Published online Jul 7, 2020. doi: 10.3748/wjg.v26.i25.3638
Peer-review started: January 10, 2020
First decision: March 6, 2020
Revised: May 8, 2020
Accepted: June 9, 2020
Article in press: June 9, 2020
Published online: July 7, 2020
Processing time: 178 Days and 20.7 Hours
Pancreatic neuroendocrine neoplasms (pNENs) that produce hormones leading to symptoms are classified as functional tumors, while others are classified as nonfunctional tumors. The traditional view is that functionality is a factor that affects the prognosis of pNEN patients. However, as the sample sizes of studies have increased, researches in recent years have proposed new viewpoints.
To assess whether functionality is an independent factor for predicting the prognosis of pNEN patients.
From January 2004 to December 2016, data of patients who underwent surgery at the primary site for the treatment of pNENs from the Surveillance, Epidemiology, and End Results (SEER) database and West China Hospital database were retrospectively analyzed.
Contemporaneous data from the two databases were analyzed separately as two cohorts and then merged as the third cohort to create a large sample that was suitable for multivariate analysis. From the SEER database, age (P = 0.006) and T stage (P < 0.001) were independent risk factors affecting the survival. From the West China Hospital database, independent prognostic factors were age (P = 0.034), sex (P = 0.032), and grade (P = 0.039). The result of the cohort consisting of the combined populations from the two databases showed that race (P = 0.015), age (P = 0.002), sex (P = 0.032) and T stage (P < 0.001) were independent prognostic factors. In the West China Hospital database and in the total population, nonfunctional pNETs and other functional pNETs tended to have poorer prognoses than insulinoma. However, functionality was not associated with the survival time of patients with pNETs in the multivariate analysis.
Functionality is not associated with prognosis. Race, age, sex, and T stage are independent factors for predicting the survival of patients with pNETs.
Core tip: Pancreatic neuroendocrine tumors (pNETs) are classified into functional and nonfunctional tumors according to the existence of hormones related symptoms or not. The traditional view is that functionality is correlated with the prognosis of pNET patients, which remains a controversial opinion. In this study, we retrospectively analyzed the clinicopathological data of 426 patients from the Surveillance, Epidemiology, and End Results database and 205 patients from the West China Hospital database. The results indicated that functionality is not associated with prognosis. Race, age, sex, and T stage are independent factors for predicting the survival of patients with pNETs.
- Citation: Chen HY, Zhou YL, Chen YH, Wang X, Zhang H, Ke NW, Liu XB, Tan CL. Functionality is not an independent prognostic factor for pancreatic neuroendocrine tumors. World J Gastroenterol 2020; 26(25): 3638-3649
- URL: https://www.wjgnet.com/1007-9327/full/v26/i25/3638.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i25.3638
Pancreatic neuroendocrine neoplasms (pNENs) account for approximately 10% of primary pancreatic tumors and the incidence of pNENs has been increasing in recent decades[1]. Compared with pancreatic ductal adenocarcinoma, pNENs are generally considered a less aggressive tumor, which occur in relatively younger patients. Neuroendocrine neoplasms (NENs) include a heterogeneous group of neoplasms, and those which produce hormones leading to symptoms (e.g., Whipple triad, Zollinger-Ellison syndrome, and carcinoid syndrome) are classified as functional tumors[2], while others that produce a series of substances without hormone related symptoms are classified as nonfunctional tumors[2,3].
The traditional view is that functionality is a factor that affects the prognosis of pNEN patients. Patients with functional tumors had a longer survival than those with nonfunctional tumors[4-6]. Tumors that secrete insulin and cause endogenous hyperinsulinemic hypoglycemia, namely, insulinomas, are believed to have a better prognosis among functional tumors, especially in the early stage[7], while patients with somatostatinoma and vipoma have been reported to have a relatively shorter survival[8].
However, as the sample sizes of studies have increased, researches in recent years have proposed new viewpoints. Due to the lack of specific symptoms, the majority of pNEN cases are diagnosed at a relatively advanced stage[1]. Therefore, nonfunctional pNENs are more likely to present with aggressive clinical manifestations[9,10], such as large diameter, increased age, high mitotic count, presence of neural invasion, extrapancreatic organ invasion or metastases, and advanced stage, which may lead to a poor prognosis[10,11]. Because of the rarity of pNENs and the low proportion of functional tumors, few studies have performed multivariate Cox regression analysis to show the effect of functionality on survival.
In the present study, we collected data from pNEN patients who underwent surgery at the primary site from the Surveillance, Epidemiology, and End Results (SEER) database and the West China Hospital database. The purpose of this study was to assess whether functionality is an independent factor to predict the prognosis of pNEN patients and explore the factors that influence the survival of these patients.
From January 2004 to December 2016, demographic, clinicopathological, and follow-up data of patients who underwent surgery for the treatment of pNENs were extracted from the SEER database using SEER*Stat software (version 8.3.5). The demographic data included age, race, and sex. The clinicopathological data included ICD-10 code, histology code, primary tumor location, tumor size, T, N, and M stages, pathologic grade, and surgery of the primary site. Survival data included survival months and vital status. Patients who underwent surgery other than pancreatectomy (local/partial resection, pancreaticoduodenectomy, or total pancreatectomy) were excluded.
Patients who underwent surgery with curative intent in West China Hospital between January 2004 and December 2016 with pathologically confirmed pNEN were included. Demographic, clinicopathological, and follow-up data of patients were retrospectively retrieved from the West China Hospital database. Patients with mixed neuroendocrine-non neuroendocrine neoplasms were not included. Patients were excluded if there was not enough information to determine the functionality of the tumor (n = 16). The follow-up deadline was August 2, 2019.
This study was approved by the West China Hospital Review Board under registration No. 2019 (124).
The pathologic grade was evaluated using mitotic count and Ki-67 index according to the World Health Organization (WHO) 2017 classification[12]. The TNM stage of tumor was assessed following the 8th edition of the American Joint Committee on Cancer (AJCC) Cancer Staging Manual[13]. Patients with pathologic grade unavailable and patients with G3 pancreatic neuroendocrine tumors (pNETs) or pancreatic neuroendocrine carcinomas (pNECs) (mitotic count > 20, Ki-67 index > 20%, and/or previously diagnosed G3 tumor) were excluded from further analysis.
Functionality was assessed according to whether hormone-related symptoms existed, regardless of the immunohistochemistry features. In addition to the nonfunctional pNEN group (N), functional pNENs were divided into two groups for further analysis: Insulinomas (I) and other functional pNENs (O).
SPSS version 23.0 (IBM Corporation, Chicago, IL) was employed to perform statistical analyses, and P < 0.05 was considered statistically significant. Continuous variables are reported in the form of the mean ± SE and were compared using the Student's t-test. Nominal data (race, primary site of tumor, sex, etc.) are presented as frequencies and percentages and were compared using χ2 tests or Fisher’s exact tests. The primary end point of this study was overall survival, which was measured from the date of tumor diagnosis to the date of last follow-up or death. Patients with (1) primary tumor unevaluated (Tx), (2) grade unevaluated (Gx), and/or (3) mitotic count higher than 20 or Ki-67 index higher than 20% (NET G3 or NEC) were not involved in the subsequent statistical analysis. After verification of the proportional hazard assumption, Cox proportional hazard models were constructed to identify factors that predicted the prognosis. All variables with a P value < 0.1 in the univariate analysis were used as input variables for the multivariate analysis which was performed using a forward stepwise method.
From the SEER database, a total of 426 patients were enrolled in this study. The baseline data are shown in Table 1. The mean age was 56.74 ± 0.67 years, and the male:female ratio was 221:205. There were 100 functional tumors (23.5%), including 52 insulinomas, 32 gastrinomas, 12 glucagonomas, 2 vipomas, and 2 somatostatinomas.
| Database | SEER database (n = 426) | West China Hospital database (n = 205) | P value |
| Race | NC | ||
| White | 357 (83.8) | 0 (0.0) | |
| Asian1 | 25 (5.9) | 205 (100) | |
| Others2 | 44 (10.3) | 0 (0.0) | |
| Tumor site | < 0.001 | ||
| Head | 104 (24.4) | 85 (41.5) | |
| Body | 51 (12) | 55 (26.8) | |
| Tail | 168 (39.4) | 44 (21.5) | |
| Unknown | 103 (24.2) | 21 (10.2) | |
| Age (yr) | < 0.001 | ||
| 0-55 | 193 (45.3) | 138 (67.3) | |
| 56- | 233 (54.7) | 67 (32.7) | |
| Sex | 0.035 | ||
| Male | 221 (51.9) | 88 (42.9) | |
| Female | 205 (48.1) | 117 (57.1) | |
| Primary tumor | 0.0303 | ||
| T1 | 121 (28.4) | 80 (39) | |
| T2 | 135 (31.7) | 72 (35.1) | |
| T3 | 94 (22.1) | 44 (21.5) | |
| T4 | 41 (9.6) | 9 (4.4) | |
| Tx | 35 (8.2) | 0 (0) | |
| Regional lymph node metastasis | 0.1713 | ||
| N0 | 188 (44.1) | 41 (20) | |
| N1 | 149 (35) | 22 (10.7) | |
| Nx | 89 (20.9) | 142 (69.3) | |
| Distant metastasis | 0.0443 | ||
| M0 | 143 (33.6) | 193 (94.1) | |
| M1 | 19 (4.5) | 12 (5.9) | |
| Mx | 264 (62) | 0 (0) | |
| Grade | < 0.0013 | ||
| pNET G1 | 229 (53.8) | 90 (43.9) | |
| pNET G2 | 45 (10.6) | 96 (46.8) | |
| pNET G3 or pNEC | 16 (3.8) | 9 (4.4) | |
| Gx | 136 (31.9) | 10 (4.9) | |
| Functionality | < 0.001 | ||
| N | 326 (76.5) | 101 (49.3) | |
| I | 52 (12.2) | 85 (41.5) | |
| O | 48 (11.3) | 19 (9.3) | |
| Surgery | < 0.0013 | ||
| Pancreaticoduodenectomy | 143 (33.6) | 39 (19) | |
| Total pancreatectomy | 39 (9.2) | 6 (2.9) | |
| Partial pancreatectomy | 203 (47.7) | 88 (42.9) | |
| Local excision | 38 (8.9) | 72 (35.1) | |
| Other surgeries | 3 (0.7) | 0 (0) |
From the West China Hospital database, the mean age of the 205 patients was 48.16 ± 0.93 years. There were 88 males (42.9%) and 117 females (57.1%). One hundred and four (50.7%) patients had functional tumors including 85 insulinomas, 9 gastrinomas, 7 glucagonomas, 1 vipoma, 1 somatostatinoma, and 1 rare pNEN secreting adrenocorticotropic hormone (ACTH)[14]. Compared with the SEER database, patients in the West China Hospital database had fewer tumors of the pancreatic tail, were younger, had a lower T stage, fewer G2 tumors, and fewer distant metastases, and had more female patients (P < 0.05). Although the N stage was comparable in the two databases, the West China Hospital database had more patients with unevaluated N stage (Nx).
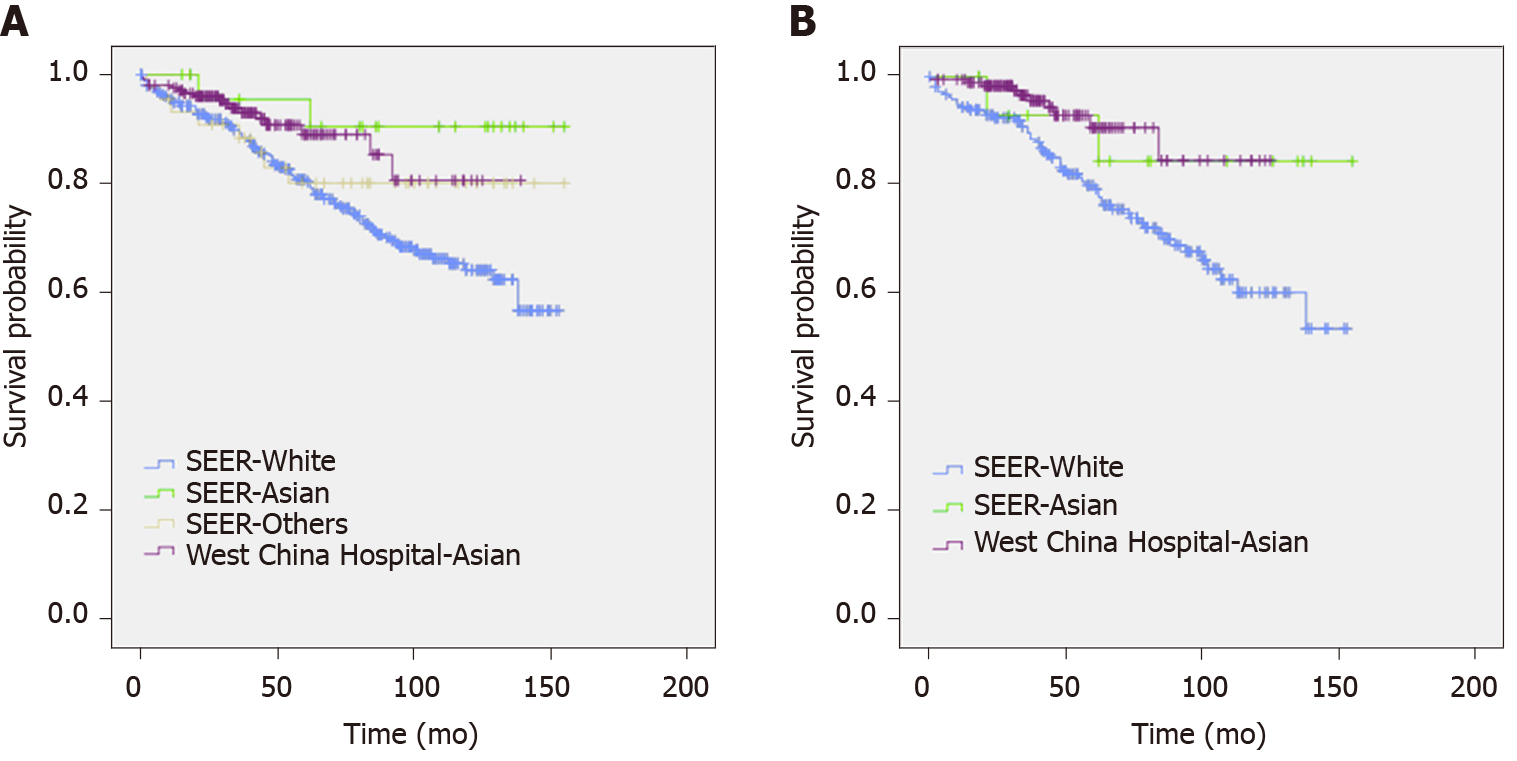
When we compared the characteristics of patients of different races (Table 2), the difference between the White and Asian populations was similar to the difference between the two datasets. In the other races, the ratio of male patients was higher, and the proportion of G3 pNETs or pNECs was higher than the respective values in the White and Asian populations. Survival curves of patients of different races from the two databases are shown in Figure 1.
| Race | White (n = 357) | Asian1 (n = 230) | Others2 (n = 44) | P value |
| Tumor site | < 0.001 | |||
| Head | 80 (22.4) | 94 (40.9) | 15 (34.1) | |
| Body | 40 (11.2) | 59 (25.7) | 7 (15.9) | |
| Tail | 153 (42.9) | 48 (20.9) | 11 (25) | |
| Unknown | 84 (23.5) | 29 (12.6) | 11 (25) | |
| Age (yr) | < 0.001 | |||
| 0-55 | 156 (43.7) | 150 (65.2) | 25 (56.8) | |
| 56- | 201 (56.3) | 80 (34.8) | 19 (43.2) | |
| Sex | 0.002 | |||
| Male | 162 (45.4) | 130 (56.5) | 30 (68.2) | |
| Female | 195 (54.6) | 100 (43.5) | 14 (31.8) | |
| Primary tumor | 0.0733 | |||
| T1 | 98 (27.5) | 89 (38.7) | 14 (31.8) | |
| T2 | 117 (32.8) | 79 (34.3) | 11 (25) | |
| T3 | 79 (22.1) | 51 (22.2) | 8 (18.2) | |
| T4 | 35 (9.8) | 10 (4.3) | 5 (11.4) | |
| Tx | 28 (7.8) | 1 (0.4) | 6 (13.6) | |
| Regional lymph node metastasis | 0.0943 | |||
| N0 | 157 (44) | 53 (23) | 19 (43.2) | |
| N1 | 128 (35.9) | 25 (10.9) | 18 (40.9) | |
| Nx | 72 (20.2) | 152 (66.1) | 7 (15.9) | |
| Distant metastasis | 0.0543 | |||
| M0 | 119 (33.3) | 202 (87.8) | 15 (34.1) | |
| M1 | 16 (4.5) | 12 (5.2) | 3 (6.8) | |
| Mx | 222 (62.2) | 16 (7) | 26 (59.1) | |
| Grade | < 0.0013 | |||
| pNET G1 | 192 (53.8) | 105 (45.7) | 22 (50) | |
| pNET G2 | 42 (11.8) | 98 (42.6) | 1 (2.3) | |
| pNET G3 or pNEC | 13 (3.6) | 9 (3.9) | 3 (6.8) | |
| Gx | 110 (30.8) | 18 (7.8) | 18 (40.9) | |
| Functionality | < 0.001 | |||
| N | 277 (77.6) | 117 (50.9) | 33 (75) | |
| I | 40 (11.2) | 93 (40.4) | 4 (9.1) | |
| O | 40 (11.2) | 20 (8.7) | 7 (15.9) | |
| Surgery | < 0.0013 | |||
| Pancreaticoduodenectomy | 111 (31.1) | 51 (22.2) | 20 (45.5) | |
| Total pancreatectomy | 33 (9.2) | 7 (3) | 5 (11.4) | |
| Partial pancreatectomy | 179 (50.1) | 96 (41.7) | 16 (36.4) | |
| Local excision | 31 (8.7) | 76 (33) | 3 (6.8) | |
| Other surgeries | 3 (0.8) | 0 (0) | 0 (0) |
Contemporaneous data from the two databases were analyzed separately as two cohorts and then merged as the third cohort to create a larger sample that was suitable for the univariate and multivariate analyses. Patients of races other than White or Asian and Pacific Islander, patients with primary tumor not assessed (Tx) or pathologic grade unevaluated (Gx), and patients who had G3 pNETs or pNECs were not enrolled in the subsequent analysis due to the limitations of the Cox regression model.
The univariate and multivariate analyses of the SEER cohort and the West China Hospital cohort are shown in Table 3. The two cohorts displayed similarities in the hazard ratios (HRs) of age, sex, T stage, regional lymph node metastasis, and distant metastasis, but showed differences in the HRs of primary site, grade, and functionality. In the multivariate analysis, the results of the SEER cohort showed that age (HR = 2.203, 95%CI: 1.249-3.884, P = 0.006) and T stage (HR = 2.589, 95%CI: 1.533-4.371, P < 0.001) were independent risk factors for predicting prognosis. The results of the West China Hospital cohort showed that age (HR = 4.558, 95%CI: 1.122-18.521, P = 0.034), sex (HR = 5.707, 95%CI: 1.161-28.057, P = 0.032), and grade (HR = 9.039, 95%CI: 1.118-73.051, P = 0.039) were independent prognostic factors.
| Study population | SEER database | West China Hospital database | All population | ||||||
| Variables | HR (95CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | |||
| Univariate analysis | |||||||||
| Country | |||||||||
| United States | 1.000 | ||||||||
| China | 0.326 | (0.159, 0.665) | 0.002 | ||||||
| Race | |||||||||
| White | 1.000 | 1.000 | |||||||
| Asian | 0.402 | (0.098, 1.652) | 0.206 | 0.326 | (0.169, 0.626) | 0.001 | |||
| Age (yr) | |||||||||
| Below 55 | 1.000 | 1.000 | 1.000 | ||||||
| Over 56 | 2.121 | (1.204, 3.738) | 0.009 | 4.224 | (1.055, 16.919) | 0.042 | 2.710 | (1.606, 4.574) | < 0.001 |
| Sex | |||||||||
| Female | 1.000 | 1.000 | 1.000 | ||||||
| Male | 1.822 | (1.065, 3.119) | 0.029 | 4.262 | (0.878, 20.686) | 0.072 | 2.067 | (1.251, 3.415) | 0.005 |
| Primary site | |||||||||
| Head | 1.000 | 1.000 | 1.000 | ||||||
| Body | 2.077 | (0.899, 4.800) | 0.087 | 0.801 | (0.146, 4.398) | 0.799 | 1.651 | (0.784, 3.479) | 0.187 |
| Tail | 1.259 | (0.616, 2.573) | 0.527 | 1.193 | (0.266, 5.349) | 0.817 | 1.445 | (0.769, 2.718) | 0.253 |
| Unknown | 1.359 | (0.598, 3.085) | 0.464 | 0.000 | 0.987 | 1.491 | (0.698, 3.187) | 0.303 | |
| Primary tumor | |||||||||
| T1-2 | 1.000 | 1.000 | 1.000 | ||||||
| T3-4 | 2.515 | (1.490, 4.246) | 0.001 | 3.663 | (0.980, 13.688) | 0.054 | 2.858 | (1.756, 4.651) | < 0.001 |
| Regional lymph node metastasis | |||||||||
| N0 | 1.000 | 1.000 | 1.000 | ||||||
| N1 | 1.602 | (0.918, 2.795) | 0.097 | 2.757 | (0.387, 19.630) | 0.311 | 1.775 | (1.040, 3.032) | 0.036 |
| Nx | 0.911 | (0.407, 2.038) | 0.820 | 0.981 | (0.186, 5.170) | 0.982 | 0.585 | (0.293, 1.167) | 0.128 |
| Distant metastasis | |||||||||
| M0 | 1.000 | 1.000 | 1.000 | ||||||
| M1 | 5.295 | (1.804, 15.543) | 0.002 | 3.423 | (0.423, 27.705) | 0.249 | 5.726 | (2.269, 14.450) | < 0.001 |
| Mx | 1.993 | (0.973, 4.079) | 0.059 | 2.773 | (1.551, 4.960) | 0.001 | |||
| Grade | |||||||||
| G1 | 1.000 | 1.000 | 1.000 | ||||||
| G2 | 1.418 | (0.734, 2.739) | 0.298 | 9.823 | (1.224, 78.806) | 0.032 | 1.266 | (0.740, 2.166) | 0.388 |
| Functionality | |||||||||
| I | 1.000 | 1.000 | 1.000 | ||||||
| N | 1.924 | (0.691, 5.353) | 0.210 | 1.473 | (0.268, 8.092) | 0.656 | 2.544 | (1.090, 5.938) | 0.031 |
| O | 2.247 | (0.600, 8.423) | 0.230 | 7.913 | (1.314, 47.670) | 0.024 | 3.925 | (1.359, 11.337) | 0.012 |
| Multivariate analysis | |||||||||
| Race | |||||||||
| White | 1.000 | ||||||||
| Asian | 0.438 | (0.225, 0.851) | 0.015 | ||||||
| Age | |||||||||
| Below 55 | 1.000 | 1.000 | 1.000 | ||||||
| Over 56 | 2.203 | (1.249, 3.884) | 0.006 | 4.558 | (1.122, 18.521) | 0.034 | 2.315 | (1.362, 3.935) | 0.002 |
| Sex | |||||||||
| Female | 1.000 | 1.000 | |||||||
| Male | 5.707 | (1.161, 28.057) | 0.032 | 1.744 | (1.049, 2.899) | 0.032 | |||
| Primary tumor | |||||||||
| T1-2 | 1.000 | 1.000 | |||||||
| T3-4 | 2.589 | (1.533, 4.371) | < 0.001 | 2.612 | (1.603, 4.254) | < 0.001 | |||
| Grade | |||||||||
| G1 | 1.000 | ||||||||
| G2 | 9.039 | (1.118, 73.051) | 0.039 | ||||||
In the cohort consisting of the combined populations from the two databases, factors that affected prognosis in the univariate analysis included country (P = 0.002), race (P = 0.001), age (P < 0.001), sex (P = 0.005), T stage (P < 0.001), regional lymph node metastasis (N1, P = 0.036), distant metastasis (P < 0.001), and functionality (nonfunctional pNETs, P = 0.031; other functional pNETs, P = 0.012). The multivariate proportional hazard model contained only race (HR = 0.438, 95%CI: 0.225-0.851, P = 0.015), age (HR = 2.315, 95%CI: 1.362-3.935, P = 0.002), sex (HR = 1.744, 95%CI: 1.049-2.899, P = 0.032), and T stage (HR = 2.612, 95%CI: 1.603-4.254, P < 0.001).
In the West China Hospital database and in the total population, nonfunctional pNETs (West China Hospital database: HR = 1.473, 95%CI: 0.268-8.092, P = 0.656; total population: HR = 2.544, 95%CI: 1.090-5.938, P = 0.031) and other functional pNETs (West China Hospital database: HR = 7.913, 95%CI: 1.314-47.670, P = 0.024; total population: HR = 3.925, 95%CI: 1.359-11.337, P = 0.012) tended to have poorer prognoses than insulinoma. However, as shown in the multivariate analysis, functionality was not associated with the survival time of patients with pNETs since it was not selected into the model.
Compared with pancreatic ductal adenocarcinoma, pNETs are characterized by a lower incidence, younger age, and better prognosis[1]. According to morphological features, the WHO 2017 guidelines divide pNENs into biologically different groups, pNETs and pNECs. pNET cells have a fairly uniform, solid, trabecular, spiral or glandular patterned nucleus with pepper-salt chromatin and granular cytoplasm, while pNECs are similar to small or large cell neuroendocrine carcinomas of the lung[15]. Only pNETs can be divided into three different prognostic groups (G1, G2, and G3) according to mitotic count and Ki-67 index. Subsequently, the AJCC updated the staging system of pancreatic tumors[13]. PNET G1 and G2 are staged in a scheme that is similar to the European Neuroendocrine Tumor Society Consensus Guidelines staging system[16,17], while G3 pNETs and pNECs share the same staging system as pancreatic exocrine tumors.
pNENs were previously classified into several groups according to the existence and type of hormone related symptoms. The group, or rather, functionality was believed to be associated with the survival of patients with pNEN. Cienfuegos et al[4] performed a log-rank survival analysis on pNEN patients, and the results showed that the nonfunctioning tumor group had a relatively poor prognosis compared with the functioning tumor group (P = 0.052). Studies have indicated that functionality is positively related to the expression of somatostatin receptor 2[18] and negatively related to aurora kinase B[19], which may contribute to the improvement in survival. Wang et al[5] and Nanno et al[6] found that functionality is a prognostic factor affecting overall survival and disease-free survival in the results of univariate Cox regression analysis. However, the multivariate analysis was not carried out in the study by Wang et al[5] (due to the small sample size) and did not include functionality as a factor in the model in the study by Nanno et al[6] (only venous invasion and grade were used as input variables).
In recent years, studies have proposed new viewpoints. Studies[20,21] that included patients with NENs in almost all the locations suggested that functionality is not associated with progression-free survival13 or disease-free survival[14]. However, there are differences in biological characteristics between NENs of lung origin and gastroenteropancreatic NENs: The majority of the functional NENs are carcinoid syndrome[20], while the functional tumors of gastroenteropancreatic NENs, especially pNENs, are mainly insulinomas[10].
Our results indicated that race, age, sex, and T stage were independent factors for predicting the survival of patients with pNETs. Although no significant differences were found in the effects of some factors on survival in the small sample cohorts, it does not mean that there is no relationship between these factors and survival. Only a sample that is large enough can reveal the real prognostic factor.
Functionality was correlated with survival in the univariate analysis, but was not associated with prognosis in the multivariate analysis. The prognosis of patients with nonfunctional tumors is generally considered to be poorer than that of patients with insulinoma. However, this is more likely related to the late diagnosis of patients with nonfunctional tumors, rather than the difference in biological properties between functional and nonfunctional tumors or the effect of hormones secreted by functional tumors. Hormone related syndrome is the only basis to distinguish between the nonfunctional neuroendocrine neoplasm and several types of functional neuroendocrine neoplasm. However, immunohistochemical staining also shows the expression of insulin/glucagon/gastrin/somatostatin in non-functional tumors. The reasonability of classification based on symptoms rather than gene expression needs to be further explored.
According to WHO guidelines, the assessment of grade depends on mitotic count and Ki-67 index, with a cutoff value of 2/10 high power fields and 3%, respectively. However, the cutoff values that make the most sense are still debatable. Some studies support that Ki-67 and mitotic count is correlated with prognosis[6,11], while there are also some studies that do not support this viewpoint[18]. In the Western China Hospital database, grade is an independent risk factor for prognosis. But in the SEER database, grade is not related to prognosis.
There was a trend of shorter survival time for patients with higher T stage in our small-sample cohort (n = 205), and T stage turned out to be an independent prognostic factor in large-sample cohorts (SEER, n = 426; total, n = 631), which is similar to the results of other studies[6,21]. On one hand, it indicated that T stage is indeed a factor that affects the prognoses of patients with pNETs; on the other hand, the results showed the importance of sample size in cohort study.
Data of the same period from the two databases were included in the analyses above. The distributions of N stage were comparable between the two datasets, but the populations from the two databases had differences in the distributions of primary tumor location, age, sex, T stage, M stage, grade, functionality, and surgery. Compared with the data in the SEER database (Table 1) and the results from other studies[4,5], the proportion of nonfunctional tumors was only approximately 50% in the West China Hospital database, indicating that some patients were not diagnosed and treated and that there is a need to advance the screening and early diagnosis of nonfunctional pNENs. Insulinomas accounted for only half of the functional tumors in the SEER database, while insulinomas accounted for the vast majority of functional pNENs in the West China Hospital database, which is similar to the results of Wang et al[5]. Local excision was performed more commonly in the West China Hospital database, especially before 2010, which led to a higher proportion of patients with no lymph nodes examined (Nx).
There were some limitations to this study: (1) Patients of the same time period were enrolled from the two databases, and the inclusion/exclusion criteria were the same. However, SEER is a multicenter database, while the West China Hospital database is a single center database. The baseline data of the two datasets had differences in the distributions of some variables. Most of the data were demographic data or objective clinical data (such as tumor size and lymph node metastasis). The tumor grade depends on the mitotic count and Ki-67 index whose implementation may vary in different centers. Cox regression of the combined dataset may not represent the relationship between grade and prognosis; and (2) This study collected data from 2004 to 2016 retrospectively, and there is not sufficient information to separate poorly-differentiated pNECs from well-differentiated G3 pNETs in the SEER database. The TNM stages of G3 pNETs and pNECs may not have the same effect on survival as those of G1 and G2 tumors since they are completely different stage systems. Therefore, we excluded all tumors with mitotic counts higher than 20 or Ki-67 indexes higher than 20% (G3 pNETs and pNECs).
Pancreatic neuroendocrine neoplasms (pNENs) that produce hormones leading to symptoms are classified as functional tumors, while others are classified as nonfunctional tumors.
The traditional view is that functionality affects the prognosis of pNEN patients. However, recent studies have proposed new viewpoints. Because of the rarity of pNENs and the low proportion of functional tumors, few studies have performed multivariate Cox regression to show the effect of functionality on survival.
To assess whether functionality is an independent factor for predicting the prognosis of pNEN patients.
From January 2004 to December 2016, data of patients who underwent surgery at the primary site for the treatment of pNENs from the Surveillance, Epidemiology, and End Results (SEER) database and West China Hospital database were retrospectively analyzed.
From the SEER database, age and T stage were independent risk factors affecting the survival. From the West China Hospital database, independent prognostic factors were age, sex, and grade. The result of the cohort consisting of the combined populations from the two databases showed that race, age, sex, and T stage were independent prognostic factors. In the West China Hospital database and in the total population, nonfunctional pNETs and other functional pNETs tended to have poorer prognoses than insulinomas. However, functionality was not associated with the survival time of patients with pNETs in the multivariate analysis.
Race, age, sex, and T stage are independent factors for predicting the survival of patients with pNETs. The results of this study do not support the opinion that hormone related syndrome is an efficacious tool to classify tumors into groups with different prognoses.
Hormone related syndrome is the only basis to assess the functionality of neuroendocrine neoplasms. Nonfunctional tumors and functional tumors were reported to have different prognoses. However, they do not have much difference in pathologic feature or gene expression. Immunohistochemical staining also displays the expression of insulin/glucagon/gastrin/somatostatin in non-functional tumors. The reasonability of classification based on symptoms rather than gene expression needs to be further explored.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and Hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Collin Y, Yang Z S-Editor: Ma YJ L-Editor: Wang TQ E-Editor: Ma YJ
| 1. | Wen J, Chen J, Liu D, Xu X, Fan M, Zhang Z. The Eighth Edition of the American Joint Committee on Cancer Distant Metastases Stage Classification for Metastatic Pancreatic Neuroendocrine Tumors Might Be Feasible for Metastatic Pancreatic Ductal Adenocarcinomas. Neuroendocrinology. 2020;110:364-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Jensen RT, Cadiot G, Brandi ML, de Herder WW, Kaltsas G, Komminoth P, Scoazec JY, Salazar R, Sauvanet A, Kianmanesh R; Barcelona Consensus Conference participants. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms: functional pancreatic endocrine tumor syndromes. Neuroendocrinology. 2012;95:98-119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 465] [Cited by in RCA: 374] [Article Influence: 28.8] [Reference Citation Analysis (1)] |
| 3. | Sorbye H, Strosberg J, Baudin E, Klimstra DS, Yao JC. Gastroenteropancreatic high-grade neuroendocrine carcinoma. Cancer. 2014;120:2814-2823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 251] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 4. | Cienfuegos JA, Rotellar F, Salguero J, Ruiz-Canela M, Núñez Córdoba JM, Sola I, Benito A, Martí-Cruchaga P, Zozaya G, Pardo F, Hernández Lizoáin JL. A single institution's 21-year experience with surgically resected pancreatic neuroendocrine tumors: an analysis of survival and prognostic factors. Rev Esp Enferm Dig. 2016;108:689-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Wang YH, Lin Y, Xue L, Wang JH, Chen MH, Chen J. Relationship between clinical characteristics and survival of gastroenteropancreatic neuroendocrine neoplasms: A single-institution analysis (1995-2012) in South China. BMC Endocr Disord. 2012;12:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Nanno Y, Toyama H, Otani K, Asari S, Goto T, Terai S, Ajiki T, Zen Y, Fukumoto T, Ku Y. Microscopic venous invasion in patients with pancreatic neuroendocrine tumor as a potential predictor of postoperative recurrence. Pancreatology. 2016;16:882-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | de Herder WW, Niederle B, Scoazec JY, Pauwels S, Kloppel G, Falconi M, Kwekkeboom DJ, Oberg K, Eriksson B, Wiedenmann B, Rindi G, O'Toole D, Ferone D; Frascati Consensus Conference; European Neuroendocrine Tumor Society. Well-differentiated pancreatic tumor/carcinoma: insulinoma. Neuroendocrinology. 2006;84:183-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 166] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 8. | Peng SY, Li JT, Liu YB, Fang HQ, Wu YL, Peng CH, Wang XB, Qian HR. Diagnosis and treatment of VIPoma in China: (case report and 31 cases review) diagnosis and treatment of VIPoma. Pancreas. 2004;28:93-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Morin E, Cheng S, Mete O, Serra S, Araujo PB, Temple S, Cleary S, Gallinger S, Greig PD, McGilvray I, Wei A, Asa SL, Ezzat S. Hormone profiling, WHO 2010 grading, and AJCC/UICC staging in pancreatic neuroendocrine tumor behavior. Cancer Med. 2013;2:701-711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Han X, Xu X, Jin D, Wang D, Ji Y, Lou W. Clinicopathological characteristics and prognosis-related factors of resectable pancreatic neuroendocrine tumors: a retrospective study of 104 cases in a single Chinese center. Pancreas. 2014;43:526-531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | You Y, Jang JY, Kim SC, Yoon YS, Park JS, Cho CK, Park SJ, Yang JD, Lee WJ, Hong TH, Ahn KS, Jeong CY, Lee HK, Lee SE, Roh YH, Kim HJ, Kim H, Han IW. Validation of the 8th AJCC Cancer Staging System for Pancreas Neuroendocrine Tumors Using Korean Nationwide Surgery Database. Cancer Res Treat. 2019;51:1639-1652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Scoazec JY, Couvelard A; Réseau TENpath. [Classification of pancreatic neuroendocrine tumours: Changes made in the 2017 WHO classification of tumours of endocrine organs and perspectives for the future]. Ann Pathol. 2017;37:444-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 13. | Bergsland EK, Woltering EA, Rindi G, O'Dorisio TM, Schilsky RL, Liu EH, Kim MK, Nakakura EK, Reidy-Lagunes DL, Strosberg JR, Tang LH, Vinik AI, Wang YZ, Asare EA, Brierley JD, Bushnell DL, Jensen RT, Pommier RF, Wolin EM, Wong RKS, Klimstra DS, Amin MB. Amin MB. Neuroendocrine Tumors of the Pancreas. In: Amin MB. AJCC Cancer Staging Manual. 8th ed. New York: Springer-Verlag, 2016: 407-419. |
| 14. | Liu X, Zou L, Yao W, Song B. Education and imaging. Hepatobiliary and Pancreatic: Pancreatic neuroendocrine neoplasm with pelvic metastases and ectopic ACTH production. J Gastroenterol Hepatol. 2014;29:1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Belotto M, Crouzillard B, Araujo KO, Peixoto RD. Pancreatic Neuroendocrine Tumors: Surgical Resection. Arq Bras Cir Dig. 2019;32:e1428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Rindi G, Klöppel G, Alhman H, Caplin M, Couvelard A, de Herder WW, Erikssson B, Falchetti A, Falconi M, Komminoth P, Körner M, Lopes JM, McNicol AM, Nilsson O, Perren A, Scarpa A, Scoazec JY, Wiedenmann B; all other Frascati Consensus Conference participants; European Neuroendocrine Tumor Society (ENETS). TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2006;449:395-401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1196] [Cited by in RCA: 1085] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 17. | Rindi G, Klöppel G, Couvelard A, Komminoth P, Körner M, Lopes JM, McNicol AM, Nilsson O, Perren A, Scarpa A, Scoazec JY, Wiedenmann B. TNM staging of midgut and hindgut (neuro) endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2007;451:757-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 680] [Cited by in RCA: 653] [Article Influence: 36.3] [Reference Citation Analysis (1)] |
| 18. | Wang Y, Wang W, Jin K, Fang C, Lin Y, Xue L, Feng S, Zhou Z, Shao C, Chen M, Yu X, Chen J. Somatostatin receptor expression indicates improved prognosis in gastroenteropancreatic neuroendocrine neoplasm, and octreotide long-acting release is effective and safe in Chinese patients with advanced gastroenteropancreatic neuroendocrine tumors. Oncol Lett. 2017;13:1165-1174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 19. | Briest F, Wang Y, Arsenic R, Elezkurtaj S, Berg E, Greshake S, Lock AC, Hörsch D, Arnold CN, Hummel M, Siegmund B, Grabowski P. Immunohistochemical Study of Mitosis-regulatory Proteins in Gastroenteropancreatic Neuroendocrine Neoplasms. Anticancer Res. 2018;38:3863-3870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Baum RP, Kulkarni HR, Singh A, Kaemmerer D, Mueller D, Prasad V, Hommann M, Robiller FC, Niepsch K, Franz H, Jochems A, Lambin P, Hörsch D. Results and adverse events of personalized peptide receptor radionuclide therapy with 90Yttrium and 177Lutetium in 1048 patients with neuroendocrine neoplasms. Oncotarget. 2018;9:16932-16950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 110] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 21. | Slagter AE, Ryder D, Chakrabarty B, Lamarca A, Hubner RA, Mansoor W, O'Reilly DA, Fulford PE, Klümpen HJ, Valle JW, McNamara MG. Prognostic factors for disease relapse in patients with neuroendocrine tumours who underwent curative surgery. Surg Oncol. 2016;25:223-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |