Published online Jun 7, 2020. doi: 10.3748/wjg.v26.i21.2831
Peer-review started: March 22, 2020
First decision: April 9, 2020
Revised: April 23, 2020
Accepted: May 13, 2020
Article in press: May 13, 2020
Published online: June 7, 2020
Processing time: 76 Days and 13 Hours
Radical resection is an important treatment method for hepatic echinococcosis. The posterosuperior segments of the liver remain the most challenging region for laparoscopic or robotic hepatectomy.
To demonstrate the safety and preliminary experience of robotic radical resection of cystic and alveolar echinococcosis in posterosuperior liver segments.
A retrospective analysis was conducted on the clinical data of 5 patients with a median age of 37 years (21-56 years) with cystic and alveolar echinococcosis in difficult liver lesions admitted to two centers from September to December 2019. The surgical methods included total pericystectomy, segmental hepatectomy, or hemihepatectomy.
Among the 5 patients, 4 presented with cystic echinococcosis and 1 presented with alveolar echinococcosis, all of whom underwent robotic radical operation successfully without conversion to laparotomy. Total caudate lobectomy was performed in 2 cases, hepatectomy of segment VII in 1 case, total pericystectomy of segment VIII in 1 case, and right hemihepatectomy in 1 case. Operation time was 225 min (175-300 min); blood loss was 100 mL (50-600 mL); and postoperative hospital stay duration was 10 d (5-19 d). The Clavien-Dindo complication grade was I in 4 cases and II in 1 case. No recurrence of echinococcosis was found in any patient at the 3 mo of follow-up.
Robotic radical surgery for cystic and selected alveolar echinococcosis in posterosuperior liver segments is safe and feasible.
Core tip: This study aimed to elucidate the safety and preliminary experience of robotic radical resection of cystic and alveolar echinococcosis in posterosuperior liver segments. Our results demonstrated that robotic transabdominal approach can be an option of treatment and feasible for resection of cystic and selected alveolar echinococcosis located in the posterosuperior hepatic segments.
- Citation: Zhao ZM, Yin ZZ, Meng Y, Jiang N, Ma ZG, Pan LC, Tan XL, Chen X, Liu R. Successful robotic radical resection of hepatic echinococcosis located in posterosuperior liver segments. World J Gastroenterol 2020; 26(21): 2831-2838
- URL: https://www.wjgnet.com/1007-9327/full/v26/i21/2831.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i21.2831
Echinococcosis is a zoonotic disease caused by Echinococcus tapeworms, which can lead to damage to tissues and organs such as the liver, lungs, and brain. Human liver echinococcosis mainly includes cystic echinococcosis (CE) caused by Echinococcus granulosus infection and alveolar echinococcosis (AE) caused by Echinococcus multilocularis infection[1]. The worldwide spread of echinococcosis has made the disease a serious threat to public health, especially in Western China. The World Health Organization (WHO) has listed echinococcosis as one of the 17 diseases to be controlled or eliminated by 2050[2].
According to the classification by the WHO Informal Working Group on Echinococcosis[3,4], the treatment methods for hepatic echinococcosis include surgery, medication, and puncture and drainage. Radical resection remains the primary treatment for CE2, CE3, and AE[5]. Since the first laparoscopic pericystectomy for hepatic echinococcosis carried out in France in 1991 by Katkhouda et al[6], there have been increasing reports on the laparoscopic treatment of CE[7-10]. The posterosuperior region of the liver – such as the caudate lobe (segment I), segment VII, and segment VIII – is considered to be the site where difficult lesions reside and remains the most challenging region for complex laparoscopic hepatectomy. Thus, extensive experience in open laparoscopic hepatectomy is imperative[11,12].
The da Vinci Surgical System (Intuitive Surgical Inc., United States) is an advanced and minimally invasive surgery tool, which has been shown to have advantages in complex hepatectomy[13,14]. Since Giulianotti et al[15] reported two cases of robotic hepatic hydatid surgery in 2011, the literature on robotic hepatic hydatid surgery in difficult lesions has mostly been presented in case reports[16]. The aim of this study was to retrospectively analyze the data on robotic surgery for hepatic echinococcosis in difficult lesions from two centers in China and to explore the experience of performing robotic surgery for CE and AE.
A retrospective analysis was conducted of the clinical data of patients with hepatic echinococcosis who underwent robotic surgery at the Second Department of Hepatopancreatobiliary Surgery, The First Medical Center, Chinese People’s Liberation Army General Hospital as well as the Department of Hepatobiliary Surgery, The People’s Hospital of Xinjiang Uygur Autonomous Region, from September to December 2019. The inclusion criteria were: (1) Patients who had pathologically confirmed CE or AE; (2) Patients who had lesions located in the caudate lobe of the liver (segment I), segment VII, and segment VIII; and (3) Patients who underwent total pericystectomy, segmental hepatectomy, or hemihepatectomy. The exclusion criteria were: (1) Patients who had multiple organ echinococcoses; and (2) Patients who could not tolerate anesthesia or surgery.
Overall, 5 patients (all men, aged 21-56 years, with a median age of 37 years) were enrolled. The body mass index was 21.22-30.1 kg/m2, with a median body mass index of 25.54 kg/m2. The classification by the American Society of Anesthesiologists was I-III. Among them, 4 cases presented with CE alongside 1 case of AE, with a median tumor diameter of 75 mm (51-80 mm) (Table 1).
| Patient No. | Age (yrs) | Gender (M/F) | BMI (kg/m2) | ASA (I/II/III) | Tumor size (mm) | Pathology (WHO-IWGE) |
| 1 | 37 | M | 23.84 | II | 75 | CE3a |
| 2 | 24 | M | 25.54 | I | 80 | CE3a |
| 3 | 31 | M | 21.22 | I | 80 | CE3a |
| 4 | 56 | M | 30.1 | III | 68 | CE3a |
| 5 | 45 | M | 27.7 | II | 51 | AE(P1N0M0) |
| Median | 37 (24, 56) | - | 25.54 (21.22, 30.1) | - | 75 (51, 80) | - |
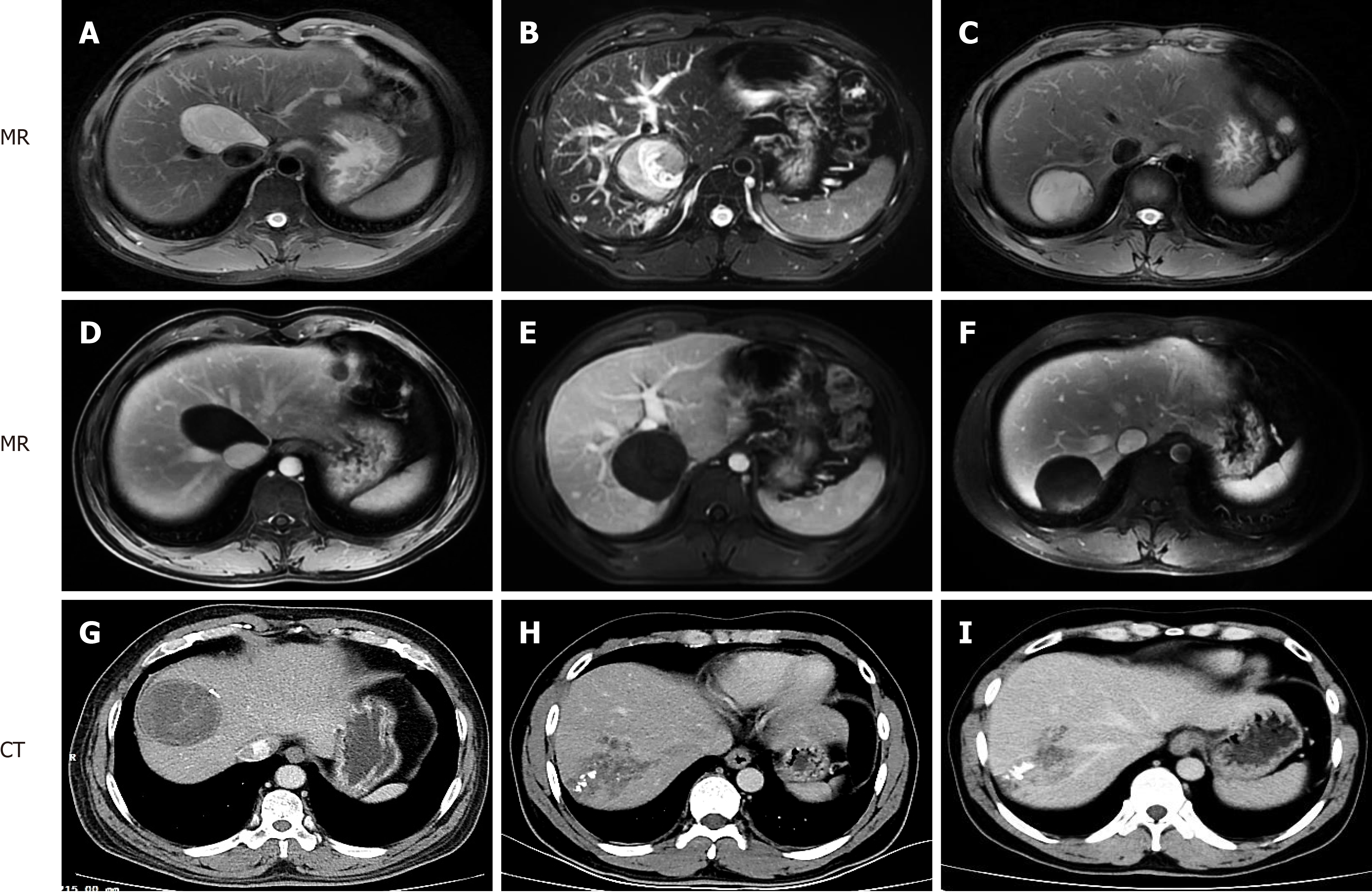
All patients completed abdominal ultrasound, computed tomography and magnetic resonance imaging examinations (Figure 1) before operation, and multidisciplinary discussion and WHO Informal Working Group on Echinococcosis classification were performed. All patients provided written informed consent. The research was approved by the hospital ethics committee, which complies with medical ethics regulations. All operations were performed by surgeons with vast experience in laparotomy and robotic hepatectomy.
Position and trocar hole layout: Tracheal intubation integrated with intravenous general anesthesia was performed in a 25° reverse Trendelenburg position and a lithotomy position. When resection of segment of VII or VIII was required, the patient was turned 45° to his left side. The CO2 pneumoperitoneum pressure was controlled at 14 mmHg, and the central venous pressure was maintained at 0-5 cm H2O[17]. Robotic procedures were performed using the da Vinci Si or Xi Surgical Systems (Intuitive Surgical Inc., United States).
The trocar layout consists of the optic port: 12 mm trocar, located at 3 cm on the right side of the umbilicus; assistant port: 12 mm trocar, located on the umbilicus (3 cm above the umbilicus when segment VII is excised); robotic arm 1:8 mm trocar, located 3-5 cm below the xiphoid processes (below the xiphoid process when segment VII is excised); robotic arm 2:8 mm trocar, located below the costal margin of the right anterior axillary line; and robotic arm 3:8 mm trocar, located below the costal margin of the left anterior axillary line.
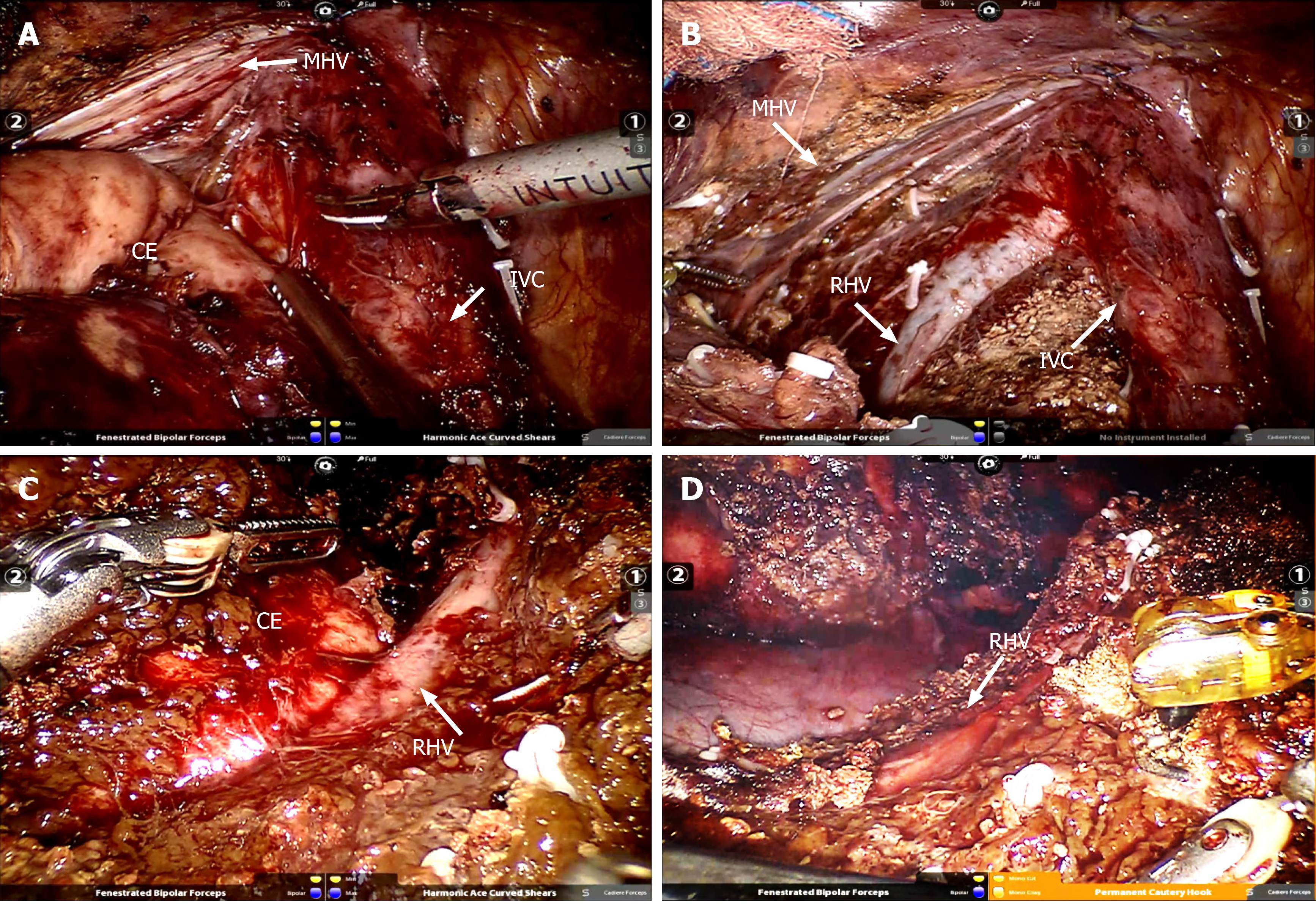
Operation methods: Intraoperative ultrasound and the Pringle maneuver were routinely used. According to previous reports[18,19], the operation methods can be summarized as follows. (1) Caudate lobectomy: The left lateral and Spiegel’s lobes were dissociated, and the short hepatic vessels of the caudate lobe were ligated using Hemolock clip (TFX Medical, RTP Durham, NC, USA). After the caudate lobe was separated from the inferior vena, the Arantius ligament and the hepatic pedicle branch of the caudate lobe were ligated using Hemolock clip. The hepatic parenchyma of the caudate lobe was detached using an ultrasonic scalpel, and the hydatid cyst was detached from the middle and the right hepatic veins. During intraoperative hepatic vein hemorrhage, the CO2 pneumoperitoneum pressure was increased to 15 mmHg at first; the hemorrhage of branches < 2 mm could be bipolarly occluded; and the ethmoidal foramina > 2 mm in hepatic veins were treated with 6-0 prolene suture. After the detachment of the hepatic parenchyma, wound surface was fully exposed in the inferior vena cava as well as the left, middle, and right hepatic veins (Figure 2A and B). (2) Hepatectomy of segment VII: The right liver needed complete mobilization. The hepatic parenchyma was detached along the cephalic approach of the right hepatic vein; the right hepatic vein branch of segment VII and the hepatic pedicle branch of segment VII were ligated using Hemolock clip; and the hydatid cyst was removed from the right hepatic vein (Figure 2B and C). (3) Pericystectomy of segment VIII: After appropriate mobilization of the right liver, the hepatic parenchyma was detached at 2 cm from the margin of the hydatid cyst; the branches of the right and middle hepatic veins as well as the hepatic pedicle branch of segment VIII were ligated using Hemolock clip; and the focus was resected completely. and (4) Right hemihepatectomy: The right gallbladder was resected, and the right hepatic artery and the right branch of the portal vein were ligated. After complete right liver mobilization, the right hepatic parenchyma was detached with an ultrasound scalpel; the branches of segment V and segment VIII of the middle hepatic vein were ligated using Hemolock clip; and the right hepatic duct and right hepatic vein were detached, respectively.
The drainage tube was routinely placed on the cross section of the liver or through the Winslow hole, which was led out from robotic arm 2. The specimens were taken using the extended assistant port in the midline of the upper abdomen.
The operation time, intraoperative blood loss and blood transfusion rate, postoperative complications, drainage tube removal time, and postoperative hospital stay were assessed. The data were expressed as median and analyzed via IBM SPSS Statistics 20 statistical software. No patient took albendazole orally after the operation, and all patients underwent abdominal ultrasound or computed tomography 1 and 3 months after operation.
All 5 patients successfully underwent robotic radical hepatectomy for hepatic echinococcosis, including total caudate lobectomy (n = 2), hepatectomy of segment VII (n = 1), total pericystectomy of segment VIII (n = 1), and right hemihepatectomy (n = 1), without conversion to laparotomy or perioperative deaths. The median operation time was 225 min (175-300 min); the median blood loss was 100 mL (50-600 mL), with 4 U of red blood cell suspension transfused in 1 patient because of an intraoperative blood loss of 600 mL; the median postoperative hospital stay was 10 d (5-19 d); and the median drainage tube removal time was 10 d (4-16 d). No bile leakage was found in any of the patients. According to the Clavien-Dindo complication grading, there were 4 cases of grade I and 1 case of grade II complications. The case of grade II complications with CE of the caudate lobe had postoperative right hepatic subcapsular hematoma; however, the patient recovered after bed rest and symptomatic treatment (Table 2). No recurrence of echinococcosis was found in any of the patients at 3 mo of follow-up.
| Patient No. | Resection | Operation time (min) | EBL (mL) | Transfusion (U) | POS (d) | Drainage (d) | Conversion | Complication grade (Clavien-Dindo) |
| 1 | I | 210 | 50 | 0 | 19 | 16 | - | II |
| 2 | I | 300 | 600 | 4 | 10 | 10 | - | I |
| 3 | VII | 175 | 100 | 0 | 11 | 10 | - | I |
| 4 | VIII | 300 | 100 | 0 | 5 | 4 | - | I |
| 5 | VII, VIII | 225 | 200 | 0 | 9 | 6 | - | I |
| Median | - | 225 (175, 300) | 100 (50, 600) | - | 10 (5, 19) | 10 (4, 16) | - | - |
Surgery is an important treatment method for hepatic echinococcosis, and the postoperative recurrence rate of hepatic echinococcosis has been reported to be 2%-25%[20]. The surgery for CE includes radical surgery and palliative surgery. In accordance with the report of Georgiou et al[21], the complication rate and 3-year postoperative recurrence rate of radical surgery are 10.95% and 6.9%, respectively, whereas the complication rate of palliative surgery is 24.13%. At present, the preferred radical resection approach for CE includes pericystectomy and segmental or partial hepatectomy[2]. The imaging findings of AE are similar to those of liver malignancies, and AE is associated with a 90% mortality rate in untreated patients. Hepatectomy is the main surgical method for AE, and liver transplantation can be considered as the last resort for end-stage AE[22].
In 2016, Di Benedetto et al[23] reported a case of caudate lobectomy (Spiegel’s lobe) for CE for the first time. The focus diameter was 5.6 cm; the operation time was 280 min; the blood loss was 200 mL; and the postoperative hospital stay was 3 d. To date, robotic total caudate lobectomy alone has not been reported in the literature. During traditional laparoscopic anatomical caudate lobectomy, due to the defects in two-dimensional visual field and endoscopic instruments, the dorsal part of the middle hepatic vein is often poorly exposed, and tearing of the small branches of the paracaval portion and the middle hepatic vein often results in catastrophic hemorrhage, which is difficult to treat under endoscopy[24]. In the present study, lesions in the caudate lobe in 2 cases of CE involved the Spiegel’s lobes, caudate processes, and paracaval portion; the focus diameter was 8 cm; and the hydatid cyst was adjacent to the hepatic vein, inferior vena cava, and hepatic pedicle. We adopted the left approach, completely removed the hydatid cyst from the surface of the hepatic vein, and successfully performed robotic total caudate lobectomy.
In 2011, Casciola et al[25] reported robotic partial hepatectomy of segments I-VIII, including pericystectomy of segment IVa in 1 case and segment VII in 1 case. The study showed that robots had certain advantages in partial hepatectomy in the posterosuperior region of the liver or in cases with the focus adjacent to the hepatic vein. In 2013, Troisi et al[26] also found that compared with laparoscopic hepatectomy, robotic surgery is safe and feasible in hepatectomy for difficult lesions of the posterosuperior region of the liver, and thus, has certain advantages in hepatectomy with the preservation of the liver parenchyma. In the current study, the focus of CE at segment VII was adjacent to the right hepatic vein in 1 case. After the focus was located by ultrasound during the operation, the hydatid cyst and the right hepatic vein were finely detached via the cephalic approach of the right hepatic vein, and segment VII was resected completely. In addition, pericystectomy was successfully performed in 1 case of CE at segment VIII. The operation time in 2 patients were 175 min and 300 min, respectively; the intraoperative blood loss was 100 mL; the postoperative hospital stay was 11 d and 5 d, respectively; and the Clavien-Dindo complication grades were grade I in both.
In 2019, Magistri et al[16] reported the results of robotic surgery in 15 cases of CE from 3 centers in Italy, including resection of the Spiegel’s lobe (as reported by Di Benedetto et al[23]), and other difficult segments. Consequently, we believe that the robot’s video imaging system, which provides a magnified high-definition three-dimensional visual field, can ensure full exposure of the deep space of the caudate lobe, segment VII, and segment VIII. With the advantages of simulating the flexibility of the human wrist, eliminating hand tremor, and providing continuous and stable traction, the robotic arm can assist in the fine separation of hydatid cyst from the hepatic vein and suturing of the hepatic vein hiatus, and reduce uncontrollable hemorrhage.
Radical hepatectomy for AE requires that the normal liver tissues should be more than 1 cm above the edge of the focus, so as to eliminate the active hyperplasia region of the focus. Laparoscopic and robotic hepatectomy for AE, however, has not been reported in the literature. In the present study, robotic right hepatectomy was successfully performed in 1 case of AE involving segment VII and segment VIII. The operation time was 225 min; the blood loss was 200 mL; the postoperative hospital stay was 9 d; and the Clavien-Dindo complication grade was grade I. As such, we believe that robotic hepatectomy is safe and feasible for AE partially confined to the hemiliver or hepatic segment.
In summary, robotic radical surgery for cystic and selected alveolar echinococcosis in difficult liver lesions is safe and feasible.
Radical resection is an important treatment method for hepatic echinococcosis. The posterosuperior segments of the liver remain the most challenging region for laparoscopic or robotic hepatectomy.
This study intended to retrospectively analyze the clinical data on robotic surgery for hepatic echinococcosis in difficult lesions from two centers in China and to explore the experience of performing robotic surgery for cystic and alveolar echinococcosis.
The aim of this study was to demonstrate the safety and preliminary experience of robotic radical resection of cystic and alveolar echinococcosis in posterosuperior liver segments.
A retrospective analysis was conducted on the clinical data of patients with hepatic echinococcosis who underwent robotic surgery from September to December 2019.
All 5 patients successfully underwent robotic radical hepatectomy for hepatic echinococcosis, including total caudate lobectomy, hepatectomy of segment VII, total pericystectomy of segment VIII, and right hemihepatectomy, without conversion to laparotomy or perioperative deaths. The operation time was 225 min; the blood loss was 100 mL; and the postoperative hospital stay duration was 10 d. The Clavien-Dindo complication grade was grade I in 4 cases and grade II in 1 case. No recurrence of echinococcosis was found in any of the patients after 3 mo of follow-up.
This study suggested that robotic radical surgery for cystic and selected alveolar echinococcosis in difficult liver lesions is safe and feasible.
Robotic transabdominal approach can be an option for resection of cystic and selected alveolar echinococcosis located in the posterosuperior hepatic segments.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lens S, Mueller S, Plessier A, Stanley A S-Editor: Wang JL L-Editor: MedE-Ma JY E-Editor: Liu MY
| 1. | McManus DP, Gray DJ, Zhang W, Yang Y. Diagnosis, treatment, and management of echinococcosis. BMJ. 2012;344:e3866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 249] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 2. | Wen H, Vuitton L, Tuxun T, Li J, Vuitton DA, Zhang W, McManus DP. Echinococcosis: Advances in the 21st Century. Clin Microbiol Rev. 2019;32:e00075-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 640] [Article Influence: 106.7] [Reference Citation Analysis (0)] |
| 3. | WHO Informal Working Group. International classification of ultrasound images in cystic echinococcosis for application in clinical and field epidemiological settings. Acta Trop. 2003;85:253-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 489] [Cited by in RCA: 501] [Article Influence: 22.8] [Reference Citation Analysis (1)] |
| 4. | Kern P, Wen H, Sato N, Vuitton DA, Gruener B, Shao Y, Delabrousse E, Kratzer W, Bresson-Hadni S. WHO classification of alveolar echinococcosis: principles and application. Parasitol Int. 2006;55 Suppl:S283-S287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 215] [Article Influence: 10.8] [Reference Citation Analysis (1)] |
| 5. | Brunetti E, Kern P, Vuitton DA, Writing Panel for the WHO-IWGE. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010;114:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1336] [Article Influence: 89.1] [Reference Citation Analysis (0)] |
| 6. | Katkhouda N, Fabiani P, Benizri E, Mouiel J. Laser resection of a liver hydatid cyst under videolaparoscopy. Br J Surg. 1992;79:560-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 81] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Kapan M, Yavuz N, Kapan S, Polat S, Goksoy E. Totally laparoscopic pericystectomy in hepatic hydatid disease. J Laparoendosc Adv Surg Tech A. 2004;14:107-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Maazoun K, Mekki M, Chioukh FZ, Sahnoun L, Ksia A, Jouini R, Jallouli M, Krichene I, Belghith M, Nouri A. Laparoscopic treatment of hydatid cyst of the liver in children. A report on 34 cases. J Pediatr Surg. 2007;42:1683-1686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Baltar Boilève J, Baamonde De La Torre I, Concheiro Coello P, García Vallejo LA, Brenlla González J, Escudero Pérez B, Solar Núñez JJ, Rivera Losada A, Folgar Villasenín L. [Laparoscopic treatment of hepatic hydatid cysts: techniques and post-operative complications]. Cir Esp. 2009;86:33-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Misra MC, Khan RN, Bansal VK, Jindal V, Kumar S, Noba AL, Panwar R, Kumar A. Laparoscopic pericystectomy for hydatid cyst of the liver. Surg Laparosc Endosc Percutan Tech. 2010;20:24-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Abu Hilal M, Aldrighetti L, Dagher I, Edwin B, Troisi RI, Alikhanov R, Aroori S, Belli G, Besselink M, Briceno J, Gayet B, D'Hondt M, Lesurtel M, Menon K, Lodge P, Rotellar F, Santoyo J, Scatton O, Soubrane O, Sutcliffe R, Van Dam R, White S, Halls MC, Cipriani F, Van der Poel M, Ciria R, Barkhatov L, Gomez-Luque Y, Ocana-Garcia S, Cook A, Buell J, Clavien PA, Dervenis C, Fusai G, Geller D, Lang H, Primrose J, Taylor M, Van Gulik T, Wakabayashi G, Asbun H, Cherqui D. The Southampton Consensus Guidelines for Laparoscopic Liver Surgery: From Indication to Implementation. Ann Surg. 2018;268:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 518] [Cited by in RCA: 496] [Article Influence: 70.9] [Reference Citation Analysis (0)] |
| 12. | Araki K, Kubo N, Watanabe A, Kuwano H, Shirabe K. Systematic review of the feasibility and future of laparoscopic liver resection for difficult lesions. Surg Today. 2018;48:659-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Nota CLMA, Molenaar IQ, van Hillegersberg R, Borel Rinkes IHM, Hagendoorn J. Robotic liver resection including the posterosuperior segments: initial experience. J Surg Res. 2016;206:133-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Chong CCN, Lok HT, Fung AKY, Fong AKW, Cheung YS, Wong J, Lee KF, Lai PBS. Robotic versus laparoscopic hepatectomy: application of the difficulty scoring system. Surg Endosc. 2020;34:2000-2006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 15. | Giulianotti PC, Coratti A, Sbrana F, Addeo P, Bianco FM, Buchs NC, Annechiarico M, Benedetti E. Robotic liver surgery: results for 70 resections. Surgery. 2011;149:29-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 206] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 16. | Magistri P, Pecchi A, Franceschini E, Pesi B, Guadagni S, Catellani B, Assirati G, Guidetti C, Guerrini GP, Tarantino G, Ballarin R, Codeluppi M, Morelli L, Coratti A, Di Benedetto F. Not just minor resections: robotic approach for cystic echinococcosis of the liver. Infection. 2019;47:973-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Egger ME, Gottumukkala V, Wilks JA, Soliz J, Ilmer M, Vauthey JN, Conrad C. Anesthetic and operative considerations for laparoscopic liver resection. Surgery. 2017;161:1191-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Ho KM, Han HS, Yoon YS, Cho JY, Choi YR, Jang JS, Kwon SU, Kim S, Choi JK. Laparoscopic Total Caudate Lobectomy for Hepatocellular Carcinoma. J Laparoendosc Adv Surg Tech A. 2017;27:1074-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Wang ZZ, Tang WB, Hu MG, Zhao ZM, Zhao GD, Li CG, Tan XL, Zhang X, Lau WY, Liu R. Robotic vs laparoscopic hemihepatectomy: A comparative study from a single center. J Surg Oncol. 2019;120:646-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Nunnari G, Pinzone MR, Gruttadauria S, Celesia BM, Madeddu G, Malaguarnera G, Pavone P, Cappellani A, Cacopardo B. Hepatic echinococcosis: clinical and therapeutic aspects. World J Gastroenterol. 2012;18:1448-1458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 241] [Cited by in RCA: 227] [Article Influence: 17.5] [Reference Citation Analysis (1)] |
| 21. | Georgiou GK, Lianos GD, Lazaros A, Harissis HV, Mangano A, Dionigi G, Katsios C. Surgical management of hydatid liver disease. Int J Surg. 2015;20:118-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Kamiyama T. Recent advances in surgical strategies for alveolar echinococcosis of the liver. Surg Today. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Di Benedetto F, Ballarin R, Tarantino G. Totally robotic isolated caudate-lobe liver resection for hydatid disease: report of a case. Int J Med Robot. 2016;12:254-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Araki K, Fuks D, Nomi T, Ogiso S, Lozano RR, Kuwano H, Gayet B. Feasibility of laparoscopic liver resection for caudate lobe: technical strategy and comparative analysis with anteroinferior and posterosuperior segments. Surg Endosc. 2016;30:4300-4306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Casciola L, Patriti A, Ceccarelli G, Bartoli A, Ceribelli C, Spaziani A. Robot-assisted parenchymal-sparing liver surgery including lesions located in the posterosuperior segments. Surg Endosc. 2011;25:3815-3824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 26. | Troisi RI, Patriti A, Montalti R, Casciola L. Robot assistance in liver surgery: a real advantage over a fully laparoscopic approach? Results of a comparative bi-institutional analysis. Int J Med Robot. 2013;9:160-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |