Published online Jun 21, 2020. doi: 10.3748/wjg.v26.i23.3201
Peer-review started: February 27, 2020
First decision: April 12, 2020
Revised: April 25, 2020
Accepted: June 5, 2020
Article in press: June 5, 2020
Published online: June 21, 2020
Processing time: 109 Days and 10.6 Hours
Pancreatic cancer has a high mortality rate with minimal proven interventions. Intraductal Papillary Mucinous Neoplasms (IPMNs) are known precursor lesions for pancreatic cancer. Identification of pancreatic cysts has improved from advances in abdominal imaging. Despite multiple revisions of the international consensus recommendations and various guidelines by other major societies, successful risk stratification of the malignant potential of mucinous pancreatic cysts remains challenging. Specifically, detection and accurate classification of advanced neoplasia (high-grade dysplasia and/or adenocarcinoma) in IPMNs is suboptimal with current diagnostic strategies. Development of interventional techniques utilizing endoscopic ultrasound include - through-the-needle microforceps biopsy, next-generation or whole genome molecular analysis of cyst fluid, and needle-based confocal laser endomicroscopy. These techniques suffer from a series of limitations in technical success, diagnostic yield, and clinical feasibility, but a combination approach may offer a solution that optimizes their cyst evaluation and risk stratification. Assessment and comparison of these techniques is restricted by lack of adequate surgical specimens for testing of diagnostic accuracy, resulting in a possible sample bias. Additional large-scale multicenter studies are needed to accumulate evidence for the utility and feasibility of their translation into clinical practice. Great strides have been made in pancreatic cyst evaluation, but further research is required to improve diagnostic accuracy and clinical management of IPMNs.
Core tip: Current International Consensus Guidelines for the assessment of pancreatic cysts are insufficient in the detection of advanced neoplasia in intraductal papillary mucinous neoplasms. This manuscript summarizes the advances in endosonographic methods aiming to address this critical need and suggests additional research is necessary for more conclusive clinical management recommendations.
- Citation: Eiterman A, Lahooti A, Krishna SG. Endosonographic diagnosis of advanced neoplasia in intraductal papillary mucinous neoplasms. World J Gastroenterol 2020; 26(23): 3201-3212
- URL: https://www.wjgnet.com/1007-9327/full/v26/i23/3201.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i23.3201
Advances in abdominal imaging techniques have led to an increase in detection and diagnosis of pancreatic cystic lesions (PCLs)[1,2]. Although the majority of pancreatic cysts are asymptomatic and do not progress to adenocarcinoma, the ability to differentiate precancerous versus benign lesions and further estimate the risk for malignant transformation of precancerous lesions remains a challenge in the field[1,3]. Among all solitary PCLs, branch duct (BD) intraductal papillary mucinous neoplasms (IPMN) are the most common type of precancerous lesions, and are often referred for surveillance due to their low chance of progressing to cancer[1,4]. However, even with the use of international consensus guidelines (ICG) for the evaluation of mucinous PCLs, advanced neoplasia (high grade dysplasia or adenocarcinoma) is frequently missed[5]. Moreover, surgery for PCLs must be highly selective since the risk of long-term significant morbidity is 30% with a 2.1% mortality rate[1,6]. Thus, a sensitive and accurate method measuring risk for advanced neoplasia in BD-IPMNs is critical to determine an optimal timeline for intervention for lesions that can potentially progress to adenocarcinoma.
Reflecting on morbidity rates for cyst resection, the recommendation of resection is becoming more heavily scrutinized. Determining the presence of low-grade or high-grade dysplasia informs the need and urgency for intervention, but evaluating dysplasia with current assessment methods remains a challenge[1]. A study by Sahora et al[7] found that an estimated 75% of resected IPMNs were evaluated as low grade dysplasia that could have instead been monitored[7]. Additional studies have reported that for patients identified as having high-grade dysplasia, early identification significantly improved survival rates and eliminated or reduced risk for recurrence[1,8,9]. Thus, advances in identifying the risk and rate of transformation to advanced neoplasia could inform the ideal point for surgical intervention[10].
Risk assessment techniques in the field are lacking methods that can be used with frequency, possess excellent sensitivity and specificity, and are minimally invasive. First line assessment includes imaging techniques, followed by more invasive imaging or biopsy methods via endoscopic ultrasound (EUS)[1]. Computed tomography and magnetic resonance imaging technology provide abdominal imaging to characterize the cyst type and preemptively assess for the presence of high-risk or concerning features[1,11]. Cyst morphology on EUS imaging has similar accuracy in diagnosing a benign from malignant cyst to that of magnetic resonance imaging but is more invasive[1]. Additional methods that use EUS-guided technology can include fine needle aspiration (FNA), through-the-needle microforceps biopsy (EUS-MFB), and needle-based confocal laser endomicroscopy (EUS-nCLE). Advances in these techniques aim to address the critical need to assess risk for malignant transformation in IPMNs. These techniques and their feasibility for clinical adaptation will be summarized in this review.
Clinical guidelines assist in the identification of advanced neoplasia in PCLs. The major society guidelines include 2012 and 2017 revised ICG, 2015 American Gastroenterological Association (AGA) guidelines, and the 2018 American College of Gastroenterology guidelines[11,12]. Changes in guidelines have improved identification of advanced neoplasia in IPMNs. Sighinolfi et al[13] retrospectively validated the 2006 ICG, 2012 ICG, and AGA criteria, where the 2012 ICG and AGA showed significantly improved specificity (58.2% and 62.4%, respectively) and diagnostic accuracy (67% and 68%, respectively) for advanced neoplasia compared to the 2006 ICG (specificity 32.7%, diagnostic accuracy 46%)[13]. Additionally, Kang et al[14] have demonstrated superior diagnostic performance of the 2017 ICG [area under the curve (AUC) 0.78] compared to 2012 ICG criteria (AUC 0.74) for the detection of malignancy in BD-or mixed-type IPMNs[14]. Yet, these consensus opinions remain suboptimal, and there is need for improved cyst surveillance methods and timing of surgical intervention.
Cystic fluid carcinoembryonic antigen (CEA) acquired from EUS-FNA reflects a pooled 66% specificity and 65% sensitivity in identifying mucinous PCLs, yet it is not helpful in differentiating low-vs high-grade dysplasia[1,15,16]. Overall, EUS-FNA has moderate diagnostic strength in identifying benign and malignant lesions, but lacks ability to monitor risk and progression of advanced neoplasia in the absence of obvious EUS-imaging morphological features of malignancy[17]. However, building on the use of this technique, as well as addressing its limitations, recent research has advanced to integrate the use of molecular and genetic testing from acquired cyst fluid as a potential solution for risk assessment[18].
Using analysis of DNA or miRNA mutations via algorithms developed with whole-genome sequencing techniques, distinct genetic profiles have been developed to identify risk for malignant transformation from cystic fluid (Table 1)[1,19,20]. Mutations in KRAS and GNAS have been specifically targeted, with 100% sensitivity and 96% specificity for the detection of IPMN. In addition, these mutations resulted in an 89% sensitivity and 100% specificity for IPMN and mucinous cystic neoplasms combined[1,18].
| Ref. | Design | Sample | Specific cyst type (NGS/surgery) | Sensitivity/ specificity | Key findings | ||||
| IPMN-Ca | IPMN-HGD | IPMN-LGD | MCN-HGD | MCN-LGD | |||||
| Springer et al[22], 2015 | Retrospective, multi-center, whole genome sequencing algorithm | n = 130 patients with surgical pathology | NA/12 | NA/22 | NA/62 | - | NA/12 | NGS identified IPMNs with 76% sensitivity and 97% specificity from presence of mutation in GNAS, RNF43, LOH in chromosome 9, or aneuploidy in chromosome 1q or 8p | Use of this molecular algorithm can avoid unnecessary surgery and is relatively sensitive for high risk cysts, Adding clinical markers and radiologic features improved sensitivity to 94% but decreased specificity to 84% |
| Singhi et al[16], 2016 | Retrospective, molecular testing of cyst fluid with novel algorithmic pathway | n = 225 patients; n = 41 patients with surgical pathology | 9/9 | 1/2 | 12/12 | - | 12/1 | Six cancer genes targeted KRAS/GNAS sensitivity 100%, specificity 100% for IPMN, TP53, PIK3CA, and/or PTEN sensitivity 91%, specificity 97% for IPMN with advanced neoplasia | Integrating molecular testing with clinical features and cytopathology into algorithm resulted in sensitivity and specificity for advanced neoplasm of 100% and 90%, respectively |
| Jones et al[49], 20161 | Prospective, NGS | n = 79 patients (92 PCL samples), n = 14 with surgical pathology | 1/4 | 0/2 | 4/4 | - | - | Thirty-nine cancer genes targeted, specificity and sensitivity for NGS was 75% and 86%, specificity and sensitivity for CEA was 100% and 57%, NGS was more sensitive but CEA was more specific for identifying mutinous etiology | A KRAS mutation reclassified 19% of cysts as mucinous that were originally identified as nonneoplastic and nonmucinous from CEA, 20% of cysts identified as nonmucinous by imaging were identified as mucinous by NGS, A KRAS/GNAS mutation correlated with nonmuciouns CEA in 71% of IPMNs |
| Rosenbaum et al[50], 2016 | Retrospective, NGS | n = 113 PCL samples (105 patients); n = 25 patients with surgical pathology | NA | NA | NA | NA | NA | Nine cancer genes targeted, Detection of a KRAS variant yielded 80% sensitivity and 88% specificity for IPMN or carcinoma. GNAS variant yielded 27% sensitivity and 100% specificity for IPMN or carcinoma | Combining cytology, CEA, and NGS yielded a 90% sensitivity and 88% specificity for IPMN or carcinoma |
| Singhi et al[18], 2018 | Prospective, FNA of cyst fluid with NGS | n = 626 PCL samples from 595 patients; n = 102 patients with surgical pathology | 13/13 | 4/4 | 39/39 | 0/2 | 2/8 | Eleven cancer genes targeted, KRAS/GNAS sensitivity 100%, specificity 96% for detection of IPMN; and 89% sensitive and 100% specific for IPMN and MCNs combined, Combination KRAS/GNAS with TP53, PIK3CA, and PTEN testing showed 88% sensitivity, 97% specificity for IPMN with advanced neoplasia, Combination KRAS/GNAS with TP53, PIK3CA, and PTEN testing showed 79% sensitive, 96% specific for all mucinous pancreatic cysts with advanced neoplasia | Mutant allele frequencies over 55% for GNAS correlated with IPMNs with HGD, even if no TP53/PIK3CA/PTEN mutations were detected, Preoperative NGS could be used to classify PCs and detect IPMNs with advanced neoplasia |
Combination KRAS/GNAS with additional mutations in TP53, PIK3CA, and PTEN showed 88% sensitivity, 97% specificity for IPMN with advanced neoplasia[18]. Additional mutations in TP53, PIK3CA, and PTEN testing showed 79% sensitivity, 96% specificity for all mucinous pancreatic cysts with advanced neoplasia, showing potential for the role of molecular analyses in risk stratification of cysts. Other genes that have proven useful in risk stratification of IPMNs include SMAD4, RNF43, and CDKN2A[18,21].
The benefits of such a technique address some of the previous sampling limitations of EUS-FNA CEA analysis, as molecular analysis uses a very small amount of cyst fluid (0.25 mL fluid) and can be helpful when aspirated volume is an issue[22]. However, challenges with this technique include – reproducibility (replicating single center results in multicenter prospective studies), and feasibility for general hospitals[23]. Further, studies to date exploring the molecular markers have often used post-resected biologic specimens, thus creating the possibility for bias in the pathology of samples. Additional studies are needed to identify an adequate panel of genes for assessment of pancreatic cysts[18]. Results were also cross-validated using standard techniques such as cytology/CEA, whose sampling errors often results in discordant pairs of specimens for correlation, given challenges in diagnostic yield[17]. Major advancements in this method have yet to solve the problem of monitoring risk for progression to advanced neoplasia, but its feasibility for repeated usage in clinical surveillance is optimistic[17].
A relatively novel procedure, EUS-MFB utilizes through-the needle microforceps to acquire a tissue biopsy from PCLs for histologic analysis (Table 2)[24]. There is paucity of large, randomized trials evaluating EUS-MFB in PCLs; however, multiple systematic reviews and meta-analyses have sought to further evaluate the utility of this device. Most of these studies (Table 2) address only diagnostic yield without the estimation of diagnostic accuracy. Tacelli et al[25] reviewed 9 studies evaluating EUS-MFB; technical success was achieved in 98.5% [95% confidence interval (CI): 97.3%-99.6%, I2 (measure of heterogeneity) = 88.6%], specimen adequacy was achieved in 86.7% (95%CI: 80.1%-93.4%, I2 = 84.3%), the pooled diagnostic yield was 69.5% (95%CI: 59.2%-79.7%, I2 = 84.7%; P < 0.001), and pooled rate of adverse events was 8.6%[25]. Another recent meta-analyses by Facciorusso et al[26], involving 11 studies, revealed that specimen adequacy was 85.3% (95%CI: 78.2%-92.5%, I2 = 41.5%), pooled diagnostic accuracy (8 of 11 studies) was 78.8% (95%CI: 73.4%-84.2%, I2 = 28.4%), and risk of adverse events was 8.98%[26]. In a study by Larghi et al[27], where EUS-MFB specimens (n = 40) were retrieved and evaluated by pathologists (n = 6), the interobserver agreement among pathologists was almost perfect for specimen adequacy (Gwet’s AC1 0.82, 95%CI: 0.79-0.98), and substantial for the diagnosis of PCL (AC1 0.62, 95%CI: 0.57-0.67)[27].
| Ref. | Design | Sample | Specific cyst type (MFB/surgery) | Results | Additional key findings | ||||
| IPMN-Ca | IPMN-HGD | IPMN-LGD | MCN-HG | MCN-LG | |||||
| Basar et al[51], 2018 | Retrospective, open label, multicenter; MFB | n = 42 patients; n = 7 surgical pathology | 2/2 | 0/0 | 1/1 | - | 0/1 | Cystic tissue acquisition yield 90% MFB vs 88.1% FNA. Diagnostic yield was 61.9% MFB vs 47.6% CFC | Specific cyst type diagnosis provided by MFB 35.7% vs CFC 4.8% |
| Kovacevic et al[31], 2018 | Retrospective, MFB | n = 31 patients; n = 18 FNA cytology; n = 4 surgical pathology | 0/1 | 0/0 | 31/2 | - | - | Technical success for MFB 87.1% vs 58.1% FNA. Diagnostic yield 71% MFB vs 35.5% CFC | MFB yielded change in clinical management in 19.4% of cases |
| Mittal et al[52], 2018 | Retrospective, EUS-MFB | n = 27 patients | 0/0 | 0/0 | 12/3 | NA | NA | Technical success 100% EUS-MFB; Diagnostic yield 88.9% EUS-MFB | MFB altered diagnosis in 26% of cases. However, cytology diagnosed 4 mucinous cysts (14.8%) that MFB missed |
| Yang et al[53], 2018 | Retrospective, EUS-MFB | n = 47 patients; n = 8 surgical pathology | 0/0 | 2/2 | 43/5 | - | - | Technical success EUS-MFB 85.1% vs 48.9% FNA | Mucinous cysts were diagnosed more often by EUS-MFB (34.3%) compared to FNA + CEA analysis combined (9.4%); FNA diagnosed adenocarcinoma for 1 patient, but was benign via EUS-MFB and surgical resection |
| Zhang et al[54], 2018 | Retrospective, MFB | n = 48 patients | 14/3 | 11/0 | 4/4 | - | 0/1 | Diagnostic yield MFB 75.0% vs 72.9% CFC | Specific cyst type diagnosis was successful 50.0% MFB vs 18.8% for FNA cytology; Three times as many IPMNs were diagnosed by MFB alone compared to CFC |
| Crinò et al[55], 2019 | Retrospective, EUS-MFB | n = 61 patients | - | - | - | 0/1 | 11/12 | Diagnostic reliability of EUS-MFB compared to surgery was 90% vs 20% CFC | Two EUS-MFB histologic samples resulted in a specific cyst diagnosis in 74% of cases; 100% histological adequacy reached with two EUS-MFB samples |
| Yang et al[24], 2019 | Prospective, Open Label, EUS-MFB | n = 114 patients; n = 23 surgical pathology | 2/2 | 2/2 | 5/5 | 3/3 | 1/1 | Specificity for EUS-MFB was 100% vs 21.4% CFC; EUS-MFB was diagnostic to the degree of dysplasia by 80% vs 0% CFC | Tissue acquisition reached 83.3% with EUS-MFB vs 37.7% with FNA; Findings from EUS-MFB were 100% concordant with analysis from resection vs 21.4% with CFC alone |
EUS-MFB likely solves the problems of low cellular cystic fluid acquisition by instead sampling tissue from the epithelium lining the cyst wall, as well as tissue beyond the epithelium[25,28]. It has been demonstrated that obtaining two macroscopically visible tissue samples reached optimal histologic adequacy and specifically, identification of stroma beyond the epithelium greatly assists in diagnosis of mucinous cystic neoplasms[29,30]. Additionally, a study by Kovacevic et al[31] posited EUS-MFB may be used to subtype IPMNs according to mucin expression, leading to more accurate risk stratification, but the scope of the study was limited and additional research and follow up is needed[31].
Although EUS-MFB carries improvements in tissue acquisition and thus advances in diagnostic yield from samples, there are increased reports of adverse events (pooled estimate of 8.6%, with limited serious adverse events), but this might be related to operator skill[25]. The benefit for obtaining tissue for histology may lead to further advantages for this technique, however it is important to note that the biopsies are obtained from focal areas in the cyst, due in part to the stiffness of the 19-g needle with the forceps[25]. It is also noted that there are concerns for gastric seeding using this technique, and thus its clinical safety and feasibility imply the need for additional testing[11].
Studies utilizing EUS-MFB have addressed the diagnostic yield, feasibility, technical success, and associated adverse events. However, there are no comparative large prospective studies with assessment of diagnostic accuracies where the reference standard is surgical histopathology and the lack of this diminishes the full impact of this novel diagnostic modality in the management of PCLs.
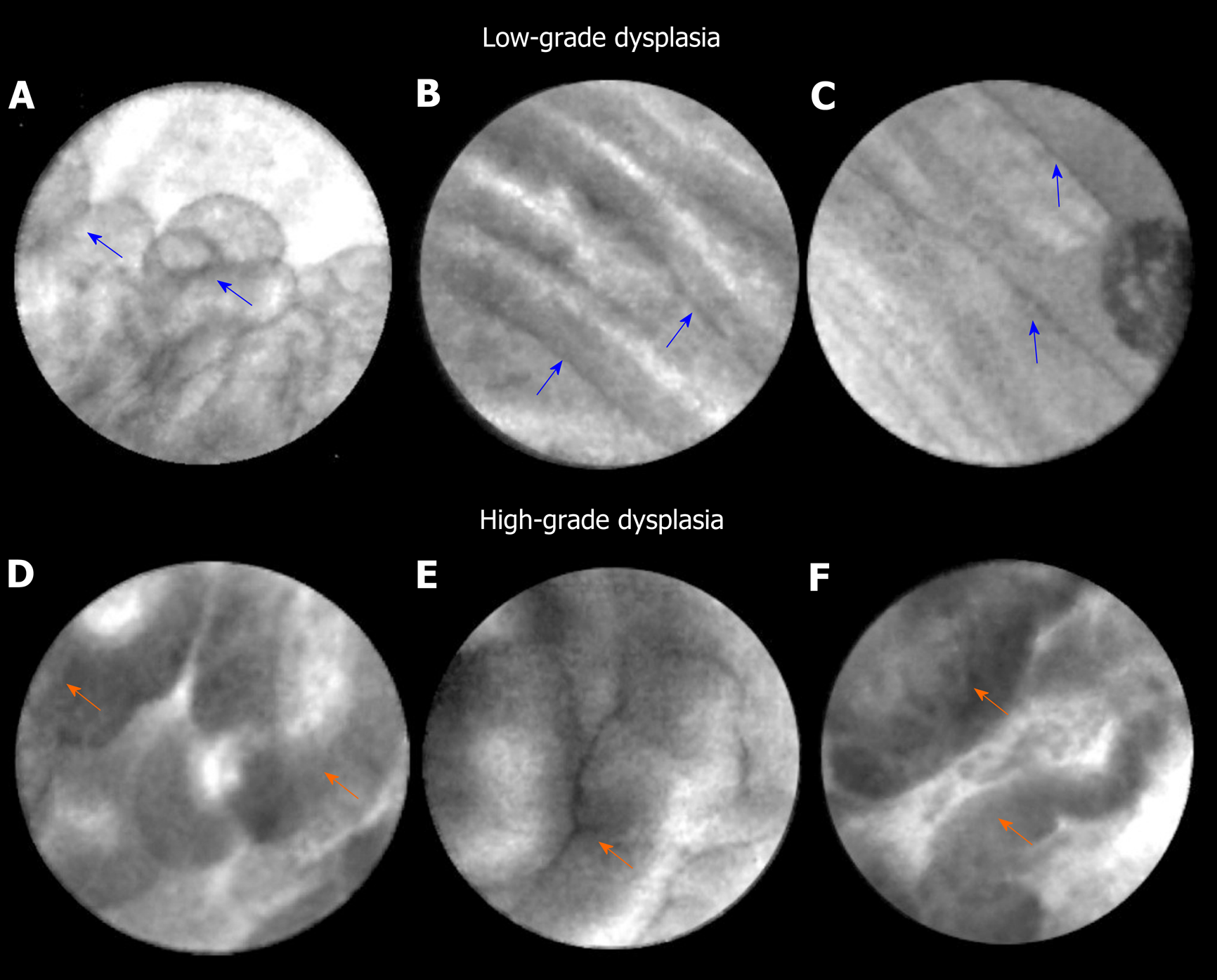
EUS guided needle confocal laser endomicroscopy is a novel in vivo imaging technique that grants real-time, high-resolution microscopic imaging of the pancreatic cyst epithelium. Major trials (INSPECT, DETECT, CONTACT, and INDEX) have established feasibility, diagnostic image standard, and characteristic CLE patterns of pancreatic cysts routinely evaluated in clinical practice[32-37]. In the INDEX study, investigators confirmed the in vivo nCLE features on ex vivo post-resection specimens of IPMNs[38,39], and also validated EUS-nCLE image patterns of mucinous PCLs including IPMNs among blinded external CLE-expert observers[40]. In a post-hoc analysis of the INDEX study, quantification of the papillary epithelial width and darkness (pixel intensity) was able to predict advanced neoplasia in IPMNs (Figure 1). The study included 26 IPMNs (high-grade dysplasia-Ca in 16) with histopathological (n = 24) or cytological (n = 2) reference standard. For diagnosing advanced neoplasia, CLE features of increased papillary epithelial “width” with cut-off value of ≥ 50 μm revealed a sensitivity, specificity, and AUC of 87.5%, 100%, and 0.95, respectively. Similar diagnostic values of sensitivity, specificity, and AUC of 87.5%, 100%, and 0.95, respectively, were observed for papillary darkness (pixel-intensity) cut-off value ≤ 90 pixels (lower values being darker). The pathology of IPMNs presents a papillary configuration that includes a spectrum of dysplastic mucinous epithelium ranging from low-to-high grade dysplasia[41,42]. While low-grade dysplasia demonstrates a single flat layer of columnar cells with retained polarity, high-grade dysplasia demonstrates loss of nuclear polarity, increased nuclear-cytoplasmic ratio, stratification of both the columnar cells and their nuclei, and complex architecture[41]. In parallel to these pathological changes, IPMNs with low-grade dysplasia reveal a thin layer of epithelium (Figure 1), whereas advanced neoplasia reveals thicker and darker epithelium since CLE imaging of the nuclei reveals dark spots, which when stratified imparts a darker hue to the epithelium. Thus, the consequence of a combination of cellular and nuclear stratification is thicker and darker epithelium of the papillae (Figure 1). Qualitative image interpretation by an endoscopist is susceptible to interobserver disagreement and quantitative estimation of papillary thickness and darkness can be laborious. Hence, computer-aided quantification utilizing machine learning and artificial intelligence would need to be adapted to reduce interobserver disagreement and endoscopist work load.
Similar to other EUS-guided evaluations, nCLE differentiation of dysplasia in IPMN needs to be reproducible and validated in prospective multi-center studies. If endomicroscopy assisted machine learning and artificial algorithms are generated, these would need to be validated in multicenter studies across multiple centers and endoscopists. Since the entire cyst is not imaged during CLE, theoretically the modality cannot be utilized to rule-out advanced neoplasia in IPMNs. Despite the limitations in the maneuverability of the EUS-FNA needle (19-g), approximately 30%-50% of the PCLs can be observed depending on the location of the neoplasm in the pancreas.
Pancreatic adenocarcinoma is a near fatal diagnosis with a < 10% 5-year overall survival rate, and there are no pragmatic investigations for screening with proven efficacy[43]. Analogous to other adenocarcinomas such as esophageal and colorectal cancer, the resection of a premalignant lesion such as IPMN should prevent development of pancreatic adenocarcinoma; however, the operative morbidity of pancreatic surgery is high, the natural history of BD-IPMNs (< 3 cm) has not been delineated, and it is challenging to diagnose BD-IPMNs with advanced neoplasia[11]. Despite multiple revisions of the international consensus guidelines and various guidelines by other major societies[11,12,44,45], there continues to be range of percentage of patients (up to 2/3rd of resections at tertiary care centers) who have low-grade dysplasia on postoperative pathology[46-48].
Improved diagnostics are needed that are sensitive and accurate in identifying dysplasia, as well as informing an appropriate timeline for intervention when considering risk of malignant transformation. Each of the methods explored in this manuscript, through-the-needle tissue biopsy/MFB, molecular analysis, and nCLE, have attempted to address this need. Collectively these methods suffer from potential sample bias given the limited amount of surgical specimen pathology available to confirm diagnostic accuracy. There is inherent need for improved sample size to refine these methods, which will likely only be attained from large-scale multisite studies. These methods each have individual limitations, and it may be prudent to evaluate a combination of techniques to optimize clinical feasibility and diagnostic accuracy. In summary, great strides have been made in the evaluation of pancreatic cysts, but there is still a critical need for further research to classify the risk for malignant potential.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Crinò SF, Pelaez-Luna M S-Editor: Zhang L L-Editor: A E-Editor: Zhang YL
| 1. | Elta GH, Enestvedt BK, Sauer BG, Lennon AM. ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am J Gastroenterol. 2018;113:464-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 423] [Article Influence: 60.4] [Reference Citation Analysis (1)] |
| 2. | Klibansky DA, Reid-Lombardo KM, Gordon SR, Gardner TB. The clinical relevance of the increasing incidence of intraductal papillary mucinous neoplasm. Clin Gastroenterol Hepatol. 2012;10:555-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 3. | Gardner TB, Glass LM, Smith KD, Ripple GH, Barth RJ, Klibansky DA, Colacchio TA, Tsapakos MJ, Suriawinata AA, Tsongalis GJ, Pipas JM, Gordon SR. Pancreatic cyst prevalence and the risk of mucin-producing adenocarcinoma in US adults. Am J Gastroenterol. 2013;108:1546-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 4. | Stark A, Donahue TR, Reber HA, Hines OJ. Pancreatic Cyst Disease: A Review. JAMA. 2016;315:1882-1893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 177] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 5. | Heckler M, Michalski CW, Schaefle S, Kaiser J, Büchler MW, Hackert T. The Sendai and Fukuoka consensus criteria for the management of branch duct IPMN - A meta-analysis on their accuracy. Pancreatology. 2017;17:255-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 6. | Scheiman JM, Hwang JH, Moayyedi P. American gastroenterological association technical review on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148:824-48.e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 295] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 7. | Sahora K, Mino-Kenudson M, Brugge W, Thayer SP, Ferrone CR, Sahani D, Pitman MB, Warshaw AL, Lillemoe KD, Fernandez-del Castillo CF. Branch duct intraductal papillary mucinous neoplasms: does cyst size change the tip of the scale? A critical analysis of the revised international consensus guidelines in a large single-institutional series. Ann Surg. 2013;258:466-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 227] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 8. | Griffin JF, Page AJ, Samaha GJ, Christopher A, Bhaijee F, Pezhouh MK, Peters NA, Hruban RH, He J, Makary MA, Lennon AM, Cameron JL, Wolfgang CL, Weiss MJ. Patients with a resected pancreatic mucinous cystic neoplasm have a better prognosis than patients with an intraductal papillary mucinous neoplasm: A large single institution series. Pancreatology. 2017;17:490-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Lennon AM, Wolfgang CL, Canto MI, Klein AP, Herman JM, Goggins M, Fishman EK, Kamel I, Weiss MJ, Diaz LA, Papadopoulos N, Kinzler KW, Vogelstein B, Hruban RH. The early detection of pancreatic cancer: what will it take to diagnose and treat curable pancreatic neoplasia? Cancer Res. 2014;74:3381-3389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 171] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 10. | Rezaee N, Barbon C, Zaki A, He J, Salman B, Hruban RH, Cameron JL, Herman JM, Ahuja N, Lennon AM, Weiss MJ, Wood LD, Wolfgang CL. Intraductal papillary mucinous neoplasm (IPMN) with high-grade dysplasia is a risk factor for the subsequent development of pancreatic ductal adenocarcinoma. HPB (Oxford). 2016;18:236-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 11. | Tanaka M, Fernández-Del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T, Salvia R, Shimizu Y, Tada M, Wolfgang CL. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017;17:738-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 868] [Cited by in RCA: 1157] [Article Influence: 144.6] [Reference Citation Analysis (1)] |
| 12. | Vege SS, Ziring B, Jain R, Moayyedi P; Clinical Guidelines Committee; American Gastroenterology Association. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148:819-22; quize12-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 629] [Cited by in RCA: 759] [Article Influence: 75.9] [Reference Citation Analysis (1)] |
| 13. | Sighinolfi M, Quan SY, Lee Y, Ibaseta A, Pham K, Dua MM, Poultsides GA, Visser BC, Norton JA, Park WG. Fukuoka and AGA Criteria Have Superior Diagnostic Accuracy for Advanced Cystic Neoplasms than Sendai Criteria. Dig Dis Sci. 2017;62:626-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Kang JS, Park T, Han Y, Lee S, Lim H, Kim H, Kim SH, Kwon W, Kim SW, Jang JY. Clinical validation of the 2017 international consensus guidelines on intraductal papillary mucinous neoplasm of the pancreas. Ann Surg Treat Res. 2019;97:58-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Ngamruengphong S, Bartel MJ, Raimondo M. Cyst carcinoembryonic antigen in differentiating pancreatic cysts: a meta-analysis. Dig Liver Dis. 2013;45:920-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Singhi AD, Zeh HJ, Brand RE, Nikiforova MN, Chennat JS, Fasanella KE, Khalid A, Papachristou GI, Slivka A, Hogg M, Lee KK, Tsung A, Zureikat AH, McGrath K. American Gastroenterological Association guidelines are inaccurate in detecting pancreatic cysts with advanced neoplasia: a clinicopathologic study of 225 patients with supporting molecular data. Gastrointest Endosc. 2016;83:1107-1117.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 118] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 17. | Maker AV, Carrara S, Jamieson NB, Pelaez-Luna M, Lennon AM, Dal Molin M, Scarpa A, Frulloni L, Brugge WR. Cyst fluid biomarkers for intraductal papillary mucinous neoplasms of the pancreas: a critical review from the international expert meeting on pancreatic branch-duct-intraductal papillary mucinous neoplasms. J Am Coll Surg. 2015;220:243-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 18. | Singhi AD, McGrath K, Brand RE, Khalid A, Zeh HJ, Chennat JS, Fasanella KE, Papachristou GI, Slivka A, Bartlett DL, Dasyam AK, Hogg M, Lee KK, Marsh JW, Monaco SE, Ohori NP, Pingpank JF, Tsung A, Zureikat AH, Wald AI, Nikiforova MN. Preoperative next-generation sequencing of pancreatic cyst fluid is highly accurate in cyst classification and detection of advanced neoplasia. Gut. 2018;67:2131-2141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 267] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 19. | Wu J, Jiao Y, Dal Molin M, Maitra A, de Wilde RF, Wood LD, Eshleman JR, Goggins MG, Wolfgang CL, Canto MI, Schulick RD, Edil BH, Choti MA, Adsay V, Klimstra DS, Offerhaus GJ, Klein AP, Kopelovich L, Carter H, Karchin R, Allen PJ, Schmidt CM, Naito Y, Diaz LA Jr, Kinzler KW, Papadopoulos N, Hruban RH, Vogelstein B. Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proc Natl Acad Sci USA. 2011;108:21188-21193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 482] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 20. | Wu J, Matthaei H, Maitra A, Dal Molin M, Wood LD, Eshleman JR, Goggins M, Canto MI, Schulick RD, Edil BH, Wolfgang CL, Klein AP, Diaz LA Jr, Allen PJ, Schmidt CM, Kinzler KW, Papadopoulos N, Hruban RH, Vogelstein B. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med. 2011;3:92ra66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 597] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 21. | Hao S, Takahashi C, Snyder RA, Parikh AA. Stratifying Intraductal Papillary Mucinous Neoplasms by Cyst Fluid Analysis: Present and Future. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Springer S, Wang Y, Dal Molin M, Masica DL, Jiao Y, Kinde I, Blackford A, Raman SP, Wolfgang CL, Tomita T, Niknafs N, Douville C, Ptak J, Dobbyn L, Allen PJ, Klimstra DS, Schattner MA, Schmidt CM, Yip-Schneider M, Cummings OW, Brand RE, Zeh HJ, Singhi AD, Scarpa A, Salvia R, Malleo G, Zamboni G, Falconi M, Jang JY, Kim SW, Kwon W, Hong SM, Song KB, Kim SC, Swan N, Murphy J, Geoghegan J, Brugge W, Fernandez-Del Castillo C, Mino-Kenudson M, Schulick R, Edil BH, Adsay V, Paulino J, van Hooft J, Yachida S, Nara S, Hiraoka N, Yamao K, Hijioka S, van der Merwe S, Goggins M, Canto MI, Ahuja N, Hirose K, Makary M, Weiss MJ, Cameron J, Pittman M, Eshleman JR, Diaz LA Jr, Papadopoulos N, Kinzler KW, Karchin R, Hruban RH, Vogelstein B, Lennon AM. A combination of molecular markers and clinical features improve the classification of pancreatic cysts. Gastroenterology. 2015;149:1501-1510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 328] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 23. | Faias S, Pereira L, Luís Â, Chaves P, Cravo M. Genetic testing vs microforceps biopsy in pancreatic cysts: Systematic review and meta-analysis. World J Gastroenterol. 2019;25:3450-3467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Yang D, Trindade AJ, Yachimski P, Benias P, Nieto J, Manvar A, Ho S, Esnakula A, Gamboa A, Sethi A, Gupte A, Khara HS, Diehl DL, El Chafic A, Shah J, Forsmark CE, Draganov PV. Histologic Analysis of Endoscopic Ultrasound-Guided Through the Needle Microforceps Biopsies Accurately Identifies Mucinous Pancreas Cysts. Clin Gastroenterol Hepatol. 2019;17:1587-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 25. | Tacelli M, Celsa C, Magro B, Barchiesi M, Barresi L, Capurso G, Arcidiacono PG, Cammà C, Crinò SF. Diagnostic performance of endoscopic ultrasound through-the-needle microforceps biopsy of pancreatic cystic lesions: Systematic review with meta-analysis. Dig Endosc. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 26. | Facciorusso A, Del Prete V, Antonino M, Buccino VR, Wani S. Diagnostic yield of EUS-guided through-the-needle biopsy in pancreatic cysts: a meta-analysis. Gastrointest Endosc. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 27. | Larghi A, Manfrin E, Fabbri C, Crinò SF, Correale L, Chiarello G, Barresi L, Van Velthuysen ML, Poley JW, Rahal D, Carrara S, Inzani F, Fornelli A. Interobserver agreement among expert pathologists on through-the-needle microforceps biopsy samples for evaluation of pancreatic cystic lesions. Gastrointest Endosc. 2019;90:784-792.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 28. | Barresi L, Tacelli M, Traina M. Endoscopic ultrasound through-the-needle biopsy diagnosis of a pancreatic lymphoepithelial cyst. Dig Endosc. 2019;31:210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Crinò SF, Bernardoni L, Gabbrielli A, Capelli P, Salvia R, Rusev BC, Scarpa A, Manfrin E. Beyond Pancreatic Cyst Epithelium: Evidence of Ovarian-Like Stroma in EUS-Guided Through-the-Needle Micro-Forceps Biopsy Specimens. Am J Gastroenterol. 2018;113:1059-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 30. | Barresi L, Tacelli M, Chiarello G, Tarantino I, Traina M. Mucinous cystic neoplasia with denuded epithelium: EUS through-the-needle biopsy diagnosis. Gastrointest Endosc. 2018;88:771-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Kovacevic B, Klausen P, Hasselby JP, Karstensen JG, Rift CV, Kalaitzakis E, Toxværd A, Hansen CP, Storkholm J, Hassan H, Vilmann P. A novel endoscopic ultrasound-guided through-the-needle microbiopsy procedure improves diagnosis of pancreatic cystic lesions. Endoscopy. 2018;50:1105-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 32. | Konda VJ, Meining A, Jamil LH, Giovannini M, Hwang JH, Wallace MB, Chang KJ, Siddiqui UD, Hart J, Lo SK, Saunders MD, Aslanian HR, Wroblewski K, Waxman I. A pilot study of in vivo identification of pancreatic cystic neoplasms with needle-based confocal laser endomicroscopy under endosonographic guidance. Endoscopy. 2013;45:1006-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 33. | Nakai Y, Iwashita T, Park DH, Samarasena JB, Lee JG, Chang KJ. Diagnosis of pancreatic cysts: EUS-guided, through-the-needle confocal laser-induced endomicroscopy and cystoscopy trial: DETECT study. Gastrointest Endosc. 2015;81:1204-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 134] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 34. | Napoléon B, Lemaistre AI, Pujol B, Caillol F, Lucidarme D, Bourdariat R, Morellon-Mialhe B, Fumex F, Lefort C, Lepilliez V, Palazzo L, Monges G, Filoche B, Giovannini M. A novel approach to the diagnosis of pancreatic serous cystadenoma: needle-based confocal laser endomicroscopy. Endoscopy. 2015;47:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 35. | Napoleon B, Lemaistre AI, Pujol B, Caillol F, Lucidarme D, Bourdariat R, Morellon-Mialhe B, Fumex F, Lefort C, Lepilliez V, Palazzo L, Monges G, Poizat F, Giovannini M. In vivo characterization of pancreatic cystic lesions by needle-based confocal laser endomicroscopy (nCLE): proposition of a comprehensive nCLE classification confirmed by an external retrospective evaluation. Surg Endosc. 2016;30:2603-2612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 36. | Napoleon B, Palazzo M, Lemaistre AI, Caillol F, Palazzo L, Aubert A, Buscail L, Maire F, Morellon BM, Pujol B, Giovannini M. Needle-based confocal laser endomicroscopy of pancreatic cystic lesions: a prospective multicenter validation study in patients with definite diagnosis. Endoscopy. 2019;51:825-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 37. | Krishna SG, Hart PA, Malli A, Kruger AJ, McCarthy ST, El-Dika S, Walker JP, Dillhoff ME, Manilchuk A, Schmidt CR, Pawlik TM, Porter K, Arnold CA, Cruz-Monserrate Z, Conwell DL. Endoscopic Ultrasound-Guided Confocal Laser Endomicroscopy Increases Accuracy of Differentiation of Pancreatic Cystic Lesions. Clin Gastroenterol Hepatol. 2020;18:432-440.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 38. | Krishna SG, Modi RM, Kamboj AK, Swanson BJ, Hart PA, Dillhoff ME, Manilchuk A, Schmidt CR, Conwell DL. In vivo and ex vivo confocal endomicroscopy of pancreatic cystic lesions: A prospective study. World J Gastroenterol. 2017;23:3338-3348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 39. | Krishna SG, Swanson B, Conwell DL, Muscarella P 2nd. In vivo and ex vivo needle-based confocal endomicroscopy of intraductal papillary mucinous neoplasm of the pancreas. Gastrointest Endosc. 2015;82:571-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 40. | Krishna SG, Brugge WR, Dewitt JM, Kongkam P, Napoleon B, Robles-Medranda C, Tan D, El-Dika S, McCarthy S, Walker J, Dillhoff ME, Manilchuk A, Schmidt C, Swanson B, Shah ZK, Hart PA, Conwell DL. Needle-based confocal laser endomicroscopy for the diagnosis of pancreatic cystic lesions: an international external interobserver and intraobserver study (with videos). Gastrointest Endosc. 2017;86:644-654.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 41. | Basturk O, Hong SM, Wood LD, Adsay NV, Albores-Saavedra J, Biankin AV, Brosens LA, Fukushima N, Goggins M, Hruban RH, Kato Y, Klimstra DS, Klöppel G, Krasinskas A, Longnecker DS, Matthaei H, Offerhaus GJ, Shimizu M, Takaori K, Terris B, Yachida S, Esposito I, Furukawa T; Baltimore Consensus Meeting. A Revised Classification System and Recommendations From the Baltimore Consensus Meeting for Neoplastic Precursor Lesions in the Pancreas. Am J Surg Pathol. 2015;39:1730-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 582] [Cited by in RCA: 578] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 42. | Cooper CL, O'Toole SA, Kench JG. Classification, morphology and molecular pathology of premalignant lesions of the pancreas. Pathology. 2013;45:286-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 43. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13300] [Cited by in RCA: 15476] [Article Influence: 2579.3] [Reference Citation Analysis (2)] |
| 44. | Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, Yamaguchi K, Yamao K, Matsuno S; International Association of Pancreatology. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1539] [Cited by in RCA: 1442] [Article Influence: 75.9] [Reference Citation Analysis (0)] |
| 45. | Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB, Schmidt CM, Shimizu M, Wolfgang CL, Yamaguchi K, Yamao K; International Association of Pancreatology. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1714] [Cited by in RCA: 1614] [Article Influence: 124.2] [Reference Citation Analysis (0)] |
| 46. | Gaujoux S, Brennan MF, Gonen M, D'Angelica MI, DeMatteo R, Fong Y, Schattner M, DiMaio C, Janakos M, Jarnagin WR, Allen PJ. Cystic lesions of the pancreas: changes in the presentation and management of 1,424 patients at a single institution over a 15-year time period. J Am Coll Surg. 2011;212:590-600; discussion 600-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 158] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 47. | Ånonsen K, Sahakyan MA, Kleive D, Waage A, Verbeke C, Hauge T, Buanes T, Edwin B, Labori KJ. Trends in management and outcome of cystic pancreatic lesions - analysis of 322 cases undergoing surgical resection. Scand J Gastroenterol. 2019;54:1051-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 48. | Vaalavuo Y, Antila A, Ahola R, Siiki A, Vornanen M, Ukkonen M, Sand J, Laukkarinen J. Characteristics and long-term survival of resected pancreatic cystic neoplasms in Finland. The first nationwide retrospective cohort analysis. Pancreatology. 2019;19:456-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 49. | Jones M, Zheng Z, Wang J, Dudley J, Albanese E, Kadayifci A, Dias-Santagata D, Le L, Brugge WR, Fernandez-del Castillo C, Mino-Kenudson M, Iafrate AJ, Pitman MB. Impact of next-generation sequencing on the clinical diagnosis of pancreatic cysts. Gastrointest Endosc. 2016;83:140-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 109] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 50. | Rosenbaum MW, Jones M, Dudley JC, Le LP, Iafrate AJ, Pitman MB. Next-generation sequencing adds value to the preoperative diagnosis of pancreatic cysts. Cancer Cytopathol. 2017;125:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 51. | Basar O, Yuksel O, Yang DJ, Samarasena J, Forcione D, DiMaio CJ, Wagh MS, Chang K, Casey B, Fernandez-Del Castillo C, Pitman MB, Brugge WR. Feasibility and safety of microforceps biopsy in the diagnosis of pancreatic cysts. Gastrointest Endosc. 2018;88:79-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 52. | Mittal C, Obuch JC, Hammad H, Edmundowicz SA, Wani S, Shah RJ, Brauer BC, Attwell AR, Kaplan JB, Wagh MS. Technical feasibility, diagnostic yield, and safety of microforceps biopsies during EUS evaluation of pancreatic cystic lesions (with video). Gastrointest Endosc. 2018;87:1263-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 53. | Yang D, Samarasena JB, Jamil LH, Chang KJ, Lee D, Ona MA, Lo SK, Gaddam S, Liu Q, Draganov PV. Endoscopic ultrasound-guided through-the-needle microforceps biopsy in the evaluation of pancreatic cystic lesions: a multicenter study. Endosc Int Open. 2018;6:E1423-E1430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 54. | Zhang ML, Arpin RN, Brugge WR, Forcione DG, Basar O, Pitman MB. Moray micro forceps biopsy improves the diagnosis of specific pancreatic cysts. Cancer Cytopathol. 2018;126:414-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 55. | Crinò SF, Bernardoni L, Brozzi L, Barresi L, Malleo G, Salvia R, Frulloni L, Sina S, Parisi A, Remo A, Larghi A, Gabbrielli A, Manfrin E. Association between macroscopically visible tissue samples and diagnostic accuracy of EUS-guided through-the-needle microforceps biopsy sampling of pancreatic cystic lesions. Gastrointest Endosc. 2019;90:933-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |