Published online Apr 28, 2020. doi: 10.3748/wjg.v26.i16.1912
Peer-review started: December 25, 2019
First decision: January 19, 2020
Revised: January 24, 2020
Accepted: April 4, 2020
Article in press: April 4, 2020
Published online: April 28, 2020
Processing time: 124 Days and 17.8 Hours
The connection between inflammatory bowel disease (IBD) and colorectal cancer (CRC) is well-established, as persistent intestinal inflammation plays a substantial role in both disorders. Cytokines may further influence the inflammation and the carcinogenesis process.
To compare cytokine patterns of active IBD patients with early and advanced CRC.
Choosing a panel of cytokines crucial for Th17/Treg differentiation and behavior, in colon specimens, as mRNA biomarkers, and their serum protein levels.
We found a significant difference between higher gene expression of FoxP3, TGFb1, IL-10, and IL-23, and approximately equal level of IL-6 in CRC patients in comparison with IBD patients. After stratification of CRC patients, we found a significant difference in FoxP3, IL-10, IL-23, and IL-17A mRNA in early cases compared to IBD, and IL-23 alone in advanced CRC. The protein levels of the cytokines were significantly higher in CRC patients compared to IBD patients.
Our findings showed that IL-6 upregulation is essential for both IBD and CRC development until the upregulation of other Th17/Treg related genes (TGFb1, IL-10, IL-23, and transcription factor FoxP3) is a crucial primarily for CRC development. The significantly upregulated IL-6 could be a potential drug target for IBD and prevention of CRC development as well.
Core tip: In our paper, we showed that IL-6 upregulation is essential for both inflammatory bowel disease and colorectal cancer (CRC) development, whereas the upregulation of other Th17/Treg related genes (TGFb1, IL-10, IL-23, and transcription factor FoxP3) is a crucial primarily for CRC development. The significantly upregulated IL-6 could be a potential drug target for inflammatory bowel disease and prevention of CRC development as well.
- Citation: Velikova TV, Miteva L, Stanilov N, Spassova Z, Stanilova SA. Interleukin-6 compared to the other Th17/Treg related cytokines in inflammatory bowel disease and colorectal cancer. World J Gastroenterol 2020; 26(16): 1912-1925
- URL: https://www.wjgnet.com/1007-9327/full/v26/i16/1912.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i16.1912
Inflammatory bowel disease (IBD), as a group of chronic relapsing inflammatory conditions of the gastrointestinal tract, is characterized by prolonged activation of the intestinal mucosal immune system, along with the system involvement, which promotes the release of biological markers, such as cytokines[1]. The initiation and aggravation of the inflammatory process seem to be due to dysregulated immune responses with a parallel increase in the expression of pro-inflammatory cytokines, and deficiency of anti-inflammatory cytokines. The dysregulated homeostasis of pro- and anti-inflammatory signals contributes to persistent intestinal inflammation[2].
Inflammation plays a substantial role in sustaining and promoting colorectal cancer (CRC) development as well. As Virchow described in 1863, cancer can arise from inflammatory sites, where the risk of CRC development may increase in the conditions of chronic intestinal inflammation, via the malignant cell transformation in the surrounding tissue. Furthermore, the inflammatory response shares various molecular mechanisms and signaling pathways with the carcinogenic process, such as apoptosis, increased proliferation rate, and angiogenesis[3]. Nonetheless, the activation of two major oncogenic transcription factors/pathways, NF-kB and STAT3, drives the process of chronic inflammation and carcinogenesis[4]. Activation of NF-kB is required for the induction of IL-6 by many cell types, such as lymphocytes, monocytes, macrophages, myeloid cells, and cancerous cells. Through STAT3 signaling, cell proliferation and survival are assured by inhibiting apoptosis, cell adhesion, angiogenesis, etc. However, STAT3 can exert opposite roles in colon carcinogenesis depending on the tumor stage[4].
Dysregulation of the immune response in patients with IBD and CRC triggers infiltration and accumulation of immune cells that provoke the release of several cytokines, chemokines, and growth factors, which may further influence the inflammation and the carcinogenesis process[2]. Immune cells, such as T regulatory (Treg) cells, Type 2 macrophages, CD4+ T-helper (Th)-17 cells, CD8+ T cells, NK, etc., can have effects either sustaining inflammation in IBD, as well as promoting or inhibiting CRC cell growth. The same is also valid for the cytokines which provide the cross-talk between immune cells and CRC cells[5]. Epithelial cells of the colon are both producers and responders to cytokines and chemokines, and those signals can modulate epithelial cell activity by affecting their proliferation, migration, and survival programs[6]. For example, cytokines, such as IL-6, IL-17, IL-21, TNFa, are believed to contribute to the formation of tumor-supportive microenvironment through mitogenic effects on the epithelial cancer cells[5]. There are many subtle mechanisms of inflammatory responses and malignancy involving cytokines: An induction of reactive oxygen species (i.e., TNFa, IL-6, TGFb), inflammation-associated tumor growth through NF-kB and STAT3 (i.e., TNFa, IL-17, IL-6, IL-23, IL-10), inflammation-associated epithelial-mesenchymal transition (i.e., TGFb, TNFa, IL-6), inflammation-associated angiogenesis (i.e., VEGF, TNFa, IL-6, TGFb), and inflammation-associated metastasis (i.e., TNFa, IL-6, TGFb, IL-10)[3,7].
This background is the reason why many investigations are concentrated on the molecular patterns and mechanisms for developing IBD and CRC. Since the recent developments for the role of cytokines in the intestinal inflammation are accumulating, there has been an increased interest in the similarities and differences in inflammation and cancer initiation and advances. We were interested in the comparing of cytokine patterns and subtle changes in cytokine milieu in inflamed tissues of IBD patients, as well as in the cancer environment in CRC patients, especially in the clinical context. However, far too little attention has been paid to opportunities for early detection of subtle changes in cytokine milieu in inflamed tissues in IBD and CRC, especially in the clinical context. As the survival of CRC affected patients depends highly on early detection, we were interested in comparing cytokine patterns of IBD patients in active disease with early and advanced CRC. To address this aim, we choose a panel of pro- and anti-inflammatory cytokines with a substantial role in Th17/Treg differentiation and behavior, like IL-6, TGFb, IL-17A, IL-23, IL-10, in colon specimens of IBD and CRC patients, as mRNA biomarkers, and their protein levels in the peripheral blood.
By employing standard clinical, laboratory, endoscopic, histopathological, and radiological criteria, two main study groups were distinguished–IBD patients and CRC patients.
A total of 18 IBD patients [13 with ulcerative colitis, ulcerative colitis (UC), and 5– with Crohn’s disease (CD)]in an active state of disease without immunosuppressive therapy, attending the University Hospital St. Ivan Rilski, Sofia, during 2011-2013, were recruited for the study. The diagnosis of patients was made according to the standard criteria of ECCO Consensus for CD (2010) and UC (2012) based on a set of anamnestic, clinical, laboratory, and instrumental studies. Criteria for the exclusion of the patients from the study were the following but not limited to: any history of colorectal cancer or hereditary cancer, or the presence of any dysplastic or lesions suggestive of tumor tissues during endoscopic and histological examination; any positive result for autoimmune disease markers (i.e., anti-nuclear antibodies), proved infectious diarrhea, any severe systemic or psychiatric illness. The mean age of the IBD patients was 38 ± 14 years, eleven (61%) were women, and seven (39%) were men.
A total of 80 patients with CRC, obtained in the University Hospital and Trakia Hospital, Stara Zagora, during 2011-2017, were included in the study. They underwent surgical resection of the tumor with curative intent. The patients had no history of prior surgery for rectal/colon tumors, and no known hereditary cancer, UC, or CD, they did not receive chemotherapy or radiation therapy prior to surgery. The diagnose of CRC was confirmed by the histopathological examination, and TNM classification was performed for tumor grading and staging. According to the TNM classification, CRC patients were stratified as the following: 40 patients with early CRC (8 with 1st stage + 32 in 2nd stage) and 40 patients with advanced CRC (19 with 3rd + 21 with 4th stages). The mean age of the CRC patients was 64.05 ± 9.6 years. The total group of CRC was composed of 49 males (61%) and 31 females (39%). Paired tumoral tissue samples and adjacent non-tumoral mucosa were obtained from 12 patients with early CRC (7 with 1st stage + 5 with 2nd stage) and 18 patients with advanced CRC (10 with 3rd + 8 with 4th stages). The mean age of the patients with early CRC was similar to that of patients with advanced CRC (67.2 ± 7.2 vs 71.1 ± 10.6; P = 0.29 t-test). Participants included 19 men (63%) and 11 women (37%).
All patients gave written consent for the study approved by the Ethical Committee of the Medical University of Sofia and University Hospital St. Ivan Rilski. All patients were informed about the purpose of the study.
A total of six intestinal samples were collected from each patient with IBD–from paired inflamed (three) and nearby macroscopically non-inflamed areas (three samples). The tissue samples were taken immediately after a routine endoscopic procedure and were stored at -80 ˚C until processing. Paired tumoral tissue samples and adjacent non-tumoral mucosa of CRC patients were also collected during the surgical procedure and stored at -80 ˚C until processing.
Three ml of peripheral venous blood from totally 80 CRC patients (40 cases of early CRC and 40 cases of advanced CRC) and 11 IBD patients in the active state of disease without immunosuppressive therapy were collected in sterile tubes, and serum samples were obtained and frozen at -80 ˚C before use.
Total RNA was isolated from tissue samples using a column-based RNA isolation kit (GeneJET RNA purification kit, Thermo scientific). The total amount of RNA was quantified by spectrophotometric analysis (GeneQuant 1300 spectrophotometer, GE Healthcare Life Sciences, Switzerland).
Reverse transcription to cDNA was accomplished manually according to the manufacturer’s instructions with the First-strand cDNA Synthesis kit (Thermo Scientific) and High Capacity cDNA Archive kit (Applied Biosystems, United States).
The qRT-PCR was performed on a 7500 Real-Time PCR system (Applied Biosystems, United States) using a TaqMan Universal PCR Mastermix, with cDNA, specific PCR primers sets for our target genes, and 6FAM-labeled TaqMan MGB probes. The ID of Taqman gene expression assays were the following: FoxP3 (Hs00203958_m1), IL-10 (Hs00174086_m1), IL-23 (Hs00372324_m1) from Thermo Scientific; IL-17A (NM_002190), IL-6 (NM_000600) and TGFb (NM_000660) from Primerdesign, United Kingdom. Eukaryotic 18S ribosomal RNA (Hs99999903_m1), from Thermo Scientific, was used as an endogenous control.
Enzyme immunoassays were performed to measure the protein levels of IL-17A, IL-6, IL-23, TGFb1, and IL-10 in patients’ sera (Human ELISA kit, Diaclone, Gene probe, France or Quantikine ELISA Kits, R and D systems, Minneapolis, MN, United States).
Triplicated PCR samples were analyzed by Sequence Detection System software v.1.3.1., and the results for mRNA expression were obtained by performing the comparative threshold cycle ΔΔCt method, after normalization to the endogenous control (as a ratio target mRNA/18S ribosomal RNA). Relative quantification analysis (RQ) represents n-fold mean difference relative to a calibrator (adjacent normal intestinal tissue). Results for local gene expression analyses were expressed as means ± SD. Cytokine levels were presented as a median and interquartile range (25th percentile and 75th percentile). Statistical differences between groups were analyzed using t-test or Mann-Whitney U-test. A value of P < 0.05 was considered significant. Statistical analysis was performed by using StatSoft software v.6. Dr. Tsvetelina Velikova from the University Hospital Lozenetz reviewed the statistical methods of this study.
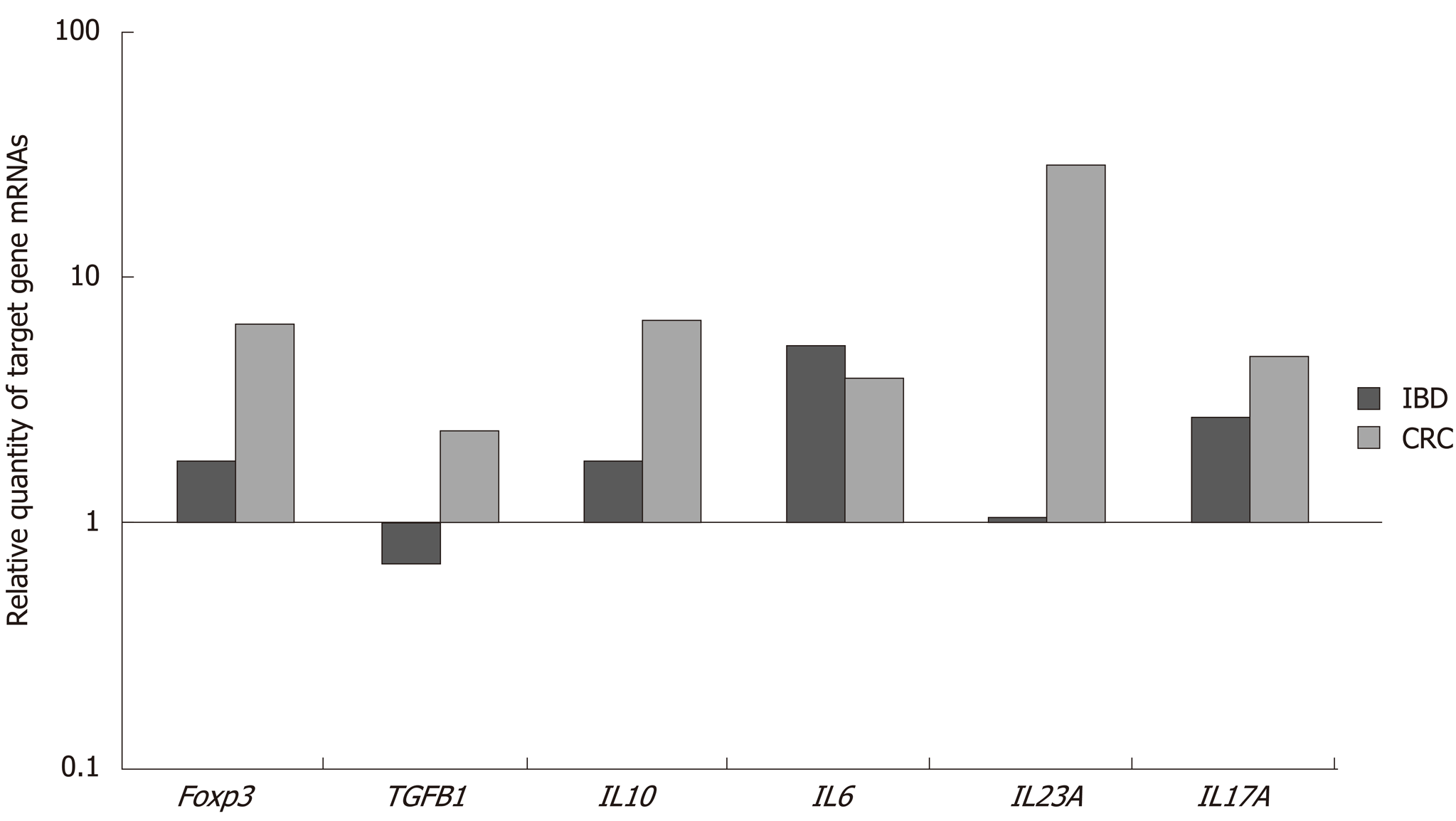
Table 1 shows the results of gene expression presented as dCt ± SD and RQ values of investigated genes in inflamed tissues of IBD patients and tumoral tissue as well. From all studied genes IL-6 and IL-17A were with an approximately equal level of upregulation in both disease–IBD and CRC, although IL-6 showed a tendency for the increased concentration in IBD and decreased IL-17A in comparison to CRC. The upregulation of IL-23 gene expression was a hallmark only for CRC, where the level of mRNA synthesis was approximately 25 times enhanced than in IBD. The higher expression was also detected for TGFb1, IL-10, and FoxP3 in colorectal tissue compared to IBD local expression.
| Genes | IBD | CRC | P value IBD vs CRC | ||||
| dCt ± SD | RQ (min-max) | dCt ± SD | RQ (min-max) | ||||
| Inflamed tissue | Non-inflamed tissue | Tumoral tissue | Non-tumoral tissue | ||||
| Foxp3 | 14.94 ± 2.65 | 15.81 ± 2.59 | 1.82 (0.11-74.85) | 18.62 ± 5.5 | 21.30 ± 4.3 | 6.42 (0.47-97.2) | 0.025 |
| TGFB1 | 9.52 ± 4.13 | 8.97 ± 4.4 | 0.68 (0.03-2.27) | 17.27 ± 2.9 | 18.63 ± 1.9 | 2.37 (0.211-93.7) | 0.007 |
| IL-10 | 16.87 ± 1.76 | 17.7 ± 1.5 | 1.78 (0.22-28.5) | 16.43 ± 5.5 | 19.17 ± 4.4 | 6.66 (0.43-202.3) | 0.023 |
| IL-6 | 17.13 ± 3.05 | 19.53 ± 2.7 | 5.28 (0.27-61.6) | 19.11 ± 3.8 | 21.07 ± 2.5 | 3.88 (0.03-512.4) | 0.784 |
| IL-23A | 18.0 ± 2.09 | 18.1 ± 2.8 | 1.07 (0.07-6.61) | 12.56 ± 2.1 | 17.55 ± 2.5 | 28.5 (2.04-219.5) | 0.0003 |
| IL-17A | 18.9 ± 2.65 | 20.3 ± 2.9 | 2.70 (0.07-20.5) | 24.7 ± 4.3 | 26.9 ± 3.0 | 4.69 (0.034-494.9) | 0.574 |
Figure 1 presents a relative quantity of mRNA levels of the investigated genes. It is visible that the highest RQ difference between IBD and CRC patients was for the IL-23 gene, as well as for the FoxP3 and IL-10 genes and the lowest – for the TGFb1, while for the gene expression of IL-6 and IL-17A the differences were not significant.
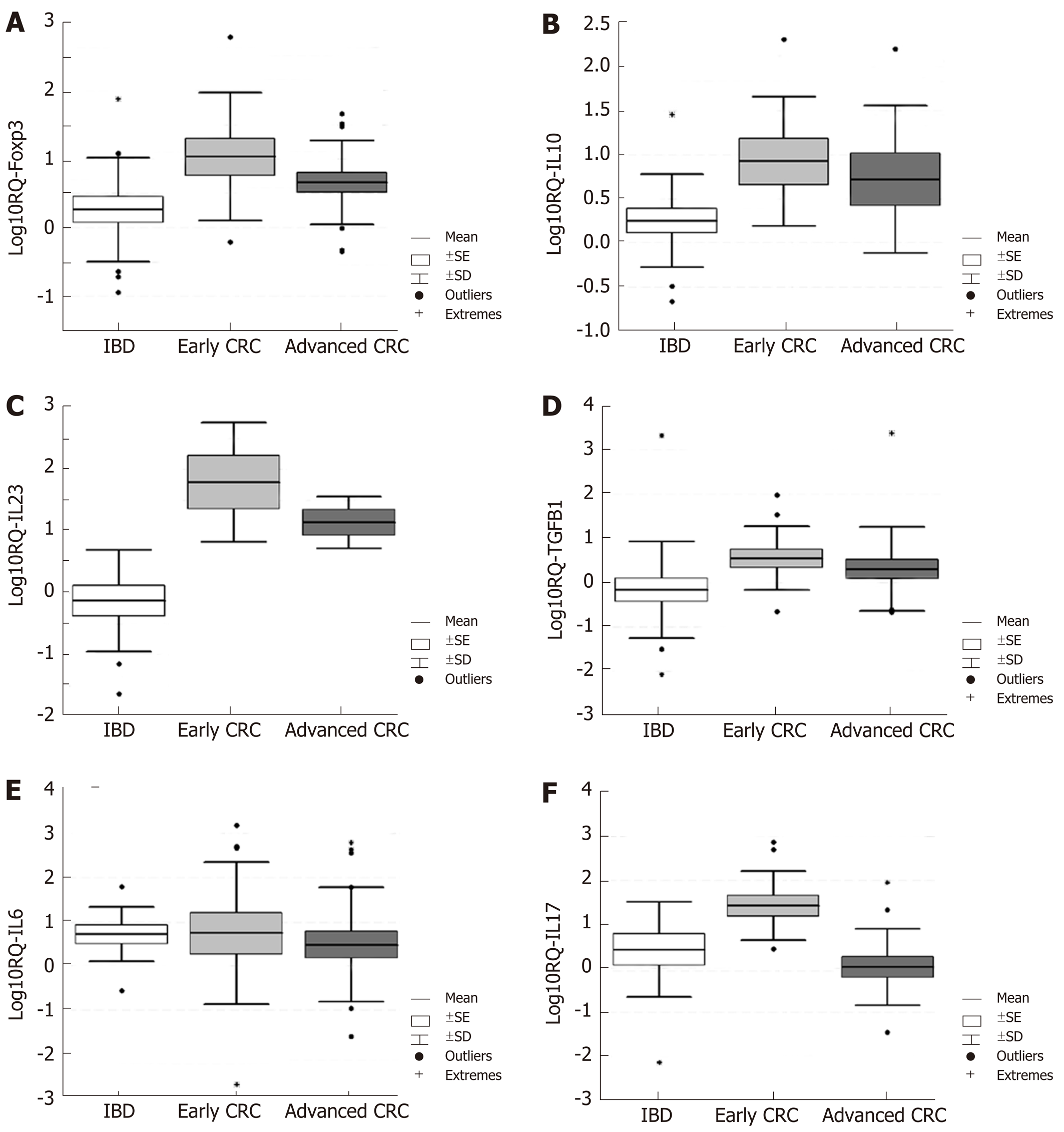
Turning now to the comparison of the local gene expression of target genes in early (1st and 2nd stages) and advanced (3rd and 4th stages) cases of CRC to that in active IBD patients, the results are shown in Figure 2A-F. The figure illustrates that the gene expression of all genes was higher in CRC cases (nevertheless early or advanced) except IL-6 and IL-17A for advanced CRC cases.
Comparing the two stages (early and advanced) of CRC patients, the t-test revealed significant elevation of Foxp3, IL-10, IL-23A, and IL-17A mRNAs in early cases of CRC compared to the IBD cases (Table 2). The TGFB1 mRNA was increased in early CRC compared to the IBD cases with borderline significance (P = 0.057). IL-6 was upregulated in both groups of patients in approximately equal amounts (Table 2). When we compared the gene expression of IBD patients and advanced cases of CRC, we saw that the gene expression of FoxP3, TGFb1, IL-10, and IL-23 was upregulated, although the statistical significance was reached only for IL-23, whereas the mRNA expression of IL-6 and IL-17 were downregulated (Table 3). Moreover, the mRNA expression of FoxP3 (RQ = 10.74), IL-10 (RQ = 8.44), IL-23A (RQ = 61.93), and IL-17A (RQ = 26.83) in early cases of CRC and IL-23A alone in advanced CRC (RQ = 13.96) were overexpressed.
| Genes | IBD | Early stages CRC (1 + 2) | P value IBD vs Early CRC | ||||
| dCt ± SD | RQ (min-max) | dCt ± SD | RQ (min-max) | ||||
| Inflamed tissue | Non-inflamed tissue | Tumoral tissue | Non-tumoral tissue | ||||
| Foxp3 | 14.94 ± 2.65 | 15.81 ± 2.59 | 1.82 (0.11-74.85) | 16.98 ± 5.8 | 20.41 ± 4.5 | 10.74 (0.615-97.2) | 0.023 |
| TGFB1 | 9.52 ± 4.13 | 8.97 ± 4.4 | 0.68 (0.03-2.27) | 16.99 ± 2.4 | 18.83 ± 2.3 | 3.59 (0.222-93.7) | 0.057 |
| IL-10 | 16.87 ± 1.76 | 17.7 ± 1.5 | 1.78 (0.22-28.5) | 16.17 ± 5 | 19.25 ± 4.1 | 8.44 (1.253-202.3) | 0.019 |
| IL-6 | 17.13 ± 3.05 | 19.53 ± 2.7 | 5.28 (0.27-61.6) | 18.12 ± 4.7 | 20.59 ± 1.9 | 5.55 (0.03-512.4) | 0.97 |
| IL-23A | 18.0 ± 2.09 | 18.1 ± 2.8 | 1.07 (0.07-6.61) | 11.69 ± 1.9 | 17.65 ± 2.9 | 61.93 (2.04-219.5) | 0.001 |
| IL-17A | 18.9 ± 2.65 | 20.3 ± 2.9 | 2.70 (0.07-20.5) | 22.42 ± 3.6 | 27.17 ± 2.9 | 26.83 (2.73-494.9) | 0.029 |
| Genes | IBD | Advanced stages CRC (3 + 4) | P value IBD vs Advanced CRC | ||||
| dCt ± SD | RQ (min-max) | dCt ± SD | RQ (min-max) | ||||
| Inflamed tissue | Non-inflamed tissue | Tumoral tissue | Non-tumoral tissue | ||||
| Foxp3 | 14.94 ± 2.65 | 15.81 ± 2.59 | 1.82 (0.11-74.85) | 19.71 ± 5.2 | 21.89 ± 4.2 | 4.55 (0.45-45.1) | 0.101 |
| TGFB1 | 9.52 ± 4.13 | 8.97 ± 4.4 | 0.68 (0.03-2.27) | 17.46 ± 3.3 | 18.5 ± 1.6 | 2.05 (0.211-14.56) | 0.176 |
| IL-10 | 16.87 ± 1.76 | 17.7 ± 1.5 | 1.78 (0.22-28.5) | 16.69 ± 6.2 | 19.09 ± 5 | 5.26 (0.43-158.8) | 0.113 |
| IL-6 | 17.13 ± 3.05 | 19.53 ± 2.7 | 5.28 (0.27-61.6) | 19.78 ± 3.1 | 21.39 ± 2.8 | 3.05 (0.025-434.7) | 0.608 |
| IL-23A | 18.0 ± 2.09 | 18.1 ± 2.8 | 1.07 (0.07-6.61) | 13.64 ± 2.1 | 17.44 ± 2.4 | 13.96 (3.93-36.28) | 0.012 |
| IL-17A | 18.9 ± 2.65 | 20.3 ± 2.9 | 2.70 (0.07-20.5) | 26.62 ± 3.9 | 26.73 ± 3.2 | 1.07 (0.034-89.26) | 0.350 |
The results from the analysis of serum cytokine levels in IBD and CRC patients are compared in Table 4. All cytokines were at a significantly higher level in CRC patients compared to IBD patients. The levels of TGFb1 were doubled in CRC compared to IBD patients, and about 20 times higher for IL-10 and almost 40 times higher for IL-23.
| Cytokines | IBD patients | CRC patients | P value (U-test) |
| TGFB1 (ng/mL) | 11.19 (9.36-20.99) | 22.57 (18.08-29.65) | 0.0008 |
| IL-10 (pg/mL) | 0.4 (0.0-2.8) | 8.29 (6.75-15.12) | 0.000001 |
| IL-6 (pg/mL) | 0.0 (0.0-2.7) | 3.21 (2.03-4.19) | 0.012 |
| IL-23A (pg/mL) | 0.7 (0.0-3.7) | 27.55 (25.35-33.0) | 0.000001 |
| IL-17A (pg/mL) | 0.0 (0.0-0.26) | 2.85 (0.0-9.0) | 0.034 |
Further, we have analyzed the cytokine serum levels in IBD patients in the active state of disease without immunosuppressive therapy (n = 11) compared to early CRC (n = 40) and advanced CRC (n = 40) (Figure 3). Non-parametric statistical analysis revealed that all cytokine levels were elevated significantly in early and advanced CRC patients compared to IBD patients, except IL-17A, which was reduced in advanced CRC to the similar to IBD levels. Moreover, the levels of IL-6, IL-10, and IL-23 were gradually elevated from IBD – to early – advanced CRC (Table 5).
| Cytokines | IBD | Early CRC | P value (U-test), IBD vs early CRC | Advanced CRC | P value (U-test), IBD vs advanced CRC |
| TGFB1 (ng/mL) | 11.19 (9.36-20.99) | 22.74 (18.17-27.98) | 0.001344 | 21.51 (15.5-31.05) | 0.0031 |
| IL-10 (pg/mL) | 0.4 (0.0-2.8) | 7.73 (6.0-12.71) | 0.000006 | 8.84 (7.2-19.81) | 0.000002 |
| IL-6 (pg/mL) | 0.0 (0.0-2.7) | 3.06 (2.03-4.19) | 0.0153 | 4.19 (2.15-8.4) | 0.044 |
| IL-23A (pg/mL) | 0.7 (0.0-3.7) | 25.7 (24.6-32.5) | 0.000024 | 30.5 (26.3-33.5) | 0.000009 |
| IL-17A (pg/mL) | 0.0 (0.0-0.26) | 3.62 (0.0-8.23) | 0.0086 | 1.2 (0.0-12.08) | 0.178 |
Our previous data on serum levels of the investigated cytokines in healthy individuals were the following: For TGFb1: 10028.82 ± 2250.15 pg/mL, for IL-6: 0.03 ± 0.02, for IL-17: 0.16 ± 0.14 pg/mL, for IL-10: 0.68 ± 0.05 pg/mL, and for IL-23: 0.33 ± 0.03 pg/mL[8].
Since the common feature between IBD and CRC-chronic inflammation was established, we aimed to compare some aspects of the molecular signature of immune cells-inflammatory in IBD and tumor-infiltrated in CRC, like the gene expression pattern for cytokines related to the Th17/Treg differentiation. Our findings showed that several cytokines were significantly upregulated in CRC patients when compared to IBD ones. In our study, the cytokines included TGFb1, IL-10, and IL-23 in the local microenvironment (tumor tissue vs. inflamed tissue), and TGFb1, IL-10, IL-17A, IL-6 and IL-23 in the systemic circulation of patients. The unequal distribution of the cytokines mentioned above supports the general hypothesis that local changes do not mirror the systemic level of inflammation. Nevertheless, a chronic inflammatory response may create an environment which is not only permissive towards cancer development, but that indeed sustains tumor promotion and progression by means of cytokines and growth factor release[9].
Along with the crucial cytokines, we investigated the mRNA expression of transcription factor FoxP3. We found that the expression of FoxP3 was significantly elevated in the whole CRC group (RQ = 6.42), as well as in the early CRC cases (RQ = 10.74), but not significantly in the advanced stages of CRC (RQ = 4.55). The role of Tregs in inflammation-associated CRC remains elusive. However, the suppression of the immune system may actively hamper host immune surveillance against tumors[5]. Nevertheless, FoxP3 expression may suppose a pivotal role for tumor growth, invasion, and spread, in addition to maintaining tumor escape from immune surveillance. Under chronic inflammatory conditions, FoxP3 + Treg are increased in circulation and accumulate in large numbers in lymph nodes and surrounding tumors. Tumor-infiltrating FoxP3 + cells are seen primarily during regression of the tumors, i.e., after biologic therapy[10].
On the other hand, Treg cells may help prevent and delay inflammation-mediated tumor growth as Treg cells exert anti-tumor activity in colitis-associated CRC. In our study, FoxP3 mRNA levels were higher in the inflamed tissue of IBD patients and even higher in tumoral tissue, especially in the early stages of CRC. This elevation suggests that in the early stages of CRC, there is a more abundant accumulation of FoxP3 + cells locally, similarly to IBD inflamed tissue.
Treg phenotype contributes significantly to CRC progression, as previously published by Miteva et al[11] colleagues in 2017. The authors claimed that the upregulation of FoxP3, IL-10, TGFb1, and IL-6 might be a transcriptional hallmark for CRC metastases, and the gene expression of Treg and Th17 related cytokines in the primary tumor and regional lymph nodes might provide suitable microenvironment sufficient for promoting metastasis[11]. Previous studies have also shown a significant upregulation of FoxP3 and IL-23 in tumor CRC tissue and elevated IL-10 mRNA on the systemic and local levels of CRC patients[12].
Recently, in human CRC patients, intratumoral FoxP3 cells were correlated with a favorable outcome. However, FoxP3 may be expressed in many cell types, including Th17 cells or tumor cells. Furthermore, Treg cells may not only have diminished efficacy during inflammation but may also differentiate directly into IL-17-producing cells[10]. This way, they may fuel carcinogenic events by contributing to hosting pro-inflammatory responses. Miteva et al[11] also suggested that the observed elevation of mRNA IL-17A with TGFb1, IL-10, and IL-6 genes in tumor tissue and regional lymph nodes could be associated with the presence of FoxP3 + Th17 cells.
Individuals with weakened IL-10 and Treg cells mediated inhibitory mechanism, and elevated IL-6 and IL-17, would be more likely to develop uncontrollable inflammation in response to proinflammatory challenges, and thus, more frequently susceptible to inflammation-associated cancers later in life[10]. In line with these are our results regarding IL-10 mRNA expression, which we found higher in CRC compared to IBD patients. However, its role remains controversial due to its pro- and anti-tumoral effects. It can be secreted not only by Tregs but also by tumor cells themselves, as well as by tumor-infiltrating macrophages[13]. IL-10 inhibits NF-Kb signaling; therefore, it can downregulate pro-inflammatory cytokine expression and acts as an anti-tumoral cytokine. IL-10 can also dampen antigen presentation, cell maturation, and differentiation, allowing tumor cells to evade immune surveillance mechanisms[14]. STAT3 can also be activated by IL-10, depending on the time frame of STAT3 activation. IL-6 leads to a temporary, rapidly declining STAT3 pho-sphorylation, whereas IL-10 induces a sustained one, leading to a pro-tumorigenic effect involving Bcl-2 upregulation and apoptosis resistance activation[15].
Some authors reported the average levels of IL-10 in CRC and CD patients. In our CRC patients, serum levels of IL-10 were significantly higher than in the IBD patients (P < 0.0001) in both early and advanced stages. We suppose that elevated IL-10 production in tumor tissue is associated with its pro-tumoral activities rather than anti-inflammatory ones. CD4+ cells insufficient in IL-10 were preferentially recruited to a Th17 phenotype when faced with a robust pro-inflammatory challenge[10]. Under conditions of low IL-10 and elevated IL-6, there is weak regulation of inflammation, which may contribute to cancer growth. Individuals with insufficient IL-10 are more susceptible to the effects of IL-6 and developing uncontrollable inflammation. In these conditions, Treg cells had increased invasion of neoplastic epithelia[2,10].
It has been reported of upregulation of IL-10 and TGFb1 cytokines in PBMCs of CRC patients preoperative with consecutive downregulation of their expression postoperative suggesting that tumor induces aberrant changes in gene expression, and indicate that the mRNA levels of cytokines were associated with the presence of CRC[16,17].
The TGFB1 expression under inflammatory conditions may be due to the down-regulation of the body`s immune responses in an attempt to control inflammation. As a critical component for both Th17 and Treg differentiation, for us, it was essential to find the expression of this cytokine locally in inflamed (IBD) and tumor (CRC) tissue samples of patients. We observed that TGFb1 was higher in CRC (early and advanced cases) compared to IBD patients. The role of TGFb1 in cancer, however, is complicated and ambiguous, varying by cell type and stages of tumorigenesis. In the early stages of CRC, TGFb1 acts as a tumor suppressor, inhibiting cell cycle progression, and promoting apoptosis. Later, TGFb1 enhances invasion and metastases by inducing epithelial-mesenchymal transition[18], as well as helps tumor growth by creating an immunotolerant tumor environment[19]. In our recent study, we showed that the expression of TGFb1 is involved in CRC development and metastasis but depends on gender and genotype[20]. Thus, we can speculate that increasing the level of TGFb1 is a marker for cancer development or progression.
In our study, the only cytokine, which was approximately equally upregulated in both CRC and IBD, was IL-6. Previously, we found a higher expression of IL-6 in inflamed mucosa of IBD patients[8] and tumor tissue and regional lymph nodes of CRC patients[10]. Here, we observed that the differences between mRNA expression of IL-6 locally in IBD and CRC were not significant. We could suggest that this crucial cytokine is of equal importance for both inflammation and tumor development.
IL-6 was shown to promote T cell accumulation in the colon lamina propria by upregulation of anti-apoptotic factors such as BCL-2 and BCL-xl[21]. IL-6 was also demonstrated to downregulate the tumor suppressor p53 simultaneously, but to upregulate the oncogene c-myc in colon epithelial cells, epithelial-mesenchymal transition, and resistance to cytotoxic stress[6], and upregulates Oct4 gene expression by activating IL-6R/JAK/STAT3 signaling pathway[3]. IL-6 and its related cytokines directly support the growth of colon epithelial cells and repair of intestinal wounds but can also promote the development of CRC[3]. In summary, IL-6 is a critical tumor promoter during early CRC. Thus, it arises as a potential target in both IBD and CRC.
Some studies showed that CRC patients presented with high levels of IL-6 and VEGF[22]. Elevated expression of IL-6, which can be detected in patient serum, is linked to increased risk of development of colorectal adenomas[23] and poor prognosis in CRC[24,25]. Our CRC patients had elevated serum levels of IL-6 (P = 0.044). However, limited studies are existing that might be used to define cut off values for IL-6 as a diagnostic tool.
TGFb1 and IL-6 as crucial cytokines for Th17 differentiation were upregulated in the inflamed tissue of IBD, as well as in tumor tissue of CRC patients. However, the expression of TGFb1 was higher in CRC patients compared to IBD patients, unlike the IL-6 expression. Based on these results, we could speculate that the additive upregulation of TGFB1 expression to the IL-6 during inflammation could drive to CRC transition. Regarding IL-17 mRNA expression, as a hallmark of Th17 cells, we found that the cytokine was upregulated in the whole CRC group (RQ = 4.69), especially in early cases in comparison with IBD (RQ = 26.83 vs 2.70; P = 0.029). Conversely, the RQ level of IL-17 in advanced CRC cases was lower (RQ = 1.07) than in the IBD group (RQ = 2.70).
It is known that IL-17A bridges the adaptive and innate immune system and plays a role in the maintenance of epithelial barrier homeostasis, but in colitis and CRC, its expression is elevated and worsens disease progression[3]. The role of IL-17A as an antitumor or tumor-promoting factor is still incompletely understood; however, increasing evidence support that IL-17A is involved in the development of CRC[26]. IL-17 family members demonstrated distinct expression patterns in CRC, suggesting a differential role exerted by each member in colon carcinogenesis[27]. It is tempting to speculate that an inflamed environment dominated by Th17 cells might facilitate cancer development. The involvement of Th17 cells in tumor growth is further sustained by the demonstrated role of IL-6 in this process[9].
IL-17A has shown a dual role in controlling neoplastic cell growth – it can inhibit tumor growth in some murine models, while it promotes malignant cells in mouse models of spontaneous intestinal cancer[28,29]. It can also stimulate tumor growth by its proangiogenic effect via enhancing the production of VEGF, basic fibroblast growth factor, and hematopoietic growth factor, and it has an impact on cancer-infiltrating stem cells[26]. IL-17A contributes to the tumor-initiating stage in the advancement of colitis-associated CRC due to the STAT3 IL-6 induced Th17 differentiation[26]. IL-17 overexpressed in tumors from colitis-associated cancer patients and is associated with angiogenesis and poor prognosis markers. It is secreted in tumors by ma-crophages/monocytes CD68+; Th17 and Treg FoxP3+IL-17+ cells[30].
The clinical implication of IL-17A is investigated by some authors, as they stated that measuring IL-17A in serum samples from CRC patients might be a valuable tumor marker[31], but it is not correlated with the TNM parameters of CRC. We have also found elevated levels of IL-17A in the serum of CRC patients compared to IBD patients (P = 0.034), which may be useful in the follow up of IBD patients and predicting CRC progression.
The blockade of IL-17A leads to a substantial modification in the microenvironment of IL-17A related inflammation and tumors[26,28,29]. And also, targeting IL-17A with anti-angiogenic therapeutics may be beneficial for the patients[26,32].
IL-6 alone can be sufficient to drive Th17 cells to secrete cytokines, whereas IL-23 is required for providing Th17 cells, a pathogenic phenotype[6]. In our study, IL-23 was the only cytokine for which mRNA expression was significantly higher in CRC (both early and advanced cases) than in IBD patients. Despite that different animal models suggest the protective role of IL-23 in induced CRC in mice, recently published papers have been shown the neoangiogenesis property of IL-23, particularly for human CRC development[16,33,34]. One possible mechanism could be the enhancement of the intestinal inflammation mediated by the Th17 axis maintained from IL-23. Moreover, IL-23 has been found to be overexpressed in a number of different human cancers compared to normal adjacent tissues, suggesting that IL-23 is essential for tumor-promoting pro-inflammatory processes and as well as the failure of the adaptive immune surveillance to infiltrate tumors[12,33]. However, IL-12p40 cytokine was evaluated as a useful prognostic marker for survival, unlike IL-23, which had no outcome prognostic value[16].
Nevertheless, we found that the level of IL-23 was about 40 times higher in CRC patients than IBD patients (P < 0.001). Recent data have shown that CRC progression was closely associated with infiltration with Th17 cells, and the central cytokines related IL-6, TGFb1, and IL-23[35,36]. CRC generates not only the local inflammatory microenvironment, named as tumor-elicited inflammation, but also promotes systemic changes that are favorable for cancer progression. Here, if we compared the serum levels of IL-23 in CRC patients to the average levels of healthy volunteers, the concentrations were 80 times higher in CRC.
Suppression of these cytokines was found to improve the symptoms of IBD to CRC progression[21,37,38]. This can be accomplished either with anti-cytokine drugs or immunosuppressive agents. Chemoprevention with anti-inflammatory agents and immunomodulatory drugs has been shown to reduce the risk of developing CRC on the grounds of inflammation by lowering the level of produced cytokines[39,40]. We have also proven the results regarding treatment with immunosuppressive drugs as more beneficial in reducing inflammation in IBD patients via driving cytokine expression to restore immune regulation[20].
The current study was limited by a relatively small number of IBD patients included. We used a convenient sample of IBD patients with no immunosuppressive therapy to avoid bias. However, caution must be applied, and the hypothesis and findings should be tested with a larger sample size.
Secondly, our study explores two cohorts of patients-IBD and sporadic CRC. Although sporadic colon carcinogenesis and colitis-associated carcinogenesis share similar immune-related mechanisms[41], we cannot exclude differences in the critical molecular mechanisms underlying these two types of CRC.
In conclusion, with this cytokine panel, we documented significant changes in genes related to Treg/Th17 development when comparing mRNA expression profiles of IBD and CRC (early and advanced) patients. Our findings showed that IL-6 upregulation is essential for both IBD and CRC, whereas the upregulation of genes related to Th17/Treg differentiation and behavior (TGFB1, IL-10, IL-23, and transcription factor FoxP3) is a crucial primarily for CRC development. Altogether we recorded marked differences in the distribution of investigated gene local expression, and the significance of these results could be used mainly in the effort to establish reliable and efficient methods for personalized therapies. Thus, the significantly upregulated IL-6 could be a potential drug target for IBD and prevention of CRC development as well.
Since various molecular mechanisms and signaling pathways are common for the carcinogenic process and inflammatory bowel disease, including accumulation of immune cells and the release of several cytokines, chemokines, and growth factors, we were interested in the comparing of cytokine patterns and subtle changes in cytokine milieu in inflamed tissues of inflammatory bowel disease (IBD) patients, as well as in the cancer environment in colorectal cancer (CRC) patients. However, far too little attention has been paid to opportunities for early detection of subtle changes in cytokine milieu in inflamed tissues in IBD and CRC, especially in the clinical context. In line with this, we were searching for mRNA cytokine patterns of IBD patients in active disease compared to early and advanced CRC, to obtain data on in the similarities and differences in inflammation and cancer initiation and advances.
Dysregulation of the immune response in patients with IBD and CRC triggers infiltration and, which may further influence the inflammation and the carcinogenesis process. Since the recent developments for the role of cytokines in the intestinal inflammation are accumulating, there has been an increased interest in their role. Furthermore, the survival of CRC affected patients depends highly on early detection. Thus, establishing some of the parameters that can be detected and followed-up and used as prognostic factors or determining the treatment options can improve the survival rate and quality of life of CRC patients.
Our objectives were: (1) To assess the mRNA cytokine levels in inflamed tissues of IBD patients, as well as in the cancer environment in CRC patients (at early and advanced stages); and (2) To compare serum protein levels of the respective cytokines in both groups of patients. The objectives were chosen to compare cytokine patterns of active IBD patients with early and advanced CRC by selecting a panel of cytokines crucial for Th17/Treg differentiation and behavior (IL-6, TGFb, IL-17A, IL-23, IL-10), as well as the transcription factor FoxP3, in colon specimens, as mRNA biomarkers, and their serum protein levels. Future directions of this study could be the follow-up of IBD patients for developing dysplastic lesions or CRC, where the proposed biomarkers can be evaluated as a prognostic factor.
To address the aims of the study, we choose a panel of pro- and anti-inflammatory cytokines with a substantial role in Th17/Treg differentiation and behavior, in colon specimens of IBD and CRC patients, as mRNA biomarkers, and their protein levels in the peripheral blood. We used RNA extraction, reverse transcription, and quantitative polymerase chain reaction, as well as enzyme immunoassays to measure the protein levels of IL-17A, IL-6, IL-23, TGFb1, and IL-10 in patients’ sera.
We found a significant difference between higher gene expression of FoxP3, TGFb1, IL-10, and IL-23, and approximately equal level of IL-6 in CRC patients in comparison with IBD patients. After stratification of CRC patients, we found a significant difference in FoxP3, IL-10, IL-23, and IL-17A mRNA in early cases compared to IBD, and IL-23 alone in advanced CRC. The protein levels of the cytokines were significantly higher in CRC patients compared to IBD patients.
Our findings contribute to the research in the field by finding that IL-6 upregulation is essential for both IBD and CRC development until the upregulation of other Th17/Treg related genes (TGFb1, IL-10, IL-23, and transcription factor FoxP3) is a crucial primarily for CRC development. The significantly upregulated IL-6 could be a potential drug target for IBD and prevention of CRC development as well. However, more research are needed regarding the role and targeting of IL-6 in CRC patients.
With the chosen cytokine panel, we documented significant changes in genes related to Treg/Th17 development when comparing mRNA expression profiles of IBD and CRC (early and advanced) patients. Our findings showed that IL-6 upregulation is essential for both IBD and CRC, whereas the upregulation of genes related to Th17/Treg differentiation and behavior (TGFB1, IL-10, IL-23, and transcription factor FoxP3) is a crucial primarily for CRC development.
In our study, the only cytokine, which was approximately equally upregulated in both CRC and IBD, was IL-6. Previously, we found a higher expression of IL-6 in inflamed mucosa of IBD patients and tumor tissue and regional lymph nodes of CRC patients. Here, we observed that the differences between mRNA expression of IL-6 locally in IBD and CRC were not significant. We could suggest that this crucial cytokine is of equal importance for both inflammation and tumor development, i.e., we hypothesized that IL-6 is a critical tumor promoter during early CRC.
Thus, the significantly upregulated IL-6 could be a potential drug target for IBD and prevention of CRC development as well, with a significant potential for the clinical practice.
However, since we used a convenient sample of IBD patients with no immunosuppressive therapy to avoid bias, caution must be applied, and the hypothesis and findings should be tested with larger sample size. Secondly, although sporadic colon carcinogenesis and colitis-associated carcinogenesis share similar immune-related mechanisms, we cannot exclude differences in the critical molecular mechanisms underlying these two types of CRC.
The most important lesson learned from this study is the crucial role of IL-6 for both IBD and CRC. It is well-known that elevated IL-6 is linked to increased risk of development of colorectal adenomas and poor prognosis in CRC, but limited data is available regarding the role of IL-6 in IBD towards CRC. Besides, inadequate studies are existing that might be used to define cut off values for IL-6 as a diagnostic tool. In line with this, future directions should include studies exploring the diagnostic and therapeutic potential of IL-6.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Bulgaria
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sugimura H, Sun X S-Editor: Zhang L L-Editor: A E-Editor: Zhang YL
| 1. | Nemeth ZH, Bogdanovski DA, Barratt-Stopper P, Paglinco SR, Antonioli L, Rolandelli RH. Crohn's Disease and Ulcerative Colitis Show Unique Cytokine Profiles. Cureus. 2017;9:e1177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 2. | Szkaradkiewicz A, Marciniak R, Chudzicka-Strugała I, Wasilewska A, Drews M, Majewski P, Karpiński T, Zwoździak B. Proinflammatory cytokines and IL-10 in inflammatory bowel disease and colorectal cancer patients. Arch Immunol Ther Exp (Warsz). 2009;57:291-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 3. | Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res. 2014;2014:149185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1033] [Cited by in RCA: 1233] [Article Influence: 112.1] [Reference Citation Analysis (0)] |
| 4. | Musteanu M, Blaas L, Mair M, Schlederer M, Bilban M, Tauber S, Esterbauer H, Mueller M, Casanova E, Kenner L, Poli V, Eferl R. Stat3 is a negative regulator of intestinal tumor progression in Apc(Min) mice. Gastroenterology. 2010;138:1003-11.e1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 129] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 5. | Monteleone G, Pallone F, Stolfi C. The dual role of inflammation in colon carcinogenesis. Int J Mol Sci. 2012;13:11071-11084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 6. | Francescone R, Hou V, Grivennikov SI. Cytokines, IBD, and colitis-associated cancer. Inflamm Bowel Dis. 2015;21:409-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 220] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 7. | Hauptman N, Glavač D. Colorectal Cancer Blood-Based Biomarkers. Gastroenterol Res Pract. 2017;2017:2195361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 8. | Velikova T, Kyurkchiev D, Spassova Z, Karakolev I, Ivanova-Todorova E, Altankova I, Stanilova S. Alterations in cytokine gene expression profile in colon mucosa of Inflammatory Bowel Disease patients on different therapeutic regimens. Cytokine. 2017;92:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Fantini MC, Pallone F. Cytokines: from gut inflammation to colorectal cancer. Curr Drug Targets. 2008;9:375-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 127] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 10. | Erdman SE, Poutahidis T. Roles for inflammation and regulatory T cells in colon cancer. Toxicol Pathol. 2010;38:76-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 11. | Miteva LD, Stanilov NS, Cirovski GМ, Stanilova SA. Upregulation of Treg-Related Genes in Addition with IL6 Showed the Significant Role for the Distant Metastasis in Colorectal Cancer. Cancer Microenviron. 2017;10:69-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Stanilov N, Miteva L, Mintchev N, Stanilova S. High expression of Foxp3, IL-23p19 and survivin mRNA in colorectal carcinoma. Int J Colorectal Dis. 2009;24:151-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Costa NL, Valadares MC, Souza PP, Mendonça EF, Oliveira JC, Silva TA, Batista AC. Tumor-associated macrophages and the profile of inflammatory cytokines in oral squamous cell carcinoma. Oral Oncol. 2013;49:216-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 14. | Braun DA, Fribourg M, Sealfon SC. Cytokine response is determined by duration of receptor and signal transducers and activators of transcription 3 (STAT3) activation. J Biol Chem. 2013;288:2986-2993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 15. | Zeng L, O'Connor C, Zhang J, Kaplan AM, Cohen DA. IL-10 promotes resistance to apoptosis and metastatic potential in lung tumor cell lines. Cytokine. 2010;49:294-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Stanilov N, Miteva L, Jovchev J, Cirovski G, Stanilova S. The prognostic value of preoperative serum levels of IL-12p40 and IL-23 for survival of patients with colorectal cancer. APMIS. 2014;122:1223-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Stanilov NS, Miteva L, Cirovski G, Stanilova SA. Increased transforming growth factor β and interleukin 10 transcripts in peripheral blood mononuclear cells of colorectal cancer patients. Contemp Oncol (Pozn). 2016;20:458-462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Morrison CD, Parvani JG, Schiemann WP. The relevance of the TGF-β Paradox to EMT-MET programs. Cancer Lett. 2013;341:30-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 165] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 19. | Feagins LA. Role of transforming growth factor-β in inflammatory bowel disease and colitis-associated colon cancer. Inflamm Bowel Dis. 2010;16:1963-1968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 20. | Stanilova S, Stanilov N, Julianov A, Manolova I, Miteva L. Transforming growth factor-β1 gene promoter -509C/T polymorphism in association with expression affects colorectal cancer development and depends on gender. PLoS One. 2018;13:e0201775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Atreya R, Mudter J, Finotto S, Müllberg J, Jostock T, Wirtz S, Schütz M, Bartsch B, Holtmann M, Becker C, Strand D, Czaja J, Schlaak JF, Lehr HA, Autschbach F, Schürmann G, Nishimoto N, Yoshizaki K, Ito H, Kishimoto T, Galle PR, Rose-John S, Neurath MF. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nat Med. 2000;6:583-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 945] [Cited by in RCA: 1027] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 22. | Middleton K, Jones J, Lwin Z, Coward JI. Interleukin-6: an angiogenic target in solid tumours. Crit Rev Oncol Hematol. 2014;89:129-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 23. | Kim S, Keku TO, Martin C, Galanko J, Woosley JT, Schroeder JC, Satia JA, Halabi S, Sandler RS. Circulating levels of inflammatory cytokines and risk of colorectal adenomas. Cancer Res. 2008;68:323-328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 234] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 24. | Grivennikov S, Karin M. Autocrine IL-6 signaling: a key event in tumorigenesis? Cancer Cell. 2008;13:7-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 288] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 25. | Naugler WE, Karin M. The wolf in sheep's clothing: the role of interleukin-6 in immunity, inflammation and cancer. Trends Mol Med. 2008;14:109-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 572] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 26. | Shi Y, Lin H, Cui J, Qi H, Florholmen J, Liu Z, Cui G. The role of interleukin-17A in colorectal tumorigenesis. Cancer Biother Radiopharm. 2013;28:429-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Al-Samadi A, Moossavi S, Salem A, Sotoudeh M, Tuovinen SM, Konttinen YT, Salo T, Bishehsari F. Distinctive expression pattern of interleukin-17 cytokine family members in colorectal cancer. Tumour Biol. 2016;37:1609-1615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, Huso DL, Brancati FL, Wick E, McAllister F, Housseau F, Pardoll DM, Sears CL. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1113] [Cited by in RCA: 1304] [Article Influence: 81.5] [Reference Citation Analysis (0)] |
| 29. | Chae WJ, Gibson TF, Zelterman D, Hao L, Henegariu O, Bothwell AL. Ablation of IL-17A abrogates progression of spontaneous intestinal tumorigenesis. Proc Natl Acad Sci U S A. 2010;107:5540-5544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 195] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 30. | Liu J, Duan Y, Cheng X, Chen X, Xie W, Long H, Lin Z, Zhu B. IL-17 is associated with poor prognosis and promotes angiogenesis via stimulating VEGF production of cancer cells in colorectal carcinoma. Biochem Biophys Res Commun. 2011;407:348-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 273] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 31. | Radosavljevic G, Ljujic B, Jovanovic I, Srzentic Z, Pavlovic S, Zdravkovic N, Milovanovic M, Bankovic D, Knezevic M, Acimovic LJ, Arsenijevic N. Interleukin-17 may be a valuable serum tumor marker in patients with colorectal carcinoma. Neoplasma. 2010;57:135-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 32. | Jauregui-Amezaga A, Somers M, De Schepper H, Macken E. Next generation of biologics for the treatment of Crohn's disease: an evidence-based review on ustekinumab. Clin Exp Gastroenterol. 2017;10:293-301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 33. | Langowski JL, Zhang X, Wu L, Mattson JD, Chen T, Smith K, Basham B, McClanahan T, Kastelein RA, Oft M. IL-23 promotes tumour incidence and growth. Nature. 2006;442:461-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 739] [Cited by in RCA: 772] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 34. | Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, Taniguchi K, Yu GY, Osterreicher CH, Hung KE, Datz C, Feng Y, Fearon ER, Oukka M, Tessarollo L, Coppola V, Yarovinsky F, Cheroutre H, Eckmann L, Trinchieri G, Karin M. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491:254-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1035] [Cited by in RCA: 1039] [Article Influence: 79.9] [Reference Citation Analysis (0)] |
| 35. | Annunziato F, Cosmi L, Liotta F, Maggi E, Romagnani S. The phenotype of human Th17 cells and their precursors, the cytokines that mediate their differentiation and the role of Th17 cells in inflammation. Int Immunol. 2008;20:1361-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 144] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 36. | Zou W, Restifo NP. T(H)17 cells in tumour immunity and immunotherapy. Nat Rev Immunol. 2010;10:248-256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 508] [Cited by in RCA: 498] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 37. | Onizawa M, Nagaishi T, Kanai T, Nagano K, Oshima S, Nemoto Y, Yoshioka A, Totsuka T, Okamoto R, Nakamura T, Sakamoto N, Tsuchiya K, Aoki K, Ohya K, Yagita H, Watanabe M. Signaling pathway via TNF-alpha/NF-kappaB in intestinal epithelial cells may be directly involved in colitis-associated carcinogenesis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G850-G859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 38. | Popivanova BK, Kitamura K, Wu Y, Kondo T, Kagaya T, Kaneko S, Oshima M, Fujii C, Mukaida N. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest. 2008;118:560-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 456] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 39. | Velayos FS, Terdiman JP, Walsh JM. Effect of 5-aminosalicylate use on colorectal cancer and dysplasia risk: a systematic review and metaanalysis of observational studies. Am J Gastroenterol. 2005;100:1345-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 379] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 40. | Rawla P, Sunkara T, Raj JP. Role of biologics and biosimilars in inflammatory bowel disease: current trends and future perspectives. J Inflamm Res. 2018;11:215-226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 105] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 41. | Stidham RW, Higgins PDR. Colorectal Cancer in Inflammatory Bowel Disease. Clin Colon Rectal Surg. 2018;31:168-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 202] [Article Influence: 28.9] [Reference Citation Analysis (1)] |