Published online Apr 21, 2020. doi: 10.3748/wjg.v26.i15.1733
Peer-review started: December 30, 2019
First decision: February 29, 2020
Revised: March 5, 2020
Accepted: April 4, 2020
Article in press: April 4, 2020
Published online: April 21, 2020
Processing time: 112 Days and 12.1 Hours
Third generation of quinolones, such as levofloxacin and moxifloxacin, -containing regimens are often used in second-line or rescue treatment of Helicobacter pylori infection. However, the increasing antibiotic resistance to quinolones affects the efficacies of quinolones-containing therapies in recent years. Therefore, there is a need to enhance the effectiveness of quinolones-containing therapies. Sitafloxacin, a fourth-generation quinolone, and vonoprazan, a novel potassium-competitive acid blocker, are now available as more effective treatment options. The aim of this paper is to summarize the current evidence of quinolone-containing therapies in rescue treatments, and to discuss the importance of drug sensitivity tests or analysis of gyrA mutation before treatments.
Core tip: The efficacies of 7-d levofloxacin or moxifloxacin, -containing regimens are becoming less effective in recent years due to the increasing antibiotic resistance, which necessitates 10-d or 14-d regimens or bismuth containing regimen are needed to achieve sufficient eradication rates. gyrA mutation is the most sensitive marker for predicting successful eradication in using quinolone-containing therapies. Thus, analysis of gyrA mutation before treatments is recommended. Seven-day sitafloxacin-amoxicillin-vonoprazan triple therapy is the best choice for third-line treatment at present.
- Citation: Mori H, Suzuki H. Update on quinolone-containing rescue therapies for Helicobacter pylori infection. World J Gastroenterol 2020; 26(15): 1733-1744
- URL: https://www.wjgnet.com/1007-9327/full/v26/i15/1733.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i15.1733
Helicobacter pylori (H. pylori) is closely related to gastric cancer, gastric ulcer, atrophic gastritis, mucosa-associated lymphoid tissue lymphoma, and H. pylori-associated dyspepsia. Thus, eradication of H. pylori is useful for treatment and prevention of these diseases[1-6]. In recent years, eradication of H. pylori has become difficult due to an increase in antibiotic resistance, thereafter selection of an efficient regimen has become increasingly important[1,7].
Levofloxacin-containing regimens are used as rescue therapy in many countries. However, in recent times, levofloxacin-amoxicillin-proton pump inhibitor (PPI) regimens were shown to be insufficient for gyrA mutation positive H. pylori strains. Moreover, prevalence of quinolone resistance combined with increased gyrA mutation positive strains have reduced the effectiveness of levofloxacin-amoxicillin-PPI regimen.
In recent years, the high effectiveness of sitafloxacin, a fourth-generation quinolone, -containing regimens to gyrA mutation positive H. pylori strains, has been demonstrated[8,9]. At this time, this article only reported the use of sitafloxacin-containing regimen in Japan. However, sitafloxacin will likely be the main quinolone-containing treatment in the future.
Vonoprazan, a novel potassium-competitive acid blocker which has a strong acid secretion inhibitory effect, has been available since 2015. The high efficacy of vonoprazan as first- and second-line H. pylori eradication therapy treatment has already been shown[10,11]. Thus, vonoprazan is expected to play a role in quinolone-containing rescue therapies.
In this article, we describe the current status of quinolone-containing rescue therapies.
We reviewed guidelines from the United States (2017), Europe (2016), Canada (2016), China (2016) and Japan (2016)[7,12-14]. Only the guideline from America suggested levofloxacin-containing triple therapy consisting of a PPI, levofloxacin, and amoxicillin as a first-line treatment option[12]. The basis of this recommendation was a network meta-analysis that showed levofloxacin-containing triple therapy for 10-14 d proved superior to clarithromycin-containing triple therapy for 7 d (90%, 95%CI: 84%-94% vs 73%, 95%CI: 71%-75%; RR 1.23, 95%CI: 1.16–1.29)[15]. The guidelines in the United States, Europe, Canada and China recommended levofloxacin-containing triple regimen as a rescue therapy[1,7,12,13]. The guidelines in Canada and China stated that increasing resistance rate of quinolones might affect the eradication rate, hence it did not recommend levofloxacin-containing regimen to be used as an initial treatment. The Japanese guideline of 2009 suggested levofloxacin-containing triple therapy as a third-line treatment option[16]. However, the 2016 guideline for Japan suggested sitafloxacin-containing triple therapy consisting of a PPI, sitafloxacin, and amoxicillin as a third-line treatment option[14]. Levofloxacin triple therapy was no longer recommended in Japan.
The most common mechanism of high-level fluoroquinolone resistance is due to mutation in one or more of the genes that encode the primary and secondary targets of these drugs, the type II topoisomerases (gyrA, gyrB, parC and parE)[17].
Mutations of gyrA within the quinolone resistance-determining regions have been found to be the main mechanism for quinolone resistance in H. pylori. The position of the gyrA mutation is usually limited to N87 or D91, both of which are in the DNA- binding region on the N-terminal domain of the gyrA protein, which includes fluoroquinolone-binding sites[18,19]. gyrA mutations in H. pylori strains correlate with phenotypic resistance of levofloxacin and sitafloxacin[8,20]. Liou et al[20] concluded that gyrA mutation in H. pylori strains is a better marker than phenotypic resistance in the prediction of levofloxacin-containing treatment outcomes[20]. We also showed that the presence of gyrA mutation is a more sensitive marker of eradication failure compared to minimum inhibitory concentrations (MICs) of sitafloxacin in using sitafloxacin-containing regimen[8]. In fact, the eradication rates of gyrA mutation-positive strains were around 70% with sitafloxacin-containing regimen, whereas most of all strains without gyrA mutation can be eradicated[9]. In meta-analysis, we found that the relative risk of the eradication failure is significantly lower in gyrA mutation at D91 compared to gyrA mutation at N87[9]. The MICs of double-mutated strains were extremely higher than those of single-mutated strains[19].
gyrB is unlikely to mutate and is thought to have little resistance[21,22], but some reports have reported resistance due to gyrB[23,24]. Since neither parC nor parE is found in the complete gene sequences of H. pylori, it is thought to be not involved in resistance[25,26].
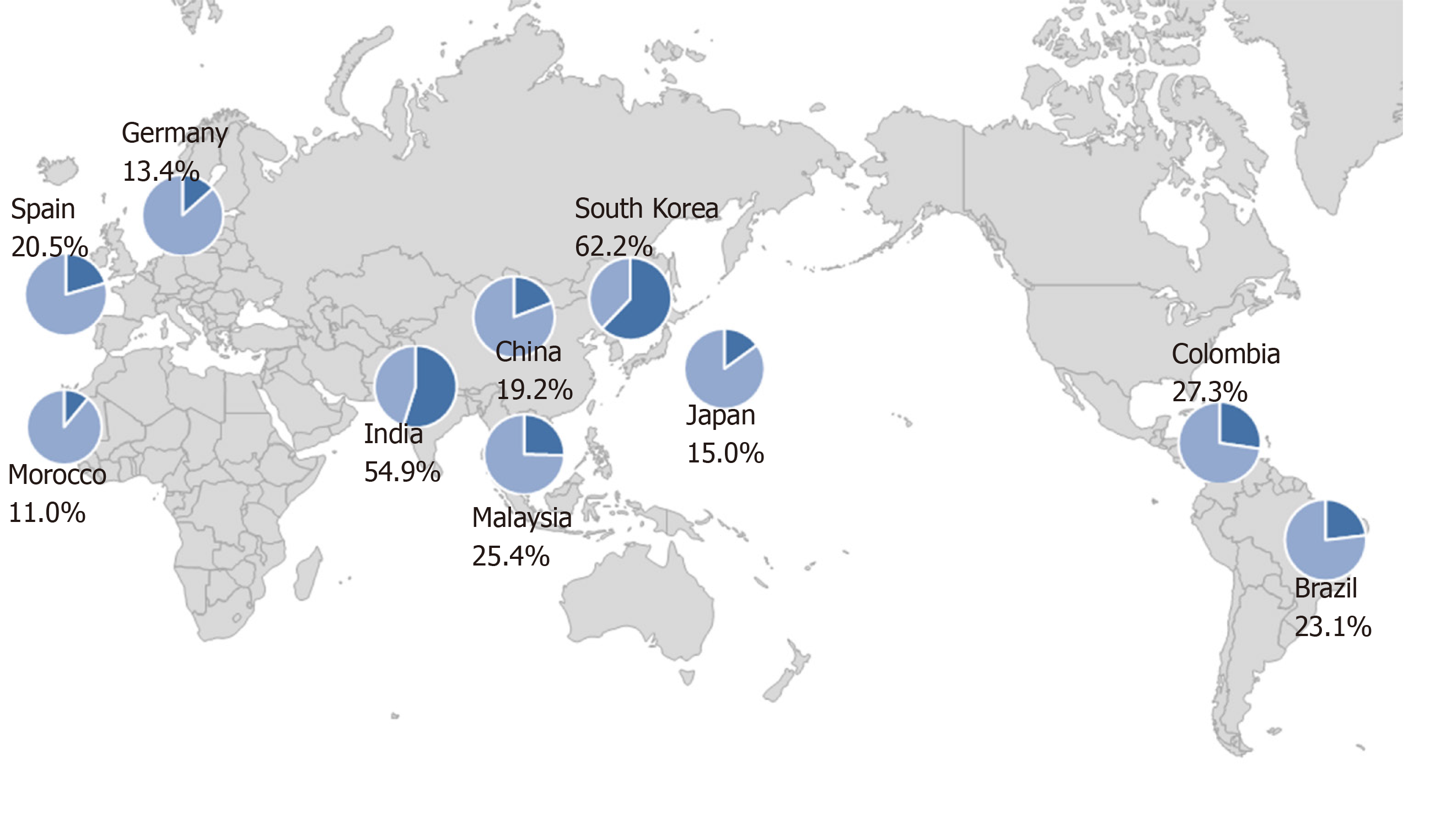
The prevalence of primary resistance of H. pylori to levofloxacin has been reported to range from 11.0% to 62.2% in different countries (Figure 1)[21,27-35]. There is no relationship between geographic factor and the resistance to levofloxacin. These data suggested that acquisition of resistance is related to high consumption rate of quinolones. Thus, the prevalence of resistance rates should be taken into consideration in selecting quinolone-containing treatments as a rescue therapy.
Levofloxacin, one of the third-generation fluoroquinolones, is available worldwide. There is abundant evidence of levofloxacin-containing rescue regimens (Table 1)[36-64]. Wong et al[36] showed the efficacy of levofloxacin-rifabutin-rabeprazole triple therapy as a rescue therapy in 2003. However, in this study, one patient developed drug-related neutropenia and thrombocytopenia, thus they concluded that rifabutin should be reserved only for resistant cases or even as a third-line therapy[36]. In addition, Zullo et al[37] and Nista et al[38] reported the efficacy of 10-d levofloxacin-amoxicillin-rabeprazole triple therapy as a third- and second-line rescue therapy, respectively, and the eradication rates seem to be sufficient (88.2% and 94.3%, respectively)[37,38]. On the other hand, Perri et al[39] and Watanabe et al[40] revealed that the efficacy of 7-d levofloxacin-amoxicillin-rabeprazole triple therapy as a second-line regimen is insufficient (66.1% and 69.7%, respectively)[39,40]. Matsumoto et al[42] showed that 7-d metronidazole-amoxicillin-lansoprazole triple therapy is significantly more effective than 7-d levofloxacin-amoxicillin-lansoprazole triple therapy as a second-line therapy in a prospective randomized trial in Japan (100.0% vs 72.4%, respectively)[42]. As a result, 7-d metronidazole-amoxicillin-lansoprazole triple therapy was confirmed as a second-line treatment in Japan[14]. The remarkable efficacy of 7-d metronidazole-amoxicillin-lansoprazole triple therapy is definitely due to the low rate of metronidazole-resistant strains in Japan, which seems to be an exceptional situation[65]. Moreover, Murakami et al[58] revealed that 7-d sitafloxacin-amoxicillin-lansoprazole triple therapy is significantly more effective than 7-d levofloxacin-amoxicillin-lansoprazole triple therapy as a third-line therapy in a prospective randomized trial in Japan (70.0% vs 43.1%, respectively). At present, levofloxacin containing triple therapy is no longer used in Japan[14].
| Ref. | Country/ Region | X-line | Publication year | Drug combination (per day) | Duration (d) | Eradication rate (%) | |
| ITT | PP | ||||||
| Wong et al[36] | China | 2nd and more | 2003 | LVFX 500 mg + RBT 300 mg + RPZ 40 mg | 7 | 91.1 | 91.1 |
| Zullo et al[37] | Italy | 3rd | 2003 | LVFX 500 mg + AMX 2000 mg + RPZ 40 mg | 10 | 83.3 | 88.2 |
| Nista et al[38] | Italy | 2nd | 2003 | LVFX 500 mg + AMX 2000 mg + RPZ 40 mg | 10 | 94.3 | 94.3 |
| Perri et al[39] | Italy | 2nd | 2003 | LVFX 500 mg + AMX 2000 mg + PPZ 80 mg | 7 | 63.8 | 66.1 |
| Watanabe et al[40] | Japan | 2nd | 2003 | LVFX 400 mg + AMX 2000 mg + LPZ 60 mg | 7 | 69.7 | 69.7 |
| Bilardi et al[41] | Italy | 2nd and more | 2004 | LVFX 500 mg + AMX 2000 mg + PPZ 80 mg | 10 | 70.5 | 75.6 |
| Matsumoto et al[42] | Japan | 2nd | 2005 | LVFX 600 mg + AMX 2000 mg + LPZ 60 mg | 7 | 70.0 | 72.4 |
| Wong et al[43] | China | 2nd and more | 2006 | LVFX 1000 mg + AMX 2000 mg + LPZ 60 mg | 7 | 57.4 | 59.6 |
| Gisbert et al[44] | Spain | 3rd | 2006 | LVFX 1000 mg + AMX 2000 mg + OPZ 40 mg | 10 | 60.0 | 66.3 |
| Perna et al[45] | Italy | 2nd | 2007 | LVFX 500 mg + AMX 2000 mg + RPZ 40 mg | 10 | 72.7 | 72.7 |
| Gisbert et al[46] | Spain | 2nd | 2008 | LVFX 1000 mg + AMX 2000 mg + OPZ 40 mg | 10 | 77.3 | 81.4 |
| Di Caro et al[47] | Italy | 2nd | 2009 | LVFX 500 mg + AMX 2000 mg + EPZ 40 mg | 7 | 65.0 | 65.0 |
| Di Caro et al[47] | Italy | 2nd | 2009 | LVFX 1000 mg + AMX 2000 mg + EPZ 40 mg | 7 | 70.0 | 70.0 |
| Di Caro et al[47] | Italy | 2nd | 2009 | LVFX 500 mg + AMX 2000 mg + EPZ 40 mg | 10 | 90.0 | 90.0 |
| Di Caro et al[47] | Italy | 2nd | 2009 | LVFX 1000 mg + AMX 2000 mg + EPZ 40 mg | 10 | 85.0 | 85.0 |
| Liou et al[48] | Taiwan | 2nd | 2010 | LVFX 750 mg + AMX 2000 mg + LPZ 60 mg | 7 | 76.9 | 80.0 |
| Liou et al[49] | Taiwan | 2nd | 2011 | Modified sequential regimen1 | 10 | 95.1 | 96.4 |
| Hu et al[50] | Taiwan | 2nd | 2011 | LVFX 500 mg + AMX 2000 mg + EPZ 80 mg | 7 | 68.9 | 75.6 |
| Ermis et al[51] | Turkey | 2nd | 2011 | LVFX 1000 mg + AMX 2000 mg + LPZ 60 mg | 7 | 37.8 | 41.2 |
| Goh et al[52] | Malaysia | 2nd | 2012 | LVFX 1000 mg + AMX 2000 mg + RPZ 40 mg | 14 | 90.3 | 90.3 |
| Chuah et al[53] | Taiwan | 2nd | 2012 | LVFX 500 mg + AMX 2000 mg + EPZ 80 mg | 7 | 78.1 | 80.3 |
| Gisbert et al[54] | Spain | 2nd | 2013 | LVFX 1000 mg + AMX 2000 mg + OPZ 40 mg | 10 | 73.8 | 75.1 |
| Calhan et al[55] | Turkey | 2nd | 2013 | Modified sequential regimen2 | 12 | 82.2 | 85.7 |
| Calhan et al[55] | Turkey | 2nd | 2013 | LVFX 500 mg + TET 2000 mg + PPZ 80 mg + bismuth 1200 mg | 10 | 90.6 | 93.1 |
| Moon et al[56] | South Korea | 2nd | 2013 | LVFX 500 mg + MTZ 1500 mg + LPZ 60 mg | 7 | 67.9 | 73.1 |
| Tai et al[57] | Taiwan | 2nd | 2013 | LVFX 500 mg + AMX 2000 mg + EPZ 80 mg | 10 | 68.0 | 75.6 |
| Tai et al[57] | Taiwan | 2nd | 2013 | LVFX 500 mg + AMX 2000 mg + EPZ 80 mg | 14 | 86.0 | 92.5 |
| Murakami et al[58] | Japan | 3rd | 2013 | LVFX 500 mg + AMX 1500 mg + LPZ 60 mg | 7 | 43.1 | 43.7 |
| Gisbert et al[59] | Spain | 2nd | 2015 | LVFX 500 mg + AMX 2000 mg + EPZ 80 mg + bismuth 480 mg | 14 | 90.0 | 91.1 |
| Cao et al[60] | China | 2nd | 2015 | LVFX 500 mg + AMX 2000 mg + LPZ 60 mg + bismuth 480 mg | 14 | 83.0 | 85.4 |
| Paoluzi et al[61] | Italy | 3rd | 2015 | LVFX 1000 mg + DOXY 200 mg + EPZ 40 mg | 7 | 46.0 | 49.0 |
| Liou et al[62] | Taiwan | 2nd | 2016 | LVFX 500 mg + AMX 2000 mg + LPZ 60 mg | 10 | 75.3 | 78.8 |
| Liou et al[62] | Taiwan | 2nd | 2016 | Modified sequential regimen3 | 10 | 84.3 | 86.3 |
| Song et al[63] | China | 2nd | 2016 | LVFX 500 mg + AMX 2000 mg + EPZ 40 mg + bismuth 440 mg | 14 | 73.5 | 78.5 |
| Hsu et al[64] | Taiwan | 2nd | 2017 | LVFX 500 mg + TET 2000 mg + EPZ 80 mg + bismuth 480 mg | 10 | 98.0 | 97.8 |
| Hsu et al[64] | Taiwan | 2nd | 2017 | LVFX 500 mg + AMX 2000 mg + EPZ 80 mg | 10 | 69.2 | 68.6 |
Di Caro et al[47] conducted a randomized study to determine dosage and length of levofloxacin-containing regimens as a second-line rescue treatment. In this study, patients were randomized into 4 groups to receive 7-d or 10-d levofloxacin 500 mg, amoxicillin 2000 mg and esomeprazole 40 mg per day or 7-d or 10-d levofloxacin 1000 mg, amoxicillin 2000 mg and esomeprazole 40 mg per day. Interestingly, based upon duration of treatment, eradication rates in the 10-d groups were significantly higher than those in the 7-d groups (87.5% vs 67.5 %, respectively); however, dosage of levofloxacin did not affect the eradication rates (77.5% vs 77.5%, respectively)[47]. Similarly, Tai et al[57] showed that the eradication rate of 14-d levofloxacin-amoxicillin-lansoprazole triple therapy was higher than that of 10-d levofloxacin-amoxicillin-lansoprazole triple therapy (92.5% vs 75.6%, respectively). A meta-analysis showed that the eradication rates of 10-d levofloxacin-containing regimens were significantly higher than those of 7-d levofloxacin-containing regimens (81.0% vs 73.0%, respectively)[66]. One other meta-analysis also showed 14-d levofloxacin-containing regimens seemed to be more effective compared to 7-d levofloxacin-containing regimens (83.4% vs 74.6%, respectively)[67]. These data suggested the dose and duration of levofloxacin-containing regimens of 500 mg per day for 10-14 d should be sufficient.
From 2013, levofloxacin and bismuth-containing regimens were reported as rescue treatments[55]. Gisbert et al[59] achieved 91.1% of eradication with 14-d levofloxacin-amoxicillin-esomeprazole-bismuth regimen as a second-line therapy. Hsu et al[64] showed 10-d levofloxacin-tetracycline-esomeprazole-bismuth regimen was more effective than levofloxacin-amoxicillin-esomeprazole triple regimen as a second-line therapy (97.8% vs 68.6%, respectively). On the other hand, Cao et al[60] revealed that 14-d levofloxacin-amoxicillin-lansoprazole-bismuth regimen was less effective compared to 14-d classical metronidazole-tetracycline-lansoprazole-bismuth quadruple therapy in areas of high quinolones resistance such as China (85.4% vs 90.6%, respectively).
Some reports showed the efficacies of modified sequential therapy containing levofloxacin[49,55,62]. Liou et al[62] revealed modified sequential therapy containing levofloxacin was more effective than 10-d levofloxacin-amoxicillin-lansoprazole triple regimen in the second-Line treatment (86.3% vs 78.8%, respectively). On the other hand, Calhan et al[55] showed that modified sequential therapy containing levofloxacin was less effective than 10-d levofloxacin-tetracyclin-pantoprazole-bismuth quadruple regimen (85.7% vs 93.1%, respectively).
Eradication rates of levofloxacin-containing regimens against levofloxacin-resistant strains or gyrA mutation-positive strains were reported to be 33.3% to 41.7%[20,45], while those of sitafloxacin-containing regimens were 68.4% to 74.4%[8,9,68]. Moreover, 7-d or 10-d sitafloxacin-containing regimens, achieved almost perfect eradication of gyrA mutation-negative strains, whereas the eradication rate of a 7-d levofloxacin-containing triple regimen was only 82.7%[9,20].
Regarding adverse effects, levofloxacin-containing triple therapies are more tolerable compared to bismuth-containing quadruple therapy[66]. 10- and 14-d levofloxacin-containing triple therapies are equally safe compared to 7-d levofloxacin-containing triple therapy[57,66].
From these data, when levofloxacin-containing regimens are used as a rescue therapy, a drug sensitivity test or an analysis of gyrA mutation should be performed before treatment. In areas of high quinolones resistance, classical bismuth-containing quadruple therapy or fourth-generation fluoroquinolone, such as sitafloxacin, -containing regimen seems to be better choices. Modified sequential therapy containing levofloxacin or 10-d levofloxacin-tetracyclin-PPI-bismuth quadruple regimen could be an option as a third-line regimen.
Moxifloxacin, one of the third-generation fluoroquinolones, is available worldwide. Di Caro et al[69] initially showed the efficacy of moxifloxacin-based therapies as a first-line therapy at first in 2002. Cheon et al[70] reported moxifloxacin-amoxicillin-esomeprazole triple therapy achieved 83.8% successful eradication, and significant superiority to bismuth- containing regimen in Korea. The reports of moxifloxacin-containing therapies were shown in Table 2. Most of the reports were published from South Korea. Interestingly, 7-d moxifloxacin-amoxicillin-PPI triple therapy achieved over 78% in PP before 2011[71-74]; from 2014 the eradication rates gradually decreased and hovered around 60%[75-79]. On the other hand, 14-d moxifloxacin-amoxicillin-PPI triple therapy is more effective than 7-d regimen, and maintains the efficacy over 80% in PP. These data suggested that 7-d moxifloxacin-amoxicillin-PPI triple therapy should not be used as a second-line regimen in Korea any longer. It is believed that the diminished effectiveness of 7-d moxifloxacin-amoxicillin-PPI triple therapy was attributed to increasing antimicrobial resistance of H. pylori to quinolones, especially in Korea[27,80]. Marušić et al[81] showed that 14-d bismuth-based quadruple therapy modified with moxifloxacin achieved 88.0 % of eradication in Croatia as a second-line treatment, thus this regimen might be useful in regions of low metronidazole resistance.
| Ref. | Country | X-line | Publication year | Drug combination (per day) | Duration (d) | Eradication rate (%) | |
| ITT | PP | ||||||
| Cheon et al[70] | South Korea | 2nd | 2006 | MOFX 400 mg + AMX 2000 mg + RPZ 40 mg | 7 | 75.6 | 83.8 |
| Kang et al[71] | South Korea | 2nd | 2007 | MOFX 400 mg + AMX 2000 mg + EPZ 40 mg | 10 | 71.9 | 82.6 |
| Bago et al[72] | Croatia | 2nd | 2009 | MOFX 400 mg + MTZ 1500 mg + OPZ 40 mg | 7 | 73.2 | 78.9 |
| Yoon et al[73] | South Korea | 2nd | 2009 | MOFX 400 mg + AMX 2000 mg + EPZ 40 mg | 7 | 75.6 | 83.8 |
| Yoon et al[73] | South Korea | 2nd | 2009 | MOFX 400 mg + AMX 2000 mg + EPZ 40 mg | 10 | 71.9 | 82.6 |
| Yoon et al[73] | South Korea | 2nd | 2009 | MOFX 400 mg + AMX 2000 mg + EPZ 40 mg | 14 | 68.0 | 79.9 |
| Miehlke et al[74] | Germany | 2nd and more | 2011 | MOFX 400 mg + AMX 2000 mg + EPZ 40 mg | 7 | 78.9 | 78.9 |
| Miehlke et al[74] | Germany | 2nd and more | 2011 | MOFX 400 mg + AMX 2000 mg + EPZ 40 mg | 14 | 95.0 | 94.4 |
| Kang et al[75] | South Korea | 2nd | 2014 | MOFX 400 mg + AMX 2000 mg + RPZ 20 mg | 7 | 58.0 | 62.5 |
| Kang et al[75] | South Korea | 2nd | 2014 | MOFX 400 mg + AMX 2000 mg + RPZ 20 mg | 14 | 71.8 | 77.5 |
| Chung et al[77] | South Korea | 2nd | 2014 | MOFX 400 mg + AMX 2000 mg + RPZ 40 mg | 7 | 62.7 | 62.7 |
| Lee et al[76] | South Korea | 2nd | 2015 | MOFX 400 mg + AMX 2000 mg + PPI | 7 | 53.1 | 55.6 |
| Lee et al[76] | South Korea | 2nd | 2015 | MOFX 400 mg + AMX 2000 mg + PPI | 14 | 73.5 | 80.6 |
| Hwang et al[78] | South Korea | 2nd | 2015 | MOFX 400 mg + AMX 2000 mg + RPZ 40 mg | 7 | 70.8 | 77.7 |
| Hwang et al[78] | South Korea | 2nd | 2015 | MOFX 400 mg + AMX 2000 mg + RPZ 40 mg | 14 | 81.4 | 90.4 |
| Lim et al[79] | South Korea | 2nd | 2015 | MOFX 400 mg + AMX 2000 mg + RPZ 40 mg | 7 | 56.7 | 59.6 |
| Lim et al[79] | South Korea | 2nd | 2015 | MOFX 400 mg + AMX 2000 mg + RPZ 40 mg | 14 | 76.3 | 80.6 |
| Marušić et al[81] | Croatia | 2nd | 2017 | MOFX 400 mg + MTZ 1000 mg + Bismuth 480 mg + PPZ 80 mg | 14 | 80.6 | 88.0 |
Few reports are available on whether levofloxacin- or moxifloxacin-containing therapy is a better rescue therapy. As a first-line treatment, Rakici et al[82] performed randomized trial between levofloxacin-amoxicillin-lansoprazole triple therapy and moxifloxacin-amoxicillin-lansoprazole triple therapy, and there was no significant difference (92.0% vs 91.8%, respectively). The side effects observed in the two groups were similar.
In conclusion, levofloxacin- or moxifloxacin-containing therapies seem to be equally effective as second-line treatments; thus, either regimens can be used at present. In regions of high quinolones resistance, 14-d moxifloxacin-amoxicillin-PPI triple therapy is better choice than 7-d regimen. Few data are available on 10-d regimen, thus future research is needed to confirm its efficacy as a second-line therapy.
Sitafloxacin, one of the fourth-generation fluoroquinolones, is only available in Japan and Thailand. The reports of sitafloxacin-containing therapies were shown in Table 3. Sánchez et al[83] initially showed that sitafloxacin was the most active fluoroquinolone compared with ciprofloxacin and moxifloxacin in vitro. Moreover, we reported that sitafloxacin exhibited the most potent activity against gyrA mutation-positive strains compared with gatifloxacin and garenoxacin, two other fourth-generation fluoroquinolones[84]. Murakami et al[85] also showed that sitafloxacin had a strong activity compared with garenoxacin and levofloxacin in vitro. Based on these in vitro data, we revealed that sitafloxacin-amoxicillin-rabeprazole triple therapy achieved 83.6% success in eradicating H. pylori as a third-line rescue treatment[8]. Moreover, even among patients with gyrA mutation-positive H. pylori, the eradication rates reached to be 74.4%. Multi-center randomized controlled study showed that sitafloxacin-amoxicillin-lansoprazole triple therapy achieved 70.0% of successful eradication as a third-line rescue treatment, whereas the eradication rates of levofloxacin-amoxicillin-lansoprazole triple therapy and high dose amoxicillin-lansoprazole dual therapy were 43.1% and 54.3%, respectively[58]. We examined randomized controlled study to assess the efficacy with extension of the duration of regimens from 7 to 10 d and the efficacy of sitafloxacin-metronidazole-esomeprazole triple therapy as a third-line rescue treatment[9]. However, there was no significant difference in the eradication rates between 10-d sitafloxacin-amoxicillin-esomeprazole triple therapy and 10-d sitafloxacin-metronidazole-esomeprazole triple therapy (82.0% vs 76.4%, P = 0.50)[9]. The 10-d regimens also could not improve eradication rates when compared with the 7-d sitafloxacin-containing regimen[9]. Furuta et al[86] compared sitafloxacin-amoxicillin-rabeprazole for 7 or 14 d or sitafloxacin-metronidazole-rabeprazole for 7 or 14 d; however, there were no significant difference between them. Recently, a randomized trial showed that 7-d sitafloxacin-amoxicillin-vonoprazan triple therapy is more effective than 7-d sitafloxacin-amoxicillin-esomeprazole triple therapy as a third-line regimen (83.3% vs 57.1%, P = 0.04)[87]. Vonoprazan, a first-in-class potassium-competitive acid blocker, exhibits more rapid, strong, and continuous gastric acid suppression was compared with conventional PPIs[10]. Vonoprazan-containing regimens showed more efficacy than PPI-containing regimens in first- and second-line treatments[10,11]. Recently, we revealed that changes in the rate of resistance to sitafloxacin were not observed from 2009 to 2015[68].
| Ref. | Country | X-line | Publication year | Drug combination (per day) | Duration (d) | Eradication rate (%) | |
| ITT | PP | ||||||
| Matsuzaki et al[8] | Japan | 3rd | 2012 | STFX 200 mg + AMX 2000 mg + RPZ 40 mg | 7 | 78.2 | 83.6 |
| Murakami et al[58] | Japan | 3rd | 2013 | STFX 200 mg + AMX 1500 mg + LPZ 60 mg | 7 | 70.0 | 72.1 |
| Furuta et al[86] | Japan | 3rd | 2014 | STFX 200 mg + AMX 2000 mg + RPZ 20-40 mg | 7 | 84.1 | 86.4 |
| Furuta et al[86] | Japan | 3rd | 2014 | STFX 200 mg + AMX 2000 mg + RPZ 20-40 mg | 14 | 88.9 | 90.9 |
| Furuta et al[86] | Japan | 3rd | 2014 | STFX 200 mg + MTZ 500 mg + RPZ 20-40 mg | 7 | 90.9 | 90.9 |
| Furuta et al[86] | Japan | 3rd | 2014 | STFX 200 mg + MTZ 500 mg + RPZ 20-40 mg | 14 | 87.2 | 91.1 |
| Mori et al[9] | Japan | 3rd | 2015 | STFX 200 mg + AMX 2000 mg + EPZ 40 mg | 10 | 81.0 | 82.0 |
| Mori et al[9] | Japan | 3rd | 2015 | STFX 200 mg + MTZ 500 mg + EPZ 40 mg | 10 | 72.4 | 76.4 |
| Sue et al[87] | Japan | 3rd | 2019 | STFX 200 mg + AMX 1500 mg + PPI | 7 | 53.3 | 57.1 |
| Sue et al[87] | Japan | 3rd | 2019 | STFX 200 mg + AMX 1500 mg + VPZ 40 mg | 7 | 75.8 | 83.3 |
Regarding adverse events to sitafloxacin-containing regimens, severe side effects are rarely reported. Mild and transient adverse effects, such as diarrhea and soft stool, were reported by 24%-80% of patients who received treatments[68,86,87].
These data suggested that 7-d sitafloxacin-amoxicillin-vonoprazan triple therapy is the best choice for third-line treatment at the current moment. However, the efficacy of 7-d sitafloxacin-amoxicillin-vonoprazan triple therapy to gyrA mutation-positive H. pylori should be evaluated in the future.
Wueppenhorst et al[88] showed that the levofloxacin/ciprofloxacin resistance occurred significantly more often in patients who had received quinolones-containing regimen when compared with patients who had not (44.5% vs 23.1%). We found that 20.8% of strains obtained double mutations in gyrA after eradication failure with stafloxacin-containing third-line treatment, which exhibited seven-fold increased MICs of sitafloxacin compared with pre-treatment. On the other hand, the MICs of sitafloxacin did not increase, when the location of the gyrA mutations did not change after treatment. Double mutations in gyrA also cause higher resistance to other fluoroquinolones[22]. If strains obtain new mutation in gyrA, quinolone-containing regimen will be less effective. Therefore, we recommend a more powerful regimen to be used, e.g., sitafloxacin-containing or extension of duration, and stronger inhibition of gastric acid secretion, when quinolone-containing regimen is used as a rescue treatment.
Quinolone-containing regimen is effective as a rescue treatment. The drug susceptibility test to quinolones and the identification of gyrA mutation are recommended before using quinolone-containing regimen. In using levofloxacin-containing regimen, 10- or 14-d regimen or bismuth containing regimen are recommended and should be restricted to regions of low levofloxacin resistance. Seven-day sitafloxacin-amoxicillin-vonoprazan triple therapy is the best choice for third-line treatment at the current moment.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and Hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El-Zahaby SA, Kravtsov V, Reddy NNR S-Editor: Dou Y L-Editor: A E-Editor: Ma YJ
| 1. | Malfertheiner P, Megraud F, O'Morain CA, Gisbert JP, Kuipers EJ, Axon AT, Bazzoli F, Gasbarrini A, Atherton J, Graham DY, Hunt R, Moayyedi P, Rokkas T, Rugge M, Selgrad M, Suerbaum S, Sugano K, El-Omar EM; European Helicobacter and Microbiota Study Group and Consensus panel. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66:6-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2220] [Cited by in RCA: 1983] [Article Influence: 247.9] [Reference Citation Analysis (1)] |
| 2. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3126] [Cited by in RCA: 3182] [Article Influence: 132.6] [Reference Citation Analysis (0)] |
| 3. | Suzuki H, Nishizawa T, Tsugawa H, Hibi T. Molecular approaches and modern clinical strategies for the management of Helicobacter pylori infection in Japan. Keio J Med. 2012;61:109-119. [PubMed] |
| 4. | Suzuki H, Mori H. Helicobacter pylori: Helicobacter pylori gastritis--a novel distinct disease entity. Nat Rev Gastroenterol Hepatol. 2015;12:556-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Matsuzaki J, Tsugawa H, Kashiwazaki Y, Mori H, Yamamoto Y, Kameyama H, Masaoka T, Kanai T, Suzuki H. Neutrophil-activating Protein Polymorphism of Helicobacter pylori Determines the Host Risk of Dyspepsia. Cell Mol Gastroenterol Hepatol. 2019;8:295-297.e6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Suzuki H. Helicobacter pylori-Associated Upper Gastrointestinal Symptoms: FD or HpD? Dig Dis Sci. 2017;62:1391-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Fallone CA, Chiba N, van Zanten SV, Fischbach L, Gisbert JP, Hunt RH, Jones NL, Render C, Leontiadis GI, Moayyedi P, Marshall JK. The Toronto Consensus for the Treatment of Helicobacter pylori Infection in Adults. Gastroenterology. 2016;151:51-69.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 695] [Cited by in RCA: 633] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 8. | Matsuzaki J, Suzuki H, Nishizawa T, Hirata K, Tsugawa H, Saito Y, Okada S, Fukuhara S, Hibi T. Efficacy of sitafloxacin-based rescue therapy for Helicobacter pylori after failures of first- and second-line therapies. Antimicrob Agents Chemother. 2012;56:1643-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Mori H, Suzuki H, Matsuzaki J, Tsugawa H, Fukuhara S, Miyoshi S, Hirata K, Seino T, Matsushita M, Masaoka T, Kanai T. Efficacy of 10-day Sitafloxacin-Containing Third-Line Rescue Therapies for Helicobacter pylori Strains Containing the gyrA Mutation. Helicobacter. 2016;21:286-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Murakami K, Sakurai Y, Shiino M, Funao N, Nishimura A, Asaka M. Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: a phase III, randomised, double-blind study. Gut. 2016;65:1439-1446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 316] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 11. | Mori H, Suzuki H, Omata F, Masaoka T, Asaoka D, Kawakami K, Mizuno S, Kurihara N, Nagahara A, Sakaki N, Ito M, Kawamura Y, Suzuki M, Shimada Y, Sasaki H, Matsuhisa T, Torii A, Nishizawa T, Mine T, Ohkusa T, Kawai T, Tokunaga K, Takahashi S. Current status of first- and second-line Helicobacter pylori eradication therapy in the metropolitan area: a multicenter study with a large number of patients. Therap Adv Gastroenterol. 2019;12:1756284819858511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 12. | Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am J Gastroenterol. 2017;112:212-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 1017] [Article Influence: 127.1] [Reference Citation Analysis (1)] |
| 13. | Liu WZ, Xie Y, Lu H, Cheng H, Zeng ZR, Zhou LY, Chen Y, Wang JB, Du YQ, Lu NH; Chinese Society of Gastroenterology, Chinese Study Group on Helicobacter pylori and Peptic Ulcer. Fifth Chinese National Consensus Report on the management of Helicobacter pylori infection. Helicobacter. 2018;23:e12475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 326] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 14. | Kato M, Ota H, Okuda M, Kikuchi S, Satoh K, Shimoyama T, Suzuki H, Handa O, Furuta T, Mabe K, Murakami K, Sugiyama T, Uemura N, Takahashi S. Guidelines for the management of Helicobacter pylori infection in Japan: 2016 Revised Edition. Helicobacter. 2019;24:e12597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 208] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 15. | Li BZ, Threapleton DE, Wang JY, Xu JM, Yuan JQ, Zhang C, Li P, Ye QL, Guo B, Mao C, Ye DQ. Comparative effectiveness and tolerance of treatments for Helicobacter pylori: systematic review and network meta-analysis. BMJ. 2015;351:h4052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 112] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 16. | Asaka M, Kato M, Takahashi S, Fukuda Y, Sugiyama T, Ota H, Uemura N, Murakami K, Satoh K, Sugano K; Japanese Society for Helicobacter Research. Guidelines for the management of Helicobacter pylori infection in Japan: 2009 revised edition. Helicobacter. 2010;15:1-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 292] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 17. | Redgrave LS, Sutton SB, Webber MA, Piddock LJ. Fluoroquinolone resistance: mechanisms, impact on bacteria, and role in evolutionary success. Trends Microbiol. 2014;22:438-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 644] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 18. | Matsuzaki J, Suzuki H, Tsugawa H, Nishizawa T, Hibi T. Homology model of the DNA gyrase enzyme of Helicobacter pylori, a target of quinolone-based eradication therapy. J Gastroenterol Hepatol. 2010;25 Suppl 1:S7-S10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Mori H, Suzuki H, Matsuzaki J, Masaoka T, Kanai T. Acquisition of double mutation in gyrA caused high resistance to sitafloxacin in Helicobacter pylori after unsuccessful eradication with sitafloxacin-containing regimens. United European Gastroenterol J. 2018;6:391-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Liou JM, Chang CY, Sheng WH, Wang YC, Chen MJ, Lee YC, Hung HW, Chian H, Chang SC, Wu MS, Lin JT. Genotypic resistance in Helicobacter pylori strains correlates with susceptibility test and treatment outcomes after levofloxacin- and clarithromycin-based therapies. Antimicrob Agents Chemother. 2011;55:1123-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Miyachi H, Miki I, Aoyama N, Shirasaka D, Matsumoto Y, Toyoda M, Mitani T, Morita Y, Tamura T, Kinoshita S, Okano Y, Kumagai S, Kasuga M. Primary levofloxacin resistance and gyrA/B mutations among Helicobacter pylori in Japan. Helicobacter. 2006;11:243-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 118] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 22. | Tankovic J, Lascols C, Sculo Q, Petit JC, Soussy CJ. Single and double mutations in gyrA but not in gyrB are associated with low- and high-level fluoroquinolone resistance in Helicobacter pylori. Antimicrob Agents Chemother. 2003;47:3942-3944. [PubMed] |
| 23. | Rimbara E, Noguchi N, Kawai T, Sasatsu M. Fluoroquinolone resistance in Helicobacter pylori: role of mutations at position 87 and 91 of GyrA on the level of resistance and identification of a resistance conferring mutation in GyrB. Helicobacter. 2012;17:36-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 24. | Chung JW, Lee GH, Jeong JY, Lee SM, Jung JH, Choi KD, Song HJ, Jung HY, Kim JH. Resistance of Helicobacter pylori strains to antibiotics in Korea with a focus on fluoroquinolone resistance. J Gastroenterol Hepatol. 2012;27:493-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Tomb JF, White O, Kerlavage AR, Clayton RA, Sutton GG, Fleischmann RD, Ketchum KA, Klenk HP, Gill S, Dougherty BA, Nelson K, Quackenbush J, Zhou L, Kirkness EF, Peterson S, Loftus B, Richardson D, Dodson R, Khalak HG, Glodek A, McKenney K, Fitzegerald LM, Lee N, Adams MD, Hickey EK, Berg DE, Gocayne JD, Utterback TR, Peterson JD, Kelley JM, Cotton MD, Weidman JM, Fujii C, Bowman C, Watthey L, Wallin E, Hayes WS, Borodovsky M, Karp PD, Smith HO, Fraser CM, Venter JC. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2635] [Cited by in RCA: 2586] [Article Influence: 92.4] [Reference Citation Analysis (0)] |
| 26. | Alm RA, Ling LS, Moir DT, King BL, Brown ED, Doig PC, Smith DR, Noonan B, Guild BC, deJonge BL, Carmel G, Tummino PJ, Caruso A, Uria-Nickelsen M, Mills DM, Ives C, Gibson R, Merberg D, Mills SD, Jiang Q, Taylor DE, Vovis GF, Trust TJ. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature. 1999;397:176-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1387] [Cited by in RCA: 1358] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 27. | Lee JY, Kim N, Nam RH, In Choi S, Lee JW, Lee DH. Primary and secondary antibiotic resistance of Helicobacter pylori in Korea from 2003 to 2018. Helicobacter. 2019;24:e12660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 28. | Liu DS, Wang YH, Zeng ZR, Zhang ZY, Lu H, Xu JM, Du YQ, Li Y, Wang JB, Xu SP, Chen Y, Lan CH, Cheng H, Jiang MD, Zhang LX, Huo LJ, Chen SY, Zhang GX, Wu KC, Zhu X, Chen YX, Zhu Y, Shu X, Xie Y, Lu NH. Primary antibiotic resistance of Helicobacter pylori in Chinese patients: a multiregion prospective 7-year study. Clin Microbiol Infect. 2018;24:780.e5-780.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 29. | Shetty V, Lamichhane B, Tay CY, Pai GC, Lingadakai R, Balaraju G, Shetty S, Ballal M, Chua EG. High primary resistance to metronidazole and levofloxacin, and a moderate resistance to clarithromycin in Helicobacter pylori isolated from Karnataka patients. Gut Pathog. 2019;11:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 30. | Hanafiah A, Binmaeil H, Raja Ali RA, Mohamed Rose I, Lopes BS. Molecular characterization and prevalence of antibiotic resistance in Helicobacter pylori isolates in Kuala Lumpur, Malaysia. Infect Drug Resist. 2019;12:3051-3061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 31. | Bluemel B, Goelz H, Goldmann B, Grüger J, Hamel H, Loley K, Ludolph T, Meyer J, Miehlke S, Mohr A, Tüffers K, Usadel H, Wagner S, Wenzel H, Wiemer L, Vorreiter J, Eisele B, Hofreuter D, Glocker EO. Antimicrobial resistance of Helicobacter pylori in Germany, 2015 to 2018. Clin Microbiol Infect. 2020;26:235-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 32. | Morilla AM, Álvarez-Argüelles ME, Duque JM, Armesto E, Villar H, Melón S. Primary antimicrobial resistance rates and prevalence of Helicobacter pylori infection in the north of Spain. A 13-year retrospective study. Gastroenterol Hepatol. 2019;42:476-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | Bouihat N, Burucoa C, Benkirane A, Seddik H, Sentissi S, Al Bouzidi A, Elouennas M, Benouda A. Helicobacter pylori Primary Antibiotic Resistance in 2015 in Morocco: A Phenotypic and Genotypic Prospective and Multicenter Study. Microb Drug Resist. 2017;23:727-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | Eisig JN, Silva FM, Barbuti RC, Navarro-Rodriguez T, Moraes-Filho JP, Pedrazzoli J. Helicobacter pylori antibiotic resistance in Brazil: clarithromycin is still a good option. Arq Gastroenterol. 2011;48:261-264. [PubMed] |
| 35. | Trespalacios-Rangél AA, Otero W, Arévalo-Galvis A, Poutou-Piñales RA, Rimbara E, Graham DY. Surveillance of Levofloxacin Resistance in Helicobacter pylori Isolates in Bogotá-Colombia (2009-2014). PLoS One. 2016;11:e0160007. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 36. | Wong WM, Gu Q, Lam SK, Fung FM, Lai KC, Hu WH, Yee YK, Chan CK, Xia HH, Yuen MF, Wong BC. Randomized controlled study of rabeprazole, levofloxacin and rifabutin triple therapy vs. quadruple therapy as second-line treatment for Helicobacter pylori infection. Aliment Pharmacol Ther. 2003;17:553-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 98] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 37. | Zullo A, Hassan C, De Francesco V, Lorenzetti R, Marignani M, Angeletti S, Ierardi E, Morini S. A third-line levofloxacin-based rescue therapy for Helicobacter pylori eradication. Dig Liver Dis. 2003;35:232-236. [PubMed] |
| 38. | Nista EC, Candelli M, Cremonini F, Cazzato IA, Di Caro S, Gabrielli M, Santarelli L, Zocco MA, Ojetti V, Carloni E, Cammarota G, Gasbarrini G, Gasbarrini A. Levofloxacin-based triple therapy vs. quadruple therapy in second-line Helicobacter pylori treatment: a randomized trial. Aliment Pharmacol Ther. 2003;18:627-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 76] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 39. | Perri F, Festa V, Merla A, Barberani F, Pilotto A, Andriulli A. Randomized study of different 'second-line' therapies for Helicobacter pylori infection after failure of the standard 'Maastricht triple therapy'. Aliment Pharmacol Ther. 2003;18:815-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Watanabe Y, Aoyama N, Shirasaka D, Maekawa S, Kuroda K, Miki I, Kachi M, Fukuda M, Wambura C, Tamura T, Kasuga M. Levofloxacin based triple therapy as a second-line treatment after failure of helicobacter pylori eradication with standard triple therapy. Dig Liver Dis. 2003;35:711-715. [PubMed] |
| 41. | Bilardi C, Dulbecco P, Zentilin P, Reglioni S, Iiritano E, Parodi A, Accornero L, Savarino E, Mansi C, Mamone M, Vigneri S, Savarino V. A 10-day levofloxacin-based therapy in patients with resistant Helicobacter pylori infection: a controlled trial. Clin Gastroenterol Hepatol. 2004;2:997-1002. [PubMed] |
| 42. | Matsumoto Y, Miki I, Aoyama N, Shirasaka D, Watanabe Y, Morita Y, Toyoda M, Mitani T, Miyachi H, Tamura T, Kasuga M. Levofloxacin- versus metronidazole-based rescue therapy for H. pylori infection in Japan. Dig Liver Dis. 2005;37:821-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 43. | Wong WM, Gu Q, Chu KM, Yee YK, Fung FM, Tong TS, Chan AO, Lai KC, Chan CK, Wong BC. Lansoprazole, levofloxacin and amoxicillin triple therapy vs. quadruple therapy as second-line treatment of resistant Helicobacter pylori infection. Aliment Pharmacol Ther. 2006;23:421-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 44. | Gisbert JP, Gisbert JL, Marcos S, Moreno-Otero R, Pajares JM. Third-line rescue therapy with levofloxacin is more effective than rifabutin rescue regimen after two Helicobacter pylori treatment failures. Aliment Pharmacol Ther. 2006;24:1469-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 45. | Perna F, Zullo A, Ricci C, Hassan C, Morini S, Vaira D. Levofloxacin-based triple therapy for Helicobacter pylori re-treatment: role of bacterial resistance. Dig Liver Dis. 2007;39:1001-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 46. | Gisbert JP, Bermejo F, Castro-Fernández M, Pérez-Aisa A, Fernández-Bermejo M, Tomas A, Barrio J, Bory F, Almela P, Sánchez-Pobre P, Cosme A, Ortiz V, Niño P, Khorrami S, Benito LM, Carneros JA, Lamas E, Modolell I, Franco A, Ortuño J, Rodrigo L, García-Durán F, O'Callaghan E, Ponce J, Valer MP, Calvet X; H. pylori Study Group of the Asociación Española de Gastroenterología. Second-line rescue therapy with levofloxacin after H. pylori treatment failure: a Spanish multicenter study of 300 patients. Am J Gastroenterol. 2008;103:71-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 47. | Di Caro S, Franceschi F, Mariani A, Thompson F, Raimondo D, Masci E, Testoni A, La Rocca E, Gasbarrini A. Second-line levofloxacin-based triple schemes for Helicobacter pylori eradication. Dig Liver Dis. 2009;41:480-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 48. | Liou JM, Lin JT, Chang CY, Chen MJ, Cheng TY, Lee YC, Chen CC, Sheng WH, Wang HP, Wu MS. Levofloxacin-based and clarithromycin-based triple therapies as first-line and second-line treatments for Helicobacter pylori infection: a randomised comparative trial with crossover design. Gut. 2010;59:572-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 49. | Liou JM, Chen CC, Chen MJ, Chang CY, Fang YJ, Lee JY, Sheng WH, Wang HP, Wu MS, Lin JT. Empirical modified sequential therapy containing levofloxacin and high-dose esomeprazole in second-line therapy for Helicobacter pylori infection: a multicentre clinical trial. J Antimicrob Chemother. 2011;66:1847-1852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 50. | Hu TH, Chuah SK, Hsu PI, Wu DC, Tai WC, Chiu YC, Wu KL, Kuo CM, Hu ML. Randomized comparison of two nonbismuth-containing rescue therapies for Helicobacter pylori. Am J Med Sci. 2011;342:177-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 51. | Ermis F, Akyuz F, Uyanikoglu A, Kurt R, Pinarbasi B, Nazik H, Kaymakoglu S, Mungan Z. Second-line levofloxacin-based triple therapy's efficiency for Helicobacter pylori eradication in patients with peptic ulcer. South Med J. 2011;104:579-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 52. | Goh KL, Manikam J, Qua CS. High-dose rabeprazole-amoxicillin dual therapy and rabeprazole triple therapy with amoxicillin and levofloxacin for 2 weeks as first and second line rescue therapies for Helicobacter pylori treatment failures. Aliment Pharmacol Ther. 2012;35:1097-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 53. | Chuah SK, Hsu PI, Chang KC, Chiu YC, Wu KL, Chou YP, Hu ML, Tai WC, Chiu KW, Chiou SS, Wu DC, Hu TH. Randomized comparison of two non-bismuth-containing second-line rescue therapies for Helicobacter pylori. Helicobacter. 2012;17:216-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 54. | Gisbert JP, Pérez-Aisa A, Bermejo F, Castro-Fernández M, Almela P, Barrio J, Cosme A, Modolell I, Bory F, Fernández-Bermejo M, Rodrigo L, Ortuño J, Sánchez-Pobre P, Khorrami S, Franco A, Tomas A, Guerra I, Lamas E, Ponce J, Calvet X; H. pylori Study Group of the Asociación Española de Gastroenterología (Spanish Gastroenterology Association). Second-line therapy with levofloxacin after failure of treatment to eradicate helicobacter pylori infection: time trends in a Spanish Multicenter Study of 1000 patients. J Clin Gastroenterol. 2013;47:130-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 55. | Calhan T, Kahraman R, Sahin A, Senates E, Doganay HL, Kanat E, Ozdil K, Sokmen HM. Efficacy of two levofloxacin-containing second-line therapies for Helicobacter pylori: a pilot study. Helicobacter. 2013;18:378-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 56. | Moon JY, Kim GH, You HS, Lee BE, Ryu DY, Cheong JH, Jung JI, Jeong JH, Song CS, Song GA. Levofloxacin, Metronidazole, and Lansoprazole Triple Therapy Compared to Quadruple Therapy as a Second-Line Treatment of Helicobacter pylori Infection in Korea. Gut Liver. 2013;7:406-410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 57. | Tai WC, Chiu CH, Liang CM, Chang KC, Kuo CM, Chiu YC, Wu KL, Hu ML, Chou YP, Chiou SS, Chiu KW, Kuo CH, Hu TH, Lin MT, Chuah SK. Ten-Day versus 14-Day Levofloxacin-Containing Triple Therapy for Second-Line Anti-Helicobacter pylori Eradication in Taiwan. Gastroenterol Res Pract. 2013;2013:932478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 58. | Murakami K, Furuta T, Ando T, Nakajima T, Inui Y, Oshima T, Tomita T, Mabe K, Sasaki M, Suganuma T, Nomura H, Satoh K, Hori S, Inoue S, Tomokane T, Kudo M, Inaba T, Take S, Ohkusa T, Yamamoto S, Mizuno S, Kamoshida T, Amagai K, Iwamoto J, Miwa J, Kodama M, Okimoto T, Kato M, Asaka M; Japan GAST Study Group. Multi-center randomized controlled study to establish the standard third-line regimen for Helicobacter pylori eradication in Japan. J Gastroenterol. 2013;48:1128-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 59. | Gisbert JP, Romano M, Gravina AG, Solís-Muñoz P, Bermejo F, Molina-Infante J, Castro-Fernández M, Ortuño J, Lucendo AJ, Herranz M, Modolell I, Del Castillo F, Gómez J, Barrio J, Velayos B, Gómez B, Domínguez JL, Miranda A, Martorano M, Algaba A, Pabón M, Angueira T, Fernández-Salazar L, Federico A, Marín AC, McNicholl AG. Helicobacter pylori second-line rescue therapy with levofloxacin- and bismuth-containing quadruple therapy, after failure of standard triple or non-bismuth quadruple treatments. Aliment Pharmacol Ther. 2015;41:768-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 60. | Cao Z, Chen Q, Zhang W, Liang X, Liao J, Liu W, Xiao S, Lu H. Fourteen-day optimized levofloxacin-based therapy versus classical quadruple therapy for Helicobacter pylori treatment failures: a randomized clinical trial. Scand J Gastroenterol. 2015;50:1185-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 61. | Paoluzi OA, Del Vecchio Blanco G, Visconti E, Coppola M, Fontana C, Favaro M, Pallone F. Low efficacy of levofloxacin-doxycycline-based third-line triple therapy for Helicobacter pylori eradication in Italy. World J Gastroenterol. 2015;21:6698-6705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 62. | Liou JM, Bair MJ, Chen CC, Lee YC, Chen MJ, Chen CC, Tseng CH, Fang YJ, Lee JY, Yang TH, Luo JC, Wu JY, Chang WH, Chang CC, Chen CY, Chen PY, Shun CT, Hsu WF, Hung HW, Lin JT, Chang CY, Wu MS; Taiwan Gastrointestinal Disease and Helicobacter Consortium. Levofloxacin Sequential Therapy vs Levofloxacin Triple Therapy in the Second-Line Treatment of Helicobacter pylori: A Randomized Trial. Am J Gastroenterol. 2016;111:381-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 63. | Song Z, Zhou L, Zhang J, He L, Bai P, Xue Y. Levofloxacin, bismuth, amoxicillin and esomeprazole as second-line Helicobacter pylori therapy after failure of non-bismuth quadruple therapy. Dig Liver Dis. 2016;48:506-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 64. | Hsu PI, Tsai FW, Kao SS, Hsu WH, Cheng JS, Peng NJ, Tsai KW, Hu HM, Wang YK, Chuah SK, Chen A, Wu DC. Ten-Day Quadruple Therapy Comprising Proton Pump Inhibitor, Bismuth, Tetracycline, and Levofloxacin is More Effective than Standard Levofloxacin Triple Therapy in the Second-Line Treatment of Helicobacter pylori Infection: A Randomized Controlled Trial. Am J Gastroenterol. 2017;112:1374-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 65. | Suzuki H, Mori H. World trends for H. pylori eradication therapy and gastric cancer prevention strategy by H. pylori test-and-treat. J Gastroenterol. 2018;53:354-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 66. | Gisbert JP, Morena F. Systematic review and meta-analysis: levofloxacin-based rescue regimens after Helicobacter pylori treatment failure. Aliment Pharmacol Ther. 2006;23:35-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 243] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 67. | Chen PY, Wu MS, Chen CY, Bair MJ, Chou CK, Lin JT, Liou JM; Taiwan Gastrointestinal Disease and Helicobacter Consortium. Systematic review with meta-analysis: the efficacy of levofloxacin triple therapy as the first- or second-line treatments of Helicobacter pylori infection. Aliment Pharmacol Ther. 2016;44:427-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 68. | Mori H, Suzuki H, Matsuzaki J, Masaoka T, Kanai T. 10-Year Trends in Helicobacter pylori Eradication Rates by Sitafloxacin-Based Third-Line Rescue Therapy. Digestion. 2019;1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 69. | Di Caro S, Ojetti V, Zocco MA, Cremonini F, Bartolozzi F, Candelli M, Lupascu A, Nista EC, Cammarota G, Gasbarrini A. Mono, dual and triple moxifloxacin-based therapies for Helicobacter pylori eradication. Aliment Pharmacol Ther. 2002;16:527-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 70. | Cheon JH, Kim N, Lee DH, Kim JM, Kim JS, Jung HC, Song IS. Efficacy of moxifloxacin-based triple therapy as second-line treatment for Helicobacter pylori infection. Helicobacter. 2006;11:46-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 71. | Kang JM, Kim N, Lee DH, Park YS, Kim YR, Kim JS, Jung HC, Song IS. Second-line treatment for Helicobacter pylori infection: 10-day moxifloxacin-based triple therapy versus 2-week quadruple therapy. Helicobacter. 2007;12:623-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 72. | Bago J, Pevec B, Tomić M, Marusić M, Bakula V, Bago P. Second-line treatment for Helicobacter pylori infection based on moxifloxacin triple therapy: a randomized controlled trial. Wien Klin Wochenschr. 2009;121:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 73. | Yoon H, Kim N, Lee BH, Hwang TJ, Lee DH, Park YS, Nam RH, Jung HC, Song IS. Moxifloxacin-containing triple therapy as second-line treatment for Helicobacter pylori infection: effect of treatment duration and antibiotic resistance on the eradication rate. Helicobacter. 2009;14:77-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 74. | Miehlke S, Krasz S, Schneider-Brachert W, Kuhlisch E, Berning M, Madisch A, Laass MW, Neumeyer M, Jebens C, Zekorn C, Knoth H, Vieth M, Stolte M, Lehn N, Morgner A. Randomized trial on 14 versus 7 days of esomeprazole, moxifloxacin, and amoxicillin for second-line or rescue treatment of Helicobacter pylori infection. Helicobacter. 2011;16:420-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 75. | Kang KK, Lee DH, Oh DH, Yoon H, Shin CM, Park YS, Kim N, Jung HC. Helicobacter pylori eradication with moxifloxacin-containing therapy following failed first-line therapies in South Korea. World J Gastroenterol. 2014;20:6932-6938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 76. | Lee ST, Lee DH, Lim JH, Kim N, Park YS, Shin CM, Jo HJ, Song IS. Efficacy of 7-Day and 14-Day Bismuth-Containing Quadruple Therapy and 7-Day and 14-Day Moxifloxacin-Based Triple Therapy as Second-Line Eradication for Helicobacter pylori Infection. Gut Liver. 2015;9:478-485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 77. | Chung KH, Lee DH, Jin E, Cho Y, Seo JY, Kim N, Jeong SH, Kim JW, Hwang JH, Shin CM. The efficacy of moxifloxacin-containing triple therapy after standard triple, sequential, or concomitant therapy failure for Helicobacter pylori eradication in Korea. Gut Liver. 2014;8:605-611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 78. | Hwang JJ, Lee DH, Lee AR, Yoon H, Shin CM, Park YS, Kim N. Efficacy of 14-d vs 7-d moxifloxacin-based triple regimens for second-line Helicobacter pylori eradication. World J Gastroenterol. 2015;21:5568-5574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 79. | Lim JH, Lee DH, Lee ST, Kim N, Park YS, Shin CM, Song IS. Moxifloxacin-containing triple therapy after non-bismuth quadruple therapy failure for Helicobacter pylori infection. World J Gastroenterol. 2015;21:13124-13131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 80. | Lee JW, Kim N, Kim JM, Nam RH, Chang H, Kim JY, Shin CM, Park YS, Lee DH, Jung HC. Prevalence of primary and secondary antimicrobial resistance of Helicobacter pylori in Korea from 2003 through 2012. Helicobacter. 2013;18:206-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 176] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 81. | Marušić M, Dominković L, Majstorović Barać K, Gulić S, Bago J, Pezerović D. Bismuth-based quadruple therapy modified with moxifloxacin for Helicobacter pylori eradication. Minerva Gastroenterol Dietol. 2017;63:80-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 82. | Rakici H, Ayaz T, Akdogan RA, Bedir R. Comparison of levofloxacin- and moxifloxacin-based triple therapies with standard treatment in eradication of Helicobacter pylori as first-line therapy. Digestion. 2014;90:261-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 83. | Sánchez JE, Sáenz NG, Rincón MR, Martín IT, Sánchez EG, Martínez MJ. Susceptibility of Helicobacter pylori to mupirocin, oxazolidinones, quinupristin/dalfopristin and new quinolones. J Antimicrob Chemother. 2000;46:283-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 84. | Suzuki H, Nishizawa T, Muraoka H, Hibi T. Sitafloxacin and garenoxacin may overcome the antibiotic resistance of Helicobacter pylori with gyrA mutation. Antimicrob Agents Chemother. 2009;53:1720-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 85. | Murakami K, Okimoto T, Kodama M, Tanahashi J, Fujioka T, Ikeda F, Muraoka H, Takigawa M, Saika T, Hasegawa M, Kobayashi I. Sitafloxacin activity against Helicobacter pylori isolates, including those with gyrA mutations. Antimicrob Agents Chemother. 2009;53:3097-3099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 86. | Furuta T, Sugimoto M, Kodaira C, Nishino M, Yamade M, Uotani T, Sahara S, Ichikawa H, Yamada T, Osawa S, Sugimoto K, Watanabe H, Umemura K. Sitafloxacin-based third-line rescue regimens for Helicobacter pylori infection in Japan. J Gastroenterol Hepatol. 2014;29:487-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 87. | Sue S, Shibata W, Sasaki T, Kaneko H, Irie K, Kondo M, Maeda S. Randomized trial of vonoprazan-based versus proton-pump inhibitor-based third-line triple therapy with sitafloxacin for Helicobacter pylori. J Gastroenterol Hepatol. 2019;34:686-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 88. | Wueppenhorst N, Stueger HP, Kist M, Glocker EO. High secondary resistance to quinolones in German Helicobacter pylori clinical isolates. J Antimicrob Chemother. 2013;68:1562-1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |