Published online Apr 21, 2020. doi: 10.3748/wjg.v26.i15.1683
Peer-review started: December 31, 2019
First decision: March 6, 2020
Revised: April 2, 2020
Accepted: April 8, 2020
Article in press: April 8, 2020
Published online: April 21, 2020
Processing time: 111 Days and 10 Hours
There has long been a recognised association between non-alcoholic fatty liver disease (NAFLD) and the composite aspects of the metabolic syndrome. Part of this association highlighted the supposed co-existence of elevated uric acid levels in those with NAFLD. There is interest in exploitation of this as a putative diagnostic and prognostic biomarker in NAFLD. Given the increased economic and health burden associated with the NAFLD epidemic, improved methods of population-based, minimally-invasive methods and biomarkers are clearly highly sought and necessary. In this opinion review we review the proposed role of uric acid in the pathogenesis of NAFLD and its potential utilisation in the diagnosis and monitoring of the disease process.
Core tip: There is significant interest in the role of uric acid as both a causative aetiological proponent in non-alcoholic fatty liver disease as well as its diagnostic utility in the diagnosis of fatty liver disease. Within this review we explore these putative molecular mechanisms which are likely implicated, in addition to exploring the most recent translational evidence of uric acid as a diagnostic tool in the clinical environ.
- Citation: Brennan P, Clare K, George J, Dillon JF. Determining the role for uric acid in non-alcoholic steatohepatitis development and the utility of urate metabolites in diagnosis: An opinion review. World J Gastroenterol 2020; 26(15): 1683-1690
- URL: https://www.wjgnet.com/1007-9327/full/v26/i15/1683.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i15.1683
Non-alcoholic fatty liver disease (NAFLD) represents a spectrum of disease ranging from simple fatty liver (steatosis; NAFL) to fatty liver with cell inflammation and ballooning (non-alcoholic steatohepatitis; NASH). Within this continuum is the propensity for progression to cirrhosis with its associated sequelae of end-stage liver failure and hepatocellular carcinoma. NASH; in particular with advanced fibrosis, represents a significant unmet medical need, with limited non-invasive screening and diagnostic and monitoring tools. The defining characteristic of NAFLD is a more than 5% lipid deposition within the liver in the absence of excess alcohol consumption, defined as > 20 g/d for women and 30 g/d for men[1].
The development of NAFLD is strongly linked to insulin resistance, diabetes and obesity. This relationship is well established and NAFLD is widely considered to be a hepatic manifestation of the metabolic syndrome[2]. There are however, clearly additional implicated factors which are as yet not completely determined. NAFLD development and progression is likely multifactorial and involve an interplay of genetic, environmental, metabolic and gut microbial factors[3]. The exact mechanisms which underlie the pathogenesis of NAFLD are not yet fully understood. Multiple research programmes are evolving towards uncovering the relevant pathological mechanisms, thereby identifying therapeutic targets in NAFLD as there is a clear unmet clinical need for pharmacotherapy in its management.
A number of studies have shown an association between elevated serum uric acid (UA) levels and the development of NAFLD[4,5]. One paper reports a prevalence of hyperuricemia of 78% in NAFLD subjects and 41% in non-NAFLD with a study group of 147 and 832 respectively[6]. The association appears to be stronger in females[7] and dose-dependent[8]. UA is the final oxidation product of purine metabolism in humans. It is a relatively insoluble waste by-product which can accumulate in the serum if there is an imbalance in its formation or excretion[9]. The majority of purines are made endogenously whilst a smaller proportion come from a dietary source. Given there is a dietary link, there is increasing evidence to support the promotion of the Mediterranean diet for the prevention and treatment of NAFLD[10].
Hyperuricemia is well known to co-exist with other “metabolic” conditions including insulin resistance, obesity, hypertension and atherosclerosis. More recently, studies have shown that having elevated levels of serum UA is an independent predictor of NAFLD[11]. It is not established however, whether these high levels are causative or represent the effect of the metabolic syndrome.
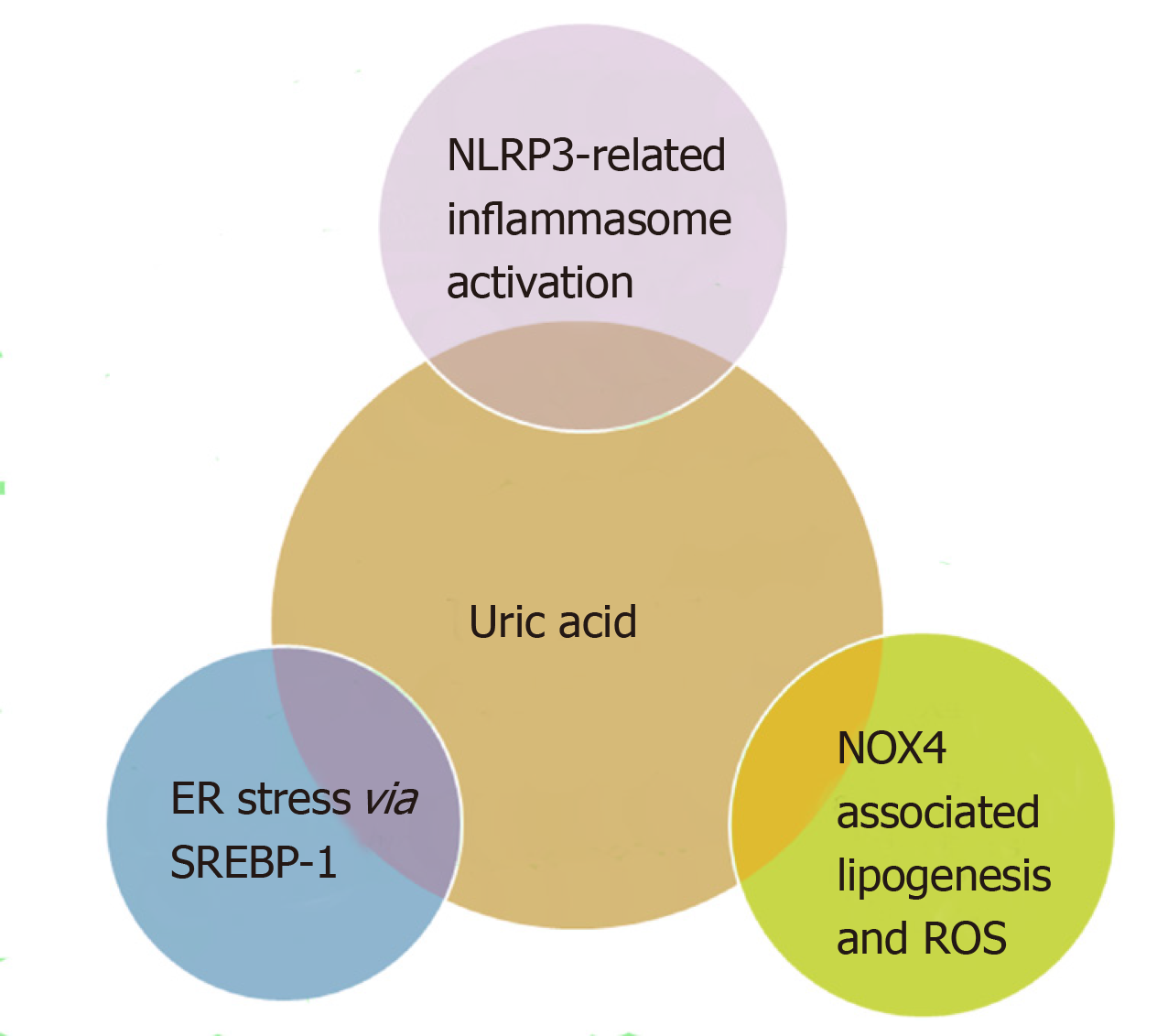
Some of the putative mechanisms for NAFLD development relating to UA are summarised in Figure 1.
Previously, it has been demonstrated that urate exerts an intracellular pro-oxidant and pro-inflammatory effect in adipose tissue[9,12,13]. Whilst there are studies that suggest that a rise in urate might be an anti-oxidant response to increased oxidative stress and adiposity, current evidence suggests that in NAFLD, it functions as a marker of xanthine oxidase (XO) activity[14]. To further substantiate this evidence, recent studies have shown a significantly raised XO levels in patients with NAFLD compared to controls[15]. A recent study in 5541 individuals showed that NAFLD significantly increased the risk of incident hyperuricemia and demonstrated in mouse models of NAFLD that XO mediates the relationship between NAFLD and hyperuricemia. The authors therefore suggested that XO inhibition may serve as a novel therapeutic target for NAFLD[4]. Urate has also been shown to increase fatty accumulation in the liver by increasing endoplasmic reticulum (ER) stress via transcriptional factors including sterol regulatory element-binding protein-1 (SREBP-1c) activation in hepatocytes[16].
It is unsurprising that XO may play an important role in NAFLD as XO is widely distributed throughout various organs including the liver, gut, lung, kidney, heart, brain and plasma[17] with the highest levels being found in the gut and the liver[18]. A fully reduced XO contains six electrons and its re-oxidation involves electron transfer to oxygen molecules which generates two molecules each of the potent reactive oxygen species, hydrogen peroxide (H2O2) and superoxide anion (O2-) species[19] for every fully reduced XO molecule, thereby generating significant oxidative stress each time a molecule of urate is formed. Superoxide anions then react with water and nitric oxide to generate further free radicals such as hydroxyl and peroxynitrite radicals.
There is evidence to support both the direct and indirect effects of UA in NAFLD pathogenesis and progression. Previously, high levels of serum UA had been regarded as beneficial, due to its ability to act as an antioxidant. However, once UA becomes intracellular it loses its favourable anti-oxidative properties and its action paradoxically; appears to be detrimental[20].
With regards to its direct effects, studies have shown that UA can stimulate fat synthesis within hepatocytes[16]. UA promotes the translocation of the NADPH oxidase subunit-4 (NOX4) into the mitochondria which increases superoxide generation. This increase in reactive oxygen species inhibits aconitase, an enzyme which catalyses the isomerisation of citrate to iso-citrate in the mitochondrial matrix during the TCA cycle. The inhibition of aconitase leads to accumulation of citrate in the mitochondria. When this citrate is released into the cytosol, it activates the ATP sensitive enzyme ATP-citrate lyase through phosphorylation at SER455. This converts citrate to acetyl-CoA for de novo lipogenesis through fatty acid synthase[20].
These observations have been supported by work which has shown that a UA driven increase in mitochondrial oxidants could be prevented with pre-administration of apocynin, an antioxidant and NOX inhibitor or by silencing NOX4[20].Consistent with these proposed mechanisms the study has also shown in hepatocytes exposed to UA, there was a 77.4% reduction aconitase activity as well as a substantial accumulation of citrate in the cytosol of hepatocytes when cells are exposed to UA[20].
Research in both HepG2 cells and primary mouse hepatocytes has also described additional mechanisms in which UA contributes to lipid accumulation in hepatocytes[16]. Reactive oxygen species produced by UA and cellular membrane NOX prompts a cascade of ER stress and release of lipogenic transcription factor SREBP-1c[16].
The ER has a key role in cellular function, responsible for the folding of proteins and synthesis of lipids. It is therefore highly sensitive to homeostatic changes and when under stress, misfolded and unfolded proteins accumulate and activate the unfolded protein response (UPR) signalling pathways in an attempt to counteract potentially harmful changes. Studies have shown that UPR regulate the expression of lipogenic enzymes through inositol-requiring enzyme 1, an ER stress sensor, and xbox binding protein 1 a transcription factor important in the cellular stress response[21]. Recent data has shown that UPR signalling and ER stress is involved in the regulation of hepatic lipid metabolism and NAFLD development[22].
The transcription factor SREBP-1 regulates the gene expression of lipogenic enzymes. Studies in both HepG2 cells and primary mouse hepatocytes show that UA can increase the cleavage of SREBP-1 into its mature form and cause nuclear translocation. Choi et al[16] have shown an upregulation of SREBP-1 and lipogenic enzymes in these cell models when exposed to UA which subsequently promotes de novo lipogenesis.
From this research it is fairly clear that in hepatocytes exposed to UA there is a pathogenic overlap between oxidative and ER stress. Both mechanisms cause an increase in expression of lipogenic genes and hepatocellular lipogenesis.
Previous research has reported that UA can activate the NOD-like receptor family pyrin domain containing 3 (NLRP3) inflammasome[23]. This is an intracellular multiprotein complex which has the ability to identify pathogenic or harmful molecular signals[8] Activation of NLRP3 has been shown to stimulate caspase-1 to cleave pro-interleukin (IL)-1b and pro-IL-18 into mature forms which causes their release from the cell. Although the specific pathogenic mechanisms underlying UA activation of the NLRP3 inflammasome and related hepatic steatosis are unknown, it is clear from the studies in both mice hepatocytes and cultured cell lines there is an association[24].
Research in mice fed a ‘hyperuricemia-inducing’ diet for eight weeks showed there was a significant increase in hepatic mRNA expression of NLPR3, caspase-1, IL-1b and IL-18. The same mice models had elevated levels of serum IL-1b and IL-18 as well as increased protein expression of IL-1b, IL-18, caspase-1 and NLPR3. Studies have also revealed elevated levels of NLRP3 expression and its components in humans with NASH[25].
To investigate the role of NLRP3 in UA induced steatohepatitis, the same research group knocked out NLRP3 in HepG2 cells[24]. They found that deletion of this inflammasome significantly reduced UA related lipogenesis in this cell line and improved insulin signalling. The researchers therefore suggest that NLRP3 has a role in impaired insulin signalling related to UA[24]. A cautionary note however, NLRP3 signalling is complex and involves a number of direct and indirect canonical pathways with multiple other known stimulatory molecules.
Given a paucity of reliable biomarkers in the diagnosis and prognostication of NAFLD, there has been an increasing interest in UA as a diagnostic utility.
As has been alluded to previously, there is increased evidence of the association of UA as an independent determinant of NAFLD, with some studies demonstrating an association even in lean/non-obese cohorts[26-28]. What is less clear is the utility of urate and its constituent degradation products in the diagnosis or prognostication of NAFLD. There would be particular attraction in incorporation of such a metabolite in to the various existing non-invasive fibrosis algorithms, particularly if it improved specificity of diagnosis.
Recently, a prospective population study in Montenegro sought to establish a diagnostic cut-off value of UA which may predict NAFLD[29]. The authors used the Fatty liver index (FLI) as a proxy reference standard for NAFLD diagnosis, with a score of > 60 established as the cut off. A total of 771 patients from the initial 1,000 screened where deemed suitable for the study. Interestingly, within this populace it was found that ALT was an independent predictor of FLI in both women and men. Contrastingly, SUA was only independently predictive of FLI in women but not in men.
Jensen et al[30] demonstrated in a Japanese cohort that serum UA (SUA) which was elevated at baseline, and those with an incrementing SUA over 5 years were both independent risk factors for developing NAFLD. This finding was substantiated in both normal and borderline liver cohorts, including those young individuals and those with increased body mass index (BMI). Whilst the study had a number of powerful aspects, it is limited by a number of elements including that of being an observational cohort and using an unselected cohort with limited adjustment for concomitant background populace disease. The authors urged caution in interpretation of the findings, since the study was again not designed to determine causality.
A comprehensive, liver-biopsy defined study in Egypt sought to develop a new non-invasive scoring modality for both NAFLD histological severity and cardiovascular morbidity[31], which examined a wide assortment of biochemical markers including SUA. The study ultimately included 61 patients across two centres with NAFLD and metabolic features. They excluded those with hepatotropic viruses, use of confounding medications, excessive alcohol consumption or patients taking insulin. The outcomes demonstrated that SUA did not offer improved diagnostic accuracy with significant fibrosis in NAFLD predicted by nine alternative parameters.
Finally, there is an encouraging study published by Culafic et al[32] whereby they proposed a model to predict NASH from simple steatosis in a NAFLD cohort. The HUFA-model incorporates; HOMA-IR, UA, Ferritin and ALT. Patients were subdivided into two cohorts: Those with simple steatosis and NASH. In total, 82 patients had simple steatosis (33 with a representative liver biopsy) and 29 individuals demonstrated biopsy-proven NASH. There was a significant difference in the SUA between those with simple steatosis and those with biopsy confirmed NASH (SUA 346.2 ± 84.7 µmol/L vs 423.5 ± 83.1 µmol/L, P = 0.001). Further analysis demonstrated that SUA levels correlated with inflammation on a histological basis. Overall, the model demonstrated that in combining all of the representative components, an area under the ROC curve of 0.94 was achieved. This corresponded to a sensitivity and sensitivity of 70.3% and 95.1% respectively for NASH. Similarly, this meant positive and negative predictive values of 83.1% and 90%.
While there are obviously parallels between elevated levels of SUA in patients with features of the metabolic syndrome; there is now significant interest in “lean-NAFLD”. This is traditionally defined as BMI < 25; with variable geographical prevalence. In the United States; studies have suggested a prevalence of 7%-9%[33,34], this is in contrast to reported prevalence rates in Hong-Kong, China (19.3%)[35] and other Asian-Pacific countries and regions with incidence purportedly of 5%-30% depending on the populations studied[36].
A large study by Zheng et al[28] and colleagues analysed 95924 patients from routine clinic appointments in Chongqing, China. The found lean-NAFLD in 7503 participants (8.16%), with 6967 having mild steatosis and 536 with moderate to severe disease. Those patients normal BMI with hyperuricaemia demonstrated an increased preponderance to NAFLD (OR = 1.718; 95%CI: 1.622-1.820) upon correction for confounding metabolic sequelae. The area under curve for identification of mild steatosis based on SUA was 0.70; whilst its utility in the detection of moderate and severe fatty liver was 0.78.
In a separate Chinese cohort[37], 9293 individuals undergoing routine health screens were assessed for evidence of lean-NAFLD using BMI < 24 kg/m2 as the threshold. Seven hundred and forty of those screened had sonographic and laboratory indices in keeping with NAFLD, thus giving an incidence of 18.5%. Again, SUA was independently associated with NAFLD. Across multiple applied regression analysis, SUA was a reproducible risk factor with a univariate OR = 1.435 (P < 0.001). The area under curve of SUA was 0.64 (95%CI: 0.63-0.66; P < 0.001), with the authors suggesting within their cohort that UA > 6 mg/dL had a useful discriminant value.
Despite the large numbers of patients within these studies there are inherent aspects that may attribute relative bias. This includes accuracy of alcohol estimates and recall biases of individuals and alcohol-related fatty liver is the most common mimic of NAFLD. Certainly, Chinese patients have a lower BMI than western counterparts, however, there may be surreptitious metabolic determinants at play, which are not entirely appreciated in the lean populace[38].
Within the Zheng et al[28] cohort, as a retrospective study is limited in only specifically excluding those individuals who had known alternative chronic liver disease, but did not account for those who had potentially undiagnosed hepatotropic viruses or lipid disorders.
Whilst there is some incipient evidence in animal models relating to NASH amelioration in response to UA lowering intervention[14,24,39,40], to date; there are no direct clinical trials establishing these outcomes in humans on NAFLD progression or mortality, and the only recommended intervention is that of 7-10% weight loss as per European Association for the Study of the Liver guidelines[41].
Whilst the evidence reviewed herein demonstrates undeniable co-existence of increased SUA across a continuum of NAFLD cohorts, the evidence for causality remains tenuous. As with a number of NAFLD studies, there is a predominance of observational and retrospective works within the literature, which suffer inherent biases. There is significant heterogeneity in the standards used for exacting NAFLD across such works including use of non-invasive modalities, all of which have relative limitations. This however, is reflective of the challenges faced in real world clinical practice, accurately mirroring the diverse NAFLD continuum and the diagnostic conundrum it poses. This is clearly illustrated by the divergent prevalence of “lean-NAFLD” and its geographical predilections and presents a further confounding entity.
Unfortunately, there is clearly no absolute evidence for incorporating UA in the diagnostic algorithms as they presently exist for large undifferentiated populous screening. Feasibly, large datasets which incorporate specific advanced diagnostic components including Enhanced Liver Fibrosis test or the algorithmic-based intelligent Liver Function Test[42] system may have a place for inclusion of UA in their respective methods.
UA is an attractive biomarker from a number of perspectives including cost basis and almost universal access to reproducible assays. There is perhaps some distinct subtleties’ emerging around the use of UA in defined subgroups, including that of the lean-NAFLD cohorts and in distinguishing mild or simple steatosis from that of moderate to severe variants.
Undoubtedly, efforts to maximise any potential role for UA should be pursued given the enormous challenges that lie ahead in NAFLD diagnosis and prognostication.
Manuscript source: Invited Manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Abenavoli L, Han T, Pellicano R, Tiribelli C, Xu CF S-Editor: Wang YQ L-Editor: A E-Editor: Ma YJ
| 1. | Dyson JK, Anstee QM, McPherson S. Non-alcoholic fatty liver disease: a practical approach to diagnosis and staging. Frontline Gastroenterol. 2014;5:211-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 239] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 2. | Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313:2263-2273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1508] [Cited by in RCA: 1744] [Article Influence: 174.4] [Reference Citation Analysis (0)] |
| 3. | Liu CQ, He CM, Chen N, Wang D, Shi X, Liu Y, Zeng X, Yan B, Liu S, Yang S, Li X, Li X, Li Z. Serum uric acid is independently and linearly associated with risk of nonalcoholic fatty liver disease in obese Chinese adults. Sci Rep. 2016;6:38605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Xu C, Wan X, Xu L, Weng H, Yan M, Miao M, Sun Y, Xu G, Dooley S, Li Y, Yu C. Xanthine oxidase in non-alcoholic fatty liver disease and hyperuricemia: One stone hits two birds. J Hepatol. 2015;62:1412-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 128] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 5. | Hediger MA, Johnson RJ, Miyazaki H, Endou H. Molecular physiology of urate transport. Physiology (Bethesda). 2005;20:125-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 210] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 6. | Cai W, Song JM, Zhang B, Sun YP, Yao H, Zhang YX. The prevalence of nonalcoholic fatty liver disease and relationship with serum uric acid level in Uyghur population. ScientificWorldJournal. 2014;2014:393628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Wu SJ, Zhu GQ, Ye BZ, Kong FQ, Zheng ZX, Zou H, Shi KQ, Lin L, Braddock M, Huang WJ, Chen YP, Zheng MH. Association between sex-specific serum uric acid and non-alcoholic fatty liver disease in Chinese adults: a large population-based study. Medicine (Baltimore). 2015;94:e802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Denoble AE, Huffman KM, Stabler TV, Kelly SJ, Hershfield MS, McDaniel GE, Coleman RE, Kraus VB. Uric acid is a danger signal of increasing risk for osteoarthritis through inflammasome activation. Proc Natl Acad Sci USA. 2011;108:2088-2093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 196] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 9. | Sautin YY, Nakagawa T, Zharikov S, Johnson RJ. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am J Physiol Cell Physiol. 2007;293:C584-C596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 567] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 10. | Abenavoli L, Di Renzo L, Boccuto L, Alwardat N, Gratteri S, De Lorenzo A. Health benefits of Mediterranean diet in nonalcoholic fatty liver disease. Expert Rev Gastroenterol Hepatol. 2018;12:873-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 11. | Ryu S, Chang Y, Kim SG, Cho J, Guallar E. Serum uric acid levels predict incident nonalcoholic fatty liver disease in healthy Korean men. Metabolism. 2011;60:860-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Baldwin W, McRae S, Marek G, Wymer D, Pannu V, Baylis C, Johnson RJ, Sautin YY. Hyperuricemia as a mediator of the proinflammatory endocrine imbalance in the adipose tissue in a murine model of the metabolic syndrome. Diabetes. 2011;60:1258-1269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 285] [Cited by in RCA: 333] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 13. | Mastrocola R, Collino M, Rogazzo M, Medana C, Nigro D, Boccuzzi G, Aragno M. Advanced glycation end products promote hepatosteatosis by interfering with SCAP-SREBP pathway in fructose-drinking mice. Am J Physiol Gastrointest Liver Physiol. 2013;305:G398-G407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Nakatsu Y, Seno Y, Kushiyama A, Sakoda H, Fujishiro M, Katasako A, Mori K, Matsunaga Y, Fukushima T, Kanaoka R, Yamamotoya T, Kamata H, Asano T. The xanthine oxidase inhibitor febuxostat suppresses development of nonalcoholic steatohepatitis in a rodent model. Am J Physiol Gastrointest Liver Physiol. 2015;309:G42-G51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 15. | Zhang J, Xu C, Zhao Y, Chen Y. The significance of serum xanthine oxidoreductase in patients with nonalcoholic fatty liver disease. Clin Lab. 2014;60:1301-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Choi YJ, Shin HS, Choi HS, Park JW, Jo I, Oh ES, Lee KY, Lee BH, Johnson RJ, Kang DH. Uric acid induces fat accumulation via generation of endoplasmic reticulum stress and SREBP-1c activation in hepatocytes. Lab Invest. 2014;94:1114-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 197] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 17. | Pacher P, Nivorozhkin A, Szabó C. Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. Pharmacol Rev. 2006;58:87-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 930] [Cited by in RCA: 841] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 18. | Parks DA, Granger DN. Xanthine oxidase: biochemistry, distribution and physiology. Acta Physiol Scand Suppl. 1986;548:87-99. [PubMed] |
| 19. | Hille R, Massey V. Studies on the oxidative half-reaction of xanthine oxidase. J Biol Chem. 1981;256:9090-9095. [PubMed] |
| 20. | Lanaspa MA, Sanchez-Lozada LG, Choi YJ, Cicerchi C, Kanbay M, Roncal-Jimenez CA, Ishimoto T, Li N, Marek G, Duranay M, Schreiner G, Rodriguez-Iturbe B, Nakagawa T, Kang DH, Sautin YY, Johnson RJ. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: potential role in fructose-dependent and -independent fatty liver. J Biol Chem. 2012;287:40732-40744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 544] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 21. | Lee AH, Scapa EF, Cohen DE, Glimcher LH. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 2008;320:1492-1496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 786] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 22. | Pagliassotti MJ. Endoplasmic reticulum stress in nonalcoholic fatty liver disease. Annu Rev Nutr. 2012;32:17-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 184] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 23. | Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1530] [Cited by in RCA: 1778] [Article Influence: 136.8] [Reference Citation Analysis (1)] |
| 24. | Wan X, Xu C, Lin Y, Lu C, Li D, Sang J, He H, Liu X, Li Y, Yu C. Uric acid regulates hepatic steatosis and insulin resistance through the NLRP3 inflammasome-dependent mechanism. J Hepatol. 2016;64:925-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 252] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 25. | Wree A, McGeough MD, Peña CA, Schlattjan M, Li H, Inzaugarat ME, Messer K, Canbay A, Hoffman HM, Feldstein AE. NLRP3 inflammasome activation is required for fibrosis development in NAFLD. J Mol Med (Berl). 2014;92:1069-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 400] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 26. | Eshraghian A, Nikeghbalian S, Geramizadeh B, Kazemi K, Shamsaeefar A, Malek-Hosseini SA. Characterization of biopsy proven non-alcoholic fatty liver disease in healthy non-obese and lean population of living liver donors: The impact of uric acid. Clin Res Hepatol Gastroenterol. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | Oral A, Sahin T, Turker F, Kocak E. Relationship Between Serum Uric Acid Levels and Nonalcoholic Fatty Liver Disease in Non-Obese Patients. Medicina (Kaunas). 2019;55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 28. | Zheng X, Gong L, Luo R, Chen H, Peng B, Ren W, Wang Y. Serum uric acid and non-alcoholic fatty liver disease in non-obesity Chinese adults. Lipids Health Dis. 2017;16:202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 29. | Klisic A, Kavaric N, Ninic A. Predictive Values of Serum Uric Acid and Alanine-aminotransferase for Fatty Liver Index in Montenegrin Population. J Med Biochem. 2019;38:407-417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Jensen T, Niwa K, Hisatome I, Kanbay M, Andres-Hernando A, Roncal-Jimenez CA, Sato Y, Garcia G, Ohno M, Lanaspa MA, Johnson RJ, Kuwabara M. Increased Serum Uric Acid over five years is a Risk Factor for Developing Fatty Liver. Sci Rep. 2018;8:11735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 31. | Hanafy AS, Seleem WM, El-Kalla F, AbdAlkhalik Basha M, Abd-Elsalam S. Efficacy of a non-invasive model in predicting the cardiovascular morbidity and histological severity in non-alcoholic fatty liver disease. Diabetes Metab Syndr. 2019;13:2272-2278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 32. | Culafic M, Vezmar Kovacevic S, Dopsaj V, Stulic M, Vlaisavljevic Z, Miljkovic B, Culafic D. A Simple Index for Nonalcoholic Steatohepatitis-HUFA-Based on Routinely Performed Blood Tests. Medicina (Kaunas). 2019;55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Younossi ZM, Stepanova M, Negro F, Hallaji S, Younossi Y, Lam B, Srishord M. Nonalcoholic fatty liver disease in lean individuals in the United States. Medicine (Baltimore). 2012;91:319-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 399] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 34. | Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2633] [Cited by in RCA: 2691] [Article Influence: 128.1] [Reference Citation Analysis (3)] |
| 35. | Wei JL, Leung JC, Loong TC, Wong GL, Yeung DK, Chan RS, Chan HL, Chim AM, Woo J, Chu WC, Wong VW. Prevalence and Severity of Nonalcoholic Fatty Liver Disease in Non-Obese Patients: A Population Study Using Proton-Magnetic Resonance Spectroscopy. Am J Gastroenterol. 2015;110:1306-14; quiz 1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 241] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 36. | Amarapurkar DN, Hashimoto E, Lesmana LA, Sollano JD, Chen PJ, Goh KL, Asia-Pacific Working Party on NAFLD. How common is non-alcoholic fatty liver disease in the Asia-Pacific region and are there local differences? J Gastroenterol Hepatol. 2007;22:788-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 292] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 37. | Hsu CL, Wu FZ, Lin KH, Chen YH, Wu PC, Chen YH, Chen CS, Wang WH, Mar GY, Yu HC. Role of Fatty Liver Index and Metabolic Factors in the Prediction of Nonalcoholic Fatty Liver Disease in a Lean Population Receiving Health Checkup. Clin Transl Gastroenterol. 2019;10:1-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 38. | Kim HJ, Kim HJ, Lee KE, Kim DJ, Kim SK, Ahn CW, Lim SK, Kim KR, Lee HC, Huh KB, Cha BS. Metabolic significance of nonalcoholic fatty liver disease in nonobese, nondiabetic adults. Arch Intern Med. 2004;164:2169-2175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 321] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 39. | Nishikawa T, Nagata N, Shimakami T, Shirakura T, Matsui C, Ni Y, Zhuge F, Xu L, Chen G, Nagashimada M, Yamashita T, Sakai Y, Yamashita T, Mizukoshi E, Honda M, Kaneko S, Ota T. Xanthine oxidase inhibition attenuates insulin resistance and diet-induced steatohepatitis in mice. Sci Rep. 2020;10:815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 40. | Chen S, Chen D, Yang H, Wang X, Wang J, Xu C. Uric acid induced hepatocytes lipid accumulation through regulation of miR-149-5p/FGF21 axis. BMC Gastroenterol. 2020;20:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 41. | European Association for the Study of the Liver (EASL). European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 3162] [Article Influence: 351.3] [Reference Citation Analysis (4)] |
| 42. | Miller MH, Fraser A, Leggett G, MacGilchrist A, Gibson G, Orr J, Forrest EH, Dow E, Bartlett W, Weatherburn C, Laurell A, Grant K, Scott K, Neville R, Dillon JF. Development and validation of diagnostic triage criteria for liver disease from a minimum data set enabling the 'intelligent LFT' pathway for the automated assessment of deranged liver enzymes. Frontline Gastroenterol. 2018;9:175-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |