Published online May 28, 2020. doi: 10.3748/wjg.v26.i20.2632
Peer-review started: December 31, 2019
First decision: April 25, 2020
Revised: May 9, 2020
Accepted: May 16, 2020
Article in press: May 16, 2020
Published online: May 28, 2020
Processing time: 149 Days and 1.3 Hours
Obese patients (Ob) with a binge eating disorders (BED) behavior pattern have a higher prevalence of postprandial distress syndrome (PDS) compared to Ob without a BED behavior pattern, while an increase of PDS has been described in Ob after sleeve gastrectomy (SG). Hedonic response to a meal is dissociable from satiation in healthy subjects. Anhedonia is the lowered ability to experience pleasure. There are no studies investigating the presence of anhedonia in Ob with and without SG and its relationship to PDS symptoms.
To assess the relationship among anhedonia, BED and upper gastrointestinal symptoms in two group of morbidly Ob with and without SG.
Eighty-one Ob without SG, 45 Ob with SG and 55 healthy controls (HC) were studied. All subjects fulfilled the binge eating scale (BES) to investigate BED, the validated 14 items Snaith-Hamilton pleasure scale (SHAPS) to assess Anhedonia as well as the Beck Depression Inventory-II (BDI II) and State Trait Anxiety Inventory (STAI) questionnaires to screen for depression and anxiety. All patients underwent a standardized questionnaire investigating the intensity-frequency scores (0-6) of upper gastrointestinal symptoms and were diagnosed for the presence of functional dyspepsia (FD) and its subtypes according to ROME IV criteria.
Ob without SG who were positive for BED had a 4.7 higher risk of FD compared to Ob without SG who were negative for BED (OR: 4.7; 95.0%CI 1.23-18.24; P = 0.02). STAI-Y2 scores were significantly higher in Ob without SG positive for BED (42.2 ± 1.5 vs Ob negative for BED: 39.6 ± 1 .0, P = 0.04), while SHAPS scores and BDI II did not differ in the two groups (1.16 ± 1.30 vs 0.89 ± 1.02, P = 0.49). A lower prevalence of BED (BES > 17: 11.4% vs 40.7%, P = 0.001) and BDI-II (6.8 ± 1.2 vs 13.8 ± 1.9, P = 0.005) was reported in Ob with SG than Ob without SG, on the contrary total mean scores of STAI-Y1 and STAI-Y2 were significantly higher in Ob with SG than Ob without SG. Thirty-five percent of Ob with SG fulfilled the diagnosis of FD. SHAPS mean scores and the prevalence of anhedonia did not differ among the two groups (18.2 vs 8.1%, P = 0.2). Fifty-four percent of Ob with SG achieved surgical success excess weight loss > 50%. Excess weight loss was negatively related to SHAPS total mean scores [adjusted B: -7. 099 (95%CI: -13.91 to -0.29), P = 0.04].
Ob without SG showed a higher prevalence of PDS, mood disorders and anxiety when positive for BE behavior compared to those negative for BE behavior, whereas no differences were found in SHAPS score. Ob with SG showed a higher prevalence of PDS compared to Ob without SG. Concerning psychological aspect, BED and depression are less frequent in the Ob with SG, while both state and trait anxiety are significantly higher. Moreover, the more an Ob with SG is anhedonic, less surgical success was achieved.
Core tip: Binge eating disorders (BED) co-occur with mood disorders and anxiety, whereas the relationship with anhedonia in obese patients undergoing sleeve gastrectomy (SG) is not known. We studied two group of morbidly obese patients with and without SG to assess the relationships among anhedonia, BED and functional dyspepsia. Our results suggest that a more regular screening for functional dyspepsia in SG candidates might help to disclose the presence of BED that may jeopardize postsurgical outcomes. Although anhedonia was not associated with BED in this study, worse surgical outcome was observed in patients with anhedonia independent of early satiety and postprandial fullness.
- Citation: Santonicola A, Gagliardi M, Asparago G, Carpinelli L, Angrisani L, Iovino P. Anhedonia and functional dyspepsia in obese patients: Relationship with binge eating behaviour. World J Gastroenterol 2020; 26(20): 2632-2644
- URL: https://www.wjgnet.com/1007-9327/full/v26/i20/2632.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i20.2632
Obesity is increasing in industrialized countries[1]. Bariatric surgery (BS) for weight loss has emerged as an effective treatment of morbid obesity (Class II and III) since it accomplishes sustained weight loss, reduces obesity-related comorbidities and improves quality of life[2-4]. Among bariatric procedures, sleeve gastrectomy (SG) has increased in popularity and in 2014 became the most performed procedure in the world[5]. The association between obesity and some psychopathological features, specifically binge eating disorder (BED) is frequently present prior to surgery[6,7]. BED is characterized by recurrent episodes of binge eating and diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-V)[8]. It has been previously demonstrated that BED occurs in a subset ranging from 27% to 47% in patients with severe obesity undergoing BS[9] and may jeopardize postsurgical outcomes[10,11]. Obese patients (Ob) with BE behavior pattern have a higher prevalence of postprandial distress syndrome (PDS), a subtype of functional dyspepsia (FD) according to Rome III criteria[12] and, an increase of PDS has been described in Ob after SG[13]. FD is a syndrome characterized by the presence of chronic or recurrent symptoms invariably referred to the epigastrium, if no structural and/or biochemical alterations are detectable. The effect of specific patterns of eating behavior such as BED on the development of FD symptoms has not yet been completely defined[14]. From a physiological perspective, excessive intake of food over a relatively short time could potentially overcome the functional accommodation and emptying, contributing to the genesis of gastrointestinal symptoms in obese individuals[15]. Conversely, after SG the new onset of PDS might be linked to the decreased gastric capacity and altered duodenal sensitivity[13]. In obesity BED co-occur with a variety of psychiatric disorders, especially mood (49%), anxiety (41%) and substance use disorders (22%)[16]. Anhedonia, defined negatively as lack of and/or decreased capacity to experience pleasure[8], is considered one of the most indicative symptoms of mood disorders and depression[14,15]. It has been recently demonstrated that the presence of anhedonia, regardless of whether the participant had received a diagnosis of major depression, was associated with uncontrolled, emotional and binge eating. Moreover, weight loss was greater among participants without anhedonia[17].
We hypothesized that a vicious circle, aided by an eating disorder (ED) behavior and the presence of anhedonia may perpetuate upper gastrointestinal (GI) symptoms prior to surgery[18-20] and, eventually, influence the outcome of BS. Therefore, the objective of this study was to evaluate the relationship among anhedonia, BED and upper GI in morbidly Ob with and without SG.
Eighty-one Ob without SG and 45 Ob with SG, similar for age and sex, were recruited from an outpatient clinic devoted to surgical therapy of obesity and related disorders at San Giovanni Bosco Hospital, Naples, Italy and run by surgeons and gastroenterologists trained in the field. The study was approved by the Ethical Committee of the ASL Napoli Centro. Informed consent was obtained from all patients.
The inclusion criteria were Caucasian females and males between the ages of 18 and 75 years having the ability to understand and the willingness to comply with the study procedures. Exclusion criteria were serious, unstable medical condition, major psychiatric disorders, previous history of drug or alcohol abuse, previous major abdominal surgery with the exception of laparoscopic SG, laparoscopic cholecystectomy and appendectomy and pregnant women.
Demographic characteristics (gender, age, smoking habits, school degree), anthropometric data [weight, height and body mass index (BMI)], and prevalence of comorbidities, such as, hypertension, dyslipidemia, type II diabetes mellitus, and respiratory diseases were individually collected. In Ob undergoing SG excess weight loss (EWL) was calculated[21] and the surgical success was defined as EWL > 50%[22]. Fifty-five healthy controls (HC) were enrolled from friends of obese patients and hospital staff as the control group. Inclusion criteria for HC were Caucasian adults, aged between 18 to 75 years without upper gastrointestinal complaints. Exclusion criteria were similar to that for Ob. Demographic characteristics (gender, age, smoking habits, school degree) and anthropometric data (weight, height and BMI) were collected for each HC.
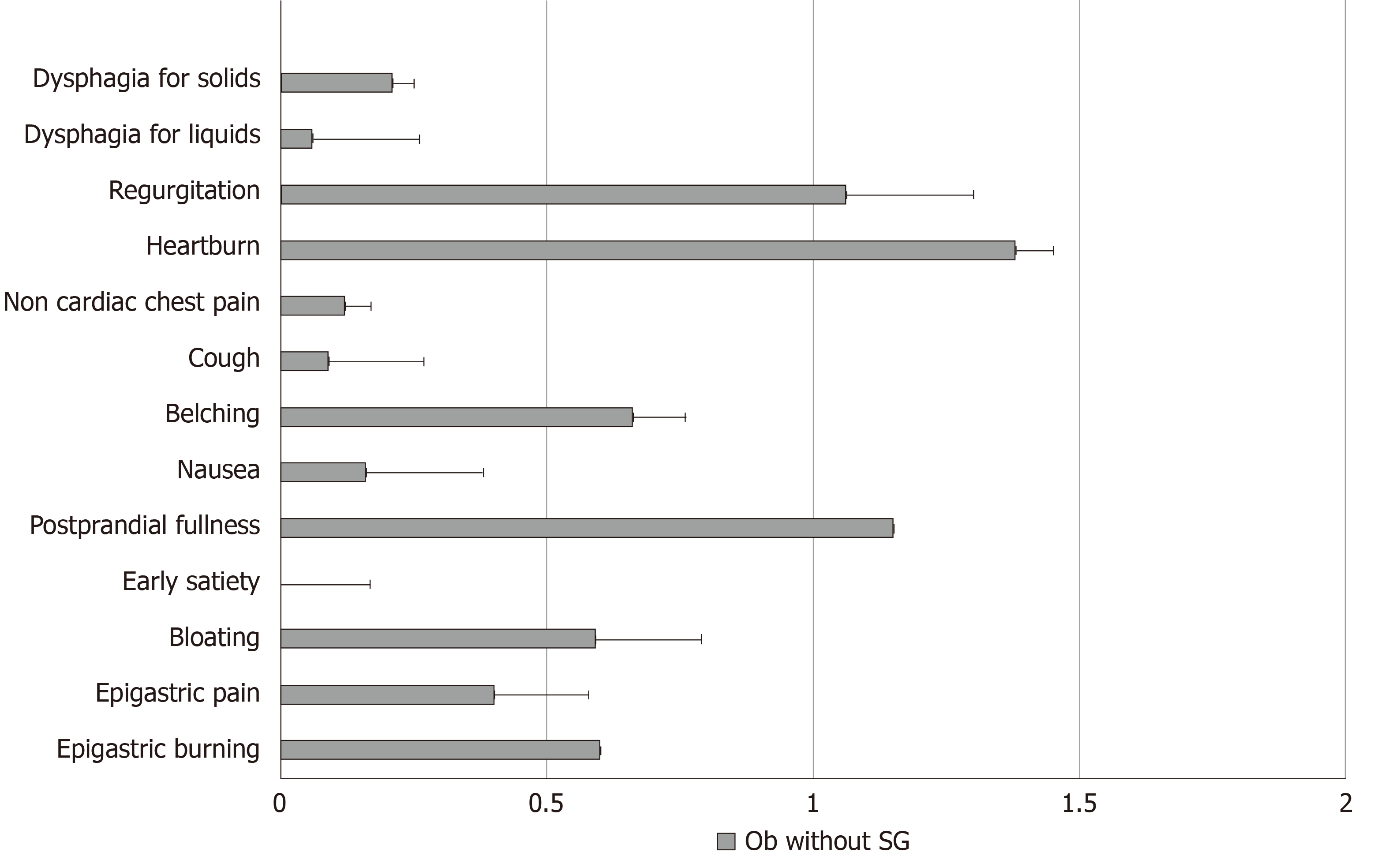
All patients were evaluated using a standardized questionnaire that assesses upper GI symptoms such as dysphagia for solids, dysphagia for liquids, regurgitation, heartburn, non-cardiac chest pain, cough, belching, nausea, bloating, early satiation, epigastric fullness, epigastric pain and epigastric burning with a scoring frequency from 0 to 3 (0 = absent, 1 = 2 d/wk; 2 = 3-5 d/wk; and 3 = 6 or 7 d/wk) and intensity from 0 to 3 (0 = absent; 1 = not very bothersome, not interfering with daily activities; 2 = bothersome, but not interfering with daily activities; and 3 = interfering with daily activities). A frequency-intensity score from 0 up to a maximum of 6 was obtained for each symptom[12].
All participants were evaluated by the Snaith–Hamilton pleasure scale (SHAPS). The SHAPS is a self-assessment tool used to measure hedonic experience or positive patient value. It is composed of 14 items that cover four domains of pleasure: Interests/pastimes, social interaction, sensory experiences and food/drink. Higher scores represent a reduction of hedonic tone. A score of three or more allows a categorical definition of anhedonia[23,24]. An Italian version of the SHAPS has been previously validated and has been used in routine clinical practice and research[25].
All participants were evaluated by the binge eating scale (BES), a specific questionnaire originally created to investigate binge-eating behavior in obese patients. The BES measures the behavioral aspects of binge eating, as well as the feelings and thoughts associated with such behavior[26]. It is a self-administered questionnaire composed of 16 multiple choice items: Eight items that describe behavioral manifestations (for example, eating fast or consuming large amounts of food) and eight items on associated feelings and cognitions (for example, fear of not stopping eating)[27]. Each item has a response range from 0 to 3 points (0 = no severity of the BES symptoms, 3 = serious problems of the BES symptoms). Based on the BES total score, individuals can be categorized into three groups according to established cut scores of binge eating severity[28]. A frequent convention is to use the BES as a screening measure to classify all participants with scores greater than or equal to 17 as “binge eaters”[29,30].
All participants filled in the Beck Depression Inventory-II (BDI-II) scale. The BDI-II is a self-report questionnaire that is integrated into routine clinical practice (as screening tools) in large managed-care organizations. BDI-II follows the criteria for depression listed in the fourth edition of the DSM. The test consists of twenty-one questions that not only assess the presence of depression, but also the severity of depression as well[31]. Each question has a set of at least four possible responses ranging in intensity, with a value of 0 to 3 is assigned for each answer and the total score compared to a key to determine severity. The standard cut-off scores are as follows: 0-9: Indicates minimal depression; 10–18: Indicates mild depression; 19–29: Indicates moderate depression; 30-63: Indicates severe depression[32]. For this study we considered a cut-off for this questionnaire of 14, considering patients positive with a score higher than 14 and negative with a score lower than or equal to 14. An Italian version of the BDI-II was previously validated[33,34].
The state trait anxiety inventory (STAI) is one of the most widely used self-report measures for the presence and grade of anxiety. The questionnaires contain 20 items, with responses related to terms of intensity (from “almost never” to “almost always”). The items are grouped into two axes, which permit a distinction between existing anxiety (STAI-Y1) and predisposition to an anxious reaction as a personality characteristic (STAI-Y2). A score ≥ 40 is the cut-off value for both scales[35].
All Ob were investigated for the presence of FD according to ROME IV criteria[36], after the exclusion of any organic disease. A complete physical examination, blood tests, upper GI endoscopy and additional tests were performed when indicated[37]. The scores were calculated for the 4 cardinal symptoms pragmatically described by the Rome IV Committee such as postprandial fullness, early satiation, epigastric pain, and epigastric burning (Table 1). The spectrum of FD includes patients suffering from the diagnostic categories of PDS which is characterized by meal-induced dyspeptic symptoms, epigastric pain syndrome (EPS) which refers to epigastric pain or epigastric burning that does not occur exclusively postprandially, can occur during fasting, and can be even improved by meal ingestion, and overlapping PDS and EPS, which is characterized by meal induced dyspeptic symptoms and epigastric pain or burning.
| Functional dyspepsia | ||
| One or more of the following: | And | No evidence of structural disease (including at upper endoscopy) that is likely to explain the symptoms |
| Bothersome postprandial fullness | ||
| Bothersome early satiation | ||
| Bothersome epigastric pain | ||
| Bothersome epigastric burning | ||
| Post Prandial distress syndrome | Epigastric pain syndrome | |
| must include one or both of the following at least 3 d/w: | Must include at least 1 of the following symptoms at least 1 day/ w: | |
| Bothersome postprandial fullness (i.e, strict enough to impact on usual activities) | Bothersome epigastric pain (i.e, severe enough to impact on usual activities) | |
| Bothersome early satiation (i.e, severe enough to prevent finishing a regular-size meal) | Bothersome epigastric burning (i.e, severe enough to impact on usual activities) | |
The primary outcomes were to evaluate upper GI symptoms, the level of anhedonia and BED in Ob with and without SG. Secondary outcomes were the evaluation of the coexistence of BED with depressive mood and anxiety as well as the relationship of anhedonia level with the outcome of BS.
The data are expressed in frequencies and percentages for qualitative variables, as mean ± standard error (mean ± SE) for quantitative ones unless otherwise indicated. Significance was expressed at P < 0.05 level. When appropriate, a χ2 test for categorical data and analysis of variance for continuous data were used. We then performed a subgroup analysis to test the risk of having PDS according to the presence or absence of BED using a logistic model. Finally, using a regression analysis, we tested how much the EWL changed according to the level of anhedonia and to the intensity-frequency scores of PDS symptoms. The SPSS for Windows version 15.0 statistical package (SPSS Inc, Chicago, IL, United States) was used for statistical analysis.
Demographic characteristics, anthropometric data, and prevalence of comorbidities in Ob without SG and HC are shown in Table 2. Thirty-three/81 (40.7%) of Ob without SG vs 3/55 (5.5%) of HC reported a patient a pathological score for BED (> 17) at BES (P < 0.001).
| Ob without SG (n = 81) | HC (n = 55) | |
| Gender (M/F) | 23/58 | 18/37 |
| Age (yr) | 36.5 ± 1.3 | 36.7 ± 1.2 |
| Weight (kg) | 122.1 ± 3.2 | 64 ± 2.5b |
| BMI (kg/m2) | 44.4 ± 0.9 | 23.2 ± 0.6b |
| Ethnic origin (Caucasian) | 100 | 100 |
| Smoking | 27.7 | 14.3 |
| Number of cigarettes per day | 22.5 ± 3.7 | 11 ± 1.0 |
| Diabetes | 8.5 | 0 |
| Hypertension | 19.1 | 7.1 |
| Dyslipidemia | 25.5 | 14.3 |
| Respiratory diseases | 42.6 | 0b |
| Musculoskeletal disorders | 19.1 | 0 |
| BES | 15.5 ± 0.9 | 5.4 ± 0.6b |
| STAI-Y1 | 39.7 ± 0.9 | 41.1 ± 1.7 |
| STAI-Y2 | 40.1 ± 0.9 | 41.2 ± 1.6 |
| BDI 2 | 13.8 ± 1.9 | 5.8 ± 0.5b |
| SHAPS | 1.0 ± 0.2 | 0.8 ± 0.1 |
STAI-Y1 and STAI-Y2 total scores were not significantly different in OB without SG and HC. Thirty nine percent of Ob without SG showed a pathological score at Beck Depression Inventory-II (BDI II) (> 14) and BDI II scores were significantly higher in Ob without SG compared to HC (Table 2). SHAPS mean scores did not differ among the two groups (Table 2) and the prevalence of anhedonia, according to DSM-V (score > 3), was similar in Ob without SG and HC (8.1% vs 6.2%, P = 0.69).
Frequency-intensity scores for selected upper GI symptoms such as dysphagia for solids, dysphagia for liquids, regurgitation, heartburn, non-cardiac chest pain, cough, belching, nausea, early satiation, epigastric fullness, epigastric pain and epigastric burning in Ob without SG are illustrated in Figure 1. According to the presence or absence of BED, the frequency intensity scores of the studied upper GI symptoms (dysphagia for solids, dysphagia for liquids, regurgitation, heartburn, non-cardiac chest pain, cough, belching, nausea, bloating) did not differ between Ob without SG who were positive or negative for BED (Table 3).
| Ob without SG positive for BED (n = 33) | Ob without SG negative for BED (n = 48) | p | |
| Dysphagia for solids | 0.34 ± 0.19 | 0.10 ± 0.07 | 0.20 |
| Dysphagia for liquids | 0 | 0.10 ± 0.07 | 0.22 |
| Regurgitation | 0.68 ± 0.24 | 1.33 ± 0.29 | 0.11 |
| Heartburn | 1.28 ± 0.39 | 1.45 ± 0.30 | 0.72 |
| Non cardiac chest pain | 0.21 ± 0.15 | 0.05 ± 0.05 | 0.28 |
| Cough | 0.14 ± 0.10 | 0.05 ± 0.05 | 0.40 |
| Belching | 0.72 ± 0.28 | 0.62 ± 0.22 | 0.76 |
| Nausea | 0.14 ± 0.10 | 0.18 ± 0.16 | 0.84 |
| Bloating | 1.52 ± 0.33 | 0.85 ± 0.26 | 0.11 |
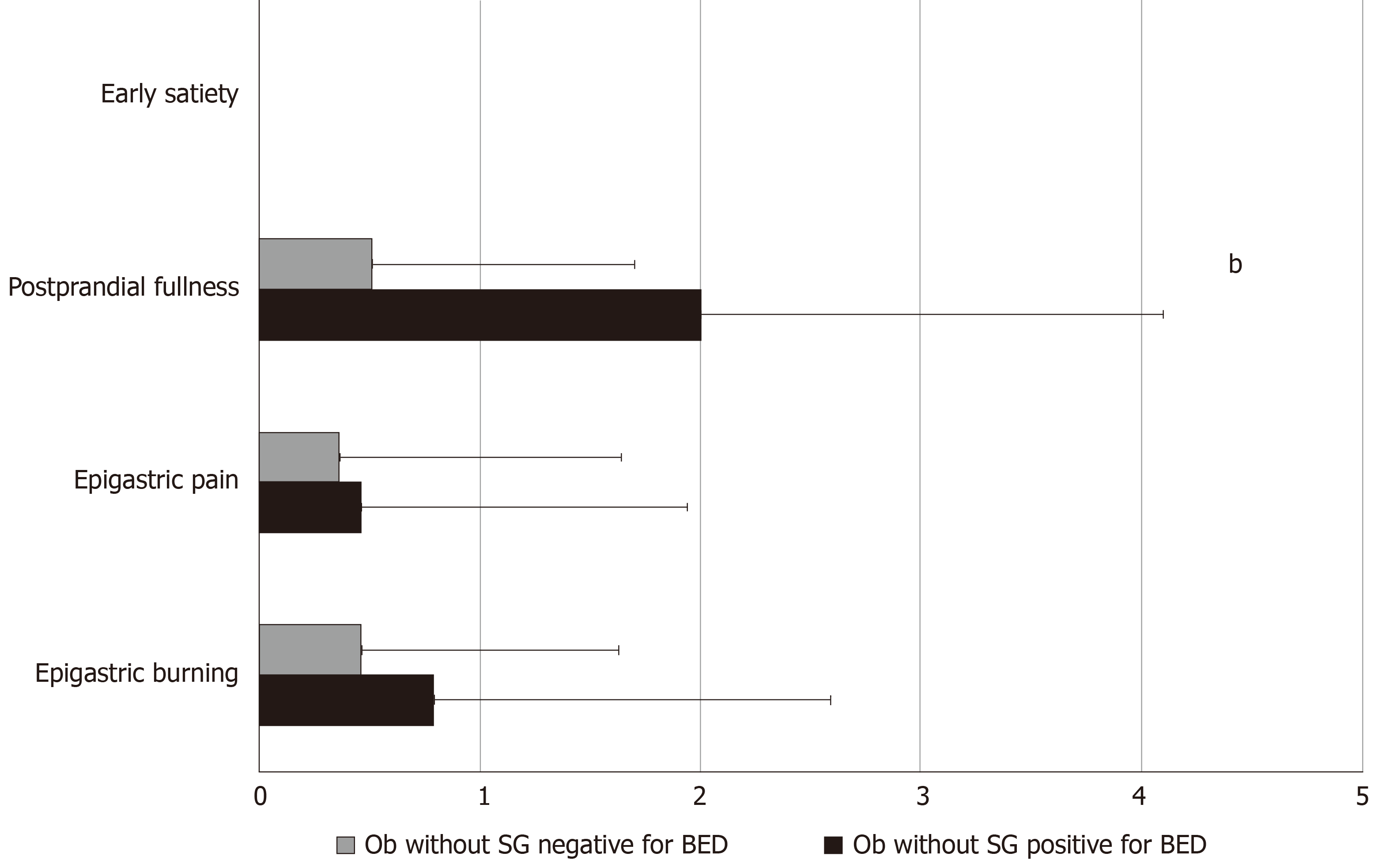
The prevalence of FD, according to Rome IV criteria, was significantly higher in Ob without SG who were positive for BED compared to those who were negative for BED (36.7% vs 10.5%, P = 0.01). Specifically, except for two patients who complained of EPS symptoms, all the remaining Ob fulfilled the diagnostic criteria for PDS. Ob without SG who were positive for BED showed 4.7 higher risk of having FD, independent of age and gender (OR: 4.7; 95.0%CI: 1.23-18.24; P = 0.02) compared to Ob without SG who were negative for BED. Figure 2 describes the frequency-intensity score of the 4 cardinal FD symptoms in Ob without SG who were positive or negative for BED.
Mean SHAPS scores and the prevalence of anhedonia, according to DSM-V (score > 3) did not differ in Ob without SG positive or negative for BED (1.16 ± 1.30 vs 0.89 ± 1.02, P = 0.49 and 10.5 vs 5.6%, P = 0.58). Also, BDI II score was similar in the two groups (10.3 ± 2.6 vs 16.7 ± 2.7, P = 0.09). Although mean STAI-Y1 scores were not significantly different among the two groups (P = 0.43), mean STAI-Y2 scores were significantly higher in Ob without SG positive for BED (42.2 ± 1.5 vs 39.6 ± 1.0, P = 0.04).
Obese patients having undergone SG (Ob with SG) were similar for age and sex to Ob without SG (Table 4). Fifty-four percent of Ob with SG achieved surgical success (EWL > 50%). A significantly lower frequency of pathological score for BED (> 17) at BES was reported in Ob with SG than Ob without SG (11.4% vs 40.7%, P = 0.001). SHAPS mean scores shown in Table 4 and the prevalence of anhedonia, according to DSM-V (score > 3) (18.2 vs 8.1%, P = 0.2) did not differ among the two groups. Total mean BES, BDI II, STAI-Y1 and STAI-Y2 in Ob with SG compared to those without SG are reported in Table 5. Frequency-intensity scores of other upper GI symptoms such as dysphagia for solids, dysphagia for liquids, regurgitation, heartburn, non-cardiac chest pain, cough, belching, nausea, bloating was not significantly different among the two groups (Table 6).
| Ob with SG (n = 45) | |
| Gender (M/F) | 7/38 |
| Age (yr) | 38.36 ± 1.6 |
| Weight (kg) | 88.47 ± 3.2 |
| BMI (kg/m2) | 32.74 ± 1.0 |
| WL | 29.61 ± 2.2 |
| EWL (%) | 53.41 ± 3.45 |
| Months since the operation | 25.67 ± 5.14 |
| Ethnic origin (Caucasian %) | 45 |
| Smoking (%) | 35.3% |
| Number of cigarettes per day | 13.17 ± 3.5 |
| Diabetes (%) | 9.1% |
| Hypertension (%) | 18.2% |
| Dyslipidemia (%) | 36.4% |
| Respiratory diseases (%) | 54.5% |
| Musculoskeletal disorders (%) | 45.5% |
| Ob without SG | Ob with SG | |
| Dysphagia for solids | 0.21 ± 0.09 | 0.15 ± 0.15 |
| Dysphagia for liquids | 0.06 ± 0.04 | 0.15 ± 0.15 |
| Regurgitation | 1.06 ± 0.2 | 1.38 ± 0.34 |
| Heartburn | 1.38 ± 0.23 | 0.96 ± 0.33 |
| Non cardiac chest pain | 0.12 ± 0.07 | 0.38 ± 0.22 |
| Cough | 0.09 ± 0.05 | 0.15 ± 0.15 |
| Belching | 0.66 ± 0.17 | 0.46 ± 0.20 |
| Nausea | 0.16 ± 0.09 | 0.42 ± 0.26 |
| Bloating | 1.13 ± 0.21 | 0.23 ± 0.17b |
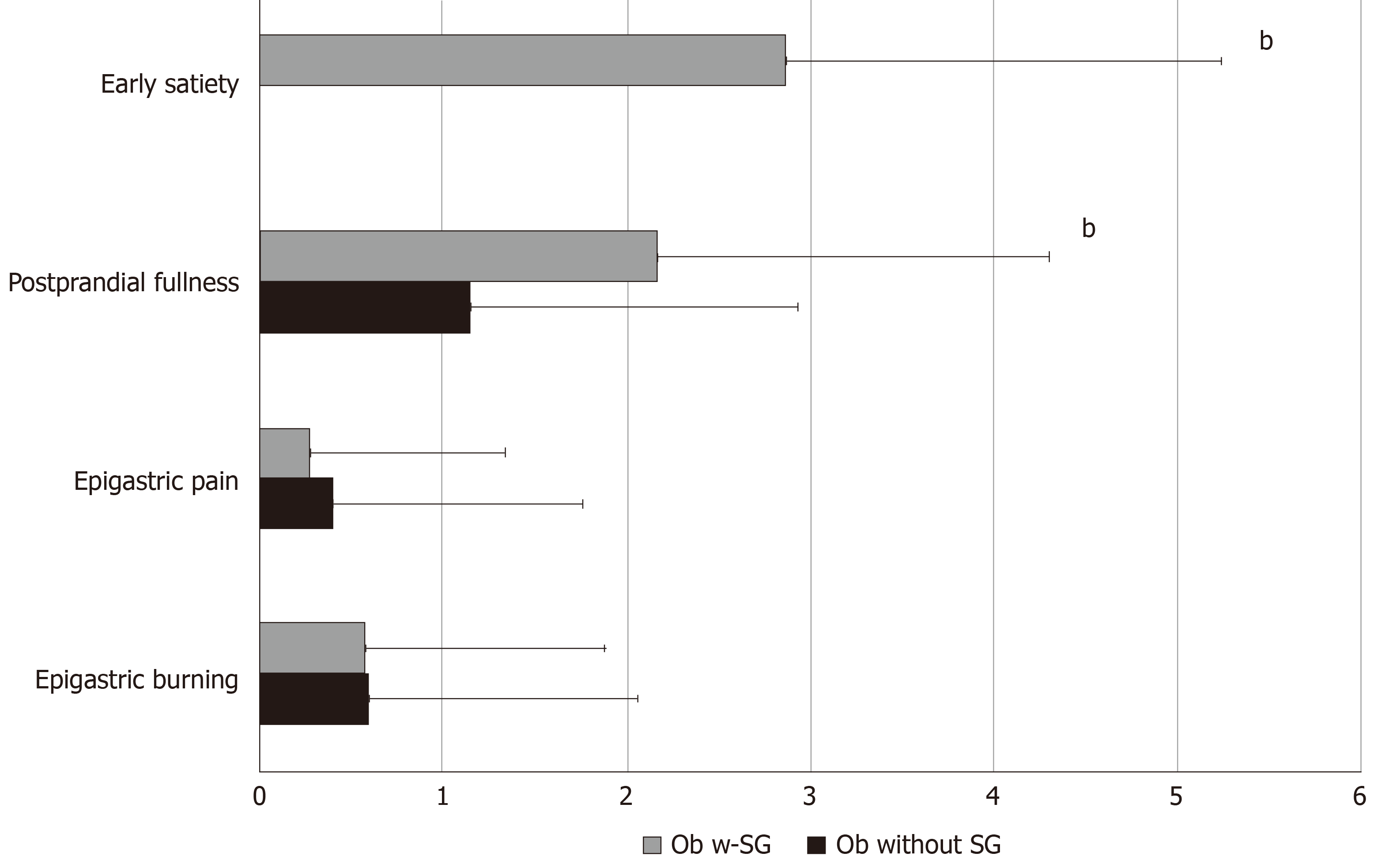
Thirty-five percent of Ob with SG fulfilled the diagnosis of FD, in particular PDS, according to Rome IV criteria; frequency-intensity scores of early satiation and postprandial fullness were significantly higher in Ob with SG compared to those without SG (Figure 3). In Ob with SG accordingly to the presence or absence of BED the frequency-intensity scores of early satiation and postprandial fullness did not differ (2.0 ± 1.4 BED positive vs 2.28 ± 0.4 BED negative). In Ob with SG the regression analysis showed that EWL is negatively related to SHAPS total mean scores [adjusted B -7. 099 (95%CI: -13.909 to -0.290), P = 0.04] independent of early satiation and postprandial fullness.
In this study two groups of Ob were evaluated. In the group without SG a higher prevalence of BED, depression and anxiety disorders but not anhedonia was observed in comparison with HC. Moreover, Ob without SG fulfilling BED criteria showed more anxiety and a higher risk of having FD. The other group of Ob with SG showed a turnabout of BED, depression and anxiety levels and a marked increase in PDS prevalence compared to Ob without SG. In addition, a higher excess weight loss was significantly associated with a lower anhedonia level.
This is, to the best of our knowledge, the first study to assess the relationships among psychiatric comorbidity and upper GI symptoms in Ob with and without SG. Depression was repeatedly associated with an overconsumption of food[38,39] and BED[40]. The presence of anhedonia, irrespective of whether the participant had received a diagnosis of major depression or dysthymia, was associated with uncontrolled, emotional eating[17]. Many studies claim that the prevalence of anxiety disorders is higher than the general population for all ED[41] as well as trait anxiety is associated with increased rates of compensatory behaviors, binge eating, and body dissatisfaction[42]. BED correlates with an increased BMI[6,7] and is a common findings in Ob undergoing BS and influences their outcome. It is already known that the frequency of FD according to Rome III criteria was similar in Ob and control subjects, while Ob with coexisting BE behavior have a higher prevalence of PDS[12]. In this study we confirmed this result with the new Rome IV criteria as well as the finding that early satiation is not a feature of Ob without SG. This finding is consistent with the datum that satiation signals that inhibit ingestion are reduced with increased BMI[43]. Conversely, the increase in frequency and severity of postprandial fullness in Ob without SG that fulfilled BED criteria might be explained by an excessive food intake over a relatively short time that could potentially impair the functional accommodation and the gastric emptying[12].
Our study cannot establish the direction of effect of these associations with regard to obesity, depression, anhedonia, anxiety, eating behavior and upper GI symptoms because of the design; however, our results suggest that in Ob without SG, the presence of BED might be a dominant player in determining FD. On the other side, concerning the psychological aspect, Ob with SG showed a reduced depression severity but an increased level of anxiety compared to Ob without SG; however, the level of anhedonia did not differ between the two Ob groups. It has been previously demonstrated that bariatric surgery might decrease depression and mental health issues post-surgery[44]. Higher rates of post-surgical depression are associated with less weight loss success[44]. Furthermore, our results demonstrated for the first time that EWL after SG was negatively related to anhedonia. In other words, the more a subject is anhedonic, less surgical success was achieved. A previous study has already suggested that weight loss was greater among participants without anhedonia that participated to a weight loss counseling intervention healthy program[17]. The prevalence of BED was lower in Ob with SG compared to Ob without SG. Since BED might negatively affect surgical outcome, it is possible that Ob with BED did not undergo BS[10,11].
In Ob with SG early satiation and postprandial fullness are more frequent than in Ob without SG, while epigastric pain and epigastric burning as well as other upper GI symptoms have the same frequency in both Ob groups. It is noteworthy that early satiation was absent in patients without SG; conversely, the reduction in gastric capacity following SG leads to a new onset of this symptom. Hence, our results suggest that in Ob with SG the increase of PDS symptoms is independent from the presence of BED.
Our data confirm a key role of epigastric symptoms in determining calorie intake in health, obesity, and dyspepsia. The complex and reciprocal interplay between biological, psychological, and social factors, rather than from linear monocausal etiopathogenetic processes is a common characteristic of both functional dyspepsia and obesity. For example, it is well known that individual assessment of obesity and development of counseling should include not only clinical factors such as body weight and dietary intake, but also an assessment of psychological factors such as psychological eating behavior traits. Rome IV criteria that are based on the biopsychosocial approach could appeared unnecessary in the daily care of these Ob but still can serve as a useful guide to help understand through symptoms the complexity of the disease and to capture also other comorbidity of the clinical condition of Ob optimizing the treatment. In fact, regarding the management a physician who acknowledges the reality of the patient’s complaints, engages in an effective physician–patient interaction, and reduces symptom severity and health care seeking. Conversely, simply managing symptoms such as postprandial fullness may lead to perform unnecessary diagnostic studies to rule out pathologic disease and is likely to promote a vicious cycle of symptom anxiety and health care seeking[45]. Another strength of the present study is that our findings offer a screenshot of the Italian obese population.
There are some limitations in this study. One is the relatively small sample size and the heterogeneity of patients that might have limited the ability to generalize the findings to wider clinical samples. Another limitation is the design of the study that weakens our findings as compared to results from a prospective longitudinal study that are needed to establish a causal relationship among anhedonia, BED and upper GI symptoms. Moreover, all data were collected using self-reported questionnaires that might reflect the participant's own perspective. However, the simplicity of the questionnaire makes it somewhat easy for the participants to give accurate information questionnaires and they were validated in Italian language.
In conclusion, the results of the current study suggest that a more regular screening of PDS symptoms accordingly to Rome Criteria when evaluating obese patients before bariatric surgery might help to disclose the presence of BED that may jeopardize postsurgical outcomes. Although anhedonia was not associated with BED in this study, worse surgical outcome was observed in patients with anhedonia independent of the higher intensity-frequency scores of early satiation and postprandial fullness. An individual assessment of psychological factors such anhedonia should be incorporated into tailoring future treatment interventions in patients with unfavorable surgical outcome. Further research is urgently required to understand the pathophysiological interactions between anhedonia, BED and the onset of upper GI symptoms in morbidly obese patients pre and post bariatric surgery.
Obesity is increasing in industrialized countries. Among bariatric procedures for weight loss sleeve gastrectomy (SG) has emerged as an effective treatment of morbid obesity. The association between obesity and some psychopathological features, specifically binge eating disorder (BED) is frequent. Anhedonia was associated with uncontrolled, emotional and binge eating. Weight loss was greater in obese patients (Ob) without anhedonia. Ob with BED have a higher prevalence of postprandial distress syndrome (PDS), a subtype of functional dyspepsia (FD) according to Rome III criteria and, an increase of PDS has been described in Ob after SG.
The effect of specific patterns of eating behavior such as BED and on the development of FD symptoms has not yet been completely defined in Ob with and without SG. There are no studies investigating the presence of anhedonia in Ob with and without SG and its relationship to PDS symptoms.
In this study we aimed to assess the relationship among anhedonia, BED and upper gastrointestinal symptoms in two group of morbidly Ob with and without SG.
Ob without SG, Ob with SG and healthy controls (HC) the binge eating scale (BES) to investigate BED, the validated 14 items Snaith-Hamilton pleasure scale (SHAPS) to assess anhedonia, the Beck Depression Inventory-II (BDI II) and state trait anxiety inventory (STAI) questionnaires to screen for depression and anxiety. They were diagnosed for the presence of functional dyspepsia (FD) and its subtypes according to ROME IV criteria.
Ob without SG who were positive for BED had a 4.7 higher risk of FD and a higher STAI-Y2 scores than Ob negative for BED, while SHAPS scores and BDI II did not differ between the two groups. Ob with SG showed a higher prevalence of PDS and STAI-Y1 and STAI-Y2 scores compared to Ob without SG. Conversely, Ob with SG had a lower prevalence of BED and BDI-II than Ob without SG. Excess weight loss was negatively related to SHAPS total mean scores [adjusted B – 7. 099 (95%CI: -13.91- -0.29), P = 0.04].
Ob without SG showed a higher prevalence of PDS, mood disorders and anxiety when positive for BE behavior. Ob with SG showed a higher prevalence of PDS compared to Ob without SG. Concerning psychological aspect, BED and depression are less frequent in the Ob with SG, while both state and trait anxiety are significantly higher. Moreover, the more an Ob with SG is anhedonic, less surgical success was achieved.
A more regular screening of PDS symptoms accordingly to Rome IV Criteria before bariatric surgery might help to disclose the presence of BED. An individual assessment of psychological factors such anhedonia should be incorporated into tailoring future treatment interventions in patients with unfavorable surgical outcome. Further research is urgently required to understand the pathophysiological interactions between anhedonia, BED and the onset of upper GI symptoms in morbidly obese patients pre and post bariatric surgery.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chiarioni G S-Editor: Wang J L-Editor: A E-Editor: Liu MY
| 1. | Friedrich MJ. Global Obesity Epidemic Worsening. JAMA. 2017;318:603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 2. | Angrisani L, Santonicola A, Hasani A, Nosso G, Capaldo B, Iovino P. Five-year results of laparoscopic sleeve gastrectomy: effects on gastroesophageal reflux disease symptoms and co-morbidities. Surg Obes Relat Dis. 2016;12:960-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Colquitt JL, Pickett K, Loveman E, Frampton GK. Surgery for weight loss in adults. Cochrane Database Syst Rev. 2014;CD003641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 497] [Cited by in RCA: 675] [Article Influence: 61.4] [Reference Citation Analysis (1)] |
| 4. | Vitiello A, Angrisani L, Santonicola A, Iovino P, Pilone V, Forestieri P. Bariatric Surgery Versus Lifestyle Intervention in Class I Obesity: 7-10-Year Results of a Retrospective Study. World J Surg. 2019;43:758-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Angrisani L, Santonicola A, Iovino P, Vitiello A, Higa K, Himpens J, Buchwald H, Scopinaro N. IFSO Worldwide Survey 2016: Primary, Endoluminal, and Revisional Procedures. Obes Surg. 2018;28:3783-3794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 791] [Cited by in RCA: 728] [Article Influence: 104.0] [Reference Citation Analysis (0)] |
| 6. | Baldofski S, Tigges W, Herbig B, Jurowich C, Kaiser S, Stroh C, de Zwaan M, Dietrich A, Rudolph A, Hilbert A. Nonnormative eating behavior and psychopathology in prebariatric patients with binge-eating disorder and night eating syndrome. Surg Obes Relat Dis. 2015;11:621-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Micanti F, Iasevoli F, Cucciniello C, Costabile R, Loiarro G, Pecoraro G, Pasanisi F, Rossetti G, Galletta D. The relationship between emotional regulation and eating behaviour: a multidimensional analysis of obesity psychopathology. Eat Weight Disord. 2017;22:105-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition, 2013. |
| 9. | Stunkard AJ. Eating disorders and obesity. Psychiatr Clin North Am. 2011;34:765-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Dawes AJ, Maggard-Gibbons M, Maher AR, Booth MJ, Miake-Lye I, Beroes JM, Shekelle PG. Mental Health Conditions Among Patients Seeking and Undergoing Bariatric Surgery: A Meta-analysis. JAMA. 2016;315:150-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 346] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 11. | Livhits M, Mercado C, Yermilov I, Parikh JA, Dutson E, Mehran A, Ko CY, Gibbons MM. Preoperative predictors of weight loss following bariatric surgery: systematic review. Obes Surg. 2012;22:70-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 415] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 12. | Santonicola A, Angrisani L, Ciacci C, Iovino P. Prevalence of functional gastrointestinal disorders according to Rome III criteria in Italian morbidly obese patients. ScientificWorldJournal. 2013;2013:532503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Carabotti M, Silecchia G, Greco F, Leonetti F, Piretta L, Rengo M, Rizzello M, Osborn J, Corazziari E, Severi C. Impact of laparoscopic sleeve gastrectomy on upper gastrointestinal symptoms. Obes Surg. 2013;23:1551-1557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Cremonini F, Camilleri M, Clark MM, Beebe TJ, Locke GR, Zinsmeister AR, Herrick LM, Talley NJ. Associations among binge eating behavior patterns and gastrointestinal symptoms: a population-based study. Int J Obes (Lond). 2009;33:342-353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Santonicola A, Gagliardi M, Guarino MPL, Siniscalchi M, Ciacci C, Iovino P. Eating Disorders and Gastrointestinal Diseases. Nutrients. 2019;11:3038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 16. | Grilo CM, White MA, Barnes RD, Masheb RM. Psychiatric disorder co-morbidity and correlates in an ethnically diverse sample of obese patients with binge eating disorder in primary care settings. Compr Psychiatry. 2013;54:209-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Keränen AM, Rasinaho E, Hakko H, Savolainen M, Lindeman S. Eating behavior in obese and overweight persons with and without anhedonia. Appetite. 2010;55:726-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Drossman DA, Creed FH, Olden KW, Svedlund J, Toner BB, Whitehead WE. Psychosocial aspects of the functional gastrointestinal disorders. Gut. 1999;45 Suppl 2:II25-II30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 136] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 19. | Jones MP, Crowell MD, Olden KW, Creed F. Functional gastrointestinal disorders: an update for the psychiatrist. Psychosomatics. 2007;48:93-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Perkins SJ, Keville S, Schmidt U, Chalder T. Eating disorders and irritable bowel syndrome: is there a link? J Psychosom Res. 2005;59:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | van de Laar A. Bariatric Outcomes Longitudinal Database (BOLD) suggests excess weight loss and excess BMI loss to be inappropriate outcome measures, demonstrating better alternatives. Obes Surg. 2012;22:1843-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 22. | Reinhold RB. Critical analysis of long term weight loss following gastric bypass. Surg Gynecol Obstet. 1982;155:385-394. [PubMed] |
| 23. | Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br J Psychiatry. 1995;167:99-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 864] [Cited by in RCA: 1102] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 24. | Carpinelli L, Bucci C, Santonicola A, Zingone F, Ciacci C, Iovino P. Anhedonia in irritable bowel syndrome and in inflammatory bowel diseases and its relationship with abdominal pain. Neurogastroenterol Motil. 2019;31:e13531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Santangelo G, Morgante L, Savica R, Marconi R, Grasso L, Antonini A, De Gaspari D, Ottaviani D, Tiple D, Simoni L, Barone P; PRIAMO Study Group. Anhedonia and cognitive impairment in Parkinson's disease: Italian validation of the Snaith-Hamilton Pleasure Scale and its application in the clinical routine practice during the PRIAMO study. Parkinsonism Relat Disord. 2009;15:576-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Gormally J, Black S, Daston S, Rardin D. The assessment of binge eating severity among obese persons. Addict Behav. 1982;7:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1310] [Cited by in RCA: 1346] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 27. | Escrivá-Martínez T, Galiana L, Rodríguez-Arias M, Baños RM. The Binge Eating Scale: Structural Equation Competitive Models, Invariance Measurement Between Sexes, and Relationships With Food Addiction, Impulsivity, Binge Drinking, and Body Mass Index. Front Psychol. 2019;10:530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 28. | Finlayson G, Arlotti A, Dalton M, King N, Blundell JE. Implicit wanting and explicit liking are markers for trait binge eating. A susceptible phenotype for overeating. Appetite. 2011;57:722-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 29. | Marcus MD, Wing RR, Hopkins J. Obese binge eaters: affect, cognitions, and response to behavioural weight control. J Consult Clin Psychol. 1988;56:433-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 67] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Grupski AE, Hood MM, Hall BJ, Azarbad L, Fitzpatrick SL, Corsica JA. Examining the Binge Eating Scale in screening for binge eating disorder in bariatric surgery candidates. Obes Surg. 2013;23:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 31. | Pop-Jordanova N. BDI in the Assessment of Depression in Different Medical Conditions. Pril (Makedon Akad Nauk Umet Odd Med Nauki). 2017;38:103-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | BECK AT, WARD CH, MENDELSON M, MOCK J, ERBAUGH J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23191] [Cited by in RCA: 23317] [Article Influence: 863.6] [Reference Citation Analysis (0)] |
| 33. | Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 920] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 34. | Montano A, Flebus GB. Presentation of the Beck Depression Inventory-Second edition (BDI‐II): Confirmation of bifactorial structure in a sample of the Italian population. 2006;52:67-82. |
| 35. | State-Trait Anxiety Inventory. The Corsini Encyclopedia of Psychology: 1-1. |
| 36. | Palsson OS, Whitehead WE, van Tilburg MA, Chang L, Chey W, Crowell MD, Keefer L, Lembo AJ, Parkman HP, Rao SS, Sperber A, Spiegel B, Tack J, Vanner S, Walker LS, Whorwell P, Yang Y. Rome IV Diagnostic Questionnaires and Tables for Investigators and Clinicians. Gastroenterology. 2016;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 441] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 37. | Stanghellini V, Chan FK, Hasler WL, Malagelada JR, Suzuki H, Tack J, Talley NJ. Gastroduodenal Disorders. Gastroenterology. 2016;150:1380-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 818] [Cited by in RCA: 973] [Article Influence: 108.1] [Reference Citation Analysis (0)] |
| 38. | Yau YH, Potenza MN. Stress and eating behaviors. Minerva Endocrinol. 2013;38:255-267. [PubMed] |
| 39. | Konttinen H, Männistö S, Sarlio-Lähteenkorva S, Silventoinen K, Haukkala A. Emotional eating, depressive symptoms and self-reported food consumption. A population-based study. Appetite. 2010;54:473-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 340] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 40. | Werrij MQ, Mulkens S, Hospers HJ, Jansen A. Overweight and obesity: the significance of a depressed mood. Patient Educ Couns. 2006;62:126-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 41. | Klump KL, Bulik CM, Kaye WH, Treasure J, Tyson E. Academy for eating disorders position paper: eating disorders are serious mental illnesses. Int J Eat Disord. 2009;42:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 292] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 42. | Rosenbaum DL, White KS. The relation of anxiety, depression, and stress to binge eating behavior. J Health Psychol. 2015;20:887-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 107] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 43. | Delgado-Aros S, Cremonini F, Castillo JE, Chial HJ, Burton DD, Ferber I, Camilleri M. Independent influences of body mass and gastric volumes on satiation in humans. Gastroenterology. 2004;126:432-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 117] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 44. | Efferdinger C, König D, Klaus A, Jagsch R. Emotion regulation and mental well-being before and six months after bariatric surgery. Eat Weight Disord. 2017;22:353-360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 45. | Van Oudenhove L, Crowell MD, Drossman DA, Halpert AD, Keefer L, Lackner JM, Murphy TB, Naliboff BD, Levy RL. Biopsychosocial Aspects of Functional Gastrointestinal Disorders. Gastroenterology. 2016;S0016-5085:27144624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 320] [Article Influence: 35.6] [Reference Citation Analysis (0)] |