Published online Jul 7, 2020. doi: 10.3748/wjg.v26.i25.3586
Peer-review started: February 3, 2020
First decision: March 24, 2020
Revised: March 26, 2020
Accepted: May 26, 2020
Article in press: May 26, 2020
Published online: July 7, 2020
Processing time: 155 Days and 4.1 Hours
Pancreatic ductal adenocarcinoma (PDAC) is one of the deadliest solid tumors. Identification of diagnostic and therapeutic biomarkers for PDAC is urgently needed. Transducin (β)-like 1 X-linked receptor 1 (TBL1XR1) has been linked to the progression of various human cancers. Nevertheless, the function and role of TBL1XR1 in pancreatic cancers are unclear.
To elucidate the function and potential mechanism of TBL1XR1 in the development of PDAC.
Ninety patients with histologically-confirmed PDAC were included in this study. PDAC tumor samples and cell lines were used to determine the expression of TBL1XR1. CCK-8 assays and colony formation assays were carried out to assess PDAC cell viability. Flow cytometry was performed to measure the changes in the cell cycle and cell apoptosis. Changes in related protein expression were measured by western blot analysis. Animal analysis was conducted to confirm the impact of TBL1XR1 in vivo.
Patients with TBL1XR1-positive tumors had worse overall survival than those with TBL1XR1-negative tumors. Moreover, we found that TBL1XR1 strongly promoted PDAC cell proliferation and inhibited PDAC cell apoptosis. Moreover, knockdown of TBL1XR1 induced G0/G1 phase arrest. In vivo animal studies confirmed that TBL1XR1 accelerated tumor cell growth. The results of western blot analysis showed that TBL1XR1 might play a key role in regulating PDAC cell proliferation and apoptosis via the PI3K/AKT pathway.
TBL1XR1 promoted PDAC cell progression and might be an effective diagnostic and therapeutic marker for pancreatic cancer.
Core tip: Transducin (β)-like 1 X-linked receptor 1 (TBL1XR1) has been linked to the progression of various human cancers. However, the function and role of TBL1XR1 in pancreatic cancers are unclear. Elucidation of the effect and potential molecular mechanism of TBL1XR1 in pancreatic cancer is important. This study showed that TBL1XR1 promoted pancreatic ductal adenocarcinoma (PDAC) cell proliferation, inhibited PDAC cell apoptosis and might regulate PDAC cell proliferation and apoptosis by the phosphatidylinositol 3-kinase/protein kinase B pathway. Therefore, TBL1XR1 might be a promising therapeutic marker for patients with advanced PDAC.
- Citation: Gu JF, Fu W, Qian HX, Gu WX, Zong Y, Chen Q, Lu L. TBL1XR1 induces cell proliferation and inhibit cell apoptosis by the PI3K/AKT pathway in pancreatic ductal adenocarcinoma. World J Gastroenterol 2020; 26(25): 3586-3602
- URL: https://www.wjgnet.com/1007-9327/full/v26/i25/3586.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i25.3586
Pancreatic carcinoma is one of the deadliest solid tumors. Currently, pancreatic carcinoma is the fourth leading cause of cancer-related death in the United States, and there were approximately 48960 new cases confirmed and 40560 deaths in 2015[1,2]. The best chance for survival is early surgical detection. However, due to the lack of effective screening tests for pancreatic carcinoma, local invasion and early metastasis, only 10%–20% of patients are diagnosed at a stage amenable to resection and curative treatment[3,4]. Therefore, the overall 5-year survival rate is lower than 5%[5]. Despite decades of efforts, there has been no significant improvement in the long-term survival of pancreatic carcinoma patients[6]. Pancreatic ductal adenocarcinoma (PDAC) is the most common type of pancreatic carcinoma. Therefore, identification of efficacious early tumor markers for the early diagnosis and treatment of PDAC has clinical value and significance[7].
As a member of the TBL1 family, transducin (β)-like 1 X-linked receptor 1 (TBL1XR1) is the key element of the SMRT/N-CoR corepressor complex[8]. TBL1XR1 is located on chromosome 3 at 3q26 and has a high degree of homology to the TBL1 protein. Recently, studies have demonstrated that TBL1XR1 could react to transcriptional regulators such as nuclear receptors by switching from gene inhibition to gene activation[9]. Moreover, TBL1XR1 plays a vital role in cell growth, cell apoptosis, inflammation and transcriptional activation, which involve estrogen receptor, androgen receptor, thyroid hormone receptor β, peroxisome proliferator-activated receptor γ, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κb), Notch and β-catenin[10]. Currently, studies focus on the effect and mechanism of TBL1XR1 in carcinogenesis and tumor progression, including those of nasopharyngeal carcinoma, colon cancer, osteosarcoma cancer, prostate cancer, breast cancer, and hepatocellular carcinoma[11-16]. Nevertheless, the role and molecular mechanism of TBL1XR1 in the progression of PDAC remain unclear.
In this study, we aimed to explore the effect of TBL1XR1 in PDAC. We found that TBL1XR1 expression was closely associated with the clinicopathologic features of PDAC, which might provide a new diagnostic and therapeutic target for PDAC.
This study was performed in 90 PDAC patients. The patients all underwent radical pancreaticoduodenectomy and were histopathologically and clinically diagnosed at Changshu No. 1 People’s Hospital Affiliated with Soochow University from 2011 to 2015. Our study complied with the ethical standards of the Declaration of Helsinki and was approved by the Ethics Committee of Changshu No. 1 People’s Hospital. Ninety pairs of PDAC and normal pancreatic epithelial samples were promptly fixed in 4% formalin and embedded in paraffin for immunohistochemical (IHC) staining after removal.
After obtaining fresh isolated specimens, we immediately fixed the specimens in 4% formalin and then embedded them in paraffin. Next, we cut them into 5 mm sections and mounted them on slides. IHC and HE staining were performed to examine the expression of TBL1XR1[17].
Immunohistochemical staining of TBL1XR1 expression in PDAC and normal pancreatic epithelial specimens was performed as described by Li et al[17]. The TBL1XR1 staining score was the summation of the staining strength and the ratio of positively stained cells. We defined TBL1XR1 staining strength as follows: 0% immunoreactive cells are scored 0; less than 5% immunoreactive cells are scored 1; 5%–50% immunoreactive cells are scored 2; and more than 50% immunoreactive cells are scored 3. Staining intensity was scored as follows: negative was scored 0; weak was scored 1; intermediate was scored 2; and strong was scored 3. For statistical convenience, we set intermediate and strong scores to be positive, and the negative and weak scores were considered to be negative[18].
The PDAC cell lines (Panc1, MiaPaCa-2, Capan1 and Aspc-1) were purchased from the Cell Bank of the Chinese Academy of Science. Panc1 and Capan1 cells were cultured in DMEM, and MiaPaCa-2 and Aspc-1 cells were cultured in RPMI-1640 medium. Streptomycin (100 μg/mL) and penicillin (100 U/ml) and 10% fetal bovine serum were added to DMEM or RPMI-1640 medium. The cells were cultivated at 37 °C in a humidified incubator with 5% CO2.
Five hundred thousand cells were inoculated into 6-well plates, and after the cells attached, we performed a cell transfection assay according to the manufacturer’s instructions. In short, 50 nmol siRNA and 6 μL of Lipofectamine 2000 were added to 200 μl of Opti-MEM medium and incubated for 5 min. Then, the two suspensions were mixed and incubated for 15 min. The suspension was added to 6-well plates and incubated for 4 h. After incubation, we replaced the medium and harvested fresh culture medium every 2 d. The siRNA sequences were as follows: CTRL siRNA: 5’-TTCTCCGAACGTGTCACGT-3’;TBL1XR1: 5’-GGAGUAGACAAGACUACAA-3’.
We performed the assay using the methods described by Li et al[17]. Total RNA was extracted from different treated cells with TRIzol reagent (Invitrogen) in accordance with the manufacturer’s instructions. The expression of TBL1XR1 and GAPDH was determined by the primers shown below: TBL1XR1: 5’-GAG GTG TTT ATT TGT GCT TGG-3’; 5’-TGC ACT TAA TAT GAA GTT GCC-3’. GAPDH: 5’-GCCGCATCTTCTTTTGCGTCGC-3’; 5’-TCCCGTTCTCAGCCTTGACGGT-3’.
The expression of TBL1XR1 was determined by normalization to the expression of the housekeeping gene GAPDH.
The lentivirus vector was constructed by the Shanghai GeneChem Company. Lentivirus-mediated RNA interference was performed in accordance with the manufacturer’s instructions. After cell adherence, a considerable volume of virus was added to the cells for 6-8 h. Then, fresh complete medium was added, and the cells were harvested for 2 d. Cells were continuously cultured and used for subsequent studies.
A CCK-8 (Beyotime, Shanghai) assay was performed to determine the viability of PDCA cells. CCK-8 is widely used in the rapid and highly sensitive detection of cell proliferation and cytotoxicity based on WST-8. PDAC cells at 1000 cells/well were inoculated into 96-well plates after the corresponding treatment and incubated for the indicated times. Then, 10 μL of CCK-8 was added to the medium and cultured for 2 h in the dark. A microplate reader (Bio-Tek, United States) was used to measure the absorbance of PDCA cells at 450 nm.
PDAC cells with different treatments (approximately 500 cells/well) were seeded in 6-well plates. The culture medium was changed every 3 d. After approximately 2 wk of culture, the cells were cleaned with PBS and fixed with 10% formalin for 20 min at ordinary temperature. Then, 0.1% crystal violet (Sigma-Aldrich) was used to stain the cells for 20 min at room temperature. After staining, the plates were washed and dried. The colonies (with more than 50 cells) were observed under a microscope (Leica, Germany).
Proteins were extracted using RIPA buffer mixed with 1% protease inhibitor cocktail (Beyotime, Shanghai, China). Ten percent sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was applied to separate the cell proteins, and polyvinylidene difluoride (PVDF) membranes were used for transfer. Five percent skim milk was used to block the membranes for 2 h. Then, the membranes were incubated with primary antibodies against TBL1XR1 (Abcam, ab228940), p-AKT (CST, 9271), p-PI3K (CST, 17366) and GAPDH (Abclonal, A19056) at 4 °C overnight. The second day, secondary antibodies were added and incubated with the PVDF membranes for 1 h at room temperature. A Gel Doc 2000 system (Bio-Rad, United States) was used to detect protein expression.
For cell cycle analysis, PDAC cells with different treatments were fixed with 75% ethanol at 4°C overnight. Then, the cells were centrifuged for precipitation and incubated with 5 μL of RNase and 5 μL of PI at room temperature. For cell apoptosis analysis, PDAC cells with different treatments were resuspended with 1 × Annexin V binding buffer and Annexin V and PI at 37 °C. After incubation for half an hour, the apoptotic cell and cell cycle ratios were detected with flow cytometric analysis.
Four- to six-week-old nu/nu nude mice were purchased from the Shanghai Laboratory Animal Centre of the Chinese Academy of Sciences (Shanghai, China). The mice were randomly divided into the Lv-shCTRL group and the Lv-shTBL1XR1 group. One hundred thousand Panc1 cells were resuspended in 0.1 mL of serum-free medium and hypodermically inoculated into the right axilla of the mice. After approximately 4 wk, the mice were sacrificed, and the subcutaneous tumors were measured and weighed. The volume of the tumors was calculated by the following formula: Volume = length (mm) × width2 (mm2)/2.
All experiments were repeated at least 3 times, and the results are expressed as the mean ± standard deviation unless otherwise stated. Student’s t test was used to compare differences between the treated groups and the corresponding control groups; P < 0.05 was considered statistically significant.
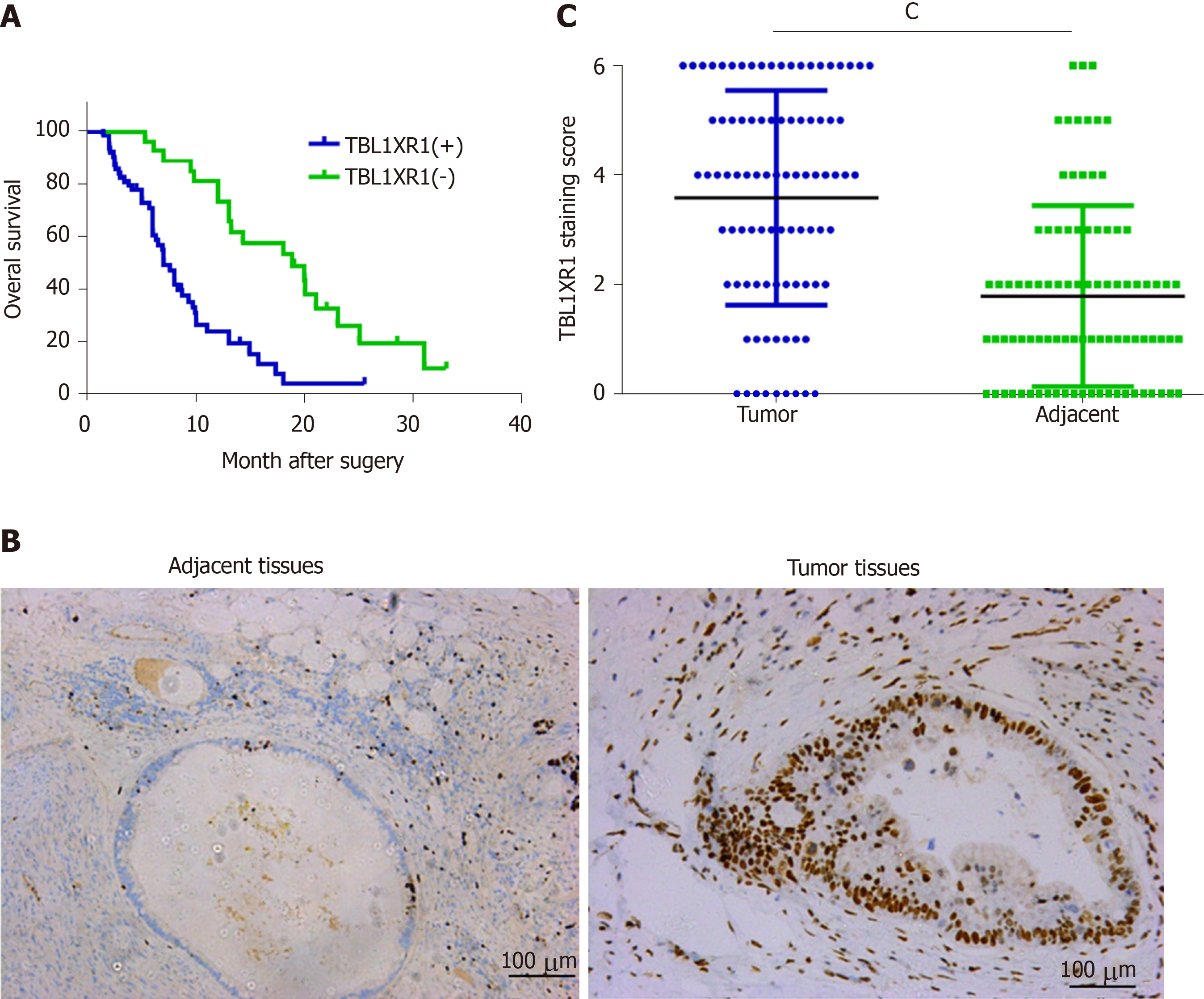
The clinicopathological features of the 90 PDAC patients are shown in Table 1. As illustrated in Table 1, the overexpression of TBL1XR1 was associated with TNM stage (P = 0.006) but not with patient age (P = 0.652), gender (P = 1.000), histopathological subtype (P = 0.929), tumor size (P = 0.465), tumor location (P = 0.065) or lymph node metastasis (P = 0.050). To additionally confirm the importance of TBL1XR1 in PDAC, we analyzed the overall survival (OS) of 90 patients by the Kaplan-Meier method (Table 2). Table 2 and Figure 1A show that patients with TBL1XR1-negative expression displayed a longer OS than those with TBL1XR1-positive expression (P < 0.001). Moreover, TNM stage (P < 0.001) and lymph node metastasis (P = 0.047) were obviously related to the average survival time. Furthermore, the Cox proportional hazards model was used in multivariate analysis. As shown in Table 3, TBL1XR1 expression, TNM stage and lymph node metastasis were found to be significant independent prognostic factors for patients with PDAC, suggesting that TBL1XR1 expression is a risk factor for PDAC.
| Parameter | Category | Case number | TBL1XR1 expression | ||
| Number of positive cases, n (%) | χ2 | P value | |||
| Age (yrs) | < 60 | 40 | 27 (67.5) | 0.214 | 0.652 |
| ≥ 60 | 50 | 36 (72.0) | |||
| Sex | Male | 59 | 41 (69.5) | 0.021 | 1.000 |
| Female | 31 | 22 (71.0) | |||
| Histopathological subtypes | High | 3 | 2 (66.7) | 0.146 | 0.929 |
| Middle | 51 | 35 (68.6) | |||
| Low | 36 | 26 (72.2) | |||
| TNM stage | 1-II | 12 | 4 (33.3) | 8.864 | 0.006b |
| III-IV | 78 | 59 (75.6) | |||
| Tumor size | < 3 cm | 60 | 42 (66.7) | 0.952 | 0.465 |
| ≥ 3 cm | 30 | 23 (76.7) | |||
| Tumor location | Head | 37 | 30 (81.1) | 3.674 | 0.065 |
| Body/tail | 53 | 33 (62.3) | |||
| Lymph node metastasis | Negative | 61 | 44 (72.1) | 0.409 | 0.624 |
| Positive | 29 | 19 (65.5) | |||
| Parameter | Category | Case number | Median survival time (mo) | P value |
| (95%CI) | ||||
| Age (yrs) | < 60 | 40 | 10.0 (4.8-15.2) | 0.413 |
| ≥ 60 | 50 | 9.7 (7.41–12.0) | ||
| Sex | Male | 59 | 9.9 (6.7-13.1) | 0.983 |
| Female | 31 | 9.9 (8.1-12.0) | ||
| Histopathological subtypes | High | 3 | 25.0 (-) | 0.760 |
| Middle | 51 | 10.3 (8.1–11.7) | ||
| Low | 36 | 8.7 (6.0-11.4) | ||
| TNM stage | 1-II | 12 | 25.0 (18.1-31.9) | < 0.001c |
| III-IV | 78 | 8.0 (5.5-10.5) | ||
| Tumor size | < 3 cm | 60 | 9.8 (8.6-11.0) | 0.251 |
| ≥ 3 cm | 30 | 8.3 (4.8-11.8) | ||
| Tumor location | Head | 37 | 9.3 (7.0-11.7) | 0.095 |
| Body/tail | 53 | 11.0 (6.0-16.0) | ||
| Lymph node metastasis | Negative | 61 | 12.0 (7.9-16.1) | 0.047a |
| Positive | 29 | 7.6 (5.3-9.8) | ||
| TBL1XR1 expression | Low | 27 | 18.9 (10.9-26.9) | < 0.001c |
| High | 63 | 7.0 (5.7-8.3) |
| Parameter | Category | Hazard ratio | 95%CI | P value |
| Age (yrs) | < 60 | 0.139 | 0.907 (0.544–1.514) | 0.709 |
| ≥ 60 | ||||
| Sex | Male | 0.013 | 1.031 (0.613–1.734) | 0.908 |
| Female | ||||
| Histopathological subtypes | High | 0.536 | 1.184 (0.754–1.859) | 0.464 |
| Middle | ||||
| Low | ||||
| TNM stage | 1-II | 5.692 | 3.046 (1.220–7.604) | 0.017a |
| III-IV | ||||
| Tumor size | < 3 cm | 1.297 | 1.378 (0.794–2.394) | 0.255 |
| ≥ 3 cm | ||||
| Tumor location | Head | 3.450 | 1.487 (0.978–2.261) | 0.063 |
| Body/tail | ||||
| Lymph node metastasis | Negative | 7.855 | 2.231 (1.273–3.909) | 0.005b |
| Positive | ||||
| TBL1XR1 expression | Low | 13.951 | 3.507 (1.815–6.774) | < 0.001c |
| High |
To investigate the possible role of TBL1XR1 in PDAC, we used IHC staining to investigate the relationship between TBL1XR1 expression and the clinicopathological features of the patients with PDAC (Figure 1B). IHC staining revealed that TBL1XR1 was mainly localized in the PDAC cell nucleus. The positive rate of TBL1XR1 staining in the tumor cells was approximately 70% (63/90) of the PDAC patients. Only 30% (27/90) of the patients possessed positive staining in the corresponding control tissues (Figure 1C, P < 0.001).
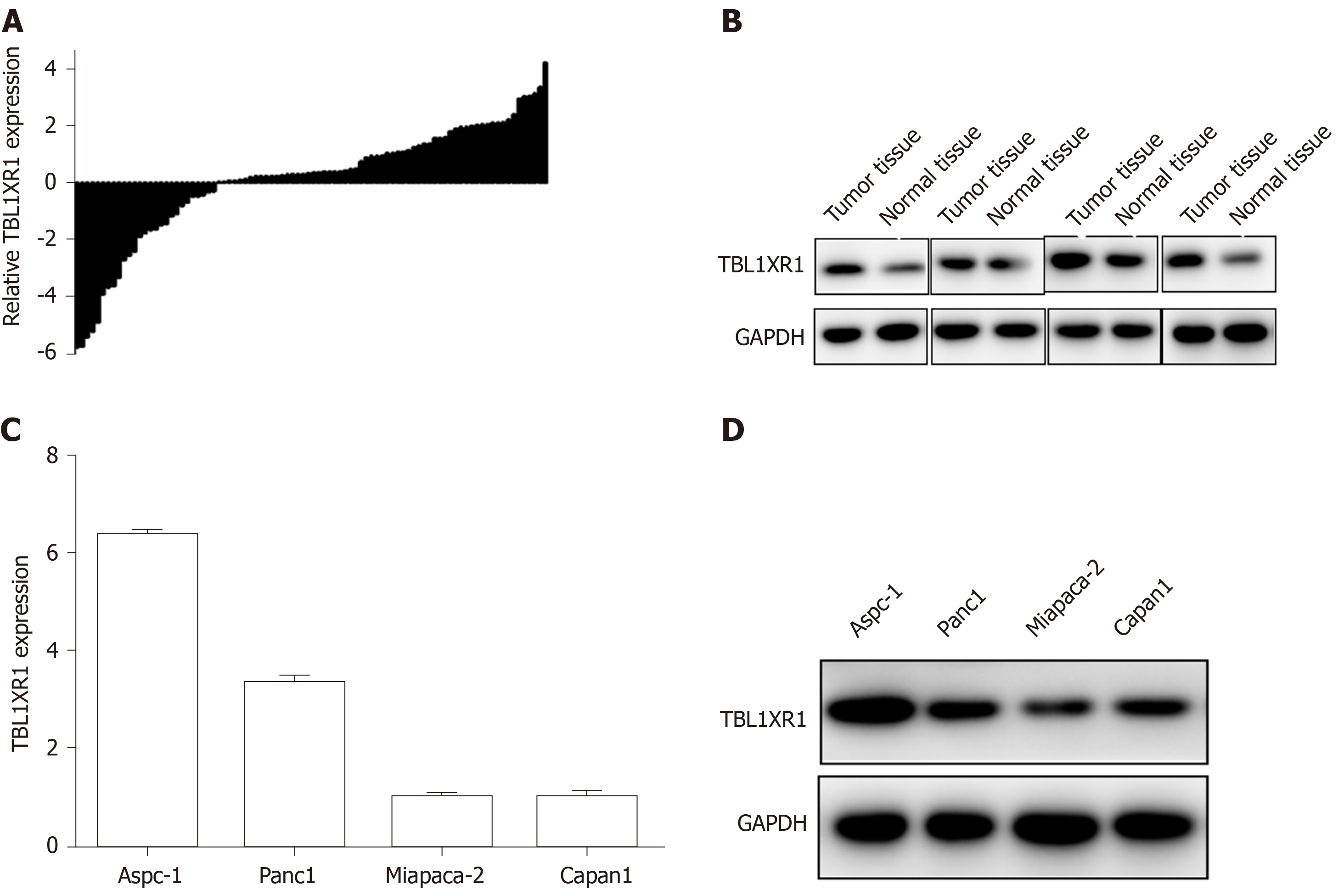
To determine the expression of TBL1XR1 in the PDAC tumor tissues and the adjacent normal tissues, we performed quantitative RT-PCR (qRT-PCR) and western blot assays. As illustrated in Figure 2A and B, enhanced expression of TBL1XR1 was observed in the PDAC tissues compared with the adjacent tissues. Then, we used MiaPaCa-2, Panc1, Aspc-1, and Capan1 cell lines to evaluate the role of TBL1XR1 in PDAC cell lines. TBL1XR1 expression at the mRNA and protein levels was examined via qRT-PCR and western blotting . We observed that TBL1XR1 was overexpressed in the PDAC cell lines, particularly in the Aspc-1 and Panc1 lines, at the mRNA (Figure 2C) and protein (Figure 2D) levels. Taken together, these data suggest that TBL1XR1 is upregulated in PDAC.
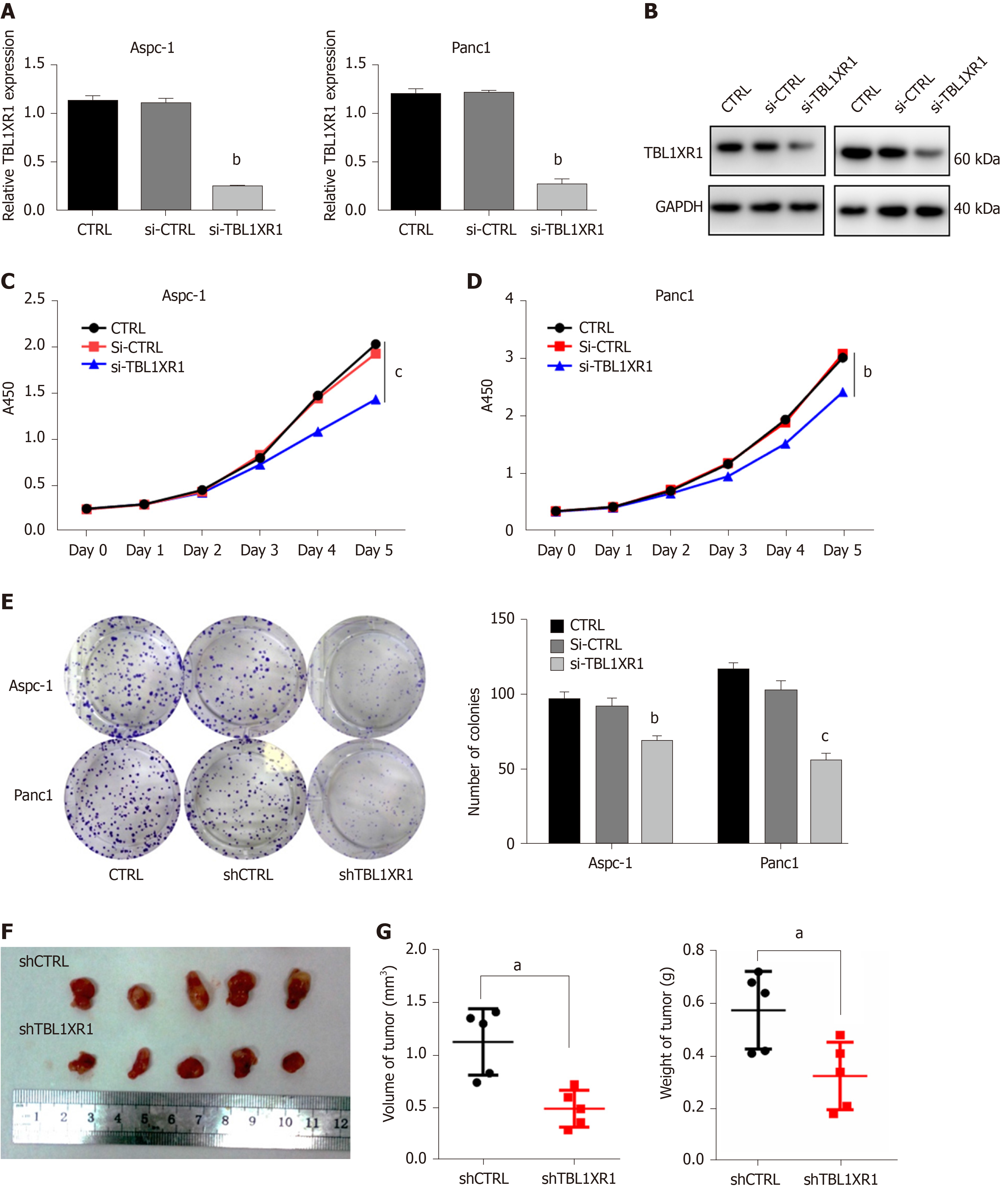
We performed CCK-8 and colony formation assays to further evaluate the effect of TBL1XR1 on the proliferation of PDAC cells. We knocked down the expression of TBL1XR1 in the Aspc-1 and Panc1 cells, and the knockdown efficiency is shown in Figure 3A and B. The CCK-8 assay showed that the downregulation of TBL1XR1 prominently suppressed PDAC cell viability compared with that of the control cells (P < 0.05, Figure 3C and D). Moreover, colony formation analysis showed that the colony formation capacity was significantly reduced in the TBL1XR1-knockdown cells compared to that of the control group (P < 0.05, Figure 3E). These results suggested that TBL1XR1 may play a vital role in PDAC cell proliferation in vitro. Furthermore, we investigated whether TBL1XR1 played a role in the proliferation of PDAC cells in vivo using xenograft mouse models. As shown, the tumor volume and weight of the TBL1XR1-knockdown group were distinctly decreased compared to those of the control group (Figure 3F and G).
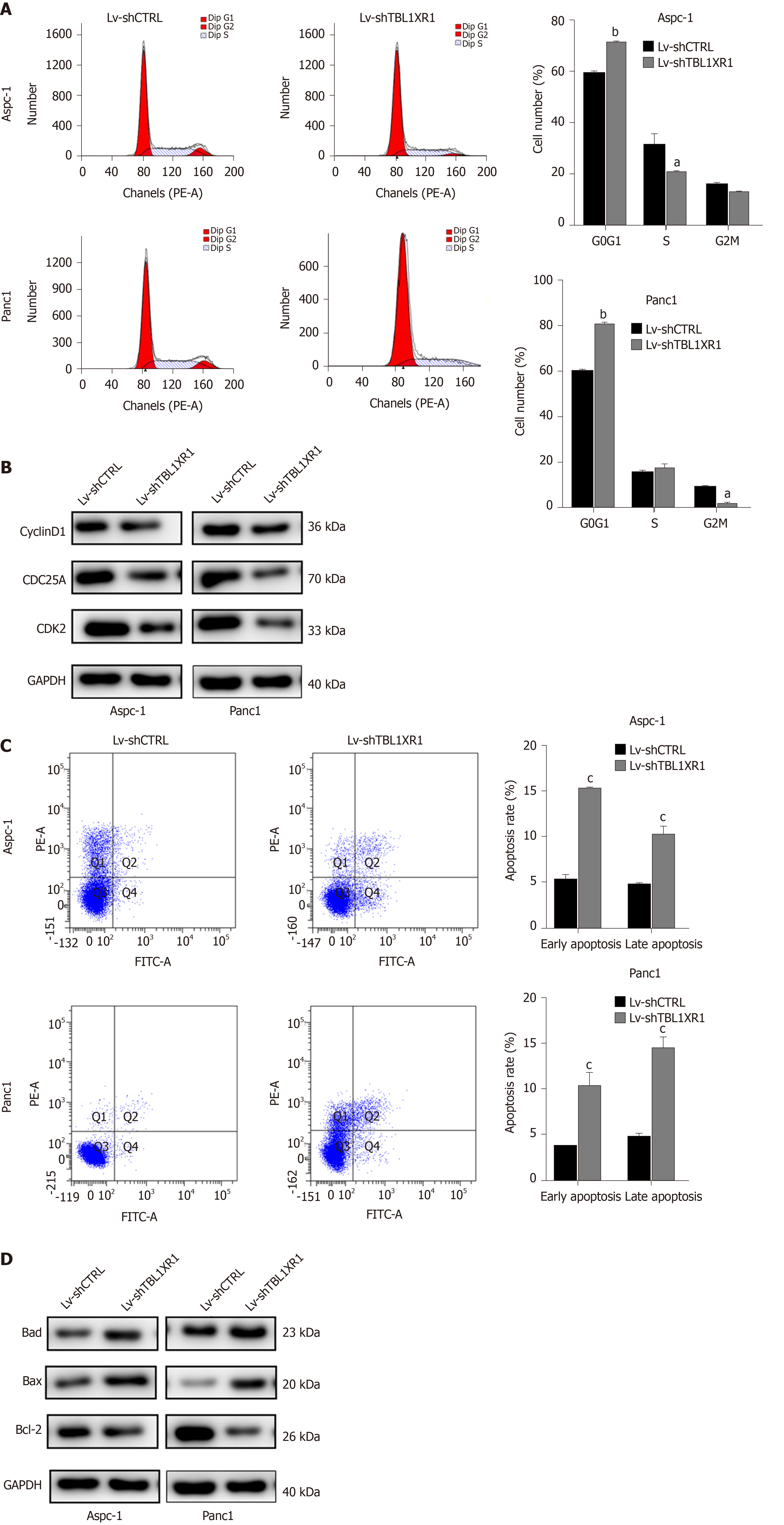
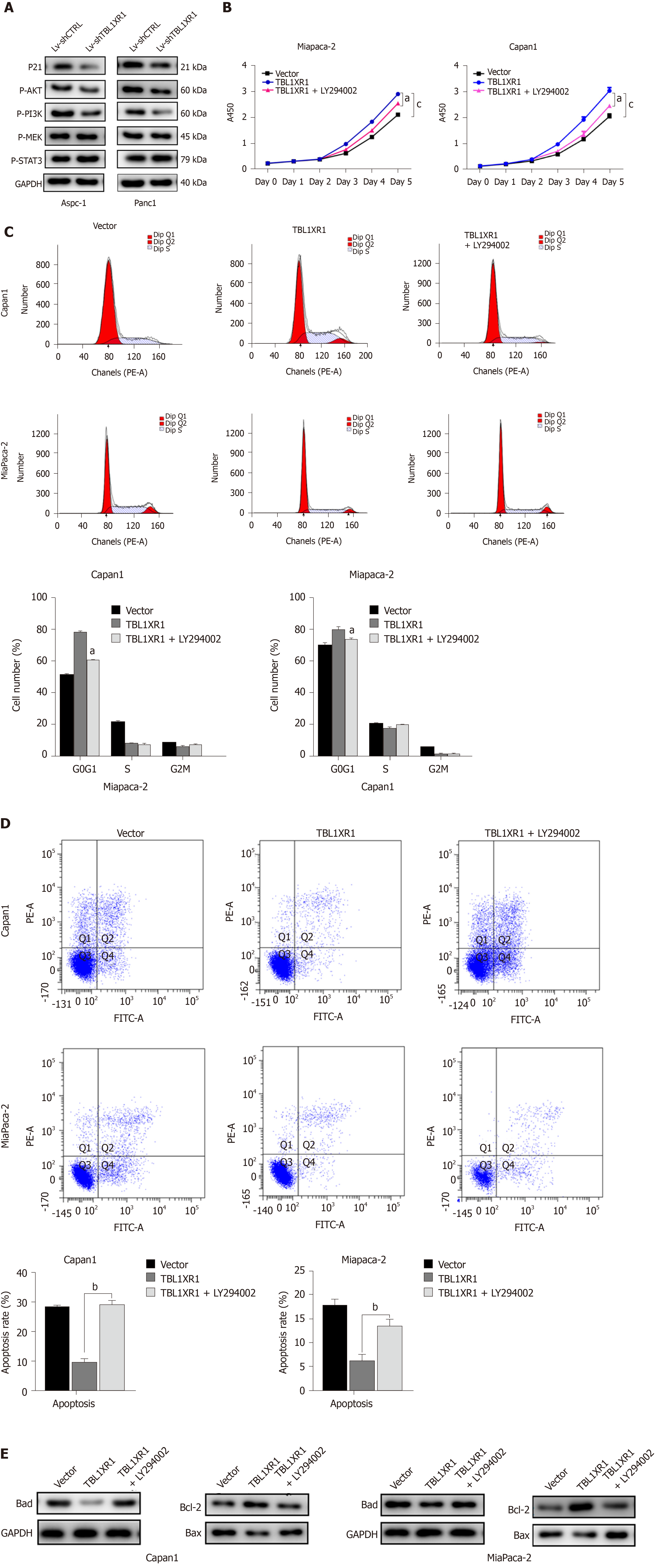
To explore the probable molecular mechanism of TBL1XR1 in PDAC cells, we detected the cell cycle and apoptotic profile of the Lv-shCTRL and Lv-shTBL1XR1 groups. As shown in Figure 4A, cell cycle analysis demonstrated that the cells treated with Lv-shTBL1XR1 exhibited cell arrest at the G0/G1 phase (Figure 4A). Moreover, we examined the protein expression of cell cycle-related regulatory genes to determine the impact of TBL1XR1 on the cell cycle by western blot assays. As depicted in Figure 4B, the protein expression levels of CDK2, CDC25A, or cyclin D1 in the Lv-shTBL1XR1 group were obviously lower than those in the control group. Therefore, we concluded that downregulation of TBL1XR1 induced PDAC cell cycle arrest at the G0/G1 phase by regulating the expression of cell cycle-related regulatory genes.
Furthermore, we performed an apoptosis assay to explore the effect of TBL1XR1 on apoptosis in PDAC cells. As shown in Figure 4C, an obvious increase in the percentage of apoptotic cells was observed in the TBL1XR1 knockdown group compared with the Lv-shCTRL group. Moreover, the knockdown of TBL1XR1 significantly enhanced the expression of Bax and Bad and reduced the expression of Bcl-2 (Figure 4D). Taken together, these results suggest that TBL1XR1 might regulate PDAC cell apoptosis and cell cycle progression.
To explore the potential mechanism by which TBL1XR1 influences proliferation and apoptosis in PDAC cells, we examined the expression changes of relevant proteins by western blotting. As shown in Figure 5A, the expression levels of p-AKT and p-PI3K were obviously reduced, while no significant effect on p-STAT3 and p-MEK was observed in the TBL1XR1 downregulation group, which indicated that TBL1XR1 might regulate PDAC cell proliferation and apoptosis via the phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT) pathway. To determine whether TBL1XR1 knockdown-induced inhibition of proliferation was regulated by the PI3K/AKT pathway, we assessed the effect of the presence or absence of LY294002 (a PI3K/Akt inhibitor) on the TBL1XR1-overexpressing cells. Unsurprisingly, overexpression of TBL1XR1 promoted proliferation and suppressed apoptosis in PDAC cells, which was consistent with previous results. LY294002 treatment reversed the TBL1XR1-mediated promotion of proliferation and inhibition of apoptosis (Figure 5B-D). Moreover, LY294002 inhibited the TBL1XR1 overexpression-induced increases in Bcl-2 and decreases in Bad and Bax (Figure 5E). Therefore, we deduced that TBL1XR1 induces PDAC cell proliferation, cell cycle arrest and apoptosis via the PI3K/AKT signaling pathway.
Although many studies have attempted to elucidate its pathogenesis, PDAC remains a deadly malignancy[19]. Hence, identification of efficacious early tumor markers for the early diagnosis and treatment of PDAC will have clinical value and significance.
TBL1XR1 (TBLR1) was first identified as a gene transcript in human CD34+CD38- cells; moreover, researchers discovered that TBL1XR1 has an F-box/WD40-repeat sequence[20]. A growing body of research has revealed that TBL1XR1 can play a vital role in tumorigenesis, invasion, metastasis, and the development of resistance to therapies[15,21]. Moreover, TBL1XR1 mRNA is highly expressed in many human tissues, such as thyroid, prostate and breast tissues[22], which indicates that TBL1XR1 may function as an oncogene. TBL1XR1 functions by activating many signal transduction pathways, such as Wnt-β-catenin, NF-κB, and Notch23. Nevertheless, it remains unclear how TBL1XR1 functions in the development of PDAC.
In this research, we discovered that the expression of TBL1XR1 was obviously enhanced in PDAC tissues compared with adjacent tissues. Moreover, we revealed that TBL1XR1 expression, TNM stage and lymph node metastasis were significant independent prognostic factors for patients with PDAC, suggesting that TBL1XR1 expression is a risk factor for PDAC. To further evaluate the function of TBL1XR1 in PDAC, we performed CCK-8 and colony formation assays, and the results showed that the downregulation of TBL1XR1 significantly suppressed PDAC cell proliferation. Moreover, we found that TBL1XR1 knockdown induced PDAC cell cycle arrest in G0/G1 phase and inhibited PDAC cell apoptosis in vitro. Additionally, the results of the in vivo animal analysis were consistent with the in vitro analysis, which indicated that TBL1XR1 might be a potential diagnostic target for PDAC.
The PI3K/AKT signaling pathway has a key impact on multiple cell processes, including cell growth, cell proliferation, angiopoiesis and survival, in both normal and tumor cells[23]. In our study, we found that TBL1XR1 can induce PDAC cell proliferation and inhibit PDAC cell apoptosis. However, whether the PI3K/AKT signaling pathway is involved in the TBL1XR1-induced promotion of PDAC cell proliferation and inhibition of apoptosis is unknown. Thus, we explored the potential mechanism by which TBL1XR1 influences proliferation and apoptosis in PDAC cells, and we found that inhibition of the PI3K/AKT pathway reversed the TBL1XR1 promotion of proliferation and inhibition of apoptosis, indicating that TBL1XR1 might play an important role in regulating PDAC cell proliferation and apoptosis. In addition, the activity of Akt could be regulated through S-nitrosylation at the kinase cysteine residues, and it was reported to be associated with reduced kinase activity[24,25], which provides us with another research direction. Overall, we illuminated the effect and mechanism of TBL1XR1 in human PDAC, and we believe that TBL1XR1 could be a promising diagnostic and therapeutic target for treating patients with advanced PDAC. Targeted therapy is an important aspect of precision therapy. We concluded that TBL1XR1 inhibitors might be promising therapeutic measures for PDAC; however, there are no inhibitors targeting TBL1XR1, which is worth investigating in the future.
In summary, our study showed that TBL1XR1 might regulate PDAC cell proliferation and apoptosis via the PI3K/AKT signaling pathway. Therefore, TBL1XR1 may be a promising diagnostic and therapeutic biomarker for patients with advanced PDAC.
Pancreatic ductal adenocarcinoma (PDAC) is one of the deadliest and most aggressive solid tumors and has a poor prognosis. Transducin (β)-like 1 X-linked receptor 1 (TBL1XR1) has been linked to the progression of various human cancers. Nevertheless, the function and role of TBL1XR1 in pancreatic cancers are unclear.
PDAC is one of the deadliest solid tumors. Identification of diagnostic and therapeutic biomarker of PDAC is urgently needed. TBL1XR1 has been linked to the progression of various human cancers. Nevertheless, the function and role of TBL1XR1 are unclear in pancreatic cancers.
To explore the potential effect and mechanism of TBL1XR1 in the development of PDAC.
Ninety histologically confirmed PDAC patients were enrolled in this study. PDAC cancer samples and cell lines were used to determine the expression of TBL1XR1. CCK-8 assays and colony formation analysis were performed to assess the viability of PDAC cells. Flow cytometry was carried out to analyze changes in the cell cycle and apoptosis. Related protein expression changes were determined by western blot analysis. Animal analysis was performed to confirm the impact of TBL1XR1 in vivo.
The results showed that patients with TBL1XR1-positive tumors had worse overall survival than those with TBL1XR1-negative tumors. Moreover, we found that TBL1XR1 obviously promoted PDAC cell proliferation and inhibited PDAC cell apoptosis. Moreover, knockdown of TBL1XR1 induced G0/G1 phase arrest. In vivo animal studies confirmed that TBL1XR1 accelerated tumor cell growth. The results of western blot analysis showed that TBL1XR1 might play a significant role in regulating PDAC cell proliferation and apoptosis via the PI3K/AKT pathway.
TBL1XR1 promotes PDAC cell progression and might be a probable effective diagnostic and therapeutic marker for pancreatic cancer.
TBL1XR1 may be an effective diagnosis and therapeutic marker for pancreatic cancer
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Chisthi MM, Fujino Y, Gassler N, Slomiany BL, Tomizawa M S-Editor: Yang Y L-Editor: MedE-Ma JY E-Editor: Ma YJ
| 1. | Garrido-Laguna I, Hidalgo M. Pancreatic cancer: from state-of-the-art treatments to promising novel therapies. Nat Rev Clin Oncol. 2015;12:319-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 443] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 2. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9172] [Cited by in RCA: 9957] [Article Influence: 995.7] [Reference Citation Analysis (0)] |
| 3. | Poruk KE, Firpo MA, Adler DG, Mulvihill SJ. Screening for pancreatic cancer: why, how, and who? Ann Surg. 2013;257:17-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 182] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 4. | Wolfgang CL, Herman JM, Laheru DA, Klein AP, Erdek MA, Fishman EK, Hruban RH. Recent progress in pancreatic cancer. CA Cancer J Clin. 2013;63:318-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 680] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 5. | Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2078] [Cited by in RCA: 2204] [Article Influence: 146.9] [Reference Citation Analysis (2)] |
| 6. | Pérez-Mancera PA, Rust AG, van der Weyden L, Kristiansen G, Li A, Sarver AL, Silverstein KA, Grützmann R, Aust D, Rümmele P, Knösel T, Herd C, Stemple DL, Kettleborough R, Brosnan JA, Li A, Morgan R, Knight S, Yu J, Stegeman S, Collier LS, ten Hoeve JJ, de Ridder J, Klein AP, Goggins M, Hruban RH, Chang DK, Biankin AV, Grimmond SM; Australian Pancreatic Cancer Genome Initiative, Wessels LF, Wood SA, Iacobuzio-Donahue CA, Pilarsky C, Largaespada DA, Adams DJ, Tuveson DA. The deubiquitinase USP9X suppresses pancreatic ductal adenocarcinoma. Nature. 2012;486:266-270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 276] [Cited by in RCA: 267] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 7. | Li M, Liu F, Zhang F, Zhou W, Jiang X, Yang Y, Qu K, Wang Y, Ma Q, Wang T, Bai L, Wang Z, Song X, Zhu Y, Yuan R, Gao Y, Liu Y, Jin Y, Li H, Xiang S, Ye Y, Zhang Y, Jiang L, Hu Y, Hao Y, Lu W, Chen S, Gu J, Zhou J, Gong W, Zhang Y, Wang X, Liu X, Liu C, Liu H, Liu Y, Liu Y. Genomic ERBB2/ERBB3 mutations promote PD-L1-mediated immune escape in gallbladder cancer: a whole-exome sequencing analysis. Gut. 2019;68:1024-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 133] [Article Influence: 22.2] [Reference Citation Analysis (1)] |
| 8. | Zhang J, Kalkum M, Chait BT, Roeder RG. The N-CoR-HDAC3 nuclear receptor corepressor complex inhibits the JNK pathway through the integral subunit GPS2. Mol Cell. 2002;9:611-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 343] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 9. | Tomita A, Buchholz DR, Obata K, Shi YB. Fusion protein of retinoic acid receptor alpha with promyelocytic leukemia protein or promyelocytic leukemia zinc finger protein recruits N-CoR-TBLR1 corepressor complex to repress transcription in vivo. J Biol Chem. 2003;278:30788-30795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Daniels G, Li Y, Gellert LL, Zhou A, Melamed J, Wu X, Zhang X, Zhang D, Meruelo D, Logan SK, Basch R, Lee P. TBLR1 as an androgen receptor (AR) coactivator selectively activates AR target genes to inhibit prostate cancer growth. Endocr Relat Cancer. 2014;21:127-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Chen SP, Yang Q, Wang CJ, Zhang LJ, Fang Y, Lei FY, Wu S, Song LB, Guo X, Guo L. Transducin β-like 1 X-linked receptor 1 suppresses cisplatin sensitivity in nasopharyngeal carcinoma via activation of NF-κB pathway. Mol Cancer. 2014;13:195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Liu H, Liu Z, Li K, Li S, Song L, Gong Z, Shi W, Yang H, Xu Y, Ning S, Ismail S, Chen Y. TBL1XR1 predicts isolated tumor cells and micrometastasis in patients with TNM stage I/II colorectal cancer. J Gastroenterol Hepatol. 2017;32:1570-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Liu F, He Y, Cao Q, Liu N, Zhang W. TBL1XR1 Is Highly Expressed in Gastric Cancer and Predicts Poor Prognosis. Dis Markers. 2016;2016:2436518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Cao Q, Wang Z, Wang Y, Liu F, Dong Y, Zhang W, Wang L, Ke Z. TBL1XR1 promotes migration and invasion in osteosarcoma cells and is negatively regulated by miR-186-5p. Am J Cancer Res. 2018;8:2481-2493. [PubMed] |
| 15. | Li X, Liang W, Liu J, Lin C, Wu S, Song L, Yuan Z. Transducin (β)-like 1 X-linked receptor 1 promotes proliferation and tumorigenicity in human breast cancer via activation of beta-catenin signaling. Breast Cancer Res. 2014;16:465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Kuang X, Zhu J, Peng Z, Wang J, Chen Z. Transducin (Beta)-Like 1 X-Linked Receptor 1 Correlates with Clinical Prognosis and Epithelial-Mesenchymal Transition in Hepatocellular Carcinoma. Dig Dis Sci. 2016;61:489-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Li M, Lu J, Zhang F, Li H, Zhang B, Wu X, Tan Z, Zhang L, Gao G, Mu J, Shu Y, Bao R, Ding Q, Wu W, Dong P, Gu J, Liu Y. Yes-associated protein 1 (YAP1) promotes human gallbladder tumor growth via activation of the AXL/MAPK pathway. Cancer Lett. 2014;355:201-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Li M, Shen J, Wu X, Zhang B, Zhang R, Weng H, Ding Q, Tan Z, Gao G, Mu J, Yang J, Shu Y, Bao R, Ding Q, Wu W, Cao Y, Liu Y. Downregulated expression of hepatoma-derived growth factor (HDGF) reduces gallbladder cancer cell proliferation and invasion. Med Oncol. 2013;30:587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Xia X, Wu W, Huang C, Cen G, Jiang T, Cao J, Huang K, Qiu Z. SMAD4 and its role in pancreatic cancer. Tumour Biol. 2015;36:111-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 20. | Zhang X, Dormady SP, Basch RS. Identification of four human cDNAs that are differentially expressed by early hematopoietic progenitors. Exp Hematol. 2000;28:1286-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Liu L, Lin C, Liang W, Wu S, Liu A, Wu J, Zhang X, Ren P, Li M, Song L. TBL1XR1 promotes lymphangiogenesis and lymphatic metastasis in esophageal squamous cell carcinoma. Gut. 2015;64:26-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 22. | Zaghlula M, Glaze DG, Enns GM, Potocki L, Schwabe AL, Suter B. Current clinical evidence does not support a link between TBL1XR1 and Rett syndrome: Description of one patient with Rett features and a novel mutation in TBL1XR1, and a review of TBL1XR1 phenotypes. Am J Med Genet A. 2018;176:1683-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Serna-Marquez N, Diaz-Aragon R, Reyes-Uribe E, Cortes-Reynosa P, Salazar EP. Linoleic acid induces migration and invasion through FFAR4- and PI3K-/Akt-dependent pathway in MDA-MB-231 breast cancer cells. Med Oncol. 2017;34:111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | Slomiany BL, Slomiany A. Helicobacter pylori Induces Disturbances in Gastric Mucosal Akt Activation through Inducible Nitric Oxide Synthase-Dependent S-Nitrosylation: Effect of Ghrelin. ISRN Gastroenterol. 2011;2011:308727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Slomiany BL, Slomiany A. Role of constitutive nitric oxide synthase S-nitrosylation in Helicobacter pylori-induced gastric mucosal cell apoptosis: effect of ghrelin. Inflammopharmacology. 2010;18:233-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |