Published online Jan 14, 2020. doi: 10.3748/wjg.v26.i2.219
Peer-review started: October 6, 2019
First decision: November 4, 2019
Revised: December 21, 2019
Accepted: January 1, 2020
Article in press: January 1, 2020
Published online: January 14, 2020
Processing time: 98 Days and 12.9 Hours
Acute liver failure (ALF) and acute-on-chronic liver (ACLF) carry high short-term mortality rate, and may result from a wide variety of causes. Plasma exchange has been shown in a randomized control trial to improve survival in ALF especially in patients who did not receive a liver transplant. Other cohort studies demonstrated potential improvement in survival in patients with ACLF.
To assess utility of plasma exchange in liver failure and its effect on mortality in patients who do not undergo liver transplantation.
Databases MEDLINE via PubMed, and EMBASE were searched and relevant publications up to 30 March, 2019 were assessed. Studies were included if they involved human participants diagnosed with liver failure who underwent plasma exchange, with or without another alternative non-bioartificial liver assist device.
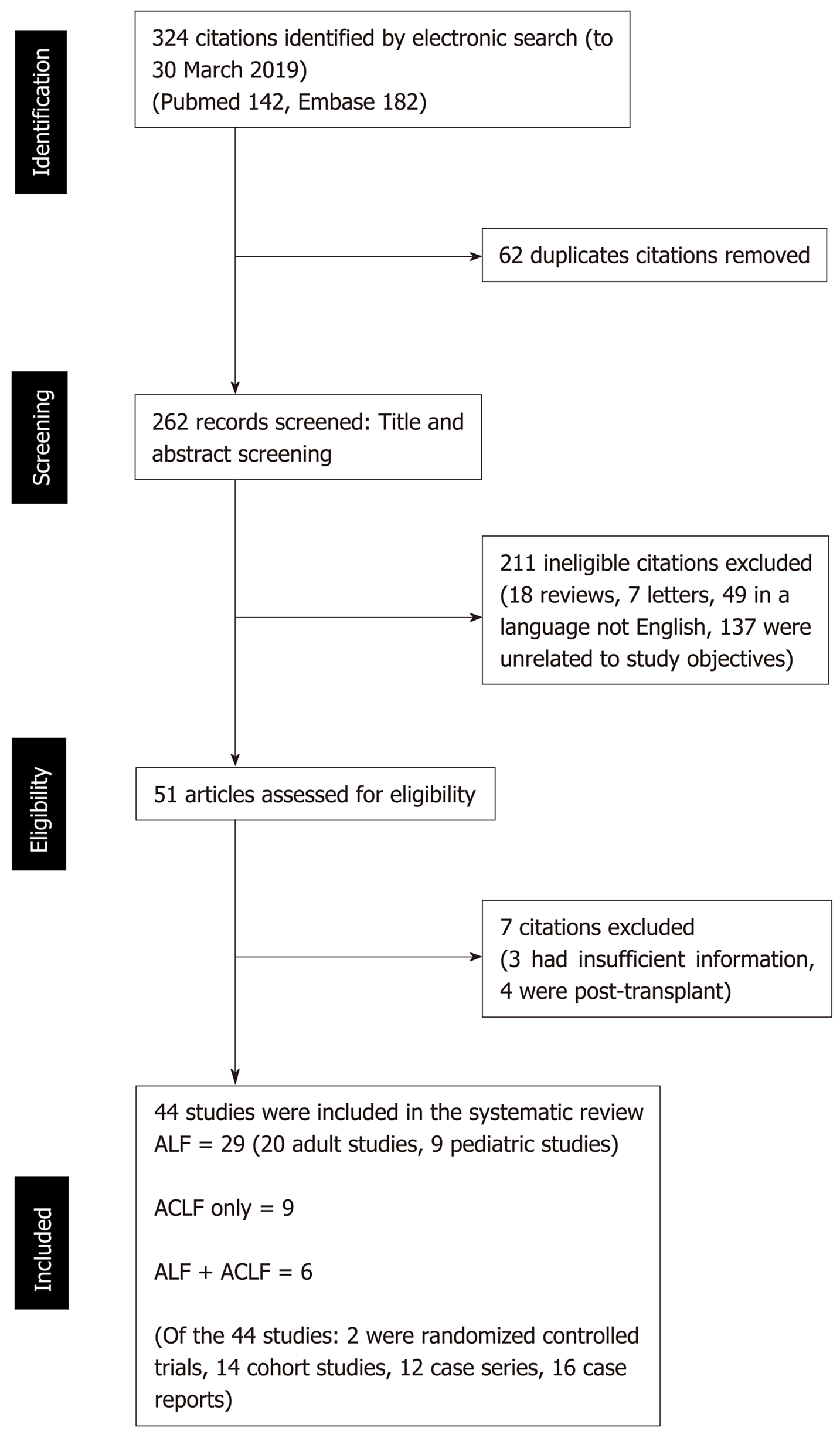
Three hundred twenty four records were reviewed, of which 62 studies were found to be duplicates. Of the 262 records screened, 211 studies were excluded. Fifty-one articles were assessed for eligibility, for which 7 were excluded. Twenty-nine studies were included for ALF only, and 9 studies for ACLF only. Six studies included both ALF and ACLF patients. A total of 44 publications were included. Of the included publications, 2 were randomized controlled trials, 14 cohort studies, 12 case series, 16 case reports. All of three ALF studies which looked at survival rate or survival days reported improvement in outcome with plasma exchange. In two out of four studies where plasma exchange-based liver support systems were compared to standard medical treatment (SMT) for ACLF, a biochemical improvement was seen. Survival in the non-transplanted patients was improved in all four studies in patients with ACLF comparing plasma exchange vs SMT. Using the aforementioned studies, plasma exchange based therapy in ACLF compared to SMT improved survival in non-transplanted patients at 30 and 90-d with a pooled OR of 0.60 (95%CI 0.46-0.77, P < 0.01).
The level of evidence for use of high volume plasma exchange in selected ALF cases is high. Plasma exchange in ACLF improves survival at 30-and 90-d in non-transplanted patients. Further well-designed randomized control trials will need to be carried out to ascertain the optimal duration and amount of plasma exchange required and assess if the use of high volume plasma exchange can be extrapolated to patients with ACLF.
Core tip: High volume plasmapheresis has been shown to improve survival in non-transplanted patients with acute liver failure. However, there has not been, to date, a review article that summarizes the different plasmapheresis regimens and its effect on mortality and improvement of liver biochemistry. This review article serves as a summary and appraisal of available literature on plasma exchange in liver failure taking into account the volume of plasma exchange, duration of plasmapheresis, and etiology of liver failure in conjunction with the study outcomes of interest and highlights potential areas which might be essential to include in future good quality randomized controlled trials.
- Citation: Tan EXX, Wang MX, Pang J, Lee GH. Plasma exchange in patients with acute and acute-on-chronic liver failure: A systematic review. World J Gastroenterol 2020; 26(2): 219-245
- URL: https://www.wjgnet.com/1007-9327/full/v26/i2/219.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i2.219
Acute and acute-on-chronic liver (ACLF) carry high short-term mortality rate, and may result from a wide variety of causes. Regardless of its underlying etiology, liver failure at its final stages results in jaundice, hepatic encephalopathy, hepato-renal syndrome, hemodynamic instability, increased susceptibility to severe infections and finally multi-organ failure[1].
Acute liver failure has been defined as a rapid decline in hepatic function characterized by jaundice, coagulopathy, and hepatic encephalopathy in patients with no prior liver disease. There are more overlaps in terminologies for ACLF, and there are currently more than ten definitions of ACLF. The two most widely used definitions are from the Asian Pacific Association for the Study of the Liver (APASL) and the European Association for the Study of the Liver (EASL) Chronic Liver Failure consortium[2]. Besides treating the underlying etiologies and supportive therapy, liver transplantation is the only definitive therapy for those with advanced disease. However, the availability of donor organ limits the availability of the patients that can be saved.
In recent years, there is increasing interest in plasma exchange for the treatment of liver failure. Since Larsen et al[3] published the first open randomized control trial of plasma exchange in patients with acute liver failure in 2016, plasmapheresis has been added to the armamentarium. High volume plasma exchange has been included in European guidelines[1] as level I, grade 1 recommendation in management of acute liver failure. Its proposed mechanism is removal of plasma cytokines and drivers of systemic inflammatory cascade through plasma exchange. Preceding the aforementioned publication, published studies on the use of plasma exchange in the setting of liver failure were mostly retrospective case series or cohort studies. These studies differed greatly in the protocols of plasma exchange. In ACLF, the data is less clear.
The objectives of this review is to provide a summary and analysis of the current evidence for the use of plasmapheresis in patients with ACLF and acute liver failure (ALF) and its effect on mortality particularly in the non-transplanted patients. In addition, the review will summarise the current literature on volume of plasma used during exchange, the duration and frequency of plasma exchange and briefly outline other available apheresis or liver support devices used in liver failure.
We included studies ranging from case reports to randomized control trials that have been published till 30 March, 2019. We excluded abstracts in this review and have restricted to only studies in English. We excluded studies with insufficient information concerning our outcomes of interest and areas of comparison: e.g., survival, the volume of plasma exchange and type of product exchanged. We included studies with only human participants diagnosed with liver failure who underwent plasma exchange, with or without other alternative liver support systems. There were no restrictions on the dose, duration, and type of plasma exchange (Table 1 for PICOS criteria). A PRISMA checklist was also used to guide the development of the systematic review.
| Variable | Description |
| Population | Humans diagnosed with liver failure (ALF/ACLF) |
| Intervention | Plasma exchange with or without other alternative liver support systems; no restrictions on dose, duration and type of plasma exchange |
| Comparator | Randomized controlled trials/Cohort studies: Standard medical treatment |
| Case series/case reports: Nil | |
| Outcome | All-cause mortality, changes in liver biochemistry, and survival in non-transplanted patients |
| Study design | Randomized Controlled Trials, Cohort studies, Case series, Case reports |
| Research question | Does plasmapheresis have an effect on all-cause mortality, changes in liver biochemistry, and survival in non-transplanted patients with ALF/ACLF, compared to standard medical treatment? |
A comprehensive search of databases and conference proceedings to identify all relevant studies up to 30 March, 2019 was performed. The following electronic databases were searched: MEDLINE via PubMed, and EMBASE. We use both text words and medical subject heading terms. The literature search strategy was adapted to suit each database.
For example, on PubMed we use the combination of the following medical subject heading terms "plasma exchange" or "plasmapheresis" and "liver failure" or "acute liver failure" or "acute on chronic liver failure”. Search was limited by “Case reports”, “Classical article”, “Clinical study”, “Clinical trial”, “Controlled clinical trial”, “Observational study”, “Randomized controlled trial”, “Review”, “Humans”, “English”, “Core clinical journals” and “MEDLINE”. The methods for data collection and analysis were based on the Cochrane Handbook of Systematic Reviews for Interventions. Where clarification of information in published data was required, corresponding authors were contacted through electronic mail for clarification.
Two review authors (Tan EXX and Lee GH) independently reviewed relevant material identified from the above search. After reading the titles and abstracts of the identified articles, full-text articles of all citations deemed to meet the inclusion criteria were sought. Duplicates were excluded. Each article was independently inspected to verify that they meet the pre-specified inclusion criteria. Study selection process is being summarized in Figure 1. Studies that were included in this systematic review are included in Tables 2-5. We created a case report form specifically for this study for systematic study review/selection and structured data extraction. Relevant study data was independently reviewed selected and extracted. Outcomes of interest such as all-cause mortality, changes in liver biochemistry, and survival in non-transplanted patients were primary outcomes of interest. The volume of plasma exchange used, duration of exchange, and etiology of liver failure were also compared in conjunction with study outcomes of interest.
| Ref. | Type of study/No. of patients recruited | Study group(s) | Plasma exchange regime | Etiology of liver failure | Results/outcome(s) of interest |
| Larsen et al[3] | Open randomized control trial; | PE + SMT vs SMT | Plasma exchange volume: Volume of plasma exchange was 15% of ideal body weight (representing 8-12 L per day per procedure); patient plasma was removed at a rate of 1-2 L per hour with replacement with fresh frozen plasma in equivalent volume | Predominantly paracetamol (59%), followed by unknown etiology, toxic hepatitis, viral hepatitis, acute Budd Chiari syndrome | HVP increases transplant free survival after 3 mo, and maximal effect of HVP was achieved in patients who did not undergo emergency transplantation |
| n = 182 (Plasma exchange + SMT 92, SMT 90) | |||||
| Overall hospital survival was 58.7% for patients treated with high volume plasma exchange vs 47.8% for control group HR: 0.56 (95%CI: 0.36-0.86), P < 0.01 | |||||
| However, HVP prior to transplantation did not improve survival compared with patients who received SMT alone P = 0.75 | |||||
| HVP procedure was undertaken on three consecutive days but with no fixed time interval between each treatment | |||||
| Bilirubin, INR, ALT and ammonia concentration decreased significantly during the first 7 d compared to SMT | |||||
| Mean number of HVP = 2.4 ± 0.8 | |||||
| Plasma exchange with donor plasma | |||||
| Nakae et al[10] | Prospective cohort study; n = 13 | PE vs PE + CHDF | Plasma exchange volume: 3.6 to 4.0 L of plasma was exchanged for the same volume of normal FFP, interval between sessions was 48h or more for both treatment methods | 7 post surgery, 4 fulminant hepatitis, 4 sepsis | Total bilirubin levels were significantly lower after treatment in both arms: Both P < 0.01 in both groups |
| No outcomes available on mortality | |||||
| Of note, decreased increase in citrate in patients with PE CHDF compared to PE alone | |||||
| Plasma exchange with FFP | |||||
| Hung et al[5] | Retrospective cohort study; n = 62 (Control 32, PE 30) | PE vs control | Average plasma exchange volume: 2916 mL (range, 1875-3750 mL), plasma exchange occurred over 2 h | 46.7% HBV, 33.3% Drug induced, 6.7% unknown, 33.3% cirrhosis. | At the end of the first week (week 1), the level of total bilirubin and grading of hepatic encephalopathy in the PE group were significantly lower than those in the control group. At the end of the second week (week 2), there were no differences in the level of total bilirubin and grading of hepatic encephalopathy between the two groups of patients |
| Difference in survival rate was not significant 66.7% in PE group vs 59.4% in control group | |||||
| No significant differences in etiology of liver failure between treatment and control groups | |||||
| Average of 6 rounds of exchange per patient (range, 2-15 rounds) | |||||
| Difference in survival days were significant, with 17.63 ± 1.86 in PE group vs 8.69 ± 0.86 in control group. P = 0.01 | |||||
| Plasma exchange with FFP | |||||
| Li et al[4] | Retrospective cohort study; n = 61 | PE + HP + CVVHDF, PE + CVVHDF and HP + CVVHDF | Plasma exchange volume: 2000-3000 mL of fresh per session. Flow rate was 80-120 mL/min, the plasma separation rate was 25-30 mL/min, the replacement time was 2.0-3.0 h | 3/61 acute viral hepatitis, 17/61 chronic toxic acute liver failure. 41 cases of non-viral induced liver injury: 5 after cardiac surgery, 7 with drug poisoning, 13 cases after pregnancy childbirth, 1 case mushroom poisoning, 10 cases with severe infection, 5 others | Treatment of the 61 patients using the artificial liver support system yielded a survival rate of 62.3% (38/61), and a viral survival rate of 35.0% (7/20); with the non-viral survival rate being 75.6% (31/41) Biochemically PE + HP + CVVHDF and PE + CVVHDF groups saw improvement in total bilirubin, ALT, PT, Albumin and HP+CVVHDF saw improvement in total and ALT (P < 0.05) |
| In the PE + HP + CVVHDF group: After completion of a single plasma exchange, the HP was carried out. After HP, the CVVHDF is carried out | |||||
| Total of 171 exchanges were done | |||||
| Nakamura et al[11] | Retrospective case series n = 49; Fulminant hepatitis 15; Severe acute hepatitis 14; Healthy controls 20 | No comparative arm | Plasma exchange volume: Approximately 2000–4000 mL of fresh-frozen plasma was substituted during each exchange | No mention | 10/15 fulminant hepatitis and all severe acute hepatitis survived |
| Significant decreases in circulating TNF-a, IL-6, and TGF-ß levels in patients with fulminant hepatitis after a single plasma exchange | |||||
| Plasma exchange with FFP | |||||
| Akdogan et al[9] | Retrospective case series; n = 39 (fulminant hepatic failure) | PE, No comparative arm | Plasma exchange volume: Total plasma volume approximately 1 | Predominantly undetermined (41%), paracetamol (28.5%), acute hepatitis B, autoimmune liver disease, vascular tumor, acute hepatitis A (in presence of cirrhosis) | Improved biochemically (coagulopathy hyperbilirubinemia, AST, Ammonia, Factor V levels): P < 0.05 |
| Plasma exchange continued on a daily basis till clinical response (subjective by ICU team) or patient expired, or transplanted | 31% underwent liver transplant, 92% of which survived at 1 year | ||||
| Overall survival 54% (21/39 patients), 37% (10/27) of non-transplanted patients survived | |||||
| No need for calcium replacement or magnesium replacement | |||||
| Plasma exchange with low volume citrate plasma | |||||
| Kondrup et al[6] | Case series; n = 11 | PE, No comparative arm | Plasma exchange volume: 20% body weight plasma exchange intended on three consecutive days, obtained a mean 2.6 exchanges and mean volume 16% body weight | 6 acetaminophen, 2 non-A/B hepatitis, Halothane, disulfurum toxicity and hepatitis B | 5/11 survivors were all acetaminophen toxicity induced ALF |
| All had improved bilirubin after treatment | |||||
| All 4 Grade IV encephalopathy patients all did not survive | |||||
| Plasma exchange with donor plasma. | Those that survived had Grade III encephalopathy or lesser | ||||
| Those that did not survive had a longer duration of coma before initiation of PE (3.5 vs 1.8 d) | |||||
| Freeman et al[13] | Case series | PE, No comparative arm | Plasma exchange volume: 3 L of plasma exchange was performed daily until conscious level improved or patient died Plasma exchange fluid: Equal volume of compatible fresh frozen plasma and plasma protein fraction (PPF) usually in the proportion of 2 units FFP:1 PPF | 4 acetaminophen, 2 nonA/B hepatitis, 1 hepatitis A, 1 mixed drug overdose, 1 ETOH in 2 | 7/9 showed improvement in coma grades, 5 achieved normal mental state, 5 were able to discharge from hospital. Of which 3 were paracetamol induced liver failure, 1 was monoamine oxidase/ tricyclic acid induced, 1 was alcohol. Survival 55% |
| n = 9 | |||||
| Improved biochemistry, bilirubin, coagulation (P < 0.01) 1 died of retroperitoneal bleeding | |||||
| Buckner et al[7] | Case series | PE, No comparative arm | Plasma exchange volume: Initial 10 L/day plasma exchange with FFP or fresh/outdated plasma | 1 Acute viral hepatitis (pediatric), 2 halothane, 1 hepatitis B viral hepatitis | 1 died (pediatric) |
| 1 patient took 37 d to awake from coma | |||||
| n = 4 (1 pediatric) | |||||
| 3/4 of patients survived | |||||
| Liu et al[31] | Case series | PE, No comparative arm | Plasma exchange volume: Each treatment lasted for 4-6 h, and the total volume exchanged was approximately 7000 mL (1.5-2x TPV) | DILI | The two patients with DILI ALF were treated with PE without need for transplant |
| n = 2 | |||||
| Biochemically improved after PE (AST ALT Bilirubin) | |||||
| Plasma exchange fluid: Maximally, 4700 mL of FFP was exchanged in each session, and the rest comprised plasma substitute consisting of 25% human albumin, pentastarch, 0.9% saline, and Ringer's solution. In each session, the plasma substitute was exchanged initially, and FFP was exchanged at the end | |||||
| Duration of PE was based on clinical improvement, both patients had intermittent PE, total 3 sessions | |||||
| Bilgir et al[15] | Case report | PE, No comparative arm | Plasma exchange volume: Each session consists of 15 units of FFP, total 4 sessions | L-asparaginase induced ALF | Patient recovered from ALF: However, no biopsy done |
| Plasma exchange fluid: FFP | |||||
| Aydemir et al[14] | Case report | PE, No comparative arm | Plasma exchange volume: 2500 mL plasma volume removed during each PE session | PTU induced ALF | Patient recovered from ALF: however, no biopsy done |
| Plasma exchange fluid: Fresh frozen plasma | |||||
| Riveiro-Barciela et al[28] | Letter to editor, case report (Ipilimumab) | PE, No comparative arm | Plasma exchange volume: 1500 mL of 5% albumin plus 4 units of plasma as replacement fluid, carried out every other day for total 5 treatments | Immunotherapy induced ALF | Patient improved. Liver tests within normal values within one month |
| Plasma exchange fluid: FFP and 5% albumin | |||||
| Damsgaard et al[8] | Case report (ALF in WD) | PE, No comparative arm | Plasma exchange volume: 8-9 L of plasma, total 12 HVP | Fulminant Wilson’s disease ALF | Even though WD ALF score was 16, patient survived without need for OLT |
| Plasma exchange fluid: Fresh frozen plasma as replacement fluid 1:1 | |||||
| Göpel et al[33] | Case report (Letter to editor) | PE, No comparative arm | Plasma exchange treatment was performed for three consecutive days | Peg-asparaginase induced ALF | Patient improved. Continuous stabilization of fibrinogen and antithrombin 3, an increase of cholinesterase, and a decrease of bilirubin. Clinical signs and symptoms such as jaundice and ascites did also rapidly improve |
| No mention of volume or type of exchange fluid | |||||
| Lin et al[16] | Case report | PE, no comparative arm | Plasma exchange was performed 2 times per week, and 2000 to 2500 mL frozen plasma was used each time | HLH | Patient’s condition deteriorated, and he died of multi-organ failure during the 6th week of hospitalization. Autopsy was declined |
| Chen et al[29] | Case report | PE, No comparative arm | Plasma exchange volume: Estimated two times the plasma volume of the patient was exchanged. At most, 40 units of FFP were exchanged, with the remainder of the infused volume consisting of plasma substitutes. The plasma substitutes consisted of 25% human albumin, pentastarch, normal saline, and Ringer’s solution | Heat stroke | On day 4 after the admission, the patient received high-volume PE (two plasma volumes exchanged). His consciousness was improved a day after PE The patient was discharged on day 16 after admission without sequelae |
| Holt et al[17] | Case report | PE, No comparative arm | Plasma exchange on post-partum days 3-5. Volume: Average of 3.2 L (1.6 estimated plasma volumes) of FFP replaced per session, followed by a tapering course of prednisone | AFLP vs HSV hepatitis associated ALF | After 3 d of TPE the patient’s mental status had returned to normal |
| Treatment with TPE was followed by biochemical and clinical improvement but during her recovery herpes simplex virus type 2 (HSV‐2) infection was diagnosed serologically and confirmed histologically | |||||
| Shen et al[18] | Case report | PE, No comparative arm | Plasma exchange: performed on days 1, 3, and 5, with 3000 mL of plasma exchanged during each session | Occupational Exposure to Tetrachloroethylene | Bilirubin, ammonia, and prothrombin time improved before hospital discharge and patients mental status gradually became normal discharged on day 26 of hospital admission |
| Pashaei et al[30] | Case report | PE, No comparative arm | Plasma exchange volume 2.5L | Wilson’s disease | 36 h after initiation of PE, encephalopathy recovered and there was no renal impairment. Copper, LDH total bilirubin decreased after the treatment |
| Plasma exchange fluid: FFP |
| Ref. | Type of study / No. of patients recruited | Study group(s) | Plasma exchange regime | Etiology; Age | Results |
| Pham et al[19] | Case series | PE, No comparative arm | Plasma volume: Targeted 1-1.25 plasma volumes | Wilsons Disease | Post TPE 9 patients underwent liver transplantation and all 10 patients had at least 6 mo survival |
| n = 10 | |||||
| Age 6-61 yr | |||||
| Median days from first to OLT was 1-53 d | |||||
| Plasma exchange fluid: 77% of procedures were performed with plasma as sole replacement fluid while 23% used the combination of plasma and 5% albumin | |||||
| Median number of TPE: 3.5 | |||||
| Chien et al[22] | Retrospective case series | PE, No comparative arm | Plasma exchange volume: Plasma exchange was usually performed daily for the first 3 d, and then shifted to every other day or every 3 d according to the patient's condition | 60% idiopathic, 17% infection, 8% metabolic and immunologic, 4% toxin | 11 (48%) had native liver recovery (NLR), 9 (39.1%) died without liver transplant, and 3 (12.9%) received liver transplantation |
| The no liver recovery group showed a lower proportion of idiopathic cases, lower peak ammonia level, higher peak alpha fetoprotein (AFP) level, and they had plasma exchange fewer times than the other groups | |||||
| n = 23 | |||||
| Age 0.29-9.25 yr | |||||
| Plasma exchange volume: 2–4 times the patient’s estimated plasma volume | |||||
| Plasma exchange fluid: FFP | |||||
| Ide et al[47] | Retrospective case series | PE/CVVHDF, No comparative arm | CVVHDF and PE were applied in all ALF patients | 2/17 viral | All laboratory results relating to liver dysfunction decreased significantly after CVVHDF + PE |
| 1/17 mitochondrial | |||||
| PE using 100 mL/kg of FFP per treatment course was implemented once daily for 6 to 8 h until the recovery of coagulopathy | Overall survival rate 88% with median follow up period of 28 mo | ||||
| 14/17 indeterminate | |||||
| n = 17 | |||||
| Age 1-11 mo [Median Weight 8.0 (2.7-10 kg)] | |||||
| Verma et al[23] | Case report | PE, No comparative arm | Plasma exchange volume: 1.5-2 h, 1.2 L plasma exchange in each session, in addition to oral D penicillamine and Zinc | Wilson’s disease | Patient improved initially but subsequently deteriorated fter developing renal failure and shock, and died from acute pulmonary hemorrhage. |
| Age 5 yr (Weight: 15 kg) | |||||
| Morgan et al[25] | Case report | PE, No comparative arm | Plasma exchange volume: 1500 mL TPE, 5 single plasma volume over 11 d in addition to trientine | Wilson’s disease | Patient had worsening bilirubin, coagulopathy despite treatment and underwent OLT 12 d after beginning TPE |
| Plasma exchange fluid: Plasma | Age 6 years | ||||
| Zhang et al[24] | Case report | PE, No comparative arm | Plasma exchange volume: 1200 mL each time, with blood flow velocity of 45–50 mL/min, plasma separation speed of 650–750 mL/h, and a replacement time of approximately 2 h. Total 9 exchanges | Wilson’s disease | CPFA started after PE. The patient had rapid recovery of consciousness, removal of copper and stabilization of serum bilirubin and hemoglobin. 9 d after last PE patient underwent liver transplant. |
| Plasma exchange fluid: FFP | Age 7 yr (Weight 21 kg) | ||||
| Yukselmis et al[26] | Case report | PE, No comparative arm | Plasma exchange volume: 1.5 times total blood volume then 1 time for the subsequent courses | Viral (Influenza) | Patient did not require transplantation in light of clinical improvement and PE resulted in complete recovery |
| Total 3 sessions PE on top of ostelmavir | Age 4 yr (Weight 16 kg) | ||||
| Plasma exchange fluid: FFP | |||||
| Ponikvar et al[27] | Case report | PE+HD, No comparative arm | Plasma exchange volume: 3 volumes of plasma (12% of body weight of 16 kg) per procedure were exchanged (1972 ± 85 mL; range, 1800–2150 mL). FFP was used as the replacement solution. An equal volume of plasma was removed and replaced | Unknown Excluded viruses and metabolic cause | Patient did not improve after 1 mo and was referred to a liver transplant center and successfully transplanted. Patient also had hyperbaric oxygen (HBO) during treatment |
| Age 3 yr (Weight 16 kg) | |||||
| A total of 13 PEs, 13 HD sessions, and 9 HBO treatments over a period of 1mo. The initial 4 PEs were followed by HD sessions while the other 8 PE treatments were given simultaneously with HD. There was no renal failure; HD was instituted to improve ammonia elimination | |||||
| Harmanci et al[48] | Case report / Letter to editor | PE, No comparative arm | Plasma exchange volume: 2.5 L per session | Wilson’s Disease | Patients mental status improved and was extubated and weaned from mechanical ventilation on the fifth day of hospitalization. The patient did not require liver transplantation. The patient was treated continously with zinc and D-penicillamine |
| Age 17 yr | |||||
| Started daily and continued for 7 consecutive days |
| Ref. | Type of study/Number of patients recruited | Study group(s) | Characteristics of study population | Plasma exchange regime | Etiology | Results |
| Meng et al[39] | Retrospective cohort study, single center; n = 158; PE group: 38; SMT group: 120 | PE vs SMT | PE group: Higher MELD score | Performed twice a week until patients’ condition was stable, additional weekly or biweekly visits were instituted if patients felt deterioration of their condition. Total duration of therapy 2-8 wk | Hepatitis B | 24/38 (63%) death in the PE group and 82% in SMT group died within 4 wks. By week 12, 71% in PE group and 86% in SMT group died |
| Baseline characteristics both groups had 26%-28% of patients with hepatic encephalopathy | ||||||
| ACLF definition: ACLF was defined as serum bilirubin ≥ 5 mg/dL and an INR 1.5 or prothrombin activity (PTA) 40 %, complicated within 4 wk by ascites and/or encephalopathy in patients with previously diagnosed or undiagnosed chronic liver diseases | 18% vs 14% transplant free survival in 3 mo comparing PE vs SMT (P < 0.01) | |||||
| Plasma exchange volume not mentioned | ||||||
| Biochemically, there is decreased bilirubin in PE arm cf SMT (P < 0.01) | ||||||
| Mao et al[38] | Retrospective cohort study, single center | PE vs SMT | ACLF definition: Acute decompensation of liver function in patients with chronic preexisting liver diseases. ACLF is defined as a syndrome with severe jaundice (total bilirubin: 171 mmol/L), coagulopathy (prolonged prothrombin time, prothrombin activity 40%), or hepatic encephalopathy (above grade II) | Plasma exchange volume: 3500 mL at 25-30 mL/min. A total of 3000–4500 mL of fresh frozen plasma (40-60 mL/kg) and 20-40 g of human albumin were supplied | Hepatitis B. Drug hepatitis, Wilson disease, alcoholic liver disease, autoimmune hepatitis excluded | 26 survivors and 36 non-survivors were in the PE group, whereas 33 survivors and 98 non-survivors were in the control group after 30 d treatment. Their survival rates were 41.9% and 25.2% for PE and medical therapy, respectively (P < 0.05) |
| Baseline characteristics 74%-77% had HE at baseline | ||||||
| PE group: 62 | ||||||
| SMT group 131 | ||||||
| No mention re: Biochemical improvement | ||||||
| Flow rate of blood was adjusted to 60–130 mL/min | ||||||
| Not randomised | ||||||
| PE was carried out 2-3 times per week for the first two weeks, then weekly, then stopped based on clinical results | ||||||
| Chen et al[42] | Retrospective cohort study multicentre (10) | PE, no comparative arm | ACLF definition: Guidelines for Diagnosis and Treatment of Liver Failure in China (2006): Early stage is defined as a progressively deepening jaundice (Bilirubin level ≥ 171 μmol/L or a daily increase of ≥ 17.1 μmol/L), PTA > 30% but ≤ 40%, and no HE or other complications. Middle-stage disease represents progression of the symptoms of the early stage, including one of the following symptoms: Grades I/II HE, ascites, or a PTA of > 20% but ≤ 30%. In the end-stage disease, the condition deteriorates further with a PTA of ≤ 20% and includes one of the following symptoms: Hepatic-renal syndrome, severe upper gastrointestinal bleeding, serious infection, and grades III/IV HE | Plasma exchange volume: 2500-3500 mL, and the PE rate was 20-25 mL/min | Hepatitis B | Forty-two of the 52 (80.8%) patients in the early stage, seventy-five of the 99 (75.8%) patients in the middle stage and thirty-seven of the 99 (37.4%) patients in the end stage survived for one month after diagnosis |
| n = 250 patients | ||||||
| Dexamethasone (5 mg) and heparin (2500 U) were injected routinely before PE | ||||||
| Authors concluded that late stage ACLF might benefit from PE as a bridge to definitive treatment-liver transplant | ||||||
| PE was repeated every 2-4 d | ||||||
| Zhou et al[45] | Retrospective, cohort | PBA + PE vs PE | ACLF was defined as acute liver injury emerging as jaundice and coagulopathy, complicated by ascites and/or encephalopathy within 4 wk in a patient with known or unknown chronic liver disease. The definition of liver failure in ACLF was as follows: Severe jaundice (total serum bilirubin ≥ 5 mg/dL) and coagulopathy (INR ≥ 1.5 or prothrombin activity < 40%) and ascites and/or encephalopathy. | Plasma exchange volume: Approximately 3000 mL of plasma was exchanged per time at a blood flow rate of 20 to 25 mL/min | HBV 56.6%, HBV+HEV 31.9% Others 11.5% | The mean overall survival for the derivation cohort was 441 d (95%CI: 379-504), and the 90- and 270-d survival probabilities were 70.3% and 58.3%, respectively |
| Derivation cohort 113 | ||||||
| Validation cohort 68 | The mean survival times of patients treated with PBA plus PE and patients treated with PE were 531 days (95%CI: 455-605) and 343 d (95%CI: 254-432), respectively (P = 0.012) | |||||
| From the derivation cohort: PE, n = 54; PE+PA, n = 59 | ||||||
| Each patient in the derivation cohort received PE 1 to 4 times | ||||||
| Predictors of survival: Age, MELD, Complication, type of ALSS No mention re: Baseline characteristics of PE vs PBA | ||||||
| Baseline population in this study is 51% cirrhotic | ||||||
| Wan et al[44] | Prospective cohort study | PE vs DPMAS | ACLF was defined as serum bilirubin 5 mg/dL and INR > 1.5 or PTA < 40%, complicated within 4 wk by ascites and/or HE in patients with previously diagnosed or undiagnosed chronic liver diseases | Plasma exchange volume: About 3000 mL of plasma exchanged at an exchange rate of 20-30 mL/min at each session. | HBV | During the study, a total of 42 patients died, with 24 in TPE group and 18 in DPMAS group. The median survival times were 12 wk in TPE group and 11 wk in DPMAS group |
| n = 60 | ||||||
| TPE 33 | PE was performed 2-3 times/week, lasting 2-3 h every session | The 4-wk and 12-wk survival rates in TPE group and DPMAS group were 87.9% and 88.9%, 34.6% and 33.3%, respectively. There was no marked difference in survival between the two groups | ||||
| DPMAS 27 | Baseline eAg positive greater in TPE group (18% vs 7.4%) | |||||
| Bilirubin removal in TPE more efficient compared to DPMAS | ||||||
| Qin et al[37] | Open label randomized controlled parallel group single-center study | PE centered ALSS vs SMT | Definition of ACLF was according to the Chinese guidelines, Bilirubin ≥ 10 mg/dL, PTA ≤ 40% and cirrhosis and multiorgan failure were not taken as mandatory criteria, according to the Chinese guidelines | PE volume: 3500 mL (40–60 mL/kg) FFP, at 25-30 mL/min | HBV | Survival rates after 90 d were 60% (62/104) in ALSS-treated patients and 47% (61/130) in the control group. (P < 0.05). The 5-year cumulative survival rates of the ALSS and control groups were 43% (45/104) and 31% (40/130), respectively (P < 0.05) |
| ALSS schedule: 3 routine treatments were performed in the first 10 d after inclusion in the study (once per 3–4 d); extra treatments were offered according to the improvement of the patients. The methods of PE-centered ALSS were chosen based on clinical conditions. For patients with coagulopathy, PE was applied; for patients with encephalopathy, PE plus hemoperfusion or continuous hemodiafiltration was used; for patients complicated with HRS or imbalance of water or electrolytes, PE plus continuous hemodiafiltration was used | ||||||
| n = 234 | ||||||
| No mention of biochemical improvement | ||||||
| Xia et al[40] | Retrospective cohort study | NBAL (all had PE) vs SMT | ACLF definition: | All of the patients were treated with PE, and most were treated with one or more additional methods, including 13/26 (50.00%) ALF patients, 16/27 (59.26%) Subacute ALF patients, and 228/407 (56.02%) ACLF patients | For ACLF: 91.24% chronic hepatitis B, 3.69% alcohol abuse, 1.01% autoimmune, 1.01% cholestasis, 3.05% other causes | Clinical outcomes were improved after NBAL treatment. The 30-d survival rates of subacute liver failure (SALF) patients were 63% among those who received NBALs and 21% among those who did not receive NBALs (P < 0.01) |
| 1 Acute deterioration of pre-existing chronic liver disease | ||||||
| n = 882 | ||||||
| 460 NBAL 422 control | 2 Extreme fatigue with severe digestive symptoms, such as obvious anorexia, abdominal distension or nausea and vomiting | |||||
| The 30-day survival rate of acute-on-chronic liver failure (ACLF) patients who received NBALs was 47%, significantly higher than that of the non-NBAL patients (P < 0.05) | ||||||
| Of which 49 ALF, 46 SALF and 787 ACLF | ||||||
| 3 Progressively worsening jaundice within a short period (serum total bilirubin ≥ 10 mg/dL or a daily elevation ≥ 1 mg/dL) | ||||||
| The choice of therapy was based on each patient’s condition: PE in combination with PP for HE was administered in 12.24% (6/49) of ALF patients, 10.77% (7/65) of SALF patients, and 7.41% (80/1079) of ACLF patients. In patients with HRS, we administered PE with CHDF in 32.65% (16/49) of ALF patients, 23.08% (15/65) sessions of SALF patients and 28.17% (304/1079) sessions of ACLF patients | ||||||
| Reported to be effective in biochemical improvement | ||||||
| 4 Obvious hemorrhagic tendency with PTA ≤ 40% (PT ≥ 18.3 s or INR > 1.50) | ||||||
| The absence of any of the above four criteria precluded a diagnosis of ACLF | ||||||
| Pts underwent 1-4 times of NBAL each | ||||||
| Li et al[49] | Prospective cohort Study | PE vs PE + UCMSCs | ACLF was defined as serum bilirubin ≥ 5 mg/dL and INR ≥ 1.5 or PTA < 40%, complicated within 4 wk by ascites and/or encephalopathy in patients with previously diagnosed or undiagnosed chronic liver disease | PE volume: About 3000 mL, and the exchange rate of plasma was 20–30 mL/min. Heparin was used as anticoagulant during PE | HBV | The cumulative survival rates at 3 mo in group A and group B were 54.5 % and 29.4 %, respectively (P = 0.015 by log rank test) |
| PE: 34 | ||||||
| INR was prominently lower in PE + UCMSCs group than in PE group (P < 0.05). At 12 mo, patients in PE+UCMSCs group showed lower levels of AST than patients in PE group (P < 0.05). | ||||||
| PE+UCMSCs: 11 | ||||||
| n = 45 | ||||||
| In PE group: MELD score: 22.5 +/- 1.4, 61.8% cirrhotic | At 24 mo, patients in PE+UCMSCs group had significantly improved levels of albumin, PT and INR than patients in PE group (P < 0.05). However, ALT, Total bilirubin, Direct bilirubin, creatinine, white blood cell, Hemoglobin, Platelet and ascites were comparable at each follow-up | |||||
| Xu et al[43] | Retrospective cohort study | PE | Definition of ACLF: Acute hepatic insult manifesting as jaundice and coagulopathy, complicated within 4 wk by ascites and/or encephalopathy in a patient with previously diagnosed or undiagnosed chronic liver disease | Total volume exchanged 3300 mL | HBV | 1-yr and 5-yr survival rates in the ALSS-LT group and LT group were 79.2% and 83%, 69.7% and 78.6% |
| n = 171 | ||||||
| Patients with coagulopathy were indicated for PE, when the patient had HE, PE + hemodiafiltration was used. For patient with hepatorenal syndrome or imbalance of water or electrolytes, PE + continuous hemodiafiltration or MARS was used | ||||||
| PE before LTx: 115 | ||||||
| Emergent LTx: 56 | ||||||
| PE group: MELD score 31+/-6 | ||||||
| Yao et al[41] | Retrospective cohort study | PE vs DPMAS + PE | Definition of ACLF: Acute hepatic insult manifesting as jaundice and coagulopathy, complicated within 4 wk by ascites and/or encephalopathy in a patient with previously diagnosed or undiagnosed chronic liver disease | PE volume: Fresh frozen plasma was 2200 to 2400 mL per treatment. Duration of single treatment was about 2 h | HBV | The total bilirubin levels immediately after treatment at 24 and 72 h after treatment were markedly decreased in DPMAS + PE group compared to that in PE group (52.3 ± 9.4% vs 42.3 ± 7.2%, P < 0.05; 24.2 ± 10.0% vs 13.5 ± 13.0%, P < 0.05; 24.8 ± 13.1% vs 14.9 ± 14.9%, P < 0.05; respectively). |
| n = 131 | ||||||
| PE group (n = 77) | Patients underwent 1-4 times of PE / PE + DPMAS | |||||
| DPMAS + PE group (n = 54) | Baseline characteristics were similar in both groups | |||||
| The 28- d survival rates was 62.3% and 72.2% in PE and DPMAS + PE groups (P = 0.146). | ||||||
| 28- d survival rates were significantly higher in DPMAS + PE group than that in PE group (57.4% vs 41.7%, P = 0.043) in the intermediate-advanced stage patients | ||||||
| Cheng et al[12] | Retrospective, cohort study single tertiary centre | PE, no comparative arm | ACLF definition: acute hepatic insult that manifests as jaundice (serum bilirubin ≥ 5 mg/dL and coagulopathy (INR ≥ 1.5), which is complicated within 4 wk by clinical ascites and/or encephalopathy in a patient with previously diagnosed or undiagnosed chronic liver disease or cirrhosis | The processed plasma volume was approximately 3000 mL for each session (1-1.5 total plasma volume); the blood flow rate was 100 mL/min; and the PE rate was 25–30 mL/min, with an equivalent volume of replacement fluid using fresh frozen plasma | Hepatitis B (75%) in ACLF group, 6% alcohol, others: HCV, AIH | Biochemical improvements seen after PE: AST/ALT/Bil/INR |
| Average 4-5 sessions of PE in ACLF group, 3-5 sessions in ALF | ||||||
| n = 55; 10 ALF, 45 ACLF | ||||||
| Initial diagnosis to PE is longer in non-survivors in ACLF and ALF though not significant | ||||||
| Survival based on etiology of ACLF: 24% HBV, 67% ETOH, 0% HBV + alcohol, 0% HCV 0% HCV + alcohol, 0% AIH | ||||||
| 79% of patients with ACLF have cirrhosis, 55% have grades III-IV HE | PE occurred daily or every other day till sustained clinical improvement, liver transplantation or no clinical response/death |
| Ref | Type of study/No. of patients recruited | Comparative arm | Plasma exchange regime | Etiology | Results |
| Xia et al[40] | n = 882; 460 NBAL 422 control; Of which 49 ALF, 46 SALF and 787 ACLF | NBAL (all had PE) vs SMT | All of the patients were treated with PE, and most were treated with one or more additional methods, including 13/26 (50.00%) ALF patients, 16/27 (59.26%) SALF patients, and 228/407 (56.02%) ACLF patients. | For ACLF: 91.24% chronic hepatitis B, 3.69% alcohol abuse, 1.01% autoimmune, 1.01% cholestasis, 3.05% other causes | Clinical outcomes were improved after NBAL treatment. The 30-d survival rates of subacute liver failure (SALF) patients were 63% among those who received NBALs and 21% among those who did not receive NBALs (P < 0.01) |
| The choice of therapy was based on each patient’s condition: PE in combination with PP for HE was administered in 12.24% (6/49) of ALF patients, 10.77% (7/65) of SALF patients, and 7.41% (80/1079) of ACLF patients. In patients with HRS, we administered PE with CHDF in 32.65% (16/49) of ALF patients, 23.08% (15/65) sessions of SALF patients and 28.17% (304/1079) sessions of ACLF patients | For ALF: 42% drug toxicity, 16% HBV, 10% surgical trauma, 30% unexplained | The 30-day survival rate of acute-on-chronic liver failure (ACLF) patients who received NBALs was 47%, significantly higher than that of the non-NBAL patients (P < 0.05) | |||
| Pts underwent 1-4 times of NBAL | For SALF: 54% drug toxicity, 30% unexplained, 4% Hepatitis E, 11% HBV | Reported to be effective in biochemical improvement | |||
| Cheng et al[12] | Retrospective, cohort study single tertiary centre; n = 55; 10 ALF, 45 ACLF | PE, no comparative arm | PE volume: About 3000 mL, and the exchange rate of plasma was 20-30 mL/min. Heparin was used as anticoagulant during PE | In ALF group: 50% HBV, 20% drug, others include ischemic hepatopathy, traumatic liver injury, HLH | 20% (1/5) of the HBV related ALF survived, 1/2 of drug related ALF survived, and 1/1 of the traumatic liver injury related ALF survived. |
| Significant improvements see in levels of serum total bilirubin, AST ALT INR PT. No significant changes in ammonia | |||||
| Nakae et al[21] | Retrospective case series; n = 21; 10 FH; 11 ALF | PDF, no comparative arm | PE volume: 1200mL of normal FFP and 50mL of 25% albumin solution was infused intravenously over 8 h | FH | 90 d survival: |
| 70% Hep B | 20% in FH patients | ||||
| 10% AIH | 54.5% in ALF patients | ||||
| 20% Drug | Overall survival 38.1% | ||||
| The PDF session lasted 8h, and the blood flow rate was 100 mL/min. Filtered replacement fluid for was infused at a dialysate flow rate of 600 mL/h and a replacement flow rate of 450 mL/h | |||||
| Lower MELD correlated to increased survival | |||||
| ALF | |||||
| No patients survived beyond 90 d with MELD > 40 | |||||
| Biochemically: Bilirubin, IL-18 statistially different when compared before and after PDF | |||||
| 3/11 Unknown | |||||
| 1/11 GVHD | |||||
| 4/11 ETOH | |||||
| 1/11 HBV | |||||
| Fluid removal was performed by reducing the replacement flow rate to 450 mL/h at most | 1/11 EBV | ||||
| 1/11 Drug | |||||
| 5/11 was labelled as AOCLF | |||||
| Pu et al[34] | Case series (excluding patients who abandoned treatment; n = 33); 8 ALF; 3 SALF; 14 ACLF | CHDF followed by sequential PE, No comparative arm | Patients underwent continuous hemofiltration on a daily basis during the daytime followed by sequential treatment with plasma exchange 1800-2400 mL or hemodialysis every 2-3 d | 29 patients with hepatitis B virus infection, 1 with Hepatitis E virus infection, and 3 patients with unknown etiology; 18 were male and 15 female; age ranged from 23 to 65 | Restoration of consciousness in 6 of 8 cases (75%) in acute liver failure (ALF) group, 3 of 3 cases (100%) in subacute liver failure (SALF) group, and 9 of 14 cases (64.29%) in acute/subacute on chronic liver failure (A/SCLF) group |
| Of all cases, 11 patients restored consciousness after 7 d in a coma. The rate of long-term survival (those who abandoned the treatment were excluded) was 3/7 (42.86%) for ALF group, 2/2 (100%) for SALF group, and 1/11 (9.09%) for A/SCLF group | |||||
| No mention of biochemical changes | |||||
| Schaefer et al[50] | Retrospective cohort study; n = 10; 8 had combined PE, HD + MARS | PE + HD + MARS vs MARS | PE volume: 1.5 plasma volume was exchanged per session within 2–3 h | Wilson’s disease in 2 patients, congenital liver fibrosis, progressive intrahepatic cholestasis, severe combined immunodeficiency, disseminated herpes simplex virus 2 infection, multi-organ failure due to mycoplasma-induced myocarditis, autoimmune hepatitis, fungal sepsis and cetirizine intoxication | MARS and PE/HD treatments were well tolerated by all patients. No bleeding episode occurred. 1 patient with multi-organ failure due to mycoplasma-induced myocarditis, 1 with cetirizine intoxication completely recovered. 3 patients were successfully transplanted, five children died with multi-organ failure and sepsis, including the three children treated with Mini-MARS |
| PE was immediately followed by a HD session in six children, using the same extracorporeal circuit with a polysulfone high-flux filter (Fresenius) | |||||
| Standard MARS treatment only slightly decreased serum bilirubin (16.3 ± 6.5-13.8 ± 5.9 mg/dL) and ammonia (113 ± 62-99 ± 68 μmol/L) and international normalized ratio (INR) tended to increase (1.5 ± 0.3 and 2 ± 1.1) | |||||
| 2 had MARS only | |||||
| Mini-MARS did not reduce serum bilirubin, ammonia slightly decreased and INR increased | |||||
| Age 0.1-18 yr | |||||
| PE/HD reduced serum bilirubin (23 ± 8.4-14.7 ± 7 mg/dL), ammonia (120 ± 60–70 ± 40 μmol/L) and INR (2.4 ± 0.8-1.4 ± 0.1, all P < 0.05). Intraindividual comparison showed a slight increase in bilirubin by 2 ± 22% with MARS and a reduction by 37 ± 11% with PE/HD (P < 0.001 vs MARS) and a decrease in ammonia of 18% ± 27% and 39% ± 23% (P < 0.05). INR increased during MARS by 26 ± 41% and decreased with PE/HD by 37 ± 20% (P < 0.01) | |||||
| Singer et al[51] | Retroespective case series | No comparative arm, TPE in all patients | Plasma volume removed per exchange was 121 ± 47 mL/kg (2.2 ± 0.6 plasma volume) of FFP | 57% FHF, 18% BA, 20% IEM, 5% other of note 43% had CLD | Coagulation profiles after TPE significantly improved compared with mean pre-exchange values |
| Spontaneous recovery was observed in three patients; the remaining either underwent transplantation (32/49) or were not considered transplant candidates because of irreversible neurologic insults (11/49) or sepsis (3/49) | |||||
| Age 10 d to 18.4 yr |
In addition, pooled odds ratios and its corresponding 95% confidence intervals were respectively calculated for 30- and 90-d mortality in ACLF patients using the random effects model. The data extracted for this calculation includes the number of events (deaths) for the respective time periods and total sample size in the intervention and control arms, and were extracted in duplicate by ET and MXW. The I2 statistic and Cochran Q test was used to evaluate statistical heterogeneity, where heterogeneity was characterized as minimal (< 25%), low (25%-50%), moderate (50%-75%) or high (> 75%) and was significant if P < 0.05. All calculations performed were 2-sided and done through Review Manager 5.3.
A total of 324 records were reviewed, of which 62 duplicates studies were removed. Of the 262 records screened, 211 studies were excluded. Fifty-one articles were assessed for eligibility, for which 7 were excluded (Figure 1). Twenty-nine studies were included for ALF only, and 9 studies for ACLF only. Six studies included patients who had both ALF and ACLF. A total of 44 publications were included (Figure 1).
A total of 35 studies included patients with ALF (Table 2, Table 3, and Table 5). Of this, 24 were studies in adults and 11 in the pediatric population (Table 3 and Table 5). In the studies that included adult subjects, 4 also included patients with ACLF. Of the 24 studies in adults, there was 1 randomised controlled trial, 4 cohort studies, 9 case series and 10 case reports. Of the 11 studies with pediatric subjects, there was 1 cohort study, 4 case series and 6 case reports; and 2 of the 11 studies included patients with both ALF and ACLF.
There is only one randomized trial to date[3] that assessed transplant-free survival comparing standard medical treatment (SMT) vs plasma exchange and SMT in patients with ALF. In the Larsen et al[3] study, high volume plasma exchange (HVP) increased survival in non-transplanted patients after three months. However, there was no significant difference in the effect of HVP in patients who received emergency liver transplantation. Of the three studies comparing plasma exchange vs SMT or alternative liver support systems, all reported an improvement in survival in patients who did not undergo liver transplant[3,4] or improvement in survival days[5].
All studies that assessed biochemical improvement pre- and post-plasma exchange, found an improvement in biochemical parameters such as coagulopathy, bilirubin, aspartate aminotransferase (AST), alanine aminotransferase or ammonia. However, biochemical improvement did not directly relate to mortality outcome. Even in the patients who did not survive, there was also biochemical improvement post- plasma exchange[5].
There is heterogeneity in the amount of plasma exchange a patient gets in ALF amongst the various studies. Two studies[3,6] used plasma exchange at least 15% ideal body weight removal at 1-2 L per hour while in Buckner et al[7]’s case series, plasma exchange with 10 L of donor plasma regardless of weight was used. Similar to Buckner et al[7], Damsgaard et al[8] in a case report used 8-9 L of plasma per session for plasma exchange for a patient with ALF from Wilson’s disease, who survived without need for liver transplant. In contrast, in case series by Akdogan et al[9], only one-plasma volume was being exchanged daily till patient expired or improved. Majority of studies used approximately 2-4 L of fresh frozen plasma at each plasma exchange[4,10-18].
While there are stark differences in amount of plasma exchanged in these aforementioned studies, in the study of the use of plasma exchange in the treatment of acute liver failure, there is only one open randomized controlled trial[3] favoring the use of high volume plasma exchange over standard medical treatment. There are no head to head studies comparing high volume to standard volume plasma exchange. Although most cohort studies or case series that used 2-4 L of plasma and or additional fluid for plasma exchange saw positive results whereby there were reported improvement in biochemical parameters such as bilirubin and coagulopathy[4,5,9,11,12,14-16,18,19], and some of which also reported increased transplant-free survival days[5], at present evidence favor large volume plasma exchange for treatment of ALF[3].
There is currently no clear evidence to support the use of other assist devices in addition to plasma exchange in management of ALF. Several studies[4,10,20] included alternative assist devices to plasma exchange and made comparisons of its efficacy in the treatment of acute liver failure. For example, comparing plasma exchange vs plasma exchange + continuous venovenous hemodiafiltration (CVVHDF), Nakae et al[10] showed that the latter resulted in a decrease in inflammatory mediators and an increase in citrate compared to the former group. Another study, also by Nakae et al[10], reported use of plasmadiafiltration, a blood purification therapy where plasma exchange is performed using a selective membrane plasma separator while the dialysate flows outside the hollow fibers for management of ALF. In that study[21], less plasma was used per cycle: 1200 mL fresh frozen plasma (FFP) and 50 mL of 25% albumin per session. However, patients had an average of 8.3 cycles of plasmadiafiltration, which is higher compared to other studies (Table 2). Transplant free survival rate was 38.1%, 54.5% in ALF, and 20% in fulminant hepatitis; there was no control arm. Pediatric studies were evaluated separately, and in the included pediatric studies[19,22-27], the amount of plasma exchange per session ranged from 1-4 plasma volumes per exchange.
Most studies used 100% FFP for plasma exchange with the exception of few studies[13,19,21,28-31], where plasma substitutes or albumin were used in conjunction with plasma. For example, in a case series by Liu et al[31], Seven liters of fluid was used for plasma exchange, but the first 4.7 L was composed of fresh frozen plasma, while the rest comprised of plasma consisting of 25% human albumin, 0.9% saline and Ringer’s solution.
There are by far no studies that use pure albumin as replacement fluid for plasma exchange in ALF. However, Collins et al[32] has described in their case report, a patient with fulminant hepatitis from Wilson's disease who underwent single-pass albumin dialysis (SPAD) with improvements in bilirubin. Although of note, the same patient underwent plasma exchange after stopping SPAD in view of serum copper rebound.
As aforementioned, only few[3,6,18,33] studies used a strict consecutive daily or every other day 3-d therapy plasma exchange regime as in the open-RCT by Larsen et al[3]. Instead, most studies continued plasma exchange till patient dies, or improves clinically, or receives a liver transplant at a range of intervals from every other day to intermittent (as and when necessary). Buckner et al[7] reported an interesting finding in their case series where a patient with halothane toxicity and acute liver failure received plasma exchange with 5-10 L of plasma almost daily for 37 d before she roused from coma. Few studies did not include detailed information on the frequency of plasma exchange[5,29,34.
Not all studies assessed included etiology of liver failure and its effect on transplant survival. In larger cohort studies, predominant causes of ALF include paracetamol, followed by unknown cause. As already been discussed in previous literature, paracetamol-induced ALF has improved prognosis compared to injury from other causes, for example, viral hepatitis causes. From the earlier studies[6,14] where in two case series survival in ALF patients receiving plasma exchange was 50%-55%, the subgroup of patients who had paracetamol-induced ALF had survival ranging from 83%-100%. However, these are from case series and the level of evidence is not strong. In a Chinese cohort[4] study comparing the efficacy of plasma exchange + hemoperfusion + CVVHDF to plasma exchange + CVVHDF and hemoperfusion + CVVHDF, treatment of the 61 patients using the artificial liver support system yielded a combined survival rate of 62.3% (38/61). When subdivided into viral versus non-viral groups, the viral group survival rate was 35.0% (7/20) while the non-viral group survival rate was 75.6% (31/41).
The use of plasma exchange in patients with fulminant liver failure from Wilson’s disease has been reported in case reports and series with encouraging out-comes[8,19,23-25,30,32,35]. EASL guidelines for Wilson’s disease[36] recommends that patients with acute liver failure due to Wilsons disease should be treated with liver transplantation when revised King’s score is 11 or higher (Grade II-2 B1 Class I, Level B). In one case report[8], a patient who met criteria for requiring liver transplant improved with plasma exchange alone, thereby averting a high-risk liver transplant. In addition, for patients who were subsequently transplanted, plasma exchange temporarily stabilized patients before liver transplant thereby allowing time to source for potential donors for liver transplant[24,25]. Nevertheless, most of the studies that included Wilson’s disease are small case series, and the level of evidence remains weak.
While there is strong evidence to use plasma exchange in ALF to improve survival[3], there has yet to be robust evidence to use plasma exchange in ACLF. Existing studies are mostly cohort studies done in Asia on patients with ACLF of predominantly hepatitis B viral etiology. Prognosis of patients with ACLF is extremely poor with mortality rates ranging from 30%-70%[2] in the absence of timely liver transplant. As there were very few studies in ACLF that only used plasma exchange solely, this review included all studies that used plasma exchange based-liver support systems in the management of ACLF.
A total of 15 studies of patients with ACLF were included (Tables 4, 5), of which 6 studies included patients with both ACLF and ALF (Table 5). 2 of the studies, which included both ACLF and ALF, were in pediatric patients. The rest of the 13 studies included adult patients only. Of the 13, 1 is a randomised controlled trial, 10 are cohort studies, 2 are case series.
Mortality: Plasma exchange with or without the use of other liver support systems improves survival in non-transplanted in patients with ACLF. An open-label randomized control study by Qin et al[37] recruited 234 patients with HBV-related ACLF not suitable for liver transplant and randomized patients to SMT vs plasma exchange centered ALSS plus SMT. In this study, survival rates in plasma exchange-based ALSS were significantly higher: 60% vs 47% in the control group. Other retrospective cohort studies[38-40] also favored plasma exchange (and or plasma exchange-based non-bioartificial liver support system) to SMT in patients with ACLF from hepatitis B infection. For example, In Yue-Meng et al[39], patients with ACLF who had plasma exchange+SMT had increased rate of survival compared to patients who had SMT only: 4-wk mortality was 82% vs 63%, P = 0.001; 12-wk mortality 86% vs 71%, P = 0.001).
Similarly, Mao et al[38] reported increased 30-day survival in patients with HBV related ACLF where survival rates were 41.9% and 25.2% for plasma exchange and medical therapy respectively (P < 0.05). In the same study[38], time from the initial diagnosis to initiation of plasma exchange was found to be longer when in non-survivors compared to survivors, although this was not statistically significant.
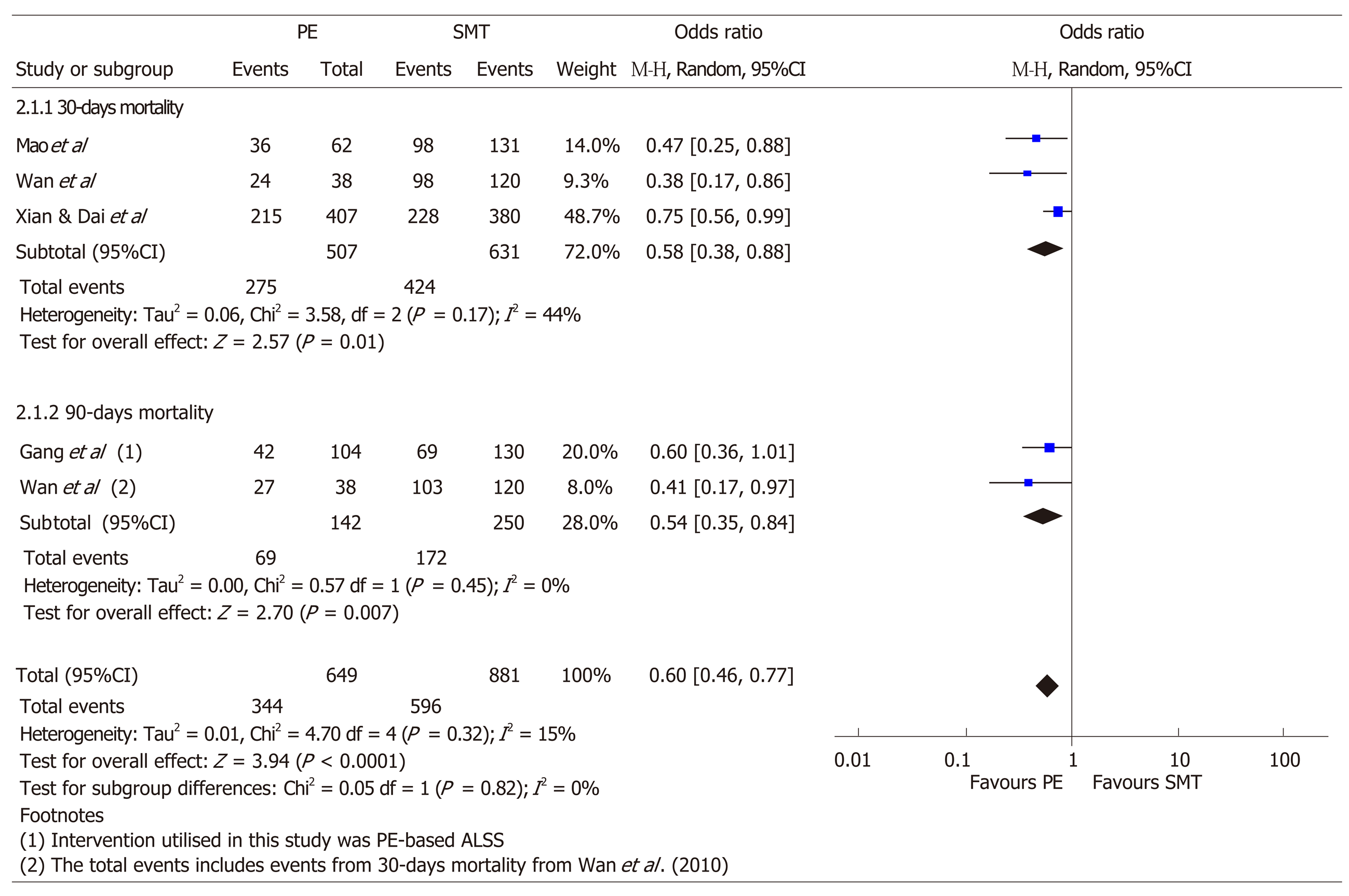
Four of the studies[37-40] that reported plasma exchange based liver support system compared to standard medical treatment were analysed for pooled mortality at 30 and 90 d and data was presented in a forest plot (Figure 2). Other studies that had no comparative standard medical treatment arms were excluded from this analysis. Using available published in the aforementioned studies, plasma exchange was superior to SMT for survival in patients with ACLF, at 30-d (OR: 0.38, 95%CI: 0.38-0.88, P = 0.01, I2 = 44%; 3 studies) and at 90-d (OR: 0.54, 95%CI: 0.35-0.84, P ≤ 0.01, I2 = 0; 2 studies). The pooled mortality at 30 and 90 d is significantly reduced in patients with ACLF who underwent plasma exchange (or plasma exchange based ALSS) vs SMT (OR: 0.60, 95%CI: 0.46-0.77, P < 0.01, I2 = 15%; 4 studies).
Several studies compared use of plasma exchange only vs plasma exchange together with other liver support system, such as the use of double plasma molecular adsorption system (DPMAS). For example, Yao et al[41] reported significantly higher 28-d survival rate in specifically intermediate-advanced stage patients with ACLF who underwent DPMAS + plasma exchange compared to plasma exchange alone (57.4% vs 41.7%, P = 0.043).
Biochemical improvement: Most studies reporting the effect of plasma exchange-based therapy on patients with ACLF which assessed biochemical improvement pre- and post-plasma exchange found an improvement in biochemical parameters such as coagulopathy, bilirubin, aspartate aminotransferase, alanine aminotransferase, or ammonia[12,39-44], though this was not always associated with clinical improvement. Zhou et al[45] reported a predictive model using baseline age, MELD score, number of complications and type of ALSS to predict survival after ALSS in patients with ACLF. In the same study, authors report that plasma bilirubin adsorption (PBA) + plasma exchange compared to plasma exchange only had better 90-day survival 70.3% vs 58.3%; although there was no mention if PBA + plasma exchange significantly decreased levels of bilirubin compared to plasma exchange alone. However when DPMAS vs plasma exchange was compared in other studies[41,44], increased clearance of bilirubin (as seen in the plasma exchange arm in Wan et al[44], and DPMAS + plasma exchange arm in Yao et al[41]) was not associated with respective significant improvements in survival. Of note, in the study by Zhou et al[45], baseline characteristics of the group of patients who underwent PBA+ plasma exchange vs plasma exchange was unavailable, essential information that could have influenced survival outcomes.
Standard vs high volume and other liver assist devices: Several studies used solely plasma exchange in management of ACLF. For the studies that included plasma exchange in management of ACLF, a range of 2000-4500 mL of plasma exchange per session[12,37,38,41,43-45] was adopted. There were no studies in ACLF group that used high volume plasma exchange. However, there were more studies in ACLF compared to ALF that use other liver assist devices in conjunction with plasma exchange – for example, DPMAS, PBA, hemofiltration, hemodiafiltration, plasma diafiltration and its combinations. As there is no head to head trial and most studies are retrospective, it is not possible to draw any conclusion as to whether one modality was superior to another.
FFP vs albumin: Most studies used FFP for plasma exchange or plasma exchange-based ALSS[12,37-39,41-45]. In addition, Mao et al[38] used additional albumin during plasma exchange. Albumin dialysis was not compared with plasma exchange in this review.
Three-day therapy vs intermittent or response guided: All studies included for review extended plasma exchange beyond three days wherever relevant based on clinical necessity[12,37-39,41-45]. In addition, most studies do not use daily plasma exchange, and instead, this was performed 2-3 times per week and were response guided, where plasma exchange often was continued till clinical improvement, transplant, or death.
Etiology specific outcome: Of the 13 included studies for plasma exchange in ACLF in adult patients, all were being conducted in Asia where hepatitis B is endemic. Thus, the majority of the patients assessed have HBV related ACLF. In comparison to non-viral causes, ACLF in the presence of viral causes tends to have a poorer survival rate. For example, Cheng et al[12] reported a 24% survival in hepatitis B related ACLF vs 67% in alcohol-related ACLF in their retrospective cohort study of 45 ACLF and 10 ALF patients. Furthermore, where there were more than two causes for chronic liver injury e.g., HCV and alcohol, HBV and alcohol or in autoimmune hepatitis, mortality was high at 100%. However, this will need to be interpreted with caution as degree fibrosis or severity of cirrhosis of each patient was not available in the published study.
Acute liver failure and acute on chronic liver failure carry a high risk of mortality in patients in the absence of a liver transplant, a scarce resource. Liver assist devices, some of which are plasma exchange-based, have been used in patients with ALF or ACLF majority of which reported showing some benefit compared to standard medical treatment. However, there remains an unmet need for good quality prospective trials to be done, to ascertain the ideal volume, type, and duration of plasma exchange in management of ALF. Additional randomized controlled studies are also required to further shed light on the utility for plasma exchange or plasma exchange-based liver support systems for ACLF.
Firstly, while there has been good evidence for the use of high volume plasma exchange in acute liver failure to improve survival, due to the paucity of good-quality studies, at present it is unknown if a lower volume or a longer (or shorter) duration (i.e., beyond the first three consecutive days) of plasma exchange will achieve equal or improved survival in ALF. This is important since donor plasma is a finite resource, and HVP is not without side effects. For example as in the above case series by Freeman et al[13] where patients with ALF were treated with standard volume plasma exchange, overall survival was 55% which on surface appears comparable to HVP in Larsen et al[3]. Nevertheless, little conclusion can be drawn as case series often seem to show benefit while well-designed clinical randomized controlled trials may fail to fulfil the hopes of initial reports. In addition, as the baseline characteristics of patients could be different from these various studies, no conclusive verdict can be made until further high-quality studies are being carried out comparing high volume plasma exchange vs standard volume plasma exchange. There is also insufficient evidence to suggest if plasma exchange with albumin or a combination of albumin and fresh frozen plasma will be non-inferior to high volume plasma exchange in management of ALF.
Furthermore, whether further doses of plasma exchange will benefit a patient with ALF beyond the third exchange will be an important question to answer with future well-designed randomized controlled trials. This is especially important in donor scarce countries, where it may take more than a few days to work up a suitable liver donor for living donor liver transplant. In Buckner et al[7], it was reported that one patient who had ALF from halothane toxicity had frequent high volume plasma exchange until she awoke from a coma 37 d later. On the other hand, Chien et al[22] reported in a case series of 23 pediatric patients that plasma exchange for more than six times probably offers the little benefit with regard to patient survival in the absence of a timely liver transplant. Mechanistically, Kondrup et al[6] has reported that on theoretical assumptions, three courses of plasma exchange on consecutive days would reduce the concentration of “toxins” distributed in extracellular water to 18% of initial concentration at the end of last exchange and an additional course will only theoretically decrease this only to 16%. While this could stand true for patients whose cause of liver failure is from drugs such as paracetamol, the same may not be the case for patients who have liver failure from other causes like autoimmune or viral.
Secondly, there remains insufficient evidence to extrapolate the findings from Larsen et al[3] to recommend plasma exchange in patients with ACLF. There is no study to our best knowledge that used consecutive three days of high volume plasma exchange for the management of ACLF. Qin et al[37] has reported the use of plasma exchange based ALSS compared to SMT in the only prospective controlled trial to date in patient with HBV-ACLF – however this has not made it to standard practice. Of note, the definition of ACLF in this study follows the Chinese definition hence does not mandate the need for cirrhosis or multi-organ failure. Thus this study population is heterogenous, ranging from patients with no cirrhosis (52%) to patients requiring intensive care and renal replacement therapy. The timing of initiation and type of antiviral was also not standardized which adds limitations and potential bias to the final results. Nevertheless, it is worthwhile to note that the baseline characteristics of both treatment groups were similar and ALSS was found to have improved 90-day and 5-year mortality compared to SMT (60% vs 47%, P = 0.016; 43% vs 31%, P = 0.013). These results are promising and thus further randomised controlled trials should be done, ideally with stratification of patients according to different etiologies and grades of ACLF (or acute decompensation in cirrhotics) and assess if there are any differences in response to plasma exchange.
Of the 13 studies on use of plasma exchange based therapy in ACLF in adult patients, 4 studies compared plasma exchange based therapy with SMT. Of the four studies, 3 studies only included patients in HBV-associated ACLF. In the one study that included patients with other etiology of ACLF, HBV-related ACLF was predominant–91.24% of the ACLF study population. Using the aforementioned studies, plasma exchange based therapy in ACLF compared to SMT improved survival at 30- and 90-d with a pooled OR of 0.60 (95%CI: 0.46-0.77, P < 0.01). However, there are limitations in that the number of studies used to generate the pooled OR estimate for mortality comparing plasma exchange vs SMT are small due to the limited number of studies reported in literature, hence the risk for bias of each study was not assessed. In addition, the definition of ACLF in these studies do not require the diagnosis of cirrhosis and or more than one organ failure. Moreover, the etiology of ACLF in these groups of patients is HBV related, and thus these findings cannot be extrapolated to ACLF caused by other etiologies.
Thirdly, it will be essential to find out if there is an objective measure of a point of no return whereby plasma exchange or plasma exchange -based ALSS will be futile, whether in ALF or ACLF. For example, Nakae et al[21] reported that while overall survival with plasma diafiltration was 54.5% in their study, there were no survivors in the MELD > 40 groups when plasma diafiltration was used in ALF. Chen et al[42] also reported a trend where less patients with late-stage ACLF who underwent plasma exchange had transplant-free survival: 80.8% patients in the early stage, 75.8% patients in the middle stage and 37.4% patients in the end-stage survived for one month after diagnosis. Mechanistically, plasma exchange acts by removing plasma cytokines and adhesion molecules, which are the drivers of the systemic inflammatory cascade from the circulation and it is possible that late in the disease course of organ failure that the utility of plasma exchange would exponentially decrease.
Finally, other than using plasma exchange for management of ALF of ACLF, in our literature search there have been reports of other extracorporeal liver support devices could potentially improve outcomes in ALF or ACLF either alone or when used in conjunction with plasma exchange. Examples include molecular adsorbents recirculating system (MARS), Fractionated Plasma Separation, Adsorption and Dialysis device, SPAD. Several of our included studies used modalities like plasma exchange + DPMAS, plasma exchange + CVVHDF, or plasma diafiltration with varying results. Of the above liver devices, MARS has been studied most widely both in ALF and ACLF and its benefits remain modest. For example, a large randomized control trial did not demonstrate benefit in 6-mo survival in patients who had ALF and underwent MARS, although a major limitation of this study was that 75% of enrolled patients received a transplant within 24 h of enrollment, thereby potentially limiting the findings of the study[46]. This will remain to be a major limitation in future studies, especially in areas where donor livers can be quickly obtained. The emerging liver support devices, such as that of bioartificial liver support device which include a bioreactor that contains hepatocytes that can replace the function of the failing liver, e.g., the Extracorporeal liver assist device has entered human clinical trials. Other artificial liver support systems that combine detoxification with techniques to attenuate liver injury include the Hepa Wash, Li-Artificial Liver Support, and the University College London-Liver Dialysis Device, which has shown efficacy in animal experiments. More studies will need to be done to allow clinical use of these devices in liver failure.
In summary, the state of art therapy for acute liver failure should include plasma exchange based on the high-quality evidence, especially in patients who do not have a donor liver in sight. Current guidelines by EASL support use of HVP in ALF. While there is an open RCT supporting for plasma exchange-centered ALSS in the management of ACLF[37], and our pooled estimates from four studies favor the use of plasma exchange in ACLF as it is associated with a decreased 30 and 90 d mortality, this has not yet made it to standard practice.
The major limitation of our study is the low number of well-designed high-quality evidence available, as most studies are case series or cohort studies. This is possibly because liver failure is not common, and patients are usually critically ill. Secondly, as most of these studies are cohort studies or case series, we are unable to assess for publication bias via a funnel plot. However, publishing bias may exist as published studies are mostly positive studies and negative studies may not be reported. Lastly, the studies that have been included in plasma exchange in ACLF are over-represented by Asian patients, and the definitions of ACLF in each of these studies vary. Furthermore, it is imperative to take into account that the definition of ACLF in most Asian studies do not mandate the need for patients to be cirrhotic and have more than one organ failure, thereby potentially selecting a different group of patients from that seen in the Western ACLF literature. Whether the benefits of HVP in ALF can be extrapolated to patients with ACLF remains uncertain. Due to limited RCTs for ALF, only a subgroup meta-analysis (Figure 2) was explored comparing mortality among non-transplanted ACLF patients who underwent plasma exchange based therapy vs SMT.
There are many unanswered questions in this field: For example, the optimal type, duration, frequency, volume and time to plasma exchange one should use for ALF, and ACLF, if at all. Future randomized control trials studying the use of plasma exchange and or other liver assist devices in liver failure should aim to answer these questions. In addition, future studies should also include the study of biomarkers that can predict the success of therapy. This might further shed light on the optimal duration, volume of plasma exchange and or alternative liver support therapy since there is vast heterogeneity in patients with ALF and ACLF, and perhaps different groups of patients will require different regimes.
Liver failure portends a high mortality without successful liver transplantation. High volume plasma exchange has been included in European guidelines as level I, grade 1 recommendation in management of acute liver failure possibly by removal of plasma cytokines and drivers of systemic inflammatory cascade through plasma exchange. In recent years, there is increasing interest in plasma exchange for the treatment of liver failure, as there is proven improvement in survival in those who do not undergo a liver transplant. Prior to this study, there were several other cohort studies reporting the benefits of plasma exchange in acute liver failure (ALF), however the volume and duration of plasma exchange varies. The evidence for use of high volume plasma (HVP) in acute-on-chronic liver (ACLF) is less robust, but the use of plasmapheresis (not high volume) has been reported in literature.
While there is good evidence to use plasmapheresis in management of acute liver failure especially when there is no liver donor in sight, the optimal volume and duration of plasma exchange is unclear. Donor plasma is a finite resource, and HVP is not without side effects such as hypocalcemia requiring rapid calcium replacement. Several cohort studies showed benefit in standard volume plasmapheresis in management of ALF however no head to head comparisons have been done. Furthermore use of plasma exchange in ACLF, while has been reported to improve survival in literature, has not been widely accepted as standard treatment due to lack of high level evidence.
This study aims to summarize and analyze the current literature for use of plasmapheresis in patients with ACLF and ALF and its effect on mortality particularly in the non-transplanted patients. In addition, the review will summarise the current literature on volume of plasma used during exchange, the duration and frequency of plasma exchange in both ALF and ACLF. It is our hope that this review will serve as a valuable resource by analyzing available literature as well as illustrate the knowledge gaps and unmet needs for future researchers in this field.
This systematic review uses guidance from the PRISMA checklist. Databases MEDLINE via PubMed, and EMBASE were searched and relevant publications up to 30 March, 2019 were assessed. Forty-four studies were shortlisted and included in Tables 2-5. In addition, pooled odds ratios and its corresponding 95% confidence intervals were respectively calculated for 30- and 90-d mortality in ACLF patients using the random effects model. We were unable to do this for ALF group due to paucity of studies and lack of critical information from eligible studies.
There is good evidence for use of high volume plasma exchange in ALF though the optimal duration and volume of plasma exchange at present is uncertain. While high quality randomized control trials are lacking, the use of plasma exchange in ACLF can be considered. Survival in non-transplanted patients was improved in all four studies in patients with ACLF comparing plasma exchange vs standard medical therapy (SMT). Using the aforementioned studies, plasma exchange based therapy in ACLF compared to SMT improved survival in non-transplanted patients at 30 and 90-d with a pooled OR of 0.60 (95%CI: 0.46-0.77, P < 0.01). There remains insufficient evidence to extrapolate the findings which recommend plasma exchange in patients with ACLF. Whether an individualized plasma exchange regime for each patient with liver failure can be personalised based on biomarkers remains unknown. More head to head trials will need to be done.
While there has been good evidence for the use of high volume plasma exchange in acute liver failure to improve survival, due to the paucity of good-quality studies, at present it is unknown if a lower volume or a longer (or shorter) duration (i.e., beyond the first three consecutive days) of plasma exchange will achieve equal or improved survival in ALF. In patients with ACLF, plasma exchange based therapy compared to SMT improves survival at 30 and 90-d in non-transplanted patients. The duration of plasma exchange therapy used in most studies in ACLF was clinical response driven and often intermittent; and not with high volume plasmapheresis. The etiology of ACLF was also mostly HBV related; the definitions of ACLF used are varied and not requiring the diagnosis of more than one organ failure and diagnosis of cirrhosis. At present, there is insufficient evidence to extrapolate the use of high volume plasmapheresis to patients with ACLF.
There are unanswered questions in use of plasma exchange in liver failure: For example, the optimal type, duration, frequency, volume and time to plasma exchange one should use for ALF, and ACLF, if at all. Future randomized control trials studying the use of plasma exchange and or other liver assist devices in liver failure should aim to answer these questions. It is also essential to find out if there is an objective measure of a point of no return whereby plasma exchange or plasma exchange-based ALSS will be futile, whether in ALF or ACLF. Subsequent clinical trials in ACLF or ALF should include study of biomarkers that can predict the success of therapy. This might further shed light on the optimal duration, volume of plasma exchange and or alternative liver support therapy since there is vast heterogeneity in patients with ALF and ACLF and one regime may not fit all. Finally, the definition of ACLF in each study needs to be clearly stated, in order to allow clinicians to assess applicability of study results to their patients.
Manuscript source: Invited Manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Singapore
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sinha R, Williams R, Zheng YW S-Editor: Wang J L-Editor: A E-Editor: Zhang YL
| 1. | European Association for the Study of the Liver; Clinical practice guidelines panel; Wendon J, Panel members, Cordoba J, Dhawan A, Larsen FS, Manns M, Samuel D, Simpson KJ, Yaron I; EASL Governing Board representative, Bernardi M. EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. J Hepatol. 2017;66:1047-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 621] [Article Influence: 77.6] [Reference Citation Analysis (1)] |
| 2. | Hernaez R, Solà E, Moreau R, Ginès P. Acute-on-chronic liver failure: an update. Gut. 2017;66:541-553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 360] [Cited by in RCA: 441] [Article Influence: 55.1] [Reference Citation Analysis (1)] |
| 3. | Larsen FS, Schmidt LE, Bernsmeier C, Rasmussen A, Isoniemi H, Patel VC, Triantafyllou E, Bernal W, Auzinger G, Shawcross D, Eefsen M, Bjerring PN, Clemmesen JO, Hockerstedt K, Frederiksen HJ, Hansen BA, Antoniades CG, Wendon J. High-volume plasma exchange in patients with acute liver failure: An open randomised controlled trial. J Hepatol. 2016;64:69-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 441] [Article Influence: 49.0] [Reference Citation Analysis (4)] |
| 4. | Li M, Sun J, Li J, Shi Z, Xu J, Lu B, Cheng S, Xu Y, Wang X, Zhang X. Clinical observation on the treatment of acute liver failure by combined non-biological artificial liver. Exp Ther Med. 2016;12:3873-3876. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Hung YM, Hung GC, Hsu PI, Hung SY, Chou KJ, Chung HM. Short-term survival advantage after plasma exchange in the treatment of acute on chronic liver failure or acute liver failure. Clin Intensive Care. 2004;15:93–99. [DOI] [Full Text] |
| 6. | Kondrup J, Almdal T, Vilstrup H, Tygstrup N. High volume plasma exchange in fulminant hepatic failure. Int J Artif Organs. 1992;15:669-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 74] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 7. | Buckner CD, Clift RA, Volwiler W, Donohue DM, Burnell JM, Saunders FC, Thomas ED. Plasma exchange in patients with fulminant hepatic failure. Arch Intern Med. 1973;132:487-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 26] [Article Influence: 0.5] [Reference Citation Analysis (2)] |
| 8. | Damsgaard J, Larsen FS, Ytting H. Reversal of Acute Liver Failure Due to Wilson Disease by a Regimen of High-Volume Plasma Exchange and Penicillamine. Hepatology. 2019;69:1835-1837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Akdogan M, Camci C, Gurakar A, Gilcher R, Alamian S, Wright H, Nour B, Sebastian A. The effect of total plasma exchange on fulminant hepatic failure. J Clin Apher. 2006;21:96-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Nakae H, Yonekawa C, Wada H, Asanuma Y, Sato T, Tanaka H. Effectiveness of combining plasma exchange and continuous hemodiafiltration (combined modality therapy in a parallel circuit) in the treatment of patients with acute hepatic failure. Ther Apher. 2001;5:471-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (5)] |
| 11. | Nakamura T, Ushiyama C, Suzuki S, Shimada N, Ebihara I, Suzaki M, Takahashi T, Koide H. Effect of plasma exchange on serum tissue inhibitor of metalloproteinase 1 and cytokine concentrations in patients with fulminant hepatitis. Blood Purif. 2000;18:50-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Cheng YL, Chang CH, Chen WT, Tsai MH, Lee WC, Tu KH, Tian YC, Chen YC, Hung CC, Fang JT, Yang CW, Chang MY. Prognostic factors and treatment effect of standard-volume plasma exchange for acute and acute-on-chronic liver failure: A single-center retrospective study. Transfus Apher Sci. 2018;57:537-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Freeman JG, Matthewson K, Record CO. Plasmapheresis in acute liver failure. Int J Artif Organs. 1986;9:433-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 37] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 14. | Aydemir S, Ustundag Y, Bayraktaroglu T, Tekin IO, Peksoy I, Unal AU. Fulminant hepatic failure associated with propylthiouracil: a case report with treatment emphasis on the use of plasmapheresis. J Clin Apher. 2005;20:235-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Bilgir O, Calan M, Bilgir F, Cagliyan G, Arslan O. An experience with plasma exchange treatment of acute lymphoblastic leukemia in a case with fulminant hepatitis related to L-asparaginase. Transfus Apher Sci. 2013;49:328-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Lin S, Li Y, Long J, Liu Q, Yang F, He Y. Acute liver failure caused by hemophagocytic lymphohistiocytosis in adults: A case report and review of the literature. Medicine (Baltimore). 2016;95:e5431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Holt EW, Guy J, Gordon SM, Hofmann JC, Garcia-Kennedy R, Steady SL, Bzowej NH, Frederick RT. Acute liver failure caused by herpes simplex virus in a pregnant patient: is there a potential role for therapeutic plasma exchange? J Clin Apher. 2013;28:426-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Shen C, Zhao CY, Liu F, Wang YD, Wang W. Acute liver failure associated with occupational exposure to tetrachloroethylene. J Korean Med Sci. 2011;26:138-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Pham HP, Schwartz J, Cooling L, Hofmann JC, Kim HC, Morgan S, Pagano MB, Schneiderman J, Winters JL, Yamada C, Wong EC, Wu Y. Report of the ASFA apheresis registry study on Wilson's disease. J Clin Apher. 2016;31:11-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 20. | Nakae H, Igarashi T, Tajimi K. Selective plasma exchange with dialysis in patients with acute liver failure. Ther Apher Dial. 2012;16:467-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 21. | Nakae H, Eguchi Y, Saotome T, Yoshioka T, Yoshimura N, Kishi Y, Naka T, Furuya T. Multicenter study of plasma diafiltration in patients with acute liver failure. Ther Apher Dial. 2010;14:444-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (3)] |
| 22. | Chien MM, Chang MH, Chang KC, Lu FT, Chiu YC, Chen HL, Ni YH, Hsu HY, Wu JF. Prognostic parameters of pediatric acute liver failure and the role of plasma exchange. Pediatr Neonatol. 2019;60:389-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 23. | Verma N, Pai G, Hari P, Lodha R. Plasma exchange for hemolytic crisis and acute liver failure in Wilson disease. Indian J Pediatr. 2014;81:498-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Zhang Y, Li L, Zhang X, Xu W, Guo Q, Zhou J. Plasmapheresis Combined with Continuous Plasma Filtration Adsorption Rescues Severe Acute Liver Failure in Wilson's Disease before Liver Transplantation. Blood Purif. 2019;47:120-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Morgan SM, Zantek ND. Therapeutic plasma exchange for fulminant hepatic failure secondary to Wilson's disease. J Clin Apher. 2012;27:282-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Yükselmiş U, Girit S, Çağ Y, Özçetin M. A child with acute liver failure associated with influenza A and resolved with plasma exchange treatment. Hong Kong J Emerg Med. 2018;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Ponikvar R, Buturović J, Cizman M, Mekjavić I, Kandus A, Premru V, Urbancic A, Zakotnik B, Bren A, Ivanovich P. Hyperbaric oxygenation, plasma exchange, and hemodialysis for treatment of acute liver failure in a 3-year-old child. Artif Organs. 1998;22:952-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Riveiro-Barciela M, Muñoz-Couselo E, Fernandez-Sojo J, Diaz-Mejia N, Parra-López R, Buti M. Acute liver failure due to immune-mediated hepatitis successfully managed with plasma exchange: New settings call for new treatment strategies? J Hepatol. 2019;70:564-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 29. | Chen KJ, Chen TH, Sue YM, Chen TJ, Cheng CY. High-volume plasma exchange in a patient with acute liver failure due to non-exertional heat stroke in a sauna. J Clin Apher. 2014;29:281-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Pashaei MR, Ajdarkosh H, Daryani NE, Habibollahi P, Beigmohammadi MT. Life saving plasmapheresis for the management of hemolytic crisis and acute liver failure in Wilson’s Disease. Hepat Mon. 2009;. |
| 31. | Liu CT, Chen TH, Cheng CY. Successful treatment of drug-induced acute liver failure with high-volume plasma exchange. J Clin Apher. 2013;28:430-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Collins KL, Roberts EA, Adeli K, Bohn D, Harvey EA. Single pass albumin dialysis (SPAD) in fulminant Wilsonian liver failure: a case report. Pediatr Nephrol. 2008;23:1013-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Göpel W, Schnetzke U, Hochhaus A, Scholl S. Functional acute liver failure after treatment with pegylated asparaginase in a patient with acute lymphoblastic leukemia: potential impact of plasmapheresis. Ann Hematol. 2016;95:1899-1901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Pu Y, Yang D, Mao Y, Zhang Y, Chen K. Therapeutic effects of blood purification in treatment of fulminant hepatic failure. Braz J Infect Dis. 2013;17:427-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Aydinli M, Harmanci O, Ersoy O, Iskit AT, Ozcebe O, Abbasoglu O, Bayraktar Y. Two unusual cases with Wilson's disease: hepatoma and fulminant hepatitis treated with plasma exchange. J Natl Med Assoc. 2006;98:1989-1991. [PubMed] |
| 36. | European Association for Study of Liver. EASL Clinical Practice Guidelines: Wilson's disease. J Hepatol. 2012;56:671-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 971] [Cited by in RCA: 783] [Article Influence: 60.2] [Reference Citation Analysis (1)] |
| 37. | Qin G, Shao JG, Wang B, Shen Y, Zheng J, Liu XJ, Zhang YY, Liu YM, Qin Y, Wang LJ. Artificial liver support system improves short- and long-term outcomes of patients with HBV-associated acute-on-chronic liver failure: a single-center experience. Medicine (Baltimore). 2014;93:e338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 38. | Mao W, Ye B, Lin S, Fu Y, Chen Y, Chen Y. Prediction value of model for end-stage liver disease scoring system on prognosis in the acute on chronic liver failure patients with plasma exchange treatment. ASAIO J. 2010;56:475-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 39. | Yue-Meng W, Yang LH, Yang JH, Xu Y, Yang J, Song GB. The effect of plasma exchange on entecavir-treated chronic hepatitis B patients with hepatic de-compensation and acute-on-chronic liver failure. Hepatol Int. 2016;10:462-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 40. | Xia Q, Dai X, Huang J, Xu X, Yang Q, Liu X, Chen Y, Li L. A single-center experience of non-bioartificial liver support systems among Chinese patients with liver failure. Int J Artif Organs. 2014;37:442-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 41. | Yao J, Li S, Zhou L, Luo L, Yuan L, Duan Z, Xu J, Chen Y. Therapeutic effect of double plasma molecular adsorption system and sequential half-dose plasma exchange in patients with HBV-related acute-on-chronic liver failure. J Clin Apher. 2019;34:392-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (1)] |
| 42. | Chen JJ, Huang JR, Yang Q, Xu XW, Liu XL, Hao SR, Wang HF, Han T, Zhang J, Gan JH, Gao ZL, Wang YM, Lin SM, Xie Q, Pan C, Li LJ. Plasma exchange-centered artificial liver support system in hepatitis B virus-related acute-on-chronic liver failure: a nationwide prospective multicenter study in China. Hepatobiliary Pancreat Dis Int. 2016;15:275-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 43. | Xu X, Liu X, Ling Q, Wei Q, Liu Z, Xu X, Zhou L, Zhang M, Wu J, Huang J, Sheng J, Zheng S, Li L. Artificial liver support system combined with liver transplantation in the treatment of patients with acute-on-chronic liver failure. PLoS One. 2013;8:e58738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 44. | Wan YM, Li YH, Xu ZY, Yang J, Yang LH, Xu Y, Yang JH. Therapeutic plasma exchange versus double plasma molecular absorption system in hepatitis B virus-infected acute-on-chronic liver failure treated by entercavir: A prospective study. J Clin Apher. 2017;32:453-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 45. | Zhou PQ, Zheng SP, Yu M, He SS, Weng ZH. Prognosis of acute-on-chronic liver failure patients treated with artificial liver support system. World J Gastroenterol. 2015;21:9614-9622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 46. | Saliba F, Camus C, Durand F, Mathurin P, Letierce A, Delafosse B, Barange K, Perrigault PF, Belnard M, Ichaï P, Samuel D. Albumin dialysis with a noncell artificial liver support device in patients with acute liver failure: a randomized, controlled trial. Ann Intern Med. 2013;159:522-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 177] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 47. | Ide K, Muguruma T, Shinohara M, Toida C, Enomoto Y, Matsumoto S, Aoki K, Fukuda A, Sakamoto S, Kasahara M. Continuous Veno-Venous Hemodiafiltration and Plasma Exchange in Infantile Acute Liver Failure. Pediatr Crit Care Med. 2015;16:e268-e274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 48. | Harmanci O, Buyukasik Y, Bayraktar Y. Successful plasma exchange treatment in hemolytic crisis of Wilson's disease preventing liver transplantation. Dig Dis Sci. 2006;51:1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 49. | Li YH, Xu Y, Wu HM, Yang J, Yang LH, Yue-Meng W. Umbilical Cord-Derived Mesenchymal Stem Cell Transplantation in Hepatitis B Virus Related Acute-on-Chronic Liver Failure Treated with Plasma Exchange and Entecavir: a 24-Month Prospective Study. Stem Cell Rev Rep. 2016;12:645-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 50. | Schaefer B, Schaefer F, Engelmann G, Meyburg J, Heckert KH, Zorn M, Schmitt CP. Comparison of Molecular Adsorbents Recirculating System (MARS) dialysis with combined plasma exchange and haemodialysis in children with acute liver failure. Nephrol Dial Transplant. 2011;26:3633-3639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 51. | Singer AL, Olthoff KM, Kim H, Rand E, Zamir G, Shaked A. Role of plasmapheresis in the management of acute hepatic failure in children. Ann Surg. 2001;234:418-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 86] [Article Influence: 3.6] [Reference Citation Analysis (0)] |