Published online Apr 21, 2020. doi: 10.3748/wjg.v26.i15.1758
Peer-review started: December 28, 2019
First decision: January 11, 2020
Revised: March 17, 2020
Accepted: March 27, 2020
Article in press: March 27, 2020
Published online: April 21, 2020
Processing time: 114 Days and 20.8 Hours
Intestinal ischemia reperfusion (I/R) occurs in various diseases, such as trauma and intestinal transplantation. Excessive reactive oxygen species (ROS) accumulation and subsequent apoptotic cell death in intestinal epithelia are important causes of I/R injury. PTEN-induced putative kinase 1 (PINK1) and phosphorylation of dynamin-related protein 1 (DRP1) are critical regulators of ROS and apoptosis. However, the correlation of PINK1 and DRP1 and their function in intestinal I/R injury have not been investigated. Thus, examining the PINK1/DRP1 pathway may help to identify a protective strategy and improve the patient prognosis.
To clarify the mechanism of the PINK1/DRP1 pathway in intestinal I/R injury.
Male C57BL/6 mice were used to generate an intestinal I/R model via superior mesenteric artery occlusion followed by reperfusion. Chiu’s score was used to evaluate intestinal mucosa damage. The mitochondrial fission inhibitor mdivi-1 was administered by intraperitoneal injection. Caco-2 cells were incubated in vitro in hypoxia/reoxygenation conditions. Small interfering RNAs and overexpression plasmids were transfected to regulate PINK1 expression. The protein expression levels of PINK1, DRP1, p-DRP1 and cleaved caspase 3 were measured by Western blotting. Cell viability was evaluated using a Cell Counting Kit-8 assay and cell apoptosis was analyzed by TUNEL staining. Mitochondrial fission and ROS were tested by MitoTracker and MitoSOX respectively.
Intestinal I/R and Caco-2 cell hypoxia/reoxygenation decreased the expression of PINK1 and p-DRP1 Ser637. Pretreatment with mdivi-1 inhibited mitochondrial fission, ROS generation, and apoptosis and ameliorated cell injury in intestinal I/R. Upon PINK1 knockdown or overexpression in vitro, we found that p-DRP1 Ser637 expression and DRP1 recruitment to the mitochondria were associated with PINK1. Furthermore, we verified the physical combination of PINK1 and p-DRP1 Ser637.
PINK1 is correlated with mitochondrial fission and apoptosis by regulating DRP1 phosphorylation in intestinal I/R. These results suggest that the PINK1/DRP1 pathway is involved in intestinal I/R injury, and provide a new approach for prevention and treatment.
Core tip: PTEN-induced kinase 1 (PINK1) is a kind of mitochondrial serine/threonine-protein kinase, which regulates mitochondrial homeostasis through regulating the phosphorylation of target proteins. Depletion of PINK1 has been proved to be associated with mitochondrial fragmentation and apoptosis in ischemic model. However, the underlying mechanism has not been clarified. By establishing an intestinal ischemia reperfusion model in mice and hypoxia/reoxygenation model in Caco-2 cells, we revealed that PINK1 inhibits mitochondrial fission and apoptosis via phosphorylating dynamin-related protein 1 on Ser637. The PINK1/dynamin-related protein 1 pathway may provide a potential target in treatment of intestinal ischemia reperfusion injury.
- Citation: Qasim W, Li Y, Sun RM, Feng DC, Wang ZY, Liu DS, Yao JH, Tian XF. PTEN-induced kinase 1-induced dynamin-related protein 1 Ser637 phosphorylation reduces mitochondrial fission and protects against intestinal ischemia reperfusion injury. World J Gastroenterol 2020; 26(15): 1758-1774
- URL: https://www.wjgnet.com/1007-9327/full/v26/i15/1758.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i15.1758
As a common and severe clinical pathophysiological condition, intestinal ischemia-reperfusion (I/R) injury occurs in gut primary diseases [e.g., bowel transplantation, and superior mesenteric artery (SMA) embolus] and is usually secondary to other serious pathologies (e.g., trauma, shock, and burn)[1-3]. Intestinal I/R inhibits the survival of intestinal epithelial cells which are indispensable components of the integrated intestinal barrier, and results in in-situ injury[4,5]. More seriously, barrier dysfunction can lead to the spread of damage-associated molecular patterns and bacterial translocation, which subsequently induces systemic inflammatory response syndrome and multiple organ dysfunction syndrome with high incidence and mortality rates[6,7]. Thus, preventing intestinal epithelial cell death is the key to attenuate intestinal I/R injury and improve the prognosis.
Our previous studies reported that excessive reactive oxygen species (ROS) and apoptosis were important factors contributing to cell injury during intestinal I/R[8-10]. Numerous studies have revealed that mitochondrial fission, a dynamic mitochondrial process by which parental mitochondria are divided into two daughter mitochondria, is closely associated with ROS production and apoptosis[11,12]. In I/R models, abnormal mitochondrial fission is increased, leading to mitochondrial fragmentation, ROS production and apoptosis[13-16]. However, whether mitochondrial fission participates in intestinal I/R injury and the regulatory mechanism are still being unexplored.
Dynamin-related protein 1 (DRP1), a member of the dynamin family of large GTPases, mediates mitochondrial fission[17,18]. Upon challenge with an apoptotic stimulus, DRP1 is recruited from the cytosol to the mitochondrial outer membrane, where it preferentially localizes to potential sites of organelle division[19,20]. Inhibition of DRP1 function, mediated by the selective inhibitor mdivi-1 or small interfering RNAs (siRNAs), blocked mitochondrial fission and apoptosis in I/R[21,22]. Phosphorylation is an important post-translational modification that can regulate the function and localization of DRP1[23,24]. In some reports on Ser637, a widely studied and highly conserved phosphorylation site of DRP1, modification of this site was found to inhibit mitochondrial division by reducing DRP1 translocation to the mitochondria[25,26]. Under I/R conditions, the phosphorylation of Ser637 was decreased[27]; however, the mechanism of this reduction is still largely unknown.
PTEN-induced kinase 1 (PINK1), a type of mitochondrial serine/threonine-protein kinase, is regarded as a protective protein for in mitochondrial homeostasis due to its regulation of target protein phosphorylation[28,29]. PINK1 has been proven to protect against cortical neuron death from ischemia by inhibiting the distribution of DRP1 in mitochondria[30]. At present, PINK1 regulation of mitochondrial fission in intestinal I/R injury is still not clear.
Accordingly, in this study, we aimed to clarify that mitochondrial fission could participate in ROS generation and apoptosis during intestinal I/R injury, and that PINK1 could regulate mitochondrial fission by dephosphorylating DRP1 at Ser637.
Adult healthy male C57BL/6 mice (aged 8 wk) weighing 20 ± 2 g were obtained from the Animal Center (SPF) of Dalian Medical University (Dalian, China). The mice were fed suitable chow food and water and were housed in an environment with controlled humidity (40%-70%), temperature (22 ± 2 °C), and light (12 h light/dark). The mice were divided randomly into two major parts First part, the mice were divided into five groups: Sham group, I/R group with reperfusion for 1 h, 2 h, 4 h and 8 h. Then, the mice were divided into four groups (sham group, sham + mdivi-1, I/R group, I/R + mdivi-1) and fasted overnight with free access to water before surgery. The intestinal I/R model was established by SMA occlusion, as previously described[5,8]. In brief, the mice were anesthetized with sodium pentobarbital via intraperitoneal injection (40 mg/kg bodyweight) before midline laparotomy. After midline laparotomy, the SMA was isolated, and an atraumatic clip was used to occlude the SMA for 45 min. Upon completion of 45 min of ischemia, the clips were removed, and reperfusion was then performed for 1 h, 2 h, 4 h and 8 h. Sham group animals underwent the same protocol without SMA occlusion. The second major part was also subjected to ischemia for 45 min and reperfusion for 4 h. In each mdivi-1-treated group, the mice received an intraperitoneal injection of mdivi-1 (1.5 mg/kg body weight, MedChem Express, United States) one hprior to I/R surgery. The mdivi-1 was dissolved in DMSO to obtain 1.5 mg/kg body weight in a final volume of 1 mL per injection. After reperfusion, the animals were sacrificed by exsanguination via the abdominal aorta, and small intestinal samples were then harvested and frozen immediately in liquid nitrogen and stored at -80 °C for analysis.
All the experimental procedures were performed in accordance with institutional guidelines for the care and use of laboratory animals and were approved by the Institutional Ethics Committee for Animal Experiments of Dalian Medical University (Dalian, China).
Caco-2 cells were obtained from the American Type Culture Collection (ATCC, Manassas, Virginia, United States) and cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Carlsbad, CA, United States) supplemented with 10% fetal bovine serum, 1% nonessential amino acids, 1% glutamine and penicillin/streptomycin. The cells were maintained in a humidified atmosphere containing 5% CO2 at 37 °C. To generate the in vitro I/R model under hypoxic conditions, Caco-2 cells were incubated in a microaerophilic system (Thermo Scientific, Marietta, GA, United States) with 5% CO2 and 1% O2 balanced with 94% nitrogen gas for 12 h. Then, the cells were cultured for 6 h under normoxic conditions to achieve reoxygenation.
Caco-2 cells were cultured and transfected with specific PINK1 siRNA (si-PINK1) (GenePharma, Shanghai, China) using Lipofectamine 3000 (Thermo Fisher Scientific United States). Si-PINK1 had the following sequence: Forward (F) 5’-GCCAGUACCUUUGUGUGAATT-3’ and reverse (R) 5’-UUCACACAAAGGUACUGGCTT-3’. PINK1 siRNA was utilized to inhibit of PINK1 expression, and all transfection methods were applied according to the manufacturer’s protocol.
Caco-2 cells were transfected at 50%-60% confluence with a plasmid encoding the PINK1 vector using Lipofectamine 3000 (Invitrogen, Carlsbad, CA, United States). At 48 h after transfection, the cells were processed with different assays.
Total protein was extracted from intestinal tissue and Caco-2 cells using a commercial protein isolation kit (KeyGEN Biotech, Nanjing, China). Equal protein amounts from the samples were analyzed using 10%-15% SDS-PAGE (Bio-Rad, Hercules, CA, United States) and then transferred to PVDF membranes (Millipore, Bedford, MA, United States). The membranes were blocked with 5% nonfat milk or 5% BSA in TBS-Tween buffer (0.1% Tween-20; pH 7.5) for 1 h at 37 °C. After blocking, the membranes were incubated with primary antibodies against total DRP1, DRP1 Ser637 (Abcam, Cambridge, United Kingdom), PINK1 (Santa Cruz Biotechnology, Santa Cruz, CA, United States), caspase-3 (Proteintech, Wuhan, China), and β-actin (ZSGB-BIO, Beijing, China) overnight at 4 °C. After washing, the membranes were incubated with the corresponding homologous horseradish peroxide-conjugated secondary antibodies for two hours at 37 °C. The bands were visualized using enhanced Chemiluminescence Plus reagents (Beyotime Institute of Biotechnology, China). Spectrophotometric analysis was performed with a BioSpectrum-510 multispectral imaging system (UVP, Upland, CA, United States) and signals were analyzed using Gel-pro Analyzer Version 5.0 (Media Cybernetics, Rockville, MD, United States).
Caco-2 cells were seeded on glass bottom cell culture dishes. Pretreated cells were incubated with 200 nmol MitoTracker Red CMXRos (ThermoFisher Scientific, Massachusetts, United States) for 30 min at 37 °C in the dark and then washed with PBS. Next, the cells were fixed using 4% paraformaldehyde for 30 min at room temperature, washed three times with PBS, and permeabilized with 0.2% Triton X-100 in PBS for 10 min at room temperature. The cells were rinsed with PBS, blocked with 2% BSA in PBS for 1 h at 37 °C and then incubated with anti-DRP1 antibodies overnight at 4 °C. After that, the cells were washed with PBS and incubated with Alexa Fluor 594-conjugated secondary antibodies (Proteintech, China) at 37 °C for 1 h. Subsequently, the cells were washed with PBS and counterstained with 4,6-diamidino-2-phenylindole (DAPI; Beyotime, Shanghai, China) nuclear stain at room temperature for 10 min. Finally, the cells were washed with PBS and examined under a laser confocal microscope (Leica, Germany).
Total protein was extracted in IP lysis buffer (20 mmol Tris-HCL, 150 mmol NaCl, 1% Triton X-100, pH 7.5). A sufficient amount of anti-DRP1 Ser637 antibody was added to 50 μL of binding buffer with already prepared protein A/G magnetic beads (Selleck Chemicals, Houston, United States). The mixtures were gently rotated for 4 h at 4 °C. Then, the tubes were placed on a magnet for 5-10 s and the supernatant was discarded. The bead-Ab complexes were resuspended in 150 μL of binding buffer and the supernatant was discarded again. This step was repeated twice. After that, 200 μg protein was added to bead-Ab complexes and the mixture was rotated for 10 min at room temperature. The precipitates were washed on the magnet four times with binding buffer and then resuspended in 1x sample buffer and boiled for 10 min. Finally, the supernatant was collected by centrifugation. Normal IgG (Beyotime Institute of Biotechnology, Shanghai, China) was used as a negative control. Samples were immunoblotted with anti-DRP1 Ser637 and anti-PINK1 antibodies.
For histological and TUNEL analyses, morphologic changes in the intestine and lung were examined by light microscopy. In brief, the intestinal tissue was fixed in 4% formalin, embedded in paraffin and sectioned. The sections (4 μm) were stained with hematoxylin and eosin, and the histopathological scores of the intestinal tissues were evaluated according to Chiu’s score. Intestinal TUNEL staining was performed using an apoptosis detection kit (Roche, Branchburg, NJ, United States).
Cell viability was assessed by CCK-8 (Dojindo Molecular Technologies, Tokyo, Japan) assays. In brief, Caco-2 cells were seeded in 96-well plates and underwent appropriate treatments. After that, 10 μL CCK-8 reagent was added to each well at 1/10 dilution and incubated for 1 h at 37 °C. The absorbance of each individual well was measured at 450 nm with a microplate reader (BioTek, Winooski, VT, United States).
Caco-2 cells were seeded on glass bottom cell culture dishes. Pretreated cells were washed twice with PBS and incubated with 5 μmol MitoSOX Red in DMEM for 10 min at 37 °C. After incubation, the cells were washed with PBS, Hoechst reagent was added, and the cells were incubated for 10 min at 37 °C. The cells were examined under laser confocal microscope to detect mitochondrial ROS levels.
A mitochondrial fission assay was performed by staining cells with MitoTracker red. Pretreated cells were washed with PBS and treated with 200 nmol MitoTracker Red for 30 min at 37 °C. Laser confocal microscopy was used to observe mitochondrial morphology.
All values are shown as the means ± SD. The data with normal distributions were compared using one-way analysis of variance (ANOVA) followed by Student-Newman-Keuls test. A two-tailed Student’s t-test was used to compare the means between two groups. Survival was assessed using the Kaplan-Meier method and compared by the log rank test. All experimental results represented at least three independent experiments. All statistical analyses were performed using GraphPad Prism 5.0 (GraphPad Prism Software, La Jolla, CA, United States) P values < 0.05 were considered statistically significant.
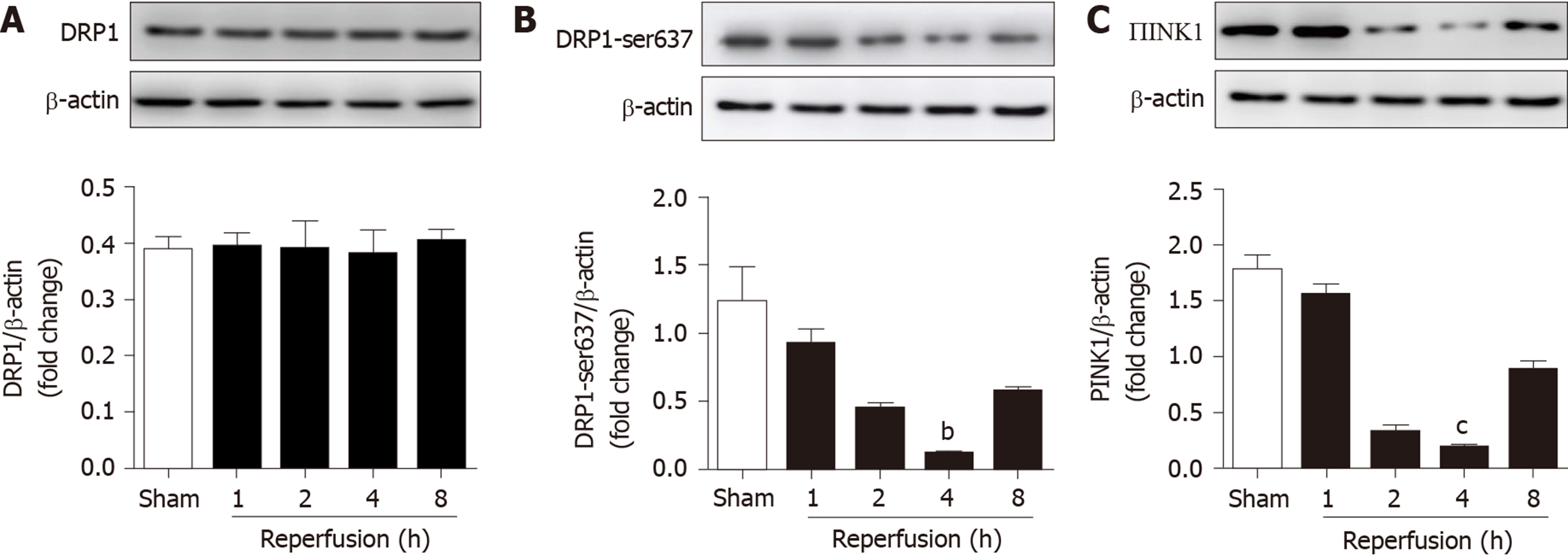
To determine whether the expression of PINK1, DRP1 and p-DRP1 Ser637 changes with the reperfusion time after intestinal ischemia, C57BL/6 mice were used to construct an intestinal I/R model. Mice in the I/R group were subjected to reperfusion for 1, 2, 4, and 8 h after 45 min ischemia. The expression of proteins in each group was detected by Western blotting. The results showed that there was no significant difference in the expression of total DRP1 after different reperfusion times (Figure 1A). However, the expression of p-DRP1 Ser637 and PINK1 was obviously decreased in the I/R groups compared with the sham group after 4 h reperfusion (Figure 1B and 1C). These results suggest that I/R decreased the mitochondrial fission-related regulators p-DRP1 Ser637 and PINK1.
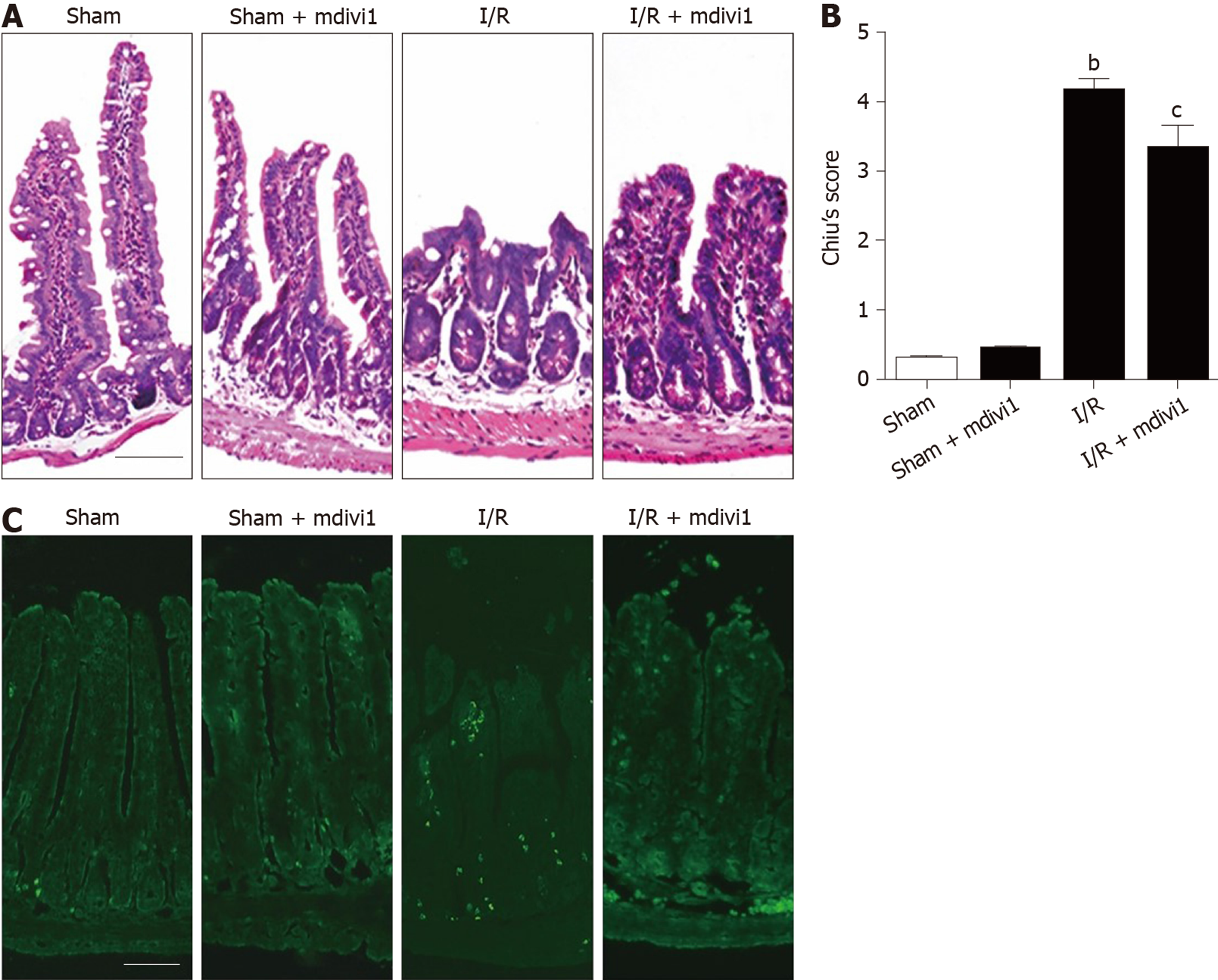
The protective effect of mitochondrial fission inhibition has been revealed in the liver, brain and kidney[13-15]. To determine whether this effect exists in intestinal I/R, we established an in vivo model (45-min ischemia and 4-h reperfusion) in mice pretreated with mdivi-1, a mitochondrial division inhibitor, as mentioned above[31]. The results showed that I/R induced intestinal epithelial injury compared with the sham conditions, and mdivi-1 administration protected the intestinal mucosa integrity (Figure 2A, B). It is well known that lung injury is generally derived from intestinal I/R injury; thus, we also assessed lung damage. As shown in Supplementary Figure 1A and 1B, mdivi-1 administration improved lung injury as detected by hematoxylin and eosin staining. Meanwhile, the TUNEL assay indicated that excessive apoptosis occurred in the I/R group, and mdivi-1 pretreatment attenuated apoptosis, as indicated by the reduced green fluorescence intensity (Figure 2C). Furthermore, we performed a 24-h survival study to evaluate the long-term protective effects of mdivi-1. As shown in Supplementary Figure 1C, the overall survival rate was significantly higher in the mdivi-1 group than in the vehicle group.
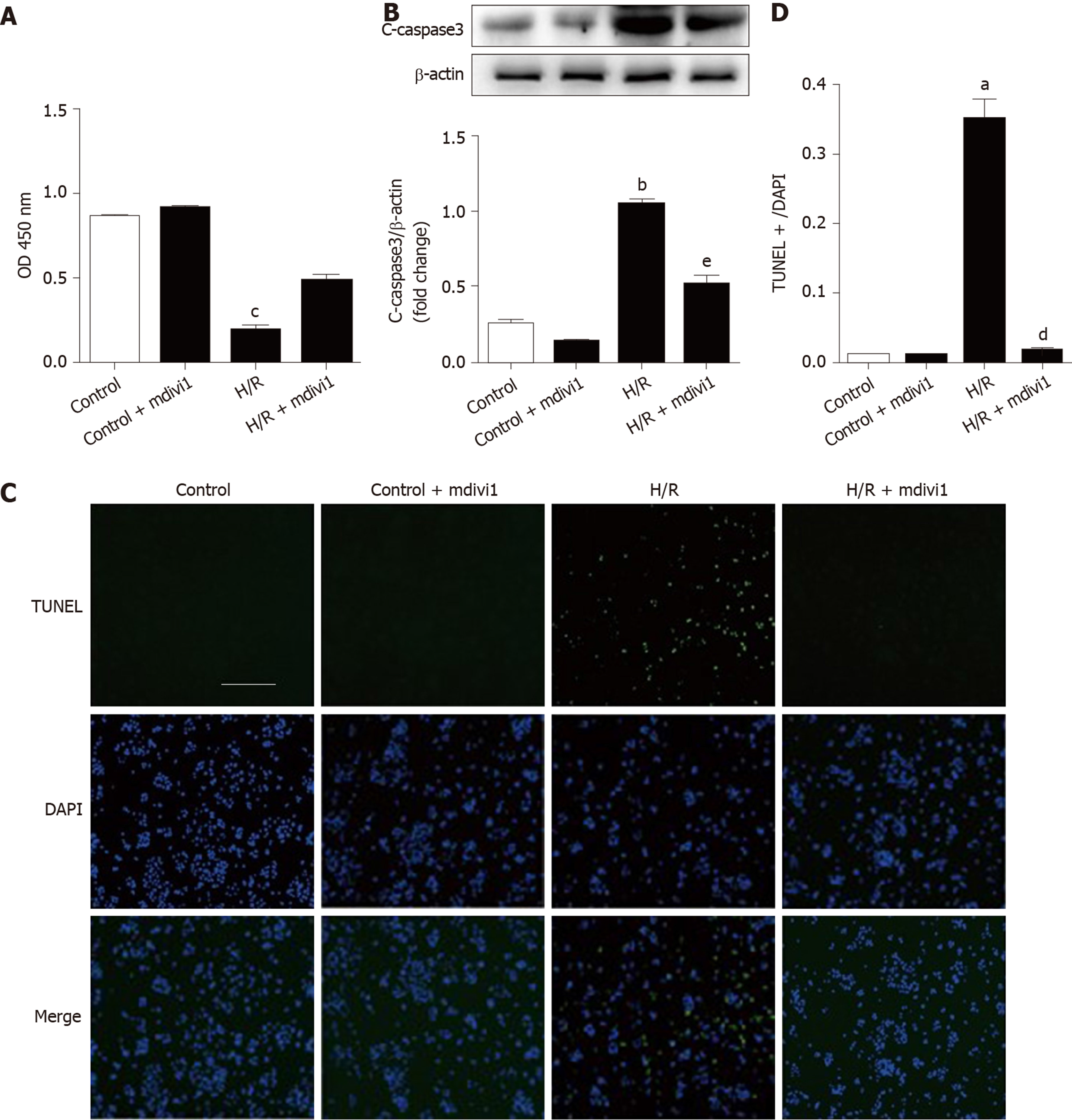
Next, Caco-2 cells were subjected to hypoxia/reoxygenation (H/R) in vitro to simulate I/R in vivo. Cell viability was evaluated to observe the effect of mdivi-1 on cell injury. Figure 3A shows that cell injury increased in the H/R group compared with the control group, while mdivi-1 reduced the injury induced by H/R. Our results indicate that inhibiting mitochondrial division by mdivi-1 can protect Caco-2 cells from H/R-induced injury. To detect apoptosis, we performed Western blotting and TUNEL assays in Caco-2 cells. The results showed that cleaved caspase 3 (c-caspase 3) protein expression and the number of apoptotic cells increased in the H/R group compared with the control group; however, mdivi-1 reduced c-caspase 3 protein expression and the number of TUNEL-positive cells (Figure 3B-3D).
Collectively, our results indicate that the mitochondrial division inhibitor mdivi-1 ameliorated intestinal I/R injury.
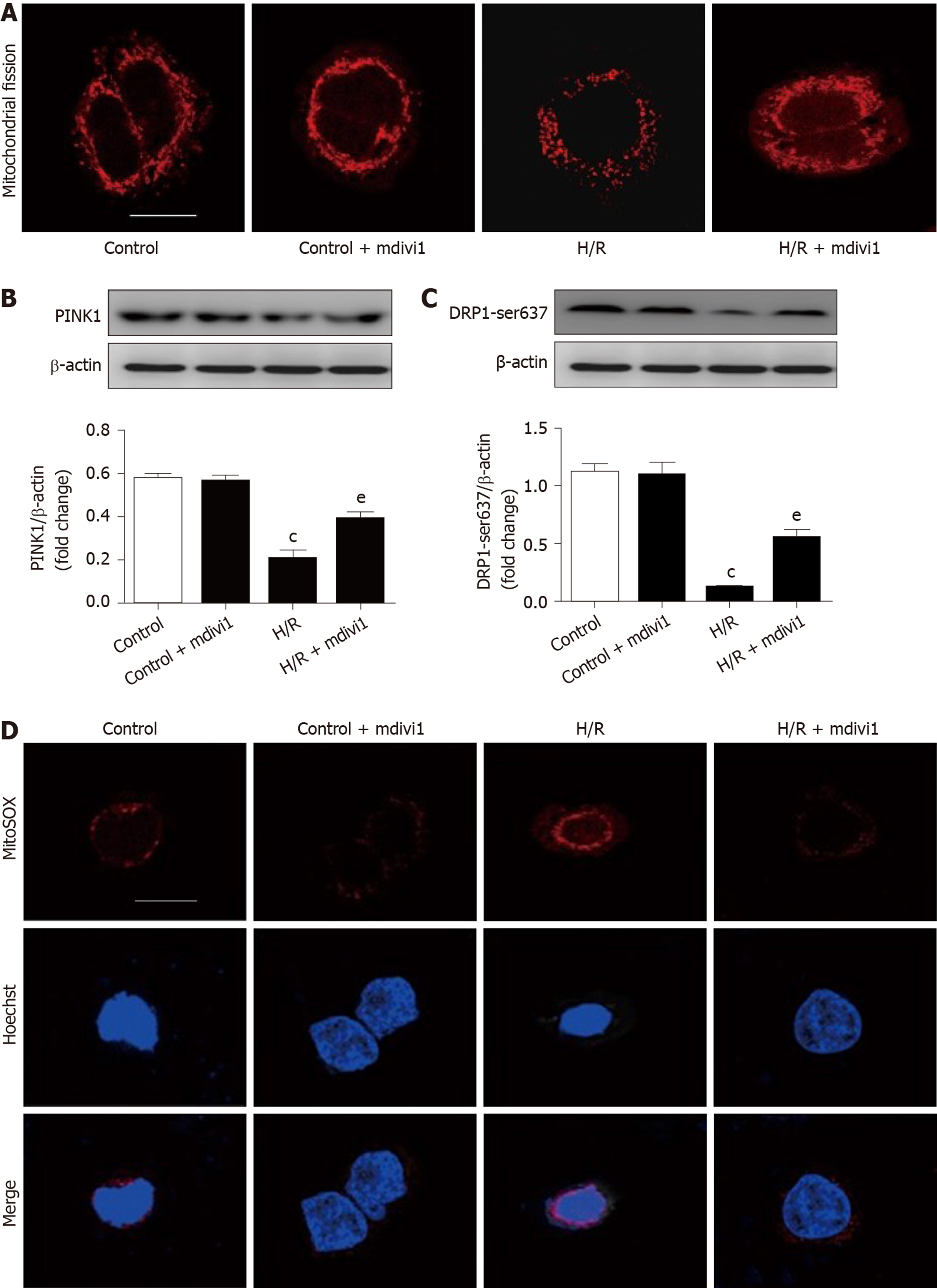
Deep Red FM was used to assay the effect of mdivi-1 on mitochondrial fission. The morphological features of the mitochondria were detected by confocal laser scanning microscopy. As shown in Figure 4A, cells in the H/R group showed more obvious mitochondrial fission with much more mitochondrial fragments than those in the control group. However, mitochondrial fission was reduced in the mdivi-1 group compared with the H/R group. Furthermore, we found that PINK1 and p-DRP1 Ser637 expression was decreased in the H/R group compared with the control group, and mdivi-1 pretreatment increased the expression of these two proteins (Figure 4B and 4C). In addition, ROS levels were increased in the H/R group compared with the control group, and mdivi-1 administration decreased ROS levels in Caco-2 cells (Figure 4D).
The results indicated that the mdivi-1 decreased mitochondrial fission and ROS accumulation after H/R in Caco-2 cells.
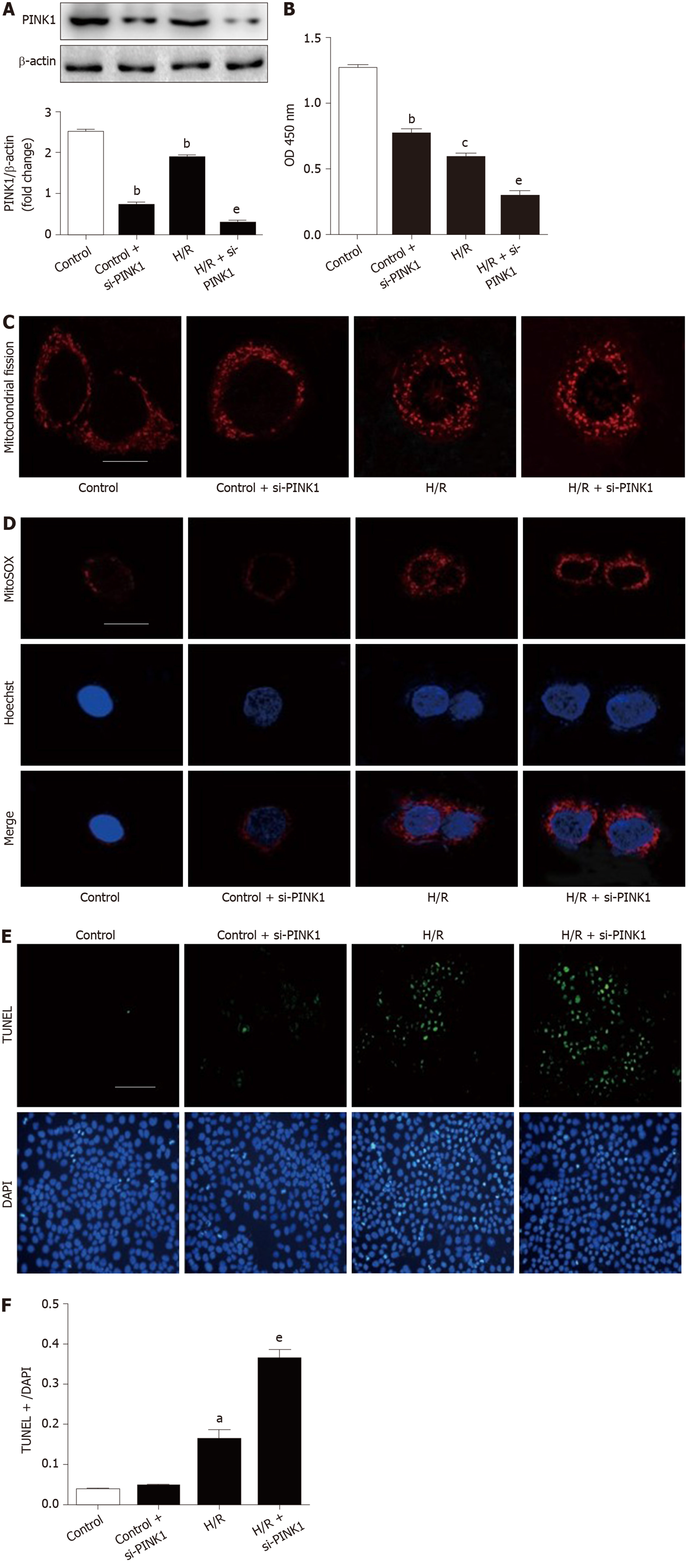
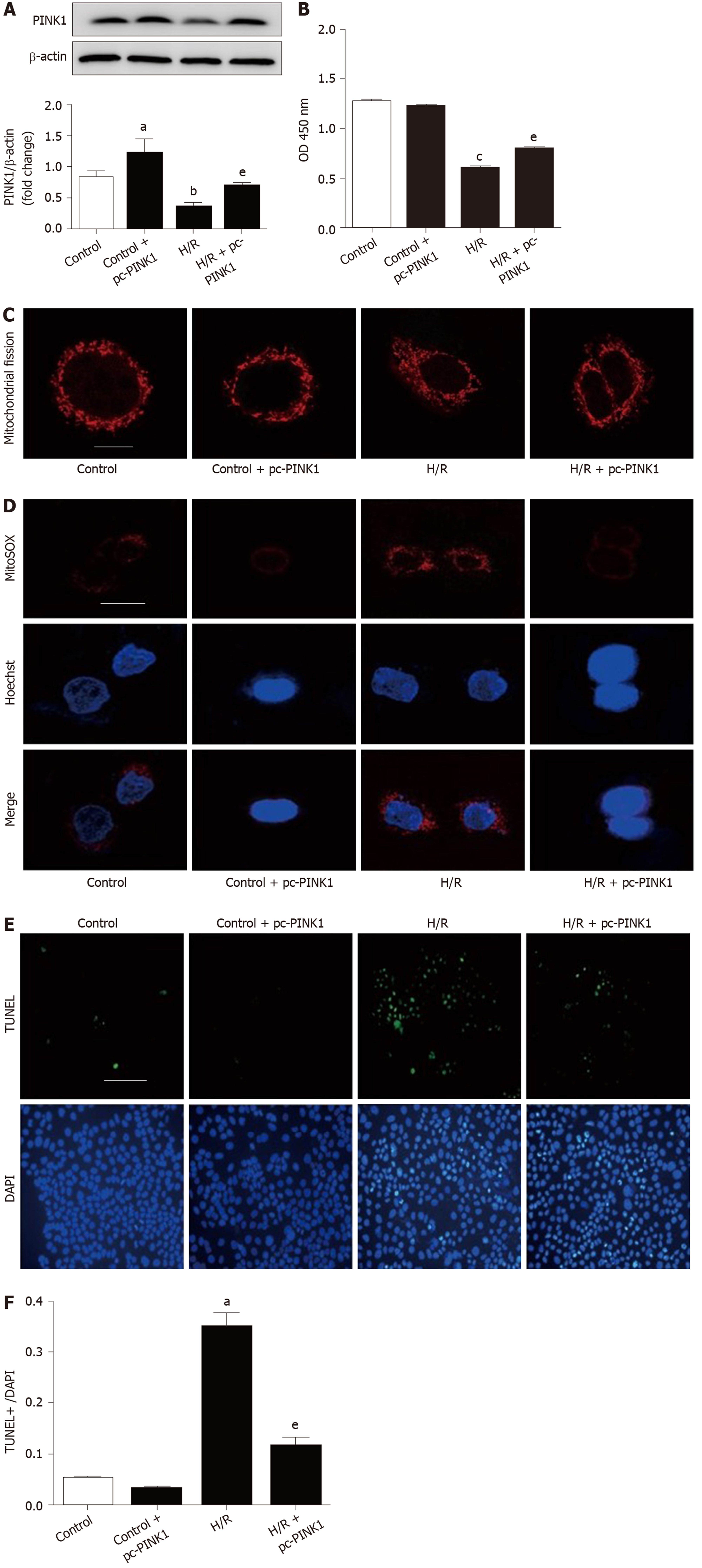
Previous studies have revealed that PINK1 can prevent I/R injury in heart, brain and liver[32-34]. To determine the effect of PINK1 in intestinal I/R, we knocked down or overexpressed PINK1 in Caco-2 cells. As shown in Figure 5A and 5B, siRNA transfection decreased PINK1 expression and cell viability compared with the H/R group. Meanwhile, mitochondrial fragmentation, ROS accumulation and apoptosis were aggravated in the PINK1 suppression group compared with the H/R group (Figure 5C-5F). In contrast, compared with H/R alone, plasmid transfection increased PINK1 expression and cell viability (Figure 6A and 6B). PINK1 upregulation inhibited mitochondrial fragmentation, ROS accumulation and apoptosis compared with H/R (Figure 6C-6F).
These results demonstrate that PINK1 plays a critical role in attenuating mitochondrial fission, ROS accumulation and apoptosis in intestinal I/R.
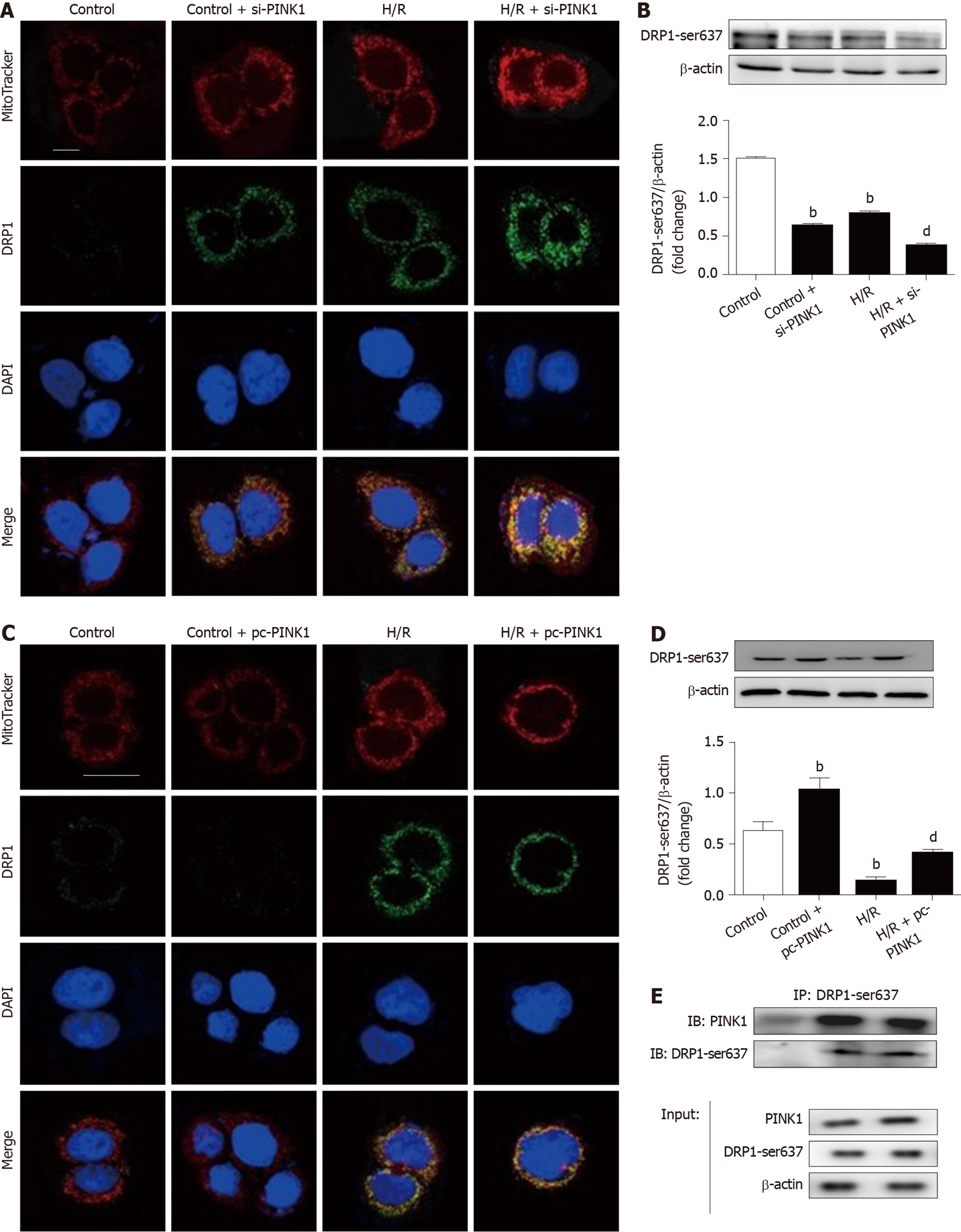
The phosphorylation of DRP1 at Ser637 has been reported to suppress DRP1 activity[25,26]. The above results demonstrated that I/R stimuli decreased the expression of PINK1 and p-DRP1 Ser637. Nevertheless, the association between PINK1 and p-DRP1 Ser637 has not been explored. Thus, we transfected a siRNA and plasmid to regulate the expression of PINK1. As shown in Figure 7A and 7B, PINK1 downregulation increased the recruitment of DRP1 to the mitochondria and decreased the expression of p-DRP1 Ser637. In contrast, PINK1 overexpression decreased the recruitment of DRP1 to the mitochondria and increased the expression of p-DRP1 Ser637 (Figure 7C and 7D). Furthermore, we performed co-IP to determine the relationship between PINK1 and p-DRP1 Ser637. Proteins isolated from intestinal tissues showed colocalization between PINK1 and p-DRP1 Ser637 in the same immunocomplex (Figure 7E).
Taken together, these results indicate that PINK1 may physically combine with DRP1 at Ser637 and inhibit mitochondrial fission.
In the present study, we created an intestinal I/R mouse model to observe mitochondrial fission induced by 45 min ischemia and different reperfusion times. Our results showed that the expression of PINK1 and phosphorylated DRP1 at Ser637 decreased and reached the lowest level at 4 h reperfusion in intestinal I/R. However, the mechanism of mitochondrial fission in intestinal I/R remains unknown. Thus, we further administrated mdivi-1 to mice, which may prevent intestinal I/R injury by regulating mitochondrial homeostasis. The protein expression levels of PINK1 and phosphorylated DRP1 Ser637 were significantly higher in the I/R + mdivi-1 group than in the I/R group, demonstrating that a mitochondrial division inhibitor prevents intestinal damage and apoptosis induced by I/R. Furthermore, mdivi-1 treatment significantly reduced mitochondrial fission, ROS levels and c-caspase 3. The results demonstrated that mitochondrial fission may play an important role in regulating intestinal I/R injury.
It was reported that upon exposure of cultured neurons to oxidative and metabolic pressure, mitochondria exhibited fragmentation and the fission protein DRP1 during multiplying stages[35]. In the human neuroblastoma cell line sh-sy5y, PINK1 overexpression reversed mitochondrial fragmentation and suppressed DRP1 translocation from the cytosol to the mitochondria in Parkinson’s disease, while PINK1 knockdown increased the severity of neuronal damage[36]. These results revealed the association between PINK1 and DRP1 during mitochondrial dynamics under stress conditions. Combined with our data, these results showed that PINK1 might play an endogenous protective role in intestinal I/R, affecting mitochondrial fission, modulating oxidative status, and influencing cell damage.
It has been shown that restoring the blood supply to the intestine (the reperfusion period) increases the damaging effects of tissue ischemic injury[2], partially due to a release of ROS[37]. Mitochondria contribute to the majority of endogenous ROS production, which causes cell injury. PINK1 is involved directly in regulating mitochondrial morphology[38] and may have a protective role against mitochondrial dysfunction. PINK1 has been demonstrated to have potential protective effects in heart I/R and spinal cord ischemia models. In this study, compared with H/R group, PINK1 knockdown decreased PINK1 protein expression and increased mitochondrial fission, as well as ROS levels and apoptosis. As expected, PINK1 overexpression showed the opposite trend. All results support that PINK1 can protect against intestinal I/R injury and that PINK1 protects cells by inhibiting mitochondrial fission. PINK1 plays a very important role in intestinal I/R injury, which may provide therapeutic treatment.
Mitochondria are dynamic organelles that maintain their shape and morphology by means of two contradicting processes, fission and fusion[39]. Mitochondrial fission participates in the constriction and cleavage of mitochondria via fission proteins, such as DRP1 and mitochondrial fission 1 protein[40]. The fusion process, on the other hand, was involved in mitochondria lengthening by combining the membrane of two nearby mitochondria. Mitofusin-1 and mitofusin-2 are mainly responsible for external membrane fusion, while Opa1 is thought to intervene in internal membrane fusion[41]. Mitochondrial fission was shown to start at 3 h after the beginning of reperfusion, sometimes long before neuronal loss, in a focal cerebral ischemia model in mice, revealing that fission is an upstream and early event in neuronal cell death[34]. Recent studies also showed the potential role of PINK1/DRP1 in cerebral ischemia[30]. As DRP1 has emerged as an important player in the anemia paradigm, PINK1, which associates with upstream kinases to modulate DRP1, may play an important role in intestinal I/R. In the present study, PINK1 knockdown increased DRP1 recruitment towards mitochondria and decreased p-DRP1 Ser637 expression. In contrast, PINK1 overexpression decreased DRP1 recruitment towards mitochondria and increased p-DRP1 Ser637 expression. Co-IP revealed that PINK1 combination with p-DRP1 Ser637 increased the phosphorylation of DRP1 at Ser637. Thus, we demonstrated that PINK1 plays a protective role in intestinal I/R injury through increasing DRP1 phosphorylation.
Collectively, our research reveals that mitochondrial fission is an important cause of ROS accumulation and apoptosis in intestine after I/R. Furthermore, PINK1 plays a critical role in mitochondrial fission inhibition. DRP1 is negatively regulated by PINK1 through binding with site Ser637. These findings indicate the potential application of mitochondrial fission inhibition in intestinal I/R treatment.
PTEN-induced putative kinase (PINK1) is a novel regulator of mitochondrial homeostasis that phosphorylates target proteins in mitochondria. PINK1 depletion has been identified to be related to mitochondrial fragmentation, ROS accumulation and apoptosis. However, the underlying regulatory mechanism of PINK1 in intestinal ischemia reperfusion (I/R) injury remains unclear. Therefore, elucidation of the protective role of PINK1 may help to improve tissue repair in I/R injury.
It is necessary to explore the function and target of PINK1 in mitochondrial regulation in intestinal I/R injury. Previous studies have demonstrated that mitochondrial dysfunction-induced apoptosis is an important cause of intestinal mucosal barrier damage and is associated with a high mortality rate in clinical practice. Moreover, the role of PINK1 in the protection against ischemic diseases has been clarified. These findings provide a basis for further study regarding the mechanism and target of PINK1 during intestinal I/R injury.
In a previous study, we investigated the effect of PINK1 on mitochondrial fission after intestinal I/R injury both in vivo and in vitro by gain- and loss-of-function approaches. Furthermore, we explored the phosphorylation site of DRP1, which is a downstream target of PINK1. Our study provides significant insight into the signaling mechanism of PINK1 during intestinal I/R injury and may contribute to the future investigation of more effective therapies in clinical practice.
Experiments used an in vivo mouse model and an in vitro Caco-2 cells models to better elucidate the pathophysiological process of intestinal I/R injury. Hematoxylin and eosin staining, and Chiu’s scoring system were used to demonstrate intestinal tissue injury. TUNEL and mitoSOX staining was carried out to display and observe apoptotic cells and ROS accumulation. Gene silencing and transfection were conducted to construct PINK1-depleted or PINK1-overexpressing cells to complete functional studies in vitro. A series of in vitro experiments, such as Western blotting, Cell Counting Kit-8 assays, and MitoTracker staining, were performed to explore the effect of PINK1 on mitochondrial fission.
Experiments in vivo showed the correlation between PINK1 and mucosal injury after intestinal I/R injury. The results of in vitro experiments showed a direct positive correlation of PINK1 with mitochondrial protection and apoptosis inhibition after hypoxia/reoxygenation. This study could be valuable as a basis for further studies on intestinal I/R injury and could potentially be utilized for therapeutic enhancement in clinical practice. Some limitations did exist: The in vivo study should be better designed to clarify causation and biological linkage, and PINK1 knockout mouse models would be helpful. Clinical samples are also needed to better support the application to human beings.
PINK1 plays a positive role in mitochondrial homeostasis and apoptosis inhibition in intestinal I/R injury. This study reveals that PINK1 could improve the phosphorylation of DRP1 at Ser637. Targeting the PINK1/DRP1 pathway may increase the therapeutic potential for intestinal I/R injury in clinical practice.
Our study illuminates the role of PINK1 in mitochondrial protection in intestinal I/R injury. Other researchers have reported that PINK1 is associated with mitophagy under I/R stress. Thus, the link between PINK1 and mitophagy in intestinal I/R needs further investigation.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Shimada S, Quarleri J, Wang Y S-Editor: Dou Y L-Editor: MedE-Ma JY E-Editor: Liu JH
| 1. | Acosta S. Epidemiology of mesenteric vascular disease: clinical implications. Semin Vasc Surg. 2010;23:4-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 191] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 2. | Mallick IH, Yang W, Winslet MC, Seifalian AM. Ischemia-reperfusion injury of the intestine and protective strategies against injury. Dig Dis Sci. 2004;49:1359-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 489] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 3. | Stone JR, Wilkins LR. Acute mesenteric ischemia. Tech Vasc Interv Radiol. 2015;18:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Cheng J, Wei Z, Liu X, Li X, Yuan Z, Zheng J, Chen X, Xiao G, Li X. The role of intestinal mucosa injury induced by intra-abdominal hypertension in the development of abdominal compartment syndrome and multiple organ dysfunction syndrome. Crit Care. 2013;17:R283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 5. | Li Y, Feng D, Wang Z, Zhao Y, Sun R, Tian D, Liu D, Zhang F, Ning S, Yao J, Tian X. Ischemia-induced ACSL4 activation contributes to ferroptosis-mediated tissue injury in intestinal ischemia/reperfusion. Cell Death Differ. 2019;26:2284-2299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 255] [Cited by in RCA: 643] [Article Influence: 107.2] [Reference Citation Analysis (0)] |
| 6. | Kannan KB, Colorado I, Reino D, Palange D, Lu Q, Qin X, Abungu B, Watkins A, Caputo FJ, Xu DZ, Semenza GL, Deitch EA, Feinman R. Hypoxia-inducible factor plays a gut-injurious role in intestinal ischemia reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2011;300:G853-G861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Wang J, Qiao L, Li S, Yang G. Protective effect of ginsenoside Rb1 against lung injury induced by intestinal ischemia-reperfusion in rats. Molecules. 2013;18:1214-1226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Feng D, Yao J, Wang G, Li Z, Zu G, Li Y, Luo F, Ning S, Qasim W, Chen Z, Tian X. Inhibition of p66Shc-mediated mitochondrial apoptosis via targeting prolyl-isomerase Pin1 attenuates intestinal ischemia/reperfusion injury in rats. Clin Sci (Lond). 2017;131:759-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 9. | Li Z, Wang G, Feng D, Zu G, Li Y, Shi X, Zhao Y, Jing H, Ning S, Le W, Yao J, Tian X. Targeting the miR-665-3p-ATG4B-autophagy axis relieves inflammation and apoptosis in intestinal ischemia/reperfusion. Cell Death Dis. 2018;9:483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 10. | Zhou W, Yao J, Wang G, Chen Z, Li Z, Feng D, Li Y, Qasim W, Tan W, Ning S, Tian X. PKCζ phosphorylates TRAF2 to protect against intestinal ischemia-reperfusion-induced injury. Cell Death Dis. 2017;8:e2935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Abate M, Festa A, Falco M, Lombardi A, Luce A, Grimaldi A, Zappavigna S, Sperlongano P, Irace C, Caraglia M, Misso G. Mitochondria as playmakers of apoptosis, autophagy and senescence. Semin Cell Dev Biol. 2020;98:139-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 366] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 12. | Panchal K, Tiwari AK. Mitochondrial dynamics, a key executioner in neurodegenerative diseases. Mitochondrion. 2019;47:151-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 13. | Kang JW, Choi HS, Lee SM. Resolvin D1 attenuates liver ischaemia/reperfusion injury through modulating thioredoxin 2-mediated mitochondrial quality control. Br J Pharmacol. 2018;175:2441-2453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Thornton C, Jones A, Nair S, Aabdien A, Mallard C, Hagberg H. Mitochondrial dynamics, mitophagy and biogenesis in neonatal hypoxic-ischaemic brain injury. FEBS Lett. 2018;592:812-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 15. | Wang Q, Xu J, Li X, Liu Z, Han Y, Xu X, Li X, Tang Y, Liu Y, Yu T, Li X. Sirt3 modulate renal ischemia-reperfusion injury through enhancing mitochondrial fusion and activating the ERK-OPA1 signaling pathway. J Cell Physiol. 2019;234:23495-23506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 16. | Yang M, Linn BS, Zhang Y, Ren J. Mitophagy and mitochondrial integrity in cardiac ischemia-reperfusion injury. Biochim Biophys Acta Mol Basis Dis. 2019;1865:2293-2302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 184] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 17. | Cerveny KL, Tamura Y, Zhang Z, Jensen RE, Sesaki H. Regulation of mitochondrial fusion and division. Trends Cell Biol. 2007;17:563-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 199] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 18. | Tilokani L, Nagashima S, Paupe V, Prudent J. Mitochondrial dynamics: overview of molecular mechanisms. Essays Biochem. 2018;62:341-360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 477] [Cited by in RCA: 935] [Article Influence: 133.6] [Reference Citation Analysis (0)] |
| 19. | Hu J, Zhang Y, Jiang X, Zhang H, Gao Z, Li Y, Fu R, Li L, Li J, Cui H, Gao N. ROS-mediated activation and mitochondrial translocation of CaMKII contributes to Drp1-dependent mitochondrial fission and apoptosis in triple-negative breast cancer cells by isorhamnetin and chloroquine. J Exp Clin Cancer Res. 2019;38:225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 108] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 20. | Zhang Q, Hu C, Huang J, Liu W, Lai W, Leng F, Tang Q, Liu Y, Wang Q, Zhou M, Sheng F, Li G, Zhang R. ROCK1 induces dopaminergic nerve cell apoptosis via the activation of Drp1-mediated aberrant mitochondrial fission in Parkinson's disease. Exp Mol Med. 2019;51:1-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 21. | Coronado M, Fajardo G, Nguyen K, Zhao M, Kooiker K, Jung G, Hu DQ, Reddy S, Sandoval E, Stotland A, Gottlieb RA, Bernstein D. Physiological Mitochondrial Fragmentation Is a Normal Cardiac Adaptation to Increased Energy Demand. Circ Res. 2018;122:282-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 22. | Maneechote C, Palee S, Kerdphoo S, Jaiwongkam T, Chattipakorn SC, Chattipakorn N. Differential temporal inhibition of mitochondrial fission by Mdivi-1 exerts effective cardioprotection in cardiac ischemia/reperfusion injury. Clin Sci (Lond). 2018;132:1669-1683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 23. | Kalia R, Wang RY, Yusuf A, Thomas PV, Agard DA, Shaw JM, Frost A. Structural basis of mitochondrial receptor binding and constriction by DRP1. Nature. 2018;558:401-405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 261] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 24. | Tsushima K, Bugger H, Wende AR, Soto J, Jenson GA, Tor AR, McGlauflin R, Kenny HC, Zhang Y, Souvenir R, Hu XX, Sloan CL, Pereira RO, Lira VA, Spitzer KW, Sharp TL, Shoghi KI, Sparagna GC, Rog-Zielinska EA, Kohl P, Khalimonchuk O, Schaffer JE, Abel ED. Mitochondrial Reactive Oxygen Species in Lipotoxic Hearts Induce Post-Translational Modifications of AKAP121, DRP1, and OPA1 That Promote Mitochondrial Fission. Circ Res. 2018;122:58-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 247] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 25. | Okada M, Morioka S, Kanazawa H, Yamawaki H. Canstatin inhibits isoproterenol-induced apoptosis through preserving mitochondrial morphology in differentiated H9c2 cardiomyoblasts. Apoptosis. 2016;21:887-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Park J, Choi H, Min JS, Park SJ, Kim JH, Park HJ, Kim B, Chae JI, Yim M, Lee DS. Mitochondrial dynamics modulate the expression of pro-inflammatory mediators in microglial cells. J Neurochem. 2013;127:221-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 225] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 27. | Quintana DD, Garcia JA, Sarkar SN, Jun S, Engler-Chiurazzi EB, Russell AE, Cavendish JZ, Simpkins JW. Hypoxia-reoxygenation of primary astrocytes results in a redistribution of mitochondrial size and mitophagy. Mitochondrion. 2019;47:244-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 28. | Lucero M, Suarez AE, Chambers JW. Phosphoregulation on mitochondria: Integration of cell and organelle responses. CNS Neurosci Ther. 2019;25:837-858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 29. | Rasool S, Trempe JF. New insights into the structure of PINK1 and the mechanism of ubiquitin phosphorylation. Crit Rev Biochem Mol Biol. 2018;53:515-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | Chen SD, Lin TK, Yang DI, Lee SY, Shaw FZ, Liou CW, Chuang YC. Roles of PTEN-induced putative kinase 1 and dynamin-related protein 1 in transient global ischemia-induced hippocampal neuronal injury. Biochem Biophys Res Commun. 2015;460:397-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Tanaka A, Youle RJ. A chemical inhibitor of DRP1 uncouples mitochondrial fission and apoptosis. Mol Cell. 2008;29:409-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 195] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 32. | Chen J, Yu W, Ruan Z, Wang S. TUG1/miR-421/PINK1: A potential mechanism for treating myocardial ischemia-reperfusion injury. Int J Cardiol. 2019;292:197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Shin JK, Lee SM. Genipin protects the liver from ischemia/reperfusion injury by modulating mitochondrial quality control. Toxicol Appl Pharmacol. 2017;328:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 34. | Wang H, Chen S, Zhang Y, Xu H, Sun H. Electroacupuncture ameliorates neuronal injury by Pink1/Parkin-mediated mitophagy clearance in cerebral ischemia-reperfusion. Nitric Oxide. 2019;91:23-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 35. | Divakaruni SS, Van Dyke AM, Chandra R, LeGates TA, Contreras M, Dharmasri PA, Higgs HN, Lobo MK, Thompson SM, Blanpied TA. Long-Term Potentiation Requires a Rapid Burst of Dendritic Mitochondrial Fission during Induction. Neuron. 2018;100:860-875.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 97] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 36. | van der Merwe C, van Dyk HC, Engelbrecht L, van der Westhuizen FH, Kinnear C, Loos B, Bardien S. Curcumin Rescues a PINK1 Knock Down SH-SY5Y Cellular Model of Parkinson's Disease from Mitochondrial Dysfunction and Cell Death. Mol Neurobiol. 2017;54:2752-2762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 37. | Bi W, Bi Y, Gao X, Li P, Hou S, Zhang Y, Bammert C, Jockusch S, Legalley TD, Michael Gibson K, Bi L. Indole-TEMPO conjugates alleviate ischemia-reperfusion injury via attenuation of oxidative stress and preservation of mitochondrial function. Bioorg Med Chem. 2017;25:2545-2568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | McWilliams TG, Muqit MM. PINK1 and Parkin: emerging themes in mitochondrial homeostasis. Curr Opin Cell Biol. 2017;45:83-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 257] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 39. | Chan DC. Mitochondrial Dynamics and Its Involvement in Disease. Annu Rev Pathol. 2020;15:235-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 881] [Article Influence: 146.8] [Reference Citation Analysis (0)] |
| 40. | Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, Smith CL, Youle RJ. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell. 2001;1:515-525. [PubMed] |
| 41. | Chandhok G, Lazarou M, Neumann B. Structure, function, and regulation of mitofusin-2 in health and disease. Biol Rev Camb Philos Soc. 2018;93:933-949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 187] [Article Influence: 23.4] [Reference Citation Analysis (0)] |