cells into C57BL/6 mice (Figure 1A). PD-L1 expression on tumor cells was quantified by the mean fluorescence intensity (MFI) (Figure 1B). Panc02 cells grown in vitro did not express PD-L1, but subcutaneous Panc02-derived tumor cells expressed PD-L1. We speculated that the increased PD-L1 expression in vivo is related to the tumor microenvironment.
We then used PT1001B (formerly known as compound 28d, DCPR049_12), a novel selective inhibitor of type I PRMTs that effectively inhibits cancer cell proliferation[19]. We wondered whether PT1001B can enhance the efficacy of anti-PD-L1 therapy in Panc02-bearing C57BL/6 mice. The tumor-bearing mice were treated with PT1001B and anti-PD-L1 mAb, alone or in combination. As shown in Figure 2A, the tumors were resistant to anti-PD-L1 monotherapy. PT1001B or anti-PD-L1 mAb therapy alone did not decrease tumor growth compared to no treatment. Interestingly, when the anti-PD-L1 mAb was combined with PT1001B, the tumors showed a better response, as assessed by tumor volume (1054.00 ± 61.37 mm3vs 555.80 ± 74.42 mm3) and weight (0.83 ± 0.06 g vs 0.38 ± 0.02 g) (Figure 2B). Importantly, treatment with PT1001B and PD-L1 blockade, alone or in combination, did not result in any overt signs of toxicity, as evidenced by weight gain in all the evaluated animal groups (Figure 2C). There was no difference in mouse body weight in different treatment groups. Therefore, these findings suggest the potential of combination therapy with PT1001B to reverse anti-PD-L1 resistance in PDAC.
To determine whether PT1001B can enhance antitumor immunity, single-cell suspensions of tumor and spleen extracts were stained for FCM analysis. As shown in Figure 3A, PT1001B decreased PD-L1 expression on cancer cells (32.74% ± 5.89% in the control group vs 17.95% ± 1.92% in the PT1001B group). PD-L1 expression was dramatically lower in the anti-PD-L1 + PT1001B group than in the control group (4.21% ± 0.82% vs 32.74% ± 5.89%). In tumors, PD-1+ leukocytes were downregulated in the three treated groups (19.93% ± 3.65% in the PT1001B group, 15.53% ± 1.71% in the anti-PD-L1 group, and 6.48% ± 1.08 % in the anti-PD-L1 + PT1001B group vs 35.77% ± 3.30% in the control group) (Figure 3B). In the spleen, PD-1+ leukocytes were downregulated to a greater extent in the anti-PD-L1 + PT1001B group than in the anti-PD-L1 group (5.98% ± 1.26% in the anti-PD-L1 + PT1001B group vs 10.35% ± 0.46% in the anti-PD-L1 group) (Figure 3C). Analysis of T cell populations from the tumors and spleens revealed that PT1001B further increased the anti-PD-L1 mAb-induced tumor infiltration of CD8+ cytotoxic T lymphocytes (CTLs) (tumor: 8.14% ± 0.82% in the anti-PD-L1 group vs 13.83% ± 0.97% in the anti-PD-L1+PT1001B group; spleen: 7.54% ± 1.09% in the anti-PD-L1 group vs 12.90% ± 0.15% in the anti-PD-L1 + PT1001B group) (Figure 3D and E). These observations suggest that PT1001B enhanced antitumor immunity by upregulating tumor infiltrating CD8+ T lymphocytes, decreasing PD-L1 expression by cancer cells, and downregulating PD-1+ leukocytes.
To estimate the effect of PT1001B on apoptosis, FCM analysis was performed after double staining with annexin V-FITC/PI. As shown in Figure 4A, the rates of early and late apoptosis were dramatically higher in the combination group than in the control group (49.00% ± 0.64% vs 24.36% ± 3.67%), while the rates showed no significant changes in the other treatment groups compared to the control. We performed immunohistochemistry analysis of tumor tissues to examine Ki67 expression and TUNEL positive cells, which are common markers of proliferation and apoptosis, respectively. Consistent with the greater degree of tumor growth inhibition, PT1001B significantly increased the efficacy of anti-PD-L1 therapy in suppressing tumor cell proliferation and inducing tumor cell apoptosis in vivo (Figure 4B and C).
Immunofluorescence was performed to detect the levels of PRMT1 and PD-L1 in tumor tissues (Figure 5). PT1001B promoted the downregulation of PRMT1 and PD-L1, as indicated by the reduction in fluorescence. In particular, the anti-PD-L1 + PT1001B group showed the most drastic effect, with the lowest intensity of PD-L1-positive staining in tumor tissue.
Immunotherapy failure in pancreatic cancer stems from the nonimmunogenic characteristic, especially the immunosuppressive microenvironment, poor T cell infiltration, and the low mutational burden, which contribute to creating an immunoprivileged environment[20]. Increased PD-L1 expression by cancer or stroma cells is a fundamental mechanism of escape from host immunity[21]. Tumor cells can inhibit the proliferation, survival, and effector function of T lymphocytes, especially CD8+ T lymphocytes, through the PD-L1/PD-1 pathway[22]. PD-L1 blockade can relieve immunosuppression, enhance antitumor immunity, and lead to the expansion of tumor-infiltrating lymphocytes[23]. Immune checkpoint blockade reverses immunosuppression to activate tumor-reactive CTLs that directly target tumor cells for apoptosis[24]. However, pancreatic cancer cannot be cured by PD-1/PD-L1 blockade alone because of the specific tumor microenvironment[25]. Therefore, the search for effective combination therapy will provide new clues that affect the efficacy of immunotherapy. It was previously reported that MEK inhibitors, BET inhibitors, and mTOR inhibitors decreased PD-L1 expression in vitro and in vivo[26-28]. In our study, we identified a novel small-molecule inhibitor of type I PRMTs that also suppressed PD-L1 expression in pancreatic cancer cells.
In the present study, we demonstrated that the combination of PD-L1 blockade and PT1001B showed increased efficacy. A limited response to anti-PD-L1 monotherapy was observed in our PDAC model. Interestingly, the resistance to anti-PD-L1 therapy was reversed by the addition of PT1001B. When analyzing the expression of key immune checkpoint proteins and immune infiltration in the tumor microenvironment and spleen, we observed that the proportion of tumor-infiltrating effector cells was increased and the proportion of PD-1+ leukocytes was decreased in the combination group. This result is significant, as limited T cell infiltration is considered a barrier to the efficacy of immunotherapy in PDAC. CTLs primarily use perforin and Fas-mediated effector mechanisms to induce tumor cell apoptosis[29]. PD-1 is mainly expressed on activated CD4+ T cells, CD8+ T cells, and B cells in the periphery[30]. PD-1 inhibition can result in the loss of peripheral tolerance and an increase in autoimmunity[31]. In addition, PT1001B amplified the inhibitory effect of anti-PD-L1 on tumor cell proliferation and enhanced the induction of tumor cell apoptosis.
PRMT1 is the major methyltransferase in mammalian cells and is usually considered an epigenetic molecular marker. A previous study suggested that PRMT1 is overexpressed in pancreatic cancer cells and that elevated PRMT1 levels predict a poor clinical outcome. Moreover, PRMT1 knockdown was shown to inhibit tumor growth in vivo[13]. However, our results indicated that PT1001B alone did not decrease tumor growth. The discrepancy between our study and previous reports might be due to the use of different mouse models, cell lines, and inhibition methods. Our study results showed that PRMT1 downregulation was correlated with PD-L1 downregulation in PDAC tumors. Studies suggest that PRMT1 regulates c-myc gene expression and function[32,33]. Current data show that c-myc, an important signaling hub and driver gene, is commonly overexpressed and aberrantly activated in PDAC[34,35]. The activation of c-myc has been shown to stimulate PD-L1 expression in some cancer cells, causing immune evasion[36], and c-myc regulates PD-L1 expression in PDAC[37]. Therefore, we speculated that PT1001B might downregulate PD-L1 expression by inhibiting the master transcription amplifier c-myc. One limitation of this study is that PT1001B inhibits four PRMTs in addition to PRMT1 (PRMT3, PRMT4, PRMT6, and PRMT8), suggesting that it is a pan inhibitor of type I PRMTs[19]. Therefore, the specificity of PT1001B as an epigenetic agent in cancer therapy remains to be further studied.
In summary, our data demonstrate that the combination of the type I PRMT inhibitor and PD-L1 blockade may be an effective therapeutic approach for PDAC. PRMT1 expression is correlated with PD-L1 expression, and PRMT1 is a potential therapeutic target. Additional research aimed at elucidating the mechanisms by which PRMT1 regulates PD-L1 protein levels is warranted.
The mechanism underlying pancreatic cancer resistance to anti-programmed death-1 (PD-1)/programmed death-ligand-1 (PD-L1) immunotherapy is poorly understood, but it has been suggested that PD-L1 expression in tumors is an important indicator of checkpoint immunotherapy efficacy. Considered an epigenetic molecular marker, protein arginine methyltransferases (PRMT)1 is the predominant type I methyltransferase responsible for the majority of cellular arginine methylation events. PRMT1 is deregulated in a wide variety of cancer types, whose biological role in tumor immunity is undefined.
We hope to search for new small-molecule inhibitors that influence PD-L1 expression and affect the efficacy of immunotherapy in pancreatic cancer.
To explore the combined antitumor effects of anti-PD-L1 and type I PRMT inhibitor on pancreatic cancer and the underlying mechanisms in vivo.
The murine pancreatic ductal adenocarcinoma (PDAC) cells Panc02 were implanted subcutaneously in C57BL/6 mice, and the tumor-bearing mice were used to assess the drug efficacy, toxic and side effects, and tumor growth in vivo. We determined the expression of key immune checkpoint proteins, detected the apoptosis in tumor tissues, and analyzed the immune cells by flow cytometry (FCM). Immunohistochemistry staining for Ki67, TUNEL assay, and PRMT1/PD-L1 immunofluorescence were exploited to elucidate the underlying molecular mechanism.
Cultured Panc02 cells did not express PD-L1 in vitro, but tumor cells derived from Panc02 transplanted tumors expressed PD-L1. Drug treatment had no obvious toxic and side effects on mice. Neither PT1001B nor anti-PD-L1 mAb alone inhibited tumor growth compared to the control. However, the therapeutic efficacy of anti-PD-L1 mAb was significantly enhanced by the addition of PT1001B. PT1001B improved antitumor immunity by inhibiting PD-L1 expression on tumor cells, upregulating tumor-infiltrating CD8+ T lymphocytes, and decreasing PD-1+ leukocytes. In addition, PT1001B amplified the inhibitory effect of anti-PD-L1 mAb in inhibiting proliferation and promoting apoptosis of tumor cells. In tumor tissues, the downregulation of PRMT1 was related to the downregulation of PD-L1.
PT1001B enhances anti-tumor immunity and combining it with anti-PD-L1 inhibits tumor growth effectively, reversing the resistance of PDAC to immunotherapy.
Our data demonstrate that PT1001B is a promising inhibitor to change the tumor microenvironment in pancreatic cancer. PRMT1 is a potential therapeutic target, and additional research aimed at elucidating the mechanisms by which PRMT1 regulates PD-L1 is warranted.
The authors would like to acknowledge Cheng Luo and Yuanyuan Zhang (Chinese Academy of Sciences Shanghai Institute of Materia Medica, Shanghai, China) for providing PT1001B.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Katuchova J, Tanase C S-Editor: Dou Y L-Editor: Wang TQ E-Editor: Zhang YL
| 1. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20504] [Article Influence: 2050.4] [Reference Citation Analysis (20)] |
| 2. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12135] [Cited by in RCA: 12986] [Article Influence: 1442.9] [Reference Citation Analysis (2)] |
| 3. | Collisson EA, Olive KP. Pancreatic Cancer: Progress and Challenges in a Rapidly Moving Field. Cancer Res. 2017;77:1060-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Goldberg MV, Maris CH, Hipkiss EL, Flies AS, Zhen L, Tuder RM, Grosso JF, Harris TJ, Getnet D, Whartenby KA, Brockstedt DG, Dubensky TW, Chen L, Pardoll DM, Drake CG. Role of PD-1 and its ligand, B7-H1, in early fate decisions of CD8 T cells. Blood. 2007;110:186-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 156] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 5. | Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, Burke MM, Caldwell A, Kronenberg SA, Agunwamba BU, Zhang X, Lowy I, Inzunza HD, Feely W, Horak CE, Hong Q, Korman AJ, Wigginton JM, Gupta A, Sznol M. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3192] [Cited by in RCA: 3319] [Article Influence: 276.6] [Reference Citation Analysis (0)] |
| 6. | Zheng L. PD-L1 Expression in Pancreatic Cancer. J Natl Cancer Inst. 2017;109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455-2465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5599] [Cited by in RCA: 6277] [Article Influence: 482.8] [Reference Citation Analysis (0)] |
| 8. | Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2655] [Cited by in RCA: 3206] [Article Influence: 320.6] [Reference Citation Analysis (0)] |
| 9. | Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2838] [Cited by in RCA: 3539] [Article Influence: 153.9] [Reference Citation Analysis (0)] |
| 10. | Bedford MT, Richard S. Arginine methylation an emerging regulator of protein function. Mol Cell. 2005;18:263-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 825] [Cited by in RCA: 895] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 11. | Smith E, Zhou W, Shindiapina P, Sif S, Li C, Baiocchi RA. Recent advances in targeting protein arginine methyltransferase enzymes in cancer therapy. Expert Opin Ther Targets. 2018;22:527-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 12. | Wang Y, Hsu JM, Kang Y, Wei Y, Lee PC, Chang SJ, Hsu YH, Hsu JL, Wang HL, Chang WC, Li CW, Liao HW, Chang SS, Xia W, Ko HW, Chou CK, Fleming JB, Wang H, Hwang RF, Chen Y, Qin J, Hung MC. Oncogenic Functions of Gli1 in Pancreatic Adenocarcinoma Are Supported by Its PRMT1-Mediated Methylation. Cancer Res. 2016;76:7049-7058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 13. | Song C, Chen T, He L, Ma N, Li JA, Rong YF, Fang Y, Liu M, Xie D, Lou W. PRMT1 promotes pancreatic cancer growth and predicts poor prognosis. Cell Oncol (Dordr). 2020;43:51-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 14. | Altan B, Yokobori T, Ide M, Mochiki E, Toyomasu Y, Kogure N, Kimura A, Hara K, Bai T, Bao P, Suzuki M, Ogata K, Asao T, Nishiyama M, Oyama T, Kuwano H. Nuclear PRMT1 expression is associated with poor prognosis and chemosensitivity in gastric cancer patients. Gastric Cancer. 2016;19:789-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Madreiter-Sokolowski CT, Győrffy B, Klec C, Sokolowski AA, Rost R, Waldeck-Weiermair M, Malli R, Graier WF. UCP2 and PRMT1 are key prognostic markers for lung carcinoma patients. Oncotarget. 2017;8:80278-80285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Zhang Y, Wang D, Zhang M, Wei H, Lu Y, Sun Y, Zhou M, Gu S, Feng W, Wang H, Zeng J, Gong A, Xu M. Protein arginine methyltransferase 1 coordinates the epithelial-mesenchymal transition/proliferation dichotomy in gastric cancer cells. Exp Cell Res. 2018;362:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Liao HW, Hsu JM, Xia W, Wang HL, Wang YN, Chang WC, Arold ST, Chou CK, Tsou PH, Yamaguchi H, Fang YF, Lee HJ, Lee HH, Tai SK, Yang MH, Morelli MP, Sen M, Ladbury JE, Chen CH, Grandis JR, Kopetz S, Hung MC. PRMT1-mediated methylation of the EGF receptor regulates signaling and cetuximab response. J Clin Invest. 2015;125:4529-4543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 123] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 18. | Cha B, Kim W, Kim YK, Hwang BN, Park SY, Yoon JW, Park WS, Cho JW, Bedford MT, Jho EH. Methylation by protein arginine methyltransferase 1 increases stability of Axin, a negative regulator of Wnt signaling. Oncogene. 2011;30:2379-2389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Wang C, Jiang H, Jin J, Xie Y, Chen Z, Zhang H, Lian F, Liu YC, Zhang C, Ding H, Chen S, Zhang N, Zhang Y, Jiang H, Chen K, Ye F, Yao Z, Luo C. Development of Potent Type I Protein Arginine Methyltransferase (PRMT) Inhibitors of Leukemia Cell Proliferation. J Med Chem. 2017;60:8888-8905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 20. | Torphy RJ, Zhu Y, Schulick RD. Immunotherapy for pancreatic cancer: Barriers and breakthroughs. Ann Gastroenterol Surg. 2018;2:274-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 119] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 21. | Mace TA, Shakya R, Pitarresi JR, Swanson B, McQuinn CW, Loftus S, Nordquist E, Cruz-Monserrate Z, Yu L, Young G, Zhong X, Zimmers TA, Ostrowski MC, Ludwig T, Bloomston M, Bekaii-Saab T, Lesinski GB. IL-6 and PD-L1 antibody blockade combination therapy reduces tumour progression in murine models of pancreatic cancer. Gut. 2018;67:320-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 394] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 22. | Xu C, Fillmore CM, Koyama S, Wu H, Zhao Y, Chen Z, Herter-Sprie GS, Akbay EA, Tchaicha JH, Altabef A, Reibel JB, Walton Z, Ji H, Watanabe H, Jänne PA, Castrillon DH, Rustgi AK, Bass AJ, Freeman GJ, Padera RF, Dranoff G, Hammerman PS, Kim CF, Wong KK. Loss of Lkb1 and Pten leads to lung squamous cell carcinoma with elevated PD-L1 expression. Cancer Cell. 2014;25:590-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 325] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 23. | Alvarez Arias DA, Kim HJ, Zhou P, Holderried TA, Wang X, Dranoff G, Cantor H. Disruption of CD8+ Treg activity results in expansion of T follicular helper cells and enhanced antitumor immunity. Cancer Immunol Res. 2014;2:207-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3965] [Cited by in RCA: 4180] [Article Influence: 380.0] [Reference Citation Analysis (0)] |
| 25. | Feng M, Xiong G, Cao Z, Yang G, Zheng S, Song X, You L, Zheng L, Zhang T, Zhao Y. PD-1/PD-L1 and immunotherapy for pancreatic cancer. Cancer Lett. 2017;407:57-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 248] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 26. | Hogg SJ, Vervoort SJ, Deswal S, Ott CJ, Li J, Cluse LA, Beavis PA, Darcy PK, Martin BP, Spencer A, Traunbauer AK, Sadovnik I, Bauer K, Valent P, Bradner JE, Zuber J, Shortt J, Johnstone RW. BET-Bromodomain Inhibitors Engage the Host Immune System and Regulate Expression of the Immune Checkpoint Ligand PD-L1. Cell Rep. 2017;18:2162-2174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 237] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 27. | Qiu XY, Hu DX, Chen WQ, Chen RQ, Qian SR, Li CY, Li YJ, Xiong XX, Liu D, Pan F, Yu SB, Chen XQ. PD-L1 confers glioblastoma multiforme malignancy via Ras binding and Ras/Erk/EMT activation. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1754-1769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 118] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 28. | Lastwika KJ, Wilson W, Li QK, Norris J, Xu H, Ghazarian SR, Kitagawa H, Kawabata S, Taube JM, Yao S, Liu LN, Gills JJ, Dennis PA. Control of PD-L1 Expression by Oncogenic Activation of the AKT-mTOR Pathway in Non-Small Cell Lung Cancer. Cancer Res. 2016;76:227-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 601] [Article Influence: 60.1] [Reference Citation Analysis (0)] |
| 29. | Lu C, Paschall AV, Shi H, Savage N, Waller JL, Sabbatini ME, Oberlies NH, Pearce C, Liu K. The MLL1-H3K4me3 Axis-Mediated PD-L1 Expression and Pancreatic Cancer Immune Evasion. J Natl Cancer Inst. 2017;109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 197] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 30. | Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887-3895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1899] [Cited by in RCA: 2198] [Article Influence: 66.6] [Reference Citation Analysis (0)] |
| 31. | Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N, Honjo T. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1292] [Cited by in RCA: 1400] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 32. | Tikhanovich I, Zhao J, Bridges B, Kumer S, Roberts B, Weinman SA. Arginine methylation regulates c-Myc-dependent transcription by altering promoter recruitment of the acetyltransferase p300. J Biol Chem. 2017;292:13333-13344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 33. | Favia A, Salvatori L, Nanni S, Iwamoto-Stohl LK, Valente S, Mai A, Scagnoli F, Fontanella RA, Totta P, Nasi S, Illi B. The Protein Arginine Methyltransferases 1 and 5 affect Myc properties in glioblastoma stem cells. Sci Rep. 2019;9:15925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 34. | Wirth M, Mahboobi S, Krämer OH, Schneider G. Concepts to Target MYC in Pancreatic Cancer. Mol Cancer Ther. 2016;15:1792-1798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 35. | Hessmann E, Schneider G, Ellenrieder V, Siveke JT. MYC in pancreatic cancer: novel mechanistic insights and their translation into therapeutic strategies. Oncogene. 2016;35:1609-1618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 36. | Casey SC, Tong L, Li Y, Do R, Walz S, Fitzgerald KN, Gouw AM, Baylot V, Gütgemann I, Eilers M, Felsher DW. MYC regulates the antitumor immune response through CD47 and PD-L1. Science. 2016;352:227-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 688] [Cited by in RCA: 1048] [Article Influence: 116.4] [Reference Citation Analysis (0)] |
| 37. | Pan Y, Fei Q, Xiong P, Yang J, Zhang Z, Lin X, Pan M, Lu F, Huang H. Synergistic inhibition of pancreatic cancer with anti-PD-L1 and c-Myc inhibitor JQ1. Oncoimmunology. 2019;8:e1581529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |