Published online Jun 14, 2020. doi: 10.3748/wjg.v26.i22.3056
Peer-review started: January 21, 2020
First decision: February 27, 2020
Revised: March 26, 2020
Accepted: May 13, 2020
Article in press: May 13, 2020
Published online: June 14, 2020
Processing time: 144 Days and 17.4 Hours
Acute pancreatitis (AP) is a pancreatic inflammatory disorder that is commonly complicated by extrapancreatic organ dysfunction. Dachengqi decoction (DCQD) has a potential role in protecting the extrapancreatic organs, but the optimal oral administration time remains unclear.
To screen the appropriate oral administration time of DCQD for the protection of extrapancreatic organs based on the pharmacokinetics and pharmacodynamics of AP rats.
This study consisted of two parts. In the first part, 24 rats were divided into a sham-operated group and three model groups. The four groups were intragastrically administered with DCQD (10 g/kg) at 4 h, 4 h, 12 h, and 24 h postoperatively, respectively. Tail vein blood was taken at nine time points after administration, and then the rats were euthanized and the extrapancreatic organ tissues were immediately collected. Finally, the concentrations of the major DCQD components in all samples were detected. In the second part, 84 rats were divided into a sham-operated group, as well as 4 h, 12 h, and 24 h treatment groups and corresponding control groups (4 h, 12 h, and 24 h control groups). Rats in the treatment groups were intragastrically administered with DCQD (10 g/kg) at 4 h, 12 h, and 24 h postoperatively, respectively, and rats in the control groups were administered with normal saline at the same time points. Then, six rats from each group were euthanized at 4 h and 24 h after administration. Serum amylase and inflammatory mediators, and pathological scores of extrapancreatic organ tissues were evaluated.
For part one, the pharmacokinetic parameters (C max, T max, T 1/2, and AUC 0 → t) of the major DCQD components and the tissue distribution of most DCQD components were better when administering DCQD at the later (12 h and 24 h) time points. For part two, delayed administration of DCQD resulted in lower IL-6 and amylase levels and relatively higher IL-10 levels, and pathological injury of extrapancreatic organ tissues was slightly less at 4 h after administration, while the results were similar between the treatment and corresponding control groups at 24 h after administration.
Delayed administration of DCQD might reduce pancreatic exocrine secretions and ameliorate pathological injury in the extrapancreatic organs of AP rats, demonstrating that the late time is the optimal dosing time.
Core tip: This study is the first to assess the optimal dosing time of Dachengqi decoction for protecting the extrapancreatic organs of acute pancreatitis rats. Based on the pharmacokinetic and pharmacodynamic experiments, we proved that delayed administration may be more appropriate in alleviating damage to multiple extrapancreatic organs in acute pancreatitis rats. In addition, this is the first time we have tried to compare the efficacy at different times after administration, and we found that a single-dose administration of the decoction leads to a rapid onset of relief but no steady-state effect, suggesting that multiple-dose administration should be considered.
- Citation: Yao JQ, Zhu L, Miao YF, Zhu L, Chen H, Yuan L, Hu J, Yi XL, Wu QT, Yang XJ, Wan MH, Tang WF. Optimal dosing time of Dachengqi decoction for protection of extrapancreatic organs in rats with experimental acute pancreatitis. World J Gastroenterol 2020; 26(22): 3056-3075
- URL: https://www.wjgnet.com/1007-9327/full/v26/i22/3056.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i22.3056
Acute pancreatitis (AP) is an inflammatory pancreatic disorder associated with substantial morbidity and mortality[1]. The overall mortality for AP can range from 1% to 15% but can reach 30% to 50% in severe cases[2-4]. The severe form of AP is commonly complicated by multiple extrapancreatic organ dysfunction, which can impact the heart, liver, lungs, kidneys, and intestines, leading to a significant increase in AP mortality[5,6]. Currently, the primary treatment for severe AP (SAP) is limited to supportive care and treatment of complications[7], and the application of traditional Chinese medicine (TCM) has a potential role in reducing AP mortality[8].
Dachengqi decoction (DCQD) was first recorded in “Shang-Han-Lun” and is one of the four classics of TCM, consisting of Dahuang (Rheum palmatum L.), Houpu (Magnolia henryi Dunn.), Zhishi (Citrus aurantium L.), and Mangxiao (Natrii Sulfas). DCQD has been widely used to alleviate AP for over 40 years in China[9]. Recent clinical research has shown that DCQD could help to restore the recovery of intestinal mucosal permeability, relieve intra-abdominal hypertension, decrease the incidence of multiple organ dysfunction syndrome (MODS), and shorten the length of hospitalization in AP patients[10-12]. Some animal experiments have demonstrated that DCQD could increase cell viability, promote the transformation of injured acinar cells from necrosis to apoptosis, and protect the pancreas from injury in vivo and in vitro[13,14]. Our previous studies have shown that DCQD could protect multiple organs (pancreas, lungs, kidneys, and intestines) from injury caused by excessive inflammatory responses and confirmed that the anti-inflammatory effects of DCQD on these organs were associated with its tissue distribution[9,15,16]. Through these studies, the therapeutic mechanism of DCQD has been further explored; however, there are few studies on the impact of different administration times on prognosis.
The timing of oral administration of TCM in AP patients has been a subject of much discussion. In China, DCQD is commonly used without food to relieve symptoms in AP patients in the early stage of AP[17]. Current AP guidelines recommend early enteral nutrition but do not provide specific guidance on the optimal time to take Chinese herbal medicine orally[18]. Our previous study proved that the oral dosing time of DCQD plays a role in the absorption of its components and its pancreatic tissue distribution. Further, administering DCQD too early may aggravate the pathological damage to the pancreas[19]. However, the effects of administration time on multiple extrapancreatic organs in AP rats are still unclear. In the current study, we evaluated the pharmacokinetics of the main components of DCQD and the related pharmacodynamics effects in heart, liver, lung, kidney, and intestinal tissues following administration of DCQD at different time points in AP rats. Based on these findings, we can suggest a better dosing time for the protection of extrapancreatic organs during SAP.
Specific-pathogen free male Sprague-Dawley rats weighing 280-300 g (aged 90 ± 5 d) were purchased from Chengdu Dashuo Experimental Animal Co., Ltd. (Chengdu, China). All the animals were raised under the same conditions, which are described in our previous article[20]. All experiments were reviewed and approved by the Institution Animal Care and Use Committee of Sichuan University (Chengdu, China; protocol number, 2019003A). After one week of adaptive feeding, the animals were fasted for 12 h before induction of the model (Supplementary Material).
The Chinese herbs used in this experiment were all drug powders obtained after spray drying and were purchased from Chengdu Green Herbal Pharmaceutical Co., Ltd. (Chengdu, China). The four drug powders, i.e., Dahuang (No. 1806013), Houpu (No. 1807029), Zhishi (No. 1810043), and Mangxiao (No. 1808009), were mixed in standard proportions (12:24:12:9, respectively) by weight and stirred with sterile double-distilled water to a concentration of 1 g/mL. This DCQD solution was stored in a 37 °C warm water bath for 30 min until use. The reagents and instruments used in this experiment are described in further detail in Supplementary Material.
This study consisted of two parts. In the first part, rats were divided into four groups (n = 6 per group) randomly: One sham-operated group (SOG1) and three model groups (MG1, MG2, and MG3). After the rats were anesthetized with 2% sodium pentobarbital (intraperitoneal injection, 40 mg/kg), the AP model was induced as described previously[14]. Briefly, 3% sodium taurocholate (1 mL/kg) was retrogradely poured into the biliopancreatic duct; the speed of administration was controlled by a micro-infusion pump at 0.1 mL/min. SOG1 underwent the same procedure but with saline. The rats were fasted and provided with water ad libitum after the operation. The four groups were orally administered with DCQD (10 g/kg) at 4h, 4 h, 12 h, and 24 h postoperatively, respectively. After administration, 0.5 mL of tail vein blood was taken at 1/6 h, 1/3 h, 2/3 h, 1 h, 2 h, 4 h, 8 h, 12 h, and 24 h, followed by administration of high dose 2% sodium pentobarbital for euthanasia (intraperitoneal injection, 200 mg/kg). After the last blood collection, heart, liver, lung, kidney, and intestinal tissues of each rat were collected and homogenized. Finally, the plasma and tissue samples were centrifuged (3000 r/min, 7 min) to obtain the supernatant and then placed in a -80°C refrigerator for further testing.
In the second part, the rats were randomly divided into a sham-operated group (SOG2), as well as three treatment groups (4 h-TG, 12 h-TG and 24 h-TG), and three corresponding control groups (4 h-CG, 12 h-CG, and 24 h-CG), with 12 rats in each group. The AP model and SOG2 were induced as described in part one. All rats were fasted and provided with water ad libitum after the operation. The rats in the treatment groups were administered with DCQD (10 g/kg) at 4 h, 12 h, and 24 h postoperatively, respectively. The rats in each control group were administered with normal saline at the same point. After a single dose, six rats from each group were euthanized at 4 h and 24 h. Meanwhile, heart blood was collected and centrifuged (3000 r/min, 7 min) to detect the IL-6, IL-10, and amylase concentrations, and extrapancreatic organ (heart, liver, lung, kidney, and intestine) sampling was performed for pathological damage assessment.
The concentrations of the ten main components of DCQD (emodin, aloe-emodin, rhein, chrysophanol, rheochrysidin, hesperidin, naringenin, naringin, magnolol, and honokiol) in serum samples and visceral organ tissues were measured by high-performance liquid chromatography-tandem mass spectroscopy (HPLC-MS/MS) as described previously[21]. All system configurations and operating conditions are described in Supplementary Material, and all operations were performed following the manufacturer’s instructions. Briefly, serum or tissue homogenate samples were extracted with ethyl acetate after adding the internal standard working fluid and hydrochloric acid buffer. Then, the mixtures were vortexed, centrifuged (3000 rpm, 7 min), warm water bathed, and incubated with the double-solvents. Finally, 20 μL of the treated supernatant was taken and tested with the HPLC-MS/MS system. The chromatographic peak areas of the serum and tissue samples, as well as the internal standard (ibuprofen), were analyzed with Analyst 1.4.2 software, and then standard curves were plotted and the concentrations of our serum and tissue samples were calculated based on the standard curves.
After obtaining the serum concentration data of each component of DCQD by HPLC-MS/MS, the corresponding pharmacokinetic parameters were determined using DAS 2.0.1 (Drug and Statistics, China), a statistical software for pharmacokinetics compiled by the Chinese Pharmacological Society. The following results were recorded and compared: The peak concentration (C max), the time to reach the peak concentration (T max), the elimination half-life (T 1/2), and the area under the concentration-time curve (AUC 0 → t).
Heart blood was collected and centrifuged (3000 r/min, 7 min) for amylase, IL-6, and IL-10 detection. Amylase levels were measured via a HITACHI automatic biochemical analyzer, as shown in Supplementary Material. IL-6 and IL-10 levels were determined with enzyme-linked immunosorbent assay kits listed in Supplementary Material.
All tissue samples were fixed (10% neutral formalin), embedded (paraffin), and sliced (5 μm) for hematoxylin and eosin staining. The stained sections were examined with an upright microscope and scored for pathological damage in a blinded manner. The degree of lung tissue damage was quantified based on a previously described scoring system[22] (0-4 points: Thickness of the alveolar wall, edema, congestion, and neutrophil infiltration in the airspace); intestinal histological damage was scored according to Wirtz et al[23]; and a previously established scoring system[9,15,24] was used to assess the severity of heart, liver, and kidney damage, including edema, neutrophil infiltration, necrosis, and hemorrhage (scores ranging from 0 (absent) to 4 (extensive)). The final histopathology score was the average of the composite scores for each component.
Graph Pad Prism 7.0 (Supplementary Material) software was used for the data analyses. All data passed the normality test and are expressed as the mean ± standard deviation. In the first part of the study, one-way analysis of variance (parametric or non-parametric) followed by pairwise comparisons was performed for statistical analysis. In part two, the Student’s t-test was employed to measure the differences of pharmacodynamic parameters between each treatment and corresponding control group. The level of statistical significance was set at P < 0.05.
In the current study, a total of ten components of DCQD were detected in tissue samples, including five components (emodin, aloe-emodin, rhein, chrysophanol, and rheochrysidin) from Dahuang, three components (naringin, naringenin, and hesperidin) from Zhishi, and two components (magnolol and honokiol) from Houpu. We failed to determine the main ingredient of Mangxiao because it is not absorbed in the small intestine. Only eight of them were successfully detected in serum at all time points after oral administration, so we only successfully fitted the concentration-time curve of eight monomers.
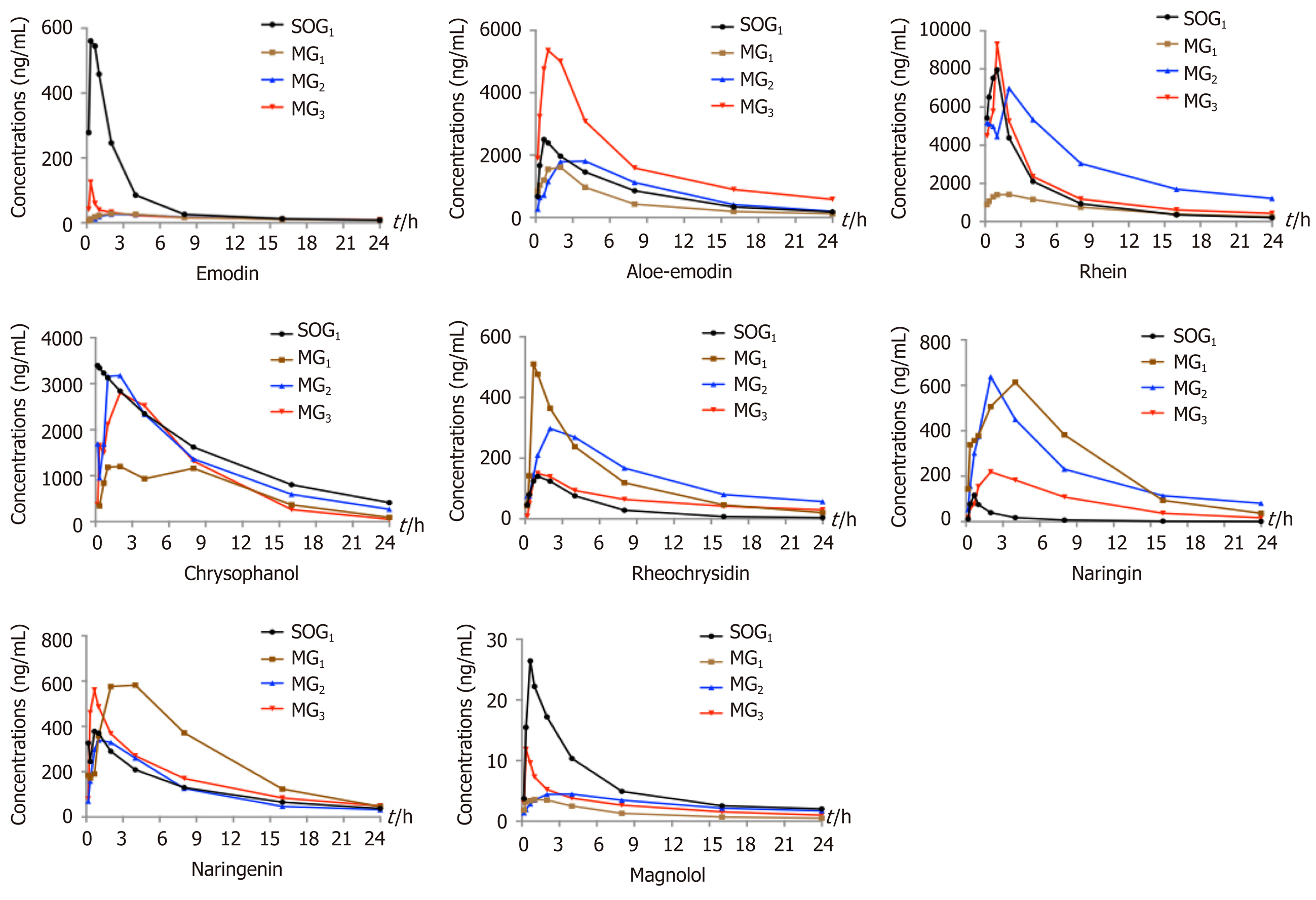
Comparison of plasma pharmacokinetic parameters: Compared with the SOG1, the T max of seven components (emodin, rhein, rheochrysidin, chrysophanol, naringin, naringenin, and magnolol) in MG1 was delayed. The C max values of emodin, aloe-emdin, rhein, chrysophanol, and magnolol in MG1 were lower, while the C max values of rheochrysidin, naringin, and naringenin were higher (Figure 1). Meanwhile, the AUC 0 → t values of chrysophanol, emodin, rhein, and magnolol in MG1 were smaller than those in SOG1, while those of naringin and naringenin were larger (P < 0.05; Table 1). The T 1/2 values of chrysophanol and naringin in MG1 were significantly shorter than those in SOG1 (P < 0.05; Table 1).
| Parameter | SOG1 | MG1 | MG2 | MG3 |
| Emodin | ||||
| AUC 0-t | 1605.0 ± 1029.5 | 359.3 ± 187.1a | 362.5 ± 67.4 | 427.5 ± 299.3 |
| T 1/2z | 5.2 ± 1.4 | 18.9 ± 12.5a | 9.0 ± 3.2 | 22.6 ± 9.0 |
| T max | 0.6 ± 0.3 | 3.5 ± 1.6a | 3.5 ± 1.6 | 0.3 ± 0dc |
| C max | 620.733 ± 477.0 | 34.1 ± 22.5a | 29.8 ± 8.8 | 125.2 ± 90.9dc |
| Aloe-emodin | ||||
| AUC 0-t | 19206.1 ± 7275.8 | 11860.0 ± 4393.20 | 20181.9 ± 7660.2 | 37303.2 ± 13970.9d |
| T 1/2z | 7.0 ± 1.9 | 5.4 ± 2.1 | 6.4 ± 0.8 | 8.1 ± 5.4 |
| T max | 1.6 ± 1.6 | 1.4 ± 0.7 | 3 ± 1.2 | 0.7 ± 0.3c |
| C max | 3797.0 ± 727.7 | 2203.9 ± 1156.1 | 2398.4 ± 708.9 | 8349.5 ± 3969.4dc |
| Rhein | ||||
| AUC 0-t | 31571.7 ± 8961.8 | 13308.8 ± 8524.4a | 61966.5 ± 18335.3b | 38030.3 ± 22227.7dc |
| T 1/2z | 4.1 ± 1.5 | 6.5 ± 2.3 | 4.8 ± 0.8 | 20.8 ± 10.0dc |
| T max | 0.8 ± 0.2 | 6.5 ± 2.3a | 1.7 ± 0.7b | 1.6 ± 1.6d |
| C max | 11807.5 ± 2771.1 | 2204.3 ± 353.4a | 9245.7 ± 1284.0b | 11113.2 ± 5342.5d |
| Chrysophanol | ||||
| AUC 0-t | 29977.3 ± 7570.0 | 15682.3 ± 5428.0a | 28337.7 ± 8880.5b | 26358.6 ± 4312.1d |
| T 1/2z | 5.7 ± 1.5 | 4.8 ± 1.0a | 4.7 ± 10.0 | 6.9 ± 4.5 |
| T max | 1.4 ± 0.7 | 3.3 ± 3.2a | 1.5 ± 0.6b | 1.8 ± 1.7 |
| C max | 7766.1 ± 2796.1 | 2018.9 ± 1227.5a | 4153.7 ± 2621.4 | 4318.1 ± 2458.9 |
| Rheochrysidin | ||||
| AUC 0-t | 867.505 ± 867.5 | 3665.5 ± 1693.3a | 3032.0 ± 1011.6 | 1510.264 ± 727.3d |
| T 1/2z | 6.1 ± 1.0 | 4.3 ± 1.2 | 4.3 ± 1.6 | 9.4 ± 1.5dc |
| T max | 1 ± 0.7 | 1.9 ± 1.5 | 2.2 ± 1.4 | 1.5 ± 0.6 |
| C max | 197.5 ± 64.0 | 728.8 ± 387.4a | 489.8 ± 176.2 | 172.9 ± 110.7d |
| Naringin | ||||
| AUC 0-t | 353.0 ± 261.0 | 6296.0 ± 1271.2a | 5449.1 ± 2183.0 | 2188.5 ± 752.5dc |
| T 1/2z | 6.4 ± 0.8 | 4.1 ± 0.7a | 5.4 ± 1.3 | 5.8 ± 5.8 |
| T max | 0.8 ± 0.2 | 1.8 ± 1.7 | 2.3 ± 1.3 | 2.5 ± 1 |
| C max | 125.6 ± 121.0 | 806.2 ± 730.9a | 682.4 ± 380.4 | 257.6 ± 26.4d |
| Naringenin | ||||
| AUC 0-t | 3064.2 ± 829.9 | 6344.8 ± 1199.2a | 3051.6 ± 932.6b | 3926.0 ± 1014.2d |
| T 1/2z | 7.368 ± 1.5 | 4.5 ± 1.7 | 10.9 ± 3.4b | 7.8 ± 1.4 |
| T max | 0.9 ± 0.7 | 2.3 ± 1.0 | 1.6 ± 1.6 | 0.6 ± 0.1d |
| C max | 634.2 ± 228.6 | 805.3 ± 113.8 | 488.9 ± 316.7 | 581.1 ± 161.7 |
| Magnolol | ||||
| AUC 0-t | 143.8 ± 35.0 | 34.8 ± 8.0a | 69.4 ± 14.8 | 64.2 ± 10.8 |
| T 1/2z | 9.0 ± 6.2 | 10.6 ± 1.4 | 13.9 ± 2.5 | 24.5 ± 9.1d |
| T max | 0.7 ± 0.3 | 0.8 ± 0.8 | 3.5 ± 1b | 0.5 ± 0.3c |
| C max | 30.6 ± 19.8 | 5.3 ± 1.7a | 5.5 ± 0.9 | 12.8 ± 3.1 |
In the three model groups, we found that the T max of emodin, aloe-emodin, rhein, chrysophanol, and naringenin in MG1 and emodin, aloe-emodin, rheochrysidin, naringenin, and magnolol in MG2 were delayed compared with MG3 (Figure 1). The C max values of emodin, aloe-emodin, rhein, chrysophanol, and magnoolol in MG1 were lower compared with those in MG3, while the values of rheochrysidin were higher (Figure 1). The AUC 0 → t values of chrysophanol, aloe-emodin, and rhein in MG1 were smaller than those in MG2 or MG3 (P < 0.05; Table 1), and the AUC 0 → t values of aloe-emodin, chrysophanol, and rhein in MG3 were larger than those in MG1 or MG2 (P < 0.05; Table 1). On the contrary, the AUC 0 → t values of naringin and naringenin in MG1 were higher compared with those in MG3 (P < 0.05; Table 1). Besides, the T 1/2 values of rhein, naringenin, and magnolol in MG1 were shorter than those in MG2 or MG3 (P < 0.05; Table 1).
Comparison of drug concentrations in tissues: In heart tissue samples, the concentrations of emodin, aloe-emodin, naringin, and honokiol in MG1 were lower than those in SOG1 (P < 0.05; Table 2). Additionally, in MG1 and MG2, the concentrations of emodin, aloe-emodin, honokiol, magnolol, naringin, naringenin, and hesperidin were lower than those in MG3 (P < 0.05; Table 2).
| Parameter | SOG1 | MG1 | MG2 | MG3 |
| Heart | ||||
| Emodin | 3.21 ± 0.13 | 1.25 ± 0.09ad | 2.24 ± 0.14c | 2.45 ± 0.21 |
| Aloe-emodin | 56.72 ± 5.67 | 21.96 ±7.21ad | 28.45 ± 8.47 | 31.53 ± 1.34 |
| Rhein | 0 | 0 | 0 | 0 |
| Chrysophanol | 15.57 ± 4.63 | 6.43 ± 1.2a | 5.65 ± 2.4 | 7.59 ± 1.98 |
| Rheochrysidin | 1.93 ± 0.89 | 0 | 0 | 2.41 ± 3.67 |
| Naringin | 40.36 ± 1.38 | 0.85 ± 0.02ad | 9.62 ± 0.37c | 10.49 ± 0.21 |
| Naringenin | 420.68 ± 67.81 | 160.09 ± 12.61a | 924.79 ± 34.67c | 270.25 ± 48.51 |
| Hesperidin | 12.22 ± 0.94 | 5.05 ± 1.2a | 10.33 ± 4.3 c | 5.24 ± 1.98 |
| Magnolol | 0.21 ± 0.04 | 0.18 ± 0.02 | 0.02 ± 0.01 c | 0.21 ± 0.10 |
| Honokiol | 0.59 ± 0.07 | 0.24 ± 0.04ad | 0.9 0 ± 0.07 c | 0.73 ± 0.09 |
| Liver | ||||
| Emodin | 14.24 ± 6.21 | 7.27 ± 2.37a | 3.97 ± 1.67 c | 8.36 ± 4.23 |
| Aloe-emodin | 506.09 ± 32.78 | 438.4 ± 123.47 | 122.87 ± 79.23 c | 383.40 ± 101.31 |
| Rhein | 45.58 ± 63.23 | 0 | 0 | 0 |
| Chrysophanol | 132.65 ± 7.43 | 40.38 ± 20.81ad | 17.10 ± 3.24 c | 18.47 ± 0.8 |
| Rheochrysidin | 20.27 ± 0.98 | 1.42 ± 0.14ad | 3.39 ± 0.29 c | 0.18 ± 0.23 |
| Naringin | 148.20 ± 8.94 | 13.19 ± 10.23a | 27.07 ± 9.81 | 23.49 ± 8.67 |
| Naringenin | 1707.40 ± 99.46 | 1459.04 ± 98.67ad | 692.88 ± 102.43c | 1855.76 ± 79.23 |
| Hesperidin | 60.03 ± 18.87 | 6.53 ± 1.23ad | 2.27 ± 0.94c | 13.65 ± 0.23 |
| Magnolol | 2.88 ± 0.41 | 3.34 ± 1.64 | 1.62 ± 0.72 c | 2.42 ± 0.23 |
| Honokiol | 3.00 ± 0.78 | 2.02 ± 0.78 | 1.64 ± 0.24 | 3.03 ± 0.31 |
| Lung | ||||
| Emodin | 3.87 ± 1.43 | 2.08 ± 0.98 | 2.99 ± 0.43 | 3.1 ± 1.02 |
| Aloe-emodin | 66.98 ± 33.62 | 56.88 ± 20.45d | 34.94 ± 13.67 c | 88.12 ± 23.21 |
| Rhein | 143.74 ± 73.64 | 164.27 ± 64.81 | 72.59 ± 42.31 c | 171.52 ± 57.84 |
| Chrysophanol | 19.05 ± 6.39 | 13.51 ± 9.43 | 15.1 ± 3.57 | 16.42 ± 4.81 |
| Rheochrysidin | 1.06 ± 0.17 | 0.18 ± 0.23a | 0.42 ± 0.02c | 0.23 ± 0.01 |
| Naringin | 12.55 ± 1.67 | 6.00 ± 3.2ad | 8.37 ± 2.37c | 24.46 ± 4.17 |
| Naringenin | 422.09 ± 203.92 | 601.12 ± 176.64 | 567.53 ± 123.67 | 427.36 ± 204.81 |
| Hesperidin | 58.67 ± 15.81 | 22.74 ± 10.13ad | 33.74 ± 10.32c | 77.35 ± 34.61 |
| Magnolol | 0.34 ± 0.19 | 0.35 ± 0.23 | 0.35 ± 0.04 | 0.46 ± 0.17 |
| Honokiol | 1.43 ± 0.19 | 1.42 ± 0.16a | 0.95 ± 0.02 c | 0.82 ± 0.04 |
| Kidney | ||||
| Emodin | 21.15 ± 10.28 | 27.75 ± 14.21 | 11.98 ± 5.67 c | 26.31 ± 13.42 |
| Aloe-emodin | 1328.18 ± 159.73 | 279.04 ± 100.04ad | 133.62 ± 92.43c | 720.74 ± 143.67 |
| Rhein | 154.59 ± 12.34 | 2.49 ± 0.98ad | 37.72 ± 1.34c | 101.55 ± 2.78 |
| Chrysophanol | 108.89 ± 7.64 | 101.18 ± 7.84ad | 46.84 ± 6.23c | 124.76 ± 4.32 |
| Rheochrysidin | 16.25 ± 2.79 | 15.23 ± 3.54 | 6.13 ± 0.98c | 13.57 ± 1.43 |
| Naringin | 208.08 ± 8.94 | 18.44 ± 3.64ad | 0.94 ± 0.17c | 59.9 ± 7.63 |
| Naringenin | 2144.99 ± 147.81 | 2506.95 ± 100.07ad | 2360.21 ± 143.64c | 1939.2 ± 127.68 |
| Hesperidin | 48.87 ± 4.81 | 9.13 ± 1.67a | 8.67 ± 1.32 | 9.90 ± 0.89 |
| Magnolol | 0.81 ± 0.2 | 0.52 ± 0.12ad | 0.30 ± 0.03 c | 1.14 ± 0.14 |
| Honokiol | 2.76 ± 0.31 | 1.56 ± 0.44ad | 1.35 ± 0.23c | 2.63 ± 0.67 |
| Intestine | ||||
| Emodin | 94.43 ± 37.23 | 19.73 ± 6.72ad | 88.3 ± 24.23 | 122.66 ± 72.61 |
| Aloe-emodin | 4515.56 ± 342.67 | 1447.33 ± 109.73ad | 975.33 ± 152.62c | 2202.32 ± 143.76 |
| Rhein | 214.49 ± 56.72 | 830.8 ± 48.63ad | 130.67 ± 65.67 | 209.09 ± 100.3 |
| Chrysophanol | 714.88 ± 300.68 | 290.97 ± 110.37ad | 410.67 ± 203.3c | 736.46 ± 45.67 |
| Rheochrysidin | 161.78 ± 80.93 | 37.9 ± 2.34ad | 27.59 ± 17.61c | 191.14 ± 59.67 |
| Naringin | 2771.61 ± 67.81 | 604.19 ± 134.6ad | 678.67 ± 57.63c | 1442.36 ± 100.75 |
| Naringenin | 1804.34 ± 307.16 | 1365.3 ± 153.62ad | 1077.59 ± 174.63 c | 3695.62 ± 483.54 |
| Hesperidin | 1156.54 ± 154.63 | 241.58 ± 72.61ad | 265.59 ± 100.32 c | 581.04 ± 201.63 |
| Magnolol | 17.13 ± 7.34 | 18.78 ± 6.43d | 18.15 ± 7.81c | 35.5 ± 5.67 |
| Honokiol | 18.7 ± 2.71 | 24.27 ± 2.43ad | 17.14 ± 3.1c | 45.48 ± 4.58 |
In liver tissue samples, the concentrations of six major components (emodin, rhein, rheochrysidin, naringin, naringenin, and hesperidin) of DCQD were obviously lower in MG1 than in SOG1 (P < 0.05; Table 2). Compared with MG3, the concentrations of emodin, aloe-emodin, naringin, magnolol, and hesperidin were lower in MG1 and MG2 (P < 0.05; Table 2).
In lung tissue samples, the concentrations of rheochrysidin, naringin, and hesperidin were lower in MG1 than in SOG1 (P < 0.05; Table 2). Meanwhile, in MG1 and MG2, the concentrations of aloe-emodin, rhein, naringin, and hesperidin were lower than those in MG3, while the concentration of honokiol was higher (P < 0.05; Table 2).
In kidney tissue samples, the concentrations of seven major components (emodin, rhein, chrysophanol, magnolol, honokiol, naringin, and hesperidin) of DCQD were clearly lower in MG1 than in SOG1 (P < 0.05; Table 2). Compared with MG3, the concentrations of aloe-emodin, rhein, chrysophanol, naringin, magnolol, and honokiol were significantly lower in MG1 and MG2; however, that of naringenin was higher (P < 0.05; Table 2).
In intestinal tissue samples, the concentrations of five major components (emodin, rhein, chrysophanol, aloe-emodin, rheochrysidin, naringin, naringenin, and hesperidin) of DCQD were significantly lower in MG1 than in SOG1 (P < 0.05; Table 2). Additionally, the concentrations of all major components of DCQD, except rhein, were significantly lower in the intestinal tissues in MG1 and MG2 than in MG3 (P < 0.05; Table 2).
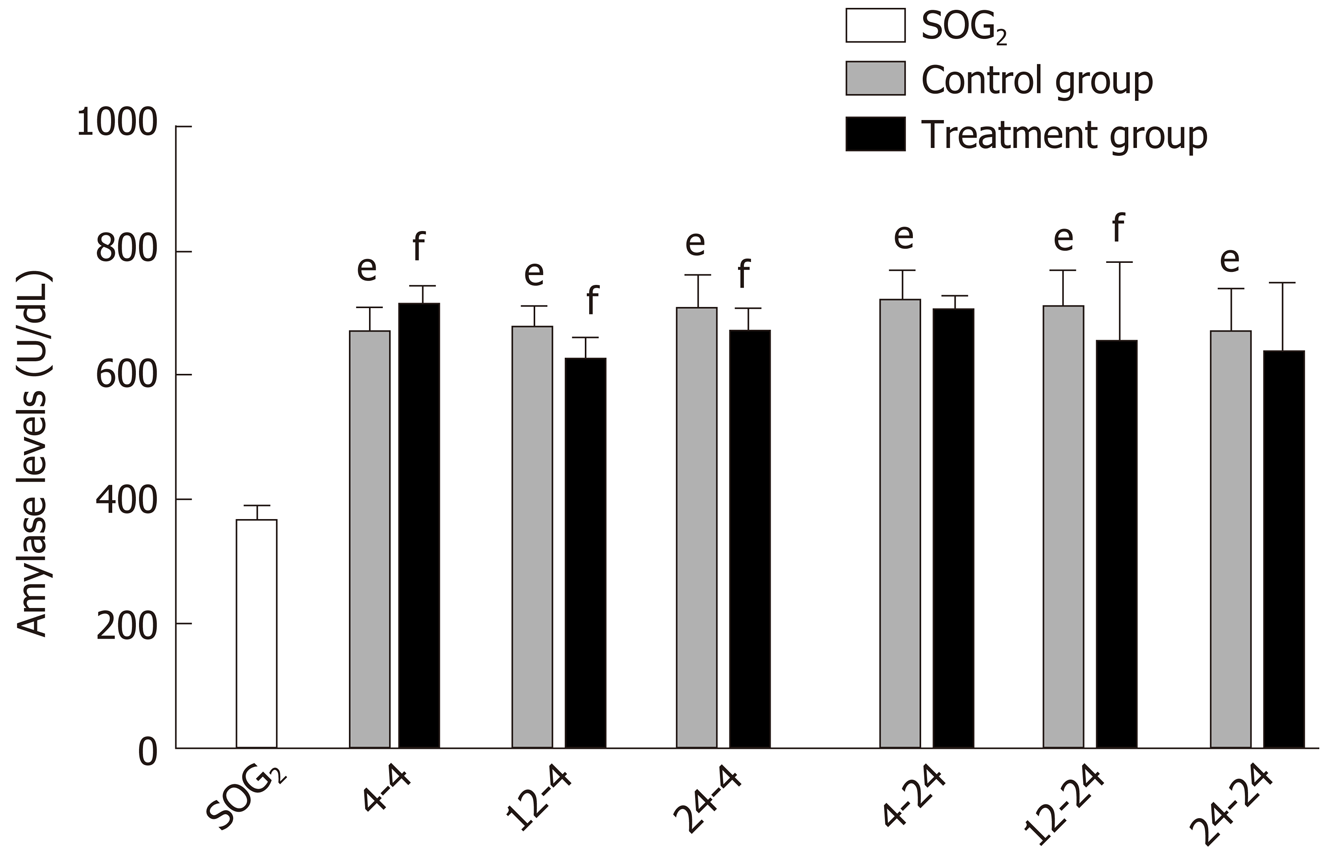
Delayed oral administration time of DCQD reduces amylase levels: Compared with SOG2, the amylase levels of all control groups were higher (P < 0.05; Figure 2), indicating that we successfully induced the AP model in rats. When rats were euthanized at 4 h after intragastric administration, the amylase levels in 4 h-TG were obviously higher than those in 4 h-CG (P < 0.05; Figure 2); however, the amylase levels in the other two treatment groups (12 h-TG and 24 h-TG) were lower compared with their respective control groups (12 h-CG and 24 h-CG, respectively) (P < 0.05; Figure 2). When rats were euthanized at 24 h after intragastric administration, the amylase levels in 12 h-TG were relatively lower than those in 12 h-CG (P < 0.05; Figure 2); however, none of the other treatment groups showed significant differences in levels compared with their corresponding control groups.
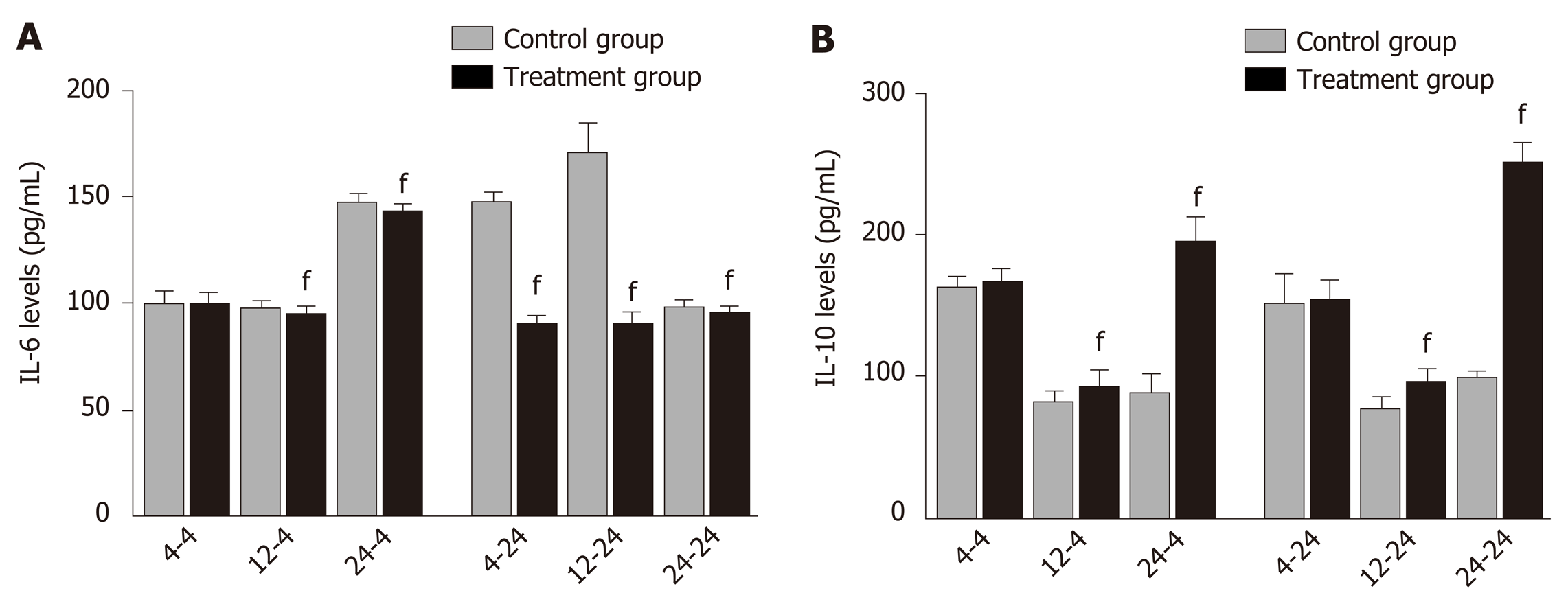
Delayed oral administration of DCQD inhibits IL-6 expression and increased IL-10 expression: When rats were euthanized at 4 h after intragastric administration, the IL-6 and IL-10 levels in 4 h-TG were similar to those in 4 h-CG (Figure 3), but the IL-6 levels in 12 h-TG and 24 h-TG were both significantly lower than those in their corresponding control groups (12 h-CG and 24 h-CG) (P < 0.05; Figure 3A). Meanwhile, the IL-10 levels were higher (P < 0.05, Figure 3B). When rats were euthanized at 24 h after intragastric administration, the IL-6 levels in each treatment group were significantly lower than those in their corresponding control group (P < 0.05; Figure 3A), and the IL-10 levels in 12 h-TG and 24 h-TG were higher than those in their respective control groups (12 h-CG and 24 h-CG) (P < 0.05; Figure 3B).
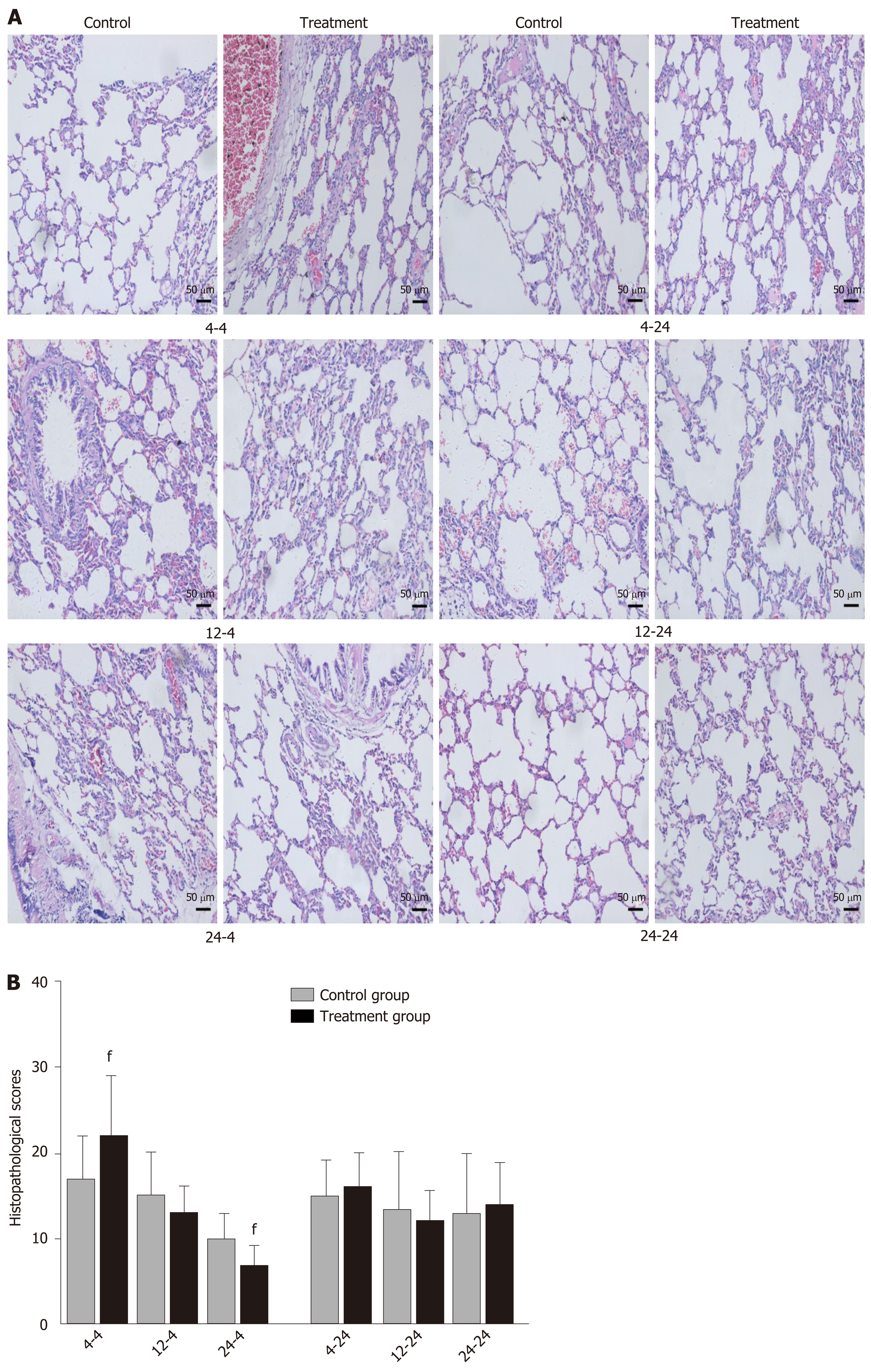
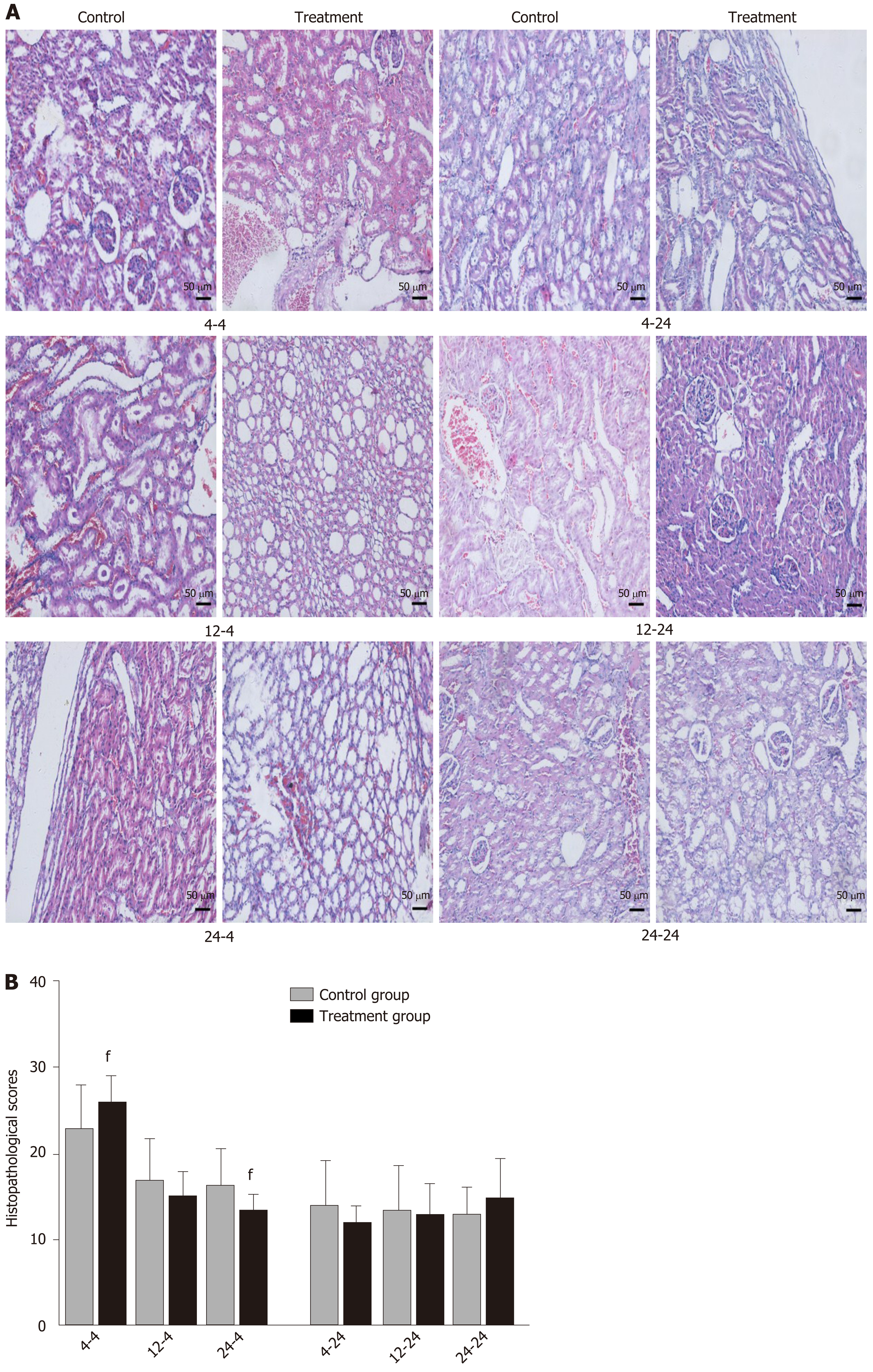
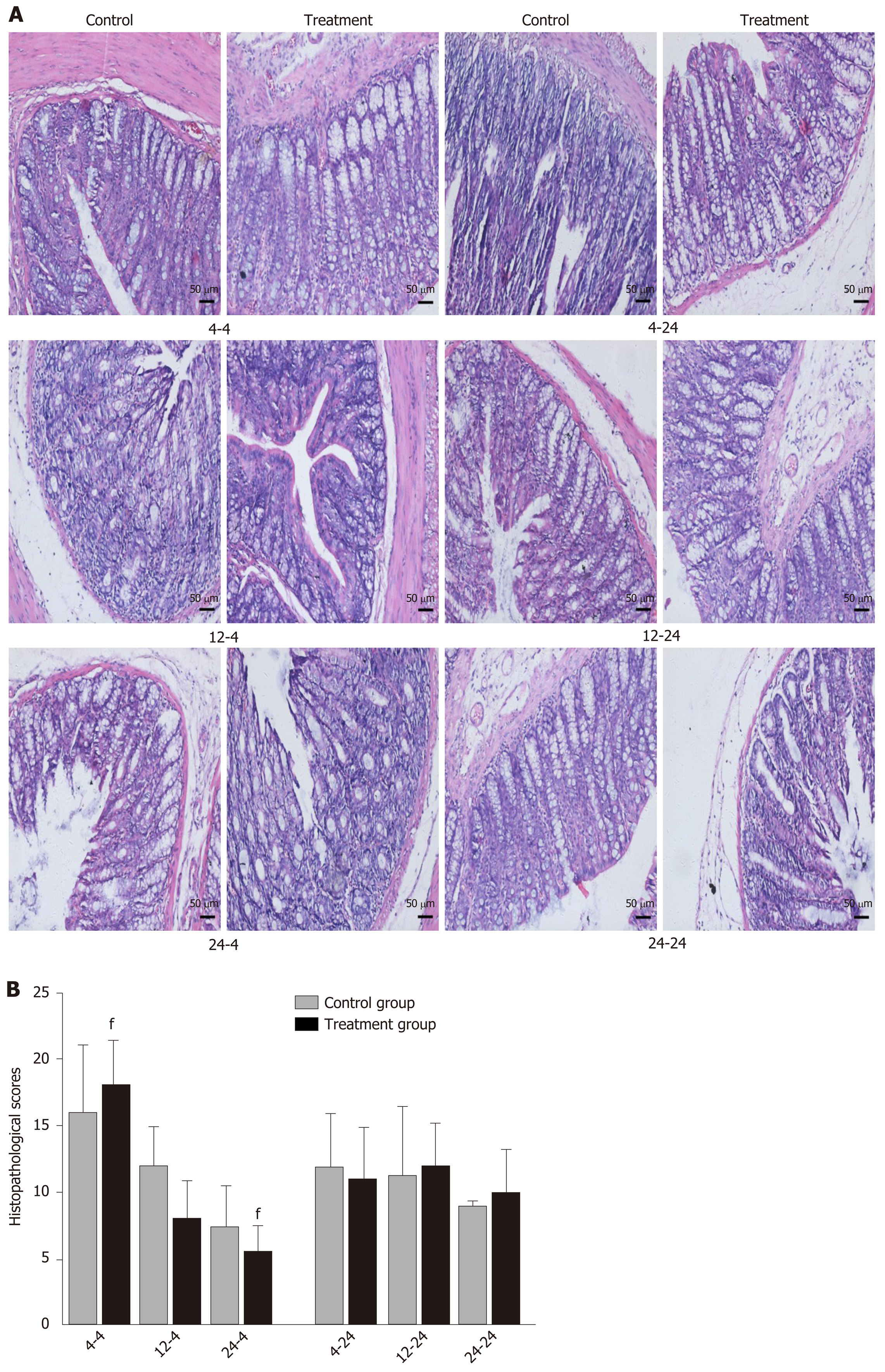
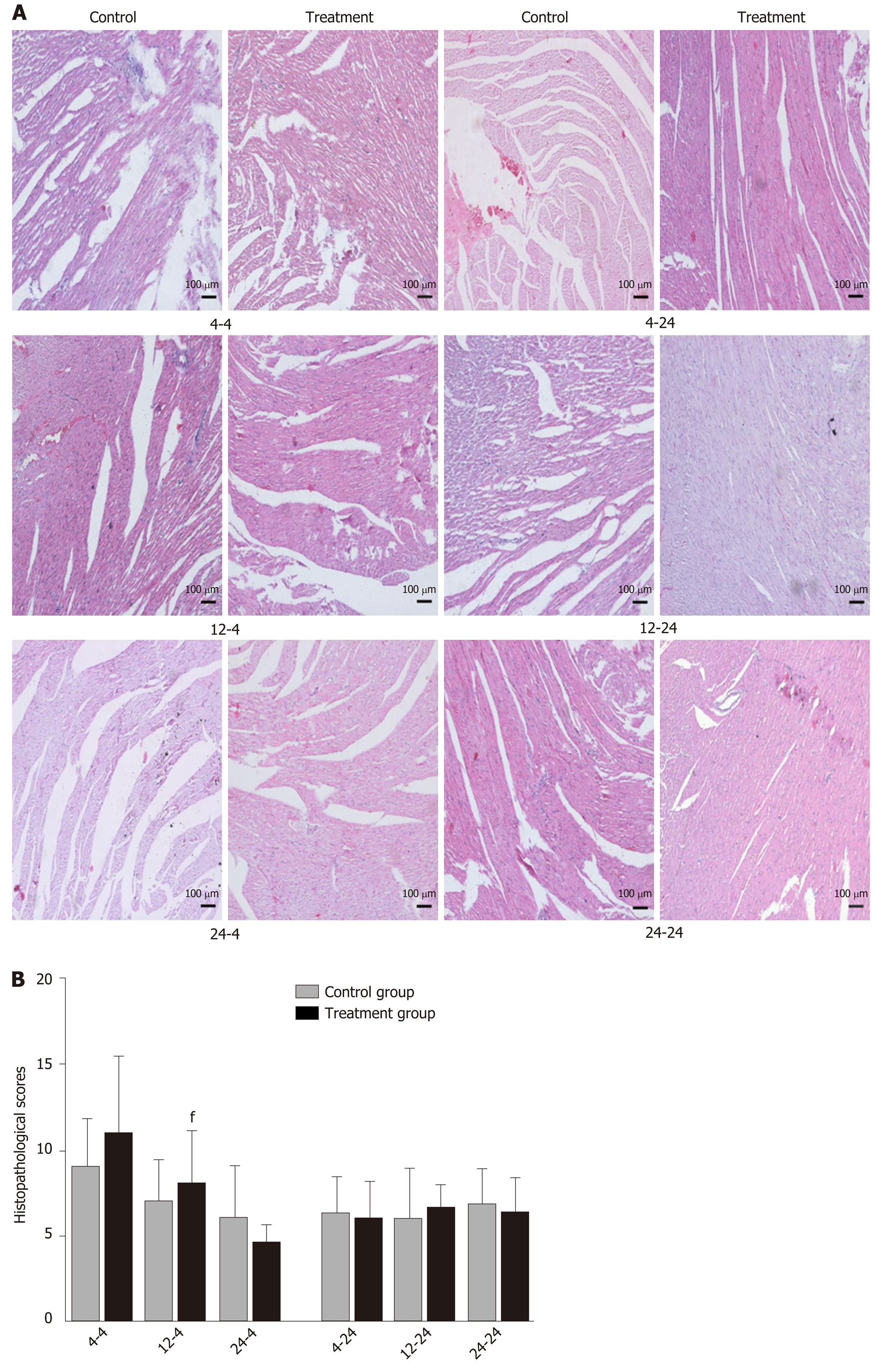
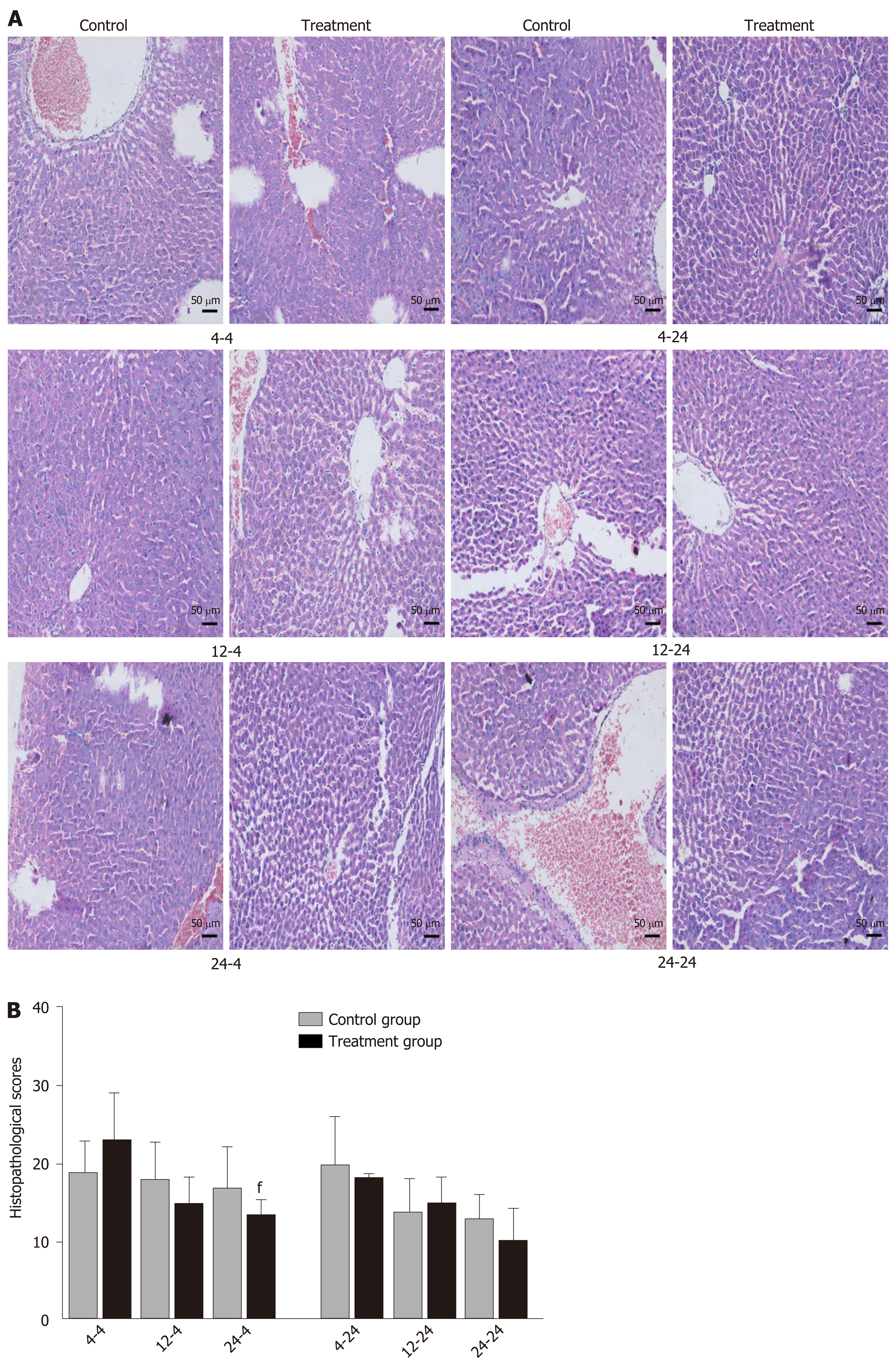
Early oral administration of DCQD aggravates the pathological damage of extr-apancreatic tissues: When rats were euthanized at 4 h after intragastric administration, the pathological injury exhibited in the lung, kidney, and intestinal tissue samples of 4 h-TG was greater than that in 4 h-CG (Figure 4A, 5A, and 6A). No obvious differences in the degree of pathological injury were found between the 12 h-TG and 12 h-CG. Furthermore, the pathological injury exhibited in the heart of 12 h-TG was less than that in 12 h-CG (Figure 7A), and the pathological injury exhibited in the lung, kidney, intestinal, and liver tissue samples of 24 h-TG was less than that in 24 h-CG (Figure 4A, 5A, 6A, and 8A).
The histopathological scores of the lung, kidney, and intestinal tissue samples of 4 h-TG were significantly higher than those of 4 h-CG (P < 0.05; Figure 4B, 5B and 6B), while the scores of the heart tissue samples of 12 h-TG were significantly lower than those of 12 h-CG (P < 0.05; Figure 7B), and the scores of the lung, kidney, intestinal, and liver tissue samples of 24 h-TG were significantly lower than those of 24 h-CG (P < 0.05; Figure 4B, 5B, 6B, and 8B).
When rats were euthanized at 24 h after intragastric administration, no obvious differences were found between the treatment groups and their respective control groups, and pathological scores also showed no significant differences (Figure 4A-8A and Figure 4B-8B).
This study is the first to assess the optimal oral administration time of DCQD for protecting the extrapancreatic organs of AP rats. Based on the pharmacokinetic and pharmacodynamics experiments, we proved that delayed administration may be more appropriate for the protection of extrapancreatic organs in AP rats. In addition, this is the first time that we have tried to compare the efficacy at different times after administration, and we found that a single-dose administration of the decoction leads to a rapid onset of relief but no steady-state effect, suggesting that multiple-dose administration should be considered.
Studies have shown that AP could affect the pharmacokinetic process of DCQD in rats[25], which may be related to insufficient effective blood volume and an excessive inflammatory response to organ damage[26]. In the early stage of AP, gastrointestinal dysfunction, including duodenal edema, paralytic ileus, increased intestinal mucosal permeability, and imbalanced intestinal flora, may inhibit the absorption of DCQD[27]. In our study, the T max and C max values of most components were lower in the AP model groups, and almost all the components from Dahuang had lower AUC and C max values in these groups. Similar results were observed in our previous study, which demonstrated that AP inhibits the absorption of herbal components from DCQD after oral administration in rats, resulting in lower C max and AUC values[28]. Additionally, the later (12 h and 24 h) time points of oral dosing with DCQD resulted in higher C max values, larger AUC 0 → t values, and longer t1/2 values for these monomers; thus, we could deduce that the inhibition caused by AP can be ameliorated by delayed administration. In contrast, the pharmacokinetic parameters of the components from Zhishi in the three model groups were better than those in the sham-operated group, indicating that the pharmacokinetics of the herbs were affected by many other factors.
First, functional homeostasis of the liver and kidney plays a role in the pha-rmacokinetics of herbs. To our knowledge, many drugs, including herbal monomers, are metabolized by the liver and kidney, and the pharmacokinetic process will change if the liver and kidney are damaged[29]. Second, the physicochemical properties of the DCQD components may also influence absorption. For example, chrysophanol is almost insoluble in water, but has high tissue permeability[30]; magnolol belongs to a first-pass metabolic model with low absorbability[31]; and naringin dissolves in water moderately and is easily decomposed into aglycon naringenin by intestinal flora during absorption[32]. Third, the molecular diameter, lipid solubility, charge amount, protein binding rate, and mode of administration may all affect drug absorption[33]. Furthermore, Gong et al[33] put forward that the compatibility of Chinese medicine could change the pharmacokinetic process of multiple ingredients in DCQD, and the effects on each ingredient are not exactly the same. Consistent with their results, our experiments showed that AP inhibited the pharmacokinetic process of Dahuang, the principal drug, while promoting the absorption of naringin and naringenin from Zhishi. Additionally, Xu et al[34] reported that there may be some drug-drug interactions between Dahuang and the other three constitutional raw materials in DCQD in the decoction procedure, which could also affect the plasma concentration of the DCQD components. These phenomena may help to clarify why the pharmacokinetic changes of the components from Zhishi were different from those of Dahuang.
Previous studies have shown that HPLC can be used to identify the main components of DCQD and their serum concentrations[21,27,35]; however, the targeting of these components to specific tissues such as heart, liver, lung, kidney and intestine tissues is still unclear. The factors affecting the distribution of drug in tissues include blood circulation, vascular permeability, physicochemical properties of drugs, affinity between drugs and tissues, and drug interactions[36]. One study demonstrated that AP could affect the pharmacokinetics of herbal components in serum and then affect their distribution in tissues[37]; therefore, it can be speculated that changes in serum pharmacokinetics can further influence the concentration of drug monomers in tissues.
Based on the hypothesis that the efficacy of TCM is related to the targeting of ingredients to specific tissues, our previous experiments have confirmed that DCQD can reduce inflammatory damage when its components target specific tissues (pancreas, lung, kidney, and liver) in rats with AP[9,15,16]. An experiment on tetrahydropalmatine showed that the increased plasma concentration and lung distribution of tetrahydropalmatine after acupoint application could exert a reinforced preventative effect on asthma[38]. Consistently, some antibiotics have been shown to have good pancreatic tissue affinity and can be used to control pancreatic infections[39]. In this study, the concentration of each component of DCQD varied greatly in different tissues. In general, compared to rats in the sham-operated group, the serum concentrations of the major components of DCQD were lower in the AP model groups, proving that AP reduces the distribution of these components to target extrapancreatic organ tissues. By comparing the concentrations of the DCQD components in the three model groups, our research showed that the later DCQD administration time points (12 h and 24 h) were associated with higher concentrations of many of the components, indicating that late dosing may promote the distribution of the monomer throughout targeted organ tissues. Based on the previous hypothesis, combined with our results, we speculate that late administration with DCQD may result in a better pharmacodynamics effect. Herein, we designed subsequent pharmacodynamics experiments to verify our hypothesis.
AP is a common and potentially fatal acute inflammatory disease characterized by an imbalance of pro-inflammatory and anti-inflammatory mediators[40]. Systemic inflammation is considered to be a key component of MODS in SAP[41]. TNF-α and IL-6 are known to be the major pro-inflammatory mediators that are associated with the onset and progression of SAP and mediate multiple types of organ damage associated with pancreatitis[42]. IL-10 is one of the most common anti-inflammatory mediators and is closely associated with the prognosis of AP; thus, the imbalance between pro-inflammatory and anti-inflammatory mediators may result in an inflammatory cascade that exacerbates the progression of AP[40,43]. We have proved that DCQD could balance the pro-inflammatory and anti-inflammatory mediators in our previous studies[9,15]. Therefore, in this research, we examined the value of IL-6 and IL-10 as predictors of inflammation in AP. Our results showed that a later time of DCQD administration (at least 12 h after AP onset) was associated with lower IL-6 levels and higher IL-10 levels in the treatment groups than those observed in the respective control groups. These results confirmed that DCQD could reduce the inflammatory response in AP rats, and we can deduce that delayed administration of DCQD may exert a better anti-inflammatory effect.
Amylase activity is an enzyme index, and serum amylase detection is a routine method for clinical diagnosis of AP. Microcirculation obstruction is a systemic reaction to pancreatic injury throughout the development of AP and is closely related to MODS[44]. Microcirculation hypoperfusion leads to Ca2+ influx and even Ca2+ overload in pancreatic cells, and Ca2+ influx activates the phospholipid cell system, leading to disruption of the lysosomal membrane, which in turn releases enzymes (including amylase) and a large number of cytotoxic substances[45,46]. In our study, the serum amylase levels in the 4-h treatment group showed an upward trend, which may be related to the microcirculation obstruction and the increased pancreatic exocrine stimulation caused by early administration with DCQD.
DCQD has cathartic functions and has been widely adopted to ameliorate diseases with symptoms of abdominal distension and constipation. It can also remove internal heat and toxins from the gastrointestinal tract[35]. A recent study demonstrated that DCQD can further reduce the risk of SIRS via decreasing the secretion of HMGB1 in SAP[47]. DCQD could also induce the pancreas to be more resistant to stress and microcirculation disorders by clearing away excessive reactive oxygen species and regulating the apoptosis/necrosis switch in pancreatic acinar cells[14,48]. Moreover, emodin, one of the most active compounds from the Chinese herb Dahuang, has been used for many years in China to treat acute severe diseases, including AP[49]. It has been reported that emodin inhibits NF-κB activation and endoplasmic reticulum stress to protect the pancreas from injury[50,51]. Naringenin is another component of DCQD that has antibacterial, antifungal, and anti-oxidative effects, and exerts a cytoprotective effect on the gastric mucosa[52]. Ge et al[53] showed that rhein could attenuate inflammation via the NF-κB/NLRP3 inflammasome pathways. In this study, delayed oral administration of DCQD could better reduce the inflammatory reaction, inhibit the excessive secretions of the pancreas, and thus reduce the pathological damage of multiple extrapancreatic organs (lung, liver, kidney, and intestine) in the early stage of AP, while early oral administration aggravated the pathological injury in lung, kidney, and intestinal tissues, further confirming that delayed oral administration is more appropriate for the protection of extrapancreatic organs.
However, there are some limitations to this study. The purpose of this study was primarily to explore the effects of different administration times on extrapancreatic organ tissues in AP and to further infer the association between tissue concentration distribution and the pharmacodynamics effects. However, a single dose of DCQD did not show obvious effects on the long-term protection of the extrapancreatic organ tissues. Therefore, multiple-dose administration should be considered in follow-up experiments. More importantly, to better validate our hypothesis, we should determine tissue drug distributions at more time points and assess the pharmacological effect simultaneously, and it may be better to examine inflammatory cytokines in each tissue sample. Thus, relevant pharmacokinetics and pharmacodynamics analysis could be further conducted.
In conclusion, AP could inhibit the pharmacokinetic process of the major DCQD components in serum and multiple extrapancreatic organ tissues, while delayed administration may ameliorate the inhibition. Importantly, early administration may aggravate the injury to the extrapancreatic organs in the early stage of AP, while delayed administration (at least 12 h after AP induction) of DCQD may reduce pancreatic exocrine secretion, balance the expression of pro- and anti-inflammatory cytokines to a greater extent, and ultimately better ameliorate the pathological injury of the extrapancreatic organs, thereby demonstrating that the late time is the optimal dosing time of DCQD for the protection of extrapancreatic organs.
Acute pancreatitis (AP) is an inflammatory pancreatic disorder associated with substantial morbidity and mortality, and the severe form of AP is commonly complicated by multiple extrapancreatic organ dysfunction. Dachengqi decoction (DCQD) is an effective prescription for the treatment of AP, however, current AP guidelines do not provide specific guidance on the optimal time to take this Chinese herbal medicine orally. Our previous study proved that administering DCQD too early may aggravate the pathological damage to the pancreas, while the effect of administration time on multiple extrapancreatic organs in AP rats is still unclear. Therefore, investigations of the optimal administration time of DCQD for the protection of multiple extrapancreatic organs are urgently required.
DCQD has been shown to protect multiple organs from injury caused by an excessive inflammatory response in AP, and we confirmed that the anti-inflammatory effect was associated with its tissue distribution. This study aimed to screen the appropriate oral administration time of DCQD for the protection of extrapancreatic organs in AP rats based on the pharmacokinetic and pharmacodynamic evidence, and to provide an experimental basis for future clinical application of DCQD.
To identify the optimal administration time of DCQD for the protection of extrapancreatic organs in experimental AP rats and observe the anti-inflammatory efficacy at different times after administration.
The current experiment was divided into pharmacokinetic and pharmacodynamic parts. The AP model was established with 3.5% sodium taurocholate. In the pharmacokinetic study, the concentrations of the DCQD components in serum and organ tissues were measured by HPLC-MS/MS, which is a sensitive, accurate, and reproducible method, and the pharmacokinetic parameters (C max, T max, T 1/2, and AUC 0 → t) were calculated with DAS 2.0.1. In the pharmacodynamic study, the levels of serum inflammatory cytokines (IL-6 and IL-10) were measured by enzyme-linked immunosorbent assay, and amylase levels were measured via a HITACHI automatic biochemical analyzer. All histopathological sections were observed and scored by two independent blinded pathologists using different scoring systems specific to different tissues. Additionally, Graph Pad Prism 7.0 software was used for the data analyses of both parts of the study.
In the pharmacokinetic study, the T max and C max values of most components were lower in the AP model groups, and the major components of DCQD had lower AUC and C max values in these groups. The later (12 h and 24 h) time points of oral dosing with DCQD resulted in higher C max values, larger AUC 0 → t values, and longer t1/2 values for these monomers, accompanied by higher concentrations of most components in the target extrapancreatic organ tissues. In the pharmacodynamic study, delayed administration of DCQD resulted in lower IL-6 and amylase levels and higher IL-10 levels, and pathological injury of multiple extrapancreatic organ (liver, lung, kidney, and intestine) tissues was slightly less at 4 h after administration, while the results were similar between the treatment and corresponding control groups at 24 h after administration.
This study provides some information on the effect of administration time on extrapancreatic organs in AP rats, but elucidation of the specific mechanism needs further study. Relevant pharmacokinetics and pharmacodynamics analysis should be considered to provide more systematic and comprehensive evidence for the clinical application of this Chinese herbal formula.
This study suggests that early administration of DCQD may inhibit the pharmacokinetic process of the major DCQD components in serum and multiple extrapancreatic organ tissues, and delayed administration time may be more helpful for alleviating the inflammatory reaction and pathological injury in multiple extrapancreatic organs. Importantly, multiple-dose administration of DCQD is well worth considering for the steady-state effect in future animal experiments or clinical applications.
Although we have found some of the potential components of DCQD in alleviating AP, and the therapeutic effect of DCQD on AP has been confirmed in a large number of in vivo and in vitro experiments, the underlying molecular mechanisms are not well established. Further investigation combing the identification of more active components, potential targets, and/or signal pathway analysis is urgently required to make a deeper and more comprehensive understanding of the therapeutic mechanism of DCQD in the treatment of AP.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Trkulja V S-Editor: Wang J L-Editor: Wang TQ E-Editor: Zhang YL
| 1. | Lee PJ, Papachristou GI. New insights into acute pancreatitis. Nat Rev Gastroenterol Hepatol. 2019;16:479-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 516] [Article Influence: 86.0] [Reference Citation Analysis (0)] |
| 2. | Li Q, Zhu B, Zhu X, Piao C, Cui W, Wang Y, Sun J, Chen W, Miao L. Treatment of necrotizing acute pancreatitis with peritoneal lavage and dialysis by a new simplified technique insert catheters: One retrospective study. Medicine (Baltimore). 2016;95:e3821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Koutroumpakis E, Wu BU, Bakker OJ, Dudekula A, Singh VK, Besselink MG, Yadav D, Mounzer R, van Santvoort HC, Whitcomb DC, Gooszen HG, Banks PA, Papachristou GI. Admission Hematocrit and Rise in Blood Urea Nitrogen at 24 h Outperform other Laboratory Markers in Predicting Persistent Organ Failure and Pancreatic Necrosis in Acute Pancreatitis: A Post Hoc Analysis of Three Large Prospective Databases. Am J Gastroenterol. 2015;110:1707-1716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 115] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 4. | Hirota M, Mayumi T, Shimosegawa T. Acute pancreatitis bundles: 10 clinical regulations for the early management of patients with severe acute pancreatitis in Japan. J Hepatobiliary Pancreat Sci. 2014;21:829-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Frossard JL, Steer ML, Pastor CM. Acute pancreatitis. Lancet. 2008;371:143-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 511] [Article Influence: 30.1] [Reference Citation Analysis (1)] |
| 6. | Mofidi R, Duff MD, Wigmore SJ, Madhavan KK, Garden OJ, Parks RW. Association between early systemic inflammatory response, severity of multiorgan dysfunction and death in acute pancreatitis. Br J Surg. 2006;93:738-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 363] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 7. | Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108:1400-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1232] [Cited by in RCA: 1380] [Article Influence: 115.0] [Reference Citation Analysis (3)] |
| 8. | Liu XB, Jiang JM, Huang ZW, Tian BL, Hu WM, Xia Q, Chen GY, Li QS, Yuan CX, Luo CX, Yan LN, Zhang ZD. [Clinical study on the treatment of severe acute pancreatitis by integrated traditional Chinese medicine and Western medicine]. Sichuan Da Xue Xue Bao Yi Xue Ban. 2004;35:204-208. [PubMed] |
| 9. | Zhao X, Zhang Y, Li J, Wan M, Zhu S, Guo H, Xiang J, Thrower EC, Tang W. Tissue Pharmacology of Da-Cheng-Qi Decoction in Experimental Acute Pancreatitis in Rats. Evid Based Complement Alternat Med. 2015;2015:283175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Wan MH, Li J, Huang W, Mukherjee R, Gong HL, Xia Q, Zhu L, Cheng GL, Tang WF. Modified Da-Cheng-Qi Decoction reduces intra-abdominal hypertension in severe acute pancreatitis: a pilot study. Chin Med J (Engl). 2012;125:1941-1944. [PubMed] |
| 11. | Chen H, Li F, Jia JG, Diao YP, Li ZX, Sun JB. Effects of traditional Chinese medicine on intestinal mucosal permeability in early phase of severe acute pancreatitis. Chin Med J (Engl). 2010;123:1537-1542. [PubMed] |
| 12. | Wan MH, Li J, Gong HL, Xue P, Zhu L, Chen GY, Xia Q, Wen-Fu T. Clinical observation on the effect of dexamethasone and Chinese herbal decoction for purgation in severe acute pancreatitis patients. Chin J Integr Med. 2011;17:141-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 13. | Li J, Zhang S, Zhou R, Zhang J, Li ZF. Perspectives of traditional Chinese medicine in pancreas protection for acute pancreatitis. World J Gastroenterol. 2017;23:3615-3623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (3)] |
| 14. | Wang J, Chen G, Gong H, Huang W, Long D, Tang W. Amelioration of experimental acute pancreatitis with Dachengqi Decoction via regulation of necrosis-apoptosis switch in the pancreatic acinar cell. PLoS One. 2012;7:e40160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Zhang YM, Ren HY, Zhao XL, Li J, Li JY, Wu FS, Su H, Tang WF. Pharmacokinetics and pharmacodynamics of Da-Cheng-Qi decoction in the liver of rats with severe acute pancreatitis. World J Gastroenterol. 2017;23:1367-1374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Yuan L, Zhu L, Zhang Y, Chen H, Kang H, Li J, Zhao X, Wan M, Miao Y, Tang W. Effect of Da-Cheng-Qi decoction for treatment of acute kidney injury in rats with severe acute pancreatitis. Chin Med. 2018;13:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Gong H, Tang W, Wang H, Xia Q, Huang X. Effects of food and gender on the pharmacokinetics of rhein and emodin in rats after oral dosing with Da-Cheng-Qi decoction. Phytother Res. 2011;25:74-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Leppäniemi A, Tolonen M, Tarasconi A, Segovia-Lohse H, Gamberini E, Kirkpatrick AW, Ball CG, Parry N, Sartelli M, Wolbrink D, van Goor H, Baiocchi G, Ansaloni L, Biffl W, Coccolini F, Di Saverio S, Kluger Y, Moore E, Catena F. 2019 WSES guidelines for the management of severe acute pancreatitis. World J Emerg Surg. 2019;14:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 538] [Cited by in RCA: 423] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 19. | Zhang YM, Zhu L, Zhao XL, Chen H, Kang HX, Zhao JL, Wan MH, Li J, Zhu L, Tang WF. Optimal timing for the oral administration of Da-Cheng-Qi decoction based on the pharmacokinetic and pharmacodynamic targeting of the pancreas in rats with acute pancreatitis. World J Gastroenterol. 2017;23:7098-7109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Zhu L, Li JY, Zhang YM, Kang HX, Chen H, Su H, Li J, Tang WF. Pharmacokinetics and pharmacodynamics of Shengjiang decoction in rats with acute pancreatitis for protecting against multiple organ injury. World J Gastroenterol. 2017;23:8169-8181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Tang W, Wan M, Zhu Z, Chen G, Huang X. Simultaneous determination of eight major bioactive compounds in Dachengqi Tang (DT) by high-performance liquid chromatography. Chin Med. 2008;3:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Kusske AM, Rongione AJ, Ashley SW, McFadden DW, Reber HA. Interleukin-10 prevents death in lethal necrotizing pancreatitis in mice. Surgery. 1996;120:284-288; discussion 289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 135] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 23. | Wirtz S, Popp V, Kindermann M, Gerlach K, Weigmann B, Fichtner-Feigl S, Neurath MF. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat Protoc. 2017;12:1295-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 538] [Cited by in RCA: 1038] [Article Influence: 129.8] [Reference Citation Analysis (0)] |
| 24. | Gibson-Corley KN, Olivier AK, Meyerholz DK. Principles for valid histopathologic scoring in research. Vet Pathol. 2013;50:1007-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 554] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 25. | Gong HL, Tang WF, Yu Q, Xiang J, Xia Q, Chen GY, Huang X, Liang MZ. Effect of severe acute pancreatitis on pharmacokinetics of Da-Cheng-Qi Decoction components. World J Gastroenterol. 2009;15:5992-5999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Wacke R, Park S, Mundkowski RG, Block N, Kuhn-Thiel A, Drewelow B. The penetration of moxifloxacin into the pancreas of male rats in experimental acute necrotizing pancreatitis. Chemotherapy. 2003;49:167-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Zhao J, Tang W, Wang J, Xiang J, Gong H, Chen G. Pharmacokinetic and pharmacodynamic studies of four major phytochemical components of Da-Cheng-Qi decoction to treat acute pancreatitis. J Pharmacol Sci. 2013;122:118-127. [PubMed] |
| 28. | Gong HL, Tang WF, Ren YY, Wan MH, Chen GY, Xia Q, Huang X. Summary of integrative medicine for severe acute pancreatitis: 26-year clinical experiences and a report of 1 561 cases. Chin J Integr Med. 2011;17:381-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Anzenbacher P, Anzenbacherová E. Cytochromes P450 and metabolism of xenobiotics. Cell Mol Life Sci. 2001;58:737-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 686] [Cited by in RCA: 634] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 30. | Singh D, Rawat MSM, Semalty A, Semalty MJJoTA, Calorimetry. Chrysophanol–phospholipid complex. J Therm Anal Calorim. 2013;111:2069-2077. [DOI] [Full Text] |
| 31. | Tsai TH, Chou CJ, Lee TF, Wang LCH, Chen CF. Pharmacokinetic and Pharmacodynamic Studies of Magnolol after Oral Administration in Rats. Pharmacy and Pharmacology Communications. 1996;2:191-193. [DOI] [Full Text] |
| 32. | Choudhury R, Chowrimootoo G, Srai K, Debnam E, Rice-Evans CA. Interactions of the flavonoid naringenin in the gastrointestinal tract and the influence of glycosylation. Biochemical and Biophysical Research Communications. 1999;265:410-415. [RCA] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 80] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 33. | Gong HL, Tang WF, Wang J, Chen GY, Huang X. Effect of formula compatibility on the pharmacokinetics of components from Dachengqi Decoction [See Text] in rats. Chin J Integr Med. 2012;18:708-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Xu F, Liu Y, Dong H, Song R, Zhang Z. Pharmacokinetic comparison in rats of six bioactive compounds between Da-Cheng-Qi decoction and its parent herbal medicines. Nat Prod Commun. 2010;5:795-800. [PubMed] |
| 35. | Yu Q, Xiang J, Tang W, Liang M, Qin Y, Nan F. Simultaneous determination of the 10 major components of Da-Cheng-Qi decoction in dog plasma by liquid chromatography tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:2025-2031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Roth K, Lehnick D, Wessels H, Höfler J, Gastl B, Jankowsky R. Pharmacokinetics, pharmacodynamics, safety, and immunogenicity of Pelmeg®, a pegfilgrastim biosimilar in healthy subjects. Pharmacol Res Perspect. 2019;7:e00503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Zhao XL, Xiang J, Wan MH, Yu Q, Chen WW, Chen GY, Tang WF. Effect of acute pancreatitis on the pharmacokinetics of Chinese herbal ointment Liu-He-Dan in anaesthetized rats. J Ethnopharmacol. 2013;145:94-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 38. | Lin YY, Wang YP, Lu HY, Guo XC, Liu X, Wu CB, Xu YH. Plasma pharmacokinetics and lung distribution of tetrahydropalmatine after topical application of cold asthma recipe extract: Feishu (BL 13) versus Non-Feishu acupoint. J Ethnopharmacol. 2014;153:454-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 39. | Uhl W, Warshaw A, Imrie C, Bassi C, McKay CJ, Lankisch PG, Carter R, Di Magno E, Banks PA, Whitcomb DC, Dervenis C, Ulrich CD, Satake K, Ghaneh P, Hartwig W, Werner J, McEntee G, Neoptolemos JP, Büchler MW; International Association of Pancreatology. IAP Guidelines for the Surgical Management of Acute Pancreatitis. Pancreatology. 2002;2:565-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 247] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 40. | Fisic E, Poropat G, Bilic-Zulle L, Licul V, Milic S, Stimac D. The Role of IL-6, 8, and 10, sTNFr, CRP, and Pancreatic Elastase in the Prediction of Systemic Complications in Patients with Acute Pancreatitis. Gastroenterol Res Pract. 2013;2013:282645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 41. | Yuan Z, Meyerholz DK, Twait EC, Kempuraj D, Williard DE, Samuel I. Systemic inflammation with multiorgan dysfunction is the cause of death in murine ligation-induced acute pancreatitis. J Gastrointest Surg. 2011;15:1670-1678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 42. | Li J, Wu Y, Zhang S, Zhang J, Ji F, Bo W, Guo X, Li Z. Baicalein protect pancreatic injury in rats with severe acute pancreatitis by inhibiting pro-inflammatory cytokines expression. Biochem Biophys Res Commun. 2015;466:664-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 43. | Zhou X, Liu Z, Jang F, Xiang C, Li Y, He Y. Autocrine Sonic hedgehog attenuates inflammation in cerulein-induced acute pancreatitis in mice via upregulation of IL-10. PLoS One. 2012;7:e44121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 44. | Tomkötter L, Erbes J, Trepte C, Hinsch A, Dupree A, Bockhorn M, Mann O, Izbicki JR, Bachmann K. The Effects of Pancreatic Microcirculatory Disturbances on Histopathologic Tissue Damage and the Outcome in Severe Acute Pancreatitis. Pancreas. 2016;45:248-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 45. | Zhou ZG, Chen YQ, Liu XB, Hu WM, Tian BL, Chen HQ. Changes of cytosolic [Ca2+]i in neutrophils in pancreatic microcirculation of rats with caerulein-induced acute pancreatitis under fluid shear stress. World J Gastroenterol. 2004;10:3185-3187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 46. | Maléth J, Hegyi P. Calcium signaling in pancreatic ductal epithelial cells: an old friend and a nasty enemy. Cell Calcium. 2014;55:337-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 47. | Chen Z, Chen Y, Pan L, Li H, Tu J, Liu C, Dai X, Zhang X, Sun G, Feng D. Dachengqi Decoction Attenuates Inflammatory Response via Inhibiting HMGB1 Mediated NF-κB and P38 MAPK Signaling Pathways in Severe Acute Pancreatitis. Cell Physiol Biochem. 2015;37:1379-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 48. | Ren YY, Gong HL, Tang WF, Wan MH, Zhao JL, Huang X. [Dachengqi Decoction induces pancreatic acinar cell apoptosis in experimental acute pancreatitis in rats]. Zhong Xi Yi Jie He Xue Bao. 2009;7:651-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 49. | Dong X, Fu J, Yin X, Cao S, Li X, Lin L, Huyiligeqi, Ni J. Emodin: A Review of its Pharmacology, Toxicity and Pharmacokinetics. Phytother Res. 2016;30:1207-1218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 409] [Cited by in RCA: 513] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 50. | Yao WY, Zhou YF, Qian AH, Zhang YP, Qiao MM, Zhai ZK, Yuan YZ, Yang SL. Emodin has a protective effect in cases of severe acute pancreatitis via inhibition of nuclear factor‑κB activation resulting in antioxidation. Mol Med Rep. 2015;11:1416-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 51. | Wu L, Cai B, Zheng S, Liu X, Cai H, Li H. Effect of emodin on endoplasmic reticulum stress in rats with severe acute pancreatitis. Inflammation. 2013;36:1020-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 52. | Dembinski A, Warzecha Z, Konturek SJ, Ceranowicz P, Dembinski M, Pawlik WW, Kusnierz-Cabala B, Naskalski JW. Extract of grapefruit-seed reduces acute pancreatitis induced by ischemia/reperfusion in rats: possible implication of tissue antioxidants. J Physiol Pharmacol. 2004;55:811-821. [PubMed] |
| 53. | Ge H, Tang H, Liang Y, Wu J, Yang Q, Zeng L, Ma Z. Rhein attenuates inflammation through inhibition of NF-κB and NALP3 inflammasome in vivo and in vitro. Drug Des Devel Ther. 2017;11:1663-1671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |