Published online Jun 28, 2020. doi: 10.3748/wjg.v26.i24.3484
Peer-review started: February 1, 2020
First decision: March 15, 2020
Revised: March 17, 2020
Accepted: May 19, 2020
Article in press: May 19, 2020
Published online: June 28, 2020
Processing time: 147 Days and 23.8 Hours
Handling of the inferior mesenteric artery (IMA) and maintaining anastomotic perfusion are important in radical resection of left-sided colorectal cancer. However, the branching of this artery and the drainage patterns of this vein vary among individuals, and the characteristics and perfusion region of this artery in elderly patients remain unclear.
To evaluate the characteristics and perfusion region of the IMA in elderly patients using angiography.
We enrolled 154 patients (> 65 years old) who underwent digital subtraction angiography of the IMA. The characteristics, bifurcation, and distribution of the IMA and termination of the anastomotic perfusion of the left colon and rectum were examined using digital subtraction angiography. Collateral arterial arches and the IMA hemoperfusion region were also recorded. Perfusion regions were cross-referenced with clinical and anatomical features by the univariate analysis.
Of 154 patients, 25 (16.2%) had IMA lesions. The left colic artery arose independently from the IMA in 44.2% of patients, shared a trunk with the sigmoid artery in 35.1%, shared an opening with the sigmoid and superior rectal arteries in 16.9%, and was absent in 5.1%. The IMA perfusion region stopped at the splenic flexure in 50 (32.5%) patients. The collateral circulation existed in the colonic perfusion region, including the marginal artery (Drummond’s artery), the ascending branch of the left colonic artery to supply the transverse colon, and the arc of Riolan with a frequency of 100%, 22.7%, and 1.9%, respectively. The IMA perfusion region was independently associated with the comorbidity of atherosclerosis, IMA atherosclerotic lesion, branching pattern, collateral circulation, and marginal artery integrity.
The IMA and its branches are prone to arteriosclerosis, and IMA perfusion may be interrupted at the splenic flexure in elderly patients. The applicability and precision of preoperative angiography for evaluating the IMA branching and perfusion patterns could facilitate geriatric laparoscopic left-sided colorectal cancer surgery with suspicion of poor IMA perfusion.
Core tip: In this study, we enrolled 154 patients (> 65 years old) who underwent digital subtraction angiography of the inferior mesenteric artery (IMA). The characteristics and perfusion patterns of the IMA were examined. Perfusion regions were cross-referenced with clinical and anatomical features. Our study demonstrated that 25 (16.2%) patients had IMA lesions and the IMA perfusion region stopped at the splenic flexure in 50 (32.5%) elderly patients. The IMA perfusion region was independently associated with IMA atherosclerotic lesion, branching pattern, collateral circulation, and marginal artery integrity. Thus, the applicability and precision of preoperative angiography for evaluating the IMA branching and perfusion patterns could facilitate geriatric laparoscopic left-sided colorectal cancer surgery with suspicion of poor IMA perfusion.
- Citation: Zhang C, Li A, Luo T, Li Y, Li F, Li J. Evaluation of characteristics of left-sided colorectal perfusion in elderly patients by angiography. World J Gastroenterol 2020; 26(24): 3484-3494
- URL: https://www.wjgnet.com/1007-9327/full/v26/i24/3484.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i24.3484
Colorectal cancer (CRC) largely affects elderly patients, and the incidence of CRC increases with age, peaking at 70 years[1,2]. Laparoscopic surgery has made it possible for elderly CRC patients to undergo surgical treatment and has become a standard procedure around the world. Handling of the inferior mesenteric artery (IMA) and vein is important in low anterior resection and abdominoperineal excision for radical resection of left-sided CRC. However, the branching pattern of the IMA varies among individuals, and the hemoperfusion region has become one of the important issues related to the anastomotic complication and the outcome of CRC resection in elderly patients. Thus, it is very important to identify these variations to ensure successful vessel ligation during surgery for left-sided CRC. Additionally, no evidence-based studies have focused on IMA perfusion and arterial communication between the superior mesenteric artery (SMA) and IMA in elderly patients. Understanding the characteristics of these vessels and applying appropriate techniques are mandatory for improving the quality of surgery and the outcomes in elderly CRC patients. To the best of our knowledge, this report is the first to address the angiographic anatomy of the left-side colorectum in elderly patients. In the present study, we aimed to demonstrate the characteristics, bifurcation, and distribution of the IMA and termination of the anastomotic perfusion of the left colon and rectum in elderly patients using digital subtraction angiography (DSA) that could facilitate safe CRC tumor resection in elderly population.
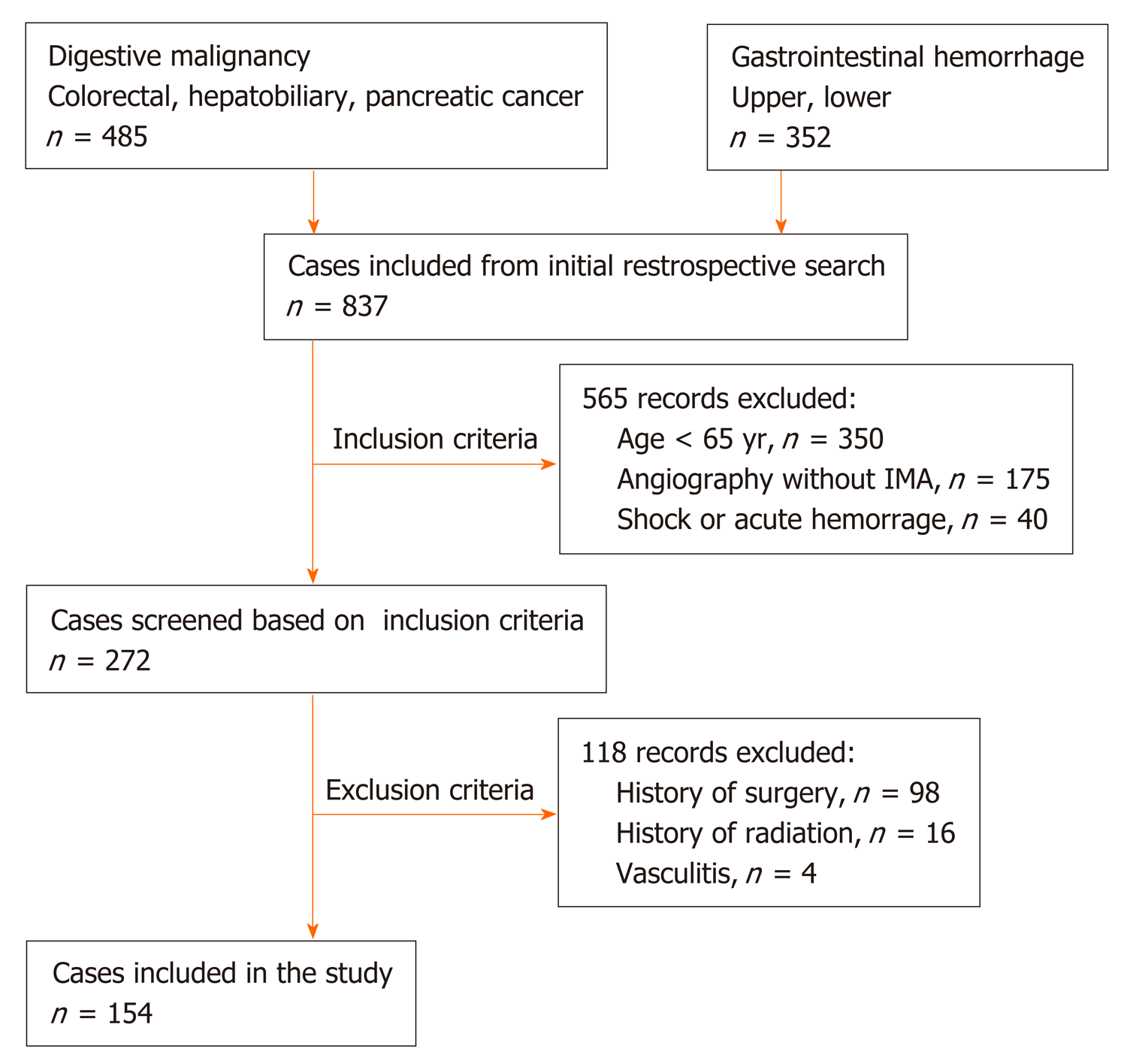
Here, we present a retrospective study (Jan 2016–Dec 2018) of 837 patients who underwent abdominal angiographic examination. This study was approved by the hospital ethical committee, and all participants signed an informed consent form. The inclusion criteria were as follows: (1) Patients aged ≥ 65 years[2,3]; (2) Patients who underwent interventional therapy and selective IMA angiography; and (3) Patients with stable vital signs without arterial spasm or excessive contraction. Patients with a history of abdominal surgery or radiotherapy, vasculitis (such as Leriche syndrome and Takayasu disease), congenital vascular malformation, acute hemorrhage, or severe shock were excluded. A total of 154 patients were finally included to be observed.
DSA was performed under local anesthesia, usually using the right femoral approach. A 5-F cobra-shaped catheter (Cordis, NJ, United States) or the SIM1 catheter (Cook, IN, United States) was introduced into the femoral artery to catheterize the abdominal aorta and the catheter tip was towards the anterior (ventral) direction. Selective DSA was performed with the catheter tip in the proximal part of the IMA using nonionic Optiray contrast medium (ioversol, 350 mg I/mL; Covidien, Dublin, Ireland; 9 mL, 3 mL/s) with the anteroposterior oblique projection. All DSA procedures were performed by abdominal interventional radiologists with over 10 years of experience (Zhang C, Li A and Luo T).
The general and IMA-specific characteristics were analyzed. The branching pattern of the IMA was classified into four different categories according to the origin of the left colic artery (LCA). The length and diameter of the IMA trunk were also measured. Collateral arterial arches and the IMA hemoperfusion region were also recorded. All data were measured and analyzed using the Philips Medical Workshop System (Philips Medical Co., NY, United States).
Student’s t-test and the chi-squared test were applied to evaluate differences in continuous variables and categorical variables for univariate analyses, respectively. All of the analyses were performed using the IBM SPSS Statistics 22 software, and differences were considered statistically significant at P < 0.05.
Between January 2016 and December 2018, a total of 154 patients were retrospectively observed, including those with digestive system malignancy, like colorectal, hepatobiliary (primary or metastasis), and pancreatic cancer, who did not underwent open or laparoscopic abdominal surgery; and gastrointestinal hemorrhage without severe shock or arterial spasm or excessive contraction (Figure 1). Out of 154 patients, 90 were male and 64 were female. The patients’ age ranged between 65 and 92 years; the average age was 72.5 ± 5.5 years. Forty (26.0%) patients had a history of atherosclerosis, including 34 coronary heart disease; 11 ischemic cerebrovascular disease; and 4 arteriosclerotic obliteration of the lower limb (Table 1).
| Number | Percent (%) | |
| Sex | ||
| Male | 94 | 61.0 |
| Female | 60 | 39.0 |
| Age, yr | 72.5 ± 5.5 | |
| BMI, kg/m2 | 23.49 ± 3.31 | |
| Comorbidity | ||
| CHD | 34 | 22.1 |
| ICVD | 11 | 7.1 |
| ASO | 4 | 2.6 |
| T2DM | 14 | 9.1 |
| IMA diameter (mm) | 3.4 ± 0.5 | |
| IMA trunk length (cm) | 3.7 ± 1.5 | |
| Type of LCA bifurcation | ||
| Type I | 68 | 44.2 |
| Type II | 54 | 35.1 |
| Type III | 26 | 16.9 |
| Type IV | 6 | 3.9 |
| Marginal artery | ||
| Integrity | 80 | 51.9 |
| Impaired integrity | 74 | 48.1 |
| aLCA and arc of Riolan | ||
| Existence | 38 | 24.7 |
| Inexistence | 116 | 75.3 |
| IMA or its branches lesion | ||
| Existence | 25 | 16.2 |
| Inexistence | 129 | 83.8 |
| IMA perfusion region | ||
| ASF | 50 | 32.5 |
| ATC | 104 | 67.5 |
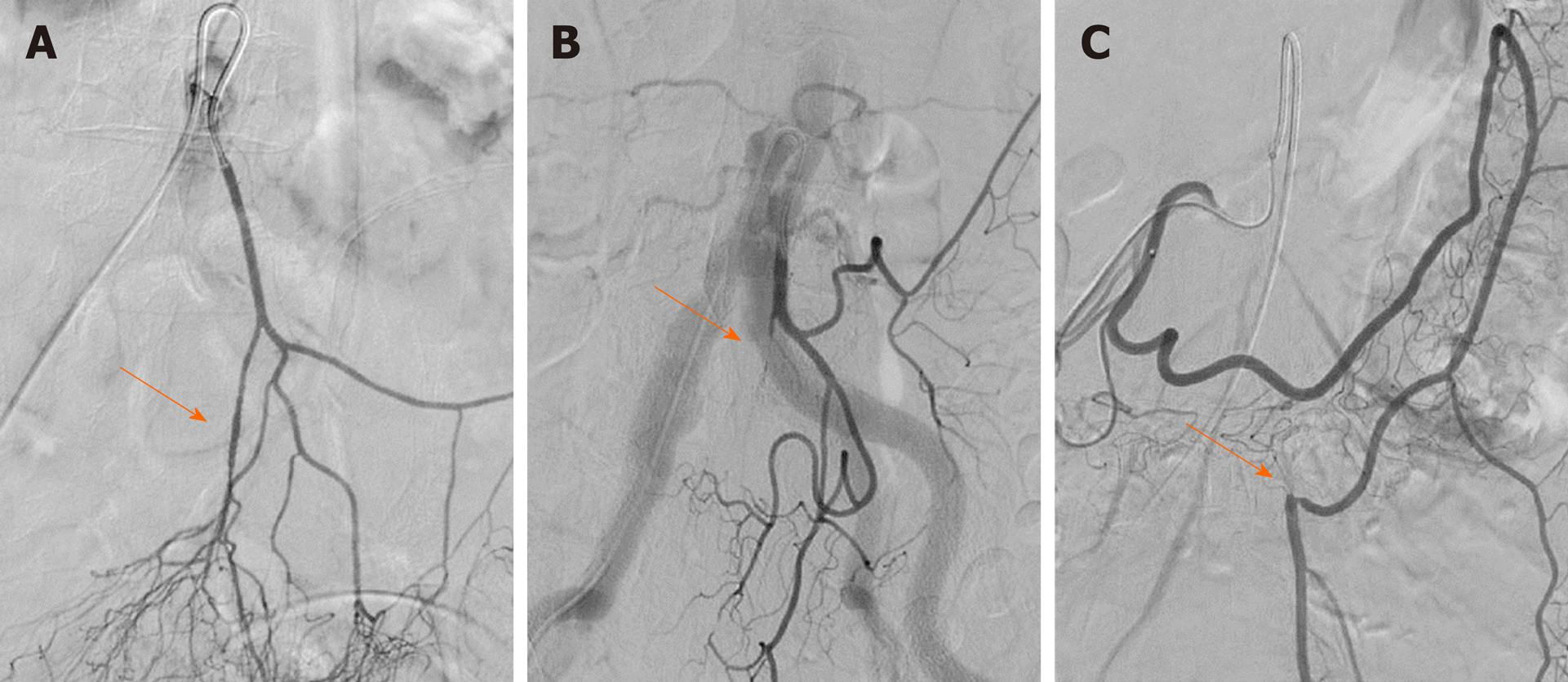
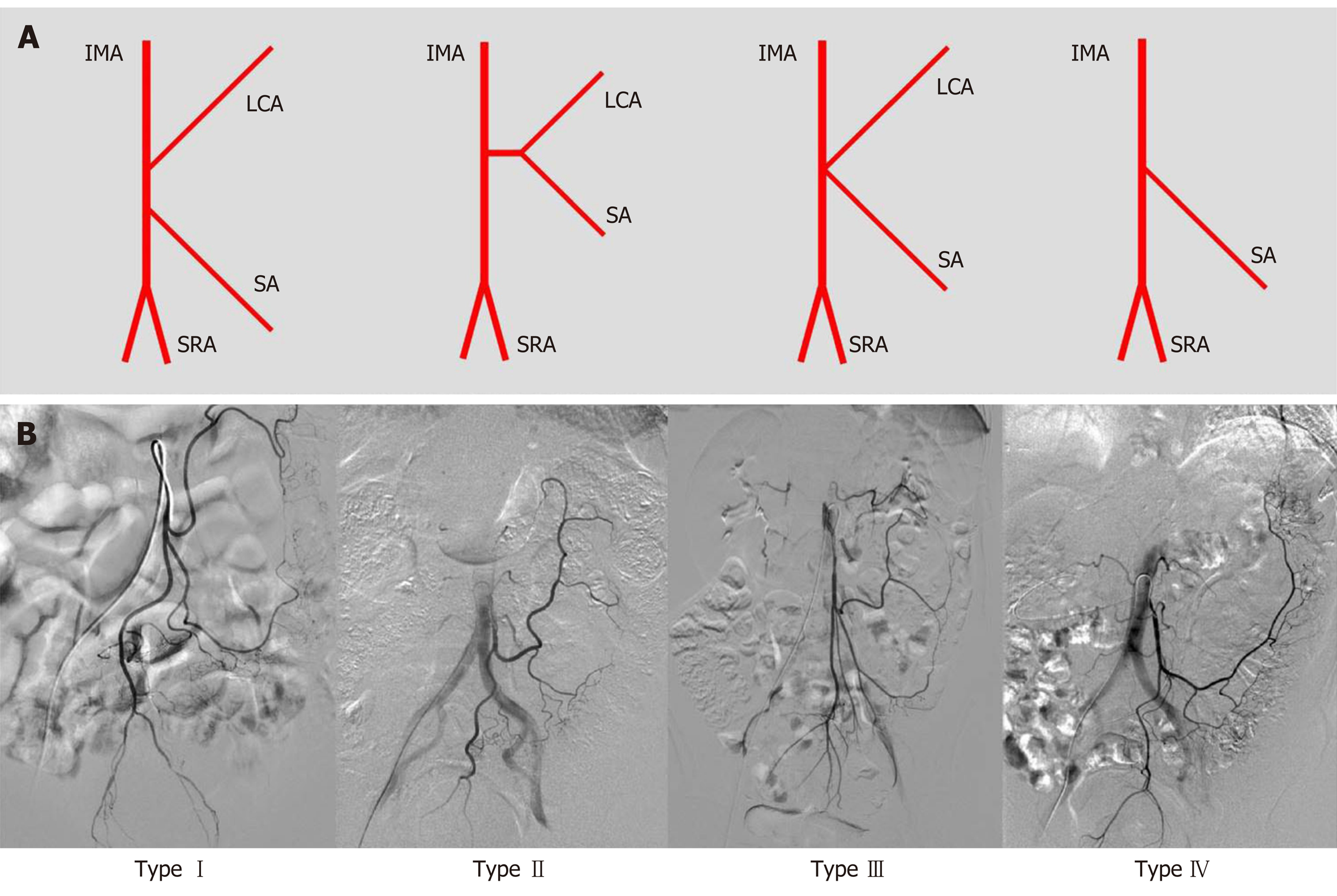
In this study, we observed that the length of the IMA trunk varied from 1.1 to 7.8 cm, with an average length of 3.7 ± 1.5 cm. The diameter of the IMA trunk was 3.4 ± 0.5 mm and varied between 2.1 and 4.8 mm (Table 1). Of all 154 patients, 25 had arteriosclerotic lesions in the IMA and its branches, including 10 with stenosis, 3 with occlusion, and 12 with atherosclerotic plaque in the arterial wall (Figure 2). We also evaluated the branches of the IMA, including the LCA, sigmoid artery (SA), and superior rectal artery (SRA). We classified the branching patterns of the LCA into four types: Type I, LCA arose independently from the IMA; type II, the LCA and SA arose from the IMA at the same point; type III, the LCA, SA, and SRA were branched from a common trunk from the IMA; and type IV, the LCA was lacking as described in a previous radiological study[4]. The patterns of the IMA are shown in Figure 3. The majority of patients were classified with either type I [n = 68 (44.2%)] or type II [n = 54 (35.1%)]; 26 (16.9%) of them had the common bifurcation of the IMA (type III), and a small number of patients [n = 6 (3.9%)] lacked the LCA (type IV).
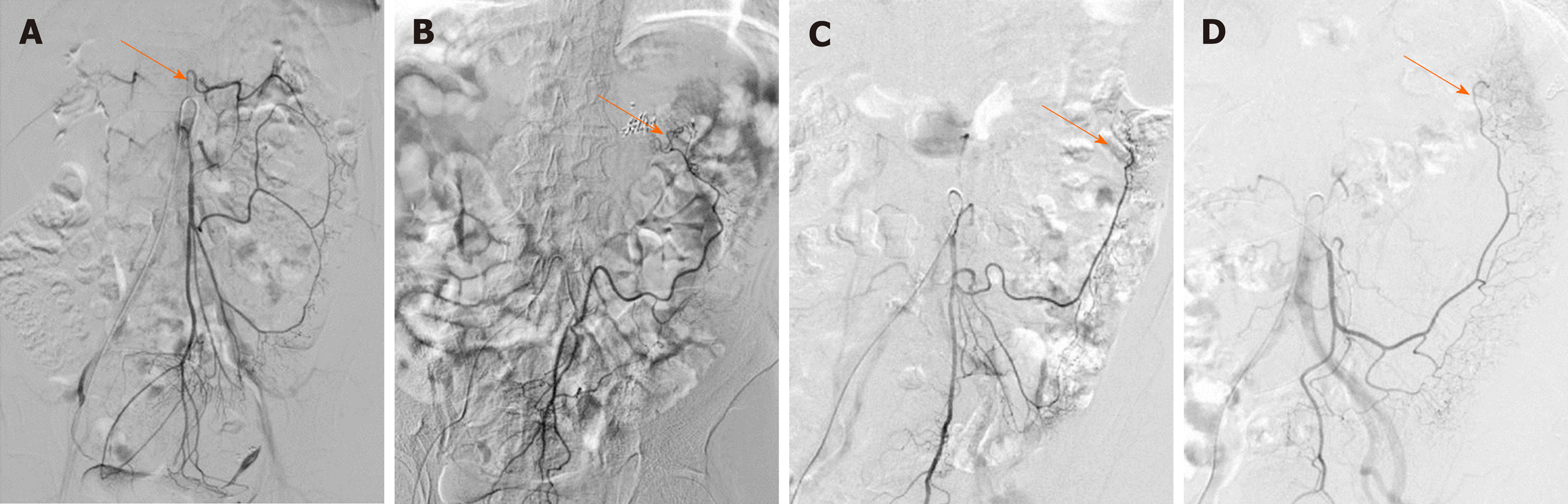
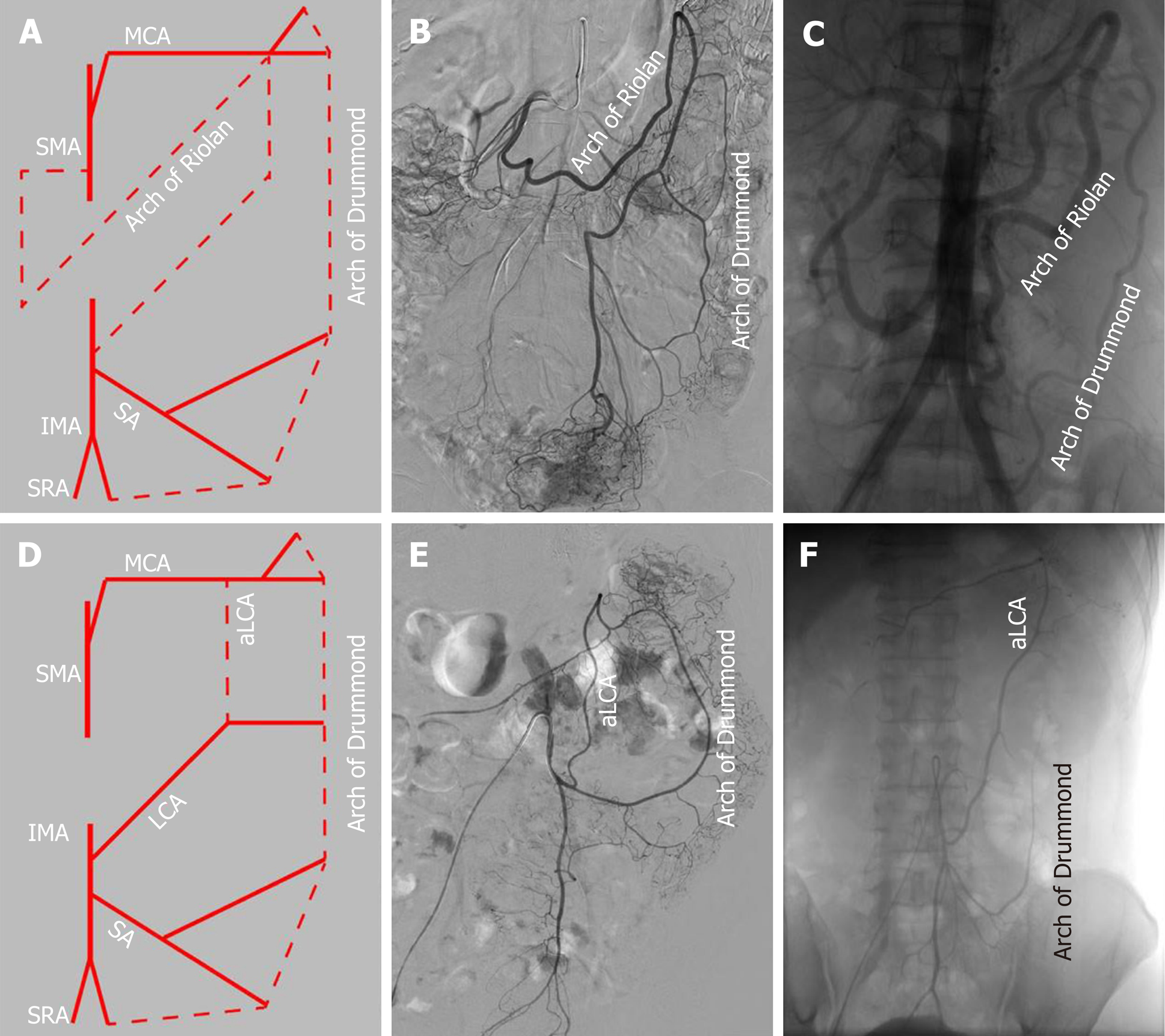
We also observed the arterial perfusion region of the IMA by angiography (Figure 4). In all cases, perfusion from the IMA to the distal region reached the middle of the rectum, and arterial perfusion to the proximal region exceeded the splenic flexure and reached the transverse colon in 104 (67.5%) cases (Figure 4A). However, in 50 (32.5%) patients, perfusion terminated at the splenic flexure (Figure 4B) and supplied only the descending colon (Figure 4C and D). The interruption of hemoperfusion in the area of the splenic flexure of the colon through the connection of the left branch of the middle colonic artery and the ascending branch of the LCA is known as Griffiths’ critical point, which demonstrates that the integrity of the marginal artery is impaired; this occurred in 74 (48.1%) of the elderly patients in our study.
The collateral circulation existed in the colonic perfusion region, including the marginal artery (Drummond’s artery), the ascending branch of the LCA (aLCA) to supply the transverse colon, and the other thick, tortuous arterial arch (arc of Riolan), which may connect the mesenteric collateral channels, with a frequency of 100%, 22.7%, and 1.9%, respectively (Figure 5). When the marginal artery or the aLCA was involved as a bypass during mesenteric occlusion, it became grossly enlarged. There were 2 cases of mesenteric artery trunk occlusion, including 1 in the SMA and 1 in the IMA. The arc of Riolan and Drummond’s artery compensated in the case of SMA occlusion (Figure 5B), and the aLCA and Drummond’s artery compensated for IMA occlusion (Figure 5C).
To determine which clinical features or IMA characteristics would be associated with the hemoperfusion region of the IMA, univariate and multivariate regression analyses were performed. The results showed that the integrity of the marginal artery and compensation of the collateral circulation, such as the aLCA and the arc of Riolan, were independently associated with the hemoperfusion region of the IMA. The collateral circulation could increase the region of IMA perfusion; however, the presence of the impaired integrity of the marginal artery decreased the IMA perfusion region, only supplying areas lower than the splenic flexure. In addition, the type IV IMA pattern could be a negative factor for IMA hemoperfusion, with a P value equal to 0.05. Meanwhile, the univariate analysis also revealed that the presence of IMA atherosclerotic lesions or comorbidity of atherosclerosis could be negative factors for IMA hemoperfusion, with a P value less than 0.05 (Table 2).
| ATC | ASF | Univariate analysis | Multivariate analysis | ||||
| n = 104 | n = 50 | t / 2 value | P value | 95%CI | OR | P value | |
| Age, mean ± SD, yr | 71.92 ± 5.41 | 73.8 ± 5.57 | 0.35 | 0.73 | - | ||
| Gender, male vs female, ratio | 1.36 | 2.12 | 1.51 | 0.219 | - | ||
| BMI, mean ± SD, kg/m2 | 23.17 ± 3.19 | 24.15 ± 3.55 | 1.73 | 0.09 | - | ||
| Comorbidity of atherosclerosis, n (%) | 22 (21.2) | 18 (36) | 3.87 | 0.049 | 0.331-5.980 | 1.407 | 0.643 |
| Comorbidity of diabetes, n (%) | 8 (7.7) | 6 (12) | 0.76 | 0.384 | - | ||
| IMA diameter, mean ± SD, mm | 3.40 ± 0.57 | 3.35 ± 0.46 | -0.51 | 0.61 | - | ||
| IMA lesion, n (%) | 13 (12.5) | 12 (24) | 3.28 | 0.036 | 0.228-7.553 | 1.314 | 0.76 |
| Type IV of IMA pattern, n (%) | 2 (1.9) | 4 (8) | 3.93 | 0.051 | 0.001-1.051 | 0.038 | 0.054 |
| Impaired integrity of marginal artery, n (%) | 28 (26.9) | 46 (92) | 57.29 | < 0.001 | 0.001-0.029 | 0.006 | < 0.001 |
| Existence of aLCA and arc of Riolan | 36 (34.6) | 2 (4) | 17.03 | < 0.001 | 14.823-457.702 | 82.368 | < 0.001 |
Anastomotic healing is an important issue in CRC surgery and could be influenced by many factors, including the ligation level of the IMA, continuity of marginal vessels, presence of atherosclerotic lesions in the mesenteric artery, and exertion of mesenteric tension on the site of anastomosis in the operative process[5]. Anastomotic ischemia is believed to be one of the major reasons and risks for the development of anastomotic leakage[6,7]. Hence, it is very important to identify variations in the IMA to ensure adequate anastomotic perfusion during left-sided colorectal operations. On the other hand, atherosclerotic diseases may also affect visceral arteries in elderly patients, such as the IMA and its branches, but no related studies have focused on evaluating the conditions of the IMA in elderly patients.
Importantly, there are two methods to divide and ligate the IMA during surgery for left-sided CRC: Ligating the IMA proximal to the origin of the LCA (high ligation); and performing ligation distal to the origin of the LCA (low ligation)[8]. To date, the region of lymphadenectomy and the frequency of anastomotic leakage in the two techniques remain controversial[9]. In high ligation, the left colon becomes more mobile and more lymph nodes are dissected[10], whereas in low ligation, possible ischemia in the proximal colon is avoided[11]. However, some studies have reported that high ligation of the IMA does not increase the frequency of anastomotic leakage[12]. Hence, understanding the anatomy and applying appropriate techniques are of instructive significance for improving the quality of surgery and the outcomes.
First, we evaluated the variations in IMA branching. In the majority of cases, the IMA was classified as type I or II (79.3%). These results are consistent with the previous results of 3D-CT angiography, which revealed frequencies of types I, II, III, and IV of 41% to 47%, 9% to 20%, 27% to 44%, and 5% to 6%, respectively[4,13]. As an anatomical variation, the low ligation procedure should theoretically correspond to the IMA pattern at different levels and the branches[14]. The LCA can easily be preserved in standard type I patients, with the ligation level below the LCA and above the SA. However, in type II patients, the vessels of the sigmoid colon are very likely to remain with retention of the LCA. Surgeons should pay more attention to hemostasis during dissection of the sigmoid colon. In type III patients, low ligation is relatively difficult, and for branch ligation, it is necessary to separate the IMA bifurcation carefully to clearly expose the LCA, SA, and even SRA. Patients suspected of having type IV variation should undergo an adequate preoperative imaging evaluation, such as by 3D-CT angiography, to determine the existence of the LCA. Excessive separation of the IMA to search for the LCA among the vessels of the left colon may cause entry of the wrong plane and lead to injury of the gonadal vessels or even the ureter.
Ensuring the best colonic perfusion remains a concern to surgeons[15,16]. The purpose of our study was to reveal the arterial characteristics of elderly patients and determine an anatomical basis for the surgical strategy in terms of the process of IMA ligation. Although multiple techniques have been used for evaluating the perfusion of the left colorectum, including angiography[17], laparoscopic ultrasonography[18], and ICG fluorescence-assisted navigation[19], only angiography can demonstrate the perfusion region of the whole colorectum, the flow direction, and the collateral arteries directly, without requiring extra time during the operation. Indeed, in our study, the hemoperfusion region of the IMA was observed. Interestingly, the range of IMA perfusion also varied, but in most cases (67.5%), the IMA could supply beyond the splenetic flexure to the transverse colon, even reaching the hepatic flexure in some cases. However, in 32.5% of cases, the hemoperfusion of the IMA split at or below the splenetic flexure. Based on the univariate and multivariate regression analyses in this study, the integrity of the marginal artery, collateral compensation, type IV IMA pattern, IMA atherosclerotic lesion, and history of atherosclerosis could be independently associated with hemoperfusion of the left-sided colorectum.
Generally, this phenomenon is interrupted when the LCA bifurcates at Griffith’s point, which is located at the splenic flexure, where the left branch of the middle colic artery meets the aLCA. This area receives poor hemoperfusion, and it is susceptible to damage and ischemia in radical resection of left-sided CRC. A previous study has reported that if the marginal artery is deficient at Griffith’s point, over-dissection and freeing of the proximal limb could result in anastomotic ischemia[20]. Our study also revealed that the hemoperfusion of the IMA only reached the descending colon, with an incomplete marginal artery at Griffith’s point. For this reason, considerable care must be taken not to disrupt the bifurcation of the LCA that replaces the function of the marginal artery in this area[21]. Collateral compensation could be a protective factor against proximal limb hemoperfusion. Three different types of collateral vessels were discerned[22]: Drummond’s artery, the aLCA, and the arc of Riolan. The marginal artery of the colon runs along the descending colon, with a prevalence of 100%. However, the other two run close to the center of the mesentery: The aLCA, representing the left arm of the SMA-IMA connection, is reported to be present in 63% to 100% of patients[23]. Nevertheless, in our study, the aLCA was present in only 24.7% of the 154 elderly patients because the aLCA was counted only when forming an arch with the Drummond’s artery at the distal transverse colon. The arc of Riolan is supposedly a thick, tortuous, uniform vessel connecting the proximal segments of the middle colic artery and LCA, representing the central point of anastomosis[24]. It can be distinguished from a normal aLCA because while this vessel is not tortuous, it runs along the descending colon and is rarely visualized by angiography.
However, IMA lesions, history of arteriosclerosis, and IMA pattern (absence of the LCA) were still identified as being associated with the hemoperfusion of the left colon based on univariate regression analysis. A previous study[25] reported that the existence of plaque increased with age, and the prevalence of plaque in the IMA was approximately 7% in all age groups; in our study, this value was 16.2% in elderly patients. Furthermore, more lesions emerged at bifurcations, such as at the opening of the IMA and SRA. Similar to the carotid and lower limb arteries, bifurcations facilitate turbulence and plaque formation, inducing stenosis or occlusion[26,27]. On angiography in our study, the lesions could be a risk factor for poor hemoperfusion of the left-side colon. Similarly, in one case report, an abnormally dilated ex-collateral artery induced colic ischemia with an imbalance in the blood flow after sigmoid colectomy[28]. When the colic ischemia is chronic, the ischemic colitis occurs. Ischemic colitis is most commonly caused by insulting to the small vessels supplying the colon, including atherosclerosis and complication of vascular and bowel surgeries. The risk factors for ischemic colitis included age greater than 60, diabetes, hypertension, atherosclerosis, and other factors[29]. Regarding the prevalence of ischemic colitis for the anatomic considerations, the left part of the colon seems to be more affected by ischemic colitis in 75%-80% of cases because of the incomplete vascular anastomosis at Griffith’s point as above mentioned, which could just demonstrate the correlation between such changes and the vessel characteristics[30,31].
The present study had some inherent limitations. Angiography could show only the anteroposterior plane of the region, and no Dyna-CT reconstruction was performed because it is difficult for elderly patients to cooperate with holding abdominal respiration. Alternatively, 3D-CT angiography could also provide helpful information, including length, branching, pattern, and the surrounding position of the IMA, to determine the optimal ligation level of the IMA in laparoscopic radical resection of left-sided CRC, which can precisely estimate the difficulties of the surgery and make the individual surgical strategy[4,13]. Moreover, because this study was not a randomized controlled study of left-sided CRC, it is difficult to determine the advantages and reveal the significance of presurgical angiography of the IMA, such as shortened operative time and reduced blood loss; additionally, anastomotic hemoperfusion after colectomy was not presented.
In conclusion, the IMA and its branches are prone to arteriosclerosis, as are the carotid and coronary arteries, in the elderly population. We concluded that IMA perfusion may be interrupted at the splenic flexure, the risk for which is increased with an incomplete marginal artery, the lack of other collateral arteries, and other factors. The applicability and precision of preoperative angiography for identification of the IMA branching pattern and perfusion region could mitigate the difficulties of the surgery and lead to individualized surgical strategies for elderly CRC patients.
Laparoscopic surgery has made it possible for elderly colorectal cancer (CRC) patients to undergo surgical treatment and has become a standard procedure around the world. Handling of the inferior mesenteric artery (IMA) and vein is important in low anterior resection and abdominoperineal excision for radical resection of left-sided CRC. The IMA characteristics and the hemoperfusion region have become one of the important issues related to the anastomotic complication and the outcome of CRC resection in elderly patients. However, the characteristics, bifurcation, and distribution and the hemoperfusion region of the IMA remain unclear in elderly patients
We demonstrated the characteristics, bifurcation, and distribution of the IMA and termination of the anastomotic perfusion of the left colon and rectum in elderly patients.
To retrospectively analyze the clinical and IMA angiographic characteristics of 154 patients over 65 years using digital subtraction angiography.
We enrolled 154 patients (> 65 years old) who underwent digital subtraction angiography of the IMA. The clinical characteristics, bifurcation, and distribution of the IMA and termination of the anastomotic perfusion of the left colon and rectum were examined. Perfusion regions were cross-referenced with clinical and anatomical features.
Of 154 patients, 25 (16.2%) had IMA lesions. The left colic artery arose independently from the IMA in 44.2% of patients, shared a trunk with the sigmoid artery in 35.1%, shared an opening with the sigmoid and superior rectal arteries in 16.9%, and was absent in 5.1%. The IMA perfusion region stopped at the splenic flexure in 50 (32.5%) patients. Collateral circulation other than the marginal artery, including the ascending left colic artery and the arc of Riolan, appeared in 38 patients. The inferior mesenteric artery perfusion region was independently associated with the IMA atherosclerotic lesion, branching pattern, collateral circulation, and marginal artery integrity.
The IMA and its branches are prone to arteriosclerosis in the elderly population. IMA perfusion may be interrupted at the splenic flexure, the risk for which is increased with an incomplete marginal artery, the lack of other collateral arteries, and the comorbidity of atherosclerosis.
The applicability and precision of preoperative angiography for the IMA branching and perfusion patterns could facilitate geriatric laparoscopic surgery, especially for the elderly left-sided colorectal cancer patients who are suspected with poor IMA perfusion.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Corresponding Author's Membership in Professional Societies: Chinese Doctor Association Surgical Branch; Beijing Medical Association Colorectal Surgical Group.
P-Reviewer: Fiori E, Kawabata H, Richardson WS, Viswanath Y S-Editor: Zhang H L-Editor: Wang TQ E-Editor: Zhang YL
| 1. | Audisio RA, Papamichael D. Treatment of colorectal cancer in older patients. Nat Rev Gastroenterol Hepatol. 2012;9:716-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Hasegawa H, Kitagawa D, Shibahara K, Funahashi S, Kitamura M. [Study of the Treatment for Colorectal Cancer(CRC)in Elderly People Aged 80 Years or Older]. Gan To Kagaku Ryoho. 2019;46:742-744. [PubMed] |
| 3. | Cassidy J, Saltz LB, Giantonio BJ, Kabbinavar FF, Hurwitz HI, Rohr UP. Effect of bevacizumab in older patients with metastatic colorectal cancer: pooled analysis of four randomized studies. J Cancer Res Clin Oncol. 2010;136:737-743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 4. | Murono K, Kawai K, Kazama S, Ishihara S, Yamaguchi H, Sunami E, Kitayama J, Watanabe T. Anatomy of the inferior mesenteric artery evaluated using 3-dimensional CT angiography. Dis Colon Rectum. 2015;58:214-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 5. | McDermott FD, Heeney A, Kelly ME, Steele RJ, Carlson GL, Winter DC. Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. Br J Surg. 2015;102:462-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 591] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 6. | Cirocchi R, Trastulli S, Farinella E, Desiderio J, Listorti C, Parisi A, Noya G, Boselli C. Is inferior mesenteric artery ligation during sigmoid colectomy for diverticular disease associated with increased anastomotic leakage? A meta-analysis of randomized and non-randomized clinical trials. Colorectal Dis. 2012;14:e521-e529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Zeng J, Su G. High ligation of the inferior mesenteric artery during sigmoid colon and rectal cancer surgery increases the risk of anastomotic leakage: a meta-analysis. World J Surg Oncol. 2018;16:157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 8. | Corder AP, Karanjia ND, Williams JD, Heald RJ. Flush aortic tie versus selective preservation of the ascending left colic artery in low anterior resection for rectal carcinoma. Br J Surg. 1992;79:680-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Hida J, Okuno K. High ligation of the inferior mesenteric artery in rectal cancer surgery. Surg Today. 2013;43:8-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Bonnet S, Berger A, Hentati N, Abid B, Chevallier JM, Wind P, Delmas V, Douard R. High tie versus low tie vascular ligation of the inferior mesenteric artery in colorectal cancer surgery: impact on the gain in colon length and implications on the feasibility of anastomoses. Dis Colon Rectum. 2012;55:515-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Park MG, Hur H, Min BS, Lee KY, Kim NK. Colonic ischemia following surgery for sigmoid colon and rectal cancer: a study of 10 cases and a review of the literature. Int J Colorectal Dis. 2012;27:671-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Rutegård M, Hemmingsson O, Matthiessen P, Rutegård J. High tie in anterior resection for rectal cancer confers no increased risk of anastomotic leakage. Br J Surg. 2012;99:127-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 13. | Ke J, Cai J, Wen X, Wu X, He Z, Zou Y, Qiu J, He X, He X, Lian L, Wu X, Zhou Z, Lan P. Anatomic variations of inferior mesenteric artery and left colic artery evaluated by 3-dimensional CT angiography: Insights into rectal cancer surgery - A retrospective observational study. Int J Surg. 2017;41:106-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Zhou J, Zhang S, Huang J, Huang P, Peng S, Lin J, Li T, Wang J, Huang M. [Accurate low ligation of inferior mesenteric artery and root lymph node dissection according to different vascular typing in laparoscopic radical resection of rectal cancer]. Zhonghua Wei Chang Wai Ke Za Zhi. 2018;21:46-52. [PubMed] |
| 15. | Cirocchi R, Trastulli S, Farinella E, Desiderio J, Vettoretto N, Parisi A, Boselli C, Noya G. High tie versus low tie of the inferior mesenteric artery in colorectal cancer: a RCT is needed. Surg Oncol. 2012;21:e111-e123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 16. | Yang Y, Wang G, He J, Zhang J, Xi J, Wang F. High tie versus low tie of the inferior mesenteric artery in colorectal cancer: A meta-analysis. Int J Surg. 2018;52:20-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 17. | Allison AS, Bloor C, Faux W, Arumugam P, Widdison A, Lloyd-Davies E, Maskell G. The angiographic anatomy of the small arteries and their collaterals in colorectal resections: some insights into anastomotic perfusion. Ann Surg. 2010;251:1092-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Sadakari Y, Nagai S, Velasquez VV, Nagayoshi K, Fujita H, Ohuchida K, Manabe T, Ohtsuka T, Nakamura M. Application of ultrasonography to high-tie and low-tie vascular ligation of the inferior mesenteric artery in laparoscopic colorectal cancer surgery: technical notes. Surg Endosc. 2019;33:309-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Gröne J, Koch D, Kreis ME. Impact of intraoperative microperfusion assessment with Pinpoint Perfusion Imaging on surgical management of laparoscopic low rectal and anorectal anastomoses. Colorectal Dis. 2015;17 Suppl 3:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | Lin PH, Chaikof EL. Embryology, anatomy, and surgical exposure of the great abdominal vessels. Surg Clin North Am. 2000;80:417-433, xiv. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Al-Asari SF, Lim D, Min BS, Kim NK. The relation between inferior mesenteric vein ligation and collateral vessels to splenic flexure: anatomical landmarks, technical precautions and clinical significance. Yonsei Med J. 2013;54:1484-1490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Renner K, Ausch C, Rosen HR, Perik E, Hochwarter G, Schiessel R, Firbas W. [Collateral circulation of the left colon: historic considerations and actual clinical significance]. Chirurg. 2003;74:575-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Predescu D, Popa B, Gheorghe M, Predescu I, Jinescu G, Boeriu M, Constantinoiu S. The vascularization pattern of the colon and surgical decision in esophageal reconstruction with colon. A selective SMA and IMA arteriographic study. Chirurgia (Bucur). 2013;108:161-171. [PubMed] |
| 24. | Lange JF, Komen N, Akkerman G, Nout E, Horstmanshoff H, Schlesinger F, Bonjer J, Kleinrensink GJ. Riolan's arch: confusing, misnomer, and obsolete. A literature survey of the connection(s) between the superior and inferior mesenteric arteries. Am J Surg. 2007;193:742-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Günenç Beşer C, Karcaaltıncaba M, Çelik HH, Başar R. The prevalence and distribution of the atherosclerotic plaques in the abdominal aorta and its branches. Folia Morphol (Warsz). 2016;75:364-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | DeBakey ME, Glaeser DH. Patterns of atherosclerosis: effect of risk factors on recurrence and survival-analysis of 11,890 cases with more than 25-year follow-up. Am J Cardiol. 2000;85:1045-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Alimohammadi M, Pichardo-Almarza C, Agu O, Díaz-Zuccarini V. A multiscale modelling approach to understand atherosclerosis formation: A patient-specific case study in the aortic bifurcation. Proc Inst Mech Eng H. 2017;231:378-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Ikeda A, Takahashi H, Miyoshi N, Haraguchi N, Hata T, Matsuda C, Yamamoto H, Mizushima T, Doki Y, Mori M. Colonic ischemia developed after laparoscopic colectomy for rectosigmoid cancer with focal infrarenal aortic stenosis. Asian J Endosc Surg. 2018;11:270-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Park CJ, Jang MK, Shin WG, Kim HS, Kim HS, Lee KS, Lee JY, Kim KH, Park JY, Lee JH, Kim HY, Nam ES, Yoo JY. Can we predict the development of ischemic colitis among patients with lower abdominal pain? Dis Colon Rectum. 2007;50:232-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Glauser PM, Wermuth P, Cathomas G, Kuhnt E, Käser SA, Maurer CA. Ischemic colitis: clinical presentation, localization in relation to risk factors, and long-term results. World J Surg. 2011;35:2549-2554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Doulberis M, Panagopoulos P, Scherz S, Dellaporta E, Kouklakis G. Update on ischemic colitis: from etiopathology to treatment including patients of intensive care unit. Scand J Gastroenterol. 2016;51:893-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |